Abstract
To evaluate the cariogenic properties of almond milk beverages, six almond milks, along with soy and whole bovine milk, were analyzed for their abilities to support Streptococcus mutans biofilm formation, acid production, and their capacity to buffer changes in pH. Biofilm formation by S. mutans was analyzed using an in vitro 96-well plate model and measured by crystal violet staining. Acid production by S. mutans was evaluated by a colorimetric L-Lactate assay and pH measurement of bacterial cultures. Buffering capacity was assessed by pH titration assay. Soy milk supported the most biofilm growth, while the least was observed with unsweetened almond milk (P<0.001, respectively). Among almond milks, sucrose sweetened milk led to the highest level of biofilm formation (P<0.001), while the least was observed with unsweetened milk (P<0.05). Sucrose sweetened almond milk yielded the lowest pH (4.56±0.66), followed by Soy milk and bovine milk; the highest pH was with unsweetened almond milk (6.48±0.5). When analyzed by pH titration, the unsweetened almond milk displayed the weakest buffering capacity while bovine milk showed the highest buffering capacity (P<0.001). These results suggest that the almond milk beverages, except those that are sweetened with sucrose, possess limited cariogenic properties, while Soy milk exhibits the most cariogenic potential. As milk alternatives become increasingly popular, dentists must counsel their patients that almond milk, especially sucrose sweetened varieties, have cariogenic potential. For patients who are lactose-intolerant or suffer from milk allergy, almond milks may be a better alternative than soy-based products.
Keywords: almond milk, dental caries, cariogenicity, Streptococcus mutans
INTRODUCTION
Bovine milk alternative beverages are on the rise. Among those available to consumers in the United States are soy, almond, cashew, and coconut milk-based beverages. Various market data report an increase in overall dairy alternative beverage sales from 2011-2014 in comparison to cow’s milk with $1 billion dollars in annual sales in the U.S. [Wang, 2016b]. Likely reasons for this surge include dietary restrictions like lactose intolerance, ethical concerns, interest in plant-base eating patterns, and other perceived health benefits [Ellis and Lieb, 2015]. While soy milk possesses nutritional benefits, concerns exist regarding its inherent phytoestrogen [Jefferson, 2010; Patisaul and Jefferson, 2010], and phytate effects [Gibson et al., 2010]. In recent years almond milk sales exceeded that of soy milk, generating over double the revenue of all other competitors in the dairy alternative category [Wang, 2016a].
One reason for the recent growth in alternative milk products is the concomitant awareness of food allergy to bovine-based products. Generally, food allergies have increased the past two decades affecting 4-6% of children in the United States [Kulis et al., 2015]. The foods most commonly associated with allergies in the United States are milk, eggs, peanuts, tree nuts, wheat, soy, fish, and shellfish [Kulis et al., 2015]. Consequently, the impact of food allergy extends beyond physiological effects, often leading to decreased quality of life including social isolation, bullying, and higher anxiety have been reported [Lieberman et al., 2010]. According to the Guidelines for the Diagnosis and Management of Food Allergies in the United States, “the only prevention option for the patient is to avoid the food allergen, and treatment involves the management of symptoms as they appear” [Boyce et al., 2010]. Thus, alternative milk products serve as nutritional and palatable alternatives for such individuals. Despite increasing consumption rates, the cariogenic potential of alternative milk products is currently unknown.
Caries remains the primary concern of dental healthcare professionals because it is still the most prevalent infectious disease worldwide. Moreover, caries rates in children ages 2-5 are on the rise in the United States [Tinanoff and Reisine, 2009]. Caries is initiated in dental plaque, a microbial biofilm grown on the tooth surfaces [Loesche, 1986]. Under conditions such as continuous consumption of high sucrose-containing diet, an ecological shift in the plaque biofilm microbiota can take place leading to overgrowth of acidogenic species resulting in eventual net loss of tooth mineral [Dashper et al., 2012; Loesche, 1986].
