Abstract
Objectives
Dysphagia, or impaired swallowing, is common in nursing home (NH) residents with dementia and contributes to malnutrition and diminished quality of life. Dysphagia also commonly leads to aspiration or passage of food or fluids into the airway, which can result in aspiration pneumonia—a leading cause of death for people with dementia. Currently available interventions for dysphagia aim to modify the risk of aspiration events primarily by modifying diet and positioning to improve the safety of an individual’s swallow. However other potentially modifiable contextual factors relevant to mealtime care within NH settings that may influence the occurrence of aspiration events, such as the nature of caregiving interactions or occurrence of dementia-related behavioral symptoms, have not been examined. To address this gap, we examined the temporal associations between caregiving approach and behavioral symptoms as antecedents to observable indicators of aspiration among nursing home (NH) residents with dementia.
Design
Secondary analysis of coded, timed-event behavioral data from 33 video-recorded observations of mealtime interactions between NH residents with dementia and caregivers.
Setting/Participants
Residents with dementia who required assistance with mealtime care (n=12) and nursing assistants (n=8) from Memory Care Units (MCU) in 2 Midwestern NHs.
Results
Observable indicators of aspiration were significantly more likely to occur during or following task-centered caregiver actions than person-centered actions (12% likelihood; Yule’s Q 0.89; OR 95% CI 12.70–23.75) and 15–30 seconds after a behavioral symptom (5% likelihood; Yule’s Q 0.65; OR 95% CI 4.18–8.57).
Conclusions
These findings provide compelling preliminary evidence that caregiver approach may influence the occurrence of aspiration. Provided the urgent need for more approaches to mitigate the complications associated with dysphagia in people with dementia, even a moderate reduction in aspiration events may be clinically meaningful. Further, well-designed observational studies with individuals with well-characterized dysphagia are needed to better understand and characterize these relationships, their temporal structures and their impacts on other relevant outcomes such as eating performance and malnutrition.
Keywords: Dementia, Dysphagia, Person-Centered Care, Mealtime, Behavioral Symptoms
INTRODUCTION
Mealtime is a universal, inherently social concept that in many cultures provides a consistent mechanism for maintaining social contact and traditions (1). The experience and quality of mealtime care are greatly impacted by both dementia and the nursing home (NH) environment (1). Research suggests that the quality of meal services in NH settings is related to nutritional status and quality of life, and the majority of NH residents, including those with cognitive impairment, express complaints about meal services when asked (2). Mealtime care for NH residents is recognized as a complex process that is influenced by various individual, social, caregiving and system-level factors (3). These complexities are compounded by the high rates of cognitive impairment in NH settings (4).
People with dementia experience a range of cognitive and functional deficits that limit their ability to carry out challenging tasks related to eating during meals including chewing and swallowing safely and efficiently (5–10). Dysphagia, or swallowing dysfunction, is present in 53 to 70% of NH residents and 32 to 84% of patients with dementia (11–12), worsening with disease progression (7–8, 10). Dysphagia can lead to a variety of adverse outcomes, including increased risk for dehydration, malnutrition, and aspiration (13–14). Aspiration, or entry of food or fluids into the airway, is a symptom of dysphagia that results from underlying swallowing impairment in the oral and/or pharyngeal stages of swallowing and commonly occurs in patients with comorbid dementia and dysphagia (6). Reducing aspiration events is an important priority for improving patient safety as these events can lead to aspiration pneumonia (13), which is a leading cause of death for people with dementia (15–16).
The majority of treatments for addressing dysphagia target modifications in either food texture or posture for individual patients with a principal goal being to reduce risk of aspiration events. These standard approaches may negatively affect quality of life resulting in poor adherence to recommendations (17); can be difficult to maintain for those with attentional issues or memory loss; and, in certain cases, can increase pneumonia risk (18). There is increasing recognition that other caregiving and contextual factors such as the qualities of a caregiver’s approach during mealtime and concurrent dementia-specific symptomatology may be relevant to improving patient safety and eating outcomes among older patients at risk for aspiration (1, 19–22). There are several potential mechanisms through which more comprehensive individual, caregiving and environmental characteristics may influence the safety of individual swallows during mealtimes and occurrence of aspiration events (9). For example, the appropriateness of caregiver’s approach to eating assistance (e.g. whether food is being provided at a pace that is too fast and may exacerbate swallowing dysfunction) (5) or whether a negative interaction occurs that may distract the patient with dementia while swallowing and increase the risk of aspiration (23).
Delivery of care to NH residents with dementia in a manner that is person-centered, commonly defined as actions that are individualized and reflective of focusing on the person rather than the care task (24), is being widely adopted in NH settings in response to calls by patients, family and funders to improve care quality and outcomes (25–27). Research has also found that nutritional intake among patients with dementia is influenced by the qualities of their interactions with caregivers (28–30); however, these studies have not explicitly explored the person-centered qualities of these interactions or their association to aspiration.
