Abstract
Background
We aimed to investigate the effects of continuous positive airway pressure (CPAP) treatment on electrocardiography (ECG), premature ventricular contraction load on 24-hour Holter recordings, and implantable cardioverter defibrillator (ICD) shocks in patients with obstructive sleep apnea syndrome (OSAS) and heart failure.
Methods
Patients with heart failure and ICD and patients with newly diagnosed OSAS were divided into two groups according to CPAP treatment. To compare the impact of CPAP on ECG parameters, both baseline and 6-month ECG, 24-hour Holter ECG, ambulatory blood pressure monitoring, echocardiography, polysomnography, and laboratory parameters were collected.
Results
CPAP treatment significantly reduced the frequency of premature ventricular contractions, T-peak to T-end, corrected QT, corrected QT dispersion, and T-peak to T-end/corrected QT ratio in the study group (p < 0.001 for all). Although the baseline NT-pro-BNP levels were similar between study and control groups, after six months, the NT-pro-BNP levels of the study group were significantly lower than that of the control group (39.18 ± 7.57 versus 46.11 ± 7.65; p < 0.001).
Conclusions
CPAP treatment in patients with heart failure and ICD and in patients with newly diagnosed OSAS may have beneficial effects on premature ventricular contractions and electrocardiographic arrhythmia indices and NT-pro-BNP levels. However, these results are needed to be clarified with further studies.
1. Introduction
Obstructive sleep apnea syndrome (OSAS) is characterized by temporary airway collapse during sleep and affects more than 4% of men and 2% of women [1]. Many cardiovascular diseases have been found to be associated with OSAS including arterial hypertension, ischemic heart disease, heart failure, arrhythmias, and stroke. Cardiovascular morbidity and mortality have been shown to be high in patients with OSAS [2, 3]. Sympathetic overactivity, activation of inflammatory cascades, vascular endothelial dysfunction, abnormalities in the coagulation pathway, and metabolic dysregulation are likely to be involved in the pathogenesis of the cardiovascular complications of OSAS.
The relation between the congestive heart failure and OSAS is somewhat complex. OSAS may affect cardiac functions and contribute to the development of heart failure in the long-term. On the other hand, heart failure itself also plays a role in the pathogenesis of OSAS [4–7]. Several studies reported that continuous positive airway pressure (CPAP) treatment in patients with congestive heart failure and OSAS reduced sympathetic activation and improved left ventricular ejection fraction [8–10]. However, the positive effect of CPAP treatment on the cardiovascular system is unclear in terms of the prognosis of patients with congestive heart failure and OSAS [11].
There are some arrhythmic changes on electrocardiography (ECG) in patients with OSAS, and some improvement with CPAP therapy has been reported [12–14]. The association between arrhythmias and OSAS is well known [15, 16]. OSAS associated with complex ventricular ectopia and ventricular tachycardia has been documented in a previous study [17]. CPAP treatment also resulted in a 58% reduction in premature ventricular beats [18]. Those findings may help to explain the pathogenesis of sudden death in OSAS patients [19]. It has also been shown that OSAS patients have a 4-fold higher frequency of atrial fibrillation if apnoea-hypopnoea index (AHI) index is greater than 30 [17].
Heart failure is a life-threatening disease with a growing incidence in developed countries due to better treatment options for heart failure, improvement of survival after myocardial infarction, and increased life expectancy. The introduction of implantable devices such as implantable cardioverter defibrillators (ICD) has improved the overall survival of patients with heart failure [20]. ICD treatment reduces mortality in patients with heart failure. However, certain conditions such as drug-resistant ventricular arrhythmias and atrial fibrillation may increase morbidity and mortality due to inappropriate and frequent shocks.
The aim of the present study is to investigate whether the regular CPAP treatment improves ECG findings and reduces premature ventricular contractions (PVC) and ICD shocks in patients with OSAS, cardiac failure, PVC, and ICD implant.
2. Materials and Methods
2.1. Study Population
This study is an observational study conducted with patients followed at the Department of Cardiology with diagnosis of chronic heart failure. Our inclusion criteria for the study population were ejection fraction < 35%, stable NYHA class ≥ II, the presence of ICD, sinus rhythm, and >30 PVC/hour on 24-hour Holter monitorization. In addition, patients had to be on stable heart failure medication for at least 4 weeks prior to enrolment and during follow-up. Patients filled Epworth Sleepiness Scale (ESS) form. Clinically suspected patients were referred to Department of Chest Diseases for possible OSAS diagnosis. After polysomnographic study in the sleep laboratory, CPAP treatment was recommended for moderate to severe OSAS patients. Patients only with OSAS were included, and patients with central sleep apnea (CSA) and mixed apnea were not included. Protocol of the study was approved by the local ethics committee, and subjects involved in the study signed informed consent approved by the institution.
