Abstract
OBJECTIVE
Adiposity and the gut microbiota are both related to the risk of type 2 diabetes. We aimed to comprehensively examine how changes induced by a weight-loss diet intervention in gut microbiota–related metabolites, such as trimethylamine N-oxide (TMAO) and its precursors (choline and l-carnitine), were associated with improvements in adiposity and regional fat deposition.
RESEARCH DESIGN AND METHODS
This study included 510 overweight and obese individuals who were randomly assigned one of four diets varying in macronutrient intake. We examined associations of 6-month changes in blood metabolites (TMAO, choline, and l-carnitine) with improvements in body weight (BW), waist circumference (WC), body fat composition, fat distribution, and resting energy expenditure (REE).
RESULTS
Individuals with a greater reduction of choline (P < 0.0001) and l-carnitine (P < 0.01) rather than TMAO showed significant losses of BW and WC at 6 months. The reduction of choline was significantly predictive of decreases in body fat composition, fat distribution, and REE. Results of sensitivity analysis showed that the baseline diabetes risk status, such as the presence of hyperglycemia (31% of the total participants) and fasting glucose levels, did not modify the associations. Early changes in choline and l-carnitine were significantly predictive of weight loss over 2 years (P < 0.05 for all). Individuals with increases in choline or l-carnitine were 2.35-times (95% CI 1.38, 4.00) or 1.77-times (1.06, 2.95) more likely to fail to lose weight (–5% or more loss) at 2 years.
CONCLUSIONS
Overweight and obese individuals who showed decreases in circulating choline or l-carnitine levels achieved greater improvements of adiposity and energy metabolism by eating a low-calorie weight-loss diet, suggesting that such metabolites are predictive of individuals’ response to the treatment. Further investigations are necessary to confirm our findings, particularly in a population with prediabetes that is more representative of the U.S. population with obesity.
Introduction
Obesity is the most important modifiable risk factors for the development of type 2 diabetes (1), and evidence has consistently demonstrated that successful weight loss contributes to reducing the risk of developing type 2 diabetes among obese patients (2,3). Emerging data have linked the gut microbiome with the host’s adiposity and type 2 diabetes risk (4,5), and studies have suggested that gut microbiome–related metabolites, such as trimethylamine N-oxide (TMAO) and its precursors of choline and l-carnitine, may play a pivotal role in regulating obesity (6–9) and obesity-related chronic metabolic diseases (10–15).
Accumulating evidence has also shown that dietary habit and macronutrient intake would influence TMAO production (13,16–20). TMAO is a small organic compound mainly derived from dietary choline and l-carnitine. Dietary choline and l-carnitine are metabolized by intestinal bacteria to produce trimethylamine (TMA) (10,13), which is in turn absorbed into the bloodstream and oxidized to TMAO by the enzyme flavin monooxygenase 3 (FMO3) in the liver (10,13,21). Hepatic TMAO production is also regulated by insulin signaling and influenced by the presence of obesity and an insulin-resistant state (7,22). Although various low-calorie weight-loss diets are effective for the treatment of obesity and the improvement of body composition and fat distribution (23,24), little has been clarified about how diet-induced changes in the gut microbiota–related metabolites are associated with the improvement of adiposity, body composition, and fat distribution.
Therefore, in the current study of overweight and obese individuals who participated in the Preventing Overweight Using Novel Dietary Strategies (POUNDS Lost) trial, we examined whether diet-induced changes in TMAO, choline, and l-carnitine were associated with the improvements of overall and central adiposity, body fat distribution, and body fat composition during the weight-loss dietary intervention trial. We also investigated whether early changes in these metabolites were significantly predictive of successful long-term weight loss.
Research Design and Methods
Study Participants
The POUNDS Lost trial was a randomized dietary intervention in which 811 individuals who were overweight or obese were assigned to one of four energy-reduced diets varying in the macronutrient composition of fat, protein, and carbohydrate to compare their effects on body weight (BW) change over 2 years (ClinicalTrials.gov, NCT00072995). The study was conducted from October 2004 through December 2007 at two sites: Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital in Boston, MA, and the Pennington Biomedical Research Center of Louisiana State University System, in Baton Rouge, LA. All participants gave written informed consent. The study was approved by each institution’s human subjects committee and by a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute. Two diets were low fat (20%), and the other two diets were high fat (40%), and two diets were average protein (15%), and the other two diets were high protein (25%), which constituted a two-by-two factorial design.
Random assignments to one of four diet groups were generated by the data manager at the coordinating center on request of a study dietitian after eligibility of a participant was confirmed. Randomization by computer occurred after the collection of baseline data and was managed by the study statistician. Investigators and staff who measured outcomes were unaware of the diet assignment of the participants (24). The study was powered to detect a 1.67-kg weight loss as an effect of the level of protein or fat in the diet over the 2-year period, assuming a withdrawal rate of 40% (24).