The numerous eating challenges experienced by NH residents with dementia are further complicated by frequently occurring behavioral symptoms, such as agitation, that interrupt meals and may also interfere with proper swallowing mechanics. Behavioral symptoms refer to non-cognitive symptoms of dementia and include actions such as verbal or physical agitation (including repetitive motor agitation and frequent calling out), verbal and physical aggression and care resistance (31–32). Despite the social and individual complexities surrounding eating for NH residents with dementia including the high prevalence and persistence of behavioral symptoms that may interrupt safe swallowing, the relationships between caregiver approach, concurrent behavioral symptoms and aspiration events during mealtime care have not been previously examined.
In order to further explore the potential relationships between caregiver approach, concurrent behavioral symptoms and aspiration, methodologies that facilitate an exploration of the temporal structures between these events (as opposed to static, cross-sectional measures) are required. Although underutilized in research on mealtime cares, sequential observational data is particularly well suited to reliably addressing the combination of verbal and non-verbal behaviors that characterize these events and the relevance of their temporal patterns to establishing antecedent-consequence relationships (20). Therefore, the current study aimed to identify temporal associations between caregiver approach, behavioral symptoms and aspiration events among NH residents with dementia to address the following question: What is the likelihood of aspiration events occurring following a person-centered caregiver action, a task-centered caregiver action or a behavioral symptom event?
METHODS
Data Sources
This secondary analysis examined data from 33 video-recorded mealtime interactions between 12 NH residents with dementia and 8 direct caregivers from Memory Care Units in 2 Midwestern NHs. All residents enrolled in the primary study were required to have medical record-documented diagnoses of dementia and to require 1:1 assistance with mealtime cares. Participants were observed for an average of 2 mealtimes (range 1–5), which was distributed across breakfast, lunch and dinner. Attempts were made to observe each resident for 3 mealtimes (breakfast, lunch and dinner), however this was ultimately not feasible due to unavailability from illness or meal refusal. In some instances, meals were interrupted due to other care needs in which event each attempt at providing the meal was coded as a unique observation. Reflective of the nature of staffing assignments in nursing homes, eating assistance for each NH resident was not provided by a consistent caregiver. To reduce risks related to observation, participant sensitization to the video-observation was facilitated by having cameras in place for 15 minutes prior to each meal and prior to residents being seated at the table, as well as observing all residents in their routine eating situation. Participants had informed consent provided by a legal guardian after which the study team evaluated assent/dissent from individual residents. The primary descriptive, observational study was approved by the University of Wisconsin-Madison Institutional Review Board (19–20).
Measures
Video observations were coded using a previously tested and executed computer-assisted coding scheme (20) which identified timed-event codes for behavioral variables of caregiver approach (classified as person-centered actions and task-centered actions; measured by the Patient-Centered Behavior Inventory and Task-Centered Behavior Inventory respectively) (33–34), behavioral symptoms (as measured by the Pittsburgh Agitation Scale) (35) and observable indicators of aspiration defined as occurrences of coughing or choking during or immediately after the swallow (36). Coughing/choking while eating or drinking are consistently included in clinical bedside evaluation protocols as sensitive indicators for the presence of aspiration (36).
Task-centered actions include observable behaviors such as outpacing, interrupting, ignoring and verbally/physically controlling actions (33–34). Person-centered actions include behaviors such as adjusting to the resident’s pace, showing approval, orientation, direct eye-gaze, asking the resident for help/cooperation and providing choices (33–34). Behavioral symptoms measured using the Pittsburgh Agitation Scale include measures of verbal and motor agitation, aggressiveness including verbal and physical threats towards self, others or property, and care resistance such as pushing away to avoid tasks or gestures of refusal (35).
The coding was carried out by four trained observers and was consistently applied to the entire duration of all mealtimes observed. Observers achieved good to very good agreement (Cohen’s k = 0.80–0.85, percent agreement 86%–90%) (19–20). Additional details regarding the nature of the observations and execution of the coding scheme in the parent study are published elsewhere (19–20).
Statistical Analysis
Because individual residents and dyads contributed varying amounts of information and dyads were not independent of one another, data were analyzed at the unit of the observation using event-sequence analysis, which is designed to examine transitions between distinct events across observations. Specifically, lag-based sequential analysis was used to determine temporal associations between caregiving, behavioral symptoms and aspiration events. To explore a broader range of temporal associations, we examined relationships with aspiration serving as both the consequence event (i.e. whether caregiver and behavioral symptom events significantly predict aspiration events within specified time frames) and as an antecedent event (whether or not aspiration events may in turn precipitate changes in caregiver response and behavioral symptom events). Specifically, we computed conditional probabilities to estimate the likelihood of aspiration events occurring following caregiver person-centered/task-centered actions or behavioral symptoms over lag intervals ranging from 0 to 30 seconds between events. The significance of sequential associations was estimated using the 95% confidence intervals associated with odds ratios. Yule’s Q (ranges from −1 to +1 with 0 indicating no effect) was computed as an index of effect size as it is less vulnerable to zero cell counts and skewed distributions than odds ratios (37).
