Abstract
Objectives
To investigate patterns of industry-sponsored educational events that focus on specific health conditions for which there are concerns about overdiagnosis and overtreatment.
Design and setting
This retrospective cohort study examines publicly reported industry-sponsored events in Australia from October 2011 to September 2015 for three conditions potentially subject to overdiagnosis and overtreatment: depression, osteoporosis and overactive bladder. We used a database of transparency reports to identify events with a focus on depression, osteoporosis and overactive bladder and compared these with other sponsored events. We hypothesised that companies marketing treatments for each condition would sponsor related events and that target audiences would mainly work in primary care, reflecting a broad patient population.
Main outcome measures
Event and attendee characteristics, sponsoring companies, related marketed treatments, cost-effectiveness ratings and dispensing rates.
Results
Over the study period, we identified 1567 events focusing on depression, 1375 on osteoporosis and 190 on overactive bladder (total n=3132, with 96 660 attendees). These events were attended by primary care doctors more often than sponsored events without a focus on these three conditions: relative risk (RR)=3.06 (95% CI 2.81 to 3.32) for depression, RR=1.48 (95% CI 1.41 to 1.55) for osteoporosis and RR=2.59 (95% CI 2.09 to 3.21) for overactive bladder. Servier, which markets agomelatine and AstraZeneca (quetiapine) sponsored 51.2% and 23.0% of depression events, respectively. Amgen and GlaxoSmithKline, which co-market denosumab, sponsored 49.5% of osteoporosis events and Astellas and Commonwealth Serum Laboratories (CSL) (mirabegron and solifenacin) sponsored 80.5% of overactive bladder events.
Conclusions
This 4-year overview of industry-sponsored events on three overdiagnosed and overtreated conditions found that primary care clinicians were often targeted, dinner was often provided and that a few companies sponsored most events. In most cases, sponsors’ products are not cost-effective choices for the specified condition. This pattern highlights the need for professional education to be free of commercial sponsorship.
Keywords: health policy, quality in health care, urinary incontinences, musculoskeletal disorders
Strengths and limitations of this study.
Australia’s transparency reports of industry funding of health professionals were unique internationally until 2015, in that the focus was on sponsored events.
This is the first data-driven national analysis of condition-specific industry educational event sponsorship for overdiagnosed and overtreated conditions.
Classification of events was blinded to sponsor, attendee characteristics and event descriptions.
For each condition, we examined trends over time in sales and dispensing of lead sponsors’ relevant marketed drugs, but we could not assess causal links between increased prescribing and event attendance, as no information was publicly available on the identity of individual event participants.
Limited detail was available on the content of sponsored events; therefore, despite a sensitive search, we may have missed some relevant events per condition.
Introduction
The role of pharmaceutical industry sponsorship of health professional education has been subject to considerable controversy. There is disagreement about whether sponsored education is primarily promotional or educational. It has been described on the one hand as ‘marketing masquerading as education’1 and on the other, if accompanied by proper controls, as able to meet the goal of ‘needs based, relevant, accessible education that is balanced and unbiased and improves healthcare outcomes.’2
This controversy extends to how treatment outcomes are discussed and how conditions are defined. Messages in commercially sponsored education may exaggerate prevalence and/or attempt to medicalise aspects of ordinary life. Identified ‘marketing messages’ in continuing medical education (CME) for low female sexual desire included statements that it is ‘very common and underdiagnosed’ and that ‘women may not be aware that they are sick or distressed.’3 Similarly, US CME sponsored by a testosterone manufacturer supported a broader definition of hypogonadism than in listed indications for testosterone.4
A sponsored CME campaign can reach many health professionals, with potential widespread effects on practice. Purdue Pharma’s launch of the opioid analgesic oxycodone in the USA included over 20 000 sponsored educational events, many of which targeted general practitioners (GPs), potentially contributing to more opioid use in primary care.5
These are product-specific examples of sponsored CME that received media attention and may not reflect broader trends. There has been little exploration of the link between sponsored CME and specific conditions prone to overdiagnosis. Overdiagnosis, the detection of conditions unlikely to lead to ill health, disability or death in the absence of treatment, has been characterised as a ‘modern epidemic’.6 7 It can lead to harm from adverse effects of treatments from which a person is unlikely to benefit, to psychological harm if a healthy person suffers from anxiety or stigma due to disease labelling and to harm to society through higher healthcare costs. There is evidence of commercial influence on overdiagnosis in a range of conditions through direct and indirect marketing aiming to establish the need for a product.7 One consequence of overdiagnosis is overtreatment, as overdiagnosis expands the pool of potentially treatable patients beyond thresholds at which treatment has been shown to be beneficial. The wide ranging influence of industries that benefit from expanded markets has been highlighted as a key driver.8
National patterns of industry sponsorship can shed light on controversies concerning the role of sponsored CME. From 2007 to late 2015, members of Medicines Australia, the national pharmaceutical industry trade association, were required by law to report on sponsored events for health professionals.9 These are described by Medicines Australia as ‘educational events’ and include both accredited CME and a large range of events without accreditation.10
Australia was one of the first countries to require the pharmaceutical industry to publicly report financing of hospitality for health professionals. In 2007, the Australian Competition Tribunal required Medicines Australia—who at the time opposed the move—to introduce mandatory disclosure of industry-sponsored events for health professionals following recommendations by the Australian Competition and Consumer Commission (ACCC). Unlike other countries relying on industry self-regulation of drug promotion via national industry associations, Australia has what could be described as a quasi-regulatory system, requiring approval of self-regulatory standards by a public regulatory body, the ACCC.11
These data provide a unique opportunity to examine the link between condition-specific sponsored events and companies marketing medicines for a condition. Over this 4-year period, 116 845 events are described, varying in scope from a half-hour journal club in a hospital meeting room to several-day conferences, sometimes held overseas.12
We report here on event sponsorship with a focus on three conditions highlighted in the medical literature as potentially subject to overdiagnosis and overtreatment: depression,13 osteoporosis14 and overactive bladder.15 We hypothesise that companies marketing drugs for these conditions are more likely to sponsor events with a focus on that condition than other companies. We also hypothesise that these events tend to target primary care practitioners who are likely to treat milder disease states than specialists. To test these hypotheses, we compare characteristics of the events focusing on these three conditions with other events sponsored by companies during the 4-year study period.