Baseline ECG, 24-hour Holter ECG, ambulatory blood pressure monitoring, echocardiography, laboratory investigations, and ICD measurements were recorded. Age, sex, body mass index (BMI), the cause of heart failure, heart rate, arterial blood pressure, left ventricular end diastolic diameter (LVEDD), left atrium (LA) diameter, polysomnography findings, levels of creatinine, and hemoglobin of the participants were also recorded. Class of drugs used by the patient was also recorded. Study group (n = 40) consisted of ICD implanted heart failure and OSAS patients who accepted CPAP treatment, and control group (n = 40) consisted of ICD implanted heart failure and OSAS patients who did not accept CPAP treatment. Chronic atrial fibrillation, bundle branch block, application of cardiac resynchronization therapy (CRT), the presence of pacemaker rhythm, estimated glomerular filtration rate of less than 60 mL/min/1.73 m2 (CKD stage ≥ III), significant lung disease, thyroid dysfunction, and New York Heart Association (NYHA) grade IV heart failure were the exclusion criteria. Six months after the initiation of CPAP therapy, ECG, 24-hour Holter monitorization, ambulatory blood pressure monitoring, and echocardiography were repeated in both groups.
2.2. Polysomnography
All referred individuals with heart failure and suspected OSAS were subjected to 1 full-night polysomnography at the sleep laboratory using the Nox A1 PSG System (Nox Medical, Iceland). Physiological variables evaluated during polysomnography included electroencephalogram, electrooculogram, electromyogram (submentonian and tibialis), electrocardiogram, oral/nasal airflow measured by oronasal thermistors, respiratory effort (thorax and abdomen), body position, and blood oxygen saturation (SpO2) (mean and minimum SpO2). The exam was performed according to specific criteria for the definition of sleep stages [21] by trained technicians who were unaware of the patient characteristics.
Sleep-related respiratory events and arousals were scored according to the American Academy of Sleep Medicine Manual for Scoring Sleep and Associated Events [22]. Central sleep apnea was defined as the absence of thoracic and abdominal movements with absence of airflow. Obstructive sleep apnea was defined as the absence of airflow in the presence of thoracic and abdominal movements. Mixed apnea was defined as apnea without any thoracoabdominal movements in whom thoracoabdominal movements reoccurred prior to reoccurrence of nasal flow. Apnea was defined as complete cessation of the airflow ≥ 10 s. Hypopnea was defined as ≥30% decrease in airflow from baseline for at least 10 seconds with a≥3% oxygen desaturation from pre-event baseline, and/or the event is associated with an arousal. Apnea/hypopnea index was determined via careful calculations. AHI score between 15 and 29 was considered as OSAS with moderate severity. Severe OSAS was defined as AHI ≥ 30.
2.3. Treatment
Autotitrating CPAP was used in the sleep laboratory within a period of less than 7 days after the diagnostic study to get a fixed CPAP pressure value. The fixed CPAP pressure was used for the rest of the study. All patients were scheduled for follow-up 2 weeks after randomization and, subsequently, at 4, 8, 12, 18, and 24 weeks. During the follow-up visits, adherence to CPAP and antihypertensive medications was documented. Compliance with CPAP therapy was based on the self-report of patients at the end of the study period. The use ratio of CPAP (%) during the study period, which was the average time with CPAP to total time spent in bed, was 91% (range 72–100%).
2.4. Electrocardiography
The 12-lead ECG (CARDIOVIT AT-102 Plus, Schiller, Switzerland) was recorded at a paper speed of 25 mm/s at 10 mm/mV amplitude in the supine position. ECGs were performed while the patient was at rest and at the morning hours (between 8:00 and 10:00 AM). All of the ECGs were scanned with a resolution of 800 dpi and transferred to a personal computer. ECG measurements of QT and Tp-e intervals at V5 were performed by experienced cardiologists who were blinded to the patient data. If V5 was not suitable, V4 or V6 were measured, respectively. A mean value of three readings was calculated for each lead. The QT interval was measured from the beginning of the QRS complex to the end of the T wave. To adjust QT for heart rate, we calculated QTc according to Bazett's formula , where RR is the RR interval in seconds. The Tp-e interval was defined as the interval from the peak of T wave to the end of T wave. The Tp-e/corrected QT ratio was calculated from these measurements. QTc dispersion is determined as the difference between the maximum and minimum QTc in all considered leads. All ECG measurements were performed by a cardiologist who was blinded to other patient information.
2.5. Echocardiographic Evaluation
We used a 2-dimensional, M-mode cardiovascular ultrasound system (Vingmed Vivid 7 system GE, Germany) for echocardiographic evaluation. The examinations were performed in the left lateral decubitus position. Parasternal long- and short-axis views and apical views were used as standard imaging windows. We measured left atrial and left ventricular end systolic diameters from parasternal long-axis view. Ejection fraction was calculated by using modified Simpson method. All echocardiographic examinations were performed by an experienced cardiologist who was blinded to patient data.
2.6. Holter Assessment
Holter ECG (medilog®AR4 plus Holter system, Schiller, Switzerland) recordings were analyzed using a MARS 8000 Holter scanner (GE Medical Systems, Milwaukee, Wisconsin). We determined all PVC, atrial fibrillation (AF), and ventricular tachycardia (VT) episodes. The percentage of PVCs was calculated as the total number of ventricular ectopic beats/the total number of beats recorded during 24 hour Holter ECG monitoring.
