Abstract
Bleeding complications arising from trauma, surgery, as well as congenital, disease-associated or drug-induced blood disorders can cause significant morbidities and mortalities in civilian and military populations. Therefore, stoppage of bleeding (hemostasis) is of paramount clinical significance in prophylactic, surgical and emergency scenarios. For externally accessible injuries, a variety of natural and synthetic biomaterials have undergone robust research, leading to hemostatic technologies including glues, bandages, tamponades, tourniquets, dressings and pro-coagulant powders. In contrast, treatment of internal non-compressible hemorrhage still heavily depends on transfusion of whole blood or blood’s hemostatic components (platelets, fibrinogen and coagulation factors). Transfusion of platelets poses significant challenges of limited availability, high cost, contamination risks, short shelf-life, low portability, performance variability and immunological side-effects, while use of fibrinogen or coagulation factors provides only partial mechanisms for hemostasis. With such considerations, significant interdisciplinary research endeavors have been focused on developing materials and technologies that can be manufactured conveniently, sterilized to minimize contamination and enhance shelf-life, and administered intravenously to mimic, leverage and amplify physiological hemostatic mechanisms. Here we provide a comprehensive review regarding the various topical, intra-cavitary and intravenous hemostatic technologies in terms of materials, mechanisms and state-of-art, and discuss challenges and opportunities to help advancement of the field.
1. Introduction
Blood, a fluid connective tissue composed of red blood cells (RBCs), white blood cells (WBCs), platelets, and non-cellular liquid (plasma) containing salts, nutrients and proteins, is present at a volume of approximately 5 liters in the average human body (70 kg body weight). Blood is responsible for transport of gases and nutrients to tissues as well as providing immune surveillance and hemostatic responses as needed. Hence, loss of blood can result in a variety of pathologic scenarios that can lead to tissue morbidities and mortalities. For example, in traumatic injuries in both battlefield and civilian conditions, significant blood loss from truncal, junctional and internal non-compressible injuries can result in significant pre-hospital (and potentially preventable) mortalities stemming from hypothermia, coagulopathy, infection, acidosis and multiple organ failure [1–6]. Also, certain congenital or disease-associated conditions (e.g. coagulation factor deficiencies and platelet dysfunctions) as well as drug-induced effects (e.g. bone marrow suppression due to chemotherapy and radiotherapy in cancer patients) can put certain patient populations at high bleeding risks [7–9]. Therefore, management of various bleeding complications remains a highly significant clinical area, and substantial interdisciplinary research efforts are focused on the development of materials and technologies for efficient hemostatic management of bleeding.
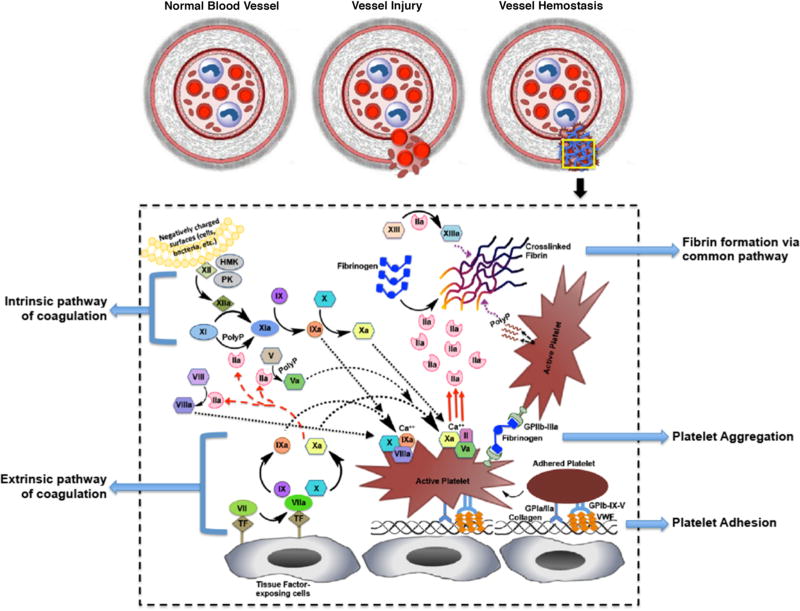
The word ‘hemostasis’ was coined by ancient Greeks from the terms haíma (meaning blood) and stasis (meaning stoppage) to describe the phenomenon of blood stagnation when alum came in contact with a wound [10]. Although scattered evidences of utilizing blood products and application of coagulation/hemostasis concepts are found in ancient Greek and Roman history as well as in reports of transfusion and organ transplants around late 18th ad early 19th centuries, the modern concept of hemostatic mechanisms is credited to the seminal report written by Paul Morawitz in 1905 [11,12]. In his report, Morawitz emphasized the role of platelets, ‘thrombokinase’ (now known as tissue factor), calcium, prothrombin and fibrinogen in promoting blood coagulation. Later in the 20th century, additional coagulation factors were identified and characterized, and the concepts of coagulation being guided by a cascade of enzymes and co-factors (i.e. the intrinsic and extrinsic coagulation cascades) leading to the final output of fibrin formation (common pathway of cascades) were put forward [13,14]. As per the current (and still evolving) understanding of the process, the body’s natural mechanisms of hemostasis are rendered by a complex, spatio-temporally regulated sequence of responses involving a combination of cellular (e.g. platelets and tissue factor-bearing cells) and plasma (e.g. coagulation factors) components, as depicted in the schematic of Figure 1.
Figure 1.
Schematic of the complex mechanism of blood vessel hemostasis. Vessel injury can lead to endothelial activation and denudation resulting in secretion and deposition of von Willebrand Factor (vWF) and exposure of collagen at the injury site, as well as, exposure of tissue factor (TF) bearing cells at the site; vWF and collagen exposure allows platelet adhesion and activation, while TF exposure allows extrinsic pathway of coagulation to propagate and produce moderate amounts of thrombin (FIIa) that activates other coagulation factors in the intrinsic pathway; activated platelets aggregate via fibrinogen (Fg) mediated interaction with platelet surface integrin GPIIb-IIIa to form a platelet plug (primary hemostasis) that staunches bleeding; the surface of aggregated active platelets exposes negatively charged phospholipids that allow co-localization and further activation of coagulation factors to form the prothrombinase (FVa + FXa + FII) complex in presence of calcium (Ca++), leading to amplified generation of thrombin (FIIa) that breaks down fibrinogen (Fg) to fibrin; fibrin self-assembles and undergoes further crosslinking by action of FXIIIa to form a dense biopolymeric mesh that forms the hemostatic clot and arrests flow of blood components (secondary hemostasis).
In the absence of injury, healthy endothelial cells lining the luminal wall of blood vessels avoid blood clotting by secretion of heparin-like molecules, thrombomodulin, nitric oxide and prostacyclin, as well as, sterically hindering adsorption of clot-relevant proteins on the vessel wall due to presence of endothelial glycocalyx. Tissue injury and bleeding result in endothelial damage, leading to vasoconstriction to reduce blood flow out of the injury site and secretion/exposure of pro-coagulant proteins and factors. Platelets rapidly respond to the bleeding site by undergoing adhesion (primarily to von Willebrand Factor and sub-endothelial collagen), activation and inter-platelet aggregation at the site to form the platelet plug [15–18], a process commonly known as ‘primary hemostasis’. In tandem, exposed sub-endothelial collagen, von Willebrand factor (VWF) secreted from injured endothelium and activated platelets, and tissue factor (Coagulation Factor III, formerly known as thrombokinase or thromboplastin) on sub-endothelial matrix and localized leukocytes lead to initiation, amplification and propagation of the coagulation cascade, culminating in thrombin-catalyzed formation of fibrin from fibrinogen, a process commonly known as ‘secondary hemostasis’ [19–21]. Critical upstream steps of this process (e.g. conversion of prothrombin to thrombin) are greatly amplified by anionic lipids (phosphatidylserines) on the membrane of active platelets as well as by anionic polymers (polyphosphates or PolyP) secreted by active platelets [22–29]. Pro-hemostatic active platelets also secrete molecules adenosine di-phosphate (ADP) and platelet factor 4 (PF4) that can modulate hemostatic mechanisms. The fibrin, formed as the final product of the coagulation cascade, associates into a cross-linked biopolymeric mesh facilitated by activated coagulation factor XIII (FXIIIa) and platelet-secreted polyphosphate (PolyP) to secure the platelet plug and other blood components at the bleeding site and form the final clot [30,31]. The clot-incorporated active platelets also facilitate clot retraction and healing mechanisms [32–35]. Post-healing of the injury site, the mature fibrin clot is lysed through the action of plasmin, which is generated from the zymogen plasminogen on the surface of the fibrin, as well as, on neighboring cell surfaces by the action of tissue plasminogen activator (tPA) or urokinase (uPA) [36]. The tPA is produced from endothelial cells while uPA is produced from monocytes, macrophages and urinary epithelium. Plasmin-induced proteolysis of fibrin results in formation of soluble fibrin degradation products (FDPs), which can have some immunomodulatory and chemotactic functions relevant to healing phases. In healthy individuals the clot formation and fibrinolytic systems are highly regulated to ensure hemostatic balance, and any dysregulation can lead impaired or weak clot formation (poor hemostasis and re-bleeding) or overly strong occlusive clot growth (thrombosis). For example, the plasminogen activators (tPA and uPA) and plasmin action are regulated by local high concentration of serine protease inhibitor (serpin) molecules like plasminogen activator inhibitors-1 (PAI-1), plasminogen activator inhibitor-2 (PAI-2) and α-2 antiplasmin [37], as well as non-serpin molecules like α-2 macroglubulin and thrombin-activated fibrinolysis inhibitor (TAFI) [38,39]. Fibrin-bound tPA shows greatly enhanced catalytic efficiency of plasminogen activation compared to solution phase tPA [40]. In parallel, plasmin is protected from inhibition by α-2 antiplasmin upon binding to fibrin [41]. Thus, a combination of feedback mechanisms of fibrin formation and fibrin destruction maintains the precise spatiotemporal regulation of hemostasis.
Based on this complex concert of mechanisms in clotting, biomaterials-based approaches to render and augment hemostasis have focused on mimicking and leveraging the various mechanistic aspects, including constriction (pressure), platelet (primary hemostasis)-relevant components and coagulation (secondary hemostasis)-relevant components. The optimum requirements for a hemostatic material (and technology) is that it (i) should be applicable and adaptable to a variety of actively bleeding wounds, (ii) should act rapidly to reduce blood loss and maintain this hemostatic condition for long durations if needed, (iii) should be easily manufacturable, sterilizable and portable, (iv) should be stable under a variety of atmospheric and ambient conditions, (v) should be easily usable by non-specialized personnel if needed and (vi) should be reasonably biocompatible so as to not induce any short-term or long-term adverse effect in the body. To this end, for externally visible and accessible (often compressible) injuries, a variety of biomaterials-based technologies in the form of powders, bandages, sprays, foams, gels, tourniquets and tamponades have been developed [42–48]. In contrast, for management of internal (often non-compressible) bleeding, the clinical gold standard is the transfusion of whole blood or blood components (RBC, plasma and platelets) [49–54], and use of fibrinogen concentrate or recombinant coagulation factors in selected groups of patients (e.g. recombinant Factor VIIa used in hemophilia patients) [55–59]. However, donor-derived blood and its components often have limited availability, require meticulous type matching, pose issues of high pathologic contamination or immunogenic risks, and have limited portability and short shelf-life. These issues are particularly challenging for transfusion of platelets, which present challenges of alloimmunization and refractoriness, have a high bacterial contamination risk and have a shelf-life of only 3–7 days [60–67]. Therefore, significant research efforts are currently being focused on minimizing contamination and improving storage life of platelets [68–71] as well as on developing in vitro bioreactor technologies for production of ‘donor-independent platelets’ from cultures of precursor cells [72–74]. In parallel, robust interdisciplinary research efforts are being directed on developing biomaterials-based technologies that can be administered intravenously and can mimic, amplify and leverage various mechanistic components of hemostasis to rapidly staunch bleeding. The current article will comprehensively and critically review the various externally used and intravenously applicable hemostatic technologies in terms of materials, clotting mechanisms and current state-of-art, and discuss the challenges and opportunities associated with these technologies to help interdisciplinary advancement of the field.
2. Hemostatic biomaterials and technologies for topical (external) applications
Externally administered hemostatic treatment of an injury or lesion is applicable to simple cuts and bruises, exposed and puncture wounds, surgical lacerations, and heterogenous blunt trauma. These scenarios result in various degrees of bleeding and tissue damage, and hence the development of appropriate hemostatic materials and technologies is driven by the need to quickly staunch bleeding, absorb shed blood, cover and protect the injury, prevent contamination and provide a suitable environment for healing. These materials and strategies include topical use of tourniquets, dressings, bandages, foams, powders and gels, as well as, administering materials intra-cavitarily. A wide variety of materials and technologies have been developed, pre-clinically evaluated and clinically translated in this area as discussed in the following sections.
2.1. Tourniquets
As described previously, a blood vessel’s first natural response to injury is spasm and construction, leading to reduction in blood flow (stypsis) and facilitating the subsequent cellular and biomolecular clotting mechanisms. Therefore, a number of hemostatic materials and technologies have focused on augmenting this process by collapsing blood vessels with application of pressure to allow platelet activity, coagulation cascade and formation of a fibrin clot. Beyond applying manual pressure with fingers and palm, a widely used technology in this category is that of tourniquets. Tourniquet is essentially a circumferentially constrictive bandage that can restrict blood supply to extremities. In ancient Roman history, Galen of Pergamon (129 – 200 AD) was known to use this method for stopping blood flow and was criticized for its use by those who feared that tourniquets would increase blood loss from a wound. In the early 16th century, famous Prussian military physician Hans von Gersdorff had described tourniquet use in amputation surgery [75]. Towards the end of the 16th century, Wilhelm Fabry of German surgical science fame, reportedly also used tourniquets in amputation surgery, utilizing mechanical tightening [76]. Historically in such uses, tourniquets have been reported to present certain negative consequences, especially in the context of denying blood supply to extremities hereby causing ischemia and infarction as well as causing mechanical nerve damage. Despite this criticism, over time tourniquets have become an adapted and accepted hemostatic technology in pre-hospital management of bleeding in the military (i.e. in combat wounds), as emphasized by documents of the Tactical Combat Casualty Care (TCCC) of the US military and reviewed recently by Lakstein et al and Kragh et al [77,78]. Before being adapted as a tactical mainstay, most tourniquets were made of an improvised stick with a cloth or a silicone elastic strip. However, due to material inferiority and risks of slippage (defective fastening), in the mid 2000s significant R&D efforts were dedicated to tourniquet design and standard-of-usage. The first result was a technology named Combat Application Tourniquet (C-A-T®, North American Rescue, USA), which is essentially a composite improvisation of the ‘cloth with turning stick for tightening’ approach (aka Spanish windlass design) that can be used with minimal effort (e.g. with one hand) [79]. Continued studies on tourniquet design, ease of usage in complex time-limiting environment, patient comfort, hemostatic efficacy and reduction of tissue morbidity and mortality have led to other tourniquet technologies such as Emergency Medical Tourniquet (EMT, Delfi Medical Innovations, Canada), Special Operations Forces Tactical Tourniquet (SOF-TT), Ratcheting Medical Tourniquet (RMT, M2 Inc, USA) etc. The EMT tourniquet is formed of circumferentially usable bladder that can go around the limb, a clamp that limits the inflated portion while holding the bladder close to the limb, and an inflator bulb equipped with a connector tube and twist cap. Hence, the design is similar to a blood pressure cuff except for the clamp component. The SOF-TT tourniquet is similar in design principle to the C-A-T system (i.e. the Spanish windlass design) with an aluminum-based stick. The RMT system requires use of a ratcheting lever instead of a windlass stick for tightening the tourniquet. The usefulness of tourniquets to reduce blood loss (and hence morbidity/mortality) by simple yet efficient application of styptic pressure is responsible for its current consideration as a standard-of-care component for emergency responders in civilian trauma [80,81].
