Significance
One of the first crucial steps of animal development is to distinguish the anterior versus the posterior pole of the embryo, i.e., the AP axis. If this process fails, embryos may develop two mirror image tails or heads. In the fly Drosophila, the mother provides the signals required for AP axis formation, while in vertebrates, gene activity of the embryo is required as well. We identified two genes whose knockdown leads to double-tail phenotypes in the beetle Tribolium, representing the insect-typical short-germ embryogenesis. Intriguingly, embryo polarity depends on zygotic gene activities and Wnt signaling. Hence, short-germ insect axis formation is more similar to vertebrates than the mechanism employed by Drosophila.
Keywords: axis formation, germ cell-less, homeobrain, Torso signaling, short-germ segmentation
Abstract
The distinction of anterior versus posterior is a crucial first step in animal embryogenesis. In the fly Drosophila, this axis is established by morphogenetic gradients contributed by the mother that regulate zygotic target genes. This principle has been considered to hold true for insects in general but is fundamentally different from vertebrates, where zygotic genes and Wnt signaling are required. We investigated symmetry breaking in the beetle Tribolium castaneum, which among insects represents the more ancestral short-germ embryogenesis. We found that maternal Tc-germ cell-less is required for anterior localization of maternal Tc-axin, which represses Wnt signaling and promotes expression of anterior zygotic genes. Both RNAi targeting Tc-germ cell-less or double RNAi knocking down the zygotic genes Tc-homeobrain and Tc-zen1 led to the formation of a second growth zone at the anterior, which resulted in double-abdomen phenotypes. Conversely, interfering with two posterior factors, Tc-caudal and Wnt, caused double-anterior phenotypes. These findings reveal that maternal and zygotic mechanisms, including Wnt signaling, are required for establishing embryo polarity and induce the segmentation clock in a short-germ insect.
One of the most important first steps in animal embryogenesis is the establishment of anterior–posterior polarity (AP polarity), distinguishing head from tail. If this process fails, mirror image duplications of embryonic structures may develop like the “double-abdomen” embryos induced by classical manipulations of insect embryos by ligation or UV-irradiation experiments (1–3). Based on these types of experiments, autonomous patterning mechanisms were proposed for the insect egg (4).
In the fly Drosophila melanogaster, mRNA of the bicoid gene is localized to the anterior pole from where the Bicoid protein diffuses to form an anterior-to-posterior gradient. An opposing gradient of Nanos protein forms from posterior to regulate Hunchback translation, which thus forms another anterior gradient. In addition, the graded activity of the Torso pathway directs patterning near both poles of the Drosophila embryo. Together, these maternal morphogenetic gradients provide positional information and initiate zygotic segmentation gene expression in a concentration-dependent manner (5, 6). Mutations in bicoid result in duplication of some posterior structures at the anterior, while symmetric larvae with “double abdomen” form after double inactivation of both Bicoid and Hunchback (7) and a number of other genotypes (8, 9) where bicoid and nanos localization is impeded. In this system, zygotic genes interpret the maternal information but are not involved in axis formation themselves. However, axis formation in Drosophila is derived and bicoid is not present outside higher dipterans (10). In the lower dipteran Chironomus, another long-germ fly, axis formation relies on maternal anterior localization of panish, a putative repressor related to the Wnt component pangolin. Knockdown of panish leads to double abdomina, while knockdown of the zygotic gene tailless leads to double heads, revealing an alternative genetic mechanism for axis formation (11). In the wasp Nasonia, a more distantly related long-germ insect, orthodenticle (otd) provides maternal morphogenetic gradients at both poles. giant, which is maternally localized at the anterior pole, substitutes for the permissive role of bicoid at the anterior (12, 13), but axis duplication phenotypes have not been described.
However, all these long-germ embryos lack the predicted autonomous posterior patterning mechanism. In contrast, typical short-germ insect embryos form posterior segments sequentially from a “growth zone” or segment addition zone (SAZ), which is specified at the posterior pole of the blastoderm. Further, in short-germ insects, extraembryonic tissue is formed at the anterior blastoderm, rather than head structures as in Drosophila. The red flour beetle Tribolium is a model for short-germ embryogenesis, where the formation of abdominal segments requires Tc-caudal expression as well as Wnt and Torso signaling at the posterior pole. Torso signaling appears to be the only conserved maternal signal. It had been suggested that Tc-otd and Tc-hunchback substitute for the anterior morphogen in the beetle Tribolium (12, 14). However, more recent data do not support a morphogenetic role of these genes in anterior patterning (15, 16). Instead, anterior localization of maternal Tc-axin mRNA represses Wnt signaling, providing a permissive environment for anterior development, as in vertebrate embryos (17–20). Ubiquitously distributed Tc-caudal mRNA is translationally repressed by anterior Tc-Mex-3 and Tc-Zen2, but their knockdown does not lead to double-abdomen phenotypes (21). In summary, the mechanism of symmetry breaking and subsequent AP-patterning in short-germ embryos is predicted to be different but has remained obscure. Notably, genetically induced mirror image phenotypes have not been observed so far. Likewise, the predicted autonomy of SAZ patterning has not been demonstrated.
Results
Unexpected Genes in AP Axis Formation.