2.7. Ambulatory Blood Pressure Monitoring
Ambulatory blood pressure monitoring was carried for 24 h using an automated sphygmomanometer (BR-102 PLUS PWA, Schiller, Switzerland). The blood pressure was recorded every 20 min during the 24 h period. Mean values were recorded before and after CPAP treatment.
2.8. Blood Sampling and Analysis
Fasting blood samples were collected from an antecubital vein in the morning and were centrifuged within one hour. Serum samples were stored at −80°C pending later measurements. N-terminal pro-brain natriuretic peptide (NT-proBNP) was measured by electrochemiluminescent immunoassay (Roche Diagnostics, Basel, Switzerland). Creatinine and hemoglobin levels were measured by standard laboratory methods. Serum NT-proBNP level was measured at baseline and at the end of 6-month follow-up in both groups. The estimated glomerular filtration rate (mL/min per 1.73 m2) was calculated to exclude renal dysfunction as a cause for increased NT-proBNP concentrations [23].
2.9. Statistical Analysis
All data were entered into a spreadsheet, and statistical analyses were performed by using R 3.3.2v (open source). Data were shown as mean ± standard deviation for continuous variables, median (minimum-maximum) for ordinal ones, and frequency with percent for categorical ones. Comparisons for continuous variables were made by using independent samples t-test and for categorical variables Fisher's exact test. Normally distributed variables were compared with paired t-test and nonnormally distributed variables were compared with Mann–Whitney U test for intragroup and intergroup differences. Two-Way Repeated Measures of ANOVA was also used for intragroup comparisons. A p value less than 0.05 was considered statistically significant.
3. Results
The mean age of the study group was 58.4 ± 8.14 years and the control group was 59.18 ± 7.97 years. The female/male ratio in the control group was 8/32 and in the control group was 9/31. There was no significant difference between the groups in terms of age and sex (p=0.67 and p=0.79). Baseline characteristics and other demographics of the study and control groups are summarized in Table 1.
Table 1.
Baseline characteristics and other demographics of the study and control groups.
| Groups | |||
|---|---|---|---|
| Study group | Control group | p value | |
| (n = 40) (%) | (n = 40) (%) | ||
| Age (years) | 58.4 ± 8.14 | 59.18 ± 7.97 | 0.67 |
| Gender | |||
| Female | 8 (20) | 9 (22.5) | 0.79 |
| Male | 32 (80) | 31 (77.5) | |
| Body mass index (kg/m) | 28.63 ± 3.16 | 27.88 ± 2.63 | 0.25 |
| Cause of congestive heart failure | |||
| Ischemic | 32 (80) | 28 (70) | 0.30 |
| Nonischemic | 8 (20) | 12 (30) | |
| Primary or secondary ICD | |||
| ICD for primary protection | 25 (62.5) | 31 (77.5) | 0.14 |
| ICD for secondary protection | 15 (37.5) | 9 (22.5) | |
| Polysomnography characteristics | |||
| Apnea/hypopnea index | 35.85 ± 8.61 | 32.45 ± 8.88 | 0.09 |
| Total sleep time (minutes) | 341.75 ± 45.45 | 337.65 ± 45 | 0.69 |
| Arousals/h sleep time | 29.25 ± 8.18 | 28.73 ± 8.95 | 0.79 |
| Mean O2 saturation (%) | 93.08 ± 1.27 | 93.35 ± 1.35 | 0.35 |
| Minimum O2 saturation (%) | 78.98 ± 2.9 | 79.95 ± 2.91 | 0.14 |
| Epworth sleepiness scale | 10.51 ± 4.82 | 10.73 ± 5.23 | 0.74 |
| Heart rate (beats per minute) | 68.3 ± 6.46 | 68.7 ± 6.31 | 0.78 |
| Blood pressure (mmHg) | |||
| Systolic | 131.13 ± 7.02 | 128.25 ± 7.39 | 0.08 |
| Diastolic | 76.5 ± 8.02 | 76 ± 7.18 | 0.77 |
| Echocardiography characteristics | |||
| Ejection fraction (%) | 30.2 ± 4.36 | 30.78 ± 4.7 | 0.57 |
| Left ventricular end diastolic diameter (mm) | 61.53 ± 4.31 | 61.68 ± 4.7 | 0.88 |
| Left atrium diameter (mm) | 39.25 ± 3.18 | 39.5 ± 3.35 | 0.73 |
| Creatinine (mg/dL) | 1.21 ± 0.28 | 1.17 ± 0.25 | 0.42 |
| Hemoglobin (g/dL) | 12.22 ± 2.03 | 12.45 ± 1.06 | 0.54 |
| Atrial fibrillation | |||
| No | 39 (97.5) | 37 (92.5) | 0.62 |
| Yes | 1 (2.5) | 3 (7.5) | |
| ICD shock appropriate | |||
| No | 35 (87.5) | 31 (77.5) | 0.24 |
| Yes | 5 (12.5) | 9 (22.5) | |
| ICD shock inappropriate | |||
| No | 39 (97.5) | 37 (92.5) | 0.62 |
| Yes | 1 (2.5) | 3 (7.5) | |
Other baseline characteristics including BMI, ejection fraction, AHI, oxygen saturation, heart rate, and blood pressure were also similar between both groups (p > 0.05 for all). Drugs used by the patients in both groups are summarized in Table 2. Drug usage characteristics of the study and control groups were also similar. No medicational change was done during follow-up especially in terms of antiarrhythmic drugs.