2.2. Naturally derived biomaterials for hemostatic applications
Mother Nature has been a dependable source for a wide variety of materials like cotton, collagen, gelatin, silk, elastin, fibrin etc., that have found extensive applications in biomedical areas of device coatings, tissue adhesives and sutures, tissue repair and regeneration, cell encapsulation, drug and gene delivery etc. [82]. A variety of naturally derived materials have also found significant applications in the area of hemostasis and bleeding management. Some of these materials have only absorption and passive interaction properties, while others have active biointeractions to promote hemostatic mechanisms. Absorptive and passively interactive materials impart partial hemostasis merely by wound site coverage, absorption of blood and exudates, and subsequent protection, but do not contain any specific component that promote or augment hemostasis or biologically protect from bacterial infection. On the other hand, bioactive materials and dressings are systems that adhere to the bleeding tissue to either render hemostasis-stimulating properties by themselves or by virtue of components embedded in them that facilitate hemostatic mechanisms and prevent infection. The following sections will provide a descriptive review of absorptive, passively interactive and bioactive materials derived from natural sources that have various hemostatic applications, and at the end Table 1 will provide an ‘at-a-glance’ summary of naturally derived hemostatic materials along with representative technology names, characteristic evaluations and current application status.
Table 1.
Topical and Externally Administered Hemostatic Biomaterials from Natural Sources
| Materials and Technologies |
Mechanism of Action | Example Evaluations and Characteristic Applications |
Characteristic Findings |
|---|---|---|---|
| Cotton and cellulose-based materials, e.g., - Cotton gauze - Open weave cloth gauze soaked in paraffin, balsam and olive oil (e.g. tulle dressings) - Oxidized Cellulose (OC) - Oxidized Regenerated Cellulose (ORC) etc. - Carboxymethyl cellulose hydrogel system etc. | Provides high absorption at the wound site in addition to possibly triggering the contact pathway of coagulation; Tulle dressings maintain a moist wound bed while allowing absorption of exudate to pass into a secondary dressing and also reduces adherence of the dressing to the wound bed; Cellulose fibers initiate clotting cascade through contact activation, and can also decrease the pH at the wound site leading to platelet activation and aggregation, degradation of interleukins, antimicrobial defense and scavenging of reactive oxygen species | Cotton gauze materials have been historically evaluated and used as absorbents, dressings and packing materials in managing all forms of bleeding; Several licensed cotton-based products are approved in clinical use as dressings and bandages; Tulle gauze has been evaluated as wound dressings in partial-thickness wound models in rats; Various FDA approved products like Traumastem and Surgicel are approved in clinical use as dressings | Effectively absorbs blood and fluids but can adhere to wound bed causing discomfort and hinder wound healing; Tulle dressings were found to capture granulocytes that enhance the antimicrobial effects, but caused disturbed pattern of epithelial growth due to being embedded in the wound; OC and ORC dressings evaluated in porcine femoral artery bleeding models revealed improved hemostatic efficiency compared to cotton gauze |
| Collagen- based materials and technologies, e.g., - AviteneTM: sheet, foam or powder of partial hydrochloric acid salt of purified bovine collagen - Helistat™: Collagen sponge material - Instat™: Microfibrillar bovine collagen etc. | Collagen promotes platelet adhesion, activation and aggregation and these aggregated active platelets further release pro-coagulant molecules (e.g. ADP, Ca++ etc.), as well as, allow co-localization and activation of coagulation factors on their membrane to augment thrombin generation and fibrin formation to accelerate clotting | In vitro studies have characterized blood interactions with collagen, platelet adhesion, activation and aggregation, platelet secretions; In vivo studies have characterized bleeding time and inflammatory responses, e.g. in canine spleen incision model and porcine transectional lacerations of spleen, liver and kidney; Approved for topical and surgical hemostat applications | In vitro, collagen based hemostats show significant adhesion and activation of platelets, leading to augmentation of platelet-mediated clotting mechanisms; In vivo, collagen-based hemostats showed significant reduction of bleeding time to allow control of bleeding within 2–5 min, with minimal immune reaction |
| Composite spray consisting of bovine collagen, bovine thrombin and autologous plasma e.g. CoStasis® | Collagen can adhere to injury site and wound bed to initiate adhesion, activation and aggregation of platelets; thrombin augments platelet activation and aggregation, and also augments conversion of fibrinogen to fibrin in situ to enhance clot formation | In vitro studies have characterized platelet adhesion, activation and aggregation; In vivo studies have evaluated the material in midline laparotomy model of liver and spleen in rabbits; Phase I and Phase II clinical evaluations performed in patients undergoing cardiac, hepatic, iliac and general surgery; Approved as surgical hemostat | In vitro studies have shown increased platelet adhesion, activation and aggregation within 2–5 minutes; In vivo, complete hemostasis achieved within 2 minutes or less in rabbit models even on 50% depletion of either platelets or fibrinogen; No detectable adverse effects associated with antibody production; Clinical studies showed cessation in intraoperative bleeding within 10 min in 90% of CoStasis treated patients compared to 58% of control patients |
| Gelatin based materials and technologies, e.g., - GelFoam: gelatin solid sponge - FloSeal: Matrix containing a mixture of crosslinked bovine gelatin granules and human thrombin - GRF Glue, GR-Dial etc.: Mixture of gelatin, resorcinol with formaldehyde and glutaraldehyde activator | Since gelatin is collagen-derived, it possibly causes activation and aggregation of platelets, accelerates clot formation and structurally supports the clot being formed; Facilitates coagulation cascade propagation via augmenting thrombin generation; Tamponande effect of swollen granular gelatin at wound site reduces bleeding and promotes formation of clotting matrix; For aldehyde-containing glue, protein crosslinking results in adherence of the glue to the wound tissue, while gelatin promotes hemostatic mechanisms and resorcinol provides bacteriostatic action | In vivo studies performed in bilateral ultrasound-guided percutaneous renal cryoablation (PRC) of swine kidney; In vivo studies performed in heparinized porcine liver abrasion model; Clinical studies performed in patients under cardiac and spinal surgeries; In vivo studies performed on bleeding control and tissue adherence in thermal injury and air-leak model on rat lung; Clinical trial carried out in patients in surgery for acute aortic dissection; GelFoam currently approved as topical hemostat, FloSeal approved for clinical use as a surgical sealant in emergency surgeries, Gelatin glues clinically approved as a surgical sealant to control intraoperative bleeding | Significantly reduced post-operative blood loss in porcine PRC kidneys; Stops bleeding within 2–3 minutes in patients undergoing cardiac, vascular, or spinal/orthopedic surgery; Glue adhered tightly to degenerated lung tissue surface and no air leaks observed 1 hr post operation at low intra-tracheal pressure; Deemed clinically safe method for acute aortic dissection surgery; In-hospital mortality in aortic surgery patients was reduced with GRF glue; Aldehyde containing materials are prone to adverse effects (dose dependence of activator) due to toxicity of formaldehyde |
| Alginate-based technologies, e.g., Algosteril | Negatively charged uronic acid chains of alginate sequester Ca++ which is a co-factor for platelet activation as well as several coagulation cascade reactions, and thus augments clotting mechanisms | Studies performed on human diabetic foot ulcer model, as well as, endo-nasal surgical procedures; Clinically approved for wound care dressing applications | Mean time to wound healing was significantly reduced compared to controls; Less severe and frequent bleeding incidents occurred compared to controls in surgical procedures |
| Chitosan-based materials and technologies, e.g., - TraumaStat: poly-ethylene fibers coated with chitosan and filled with precipitated silica - HemCon, ChitoFlex: bandages from lyophilized chitosan material - Celox: granular chitosan | High surface area to allow robust interaction with platelets and coagulation factors increasing the pace and strength of the resulting clot; Possible mobilization of Ca++ to augment platelet activation and coagulation factor activation for clot formation | Rigorous in vivo evaluation in porcine complex groin injury models, porcine femoral artery and vein transection hemorrhage model, porcine liver injury hemorrhage model etc.; Approved for bleeding management in civilian and military trauma | Significant reduction of blood loss in hemorrhagic models; Decreased post-compression blood loss and decreased fluid requirement; Improved survival rates in trauma models, compared to control treatment |
| Zeolite and kaolin powder based materials and technologies, e.g., QuikClot powder, QuikClot-modified gauze (Combat Gauze), Advanced Clotting Sponge, WoundStat etc. | Aluminosilicate inorganic powder material can rapidly absorb water from blood (hygroscopic action) to concentrate coagulation factors; The powder can release Ca++ in blood and can activate FXII to trigger the intrinsic coagulation pathway; The powder can possibly induce contact activation of platelets | Rigorous evaluation in porcine complex groin injury, porcine grade 4 and 5 liver injury, porcine partial nephrectomy, porcine femoral artery hemorrhage, rabbit complex groin injury, porcine and rabbit extremity hemorrhage models etc.; Approved for selective management of traumatic bleeding in civilian and military applications | Significant reduction in blood loss, robust clot formation, significant reduction in re-bleeding, significant improvement in survival rate compared to control treatments; Persistent issue of thermal damage to tissue due to the highly exothermic nature of hygroscopic action |
| Blood-derived or recombinant hemostatic factors and materials, e.g., Composite fibrin glues and adhesives (Tissel, Evicel, Vitagel etc.), Fibrinogen and thrombin mixture for in situ fibrin formation, Fibrin fleece (e.g. TachSeal), Fibrin fleece with aprotinin (e.g. TachoComb), Autologous plasma sealants (e.g. CryoSeal, Vivostat etc.) | Fibrin is the major physiological crosslinked biopolymer component of clot and therefore it is capable of hemostasis, platelet and other blood cells arrest, further activation and enhancement of coagulation mechanisms; Thrombin component can augment in situ conversion of fibrinogen into fibrin to enhance clot formation and clot mechanical stability; Components like aprotinin can prevent fibrinolysis and thereby maintain clot strength; Matrix can further contain other components like microfibrillar collagen etc. for added effect | Rigorous evaluation in rat femoral artery repair model, canine dura puncture and spinal surgery models, rat and pig non-cardiac thoracic surgery models, porcine liver injury model, porcine aortic injury model, etc. Clinical studies carried out for use as tissue sealant and hemostat in ophthalmology, hernia repair, non-cardiac thoracic surgery, fistula repair, orthopedic surgery, gastro-intestinal surgery, dental procedures etc.; Currently approved clinically as tissue sealant, tissue adhesive and hemostat in a variety of surgical procedures | Increased tissue adhesion, decreased blood loss, high hemostatic efficacy, reduced risks of re-bleeding; Presence of non-human sourced components (bovine, equine etc.) may result in various degrees of immunogenic effects |
2.2.1 Absorptive and passively interactive materials in hemostatic technologies
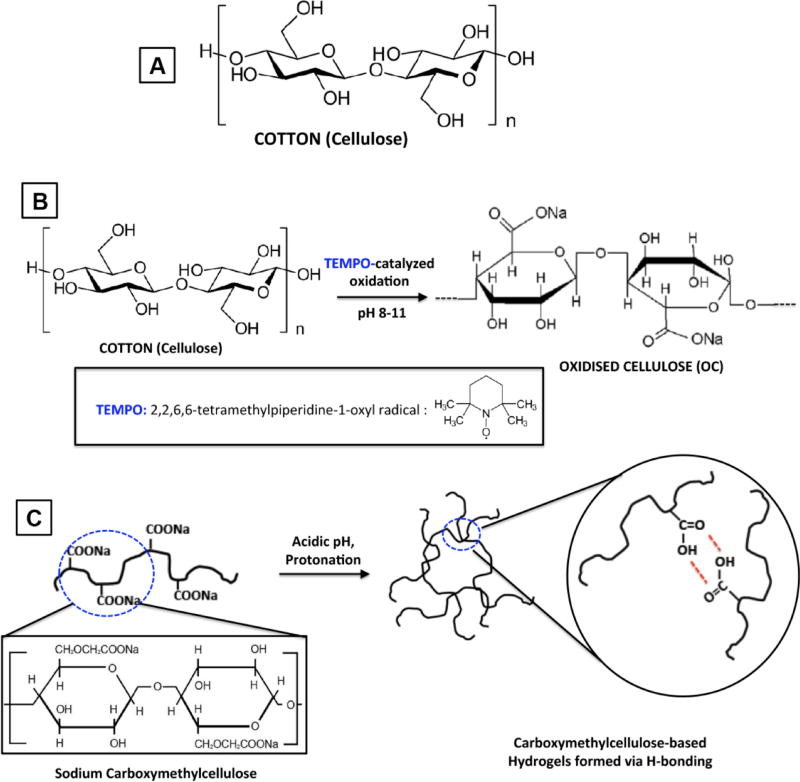
While the function of a tourniquet is to augment the vasoconstriction and stypsis at the bleeding site, the function of a bandage or dressing is to directly cover and protect the site from further contamination and tissue damage, allow exchange of gas and fluids (blood and exudate absorption and removal), potentially prevent infection and allow healing in the long term. To this end, sterile wound dressings and absorbent pads have been made of various materials including cotton gauze or cotton pad, oxidized cellulose material, nylon/rayon/polyester variants, tulle gauze, semi-permeable porous polymer membranes and foams, hydrocolloidal and hydrofiber materials, and amorphous hydrogels. Cotton is a cellulosic polymer that differs from wood cellulose by the fact that it has a much higher degree of polymerization and crystallinity than wood cellulose. Cellulose is a homopolysaccharide of glucopyranose, polymerized through β-glucosidic bonds [83,84]. Cotton contains about 90% of this cellulosic polymer along with a small amount of hydrophobic waxes and pectin, while wood contains about 40–50% cellulosic polymer. The US military standard field dressing consists of two layers of gauze wrapped over densely packed cotton, such that it can absorb a large volume of blood while the cotton strands are thought to trigger platelet activation and aggregation due to high hydrophilicity, negative surface charge and surface energy [85]. The cotton gauze or pad, when directly placed over a bleeding injury, may provide high blood absorption, partial hemostatic reaction (thrombin generation via contact pathway) and additional tamponade effect with adjunctive compression components, but they can adhere to the wound tissue which may be an issue during their subsequent removal. Nonetheless, in recent years interesting research is being focused on modulating the hemostatic characteristics of cotton dressings and gauze by modifying them with other hemostatic or strengthening materials like kaolin mineral, chitosan, viscose, rayon etc. [85,86]. Oxidized cellulose (OC) derived from cotton and oxidized regenerated cellulose (ORC) derived usually from wood pulp, refer to manipulation of the cellulose structure where primary and secondary alcohol moieties are oxidatively converted to aldehyde, ketone or carboxyl groups, which significantly changes the physico-chemical and mechanical properties of OC and ORC compared to native cellulose [87–91]. The use of OC and ORC as hemostatic dressing materials in surgery was reported around WWII [92–94], and since then its widespread application as a surgical wound dressing material has established its biocompatibility, bactericidal and hemostatic properties [84, 95–100]. The acidic pH of these oxidized cellulose materials and the negative charge are thought to impart platelet activation, aggregation and intrinsic pathway of coagulation mechanisms. These materials are also reported to be biodegradable via enzymatic (glycosidase-based) and macrophagic processes [101,102]. Recent advancement of these materials involves modification of the material matrix with other hemostatic agents like fibrin [103]. Like cotton gauze and pads, the OC and ORC materials can present the issue of adherence to the bleeding tissue, that may pose some logistical difficulties during their subsequent removal.