Several studies have analyzed the cariogenic properties of bovine milk [Bowen et al., 1997; Giacaman and Munoz-Sandoval, 2014; Munoz-Sandoval et al., 2012; Prabhakar et al., 2010; Sheik and Erickson, 1996]. Using in vitro caries and biofilm models, plain and sweetened bovine milk have been found to have a high buffering capacity and support the formation of S. mutans biofilms, indicative of caries potential; sugar supplementation further enhance such potential [Giacaman and Munoz-Sandoval, 2014; Prabhakar et al., 2010]. In studies of dairy milk alternatives, Daspher et al. reported that soy milk supported significantly higher quantities of organic acid production by S. mutans fermentation and had a lower buffering capacity in comparison to bovine milk, which indicates soy milk has a higher cariogenic potential than bovine milk [Dashper et al., 2012].
Despite recent increases in consumption of almond milks, currently there is no information available concerning the cariogenic potential of these beverages. The aim of this study was to analyze the cariogenic potential of six different almond milk beverages in regards to biofilm formation, acid production by S. mutans, and buffering capacity, with soy milk and a bovine whole milk serving as controls.
METHODS
Milk beverages
Six almond milk beverages were analyzed in this study as shown in Table 1. They included two original unsweetened almond milks from Diamond Breeze® products (AU) and Silk® products (SU), two original sweetened almond milks from Diamond Breeze products (AO) and Silk products (SO), one Silk® Vanilla Almond (VAN) and one Silk® Chocolate Almond (CHOC). They were analyzed along with bovine whole milk (COW) and Soy milk (SOY) as controls.
Table 1.
Beverages tested and their major nutritional characteristics:
| Milk Type | Abbreviation | Sugars (grams)# |
Sugars Added## | Protein (grams)# |
|---|---|---|---|---|
| Silk Original Almond* | SO | 7 | Cane sugar | 1 |
| Silk Original Unsweetened Almond* | SU | 0 | None | 1 |
| Silk Vanilla Almond* | VAN | 16 | Cane Sugar | 1 |
| Silk Chocolate Almond* | CHOC | 17 | Cane Sugar Cocoa (Dutch Process) |
1 |
| Almond Breeze Original Almond** | AO | 7 | Evaporated Cane Juice | 1 |
| Almond Breeze Original Unsweetened Almond** | AU | 0 | None | 1 |
| Horizon Whole Milk*** | COW | 11 | None | 8 |
| Silk Soy milk* | SOY | 6 | None | 8 |
Note:
WhiteWave Foods, Denver, CO;
Blue Diamond® Growers, Sacramento, CA;
WhiteWave Foods, Broomfield, CO;
indicates the amount of sugars or proteins per servings;
indicates the major source of the sugars.
Bacterial strain and cultivation
S. mutans UA159, a major cariogenic bacterial strain commonly used in in vitro and in vivo caries models, was maintained on Brain Heart Infusion (BHI) (Difico) agar plates and transferred in BHI broth at 37°C in an aerobic chamber with 5% CO2 [Wen and Burne, 2002]. For biofilm formation, Todd Hewitt broth (Difico) plus 0.2% (w/v) yeast extract (THY) was also used either together with milk beverages in a 1:2 ratio or alone as controls.
Biofilm formation
S. mutans UA159 cultures were transferred to fresh BHI medium, and allowed to grow until the mid-exponential phase (OD600nm ~0.5). For biofilm formation [Loo et al., 2000; Wen and Burne, 2002], actively growing S. mutans cultures were diluted 1:100 with various milk beverages, and 200 μL aliquots were then transferred to wells of 96 well culture plates (Corning, New York) in triplicate. To evaluate the dilution effects by saliva and/or other beverages in the oral cavity, a set of milk beverages diluted by 1:2 ratio with sterile deionized water or THY broth and inoculated with actively growing S. mutans similarly as described above. For controls, milk beverages with no bacterial inoculum as well as THY with and without inoculum were used. Plates were incubated at 37°C in an aerobic chamber with 5% CO2 to cultivate biofilms. After 24 hours, the 96 well plates were agitated at 200 RPM for 5 minutes on a shaker (Shaker 20E; Labner International, Edison, N.J.), and the non-adherent cells were removed by gentle rinsing three times in water. The adherent biofilm cells were stained with 0.1% crystal violet for 30 minutes, washed properly in water, and dye extracted using an ethanol-acetone mix (4:1). Biofilm formation was then quantified by measuring the absorbance of the extracted solution with a spectrophotometer at 575 nm. The absorbance was further normalized using proper dilution factors, when needed [Loo et al., 2000; Wen and Burne, 2002].
