Abstract
OBJECTIVE
The goal of this study was to determine whether plasma levels of advanced glycation end products (AGE) and oxidation products (OP) predict the incidence of cardiovascular disease (CVD) in type 2 diabetes.
RESEARCH DESIGN AND METHODS
Five specific AGE (methylglyoxal hydroimidazolone, carboxymethyl lysine, carboxyethyl lysine, 3-deoxyglucosone hydroimidazolone, and glyoxal hydroimidazolone) and two OP (2-aminoadipic acid and methionine sulfoxide [MetSO]) were measured at baseline in two intensive glucose-lowering studies: 1) a subcohort of the Veterans Affairs Diabetes Trial (VADT) (n = 445) and 2) a nested case-control subgroup from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study (n = 271).
RESULTS
Increased levels of several AGE and OP were associated with older age, decreased kidney function, previous CVD, and longer diabetes duration, but not with hemoglobin A1c. In the VADT, increased risk of incident CVD events (n = 107) was associated with lower MetSO after adjusting for age, race/ethnicity, sex, prior CVD event, kidney function, treatment assignment, and diabetes duration (hazard ratio [HR] 0.53; 95% CI 0.28–0.99; P = 0.047). Individuals with both low MetSO and high 3-deoxyglucosone hydroimidazolone concentrations were at highest risk for CVD (HR 1.70; P = 0.01). In the ACCORD study, those with incident CVD events (n = 136) had lower MetSO (by 14%; P = 0.007) and higher glyoxal hydroimidazolone and carboxymethyl lysine (by 18% and 15%, respectively; P = 0.04 for both); however, only the difference in MetSO remained significant after adjustment for prior CVD event (P = 0.002).
CONCLUSIONS
Lower levels of MetSO and higher levels of select AGE are associated with increased incident CVD and may help account for the limited benefit of intensive glucose lowering in type 2 diabetes.
Introduction
Cardiovascular diseases (CVD) are the major cause of mortality in patients with type 2 diabetes (T2D). Despite a well-established role for chronic hyperglycemia in CVD risk (1), intensive glycemic control for up to 6 years at best modestly reduces the development of CVD in people with longstanding T2D (2–4). These incongruent results raise the possibility that vascular changes resulting from years of chronic hyperglycemia might lessen the effect of intensive glucose lowering in these studies.
Advanced glycation end products (AGE) and related oxidative end products (OP) are reactive intermediates of chronic hyperglycemia with proteins, lipids, and nucleic acids, resulting in long-lasting modification of these substrates (5–7). The formation of AGE and OP is intertwined; increased AGE may lead to oxidative stress (8) and, vice versa, reactive oxygen species (ROS) may facilitate AGE formation (9). Both AGE and OP are increased in diabetes and have been thought to induce, in part, the cardiovascular complications of diabetes (6,7)
In line with in vitro and preclinical evidence linking AGE and OP to atherosclerosis and CVD (reviewed by Shah and Brownlee [5]), several clinical and epidemiological studies indicate an association between various AGE and CVD events (10–19). We recently found a significant association between several plasma AGE and OP and progression of carotid intima media thickness and calcified coronary atherosclerosis in patients with T2D participating in the Veterans Affairs Diabetes Trial (VADT) (20).
In this study we demonstrate that plasma levels of AGE and OP were also associated with the risk of clinical CVD events in these same VADT participants, and we confirm these findings in a nested case-control cohort of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) lipid trial (21).
Research Design and Methods
The samples and the data for this study came from the baseline visits from two independent studies of intensive glucose lowering in patients with T2D. The main cohort was a subset of 445 participants of the VADT (4), which was previously used to study the long-term effects (after VADT) of glucose-lowering therapy on measures of carotid and coronary atherosclerosis (22). The primary CVD outcome for the current study was the first occurrence of any one of a composite of nonfatal macrovascular events, including myocardial infarction; stroke; new or worsening congestive heart failure; surgical intervention for cardiac, cerebrovascular, or peripheral vascular disease; inoperable coronary artery disease; and amputation for ischemic gangrene (4). The confirmatory cohort was a nested case-control subgroup of 271 participants (135 cases, 136 controls) from the ACCORD lipid trial (21). The primary outcome (cases) in the ACCORD trial was the first occurrence of nonfatal myocardial infarction or nonfatal stroke or death from cardiovascular causes (3). Cases and controls were matched by age, sex, race/ethnicity, and study treatment. All participants provided written informed consent to participate in the VADT and ACCORD studies, both of which are registered at clinicaltrials.gov (identifiers NCT00032487 and NCT00000620, respectively).
