Abstract
Self-renewal and differentiation are defining characteristics of hematopoietic stem and progenitor cells, and their balanced regulation is central to lifelong function of both blood and immune systems. In addition to cell-intrinsic programs, hematopoietic stem and progenitor cell fate decisions are subject to extrinsic cues from within the bone marrow microenvironment and systemically. Yet, many of the paracrine and endocrine mediators that shape hematopoietic function remain to be discovered. Extracellular vesicles serve as evolutionarily conserved, constitutive regulators of cell and tissue homeostasis, with several recent reports supporting a role for extracellular vesicles in the regulation of hematopoiesis. We review the physiological and pathophysiological effects that extracellular vesicles have on bone marrow compartmental function while highlighting progress in understanding vesicle biogenesis, cargo incorporation, differential uptake, and downstream effects of vesicle internalization. This review also touches on the role of extracellular vesicles in hematopoietic stem and progenitor cell fate regulation and recent advances in therapeutic and diagnostic applications of extracellular vesicles in hematologic disorders.
Introduction
To fulfill its critical systemic functions in oxygen delivery, coagulation and immune defense, hematopoiesis is regulated via integration of cell-intrinsic programs with extrinsic cues from the surrounding bone marrow (BM) microenvironment.1,2 Recent studies from infectious diseases, cardiovascular, and cancer fields demonstrate the existence of systemic crosstalk with BM cells which adds to the complexity of compartmental signaling, especially during injury responses.1,3 Cytokines, chemokines and other growth factors act as important mediators in a reasonably well-understood system by which the extrinsic ligands act on cells expressing the cognate receptor (Figure 1A). These in turn transmit signals to a network of cellular signaling pathways regulating hematopoiesis, including Wnt, Notch, transforming growth factor beta (TGF-β), phosphatidylinositol-3 kinase, and the mammalian target of rapamycin.4–7 Signaling by extrinsic mediators through any one of these pathways triggers activation of quiescent long-lived hematopoietic stem cells (HSCs). More recent studies of the leukemic microenvironment have revealed that tumor-derived paracrine factors also act on mesenchymal stromal cells, osteoprogenitors and endothelial cells within the BM, indirectly suppressing hematopoietic stem and progenitor cells (HSPCs).1,3,8 Thus, dynamic compartmental interactions shape physiological and pathophysiological regulation of BM function.
Figure 1.
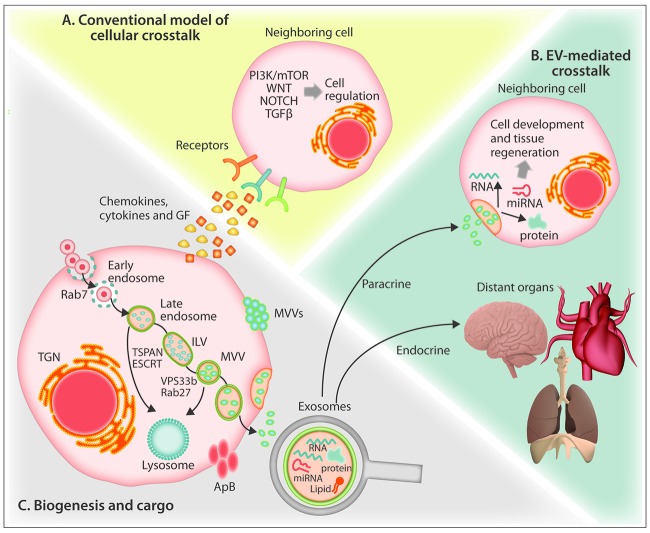
Schematic representation of biogenesis of extracellular vesicles and unique aspects of their trafficking. (A) The conventional model of cellular crosstalk involves receptor-ligand interactions between secreted chemokines, cytokines and growth factors and cellular surface receptors. (B) EV-mediated crosstalk occurs through the trafficking of vesicle-associated protein, lipid and RNA components to proximal cells or to distal organs via the bloodstream in a “paracrine” or “endocrine” manner, respectively. (C) Exosomes are formed from the maturation of early endosomes into Rab7-containing late endosomes leading to the generation of intraluminal vesicles via the action of tetraspanin and ESCRT proteins which sort the endosomal constituents into distinct multivesicular bodies. Through the action of Rab27 and VPS33b, multivesicular bodies evade lysosome degradation and fuse with the plasma membrane to release 30–125 nm exosomes. Cells also release 50–1000 nm microvesicles that form through calcium-mediated budding of the plasma membrane, and during programed cell death, large (>1000 nm) apoptotic bodies. ApB: apoptotic bodies ESCRT: endosomal-sorting complex required for transport; GF: growth factors; ILV: intraluminal vesicle; MV: microvesicle; MVB: multivesiclular bodies; mTOR: mammalian target of rapamycin; PI3K; phosphatidylinositol-3 kinase; TGF-β: transforming growth factor beta; TGN: trans-Golgi network; TSPAN: tetraspanin; VPS33B: vacuolar protein sorting-associated protein 33B.
Extracellular vesicle (EV) biogenesis is a constitutive cellular process, broadly conserved across evolution, with a role in development, homeostatic organismal function and tissue regeneration.9–11 EVs of various shapes and sizes have been demonstrated in every biofluid tested to date, with substantial variation in their structure, content and function.12 Protein, lipid and RNA components contribute to cell-cell crosstalk at a short distance, in a paracrine or endocrine manner via the blood-stream (Figure 1B).10,12 However, given their complex cargo and poorly understood selectivity for cellular uptake, many phenotypic outcomes are not easily explained by conventional models of cell-cell crosstalk. The consequences of simultaneously transferring an unknown number of non-randomly assembled proteins and RNA to another cell defy the clear predictions that apply to more conventional receptor-ligand signaling. However, while an understanding of the molecular basis for EV crosstalk is in its infancy, the key principles of how EVs shape tissue function are beginning to emerge.12 Several groups have recently demonstrated that EVs contribute to the compartmental regulation of hematopoiesis in the BM.13,14 In this review, we present current evidence for the role of EVs in both homeostatic and pathogenic hematopoietic niches with emphasis on regulatory mechanisms, experimental outcomes and the critical open questions in the field.
Extracellular vesicles
EVs are membrane-enclosed structures of varying size (30–10,000 nm) released from cells to mediate both local and distant intercellular communication. Platelet-derived vesicles were first identified by electron microscopy over 50 years ago,15 yet the full spectrum of subtypes and activities of EVs have only become a major focus of interest in recent years. In the early 1980s, it was reported that sheep reticulocytes selectively release transferrin receptor within EVs during programmed enucleation of the maturing red cell and were generally considered to simply reflect the export of cellular waste.16 Recent studies of EVs in the BM have shown that these vesicles serve to regulate hematopoiesis, participate in immune cell activation, and act as mediators of hemostatic functions.11,17,18 Hematologic malignancies such as leukemia, multiple myeloma or viral infections can coopt EV trafficking mechanisms, upend these homeostatic processes and use EVs to reinforce tumor growth, chemotherapeutic resistance, invasion, metastasis and relapse.19–21
EVs can be broadly classified into four subtypes (Table 1) based upon vesicle size and method of cellular release: exosomes (30–150 nm), microvesicles (50–1000 nm), large vesicles (>1000 nm) and apoptotic bodies (>1000 nm).22 It is technically challenging to separate vesicle types, and no standardized method exists to date. Techniques utilized for EV purification often rely on size or density.12 However, there is overlap between exosomes and microvesicles in composition and function, and neither size-exclusion chromatography nor ultracentrifugation in density gradients for separation will yield pure populations.22 Moreover, due to overlap between these vesicles in size and miRNA carrier function with plasma abundant chylomicrons and very low density lipoproteins, EV dimension should be considered an arbitrary surrogate metric, and a more biologically informed classification would likely enhance reproducibility in the field, advance their detection and inform treatment strategies.
Table 1.
Types of extracellular vesicles.