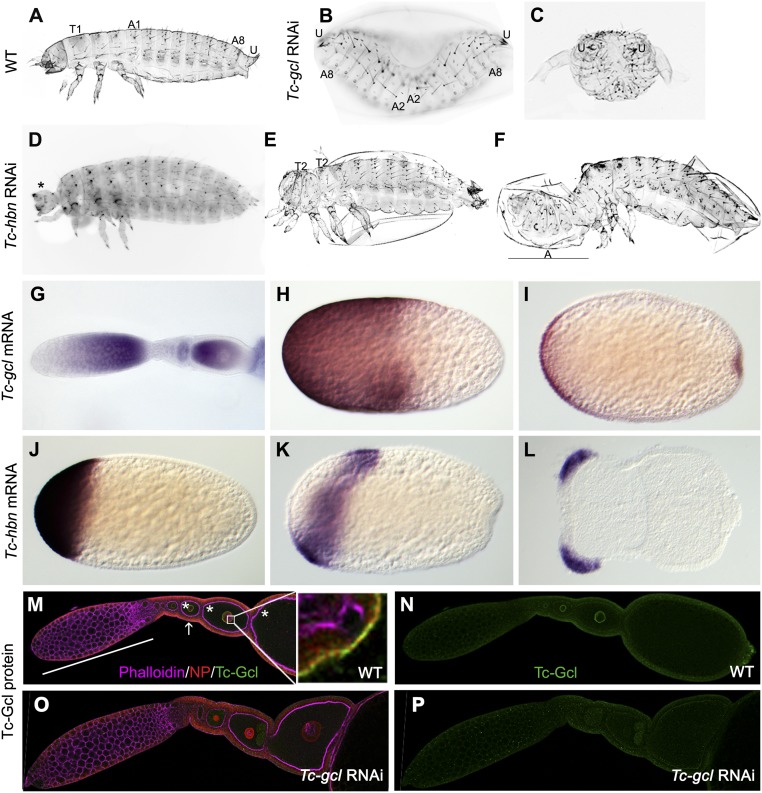
In an ongoing genome-wide RNAi screen we searched for cuticle phenotypes affecting the anterior–posterior body axis (22, 23). We found two genes whose knockdown led to double-abdomen phenotypes. One of these dsRNAs, iB_02693, targeted the ortholog of the Drosophila germ cell-less gene (gcl), which in the fly is required at the posterior pole for germ cell development. It encodes a BTB domain protein localized at the nuclear lamina in both Drosophila and vertebrates, where it appears to be involved in transcriptional regulation (24–26) or cytoskeleton organization (27, 28). Knockdown of Tribolium germ cell-less (Tc-gcl) results in double-abdomen phenotypes with high penetrance (>95%), where head and thorax are replaced by a mirror image abdomen (Fig. 1 B and C and SI Appendix, Fig. S1). Interestingly, in some specimens the total number of segments of these two abdomina (2 × 10 abdominal segments) exceeds the normal number of segmentation-clock–derived segments (16 segments), demonstrating the autonomy of the duplicated SAZ. Some segmentation and dorsal closure defects occur as well.
Fig. 1.
Tc-gcl and Tc-hbn RNAi phenotypes and expression. (A) Wild-type first-instar larval cuticle with thoracic (T) and abdominal (A) segments and urogomphi (U). (B and C) Tc-gcl RNAi larvae lacking the thorax but displaying mirror image abdomina. (D–F) Tc-hbn RNAi phenotypes range from loss of the labrum and antennae (asterisk in D) to specimen with partially duplicated thorax (E) and incomplete double abdomen (F). (G–I) Tc-gcl mRNA is found in nurse cells and in the anterior of developing oocytes (G) and in the anterior half of freshly laid eggs (H). In differentiated blastoderm embryos, expression is found at the anterior and posterior poles (I). (J–L) Zygotic Tc-hbn mRNA is expressed in an anterior cap (J) but later retracts from the pole (K) to form domains in the head lobes of germ rudiments (L). (M and N) Tc-Gcl protein distribution in ovaries. Tc-Gcl (green signal; single channel shown in N) is found at the nuclear envelope, which is marked by a nuclear pore protein (red signal; see Inset for blow-up). White bar, nurse cells; stars, oocytes; white arrow, follicle cells. (O and P) After Tc-gcl RNAi, the nuclear staining is reduced (single channel shown in P). Anterior is to the Left in A–L; A–F are not in the same scale.
We found maternal Tc-gcl transcripts to be localized to the anterior half of the developing oocyte and the freshly laid egg (Fig. 1 G–I). We generated an antibody detecting Tc-Gcl. As in Drosophila, we found the protein at the nuclear envelope, which was marked with a nuclear pore protein (Fig. 1 M and N). Later, a posterior domain with similarity to Tc-vasa expression developed de novo (Fig. 1I and SI Appendix, Fig. S2 C, G, and H). However, neither dsRNA injected into embryos, nor into L5 larvae, resulted in strongly reduced egg production, suggesting that in Tribolium, gcl may be dispensable for germ cell development. dsRNA injection into early embryos did not lead to lethality or cuticle phenotypes, indicating a predominantly maternal function of Tc-gcl.
The second dsRNA (iB_04564) causing double abdomina targeted the ortholog of homeobrain, which is a homeodomain transcription factor expressed in the head and brain primordium of Drosophila, but for which a function has not been described (29). Knockdown of Tribolium homeobrain (Tc-hbn) resulted in anterior deletions or double-abdomen phenotypes (Fig. 1 D–F). Both penetrance and expressivity of double abdomina were lower compared with Tc-gcl knockdown (SI Appendix, Fig. S1 O and P), and thoracic segments remained present even in the strongest phenotypes (SI Appendix, Fig. S1). Tc-hbn is expressed as one of the earliest zygotic genes in an anterior cap of the blastoderm, which later retracts from the pole and eventually forms bilateral domains in the head anlagen (Fig. 1 J–L and SI Appendix, Fig. S2). Both phenotypes were reproduced by RNAi using nonoverlapping dsRNA fragments in the same genetic background. It was unexpected to find axis duplication upon knockdown of a zygotic gene since the current insect paradigm holds that maternal morphogens establish the axes, while zygotic genes then interpret them.