Major exclusion criteria in this trial were the presence of diabetes or unstable cardiovascular disease, the use of medications that affect BW, and insufficient motivation (24). More details of this trial, such as randomization, blinding, and adherence, have been described in detail elsewhere (24).
Of the total 811 individuals, the current study included 510 participants on the basis of availability of blood samples and measurements of TMAO, choline, and l-carnitine at the baseline examination and at 6 months during the intervention. The baseline BMI was not statistically different between participants who were included (n = 510, BMI 32.6 kg/m2) or were not included (n = 301, BMI 32.9 kg/m2) in the present analysis. Most of the participants were considered normoglycemic based on fasting glucose levels (82% had fasting glucose <5.5 mmol/L) or HbA1c levels (77% had HbA1c <5.7% [39 mmol/mol]). Of the 510 participants, data on weight changes at 2 years were available for 85% (n = 433), which was comparable to the 80% rate of original study participants, as reported previously (24).
Measurements of TMAO, Choline, and l-carnitine
Fasting blood samples were obtained at baseline and 6 months and stored at −80°C. Circulating levels of TMAO, choline, and l-carnitine were measured at Preventive Research Laboratory and Laboratory Diagnostic Core, Cleveland Clinic (Cleveland, OH), and details of the measurements were addressed elsewhere (10–13). Plasma TMAO, choline, and l-carnitine concentrations were measured using stable isotope dilution high-performance liquid chromatography with electrospray ionization tandem mass spectrometry.
Measurements of Adiposity and Energy Expenditure
Height was measured at the baseline examination. BW and waist circumference (WC) were measured in the morning before breakfast at baseline and at 6, 12, 18, and 24 months during the intervention. Body weight was measured by calibrated hospital scales, and WC was measured using a nonstretchable tape measure, 4 cm above the iliac crest. BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2). For the assessment of body composition, a random sample of ∼50% of the total study participants were selected to undergo DEXA scans using a Hologic QDR 4500A (Hologic) after an overnight fast. Total fat mass, total lean mass, whole-body total fat mass percentage, and trunk fat percentage were measured at baseline and at 6 and 24 months during the intervention. Computed tomography (CT) was performed in 50% of a random sample of those participants who had DEXA scans, resulting in a sample of 25% of the total participants. After CT scans were performed, a series of eight single-slice images were obtained every 10 cm from 2 cm below and 5 cm above the fourth and fifth lumbar vertebral interspaces. These contiguous cross-sectional images were analyzed, and the total volume was calculated from the individual slices. Total adipose tissue mass, visceral adipose tissue mass, deep subcutaneous adipose tissue mass, and superficial adipose tissue mass within the abdomen were assessed by standard methods at baseline and at 6 and 24 months. Measurements of resting energy expenditure (REE) were performed for all trial participants at baseline and at 6 and 24 months. Details of the assessment of REE have been reported previously (25).
To investigate how initial (6-month) changes in the metabolites during the intervention were predictive of successful long-term weight loss, we used a cutoff point of –5% of weight loss to define successful or unsuccessful weight loss at 1 year and 2 years based on a previous publication of the POUNDS Lost trial (26). Weight loss of at least 5% of BW has been established as the minimal lower weight-loss bound to obtain clinically meaningful health benefits, including the prevention and treatment of type 2 diabetes and the improvement of cardiometabolic abnormalities (1,27).
Other Measurements
Dietary intake was assessed in a random sample of 50% of the total participants by a review of 5-day diet records at baseline and by 24-h recall during a telephone interview on 3 nonconsecutive days at 6 months and at 2 years to assess the adherence to the dietary intervention. Fasting glucose and fasting insulin concentrations (Diagnostic Products Corporation) were measured at the Pennington Biomedical Research Center Clinical Laboratory, and the HOMA for assessing insulin resistance was calculated.
Statistical Analysis
Data on TMAO, choline, and l-carnitine were log-transformed to improve normality. We first analyzed associations of TMAO, choline, or l-carnitine with adiposity measurements at the baseline examination per 1 log-transformed increase in TMAO, choline, or l-carnitine using general linear models adjusted for age, sex, ethnicity, and diet group. Our primary outcomes were changes in BW and WC during the 2-year intervention. Secondary outcomes were changes in body fat composition, body fat distribution, and REE. We calculated changes in TMAO, choline, and l-carnitine from baseline to 6 months during the intervention and used general linear models to analyze the effect of each 1 log-transformed decrease in these measurements on changes in the outcome measurements. Multivariate-adjusted models were performed, including age, sex, ethnicity, diet group, BMI, value for the respective outcome traits at baseline (except for the outcome of BW), and TMAO, choline, or l-carnitine levels at baseline. We also performed a sensitivity analysis using a multivariate-adjusted model that further included a parental history of diabetes and baseline fasting glucose levels. We tested interactions between the presence of hyperglycemia (elevated fasting glucose ≥5.5 mmol/L or elevated HbA1c concentrations ≥5.7% [39 mmol/mol]) and changes in the metabolite levels for the outcomes at 6 months to investigate whether the baseline diabetes risk status modified the associations. A logistic regression model was performed to calculate odds ratios (ORs) and 95% CIs for a failure of achieving successful weight loss according to 1 SD log-transformed or tertile categories of changes in the metabolites. Statistical analyses were performed with SAS 9.3 software (SAS Institute). All P values were nominal and two-sided, and a P value <0.05 was considered statistically significant.