Table 2.
Drug usage characteristics of the groups.
| Groups | p value | ||
|---|---|---|---|
| Study group (n = 40) | Control group (n = 40) | ||
| β blocker | |||
| Not using | 3 (7.5) | 3 (7.5) | 1.0 |
| Using | 37 (92.5) | 37 (92.5) | |
| Renin-angiotensin system blocker | |||
| Not using | 2 (5) | 2 (5) | 1.0 |
| Using | 38 (95) | 38 (95) | |
| Aldosterone antagonist | |||
| Not using | 9 (22.5) | 9 (22.5) | 1.0 |
| Using | 31 (77.5) | 31 (77.5) | |
| Class III antiarrhythmic agents | |||
| Not using | 25 (62.5) | 30 (75) | 0.23 |
| Using | 15 (37.5) | 10 (25) | |
| Diuretics | |||
| Not using | 5 (12.5) | 3 (7.5) | 0.71 |
| Using | 35 (87.5) | 37 (92.5) | |
ESS values were similar between both groups (10.51 ± 4.82 versus 10.73 ± 5.23, p=0.74).
At the end of 6 months of CPAP treatment, frequency of PVC, Tp-e, QTc, QTc dispersion, and Tp-e/QTc ratio decreased significantly in the study group (p < 0.001 for all). These parameters did not change significantly in the control group after 6 months (p > 0.05 for all). Table 3 summarizes the chances of frequency of PVC, Tp-e, QTc, QTc dispersion, and Tp-e/QTc ratio during the follow-up period in both groups.
Table 3.
The chances of frequency of PVC, Tp-e, QTc, QTc dispersion, and Tp-e/QTc ratio during the follow-up period in both groups.
| Study group | Control group | Study group versus control group | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6th month | |||||||
| Baseline | 6th month | p value∗ | Baseline | 6th month | p value∗ | p value | p value | |
| Frequency of PVC | 1290 (865–1665) | 945 (685–1330) | <0.001 | 1360 (960–1765) | 1325 (990–1740) | 0.57 | 0.82 | 0.004∗∗ |
| Tp-e interval (ms) | 79.7 ± 7.02 | 71.85 ± 6.07 | <0.001 | 79.18 ± 8.01 | 79.13 ± 7.97 | 0.89 | 0.76 | <0.001 |
| QTc interval (ms) | 410.05 ± 11.2 | 397.2 ± 11.64 | <0.001 | 410.43 ± 12.11 | 414.25 ± 11.56 | 0.004 | 0.89 | <0.001 |
| QTc dispersion | 53.28 ± 8.51 | 37.95 ± 8.31 | <0.001 | 55.48 ± 9.97 | 54.83 ± 9.58 | 0.16 | 0.29 | <0.001 |
| Tp-e/QTc ratio | 194.18 ± 14.99 | 180.48 ± 13.89 | <0.001 | 192.15 ± 15.03 | 190.48 ± 16.81 | 0.13 | 0.55 | <0.001 |
∗Two-way repeated measures ANOVA was used; ∗∗frequency of premature ventricular contractions were compared between the groups using Mann–Whitney U test; PVC: premature ventricular contractions; Tp-e: T-peak to T-end interval; QTc: corrected QT interval.
CPAP treatment significantly reduced the heart rate and systolic and diastolic blood pressure and increased the ejection fraction in the study group (p < 0.05 for all). BMI and LVEDD did not change significantly after 6 months of CPAP treatment. Table 4 summarizes the BMI, ejection fraction, heart rate, blood pressure, and LVEDD measures of the groups in detail.
Table 4.
The comparisons of certain parameters of the groups during the follow-up period.
| Study group | Control group | |||||
|---|---|---|---|---|---|---|
| Baseline | 6th month | p value | Baseline | 6th month | p value | |
| Body mass index (kg/m2) | 28.63 ± 3.16 | 28.65 ± 2.92 | 0.812 | 27.88 ± 2.63 | 27.83 ± 2.54 | 0.534 |
| Ejection fraction (%) | 30.2 ± 4.36 | 30.98 ± 4.19 | <0.001 | 30.78 ± 4.7 | 30.75 ± 4.49 | 0.860 |
| Heart rate (beats per minute) | 68.3 ± 6.46 | 67.38 ± 6.59 | 0.002 | 68.7 ± 6.31 | 68.48 ± 6.26 | 0.060 |
| Systolic blood pressure (mmHg) | 131.13 ± 7.02 | 127.5 ± 5.66 | <0.001 | 128.25 ± 7.39 | 127.75 ± 7.16 | 0.160 |
| Diastolic blood pressure | 76.5 ± 8.02 | 74.13 ± 6.59 | <0.001 | 76 ± 7.18 | 76 ± 7.18 | 0.999 |
| Left ventricular end diastolic diameter (mm) | 61.53 ± 4.31 | 61.68 ± 4.32 | 0.262 | 61.68 ± 4.7 | 62.03 ± 4.65 | 0.001 |
Appropriate ICD shocks during follow-up in study and control groups due to VT/VF were seen in 5 patients and 9 patients, respectively (%12.5 versus %22.5, p=0.24). Inappropriate ICD shocks were much more in control group (%2.5 versus %7.5, p=0.62) but did not reach statistical significance. Also atrial fibrillation rate detected by ICD devices was higher in control group but again did not have statistical significance.