As described previously (20,23), five dicarbonyl-derived AGE—Nɛ-carboxymethyl lysine (CML), Nɛ-carboxyethyl lysine (CEL), glyoxal hydroimidazolone (G-H1), methylglyoxal hydroimidazolone (MG-H1), and 3-deoxyglucosone hydroimidazolone (3DG-H1)—and two OP, methionine sulfoxide (MetSO) and 2-aminoadipic acid (2-AAA), were measured by liquid chromatography (LC)–mass spectrometry (MS) using internal stable heavy isotope substituted standards (PreventAGE Healthcare Technology). Analysis was performed in a blinded fashion on the plasma filtrate following centrifugation through 10,000 cutoff Amicon filters. This fraction contains free AGE and OP, as well as peptides of various sizes, and the analytical method measured the free products. An Agilent model 6490 Triple Quadrupole MS system with a 1290 Rapid Resolution LC system was used to detect analytes. All AGE and OP were separated and analyzed in a single run using a single Waters X-select HSS T3 column (2.5 μm, 2.1 × 150 mm) with a mobile phase gradient of methanol and water with 0.20% heptafluorobutyric acid. Total analysis time ran 19 min. Interassay coefficients of variation varied from 3.6% (2-AAA) to 9.6% (G-H1).
Statistical analyses were performed with the SAS statistical package version 9.4 (SAS Institute, Cary, NC). Data were log10 transformed if they were not normally distributed. P values <0.05 were considered statistically significant. Between-group differences were assessed with the Student t test for continuous variables and the χ2 test for categorical outcomes. Univariate associations between the continuous variables were assessed by Spearman correlation. Mixed-model ANCOVA was used to test the differences between the groups after adjusting for subject-specific random effects and fixed effects of potential confounders. Risk of incident cardiovascular events in the VADT was analyzed by Cox proportional hazards models adjusted for treatment assignment. Additional adjustments were made for age, race/ethnicity, sex, prior CVD event, kidney function, treatment assignment, and diabetes duration. Combinations of AGE and OP predicting CVD events were selected by stepwise Cox regression, with P < 0.2 for entry into and P < 0.1 for retention in the model. Cumulative probability curves constructed using the Kaplan–Meier method compared subjects at low and high risk, as defined by selected median AGE and OP or their combinations.
Results
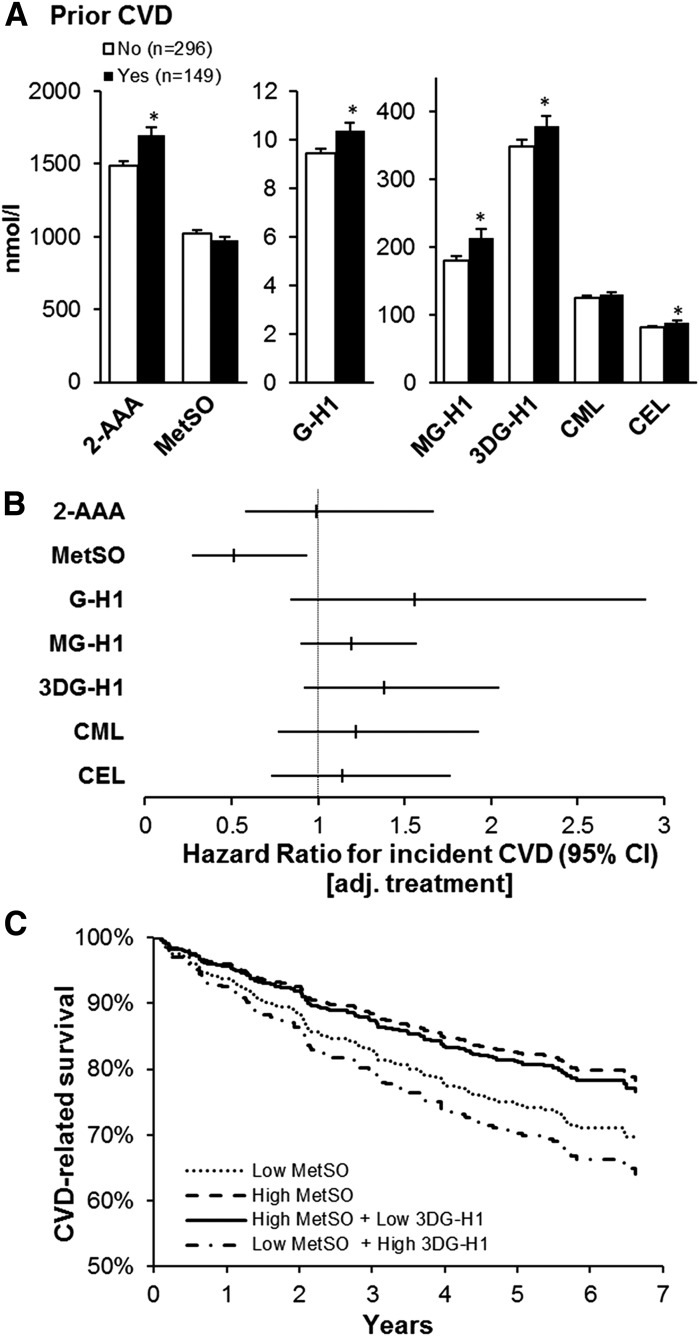