The adherence issue can be resolved by making materials non-adherent yet absorbent in dressings, e.g., paraffin-soaked tulle gauze and various polymer-based, hydrogel-based, hydrofiber-based and hydrocolloid-based dressings. Tulle dressings (Tulle Gras in French, meaning ‘oily tulle’) are essentially open weave cloth gauze soaked in paraffin (petroleum jelly), balsam and olive oil to impart hydrophobicity and non-adherence to bleeding wound site [104]. These materials are usually used for low exudate wounds and can be further modified by impregnating with anti-septic agents like chlorhexidine gluconate to impart infection-protection. The cotton pad, gauze and tulle materials are traditionally considered as ‘passive wound dressing’ materials since their primary function is to fully cover the wound for physical and mechanical protection, while allowing exudate absorption and fluid exchange. For high exudate situation, cellulose-based hydrogels have also been developed. For example, amorphous hydrogel materials consisting of mostly water with about 2–3% of a gel-forming polymer such as sodium carboxymethylcellulose, modified starch or sodium alginate, along with about 15–20% propylene glycol (a humectant and preservative), have been developed for wound dressing and hemostatic applications [105,106]. These dressings, along with synthetic polymer based hydrocolloid and hydrofiber systems (discussed later in this review), are highly suitable for burn wounds and necrotic or sloughy wound beds, since the water content can cool down the injury site to aid in comfort and healing. The gels are often used with a secondary dressing material like perforated or gas-permeable plastic film that prevents the water content of the hydrogel from evaporating outward but rather donated towards the wound. Similar hydrated water-donating property can be achieved with corboxymethylcellulose-based hydrogelic particles dispersed within polyurethane film or foam (for hydrocolloids) or carboxymethylcellulose-based fibers manufactured into non-woven pads or ribbons [107]. Both of these systems are useful for hemostatic action, protection and healing maintenance of heavily exudating deep wounds. Figure 2 shows representative chemical structures of common cellulose-based materials that are used in hemostatic wound-dressing applications. It is important to note here that although cotton and oxidized cellulose system are traditionally included in the ‘passive dressing’ group, they can be argued to possess a degree of bioactivity due to their ability to potentially stimulate primary (platelet activation and/or aggregation) and secondary (contact activation of intrinsic coagulation pathway) hemostatic mechanism components. However, a systematic study of the hemostatic mechanisms triggered by these materials is yet to be reported, and most studies report performance output in terms of extent of hemostasis, blood loss and tissue morbidity evaluation.
Figure 2.
Representative chemical structures of cotton (cellulose) biopolymers and its derivatives that have undergone extensive research in the development of hemostatic technologies like gauze and wound dressings.
2.2.2. Bioactive materials in hemostatic technologies
Thrombin, Fibrinogen and Fibrin
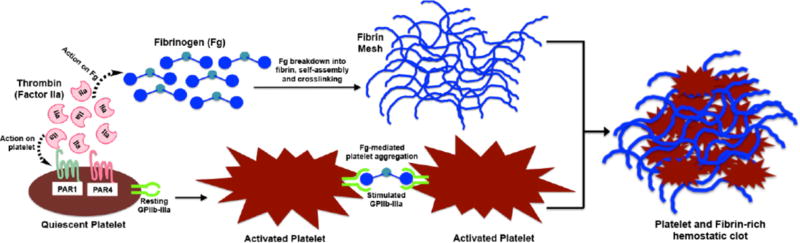
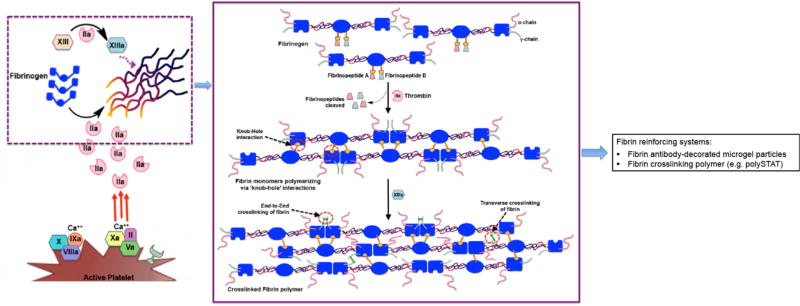
Thrombin, fibrinogen and fibrin (along with active platelets), are the critical components of the forming the hemostatic clot, as shown in Figure 3. Consequently, fibrin which is the protein formed as the end product of the coagulation cascade via reaction of thrombin on fibrinogen, has become an important bioactive material to be used in hemostatic applications. Reports from the early 20th century suggest fibrin to be superior to cotton in terms of hemostatic capacity [108–110], and since then fibrin dressings and sealants have become one of the most-studied hemostatic material, especially in the surgery field [111–115]. In early reports by Grey and Harvey, fibrin was used as pre-polymerized material processed into tamponade and plaque-like devices for treatment of bleeding in parenchymal organs [108,109]. These products demonstrated hemostatic capacity but had reduced capacities of tissue-integration since the fibrin was already pre-polymerized. Around WWII, Cronkite et al and Tedrick et al reported the usage of fibrinogen with thrombin to produce fibrin in situ in relevance to skin transplant procedures [116,117], which has led to the modern day usage of this precursor mixture for fibrin-based hemostatic products. Fibrin can be used in dry condition where animal- or human-sourced thrombin and fibrinogen are freeze-dried, processed into powder, foam, fleece, etc. and impregnated into a secondary bandage or carrier dispersant system to be applied directly onto the bleeding site [115, 118–123]. The secondary dressing can be a passive polymeric strip like silicone with an absorbent vicryl mesh backing or another bioactive but mechanically more robust material like collagen sheet [124–126]. The materials design rationale for all these systems is that upon contact with an actively bleeding site, the fibrinogen and thrombin in the dressing will interact to form fibrin in situ, leading to a hemostatic effect. In all evaluations so far, these fibrin(-generating) dressings have shown superior hemostatic performance compared to passive dressings, possibly because of their pro-coagulant bioactivity [123, 127–132]. Fibrin adhesives can also be used topically in a liquid form, where the freeze-dried fibrinogen and thrombin components are reconstituted in sterile saline immediately before administration, often through a specially manufactured dual-barrel injection device [111,112,133,134]. These liquid sealants can have high degree of tissue adherence due to physico-chemical (electrostatic, hydrogen and covalent bonding) interactions as well as mechanical integration into the tissue and can be applied to heterogenous injury sites due to their form-filling nature. The dry fibrin dressings and the liquid fibrin adhesive sealants have been reported to contain varying degrees of fibrinogen and thrombin, depending upon products from various companies and laboratories [135,136]. These compositionally different products have been tested for assessing variations in hemostatic performance, tensile strength, tissue adhesiveness etc. In some cases, no significant difference was found in the output performances, while in other cases changing the fibrinogen concentration showed some effect in mechanical strength and tissue adherence [137–140]. The fibrin-forming precursor systems (dry or liquid) can also contain other pro-coagulant materials like Factor XIII (a fibrin cross-linking transglutaminase enzyme) as well as anti-fibrinolytic agents like aprotinin and tranexamic acid (TXA, discussed in detail later in this review) to modulate clot formation speed and clot strength/stability [141–143]. Although such modifications may provide marginal benefits, the significance of such benefits have not been systematically evaluated and statistically established.
Figure 3.
Schematic of concomitant roles of thrombin and fibrin(ogen) in propagating the formation of hemostatic clots via activation of platelets and formation of fibrin mesh.
The lingering issue for a long time with such fibrinogen and thrombin based products has been the risk of immunogenicity and viral contamination, since the components are sourced from animal (bovine, porcine) or human pooled blood. Bovine thrombin preparations have been implicated in immunogenic reactions and increased risk of adverse clinical outcomes following its use in surgical procedures [144]. Fibrin based products that were mass-produced around 1944–45 to meet the needs during WWII were withdrawn in 1946 because of reports of hepatitis transmission [145]. As a progression of that, in the late 1970s many plasma-sourced fibrinogen-based products, originally clinically approved for hemostatic and surgical procedures, were recalled by the FDA. In the last decade, due to the emergence and establishment of rigorous blood screening, serological testing and pathogen (bacteria, virus) reduction/inactivation technologies, plasma-sourced products including fibrin systems have undergone a revival [110,115,145]. Through rigorous research conducted at several laboratories, including studies led by the US Army Institute of Surgical Research (USAISR), products like TachoComb and TachoSil (Nycomed, Austria) and Dry Fibrin Sealant Dressing (DFSD, developed by American Red Cross with USAISR) have become an important component in the current state-of-art toolbox for hemostatic management of traumatic and surgical bleeding [110,115,123]. Fibrin’s natural spatio-temporally regulated characteristics of biodegradation and wound healing are also responsible for its continued popularity in the milieu of hemostatic materials [146]. In recent years, there have also been reports on sourcing the fibrinogen and thrombin from salmon [147–151] as well as developing recombinant versions of such coagulation proteins [152–155]. For example, Recothrom™ (ZymoGenetic Inc, USA) is a fully recombinant human thrombin that has been clinically approved as a topical hemostatic agent to treat oozing blood and capillary bleeding and can be used in conjunction with other wound dressings. A transgenic approach has also been utilized to develop proteins like fibrinogen in the milk of other mammals for potential pharmacotherapeutic use [156]. Recombinant and transgenic versions of thrombin and fibrinogen are under pre-clinical and clinical evaluation, and can potentially resolve the availability and immunogenicity issues that are otherwise associated with human or animal plasma-resourced products. As for liquid fibrin sealants and adhesive products, although they remain a relevant material in surgery, their widespread pre-hospital use (e.g. in the battlefield) has been somewhat restricted due to time-consuming rehydration and mixing process of the lyophilized powders for in situ delivery. Furthermore, the resultant fibrin is capable of hemostasis in low volume and pressure bleeding scenarios but not heavy traumatic bleeding. In recent years, autologous fibrin generation technologies like CryoSeal (Asahi Kasei Medical Co., Tokyo, Japan) and Vivostat (Vivostat A/S, Denmark) have been reported that utilize small volumes of patient’s own plasma to generate fibrin sealant in the operating room [157]. These sealants have shown significant clinical promise as hemostatic materials during spine and sternum surgeries [158–161]. Fibrin has also be developed into ‘foam’ technologies for spray-based hemostatic use as sealants for heavy parenchymal bleeding [162–164]. Fibrin sealants have also been combined with other hemostatic materials like oxidized cellulose-based Surgicel® (Ethicon, USA) to cumulatively enhance hemostatic capability in surgical applications [165]. Due to their potential for externally injectable and space-filling properties, the liquid versions of fibrin sealant products may find use in intra-cavitary hemostatic applications.
Collagen and Gelatin
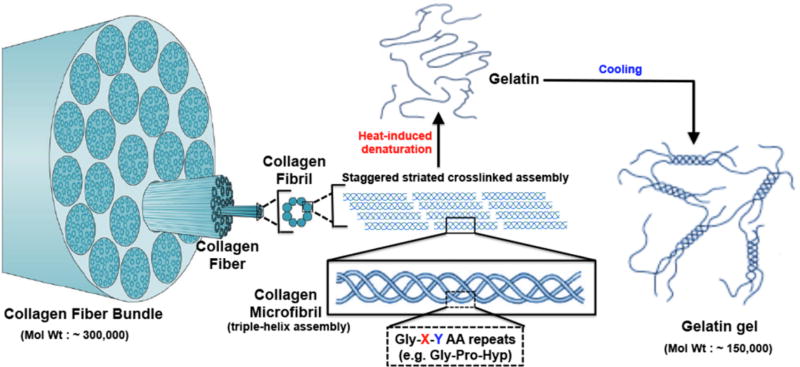
Another important bioactive material in hemostatic applications is collagen and its denatured variant, gelatin (multiscale schematic structure shown in Figure 4). Collagen is the most abundant structural protein found in the extracellular matrices of many connective tissues in mammals, making up about 25–35% of the whole-body protein content [166,167]. Fibrous collagen is present in the sub-endothelial matrix (Type III collagen mainly, with Type IV in the basement membrane) and upon injury (including endothelial disruption and denudation), collagen is exposed to circulating blood components. VWF secreted from injured endothelium and activated platelets can bind and self-associate on exposed collagen. Platelets can bind to self-associated VWF via platelet surface GPIbα receptor protein, and can also bind to collagen directly via platelet surface GPIa/IIa and GPVI receptor proteins [15,168,169]. These adhesion mechanisms of platelets at the injury site lead to further activation of localized platelets, ultimately leading to active platelet aggregation at the site (primary hemostasis) as well as augmentation of coagulation cascade events on the active platelet membrane (secondary hemostasis) [19,21]. Direct effect of collagen on coagulation factor localization and activation has also been reported, e.g. for FXII and FIX [170]. These physiological mechanisms have inspired the utilization of bio-derived collagen as a topical hemostatic material. In 1960s and 1970s, Hait et al had reported on the capability of isolated bovine collagen to adhere effectively to bleeding surfaces and promote hemostasis [171–173]. Such properties have eventually led to development of a wide variety of collagen-based products in sheet, powder, foam and fiber forms to be utilized as a topical hemostatic material, especially in surgical applications [174–179]. Representative commercially available technologies that utilize such collagen forms are Avitene™ (Davol Inc, USA), Helistat™ (Integra LifeSciences, USA), Instat™ (Ethicon, J & J, USA) etc. Collagen-based materials and technologies have also been studied for liquid form sealants. For example, a liquid composite spray consisting of microfibrillar bovine collagen, bovine thrombin and autologous plasma named CoStasis® (Cohesion Technologies, USA) was reported to render efficient hemostasis when externally administered in pre-clinical animal bleeding models (e.g. liver, spleen and kidney bleeding) and was subsequently clinically approved for surgical applications [180–183]. As with animal-sourced fibrin, animal-sourced collagen can pose immunogenic risks, and therefore research into reducing immunogenicity and infectivity has led to the popularity of a low-immunogenic collagen-derived material called gelatin [184–188]. Gelatin can be formed by thermal denaturation or irreversible hydrolysis of collagen and is extensively used in the food industry [189]. Gelatin was also been found to retain hemostatic properties like collagen [190,191]. Gelatin has been mixed into fibrin dressings to enhance the mechanical stability of the material [146]. Gelatin-based solid materials in spongy and powder form (e.g. GelFoam®, Pfizer, USA) have been evaluated for hemostatic dressings in surgical procedures but have shown limited efficacy in controlling severe bleeding [192–194]. Gelatin has also been evaluated as a material component in liquid hemostatic sealants, e.g. in products like Floseal® (Baxter, USA) where it was combined with thrombin to form a composite hemostatic sealant matrix [195]. In these composite gelatin-based sealants, the gelatin component is made up of collagen-derived gelatin cross-linked by glutaraldehyde and ground into macroscopic particles, which is mixed with bovine thrombin in a special syringe for intra-operative administration [196–199]. Another gelatin-based liquid sealant material has been reported as gelatin-resorcin-formalin glue (GRF glue), where gelatin, resorcinol, formaldehyde and glutaraldehyde are mixed in an aqueous medium for application to a bleeding site such that the aldehydes promote reaction and integration with tissue, gelatin promotes hemostatic mechanisms and resorcinol provides bacteriostatic action [200–202]. This material has been reported in extensive use in acute aortic dissection procedures but has also raised issues of formaldehyde-associated toxicity. Similar to liquid fibrin-based sealants, collagen-based and gelatin-based liquid sealants have also been evaluated for heavy hemorrhage treatment and these may also find use in intra-cavitary hemostasis applications. In recent years, there is also growing interest in recombinant collagen and gelatin materials that may potentially find application in future hemostatic technologies that pose reduced immunogenic risks [203–205].