Baseline NT-Pro BNP levels were 43.88 ± 7.79 pg/mL in the study group and 44.65 ± 7.79 pg/mL in the control group. The NT-Pro BNP levels at 6th month were 39.18 ± 7.57 pg/mL in the study group and 46.11 ± 7.65 pg/mL in the control group. There was a statistically significant difference between baseline and 6th-month measurements in the study group in terms of NT-Pro BNP (p < 0.001). There was also a statistically significant difference between the study and control groups at 6th month (p < 0.001), but not baseline (p=0.112) NT-Pro BNP measurements. Figure 1 shows the baseline and 6th-month NT-pro-BNP levels in the groups.
Figure 1.
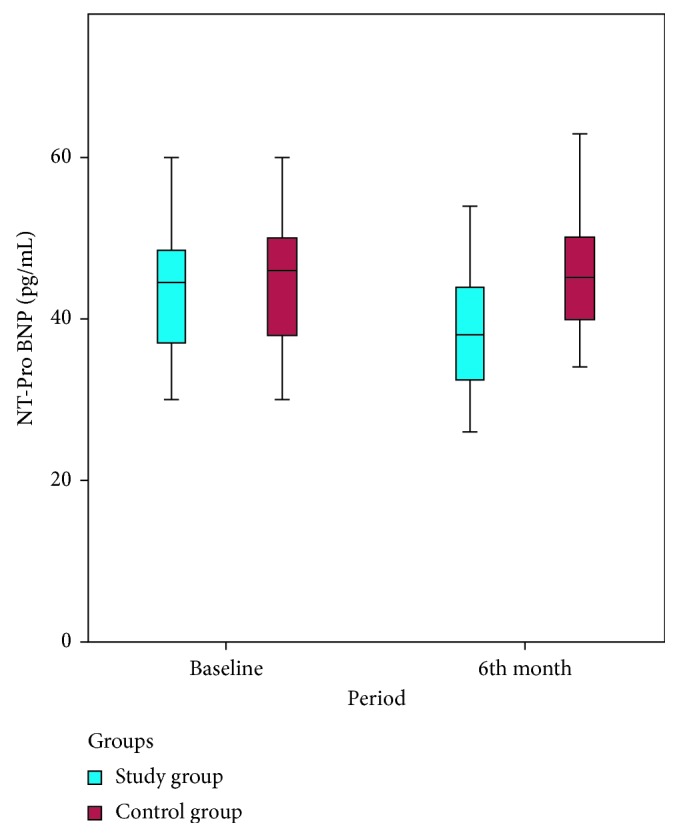
The baseline and 6-month NT-pro BNP levels in both groups.
4. Discussion
Individuals with OSAS have frequent cessation or reduction of airflow during sleep that results in hypoxemia and arousals from sleep. Continuous positive airway pressure (CPAP) is the standard first-line treatment for OSAS [24]. One metaanalysis from consistent evidence from good- and fair-quality randomized controlled trials reported that CPAP effectively reduces AHI and excessive sleepiness in patients with OSAS [25]. Several studies showed the beneficial effects of CPAP on cardiac functions in OSAS patients [26, 27]. The possible mechanism of action of CPAP may include improved myocardial oxygen delivery, decreased sympathetic activity, left ventricular transmural pressure, and afterload [28]. In the present study, we have found beneficial effects of CPAP treatment in patients with OSAS and heart failure in terms of reduction in premature ventricular contractions and ventricular wall stress.
The issue of whether OSAS is an independent risk factor for patients with heart failure is controversial. The results of published studies on this subject vary significantly [29, 30]. Lanfranchi et al. [29] reported approximately 4-fold increased mortality rates in patients with OSAS and heart failure compared to heart failure patients without OSAS. Fries et al. [30] investigated the prognostic value of OSAS in congestive heart failure patients with ICD implants and found that mortality rates were 44% in central sleep apnea and 13% in the absence of apnea at the 2 years of follow-up (p < 0.05), but this study found no association between sleep apnea and appropriate ICD therapy for VT or VF. Adlakha and Shepard [31] speculated that hypoxia in the obstructive sleep apnea leads to cardiac arrhythmias. Sudden deaths, particularly at night, in OSAS may be due to these arrhythmias [19, 32]. Clay et al. [33] reported that OSAS patients with ICD had higher rates of ventricular ectopia and nonsustained ventricular tachycardia. Javaheri [34] found an increased frequency of VT and VES in patients with congestive heart failure and OSAS. Bitter et al. [35] speculated that OSAS is an independent risk factor for malignant ventricular arrhythmias that require ICD treatment. We found reduced premature ventricular contraction rates after 6 months of CPAP treatment in patients with OSAS and heart failure compared to untreated controls. We did not make major changes in the heart failure treatment of patients. So, favorable results would be attributable to CPAP treatment.
