Abstract
Reversible silencing of neuronal activity is a powerful approach for isolating the roles of specific neuronal populations in circuit dynamics and behavior. In contrast with neuronal excitation, for which the majority of studies have used a limited number of optogenetic and chemogenetic tools, the number of genetically encoded tools used for inhibition of neuronal activity has vastly expanded. Silencing strategies vary widely in their mechanism of action and in their spatial and temporal scales. Although such manipulations are commonly applied, the design and interpretation of neuronal silencing experiments present unique challenges, both technically and conceptually. Here, we review the most commonly used tools for silencing neuronal activity and provide an in-depth analysis of their mechanism of action and utility for particular experimental applications. We further discuss the considerations that need to be given to experimental design, analysis, and interpretation of collected data. Finally, we discuss future directions for the development of new silencing approaches in neuroscience.
Introduction
Perturbation of neuronal activity has always played a key role in neuroscience research, complementing electrophysiological and anatomical experiments to gain insight into the functional roles of particular brain regions, circuits, and cells. Genetically encoded tools designed for acute and chronic manipulations of circuit function are now commonly used to assess the contribution of defined brain structures and individual neural circuit components to computation and animal behavior. Before genetically encoded tools were available, reversible silencing could be achieved only with relatively low spatial and temporal specificity (Figure 1A), for example, by local cooling (Ferster et al., 1996; Long and Fee, 2008; Ponce et al., 2008) or by pharmacological agents such as GABA receptor agonists, neurotransmitter receptor antagonists, or sodium channel blockers. While these approaches have led to many insights into the role of defined brain structures in behavioral and cognitive processes, precise control over the activity of genetically defined neurons is a crucial advantage conferred by the new generation of genetically encoded tools. Early genetically encoded approaches capitalized on several different strategies, including temperature-sensitive Drosophila mutants (Kitamoto, 2001), first-generation chemogenetic approaches (Lechner et al., 2002; Lerchner et al., 2007), or the light-activated microbial rhodopsins halorhodopsin (Han and Boyden, 2007; Zhang et al., 2007) and archaerhodopsin (Chow et al., 2010). Further development has expanded this initial variety to include a wider palette of ion-pumping microbial rhodopsins (Chuong et al., 2014), anion-conducting channelrhodopsins (Berndt et al., 2016; Govorunova et al., 2015; Wietek et al., 2015) and chemogenetic tools (reviewed in Burnett and Krashes, 2016; Lerner et al., 2016; Roth, 2016; Sjulson et al., 2016; Sternson and Roth, 2014).
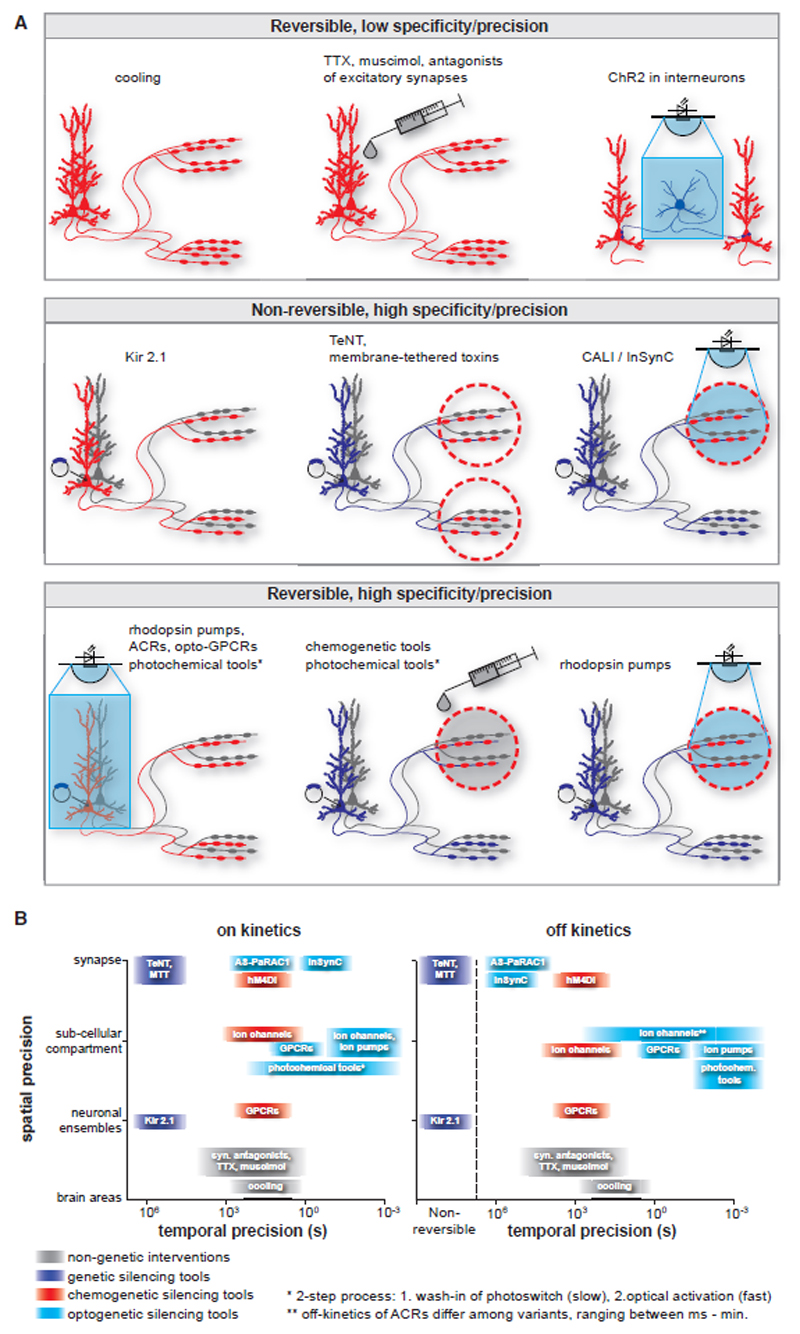
Figure 1. Silencing Strategies in Neurons.
(A) Silencing strategies are depicted with regard to reversibility, cellular specificity, and spatiotemporal precision. Principal neurons are shown with axonal projections terminating in two distinct target regions. Silenced neurons or subcellular compartments are red. Unaffected neurons or subcellular compartments are gray. Neurons expressing a genetically encoded silencing tool (small circular symbol) are blue where they are not silenced. Target regions are circled red when silencing effect is specific to terminals.
(B) Temporal precision of onset (left) and offset (right) versus spatial precision of silencing effect mediated by various strategies. Note the logarithmic scaling of the axes. Colors indicate different classes of tools or strategies.
This growing cadre of engineered, genetically encoded tools now provides scientists with a range of cell-type-specific manipulations that widely diverge in their biophysical mechanisms, their mode of operation and the time-scale at which they act (Figure 1B). Advances in gene delivery technologies have made it possible to apply these tools to specific populations of neurons defined by their unique genetic profiles and physiological properties with increasing specificity (Sjulson et al., 2016). While optogenetic tools allow exquisite temporal and spatial specificity in the control of neuronal firing, chemogenetic tools provide complementary manipulations, typically acting over longer temporal and larger spatial scales. These techniques have facilitated a systematic investigation of neuronal circuits but also present new challenges involving the selection of appropriate tools and gene targeting strategies to match diverse experimental demands.
Channelrhodopsins (ChRs) have been used in a wide variety of genetically identified neuronal cell types and model organisms for light-induced excitation of targeted neurons (Fenno et al., 2011). ChR2 (Nagel et al., 2003) and several closely related variants such as the enhanced ChR2(H134R) mutant (Nagel et al., 2005) have become the workhorses in many neuroscience laboratories and are routinely used for neuronal excitation. Optogenetic stimulation paradigms, typically consisting of brief light pulses that trigger neuronal firing at desired frequencies, can be maintained over long time periods with minimal off-target effects (Lerner et al., 2016; Yizhar et al., 2011). In contrast, neuronal silencing strategies must ensure that action potential initiation or propagation is suppressed for the entire duration of the experiment or that synaptic transmission is blocked with sufficient efficacy. Additionally, while optogenetic excitation typically acts through a universal mechanism of increased cation conductance and depolarization, optogenetic and chemogenetic inhibition utilize a wide range of cellular mechanisms (Figure 2A), each dictating unique experimental requirements and constraints. In view of the large variety of available silencing strategies, some tools are better suited for particular applications than for others. Light-activated tools, for example, are ideal for precisely timed silencing over seconds to minutes. On the other hand, silencing tools activated by a chemical ligand are better suited for experiments spanning longer time periods and allow less-invasive, hardware-free experimentation.
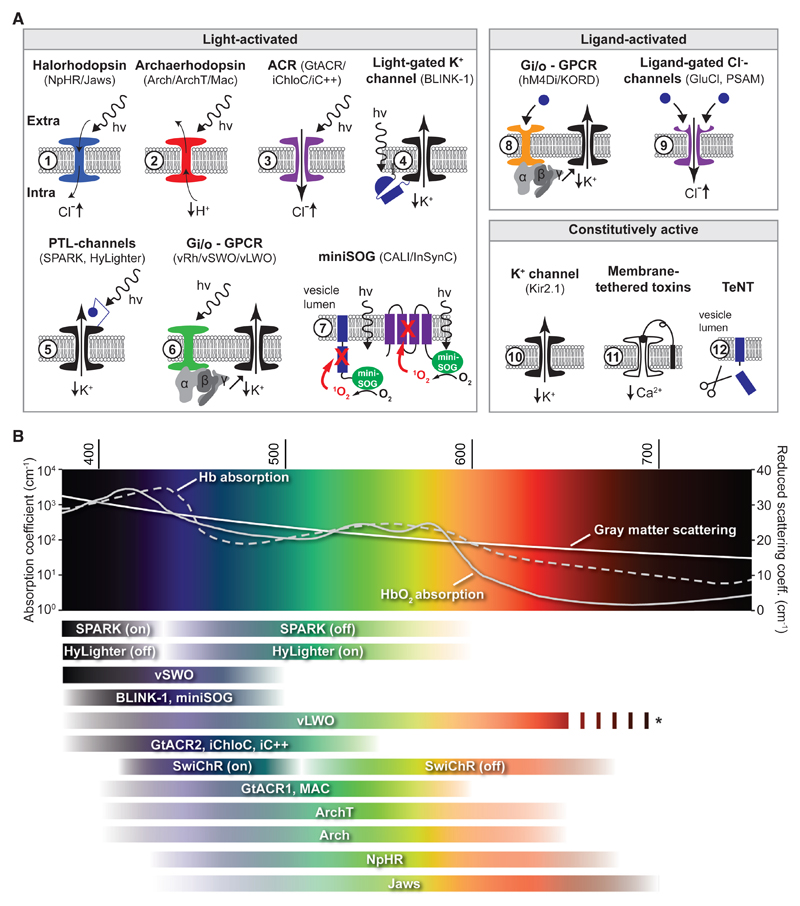
Figure 2. Genetically Encoded Silencing Tools.
(A) Overview of genetically encoded silencing tools, grouped according to their mode of activation. The group of light-activated silencing tools comprises rhodopsin ion pumps (1–2), anion channelrhodopsins (ACRs) (3), LOV-domain-activated potassium channels (4), ion channels coupled to photoswitched tethered ligands (PTL) (5), potassium channels downstream of G-protein-coupled receptor (GPCR) signaling (6), and the synaptic vesicle proteins VAMP-2 (blue) or synaptophysin-1 (violet) (7). In the latter case, chromophore-assisted light inactivation (CALI) of those proteins is achieved via singlet-oxygen generation through miniSOG. The ligand-activated tools consist of GPCRs (8) and chloride channels activated by highly selective, otherwise inert ligands (9). Although GPCRs mainly act through G-protein-coupled inward-rectifying potassium (GIRK) channels, they also recruit other mechanisms (see Main Text). The third group consists of tools that are permanently active when expressed (10–12).
(B) Approximate action spectra of light-activated silencing tools. Hemoglobin light absorption (μ10) and gray matter scattering drastically decrease toward longer wavelengths. Values for hemoglobin absorption from: http://omlc.org/spectra/hemoglobin/summary.html. Gray matter scattering was estimated using the following function: 2.37*(λ/500 nm)−1.15 (from Liu et al., 2015). Below are action spectra of the indicated tools. Color saturation of the horizontal bars scales approximately with relative absorption. * The action spectrum of vLWO was maximal at the highest wavelength tested and is likely to extend farther into the near-infrared range.
As silencing neuronal activity requires constant function of the silencing tool, it might entail various undesired effects. Prolonged optogenetic inhibition can lead to tissue heating, while chronic chemogenetic inhibition could result in desensitization and homeostatic adaptations. The choice of tool for a particular experiment must therefore take such factors into consideration, beyond the simple considerations of spatiotemporal scale and genetic targeting. Here, we review several of the most commonly used silencing strategies in neural systems. We provide an in-depth analysis of their mechanism of action, limitations, and utility for particular experimental applications. The review is structured with temporal precision as a guiding principle, such that the most temporally precise methods are followed by slower tools and chronic manipulations. We separately discuss experimental approaches for somatodendritic silencing and for silencing of axonal terminals given the unique constraints associated with each. We finally discuss general considerations that need to be given to planning, execution, and interpretation of silencing experiments in neuronal systems.
Silencing Electrical Activity in the Somatodendritic Compartment
Optogenetic Tools for Silencing Neurons at the Millisecond-to-Second Timescale
Optogenetic inhibition of neuronal activity relies on the simple single-component nature of the rhodopsin family of proteins (Figure 2A). These proteins, naturally expressed in archaea, fungi, and eubacteria, all share a seven-transmembrane architecture and utilize a covalently bound all-trans retinal as their light-sensing chromophore (Ernst et al., 2014; Zhang et al., 2011). Retinal is synthesized in most vertebrate tissues, including the brain, and can be easily provided through food to animals that do not synthesize it naturally (Mohammad et al., 2017; Nagel et al., 2005; Schroll et al., 2006). The millisecond kinetics of the microbial rhodopsins permit neuronal silencing with precise onset and offset, suitable for experiments in which silencing is targeted at defined time points such as the delivery of sensory stimuli or behavioral events. The most direct approaches utilize light-activated ion pumps and channels that can rapidly and reversibly hyperpolarize neurons in response to light. Additional optogenetic tools have been coupled to the second messenger signaling machinery or act as biochemical modulators (Figure 2A). Optimal use of these powerful tools requires an understanding of their mechanism of action and their impact on membrane potential and on the intracellular milieu.
Light-Driven Ion Pumps
Light-driven ion pumps can serve as powerful tools for suppression of neuronal activity with high temporal and spatial resolution (Chow et al., 2010; Chuong et al., 2014; Zhang et al., 2007). Ion-pumping microbial rhodopsins have evolved with high ion selectivity to efficiently generate electrochemical gradients for ATP production (Danon and Stoeckenius, 1974). Some of the organisms expressing these rhodopsins thrive in extreme ionic environments, where light-driven ion pumps function to counteract osmotic pressure (Bodaker et al., 2012). Naturally occurring, light-driven ion pumps described to date conduct protons, chloride, or sodium (Inoue et al., 2013; Oesterhelt and Stoeckenius, 1971; Schobert and Lanyi, 1982), while recent structure-guided protein engineering has further led to the development of the first potassium pumping rhodopsin (Gushchin et al., 2015). Despite remarkable differences in their mechanisms of ion transport, light-driven ion pumps share many structural and functional similarities. The ion transport process is initiated by photon absorption, leading to isomerization of the retinal chromophore molecule. The resulting structural rearrangements of distinct amino acids alter their protonation states or change their affinity to the transported ion. Subsequently, a broader structural rearrangement leads to a stepwise release of one ion and an uptake of another ion on the opposing side of the membrane (Inoue et al., 2015). At no point during this cyclic sequence of states (photocycle) does the pump form a continuous, water-filled pore between the intracellular and extracellular compartments. In addition to these mechanistic similarities, the different light-driven pumps also possess similar photophysical properties. Their molecular extinction coefficients are in the range of 50–70 mM–1 cm–1, similar to the extinction coefficients of fluorescent proteins; their quantum efficiency lies between 0.3 and 0.8, and their turnover rates range from 5 to 25 ms, making these microbial ion-pumping rhodopsins attractive tools for millisecond-precision optogenetic silencing (Chow et al., 2010; Inoue et al., 2013; Mattis et al., 2011).