pH analysis and lactic acid estimation
The pH of all milk beverages before and after S. mutans fermentation was assessed using a micro-pH probe (Thermo Fisher Scientific, Waltham, MA). For post-fermentation pH reduction and lactic acid estimation, a set of cultures were set up using 5 ml Falcon tubes similarly as described above. After 24 hours, the pH of the culture medium was recorded, and following centrifugation at 4°C at 3,000×g for 10 minutes, the concentration of lactic acid in the cell-free culture medium was estimated using the colorimetric EnzyChrome™ L-Lactate Assay Kit (BioAssay Systems, Hayward, CA) according to the manufacturer instructions.
Buffering capacity
Buffering capacity of the milk beverages was analyzed by pH titration with a micro-pH probe and calculating the amount of 1 M HCl required for a pH change of one unit was recorded as a correlate of buffering capacity.
Statistical analysis
Analysis of Variance (ANOVA) was first used to determine if significant differences exist among different milk products, and then pairwise Tukey’s test and sometimes, t-test were used to analyze the differences between different milk products. All statistical analyses were conducted using SAS 9.0 (SAS Institute Inc., Cary, NC). A difference at P≤0.05 is considered statistically significant.
RESULTS
Biofilm growth in milk and milk alternatives
S. mutans exhibited the most biofilms when grown in SOY, with an average absorbance of 10.88 ± 1.73 (P<0.001 vs. all except COW) (Fig. 1). COW also yielded significantly more biofilms than the other milk beverages except SOY (P<0.001), and the least amount of biofilm was observed when S. mutans was grown in unsweetened AU and SU, with an averaging absorbance of 0.33±0.05 and 0.53±0.15, respectively. When compared among the almond milk beverages, AO supported significantly more biofilm growth than the others (average of 5.29 ± 1.63 (P<0.001), while the unsweetened AU and SU yielded significantly less biofilm (P ≤ 0.05). Similar results were obtained with the milk and milk alternatives that were diluted with deionized water, except the diluted SOY (SOYd) and the diluted AO (AOd) (Fig. S1). When grown in diluted SOYd, S. mutans reduced biofilm formation by 1.98-fold (P<0.01) as compared to growth in undiluted SOY. On the other hand, S. mutans increased biofilm formation by 1.87-fold in diluted AOd (P<0.05), when compared to the undiluted milk. As a control, S. mutans generated only limited biofilms, with an average A575nm of 0.39± 0.17, when grew on THY alone.
Figure 1. Biofilm formation in milk and milk alternatives.
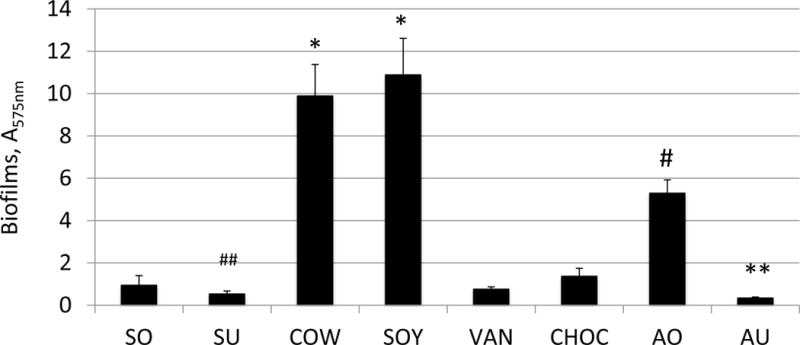
S. mutans was grown in 96-well polystyrene plates in milk beverages for 24 h and biofilms were assessed by crystal violet staining. Data presented are results from biofilms grown in milk alone. Similar results were also obtained with milks diluted by 1:2 ratio with sterile deionized water (Data not shown). Data represent averages (±SD in error bars, N=9) of three sets of separate experiments. *#, P<0.001 vs all others; ##P<0.001 vs AO, <0.05 vs other almonds except SO; ** P<0.05 vs SO and SU, <0.01 vs VAN and CHOC, <0.001 vs AO.