Figure 4.
Multiscale schematic representation of fibrillar collagen structure where triple-helical microfibrils formed of Gly-X-Y amino acid repeat units assemble in staggered orientation to form collagen fibrils, which in turn further assemble to form high molecular weight collagen fibers and bundles; denaturation of collagen disrupts this assembled structures to form gelatin, which can be partly reassembled into helical components to form lower molecular weight gels.
Polysaccharide derivatives
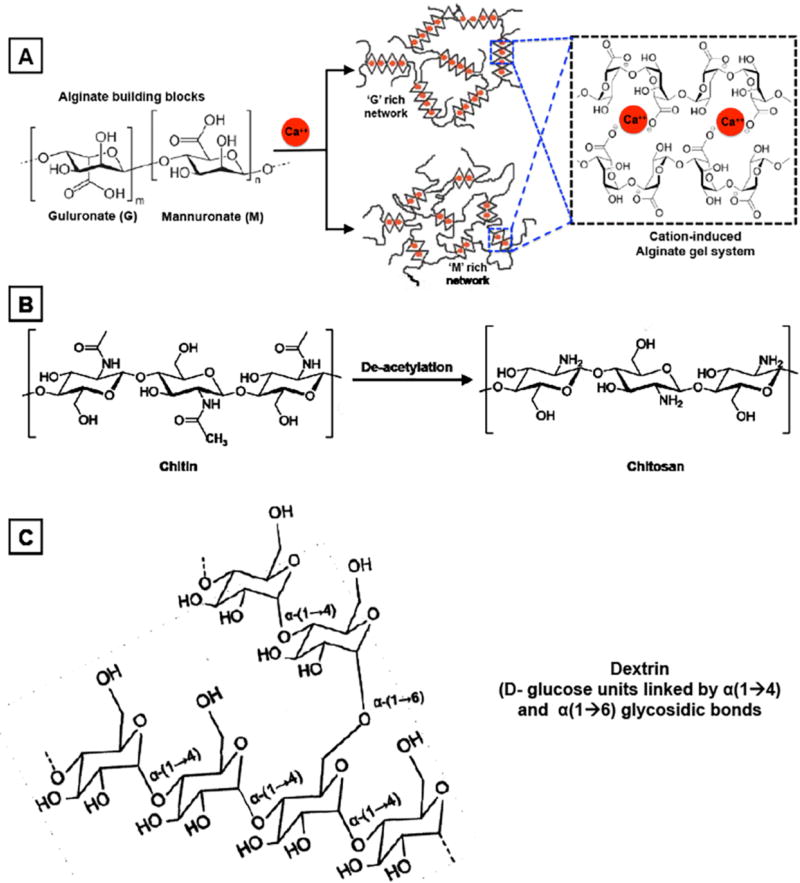
Aside from materials and technologies based on fibrin (fibrinogen + thrombin), collagen and gelatin, a third prominent category of bioactive materials in hemostatic applications is that of natural polysaccharides in native or modified forms [206]. One prominent material in this category is alginate, which is a linear polysaccharide derived from brown algae and is comprised of blocks of (1,4)-linked β-D-mannuronate (M) and α-L-guluronate (G) residues where the blocks may be consecutive G (i.e. GGGGGG), consecutive M (i.e. MMMMMM) or alternating G and M residues (i.e. GMGMGM) [207, 208]. The negatively charged uronic acid chains of alginate can gel in presence of a cation like calcium (Ca++), and this sequestration of Ca++ is thought to be responsible for the hemostatic property of these gels since Ca++ is a co-factor for platelet activation as well as several coagulation cascade reactions [209, 210]. In pre-clinical animal model studies as well as in clinical investigation, alginate-based dressings have shown superior hemostatic performance compared to traditional gauze in low-to-moderate bleeding scenarios [211–213]. The hydrogelic state of alginate dressings is suitable to keep the wound-bed moist for healing and provide comfort during dressing changes. Besides the traditional hydrogelic form, the alginate material can also be made into micro/nano particles as well as micro/nano fiber using suitable processing techniques, and these solid forms have been recently evaluated in hemostatic applications. Alginate microspheres loaded with the anti-fibrinolytic agent tranexamic acid or the pro-coagulant agent thrombin, have shown promising hemostatic capabilities in vitro and in vivo in pre-clinical models [214, 215]. Currently a large number of alginate-based wound dressings are clinically approved for surgical and hemostatic applications, e.g. Algosteril® (Johnson & Johnson, USA) and KALTOSTAT® (Conva Tec, UK). Alginate material has also been combined with other hemostatic materials (collagen, gelatin, oxidized cellulose, other polysachharides etc.) for the development of composite hemostat technologies that may provide enhanced treatment capabilities compared to mono-component systems. For example, highly absorbent collagen-alginate and gelatin-alginate dressings have shown superior wound treatment capabilities [216–218]. Alginate fibers have also been combined or co-spun with other polysachharides (e.g. chitosan) and natural polymers (e.g. gelatin), to enhance hemostatic and anti-bacterial effects [208, 219].
Along with alginate, chitosan itself has attained prominence in the area of hemostatic dressings and technologies. Chitin is a hard nitrogenous polysaccharide composed of β (1,4)-linked 2-acetamido-2-deoxy-β-D-glucose (N-acetylglucosamine), and it is the second most ubiquitous natural polysaccharide on earth (after cellulose), usually found in the exoskeleton as well as in the internal structure of invertebrates (crustaceans, shellfish etc.) [220]. Enzymatic or alkaline de-acetylation of chitin results in the linear polysaccharide chitosan, which can essentially remain as a co-polymer of N-acetylglucosamine and glucosamine depending on the degree of de-acetylation. The ability of chitosan to facilitate coagulation was reported first in the early 1980s by Malette et al [221], and this has led to extensive investigation of chitosan in various forms for hemostatic materials and technologies. The hemostatic ability of positively charged chitosan is thought to stem from its electrostatic interaction with negatively charged cell membranes of RBCs, resulting in RBC agglutination as a ‘physical’ mechanism of hemostatic plug formation [222, 223]. Chitosan has also been reported to enhance adhesion, activation and aggregation of platelets (i.e. enhancement of primary hemostatic mechanisms) as well as to be able to adsorb fibrinogen from plasma, as well as, to trigger complement activation [224–228]. The platelet stimulating effect of chitosan has been attributed to Ca++ mobilization. Chitosan is an acidic polyelectrolyte where about 50% de-acetylation of chitin results in a chitosan system with pKa of ~7.5 (i.e. soluble in water). Therefore, modulating the de-acetylation degree provides a control to modulate chitosan physico-mechanical as well as chemical properties. Chitosan can be made into films, fibers, hydrogels, lyophilized particulates and solutions, and all of these forms have demonstrated hemostatic capabilities in various studies. For example, chitosan-based layered materials with interconnected open porous structures and high specific surface area were used to develop a highly effective hemostatic dressing named HemCon® (HemCon Medical Technologies, Oregon, USA) that was extensively evaluated by USAISR investigators for management of traumatic hemorrhage, resulting in the technology being added to the metric of hemostatic strategies for US military [145, 229, 230]. This material however is rigid and inflexible, which may make it difficult to be applied over complex injuries. In further progress of this technology, the material has shown great clinical success in both military and civilian populations and a flexible version (Chitoflex®, HemCon Medical Technologies) has been developed for further evaluation [231–233]. Particulate (granulated, powder) forms of chitosan have also been utilized in developing hemostatic technologies. A prominent example is a technology named Celox® (MedTrade Products Ltd, UK), which utilizes chitosan granules and flakes to provide high contact surface area for interact with blood [234]. Upon direct administration at the wound site, blood interaction with this material is known to result in swelling of the granules for hydrogelic absorptive effect, and the chitosan contact promotes multiple mechanistic components of hemostasis. This material has been extensively evaluated for hemostatic management of heavy bleeding wounds (liver blunt trauma, arterial puncture bleeding, groin laceration etc.), showing highly effective hemostasis and reduced occurrence of re-bleeding [235–238]. Along with HemCon®, Celox® is now a prominent component of hemostatic management in pre-hospital and hospital scenarios. Chitosan has also been utilized to make hydrogel systems in situ by reacting thiol-modified chitosan with maleimide-modified ε-polylysine, and these materials have shown promising hemostatic capabilities added with tissue-adhesive properties of polylysine [239]. There are also several recent reports on developing foams with chitosan or mixture of chitosan with other materials (e.g. gelatin etc.), for potential application in hemostatic technologies [240–242]. Chitosan gauze has also been recently reported to be coated with N-(2-Hydroxypropyl)methacrylamide (HPMA) based synthetic fibrin-strengthening polymer (e.g. PolySTAT) for enhanced hemostatic action [223]. The PolySTAT material itself will be discussed in the context of fibrin-strengthening materials, later in this review. A chitosan analog, poly(-N-acetyl glucosamine), produced by a fermentation process and isolated from controlled, aseptic, microalgal cultures grown on a defined culture medium, has also gained reputation as a hemostatic material in gel form, fiber slurry and membrane form [243–245]. Thromboelastographic evaluation of this material, along with other analogous materials like chitin, chitosan etc. demonstrated reduced clot induction time when mixed with blood. The membrane form prepared by lyophilization and sheet-pressing of this material was added to a backing of standard hemostatic gauze, resulting in a hemostatic technology named Rapid Deployment Gauze (RDH, Marine Polymer Technologies USA) that was evaluated extensively for its hemostatic capability in severe traumatic bleeding [246, 247]. The hemostatic efficacy shown by this technology in pre-clinical and clinical studies has resulted in its clinical approval and its adaptation as an important component in the bleeding injury management strategies in pre-hospital scenarios. Porous polysaccharide microparticles developed from processed potato starch (dextrin) have also been studied for potential hemostatic applications, especially as a technology named TraumaDEX® (Medafor Inc., USA) that can be directly applied to bleeding wounds. When evaluated in pre-clinical bleeding models, this material and technology has demonstrated hemostatic efficacy comparable to standard gauze dressing. In similar studies, the hemostatic efficacy of TraumaDEX® was also found to have no statistical difference compared to Celox® [248, 249]. Altogether, bio-derived polysaccharides, like alginate, chitosan and dextrin, form an important materials category for hemostatic applications, and a large variety of technologies comprising of films, sheets, membranes, powders, microparticulates and liquid forms have been developed from these materials, many of which have become clinically approved systems for bleeding management in pre-hospital as well as hospital scenarios in military and civilian settings. Figure 5 shows representative chemical structures of relevant polysaccharide materials used in hemostatic dressings described above.
Figure 5.
Chemical structures of some polysaccharide polymers, namely alginate, chitosan and dextrin that have been extensively used in development of hemostatic bandages and dressings.
Minerals and zeolites
As mentioned previously, the ability of alum to facilitate hemostasis is by augmenting vasoconstriction and stypsis, which essentially is a mechanical augmentation of hemostasis. Certain microporous aluminosilicate minerals (also called zeolites) have shown the ability for bioactive augmentation of hemostatic mechanisms. The most famous material of this category is Quikclot® (Z-Medica Inc., USA), a granular zeolite technology, that has been shown to render efficient hemostasis in severe hemorrhage from arteries, liver injuries and groin injuries in multiple animal models [250–253]. The high hemostatic efficacy of this material was also demonstrated in clinical studies and this has resulted in its approval for use in combat casualties with severe bleeding [254]. The material is thought to promote rapid hemostasis via a combination of super-absorbent property (which removes aqueous volume from blood and concentrates clotting factors), platelet activation capacity and coagulation factor activation capability (contact activation of intrinsic hemostatic pathway) [255–257]. However, the interaction of this zeolite material with blood (aqueous medium) is substantially exothermic, resulting in sudden rise in local temperature, which was found to cause tissue damage and debilitation [258, 259]. In this aspect some research is being directed towards modulating the composition and structure of these zeolite minerals to retain its hemostatic property while reducing the exothermic side-effects [260–263]. In recent years, Quikclot® has been used to modify the matrix of standard gauze and sponge used in US military, resulting in a technology named Quikclot Combat Gauze (QCG) or Quikclot-Advanced Clotting Sponge (QC-ACS), that is considered to reduce the exothermic side effect of the mineral because of co-absorption by the gauze [264–266]. This new mineral-impregnated dressing material has shown similar hemostatic effect as the mineral itself and is currently an important component of tactical hemostatic strategies for the US military [267]. Another mineral based technology is WoundStat® (TraumaCare Inc., USA) that uses a smectite mineral and super-absorbent polymers [268, 269]. This technology has shown highly efficient hemostasis in topical treatment of arterial bleeding and had gained much popularity as a field hemostat in US military [270, 271]. However, further safety studies on the material revealed several systemic side effects, including vascular endothelial injury, transmural damage, systemic thrombotic and embolic risks, due to microscopic residues of WoundStat remaining in the wound and blood vessels [233, 271]. This has resulted in the military dropping the usage of this material and replacing it with the QCG technology mentioned previously.
