Palbociclib is a cyclin-dependent kinase (CDK) inhibitor of CDK4 and CDK6 that has been approved by the US Food and Drug Administration (FDA) for the treatment of estrogen receptor (ER)-positive, HER2-negative metastatic breast cancer (MBC), demonstrating an improvement in progression-free survival (PFS) when added to anti-estrogen therapy in two large phase III trials.1,2 The CDK 4/6 proteins are important activators of cell-cycle progression and phosphorylate the retinoblastoma protein, thereby pushing resting cells into the S-phase of the cell cycle.3,4 Increased signaling of the CDK4/CDK6/E2F axis is a common feature of luminal ER+ breast cancer.5 Hematopoiesis is a dynamic process that relies on active proliferation and differentiation of stem cells into erythrocytes, myelocytes, and platelets, processes that rely on co-ordinated progression through various phases of the cell cycle. Neutropenia is a well-recognized adverse effect of palbociclib, and is rapidly reversible when the drug is interrupted, but nevertheless occurs in 80% of patients treated with palbociclib, whereas anemia (24%) and thrombocytopenia (16%) are less common.1 Literature on the clinical characteristics of anemia associated with use of palbociclib or of other CDK4/6 inhibitors is scarce. We report 3 patients who developed dysplastic hematopoiesis with macrocytic anemia that developed during palbociclib treatment of breast cancer, findings that mimicked a clinical diagnosis of myelodysplastic syndrome (MDS) based on the morphology of peripheral blood (PB) smear and bone marrow (BM) samples. We then aimed to evaluate changes in hematologic parameters during palbociclib therapy in a cohort of patients with MBC. Parameters included: mean corpuscular volume (MCV), hemoglobin (Hb), and red cell distribution width (RDW). We assessed the use of change in MCV as a pharmacodynamic biomarker of CDK4/6 inhibition. Furthermore, we performed in vitro experiments to evaluate CDK4 and CDK6 levels in BM samples derived from patients with MDS and healthy controls, and investigated the effect of palbociclib on red cell maturation and differentiation in vitro.
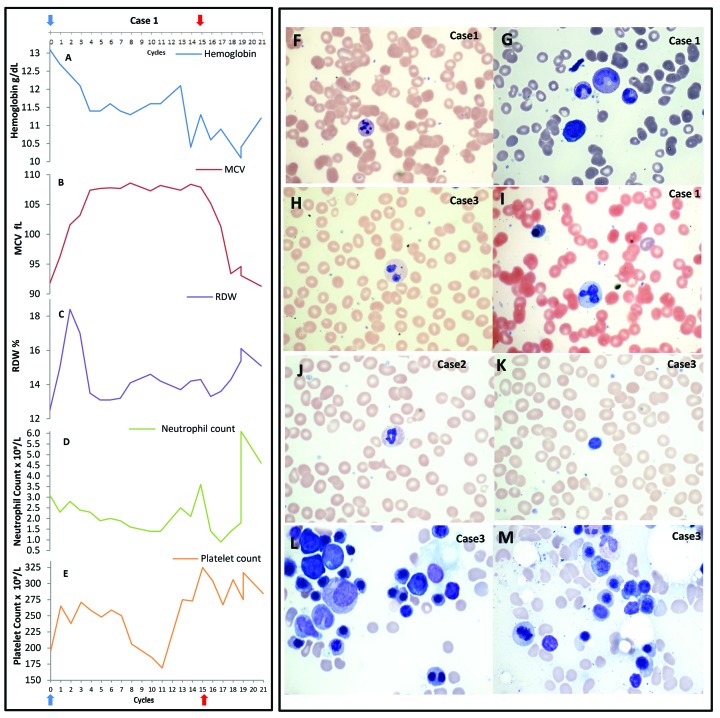
Three patients between the ages of 68 to 76 years who received palbociclib for MBC developed macrocytic, dysplastic anemia resembling MDS (Figure 1A–E and Online Supplementary Figure S1). The anemia plateaued 4 cycles after starting palbociclib (1 cycle = 28 days). PB smears demonstrated macrocytosis, dysplastic maturation with hypolobated, hypogranulated pseudo Pelger-Huet neutrophils, dysplastic granulation, and giant platelets (Figure 1F–M and Online Supplementary Figure S2). No other common causes for macrocytosis, such as B12/folate deficiency, liver insufficiency, or hypothyroidism, were observed (Online Supplementary Table S1). Unilateral BM aspirate/biopsy in all 3 cases revealed moderately hypocellular marrow (approx. 10% cellularity) with mildly decreased myeloid/erythroid ratio, dysplastic erythroid forms including binucleated forms, nuclear budding, small hypolobated megakaryocytes, and adequate stainable iron sores (Figure 1F–M and Online Supplementary Figure S2). Due to pathological similarities to MDS, cytogenetic/mutational analysis was conducted; this did not reveal any pathogenic chromosomal alterations or mutations (GENOPTIX 40-gene myeloid molecular profile). Flow cytometry did not reveal any increase in leukemic blasts.