The clinical and demographic characteristics within each subset of participants were comparable with those of the full VADT and ACCORD lipid cohorts, respectively; they comprised predominantly white, overweight or obese men with a median 10-year history of diabetes and suboptimal glycemic control (Supplementary Table 1). In both subset cohorts, all AGE and 2-AAA negatively correlated with kidney function, and several of them positively correlated with age. All AGE and 2-AAA positively correlated with diabetes duration but not with hemoglobin A1c (Table 1). Those with a history of CVD events before enrolling in the VADT had significantly higher 2-AAA (mean ± SD 1,699 ± 639 vs. 1,484 ± 551 nmol/L; P = 0.0002), GH-1 (10.4 ± 4 vs. 9.5 ± 3 nmol/L; P = 0.003), MG-H1 (214 ± 166 vs. 179 ± 135 nmol/L; P = 0.01), 3DG-H1 (379 ± 182 vs. 348 ± 177 nmol/L; P < 0.05), and CEL (88 ± 51 vs. 81 ± 37 nmol/L; P = 0.03) (Fig. 1A). However, only the significant difference in 2-AAA levels persisted after adjusting for age, race/ethnicity, sex, diabetes duration, and kidney function (P = 0.0002).
Table 1.
Association of AGE and OP with clinical characteristics at baseline
| Characteristics, by cohort | 2-AAA | MetSO | G-H1 | MG-H1 | 3DG-H1 | CML | CEL |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| VADT | −0.03 | 0.12* | 0.31§ | 0.23§ | 0.18§ | 0.16‡ | 0.26§ |
| ACCORD | −0.18* | 0.02 | 0.14* | 0.12 | 0.11 | 0.09 | 0.06 |
| BMI | |||||||
| VADT | 0.22§ | −0.03 | −0.01 | 0.03 | 0.01 | 0.02 | 0.00 |
| ACCORD | 0.01 | −0.12 | −0.04 | −0.01 | −0.004 | 0.07 | 0.08 |
| Diabetes duration | |||||||
| VADT | 0.02 | 0.08 | 0.18‡ | 0.17‡ | 0.17‡ | 0.14† | 0.13† |
| ACCORD | −0.11 | 0.00 | 0.11 | 0.12* | 0.08 | 0.18* | 0.17* |
| Hemoglobin A1c | |||||||
| VADT | 0.02 | −0.04 | −0.02 | −0.05 | −0.01 | 0.01 | −0.08 |
| ACCORD | 0.00 | 0.03 | −0.09 | −0.07 | 0.00 | −0.09 | −0.06 |
| GFR | |||||||
| VADT | −0.14† | −0.09 | −0.43§ | −0.29§ | −0.30§ | −0.35§ | −0.45§ |
| ACCORD | −0.15* | −0.11 | −0.50§ | −0.37* | −0.37* | −0.51* | −0.39* |
Data are Spearman correlation coefficients. GFR, glomerular filtration rate (Modification of Diet in Renal Disease formula).
*P < 0.05, †P < 0.01, ‡P < 0.001, §P < 0.0001.
Figure 1.
Baseline plasma OP and AGE, and prevalent and incident CVD, in the VADT. A: OP and AGE by CVD history. Data are mean ± SE. *P < 0.05 between groups. B: Cox proportional HR and 95% CI for treatment-adjusted (adj.) effects of OP and AGE for incident CVD. C: Kaplan-Meier curves of CVD-related survival stratified by high and low MetSO alone or in combination with low and high 3DG-H1, respectively (both stratified by median).