RESULTS
All study participants were Caucasian, half were female and the mean age of study participants was 84 (range 71–98) (19). Half of participants were on a modified diet meaning that they received either pureed foods or thickened liquids. Observable indicators of aspiration were present in 15 observations with a range of 1 to 10 coded aspiration events per observation and an average duration of 6.6 seconds per event. Behavioral symptoms occurred in 13 observations with a range of 1–26 symptoms. Caregiver actions were largely person-centered, with 96% of all caregiver events being classified as person-centered actions (19).
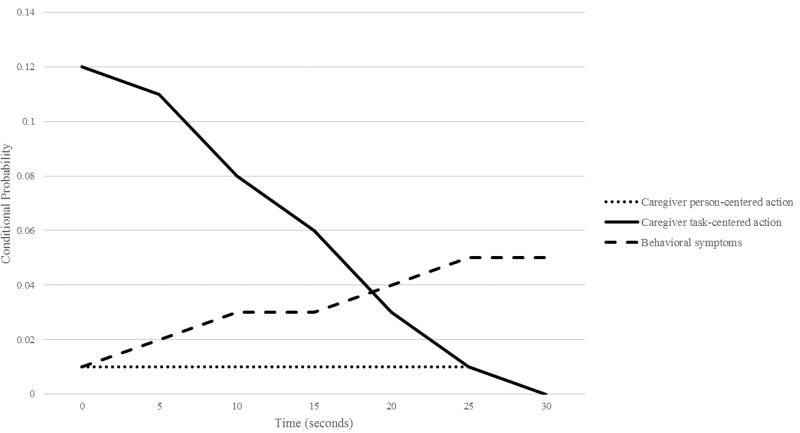
Sequential analysis for caregiver and behavioral symptoms as antecedents to aspiration events revealed a significant association between caregiver task-centered actions and aspiration events. The effect of this association was strongest at a lag interval of 0 indicating co-occurrence between task-centered caregiver actions and aspiration events (12% likelihood; Yule’s Q 0.89; OR 95% CI 12.70–23.75). The association remained significant through lag intervals up to 20 seconds but with diminishing strength and probability (Table 1; Figure 1). As compared to task-centered caregiver actions, aspiration events were less likely to occur following person-centered caregiver actions; however, this association was largely minimal/not present (0–1% likelihood) and was non-significant for most lag intervals. Aspiration events were also significantly more likely to occur following behavioral symptoms at the 10 to 30 second lag interval (3–5% likelihood; Yule’s Q 0.55–0.71; OR 95% CI 2.22–5.40; 4.18–8.57).
Table 1.
Lag-Sequential Analysis for Caregiver Person-Centered Actions, Task-Centered Actions, and Behavioral Symptoms as Antecedents of Aspiration Events
| Antecedent | Conditional probability | Yule’s Q | Odds ratio |
|---|---|---|---|
| Lag interval—0 s | |||
| Caregiver person-centered action | 0.01 | −0.18 | 0.7 [0.46, 1.07] |
| Caregiver task-centered action | 0.12 | 0.89 | 17.37 [12.70, 23.75] |
| Behavioral symptoms | 0.01 | −0.24 | 0.61 [0.23, 1.65] |
|
| |||
| Lag interval—5 s | |||
| Caregiver person-centered action | 0.01 | −0.13 | 0.78 [0.52, 1.17] |
| Caregiver task-centered action | 0.11 | 0.87 | 14.95 [10.76, 20.77] |
| Behavioral symptoms | 0.02 | 0.38 | 2.23 [1.30, 3.82] |
|
| |||
| Lag interval—10 s | |||
| Caregiver person-centered action | 0.01 | 0.03 | 1.06 [0.74, 1.52] |
| Caregiver task-centered action | 0.08 | 0.83 | 11.08 [7.69, 15.97] |
| Behavioral symptoms | 0.03 | 0.55 | 3.46 [2.22, 5.40] |
|
| |||
| Lag interval—15 s | |||
| Caregiver person-centered action | 0.01 | 0.05 | 1.1 [0.78, 1.57] |
| Caregiver task-centered action | 0.06 | 0.78 | 8.18 [5.43, 12.33] |
| Behavioral symptoms | 0.03 | 0.62 | 4.22 [2.80, 6.37] |
|
| |||
| Lag interval—20 s | |||
| Caregiver person-centered action | 0.01 | 0.05 | 1.11 [0.78, 1.58] |
| Caregiver task-centered action | 0.03 | 0.59 | 3.86 [2.20, 6.77] |
| Behavioral symptoms | 0.04 | 0.63 | 4.43 [2.96, 6.63] |
|
| |||
| Lag interval—25 s | |||
| Caregiver person-centered action | 0.01 | −0.16 | 0.73 [0.48, 1.12] |
| Caregiver task-centered action | 0.01 | 0.07 | 1.14 [0.42, 3.08] |
| Behavioral symptoms | 0.05 | 0.71 | 5.99 [4.18, 8.57] |
|
| |||
| Lag interval—30 s | |||
| Caregiver person-centered action | 0 | −0.39 | 0.44 [0.26, 0.75] |
| Caregiver task-centered action | 0 | −1 | 0 [88, 88] |
| Behavioral symptoms | 0.04 | 0.62 | 4.25 [2.82, 6.41] |
Figure 1.