Ventricular repolarization and arrhythmogenesis can be assessed by several electrocardiographic parameters including QTc interval, QT dispersion, and T wave measurements [36]. Tp-e is used as an indicator of transmural dispersion of ventricular repolarization [37]. Prolonged Tp-e interval may predict ventricular arrhythmias and mortality [38, 39]. Thus, Tp-e/QTc ratio has been proposed to be a more specific index of ventricular arrhythmogenesis [40, 41]. Kilicaslan et al. [36] reported prolonged Tp-e interval and increased Tp-e/QTc ratio in patients with moderate and severe OSAS as compared to healthy controls. Their finding of Tp-e and QTc interval in OSAS patients were comparable with the present study results. We found that, CPAP treatment significantly reduced the frequency of PVC, Tp-e interval, QTc interval, QTc dispersion, and Tp-e/QTc ratio in the study group, whereas the control group did not show any significant difference for the mentioned parameters.
Myocytes of cardiac ventricle constitute the major source of BNP-related neurohormones. B-type natriuretic peptide (BNP) is a 32 amino acid peptide primarily synthesized, stored, and secreted from left ventricle. NT proBNP is derived from the BNP and secreted when the ventricular wall stress increased [42, 43]. It has been used as an indicator of heart failure and symptomatic idiopathic PVC [42, 44, 45]. NT proBNP suggested as a sensitive indicator of PVC-induced increased ventricular wall stress [44, 45]. Skranes et al. [46] reported that the higher levels of NT-proBNP levels are independently associated with the incidence of frequent ventricular ectopy and complex ventricular ectopy in a moderately large, community-based population. Another study [47] found that fatigue was associated with higher baseline NT-proBNP in patients with frequent PVCs and preserved LV function. Tasci et al. [48] reported that nasal CPAP treatment significantly reduced NT-proBNP in patients with normotensive and hypertensive OSAS. Similarly, Strehmel et al. reported reduced NT-proBNP levels after CPAP treatment in patients with OSAS and coronary artery disease [49]. In the present study, we have found that 6 months CPAP treatment for OSAS significantly reduced the NT-proBNP levels which means reduced ventricular wall stress.
The present study has some limitations. We did not evaluate the AHI index using polysomnography at the 6-month follow-up. Therefore, an association between AHI and cardiac functions remained unclarified. The randomization in this study depended on the patient preferences (whether or not CPAP treatment is desired). However, real randomization in this context carries ethical considerations. The relatively small number of patients in our study is another limitation. A larger patient population with a longer follow-up would provide more precise results.
5. Conclusions
We have found that the frequency of PVC and NT-proBNP levels can be reduced by CPAP treatment in patients with heart failure and OSAS. CPAP therapy has been shown to significantly improve cardiac functions. Further studies are needed to clarify the possible relationships between CPAP and cardiac functions in detail.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Young T., Palta M., Dempsey J., et al. The occurrence of sleep-disordered breathing among middle-aged adults. New England Journal of Medicine. 1993;328(17):1230–1235. doi: 10.1056/nejm199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Butt M., Dwivedi G., Khair O., Lip G. Y. H. Obstructive sleep apnea and cardiovascular disease. International Journal of Cardiology. 2010;139(1):7–16. doi: 10.1016/j.ijcard.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 3.McNicholas W. T., Bonsigore M. R. Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. European Respiratory Journal. 2007;29(3):156–178. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 4.Pinski M. R. Sleeping with the enemy: the heart in obstructive sleep apnea. Chest. 2002;121(4):1022–1024. doi: 10.1378/chest.121.4.1022. [DOI] [PubMed] [Google Scholar]
- 5.Bradley T. D. Floras, sleep apnea and heart failure. Part I: obstructive sleep apnea. Circulation. 2003;107(12):1671–1678. doi: 10.1161/01.cir.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- 6.Naughton M. T. Heart failure and obstructive apnea. Sleep Medicine Reviews. 1998;2(2):93–103. doi: 10.1016/s1087-0792(98)90002-8. [DOI] [PubMed] [Google Scholar]
- 7.Caples S. M., Wolk R., Somers V. K. Influence of cardiac function and failure on sleep- disordered breathing: evidence for a causative role. Journal of Applied Physiology. 2005;99(6):2433–2439. doi: 10.1152/japplphysiol.00676.2005. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko Y., Floras JS., Usui K., et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apneas. New England Journal of Medicine. 2003;348(13):1233–1241. doi: 10.1056/nejmoa022479. [DOI] [PubMed] [Google Scholar]
- 9.Mansfield D. R., Gollogly N. C., Kaye D. M., et al. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. American Journal of Respiratory and Critical Care Medicine. 2004;169(3):361–366. doi: 10.1164/rccm.200306-752oc. [DOI] [PubMed] [Google Scholar]