During the VADT, a total of 107 patients within this cohort subset developed a CVD event after a median of 2.3 years (range 0.1–6.6 years). These patients with incident CVD events were older, more likely to be non-Hispanic white, and had a history of a previous CVD event and a longer diabetes duration (Table 2). Higher plasma MetSO concentrations were associated with a lower risk of incident CVD events (Fig. 1B), and this association was independent of age, race/ethnicity, sex, prior CVD event, kidney function, treatment assignment, and diabetes duration (hazard ratio [HR] 0.53 [95% CI 0.28–0.99]; P = 0.047). Although a trend was seen for increased CVD risk for several AGE in the treatment-adjusted models, these did not reach statistical significance (Fig. 1B). In stepwise modeling evaluating all AGE and OP, however, both 3DG-H1 (HR 1.51 [95% CI 1.003–2.27]; P < 0.05) and MetSO (HR 0.45 [95% CI 0.27–0.85]; P = 0.01) were associated with incident CVD. The combination of both low MetSO and high 3DG-H1 further increased the risk for CVD beyond low MetSO alone (Fig. 1C). This combination remained a significant predictor of CVD after adjusting for age, race/ethnicity, sex, prior CVD event, kidney function, treatment assignment, and diabetes duration (HR 1.70 [95% CI 1.12–2.57]; P = 0.01).
Table 2.
Baseline demographic and clinical characteristics by incident CVD events in the VADT and the ACCORD subcohorts included in these analyses
| VADT |
ACCORD |
|||
|---|---|---|---|---|
| CVD (n = 107) | No CVD (n = 338) | CVD (n = 135) | No CVD (n = 136) | |
| Participants receiving intensive treatment | 50 | 52 | 47 | 51 |
| Age (years) | 60 ± 8† | 58 ± 8 | 64 ± 6 | 64 ± 6 |
| Male sex | 99 | 96 | 76 | 75 |
| Non-Hispanic white | 72* | 60 | 72 | 68 |
| Prior CVD | 59§ | 25 | 57§ | 33 |
| BMI (kg/m2) | 31 ± 5 | 31 ± 4 | 32 ± 5 | 32 ± 5 |
| Diabetes duration (years) | 13 ± 8‡ | 10 ± 7 | 12.7 ± 7.3 | 10.5 ± 7 |
| Hemoglobin A1c (% [mmol/mol]) | 9.3 ± 1.3 (79 ± 14) | 9.5 ± 1.5 (80 ± 17) | 8.2 ± 0.9 (67 ± 10) | 8.3 ± 1.0 (67 ± 11) |
| Cholesterol (mmol/L) | ||||
| Total | 4.8 ± 1.5 | 4.7 ± 0.9 | 4.5 ± 0.9 | 4.7 ± 1.0 |
| LDL | 2.8 ± 0.8 | 2.7 ± 0.8 | 2.4 ± 1.0 | 2.7 ± 0.8 |
| HDL | 0.91 ± 0.21 | 0.95 ± 0.26 | 0.93 ± 0.17 | 0.96 ± 0.19 |
| Triglycerides (mmol/L) | 1.8 ± 0.8 | 1.9 ± 0.9 | 2.2 ± 1.1 | 2.4 ± 1.4 |
| GFR (mL/min/1.73 m2) | 80 ± 22 | 83 ± 18 | 86 ± 25 | 90 ± 21 |
Data are mean ± SD or percentages. Incident composite CVD events in the VADT included myocardial infarction; stroke; new or worsening congestive heart failure; surgical intervention for cardiac, cerebrovascular, or peripheral vascular disease; inoperable coronary artery disease; and amputation for ischemic gangrene. Incident composite CVD in the ACCORD included myocardial infarction, stroke, and cardiovascular death. GFR, glomerular filtration rate (Modification of Diet in Renal Disease formula).
*P < 0.05, †P < 0.01, ‡P < 0.001, §P < 0.0001, independent samples t test or χ2 test, as appropriate.