Results
Median (25th, 75th) values of TMAO, choline, and l-carnitine at the baseline examination were 2.7 (1.8, 3.8) µmol/L, 8.6 (7.4, 10.3) µmol/L, and 34.5 (30.0, 39.3) µmol/L, respectively. Of the total participants, 81% were white and 2.6% were nonsmokers. Mean (SD) values of age (51.5 [9.0] years) and BMI (32.6 [3.8] kg/m2) were comparable to that of the original study participants (24). Mean (SD) values of fasting glucose and HbA1c were 5.1 (0.7) mmol/L and 5.4% (0.4%) (International Federation of Clinical Chemistry and Laboratory Medicine-HbA1c, 36 [5] mmol/mol), respectively. The prevalence of hyperglycemia (fasting glucose ≥5.6 mmol/L or HbA1c ≥5.7%) was 31.2% at the baseline examination. Other characteristics of total study participants are reported in Supplementary Table 1.
At the baseline examination before the diet intervention, higher levels of TMAO were related to higher degrees of trunk fat percentage (P = 0.03) and whole-body total fat mass percentage (P = 0.05) (Table 1). Higher levels of choline were significantly associated with higher degrees of baseline BMI (P = 0.02), WC (P = 0.006), body fat composition (trunk fat percentage [P = 0.009] and whole-body total fat mass percentage [P = 0.01]), and body fat distribution (superficial adipose tissue mass [P = 0.02], visceral adipose tissue mass [P = 0.03], and total adipose tissue mass [P = 0.002]). l-carnitine levels were also positively associated with BMI (P = 0.02), WC (P < 0.001), trunk fat percentage (P = 0.004), whole-body total fat mass percentage (P = 0.02), and visceral adipose tissue mass (P = 0.04) at the baseline examination.
Table 1.
Associations of adiposity measures and TMAO, choline, or l-carnitine before the weight-loss dietary intervention among overweight and obese individuals
| Outcomes | Baseline TMAO |
Baseline choline |
Baseline l-carnitine |
|||
|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | |
| BMI | 0.09 (0.27) | 0.75 | 1.70 (0.74) | 0.02 | 2.01 (0.82) | 0.02 |
| WC | 0.06 (0.76) | 0.94 | 5.78 (2.10) | 0.006 | 8.25 (2.32) | <0.001 |
| Body fat composition | ||||||
| Trunk fat % | 1.06 (0.49) | 0.03 | 3.19 (1.22) | 0.009 | 3.94 (1.35) | 0.004 |
| Whole-body total fat mass % | 0.86 (0.44) | 0.05 | 2.79 (1.09) | 0.01 | 2.88 (1.21) | 0.02 |
| Body fat distribution | ||||||
| Adipose tissue mass | ||||||
| Deep subcutaneous | 0.05 (0.25) | 0.84 | 1.06 (0.54) | 0.05 | 0.73 (0.67) | 0.28 |
| Superficial | −0.04 (0.48) | 0.93 | 2.50 (1.01) | 0.02 | 0.96 (1.27) | 0.45 |
| Visceral | 0.24 (0.29) | 0.41 | 1.35 (0.61) | 0.03 | 1.53 (0.75) | 0.04 |
| Total | 0.10 (0.66) | 0.88 | 4.35 (1.37) | 0.002 | 2.79 (1.74) | 0.11 |
β (SE) per 1 increase of log-transformed TMAO, choline, or l-carnitine for differences in the adiposity measures after adjustment for age, sex, ethnicity, and diet group.
During the 6 months after the diet intervention, we found that there was a large interindividual variability of 6-month changes in TMAO (range –24.4 to 22.4 μmol/L; median [25th, 75th], 0 [–1.2, 1.3] µmol/L), choline (range –8.8 to 21.5 μmol/L; median [25th, 75th], –0.2 [–1.4, 1.0] µmol/L), and l-carnitine (range –27.3 to 28.4 μmol/L; median [25th, 75th], 0.2 [–3.4, 3.2] µmol/L) across the study participants. No significant differences were found in mean or median values of changes in TMAO, choline, or l-carnitine across the different diets varying in fat, protein, or carbohydrate (Supplementary Table 2). Individuals who showed increases in l-carnitine were more likely to have a higher intake of total energy and higher values of urinary nitrogen excretion at 6 months (Supplementary Table 3).