To investigate clinical implications, we examine whether sponsors’ products were judged to be cost-effective and are covered under Australia’s Pharmaceutical Benefits Schemes (PBS). The PBS was introduced in 1948 to subsidise the costs of outpatient medicines for the entire Australian population. The aim is to provide affordable access to needed medicines. An expert committee, the Pharmaceutical Benefits Advisory Committee (PBAC), recommends listing of medicines based on cost-effectiveness considerations that include both therapeutic gains and price. Medicines not listed on the PBS tend to have very limited sales.
We assess sponsorship patterns per condition in terms of audience, clinical versus non-clinical setting and provision of meals. We examine how often events included company-sponsored dinners as events with dinners provided are often held at restaurants and represent a higher value gift. For products for which there was a shift in which company held distribution rights over the study period, we also examine timing of event sponsorship in relation to distribution rights.
Methods
Data sources
We downloaded 301 publicly available company reports covering the period from October 2011 to September 2015 from the Medicines Australia website (www.medicinesaustralia.com.au), converted them from PDF into Excel files, cleaned the data and resolved discrepancies. For example, we removed text from columns that should have contained numeric values only (eg, total cost) and, for a small minority of events, corrected totals equal to less than reported component costs.
These reports include the sponsoring company, timing, event description, venue type, number and profession of attendees, hospitality costs and total event costs. Coding methods are described in detail elsewhere.12 We developed a retrospective cohort of sponsored events based on timing of sponsorship per company over time. A descriptive overview of the data on sponsored events has been published,12 and the dataset used for this analysis is available at http://dx.doi.org/10.4227/11/592631edbd9d5.
We obtained a list of brands sold in Australia for each condition from the Australian Medicines Handbook16 and manufacturers’ websites and annual dispensing data for publicly reimbursed drugs through Australia’s PBS at http://www.pbs.gov.au/info/browse/statistics#AS. For non PBS-subsidised drugs, sales volume data were obtained through Quintiles IMS.
We examined volume of use using annual numbers of dispensed prescriptions for PBS-subsidised drugs and numbers of units sold to retail and hospital pharmacies for non PBS-subsidised drugs.
Selection of targeted conditions
We chose depression, osteoporosis and overactive bladder as illustrative case studies of conditions for which diagnostic thresholds and treatment have extended beyond levels at which patients are likely to benefit. We selected these conditions a priori before carrying out any analyses.
Depression screening leads to many false positives,17 18 and many patients prescribed antidepressants in primary care fail to meet diagnostic criteria for major depression,19 a phenomenon that has been described as ‘medicalising sadness’.13 In 2013, Australia had one of the highest rates of antidepressant use among Organisation for Economic Cooperation and Development (OECD) countries.20
Questions have also been raised about diagnostic criteria for osteoporosis and the role of bone densitometry in greatly expanding the treatable population, primarily when used in screening of asymptomatic postmenopausal women and as a diagnostic tool for women with low-trauma fractures. Bone density screening is poorly predictive of clinical fractures, and a focus on bone density rather than fragility fractures has led to many more diagnoses.14 Further treatment expansion has occurred through lowered thresholds for ‘pre-osteoporosis’ and ‘osteopenia’, which further extend disease labelling to populations that fail to meet established criteria for a diagnosis of osteoporosis.
An ‘imprecise’ symptom-based definition of overactive bladder, largely linked to commercial interests, has replaced urodynamically confirmed bladder instability.15 Tolterodine, marketed by Pharmacia, was the first drug approved for overactive bladder symptoms. In 2002, Pharmacia’s vice president described a threefold expansion of the treatable population through a definition of overactive bladder no longer requiring urinary incontinence.21
Drug treatments for these three conditions have been heavily advertised to the public in the USA, with advertising that relies heavily on emotional appeals, targets women and tends to blur the boundaries between normal life and medical conditions requiring treatment.22
Coding of Medicines Australia data on sponsored events
An initial coding scheme for industry-sponsored events included sponsoring company, location, attendee profession, clinical focus, type of hospitality (such as whether meals or travel and accommodation were provided) and a set of relevant keywords to search unstructured text.12 We designed an additional coding scheme to identify events focusing on the three included conditions. The research team iteratively developed keywords based on disease names/symptoms and drug classes and products sold in Australia (generic and brand names) for each condition. Keywords were used to search unstructured text in the ‘Description of function’ column of reports. All relevant keywords associated with one or more events listed in the database were retained in the final coding scheme (see online supplementary appendix tables 1 and 2). During coding, we concealed other variables (using Excel’s ‘Column Hide’ function) to blind the coder (SS) to sponsor, attendee characteristics and event descriptors.
bmjopen-2017-019027supp001.pdf (198KB, pdf)
Analysis
For each included condition, we provide a detailed analysis for all companies sponsoring at least 5% of events. We examined whether these companies market drugs to treat the condition and PBS reimbursement status for these drugs. We present frequency tables for event and attendee characteristics. Costs are reported in Australian dollars. We performed χ2 analyses to compare events per condition with other sponsored events using SPSS V.22.