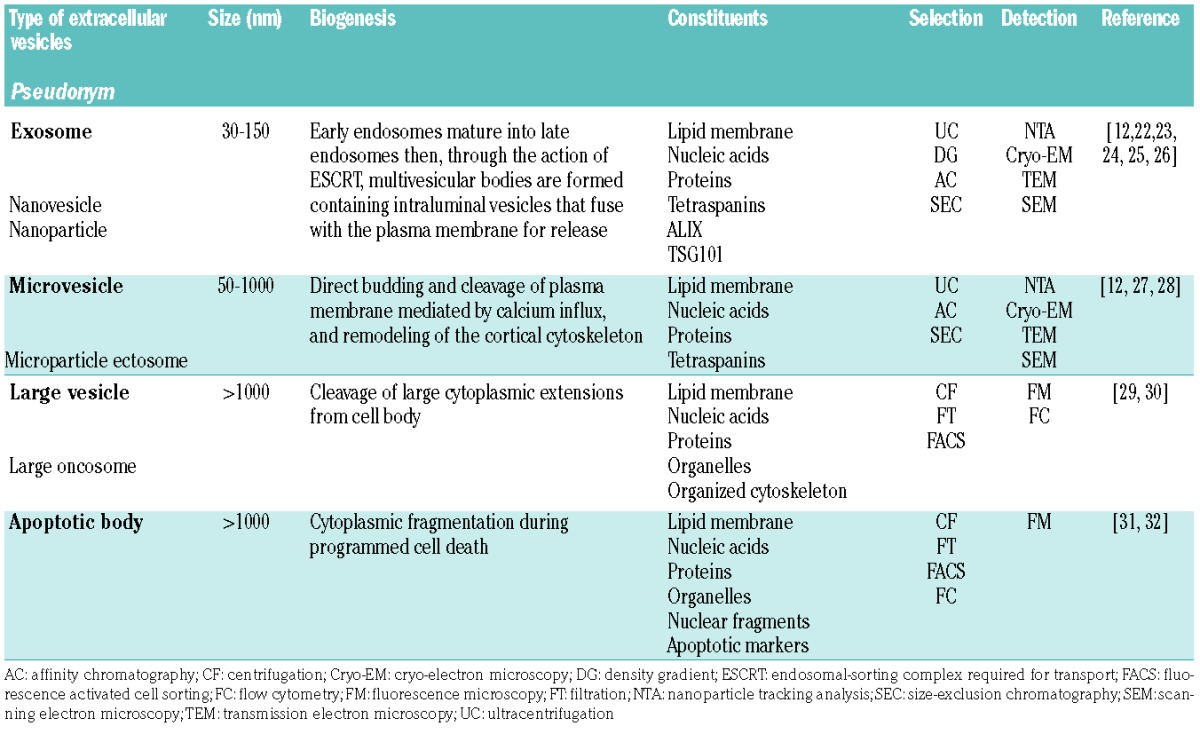
Exosomes
The biogenesis of exosomes, the smallest type of EV, begins with the inward cleavage of the plasma membrane to form an endosome containing selectively enclosed cytoplasmic components within the lumen. As illustrated in Figure 1C, early endosomes, characterized by the presence of Rab5 protein, undergo maturation into Rab7 containing late endosomes which generate multiple intraluminal vesicles through the action of tetraspanins and endosomal sorting complex required for transport (ESCRT) proteins.25 Together these proteins function to facilitate further inward cleavage and sorting of endosomal constituents into discrete intraluminal vesicles. These multivesicular bodies, through RAB27- and VPS33b-dependent mechanisms, evade lysosome degradation and fuse with the plasma membrane to release intraluminal vesicles as exosomes.23,24 Through this highly regulated endosomal process of formation, the size of exosomes is relatively constant as compared to the larger types of vesicle. In addition to tetraspanins, proteins ALG-2 interacting-protein X and tumor susceptibility gene 101 (ALIX and TSG101, respectively) are reported to be involved in the endosomal process, and are frequently used as markers for exosomes.12,22 Different cell types can release discrete subpopulations of exosomes, each with different proteomic properties and RNA cargo and divergent membrane protein composition.25,26
Microvesicles
Intermediate-sized EVs are most frequently referred to as microvesicles, ectosomes, or if tumor-derived, oncosomes, which arise via direct budding and cleavage of the plasma. Microvesicles are spherical and span a broad range of sizes, being between 50 nm to 1000 nm in diameter. They are distinguished based on their formation and release, and do not utilize the endosomal/multivesicular body pathway.27 Instead, microvesicles are formed through a process that involves calcium influx and remodeling of the cortical cytoskeleton to release the membrane-enclosed cytosolic cargo.12 Viewed broadly, microvesicles do not appear to be formed in a consistent manner like exosomes. However, when restricted to a specific cell type, microvesicles may form in a uniform manner, as illustrated in one recent study of neutrophils that consistently shed two distinct narrowly defined vesicle populations of ~100 nm and ~500 nm, both budding at the limiting membrane.28
Large vesicles
Large vesicles, also referred to as large oncosomes due to their tumor-derived origin, are a class of EVs that can reach up to 10 microns in size and contain intact organelles and an ordered cytoskeletal structure.29 Large vesicles are similar to apoptotic bodies in size and composition; however, unlike apoptotic bodies, large vesicles are formed from cleavage of cytoplasmic extensions from intact living cells. Large vesicles have been described in B-cell acute lymphoblastic leukemia and prostate cancer, and have been demonstrated within patients’ samples and in vitro cultures of cancer cell lines.29,30
Apoptotic bodies
Apoptotic bodies emerge during the course of programmed cell-death, as nuclear karyorrhexis occurs with cytoplasm and surrounding plasma membrane beginning to bleb into fragments.31 Apoptotic bodies consist of an intact plasma membrane enclosing cytosolic components and can contain both organelles and nuclear fragments. These bodies are subsequently eliminated through phagocytosis by surrounding cells and degraded in phagolysosomes.31 It has been reported that apoptotic bodies can horizontally transfer DNA to phagocytic recipient cells. As an example of this, one study showed that Epstein-Barr virus-infected B-lymphocytes generate apoptotic bodies that carry viral DNA and aid in the transfer of the virus to uninfected cells.32
Vesicle fate
Once released from the parent cell, EVs can follow multiple routes. Some cancer cells generate EVs that rupture soon after release from their parent cells, distributing enzymes such as vascular endothelial growth factor and matrix metalloproteases into the surrounding interstitial space in order to promote angiogenesis and support cancer invasion through metastatic dissemination.33,34 EVs released into the blood appear to have a short half-life in circulation. In one representative study of B16-BL6 melanoma-derived EVs packaged with luciferase and lactadherin, luciferase activity was lost within minutes of intravenous injection with an observed serum half-life of approximately 2 minutes followed by rapid redistribution into tissues.35
A broad range of mechanisms for cellular uptake have been identified for EVs, including membrane fusion, phagocytosis or receptor-mediated caveolin-, clathrin- or lipid raft-mediated endocytosis, all culminating with transport of the EV cargo directly into the intracellular compartment.36 The differences from study to study suggest that EV uptake is a variable process and likely dependent on the type of EV and the parent and recipient cells involved. Experiments have shown that uptake is prevented at lower temperatures, suggesting that internalization is energy dependent and does not occur as a passive process.37 The uptake of EVs can be partially blocked by treating vesicles with either heparan sulfate or proteinase K, indicating a role for proteoglycans and surface proteins, respectively, in gaining entry into the cell.37,38 Pre-treatment of cells with the actin-depolymerizing drug cytochalasin D prior to EV exposure prevents cytoskeletal remodeling and reduces EV internalization,39 The use of the dynamin 2 inhibitor dynasore, which abrogates caveolin/clathrin-mediated endocytosis, has also been shown to inhibit uptake of reticulocyte-derived exosomes by macrophages.40 These data taken together are suggestive of an endocytic process mediating vesicle internalization. Little is known about specific mechanisms of uptake within the hematopoietic niche, although one study reported that megakaryocyte-derived EVs gain entry into hematopoietic progenitors cells via lipid raft mediated endocytosis, macropinocytosis and membrane fusion.61 Further study is warranted in order to understand the cellular events by which HSPC and supportive cells of the bone marrow differentially regulate the process of EV entry.
How EVs are specifically targeted to different cell types within the hematopoietic niche in order to regulate hematopoiesis remains largely unknown. Among the most abundant membrane-associated proteins found on EVs are tetraspanins, a large cell-surface protein superfamily that interacts with transmembrane proteins and cytosolic signaling molecules to facilitate the organization of these structures into microdomains.41 Tetraspanins have been linked to many functions: intracellular signaling through G-protein coupled receptors and protein kinase C; migration and metastasis by interacting with integrins and vascular cell adhesion molecule; cell morphogenesis by direct binding of alpha-actinin and the induction of actin polymerization.42,43 EV-embedded tetraspanins are dependent on the type of their parent cell; however, CD9, CD63, CD81, CD82, and CD151 are enriched in EVs derived from a range of sources.22 CD9, a common tetraspanin used to identify EVs was previously described in association with c-kit/CD117, a tyrosine kinase receptor that is highly expressed on HSPCs.44 Tetraspanins such as CD37, CD53 and TSSC6 have been found exclusively on hematopoietic cells. It is known that these tetraspanins interact with hematopoietic-specific targets such as Src homology region 2 domain-containing phosphatase-1, the pattern recognition receptor dectin-1, MHC-I/II, integrin α4β1, T-cell/NK-cell co-stimulatory CD2, as well as common signal transducers including phosphatidylinositol-3 kinase and protein kinase C.45 Hematopoietic-specific tetraspanins and integrins on the EV surface remain strong candidates in targeting vesicles to specific cell types within the hematopoietic niche.