When we investigated associations between changes in the metabolite levels and the improvement of adiposity, we found that greater decreases in choline and l-carnitine, rather than TMAO, were significantly associated with greater reductions in BW and WC at 6 months after adjusting for covariates (Table 2). On one hand, each 1 log-transformed decrease in choline was associated with a greater reduction of BW (β [SE] –7.64 [1.12], P < 0.0001) and WC (β [SE] –6.48 [1.22], P < 0.0001) at 6 months. On the other hand, greater decreases in TMAO tended to be related to increases in BW and WC at 6 months, although the results were not statistically significant. No significant interactions were found between diet groups (high- or low-protein diets, and high- or low-fat diets) and changes in these metabolites on the improvements of BW and WC at 6 months. For the changes in body fat composition and fat distribution, decreases in choline levels were particularly significantly associated with greater reductions of whole-body total fat mass percentage, trunk fat percentage, deep subcutaneous adipose tissue mass, superficial adipose tissue mass, visceral adipose tissue mass, and total adipose tissue mass, as well as REE. Interestingly, greater decreases in TMAO were associated with increases in whole-body total fat mass percentage, trunk fat percentage, and total adipose tissue at 6 months (P < 0.05 for all).
Table 2.
Changes (Δ) in obesity measurements and energy expenditure at 6 months per 1 log-transformed decreases in TMAO, choline, and l-carnitine levels
| Outcomes | ΔTMAO |
ΔCholine |
Δl-carnitine |
|||
|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | |
| ΔBW | 0.52 (0.41) | 0.21 | −7.64 (1.12) | <0.0001 | −5.39 (1.45) | 0.0002 |
| ΔWC | 0.66 (0.44) | 0.13 | −6.48 (1.22) | <0.0001 | −4.43 (1.55) | 0.004 |
| ΔWhole-body total fat mass % | 0.77 (0.31) | 0.01 | −2.71 (0.85) | 0.002 | −1.68 (0.97) | 0.09 |
| ΔTrunk fat % | 1.07 (0.42) | 0.01 | −3.64 (1.13) | 0.002 | −2.35 (1.31) | 0.07 |
| ΔΑdipose tissue mass | ||||||
| ΔDeep subcutaneous | 0.27 (0.15) | 0.07 | −1.01 (0.39) | 0.01 | −0.59 (0.42) | 0.16 |
| ΔSuperficial | 0.58 (0.29) | 0.05 | −1.88 (0.75) | 0.01 | −0.61 (0.73) | 0.4 |
| ΔVisceral | 0.22 (0.16) | 0.18 | −1.01 (0.42) | 0.02 | −0.42 (0.45) | 0.36 |
| ΔTotal | 0.94 (0.45) | 0.04 | −3.14 (1.18) | 0.009 | −1.19 (1.13) | 0.29 |
| ΔREE | −13.2 (10) | 0.19 | −71.5 (27.9) | 0.01 | −36.5 (35.4) | 0.3 |
β (SE) represents changes of the outcomes when the circulating metabolite levels were decreased during the diet intervention.
Data after adjustment for age, sex, ethnicity, diet group, BMI, value for the respective outcome traits at the baseline examination (except for the outcome ΔBW), and TMAO, choline, or l-carnitine levels at baseline.
Results were fundamentally the same when we further adjusted for a parental history of diabetes and baseline fasting glucose levels in the sensitivity analysis (Supplementary Table 4). When we examined whether the baseline diabetes risk status modified the associations, there were no significant interactions between the presence of hyperglycemia (elevated fasting glucose or HbA1c concentrations) and changes in the metabolite levels on the outcomes (6-month changes in adiposity measures or REE) (Supplementary Table 5).