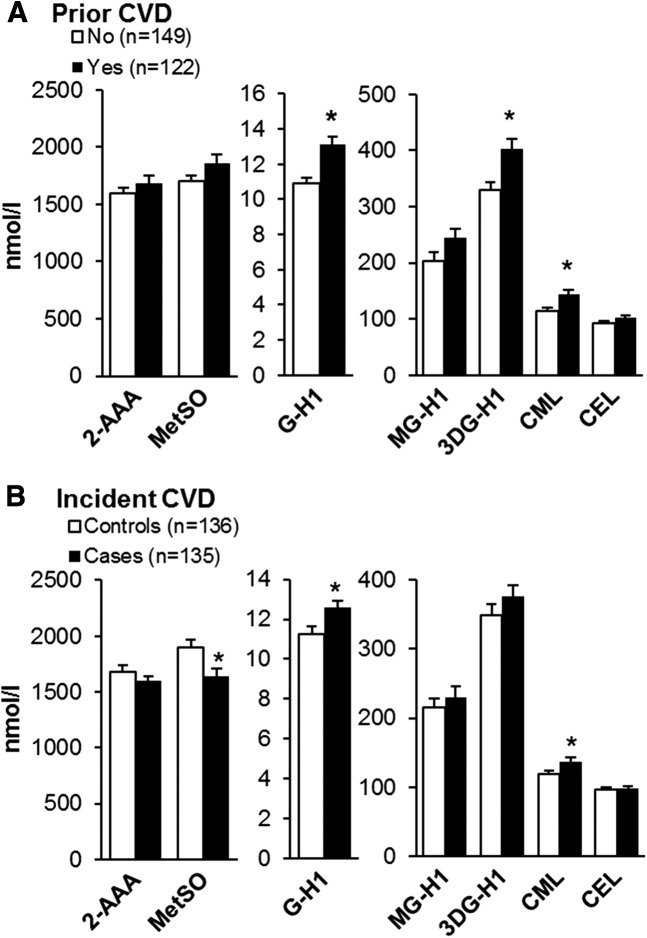
In the ACCORD study, those with prior CVD had higher G-H1 (mean ± SD 13 ± 4 vs. 11 ± 4 nmol/L; P < 0.0001), 3DG-H1 (402 ± 205 vs. 330 ± 165 nmol/L; P = 0.002), and CML (144 ± 72 vs. 115 ± 63 nmol/L; P = 0.0004) (Fig. 2A); however, only the higher G-H1 remained significant (P = 0.007) after adjusting for age, race/ethnicity, sex, diabetes duration, and kidney function. History of CVD was also more common in those with incident CVD (i.e., cases; median time to an event 3 years [range 1–7 years]) than in controls (Table 2). Incident CVD cases had lower MetSO (1,637 ± 802 vs. 1,901 ± 801 nmol/L; P = 0.007) and higher G-H1 (13 ± 4 vs. 11 ± 4 nmol/L) and CML (137 ± 79 vs. 119 ± 56 nmol/L) (P = 0.04 both) (Fig. 2B); however, only the difference in MetSO remained significant after adjustment for CVD history (P = 0.002).
Figure 2.
Baseline plasma AGE and OP in an ACCORD nested case subcohort by history of CVD (A) and incident CVD (B) during the study. Data are mean ± SE. *P < 0.05 between groups.
Conclusions
We found that higher levels of MetSO in both the VADT and ACCORD subset cohorts remained strongly associated with reduced incident CVD events in middle-aged and older patients with a relatively long history of T2D. Several other AGE and OP were also associated with prevalent and incident CVD. Although most AGE associations did not remain significant after adjusting for major risk factors or history of CVD, higher concentrations of 3DG-H1 in combination with lower MetSO levels further enhanced risk for incident CVD in the VADT.
Thus, in two separate cohorts, we observed the novel finding that a significant association exists between lower MetSO and incident CVD. Consistent with these results, a recent preliminary report of type 1 diabetes from the Epidemiology of Diabetes Interventions and Complications (EDIC)/Diabetes Control and Complications Trial (DCCT) cohort showed an inverse association between plasma MetSO and incident CVD (24). Because MetSO is formed by ROS-mediated oxidation of methionine residues of multiple proteins, its inverse association with CVD events seems to contradict the notion of a deleterious effect of oxidative stress on cardiovascular health (5). However, methionine residues on protein molecules have been proposed to constitute an important antioxidant defense mechanism, as a variety of oxidants react readily with methionine to form the less reactive methionine sulfoxide (25). Surface-exposed methionine residues provide an efficient scavenger system and protect other functionally essential residues from oxidative damage. Furthermore, MetSO can be reduced back to methionine by the enzyme methionine sulfoxide reductase, providing a catalytic amplification of the antioxidant potential of each methionine residue (26). Our findings can be interpreted as showing that those with lower MetSO levels have less effective protection against oxidative stress through this pathway, resulting in greater oxidative damage to vascular cells. MetSO formation can also be an important autoregulatory component in cell signaling. For example, methionine sulfoxidation of calcium-calmodulin has been identified to contribute to downregulation of cellular metabolism and ATP utilization and, as a consequence, reduced ROS generation under stress conditions (27).