Figure 1.
Clinical cases: hematologic changes in 3 patients with dysplastic, macrocytic anemia. (A–E) Hematologic parameters of Case 1 are shown. Blue arrow: palbociclib start; red arrow: last date of palbociclib treatment. (A) Hemoglobin decreases with palbociclib use. (B) Mean corpuscular volume (MCV) increases with palbociclib (plateau after 4–5 cycles and correlates with lower hemoglobin level), and returns to baseline 4 cycles after palbociclib discontinuation. (C) Red cell distribution width (RDW) initially increases but returns to normal after 4 cycles of palbociclib initiation, and increases upon palbociclib discontinuation. (D) Absolute neutrophil count with slight decrease over time with palbociclib use. (E) Platelet count shows normal values during palbociclib treatment. (F–M) Peripheral smears and bone marrow aspirates from our 3 index cases (Case 1, Case 2, and Case 3). (F) Granulocytes with karyorrhexis 100X, (G) giant band 100X, (H) bilobed neutrophil with hypogranular cytoplasm, Wright-Geimsa 100X, (I) nucleated red cell and dysplastic neutrophil, Wright-Geimsa 100X, (J) dysplastic hypogranular neutrophils, Wright-Geimsa100X, and (K) macrocytic red blood cells and dysplastic neutrophils, Wright-Geimsa100X. (L) Bone marrow aspirate with binucleated red cell and nuclear irregularity in erythroid precursors, Wright stain 100X. (M) Beginning of erythroid nuclear fragmentation, Wright stain 100X.
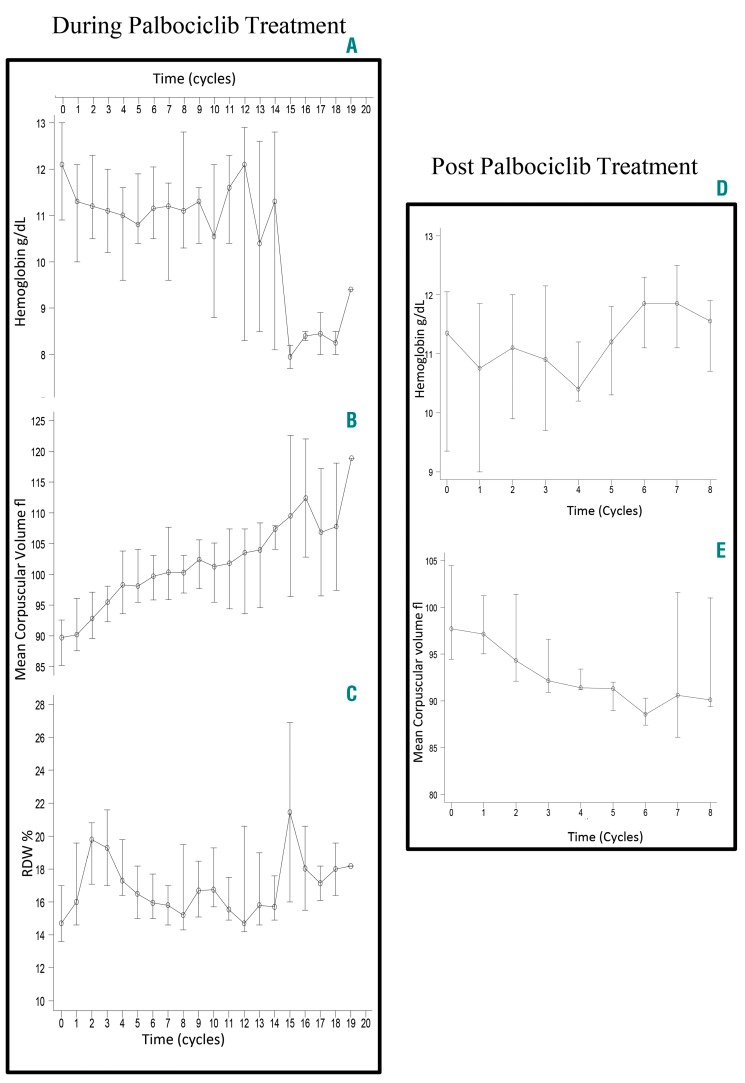
To determine the extent of macrocytosis in a larger cohort, we evaluated hematologic parameters in 34 patients who had received palbociclib for ER+ MBC between January 1st 2015 and March 1st 2017 (n=34). Hematologic parameters during palbociclib treatment, maximum MCV and minimum Hb were compared to baseline values using the Wilcoxon signed-rank test. Significant change in MCV (delta MCV) was defined as 10 fL or more. The Kaplan-Meier method was used to estimate the median PFS. All patients displayed an increase in MCV with palbociclib use (range 0.2–27.4 fL). The median baseline MCV was 89.8 fL [Interquartile Range (IQR): 85–93] compared to 100.1 fL (IQR: 97.1–107.9) with palbociclib use (P<0.0001). A total of 16 (47%) patients developed macrocytosis (MCV≥100 fL) during the study period. The median decrease in Hb was 1.6 g/dL; moreover, 18 out of 20 patients with normal baseline Hb achieved low Hb levels (<12.3 g/dL) with palbociclib (Online Supplementary Table S2). Longitudinal follow up of MCV over time for the entire cohort