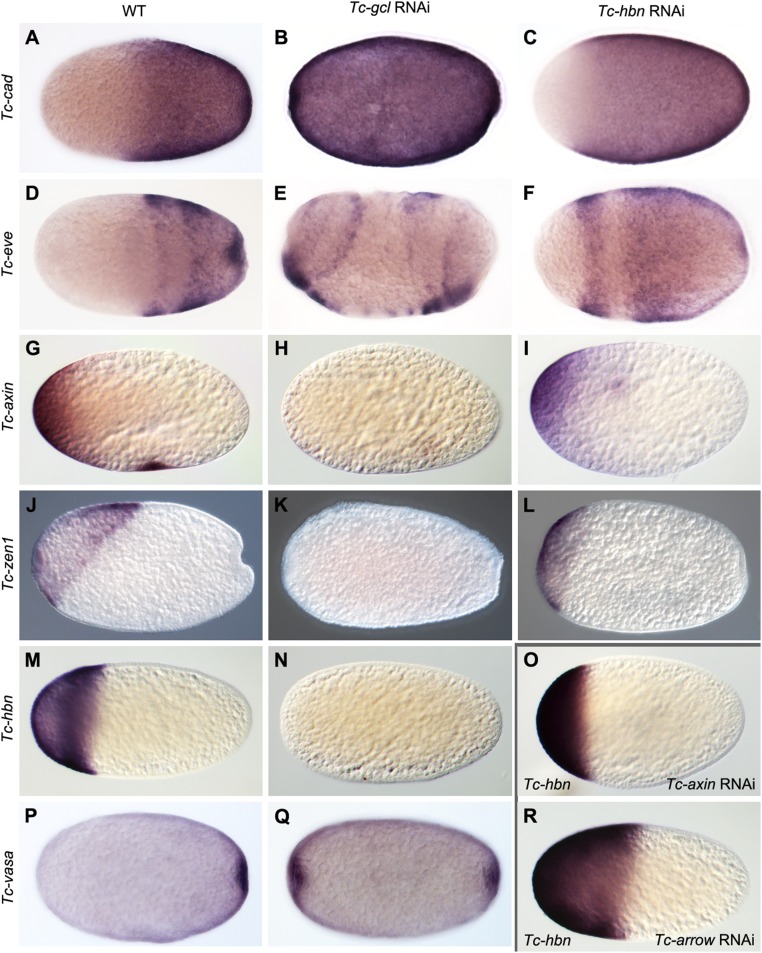
To characterize the mechanism of axis duplication we scrutinized the expression of SAZ markers in RNAi embryos (Fig. 2). Tc-gcl RNAi induced a mirror image duplication of Tc-even-skipped (Tc-eve) and Tc-vasa expression from early blastoderm stages onward, while Tc-caudal (Tc-cad) was expressed ubiquitously (Fig. 2 B, E, and Q). Early Tc-wingless (Tc-wg) expression in the SAZ was duplicated as well (SI Appendix, Fig. S3 A and B), while its segmental pattern prefigured the duplication seen in the cuticles (SI Appendix, Fig. S3). In Tc-hbn knockdown embryos, in contrast, the anterior expression boundary of these genes was only shifted anteriorly during blastoderm stages (Fig. 2 C and F), while Tc-vasa expression was not altered. Later, in germ band embryos, duplicated expression domains of Tc-cad and Tc-wg arose in the head, which developed into a mirror image SAZ (SI Appendix, Fig. S4 A–I).
Fig. 2.
Change of embryo fate map after Tc-gcl and Tc-hbn RNAi. (A–C) Tc-cad mRNA is expressed in the posterior half of WT blastoderm embryos (A) but is ubiquitously distributed after Tc-gcl RNAi (B). It is shifted anteriorly after Tc-hbn RNAi (C). (D–F) Striped Tc-eve mRNA expression at late WT blastoderm embryos (D) is duplicated after Tc-gcl RNAi (E) but shifted anteriorly in Tc-hbn RNAi (F). (G–I) Maternal Tc-axin mRNA at the anterior pole (G) is lost after Tc-gcl RNAi (H) but not affected after Tc-hbn RNAi (I). (J–L) Tc-zen1 expression in the serosa (J) is lost after Tc-gcl RNAi (K) and reduced upon Tc-hbn RNAi (L). (M –O and R) Tc-hbn expression at the anterior pole (M) is absent after Tc-gcl RNAi (N). In Tc-axin RNAi (Wnt derepression) the boundary is shifted toward anterior (O), while it is shifted posteriorly after Tc-arrow RNAi (Wnt repression; R). (P and Q) Tc- vasa mRNA expression marking the posterior pit in WT (P) is duplicated after Tc-gcl RNAi (Q).
Time and location of ectopic SAZ formation in Tc-gcl knockdown embryos corresponds to a region with active Torso signaling, in line with the notion that Torso signaling is required for SAZ formation (20, 30). The fact that a functional SAZ can form at both poles of the blastoderm as well as later in the head of postblastodermal embryos, strongly supports the idea of autonomy of SAZ patterning; i.e., the SAZ has self-organizing capabilities.
Tc-Gcl Acts Upstream in Anterior Patterning.
Next, we sought to establish the genetic interactions involved in ectopic SAZ formation. The maternal expression of the anteriorly localized morphogen Tc-axin (18), a negative regulator of Wnt signaling, was strongly reduced or absent upon Tc-gcl knockdown (Fig. 2 G and H). This was not a general effect on maternally localized mRNAs because Tc-pangolin (Tc-pan) remained properly localized at the anterior pole. Both the expressions of Tc-zen1, a marker for extraembryonic serosa (Fig. 2 J and K), and of Tc-hbn were abolished (Fig. 2 M and N). Conversely, Tc-gcl expression was not altered in Tc-axin, Tc-pan, or Tc-hbn knockdown. These results place Tc-gcl upstream of both maternally localized Tc-axin and of the earliest known zygotic anterior markers, Tc-zen1 and Tc-hbn.
The lack of anteriorly localized Tc-axin could be due to reduction of transcription in the nurse cells, reduced transport of Tc-axin mRNA to the oocyte, or due to lack of anterior localization within the oocyte. To distinguish between these possibilities, we performed in situ hybridization in wild-type and Tc-gcl RNAi ovaries. We found that in RNAi ovaries, the signal in oocytes was reduced compared with the one in nurse cells (Fig. 3 J–M). qPCR confirmed that the Tc-axin message was significantly reduced 3.5-fold in both freshly laid eggs and ovaries (which included late oocytes; SI Appendix, Fig. S6). Hence, Tc-Gcl appears to act in transport either by a nontranscriptional role in cytoskeletal regulation or by transcriptional regulation of another factor required for mRNA transport (27, 28).