Results
Over the 4-year study period, we identified 3132 events focusing on the three conditions, with 96 620 attendees. This included 1567 events with a focus on depression, with 41 474 attendees; 1375 on osteoporosis with 33 916 attendees and 190 on overactive bladder with 21 270 attendees. As no individuals are named, we could not ascertain numbers of repeat attendees. Table 1 summarises event characteristics. Events focusing on these conditions represent 2.7% of sponsored events (n=116 845) over the 4-year period and 2.8% of attendees (n=3 481 750).12
Table 1.
Characteristics of sponsored events for the three conditions
| Total events (n=116 845) | Depression (n=1567) | Osteoporosis (n=1375) | Overactive bladder (n=190) | |
| Attendees | ||||
| Total number of attendees (% of total) | 3 481 750 | 41 472 (1.2) | 33 916 (1.0) | 21 270 (0.6) |
| Median/event (IQR) | 18 (12–25) | 19 (12–28) | 19 (12–27) | 20 (13–34) |
| Clinicians present (% of events) | ||||
| Medical specialists | 80 060 (68.5) | 839 (53.5) | 921 (67.0) | 50 (26.3) |
| Primary care doctors | 24 662 (21.1) | 1159 (74.0) | 638 (46.4) | 132 (69.5) |
| Trainees | 44 774 (38.3) | 222 (14.2) | 531 (38.6) | 18 (9.5) |
| Nurses | 46 214 (39.6) | 357 (22.8) | 359 (26.1) | 46 (24.2) |
| Types of medical specialists present (% of events) | ||||
| Most frequent | Oncology 19 723 (16.9) | Psychiatry 804 (51.3) | Endocrinology 516 (37.5) | Urology 30 (15.9) |
| Second | Surgery 10 670 (9.1) | Geriatrics 55 (3.5) | Rheumatology 190 (13.8) | Ob/Gyn 13 (6.8) |
| Expenses ($A) | ||||
| Total cost of events | $A286 117 928 | $A6 259 581 (2.2%) | $A6 073 333 (2.1%) | $A568 332 (0.2%) |
| Median cost per event (IQR) | $A263 ($A153–1195) | $A1941 ($A659–3264) | $A686 ($A217–2500) | $A2012 ($A765–3370) |
| Median cost per head (IQR) | $A14 ($A10–68) | $A104 ($A48–141) | $A52 ($A13–119) | $A85 ($A31–90) |
| Food and drink cost (% of total cost) | $A84 862 791 (30) | $A2 441 950 (39) | $A2 314 319 (38) | $A2 33 548 (41) |
| Median per event food and drug cost (IQR) | $A197 ($A107–405) | $A911 ($A135–1712) | $A337 ($150–1478) | $A1115 ($A91–1868) |
| Median per head food and drink cost (IQR) | $A12 ($A8–20) | $A57 ($A11–77) | $A17 ($A11–75) | $A55 ($A11–80) |
| Event characteristics (%) | ||||
| Clinical setting | 74 998 (64.2) | 487 (31.1) | 692 (50.3) | 44 (23.2) |
| Any food provided | 1 05 667 (90.4) | 1441 (92.0) | 1298 (94.4) | 158 (83.2) |
| Dinner | 19 873 (17.0) | 811 (51.7) | 512 (37.2) | 41 (21.6) |
| Lunch | 25 935 (22.2) | 241 (15.4) | 485 (35.3) | 28 (14.7) |
| Tea | 14 067 (12.0) | 15 (1.0) | 69 (5.0) | 2 (1.1) |
| Breakfast | 12 806 (11.0) | 24 (1.5) | 77 (5.6) | 7 (3.7) |
| All-day event meals | 3113 (2.7) | 62 (4.0) | 58 (4.2) | 1 (0.5) |
| Unspecified | 29 873 (25.6) | 288 (18.4) | 97 (7.1) | 79 (41.6) |
Ob/Gyn, obstetrics and gynaecology.
For all three conditions, the median number of event attendees (19 to 20) was similar to sponsored events in general.12 For all three conditions, events were held less often in a clinical setting (hospital or clinic) than other sponsored events: relative risk (RR)=0.51 (95% CI 0.50 to 0.53) for depression; RR=0.72 (95% CI 0.68 to 0.76) for osteoporosis; RR=0.47 (95% CI 0.43 to 0.50) for overactive bladder. Nurses were only at 24.4% of condition-focused events compared with 39.6% of total events,12 likely reflecting the less frequent hospital setting. However, attendees were more likely to be primary care physicians (GPs or family medicine) than at other events: RR=3.06 (95% CI 2.81 to 3.32) for depression, RR=1.48 (95% CI 1.41 to 1.55) for osteoporosis, and RR=2.59 (95% CI 2.09 to 3.21) for overactive bladder. Depression and osteoporosis events were also more likely to feature a dinner than other events: RR=1.73 (95% CI 1.64 to 1.82) for depression and RR=1.33 (95% CI 1.27 to 1.38) for osteoporosis. This trend was not seen for overactive bladder.