demonstrated a rapid increase in MCV during the first 4–5 cycles of palbociclib. The median MCV after 4 cycles of palbociclib was 98.3 fL (IQR: 93.6–103.8) compared to 89.8 fL (IQR: 85.2–92.6) at baseline (P=0.0001). RDW also increased rapidly after palbociclib was started and returned to normal after 4–5 cycles. When compared to baseline RDW (14.7%, IQR: 13.6–17.0), the RDW peaked after 2 cycles of palbociclib (19.8%; IQR: 17.1–20.8; P<0.0001), and returned to baseline after 5 cycles (16.5%; IQR: 15.0–18.2; P=0.09). MCV decreased after palbociclib discontinuation and plateaued after 4–5 cycles (Figure 2 and Online Supplementary Figure S3).
Figure 2.
Hematologic parameters change over time in a retrospective cohort of patients who received palbociclib for advanced breast cancer. (A–C) Red blood cell (RBC) parameters [median, interquartile range (IQR)]) in entire cohort (n=34) during palbociclib treatment. Time 0 is palbociclib start; patients censored upon palbociclib discontinuation. (A) Hemoglobin change over time. Hemoglobin decreases over time with a median decrease of 1.6 g/dL. (B) Mean corpuscular volume (MCV) change over time. All patients had an increase in MCV with a median increase of 12.4 fL. (C) Red cell distribution width (RDW) change over time. RDW increases and returns to baseline after 4–5 cycles, reflecting production of a new population of RBCs with different morphology (larger) upon palbociclib start leading to uniformly large RBCs after 4–5 cycles. (D and E) Hematologic parameters for patients who discontinued palbociclib (n=14). Time 0 is date of palbociclib discontinuation. Longitudinal data reflecting cohort median and IQR over time. Hemoglobin starts to recover 4–5 months after palbociclib discontinuation. MCV decreases after palbociclib discontinuation and plateaus after 4–5 months from palbociclib discontinuation.
Delta MCV 10 fL or more, achieved in 24 patients, was associated with longer duration of palbociclib treatment (10 vs. 5 cycles; P=0.006) and a greater reduction in Hb (1.95 vs. 1.0 g/dL; P=0.001) compared to those with delta MCV less than 10 fL (Online Supplementary Table S3). Median PFS was significantly longer for patients who achieved delta MCV 10 fL or more compared to those with delta MCV less than 10 fL [422 vs. 216 days; Hazard Ratio (HR) 0.29; P=0.035] (Online Supplementary Figure S4); however, when delta MCV 10 fL or more was included as time-dependent variable, this difference was not statistically significant (HR 0.59; P=0.4).