Fig. 3.
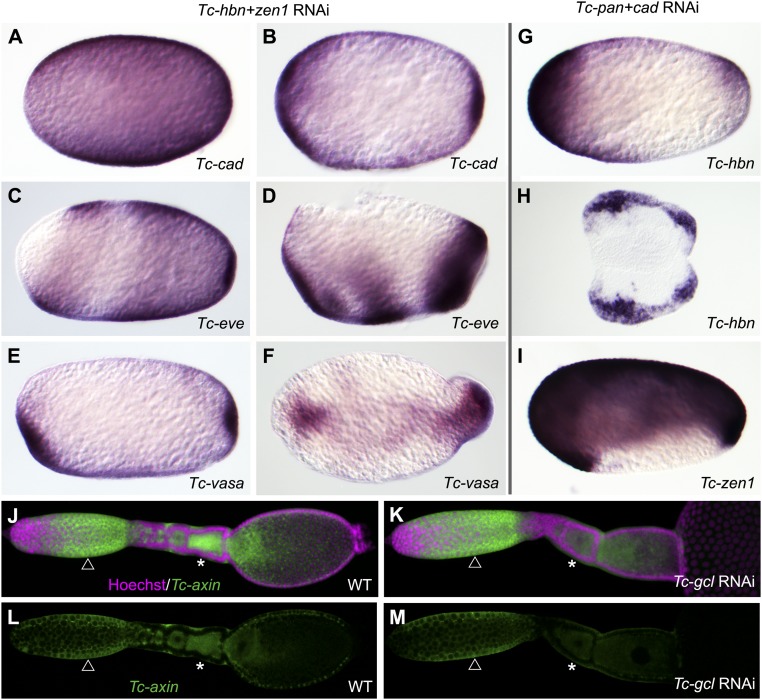
Zygotic genes required for anterior and posterior structures. (A–F) Tc-hbn+Tc-zen1 double RNAi leads to mirror image expression of SAZ markers like Tc-cad (A and B), Tc-eve (C and D), and Tc-vasa (E and F). (G–I) Upon Tc-pan+Tc-cad double RNAi, anterior markers become expressed at the posterior pole. Tc-hbn mRNA develops an additional posterior domain (G) and marks duplicated posterior head lobes in germ rudiments (H). Tc-zen1 expression covers both poles and dorsal tissue (I). (J–M) In Tc-gcl RNAi ovaries, Tc-axin mRNA remains expressed in nurse cells (compare arrow in J and L with K and M) but is reduced in oocytes (compare stars in J and L with K and M). This indicates that Tc-axin transport is affected in Tc-gcl RNAi. Respective WT embryos are shown in Fig. 1.
We wondered if the gcl phenotype can be phenocopied through direct interference with early Wnt signaling. Knocking down the Wnt pathway components Tc-axin, Tc-arrow, or Tc-pan did not produce double abdomina; using molecular markers to probe early embryos, we found that the expression boundaries of Tc-hbn and Tc-zen1 were shifted posteriorly in Wnt-low and anteriorly in Wnt-high situations (SI Appendix, Fig. S5). However, none of the treatments completely abolished their expression. Further, the shift of Tc-hbn was small in early blastoderm embryos but became prominent subsequently (SI Appendix, Fig. S5 B–D and T–V). Hence, interference with early Wnt signaling alone did not phenocopy the Tc-gcl phenotype in our hands, while recently, double-abdomen phenotypes have been described upon Tc-axin RNAi (31).
We next tested whether Tc-axin might cooperate with some other gcl-dependent factor to repress anterior SAZ formation. However, double-RNAi treatments of Tc-axin combined with either Tc-zen1, Tc-zen2, Tc-hbn, Tc-tll, or Tc-pan also did not produce double abdomina. Only in very few Tc-axin/Tc-zen1 double-RNAi embryos did we observe a small ectopic Tc-cad domain in the head anlagen of posterior pit-stage embryos (SI Appendix, Fig. S4P). Likewise, double knockdown of Tc-pan with either Tc-zen1 or Tc-hbn did not lead to double abdomen on the cuticle level.
Drosophila gcl dampens Torso signaling by targeting the receptor for degradation (32). Hence, we tested for a similar role in anterior patterning in Tribolium. In wild-type embryos, activated MAPK emerges first at the anterior pole and remains more active than at the posterior pole throughout the blastoderm, suggesting that anterior Torso signaling is not negatively affected by anteriorly localized Tc-Gcl in the embryo. Subsequently, the anterior domain expands and retracts into a subterminal ring, while the activity directly at the anterior pole becomes weaker compared with the posterior domain (SI Appendix, Fig. S7). This late effect might reflect dampening of anterior Torso signaling by accumulating Tc-Gcl protein translated from maternally contributed Tc-gcl mRNA. In Tc-gcl RNAi embryos, the early pattern of activated MAPK is comparable to wild type, while from blastoderm stages onward, symmetric MAPK activity reflects the mirror image symmetry of the embryo (SI Appendix, Fig. S7).
Zygotic Genes Contribute to Axis Formation.
It was unexpected that RNAi targeting zygotic Tc-hbn resulted in axis duplication. However, this phenotype was weaker and less penetrant than the one of maternal Tc-gcl, suggesting Tc-hbn might be only one of several zygotic effectors. Indeed, combining Tc-hbn with Tc-zen1 (but not Tc-zen2) resulted in a substantially more penetrant and stronger double-abdomen phenotype (SI Appendix, Fig. S1Q), including some additional segmentation and dorsal closure defects similar to Tc-gcl RNAi (SI Appendix, Fig. S4 Q and R). Moreover, we observed near-homogeneous expression of Tc-cad and an increased anterior shift of Tc-eve expression in blastoderm embryos (Fig. 3 A–D), while Tc-vasa displayed mirror image expression at both poles (Fig. 3 E and F). Further, we found mutual activation of Tc-hbn and Tc-zen1 RNAi (Fig. 2L and SI Appendix, Fig. S4 S–U). In summary, the zygotic genes Tc-hbn and Tc-zen1 act synergistically in suppressing an anterior SAZ, thereby preventing axis duplication.