A recent study demonstrated that, once inside the target cell, EVs are sorted into the endosomal pathway, move quickly through the cytoplasm and then stall at the endoplasmic reticulum, before eventually fusing with lysosomes for degradation.46 The process of cargo release by internalized EVs remains to be clarified. As the principal compartment for translation within the cell, the endoplasmic reticulum is a likely site for the deposition of mRNA and miRNA cargo. This and the assembly of the RNA interference-silencing complex in the endoplasmic reticulum may potentially explain how EVs alter protein synthesis and change cellular behavior. The half-life of internalized EVs has not been well defined. In the same study, 293T-derived EVs remained intact for hours to days once inside primary fibroblasts, with 50–60% merging with lysosomes by 48 hours.46
Physiological regulation of hematopoiesis by extracellular vesicles
The BM comprises hematopoietic and non-hematopoietic cells organized into specialized microenvironments that provide dynamic regulation of hematopoiesis to assure the adequate formation and function of mature blood cells from HSCs.1 Mesenchymal stem cells (MSCs), their osteoprogenitor cell progeny, as well as endothelial cells and adipocytes coordinately maintain hematopoiesis by regulating proliferation, quiescence, differentiation, and apoptosis of HSPCs through juxtacrine and paracrine activity.2 Changes in compartmental oxygen concentration, hemorrhage, chemotherapy and irradiation can all prompt the emergence of HSCs from quiescence,47,48 and several lines of evidence suggest that EVs are involved in regulating BM function during homeostasis and in response to injury (Table 2A, Figure 2).
Table 2A.
Physiological regulation of hematopoiesis by extracellular vesicles.
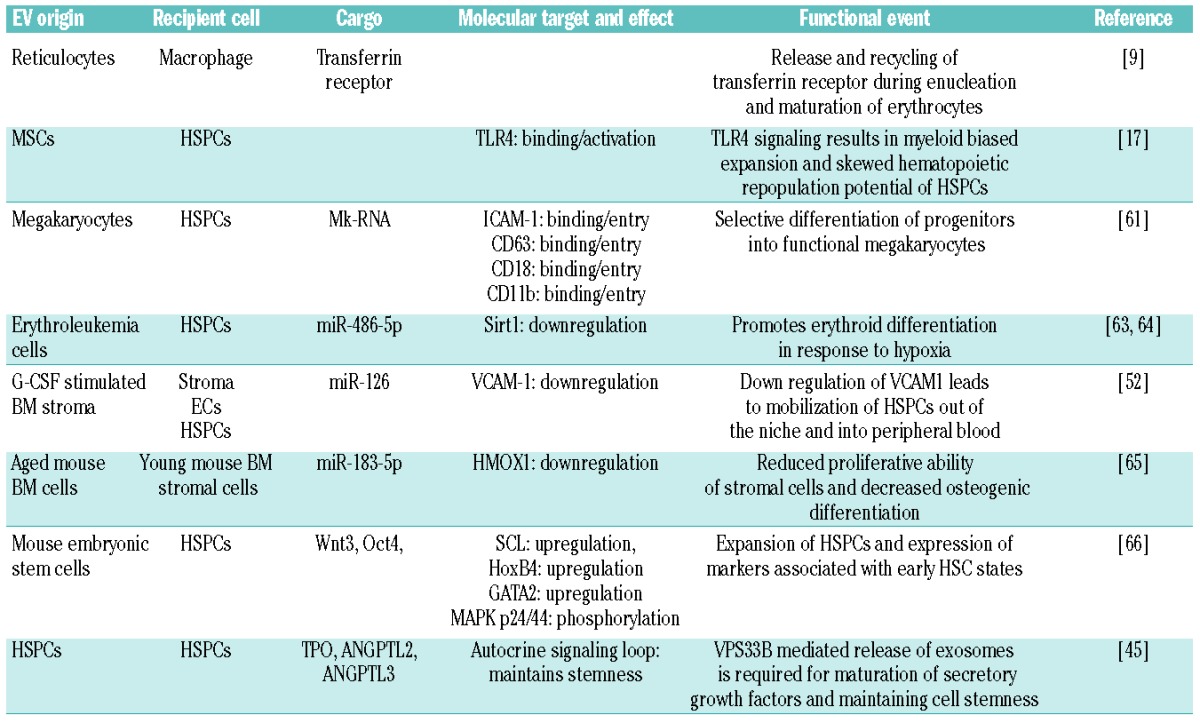
Figure 2.
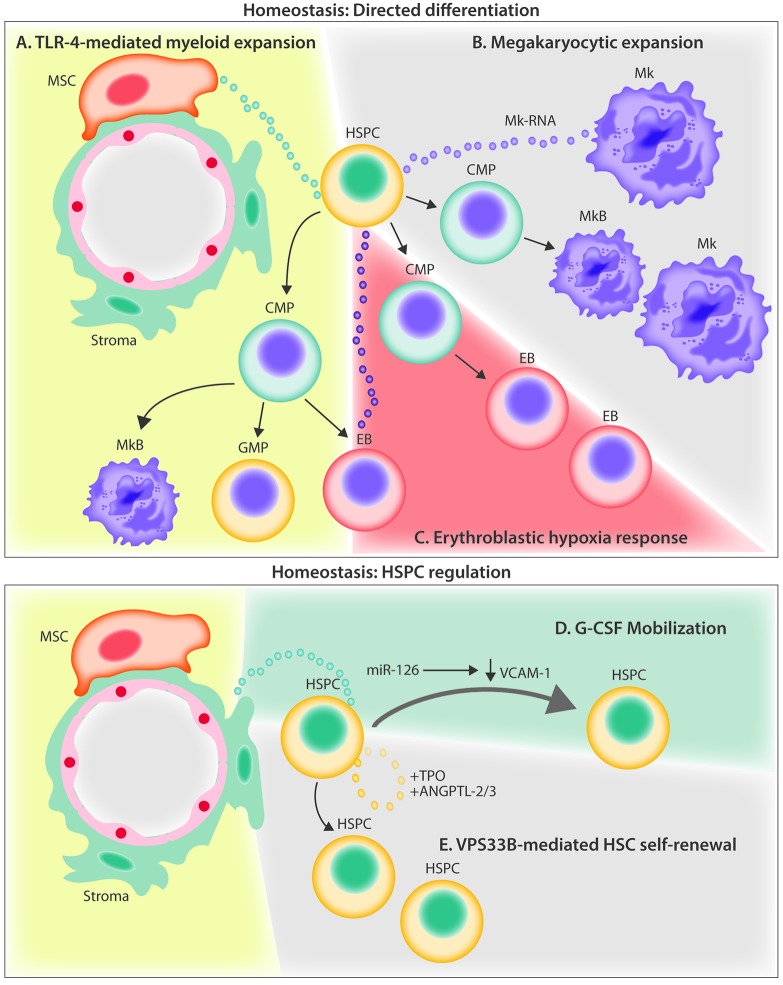
Current evidence for extracellular vesicle crosstalk in the homeostatic bone marrow microenvironment. (A) MSC-derived EVs signal to HSPCs through the TLR-4 pathway, resulting in myeloid biased expansion. (B) Megakaryocyte-derived MVs are internalized by HSPCs and increase differentiation of new megakaryocytes through RNA-mediated signaling. (C) Hypoxia induces erythroleukemia cells to release EVs containing miR-486 which increases erythroblastic differentiation by targeting Sirt1 in HSPCs. (D) G-CSF infusion stimulates the release of EVs containing miR-126 that act to down-regulate VCAM-1 in HSPCs, resulting in their mobilization out of the BM. (E) HSPCs autoregulate stem potential by packaging and releasing critical secretory proteins through the exosomal pathway via the action of VPS33B. ANGPTL-2/3; angiopoietin-like protein 2 and 3; BM: bone marrow; CMP: common myeloid progenitor; EB: erythroblast; EVs: extracellular vesicles; G-CSF: granulocyte colony-stimulating factor; GMP; granulocyte monocyte progenitor; HSPC: hematopoietic stem and progenitor cell; Mk: megakaryocytes; MkB: megakaryoblast; MSC: mesenchymal stem cell; miR: microRNA; MV: microvesicles; TLR-4: Toll-like receptor 4; TPO: thrombopoietin; VCAM-1: vascular cell adhesion molecule; VPS33B: vacuolar protein sorting-associated protein 33B.