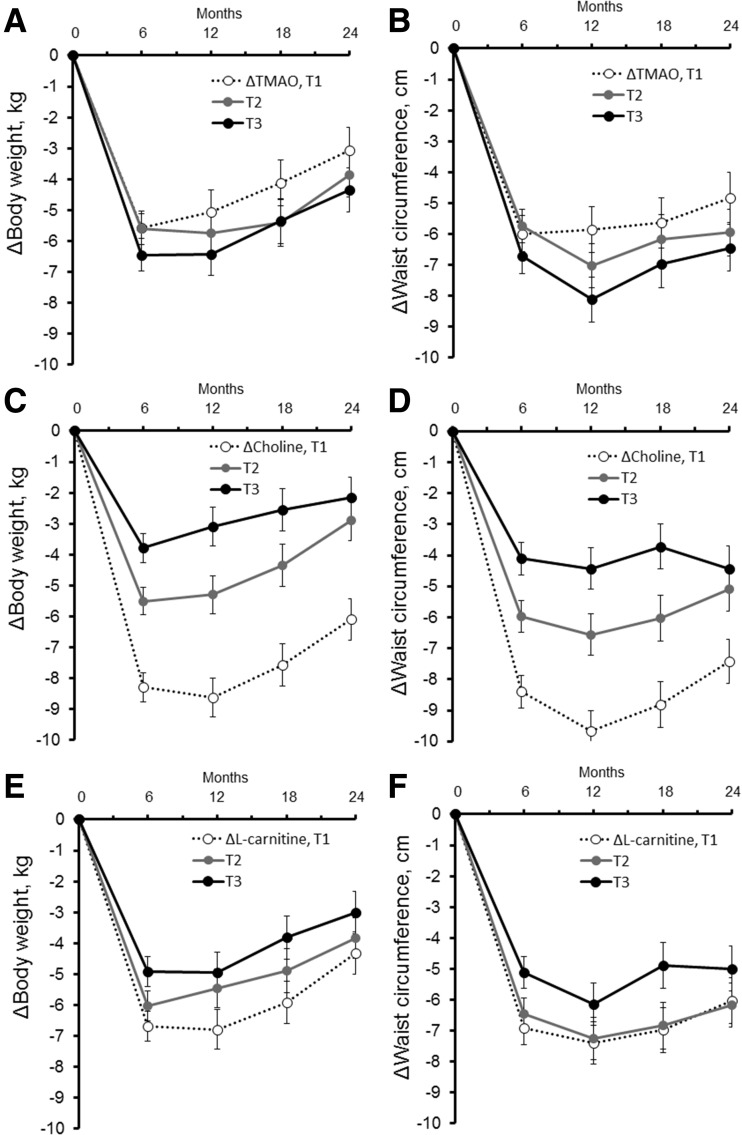
We then investigated whether the initial (6-month) changes in these metabolites were significantly predictive of long-term (12-, 18-, and 24-month) improvement of adiposity (Fig. 1 and Supplementary Table 6). We observed that individuals with decreases of choline (tertile 1: median [25th, 75th], –2.0 [–2.9, –1.4] µmol/L) particularly showed large reductions in BW (Fig. 1C) and WC (Fig. 1D) over 2 years. The initial decreases in choline were significantly predictive of 2-year improvement of body fat composition and visceral adipose tissue (Supplementary Table 6). Also, initial decreases in l-carnitine were significantly predictive of 12- and 18-month changes in BW and WC as well as of 2-year improvement of BW.
Figure 1.
Trajectories of changes in BW and WC according to initial changes (Δ) in TMAO (panels A and B), choline (panels C and D), and l-carnitine (panels E and F). Data were adjusted for age, sex, ethnicity, diet group, value for the respective outcome traits (BW or WC) at the baseline examination, and value for the respective metabolites (TMAO, choline, or l-carnitine) at the baseline examination. The lowest tertile (T) category indicates the largest reduction of circulating metabolites from the baseline to 6 months. For ΔTMAO (from baseline to 6 months), median (25th, 75th) values were T1 (n = 170): –2.0 (–3.5, –1.2) µmol/L; T2 (n = 170): 0 (–0.3, 0.3) µmol/L; and T3 (n = 170): 1.9 (1.3, 4.0) µmol/L, respectively. For Δcholine, median (25th, 75th) values were T1 (n = 170): –2.0 (–2.9, –1.4) µmol/L; T2 (n = 170): –0.2 (–0.5, 0.2) µmol/L; and T3 (n = 170): 1.6 (1.0, 2.1) µmol/L. For Δl-carnitine, median (25th, 75th) values were T1 (n = 169): –5.0 (–7.5, –3.4) µmol/L; T2 (n = 171): 0.2 (–1.0, 1.1) µmol/L; and T3 (n = 170): 4.7 (3.2, 6.7) µmol/L.
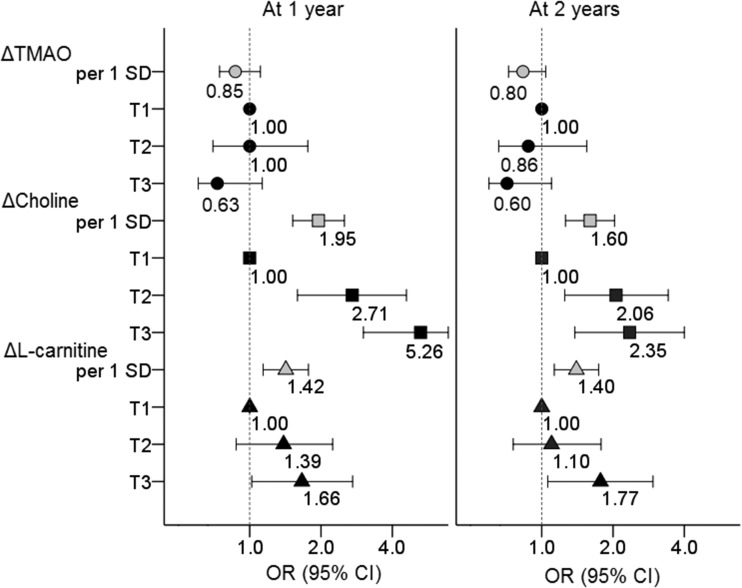
Finally, we investigated whether the initial changes (Δ) in TMAO, choline, and l-carnitine were significantly predictive of successful weight loss at 1 year and at 2 years (Fig. 2). Of the study participants, 43.6% (n = 199 of 456) failed to achieve successful weight loss at 1 year and 58.7% (n = 254 of 433) failed at 2 years. On one hand, we did not find a significant association between ΔTMAO and the probability of successful weight loss. On the other hand, we found that the initial changes in choline and l-carnitine were significantly predictive of weight-loss failure over 2 years during the intervention. Compared with participants in the lowest tertile (T1) group of Δcholine (median [25th, 75th], –2.0 [–2.9, –1.4] µmol/L), those in the T2 group (–0.2 [–0.5, 0.2] µmol/L) or the T3 group (1.6 [1.0, 2.1] µmol/L) of Δcholine showed an elevated of OR of 2.06 (95% CI 1.25, 3.42) or an OR of 2.35 (95% CI 1.38, 4.00), respectively, for weight-loss failure at 2 years. For associations of Δl-carnitine with weight loss, compared with the T1 group of Δl-carnitine (median [25th, 75th], –5.0 [–7.5, –3.4] µmol/L), the T3 group of Δl-carnitine (4.7 [3.2, 6.7] µmol/L) showed a significantly increased risk of weight-loss failure at 1 year (OR 1.66; 95% CI 1.02, 2.72) and also at 2 years (OR 1.77; 95% CI 1.06, 2.95).