- 10.Malone S., Liu P. P., Holloway R., Rutherford R., Xie A., Douglas Bradley T. Obstructive sleep apnoea in patients with dilated cardiomyopathy: effects of continuous positive airway pressure. The Lancet. 1991;338(8781):1480–1484. doi: 10.1016/0140-6736(91)92299-h. [DOI] [PubMed] [Google Scholar]
- 11.Roebuck T., Solin P., Kaye D. M., Bergin P., Bailey M., Naughton M. T. Increased long-term mortality in heart failure due to sleep apnoea is not yet proven. European Respiratory Journal. 2004;23(5):735–740. doi: 10.1183/09031936.04.00060404. [DOI] [PubMed] [Google Scholar]
- 12.Can I., Aytemir K., Demir A. U., et al. P-wave duration and dispersion in patients with obstructive sleep apnea. International Journal of Cardiology. 2009;133(3):e85–e89. doi: 10.1016/j.ijcard.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 13.Maeder M. T., Münzer T., Rickli H., et al. Association between heart rate recovery and severity of obstructive sleep apnea syndrome. Sleep Medicine. 2008;9(7):753–761. doi: 10.1016/j.sleep.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Sumi K., Chin K., Takahashi K., et al. Effect of nCPAP therapy on heart rate in patients with obstructive sleep apnoea-hypopnoea. QJM. 2006;99(8):545–553. doi: 10.1093/qjmed/hcl074. [DOI] [PubMed] [Google Scholar]
- 15.Phillips B. Sleep-disordered breathing and cardiovascular disease. Sleep Medicine Reviews. 2006;9(2):131–140. doi: 10.1016/j.smrv.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Shamsuzzaman A. S., Gersh B. J., Somers V. K. Obstructive sleep apnea. Implications for cardiac and vascular disease. JAMA. 2003;290(14):1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 17.Mehra R., Benjamin E. J., Shahar E., et al. Association of nocturnal arrhythmias with sleep- disordered breathing: the sleep heart health study. American Journal of Respiratory and Critical Care Medicine. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan C. M., Usui K., Floras J. S., et al. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax. 2005;60(9):781–785. doi: 10.1136/thx.2005.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gami A. S., Howard D. E., Olson E. J., Somers V. K. Day-night pattern of sudden death in obstructive sleep apnea. New England Journal of Medicine. 2005;352(12):1206–1214. doi: 10.1056/nejmoa041832. [DOI] [PubMed] [Google Scholar]
- 20.Verschure D. O., van Eck-Smit B. L., Somsen G. A., Knol R. J., Verberne H. J. Cardiac sympathetic activity in chronic heart failure: cardiac (123)I-mIBG scintigraphy to improve patient selection for ICD implantation. Netherlands Heart Journal. 2016;24(12):701–708. doi: 10.1007/s12471-016-0902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rechtschaffen A., Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA, USA: University of California; 1968. [Google Scholar]
- 22.Iber C., Ancoli-Israel S., Chesson A. L., Quan S. F. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st. Westchester, IL, USA: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 23.Vickery S., Price C. P., John R. I., et al. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy. American Journal of Kidney Diseases. 2005;46(4):610–620. doi: 10.1053/j.ajkd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Avidan A. Y. The development of central sleep apnea with an oral appliance. Sleep Medicine. 2006;7(1):85–86. doi: 10.1016/j.sleep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Jonas D. E., Amick H. R., Feltner C., et al. Screening for Obstructive Sleep Apnea in Adults: An Evidence Review for the U.S. Preventive Services Task Force. Rockville, MD, USA: Agency for Healthcare Research and Quality; 2017. Evidence Synthesis No. 146. AHRQ Publication No. 14-05216-EF-1. [PubMed] [Google Scholar]
- 26.Bradley T. D., Floras J. S. Pathophysiologic and therapeutic implications of sleep apnea in congestive heart failure. Journal of Cardiac Failure. 1996;2(3):223–240. doi: 10.1016/s1071-9164(96)80045-5. [DOI] [PubMed] [Google Scholar]
- 27.Fung J. W., Li T. S. T., Choy D. K. L., et al. Severe obstructive sleep apnea is associated with left ventricular diastolic dysfunction. Chest. 2002;121(2):422–429. doi: 10.1378/chest.121.2.422. [DOI] [PubMed] [Google Scholar]
- 28.Dursunoglu N., Dursunoglu D., Ozkurt S., et al. Effects of CPAP on right ventricular myocardial performance index in obstructive sleep apnea patients without hypertension. Respiratory Research. 2006;7(1):p. 22. doi: 10.1186/1465-9921-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanfranchi P. A., Braghiroli A., Bosimini E., et al. Prognostic value of nocturnal Cheyne- Stokes respiration in chronic heart failure. Circulation. 1999;99(11):1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 30.Fries R., Bauer D., Heisel A., et al. Clinical significance of sleep-related breathing disorders in patients with implantable cardioverter defibrillators. Pacing and Clinical Electrophysiology. 1999;22(1):223–227. doi: 10.1111/j.1540-8159.1999.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 31.Adlakha A., Shepard J. W. Cardiac arrhythmias during normal sleep and in obstructive sleep apnea syndrome. Sleep Medicine Reviews. 1998;2(1):45–60. doi: 10.1016/s1087-0792(98)90053-3. [DOI] [PubMed] [Google Scholar]