pH of milk and milk alternatives after S. mutans growth
Almond milk beverages and the Soy milk all showed high initial pHs with the highest recorded being AO at 8.44 ± 0.07 (P≤0.01). COW had the lowest initial pH of 6.80 ± 0.02 (P<0.001) (Fig. 2). Following bacterial fermentation for 24 hours, AO had the lowest culture medium pH at 4.56 ± 0.66 (P<0.001 vs SU and AU only), and had the biggest pH reduction by 3.88 units (P<0.001). Similar results were also observed with SOY and COW.
Figure 2. Analysis of initial pH and following bacterial fermentation.
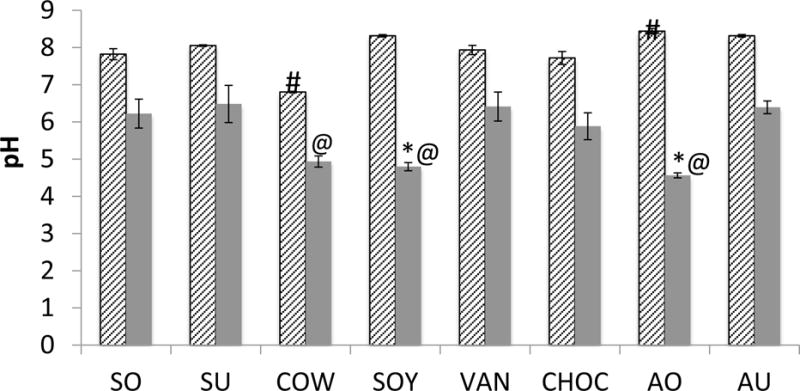
The initial pHs of the milk beverages (hatched bars) and the culture medium after 24 hours of bacterial fermentation (solid bars) were measured using a micro-pH prob. Data represent averages (±SD in error bars, N=9) of three sets of separate experiments. The highest initial pH was measured with AO and the lowest with COW (#, P<0.001). After 24 hours, the lowest pH was shown in AO, followed by SOY and COW (*, P<0.001 vs SU and AU). The biggest pH drop was measured with AO and SOY (@ P<0.001).
In the cell-free culture medium, the highest amount of lactic acid was measured with COW, with an average of 16.00±0.91 mM (P<0.001 vs SU and AU, >0.05 vs the rest), and the least amount of lactic acid was shown in SU averaging 4.12±1.63 mM (P<0.001 vs COW) (Fig. 3). Of the almond milks analyzed, VAN supported the most lactic acid production, followed by SO, CHOC and AO, and the least lactic acid was measured with SU and AU. Similar trends, but in slightly reduced levels (P>0.05), were measured with milk and milk alternatives diluted with deionized water (data not shown).
Figure 3. Lactic acid concentration of milk and milk alternatives following bacterial growth.
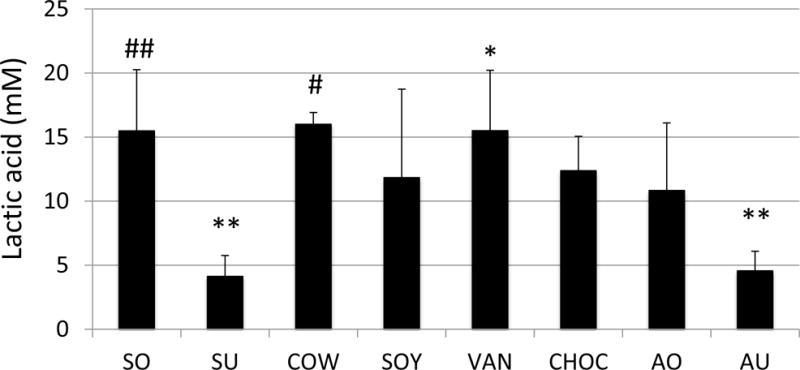
Lactic acid in the 24 hour culture medium was analyzed using a colorimetric assay. Data represent averages (±SD in error bars, N=9) of three sets of separate experiments. ##P<0.001 vs SU and AU, <0.05 vs SOY, CHOC and AO; **P<0.001 vs COW, VAN and SO, <0.01 vs CHOC, and <0.05 vs SOY and AO; #P<0.001 vs SU and AU, and <0.05 vs CHOC; *P<0.001 vs SU and AU, <0.01 vs AO, <0.05 vs SOY.