Some of the earliest descriptions of EVs revealed their role as platelet-derived anti-hemophilic particles and in transferrin receptor release from sheep reticulocytes.9 Additionally, more recent evidence points to EVs as important physiological mediators of signaling across the immunological synapse.11,49 Yet, much less is known about how vesicles might contribute to steady-state hematopoietic function or during a regenerative BM response. EV release is very clearly subject to a range of cellular stimuli, including cytokine activation, ionizing radiation, and differences in tissue oxygen tension.50,51 Granulocyte colony-stimulating factor mobilization is one such stimulus that appears to increase vesicle release from hematopoietic progenitors.52 Following injury, EV release may promote the selective delivery of miRNAs and other cargo, and may explain the enhanced angiogenic and regenerative activity after hypoperfusion injury in distant tissues.53
Several groups have studied the release and function of EVs by BM stroma, such as endothelial cells and MSCs. Endothelial cells have been shown to generate EVs with pro-angiogenic effects through the actions of miR-126,54 and have been linked to age-related downregulation of osteogenic differentiation within the BM.55,56 More literature exists on the release and function of EVs from BM-derived MSCs. Our group recently demonstrated the trafficking of EVs from BM-derived MSCs to hematopoietic cells influencing progenitor commitment.17 Other groups showed that MSC-derived EVs selectively promoted tumor growth in patients with multiple myeloma.21 Additionally MSC-derived EVs have been shown to regulate angiogenic activity in endothelial cells, supporting the notion that BM MSC-derived EVs can regulate specific cell populations both within and outside of the hematopoietic compartment.57
In a recent study we showed that murine HSPCs (KSL: c-kit+/sca-1+/lineage-depleted) exposed to BM MSC-derived EVs in vitro prompted activation with myeloid progenitor biased expansion and a skewed hematopoietic repopulation potential.17 Remarkably, this process seemed to be dependent on Toll-like receptor signaling and could be specifically abrogated in HSPCs from TLR4 knockout or MyD88 knockout animals (Figure 2A). EVs of all classes are also rich in lipid components, especially products of arachidonic acid metabolism, including prostaglandin E2.58 Considering the potent activity of prostaglandin E2 in regulating HSC expansion and engraftment,59 it is tempting to speculate that EV-bound prostaglandin E2 released by MSCs contributes to this activity.60
The EV-mediated influence on hematopoiesis is not limited to supportive stromal cells alone. Megakaryocytes have also been shown to impart regulatory control on HSPCs by releasing microvesicles to orchestrate specific cell-type commitment. Megakaryoctye-derived microvesicles are among the most abundant microvesicles in the circulation, and attach to HSPCs by interacting with ICAM-1, CD43, CD18 and CD11b epitopes. Upon cell surface contact, these microvesicles become internalized where megakaryocyte RNA appears to serve as the mediator of biological effects, as evidenced by a loss of function of megakaryocyte microvesicles following RNAase treatment. Functionally, the internalization of these megakaryo cyte microvesicles was found to redirect the differentiation of HSPCs toward functional megakaryocytes with limited effects on the phenotype of endothelial or stromal cells (Figure 2B).61
Several studies have demonstrated the importance of EV miRNA in regulating erythropoietic differentiation of HSPCs in both murine and human models.62 One recent report described that erythroleukemia cells respond to hypoxia by rapidly releasing exosomes containing miR-486, a known regulator of erythroid differentiation, which targets Sirt1 in CD34+ HSPCs (Figure 2C).63 This confirmed and extended previous studies that had implicated the increased expression of miR-486-5p in supporting erythroid differentiation of CD34+ cells in vitro.64 Conversely, the inhibition of miR-486-5p has been found to suppress CD34+ cell growth in vitro and in vivo, and decrease erythroid differentiation and survival of erythroid cells. It is possible that a similar physiological mechanism might exist to regulate hypoxia-responsive erythropoiesis in order to increase the delivery of oxygen to starved tissues.
EVs within the BM microenvironment have been shown to modulate the behavior of HSCs in other ways. For example, treatment with pharmacological concentrations of granulocyte colony-stimulating factor used to mobilize stem and progenitor cells for collection and subsequent transplantation causes an increase in EVs containing high levels of miR-126 within the BM. These EVs are internalized by stroma, HSPCs and endothelial cells, delivering miR-126 into the cell, where it acts to translationally suppress vascular cell adhesion molecule-1 (Figure 2D). This decrease in vascular cell adhesion molecule-1, along with other signaling events, results in reduced HSPC adhesion and a shift into the peripheral blood, for collection by leukapheresis.52 Experimentally, EV-contained miR-126 released from mobilized human CD34 cells conferred pro-angiogenic activity and promoted hindlimb ischemia repair.53 Another recent study found that aging and oxidative stress alter the miRNA content of EVs in the BM microenvironment leading to age-related stem cell dysfunction. The investigators showed that BM-derived EVs from aged mice contain abundant miR-183-5p which, when endocytosed by primary BM stromal cells from young mice, decreased proliferation and inhibited osteogenic differentiation by reducing heme oxygenase 1, an enzyme essential in heme catabolism.65 Microvesicles derived from mouse embryonic stem cells were found to contain high levels of transcripts associated with pluripotency (Wnt-3 and Oct-4), and when exposed to hematopoietic progenitors led to their expansion.66 Additionally, hematopoietic progenitors exposed to the microvesicles derived from mouse embryonic stem cells were found to upregulate the expression of early HSC markers (SCL, HoxB4 and GATA2) and showed phosphorylation of MAPK p24/44 and serine-threonine kinase AKT.66
Finally, HSCs may contribute to their own stemness in part through secretory signaling and autocrine loops, involving vacuolar protein sorting protein 33b (VPS33B)-mediated release of exosomes as carriers of thrombopoietin and angiopoietin-like protein 2 and 3 (Figure 2E). Herein, the loss of VPS33B compromised HSC potential and reduced leukemogenicity in cancer models.23 This and other studies discussed in this section support the view that within the physiological BM microenvironment, HSPCs release and internalize EVs, and are broadly responsive to regulation by vesicle trafficking in order to maintain hematopoiesis.
Pathophysiological regulation of hematopoiesis by extracellular vesicles
Aside from the role of EVs in the cellular crosstalk in the BM under physiological conditions, EV trafficking also plays a distinct role in deregulating hematopoiesis in injury and disease states, such as hematologic malignancies and extramedullary cancers (Table 2B).13,14,48 For example, MSC-derived EVs appear to contribute to marrow repair after radiation damage, restoring HSPC proliferation and engraftment with partial restoration of peripheral blood counts after intravenous injection of MSC-derived EVs.48
Table 2B.
Pathophysiological regulation of hematopoiesis by extracellular vesicles.