Lag Sequential Analysis for Caregiver Person-Centered Actions, Task-Centered Actions and Behavioral Symptoms as Antecedents of Aspiration Events
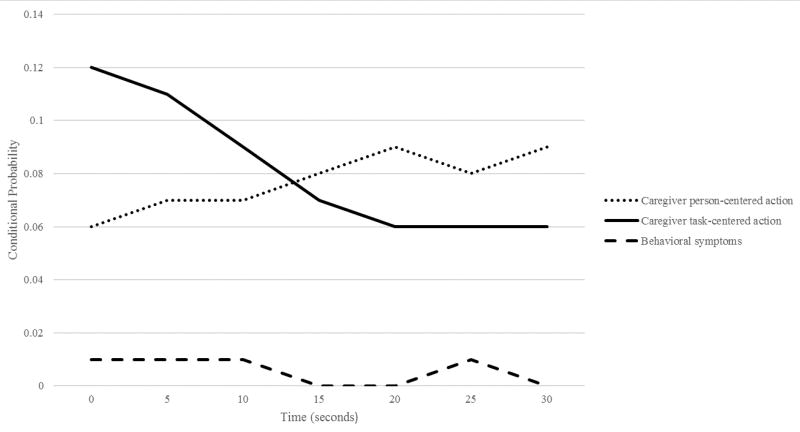
Sequential analysis examining aspiration as an antecedent variable demonstrated a statistically significant association between aspiration events and subsequent task-centered caregiver actions which was significant across all lag intervals but was stronger between the 10 and 5 second lag (9–11% likelihood; Yule’s Q 0.86–0.89; OR 95% CI 9.14–18.54; 11.88–22.70 respectively). Conditional probabilities between aspiration and person-centered actions were similar to task-centered actions, however these associations were non-significant. There were no significant associations in aspiration events as antecedents to behavioral symptoms (Table 2; Figure 2).
Table 2.
Lag-Sequential Analysis for Caregiver Person-Centered Actions, Task-Centered Actions, and Behavioral Symptoms as Consequences of Aspiration Events
| Consequences | Conditional probability | Yule’s Q | Odds ratio |
|---|---|---|---|
| Lag interval—0 s | |||
| Caregiver person-centered action | 0.06 | −0.18 | 0.7 [0.46–1.07] |
| Caregiver task-centered action | 0.12 | 0.89 | 17.37 [12.70–23.75] |
| Behavioral symptoms | 0.01 | −0.24 | 0.61 [0.23–1.65] |
|
| |||
| Lag interval—5 s | |||
| Caregiver person-centered action | 0.07 | −0.03 | 0.94 [0.65–1.38] |
| Caregiver task-centered action | 0.11 | 0.89 | 16.42 [11.88–22.70] |
| Behavioral symptoms | 0.01 | −0.22 | 0.64 [0.24–1.71] |
|
| |||
| Lag interval—10 s | |||
| Caregiver person-centered action | 0.07 | −0.07 | 0.86 [0.58–1.28] |
| Caregiver task-centered action | 0.09 | 0.86 | 13.02 [9.14–18.54] |
| Behavioral symptoms | 0.01 | −0.34 | 0.49 [0.16–1.52] |
|
| |||
| Lag interval—15 s | |||
| Caregiver person-centered action | 0.08 | 0.03 | 1.06 [0.73–1.53] |
| Caregiver task-centered action | 0.07 | 0.81 | 9.6 [6.45–14.30] |
| Behavioral symptoms | 0 | −0.72 | 0.16 [0.02–1.17] |
|
| |||
| Lag interval—20 s | |||
| Caregiver person-centered action | 0.09 | 0.12 | 1.28 [0.91–1.81] |
| Caregiver task-centered action | 0.06 | 0.78 | 7.95 [5.15–12.27] |
| Behavioral symptoms | 0 | −1 | 0 [88.88] |
|
| |||
| Lag interval—25 s | |||
| Caregiver person-centered action | 0.08 | 0.06 | 1.12 [0.78–1.63] |
| Caregiver task-centered action | 0.06 | 0.78 | 8.29 [5.37–12.80] |
| Behavioral symptoms | 0.01 | −0.31 | 0.53 [0.17–1.64] |
|
| |||
| Lag interval—30 s | |||
| Caregiver person-centered action | 0.09 | 0.07 | 1.16 [0.80–1.68] |
| Caregiver task-centered action | 0.06 | 0.79 | 8.55 [5.54–13.21] |
| Behavioral symptoms | 0 | −1 | 0 [88.88] |
Figure 2.