Figure 2.
Probability of a failure of successful weight loss at 1 year and 2 years according to initial changes (Δ) in TMAO, choline, and l-carnitine. ORs after adjustment for age, sex, ethnicity, BMI, diet group, and baseline value of TMAO, choline, or l-carnitine. The lowest tertile (T) category indicates the largest reduction of circulating metabolites from the baseline to 6 months. The gray symbols represent ORs for the outcomes per 1 SD log-transformed changes in the metabolites; the black symbols represent ORs for the outcomes across tertile categories of changes in the metabolites. For ΔTMAO (from baseline to 6 months), median (25th, 75th) values were T1 (n = 170): –2.0 (–3.5, –1.2) µmol/L; T2 (n = 170): 0 (–0.3, 0.3) µmol/L; and T3 (n = 170): 1.9 (1.3, 4.0) µmol/L, respectively. For Δcholine, median (25th, 75th) values were T1 (n = 170): –2.0 (–2.9, –1.4) µmol/L; T2 (n = 170): –0.2 (–0.5, 0.2) µmol/L; and T3 (n = 170): 1.6 (1.0, 2.1) µmol/L. For Δl-carnitine, median (25th, 75th) values were T1 (n = 169): –5.0 (–7.5, –3.4) µmol/L; T2 (n = 171): 0.2 (–1.0, 1.1) µmol/L; and T3 (n = 170): 4.7 (3.2, 6.7) µmol/L.
Conclusions
In this long-term dietary intervention trial, we found that circulating choline and l-carnitine levels decreased among participants who had greater improvements of adiposity after eating a low-calorie weight-loss diet. Further, more significant decreases in choline were strongly associated with larger reductions in body fat composition, fat distribution, and energy expenditure. Our results indicated that initial (6-month) changes in circulating precursors of TMAO, rather than TMAO itself, were significantly predictive of successful long-term weight loss, suggesting that such metabolites of l-carnitine and choline could be markers for assessing the effectiveness of a long-term dietary intervention.
At the baseline examination, we observed a positive correlation between adiposity and TMAO, choline, and l-carnitine, partly in line with findings of previous studies that reported higher levels of choline or l-carnitine were related to higher BMI and greater visceral adiposity (6,16,17). A positive correlation between TMAO levels and visceral adiposity was also reported (7), and a recent animal study showed that the TMAO-generating enzyme FMO3 regulates obesity and beiging of white adipose tissue (8). We, however, observed that decreases in choline or l-carnitine levels, but not TMAO levels, were significantly associated with the improvements in BW, WC, body fat composition, and fat distribution at 6 months. Studies in animal models suggest important links between choline/1-carbon metabolism and energy homeostasis (9), and mice fed a choline-deficient diet became hypermetabolic and lost weight (28) and also showed an improvement of insulin sensitivity (29). We found, particularly, that changes in choline were correlated with changes in REE, and previous analyses in the POUNDS Lost trial showed that REE fell after weight loss with an adaptive thermogenesis (25).
Accumulating evidence has also shown that gut microbiota may affect body adiposity of the host (4,30), probably through regulating the metabolism of ingested food and energy balance (30,31). Dietary choline and l-carnitine are most abundant in animal foods, such as eggs, red meats, and fish, that are rich in dietary protein, and we observed that urinary nitrogen excretion, which is a biomarker of protein intake and density, was significantly higher at 6 months in individuals with increases in choline and l-carnitine. Markers of dietary protein were also associated with successful weight loss in previous study results of the POUNDS Lost trial (32). The production of TMAO is regulated by different pathways such as metabolism by the gut microbiome of dietary quaternary amines (choline, l-carnitine, betaine, and phosphatidylcholine) and host hepatic FMOs (13,33). Therefore, we speculate that the diet-induced weight loss might have been more clearly reflected by changes in precursors of TMAO (choline and l-carnitine) rather than TMAO itself. Previous studies reported that the hepatic TMAO production was regulated by insulin signaling and influenced by the presence of obesity and an insulin-resistant state (7,22), and thus, we also speculated that improvement of insulin sensitivity along with weight loss might contribute to the increases in TMAO production among our study participants. Increases in TMAO levels might reflect the interesting adaptive effect on the gut microbiota composition; however, these results were hard to determine in this current study because the associations were not significant and gut microbiota samples were not available. Similar to our findings, a few studies of morbidly obese patients showed that levels of choline (6,34) and l-carnitine (6) decreased after bariatric surgery along with weight loss and also that circulating levels of TMAO significantly increased after the bariatric surgery (6,34).