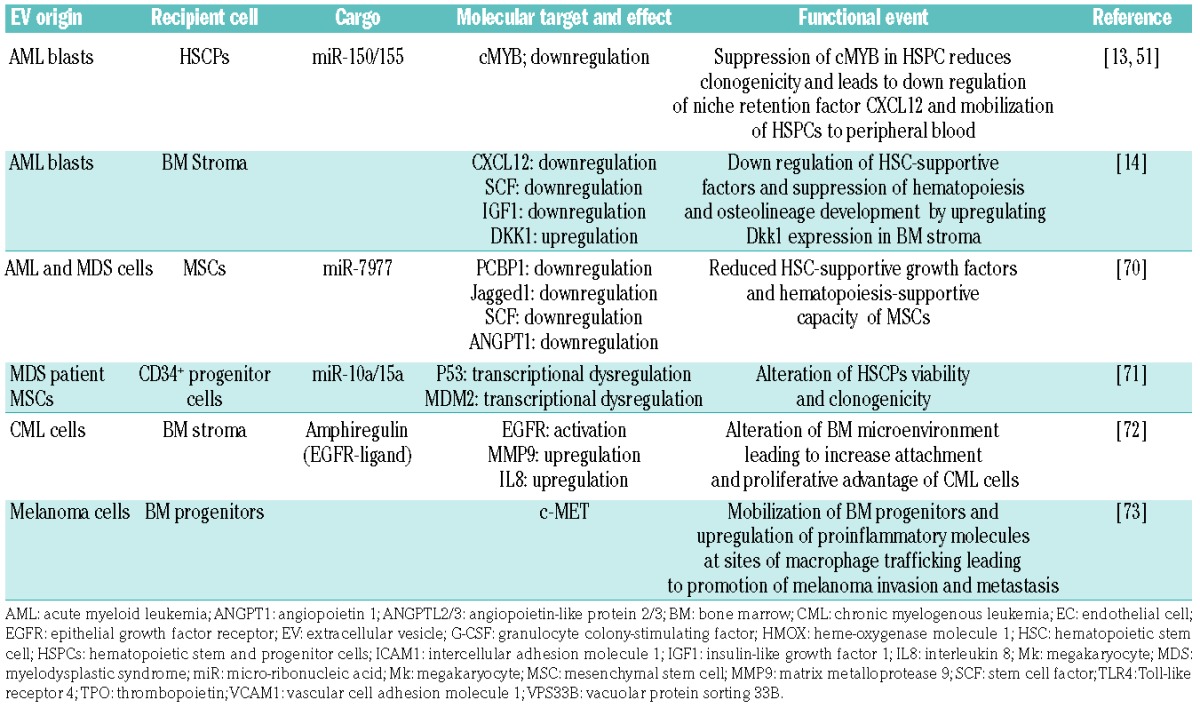
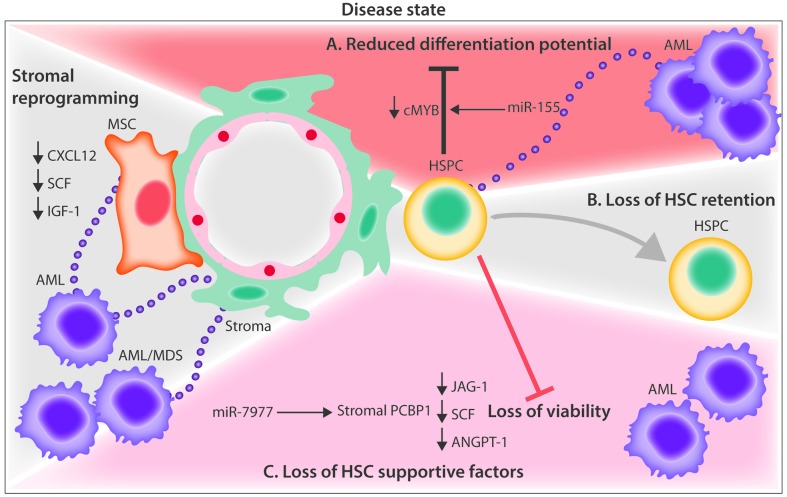
We reported that acute myeloid leukemia (AML) blasts rely on EVs for the transfer of miR-150 and miR-155, which target cMyb, a highly expressed transcription factor in progenitor cells to suppress HSPC clonogenicity. The coincident downregulation of the niche retention factor CXCL12 in those studies led to HSPC mobilization into the peripheral blood (Figure 3A).13,51 These observations were extended more recently by others showing that AML EVs not only downregulate HSC-supporting factors (CXCL12, stem cell factor, and insulin-like growth factor 1) (Figure 3B), but simultaneously suppress hematopoiesis and osteolineage development by upregulating Dkk1 expression in BM stromal cells.14 On the other hand, one study showed that AML EVs increased the number of HSCs by enhancing their survival while retaining their clonogenicity and stemness with no change in the hematopoietic CD34+, CD34+CD38−, CD90+, and CD117+ phenotypes.67 Illustrating one of the key challenges in understanding HSPC regulation by EVs, neither of the two latter studies identified the specific EV component responsible. We and others previously showed that EVs released by steady-state or reprogrammed malignant stroma carry cytokines.17,68,69 Because most analyses of secreted cytokines do not separate vesicle-bound and vesicle-free cytokine activity it is entirely possible that some of the known cytokine activities that regulate HSPC in the leukemic niche reflect EV-mediated trafficking.
Figure 3.
Current evidence for extracellular vesicle crosstalk in the leukemic microenvironment (A) EVs from AML blasts traffic miR-155 to HSPCs and down-regulate critical transcription factor, c-MYB, resulting in reduced differentiation potential. (B) AML EVs reprogram MSCs and stromal cells, and downregulate niche retention factor CXCL12 resulting in mobilization of HSPCs from the BM. (C) AML and MDS EVs promote the loss of HSPC supportive factors, CXCL12, SCF, IGF-1 through the trafficking of miR-7797 to supportive stroma, leading to reduced HSPC viability and hematopoietic potential. AML: acute myelogenous leukemia; ANGPT-1: angiopoietin 1; BM: bone marrow; CXCL12: C-X-C motif chemokine 12; EVs: extracellular vesicles; HSPCs: hematopoietic stem and progenitor cells; IGF-1: insulin-like growth factor 1; MDS: myelodysplastic syndrome; miR: microRNA; MSC: mesenchymal stem cell; PCBP1: poly(rc) binding protein 1; SCF: stem cell factor.
Other hematologic disorders affect hematopoiesis indirectly by altering the function of the supportive non-hematopoietic stroma. Both AML and myelodysplastic syndrome cells were shown to reduce the hematopoiesis-supportive capacity of MSCs by delivering miR-7977 via EVs. After uptake by MSCs, the EV-trafficked miR-7797 suppresses hematopoietic growth factors (jagged-1, stem cell factor and angiopoietin-1) by targeting the poly (rC) binding protein 1 post-transcriptional regulator (Figure 3C).70 MSCs from patients with myelodysplastic syndrome were also shown to release EVs that traffic miR-10a and miR-15a to CD34+ progenitor cells, causing the transcriptional regulation of MDM2 and P53 genes, altering HSPC viability and clonogenicity.71 EVs released from chronic myelogenous leukemia cells have also been implicated in altering the BM microenvironment by activating epithelial growth factor receptor signaling in stromal cells. Chronic myelogenous leukemia exosomes were shown to contain amphiregulin, an epithelial growth factor receptor-activating ligand that leads to the downstream expression of matrix metalloproteinase-9 and interleukin-8, giving leukemic cells an adhesive and proliferative advantage within the hematopoietic niche.72
Extramedullary cancers, such as melanoma, also use EVs for the endocrine regulation of BM progenitors. For example, one study showed that melanoma EVs mobilize BM progenitors by targeting the receptor tyrosine kinase, c-MET, in turn upregulating pro-inflammatory molecules at sites of macrophage trafficking to promote their invasion and metastasis in distant organs.73
Diagnostic and therapeutic application of extracellular vesicles in hematologic disorders
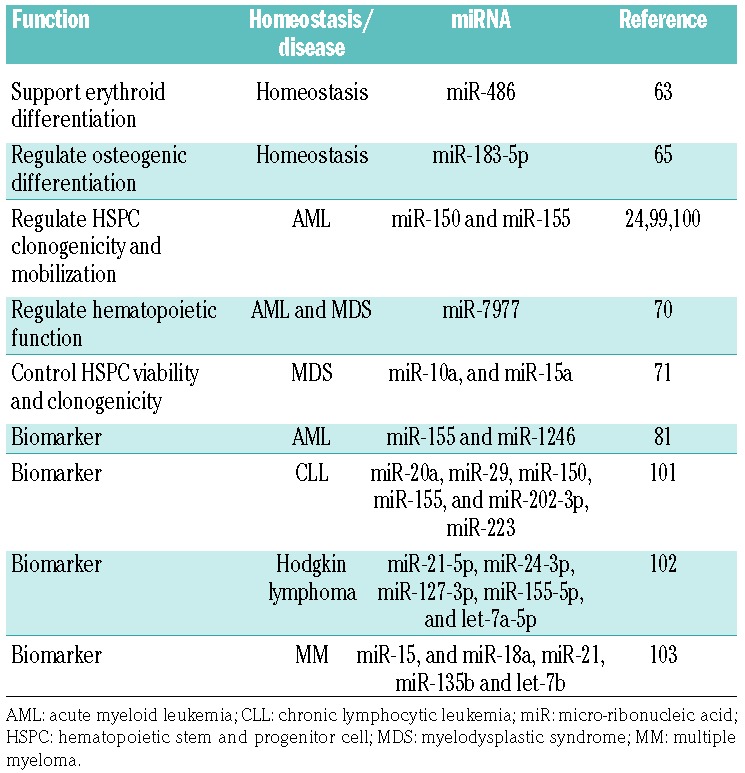
Vesicles are continuously shed by a wide range of blood cells.74 In addition to their short-range biological effects, the small size and rapid equilibration of EVs between tissues and the bloodstream have fueled interest in developing minimally invasive biomarkers based on EVs and their cargo. Identification of disease-specific markers based on circulating EVs may aid in early detection and post-remission monitoring for several types of hematologic disorders (Figure 4C). A number of studies indicate the feasibility of developing circulating EVs as a minimally invasive platform for the analysis of miRNA and protein content profiles to detect and classify hematologic malignancies and non-malignant hematopoietic disorders.75,76 For example, antibody microarray profiling of the membrane protein content of plasma EVs from patients with chronic lymphoid leukemia showed elevated levels of CD5, CD19, CD31, CD44, CD55, CD62L, CD82, HLA-A, HLA-B, HLA-C, and HLA-DR and low levels of CD21, CD49c, and CD63.77 The utility of EVs as biomarkers in chronic lymphoid leukemia is also supported by the observation of high levels of CD19 and CD37 relative to the levels in healthy controls.78 In patients with newly diagnosed AML, plasma EVs were rich in myeloblastic markers (CD34, CD33 and CD117), but also TGF-β1 protein, and MHC class I chain-related genes (MICA and MICB).69 The dynamic range of plasma EV-TGF-β1 readily separated AML patients by diagnosis, early and late remission status. In multiple myeloma, EVs bearing CD38, CD138, CD44 and CD147 allowed stratification of patients by disease phase and therapy response.79 There is a range of platforms for RNA amplification and the high incidence of relapse in AML patients has driven efforts to mine EV RNA content for highly sensitive detection of minimal residual disease and emergent drug resistance. EV miRNA appears to be a particularly promising minimally invasive biomarker platform. When EV cargo loading leads to the selective enrichment of some and exclusion of other cellular miRNA, the resulting highly selective vesicle miRNA profiles offer a potentially significant advantage over analysis of more diverse and abundant “free” circulating miRNA, which is often complexed with small lipoprotein particles. This improved signal to noise ratio led a number of groups to survey EV miRNA as a highly dynamic biomarker tool in hematologic malignancies (Table 3), and several studies have shown that AML EVs contain characteristic miRNA profiles.80,81 A particularly intriguing aspect of circulating EV miRNA is that it represents contributions of (occasionally identical) miRNA contained in EVs from multiple cellular sources. Such a compartmental biomarker reflects EV miRNA contributions from leukemic clones and the surrounding BM stromal cells.68,81
Figure 4.