As a result of their potential to accumulate in tissue over the long term and the ability to initiate and promote vascular damage (28), AGE and select OP are considered important contributors to the development of microvascular and macrovascular complications in T2D (5). In support of this concept, several studies showed increases in various AGE or glycoxidation products in T2D and CVD (10–14). In this analysis using LC-MS in two different cohorts, plasma levels of multiple free AGE and OP adducts were indeed increased in individuals with preexisting CVD; however, these differences seemed to be largely explained by standard CVD risk factors and demographic characteristics. In fact, only 2-AAA in the VADT and G-H1 in the ACCORD cohorts remained significantly higher in those with prevalent CVD after adjusting for these potential confounders. Our results show some similarity to a previous report by Hanssen et al. (10), who detected associations between several AGE and prevalent CVD in individuals with normal glucose metabolism, impaired glucose metabolism, or T2D, although none of these were independent of traditional CVD risk factors.
Consistent with several previous longitudinal studies that have reported associations between plasma AGE and incident CVD (15,18,19,29), we found a nonsignificant trend for an increased risk for CVD for most AGE in the VADT. 3DG-H1 did become a significant predictor of CVD events after inclusion with MetSO in a combined model. This supports the concept that AGE may contribute to CVD; we previously observed a significant positive association between several AGE and OP and long-term progression of atherosclerosis, defined by vascular calcification or carotid intima media thickness, in this same VADT subcohort (20). In the ACCORD subcohort, G-H1 and CML were higher in those who developed incident CVD; however, this increase was partially explained by preexisting CVD. It is possible that median follow-up times of 5.6 and 4.3 years, respectively, may be too short to detect a stronger association between higher AGE and OP and clinical CVD outcomes. In fact, the follow-up time in a previous study demonstrating a significant association between plasma AGE and incident CVD events was nearly 10 years (29).
Weak associations of plasma AGE with clinical markers of glycemic control, as observed in this study, have been noted previously in patients without diabetes (30). This is not surprising, as the majority of free AGE in the circulation originate from long-lived tissue and cellular stores in the body (31) and thus are not readily influenced by ambient glucose control. Consistent with the prolonged accumulation of these products with persistent hyperglycemia and oxidative stress, plasma concentrations of several AGE and OP were directly associated with duration of diabetes. The poor association between AGE and OP with hemoglobin A1c also emphasizes the minor effect of short- to moderate-term glycemia changes on these adducts in the setting of advanced T2D. This may also partly explain the modest protective effect of intensive glucose lowering in patients with advanced T2D observed during both the VADT and ACCORD trial (3,4).
Our study has several potential limitations. Despite a moderate number of CVD events in both study subsets, our cohorts may not have had adequate statistical power to detect small effects of some AGE and OP. In addition, because the original VADT subcohort used in this analysis was designed to examine long-term atherosclerosis changes, it did not include individuals who died during the VADT (20). This may have modified the CVD risk associated with AGE and OP. Because age and renal function are important determinants of death and were associated with higher AGE concentrations, which are in turn associated with all-cause and CVD mortality (15,18), excluding these individuals may have underestimated the association of AGE with CVD. We believe it is important that we found similar associations of CVD with AGE and MetSO in the confirmatory ACCORD subcohort that included CVD death within its primary outcome. Further studies of the full VADT and ACCORD cohorts and their ongoing long-term observational follow-up studies will clarify these issues. Although lower glomerular filtration rate was associated with higher levels of most AGE and 2-AAA in our study, consistent with prior reports (32,33), its association with MetSO was weak and does not account for relationships between MetSO and incident CVD. Although it does not lessen the importance of the associations between AGE and CVD, in this study we could not account for dietary AGE as a source of free AGE in plasma (34,35). However, dietary AGE modestly increase free AGE in plasma, mostly during the postprandial period, and they are cleared relatively quickly via the kidneys (34,36). Thus their contribution to plasma levels was likely small after an overnight fast in our study subjects with preserved kidney function. Finally, the VADT cohort comprised largely white men; thus our results cannot be generalized to the entire population. Of note, however, some previous studies suggest the possibility of a stronger association of selected AGE and incident CVD in women compared with men (15,19).