Despite the similarities described above, light-evoked photocurrents carried by microbial ion pumps expressed in mammalian cells are variable. Photocurrent magnitudes strongly depend both on the expression level and on the efficiency of membrane targeting (Gradinaru et al., 2010). Current versions of light-driven ion pumps for optogenetic silencing have undergone extensive optimization, leading to enhanced versions with altered biophysical properties such as a red-shifted, high-current chloride pump (Jaws, Chuong et al., 2014) or a blue-shifted version of the proton pump Arch (Sudo et al., 2013) (see Table 1 for additional variants). Nevertheless, these enhanced tools still exhibit a decline in photocurrent amplitudes when illuminated for prolonged periods, leading to a decline in the efficacy of silencing over time. This so-called inactivation can cause a reduction of 50%–90% of peak activity during a 60-s illumination period (Mattis et al., 2011). The recovery time of photocurrent amplitudes to the initial value after prolonged illumination (>15 s) can be in the range of several seconds (Table 1, see Mattis et al., 2011). One cause for this inactivation is intrinsic to the microbial rhodopsin protein, stemming from a branched photocycle, which contains intermediates with slower recovery kinetics (Groma and Dancshazy, 1986; Mattis et al., 2011). Another source of inactivation is the change in the intracellular concentration of the conducted ion: Mahn et al. demonstrated that adding a membrane-permeant proton carrier can greatly reduce the inactivation rate of proton-pumping eArch3.0, suggesting that reduced intracellular proton concentrations induced by proton pumping can alter the efficacy of continued transport (Mahn et al., 2016). The inward-pumping chloride pump eNpHR3.0 has also been shown to alter intracellular chloride concentrations (Alfonsa et al., 2015). Indeed, Raimondo et al. showed that illumination of CA3 neurons expressing eNpHR3.0 leads to a significant change in the GABAA reversal potential within <1 s of illumination (Raimondo et al., 2012). An additional consequence of strong ion-pumping activity is an increased firing rate when illumination is abruptly terminated. This rebound activity can be directly due to either the release from hyperpolarization, because of increased intracellular chloride, or decreased intracellular proton concentrations. These changes in intracellular ion levels could trigger either efflux of chloride or influx of protons after light offset, which can generate fast membrane depolarization, especially when these highly potent ion pumps are expressed at high levels. Ramp-like termination of the light pulse can be used to alleviate such light-off rebound effects (Chuong et al., 2014; Mahn et al., 2016).
Table 1. Genetically Encoded Silencing Tools Applied in Neurons.
| Tool | Modality | Kinetics/Time Domain | Ligand/Peak λa (nm) | Inactivation | Multiplexing Ligand/Wavelengtha | References | Examples for Applications in Model Systemsb |
|---|---|---|---|---|---|---|---|
| Constitutively Expressed/Non-regulated Silencing Tools | |||||||
| Kir2.1 | inward-rectifying potassium channel | on: days off: none constitutively active |
none | none | all light wavelengths and chemogenetic agonists | Burrone et al., 2002; Johns et al., 1999 | cultured neurons (Burrone et al., 2002); fruit fly (Baines et al., 2001); zebrafish (Hua et al., 2005); mouse (Yu et al., 2004) |
| TeNT | proteolysis of presynaptic VAMP2 | on: days off: none constitutively active |
none | none | all light wavelengths and chemogenetic agonists | Harms and Craig, 2005; Yamamoto et al., 2003 | cultured neurons (Lee et al., 2010); fruit fly (Sweeney et al., 1995); mouse (Murray et al., 2011) |
| Membrane-tethered toxins | genetically encoded calcium channel blocker | on: days off: none constitutively active |
none | none | all light wavelengths and chemogenetic agonists | Auer et al., 2010 | mouse (Christoffel et al., 2015) |
| Optogenetic Tools | |||||||
| Ion Pumps | |||||||
| H+ Pumps | |||||||
| Archaerhodopsin-3 (Arch); eArch3.0 | microbial light-driven proton pump | on: ms off: ms usable time domain: ms – s |
550 | light off | >670 nm, chemical ligands | Chow et al., 2010; Mattis et al., 2011 | mouse (Lucas et al., 2016); roundworm (Husson et al., 2012) |
| ArchT; eArchT3.0 | microbial light-driven proton pump | on: ms off: ms usable time domain: ms – s |
570 | light off | >670 nm, chemical ligands | Han et al., 2011; Mattis et al., 2011 | mouse (Trouche et al., 2016); rat (Basting et al., 2015); ferret (Zhou et al., 2016); macaque monkey (Han et al., 2011); roundworm (Okazaki and Takagi, 2013) |
| Blue-shifted Archaerhodopsin-3 | microbial light-driven proton pump (blue-shifted) | on: ms off: ms usable time domain: ms – s |
500 | light off | >600 nm, chemical ligands | Sudo et al., 2013 | – |
| Mac, eMac3.0 | eukaryotic light-driven proton pump | on: ms off: ms usable time domain: ms – s |
550 | light off | >630 nm, chemical ligands | Chow et al., 2010; Mattis et al., 2011; Waschuk et al., 2005 | roundworm (Husson et al., 2012) |
| Cl– Pumps | |||||||
| eNpHR(2.0, 3.0) | microbial light-driven chloride pump | on: ms off: ms usable time domain: ms – s |
570 | light off | >670 nm, chemical ligands | Gradinaru et al., 2010 | roundworm (Husson et al., 2012; Zhang et al., 2007); zebrafish (Arrenberg et al., 2009); mouse (Gradinaru et al., 2010); rat (Stefanik et al., 2013); macaque monkey (Diester et al., 2011) |
| Jaws | microbial light-driven chloride pump | on: ms off: ms usable time domain: ms – s |
584 | light off | >680 nm, chemical ligands | Chuong et al., 2014 | rat (Robinson et al., 2017); macaque monkey (Acker et al., 2016) |
| Na+ Pumps | |||||||
| KR2 | microbial sodium pump | on: ms off: ms usable time domain: ? |
525–550 | light off | >650 nm, chemical ligands | Kato et al., 2015 | – |
| I1K6NaR | chimeric microbial sodium pump | on: ms off: ms usable time domain: ? |
528 | light off | >650 nm, chemical ligands | Hoque et al., 2016 | – |
| Ion Channels | |||||||
| eACRsc | |||||||
| iChloC | light-gated anion channel based on chr2 | on: ms off: ms usable time domain: s – min |
494 | light off | >630 nm, chemical ligands | Wietek et al., 2015 | mouse (Takahashi et al., 2016) |
| iC++ | light-gated anion channel based on c1c2 | on: ms off: s usable time domain: ms – s |
488 | light off | >600 nm, chemical ligands | Berndt et al., 2016 | mouse (Park et al., 2016) |
| SwiChR++ | light-gated, bistable anion channel based on c1c2 | on: ms off: min (spontaneous), s (light-accelerated) usable time domain: s – hr |
488 | light off (slow) >600 nm (fast) | >600 nm, chemical ligands | Berndt et al., 2016 | mouse (Iyer et al., 2016; Kim et al., 2016) |
| nACRsd | |||||||
| GtACR1 | natural light-gated anion channel | on: ms off: ms – s usable time domain: ms – s |
520 | light off | >630 nm, chemical ligands | Govorunova et al., 2015 | cultured neurons (Mahn et al., 2016); fruit fly (Mohammad et al., 2017) |
| GtACR2 | natural light-gated anion channel | on: ms off: ms – s usable time domain: ms – s |
470 | light off | >550 nm, chemical ligands | Govorunova et al., 2015 | mouse (brain slices) (Alfonsa et al., 2016); fruit fly (Mohammad et al., 2017) |
| PsChR1/PsuACR1 | natural light-gated anion channels | on: ms off: ms – s usable time domain: ms – s |
540 | light off | >640 nm, chemical ligands | Govorunova et al., 2016; Wietek et al., 2016 | – |
| K+ Channels | |||||||
| BLINK-1 | miniature viral potassium channel Kcv fused to LOV2 domain | on: s off: min usable time domain: min |
450 | light off | >510 nm, chemical ligands | Cosentino et al., 2015 | – |
| GPCRs | |||||||
| opto-MOR | signaling domain of rat mu-opioid receptor fused to rat rhodopsin 4 | on: s off: s – min usable time domain: s – min |
470 | light off | – | Siuda et al., 2015a | – |
| vRh | vertebrate cone opsin coupling to Gi/o pathway | on: s off: s usable time domain: s – min |
485 | light off | >600 nm | Li et al., 2005; Masseck et al., 2014 | – |
| vSWO | vertebrate cone opsin coupling to Gi/o pathway | on: s off: s usable time domain: s – min |
<400 | light off | >520 nm, chemical ligands | Masseck et al., 2014 | mouse (Hasegawa et al., 2017) |
| vLWO | vertebrate cone opsin coupling to Gi/o pathway | on: s off: s usable time domain: s – min |
>450 | light off | <400 nm, chemical ligands | Masseck et al., 2014 | – |
| Chemogenetic Tools | |||||||
| Ligand-Gated G Proteins | |||||||
| Alstr | insect allatostatin neuropeptide receptor coupling to Gi/o pathway | on: min off: min usable time domain: min – hr |
allostatin | wash-out/metabolization of ligand | light, other chemical ligands | Tan et al., 2006 | mouse, rat, ferret, and macaque monkey (Tan et al., 2006; Wehr et al., 2009) |
| RASSLs | κ-opioid receptor based GPCR coupling to Gi/o pathway | on: min off: min usable time domain: min – days (chronic administration) |
spiradoline | wash-out/metabolization of ligand | light, other chemical ligands | Coward et al., 1998 | not used in the CNS (Sternson and Roth, 2014) |
| hM4Di | DREADD-based on human muscarinic receptor 4 coupling to Gi/o pathway | on: min off: min usable time domain: min – days (chronic administration) |
CNO/compound 21 | wash-out/metabolization of ligand | light, other chemical ligands | Armbruster et al., 2007 | mouse (Atasoy et al., 2012); rat (Kätzel et al., 2014); macaque monkey (Eldridge et al., 2016) |
| KORD | κ-opioid receptor based GPCR coupling to Gi/o pathway | on: min off: min usable time domain: min – days (chronic administration) |
salvinorin B | wash-out/metabolization of ligand | light, other chemical ligands | Vardy et al., 2015 | mouse (Lemos et al., 2016); rat (Marchant et al., 2016) |
| Ligand-Gated Ion Channels | |||||||
| GluCl | invertebrate glutamate-activated chloride channel | on: min off: min usable time domain: hr – days (chronic administration) |
ivermectin | wash-out (slow)/metabolization of ligand | light, other chemical ligands | Cully et al., 1994; Slimko et al., 2002 | restricted to vertebrate/mammalian systems; mouse (Lerchner et al., 2007) |
| PSAM | chimeric channels selective for particular ions, exclusively gated by an exogenous ligand | on: min off: min usable time domain: hr – days |
pharmacologically selective effector molecules (PSEMs) | wash-out/metabolization of ligand | light, other chemical ligands | Magnus et al., 2011 | mouse (Basu et al., 2013) |
| Photopharmacogenetic Tools | |||||||
| SPARK | genetically modified Shaker potassium channel selectively binding a photoswitchable tethered ligand (PTL) | on: s off: s usable time domain: s – hr |
MAQ + 380 light | 500 | other chemical ligands | Banghart et al., 2004 | – |
| HyLighter | potassium selective pore of a bacterial glutamate receptor (sGluR0) fused to ligand-binding domain of a mammalian glutamate receptor (iGluR6) binding a PTL | on: s off: s usable time domain: s – hr |
MAQ + 380 light | 500 | other chemical ligands | Janovjak et al., 2010 | – |
| Kv1.3, Kv3.1, Kv7.2 and SK2 | genetically modified potassium channel subunits selectively binding a PTL | on: s off: s usable time domain: s – hr |
MAQ + 380 light | 500 | other chemical ligands | Fortin et al., 2011 | – |
Estimated from published action spectra.
We provide representative examples of applications of the respective tools in studies other than the original publication. This is not an exhaustive list encompassing all studies but rather a selection demonstrating a variety of applications.
eACRs, engineered anion-conducting ChRs. First-generation eACRs (ChloC, slowChloC, iC1C2, SwiChR; Berndt et al., 2014; Wietek et al., 2014) are not considered due to imperfect anion selectivity.
nACRs, naturally occurring anion-conducting ChRs.
In summary, light-driven ion pumps have been shown to effectively function as silencers of neuronal activity when activated for brief periods of time (typically <15 s, but see Goshen et al., 2011). Due to their pumping mechanism, their activity is robust to changes in ionic gradients, and their net effect on membrane potential is constant across a wide range of conditions. These tools are limited, however, in situations requiring long-lasting inhibition and could potentially influence the cellular ion homeostasis. Moreover, the one-to-one stoichiometry between photon absorption and ion pumping necessitates constant illumination with relatively high light flux to achieve continued hyperpolarization, setting an upper bound on the volume of the modulated area due to risks of heating and photodamage (Figure 3C).
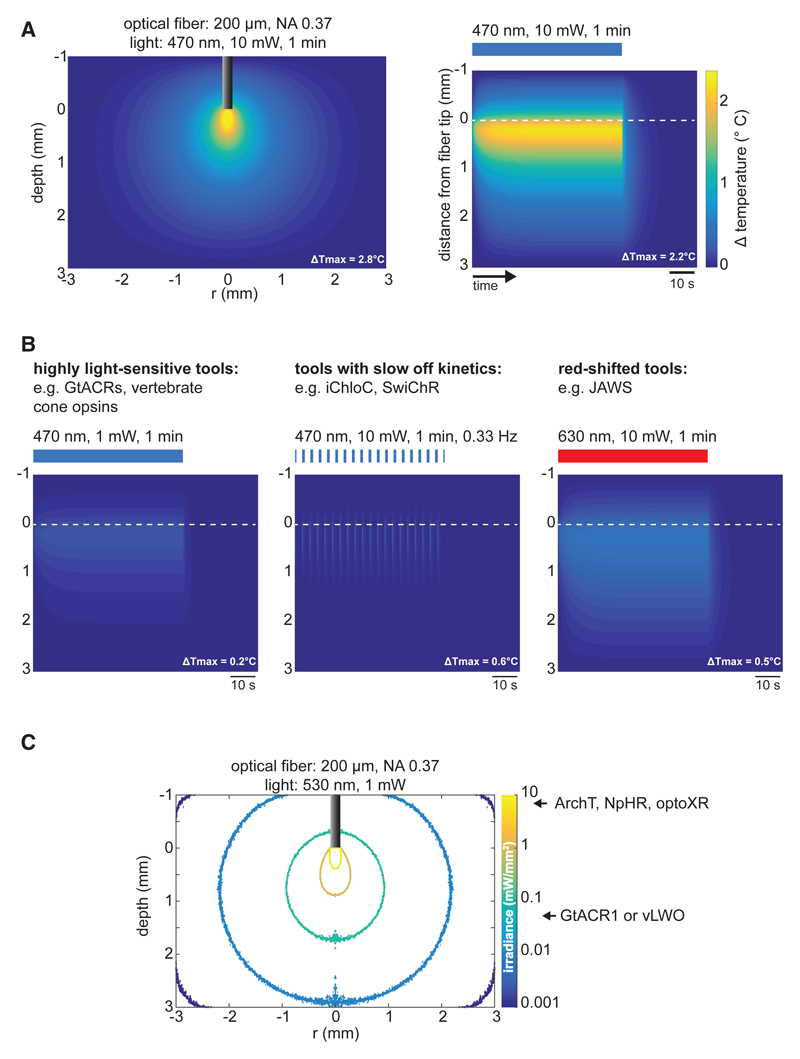
Figure 3. Monte Carlo Simulation of Light-Induced Heat Distribution and Light Propagation in Neuronal Tissue.
(A) Simulation of tissue heating in a “typical” in vivo optogenetic experiment using continuous blue light for ChR2 activation. Tissue heating is modeled for optical stimulation with 470 nm light for 1 min through a fiber with 200 μm diameter and a numerical aperture of 0.37. Left: spatial heat distribution in 2D cross-section under the optical fiber. Right: average temperature change over time within a radial distance of 0.2 mm from the fiber tip. The location of the fiber tip is indicated by the white dashed line.
(B) Strategies to reduce tissue heating in optogenetic silencing experiments. Left: various optogenetic silencing tools are orders of magnitudes more light sensitive than the classical rhodopsin ion pumps NpHR or Arch. Therefore, lower light intensities can be used to silence neurons within a given volume. Middle: silencing tools with slow off/closing kinetics can sustain their silencing activity after light is off. Using short light pulses of high intensities avoids heat buildup. Right: long-wavelength light is absorbed less strongly in brain tissue and can be used to activate red-shifted silencing tools. Scales are as in (A).
(C) Monte Carlo simulation of light penetration under illumination conditions considered not to increase spiking activity due to tissue heating (see Stujenske et al., 2015). Colored contours circumcise volumes where photon flux has dropped 10-fold starting at 10 mW/mm2. 530 nm light can be used to activate various opsins such as Arch, NpHR, optoXRs, GtACR1, or vLWO. Required photon flux to achieve maximal silencing is several orders of magnitude lower for GtACR1 or vLWO compared to the other opsins. Thus, large volumes can be efficiently silenced with such highly sensitive tools without significantly heating the tissue.
Light-Gated Anion Channels
As discussed above, microbial ion-pumping rhodopsins such as eNpHR3.0 and eArch3.0 hyperpolarize the membrane independently of cellular activity, limiting their utility under circumstances when silencing is required for more than a few seconds. One alternative could be light-gated ion channels selective for potassium or chloride, which would ideally conduct many ions per absorbed photon. In contrast to ion-pumping rhodopsins, ion flux through such light-gated channels depends on the membrane potential and can efficiently “shunt” membrane depolarization to the reversal potential of potassium or chloride, which in both cases is close to the resting membrane potential of most neurons. Until recently, all of the known naturally occurring light-gated ion channels were cation selective. Recent engineering efforts, along with the identification of naturally occurring anion-conducting channelrhodopsins, have generated a completely new class of optogenetic silencing tools.