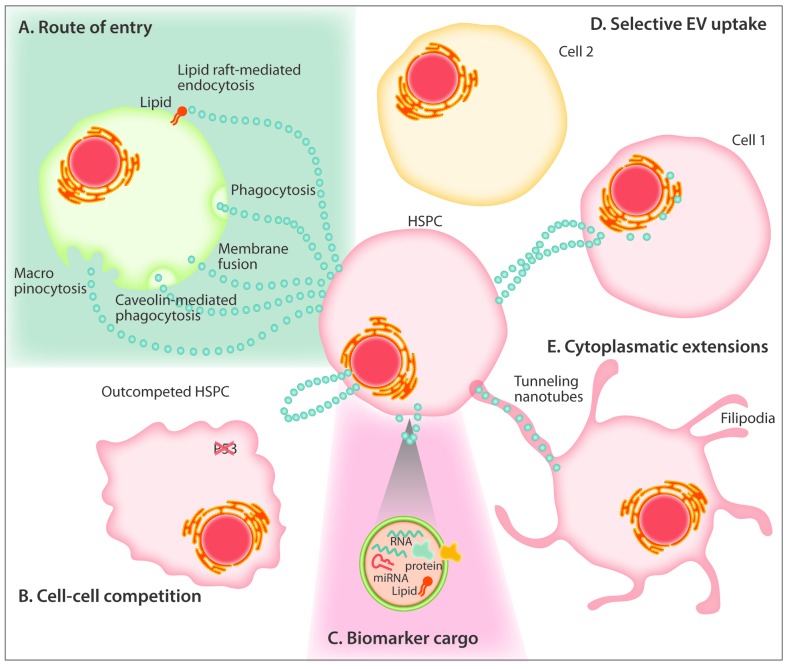
Unresolved aspects of extracellular vesicle biology in the regulation of hematopoiesis. (A) EVs have been proposed to enter recipient cells through lipid raft-mediated internalization, endocytosis, phagocytosis, membrane fusion, caveolin-mediated endocytosis and macropinocytosis. (B) Exosome-mediated crosstalk may explain the intercellular competition of neighboring cells where the “winner” HSPC outcompetes the less fit HSPC through a P53-dependent mechanism. (C) Vesicles contain cargo comprised of uniquely packaged proteins, miRNAs and RNAs which serve as promising biomarkers for disease detection. (D) Vesicles from HSPCs and other cells of the bone marrow niche have been shown to exhibit preferential targeting to specific recipient cells for entry. (E) Cytonemes (filopodia, invadopodia, tunneling nanotubes) are cytoplasmic extensions that serve as modes of exosomal transfer to adjacent bystander cells. EVs: extracellular vesicles; HSPC: hematopoietic stem and progenitor cell.
Table 3.
Extracellular vesicle miRNA, selective roles in homeostasis and hematologic malignancies.
The use of EVs as biomarkers is not limited to hematologic malignancies; it may also be prognostically useful for benign hematologic disorders such as sickle-cell anemia. Plasma EVs from patients with sickle-cell anemia showed a distinct signature of miRNA that not only distinguished patients from healthy donors, but also coincided with the stage of the disease in the patients.82 In a study of patients with BM failure, investigators showed distinct profiles in patients with aplastic anemia and myelodysplastic syndrome and a biomarker response to successful immunosuppressive therapy.83 While many believe that EV miRNA under consideration as hematologic biomarkers may primarily serve a biological role in endocrine cell-cell communication between distant tissues and cells, an alternative, and no less intriguing, possibility is the deliberate secretion of protein and RNA into EVs as a way of preventing regulation of the originating cell.84
Finally, autologous EVs from any number of accessible and in vitro expandable cells, including MSCs, T cells, and NK cells, can be a potentially exciting prospect for EV therapeutics, especially if approaches for selective loading and organ targeting can be developed. Human MSC EVs, for example, were shown in vitro to shuttle miR-155 and miR-146 that exerted immunomodulatory effects through suppression of NK-, B-, and T-cell activity.85 In a murine model, CD73-bearing MSC EVs effectively reversed graft-versus-host disease through the promotion of adenosine metabolism that in turn suppressed Th1-mediated inflammation immune suppression.86 As noted earlier, CD34-derived EVs appear to reverse hindlimb ischemia in animal models.53 A recent human study relied on MSC-derived EVs to treat graft-versus-host disease, based on prior successful work using pooled MSCs as a promising therapy for refractory graft-versus-host disease, a complication of allogeneic HSC transplantation.87 Presumably based on the high concentration of interleukin-10, TGF-β, and HLA-G, EV injection resulted in a significant reduction of the patient’s inflammatory response and improved the symptoms of graft-versus-host disease in multiple organ systems.
One recent study showed that Rab27 alpha/beta double knockout (RAB27DKO) mice, with impaired exosome release, have increased levels of cytokines and myeloproliferation, consistent with chronic inflammation. Grafting these mice with wild-type HSCs, or injecting EVs produced by granulocyte-macrophage colony-stimulating factor-expanded wild-type HSCs ameliorated the inflammatory state. While Rab27DKO mice showed no response to lipopolysaccharide, response could be restored following exposure to wild-type hematopoietic EVs, but not EVs harvested from miR-155−/− cells, indicating that vesicle trafficking of miR-155 regulates the innate immune response.88
Perspective and open questions
HSCs are competitively displaced from the hematopoietic niche in several types of cancer. Yet, the successive loss of HSCs from the BM is not explained by mere physical displacement, but rather occurs even at a disproportionately low tumor burden or with extramedullary tumor location. The underlying mechanisms of how cancer cells are able to disrupt the hematopoietic niche still remain unclear.8,89,90 Cellular competition was historically characterized in Drosophila as a non-cell autonomous mechanism involving p53 as a rheostat, whereby healthy (low p53) cells can competitively eliminate damaged and functionally compromised neighboring cells (Figure 4B).91,92 It is thus tempting to speculate that a process such as cell competition between healthy and leukemic cells, the senescence-associated secretory phenotype, or even submicroscopic pre-cancerous changes in apparently healthy cells adjacent to tumor tissue (the so called “field effects”), result from EV trafficking.92,93 The described interaction between EVs and other cells through integrin receptor on the cell surface constitutes a potential candidate mechanism for the disruption of HSC retention in the BM niche.3,13,94
More broadly, the cellular context of vesicle transfer between cells in the hematopoietic niche, and how different routes of delivery affect target cell response are areas requiring urgent clarification. For example, it is evident that EV crosstalk occurs through the release of free vesicles into the interstitial space to interact with target cells in a paracrine or endocrine manner. However, vesicle transfer also appears to utilize cytoplasmic extensions (variously referred to as cytonemes, nanotubes, or invadopodia) which deliver contents directly into adjacent cells. These alternative modes of delivery make it difficult to cleanly segregate contact-dependent effects from those relying on “free” vesicle exchange (Figure 4E).46,95 Clearly, the development of experimental approaches that more closely resemble the physiological context should be a priority for future studies.