In conclusion, our results indicate that lower levels of MetSO and higher levels of select AGE are associated with incident cardiovascular events over 3–7 years of follow-up in patients with long-standing T2D. This may help explain the limited benefit of intensive glucose-lowering therapy in these moderate-duration studies.
Supplementary Material
Article Information
Acknowledgments. The authors thank the VADT study participants and study staff and the investigators at the Phoenix, San Diego, Long Beach, Hines, Pittsburgh, Tucson, and Miami VA Medical Centers for participating in this study. The authors also acknowledge the contributions of the Hines VA Cooperative Studies Program Coordinating Center.
Funding. This work was supported by the Veterans Affairs Cooperative Studies Program, Department of Veterans Affairs Office of Research and Development. Additional support was received from the National Institutes of Health (R01-HL110418 [to H.G.] and R01-067690 and 5R01-094775 [to P.D.R.]), the American Diabetes Association (to P.D.R.), and the National Institute of Diabetes and Digestive and Kidney Diseases (Small Business Innovation Research grant 4R44-DK101226-01A1 [to P.J.B.]). B.D.C. is supported by a National Heart Foundation Future Leader Fellowship (no. 100864).
Duality of Interest. S.H. and P.J.B. are employed by PreventAGE Healthcare, where the assays of AGE and OP were performed. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.K., A.S., and P.D.R. conceived and designed the study, analyzed and interpreted the data, and wrote the manuscript. S.H. and P.J.B. measured AGE and OP values. G.B. advised on statistical analysis methods and acquired the data. B.D.C. reviewed and edited the manuscript. H.G. designed the study and reviewed the manuscript. P.D.R. was the principal investigator. All authors reviewed and edited the manuscript, approved the final version, and are accountable for all aspects of the work. J.K. and P.D.R. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Part of this study was presented in abstract form for a late-breaking poster presentation at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1740/-/DC1.
The VADT Investigators are listed in N Engl J Med 2009;360:129–139.
The contents of this study do not represent the views of the Department of Veterans Affairs or the U.S. Government.
References
- 1.Sarwar N, Gao P, Seshasai SR, et al.; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies [published correction appears in Lancet 2010;376:958]. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 3.Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 5.Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016;118:1808–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 8.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 1999;48:1–9 [DOI] [PubMed] [Google Scholar]
- 9.Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 2010;59:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanssen NM, Engelen L, Ferreira I, et al. Plasma levels of advanced glycation endproducts Nε-(carboxymethyl)lysine, Nε-(carboxyethyl)lysine, and pentosidine are not independently associated with cardiovascular disease in individuals with or without type 2 diabetes: the Hoorn and CODAM studies. J Clin Endocrinol Metab 2013;98:E1369–E1373 [DOI] [PubMed] [Google Scholar]
- 11.Kilhovd BK, Berg TJ, Birkeland KI, Thorsby P, Hanssen KF. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care 1999;22:1543–1548 [DOI] [PubMed] [Google Scholar]
- 12.Lapolla A, Piarulli F, Sartore G, et al. Advanced glycation end products and antioxidant status in type 2 diabetic patients with and without peripheral artery disease. Diabetes Care 2007;30:670–676 [DOI] [PubMed] [Google Scholar]
- 13.Dworacka M, Winiarska H, Szymanska M, Szczawinska K, Wierusz-Wysocka B. Serum N-epsilon-(carboxymethyl)lysine is elevated in nondiabetic coronary heart disease patients. J Basic Clin Physiol Pharmacol 2002;13:201–213 [DOI] [PubMed] [Google Scholar]
- 14.Yoshida N, Okumura K, Aso Y. High serum pentosidine concentrations are associated with increased arterial stiffness and thickness in patients with type 2 diabetes. Metabolism 2005;54:345–350 [DOI] [PubMed] [Google Scholar]