The high-resolution crystal structure of the channelrhodopsin chimera C1C2 (Kato et al., 2012) allowed engineering-based approaches aimed at selectively altering the electrostatic properties of the channel’s ion-conducting pore in order to convert cation-selective ChR variants into anion-selective light-gated ChRs. Two different strategies have led to ChR variants with enhanced anion selectivity (Berndt et al., 2014; Wietek et al., 2014). One strategy was aimed at systematically replacing negative charges in the extracellular outer pore with positive or neutral charges, without compromising the photocycle or protein stability of C1C2 (Berndt et al., 2014). The second strategy was based on the exchange of a single acidic amino acid for a basic one in the central gate of ChR2 (Wietek et al., 2014). While both approaches led to increased anion selectivity, in both cases a significant portion of protons was still conducted by these engineered variants. This residual proton conductance rendered these initial engineered anion-conducting ChRs (eACRs) of limited use as silencing tools. In a second round of optimization, both groups eliminated this residual proton conductance, yielding iC++ and iChloC, two highly anion-selective eACRs (Berndt et al., 2016; Wietek et al., 2015). One advantage to using engineered ChR-based ACRs is the large array of known ChR variants with diverse spectral and kinetic properties (Yizhar et al., 2011). Based on these ChR variants, targeted introduction of point mutations was used to slow down the closing kinetics of the eACRs. This drastically increased the number of conducted ions per absorbed photon, yielding effective inhibition with greatly reduced light power demand (Figure 3C; Berndt et al., 2014, 2016; Wietek et al., 2014, 2015). In addition, spectrally shifted versions of eACRs based on existing ChR variants with distinct action spectra may become available in the future.
In parallel, new screening efforts have unveiled a new class of natural ACRs (nACRs) from the cryptophyte alga Guillardia theta (Govorunova et al., 2015). These rhodopsin channels, named GtACR1 and GtACR2, have near-perfect anion selectivity and produce several-fold larger photocurrents in mammalian cells than any of the eACRs, owing to a higher single-channel conductance than that of the known cation-conducting ChRs (Govorunova et al., 2015; Sineshchekov et al., 2015). A more recent screen expanded the family of cryptophyte ACRs to 20 members (Govorunova et al., 2017), and a previously discovered ChR from Proteomonas sulcate (Klapoetke et al., 2014) was identified as another naturally occurring ACR (Govorunova et al., 2016; Wietek et al., 2016).
Light-gated ACRs with slow off kinetics are of particular interest when silencing is required for a period of seconds to minutes. By keeping the channel in its open state with brief light pulses, a constant chloride conductance can be maintained to inhibit any depolarizing events. Previous work has shown that ChR variants with slow off-kinetics enable greater effective light sensitivity due to the accumulation of open channels with a longer light pulse at lower photon flux (Berndt et al., 2009; Mattis et al., 2011). Step-function ACRs therefore allow lower overall photon flux, thus eliminating potential artifacts caused by excessive light exposure (Figure 3B). Although this comes at the cost of dramatically decreased closing kinetics and a conducting state that can last for minutes, some step-function mutations allow acceleration of channel closure with red-shifted light. Thus, with sufficient light power, the silencing period can be terminated within a few seconds (Berndt et al., 2016).
A second advantage of ACRs is their mode of inhibition that, like GABAA receptors, results in shunting of the membrane potential. In contrast to hyperpolarization, which is a subtractive mode of inhibition, shunting inhibition is divisive by nature. Shunting is energetically efficient since it minimizes ionic flux in quiescent neurons and could therefore prevent non-physiological changes in intracellular chloride concentration, making ACRs attractive for in vivo applications (Berndt et al., 2016; Chung et al., 2017; Iyer et al., 2016; Kim et al., 2016; Takahashi et al., 2016; Wietek et al., 2015). However, since the action of anion channels is directly dependent on the chloride gradient, their physiological effect might be complex in some cell types or neuronal compartments (Figure 4B). Under conditions where the intracellular chloride concentration is high, ACR activation may lead to excitation rather than inhibition. In immature neurons, for example, where the potassium-chloride co-transporter KCC2 is not yet expressed, intracellular chloride concentration is elevated (Kaila et al., 2014). Activation of ACRs may therefore lead to excitation rather than inhibition of these immature neurons. Yet, long-lasting, large photocurrents may shunt action potentials in such neurons (Heigele et al., 2016). In addition, some axons and presynaptic terminals have also been suggested to contain elevated chloride levels (Pugh and Jahr, 2011; Szabadics et al., 2006; Turecek and Trussell, 2001). Consistent with these reports, activation of axonal GtACRs in acute brain slices was shown to cause presynaptic release (Mahn et al., 2016) and evoked antidromic spikes (Malyshev et al., 2017). This example demonstrates that the action of ACRs may be difficult to predict in situations where the local chloride gradient is unknown. The use of ACRs as a silencing tool is strongly dependent on the natural ability of chloride to shunt membrane depolarization or inhibit excitation in the system under investigation. Whereas most adult neurons would be effectively silenced using these channels, effective silencing should always be validated in vivo, and alternative silencing strategies should be considered in cases where the chloride reversal potential is depolarizing (e.g., in cerebellar granule cells, Pugh and Jahr, 2011), variable over time, or uncharacterized.
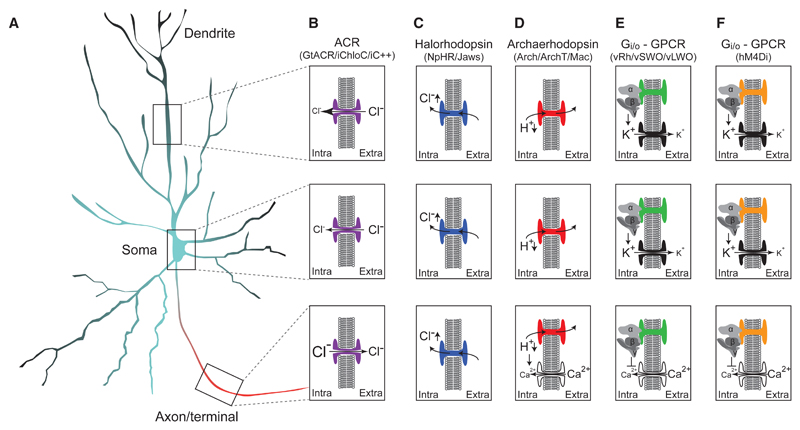
Figure 4. Compartment-Specific Action of Optogenetic and Chemogenetic Silencing Tools.
(A) Schematic depiction of an archetypical neuron. Color indicates intracellular chloride concentration, from lower (black) to higher (red) chloride concentration than in the soma (blue, Kole and Stuart, 2012).
(B) ACR activation throughout the neuron leads to light-activated chloride conductance, which is predominantly hyperpolarizing in soma and dendrites, but could be depolarizing in the axon due to an elevated intra-axonal chloride concentration (Mahn et al., 2016; Malyshev et al., 2017).
(C) Halorhodopsins such as eNpHR3.0 and Jaws carry chloride into the cytoplasm uniformly across the axo-dendritic gradient due to their active ion-pumping function. While these tools can effectively silence both somatic and axonal activity, they can also lead to an increase in the chloride concentration and result in a depolarizing effect of GABAA receptors (Raimondo et al., 2012).
(D) Proton-pumping rhodopsins hyperpolarize the neuron through active proton pumping but might also cause drastic changes in pH and elevated intracellular Ca2+, which in the synaptic terminals could lead to increased spontaneous release of neurotransmitter (Mahn et al., 2016).
(E) Gi/o-coupled vertebrate rhodopsins inhibit the somatodendritic compartment through recruitment of GIRK channels (Masseck et al., 2014). These rhodopsins were reported to inhibit neurotransmitter release, potentially through direct action on Ca2+ channels (Li et al., 2005).
(F) Gi/o-coupled DREADDs inhibit the somatodendritic compartment through recruitment of GIRK channels. Inhibition of synaptic transmission is probably GIRK independent but rather occurs through G-protein-mediated silencing of Ca2+ channel activity (Stachniak et al., 2014; Vardy et al., 2015).
Light-Activated G-Protein-Coupled Receptors
Light-activated G-protein-coupled receptors (GPCRs) are promising candidates to circumvent some of the limitations discussed above for rhodopsin pumps and ACRs. GPCRs comprise the largest class of neuronal signaling membrane proteins (Premont and Gainetdinov, 2007; Vassilatis et al., 2003). All known mammalian GPCRs are seven-transmembrane proteins that couple to heterotrimeric G proteins or to β-arrestin. The α subunits of the G proteins can be broadly subdivided into four classes: Gi/o, Gs, Gq/11, and G12/13 (Wettschureck and Offermanns, 2005). Their selective coupling to canonical signaling pathways makes these receptors powerful targets for engineering approaches to render them controllable via exogenous stimulants. While Gs, Gq/11, and G12/13 trigger signaling cascades that putatively lead to enhanced neuronal activity, Gi/o reduces adenylyl cyclase activity, inactivates P/Q- and N-type calcium channels and activates endogenous mammalian G-protein-coupled inwardly rectifying K+ (GIRK) channels (Wettschureck and Offermanns, 2005).
Vertebrate rhodopsins (vRh), the visual pigments of vertebrate vision, are well characterized and are orders of magnitude more light sensitive than channelrhodopsins (Kleinlogel, 2016; Masseck et al., 2014). In retinal rod cells, vRh couples to the G protein transducin (Gt). Although transducin is not expressed in neurons, its α subunit belongs to the Gi/o subfamily, and therefore vertebrate rhodopsins can couple to other Gi/o family members when heterologously expressed. Indeed, the vertebrate rhodopsin was one of the first light-sensitive proteins utilized to achieve light-based modulation of activity in mammalian neurons (Li et al., 2005). One drawback of vRh, however, is that its light-induced signaling responses strongly adapt under repetitive stimulation—a limitation that was partially overcome by using the murine cone short or long wavelength opsins (vSWO and vLWO; Masseck et al., 2014). Unlike in the case of microbial rhodopsins, activation of vertebrate rhodopsins occurs through light-mediated conformational change of retinal from 11-cis to 11-trans and leads to a stable photocycle intermediate. The rhodopsin protein cannot be reactivated until the bound retinal, now in all-trans conformation, dissociates and a new 11-cis retinal binds. This effect, known as bleaching, renders the opsin photocycle directly dependent on the availability of a new 11-cis-retinal molecule. However, this seems not to be an issue in the mammalian brain, where sufficient amounts of cis-retinal are present (Masseck et al., 2014). Nevertheless, this limitation could restrict the magnitude and reproducibility of vertebrate rod and cone opsin activation (Bailes et al., 2012) and necessitates the addition of cis-retinal to the medium for in vitro applications or to the food of animals that do not synthesize it naturally (Mohammad et al., 2017; Nagel et al., 2005; Schroll et al., 2006).
The highly conserved seven-transmembrane domain structure of GPCRs has motivated the generation of chimeric proteins, where the intracellular loops of light-activated GPCRs were replaced with the intracellular loops of ligand-activated GPCRs, such as adrenergic and dopamine receptors. Indeed, this approach was successful (Kim et al., 2005) and is generalizable to other GPCRs, leading to the development of various “optoXRs” (Airan et al., 2009; Oh et al., 2010; Siuda et al., 2015a) (Figure 2A). A light-sensitive μ-opioid-like receptor (opto-MOR) was recently generated using the same approach (Siuda et al., 2015a). Activation with light triggered Gi/o protein signaling, which reduced firing rates in expressing neurons in vitro and elicited behavioral effects in vivo. It has been shown that opto-MOR and other optoXRs (Siuda et al., 2015a, 2015b) internalize with kinetics similar to those of the native receptors. This can be an advantage when using these receptors to investigate endogenous signaling but could also pose a limitation when efficient neuronal silencing is required over longer periods. This limitation might be overcome by sufficient expression levels of the transgene leading to saturation of the machinery responsible for internalization of the activated receptors (Roth, 2016).
Light-based control of Gi/o signaling downstream of opioid receptors has also been achieved with selective caged agonists that can be released by UV light. Caged agonists of the opioid receptors Leu5-enkephalin (LE) and Dynorphin A (Dyn-8) were developed and used to silence neurons of the locus coeruleus (Banghart and Sabatini, 2012). This strategy circumvents the requirement for overexpression of heterologous channels but requires the delivery of caged molecules into the brain region under investigation. Moreover, the endogenous opioid receptors can still be activated by their natural ligands and are therefore not under rigorous control. In summary, Gi/o-coupled receptors are suitable targets for neuronal silencing strategies. Since GPCRs operate on a slower timescale compared to ion pumps and channels (see Table 1), their use is limited to applications that do not require millisecond precision. Additionally, it should be taken into account that while these tools can indeed influence neuronal excitability, the activation of such canonical signaling pathways could also be associated with changes in additional downstream targets such as post-translational modifications and gene expression.
Silencing by Excitation
The generation of transgenic mice expressing ChR2 in interneurons (Madisen et al., 2012) and the development of viral vectors with high interneuron specificity (Dimidschstein et al., 2016) has enabled a new type of optogenetic method for inhibition of circuit activity, utilizing the robust effects of GABA-mediated inhibition in cortical circuits. Analogous to silencing with the GABAA receptor agonist muscimol, illumination of cortical regions containing ChR2-expressing inhibitory interneurons yields highly robust inhibition of local-circuit activity and allows meso-scale silencing experiments in which large portions of cortical subfields are silenced in behaving animals (Guo et al., 2015; Guo et al., 2014). Since this method uses ChR2 to increase spiking of interneurons, it employs the natural circuitry of the brain to acutely attenuate spiking in the local circuit and thus silence excitatory output from principal cells (Figure 1A). Consequently, local but also long-range excitatory projections from the circuit under illumination can be silenced transiently and reversibly with high temporal precision. An additional benefit, stemming from the kinetics of GABA receptors employed in this approach, is that efficient silencing can be maintained using pulsed light, avoiding the continuous illumination required for microbial ion-pumping rhodopsins and some ACRs (Figure 3B). The main limitation of this indirect silencing approach is that it does not permit silencing of selected populations or specific subtypes of neurons. To inhibit a specific population of neurons, defined for instance by cortical layer or projection target, direct silencing is still the most effective approach.
Chemogenetic Tools for Silencing Neurons at the Minute-to-Hour Timescale
Chemically Controlled GPCR Signaling
In recent years, chemically controlled GPCRs have gained much attention as a means of controlling neuronal activity. One of the first strategies utilized to reversibly silence genetically identified sets of mammalian neurons exploited the Gi/o signaling cascade by overexpression of the Drosophila allatostatin (AL) neuropeptide receptor (AlstR) (Lechner et al., 2002). Neither AL nor AlstR are naturally expressed in mammalian cells. Activation of exogenously expressed AlstR with AL will mainly activate endogenous GIRK channels, leading to membrane hyperpolarization and a concomitant reduction of action potential firing in most neurons. This system was successfully used in mammals to silence neurons in various brain regions, including the lateral geniculate nucleus, visual and barrel cortices (Tan et al., 2006), preBötzinger complex (Tan et al., 2008), auditory cortex (Wehr et al., 2009), amygdala (Zhou et al., 2009), and hippocampus (Haettig et al., 2013). This system, like other ligand-gated approaches, is limited temporally by the pharmacokinetics of its ligand, AL. An additional limitation lies in the need to infuse AL directly into the brain, as it does not cross the blood-brain barrier. Thus, taking into account AL’s short half-life of 1–2 hr in vivo (Tan et al., 2006), repeated silencing of the same set of neurons in chronic experiments is difficult to achieve.
The first efforts to genetically engineer GPCRs controlled by non-natural ligands date back more than 25 years. The binding site of the β2-adrenergic receptor was mutated so that it could bind the small molecule 1-(3′,4′-dihydroxyphenyl)-3-methyl-L-butanone (L-185,870) instead of its natural ligand adrenaline (Strader et al., 1991). While these receptors had only limited potency, a second class of engineered receptors termed RASSLs (receptors activated solely by a synthetic ligand) was later developed. For example, a κ-opioid receptor (KOR) was engineered to be solely activated by spiradoline (Coward et al., 1998). However, this approach was also of limited use for controlling neuronal activity since most RASSLs were constitutively active to some degree (Chang et al., 2007; Sweger et al., 2007), and spiradoline could also activate endogenous KORs. Additional chemogenetic attempts to exclusively control engineered GPCRs—such as the adenosine or serotonin receptors—were hampered by the off-target activation of endogenous GPCRs through the synthetic ligands or by the low potency of these ligands (Sternson and Roth, 2014).
Due to these limitations, a new strategy was developed to generate designer receptors exclusively activated by designer drugs (DREADDs). Directed molecular evolution of GPCRs was used to generate receptors selective for specific small-molecule ligands (Armbruster et al., 2007). Clozapine-N-oxide (CNO) was chosen as agonist since it penetrates the blood-brain barrier, is pharmacologically inert in mice, and has optimal pharmacokinetics. This approach was highly successful, yielding various DREADDs that are based on the different human muscarinic receptors, which are inactive in absence of CNO, insensitive to acetylcholine (Ach), and are sensitive to nanomolar concentrations of CNO. Importantly, the Gαi-coupled M2 and M4 DREADDs are useful for neuronal inhibition. The M4 variant hM4Di has become a widely used chemogenetic tool for inhibition of defined neuronal circuits. Its success can be attributed to minimal basal activity of the DREADDs, their high selectivity for CNO, and the properties of CNO itself. However, in some systems, overexpression of hM4Di was shown to alter the biophysical properties of neurons and the expression of native GPCRs (Saloman et al., 2016). Constitutive activity of GPCRs is therefore a potential issue and expression levels should be kept at a minimum (Roth, 2016).