We wondered whether zygotic genes might likewise be involved in specifying the posterior pole. In Tribolium, both posterior Tc-cad expression and Wnt signaling are required for SAZ formation (17, 21, 33). Hence, we hypothesized that Tc-cad activity at the posterior pole cooperates with Wnt signaling to repress anterior patterning. Indeed, in the double knockdown of Tc-cad and Tc-pan, we found Tc-hbn and Tc-zen1 to become ectopically expressed at the posterior pole of the blastoderm, indicating the ectopic specification of anterior tissues (Fig. 3 G and I). Such ectopic expression was not found in Tc-pan single knockdown (SI Appendix, Fig. S5), while Tc-cad single RNAi did not lead to the formation of extraembryonic tissue at the posterior pole (34). In germ-rudiment embryos, both Tc-hbn expression and embryo morphology indicated that a second head anlage had developed at the posterior (Fig. 3H and SI Appendix, Fig. S4 J–O; these embryos did not produce cuticles).
Discussion
A Model for Axis Formation in Short-Germ Embryos.
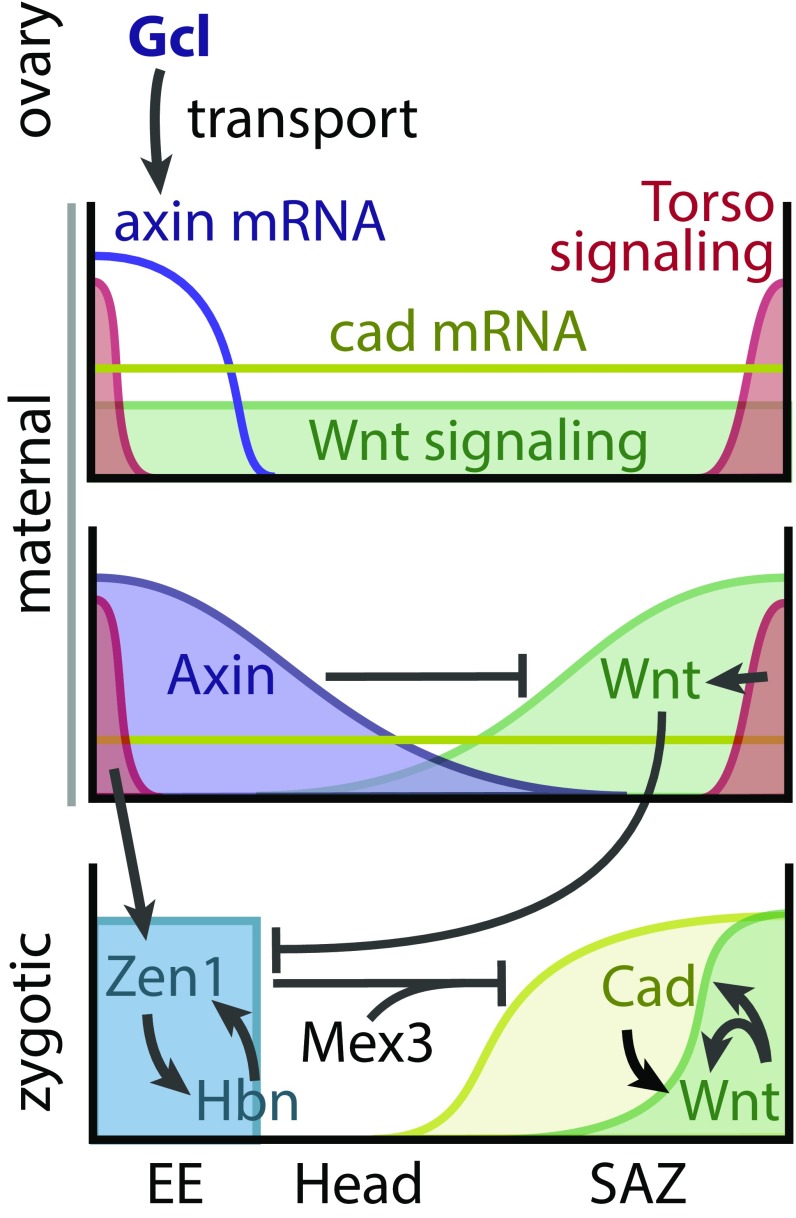
In the freshly laid egg (Fig. 4, Top), maternally contributed Tc-cad mRNA and Tc–β-Catenin protein are initially evenly distributed (35). Torso signaling is active at both poles, being required for the formation of the serosa at the anterior and the SAZ at the posterior pole (20, 30, 36). Symmetry breaking is initiated by maternal Tc-Gcl, which is required to establish Tc-axin mRNA at the anterior pole. There, Tc-Axin protein leads to degradation of Tc–β-Catenin, resulting in repression of canonical Wnt signaling at the anterior pole (Fig. 4, Middle and SI Appendix, Fig. S8). In the absence of Wnt signaling, Torso signaling contributes to Tc-zen1 expression (36) and allows for expression of zygotic Tc-hbn at the anterior pole (Fig. 4, Bottom). Once activated, Tc-hbn and Tc-zen1 form a zygotic feedback loop, which further promotes anterior fates by repressing Tc-cad. Two factors repress translation of maternal Tc-cad mRNA: Tc-zen2, which is activated by Tc-zen1, and Tc-mex3 (21, 37). Later, Tc-zen1 is required for specifying the extraembryonic serosa. Hence, our data indicate that Tc-gcl acts predominantly via the Wnt pathway, although we cannot exclude additional functions. At the posterior pole, Wnt and Torso signaling together with maternal Tc-cad initiate a feedback loop between Wnt signaling and Tc-cad to form a signaling center that specifies the SAZ (Fig. 4, Bottom and SI Appendix, Fig. S8). Indeed, Wnt activates Tc-cad (38) and Tc-wg (38) expression in Tribolium, but it remains unclear whether Tc-cad activates the expression of Wnt ligands like it does in spiders (39) but not in Gryllus (40).