The median cost per attendee was higher than for events in general ($A14): $A104 for depression, $A52 for osteoporosis and $A85 for overactive bladder (table 1).
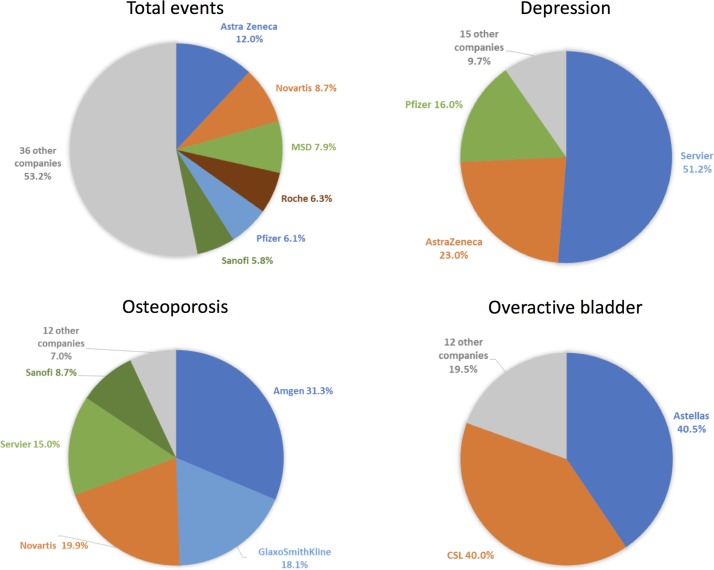
A few companies sponsored most of these condition-focused events. Figure 1 provides an overview of companies sponsoring ≥5% of events. All sell at least one drug for the relevant indication. Table 2 provides an overview of these drugs’ PBS funding status. We present illustrative event descriptions featuring brand names in table 3, with added details on sponsored events per company in online supplementary appendix table 3.
Figure 1.
Per cent of events sponsored by each company, in total and per condition. Companies that sponsored at least 5% of events are listed.
Table 2.
PBS subsidy of drugs marketed for depression, osteoporosis and overactive bladder marketed by sponsoring companies
| Company | Drug for condition (brand) |
PBS subsidy?(Y/N) | PBAC decisions and rationale for restrictions | Notes |
| Depression | ||||
| Servier | Agomelatine (Valdoxan) | No | November 2010: uncertainty; inappropriate comparator July 2011, March 2012: superior clinical effectiveness and safety over SSRIs not demonstrated |
|
| AstraZeneca | Quetiapine (Seroquel XR)* | No for MDD, treatment-resistant depression or anxiety disorders | November 2011: inadequate clinical evidence to support superiority July 2013: non-inferior comparative safety and effectiveness not established |
Quetiapine is PBS-funded for schizophrenia; acute mania and bipolar disorder |
| Pfizer | Desvenlafaxine (Pristiq) | Yes | November 2008: cost minimisation† versus venlafaxine; no evidence of therapeutic advantage | |
| Venlafaxine (Efexor-XR, Altven), sertraline (Zoloft), reboxetine (Edronax), doxepin (Sinequan) | Yes | General schedule‡ listings, major depressive disorder | ||
| Osteoporosis | ||||
| Amgen and GSK | Denosumab (Prolia) | Yes | July 2010: cost-minimisation† versus zoledronic acid November 2011: streamlined authority§, postmenopausal osteoporosis, age 70+, BMD T-score ≤−2.5; cost-minimisation versus alendronate July 2013: superiority versus zoledronic acid rejected; non-inferiority accepted |
2009: co-commercialised by Amgen and GSK; Dec 2015: Amgen reacquires all marketing rights in Australia |
| Novartis | Zoledronic acid (Aclasta) | Yes | July 2008: authority required¶ cost-minimisation versus alendronate; November 2008: listing extended: women aged 70+; BMD T-score≤−3.0 November 2009: extended to men July 2011: 3-year limit removed; listing changed to streamlined authority |
|
| Oestradiol/norethisterone (Estalis Continuous) | Yes | General schedule‡ | ||
| Servier | Strontium (Protos) | No (previously subsidised) | July 2015: restricted to severe established osteoporosis, patients unable to use other drugs, without cardiovascular contraindications August 2016: delisted due to cardiac risks |
|
| Sanofi | Risedronic acid (Actonel, Actonel Ec, Actonel Ec Combi, Actonel Ec Combi D) | Yes | February 2001: postmenopausal osteoporosis; minimal fracture trauma; cost-minimisation versus alendronate December 2001— extended to corticosteroid-induced osteoporosis March 2013: extended to patients aged 70+BMD T-score≤−2.5 |
December 2014: Sanofi transfers marketing rights to Actavis |
| Overactive bladder | ||||
| Astellas | Mirabegron (Betmiga) | No | NA. No request made for PBS listing | |
| CSL and Astellas | Solifenacin (Vesicare) | No | July 2007: uncertain clinical benefit and cost-effectiveness | February 2011—October 2014: marketed by CSL October 2014: Astellas regains marketing rights |
*Immediate release quetiapine products are not indicated for MDD or general anxiety disorder, only Seroquel XR.