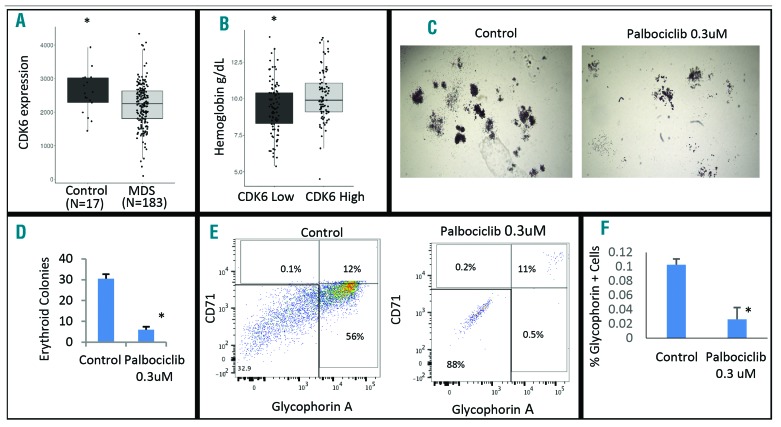
These data suggest that palbociclib use led to a macrocytic, dysplastic anemia with bone marrow changes that mimic human MDS. Therefore, CDK4 and CDK6 expression by qRT-PCR was evaluated using MDS-derived (n=183) and healthy (n=17) CD34+ hematopoietic stem cells (HSCs) obtained from a large cohort of gene expression profiles. CDK6 expression was significantly lower in MDS HSCs when compared to healthy controls (P=0.04), while there was no statistical difference in CDK4 expression between MDS and control samples (P=0.64) (Figure 3A). Furthermore, MDS patients with lower CDK6 levels (< median expression) presented with worse anemia than patients with higher CDK6 levels (mean Hb 9.3 vs. 10.3 g/dL; P=0.001) (Figure 3B). There was no difference in neutrophil count (2.4 k/uL vs. 3.2 k/uL; P=0.16) or in platelet count (208 k/uL vs. 195 k/uL; P=0.66) between low-CDK6 and high-CDK6 expression groups.
Figure 3.
Reduced expression of CDK6 is seen in human myelodysplastic syndrome (MDS), and palbociclib causes reduced erythroid differentiation in vitro. (A) Significantly reduced expression of CDK6 was seen in 183 MDS patient-derived CD34+ stem and progenitor cells when compared to healthy marrow CD34+ controls (P=0.04). (B) MDS samples were separated on the basis of high (>median expression) and low CDK6 expression. Patients with lower CDK6 expression had significantly worse hemoglobin values (mean 9.3g/dL in low vs. 10.3 g/dL in high CDK6 group; P=0.001). (C and D) Healthy CD34+ stem cells were grown in methylcellulose for 14 days with and without palbociclib (0.3 uM). Palbociclib exposure led to a reduced number and size of erythroid colonies. (E) Colonies were picked and analyzed by flow cytometry. Palbociclib treatment led to reduced numbers of erythroid cells (CD71 and Glycophorin A positive). (F) Numbers of Glycophorin A positive cells were significantly reduced in replicates after palbociclib treatment (n=2, t-test <0.05).
Lastly, we treated healthy CD34+ HSCs with palbociclib in vitro, and performed flow cytometry to analyze erythroid maturation after 14 days of culture in methylcellulose. We determined that palbociclib treatment led to a decrease in erythroid colony formation and reduced erythroid differentiation by significantly inhibiting the number of glycophorin-A positive erythroid cells (Figure 3C–F).
To our knowledge, this is the first report of macrocytosis and dysplastic erythropoiesis with palbociclib use in human subjects. Our RDW/MCV changes correlate with the hypothesis that palbociclib induces development of a new population of large erythrocytes; this effect continues during palbociclib treatment and resolves after palbociclib discontinuation. Since palbociclib affects cancer cell growth/proliferation by CDK4/6 inhibition, and CDK4/6 inhibition seems to correlate with change in MCV in a time-dependent fashion, change in MCV is a potential pharmacodynamic biomarker of functional CDK4/6 inhibition in vivo. While macrocytosis (MCV≥100 fL) has clinical significance in several hematologic diseases,6 we believe that a more important pharmacodynamic biomarker of palbociclib effect would be change in MCV, which not only accounts for post-treatment MCV, but also for the pre-treatment MCV. In fact, larger studies are required to assess whether delta MCV 10 fL or more is associated with PFS in MBC.
Neutrophils have a short life span (8 hours-5 days),7 and neutrophil count can be affected by shifts with the marginated neutrophil pool due to circadian rhythm or stress responses.8 The palbociclib dose-limiting toxicity9 neutropenia is rapidly reversible and does not seem to have a detrimental effect on palbociclib efficacy in MBC.10 In the phase III PALOMA-3 trial, there was no difference in PFS for patients who had grade 3 or higher neutropenia compared to those with grade 2 or less neutropenia (median PFS 11.1 vs. 11.0 months; P=0.93).11 The effect of palbociclib on erythroid lineage might provide additional pharmacodynamic information on the effect on myeloid lineage. In the PALOMA-3 trial, palbociclib dosage was adjusted mainly for neutropenia or thrombocytopenia but not for anemia, and palbociclib treatment led to anemia (any grade) in 24% patients. We identified a higher rate of any grade anemia (37%), suggesting that palbociclib frequently affects erythropoiesis. In addition, given our findings suggesting a time-dependent correlation between MCV and palbociclib use, along with a long half life of red blood cells (115 days),12 change in MCV could be a more attractive pharmacodynamic biomarker of in vivo CDK4/6 inhibition with palbociclib use than neutrophil count.