Fig. 4.
Model for axis formation Tribolium castaneum. See text for details. EE, extraembryonic tissue; SAZ, segment addition zone.
This model explains the phenotypes we observe: Removing localized Tc-axin by Tc-gcl RNAi results in derepression of Wnt signaling and Tc-cad translation at the anterior, which together with Torso signaling initiate a SAZ at the anterior pole like they do at the posterior pole (33, 36, 38, 41). In this model, all three components are required for specifying the SAZ, while Wnt signaling and Tc-cad are sufficient to suppress the expression of Tc-hbn and Tc-zen1 in the posterior Torso domain.
Zygotic Regulation Instead of Maternal Gradients.
In the long-germ embryos of both Drosophila and Nasonia, maternal morphogenetic gradients of transcription factors regulate zygotic genes in a concentration-dependent manner (6, 12, 42). Zygotic genes are not involved in axis specification, and respective mutants lead to loss of body regions but not to axis duplications. Our data suggest that this paradigm does not hold true for short-germ insects. Here, initial asymmetry provided by maternal signals needs to be reinforced by two zygotic signaling centers located at both poles, respectively. Hence, in contrast to Drosophila, knockdown of zygotic genes (zen1 and hbn) does lead to posterior axis duplications. Wnt and cad probably form another zygotic feedback loop required for maintaining the growth zone. A more central role of zygotic control in AP axis formation is corroborated by results in the dipteran Chironomus, where knockdown of the zygotic gene tailless is sufficient to produce double heads (11). Likewise, dorsal-ventral axis formation is based on zygotic self-regulatory circuits in Tribolium and on a self-organizing gene network in the hemimetabolan insect Oncopeltus, contrasting the hierarchical maternal control in Drosophila (43).
Taken together, these insights indicate that insect and vertebrate axis formation have more in common than had been assumed based on the Drosophila system. First, initial symmetry breaking based on maternal molecules leads to zygotic interactions, which are required to establish the axes (44–46). Second, the repression of anterior Wnt signaling is required in the vertebrate anterior neural plate (47, 48), the anterior of ancestral animal embryos (49) and Tribolium (18), but not in Drosophila. It is tempting to speculate that the same is true for Chironomus, where the anterior determinant has similarity to a Wnt pathway component (11). Intriguingly, Tribolium uses a Drosophila-like mechanism (anterior localization of maternal mRNAs) to realize a vertebrate-like situation (i.e., anterior repression of Wnt signaling).
Materials and Methods
RNAi.
Initial validation was performed in the same genetic background as in the iBeetle screen (23). All further experiments were done in the pig19 strain (50). dsRNA was synthesized with the Ambion T7 MEGAscript kit (Life Technologies), and pupae, larvae and embryos were injected as described previously (51, 52) using FemtoJet (Eppendorf). Off-target effects for Tc-gcl and Tc-hbn were tested by nonoverlapping fragments (SI Appendix, Fig. S1). dsRNA concentrations are listed in SI Appendix, Table S2.
Generation of a Tc-Gcl Polyclonal Antibody.
The N terminus (amino acids 1–57) was cloned into pET SUMO vector, generating a fusion with a His-SUMO tag and expressed in BL21-DE3 Rosetta cells. Antibodies were produced and affinity purified in guinea pigs by Eurogentec.
Ovary WMISH and Antibody Staining.
Embryonic and ovary WMISH were performed as described previously (38, 53, 54). Phospho-p44/42 MAPK Rabbit mAb (Cell Signaling Technology, Inc.) with 1:1,000 dilution was used to analyze the Torso signaling during the early embryogenesis. Images were acquired with a Zeiss Axioplan 2 LSM510.
Real-Time qPCR.
Total RNAi was extracted from 0- to 4-h-old RNAi and WT embryos and RNAi and WT ovaries using TRIzol (Ambion) (55). One microgram for embryos and 500 ng for ovaries was used for the first strand cDNA synthesis (SuperScript III; Thermo Scientific). qRT PCR was performed in CFX96 Real-Time PCR System (Bio-Rad Laboratories) with HOT FIREpol EvaGreen qPCR Mix Plus (ROX) (Solis BioDyne). Three biological and three technical replicates and no-RT and no template control were performed. Relative fold-change expression between WT and RNAi tissues was calculated by double-delta Ct method with RPS18.3 as ref. 56. Significance was tested with the Welch Two Sample t test.
See SI Appendix for more details on the methods used.
Supplementary Material
Acknowledgments
We thank Claudia Hinners and Elke Küster for technical assistance, Mathias Teuscher and Michael Schoppmeier for help with ovary in situs, and Laurin Tomasek for preliminary work on the hbn/zen1 synergism. We thank Achim Dickmanns and Max Farnworth for help with expressing the protein for antibody production, using the expression system provided by Christopher D. Lima, Cornell University. We thank Deutsche Forschungsgemeinschaft (DFG) for funding the iBeetle RNAi screen (FOR1234) and this analysis (Grants KL 656/7-1; BU1443/8-2 and BU1443/11).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716512115/-/DCSupplemental.