†Cost-minimisation: product is considered non-inferior in safety and efficacy to listed comparator; no higher pricing allowed.
‡General schedule: no prior authority required.
§Streamlined authority: no prior approval required, but a streamlined authority code is required on the prescription; if quantities and/or repeats exceed specified levels, treated as authority required.
¶Authority required: telephone or written approval required from Department of Health prior to prescribing.
BMD, bone mineral density; GSK, GlaxoSmithKline; MDD, major depressive disorder; NA, not applicable; PBAC, Pharmaceutical Benefits Advisory Committee; PBS, Pharmaceutical Benefits Scheme; SSRI, selective serotonin reuptake inhibitor.
Table 3.
Illustrative examples of verbatim descriptions of sponsored events
| Sponsor | Date | Event content | Venue | Professionals present | Hospitality | Total cost |
| Servier | May 2012 | 120 minute presentation including a discussion on Valdoxan-specific patient cases | The Sebel Resort, Windsor, NSW | 26 advanced trainees, hospital pharmacists, psychiatrists | Dinner, including alcoholic and non- alcoholic beverages | $A4138.84 |
| Astrazeneca | August 2014 | Educational dinner meeting for GPs with a specialist presentation on ‘The Anxious Depressed Patient’. 1 hour educational content |
Kimberly Gardens St Kilda East VIC |
35 GPs, Psychiatrists | Dinner with alcoholic and non alcoholic beverages | $A3750.00 |
| GSK | June 2015 | HCP osteoporosis presentation. Providing HCPs with the confidence to switch appropriate patients from bisphosphonates to Prolia. GSK was not responsible for organising the educational content. Duration of educational content: 2 hours | GG Restaurant, 105 Yarra St, Geelong VIC 3220 | 38 GPs, Endocrinologists | Three-course dinner, juice/water, non-alcoholic beverage, alcoholic beverage | $A4136.5 |
| Astellas | April 2014 | Educational dinner meeting. Prof Philip Van Kerrebroeck giving educational launch presentation on BETMIGA, the new oral treatment in the management of overactive bladder. One hour educational content. | The Terrace Room (Private Dining), L’Aqua, Sydney, NSW | 10 leading specialists with an interest in OAB—especially Urologists | Food and beverages | $A11 742.95 |
GP, general practitioner; GSK, GlaxoSmithKline; HCP, health care professional; NSW, New South Wales; OAB, overactive bladder; VIC, Victoria.
Depression-related events
Two companies sponsored >80% of depression-related events: Servier (51.2% of events), which markets the antidepressant agomelatine and AstraZeneca (23.0% of events), which markets the antipsychotic quetiapine. The extended-release formulation of quetiapine (brand name Seroquel XR) is approved for depression treatment in patients intolerant to other therapies or with inadequate response. Neither agomelatine nor the depression indication for quetiapine are PBS subsidised. Pfizer, which markets five PBS-subsidised antidepressants (desvenlafaxine, venlafaxine, sertraline, reboxetine, doxepin), was the next most frequent sponsor (16.0% of events).
We examined agomelatine and quetiapine sales volumes over the study period. Agomelatine sales tripled, from 99 625 units in 2012 to 300 103 units in 2015 (28 days treatment/unit). Sales of the extended-release formulation of quetiapine increased from 247 374 units in 2012 to 374 917 in 2015 (60 days treatment/unit). Sales of other AstraZeneca quetiapine formulations decreased over the same period, from 499 445 units sold in 2012 to 202 783 in 201523 (see online supplementary appendix figure 1.1).
Seventy-nine AstraZeneca events focused on ‘the anxious depressed patient’. Figure 2 is an invitation for one of these events, featuring the same image that was used in an advertisement for extended-release quetiapine that appeared in the Medical Journal of Australia. This formulation of quetiapine is also indicated for generalised anxiety disorder.
Figure 2.
Invitation for an AstraZeneca sponsored event.
Osteoporosis-related events
Osteoporosis event sponsorship, similarly, was highly concentrated: Amgen and GSK, which comarket denosumab, sponsored 31.3% and 18.1% of events respectively (in total, 49.4%). Novartis, which markets zoledronic acid, and oestradiol/norethisterone, a hormone therapy approved for osteoporosis prevention in high-risk women intolerant of other products, sponsored 19.9% of events; Servier, which markets strontium, sponsored 15.0%; and Sanofi, which marketed risedronic acid until December 2014, sponsored 8.7%. Denosumab, zoledronic acid, oestradiol/norethisterone and risedronic acid are PBS subsidised; strontium was delisted in August 2016 due to cardiac risks.
Sanofi transferred its marketing rights for risedronate to Actavis in December 2014.24 Sanofi sponsored no osteoporosis events from October 2014 onwards (see online supplementary appendix figure 1.2).
Denosumab dispensations increased nearly sevenfold over the study period, from 45 220 in 2012 to 309 350 in 2015.25 Risedronate, zoledronic acid and strontium dispensations all decreased (see online supplementary appendix figure 1.3) Of 193 events mentioning denosumab’s brand name, Prolia, 104 were sponsored by Amgen and 88 by GSK.