2.3. Synthetically derived hemostatic materials
While a large volume of research has been conducted on naturally derived materials for developing bleeding management technologies, a small number of synthetically derived systems, especially certain polymers and peptides, have also been developed and evaluated for hemostatic applications. One such polymer category is that of basic poly(amino acids) like polylysine. During the 1950s DeVries et al reported that basic poly(amino acids) like polylysine, polyarginine etc. augment generation of thrombin and fibrin and also retard fibrinolysis [272–274]. It was postulated that the cationic nature of polylysine facilitates complexation and activation of certain coagulation factors [275]. This kind of report has possibly guided the use of polylysine as a cationic polyeletrolyte to be mixed with chitosan for hemostatic applications, but stand-alone polylysine based technologies have not undergone much development possibly because of biocompatibility and cytotoxicity issues of cationic polymers [276]. Poly(alkylene oxides), e.g. poly (ethylene oxide) (PEO) and poly(propylene oxide) (PPO), have been investigated as hemostatic materials since they are already well-established biocompatible synthetic polymers in biomaterials applications. For example, Wang et al and Wellisz et al have reported on the hemostatic capability of a PEO-PPO-PEO block copolymer based waxy material (now marketed as Ostene®, Baxter USA) to render hemostasis in orthopedic surgeries [277, 278]. This material acts much like natural bone wax (a mixture of beeswax with paraffin or petroleum jelly) in the context of facilitating hemostasis by a tamponade mechanical effect rather than a biochemical augmentation of coagulation mechanisms. A similar block copolymer made of poly(ethylene glycol)-b-poly(dihydroxyacetone) has also been reported recently by Spector et al. to have promising mechanical hemostatic properties [279]. A mixture of tetra-succinimidyl-derivatized and tetra-thiol-derivatized poly(ethylene glycol) has been recently evaluated as a hydrogel material for hemostatic liquid sealant [280]. In these studies, it was shown that this material can crosslink with tissues and is capable of rendering a mechanical hemostat effect by virtue of sealing a puncture hole in a rabbit artery bleed model. Several successive evaluations in pre-clinical models and in clinical trails have led to approval of this material as a surgical sealant named CoSeal® (Baxter, USA) [281, 282]. A similar material has been developed by co-polymerizing poly(ethylene glycol) with poly(α-hydroxy acid) diacrylate, and after evaluation in vitro and in vivo, this material is currently marketed as AdvaSeal® (Ethicon Inc., USA). Thus, poly(ethyelene oxide) based synthetic external hemostatic sealants have undergone considerable clinical translation, especially in surgical bleeding applications.
Another class of synthetic polymers that has gained clinical significance in tissue sealant applications is poly(cyanoacrylates). This class of polymers was reported to have excellent tissue-adhesive properties via polar interactions with the tissue and was therefore used as a sutureless tissue sealant as early as the 1940s. Synthetic cyanoacrylates (e.g. 2-octyl cyanoacrylate) under the product name Dermabond® (Ethicon Inc., New Jersey, USA), was approved in the 1990s by the FDA for skin closure. Since then, this class of tissue sealant has been widely used for surgical wound closure and hemostat applications [283–287]. Long chain poly(cyanoacrylates) sealants have shown reduced tissue toxicity and have progressed into clinically approved technologies like Histoacryl® (TissueSeal LLC, USA) and GLUture® (World Precision Instruments) for topical applications in surgeries [287, 288]. Cyanoacrylate based tissue sealants have also been combined with tourniquet-based procedures to evaluate their ability to staunch bleeding from larger hemorrhagic injuries [289]. It should be noted that cyanoacrylates technically do not possess inherent ‘hemostatic’ property in the classical sense, but rather their mode of action is through physical sealing, mechanical barrier and wound closure. Several other well-known polymeric biomaterials used in biomedical devices like sutures, contact lenses, coatings, drug delivery systems etc. have been investigated for hemostatic applications. For example, poly(glycolic acid) or PGA is an established biomaterial that is used in a variety of clinically approved bioerodible devices like sutures and implants, and this polymer has been used to develop a hemostatic felt dressing. This felt (Soft PGA Felt, Aventis Behring, Germany) was evaluated in surgical hemostatic treatment of liver resection surgeries in patients, showing promising results as a hemostatic technology option combined with fibrin glue [290]. Poly(2-hydroxyethyl methacrylate) or poly-HEMA is an established biomaterial which had originally become known for its use in contact lens devices. Porous particles made from this polymer has been reported to have the capability of reducing blood loss in endovascular occlusion surgeries [291]. Poly(acrylic acid) is another synthetic polymer biomaterial which was used to develop a hemostatic technology named Feracryl® by Russian scientists in the 1980s [292]. This material was developed by reaction of acrylic acid with Mohr’s salt in an aqueous medium and had a small percentage of iron(III) coordinated with the polymer. In evaluation in vitro and in vivo this material was found to be non-toxic with promising hemostatic ability and this has led to development of several acrylic acid based hemostatic technologies in Russia but not much has been reported elsewhere globally. Carr et al have reported on a microporous poly(acrylamide) gel in the development of a super-absorbent hemostatic technology named BioHemostat® (Hemodyne Inc., USA), which showed great promise in the treatment of heavy bleeding injuries [293]. The hemostatic ability of this material was attributed to its capacity of absorbing high amount of fluids from the wound and expanding to exert a mechanical tamponade effect on the wound. Casey et al. have recently reported on the development of a series of cationic acrylamide hydrogels that demonstrated the capability of activating coagulation factors that may aid in clot formation and hemostasis [294]. Another tissue sealant technology based on albumin-glutaraldehyde (e.g. BioGlue®, CryoLife Inc., Georgia, USA), is available in the United States for surgical adhesive applications in open surgical repair of large vessels (such as aorta, femoral and carotid arteries) [295]. In recent years, mussel-inspired biomimetic materials design approaches have led to several interesting novel classes of tissue-adhesive synthetic polymers, including citric-acid based, catechol based and hyperbranched poly(aminoester) based systems, which may find potential use as hemostats and sealants [296–298]. These studies and reports establish the promise of tissue-interactive synthetic polymeric systems for the engineering of hemostatic technologies, that can staunch bleeding through mechanical as well as biochemical mechanisms. Recently, several research groups have reported on a synthetic peptide termed RADA16-I (essentially a 4-mer repeat of Arginine-Alaninine-Aspartate-Alanine) that can self-assemble into supramolecular structures and can gel in presence of blood to arrest blood cells and promote a coagulatory effect [299–302]. The molecular mechanism of such hemostatic action of this synthetic peptide material is thought to be more due to gelation and tissue adhesion, and the presence of any additional biochemical involvement in the coagulation cascade is currently unclear. Besides being used directly as absorbent dressing, sealant or tamponade systems, synthetic polymers are also used as scaffold or backing components in many hemostat technologies. Examples of these were previously described in technologies where polyurethane films and foams were used as scaffold material for dressings. In other composite applications, polymers like polypropylene and polyurethane have been used as scaffold materials for impregnating chitosan material [303, 304]. Dressings made of polymer films, foams and amorphous hydrogels are considered to be ‘interactive wound dressings’ as they are mostly transparent to allow monitoring of wound status, while maintaining non-adherent and absorbent properties. The polymer film wound dressings are usually made of semi-permeable polyurethane membrane with an acrylic adhesive backing, while he polymer foam wound dressings are formed of soft, open cell, hydrophobic polyurethane foam sheet, approximately 6–8mm thick, with a high capacity of exudate absorption [305]. The film systems are usually used for low exudate wounds while the foam systems can be used for heavy bleeding and high exudate wounds. Polymer-based semi-permeable dressings and foams also have the advantage of preventing bacteria and fluid transfer at the injury site while allowing regulated transfer of air and moisture, such that the wound bed can be kept optimally moist to aid comfort and healing. However, if the wound site produces high amount of exudate, its build-up may negatively affect the adjoining tissue (e.g. maceration). Altogether, a variety of synthetic polymeric biomaterials have become stand-alone or integrative components of many hemostatic materials and technologies, as they provide advantages of customizing chemistry for stimulating pro-coagulant mechanisms while reducing immunogenicity risks otherwise associated with some of the bio-derived materials.
2.4. Compression bandage technologies
The aspect of applying pressure to cause ‘stypsis’ and the aspect of applying bandages and dressings (made from natural or synthetic biomaterials) to allow absorptive and interactive mechanisms at the bleeding site, have been integrated to result in compression bandage technologies. The simplest versions of such technologies consist of cotton gauze pads placed with manual pressure over the bleeding wound, but this material as well as procedure is unreliable due to variations in severity of injuries and external manual pressure needed for efficient hemostatic compression [48, 306]. A marginal improvement of this technology is found in the Army Field Bandage where a thick pad of cotton is contained within layers of gauze and tying straps are attached to this material to help fastening and tightening of pressure [307]. A further improvement is found in the emergency field bandage (aka ‘the Israeli bandage’, First Care Products) originally designed and developed by Israeli military serviceman Ben Bar-Natan, which consists of an elastic bandage sewn over a sterile non-adherent absorbent pad material. The bandage is equipped with a pressure bar through which the wrapping material may be inserted, reversed in direction and tightened over the bleeding site to apply tourniquet-like compression [308]. Unlike the direct placement of gauze or gauze-wrapped cotton pad on the bleeding injury, which can make removal of the bandage material cumbersome and risky, the utilization of non-adherent material along with the compression bar made this bandage design a welcome improvement in management of traumatic wounds in the military. Another military-tested compression bandage is the CinchTight bandage system (H&H Medical Corporation, USA), which is available in a variety of sizes and consists of long elastic wrapping sewn over a sterile non-adherent absorbent pad and equipped with a metal hook (instead of the pressure bar found in the Israeli bandage) through which the elastic wrap can be manipulated to wrap-around and tighten for compressive force on the wound site. Another relatively recent technology in the mix of compression bandages is the Elastic Adhesive (ELAD) bandage developed by Dr. Sody Naimer in Israel [309]. In this design, the non-adherent sterile absorbent pad is laminated over by a long self-adherent polyethylene strip that allows for flexible wrapping around various tissue/organ morphologies while the transparency of the strip allows for direct observation of the dressing-covered wound site to ensure that bleeding has been reduced. In all the above designs, the main purpose is to provide a combination of compression plus sterile coverage of the wound that can reduce bleeding to some extent, and this reduction may be sufficient to reduce the tissue morbidity or mortality risk until the patient can be attended to in a medical facility. The elastic wrap-around materials in many cases are proprietary in composition, but it is apparent that most of these materials are possibly latex or nylon or Coban™ (3M, USA) type non-woven elastic composites or polyethylene-based stretchable polymers. Table 2 provides an ‘at-a-glance’ summary of synthetically derived hemostatic biomaterials along with representative technology names, characteristic evaluations and current application status.
Table 2.
Topical and Externally Administered Hemostatic Biomaterials from Synthetic Sources
| Materials and Technologies | Mechanism of Action | Example Evaluations and Characteristic Applications |
Characteristic Findings |
|---|---|---|---|
| Polyurethane-based systems, e.g., - semi-permeable polyurethane membrane with an acrylic adhesive backing in wound dressings (e.g. Bioclusive, Mefilm, OpSite, Flexigrid, Tegaderm etc.) - soft, open cell, hydrophobic, polyurethane foam sheet for wound dressings (e.g. Allevyn, Lyofoam, Tielle etc.) | Polyurethane material can create a moist wound environment owing to their permeability to air and water vapor and impermeability to fluids and bacteria; Moisture and Oxygen are transmitted in addition to providing thermal insulation to the wound; Promote autolytic debridement; Many polyurethane dressings are highly absorbent due to the presence of hydrophilic contact surface and microporous foam combined with hydrophobic backing | Polyurethane film dressings evaluated clinically for split-thickness skin grafting; Polyurethane foam based dressings evaluated clinically for management of leg ulcers and other exudative wounds; Comparative clinical data are under-reported and randomized clinical trials across the various technologies are lacking, but several polyurethane-based dressings remain currently clinically approved | Protects wound from bacteria, foreign debris and shearing injuries; High absorptive capacity of microporous polyurethane foams allow for significant removal of exudate; Polyurethane dressings are easy to remove, allowing convenient changing of wound dressings without injury; |
| Polylysine based systems, e.g., - poly(amino acid) polymer of lysine - Hydrogels composed of ε-polylysine grafted unto functionalized chitosan - Hydrogels of ε-polylysine grafted polyethylene glycol | Cationic polylysine can complex and activate coagulation factors, increase thrombin and fibrin generation, and retards fibrinolysis | In vitro fibrinolytic activity tested on human plasma, coagulation factor activation, Cytotoxicity (MTT, LDH); In vivo hemostatic ability assays was evaluated by assessing total blood loss in a rat liver injury model; Polylysine materials also tested for evaluation of wound closure on the back of male Sprague-Dawley rats; No stand-alone polylysine technology for externally administered hemostatic application exists, but the material has been investigated as a co-polymer component (e.g. with alginate). | Polylysine has shown conflicting results of resisting fibrinolysis, but also acting as a co-factor to activate plasminogen that can cause fibrinolysis; Polylysine-based materials have shown significantly reduced blood loss compared to untreated liver injury; Polylysine materials showed greater capabilities for wound closure compared to suture, fibrin glue and cytotoxic cyanoacrylate treatment after 7 and 14 days post treatment; Evidence exists for polylysine-induced activation of FVII for increased thrombin and fibrin generation; Polylysine may impart cytotoxic effects dependent on polymer concentration, incubation times and charge density |
| Water soluble Polyethylene oxide and polypropylene oxide (PEO-PPO-PEO) block copolymer for bone hemostasis, e.g. Ostene | Facilitate hemostasis by tamponade mechanical effect | Evaluated for soft tissue response in paravertebral muscles incision in rabbits; Evaluated in Femur defect model hemostasis in rabbits; Clinically approved for orthopedic application | Ostene showed no fibrous response at the bone defect site 2 weeks after implantation in animal models; No adverse effects of Ostene after 4 and 8 weeks with evidence of new bone formation in animal models |
| Poly(ethylene glycol)-b-poly(dihydroxyacetone) MPEG-pDHA block copolymer | Facilitate hemostasis by tamponade mechanical effect; Can be resorbed and metabolized in the body | In vivo evaluation for blood loss and bleeding time from partial and survival hepatectomy in rodent models; Only pre-clinical evaluation reported | Significantly reduced bleeding time and blood loss compared to saline treatment; No abnormal inflammatory response was detected for MPEG-pDHA compared to saline control |
| Gels formed from reaction of tetra-succinimidyl and tetra-thiol-derivatized polyethylene glycol (PEG), e.g. CoSeal | Tetra-succinimidyl and tetra-thiol-derivatized polyethylene glycol (PEG) can crosslink with tissue, as well as, the hydrogel can form a mechanical barrier to prevent bleeding at the wound site. | In vitro adhesion capability evaluated in biological tissue, e.g. porcine carotid artery model; In vivo blood loss and compatibility with biological tissue evaluated in rabbit carotid artery models; In vivo suture line bleeding evaluated in canine iliac artery PTFE graft model; Clinical trials performed on patients undergoing placement of peripheral vascular grafts to seal anastomotic suture lines; Clinically approved as a surgical sealant material | Favorable adhesion to collagen membrane, grafts and carotid artery; In rabbit and canine models, Coseal caused rapid cessation in bleeding, reduced blood loss, reduced time to hemostasis and showed compatibility with biological tissue; Clinical studies showed successful sealing within 10 minutes across surgical groups (>80% of patients), and immediate sealing in 32% of patients; |
| Co-polymer of poly(ethylene glycol) with poly(α-hydroxy acid) diacrylate, e.g. AdvaSeal, FocalSeal etc. | The photopolymerized hydrogel adheres to the wound tissue and mechanically seals the wound | In vivo hemostatic capability evaluated in canine models; Clinical studies showed promising results in sealing of pulmonary leaks; Limited clinical use as surgical sealant | In vivo canine models showed improved cessation of blood flow; Clinical studies showed sealing of pulmonary air leaks immediately; Hydrogel swelling may induce unwanted pressure on surrounding tissue |