Lag Sequential Analysis for Caregiver Person-Centered Actions, Task-Centered Actions and Behavioral Symptoms as Consequences of Aspiration Events
DISCUSSION
We identified significant temporal associations between caregiver task-centered actions and observable indicators of aspiration events in NH residents with dementia during mealtimes. Provided the importance of effective and acceptable treatment approaches for persons with dementia and comorbid dysphagia, even a moderate reduction in aspiration events—such as the 12% likelihood found in the current analysis—may be clinically meaningful. Conversely, despite a much higher prevalence of person-centered caregiver actions overall (96% of all caregiver actions were person-centered), either no association or a negative association was found between these actions and observable signs of aspiration. Findings also suggest that caregivers may respond to aspiration events with task-centered approaches. These responses to aspiration events may represent attempts to take control of the situation or interrupt the individual’s eating upon witnessing choking or difficulty with swallowing. The reciprocal temporal association between aspiration events and task-centered actions may also be reflective of the dynamic nature of mealtime interactions or challenging points during the eating process where both the caregiver and resident are struggling to accomplish feeding tasks.
Findings provide some evidence that behavioral symptoms may precipitate aspiration events. However, this association was not as strong across all lag intervals and the clinical relevance of the 3 to 5% increased likelihood of aspiration events is unclear; these estimates also do not account for any interaction effects between caregiver approach and the development of behavioral symptoms. In conclusion, these preliminary results suggest that caregiver approach during mealtimes may precipitate or mitigate aspiration events during mealtime cares. A better understanding of these relationships may ultimately inform the development of more comprehensive dysphagia treatment approaches for people with dementia.
There are several plausible explanations for the observed associations. Mealtime is commonly rushed in NH settings and caregivers may outpace the functional abilities of individuals with dementia or exhibit physically or verbally controlling actions while trying to accomplish these complex feeding tasks (38), which may distract from or hinder swallowing actions. Behavioral symptoms are widely understood to be representative of meaningful communication that become more frequent as people with dementia experience progressive deficits in language and communication and rely heavily on behaviors to communicate their unmet needs (32, 39). We observed behavioral symptoms to be more common in the longer lag interval which may represent meaningful communication about dissatisfaction or unmet needs with something specific to the mealtime environment or eating process, such as the experience of pain during swallowing. Because these symptoms are a form of communication, they may distract from eating through increases in selective attention aimed at addressing the need or as a result of emotional distress if the needs remain unaddressed, both of which may interrupt the person with dementia from focusing on the already cognitively demanding task of navigating steps in the eating process including the swallow (23, 40). Strengths of the present study include the integration of timed-event variables in order to detect temporal patterns and event transitions, and the use of measures for behavioral symptoms and caregiver approach that incorporate both verbal and non-verbal behaviors.
This study does have important limitations. The analysis does not account for differences in individual resident or dyadic characteristics. Furthermore, provided the exploratory nature of this study, study sensitivity analyses for exploring individual differences across observations were not carried out due to limited statistical power. Participating NHs in the parent study were purposefully chosen for their focus on providing person-centered dementia care, which is a potential source of bias.
Additionally, the primary study was not designed specifically to study dysphagia and as a result formal dysphagia evaluations (clinical bedside examinations and/or instrumental evaluations, including videofluoroscopy or endoscopy) were not performed. Both clinical and instrumental evaluations of swallowing provide relevant contextual information about observed aspiration events but neither were available to inform interpretation of the findings of this study at a patient level. Although clinical bedside evaluation of swallowing is the most widely used assessment approach, particularly in NH settings, this approach does not consistently identify occurrences of silent aspiration (aspiration events with no patient response such as coughing or choking) or other aspects of dysphagia (41). In future studies, incorporation of instrumental examination would provide more rigorous confirmation of the presence or absence of aspiration, the overall severity of dysphagia, and specific biomechanical changes within the swallow, which would support improved interpretation of the clinical significance of these findings for patients with different types or degrees of swallowing impairment. Furthermore, this study did not characterize transitions between specific task-centered actions or behavioral symptoms and aspiration events due to sample size limitations.