Overactive bladder-related events
Two companies dominated sponsorship of overactive bladder events: Astellas (40.5% of events), which markets mirabegron and solifenacin, the latter after October 2014 and CSL (40.0% of events), which marketed solifenacin from February 2011 to October 2014. Neither drug is PBS subsidised. Astellas did not request PBS reimbursement for mirabegron. PBAC rejected solifenacin in 2007, judging benefits and cost-effectiveness to be uncertain.
All CSL-sponsored overactive bladder events occurred while the company held distribution rights for solifenacin to October 2014. Most Astellas-sponsored events were held from 2014 onwards, when it obtained marketing rights (see online supplementary appendix figure 1.4). Both solifenacin and mirabegron sales increased over the study period (see online supplementary appendix figure 1.5).
Discussion
In this analysis of 3132 Australian pharmaceutical industry-sponsored events with 96 660 attendees, focusing on three clinical conditions prone to overdiagnosis, we found a strong concentration of sponsorship among few companies. Two companies sponsored over 70% of depression events; another two companies over 80% of overactive bladder events. For osteoporosis, the two companies that comarket denosumab sponsored nearly 50% of events.
Several products marketed by key event sponsors were considered unacceptable for PBS reimbursement, and are associated with cost, efficacy and safety concerns that have been flagged internationally. Servier, which sponsored over half of depression-related events, sells agomelatine which is not PBS subsidised. Agomelatine is not approved in the USA or Canada. A French independent drug bulletin, Prescrire, characterised the drug as ‘more dangerous than useful’ and called for its withdrawal in 2015.26 A Spanish bulletin, similarly, considered it ‘worse than first-line antidepressants, up to 15-fold more expensive and a worrying hepatic safety profile.’27
A 2012 Cochrane systematic review found that AstraZeneca’s atypical antipsychotic quetiapine had limited efficacy evidence for depression.28 An updated systematic review, published in 2015, concluded that quetiapine had not been shown to improve function and that methodological biases had exaggerated benefits and minimised harm.29
Like agomelatine, denosumab is on the French bulletin Prescrire’s list of 71 drugs to avoid in 2016 because of ‘a disproportionate risk of adverse events’ including serious infections due to immunosuppression, with only modest efficacy.30 In 2015, half of all new Australian osteoporosis prescriptions were for denosumab.31
All anticholinergic overactive bladder drugs, including solifenacin, have modest benefits, preventing one incontinence episode on average every 2 days, with frequent dry mouth and constipation, and there is observational evidence of dementia risk with longer term use.32 Mirabegron has similar efficacy to anticholinergics32 and can lead to severe hypertension.33
This analysis is limited by the data available. Our analysis only includes 2.7% of events, a likely underestimate as not all event descriptions mention a condition. These three conditions are illustrative case studies and cannot be assumed to represent all condition-related sponsored events. A variety of influences are expected to affect sales trends, including a large range of promotional activities. Sponsored events represent only one aspect of broader promotional campaigns to promote sales.34 However, a strength of this analysis is that it covers all sponsored events in Australia over 4 years, and coding was blinded to sponsor identity, types of attendees, gifts and costs. Due to the unique Australian dataset, this is the first such data-driven national analysis to examine condition-specific event sponsorship.
Company reports on financing of sponsored events provided limited information on content, leaving many questions unanswered. More research is needed on the messages in sponsored education, including on thresholds for disease diagnosis and treatment. Additionally, as individuals were not named, we could not directly evaluate the link between event attendance and individual prescribing patterns.
We could only examine potential contributions to overdiagnosis and overtreatment indirectly. We had hypothesised that events would focus on primary care, reflecting milder disease states. This is a hypothesised association only; we could not directly assess whether the messages in these events promote overdiagnosis or overtreatment. However, nearly two-thirds of events, 62%, for the three conditions were attended by primary care doctors, versus 21% of other events. The focus on primary care was most pronounced for depression events: 74%.
The concentration of sponsorship by companies marketing products subject to safety, cost and efficacy concerns raises questions about influences on prescribing choice. This pattern is consistent with Brody and Light’s hypothesis of an ‘inverse benefit law’, in which intense marketing of drugs that may benefit a small proportion of patients is harmful to public health because a broader patient population is targeted than is likely to benefit.35
Many of these condition-focused events included dinner and were held in non-clinical settings such as restaurants. Costs per person were higher than for events in general. Even small gifts, such as food and drink, can affect behaviour.36 An analysis of US transparency reports found that physicians who receive one or more sponsored meals with a mean value of <US$20 were more likely to prescribe the promoted product, with larger effects observed the more meals received.37 We examined whether overseas travel may have been responsible for higher median costs. In total (n=1 17 845), 1.9% of events were held overseas, and as expected, these events had the highest per person costs. However, only 0.1% of depression-related events, 0.4% of osteoporosis-related events and no overactive bladder events were held overseas. Therefore, this is an unlikely explanation. Travel costs within Australia are not reported separately from other hospitality costs, so we could not examine their contribution to overall costs.