Another concern relates to CNO and its partial conversion to clozapine, which can bind to endogenous GPCRs in various species such as guinea pigs, primates (including humans; Jann et al., 1994) and rats (MacLaren et al., 2016). Since DREADDs bind clozapine with much higher affinity than they do CNO (Armbruster et al., 2007), it is possible that retro-converted clozapine contributes to the effects of CNO on DREADD activity. Thus, CNO dosage has to be carefully considered, and controls with CNO administration in DREADD-negative animals are essential. Although CNO was reported to be stable in mice (Guettier et al., 2009), new chemical actuators are now available (e.g., Compound 21 or perlapine, Chen et al., 2015), which cannot be metabolized to active substances in mammals (Roth, 2016).
How hM4Di exactly mediates silencing of neuronal circuits remains a matter of debate. While initial studies suggested that this receptor mainly suppresses neuronal action potential firing via GIRK-mediated membrane hyperpolarization (Armbruster et al., 2007; Ferguson et al., 2011; Kozorovitskiy et al., 2012), recent studies have indicated that inhibitory DREADDs can strongly attenuate synaptic neurotransmitter release without affecting action potential firing (Mahler et al., 2014; Stachniak et al., 2014; Vardy et al., 2015; Zhu and Roth, 2014). GIRK channels are not the sole downstream actuators of Gαi/o signaling, and are most likely not the targets of GPCR-mediated Gαi/o signaling at the presynaptic compartment (Figure 4F) (Drake et al., 1997; Lüscher et al., 1997). Other mechanisms might act to suppress presynaptic neurotransmitter release, including the inhibition of N-type Ca2+ channels (Delaney and Crane, 2016) or interference with components of the presynaptic release machinery, such as RIM1α (Chevaleyre et al., 2007). Whether reduced spiking or inhibition of neurotransmitter release dominates the DREADD silencing effect may ultimately depend on the composition of the cellular signaling machinery and its downstream targets present in the DREADD-expressing neurons (Zhu and Roth, 2014).
Multiplexed Chemogenetic Silencing of Distinct Neuronal Populations
It is often advantageous to independently control the activity of more than one neuronal population (e.g., different types of excitatory cells, excitatory and inhibitory cells, etc.) or more than one cell type in the same brain volume (e.g., neurons and astrocytes). In addition, independent up- and downregulation of neuronal activity, either of the same neurons or of different neurons, might be desired in some experimental conditions. These can be achieved by using multiple tools in the same experimental system. In these cases, it is essential that activation of one tool does not influence the activity or performance of the other. Chemogenetic tools are potentially suitable for such multiplexed applications due to their ligand selectivity and owing to the fact that they can couple to distinct downstream signaling pathways, which can either up- or downregulate neuronal activity.
The DREADD strategy has been extended to include a Gi-coupled KOR-based DREADD (KORD), yielding a variant selectively activated by the highly specific compound salvinorin B (Vardy et al., 2015). This latest addition of KORD to the DREADD family allows the combination of chemogenetic silencing and activation in the same cell or silencing of one neuronal population and activation or silencing of a second, independent population (Burnett and Krashes, 2016). Using two GPCRs has the advantage that they both operate on similar temporal and spatial scales (Figure 1B). Thus, for experiments requiring concurrent manipulation of distinct cell populations over a similar time frame, such combination is advantageous. For example, Vardy et al. combined the excitatory Gs-coupled hM3Dq with the Gi-coupled KORD in the same population of VTA/SNCVGAT neurons to bidirectionally control locomotion. In mice expressing these engineered receptors, injection of salvinorin B increased, while CNO injection decreased locomotor activity. In addition, when salvinorin B was administered shortly after CNO, reduced locomotor activity was rescued to normal levels (Vardy et al., 2015).
Multiplexed manipulation of neuronal activity has also been achieved by combining two different optogenetic tools (Carus-Cadavieco et al., 2017; Chow et al., 2010; Han and Boyden, 2007; Klapoetke et al., 2014; Zhang et al., 2007). However, completely independent control of such optogenetic tools is difficult to achieve as action spectra of all optically controlled tools are very broad and are usually somewhat overlapping (Figure 2B). Thus, despite the lack of spatiotemporal precision, chemogenetic tools are superior with respect to their selective activation. Under some circumstances, the long-lasting effects of DREADD-based manipulations can be multiplexed with millisecond-scale optogenetic control of the same or different neurons. A study by Stachniak et al. elegantly combined acute stimulation of arcuate nucleus Agouti-related peptide (AgRP) neurons via ChR2 with selective silencing of their terminals in the paraventricular hypothalamus via hM4Di (Stachniak et al., 2014). A more recent study used a combination of ChR2 and hM3Dq to unravel how inhibitory projections from the central amygdala to interneurons in the parvocellular reticular formation (PCRt) regulate prey capture behavior in mice (Han et al., 2017). In this case, the effects of ChR2-evoked disinhibition in PCRt were countered by direct chemogenetic activation of downstream inhibitory neurons in PCRt.
Kinetics of DREADD-Mediated Excitation and Inhibition
Despite the widespread use of DREADDs for inhibition and excitation of neuronal populations, a systematic characterization of their kinetics is somewhat lacking. The timescale of effective DREADD-mediated modulation of neuronal activity is mainly determined by CNO pharmacokinetics, which can vary widely in vitro and in vivo, and by the potential desensitization kinetics of the receptor-signaling cascade. Indeed, desensitization is known to occur after activation of GPCRs. Specifically, the human muscarinic receptor 4, on which hM4Di is based, has been shown to undergo ligand-mediated sequestration on timescales of tens of minutes (Tsuga et al., 1998). However, models of GPCR function suggest that high levels of expression, as achieved with viral vectors, make them less sensitive to desensitization due to downstream amplification of the signal cascade (Roth, 2016).
While many behavioral studies have established that DREADD-mediated effects can last for many hours (Burnett and Krashes, 2016; Roth, 2016), studies that tested the effect of hM4Di on neuronal excitability were typically conducted on a shorter timescale of up to 30 min (Isosaka et al., 2015; Kozorovitskiy et al., 2012; Mahler et al., 2014; Zhu and Roth, 2014). One recent study reported that 30 min following injection of CNO, the firing rate of hM4Di-expressing cells reduced significantly, and returned to pre-CNO baseline after 12 hr (Miao et al., 2015). The efficacy of inhibition during intermediate time points, in the continuous presence of CNO, has not been reported. On the other hand, two studies have conducted measurements of the long-term effects of hM3Dq activation on neuronal activity. In one study, local field potential (LFP) recording from the hippocampus in mice expressing hM3Dq (Alexander et al., 2009) revealed that CNO administration increased gamma-band power, an effect that peaked within ~1 hr after CNO injection and decayed to baseline within ~9 hr. The second study examined the behavior of mice that expressed hM3Dq in serotonergic neurons and consumed CNO in their drinking water for 3–4 weeks (Urban et al., 2016). Whole-cell recording in the acute slice preparation revealed that following this prolonged period of continuous CNO exposure, CNO still induced depolarization, suggesting that no desensitization occurs over weeks.
In summary, the timescale of the behavioral or physiological phenomenon under investigation should be taken into consideration when choosing an inhibitory tool, since effective inhibition over a short time window does not necessarily predict long-lasting efficacy.
Ligand-Gated Chloride Channels
Before optogenetic tools became available, genetically engineered ligand-gated silencing tools were used to reversibly silence defined populations of neurons with some spatiotemporal control (Figure 2A). One of the first strategies exploited invertebrate glutamate-activated chloride channels (GluCls), which do not naturally occur in mammals. These channels are highly sensitive to nanomolar concentrations of the selective allosteric agonist ivermectin (IVM) (Cully et al., 1994), which is inert in mammals. IVM is an approved anti-parasitic drug, which is applied in low doses as medication in humans and therefore makes exogenously expressed GluCl a highly attractive target for selective silencing strategies. Application of IVM to cultured wild-type hippocampal neurons had no detectable effect, while excitatory currents were strongly shunted and action potentials were completely blocked in neurons co-expressing the α and β subunits of GluCl (Slimko et al., 2002). The affinity of GluCls for glutamate, their natural agonist that is highly abundant in the CNS of most mammals, was strongly attenuated by a point mutation in the glutamate binding pocket of the β subunit with negligible effects on IVM affinity (Li et al., 2002). This engineered GluCl made its use in mammals more specific due to the higher selectivity for IVM. Due to its lipophilicity, IVM can readily penetrate the blood-brain barrier and enter the brain, making in vivo applications straightforward. For example, GluCl was used in mice to reversibly silence striatal neurons in vivo (Lerchner et al., 2007). The behavioral effects were robust and reproducible with repeated injections of IVM. However, the onset of the behavioral effects after a single injection of IVM was delayed by hours and declined only slowly over days (Figure 1B). This is probably due to uptake of IVM by fatty tissue and slow release and metabolism. More recent engineering efforts were aimed at generating modified GluCls with higher affinity for IVM and better membrane trafficking in order to avoid potential side effects from high doses of IVM and to achieve more efficient and homogeneous neuronal GluCl expression (Frazier et al., 2013). In summary, GluCls may serve as an excellent reversible silencing tool under conditions where Cl– currents inhibit neuronal activity. Their two main limitations are similar to those for DREADDS: the poor temporal control, which does not allow acute interventions as required in many behavioral paradigms, and the difficulty to achieve precise dosage of IVM in the brain.
More recently, a different approach was taken to silence neuronal activity with ligand-gated ion channels (LGICs). This approach hinges on previous findings that ligand-binding domains (LBDs) of the α7 nicotinic acetylcholine receptor (nAChR) can be fused to functionally diverse ion pore domains (IPDs) to generate chimeric channels whose ligand gating is determined by the LBD and ion selectivity is determined by the IPD (Eiselé et al., 1993; Grutter et al., 2005). Mutating the nAChR LBD yielded a pharmacologically selective actuator module (PSAM). This module, as the name implies, does not bind the natural agonist acetylcholine but is exclusively activated by pharmacologically selective effector molecules (PSEMs) (Magnus et al., 2011). Various exclusive PSAM/PSEM modules were developed, which can be combined with IPDs from different ligand-gated ion channels. This system is therefore highly versatile as it allows orthogonal silencing of distinct defined neuronal populations. For example, the IPD of a glycine receptor can be coupled to two different PSAMs to generate two genetically encoded chloride channels that can be exclusively activated by their respective PSEM. Upon activation, these channels produce large chloride conductances, shunting even strong excitatory currents (Magnus et al., 2011). The PSEMs used for the evolution of specific PSAM/PSEM pairs are based on the α7 nAChR agonist quinuclidinyl benzamide PNU-282987, which readily crosses the blood-brain barrier, making engineered LGICs favorable tools for silencing neuronal activity in vivo (Basu et al., 2013; Lovett-Barron et al., 2014).
Combined Light- and Ligand-Gated Inhibitory Ion Channels
As opposed to chloride, potassium conductance hyperpolarizes almost all types of CNS neurons since the reversal potential for potassium is typically well below the resting membrane potential. While the inward-rectifying potassium channel Kir2.1 proved to be an efficient tool to reduce neuronal excitability, its constitutive conductance narrows its use to chronic, irreversible silencing (Burrone et al., 2002; Johns et al., 1999). Engineered potassium channels, controlled by covalently attached synthetic photoswitched tethered ligands (PTLs), could overcome such limitations, allowing reversible activation and inactivation of a hyperpolarizing potassium leak current. An initial strategy was termed SPARK (synthetic photoisomerizable azobenzene-regulated potassium [K+] channels) and is based on a genetically modified Shaker potassium channel, which selectively binds an azobenzene moiety that, in turn, is linked to a pore blocker (Banghart et al., 2004). Various other K+ channels have been designed following the same engineering approach (Fortin et al., 2011). In all of them, a photoactive ligand, termed MAQ, consists of a maleimide (M), which binds to a genetically introduced cysteine on an extracellular domain of the channel, an azobenzene residue (A) that constitutes the light-sensitive switch, and a quaternary ammonium (Q) group that blocks the channel. Illumination with UV light photoconverts the A residue to the cis configuration, pulling the Q residue from the pore to unblock the channel. Green light switches the A moiety back to the trans-configuration, allowing the Q residue to re-enter and block the pore (Figure 2A). This elegant approach allows bistable photoswitching of a potassium channel and thereby, in principle, enables temporally precise control of neuronal excitability with light. One limitation of this system lies in the requirement for the chemical ligand to prevent the modified potassium channels from hyperpolarizing the neurons as soon as they are expressed. Thus, in theory, the ligand has to be present at all times in sufficient concentrations from the onset of channel expression until the actual experiment, if neuronal silencing is not desired. Another limitation for in vivo applications is that the A group relaxes to the cis conformation in the dark, necessitating constant illumination with green light to block the pore.
A more complex approach toward a light-gated potassium channel, which is naturally closed involves fusion of sequences from the potassium-selective pore of a bacterial glutamate receptor (sGluR0) and the ligand-binding domain of a mammalian glutamate receptor (iGluR6) containing the PTL binding site. In this case, a glutamate is attached to a maleimide azobenzene residue (Janovjak et al., 2010). This hybrid channel was termed HyLighter. Photoswitching to the cis configuration brings the glutamate to the binding site of the receptor, opening the channel. Thus, addition of the photoswitch is only required at the time of the experiment, as the channel is normally closed in its absence.
The azobenzene switch has been exploited to generate large numbers of additional photoswitches that can either tether covalently to engineered target molecules (PTLs) or can act as freely diffusible photochromic ligands (PCLs). PCLs have the advantage that they do not require engineered target molecules, but rather act as highly specific, photoswitchable small-molecule drugs. For example, QAQ is a PCL based on an azobenzene switch flanked by two quaternary ammonium groups that acts as a diffusible light-switched blocker for voltage-gated Na+, Ca2+, and K+ channels. This tool was used to control nociception with light by silencing sensory neurons in mice (Mourot et al., 2012). Another example for a PCL is a light-operated GIRK channel opener (LOGO). Here, a photoswitch was developed that selectively binds GIRK1 subunits and opens the channel upon UV illumination and was used to silence hippocampal neurons and alter motility of zebrafish larvae (Barber et al., 2016). However, many more PTLs and PCLs exist that have ambiguous effects on neuronal activity, depending on the type of neuron and the identity of their molecular targets. We will not discuss these tools extensively here as they are covered by several excellent recent reviews (Fehrentz et al., 2011; Kramer et al., 2013; Reiner et al., 2015).
One disadvantage of the azobenzene-based photoswitches is their requirement for UV light, which is strongly absorbed in brain tissue (Figure 2B; Eggert and Blazek, 1987) and could have detrimental effects on cell health. New, red-shifted generations of azobenzene switches may overcome the UV dependence (Dong et al., 2015; Kienzler et al., 2013). Another way around UV illumination is two-photon switching of azobenzenes with pulsed near-infrared light, which penetrates brain tissue with high efficiency (Carroll et al., 2015), and can allow spatially confined photoswitching at the microscope focal plane. Irrespective of light delivery, the PTLs or PCLs themselves have to be delivered to the neurons under investigation. While this is relatively simple in cell culture and small model organisms such as zebrafish larvae (Beharry et al., 2011; Carroll et al., 2015; Rovira et al., 2016), delivery to the mammalian brain may be more complex (although feasible, Lin et al., 2015). Novel tools for fluid delivery to the mammalian brain through a combined microfluidic channel-waveguide implant might make this approach more practicable (Jeong et al., 2015; Park et al., 2017).
Silencing Synapses
Silencing synaptic transmission in defined projection pathways can help elucidate their contribution to neuronal network dynamics and animal behavior. Ideally, one would transiently inhibit synaptic transmission only from a selected source population onto one defined target region or neuronal population. In many cases, projections from multiple neuronal populations converge onto a given brain region. These projections are often spatially intermingled with other long-range inputs and with local axon collaterals. While pharmacological and photochemical interventions are useful for bulk inhibition of specific neurotransmitter inputs to a defined brain region (Figure 1), optogenetic and chemogenetic tools can potentially offer a critical advantage in cell-type and spatio-temporal specificity, allowing local inhibition of synaptic terminal function without compromising the function of the upstream cell bodies or their axonal collaterals to other brain regions. In other cases, silencing of postsynaptic units (e.g., spines or dendritic branches) with high spatiotemporal control is needed to address the computation of inputs onto a given neuron. Yet, silencing of synaptic terminals appears to be substantially more complex than somatic silencing, for several reasons: (1) targeting of the actuator protein (opsin or chemogenetic tool) to the terminal can require longer expression time or the addition of specific targeting motifs to enhance axonal transport (Rajasethupathy et al., 2015; Stachniak et al., 2014); (2) optogenetic manipulation of axons appears to require higher light power than manipulation of neuronal somata (Jackman et al., 2014); (3) ionic composition of the axonal cytosol may differ from the somatodendritic cytosol and effects of silencing tools are therefore difficult to predict (Mahn et al., 2016); (4) recording the activity of presynaptic terminals is more challenging than somatic recording (Bischofberger et al., 2006; Hu and Shu, 2012; Schneggenburger and Forsythe, 2006). These constraints limit the feasibility of validating the physiological impact of manipulations on synaptic terminal function, such that these effects are typically evaluated indirectly through recordings from postsynaptic neurons and optical imaging.