References
- 1.Yajima H. Studies on embryonic determination of the harlequin-fly, Chironomus dorsalis. I. Effects of centrifugation and of its combination with constriction and puncturing. J Embryol Exp Morphol. 1960;8:198–215. [PubMed] [Google Scholar]
- 2.Sander K. [Reversal of the germ band polarity in egg fragments of Euscelis (Cicadina)] Experientia. 1961;17:179–180. doi: 10.1007/BF02160369. [DOI] [PubMed] [Google Scholar]
- 3.Kalthoff K, Sander K. Der Entwicklungsgang der Mi\s sbildung „Doppelabdomen” im partiell UV-bestrahlten Ei von Smittia parthenogenetica (Dipt., Chironomidae) Dev Genes Evol. 1968;161:129–146. doi: 10.1007/BF00585968. [DOI] [PubMed] [Google Scholar]
- 4.Meinhardt H. A model of pattern formation in insect embryogenesis. J Cell Sci. 1977;23:177–39. [PubMed] [Google Scholar]
- 5.Nüsslein-Volhard C, Frohnhöfer HG, Lehmann R. Determination of anteroposterior polarity in Drosophila. Science. 1987;238:1675–1681. doi: 10.1126/science.3686007. [DOI] [PubMed] [Google Scholar]
- 6.Driever W, Nüsslein-Volhard C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell. 1988;54:95–104. doi: 10.1016/0092-8674(88)90183-3. [DOI] [PubMed] [Google Scholar]
- 7.Hülskamp M, Pfeifle C, Tautz D. A morphogenetic gradient of hunchback protein organizes the expression of the gap genes Krüppel and knirps in the early Drosophila embryo. Nature. 1990;346:577–580. doi: 10.1038/346577a0. [DOI] [PubMed] [Google Scholar]
- 8.Nüsslein-Volhard C. Genetic analysis of pattern-formation in the embryo of Drosophila melanogaster. Dev Genes Evol. 1977;183:249–268. doi: 10.1007/BF00867325. [DOI] [PubMed] [Google Scholar]
- 9.Mohler J, Wieschaus EF. Dominant maternal-effect mutations of Drosophila melanogaster causing the production of double-abdomen embryos. Genetics. 1986;112:803–822. doi: 10.1093/genetics/112.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stauber M, Jäckle H, Schmidt-Ott U. The anterior determinant bicoid of Drosophila is a derived Hox class 3 gene. Proc Natl Acad Sci USA. 1999;96:3786–3789. doi: 10.1073/pnas.96.7.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klomp J, et al. Embryo development. A cysteine-clamp gene drives embryo polarity in the midge Chironomus. Science. 2015;348:1040–1042. doi: 10.1126/science.aaa7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch JA, Brent AE, Leaf DS, Pultz MA, Desplan C. Localized maternal orthodenticle patterns anterior and posterior in the long germ wasp Nasonia. Nature. 2006;439:728–732. doi: 10.1038/nature04445. [DOI] [PubMed] [Google Scholar]
- 13.Brent AE, Yucel G, Small S, Desplan C. Permissive and instructive anterior patterning rely on mRNA localization in the wasp embryo. Science. 2007;315:1841–1843. doi: 10.1126/science.1137528. [DOI] [PubMed] [Google Scholar]
- 14.Schröder R. The genes orthodenticle and hunchback substitute for bicoid in the beetle Tribolium. Nature. 2003;422:621–625. doi: 10.1038/nature01536. [DOI] [PubMed] [Google Scholar]
- 15.Marques-Souza H, Aranda M, Tautz D. Delimiting the conserved features of hunchback function for the trunk organization of insects. Development. 2008;135:881–888. doi: 10.1242/dev.018317. [DOI] [PubMed] [Google Scholar]
- 16.Kotkamp K, Klingler M, Schoppmeier M. Apparent role of Tribolium orthodenticle in anteroposterior blastoderm patterning largely reflects novel functions in dorsoventral axis formation and cell survival. Development. 2010;137:1853–1862. doi: 10.1242/dev.047043. [DOI] [PubMed] [Google Scholar]
- 17.Copf T, Schröder R, Averof M. Ancestral role of caudal genes in axis elongation and segmentation. Proc Natl Acad Sci USA. 2004;101:17711–17715. doi: 10.1073/pnas.0407327102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu J, et al. Asymmetrically expressed axin required for anterior development in Tribolium. Proc Natl Acad Sci USA. 2012;109:7782–7786. doi: 10.1073/pnas.1116641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 20.Schoppmeier M, Schröder R. Maternal torso signaling controls body axis elongation in a short germ insect. Curr Biol. 2005;15:2131–2136. doi: 10.1016/j.cub.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Schoppmeier M, Fischer S, Schmitt-Engel C, Löhr U, Klingler M. An ancient anterior patterning system promotes caudal repression and head formation in ecdysozoa. Curr Biol. 2009;19:1811–1815. doi: 10.1016/j.cub.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 22.Dönitz J, et al. iBeetle-Base: A database for RNAi phenotypes in the red flour beetle Tribolium castaneum. Nucleic Acids Res. 2015;43:D720–D725. doi: 10.1093/nar/gku1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt-Engel C, et al. The iBeetle large-scale RNAi screen reveals gene functions for insect development and physiology. Nat Commun. 2015;6:7822. doi: 10.1038/ncomms8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jongens TA, Ackerman LD, Swedlow JR, Jan LY, Jan YN. Germ cell-less encodes a cell type-specific nuclear pore-associated protein and functions early in the germ-cell specification pathway of Drosophila. Genes Dev. 1994;8:2123–2136. doi: 10.1101/gad.8.18.2123. [DOI] [PubMed] [Google Scholar]
- 25.Leatherman JL, Levin L, Boero J, Jongens TA. Germ cell-less acts to repress transcription during the establishment of the Drosophila germ cell lineage. Curr Biol. 2002;12:1681–1685. doi: 10.1016/s0960-9822(02)01182-x. [DOI] [PubMed] [Google Scholar]
- 26.de la Luna S, Allen KE, Mason SL, La Thangue NB. Integration of a growth-suppressing BTB/POZ domain protein with the DP component of the E2F transcription factor. EMBO J. 1999;18:212–228. doi: 10.1093/emboj/18.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cinalli RM, Lehmann R. A spindle-independent cleavage pathway controls germ cell formation in Drosophila. Nat Cell Biol. 2013;15:839–845. doi: 10.1038/ncb2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerit DA, et al. Germ cell-less promotes centrosome segregation to induce germ cell formation. Cell Rep. 2017;18:831–839. doi: 10.1016/j.celrep.2016.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walldorf U, Kiewe A, Wickert M, Ronshaugen M, McGinnis W. Homeobrain, a novel paired-like homeobox gene is expressed in the Drosophila brain. Mech Dev. 2000;96:141–144. doi: 10.1016/s0925-4773(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 30.Schröder R, Eckert C, Wolff C, Tautz D. Conserved and divergent aspects of terminal patterning in the beetle Tribolium castaneum. Proc Natl Acad Sci USA. 2000;97:6591–6596. doi: 10.1073/pnas.100005497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prühs R, Beermann A, Schröder R. 2017. The roles of the Wnt-antagonists axin and Lrp4 during embryogenesis of the red flour beetle Tribolium castaneum. J Dev Biol 5:10. doi:10.3390.