Timing of sponsorship was linked to when a company sold a drug to treat the included condition, consistent with a sales orientation. Companies discontinued event sponsorship of overactive bladder and osteoporosis events when they no longer had marketing rights for a product for these conditions. This promotional orientation is consistent with internal documents released during the US legal case on gabapentin, which described the use of CME to market off-label use.38
In this 4-year overview of industry-sponsored events focusing on depression, osteoporosis and overactive bladder, we found concentrated sponsorship among few companies per condition. These companies mainly market products that are not considered cost-effective choices for the specified conditions. This raises concerns about impacts on prescribing quality and on national prescribing trends. There was a strong focus on primary care physicians, frequent provision of dinner and non-clinical settings. Although a focus on primary care does not necessarily imply promotion of overdiagnosis, promotion in primary care is consistent with a focus on a broader rather than narrower patient population. This observed pattern of event sponsorship raises concerns about the role of industry-sponsored education in conditions identified as prone to overdiagnosis and highlights the need to ensure that professionals have ready access to continuing professional education that is free of commercial sponsorship.
Supplementary Material
Acknowledgments
The authors would like to thank Quintiles IMS for their assistance in providing data on pharmaceutical sales volumes in Australia.
Footnotes
Contributors: BM initiated the study, designed the data coding approach and plans for analysis, assisted with the development of the coding scheme, carried out analyses and drafted and revised the paper. She is the guarantor. SS and AF contributed to the study design and coding scheme, carried out data coding and analysis and revised the draft paper. QG, RM and LB contributed to the study design and coding scheme and revised the draft paper.
Funding: Faculty of Pharmacy, The University of Sydney, provided summer scholarship funding for SS for the work contributed to this study.
Competing interests: BM reported that she was an expert witness on behalf of plaintiffs in a Canadian class action suit concerning cardiovascular risks of a testosterone gel.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data have been made publicly available and can be accessed at http://dx.doi.org/10.4227/11/592631edbd9d5.
All data have been made publicly available and can be accessed at http://dx.doi.org/10.4227/11/592631edbd9d5.
Presented at: Preliminary study results were presented at the Preventing Overdiagnosis Conference in Barcelona, Spain, September 2016.
References
- 1.Moynihan R. Doctors′ education: the invisible influence of drug company sponsorship. BMJ 2008;336:416–7. 10.1136/bmj.39496.430336.DB [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marlow B. Is continuing medical education a drug-promotion tool?: no. Can Fam Physician 2007;532:16504–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Meixel A, Yanchar E, Fugh-Berman A. Hypoactive sexual desire disorder: inventing a disease to sell low libido. J Med Ethics 2015;41:859–62. 10.1136/medethics-2014-102596 [DOI] [PubMed] [Google Scholar]
- 4.Fauber J, Jones C, Fiore K. Slippery slope: testosterone muscles its way to profits. Medpage Today 2015. http://www.medpagetoday.com/special-reports/slipperyslope/54156 (accessed 17 Jan 2017). [Google Scholar]
- 5.Spithoff S. Industry involvement in continuing medical education: time to say no. Can Fam Physician 2014;60:694700–3. [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman JR, Cooper RJ. Overdiagnosis of disease: a modern epidemic. Arch Intern Med 2012;172:1123–4. 10.1001/archinternmed.2012.3319 [DOI] [PubMed] [Google Scholar]
- 7.Moynihan R, Heath I, Henry D. Selling sickness: the pharmaceutical industry and disease mongering. BMJ 2002;324:886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ 2012;344:e3502 10.1136/bmj.e3502 [DOI] [PubMed] [Google Scholar]
- 9.Monk D. Improving transparency in the pharmaceutical industry. Aust Prescr 2016;39:110–1. 10.18773/austprescr.2016.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson J, Moynihan R, Walkom E, et al. . Mandatory disclosure of pharmaceutical industry-funded events for health professionals. PLoS Med 2009;6:e1000128 10.1371/journal.pmed.1000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medicines Australia. Code of Conduct. 18th edn, 2015. https://medicinesaustralia.com.au/wp-content/uploads/sites/52/2010/01/20150617-PUB-Code-Edition-18-FINAL.pdf
- 12.Fabbri A, Grundy Q, Mintzes B, et al. . Pharmaceutical industry-funded events for health professionals: an analysis of data released under Australian transparency rules. BMJ Open 2017;7:e016701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowrick C, Frances A. Medicalising unhappiness: new classification of depression risks more patients being put on drug treatment from which they will not benefit. BMJ 2013;347:f7140 10.1136/bmj.f7140 [DOI] [PubMed] [Google Scholar]
- 14.Järvinen TL, Michaëlsson K, Jokihaara J, et al. . Overdiagnosis of bone fragility in the quest to prevent hip fracture. BMJ 2015;350:h2088 10.1136/bmj.h2088 [DOI] [PubMed] [Google Scholar]
- 15.Tikkinen KA, Auvinen A. Does the imprecise definition of overactive bladder serve commercial rather than patient interests? Eur Urol 2012;61:746–8. 10.1016/j.eururo.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 16.AMH. Australian Medicines Handbook. Adelaide, Australia: AMH, 2016. [Google Scholar]
- 17.Jerant A, Kravitz RL, Fernandez Y Garcia E, et al. . Potential antidepressant overtreatment associated with office use of brief depression symptom measures. J Am Board Fam Med 2014;27:611–20. 10.3122/jabfm.2014.05.140038 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell AJ, Vaze A, Rao S. Clinical diagnosis of depression in primary care: a meta-analysis. Lancet 2009;374:609–19. 10.1016/S0140-6736(09)60879-5 [DOI] [PubMed] [Google Scholar]
- 19.Wong J, Motulsky A, Eguale T, et al. . Treatment indications for antidepressants prescribed in primary care in Quebec, Canada, 2006-2015. JAMA 2016;315:2230–1. 10.1001/jama.2016.3445 [DOI] [PubMed] [Google Scholar]
- 20.Organisation for Economic Cooperation and Development (OECD). Health at a Glance 2015: how does Australia compare? https://www.oecd.org/australia/Health-at-a-Glance-2015-Key-Findings-AUSTRALIA.pdf (accessed 16 Jan 2017).