Carcinogenicity studies have not been conducted with palbociclib.13 Dysplastic changes in our 3 index cases improved upon palbociclib discontinuation, suggesting that dysplastic changes are transient and are due to CDK4/6 inhibition; however, these findings raise concerns about long-term safety of CDK4/6 inhibition. Further research is required to investigate dysplastic changes induced by CDK4/6 inhibition, especially because CDK 4/6 inhibitors are being evaluated in the management of early breast cancer14,15 which could lead to a larger population being exposed to these drugs.
In conclusion, we report reversible macrocytosis and dysplastic hematopoiesis with palbociclib use that mimics MDS. Nearly all patients receiving palbociclib treatment have an increase in MCV, and this is a potential in vivo pharmacodynamic biomarker of CDK4/6 inhibition in patients with metastatic breast cancer receiving palbociclib. CDK6 expression is lower in HSCs derived from MDS compared to healthy controls, and low CDK6 expression in MDS correlates with a worse anemia phenotype. While long-term follow up of palbociclib trials is awaited, continued safety monitoring of patients on palbociclib is suggested.
Supplementary Material
Footnotes
Funding: the manuscript development is supported by The Einstein Paul Calabresi Career Development Program (NIH 5K12CA132783-08) and NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA (Grant n. UL1TR001073).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375(20):1925–1936. [DOI] [PubMed] [Google Scholar]
- 2.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. [DOI] [PubMed] [Google Scholar]
- 3.Goodrich DW, Wang NP, Qian YW, Lee EY, Lee WH. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell. 1991;67(2):293–302. [DOI] [PubMed] [Google Scholar]
- 4.Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98(6):859–869. [DOI] [PubMed] [Google Scholar]
- 5.Miller TW, Balko JM, Fox EM, et al. ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011;1(4):338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green R, Dwyre DM. Evaluation of Macrocytic Anemias. Semin Hematol. 2015;52(4):279–286. [DOI] [PubMed] [Google Scholar]
- 7.Pillay J, den Braber I, Vrisekoop N, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116(4):625–627. [DOI] [PubMed] [Google Scholar]
- 8.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31(8):318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flaherty KT, Lorusso PM, Demichele A, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res. 2012;18(2):568–576. [DOI] [PubMed] [Google Scholar]
- 10.Sun W, O’Dwyer PJ, Finn RS, et al. Characterization of Neutropenia in Advanced Cancer Patients Following Palbociclib Treatment Using a Population Pharmacokinetic-Pharmacodynamic Modeling and Simulation Approach. J Clin Pharmacol. 2017;57(9):1159–1173. [DOI] [PubMed] [Google Scholar]
- 11.Verma S, Bartlett CH, Schnell P, et al. Palbociclib in Combination With Fulvestrant in Women With Hormone Receptor-Positive/HER2-Negative Advanced Metastatic Breast Cancer: Detailed Safety Analysis From a Multicenter, Randomized, Placebo-Controlled, Phase III Study (PALOMA-3). Oncologist. 2016;21(10):1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franco RS. Measurement of red cell lifespan and aging. Transfus Med Hemother. 2012;39(5):302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inc PLDP. IBRANCE (R) [package insert]. Pfizer Laboratories Div Pfizer Inc, NY, NY; 2016. [cited March 19th, 2017; Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=2191] [Google Scholar]
- 14.Alliance Foundation Trials L. PALbociclib CoLlaborative Adjuvant Study: A Randomized Phase III Trial of Palbociclib With Standard Adjuvant Endocrine Therapy Versus Standard Adjuvant Endocrine Therapy Alone for Hormone Receptor Positive (HR+) / Human Epidermal Growth Factor Receptor 2 (HER2)-Negative Early Breast Cancer (PALLAS). 2017. [cited 2017 March, 19th 2017; Available from: https://clinicaltrials.gov/show/NCT02513394]
- 15.Institute JB. Neoadjuvant Biomarker ResearcH Study of Palbociclib Combined With Endocrine Therapy in Estrogen Receptor Positive/HER2 Negative Breast CAncer (NeoRHEA). 2017. [cited 2017 March, 19th 2017; Available from: https://clinicaltrials.gov/show/NCT03065621]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.