| Cyanoacrylate base materials and technologies, e.g., - Synthetic 2-octyl cyanoacrylate glue, e.g. Dermabond - Synthetic n-butyl-2- cyanoacrylate glue, e.g. Histoacryl - Synthetic cyanoacrylate glue containing 60% 2-octyl and 40% N-butyl cyanoacrylate, e.g. GLUture etc. | Cyanoacrylate materials can adhere to wound tissue by polymerization upon contact with tissue fluid, to form a glue effect and also an antimicrobial barrier that closes the wound for protection; Resorbable material | In vitro evaluation performed for antimicrobial activity; In vivo studies evaluated inflammation, micro-calcification and encrustation in porcine and rabbit transverse cystotomy models; In vivo time to hemostasis evaluated in porcine epistasis model; Tissue adhesion studied in small skin lacerations (<5 cm), declawing and tail docking procedures in animals; Clinical trial performed on warfarin treated patients under oral surgery; Cyanoacrylate-based systems clinically approved for surgical glue for topical and local hemostasis | Cyanoacrylate material provided antimicrobial barrier with 99% efficacy for at least 72 hours; Sufficiently seals large cystotomy, with similar inflammatory response as normal suture, and reabsorbed after 4 weeks, preventing encrustation; Decreased time to complete and sustained hemostasis observed in porcine epistasis models; |
| Woven textile of polyglycolic acid (PGA) as felt material, e.g. Neoveil | Compresses the edge of the wound and acts as a sealant; Resorbable material | Clinical trial performed on patients requiring repair of a lacerated liver and prevention of biliary leakage; Selective clinical use as a dressing in combination with other materials | PGA felt used with a matrix of fibrin glue rendered complete hemostasis and wound sealing |
| Acrylate-based materials and technologies, e.g., - Poly-2-hydroxy ethyl methacrylate - Aqueous solution (1%) of iron salt of polyacrylic acid (PAA) containing 0.05—2.5% Fe, e.g. Feracryl | Acrylate hydrogels can swell to allow adsorption of protein, absorption of blood components and assisting in thrombus formation and clot stabilization in specific applications | In vitro studies done on effect on microbial samples from patients with purulent septic diseases; In vivo studies done on bleeding time analysis in rat models with skin, liver or spleen wounds; Clinical studies done on patients with dental pathologies; Clinical trials done on children treated for non-operable hemangiomas of the liver and angiodysplasias; Widespread clinical use not reported | Poly HEMA hydrogel embolization found effective as preoperational treatment to prevent hemorrhage during surgery and provide stable endovascular occlusion for safe removal of tumor; Acrylate gels can be modified with antibacterial agents like metronidazole and dioxidine for added benefit; |
| Flexible outer layer of electrospun ethylene-vinyl acetate copolymer surrounding a hydrophilic absorbent layer of polyacrylic acid: BioHemostat | When exposed to fluids at the wound site, the material expands and swells, filling the wound and stopping bleeding while developing significant pressure within the confined space to enhance clotting; | In vitro evaluation of fluid absorption in device, expansion, swelling and pressure development from device; No in vivo evaluation reported | BioHemostat absorbs and retains up to 140 times the weight of the aqueous component in blood, expands to occupy up to 8 times its original volume and is capable of developing significant pressure (90 mm Hg in 180 seconds) within a confined space to staunch bleeding; Addition of other hemostatic agents like thrombin to the dressing can be utilized to accelerate clot formation |
| Bovine serum albumin and glutaraldehyde based tissue sealant: e.g. BioGlue | Sealant formed from reaction of amine groups of albumin to extracellular matrix proteins of the target tissue by the crosslinking action of glutaraldehyde; Resorbable material | In vivo evaluation performed regarding blood loss, inflammatory response and absorption of device in vascular anastomoses models in sheep; Clinical trial performed regarding apical hemostasis in patients undergoing transapical aortic valve implantation (TAVI); Clinically approved as surgical adhesive material | Material found to reduce post-surgical bleeding, rate of blood loss and total blood loss, and remained inert after 3 months with minimal inflammation; Material found to effectively seal myocardial holes of polypropylene stitches at the heart apex during TAVI; Some late wound healing complications reported in patients, characterized by wound swelling and secretion, possibly due to cytotoxic effect of glutaraldehyde; |
| Materials based on 4-mer repeat of synthetic peptide Arginine-Alanine-Aspartate-Alanine: RADA-16 | Peptide self-assembles into β-sheet based supramolecular structures that gel in the presence of blood to arrest blood cells and promote coagulatory effect | In vitro clotting characterization performed using anticoagulated whole human blood; In vivo studies performed in hemostasis in rat and pig skin punch models, rat liver injury models, and rat spinal chord transection models; Currently in pre-clinical development | Effect of the material on promoting hemostasis is correlated to rapid self-assembly and gelation to render high absorption of blood fluid, concentrating of coagulation factors and entrapment of blood cellular components; No evidence on direct promotion of coagulatory biochemical reactions has been reported yet; All animal model-based studies have shown rapid hemostasis through a gel formation within minutes of application |
2.5. Combination systems and advanced technologies
The biggest challenge in external management of bleeding is the treatment of severely bleeding wounds where the injury is heterogenous and the hemorrhage is often non-compressible. This is significant in both civilian and military trauma cases where severe exsanguination results in high mortality [310]. Therefore, while a staggering volume of research endeavors, technology development and clinical translation have been dedicated to development of hemostatic systems over the past century, the current focus is on efficient utilization of these systems as well as development of new systems to specifically treat non-compressible (often intra-cavitary) hemorrhage. This has not only prompted research in new materials and technologies, but also establishment of suitable pre-clinical animal models where the efficacies of these technologies can be evaluated and compared in a standardized way in terms of clotting mechanisms, time-to-clot, clot strength and stability, and survival (damage control resuscitation) [45, 311, 312]. The recent comparison studies using such established animal models have provided interesting insight in the pros and cons of various hemostatic materials. For example, in a comparison of WoundStat® (mineral powder) verus Celox® (chitosan powder) using a heavy-bleeding arteriotomy model in pigs, it was found that WoundStat® conferred better hemostasis, but Celox® conferred better tissue-compatibility [233]. This can have potential impact on using these materials to treat heavy-bleeding intra-cavitary wounds where the material is supposed to interact with heterogenous tissue beds and would either biodegrade (e.g. for chitosan) or need to be removed (e.g. for mineral) over time. In another study comparing QCG versus Celox® versus chitosan-impregnated gauze versus HemCon® in porcine bleeding model, the QCG gauze was shown to have a superior performance than the rest, and it was also evident that such technologies used by a trained medical personnel (e.g. military trained in tactical combat casualty care or TCCC course) results in improved hemostasis compared to non-medical personnel [313]. Combining two different hemostatic materials/mechanisms may provide a cumulative advantage over using each material by itself. This is evident in the superior performance of DFSD (fibrinogen and thrombin impregnated dressing on polymer mesh backing) and QCG (Quikclot zeolite impregnated gauze) compared to fibrin only or Quikclot® zeolite only [123, 255]. Another combination material example is a technology named TraumaStat® (OreMedix, USA), where chitosan was combined with silica and polyethylene to form a flexible gauze that integrated the tissue adhesiveness of chitosan with the coagulation factor activating capacity (contact activation) of silica, to render superior hemostatic capability compared to just chitosan-based systems [314, 315].
3. Hemostatic materials and technologies for intra-cavitary applications
Hemostatic materials in gel, particulate, pelleted and other flexible forms may be more useful in treating certain highly lethal hemorrhagic injuries like deep ballistic puncture wounds and intra-cavitary blunt injuries where the standard-of-care is to rapidly administer ‘packing and absorbent’ material into the injury volume to staunch bleeding. The benefit of such ‘space-filling’ hemostatic materials was demonstrated by Kheirabadi et al using fibrin sealant foam and FloSeal® in a liver hemorrhage model (bleeding within abdominal cavity) model [316]. To this end, in recent years several interesting biomaterials and advanced technologies for intra-cavitary (or complex wound) hemostatic applications have been reported. One such technology is made of plant-derived cellulose sponge pellets coated with chitosan that are compressed as a starting form and upon absorption of blood (aqueous medium), expand axially within a very short period of time (~ 20 seconds) [317]. As a result this material is capable of combining pro-coagulant (because of chitosan), absorptive (because of cellulose) and tamponade (because of expanding) effects, to render efficient hemostasis. After rigorous evaluation, this technology has recently undergone FDA-approval under the name XSTAT® (RevMedX, Orgeon, USA) [318]. Self-expanding foams made of in situ forming polyurethane mixtures have also been reported in the context of evaluating hemostatic capability in heavy bleeding wounds [319, 320]. In another recent approach, an algae-based gel product developed by Landolina et al from alginate and other polysachharides was shown to rapidly staunch bleeding in open wounds in soft tissue and is being currently marketed in the veterinary as well as prospective human trauma hemostat uses under the names Vetigel® and Traumagel® (Cresilon Inc. formerly Suneris Inc., USA) [321]. Translation of this material in hemostatic treatment of bleeding injuries in civilian and military population would require further testing in established animal models, along with determination of the biocompatibility and spatio-temporal fate of the material at the injury site.
4. Hemostatic materials and technologies for intravenous applications
As emphasized previously, bleeding can cause a variety of debilitating health issues, especially in traumatic and surgical settings. The majority of hemorrhage-related deaths in the military and civilian populations occur from non-compressible and heavy intra-cavitary hemorrhage that cannot be completely managed by tourniquets, external dressings and bandages [1, 316]. Certain dressings and bandages have undergone advancement in materials components as well as technology designs to improve the treatment options for such heavy bleeding, especially in intra-cavitary applications, as reviewed in previous sections. However, intravenous fluid resuscitation and subsequent blood component transfusion currently remains the primary option for improving survival of civilian and battlefield casualties with heavy truncal and junctional hemorrhage and traumatic brain injuries [322–324]. Unfortunately, such procedures are often logistically challenging in austere pre-hospital (e.g. first responder) conditions, as well as, in medical facilities without access to blood components, due to the limitations of blood availability and portability, lack of appropriately trained personnel, high contamination risk, short shelf-life etc. Especially for platelets, the major hemostatic cellular component of blood, these issues present severe functional and logistical barriers, and therefore the development of intravenously transfusable semi-synthetic or synthetic platelet analogues that can provide platelet’s hemostatic functions without its biologic issues, has become an exciting area of biomaterials research. In parallel, synthetic mimicry of certain other non-cellular coagulatory components of blood have also gained momentum in recent years. All of these materials and technologies are to be intravenously administered and are currently in the pre-clinical evaluation stage, with anticipated translation not only for pre-hospital management of civilian and military trauma but also for transfusion applications in surgery and hematology/oncology areas in hospital settings.
4.1. Naturally derived intravascular pro-coagulant materials
As described in the introduction section, overall hemostasis is a combined output of platelet plug formation (primary hemostasis) and coagulation cascade leading to crosslinked fibrin biopolymer formation (secondary hemostasis) at the bleeding site. Therefore, while one arm of materials research has focused on technologies that leverage and mimic platelet functions, the other arm has focused on various molecular aspects of modulating the initiation, propagation or stabilization of the coagulation cascade components and final fibrin clot. In modulating the molecular aspects of the coagulation cascade, the most obvious approach is to intravenously administer fibrinogen as well as coagulation factors. In a bleeding patient, fibrinogen levels can be replenished by directly administering fibrinogen concentrate, or cryoprecipitate (a mixed product containing fibrinogen, factor VIII, von Willebrand factor, and FXIII). These fibrinogen products have been extensively studied in pre-clinical animal models as well as in selected clinical trials and significant benefit of these products in hemostatic management of traumatic and surgical bleeding have been demonstrated [57–59, 325]. Further clinical studies are needed to establish the short-term and long-term systemic safety of such products. In the field of coagulation factors, FVIII and FIX concentrates isolated from pooled donor plasma became commercially available in the 1970s for the treatment of hemophilia, but were found to transmit the risk of viral hepatitis [155, 326]. In transfusion medicine, the current clinical mainstay of factor replenishment is the use of fresh frozen plasma (FFP), which is rich in multiple coagulation factors (prothrombin or FII, FV, FVII, FIX and FX) [327–329]. However, FFP also suffers from issues of availability, pathogenic contamination risks and immunologic side-effect risks [155, 330, 331]. These issues have led to the research and development of a variety of coagulation factors using recombinant technologies during the past two decades [155, 332–336]. For example, following the cloning of the human FVIII mRNA in 1984, a variety of recombinant FVIII proteins have been developed, such as Kogenate® (Bayer), Recombinate® (Baxter), Refacto® (Wyeth Pharmaceuticals), and ADVATE® (Baxter) [337, 338]. All these recombinant FVIII preparations have demonstrated significant hemostatic efficacy in treating bleeding risks in patients with hemophilia A. Similarly, the cloning of FIX from a human liver cDNA library has led to development of recombinant FIX (e.g. BeneFix®, Wyeth Pharmaceuticals) for bleeding risk treatment in haemophilia B patients [339–341]. It is important to note here that the use of Factor VIII and Factor IX have been reported to induce development of inhibitory alloantibodies in at least 30% of treated patients after multiple prophylactic dosages, and this has led to the development of one of the most notable recombinant coagulation factor product, the recombinant Factor VIIa (FVIIa) [332, 333]. As was depicted in Figure 1, this coagulation factor can interact with Tissue Factor to generate moderate amounts of thrombin (Factor IIa) in the extrinsic pathway of coagulation cascade. FVIIa (and thrombin) can also reportedly activate FIX to FIXa, which subsequently takes part in activation and complexation with other coagulation factors (FXa and FVIIIa) on the surface of activated platelet membrane in presence of Ca++ to form the prothrombinase (FXa + FVa + FII) complex, further amplifying the generation of thrombin (Figure 1) via the common pathway of coagulation [342, 343]. Thrombin further activates localized platelets and also breaks down fibrinogen to form fibrin, the final coagulation cascade product. Therefore, the central theme in utilization of recombinant or plasma-derived FVIIa to improve hemostasis is to augment the generation of thrombin while bypassing the tenase amplification step, and this has proven highly beneficial in hemostatic treatment of patients with congenital coagulation factor defects, hematologic malignancies, thrombocytopenia and traumatic bleeding. FVII was originally cloned in the late 1980s from human liver and Hep G2 cells and has subsequently led to the development of the recombinant FVIIa product NovoSeven® (Novo Nordisk) [344–346]. This product was originally clinically approved to treat hemophilia patients where lack of FVIII (Hemophilia A) or FIX (Hemophilia B) affects the intrinsic pathway of thrombin generation, and therefore intravenous administration of FVIIa was postulated to compensate for this by generating thrombin via the tissue factor (extrinsic) pathway [347, 348]. NovoSeven® has also been recently approved for use in patients with Glanzmann Thrombasthenia where patient’s platelets have qualitative or quantitative deficiencies that affect their ability to bind to fibrinogen via integrin GPIIb/IIIa. For patients with thrombocytopenia (platelet deficiencies), it has been demonstrated that combining FVIIa therapy with platelet transfusion may provide additive benefit, since the common pathway of coagulation cascade that leads to amplified thrombin generation and fibrin formation is facilitated by the presence of activated platelet membrane [333]. It is important to note here that the hemostatic promise shown by intravenous FVIIa has also led to its incorporation in externally applicable dressings like gelatin sponge or microporous polysaccharide spheres, to improve the overall hemostatic capacity of these technologies [349, 350]. The clinical benefit of recombinant FVIIa in trauma, intracranial bleeding, and other heavy bleeding surgeries are also being explored [351–354]. For example, two randomized, placebo-controlled, double-blind trials (one in blunt trauma and one in penetrating trauma) have been carried out to evaluate the efficacy and safety of recombinant FVIIa as adjunctive therapy for hemostatic management of trauma patients, and the results have indicated a reduction in need of blood component transfusion in the patients, along with systemic safety of the FVIIa in the dose ranges studied [353].