- 32.Pae J, Cinalli RM, Marzio A, Pagano M, Lehmann R. GCL and CUL3 control the switch between cell lineages by mediating localized degradation of an RTK. Dev Cell. 2017;42:130–142.e7. doi: 10.1016/j.devcel.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolognesi R, Farzana L, Fischer TD, Brown SJ. Multiple Wnt genes are required for segmentation in the short-germ embryo of Tribolium castaneum. Curr Biol. 2008;18:1624–1629. doi: 10.1016/j.cub.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benton MA, Akam M, Pavlopoulos A. Cell and tissue dynamics during Tribolium embryogenesis revealed by versatile fluorescence labeling approaches. Development. 2013;140:3210–3220. doi: 10.1242/dev.096271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz C, Schröder R, Hausdorf B, Wolff C, Tautz D. A caudal homologue in the short germ band beetle Tribolium shows similarities to both, the Drosophila and the vertebrate caudal expression patterns. Dev Genes Evol. 1998;208:283–289. doi: 10.1007/s004270050183. [DOI] [PubMed] [Google Scholar]
- 36.Pridöhl F, et al. Transcriptome sequencing reveals maelstrom as a novel target gene of the terminal system in the red flour beetle Tribolium castaneum. Development. 2017;144:1339–1349. doi: 10.1242/dev.136853. [DOI] [PubMed] [Google Scholar]
- 37.van der Zee M, Berns N, Roth S. Distinct functions of the Tribolium zerknüllt genes in serosa specification and dorsal closure. Curr Biol. 2005;15:624–636. doi: 10.1016/j.cub.2005.02.057. [DOI] [PubMed] [Google Scholar]
- 38.Oberhofer G, Grossmann D, Siemanowski JL, Beissbarth T, Bucher G. Wnt/β-catenin signaling integrates patterning and metabolism of the insect growth zone. Development. 2014;141:4740–4750. doi: 10.1242/dev.112797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schönauer A, et al. The Wnt and delta-notch signalling pathways interact to direct pair-rule gene expression via caudal during segment addition in the spider Parasteatoda tepidariorum. Development. 2016;143:2455–2463. doi: 10.1242/dev.131656. [DOI] [PubMed] [Google Scholar]
- 40.Shinmyo Y, et al. Caudal is required for gnathal and thoracic patterning and for posterior elongation in the intermediate-germband cricket Gryllus bimaculatus. Mech Dev. 2005;122:231–239. doi: 10.1016/j.mod.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 41.El-Sherif E, Zhu X, Fu J, Brown SJ. Caudal regulates the spatiotemporal dynamics of pair-rule waves in Tribolium. PLoS Genet. 2014;10:e1004677. doi: 10.1371/journal.pgen.1004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.St Johnston D, Nüsslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992;68:201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- 43.Nunes da Fonseca R, et al. Self-regulatory circuits in dorsoventral axis formation of the short-germ beetle Tribolium castaneum. Dev Cell. 2008;14:605–615. doi: 10.1016/j.devcel.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Cho KW, Blumberg B, Steinbeisser H, De Robertis EM. Molecular nature of Spemann’s organizer: The role of the Xenopus homeobox gene goosecoid. Cell. 1991;67:1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Robertis EM. Spemann’s organizer and the self-regulation of embryonic fields. Mech Dev. 2009;126:925–941. doi: 10.1016/j.mod.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubrulle J, Pourquié O. Coupling segmentation to axis formation. Development. 2004;131:5783–5793. doi: 10.1242/dev.01519. [DOI] [PubMed] [Google Scholar]
- 47.Lewis SL, et al. Dkk1 and Wnt3 interact to control head morphogenesis in the mouse. Development. 2008;135:1791–1801. doi: 10.1242/dev.018853. [DOI] [PubMed] [Google Scholar]
- 48.Niehrs C. Head in the WNT: The molecular nature of Spemann’s head organizer. Trends Genet. 1999;15:314–319. doi: 10.1016/s0168-9525(99)01767-9. [DOI] [PubMed] [Google Scholar]
- 49.Guder C, et al. An ancient Wnt-Dickkopf antagonism in Hydra. Development. 2006;133:901–911. doi: 10.1242/dev.02265. [DOI] [PubMed] [Google Scholar]
- 50.Lorenzen MD, et al. piggyBac-based insertional mutagenesis in Tribolium castaneum using donor/helper hybrids. Insect Mol Biol. 2007;16:265–275. doi: 10.1111/j.1365-2583.2007.00727.x. [DOI] [PubMed] [Google Scholar]
- 51.Bucher G, Scholten J, Klingler M. Parental RNAi in Tribolium (Coleoptera) Curr Biol. 2002;12:R85–R86. doi: 10.1016/s0960-9822(02)00666-8. [DOI] [PubMed] [Google Scholar]
- 52.Posnien N, et al. RNAi in the red flour beetle (Tribolium) Cold Spring Harb Protoc. 2009;2009:pdb.prot5256. doi: 10.1101/pdb.prot5256. [DOI] [PubMed] [Google Scholar]
- 53.Schinko J, Posnien N, Kittelmann S, Koniszewski N, Bucher G. Single and double whole-mount in situ hybridization in red flour beetle (Tribolium) embryos. Cold Spring Harb Protoc. 2009;2009:pdb.prot5258. doi: 10.1101/pdb.prot5258. [DOI] [PubMed] [Google Scholar]
- 54.Bäumer D, Trauner J, Hollfelder D, Cerny A, Schoppmeier M. JAK-STAT signalling is required throughout telotrophic oogenesis and short-germ embryogenesis of the beetle Tribolium. Dev Biol. 2011;350:169–182. doi: 10.1016/j.ydbio.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 55.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 56.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.