Spatiotemporally Precise Silencing of Presynaptic Terminals
In principle, silencing of synaptic transmission could be achieved by interfering with action potential propagation or by directly blocking synaptic vesicle release through light application to the axonal terminals (Figure 1A). By expressing proton or chloride pumps and delivering light to axonal projection targets, several studies have achieved successful inhibition of axon terminal function (Mahn et al., 2016; Spellman et al., 2015; Stuber et al., 2011; Tye et al., 2011). Hyperpolarization of the membrane potential with electrogenic light-driven ion pumps is inevitably linked to active transport of ions and hence a change in ion composition of intracellular and extracellular space (El-Gaby et al., 2016; Ferenczi et al., 2016; Mahn et al., 2016; Raimondo et al., 2012). In the case of proton pumps, this has a direct impact on the intracellular pH. The magnitude of an increase in intracellular pH and thereby the cellular effects depend strongly on the geometry of the modulated compartment (Figure 4D). For example, in hippocampal CA1 neurons with an estimated buffer capacity of 20 mM (Bevensee et al., 1996), translocation of approximately 10 million protons across the membrane of an axon with a diameter of 1 μm and a length of 10 μm is predicted to change the intracellular pH by 0.1. In such small cylindrical compartments, where membrane surface area is large compared to the corresponding volume, a sufficient number of opsin molecules can be integrated into the membrane to achieve such proton efflux within several seconds (Zimmermann et al., 2008). Indeed, in the acute slice preparation, extended activation of proton pumps in axonal terminals led to alkalization-mediated calcium influx and thus increased spontaneous vesicle release rates in addition to the intended decrease in evoked release (El-Gaby et al., 2016; Mahn et al., 2016). Although an increase in spontaneous release is a potential confound due to the possible recruitment of local-circuit inhibition at the site of illumination, elimination of spiking-evoked release could be sufficient for “scrambling” the information content of the synapse, effectively disrupting the transmission of information through the modulated pathway. The net effect of such manipulations might, however, be highly circuit-specific and should be carefully validated (Wiegert and Oertner, 2016).
Given the limitations of the ion-pumping rhodopsins in silencing presynaptic terminal activity, it is interesting to consider the use of ACRs for this purpose. While this is potentially feasible due to the hyperpolarizing action of chloride in neurons, ACR-mediated inhibition of synaptic release would rely on the chloride reversal potential in the axonal compartment (Figure 4B), which has been shown to be depolarizing in some projection pathways (Price and Trussell, 2006; Pugh and Jahr, 2011; Szabadics et al., 2006; Turecek and Trussell, 2001) and can change with age (Ferando and Mody, 2015). While it is still possible that a large chloride conductance would effectively shunt incoming action potentials, such an effect should be rigorously validated (Spellman et al., 2015). Further optimization of newly described tools such as light-gated potassium channels (Cosentino et al., 2015) or optoGPCRs (Figure 4E) (Airan et al., 2009; Kleinlogel, 2016; Li et al., 2005; Masseck et al., 2014; Siuda et al., 2015a) might eventually provide improvements in the efficacy of fast optogenetic inhibition of neurotransmitter release. OptoGPCRs are especially promising, since they can inhibit presynaptic P/Q type calcium channels, which are required for presynaptic neurotransmitter vesicle fusion (Li et al., 2005). For the time being, it seems that chloride pumps are the most suitable tool for brief synaptic terminal silencing, although their use should be carefully controlled to account for changes in chloride reversal potential (Raimondo et al., 2012), light-off rebound responses and the efficacy of silencing during prolonged activation (Mahn et al., 2016).
Spatiotemporally Precise Postsynaptic Silencing
Investigating the integration of synaptic inputs onto a given dendrite, or dissecting the interaction between different dendrites on a postsynaptic cell, requires high-precision silencing of specific post-synaptic sites. Presynaptic strategies are often not useful for this type of experiment due to the complex connectivity patterns and the multitude of presynaptic neurons innervating a single postsynaptic cell. Postsynaptic silencing of synaptic inputs without affecting somatic properties or axonal function poses unique challenges due to the electrotonic proximity of dendritic structures with the neuronal soma. In principle, different strategies can be exploited to achieve subcellular postsynaptic silencing. Local application of glutamate receptor antagonists or GABA receptor agonists can silence dendritic segments in large polarized neurons, such as L5 pyramidal cells, without compromising postsynaptic function on distant dendrites of the same cell (Takahashi et al., 2016). Higher spatiotemporal precision can be achieved, in principle, with local photolysis of caged inhibitory neurotransmitters such as NI-caged GABA and glycine (Canepari et al., 2001) or RuBi-caged GABA (Fortin et al., 2011).
A recent study achieved optogenetic manipulation of synaptic strength by selectively targeting AS-PaRac1, a photoactivatable GTPase, to spines of recently activated synapses. Selective spine targeting was achieved by fusing three components: an improved version of PaRac1 (Wu et al., 2009); PSDΔ1.2, which binds to the postsynaptic density; and an mRNA dendritic targeting element (Hayashi-Takagi et al., 2015). Illumination with blue light triggered weakening of the targeted synapses by increasing GTPase activity, presumably altering actin polymerization in spines (Hall, 1994; Luo et al., 1996). While this strategy is useful for weakening or de-potentiating synapses, it is not a silencing tool per se. Moreover, once weakened, modulated spines need to recruit the endogenous molecular machinery for the recovery of synaptic strength. Thus, the effect of AS-PaRac1 is not reversible in a simple sense.
Subcellular compartments, such as particular dendrites, can be silenced with cell-wide-expressed optogenetic tools by applying local illumination to the subcellular compartment of interest. A recent study used superficial blue-light illumination of the somatosensory cortex to silence mainly the apical tufts of L5 pyramidal cells expressing iChloC (Takahashi et al., 2016). Due to the architecture of L5 pyramidal neurons, somata, basal dendrites, and main apical branches are located relatively deep below the surface of the cortex and are therefore exposed to substantially lower light power (Al-Juboori et al., 2013). New developments in light delivery hardware can theoretically allow simultaneous local illumination of hundreds of subcellular targets in the mammalian brain with micrometer resolution (Szalay et al., 2016). Holographic two-photon excitation of various ChR variants has already been achieved (Bègue et al., 2013; Bovetti et al., 2017). Combined with inhibitory optogenetic tools of appropriate light sensitivity and two-photon absorption cross-section, such techniques can be used to deconstruct postsynaptic computation with unprecedented resolution.
Chemogenetic Silencing of Presynaptic Terminals
To date, inhibitory DREADDs are probably the most reliable tools for reversible silencing of synaptic transmission. Although limited in their temporal and spatial resolution by ligand diffusion and biochemical pathway time constants, the inhibitory DREADDs hM4Di and KORD potently inhibit presynaptic release in addition to their GIRK-mediated effects on intrinsic excitability (Figure 4E) (Stachniak et al., 2014; Vardy et al., 2015). Local injection of CNO into the target area of presynaptic terminals has been used successfully in various studies to locally shut down synaptic input from a defined population of neurons (Franklin et al., 2017; Gremel et al., 2016; Mahler et al., 2014; Stachniak et al., 2014; Ye et al., 2017). An axon-localized version of hM4Di was developed in order to avoid hyperpolarization of the somatodendritic compartment while silencing synaptic release, utilizing an intracellular neurexin signaling motif that facilitates internalization of non-axonal protein (Stachniak et al., 2014). This strategy could in principle be applied to other transmembrane silencing tools such as microbial rhodopsin ion pumps or light-gated GPCRs if they are sufficiently expressed in the axon. The PSAM approach was also used to manipulate synaptic release through local application of a PSEM ligand to axons expressing the PSAM (L141F)-GlyR (Basu et al., 2013). Despite evidence indicating that axonal chloride concentrations are higher, leading to a depolarizing chloride reversal potential in this compartment (Figure 4B), the shunting effect of a slow-acting ligand-gated chloride channel might be able to overcome this constraint. Nevertheless, recordings from postsynaptic neurons should accompany any such manipulation, to characterize potential side effects (Mahn et al., 2016).
Chronic Silencing of Presynaptic Function
Chronic silencing of specific long-range projections can be achieved through expression of potassium channels such as Kir2.1 (Figure 1A; Burrone et al., 2002; Johns et al., 1999; Xue et al., 2014), which can prevent action potential firing via constitutive membrane hyperpolarization, or the tetanus toxin light chain (TeNT; Sweeney et al., 1995), which selectively eliminates synaptic vesicle exocytosis in the targeted neurons (Figure 1A). Although TeNT is less temporally precise than the chemogenetic approaches described above, it can be highly efficient in chronic silencing (Figure 1B), and when the targeted neurons project mainly to one downstream target (collaterals will also be silenced by the expression of TeNT). Chronic silencing of synaptic transmission with spatial precision can also be achieved with a method called InSynC (Figures 1 and 2; Lin et al., 2013). In this technique, light absorption by light-oxygen-voltage (LOV) domains leads to the formation of a flavin-cofactor radical, which was utilized to engineer a genetically encoded tool for reactive oxygen species generation (miniSOG; Shu et al., 2011). When targeted to presynaptic terminals, miniSOG can be used to damage the vesicle release machinery by oxidation of surrounding proteins, leading to long-term inhibition of neurotransmission (Figures 1B and 2A; Lin et al., 2013). The drawback of this approach is that vesicle release inhibition only recovers on the timescale of synaptic protein turnover, and long-lasting effects on synaptic strength are unknown. Further characterization is needed of the dark activity, stability, and kinetic properties of these tools in mammalian neurons to fully evaluate their utility for silencing neurons or synaptic compartments. Although LOV-domain-based tools show great promise for neuroscience research, they have yet to be shown to work as reliably, robustly, and specifically as the microbial opsin-based approaches for silencing neurons.
Experimental Considerations and Constraints Associated with Neuronal Silencing Experiments
Silencing of neural activity can be a powerful tool in the neuroscientist’s arsenal. However, each of the silencing approaches described in this review carries its own unique constraints. Neuronal excitability can vary greatly between the dissociated primary culture, acute slice preparations, anesthesia, and wakefulness. Inactivation of tonic baseline firing (for example, in an anesthetized animal or in dissociated culture) might not be indicative for inhibition of task-related activity patterns in the behaving animal. These differences in neuronal excitability highlight the need for careful validation of the silencing approach under settings that are as similar as possible to the ones used in the actual experiment. In the sections below, we detail some of the major experimental constraints that should be considered when designing silencing experiments.
Light and Heating-Related Considerations
Due to the intrinsic light requirements of most microbial opsins used as optogenetic tools, in vivo optogenetic silencing approaches utilize high-power light sources to reach sufficiently high light intensities in the region of interest (Rajasethupathy et al., 2016; Yizhar et al., 2011). However, delivery of high-intensity light through an optical fiber can elevate neuronal firing rates (Christie et al., 2013; Han, 2012; Kim and Connors, 2012; Wang et al., 2011) and change blood flow (Rungta et al., 2017) locally through tissue heating. Depending on the fiber diameter and the wavelength used, this can become a serious concern, necessitating empirical and theoretical methods for estimating light-induced heating in the target tissue (Acker et al., 2016). This problem has been addressed with Monte Carlo simulations of light propagation from the fiber tip in brain tissue (Liu et al., 2015; Stujenske et al., 2015; Yona et al., 2016). The widely used ion pumps halorhodopsin and archaerhodopsin require particularly high light intensities since constant photon flux is necessary for continued ion transport. Compared to the most widely used ChRs (e.g., ChR2-H134R) the red-shifted action spectrum of both ion pumps allows for somewhat higher light intensities since the lower absorption coefficient of red-shifted light leads to lower tissue heating (Figure 3; Stujenske et al., 2015). However, according to current models for light absorption in brain tissue, light intensities in the order of 10 mW delivered through an optical fiber will cause significant heating of large volumes of brain tissue during continuous illumination, increasing local temperatures by several degrees Celsius (peak temperatures >4°C and >2°C for 532 and 593 nm, respectively; Stujenske et al., 2015). Recent work suggested that these Monte Carlo results might in fact be an underestimate of in vivo temperature rise (Arias-Gil et al., 2016, but see also Suhan et al., 2017).
With respect to heating, optogenetic silencing tools with slow off-kinetics or higher light sensitivity may be favorable to the widely used ion pumps (Figure 3). Several members of the new class of light-gated anion channels are promising candidates. Slow variants of nACRs and eACRs were recently developed, based on previously identified mutations in ChR2 (Berndt et al., 2014, 2016; Sineshchekov et al., 2015; Wietek et al., 2014, 2015). Indeed, some of these slow ACRs were successfully used in vivo to elicit long-lasting silencing effects with short light pulses (Iyer et al., 2016; Kim et al., 2016; Takahashi et al., 2016; Wietek et al., 2015). In principle, bistable ACRs can allow the temporal dissociation of light stimulation and the experimental condition under which silencing is examined. In this case, potential heat effects should have dissipated at the start of the actual experimental paradigm.
To illustrate these points, we used the model developed by Stujenske et al. (2015) to estimate tissue heating under several commonly used light intensities, stimulation patterns, and wavelengths compatible with recently developed silencing tools (Figure 3). Some of these tools require luminous flux that is three to four orders of magnitude lower compared to the classical ion pumps to exert a full shutdown of neuronal spiking (Figure 4C; Govorunova et al., 2016; Masseck et al., 2014). Thus, aiming for minimal tissue heating, large brain areas (several cubic millimeters) can be silenced under illumination conditions that produce a maximal temperature increase of 0.2°C (Figure 3C). With regard to the efficacy of silencing at a given light power density, it is important to note that most published reports of the light sensitivity of optogenetic tools calculate these values as a fraction of maximal current (Mattis et al., 2011; Wietek et al., 2016). While these values are biophysically correct, maximal photocurrents are often not required for complete silencing of the targeted population, particularly with high-conductance ACRs. Therefore, lower light power densities could potentially be used, as silencing efficiency depends not only on the intrinsic light sensitivity, but also on the maximal photocurrent of the optogenetic tool being used, which is determined by its expression level and conductance.
Synaptic Scaling and Homeostatic Plasticity
One potential concern that should be considered with regard to any long-term synaptic silencing manipulation is the ability of synapses to adapt to changes in their activity rate. This form of compensation is termed homeostatic plasticity. Synaptic scaling is one form of homeostatic plasticity, where synaptic transmission is globally adjusted to compensate for up- or downregulation of neuronal activity levels (Goold and Nicoll, 2010; Turrigiano et al., 1998). Thus, when circuits are silenced with pharmacological blockers of synaptic transmission or neuronal activity such as TTX, synaptic scaling can potentially act to restore the circuit into the state of activity it was in before the inhibitors were applied. Removing the inhibitor may then result in an overactive circuit. Notably, homeostatic plasticity is not limited to synapses but can occur at various cellular and subcellular levels. For example, increased intrinsic excitability of the plasma membrane may serve to compensate for reduced spiking activity (Sokolova and Mody, 2008; Stemmler and Koch, 1999). Such compensatory mechanisms are usually invoked at timescales of hours to days. Therefore, transient silencing strategies may be applied safely, while the precise mechanism of action of chronic silencing strategies should be considered in order to avoid undesired side effects and misinterpretation. As an example, expression of the inward rectifier potassium channel Kir2.1 hyperpolarizes neurons by increasing the potassium leak current (Burrone et al., 2002; Johns et al., 1999; Xue et al., 2014). However, since Kir2.1 is constitutively expressed, the resulting constant hyperpolarization leads to homeostatic upregulation of synaptic input (Burrone et al., 2002) and neuronal excitability. In the latter case, chronically increased potassium conductance may be counteracted over time by the neuron’s own homeostatic machinery. An elegant behavioral demonstration of differential effects between temporally precise and prolonged optogenetic inhibition involved the inhibition of hippocampal activity using eNpHR3.1 (Goshen et al., 2011). Inhibiting the hippocampal CA1 region during remote contextual fear recall resulted in elimination of freezing behavior, while 30-min-long inhibition had no effect, consistent with previously published work that utilized pharmacological blockade of this region (Kitamura et al., 2009).
Unlike cell-wide neuronal silencing, silencing tools expressed at presynaptic terminals will not alter the neuronal membrane potential and thus its firing properties. Rather, they selectively prevent neurotransmitter release and synaptic transmission. Yet, despite the circumvention of cell-wide homeostatic effects, constitutive synaptic silencing may lead to presynaptic or post-synaptic scaling. For example, TeNT selectively cleaves the presynaptic vesicle SNARE protein VAMP2 and thereby completely and irreversibly abolishes neurotransmitter vesicle release from cells expressing this protein (Harms and Craig, 2005; Yamamoto et al., 2003). While exogenous bath application or injection of purified TeNT into the brain inhibits transmission at all synapses that are reached by diffusion, genetic overexpression of TeNT allows for selective silencing of neurotransmitter release from terminals of selected cells (Ehlers et al., 2007; Lee et al., 2010). Sparse expression of TeNT in a small set of neurons was used to investigate metaplasticity, another form of homeostatic plasticity at single synapses (Lee et al., 2010). Metaplasticity is described as the mechanism that adjusts the ability to strengthen and weaken synapses based on the recent history of neuronal activity (Abraham, 2008). In essence, this means that synapses change their state in a long-lasting way according to their past activity by adapting their ability to undergo plasticity. Thus, metaplasticity—as the term implies—is a plastic change of the synaptic plasticity mechanisms (Slutsky et al., 2004). While many studies show that various stimulation paradigms can prime synapses to be efficiently potentiated by altering the NMDA receptor (NMDAR) content of the synapses, it has also been demonstrated that silencing presynaptic terminals can prime NMDAR insertion in postsynaptic spines (Lee et al., 2010). Therefore, any chronic silencing strategy, be it at the level of neuronal ensembles or at a sparse set of synapses, will come with the cost of homeostatic adaptations.