- 15.Semba RD, Ferrucci L, Sun K, et al. Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging Clin Exp Res 2009;21:182–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semba RD, Bandinelli S, Sun K, Guralnik JM, Ferrucci L. Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J Am Geriatr Soc 2009;57:1874–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kizer JR, Benkeser D, Arnold AM, et al. Advanced glycation/glycoxidation endproduct carboxymethyl-lysine and incidence of coronary heart disease and stroke in older adults. Atherosclerosis 2014;235:116–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilhovd BK, Juutilainen A, Lehto S, et al. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: a population-based 18 year follow-up study. Diabetologia 2007;50:1409–1417 [DOI] [PubMed] [Google Scholar]
- 19.Kilhovd BK, Juutilainen A, Lehto S, et al. High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: a population-based 18-year follow-up study. Arterioscler Thromb Vasc Biol 2005;25:815–820 [DOI] [PubMed] [Google Scholar]
- 20.Saremi A, Howell S, Schwenke DC, Bahn G, Beisswenger PJ, Reaven PD; VADT Investigators . Advanced glycation end products, oxidation products, and the extent of atherosclerosis during the VA Diabetes Trial and Follow-up Study. Diabetes Care 2017;40:591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginsberg HN, Elam MB, Lovato LC, et al.; ACCORD Study Group . Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saremi A, Anderson RJ, Luo P, et al.; VADT . Association between IL-6 and the extent of coronary atherosclerosis in the Veterans Affairs Diabetes Trial (VADT). Atherosclerosis 2009;203:610–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beisswenger PJ, Howell SK, Russell G, Miller ME, Rich SS, Mauer M. Detection of diabetic nephropathy from advanced glycation endproducts (AGEs) differs in plasma and urine, and is dependent on the method of preparation. Amino Acids 2014;46:311–319 [DOI] [PubMed] [Google Scholar]
- 24.Beisswenger P, Bebu I, Howell S, Pan H, Lachin J; Group TDER . Oxidative and advanced glycation end products predict cardiovascular disease in type 1 diabetes. Diabetes 2017;66(Suppl. 1):A117 [Google Scholar]
- 25.Levine RL, Berlett BS, Moskovitz J, Mosoni L, Stadtman ER. Methionine residues may protect proteins from critical oxidative damage. Mech Ageing Dev 1999;107:323–332 [DOI] [PubMed] [Google Scholar]
- 26.Levine RL, Moskovitz J, Stadtman ER. Oxidation of methionine in proteins: roles in antioxidant defense and cellular regulation. IUBMB Life 2000;50:301–307 [DOI] [PubMed] [Google Scholar]
- 27.Bigelow DJ, Squier TC. Redox modulation of cellular signaling and metabolism through reversible oxidation of methionine sensors in calcium regulatory proteins. Biochim Biophys Acta 2005;1703:121–134 [DOI] [PubMed] [Google Scholar]
- 28.Hanssen NM, Wouters K, Huijberts MS, et al. Higher levels of advanced glycation endproducts in human carotid atherosclerotic plaques are associated with a rupture-prone phenotype. Eur Heart J 2014;35:1137–1146 [DOI] [PubMed] [Google Scholar]
- 29.Hanssen NM, Beulens JW, van Dieren S, et al. Plasma advanced glycation end products are associated with incident cardiovascular events in individuals with type 2 diabetes: a case-cohort study with a median follow-up of 10 years (EPIC-NL). Diabetes 2015;64:257–265 [DOI] [PubMed] [Google Scholar]
- 30.Teichert T, Hellwig A, Peßler A, et al. Association between advanced glycation end products and impaired fasting glucose: results from the SALIA Study. PLoS One 2015;10:e0128293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thornalley PJ, Battah S, Ahmed N, et al. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem J 2003;375:581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieuw-A-Fa ML, van Hinsbergh VW, Teerlink T, et al. Increased levels of N(epsilon)-(carboxymethyl)lysine and N(epsilon)-(carboxyethyl)lysine in type 1 diabetic patients with impaired renal function: correlation with markers of endothelial dysfunction. Nephrol Dial Transplant 2004;19:631–636 [DOI] [PubMed] [Google Scholar]
- 33.Saulnier PJ, Wheelock KM, Howell S, et al. Advanced glycation end products predict loss of renal function and correlate with lesions of diabetic kidney disease in American Indians with type 2 diabetes. Diabetes 2016;65:3744–3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koschinsky T, He CJ, Mitsuhashi T, et al. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci U S A 1997;94:6474–6479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faist V, Erbersdobler HF. Metabolic transit and in vivo effects of melanoidins and precursor compounds deriving from the Maillard reaction. Ann Nutr Metab 2001;45:1–12 [DOI] [PubMed] [Google Scholar]
- 36.Ahmed N, Babaei-Jadidi R, Howell SK, Thornalley PJ, Beisswenger PJ. Glycated and oxidized protein degradation products are indicators of fasting and postprandial hyperglycemia in diabetes. Diabetes Care 2005;28:2465–2471 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.