In addition to hematopoietic regulation, the vesicles released by BM cells may play a central role in the establishment and propagation of pathophysiological events outside the medullary space, ranging from promoting metastatic dissemination of melanoma cells, to priming inflammatory responses after cardiac injury.73,96 A mechanistic understanding of the EV-mediated crosstalk between tissues is currently lacking, and EV as carriers of cytokines, bioactive lipids and several classes of RNA may deserve greater consideration when systemic conditions affect BM function. For example, given the EV-mediated crosstalk between lymphocytes and antigen-presenting cells,11 or priming of an inflammatory phenotype in the BM by EV miRNA,97 it is not inconceivable that systemic inflammatory effects after cardiovascular injury or chronic stress conditions are similarly induced by EVs and their cargo.96 Such a systemic communication model finds further support in reports of BM-derived EV trafficking to the brain during experimentally induced systemic inflammation.98
Finally, it is now widely accepted that EVs contribute to pathophysiological regulation, and the suppression of EV release in disease states may offer therapeutic benefit. However, while several of the molecular mechanisms involved in EV release have been described,24,99 broad suppression of EV release is an unlikely therapeutic goal given the role EVs play in maintaining homeostasis. Rather, a nuanced understanding of cell-specific biogenesis, cargo incorporation, and EV-recipient cell affinity may offer the insight necessary for more targeted and disease-specific approaches. Further research into the identity of vesicle surface molecules that govern target cell specificity and route of cellular uptake will be critical in mapping the role that EVs play in regulating hematopoiesis (Figure 4A,D). Classifying these surface molecules may also prove useful for harnessing the potential of vesicles to deliver targeted therapeutics within the BM, or blocking the action of cancer-derived EVs, in order to advance the treatment of hematologic disease. Additionally, the intracellular events that follow uptake are particularly poorly understood. Answering questions about miRNA copy number per EV, or intracellular EV processing, cargo unloading and vesicle degradation will be crucial to realize the therapeutic potential for modified EVs. Finally, understanding how different components of a given vesicle cooperatively alter the behavior of a cell, and whether vesicles with an identical cell origin can differentially regulate multiple cell types in the niche, will be key to utilizing this complex biological process in order to create realistic therapeutics.
In sum, EVs offer fundamental new insight into the biology of HSC regulation, as well as translational opportunities for mitigating injury, and opposing malignancy. Due to their constitutive role in regulating specific cell populations within the marrow niche, and unique signatures, EVs could prove to be a powerful tool for advancing hematology, and be exploited to improve diagnosis, disease monitoring and therapy.
Supplementary Material
Acknowledgments
We apologize to those investigators whose work we were unable to cite for space limitations. We recognize funding through the Hyundai Hope on Wheels Program (PK), Max Blue Butterfly Campaign (PK), the Medical Research Foundation of Oregon (SA) and we gratefully acknowledge contributions from Ben Doron.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/3/382
References
- 1.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietras EM, Warr MR, Passegué E. Cell cycle regulation in hematopoietic stem cells. J Cell Biol. 2011;195(5):709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schepers K, Campbell TB, Passegué E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 2015;16(3):254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blank U, Karlsson S. TGF-β signaling in the control of hematopoietic stem cells. Blood. 2015;125(23):3542–3550. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki S, Iwama A, Takayanagi S-I, et al. Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO. 2006;25(15):3515–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming HE, Janzen V, Celso Lo C, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2(3):274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yilmaz OH, Morrison SJ. The PI-3kinase pathway in hematopoietic stem cells and leukemia-initiating cells: a mechanistic difference between normal and cancer stem cells. Blood Cells Mol Dis. 2008;41(1):73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322(5909):1861–1865. [DOI] [PubMed] [Google Scholar]
- 9.Johnstone RM. Exosomes biological significance: a concise review. Blood Cells Mol Dis. 2006;36(2):315–321. [DOI] [PubMed] [Google Scholar]
- 10.Thomou T, Mori MA, Dreyfuss JM, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2017;2:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yáñez-Mó M, Siljander PR-M, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(0):27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornick NI, Doron B, Abdelhamed S, et al. AML suppresses hematopoiesis by releasing exosomes that contain microRNAs targeting c-MYB. Sci Signal. 2016;9(444):ra88. [DOI] [PubMed] [Google Scholar]
- 14.Kumar B, Garcia M, Weng L, et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia. 2017. August 17 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–288. [DOI] [PubMed] [Google Scholar]
- 16.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. [DOI] [PubMed] [Google Scholar]
- 17.Goloviznina NA, Verghese SC, Yoon YM, Taratula O, Marks DL, Kurre P. Mesenchymal stromal cell-derived extracellular vesicles promote myeloid-biased multipotent hematopoietic progenitor expansion via Toll-like receptor engagement. J Biol Chem. 2016;291(47):24607–24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geddings JE, Hisada Y, Boulaftali Y, et al. Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice. J Thromb Haemost. 2016;14(1):153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci USA. 2009;106(10):3794–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshino A, Costa-Silva B, Shen T-L, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123(4):1542–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113(8):E968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu H, Chen C, Hao X, et al. Sorting protein VPS33B regulates exosomal autocrine signaling to mediate hematopoiesis and leukemogenesis. J Clin Invest. 2016;126(12):4537–4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30 [DOI] [PubMed] [Google Scholar]
- 25.Willms E, Johansson HJ, Mäger I, et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 2016;6(1):22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith ZJ, Lee C, Rojalin T, et al. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J Extracell Vesicles. 2015;4:28533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timár CI, Lőrincz ÁM, Csépányi-Kömi R, et al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood. 2013;121(3):510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson SM, Dempsey C, Parker C, Mironov A, Bradley H, Saha V. Acute lymphoblastic leukaemia cells produce large extracellular vesicles containing organelles and an active cytoskeleton. J Extracell Vesicles. 2017;6(1):e1294339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Vizio D, Morello M, Dudley AC, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181(5):1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9(3):231–241. [DOI] [PubMed] [Google Scholar]
- 32.Holmgren L, Szeles A, Rajnavölgyi E, et al. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood. 1999;93(11):3956–3963. [PubMed] [Google Scholar]
- 33.Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J Cell Biochem. 2008;105(5):1211–1218. [DOI] [PubMed] [Google Scholar]
- 34.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in ccncer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi Y, Nishikawa M, Shinotsuka H, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165(2):77–84. [DOI] [PubMed] [Google Scholar]
- 36.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3(1):24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christianson HC, Svensson KJ, van Kuppevelt TH, Li J-P, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci USA. 2013;110(43):17380–17385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svensson KJ, Christianson HC, Wittrup A, et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288(24):17713–17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrès C, Blanc L, Bette-Bobillo P, et al. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115(3):696–705. [DOI] [PubMed] [Google Scholar]
- 41.Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nature. 2009;9(1):40–55. [DOI] [PubMed] [Google Scholar]
- 42.Yáñez-Mó M, Barreiro O, Gordon-Alonso M, Sala-Valdés M, Sánchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19(9):434–446. [DOI] [PubMed] [Google Scholar]
- 43.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6(10):801–811. [DOI] [PubMed] [Google Scholar]
- 44.Anzai N, Lee Y, Youn B-S, et al. C-kit associated with the transmembrane 4 superfamily proteins constitutes a functionally distinct subunit in human hematopoietic progenitors. Blood. 2002;99(12):4413–4421. [DOI] [PubMed] [Google Scholar]