There is a growing body of evidence aimed at addressing the myriad of challenges experienced by people with dementia during mealtime cares. This research addresses a range of interrelated care processes, interventions and outcomes including the quality of food service, social interactions, mealtime difficulties, aversive feeding behaviors, behavioral symptoms, eating performance and aspiration (2, 10, 42–44). It has been shown that individuals with dementia are more likely than those without dementia to receive self-feeding cues or direct assistance from caregivers (5). The appropriate use of cueing and direct assistance for patients with dementia during mealtime is critical as it can either aid in maintenance of independence or result in assistance beyond what is necessary thereby leading to more dependence (5, 9). When volunteers feeding older hospitalized patients with dysphagia were trained in how to provide optimal support, targeted feeding assistance was found to result in increased oral intake (21). Given that person-centered qualities of a caregiver’s approach are sequentially and temporally related to mealtime behavioral symptoms (5, 19), it is important to design research that systematically elucidates interactions among caregiver feeding approaches and swallowing-related outcomes, including the occurrence of aspiration. An improved understanding of which specific care behaviors may be more or less effective in encouraging swallowing safety and minimizing aspiration events will inform the development and refinement of training programs for caregivers feeding patients with dementia.
Few empiric studies explicitly address potential linkages between social, individual, behavioral and physiological challenges specific to mealtime cares for people with dementia. The present study provides a novel example of the types of new research questions that can be answered through the use of sequential observation data and integration of concepts from social, behavioral and physiologic domains. Greater integration across these conceptually interrelated areas may provide new insights into how we understand these different experiences and events, and may ultimately inform new directions for targeting clinical interventions.
In conclusion, we found that task-centered caregiver actions and behavioral symptoms are temporally associated with observable indicators of aspiration in NH residents with dementia. Future research is needed to explore these relationships in larger, more diverse samples with well-characterized dysphagia, and to identify which specific caregiving actions and behavioral symptoms are most likely to lead to the occurrence of aspiration events. Additional well-designed observational studies are needed to examine potential interaction effects and incorporate other clinical outcomes such as eating performance.
Acknowledgments
Conflict of Interest Disclosure: Dr. Gilmore-Bykovskyi reports receiving support from the National Hartford Centers of Gerontological Nursing Excellence and the American Nurses Foundation Virginia Stone Research Award in Clinical Gerontology. Dr. Rogus-Pulia reports receiving grant funding from the American Speech-Language-Hearing Foundation, the Wisconsin Alzheimer’s Disease Research Center, and the Veterans Health Administration’s Office of Geriatrics and Extended Care. Dr. Rogus-Pulia has been remunerated for presentations through the American Speech-Language-Hearing Association (ASHA) and the National Foundation of Swallowing Disorders (NFOSD).
The authors would also like to acknowledge Laura Block for assistance with manuscript preparation. The authors have obtained written consent from all contributors who are not authors and are named in this section. This manuscript was prepared at the William S. Middleton Veteran Affairs Hospital in Madison, Wisconsin; Geriatric Research, Educational and Clinical Center manuscript 2017– 009. The views and content expressed in this article are solely the responsibility of the authors and do not necessarily reflect the position, policy, or official views of the Department of Veteran Affairs or the U.S. government.
Footnotes
Ethical Standards: The authors confirm that the present study was conducted in a manner consistent with established ethical regulations within the United States and that the present study was reviewed and approved by a Human Subjects Institutional Review Board.
Author Contributions
Study conception: Gilmore-Bykovskyi
Study design: Gilmore-Bykovskyi
Data acquisition: Gilmore-Bykovskyi
Data analysis and interpretation: Gilmore-Bykovskyi, Rogus-Pulia
Drafting of manuscript: Gilmore-Bykovskyi, Rogus-Pulia
Critical revision and approval of final manuscript to be published: Gilmore-Bykovskyi, Rogus-Pulia
References
- 1.Chang CC, Roberts BL. Feeding difficulty in older adults with dementia. J Clin Nurs. 2008;17:2266–2274. doi: 10.1111/j.1365-2702.2007.02275.x. [DOI] [PubMed] [Google Scholar]