4.2. Synthetically derived pro-coagulant systems
In recent years, research approaches have also focused on mimicking and leveraging the body’s coagulation mechanisms using intravascularly applicable synthetic biomaterials. For example, in the work of Morrissey and colleagues, it was demonstrated that inorganic polyphosphate polymers (PolyP) secreted from activated platelets is capable of facilitating activation of FXI to FXIa and FV to FVa, which subsequently results in the prothrombinase complex leading to thrombin generation and back-activation of other coagulation factors [27, 29, 30]. This property of platelet-derived PolyP was recently leveraged by Kudela et al to create silica nanoparticles coated with this PolyP polymer (PolyP-SNP), which demonstrated promising hemostatic characteristics in vitro in the context of augmenting thrombin generation and reducing clotting time in thromboelastographic evaluation with pooled plasma [355]. To advance this technology towards safe clinical translation as an intravenous hemostat, an important design requirement would be to control the exposure of the PolyP-coated surface to coagulation factors selectively at the bleeding site such that the thrombin and fibrin augmentation can be localized at that site while avoiding systemic off-target pro-coagulant effects. An additional challenge may be the maintenance of PolyP activity over reasonable periods of time, since these polyphosphate polymers are known to be rapidly deactivated by phosphatases in circulation. Masking the PolyP coating on particles from phosphatase action in circulation and then programming in a mechanism to have it exposed (unmasked) selectively at the bleeding site can lead to a unique target-responsive hemostatic particle design. The PolyP material itself has shown additional interesting properties regarding enhancement of fibrin clot strength and stability, in that it can inhibit fibrinolysis by affecting thrombin-activated fibrinolysis inhibitor (TAFI), as well as, increase fibrin fiber thickness [27]. This property may be beneficial to intravenous hemostasis applications if the clot formation can be directed selectively at the bleeding site, especially in non-compressible hemorrhage (e.g. in trauma). In another interesting recent report, Baylis et al have reported on ‘self-propelled’ particles made from calcium carbonate (CaCO3) mixed with protonated tranexamic acid (TXA-NH3+), where the propulsion occurs due to carbon dioxide generation via the reaction between CaCO3 and TXA-NH3+ in the milieu [356]. These particles were reported to be capable of being propelled through blood against convective forces, and external application of these particles in several pre-clinical bleeding models (mice and pigs) demonstrated promising tissue-penetration to deliver loaded thrombin for hemostatic clot promotion. The particles were reported to have safe biodistribution when intravenously injected in mice. However, it was unclear from the report whether the particles, reportedly about 10µ in diameter, were designed for actual safe intravascular applications systemically, since solid particles of such large diameter are known to pose occlusive risks in the microvasculature.
Figure 6 shows selected results from hemostasis-relevant studies carried out with PolyP-coated silica particle systems (6A and 6B) and the ‘self-propelled’ CaCO3 particles mixed with TXA-NH3+ and thrombin payloads (6C, 6D and 6E). Figure 6A demonstrates that PolyP loaded in silica nanoparticles (PolyP-SNP) accelerates thrombin generation compared to SNP alone while Figure 6B demonstrates that PolyP delivered via SNP particles (PO3 in SNP) reduces clotting time of blood (i.e. speeds up coagulation) compared to PolyP in solution. Figure 6C shows representative fluorescence and scanning electron micrograph images of CaCO3 particles along with a schematic of administration of such ‘self-propelled’ particles loaded with thrombin in TXA-NH3+ medium to a liver puncture site. Figure 6D shows fluorescence-based confirmation (green fluorescence) of particles in the liver injury model in mice, while Figure 6E shows significant reduction of blood loss in the mouse liver injury model when such ‘self-propelled’ particles were used to deliver thrombin deep into the injury site.
Figure 6.
Selected results from hemostasis-relevant studies carried out with PolyP-coated silica particle systems (6A and 6B) and thrombin-loaded CaCO3-based particles mixed in TXA-NH3+ that can self-propel themselves into wound depth (6C, 6D and 6E). 6A demonstrates that PolyP loaded in silica nanoparticles (PolyP-SNP) accelerates thrombin generation compared to SNP alone while 6B demonstrates that PolyP delivered via SNP particles (PO3 in SNP) reduces clotting time of blood (i.e. speeds up coagulation) compared to PolyP in solution; 6C shows representative fluorescence and scanning electron micrograph images of CaCO3 particles along with a schematic of experimental set-up to administer thrombin-loaded ‘self-propelled’ particles in TXA-NH3+ medium to a liver puncture site; 6D shows presence of green fluorescent particles deep within the liver injury site in mice; 6E demonstrates that liver injury site-localized delivery of thrombin-loaded ‘self-propelled’ CaCO3 particles in TXANH3+ results in significant reduction of blood loss, compared to non-propelled thrombin delivery or control treatment. Figure components adapted and reproduced with permission.[355,356] Copyright 2015, John Wiley & Sons Inc. and 2015, AAAS Science Advances CC-BY NC.
4.3. Materials and technologies to enhance clot strength and stability
As described previously in the introduction section, in physiological scenarios hemostasis is a highly spatiotemporally regulated balance between clot formation and clot lysis [36]. However, in pathological scenarios, dysregulated fibrinolysis can cause substantial hemostatic abnormality (e.g. trauma-induced coagulopathy) leading to sub-optimal stoppage of bleeding (weak fibrin clot and hyper-fibrinolysis) as well as disseminated intravascular coagulopathy (DIC) risks [357]. In such cases, drugs, biomaterials and technologies that can reduce fibrinolysis or increase fibrin strength (and stability) can provide significant therapeutic benefit. One such drug is the antifibrinolytic agent tranexamic acid (TXA), which is a synthetic derivative of the amino acid lysine that inhibits fibrinolysis by blocking the lysine binding sites on plasminogen [358, 359]. TXA has recently undergone extensive clinical studies as an intravenous hemostat, e.g. in the Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage 2 (CRASH-2) trial [360, 361]. These studies have established that TXA administration can reduce exsanguination and thus the number of transfusions, and also reduce mortality risks in trauma and surgery patients. However, CRASH-2 trial studies were performed in civilian hospitals and did not have specificity towards measures of coagulopathy, injury severity or mechanism of injury, which may not be fully applicable to combat trauma. Therefore TXA has been further studied for hemostatic potential in severe combat trauma, e.g. in the Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study [362]. These studies have further concluded that the use of TXA with blood component–based resuscitation in treating combat injury results in improved coagulation outcomes and survival in patients requiring massive transfusion. Based on such promising findings, further studies with TXA for mitigating hemorrhagic complications are currently ongoing, e.g. the Pre-hospital Anti-fibrinolytics for Traumatic Coagulopathy and Haemorrhage (PATCH) trial, the Study of Tranexamic Acid during Air Medical Prehospital Transport (STAAMP) trail and the efforts of the Trans-agency Collaboration for Trauma Induced Coagulopathy (TACTIC) consortium [363], that are expected to enhance the optimal therapeutic approaches involving TXA.
It has also been well established that when platelets interact with fibrin polymers within blood clots, the biomechanical contraction of clot-associated active platelet cytoskeleton facilitates the retraction and stiffening of clots [35, 364]. This property of platelets was used as an inspiration to produce synthetic platelet-like particles (PLPs) that were formed of deformable ultra-low crosslinked poly(N-isopropylacrylamide-co-acrylic acid) (pNIPAm-AAc) gel microparticles surface-modified with fibrin protofibril-specific antibody motifs [365]. These PLPs were tested in vitro by flowing them in thrombin-activated platelet-poor plasma (PPP) within endothelialized microfluidic devices, and the particles demonstrated formation of fibrin. It is to note that in this study the PPP was already thrombin-activated, and classically it is the thrombin (and not platelets) that break down the plasma fibrinogen to fibrin. Platelets essentially augment this process by providing the active platelet surface for the formation of prothrombinase complex and PLPs were not reported to have such active ‘fibrin-generating’ surface but rather a ‘fibrin-binding’ surface. To that end, it was not clear from the studies how the thrombin-activated PPP itself could not form sufficient fibrin, but the presence of the PLP particles in that thrombin-activated PPP somehow resulted in fibrin formation at levels similar to that of platelet-rich plasma. Nonetheless, the PLPs demonstrated fibrin-retracting property when incorporated in clots, although this action occurred over much longer time-scales compared to retraction time-scales shown by natural platelets. For in vivo studies, the PLPs were injected in rats and 5 min after injection the rat femoral vein was injured to bleed. The PLPs were found to get incorporated in the clots at the vein injury site and the bleeding time for PLP-injected animals were found to be only slightly lower (~120 sec) than non-injected animals (~150 sec) and comparable to FVIIa-injected animals. Further evaluation of this technology will be necessary regarding evaluation of long-term biodistribution (higher than 5 min circulation), systemic side-effects and performance in animal models with platelet dysfunctions (e.g. thrombocytopenia, coagulopathy etc.).
Another recently reported polymeric material for intravenous hemostat application was inspired by the physiological action of the transglutaminase coagulation factor FXIIIa, which strengthens and stabilizes the fibrin clot by creating intra- and inter-fiber cross-links through amide bond formation between the lysine and glutamic acid residues of self-assembled fibrin protofibrils (native mechanism schematic shown in Figure 7) [366, 367]. This polymer, termed polySTAT, was formed by incorporating fibrin-binding peptide domains on a linear water-soluble (hydroxyethyl)methacrylate (HEMA) and N-hydroxysuccinimide methacrylate (NHSMA) co-polymer backbone [p(HEMA-co-NHSMA)] [368]. The fibrin-binding peptide itself was adapted from the work previously reported by Kolodziej et al regarding phage display-based development of fibrin-specific peptides [369]. The polySTAT polymer was able to incorporate homogenously within fibrin clots when added to fibrinogen in presence of thrombin. Rheometric and thromboelastographic characterization of mechanical properties of polySTAT-enriched fibrin clot compared against fibrin clots enriched with recombinant FXIIIa or with polySCRAM (control polymer containing scrambled non-specific peptide) showed that the polySTAT polymer enhanced clot formation, strengthening and stabilization capacities at levels significantly higher than the control polymer and comparable to the physiologically relevant recombinant FXIIIa activity. In a rat femoral artery bleeding model of trauma-induced coagulopathy, intravenous administration of the polySTAT polymer significantly enhanced clotting strength and stability, reduced re-bleeding and blood loss and rendered efficient stabilization of blood pressure with reduced need of volume resuscitation. Biodistribution of the polymer indicated that the material is mostly cleared from plasma and sequestered in the kidney over time, which may be indicative of its circulation safety regarding off-target effects. Dose response effects of this polymer as well as its long-term effects from accumulation in the kidney need to be further investigated. Figure 8 shows representative results from hemostatic studies carried out with PLP particles (8A–8D) and with polySTAT polymer systems (8E–8G). Figure 8A shows magnified images of fibrin-binding soft microgel platelet-like particles (PLPs), natural platelets (PRP), non-binding control microgel particles (S11–ULCs), and fibrin-binding rigid polystyrene particles (H6-PS) within fibrin matrices 1 hr post fibrin-polymerization while Figure 8B shows the calculated spread area of PLPs, natural platelets, S11-ULCs and H6-PS particles within these fibrin matrices, demonstrating that soft PLPs have platelet-like spreading behavior (compared to control non-binding and control rigid particles). Figure 8C and 8D show bleeding time and blood loss analysis data in rat femoral vein injury model where administration of PLPs resulted in reduced bleeding time as well as reduced blood loss similar to administration of FVIIa (compared to saline vehicle or control particles). Figure 8E shows the homogenous incorporation of the fibrin-strengthening polySTAT polymer (green fluorescence) within native fibrin matrix (red fluorescence). Figure 8F shows that polySTAT incorporation and strengthening within fibrin results in reduced clot lysis over time (i.e. increased clot strength and stability) compared to control saline and control polymer (polySCRAM). Figure 8G demonstrates that intravenous administration of polySTAT results in significant reduction of blood loss rate in a rat femoral artery hemorrhage model.
Figure 7.
Schematic of fibrinogen conversion to fibrin and assembly of cross-linked fibrin biopolymeric mesh catalyzed by thrombin (FIIa) and FXIIIa, and hemostatic materials and technologies inspired by these mechanisms.
Figure 8.
Selected results from hemostatic studies carried out with platelet-like microgel particles (PLPs, 8A–8D) and with polySTAT injectable polymer systems (8E–8G). 8A shows magnified images of fibrin-binding soft ultralow crosslinked microgel particles (PLPs), natural platelets (PRP), non-binding control microgel particles (S11–ULCs), and fibrin-binding rigid polystyrene particles (H6-PS) within fibrin matrices 1 hr post fibrin polymerization and 8B shows the calculated spread area of PLPs, PRP platelets, S11-ULCs and H6-PS particles within the fibrin matrices, demonstrating that soft PLPs have spreading behavior comparable to natural platelets (PRP) and greater than control particles; 8C and 8D show bleeding time and blood loss analysis data in rat femoral vein injury model where administration of PLPs resulted in reduced bleeding time and blood loss comparable to administration of FVIIa and significantly lower than treatment with vehicle or control particles; 8E shows the homogenous incorporation of the fibrin-binding polySTAT polymer (green fluorescence) within native fibrin matrix (red fluorescence); 8F shows that polySTAT incorporation within fibrin results in crosslinking based strengthening of fibrin and thus reduction of clot lysis over time (i.e. increased clot stability) compared to treatment with control saline and control polymer (polySCRAM); 8G demonstrates that intravenous administration of polySTAT results in significant reduction of blood loss rate in a rat femoral artery hemorrhage model. Figure components adapted and reproduced with permission.[365,368] Copyright 2014, Macmillan Publishers Ltd and 2015, AAAS.