Understanding and Interpreting the Experimental Outcomes of Neuronal Silencing
Inactivation of neural activity is often carried out to complement experiments in which the same neuronal population is excited using genetically encoded tools. The often-cited view of these manipulations reflecting “necessity” and “sufficiency” stems from the intuitive assumption of symmetry in the physiological impact of these manipulations on the neural circuit. While in some cases this might indeed be true, many factors could influence the degree of symmetry, including the spontaneous firing rates of the modulated neurons and their state-dependent activity patterns (Phillips and Hasenstaub, 2016). Recent work has highlighted the difficulty of elucidating the dependence of a downstream target on the information provided by an upstream brain area (Otchy et al., 2015). Using transient silencing approaches, the sudden removal of a permissive but not truly necessary input could diminish the overall excitatory drive in the target area, thus compromising its function in a manner that does not reflect the true role of the manipulated region under steady-state conditions. In contrast, homeostatic regulation of neuronal activity can compensate for the missing input if the transmitted information was indeed not necessary for the underlying computation. Work in hippocampal circuits has shown that, upon silencing of a subpopulation of CA1 neurons, other sets of neurons can rapidly increase their activity, triggering a redistribution in the firing rates of local-circuit interneurons (Trouche et al., 2016). Inhibition of neurotransmitter release in any of the above-mentioned ways cannot be used to differentiate between a permissive and an instructive input. To tackle this question, a method that does not change the overall amount of released neurotransmitter, but rather scrambles the information transmitted by the targeted region, would be desirable. One potential approach by which such scrambling could be achieved would be through an optogenetic tool capable of altering the axonal conductance speed or decreasing the reliability of synaptic transmission at a given synapse. Until such advanced approaches exist, manipulations of neural circuit activity should take into account the range of natural activity patterns in the circuit, since any manipulation that shifts the circuit into a state that is outside its natural “manifold” can be difficult to interpret (Jazayeri and Afraz, 2017).
Future Perspectives
Light-Gated Potassium Channels for Somatic or Axonal Silencing
Given the disadvantages associated with photoswitched tethered ligands, a purely light-gated potassium channel would be beneficial. To date, no naturally occurring light-gated potassium channel has been reported. At first glance, a light-gated potassium channel derived from ChR—analogous to the recently reported engineered ACRs—would be an obvious addition to the existing palette of optogenetic silencing tools. However, unlike highly specialized ion channels, in which ion selectivity is determined by a complex pore architecture (Gouaux and Mackinnon, 2005), ChR2 is not very selective for any particular cation (Nagel et al., 2003). Rather, its photocurrents show a strong dependency on the atomic radius of the conducted cation. Despite the inverse relationship between atomic radius and conductance, sodium and potassium ions traverse the ChR2 pore with relatively similar efficiency (Nagel et al., 2003). Although the high-resolution crystal structure of the ChR chimera C1C2 guided the transformation of C1C2 into a more potassium-selective form, pure potassium conductance could not be achieved (Kato et al., 2012). Thus, conversion of ChR into a form purely selective for a particular cation would require more extensive engineering efforts than the generation of eACRs necessitated, where the overall charge selectivity was inverted.
While ChR-based strategies have yet to succeed, fusion of the miniature viral potassium channel Kcv to a LOV2 module yielded the first light-gated potassium channel, which does not require additional components or co-factors (Cosentino et al., 2015). Although this “blue-light-induced potassium channel 1 (BLINK1)” was shown to alter behavior in zebrafish larvae, it did not show robust expression in mammalian neurons. Further improvement of this initial variant might prove useful in the future.
High-Efficacy Tools for Silencing Presynaptic Terminals
As discussed throughout this article, temporally and spatially precise synaptic terminal inhibition has been achieved using expression of proton or chloride pumps in combination with light delivery to axonal projection targets. This approach, however, is subject to limitations in terms of inhibition efficiency over extended time periods, relatively high light power requirements and altered ion concentrations (Mahn et al., 2016; Raimondo et al., 2012). Where the experimental paradigm allows, these limitations have led researchers to utilize the chemogenetic approach of hM4Di expression combined with local CNO infusion at the synaptic terminal field, and to develop ChR-mediated stimulation protocols for inducing synaptic depression (Creed et al., 2015; Klavir et al., 2017; Nabavi et al., 2014). However, the timescales of synaptic plasticity and the rates of CNO diffusion and clearance limit the spatial and temporal control afforded by these approaches. Furthermore, overexpression of hM4Di in itself may alter neuronal properties (Saloman et al., 2016).
Continued efforts are therefore under way to develop new optogenetic tools that allow for efficient synaptic silencing over time-scales of seconds to hours. Such tools should target different stages along the synaptic vesicle release process. The earliest point of interference would be to suppress action potential propagation in the axon. One possible avenue could be the further optimization of light-gated potassium channels, as discussed above (Cosentino et al., 2015), or the generation of multicomponent systems where a potassium-channel ligand, e.g., a cyclic nucleotide, is produced in a light-dependent manner (Gao et al., 2015; Kim et al., 2015; Ryu et al., 2010; Stierl et al., 2011). One common difficulty of any approach attempting to block the propagation of action potentials is that the high sodium channel density in the axonal membrane presents a very high safety factor (Hille, 2001). Overcoming this safety factor might not be easily achieved. Other points of interference are the action potential-evoked activation of voltage-sensitive calcium channels or the synaptic release machinery. The presynaptic mode of action of hM4Di might be governed by Gi/o-mediated reduction of the opening probability of N-type calcium channels and/or interference with proteins of the vesicle-fusion machinery (Figure 4F; Chevaleyre et al., 2007; Delaney and Crane, 2016), suggesting that naturally occurring or engineered Gi/o-coupled opsins might trigger a light-induced hM4Di-like effect (Figure 4E). Several other tools, such as InSynC, have been designed to target the vesicle release machinery (Lin et al., 2013), but highly specific, reversible tools are still lacking. One common difficulty of most of these approaches is the need for selective enrichment of these tools at the pre-synapse, as targeting to the presynaptic plasma membrane has proved challenging. Modest axonal enrichment of ChR2 was achieved by coupling it with myosin VI (Lewis et al., 2011), while axon-specific expression of hM4Di was based on reduction of somatodendritic expression (Stachniak et al., 2014). In summary, silencing of presynaptic terminals is an area of optogenetic technology that still awaits a refined, highly effective, and universal solution.
Summary
Reversible silencing of neural activity is a powerful approach that has been increasingly used in systems neuroscience for testing the roles of defined brain structures, neuronal populations, and projection pathways in a wide range of brain functions and behaviors. However, in contrast with optogenetic excitation for which the majority of studies have used a limited number of channelrhodopsin-based tools, the number of genetically encoded tools used for inhibition of neural activity has vastly expanded over the last few years, and many different silencing strategies have been employed. Although such manipulations of neural activity are widely used, the design and interpretation of neural silencing experiments presents unique challenges, both technically and conceptually. Thus, the choice of an appropriate silencing strategy should be carefully considered with respect to the experimental requirements and to the limitations of both the tools themselves and the experimental systems in which they are applied.
Acknowledgments
We thank Lief E. Fenno, Jonas Wietek, Laura Laprell, Paul Lamothe, and all Wiegert and Yizhar lab members for critical comments and discussion. This work was funded by grants from the European Research Council (ERC-2016-StG 714762) and the German Research Foundation (SPP 1926 and FOR 2419) to J.S.W. and from the Israel Ministry of Science Technology and Space, the Israel Science Foundation (ISF #1351-12), the European Research Council (ERC-StG #337637), the Human Frontier Science Program, the I-CORE program of the Planning and Budgeting Committee and the Israel Science Foundation (grant no. 51/11), and the Gertrude and Philip Nollman Career Development Chair to O.Y. Work in the Yizhar lab is supported by the Adelis Foundation, the Candice Appleton Family Trust, and the Lord Sieff of Brimpton Memorial Fund. M.P. was supported by the Koshland Foundation.
References
- Abraham WC. Metaplasticity: Tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Acker L, Pino EN, Boyden ES, Desimone R. FEF inactivation with improved optogenetic methods. Proc Natl Acad Sci USA. 2016;113:E7297–E7306. doi: 10.1073/pnas.1610784113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- Al-Juboori SI, Dondzillo A, Stubblefield EA, Felsen G, Lei TC, Klug A. Light scattering properties vary across different regions of the adult mouse brain. PLoS ONE. 2013;8:e67626. doi: 10.1371/journal.pone.0067626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonsa H, Merricks EM, Codadu NK, Cunningham MO, Deisseroth K, Racca C, Trevelyan AJ. The contribution of raised intraneuronal chloride to epileptic network activity. J Neurosci. 2015;35:7715–7726. doi: 10.1523/JNEUROSCI.4105-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonsa H, Lakey JH, Lightowlers RN, Trevelyan AJ. Cl-out is a novel cooperative optogenetic tool for extruding chloride from neurons. Nat Commun. 2016;7 doi: 10.1038/ncomms13495. 13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Gil G, Ohl FW, Takagaki K, Lippert MT. Measurement, modeling, and prediction of temperature rise due to optogenetic brain stimulation. Neurophotonics. 2016;3 doi: 10.1117/1.NPh.3.4.045007. 045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrenberg AB, Del Bene F, Baier H. Optical control of zebrafish behavior with halorhodopsin. Proc Natl Acad Sci USA. 2009;106:17968–17973. doi: 10.1073/pnas.0906252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer S, Stürzebecher AS, Jüttner R, Santos-Torres J, Hanack C, Frahm S, Liehl B, Ibañez-Tallon I. Silencing neurotransmission with membrane-tethered toxins. Nat Methods. 2010;7:229–236. doi: 10.1038/nmeth.1425. [DOI] [PubMed] [Google Scholar]
- Bailes HJ, Zhuang LY, Lucas RJ. Reproducible and sustained regulation of Gas signalling using a metazoan opsin as an optogenetic tool. PLoS ONE. 2012;7:e30774. doi: 10.1371/journal.pone.0030774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banghart MR, Sabatini BL. Photoactivatable neuropeptides for spatiotemporally precise delivery of opioids in neural tissue. Neuron. 2012;73:249–259. doi: 10.1016/j.neuron.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DM, Schönberger M, Burgstaller J, Levitz J, Weaver CD, Isacoff EY, Baier H, Trauner D. Optical control of neuronal activity using a light-operated GIRK channel opener (LOGO) Chem Sci (Camb) 2016;7:2347–2352. doi: 10.1039/c5sc04084a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basting TM, Burke PG, Kanbar R, Viar KE, Stornetta DS, Stornetta RL, Guyenet PG. Hypoxia silences retrotrapezoid nucleus respiratory chemoreceptors via alkalosis. J Neurosci. 2015;35:527–543. doi: 10.1523/JNEUROSCI.2923-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Srinivas KV, Cheung SK, Taniguchi H, Huang ZJ, Siegelbaum SA. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron. 2013;79:1208–1221. doi: 10.1016/j.neuron.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bègue A, Papagiakoumou E, Leshem B, Conti R, Enke L, Oron D, Emiliani V. Two-photon excitation in scattering media by spatiotemporally shaped beams and their application in optogenetic stimulation. Biomed Opt Express. 2013;4:2869–2879. doi: 10.1364/BOE.4.002869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beharry AA, Wong L, Tropepe V, Woolley GA. Fluorescence imaging of azobenzene photoswitching in vivo. Angew Chem Int Ed Engl. 2011;50:1325–1327. doi: 10.1002/anie.201006506. [DOI] [PubMed] [Google Scholar]
- Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nat Neurosci. 2009;12:229–234. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science. 2014;344:420–424. doi: 10.1126/science.1252367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Lee SY, Wietek J, Ramakrishnan C, Steinberg EE, Rashid AJ, Kim H, Park S, Santoro A, Frankland PW, et al. Structural foundations of optogenetics: Determinants of channelrhodopsin ion selectivity. Proc Natl Acad Sci USA. 2016;113:822–829. doi: 10.1073/pnas.1523341113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevensee MO, Cummins TR, Haddad GG, Boron WF, Boyarsky G. pH regulation in single CA1 neurons acutely isolated from the hippocampi of immature and mature rats. J Physiol. 1996;494:315–328. doi: 10.1113/jphysiol.1996.sp021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Li L, Geiger JR, Jonas P. Patchclamp recording from mossy fiber terminals in hippocampal slices. Nat Protoc. 2006;1:2075–2081. doi: 10.1038/nprot.2006.312. [DOI] [PubMed] [Google Scholar]
- Bodaker I, Suzuki MT, Oren A, Béjà O. Dead Sea rhodopsins revisited. Environ Microbiol Rep. 2012;4:617–621. doi: 10.1111/j.1758-2229.2012.00377.x. [DOI] [PubMed] [Google Scholar]
- Bovetti S, Moretti C, Zucca S, Dal Maschio M, Bonifazi P, Fellin T. Simultaneous high-speed imaging and optogenetic inhibition in the intact mouse brain. Sci Rep. 2017;7:40041. doi: 10.1038/srep40041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett CJ, Krashes MJ. Resolving behavioral output via chemogenetic designer receptors exclusively activated by designer drugs. J Neurosci. 2016;36:9268–9282. doi: 10.1523/JNEUROSCI.1333-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, O’Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Canepari M, Nelson L, Papageorgiou G, Corrie JE, Ogden D. Photochemical and pharmacological evaluation of 7-nitroindolinyl-and 4-methoxy-7-nitroindolinyl-amino acids as novel, fast caged neurotransmitters. J Neurosci Methods. 2001;112:29–42. doi: 10.1016/s0165-0270(01)00451-4. [DOI] [PubMed] [Google Scholar]
- Carroll EC, Berlin S, Levitz J, Kienzler MA, Yuan Z, Madsen D, Larsen DS, Isacoff EY. Two-photon brightness of azobenzene photoswitches designed for glutamate receptor optogenetics. Proc Natl Acad Sci USA. 2015;112:E776–E785. doi: 10.1073/pnas.1416942112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carus-Cadavieco M, Gorbati M, Ye L, Bender F, van der Veldt S, Kosse C, Börgers C, Lee SY, Ramakrishnan C, Hu Y, et al. Gamma oscillations organize top-down signalling to hypothalamus and enable food seeking. Nature. 2017;542:232–236. doi: 10.1038/nature21066. [DOI] [PubMed] [Google Scholar]
- Chang WC, Ng JK, Nguyen T, Pellissier L, Claeysen S, Hsiao EC, Conklin BR. Modifying ligand-induced and constitutive signaling of the human 5-HT4 receptor. PLoS ONE. 2007;2:e1317. doi: 10.1371/journal.pone.0001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Choo H, Huang XP, Yang X, Stone O, Roth BL, Jin J. The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem Neurosci. 2015;6:476–484. doi: 10.1021/cn500325v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Heifets BD, Kaeser PS, Südhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie IN, Wells JA, Southern P, Marina N, Kasparov S, Gourine AV, Lythgoe MF. fMRI response to blue light delivery in the naïve brain: Implications for combined optogenetic fMRI studies. Neuroimage. 2013;66:634–641. doi: 10.1016/j.neuroimage.2012.10.074. [DOI] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK, Dey A, Smith M, Rebusi N, Pfau M, et al. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat Neurosci. 2015;18:962–964. doi: 10.1038/nn.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Weber F, Zhong P, Tan CL, Nguyen TN, Beier KT, Hörmann N, Chang WC, Zhang Z, Do JP, et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature. 2017;545:477–481. doi: 10.1038/nature22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong AS, Miri ML, Busskamp V, Matthews GA, Acker LC, Sørensen AT, Young A, Klapoetke NC, Henninger MA, Kodandaramaiah SB, et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat Neurosci. 2014;17:1123–1129. doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino C, Alberio L, Gazzarrini S, Aquila M, Romano E, Cermenati S, Zuccolini P, Petersen J, Beltrame M, Van Etten JL, et al. Optogenetics. Engineering of a light-gated potassium channel. Science. 2015;348:707–710. doi: 10.1126/science.aaa2787. [DOI] [PubMed] [Google Scholar]
- Coward P, Wada HG, Falk MS, Chan SD, Meng F, Akil H, Conklin BR. Controlling signaling with a specifically designed Gi-coupled receptor. Proc Natl Acad Sci USA. 1998;95:352–357. doi: 10.1073/pnas.95.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed M, Pascoli VJ, Lüscher C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347:659–664. doi: 10.1126/science.1260776. [DOI] [PubMed] [Google Scholar]
- Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, Arena JP. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- Danon A, Stoeckenius W. Photophosphorylation in Halobacterium halobium. Proc Natl Acad Sci USA. 1974;71:1234–1238. doi: 10.1073/pnas.71.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney AJ, Crane JW. Presynaptic GABAB receptors reduce transmission at parabrachial synapses in the lateral central amygdala by inhibiting N-type calcium channels. Sci Rep. 2016;6:19255. doi: 10.1038/srep19255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K, Shenoy KV. An optogenetic toolbox designed for primates. Nat Neurosci. 2011;14:387–397. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidschstein J, Chen Q, Tremblay R, Rogers SL, Saldi GA, Guo L, Xu Q, Liu R, Lu C, Chu J, et al. A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat Neurosci. 2016;19:1743–1749. doi: 10.1038/nn.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Babalhavaeji A, Samanta S, Beharry AA, Woolley GA. Red-shifting azobenzene photoswitches for in vivo use. Acc Chem Res. 2015;48:2662–2670. doi: 10.1021/acs.accounts.5b00270. [DOI] [PubMed] [Google Scholar]
- Drake CT, Bausch SB, Milner TA, Chavkin C. GIRK1 immunoreactivity is present predominantly in dendrites, dendritic spines, and somata in the CA1 region of the hippocampus. Proc Natl Acad Sci USA. 1997;94:1007–1012. doi: 10.1073/pnas.94.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert HR, Blazek V. Optical properties of human brain tissue, meninges, and brain tumors in the spectral range of 200 to 900 nm. Neurosurgery. 1987;21:459–464. doi: 10.1227/00006123-198710000-00003. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiselé JL, Bertrand S, Galzi JL, Devillers-Thiéry A, Changeux JP, Bertrand D. Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature. 1993;366:479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- El-Gaby M, Zhang Y, Wolf K, Schwiening CJ, Paulsen O, Shipton OA. Archaerhodopsin selectively and reversibly silences synaptic transmission through altered pH. Cell Rep. 2016;16:2259–2268. doi: 10.1016/j.celrep.2016.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge MA, Lerchner W, Saunders RC, Kaneko H, Krausz KW, Gonzalez FJ, Ji B, Higuchi M, Minamimoto T, Richmond BJ. Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nat Neurosci. 2016;19:37–39. doi: 10.1038/nn.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst OP, Lodowski DT, Elstner M, Hegemann P, Brown LS, Kandori H. Microbial and animal rhodopsins: Structures, functions, and molecular mechanisms. Chem Rev. 2014;114:126–163. doi: 10.1021/cr4003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrentz T, Schönberger M, Trauner D. Optochemical genetics. Angew Chem Int Ed Engl. 2011;50:12156–12182. doi: 10.1002/anie.201103236. [DOI] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferando I, Mody I. In vitro gamma oscillations following partial and complete ablation of δ subunit-containing GABAA receptors from parvalbumin interneurons. Neuropharmacology. 2015;88:91–98. doi: 10.1016/j.neuropharm.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi EA, Vierock J, Atsuta-Tsunoda K, Tsunoda SP, Ramakrishnan C, Gorini C, Thompson K, Lee SY, Berndt A, Perry C, et al. Optogenetic approaches addressing extracellular modulation of neural excitability. Sci Rep. 2016;6:23947. doi: 10.1038/srep23947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D, Chung S, Wheat H. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature. 1996;380:249–252. doi: 10.1038/380249a0. [DOI] [PubMed] [Google Scholar]
- Fortin DL, Dunn TW, Fedorchak A, Allen D, Montpetit R, Banghart MR, Trauner D, Adelman JP, Kramer RH. Optogenetic photochemical control of designer K+ channels in mammalian neurons. J Neurophysiol. 2011;106:488–496. doi: 10.1152/jn.00251.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Silva BA, Perova Z, Marrone L, Masferrer ME, Zhan Y, Kaplan A, Greetham L, Verrechia V, Halman A, et al. Prefrontal cortical control of a brainstem social behavior circuit. Nat Neurosci. 2017;20:260–270. doi: 10.1038/nn.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier SJ, Cohen BN, Lester HA. An engineered glutamategated chloride (GluCl) channel for sensitive, consistent neuronal silencing by ivermectin. J Biol Chem. 2013;288:21029–21042. doi: 10.1074/jbc.M112.423921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Nagpal J, Schneider MW, Kozjak-Pavlovic V, Nagel G, Gottschalk A. Optogenetic manipulation of cGMP in cells and animals by the tightly light-regulated guanylyl-cyclase opsin CyclOp. Nat Commun. 2015;6 doi: 10.1038/ncomms9046. 8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goold CP, Nicoll RA. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron. 2010;68:512–528. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. Dynamics of retrieval strategies for remote memories. Cell. 2011;147:678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Gouaux E, Mackinnon R. Principles of selective ion transport in channels and pumps. Science. 2005;310:1461–1465. doi: 10.1126/science.1113666. [DOI] [PubMed] [Google Scholar]
- Govorunova EG, Sineshchekov OA, Janz R, Liu X, Spudich JL. NEUROSCIENCE. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science. 2015;349:647–650. doi: 10.1126/science.aaa7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorunova EG, Sineshchekov OA, Spudich JL. Proteomonas sulcata ACR1: A fast anion channelrhodopsin. Photochem Photobiol. 2016;92:257–263. doi: 10.1111/php.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorunova EG, Sineshchekov OA, Rodarte EM, Janz R, Morelle O, Melkonian M, Wong GK, Spudich JL. The expanding family of natural anion channelrhodopsins reveals large variations in kinetics, conductance, spectral sensitivity. Sci Rep. 2017;7 doi: 10.1038/srep43358. 43358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Chancey JH, Atwood BK, Luo G, Neve R, Ramakrishnan C, Deisseroth K, Lovinger DM, Costa RM. Endocannabinoid modulation of orbitostriatal circuits gates habit formation. Neuron. 2016;90:1312–1324. doi: 10.1016/j.neuron.2016.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groma GI, Dancshazy Z. How many M forms are there in the bacteriorhodopsin photocycle? Biophys J. 1986;50:357–366. doi: 10.1016/S0006-3495(86)83469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutter T, de Carvalho LP, Dufresne V, Taly A, Edelstein SJ, Changeux JP. Molecular tuning of fast gating in pentameric ligandgated ion channels. Proc Natl Acad Sci USA. 2005;102:18207–18212. doi: 10.1073/pnas.0509024102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guettier JM, Gautam D, Scarselli M, Ruiz de Azua I, Li JH, Rosemond E, Ma X, Gonzalez FJ, Armbruster BN, Lu H, et al. A chemicalgenetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci USA. 2009;106:19197–19202. doi: 10.1073/pnas.0906593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZV, Li N, Huber D, Ophir E, Gutnisky D, Ting JT, Feng G, Svoboda K. Flow of cortical activity underlying a tactile decision in mice. Neuron. 2014;81:179–194. doi: 10.1016/j.neuron.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JZ, Graves AR, Guo WW, Zheng J, Lee A, Rodríguez-González J, Li N, Macklin JJ, Phillips JW, Mensh BD, et al. Cortex commands the performance of skilled movement. eLife. 2015;4:e10774. doi: 10.7554/eLife.10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gushchin I, Shevchenko V, Polovinkin V, Kovalev K, Alekseev A, Round E, Borshchevskiy V, Balandin T, Popov A, Gensch T, et al. Crystal structure of a light-driven sodium pump. Nat Struct Mol Biol. 2015;22:390–395. doi: 10.1038/nsmb.3002. [DOI] [PubMed] [Google Scholar]
- Haettig J, Sun Y, Wood MA, Xu X. Cell-type specific inactivation of hippocampal CA1 disrupts location-dependent object recognition in the mouse. Learn Mem. 2013;20:139–146. doi: 10.1101/lm.027847.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Han X. Optogenetics in the nonhuman primate. Prog Brain Res. 2012;196:215–233. doi: 10.1016/B978-0-444-59426-6.00011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R, Boyden ES. A high-light sensitivity optical neural silencer: Development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Rangel MJ, Jr, Motta SC, Zhang X, Perez IO, Canteras NS, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE. Integrated control of predatory hunting by the central nucleus of the amygdala. Cell. 2017;168:311–324. doi: 10.1016/j.cell.2016.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms KJ, Craig AM. Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. J Comp Neurol. 2005;490:72–84. doi: 10.1002/cne.20635. [DOI] [PubMed] [Google Scholar]
- Hasegawa E, Maejima T, Yoshida T, Masseck OA, Herlitze S, Yoshioka M, Sakurai T, Mieda M. Serotonin neurons in the dorsal raphe mediate the anticataplectic action of orexin neurons by reducing amygdala activity. Proc Natl Acad Sci USA. 2017;114:E3526–E3535. doi: 10.1073/pnas.1614552114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature. 2015;525:333–338. doi: 10.1038/nature15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heigele S, Sultan S, Toni N, Bischofberger J. Bidirectional GABAergic control of action potential firing in newborn hippocampal granule cells. Nat Neurosci. 2016;19:263–270. doi: 10.1038/nn.4218. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes (Sinauer) 2001 [Google Scholar]
- Hoque MR, Ishizuka T, Inoue K, Abe-Yoshizumi R, Igarashi H, Mishima T, Kandori H, Yawo H. A chimera Na+-pump rhodopsin as an effective optogenetic silencer. PLoS ONE. 2016;11:e0166820. doi: 10.1371/journal.pone.0166820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Shu Y. Axonal bleb recording. Neurosci Bull. 2012;28:342–350. doi: 10.1007/s12264-012-1247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–1026. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- Husson SJ, Liewald JF, Schultheis C, Stirman JN, Lu H, Gottschalk A. Microbial light-activatable proton pumps as neuronal inhibitors to functionally dissect neuronal networks in C. elegans. PLoS ONE. 2012;7:e40937. doi: 10.1371/journal.pone.0040937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Ono H, Abe-Yoshizumi R, Yoshizawa S, Ito H, Kogure K, Kandori H. A light-driven sodium ion pump in marine bacteria. Nat Commun. 2013;4:1678. doi: 10.1038/ncomms2689. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kato Y, Kandori H. Light-driven ion-translocating rhodopsins in marine bacteria. Trends Microbiol. 2015;23:91–98. doi: 10.1016/j.tim.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Isosaka T, Matsuo T, Yamaguchi T, Funabiki K, Nakanishi S, Kobayakawa R, Kobayakawa K. Htr2a-expressing cells in the central amygdala control the hierarchy between innate and learned fear. Cell. 2015;163:1153–1164. doi: 10.1016/j.cell.2015.10.047. [DOI] [PubMed] [Google Scholar]
- Iyer SM, Vesuna S, Ramakrishnan C, Huynh K, Young S, Berndt A, Lee SY, Gorini CJ, Deisseroth K, Delp SL. Optogenetic and chemogenetic strategies for sustained inhibition of pain. Sci Rep. 2016;6:30570. doi: 10.1038/srep30570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Beneduce BM, Drew IR, Regehr WG. Achieving high-frequency optical control of synaptic transmission. J Neurosci. 2014;34:7704–7714. doi: 10.1523/JNEUROSCI.4694-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann MW, Lam YW, Chang WH. Rapid formation of clozapine in guinea-pigs and man following clozapine-N-oxide administration. Arch Int Pharmacodyn Ther. 1994;328:243–250. [PubMed] [Google Scholar]
- Janovjak H, Szobota S, Wyart C, Trauner D, Isacoff EY. A light-gated, potassium-selective glutamate receptor for the optical inhibition of neuronal firing. Nat Neurosci. 2010;13:1027–1032. doi: 10.1038/nn.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri M, Afraz A. Navigating the neural space in search of the neural code. Neuron. 2017;93:1003–1014. doi: 10.1016/j.neuron.2017.02.019. [DOI] [PubMed] [Google Scholar]
- Jeong JW, McCall JG, Shin G, Zhang Y, Al-Hasani R, Kim M, Li S, Sim JY, Jang KI, Shi Y, et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell. 2015;162:662–674. doi: 10.1016/j.cell.2015.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DC, Marx R, Mains RE, O’Rourke B, Marbán E. Inducible genetic suppression of neuronal excitability. J Neurosci. 1999;19:1691–1697. doi: 10.1523/JNEUROSCI.19-05-01691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cationchloride cotransporters in neuronal development, plasticity, and disease. Nat Rev Neurosci. 2014;15:637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HE, Zhang F, Yizhar O, Ramakrishnan C, Nishizawa T, Hirata K, Ito J, Aita Y, Tsukazaki T, Hayashi S, et al. Crystal structure of the channelrhodopsin light-gated cation channel. Nature. 2012;482:369–374. doi: 10.1038/nature10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HE, Inoue K, Abe-Yoshizumi R, Kato Y, Ono H, Konno M, Hososhima S, Ishizuka T, Hoque MR, Kunitomo H, et al. Structural basis for Na(+) transport mechanism by a light-driven Na(+) pump. Nature. 2015;521:48–53. doi: 10.1038/nature14322. [DOI] [PubMed] [Google Scholar]
- Kätzel D, Nicholson E, Schorge S, Walker MC, Kullmann DM. Chemical-genetic attenuation of focal neocortical seizures. Nat Commun. 2014;5:3847. doi: 10.1038/ncomms4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienzler MA, Reiner A, Trautman E, Yoo S, Trauner D, Isacoff EY. A red-shifted, fast-relaxing azobenzene photoswitch for visible light control of an ionotropic glutamate receptor. J Am Chem Soc. 2013;135:17683–17686. doi: 10.1021/ja408104w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Connors BW. High temperatures alter physiological properties of pyramidal cells and inhibitory interneurons in hippocampus. Front Cell Neurosci. 2012;6:27. doi: 10.3389/fncel.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Hwa J, Garriga P, Reeves PJ, RajBhandary UL, Khorana HG. Light-driven activation of beta 2-adrenergic receptor signaling by a chimeric rhodopsin containing the beta 2-adrenergic receptor cytoplasmic loops. Biochemistry. 2005;44:2284–2292. doi: 10.1021/bi048328i. [DOI] [PubMed] [Google Scholar]
- Kim T, Folcher M, Doaud-El Baba M, Fussenegger M. A synthetic erectile optogenetic stimulator enabling blue-light-inducible penile erection. Angew Chem Int Ed Engl. 2015;54:5933–5938. doi: 10.1002/anie.201412204. [DOI] [PubMed] [Google Scholar]
- Kim H, Ährlund-Richter S, Wang X, Deisseroth K, Carlén M. Prefrontal parvalbumin neurons in control of attention. Cell. 2016;164:208–218. doi: 10.1016/j.cell.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, et al. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavir O, Prigge M, Sarel A, Paz R, Yizhar O. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat Neurosci. 2017;20:836–844. doi: 10.1038/nn.4523. [DOI] [PubMed] [Google Scholar]
- Kleinlogel S. Optogenetic user’s guide to Opto-GPCRs. Front Biosci (Landmark Ed.) 2016;21:794–805. doi: 10.2741/4421. [DOI] [PubMed] [Google Scholar]
- Kole MH, Stuart GJ. Signal processing in the axon initial segment. Neuron. 2012;73:235–247. doi: 10.1016/j.neuron.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Saunders A, Johnson CA, Lowell BB, Sabatini BL. Recurrent network activity drives striatal synaptogenesis. Nature. 2012;485:646–650. doi: 10.1038/nature11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer RH, Mourot A, Adesnik H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat Neurosci. 2013;16:816–823. doi: 10.1038/nn.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner HA, Lein ES, Callaway EM. A genetic method for selective and quickly reversible silencing of mammalian neurons. J Neurosci. 2002;22:5287–5290. doi: 10.1523/JNEUROSCI.22-13-05287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Yasuda R, Ehlers MD. Metaplasticity at single glutamatergic synapses. Neuron. 2010;66:859–870. doi: 10.1016/j.neuron.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Friend DM, Kaplan AR, Shin JH, Rubinstein M, Kravitz AV, Alvarez VA. Enhanced GABA transmission drives bradykinesia following loss of dopamine D2 receptor signaling. Neuron. 2016;90:824–838. doi: 10.1016/j.neuron.2016.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJ. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl- channel. Neuron. 2007;54:35–49. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Lerner TN, Ye L, Deisseroth K. Communication in neural circuits: Tools, opportunities, and challenges. Cell. 2016;164:1136–1150. doi: 10.1016/j.cell.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Jr, Mao T, Arnold DB. A role for myosin VI in the localization of axonal proteins. PLoS Biol. 2011;9:e1001021. doi: 10.1371/journal.pbio.1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Slimko EM, Lester HA. Selective elimination of glutamate activation and introduction of fluorescent proteins into a Caenorhabditis elegans chloride channel. FEBS Lett. 2002;528:77–82. doi: 10.1016/s0014-5793(02)03245-3. [DOI] [PubMed] [Google Scholar]
- Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci USA. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Sann SB, Zhou K, Nabavi S, Proulx CD, Malinow R, Jin Y, Tsien RY. Optogenetic inhibition of synaptic release with chromophore-assisted light inactivation (CALI) Neuron. 2013;79:241–253. doi: 10.1016/j.neuron.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WC, Tsai MC, Davenport CM, Smith CM, Veit J, Wilson NM, Adesnik H, Kramer RH. A Comprehensive optogenetic pharmacology toolkit for in vivo control of GABA(A) receptors and synaptic inhibition. Neuron. 2015;88:879–891. doi: 10.1016/j.neuron.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jacques SL, Azimipour M, Rogers JD, Pashaie R, Eliceiri KW. OptogenSIM: A 3D Monte Carlo simulation platform for light delivery design in optogenetics. Biomed Opt Express. 2015;6:4859–4870. doi: 10.1364/BOE.6.004859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barron M, Kaifosh P, Kheirbek MA, Danielson N, Zaremba JD, Reardon TR, Turi GF, Hen R, Zemelman BV, Losonczy A. Dendritic inhibition in the hippocampus supports fear learning. Science. 2014;343:857–863. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas EK, Jegarl AM, Morishita H, Clem RL. Multimodal and site-specific plasticity of amygdala parvalbumin interneurons after fear learning. Neuron. 2016;91:629–643. doi: 10.1016/j.neuron.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Hensch TK, Ackerman L, Barbel S, Jan LY, Jan YN. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, Clark SD. Clozapine N-oxide administration produces behavioral effects in long-evans rats: Implications for designing DREADD Experiments. eNeuro. 2016;3 doi: 10.1523/ENEURO.0219-16.2016. ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM. Chemical and genetic engineering of selective ion channel-ligand interactions. Science. 2011;333:1292–1296. doi: 10.1126/science.1206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014;17:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahn M, Prigge M, Ron S, Levy R, Yizhar O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat Neurosci. 2016;19:554–556. doi: 10.1038/nn.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyshev AY, Roshchin MV, Smirnova GR, Dolgikh DA, Balaban PM, Ostrovsky MA. Chloride conducting light activated channel GtACR2 can produce both cessation of firing and generation of action potentials in cortical neurons in response to light. Neurosci Lett. 2017;640:76–80. doi: 10.1016/j.neulet.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Whitaker LR, Harvey BK, Kaganovsky K, Adhikary S, Hope BT, Heins RC, Prisinzano TE, Vardy E, et al. Role of ventral subiculum in context-induced relapse to alcohol seeking after punishment-imposed abstinence. J Neurosci. 2016;36:3281–3294. doi: 10.1523/JNEUROSCI.4299-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masseck OA, Spoida K, Dalkara D, Maejima T, Rubelowski JM, Wallhorn L, Deneris ES, Herlitze S. Vertebrate cone opsins enable sustained and highly sensitive rapid control of Gi/o signaling in anxiety circuitry. Neuron. 2014;81:1263–1273. doi: 10.1016/j.neuron.2014.01.041. [DOI] [PubMed] [Google Scholar]
- Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O’Shea DJ, Prakash R, Gunaydin LA, Hyun M, Fenno LE, Gradinaru V, et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods. 2011;9:159–172. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao C, Cao Q, Ito HT, Yamahachi H, Witter MP, Moser MB, Moser EI. Hippocampal Remapping after Partial Inactivation of the Medial Entorhinal Cortex. Neuron. 2015;88:590–603. doi: 10.1016/j.neuron.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Mohammad F, Stewart JC, Ott S, Chlebikova K, Chua JY, Koh TW, Ho J, Claridge-Chang A. Optogenetic inhibition of behavior with anion channelrhodopsins. Nat Methods. 2017;14:271–274. doi: 10.1038/nmeth.4148. [DOI] [PubMed] [Google Scholar]
- Mourot A, Fehrentz T, Le Feuvre Y, Smith CM, Herold C, Dalkara D, Nagy F, Trauner D, Kramer RH. Rapid optical control of nociception with an ion-channel photoswitch. Nat Methods. 2012;9:396–402. doi: 10.1038/nmeth.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, Sauer JF, Riedel G, McClure C, Ansel L, Cheyne L, Bartos M, Wisden W, Wulff P. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D, Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971;233:149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Oh E, Maejima T, Liu C, Deneris E, Herlitze S. Substitution of 5-HT1A receptor signaling by a light-activated G protein-coupled receptor. J Biol Chem. 2010;285:30825–30836. doi: 10.1074/jbc.M110.147298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki A, Takagi S. An optogenetic application of proton pump ArchT to C. elegans cells. Neurosci Res. 2013;75:29–34. doi: 10.1016/j.neures.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Otchy TM, Wolff SB, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SM, Ölveczky BP. Acute off-target effects of neural circuit manipulations. Nature. 2015;528:358–363. doi: 10.1038/nature16442. [DOI] [PubMed] [Google Scholar]
- Park S, Kramer EE, Mercaldo V, Rashid AJ, Insel N, Frankland PW, Josselyn SA. Neuronal Allocation to a Hippocampal Engram. Neuropsychopharmacology. 2016;41:2987–2993. doi: 10.1038/npp.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Guo Y, Jia X, Choe HK, Grena B, Kang J, Park J, Lu C, Canales A, Chen R, et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat Neurosci. 2017;20:612–619. doi: 10.1038/nn.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips EA, Hasenstaub AR. Asymmetric effects of activating and inactivating cortical interneurons. eLife. 2016 doi: 10.7554/eLife.18383. Published online October 10, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce CR, Lomber SG, Born RT. Integrating motion and depth via parallel pathways. Nat Neurosci. 2008;11:216–223. doi: 10.1038/nn2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- Price GD, Trussell LO. Estimate of the chloride concentration in a central glutamatergic terminal: A gramicidin perforated-patch study on the calyx of Held. J Neurosci. 2006;26:11432–11436. doi: 10.1523/JNEUROSCI.1660-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh JR, Jahr CE. Axonal GABAA receptors increase cerebellar granule cell excitability and synaptic activity. J Neurosci. 2011;31:565–574. doi: 10.1523/JNEUROSCI.4506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo JV, Kay L, Ellender TJ, Akerman CJ. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat Neurosci. 2012;15:1102–1104. doi: 10.1038/nn.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasethupathy P, Sankaran S, Marshel JH, Kim CK, Ferenczi E, Lee SY, Berndt A, Ramakrishnan C, Jaffe A, Lo M, et al. Projections from neocortex mediate top-down control of memory retrieval. Nature. 2015;526:653–659. doi: 10.1038/nature15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasethupathy P, Ferenczi E, Deisseroth K. Targeting neural circuits. Cell. 2016;165:524–534. doi: 10.1016/j.cell.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Levitz J, Isacoff EY. Controlling ionotropic and metabotropic glutamate receptors with light: Principles and potential. Curr Opin Pharmacol. 2015;20:135–143. doi: 10.1016/j.coph.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NTM, Priestley JB, Rueckemann JW, Garcia AD, Smeglin VA, Marino FA, Eichenbaum H. Medial entorhinal cortex selectively supports temporal coding by hippocampal neurons. Neuron. 2017;94:677–688. doi: 10.1016/j.neuron.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL. DREADDs for Neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira X, Trapero A, Pittolo S, Zussy C, Faucherre A, Jopling C, Giraldo J, Pin JP, Gorostiza P, Goudet C, Llebaria A. OptoGluNAM4.1, a photoswitchable allosteric antagonist for real-time control of mGlu4 receptor activity. Cell Chem Biol. 2016;23:929–934. doi: 10.1016/j.chembiol.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Rungta RL, Osmanski BF, Boido D, Tanter M, Charpak S. Light controls cerebral blood flow in naive animals. Nat Commun. 2017;8:14191. doi: 10.1038/ncomms14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu MH, Moskvin OV, Siltberg-Liberles J, Gomelsky M. Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. J Biol Chem. 2010;285:41501–41508. doi: 10.1074/jbc.M110.177600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloman JL, Scheff NN, Snyder LM, Ross SE, Davis BM, Gold MS. Gi-DREADD expression in peripheral nerves produces ligand-dependent analgesia, as well as ligand-independent functional changes in sensory neurons. J Neurosci. 2016;36:10769–10781. doi: 10.1523/JNEUROSCI.3480-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Forsythe ID. The calyx of Held. Cell Tissue Res. 2006;326:311–337. doi: 10.1007/s00441-006-0272-7. [DOI] [PubMed] [Google Scholar]
- Schobert B, Lanyi JK. Halorhodopsin is a light-driven chloride pump. J Biol Chem. 1982;257:10306–10313. [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Völler T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Shu X, Lev-Ram V, Deerinck TJ, Qi Y, Ramko EB, Davidson MW, Jin Y, Ellisman MH, Tsien RY. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011;9:e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sineshchekov OA, Govorunova EG, Li H, Spudich JL. Gating mechanisms of a natural anion channelrhodopsin. Proc Natl Acad Sci USA. 2015;112:14236–14241. doi: 10.1073/pnas.1513602112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuda ER, Copits BA, Schmidt MJ, Baird MA, Al-Hasani R, Planer WJ, Funderburk SC, McCall JG, Gereau RW, 4th, Bruchas MR. Spatiotemporal control of opioid signaling and behavior. Neuron. 2015a;86:923–935. doi: 10.1016/j.neuron.2015.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuda ER, McCall JG, Al-Hasani R, Shin G, Park S, II, Schmidt MJ, Anderson SL, Planer WJ, Rogers JA, Bruchas MR. Optodynamic simulation of β-adrenergic receptor signalling. Nat Commun. 2015b;6:8480. doi: 10.1038/ncomms9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjulson L, Cassataro D, DasGupta S, Miesenböck G. Cell-specific targeting of genetically encoded tools for neuroscience. Annu Rev Genet. 2016;50:571–594. doi: 10.1146/annurev-genet-120215-035011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slimko EM, McKinney S, Anderson DJ, Davidson N, Lester HA. Selective electrical silencing of mammalian neurons in vitro by the use of invertebrate ligand-gated chloride channels. J Neurosci. 2002;22:7373–7379. doi: 10.1523/JNEUROSCI.22-17-07373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky I, Sadeghpour S, Li B, Liu G. Enhancement of synaptic plasticity through chronically reduced Ca2+ flux during uncorrelated activity. Neuron. 2004;44:835–849. doi: 10.1016/j.neuron.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Sokolova IV, Mody I. Silencing-induced metaplasticity in hippocampal cultured neurons. J Neurophysiol. 2008;100:690–697. doi: 10.1152/jn.90378.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522:309–314. doi: 10.1038/nature14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron. 2014;82:797–808. doi: 10.1016/j.neuron.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, LaLumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmler M, Koch C. How voltage-dependent conductances can adapt to maximize the information encoded by neuronal firing rate. Nat Neurosci. 1999;2:521–527. doi: 10.1038/9173. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- Stierl M, Stumpf P, Udwari D, Gueta R, Hagedorn R, Losi A, Gärtner W, Petereit L, Efetova M, Schwarzel M, et al. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J Biol Chem. 2011;286:1181–1188. doi: 10.1074/jbc.M110.185496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader CD, Gaffney T, Sugg EE, Candelore MR, Keys R, Patchett AA, Dixon RA. Allele-specific activation of genetically engineered receptors. J Biol Chem. 1991;266:5–8. [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stujenske JM, Spellman T, Gordon JA. Modeling the spatio-temporal dynamics of light and heat propagation for in vivo optogenetics. Cell Rep. 2015;12:525–534. doi: 10.1016/j.celrep.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo Y, Okazaki A, Ono H, Yagasaki J, Sugo S, Kamiya M, Reissig L, Inoue K, Ihara K, Kandori H, et al. A blue-shifted light-driven proton pump for neural silencing. J Biol Chem. 2013;288:20624–20632. doi: 10.1074/jbc.M113.475533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhan S, Ilona S, Chih-Chieh C, Isabelle D, Stéphane P, Antoine C, Claire M, Frédéric P. Experimental assessment of the safety and potential efficacy of high irradiance photostimulation of brain tissues. Sci Rep. 2017;7:43997. doi: 10.1038/srep43997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Sweger EJ, Casper KB, Scearce-Levie K, Conklin BR, McCarthy KD. Development of hydrocephalus in mice expressing the G(i)-coupled GPCR Ro1 RASSL receptor in astrocytes. J Neurosci. 2007;27:2309–2317. doi: 10.1523/JNEUROSCI.4565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnár G, Oláh S, Barzó P, Tamás G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Szalay G, Judák L, Katona G, Ócsai K, Juhász G, Veress M, Szadai Z, Fehér A, Tompa T, Chiovini B, et al. Fast 3D imaging of spine, dendritic, and neuronal assemblies in behaving animals. Neuron. 2016;92:723–738. doi: 10.1016/j.neuron.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Oertner TG, Hegemann P, Larkum ME. Active cortical dendrites modulate perception. Science. 2016;354:1587–1590. doi: 10.1126/science.aah6066. [DOI] [PubMed] [Google Scholar]
- Tan EM, Yamaguchi Y, Horwitz GD, Gosgnach S, Lein ES, Goulding M, Albright TD, Callaway EM. Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron. 2006;51:157–170. doi: 10.1016/j.neuron.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Perestenko PV, van de Ven GM, Bratley CT, McNamara CG, Campo-Urriza N, Black SL, Reijmers LG, Dupret D. Recoding a cocaine-place memory engram to a neutral engram in the hippocampus. Nat Neurosci. 2016;19:564–567. doi: 10.1038/nn.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuga H, Okuno E, Kameyama K, Haga T. Sequestration of human muscarinic acetylcholine receptor hm1-hm5 subtypes: Effect of G protein-coupled receptor kinases GRK2, GRK4, GRK5 and GRK6. J Pharmacol Exp Ther. 1998;284:1218–1226. [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban DJ, Zhu H, Marcinkiewcz CA, Michaelides M, Oshibuchi H, Rhea D, Aryal DK, Farrell MS, Lowery-Gionta E, Olsen RH, et al. Elucidation of the behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons. Neuropsychopharmacology. 2016;41:1404–1415. doi: 10.1038/npp.2015.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy E, Robinson JE, Li C, Olsen RH, DiBerto JF, Giguere PM, Sassano FM, Huang XP, Zhu H, Urban DJ, et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron. 2015;86:936–946. doi: 10.1016/j.neuron.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, Brown A, Rodriguez SS, Weller JR, Wright AC, et al. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci USA. 2003;100:4903–4908. doi: 10.1073/pnas.0230374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Qin J, Han Y, Cai J, Xing GG. Hyperthermia induces epileptiform discharges in cultured rat cortical neurons. Brain Res. 2011;1417:87–102. doi: 10.1016/j.brainres.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Waschuk SA, Bezerra AG, Jr, Shi L, Brown LS. Leptosphaeria rhodopsin: Bacteriorhodopsin-like proton pump from a eukaryote. Proc Natl Acad Sci USA. 2005;102:6879–6883. doi: 10.1073/pnas.0409659102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Hostick U, Kyweriga M, Tan A, Weible AP, Wu H, Wu W, Callaway EM, Kentros C. Transgenic silencing of neurons in the mammalian brain by expression of the allatostatin receptor (AlstR) J Neurophysiol. 2009;102:2554–2562. doi: 10.1152/jn.00480.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- Wiegert JS, Oertner TG. How (not) to silence long-range projections with light. Nat Neurosci. 2016;19:527–528. doi: 10.1038/nn.4270. [DOI] [PubMed] [Google Scholar]
- Wietek J, Wiegert JS, Adeishvili N, Schneider F, Watanabe H, Tsunoda SP, Vogt A, Elstner M, Oertner TG, Hegemann P. Conversion of channelrhodopsin into a light-gated chloride channel. Science. 2014;344:409–412. doi: 10.1126/science.1249375. [DOI] [PubMed] [Google Scholar]
- Wietek J, Beltramo R, Scanziani M, Hegemann P, Oertner TG, Wiegert JS. An improved chloride-conducting channelrhodopsin for light-induced inhibition of neuronal activity in vivo. Sci Rep. 2015;5:14807. doi: 10.1038/srep14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietek J, Broser M, Krause BS, Hegemann P. Identification of a natural green light absorbing chloride conducting channelrhodopsin from Proteomonas sulcata. J Biol Chem. 2016;291:4121–4127. doi: 10.1074/jbc.M115.699637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Atallah BV, Scanziani M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature. 2014;511:596–600. doi: 10.1038/nature13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Wada N, Kitabatake Y, Watanabe D, Anzai M, Yokoyama M, Teranishi Y, Nakanishi S. Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J Neurosci. 2003;23:6759–6767. doi: 10.1523/JNEUROSCI.23-17-06759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kapeller-Libermann D, Travaglia A, Inda MC, Alberini CM. Direct dorsal hippocampal-prelimbic cortex connections strengthen fear memories. Nat Neurosci. 2017;20:52–61. doi: 10.1038/nn.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Yona G, Meitav N, Kahn I, Shoham S. Realistic numerical and analytical modeling of light scattering in brain tissue for optogenetic applications(1,2,3) eNeuro. 2016;3 doi: 10.1523/ENEURO.0059-15.2015. NEURO.0059-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CR, Power J, Barnea G, O’Donnell S, Brown HE, Osborne J, Axel R, Gogos JA. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–566. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, Prigge M, Berndt A, Cushman J, Polle J, et al. The microbial opsin family of optogenetic tools. Cell. 2011;147:1446–1457. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZC, Yu C, Sellers KK, Fröhlich F. Dorso-lateral frontal cortex of the ferret encodes perceptual difficulty during visual discrimination. Sci Rep. 2016;6:23568. doi: 10.1038/srep23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Roth BL. Silencing synapses with DREADDs. Neuron. 2014;82:723–725. doi: 10.1016/j.neuron.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann D, Zhou A, Kiesel M, Feldbauer K, Terpitz U, Haase W, Schneider-Hohendorf T, Bamberg E, Sukhorukov VL. Effects on capacitance by overexpression of membrane proteins. Biochem Biophys Res Commun. 2008;369:1022–1026. doi: 10.1016/j.bbrc.2008.02.153. [DOI] [PubMed] [Google Scholar]