- 2.Simmons SF, Cleeton P, Porchak T. Resident Complaints About the Nursing Home Food Service: Relationship to Cognitive Status. J Gerontol B Psychol Sci Soc Sci. 2009;64B:324–327. doi: 10.1093/geronb/gbp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller H, Carrier N, Duizer L, et al. Making the most of mealtimes (M3): Grounding mealtime interventions with a conceptual model. J Am Med Dir Assoc. 2014;15:158–161. doi: 10.1016/j.jamda.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein AB, Remsburg RE. Estimated Prevalence of People With Cognitive Impairment: Results From Nationally Representative Community and Institutional Surveys. Gerontologist. 2007;47:250–254. doi: 10.1093/geront/47.3.350. [DOI] [PubMed] [Google Scholar]
- 5.Priefer BA, Robbins J. Eating changes in mild-stage Alzheimer’s disease: a pilot study. Dysphagia. 1997;12:212–221. doi: 10.1007/PL00009539. [DOI] [PubMed] [Google Scholar]
- 6.Rogus-Pulia N, Malandraki GA, Johnson S, Robbins J. Understanding Dysphagia in Dementia: The Present and the Future. Curr Phys Med Rehabil Rep. 2015;3:86–97. doi: 10.1007/s40141-015-0078-1. [DOI] [Google Scholar]
- 7.Horner J, Alberts MJ, Dawson DV, Cook GM. Swallowing in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 1994;8(3):177–189. doi: 10.1097/00002093-199408030-00004. [DOI] [PubMed] [Google Scholar]
- 8.Volicer L, Seltzer B, Rheaume Y, et al. Eating difficulties in patients with probable dementia of the Alzheimer type. J Geriatr Psychiatry Neurol. 1989;2(4):188–195. doi: 10.1177/089198878900200404. [DOI] [PubMed] [Google Scholar]
- 9.Edahiro A, Hirano H, Yamada R, Chiba Y, Watanabe Y, Tonogi M, et al. Factors affecting independence in eating among elderly with Alzheimer’s disease. Geriatr Gerontol Int. 2012;12(3):481–90. doi: 10.1111/j.1447-0594.2011.00799.x. [DOI] [PubMed] [Google Scholar]
- 10.Alagiakrishnan K, Bhanji RA, Kurian M. Evaluation and management of oropharyngeal dysphagia in different types of dementia: A systematic review. Arch Gerontol Geriatr. 2013;56:1–9. doi: 10.1016/j.archger.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Park YH, Han HR, Oh BM, et al. Prevalence and associated factors of dysphagia in nursing home residents. Geriatr Nurs. 2013;34:212–217. doi: 10.1016/j.gerinurse.2013.02.01. [DOI] [PubMed] [Google Scholar]
- 12.Sarabia-Cobo CM, Pérez V, de Lorena P, et al. The incidence and prognostic implications of dysphagia in elderly patients institutionalized: A multicenter study in Spain. Appl Nurs Res. 2016;30:e6–9. doi: 10.1016/j.apnr.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Cabré M, Serra-Prat M, Force L, et al. Oropharyngeal Dysphagia is a Risk Factor for Readmission for Pneumonia in the Very Elderly Persons: Observational Prospective Study. J Gerontol A Biol Scie Med Sci. 2013;69:330–7. doi: 10.1093/gerona/glt099. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi K, Aida J, Ito K, et al. Nutritional status and dysphagia risk among community-dwelling frail older adults. J Nutr Health Aging. 2014;18:352–357. doi: 10.1007/s12603-014-0025-3. [DOI] [PubMed] [Google Scholar]
- 15.Paranji S, Paranji N, Wright S, Chandra SA. Nationwide Study of the Impact of Dysphagia on Hospital Outcomes Among Patients With Dementia. Am J Alzheimers Dis Other Demen. 2017;32(1):5–11. doi: 10.1177/1533317516673464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunnström HR, Englund EM. Cause of death in patients with dementia disorders. Eur J Neurol. 2009;16(4):488–492. doi: 10.1111/j.1468-1331.2008.02503.x. [DOI] [PubMed] [Google Scholar]
- 17.Colodny N. Dysphagic independent feeders’ justifications for noncompliance with recommendations by a speech-language pathologist. Am J Speech Lang Pathol. 2005;14(1):61–70. doi: 10.1044/1058-0360(2005/008). [DOI] [PubMed] [Google Scholar]
- 18.Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509–518. doi: 10.7326/0003-4819-148-7-200804010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmore-Bykovskyi AL, Roberts TJ, Bowers BJ, et al. Caregiver Person-Centeredness and Behavioral Symptoms in Nursing Home Residents with Dementia: A Timed-Event Sequential Analysis. Gerontologist. 2015;55:S61–S66. doi: 10.1093/geront/gnu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilmore-Bykovskyi AL. Caregiver Person-Centeredness and Behavioral Symptoms during Mealtime Interactions: Development and Feasibility of a Coding Scheme. Geriatr Nurs. 2015;36:S10–S15. doi: 10.1016/j.gerinurse.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright L, Cotter D, Hickson M. The effectiveness of targeted feeding assistance to improve the nutritional intake of elderly dysphasic patients in hospital. J Hum Nutr Diet. 2008;21(6):555–562. doi: 10.1111/j.1365-277X.2008.00915.x. [DOI] [PubMed] [Google Scholar]