- 45.Beckwith KA, Byrd JC, Muthusamy N. Tetraspanins as therapeutic targets in hematological malignancy: a concise review. Front Physiol. 2015;6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heusermann W, Hean J, Trojer D, et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol. 2016;213(2):173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kucharzewska P, Christianson HC, Welch JE, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA. 2013;110(18):7312–7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wen S, Dooner M, Cheng Y, et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia. 2016;30(11):2221–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gutiérrez-Vázquez C, Villarroya-Beltri C, Mittelbrunn M, Sánchez-Madrid F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol Rev. 2013;251(1):125–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288(48):34343–34351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huan J, Hornick NI, Goloviznina NA, et al. Coordinate regulation of residual bone marrow function by paracrine trafficking of AML exosomes. Leukemia. 2015;29(12):2285–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salvucci O, Jiang K, Gasperini P, et al. MicroRNA126 contributes to granulocyte colony-stimulating factor-induced hematopoietic progenitor cell mobilization by reducing the expression of vascular cell adhesion molecule 1. Haematologica. 2012;97(6):818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathiyalagan P, Liang Y, Kim D, et al. Angiogenic mechanisms of human CD34(+) stem cell exosomes in the repair of ischemic hindlimb. Circ Res. 2017;120(9):1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lombardo G, Dentelli P, Togliatto G, et al. Activated Stat5 trafficking via endothelial cell-derived extracellular vesicles controls IL-3 pro-angiogenic paracrine action. Sci Rep. 2016;6(1):25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weilner S, Schraml E, Wieser M, et al. Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell. 2016;15(4):744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hromada C, Mühleder S, Grillari J, Redl H, Holnthoner W. Endothelial extracellular vesicles-promises and challenges. Front Physiol. 2017;8:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong M, Yu B, Wang J, et al. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 2017;8(28):45200–45212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841(1):108–120. [DOI] [PubMed] [Google Scholar]
- 59.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113(22):5444–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang J, Kao C-Y, Papoutsakis ET. How do megakaryocytic microparticles target and deliver cargo to alter the fate of hematopoietic stem cells? J Control Release. 2017;247:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Connell RM, Chaudhuri AA, Rao DS, Gibson WSJ, Balazs AB, Baltimore D. MicroRNAs enriched in hematopoietic stem cells differentially regulate long-term hematopoietic output. Proc Natl Acad Sci USA. 2010;107(32):14235–14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi X-F, Wang H, Kong F-X, et al. Exosomal miR-486 regulates hypoxia-induced erythroid differentiation of erythroleukemia cells through targeting Sirt1. Exp Cell Res. 2017;351(1):74–81. [DOI] [PubMed] [Google Scholar]
- 64.Wang L-S, Li L, Li L, et al. MicroRNA-486 regulates normal erythropoiesis and enhances growth and modulates drug response in CML progenitors. Blood. 2015;125(8):1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davis C, Dukes A, Drewry M, et al. MicroRNA-183-5p increases with age in bone-derived extracellular vesicles, suppresses bone marrow stromal (stem) cell proliferation, and induces stem cell senescence. Tissue Eng Part A. 201623(21–22);1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–856. [DOI] [PubMed] [Google Scholar]
- 67.Razmkhah F, Soleimani M, Mehrabani D, et al. Leukemia microvesicles affect healthy hematopoietic stem cells. Tumor Biol. 2017;39(2):101042831769223. [DOI] [PubMed] [Google Scholar]
- 68.Viola S, Traer E, Huan J, et al. Alterations in acute myeloid leukaemia bone marrow stromal cell exosome content coincide with gains in tyrosine kinase inhibitor resistance. Br J Haematol. 2015;172(6):938–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011;96(9):1302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horiguchi H, Kobune M, Kikuchi S, et al. Extracellular vesicle miR-7977 is involved in hematopoietic dysfunction of mesenchymal stromal cells via poly(rC) binding protein 1 reduction in myeloid neoplasms. Haematologica. 2016;101(4):437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muntión S, Ramos TL, Diez-Campelo M, et al. Microvesicles from mesenchymal stromal cells are involved in HPC-microenvironment crosstalk in myelodysplastic patients. Wagner W, editor. PLoS One. 2016;11(2):e0146722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corrado C, Saieva L, Raimondo S, Santoro A, De Leo G, Alessandro R. Chronic myelogenous leukaemia exosomes modulate bone marrow microenvironment through activation of epidermal growth factor receptor. J Cell Mol Med. 2016;20(10):1829–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peinado H, Alečković M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burnouf T, Chou M-L, Goubran H, Cognasse F, Garraud O, Seghatchian J. An overview of the role of microparticles/microvesicles in blood components: are they clinically beneficial or harmful? Transfus Apher Sci. 2015;53(2):137–145. [DOI] [PubMed] [Google Scholar]
- 75.Caivano A, Laurenzana I, De Luca L, et al. High serum levels of extracellular vesicles expressing malignancy-related markers are released in patients with various types of hematological neoplastic disorders. Tumour Biol. 2015;36(12):9739–9752. [DOI] [PubMed] [Google Scholar]
- 76.Caivano A, La Rocca F, Laurenzana I, et al. Extracellular vesicles in hematological malignancies: from biology to therapy. Int J Mol Sci. 2017;18(6):1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Belov L, Matic KJ, Hallal S, Best OG, Mulligan SP, Christopherson RI. Extensive surface protein profiles of extracellular vesicles from cancer cells may provide diagnostic signatures from blood samples. J Extracell Vesicles. 2016;5(1):e25355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Luca L, D’Arena G, Simeon V, et al. Characterization and prognostic relevance of circulating microvesicles in chronic lymphocytic leukemia. Leuk Lymphoma. 2017;58(6):1424–1432. [DOI] [PubMed] [Google Scholar]
- 79.Harshman SW, Canella A, Ciarlariello PD, et al. Proteomic characterization of circulating extracellular vesicles identifies novel serum myeloma associated markers. J Proteomics. 2016;136:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. [DOI] [PubMed] [Google Scholar]
- 81.Hornick NI, Huan J, Doron B, et al. Serum exosome microRNA as a minimally-invasive early biomarker of AML. Sci Rep. 2015;5:11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khalyfa A, Khalyfa AA, Akbarpour M, et al. Extracellular microvesicle microRNAs in children with sickle cell anaemia with divergent clinical phenotypes. Br J Haematol. 2016;174(5):786–798. [DOI] [PubMed] [Google Scholar]
- 83.Hosokawa K, Kajigaya S, Feng X, et al. A plasma microRNA signature as a biomarker for acquired aplastic anemia. Haematologica. 2017;102(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin M-C, Chen S-Y, Tsai H-M, et al. PGE2/EP4 signaling controls the transfer of the mammary stem cell state by lipid rafts in extracellular vesicles. Stem Cells. 2017;35(2):425–444. [DOI] [PubMed] [Google Scholar]
- 85.Di Trapani M, Bassi G, Midolo M, et al. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep. 2016;6(1):24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amarnath S, Foley JE, Farthing DE, et al. Bone marrow-derived mesenchymal stromal cells harness purinergenic signaling to tolerize human Th1 cells in vivo. Stem Cells. 2015;33(4):1200–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kordelas L, Rebmann V, Ludwig A-K, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–973. [DOI] [PubMed] [Google Scholar]
- 88.Alexander M, Ramstead AG, Bauer KM, et al. Rab27-dependent exosome production inhibits chronic inflammation and enables acute responses to inflammatory stimuli. J Immunol. 2017;199(10):3559–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boyd AL, Campbell CJ, Hopkins CI, et al. Niche displacement of human leukemic stem cells uniquely allows their competitive replacement with healthy HSPCs. J Exp Med. 2014;211(10):1925–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shiozawa Y, Pedersen EA, Havens AM, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121(4):1298–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66(9):4795–4801. [DOI] [PubMed] [Google Scholar]
- 92.Bondar T, Medzhitov R. p53-mediated hematopoietic stem and progenitor cell competition. Stem Cell. 2010;6(4):309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Slaughter DP, Southwick HW, Smekjal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–968. [DOI] [PubMed] [Google Scholar]
- 94.Dhawan A, Bonin von M, Bray LJ, et al. Functional interference in the bone marrow microenvironment by disseminated breast cancer cells. Stem Cells. 2016;34(8):2224–2235. [DOI] [PubMed] [Google Scholar]
- 95.Jung Y, Wang J, Havens A, et al. Cell-to-cell contact is critical for the survival of hematopoietic progenitor cells on osteoblasts. Cytokine. 2005;32(3–4):155–162. [DOI] [PubMed] [Google Scholar]
- 96.Heidt T, Sager HB, Courties G, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alexander M, Hu R, Runtsch MC, et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ridder K, Keller S, Dams M, et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12(6):1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dai J, Lu Y, Wang C, et al. Vps33b regulates Vwf-positive vesicular trafficking in megakaryocytes. J Pathol. 2016;240(1):108–119. [DOI] [PubMed] [Google Scholar]
- 100.Zhi F, Cao X, Xie X, et al. Identification of circulating microRNAs as potential biomarkers for detecting acute myeloid leukemia. PLoS One. 2013;8(2):56718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yeh Y-Y, Ozer HG, Lehman AM, et al. Characterization of CLL exosomes reveals a distinct microRNA signature and enhanced secretion by activation of BCR signaling. Blood. 2015;125(21):3297–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Eijndhoven MA, Zijlstra JM, Groenewegen NJ, et al. Plasma vesicle miRNAs for therapy response monitoring in Hodgkin lymphoma patients. JCI Insight. 2016;1(19):89631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Manier S, Liu C-J, Avet-Loiseau H, et al. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood. 2017;129(17):2429–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.