How to predict successful long-term weight-loss based on information obtained in the early period of intervention is a topic of debate. Moderate weight loss (5–7%) is beneficial to preventing the progression from prediabetes to type 2 diabetes, improving glycemic control, and reducing the need for glucose-lowering medications (2,3,35,36). Sustaining weight loss may be challenging, however, and we observed that more than half of the participants regained BW from 6 months to 2 years during the intervention (24). Previous studies reported that early weight loss and behavioral adherence were predictive of long-term weight-loss outcomes (26,37,38), and our study newly showed that early changes in the metabolites of choline and l-carnitine were significantly predictive of a failure for achieving successful weight loss over 2 years. Although we could not determine whether the effect of changes in the metabolites on the long-term weight-loss success was causal in this study of obese participants, our study suggested that there was large interindividual variability in changes of the metabolites after the intervention and that such variability was significantly predictive of successful long-term weight loss. Further studies would be warranted to investigate associations of changes in TMAO and its precursors with the risk of type 2 diabetes.
Our study has several strengths. We assessed changes in circulating TMAO, choline, and l-carnitine levels in thus far the largest and longest weight-loss diet-intervention trial. Our study participants were free of diagnosed diabetes or unstable cardiovascular diseases that may affect the levels of these metabolites. The robust results of the metabolites and adiposity measurements at multiple follow-up assessments strength our conclusion.
Several limitations merit comment. We did not collect data on gut microbiota in this study and could not assess the role of microbiota itself in the associations of the metabolites and adiposity. There might be endogenous or exogenous nutrients sources that would affect the metabolite levels. The association between these metabolites and weight loss and its relationship with microbiome should be further investigated. Our study included only individuals who were overweight or obese who participated in the clinical trial, and how changes in these metabolites are associated with weight-loss outcomes in the general population needs to be further confirmed in other studies. Further, compared with characteristics of a general population of the U.S., such as among participants in the National Health and Nutrition Examination Survey (NHANES) (39), our study participants were mainly white, well-educated (70% were college graduate or beyond), and nonsmokers, which might limit the generalizability of our findings to other populations. In addition, although the baseline diabetes risk status did not modify our main findings, the prevalence of prediabetes in the current study (31% of the total overweight and obese participants in this study) was lower compared with an estimate of the NHANES (∼37–38% of the overall population) (40). Therefore, further research would be necessary to confirm our findings, especially in a population that is more representative of the U.S. population with overweight and obesity.
In conclusion, decreases in circulating choline and l-carnitine levels were significantly predictive of greater improvement of adiposity after eating a low-calorie weight-loss diet. Early changes in circulating choline or l-carnitine levels were significantly predictive of long-term successful weight loss and individuals’ response to the treatment, regardless of the different diet intervention.
Supplementary Material
Article Information
Acknowledgments. The authors thank all of the participants in the study for their dedication and contribution to the research. The authors also thank the Preventive Research Laboratory and Laboratory Diagnostic Core, Cleveland Clinic, for the measurements.
Funding. The study is supported by National Institutes of Health grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, and HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK-115679, DK-091718, DK-100383, and DK-078616), the Boston Obesity Nutrition Research Center (DK-46200), and United States–Israel Binational Science Foundation grant 2011036. Y.H. is a recipient of a Grant-in-Aid for Scientific Research and Postdoctoral Fellowship for Research Abroad from the Japan Society for the Promotion of Science. L.Q. was a recipient of the American Heart Association Scientist Development Award (0730094N).
The sponsors had no role in the design or conduct of the study.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.H. contributed to the study concept and design, analysis and interpretation of data, drafting and revising the manuscript, statistical analysis, and study supervision. D.S. contributed to analysis and interpretation of data and to drafting and revising the manuscript. S.R.S., G.A.B., and F.M.S. contributed to acquisition and interpretation of data and to drafting and revising the manuscript. L.Q. contributed to the study concept and design, acquisition of data, analysis and interpretation of data, drafting and revising the manuscript, statistical analysis, and funding and supervised the study. L.Q. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00072995, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-2108/-/DC1.