Buffering capacity of milk and milk alternatives
pH titration results demonstrated that COW had the highest buffering capacity, with the average amount of HCl required for reduction of pH for 1 unit at 52.67 ± 15.04 µmoles (P<0.001) (Fig. 4). AU displayed the weakest capacity of buffering with just over 6.67 ± 2.08 µmoles of HCl needed to alter the pH by a similar amount (P<0.05).
Figure 4. Buffering capacity of milk and milk alternatives.
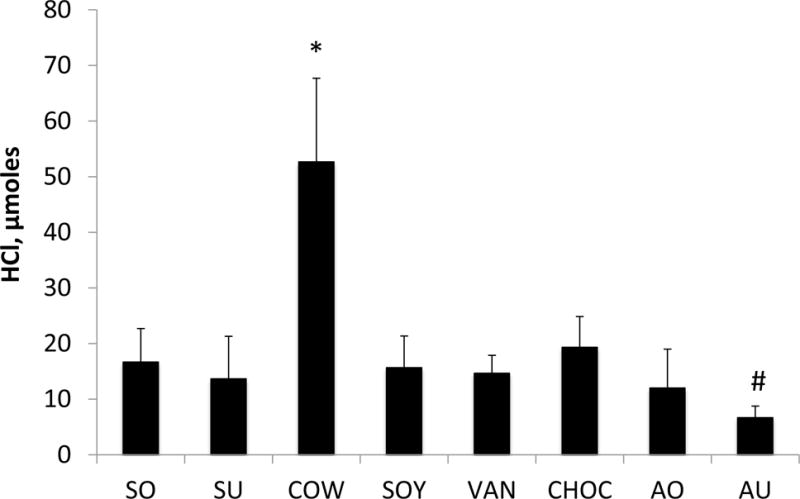
The buffering capacity of the milk beverages was measured by pH titration and is expressed as μmoles of HCl needed for titrating the pH by one unit. Data represent averages (±SD in error bars, N=3) of three sets of separate experiments. *P<0.001, COW vs. all others; AU # P<0.05 vs. all others except SU and AO.
DISCUSSION
Dental caries is a disease that is directly associated with the acidic metabolites produced by microbes within plaque biofilm communities [Burne, 1998]. The ability to support cariogenic bacterium to form biofilms, to produce acids from sugar fermentation, and the buffering capacity are common indicators used to analyze the cariogenic potential of various foods and beverages [Bowen et al., 1997; Dashper et al., 2012; Giacaman and Munoz-Sandoval, 2014; Munoz-Sandoval et al., 2012; Prabhakar et al., 2010]. In this study, the cariogenic properties of six popular almond milk beverages, along with bovine whole milk and soy milk, were examined for the first time using cariogenic S. mutans grown in a commonly used in vitro biofilm model [Wen and Burne, 2002]. Our results demonstrate that, of the milk beverages analyzed, Soy milk (SOY) and bovine whole milk (COW) supported the most biofilm formation by S. mutans and displayed some of the lowest culture pHs after 24 hours. Consistent with recent findings by Daspher et al., [Dashper et al., 2012], soy milk had one of the biggest reductions in culture medium pH, as also reflected by its ability to promote cariogenic biofilm formation and its poor buffering capacity. These results suggest that soy milk is a highly cariogenic beverage [Dashper et al., 2012]. Bovine milk, a major food product for humans of all age, is rich in calcium, phosphate, and casein that are known to be beneficial to healthy tooth enamel and exhibit anti-cariogenic activity [Bowen et al., 1997; Munoz-Sandoval et al., 2012]. Bovine milk does have strong buffering capacity, actually the highest among the milk beverages tested, but the significant amount of bacterial biofilm accumulated after 24 h also suggests major acid production from sugar fermentation, as supported by the results of lactic acid and pH analysis. These results also suggest that bovine milk is potentially cariogenic, consistent with recent findings by Prabhakar et al. and several others [Dashper et al., 2012; Giacaman and Munoz-Sandoval, 2014; Munoz-Sandoval et al., 2012; Prabhakar et al., 2010] .