- 22.Niezgoda H, Keller H, Steele C, Chambers L. What should a case-finding tool for dysphagia in long-term care residents with dementia look like? J Am Med Dir Assoc. 2014;15(4):296–298. doi: 10.1016/j.jamda.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troche MS, Okun MS, Rosenbek JC, Altmann LJ, Sapienza CM. Attentional resource allocation and swallowing safety in Parkinson’s disease: a dual task study. Parkinsonism Relat Disord. 2014;20(4):439–443. doi: 10.1016/j.parkreldis.2013.12.011. doi: 10.1016/j.parkreldis.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edvardsson D, Winblad B, Sandman PO. Person-centered care of people with severe Alzheimer’s disease: current status and ways forward. Lancet Neurol. 2008;4:362–367. doi: 10.1016/S1474-4422(08)70063-2. [DOI] [PubMed] [Google Scholar]
- 25.Simmons SF, Rahman AN. Next steps for achieving person-centered care in nursing homes. J Am Med Dir Assoc. 2014;15:615. doi: 10.1016/j.jamda.2014.06.008. http://dx.doi.org/10.1016/j.jamda.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Miller SC, Looze J, Shield R, et al. Culture change practice in US nursing homes: Prevalence and variation by state Medicaid reimbursement policies. Gerontologist. 2014;54:434–445. doi: 10.1093/geront/gnt020. https://doi.org/10.1093/geront/gnt020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koren MJ. Person-centered care for nursing home residents: The culture-change movement. Health Aff. 2010;29:312–317. doi: 10.1377/hlthaff.2009.0966. https://doi.org/10.1377/hlthaff.2009.0966. [DOI] [PubMed] [Google Scholar]
- 28.Simmons SF, Osterweil D, Schnelle JF. Improving food intake in nursing home residents with feeding assistance: a staffing analysis. J Gerontol A Biol Sci Med Sci. 2001;56(12):790–794. doi: 10.1093/gerona/56.12.m790. doi: https://doi.org/10.1093/gerona/56.12.M790. [DOI] [PubMed] [Google Scholar]
- 29.Mamhidir AG, Karlsson I, Norberg A, Mona K. Weight increase in patients with dementia, and alteration in meal routines and meal environment after integrity promoting care. J Clin Nurs. 2007;16(5):987–996. doi: 10.1111/j.1365-2702.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- 30.Watson R, Green SM. Feeding and dementia: a systematic literature review. J Adv Nurs. 2006;54(1):86–93. doi: 10.1111/j.1365-2648.2006.03793.x. [DOI] [PubMed] [Google Scholar]
- 31.Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimer's Dement. 2011;7(5):532–9. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kales HC, Gitlin LN, Lyketosos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369. doi: https://doi.org/10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman CK, Medvene LJ, Van Haitsma K. A person-centered care intervention for geriatric certified nursing assistants. Gerontologist. 2013;53:687–698. doi: 10.1093/geront/gns135. [DOI] [PubMed] [Google Scholar]
- 34.Lann-Walcott H, Medvene LJ, Williams K. Measuring the person-centeredness of caregivers working with nursing home residents with dementia. Behav Ther. 2011;42:89–99. doi: 10.1016/j.beth.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen J, Burgio L, Kollar M, et al. The Pittsburgh Agitation Scale: A user-friendly instrument for rating agitation in dementia patients. Am J Geriatr Psychiatry. 1994;2:52–59. doi: 10.1097/00019442-199400210-00008. [DOI] [PubMed] [Google Scholar]
- 36.Carnaby-Mann G, Lenius K. Bedside examination of dysphagia. Phys Med Rehabil Clin N Am. 2008;19:747–68. doi: 10.1016/j.pmr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 37.McComas JJ, Moore T, Dahl N, et al. Calculating contingencies in natural environments: Issues in the application of sequential analysis. J Appl Behav Anal. 2009;42:413–423. doi: 10.1901/jaba.2009.42-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reimer HD, Keller HH. Mealtimes in Nursing Homes: Striving for Person-Centered Care. J Nutr Elder. 2009;28:327–347. doi: 10.1080/01639360903417066. [DOI] [PubMed] [Google Scholar]
- 39.Algase D, Beck C, Kolanowski A, et al. Need-driven dementia-compromised behavior: An alternative view of disruptive behavior. Am J Alzheimers Dis Other Demen. 1996;11(6):10–9. [Google Scholar]
- 40.Leder SB, Suiter DM, Lisitano Warner H. Answering orientation questions and following single-step verbal commands: effect on aspiration status. Dysphagia. 2009;24(3):290–295. doi: 10.1007/s00455-008-9204-x. [DOI] [PubMed] [Google Scholar]
- 41.O’Horo J, Rogus-Pulia N, Garcia-Arguello L, Robbins J, Safdar N. Bedside Diagnosis of Dysphagia: A Systematic Review. J Hosp Med. 2015;10(4):256–65. doi: 10.1002/jhm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aselage MB. Measuring mealtime difficulties: eating, feeding and meal behaviours in older adults with dementia. J Clin Nursing. 2010;19:621–631. doi: 10.1111/j.1365-2702.2009.03129.x. [DOI] [PubMed] [Google Scholar]
- 43.Liu W, Cheon J, Thomas SA. Interventions on mealtime difficulties in older adults with dementia: a systematic review. Int J Nurs Stud. 2014;51:14–27. doi: 10.1016/j.ijnurstu.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 44.Whear R, Abbott R, Thompson-Coon J, Bethel A, Roger M, Hemsley A, Stahl-Timmins W, Stein K. Effectiveness of mealtime interventions on behavior symptoms of people with dementia living in care homes: a systematic review. J Am Med Dir Assoc. 2014;15:185–93. doi: 10.1016/j.jamda.2013.10.016. [DOI] [PubMed] [Google Scholar]