References
- 1.Jensen MD, Ryan DH, Apovian CM, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society . 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014;129(Suppl. 2):S102–S138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merlotti C, Morabito A, Ceriani V, Pontiroli AE. Prevention of type 2 diabetes in obese at-risk subjects: a systematic review and meta-analysis. Acta Diabetol 2014;51:853–863 [DOI] [PubMed] [Google Scholar]
- 3.Schwarz PE, Greaves CJ, Lindström J, Yates T, Davies MJ. Nonpharmacological interventions for the prevention of type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:363–373 [DOI] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. . A core gut microbiome in obese and lean twins. Nature 2009;457:480–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin J, Li Y, Cai Z, et al. . A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012;490:55–60 [DOI] [PubMed] [Google Scholar]
- 6.Trøseid M, Hov JR, Nestvold TK, et al. . Major increase in microbiota-dependent proatherogenic metabolite TMAO one year after bariatric surgery. Metab Syndr Relat Disord 2016;14:197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randrianarisoa E, Lehn-Stefan A, Wang X, et al. . Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci Rep 2016;6:26745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schugar RC, Shih DM, Warrier M, et al. . The TMAO-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Reports 2017;20:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeisel SH. Metabolic crosstalk between choline/1-carbon metabolism and energy homeostasis. Clin Chem Lab Med 2013;51:467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Klipfell E, Bennett BJ, et al. . Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Tang WH, Buffa JA, et al. . Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 2014;35:904–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang WH, Wang Z, Levison BS, et al. . Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koeth RA, Wang Z, Levison BS, et al. . Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shan Z, Sun T, Huang H, et al. . Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr 2017;106:888–894 [DOI] [PubMed] [Google Scholar]
- 15.Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc 2017;6:e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malinowska AM, Szwengiel A, Chmurzynska A. Dietary, anthropometric, and biochemical factors influencing plasma choline, carnitine, trimethylamine, and trimethylamine-N-oxide concentrations. Int J Food Sci Nutr 2017;68:488–495 [DOI] [PubMed] [Google Scholar]
- 17.Rohrmann S, Linseisen J, Allenspach M, von Eckardstein A, Müller D. Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. J Nutr 2016;146:283–289 [DOI] [PubMed] [Google Scholar]
- 18.Boutagy NE, Neilson AP, Osterberg KL, et al. . Short-term high-fat diet increases postprandial trimethylamine-N-oxide in humans. Nutr Res 2015;35:858–864 [DOI] [PubMed] [Google Scholar]
- 19.Boutagy NE, Neilson AP, Osterberg KL, et al. . Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity (Silver Spring) 2015;23:2357–2363 [DOI] [PubMed] [Google Scholar]
- 20.Miller CA, Corbin KD, da Costa KA, et al. . Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am J Clin Nutr 2014;100:778–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. . Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 2013;17:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao J, Ling AV, Manthena PV, et al.; Morbid Obesity Study Group . Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun 2015;6:6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston BC, Kanters S, Bandayrel K, et al. . Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA 2014;312:923–933 [DOI] [PubMed] [Google Scholar]
- 24.Sacks FM, Bray GA, Carey VJ, et al. . Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Jonge L, Bray GA, Smith SR, et al. . Effect of diet composition and weight loss on resting energy expenditure in the POUNDS LOST study. Obesity (Silver Spring) 2012;20:2384–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas DM, Ivanescu AE, Martin CK, et al. . Predicting successful long-term weight loss from short-term weight-loss outcomes: new insights from a dynamic energy balance model (the POUNDS Lost study). Am J Clin Nutr 2015;101:449–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Diabetes Association Lifestyle management. Sec. 4. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(Suppl. 1):S33–S43 [DOI] [PubMed] [Google Scholar]
- 28.Rizki G, Arnaboldi L, Gabrielli B, et al. . Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res 2006;47:2280–2290 [DOI] [PubMed] [Google Scholar]
- 29.Raubenheimer PJ, Nyirenda MJ, Walker BR. A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes 2006;55:2015–2020 [DOI] [PubMed] [Google Scholar]
- 30.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–1031 [DOI] [PubMed] [Google Scholar]
- 31.Jumpertz R, Le DS, Turnbaugh PJ, et al. . Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr 2011;94:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bray GA, Ryan DH, Johnson W, et al. . Markers of dietary protein intake are associated with successful weight loss in the POUNDS Lost trial. Clin Obes 2017;7:166–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warrier M, Shih DM, Burrows AC, et al. . The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Reports 2015;10:326–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narath SH, Mautner SI, Svehlikova E, et al. . An untargeted metabolomics approach to characterize short-term and long-term metabolic changes after bariatric surgery. PLoS One 2016;11:e0161425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet 2015;115:1447–1463 [DOI] [PubMed] [Google Scholar]
- 36.Pastors JG, Warshaw H, Daly A, Franz M, Kulkarni K. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care 2002;25:608–613 [DOI] [PubMed] [Google Scholar]
- 37.Williamson DA, Anton SD, Han H, et al. . Early behavioral adherence predicts short and long-term weight loss in the POUNDS LOST study. J Behav Med 2010;33:305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unick JL, Neiberg RH, Hogan PE, et al.; Look AHEAD Research Group . Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity (Silver Spring) 2015;23:1353–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.