- 21.Elliott C, Coat W, Hat B. Adventures on the dark side of medicine. Boston, MA, USA: Beacon Press, 2010. [Google Scholar]
- 22.Mintzes B, doctor’ ‘Ask your. ‘Ask your doctor’. Women and direct to consumer advertising : Ford AR, Sabil D, The push to prescribe: women and Canadian drug policy. Toronto: Women’s Press, 2010:15–46. [Google Scholar]
- 23.QuintilesIMS. Quetiapine Fumarate unit sales in Australia, 2012 to 2015. Data provided to authors on request by QuintilesIMS. 2016.
- 24.Pharma Dispatch. Actavis takes back Actonel. 2014. https://pharmadispatch.com/news/actavis-takes-back-actonel (accessed 9 Jan 2017).
- 25.Pharmaceutical Benefits Scheme. Australian Department of Health. ASM_Table_1. Australian Statistics on Medicine. 2012 to 2015. 2016. https://www.pbs.gov.au/info/browse/statistics (accessed 25 Jan 2017).
- 26.Prescrire Editorial Staff. Pour Mieux Soigner: des médicaments à écarter: bilan 2015. La Revue Prescrire 2015;35:144–51. [Google Scholar]
- 27.Anonymous. Worse than first-line antidepressants, up to 15-fold more expensive, and a worrying hepatic safety profile. Drug assessment report drug and therapeutics bulletin of navarre. 2010. DAR no. 3 http://www.navarra.es/home_en/Temas/Portal+de+la+Salud/Profesionales/Documentacion+y+publicaciones/Publicaciones+tematicas/Medicamento/FET/2010/DAR+No+3+Agomelatine.htm
- 28.Komossa K, Depping AM, Gaudchau A, et al. . Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev 2010;12 CD008121 10.1002/14651858.CD008121.pub2 [DOI] [PubMed] [Google Scholar]
- 29.Therapeutics Initiative. Antipsychotics should not be used for non-psychotic depression. 2015. Therapeutics Letter 95 http://www.ti.ubc.ca/2015/09/30/antipsychotics-should-not-be-used-for-non-psychotic-depression/ (accessed 16 Jan 2017). [PubMed]
- 30.Prescrire Editorial Staff. Towards better patient care: drugs to avoid in 2016. Prescrire Int 2016;25:105–11. [PubMed] [Google Scholar]
- 31.McColl G. Chair, drug utilisation sub committee. Drug utilisation committee outcome statement 29-30 september 2016. Canberra, Australia: Pharmaceutical Benefits Advisory Committee, Pharmaceutical Benefits Service, 2016. http://www.pbs.gov.au/industry/listing/elements/dusc-meetings/dos/dusc-dos-sep-2016.pdf [Google Scholar]
- 32.Therapeutics Initiative. Are claims for newer overactive bladder drugs warranted? 2015. Therapeutics Letter 93 http://www.ti.ubc.ca/2015/04/22/are-claims-for-newer-drugs-for-overactive-bladder-warranted/ (accessed 26 Jan 2017). [PubMed]
- 33.Medicines and Health Products Regulatory Agency. Mirabegron (Betmiga): risk of severe hypertension and associated cerebrovascular and cardiac events. Drug Safety Update. 2015. https://www.gov.uk/drug-safety-update/mirabegron-betmiga-risk-of-severe-hypertension-and-associated-cerebrovascular-and-cardiac-events (accessed 20 Jan 2017).
- 34.Gagnon MA, Lexchin J. The cost of pushing pills: a new estimate of pharmaceutical promotion expenditures in the United States. PLoS Med 2008;5:e1 10.1371/journal.pmed.0050001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brody H, Light DW. The inverse benefit law: how drug marketing undermines patient safety and public health. Am J Public Health 2011;101:399–404. 10.2105/AJPH.2010.199844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dana J, Loewenstein G. A social science perspective on gifts to physicians from industry. JAMA 2003;290:252–5. 10.1001/jama.290.2.252 [DOI] [PubMed] [Google Scholar]
- 37.DeJong C, Aguilar T, Tseng CW, et al. . Pharmaceutical industry-sponsored meals and physician prescribing patterns for medicare beneficiaries. JAMA Intern Med 2016;176:1114–10. 10.1001/jamainternmed.2016.2765 [DOI] [PubMed] [Google Scholar]
- 38.Steinman MA, Bero LA, Chren MM, et al. . Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med 2006;145:284–93. 10.7326/0003-4819-145-4-200608150-00008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-019027supp001.pdf (198KB, pdf)