Of the almond based products, AO supported significantly more biofilm than all others, had the lowest culture pH, and the biggest pH reduction after 24 h of bacterial fermentation. Relative to the unsweetened almond milk AU, this sweetened almond brand is supplemented with Evaporated Cane Juice (mainly sucrose) and contains more than seven grams of sugars per serving (23.33%, w/v) (Table 1). As a highly cariogenic sugar, sucrose is known to stimulate S. mutans biofilm formation. Therefore, it is not surprising that sweetened almond AO produced more biofilms and resulted in more culture pH reduction than the unsweetened AU. Interestingly, the amount of lactic acid detected in the culture medium of AO was not as much as some other almond milks, such as VAN and CHOC, which also contain lots of sugar and Cane sugars but did not yield as much biofilms as AO. S. mutans is known for its ability to utilize various sugars at micro-concentrations, although the metabolic pathways of glucose by this bacterium vary, depending on the environmental conditions, such as glucose availability, oxygen tension and pH. When glucose is available in excess or during aerobic growth, S. mutans catabolizes glucose through homofermentation pathways, yielding primarily lactate, the most acidic of all organic acids. However, under anaerobic conditions it undergoes heterofermentation, generating also formate, acetate, acetone and ethanol [Bitoun et al., 2012; Loesche, 1986; Yamada et al., 1985]. When grown in this sucrose sweetened AO, S. mutans generated significantly more biofilm, which likely results in reduction of oxygen availability, especially for the bacterial cells deep in the biofilm clusters. The reduced oxygen availability could lead to a shift of metabolic pathway to heterofermentation by S. mutans, partly contributing to the reduced lactic acid production. However, other not yet identified factors in these beverages could also have played a role influencing the metabolic pathways and acid production. The increased biofilm by S. mutans formation following 1:2 ratio dilutions of AO with deionized water (AOd, Fig. S1) also support such a notion, although the nature of the factors and the underlying mechanisms await further investigation.
As shown in Table 1, the Silk brand Original Almond (SO) contains similar amount of carbohydrates/sugars as AO, but interestingly, it yielded a lot less biofilm than AO, and unlike AO, had no significant reduction of culture pH following 24 h of bacterial fermentation. As is indicated in the labels, one major difference between these two almond milks is the nature of the supplemental sugars with SO being cane sugar and AO as evaporated cane juice. According to a US Patent awarded in 2001, the “Evaporated Cane Juice” is “not a syrup or a sugar but actually a juice concentrate” from sugar cane sticks. However, it awaits further investigation whether the evaporated cane juice or other factor(s) in this brand of almond milk contribute to the differences observed between these two milk alternatives.
Silk Vanilla Almond (VAN) contains the most carbohydrates among the milk beverages analyzed, and is also added with cane sugar. Not surprisingly, it supported more acids from bacterial fermentation than the other milk beverages. In 96 well biofilm model, however, VAN did not support much S. mutans biofilm formation as compared to some of the other almond milks tested.
It is apparent that the sweetened almond brands led to enhanced biofilm formation by S. mutans and more acid production leading to lower culture medium pH than the unsweetened brands. While a direct comparison cannot be made, similar patterns in biofilm formation, acid production and pH reduction in sweetened and unsweetened cow’s milk have been noted. For example, our results are consistent with findings of Giacman et al., which showed that addition of 10% sucrose to whole milk greatly enhances S. mutans biofilms and acid production, making it as cariogenic as a 10% sucrose solution [Giacaman and Munoz-Sandoval, 2014; Prabhakar et al., 2010]. On the other hand, both unsweetened almond brands (SU and AU) had a pH above 5.5 after 24 h of fermentation, the critical pH for demineralization. Both SU and AU brands have no sugar according to the manufacturer’s labeling. However, cognizance of consumption frequency of no-sugar added milk alternatives with sucrose-laden drinks is warranted.