- 32.Seppälä T., Partinen M., Penttilä A., Aspholm R., Tiainen E., Kaukianen A. Sudden death and sleeping history among Finnish men. Journal of Internal Medicine. 1991;229(1):23–28. doi: 10.1111/j.1365-2796.1991.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 33.Clay R., Kapur V., Gronquist J. M., et al. Risk of obstructive sleep apnea in patients with pacemakers and implantable cardioverter defibrillators and incidence of ventricular arrhythmia. American Journal of Respiratory and Critical Care Medicine. 2016;193:p. A4206. [Google Scholar]
- 34.Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients. The final report. International Journal of Cardiology. 2006;106(1):21–28. doi: 10.1016/j.ijcard.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 35.Bitter T., Westerheide N., Prinz C., et al. Cheyne-Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. European Heart Journal. 2011;32(1):61–74. doi: 10.1093/eurheartj/ehq327. [DOI] [PubMed] [Google Scholar]
- 36.Kilicaslan F., Tokatli A., Ozdag F., et al. Tp-e interval, Tp-e/QT ratio, and Tp-e/QTc ratio are prolonged in patients with moderate and severe obstructive sleep apnea. Pacing and Clinical Electrophysiology. 2012;35(8):966–972. doi: 10.1111/j.1540-8159.2012.03439.x. [DOI] [PubMed] [Google Scholar]
- 37.Topilski I., Rogowski O., Rosso R., et al. The morphology of the QT interval predicts torsade de pointes during acquired bradyarrhythmias. Journal of the American College of Cardiology. 2007;49(3):320–328. doi: 10.1016/j.jacc.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 38.Castro Hevia J., Antzelevitch C., Tornés Bárzaga F., et al. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. Journal of the American College of Cardiology. 2006;47(9):1828–1834. doi: 10.1016/j.jacc.2005.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erikssen G., Liestøl K., Gullestad L., et al. The terminal part of the QT interval (T peak to T end): a predictor of mortality after acute myocardial infarction. Annals of Noninvasive Electrocardiology. 2012;17(2):85–94. doi: 10.1111/j.1542-474x.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X., Xie Z., Chu Y., et al. Association between Tp-e/QT ratio and prognosis in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Clinical Cardiology. 2012;35(9):559–564. doi: 10.1002/clc.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimizu M., Ino H., Okeie K., et al. T-peak to T-end interval may be a better predictor of high-risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clinical Cardiology. 2002;25(7):335–339. doi: 10.1002/clc.4950250706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knebel F., Schimke I., Pliet K., et al. NT-ProBNP in acute heart failure: correlation with invasively measured hemodynamic parameters during recompensation. Journal of Cardiac Failure. 2005;11(5):S38–S41. doi: 10.1016/j.cardfail.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Krittayaphong R., Boonyasirinant T., Saiviroonporn P., et al. Correlation Between NT-pro BNP levels and left ventricular wall stress, sphericity index and extent of myocardial damage: a magnetic resonance imaging study. Journal of Cardiac Failure. 2008;14(8):687–694. doi: 10.1016/j.cardfail.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Sekiguchi Y., Aonuma K., Yamauchi Y., et al. Chronic hemodynamic effects after radiofrequency catheter ablation of frequent monomorphic ventricular premature beats. Journal of Cardiovascular Electrophysiology. 2005;16(10):1057–1063. doi: 10.1111/j.1540-8167.2005.40786.x. [DOI] [PubMed] [Google Scholar]
- 45.Tada H., Ito S., Shinbo G., et al. Significance and utility of plasma brain natriuretic peptide concentrations in patients with idiopathic ventricular arrhythmias. Pacing and Clinical Electrophysiology. 2006;29(12):1395–1403. doi: 10.1111/j.1540-8159.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- 46.Skranes J. B., Einvik G., Namtvedt S. K., et al. Biomarkers of cardiovascular injury and stress are associated with increased frequency of ventricular ectopy: a population-based study. BMC Cardiovascular Disorders. 2016;16:p. 233. doi: 10.1186/s12872-016-0407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Huls van Taxis C. F., Piers S. R., de Riva Silva M., et al. Fatigue as presenting symptom and a high burden of premature ventricular contractions are independently associated with increased ventricular wall stress in patients with normal left ventricular function. Circulation: Arrhythmia and Electrophysiology. 2015;8:1452–1459. doi: 10.1161/circep.115.003091. [DOI] [PubMed] [Google Scholar]
- 48.Tasci S., Manka R., Scholtyssek S., et al. NT-pro-BNP in obstructive sleep apnea syndrome is decreased by nasal continuous positive airway pressure. Clinical Research in Cardiology. 2006;95(1):23–30. doi: 10.1007/s00392-006-0315-9. [DOI] [PubMed] [Google Scholar]
- 49.Strehmel R., Valo M., Teupe C. Natriuretic peptide and high-sensitive troponin t concentrations correlate with effectiveness of short-term cpap in patients with obstructive sleep apnea and coronary artery disease. Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine. 2016;10:33–39. doi: 10.4137/ccrpm.s40939. [DOI] [PMC free article] [PubMed] [Google Scholar]