Almond as well as Coconut contains phytochemicals, such as lauric acid, capric acid and polyphenols that have been shown to possess antimicrobial activities [Bergsson et al., 2001; Mandalari et al., 2010a; Mandalari et al., 2010b]. Although no detailed information is available concerning the content of these phytochemicals in the almond milk products, their presence will likely have an impact on the ability of S. mutans to grow and/or form biofilms. This may partly explain why overall almond milk alternatives produced a lot less biofilms than Soy milk. The increased biofilm formation by S. mutans when grown in diluted almond milks, especially, AOd as compared to the un-diluted counterparts could also be in part attributed to the reduced concentrations of antimicrobials.
These results suggest that almond milk beverages have cariogenic potential, especially when supplemented with sugars, but are not as cariogenic as soy milk. The information generated from this study will be useful for dentists when they council patients and their parents on diet selection and preventive care strategies. However, a more definitive conclusion on the comparative cariogenicity of milk alternatives to traditional milk awaits further studies using in vivo caries models and clinical trials.
Conclusions
Almond milk beverages, especially the sweetened almond milks, supported significant amount of S. mutans biofilms, indicative of cariogenic properties, although they do not support as much cariogenic biofilm or acid production as soy milk. When choosing an almond milk product, patients should be advised that sweetened almond milks have increased cariogenic properties compared to the unsweetened varieties. As these products are increasingly consumed, dentists should include this information in their dietary counseling discussions.
Supplementary Material
Acknowledgments
This study was supported in part by residents research fund from LSU School of Dentistry.
Footnotes
Author contributions
JAT, ZTW, JL and BMP conceived the project and wrote the manuscript. JL did most of the experiments and some writing. TT, AD, and TG helped with some of the experiments. QY did the data analysis.
Declaration of conflict of interest
All authors acknowledge that there is no conflict of interest with the content of the manuscript and have noting to disclose.
References
- Bergsson G, Arnfinnsson J, Steingrimsson O, Thormar H. In vitro killing of candida albicans by fatty acids and monoglycerides. Antimicrobial agents and chemotherapy. 2001;45:3209–3212. doi: 10.1128/AAC.45.11.3209-3212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitoun JP, Liao S, Yao X, Xie GG, Wen ZT. The redox-sensing regulator rex modulates central carbon metabolism, stress tolerance response and biofilm formation by streptococcus mutans. PLoS One. 2012;7:e44766. doi: 10.1371/journal.pone.0044766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WH, Pearson SK, Rosalen PL, Miguel JC, Shih AY. Assessing the cariogenic potential of some infant formulas, milk and sugar solutions. Journal of the American Dental Association. 1997;128:865–871. doi: 10.14219/jada.archive.1997.0336. [DOI] [PubMed] [Google Scholar]
- Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA, Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM. Panel NI-SE: Guidelines for the diagnosis and management of food allergy in the united states: Summary of the niaid-sponsored expert panel report. The Journal of Allergy and Clinical Immunology. 2010;126:1105–1118. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne RA. Oral streptococci…Products of their environment. J Dent Res. 1998;77:445–452. doi: 10.1177/00220345980770030301. [DOI] [PubMed] [Google Scholar]
- Dashper SG, Saion BN, Stacey MA, Manton DJ, Cochrane NJ, Stanton DP, Yuan Y, Reynolds EC. Acidogenic potential of soy and bovine milk beverages. Journal of Dentistry. 2012;40:736–741. doi: 10.1016/j.jdent.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Ellis D, Lieb J. Hyperoxaluria and genitourinary disorders in children ingesting almond milk products. The Journal of Pediatrics. 2015;167:1155–1158. doi: 10.1016/j.jpeds.2015.08.029. [DOI] [PubMed] [Google Scholar]
- Giacaman RA, Munoz-Sandoval C. Cariogenicity of different commercially available bovine milk types in a biofilm caries model. Pediatric Dentistry. 2014;36:1E–6E. [PubMed] [Google Scholar]
- Gibson RS, Bailey KB, Gibbs M, Ferguson EL. A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income contries and implications for bioavailability. Food Nutri Bull. 2010;31:S314–346. doi: 10.1177/15648265100312S206. [DOI] [PubMed] [Google Scholar]
- Jefferson WN. Adult ovarian function can be affected by high levels of soy. The Journal of Nutrition. 2010;140:2322S–2325S. doi: 10.3945/jn.110.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulis M, Wright BL, Jones SM, Burks AW. Diagnosis, management, and investigational therapies for food allergies. Gastroenterology. 2015;148:1132–1142. doi: 10.1053/j.gastro.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Weiss C, Furlong TJ, Sicherer M, Sicherer SH. Bullying among pediatric patients with food allergy. Ann Allergy Asthma Immunol. 2010;105:282–286. doi: 10.1016/j.anai.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Loesche WJ. Role of streptococcus mutans in human dental decay. Microbiological Reviews. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo CY, Corliss DA, Ganeshkumar N. Streptococcus gordonii biofilm formation: Identification of genes that code for biofilm phenotypes. Journal of Bacteriology. 2000;182:1374–1382. doi: 10.1128/jb.182.5.1374-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandalari G, Bisignano C, D’Arrigo M, Ginestra G, Arena A, Tomaino A, Wickham MS. Antimicrobial potential of polyphenols extracted from almond skins. Letters in Applied Microbiology. 2010a;51:83–89. doi: 10.1111/j.1472-765X.2010.02862.x. [DOI] [PubMed] [Google Scholar]
- Mandalari G, Faulks RM, Bisignano C, Waldron KW, Narbad A, Wickham MS. In vitro evaluation of the prebiotic properties of almond skins (amygdalus communis l) FEMS Microbiology Letters. 2010b;304:116–122. doi: 10.1111/j.1574-6968.2010.01898.x. [DOI] [PubMed] [Google Scholar]
- Munoz-Sandoval C, Munoz-Cifuentes MJ, Giacaman RA, Ccahuana-Vasquez RA, Cury JA. Effect of bovine milk on streptococcus mutans biofilm cariogenic properties and enamel and dentin demineralization. Pediatric Dentistry. 2012;34:e197–201. [PubMed] [Google Scholar]
- Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front Neuroendocrinol. 2010;31:400–419. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar AR, Kurthukoti AJ, Gupta P. Cariogenicity and acidogenicity of human milk, plain and sweetened bovine milk: An in vitro study. The Journal of Clinical Pediatric Dentistry. 2010;34:239–247. doi: 10.17796/jcpd.34.3.lk08l57045043444. [DOI] [PubMed] [Google Scholar]
- Sheik C, Erickson PR. Evaluation of plaque ph changes following oral rinse with eight infant formulas. Pediatr Dent. 1996;18:200–2004. [PubMed] [Google Scholar]
- Tinanoff N, Reisine S. Update on early childhood caries since the surgeon general’s report. Academic Pediatrics. 2009;9:396–403. doi: 10.1016/j.acap.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang V. America’s almind milk boom tops $700 million in sales. 2016a Accessed nov. 26, 2016 Http://www.Bloomberg.Com/news/articles/2014-08-08/the-almond-milk-boom-silks-huge-sales-lead-the-way-trounce-soys (archived by webcite@ at http://www.Webcitation.Org/6mlvwuvep)
- Wang V. Soly milk fades as americans opt for drinkable almonds. 2016b Accessed nov. 26, 2016 Http://www.Bloomberg.Com/news/articles/2013-08-21/soy-milk-fades-as-americans-opt-for-drinkable-almonds (archived by webcite@ at http://www.Webcitation.Org/6mlxu3ve5).
- Wen ZT, Burne RA. Functional genomics approach to identifying genes required for biofilm development by streptococcus mutans. Appl Environ Microbiol. 2002;68:1196–1203. doi: 10.1128/AEM.68.3.1196-1203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Takahashi-Abbe S, Abbe K. Effects of oxygen on pyruvate formate-lyase in situ and sugar metabolism of streptococcus mutans and streptococcus sanguis. Infection and Immunity. 1985;47:129–134. doi: 10.1128/iai.47.1.129-134.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