4.4. Materials and technologies for vascular embolization
Another interesting area of using synthetic materials in intravascular hemostatic applications, is the area of blood vessel embolization that is often used to staunch internal bleeding in aneurysms, gastro-intestinal bleeds etc. [370–372]. Catheter-mediated deployment of permanent metallic coils or synthetic liquid polymers (e.g. Onyx, an ethylene-vinyl alcohol copolymer dispersed in dimethyl sulfoxide solvent) has already been clinically approved for such site-localized embolic applications [370, 373, 374]. Metallic coils and Onyx polymers have been reported to present issues of material migration, secondary tissue damage, off-target embolization, incomplete occlusion, biocompatibility issues etc. [371, 375]. To resolve some of these issues, Avery et al recently reported on development of a shear-thinning polymer system formed from a nanocomposite of gelatin and silica nanoparticles, that can be injected into vascular compartment for embolic effect [376, 377]. The unique rheological properties of this material, rendered by charge based ‘physical hydrogel’ interactions between the silica and gelatin materials, allows for shear-driven (e.g. through a pressurized catheter) introduction into blood vessel due to shear-thinning of the material and upon release of pressure the nanocomposite can quickly recover its solid like behavior to render embolization. This unique polymeric system has shown promising embolic efficacy in small and large animal models, to staunch bleeding. Gelatin is a collagen-derived degradation product and therefore can directly activate platelets [378]. Silica, because of its charge, is known to activate coagulation factors [379]. Therefore, while these materials attributes may be beneficial in augmentation of embolic and hemostatic effects selectively at the injury site, presence of these materials in systemic circulation as a result of polymer nanocomposite degradation, may pose systemic pro-thrombotic and pro-coagulant risks. Therefore, future advancement of this material and embolic technology would require in depth evaluation of its long-term systemic safety (including degradation products), clearance mechanisms, off-target effects on systemic hemostatic components (e.g. platelets and coagulation factors) and effects on immune and complement system.
4.5. Natural platelet-derived systems
The platelet membrane presents multiple macromolecular entities that participate in various aspects of primary (platelet adhesion and aggregation) and secondary (fibrin mesh formation) hemostasis. Figure 9 shows a schematic of platelet’s hemostatically relevant surface-interactive adhesion and aggregation mechanisms and the technologies inspired therefrom. The GPIb-IX-V complex as well as GPIa/IIa and GPVI are responsible for platelet adhesion at the bleeding site by binding to VWF and collagen, respectively [15, 16, 380, 381]. The platelet surface integrin GPIIb-IIIa, in its activated conformation, is responsible for binding blood protein fibrinogen by which platelets can bridge with each other to form a platelet plug [382, 383]. Active platelet surface also expresses P-selectin (by secretion from platelet α granules), which can bind to glycoprotein ligands on other platelets and leukocytes at sites of platelet aggregation and inflammation [384, 385]. The surface of active platelets also present a high extent of anionic phospholipids (e.g. phosphatidylserines translocated from inner leaflet to outer leaflet of the membrane) that, in presence of calcium ion (Ca++), facilitates co-localization and activation of several coagulation factors to form the extrinsic and intrinsic tenase as well as the prothrombinase complex, ultimately leading to the conversion of Factor II (prothrombin) to Factor IIa (thrombin) which in turn breaks down fibrinogen to fibrin. Activated platelets also secrete several granule contents, e.g. PolyP and vWF that can modulate and amplify various aspects of coagulation. Because of such multifactor role of platelets in clot promotion, transfusion of platelet concentrates (PC) has become an important clinical component in the prophylactic management of bleeding risks, as well as, emergency treatment of traumatic hemorrhage [54, 61]. However, the PC products at room temperature pose high risks of bacterial contamination, and also are known to undergo activation and degranulation during transport and storage, resulting in very short shelf-life (3–5 days) [386, 387]. Therefore, a significant volume of research is currently being directed towards reducing contamination risks by pathogen reduction technologies [68, 69, 388], as well as, enhancing the platelet storage stability and shelf-life via cold-storage (4°C), freezing and lyophilization [389–396]. In parallel, several research efforts have been directed towards development of hemostatic technologies that use a variety of platelet-derived biological components.
Figure 9.
Schematic of platelet’s injury site-selective adhesion mechanisms (platelet GPIbα of the GPIb-IX-V complex binding to vWF, and GPIa-IIa as well as GPVI binding to collagen) and aggregation mechanism (fibrinogen-mediated bridging of active platelet surface integrin GPIIb-IIIa), and various hemostatic technologies inspired by these mechanisms.
Since platelet’s hemostatic properties are significantly mediated by various membrane components, platelet membranes isolated from outdated platelet concentrates have been utilized to develop phospholipid vesicles, about 0.5 µ in diameter, through a serial process of differential centrifugation, freeze-thawing, pasteurization and sonication, and these vesicles have been evaluated as intravenously administered hemostat under the product name ‘infusible platelet membrane’ (IPM Cyplex™, Cypress Bioscience, San Diego, California) [397]. Evaluation of IPM has been carried out in vitro, in vivo in pre-clinical models and in multiple phases of clinical studies, but it is yet to be approved by the FDA for clinical use, due to certain issues. For example, since the membrane is isolated from allogeneic platelet concentrates, the pathogenic contamination risks associated with platelet concentrates may also be present in IPM products, which in turn require rigorous purification and treatment to ensure safety. It has been reported that such treatments can lead to loss of functional components in the membrane that are otherwise needed for hemostatic mechanisms. Several antigens (HLA I and II) and integrin GPIIb-IIIa have been reported to be lost in IPM due to purification and pathogen reduction treatments [398]. While loss of HLA can be viewed as an advantage in the context of the ability to use IPM without the need for blood type matching, the loss of GPIIb-IIIa renders inability to form the fibrinogen-mediated platelet plug and thereby affects primary hemostatic capability of IPM. Interestingly, presence of pro-coagulant phospholipid function was reported to be retained on IPM surface, implying that secondary hemostatic mechanisms may still be rendered by these vesicles [399]. Also, partial functionality of the GPIb-IX-V complex and P-selectin was reported to be retained in flow cytometric analysis of IPM, suggesting that primary hemostatic function may be partly possible by this product [398]. In pre-clinical animal models, IPM has shown promising hemostatic efficacy (usually measured by reduction in blood volume loss or improvement in time for blood to clot from a controlled injury), without systemic toxicity and thromboembolic risks [400–402]. These promising studies have led to clinical evaluation of IPM in healthy as well as thrombocytopenic patients. In early phase clinical studies, transfusion of IPM showed hemostatic capability comparable to transfusion of allogeneic platelet concentrates and did not show major adverse effects of immune response and refractoriness. Clinical efficacy of IPM products, especially in patients who show refractory immune response to repeated platelet transfusions as well as in patients who are thrombocytopenic with multiple other indications, is yet to be established. Efficacy in non-compressible hemorrhage (e.g. in trauma) is also yet to be established. A variant of the IPM strategy, named Thrombosomes® (CellPhire, Maryland USA), was recently reported where freeze-dried platelets stabilized in trehalose were prepared from a pool of Group O in-date leukoreduced apheresis platelet units [403, 404]. This product was tested for its physical and surface characteristics, systemic distribution, toxicity, sequestration and hemostatic efficacy in vitro as well as in vivo in pre-clinical animal models of bleeding (in rabbits, beagles and rhesus macaque), and the results reported were quite promising in terms of in vitro platelet aggregatory and pro-coagulant capacity and in vivo systemic circulation and safety. In vivo hemostatic efficacy has been reported for this product in terms of reducing bleeding in pre-clinical animal models. Interestingly, isolated platelet membrane based technologies have also been recently reported by several research groups in the context of coating drug delivery vehicles (e.g. polymeric and silica nano/microparticles) to demonstrate targeted action in treating cancer and bacterial infection, thereby emphasizing the potential utilization of platelet’s role in multiple pathologies for unique treatment strategies [405–408]. Figure 10 shows selected results from hemostatic studies with IPM, where addition of IPM in thrombocytopenic blood perfused ex vivo in rabbit aorta segments dose-dependently increased fibrin deposition at low and high shear rates (shown in Figure 10A) and in vivo administration of IPM in thrombocytopenic rabbits resulted in reduction of bleeding time (shown in Figure 10B).
Figure 10.
Selected results from hemostatic studies with infusible platelet membrane (IPM) technology, where (10 A) addition of IPM in thrombocytopenic blood perfused ex vivo in rabbit aorta segments dose-dependently increased fibrin deposition at low and high shear rates and (10B) in vivo administration of IPM in thrombocytopenic rabbits resulted in significant reduction of bleeding time. Figure components adapted and reproduced with permission.[401,402] Copyright 2000, John Wiley & Sons Inc. and 2012, IJPSR.
Instead of using intact isolated platelet membrane to form freeze-dried vesicles or coated particle systems, some approaches have focused on incorporating specific platelet surface protein and receptor components in lipidic or polymeric particle systems. The earliest report of this approach was in a product called ‘plateletsome’, where deoxycholate-induced extraction of platelet membrane fraction with multiple protein components including GPIb, GPIIb/IIIa and GPIV was incorporated into the membrane of liposomal vesicles formed of sphingomyelin, phosphatidylcholine and monosialylganglioside using reverse-phase evaporation sonication technique [409]. This product was tested for hemostatic capability and demonstrated substantial reduction of tail-bleeding in thrombocytopenic rats while showing systemic safety. However, no further reports have been available about this product after the mid 1990s. In an analogous approach, Takeoka and colleagues from Keio University and Waseda University in Japan developed recombinant GPIbα (rGPIbα) and GPIa-IIa (rGPIa-IIa) proteins and conjugated them on the surface of liposomes, latex beads or albumin particles to render synthetic particulate systems with platelet-mimetic VWF-binding and collagen-binding capabilities [410–413]. In vitro, these designs have shown efficient binding to VWF-coated surfaces as well as collagen surfaces in presence of soluble VWF, with more binding evident at higher shear rates, thereby closely mimicking the mechanisms of natural platelet adhesion at high shear. Takeoka and colleagues have also investigated leveraging the co-operative activity of platelet surface motifs that facilitate hemostasis in a physiological scenario. Platelets render primary hemostasis by a co-operative interplay between pro-adhesive (VWF-binding and collagen-binding) and pro-aggregatory (fibrinogen-mediated GPIIb-IIIa binding) functionalities [17, 19]. Takeoka et al demonstrated that platelet’s pro-adhesive mechanisms of VWF-binding and collagen-binding could be combined on a synthetic particle surface by conjugating rGPIbα and rGPIa-IIa motifs together on the outer leaflet of liposomes [411, 412]. The rGPIbα motif was utilized to enable reversible binding to VWF under shear flow, while the rGPIa-IIa motif was utilized to enable adhesion stabilization by concomitant binding to collagen. Compared to liposomes bearing rGPIbα only or rGPIa-IIa only, those bearing a combination of these motifs showed significantly higher adhesion and retention on collagen-coated surfaces in presence of soluble VWF under high shear flow conditions. In another recent interesting approach, Doshi et al created spherical polystyrene as well as crosslinked discoid albumin particles, surface-decorated either with recombinant VWF-A1 domain fragment or recombinant platelet GPIbα domain fragment to mimic platelet-to-VWF interaction under flow [414]. These particle systems were evaluated in vitro where the discoid particles showed better adhesion than spherical particles on target surfaces, and the particles could get incorporated within platelet thrombi on collagen-coated surfaces in microfluidic chambers. Figure 11 shows selected study results of the above-described technologies that leverage platelet’s adhesion mechanisms. Aggregometry studies shown in Figure 11A demonstrates that addition of rGPIbα-decorated albumin microsphere particles significantly enhances that percent (%) aggregation of platelets in presence of vWF and ristocetin, compared to without ristocetin, thereby confirming that the aggregation enhancement is due to the binding of rGPIbα to ristocetin-induced unfolded vWF. Figure 11B shows that polystyrene microparticles surface-decorated with vWF’s A1 domain or GPIbα fragment can undergo shear-responsive enhanced adhesion on GPIbα –coated or vWF-coated microfluidic surfaces respectively. Figure 11C shows a schematic of rGPIbα-coated red fluorescent liposomes flowed over vWF-coated surfaces, with the results demonstrating enhanced adhesion and accumulation of these liposomes over time at higher shear, mimicking the shear-responsive interaction of platelet GPIbα to vWF in platelet’s adhesion mechanism.
Figure 11.
Selected results from studies involving technologies that leverage platelet’s bleeding site-selective adhesion mechanisms. 11A shows platelet aggregometry studies to demonstrate that addition of rGPIbα-decorated albumin microsphere particles significantly enhances the aggregation level of platelets in presence of vWF and ristocetin, compared to without ristocetin, thereby confirming that the aggregation enhancement is due to the binding of rGPIbα to ristocetin-induced unfolded vWF; 11B shows that polystyrene microparticles surface-decorated with vWF’s A1 domain or platelet’s GPIbα fragment can undergo shear-responsive enhanced adhesion on GPIbα–coated or vWF-coated surfaces respectively, under flow, mimicking platelet-relevant adhesion mechanisms; 11C shows an experimental schematic of flowing rGPIbα-decorated red fluorescent liposomes over vWF-coated surfaces, with the corresponding fluorescence microscopy results demonstrating enhanced adhesion and accumulation of these liposomes over time at higher shear, mimicking the shear-responsive interaction of platelet GPIbα to vWF. Figure components adapted and reproduced with permission[413,414]. Copyright 2012, John Wiley & Sons Inc. and 2000, American Chemical Society.
The designs described above represent primarily the adhesive component of platelet function in hemostasis, but do not have the ability to aggregate among themselves or site-selectively amplify the aggregation of active platelets. Platelet aggregation is a function of fibrinogen-mediated bridging of integrin GPIIb-IIIa on active platelets and therefore certain designs have focused on leveraging this mechanism by coating particles with fibrinogen. In the 1980s, Coller et al reported conjugation of fibrinogen on acrylonitrile beads, where the resultant beads enhanced agglomeration of ADP-activated platelets in vitro [415]. Fibrinogen-coated systems were also reported in the early 1990s by Agam and Livne, where erthyrocytes (RBCs) were coated with fibrinogen, and these modified RBCs showed induction of platelet aggregation in vitro and reduction of prolonged bleeding time in thrombocytopenic rats in vivo [416]. In the mid 1990s, Yen et al reported a biomaterials technology named ‘Thrombospheres™’ consisting of albumin microspheres coated with fibrinogen, and these particles rendered aggregation of active platelets in vitro as well as reduction of bleeding time in an ear-punch model in rabbits in vivo [417, 418]. A similar design termed ‘Synthocytes™’ was reported by Levi et al in the late 1990s, where fibrinogen-coated albumin microcapsules were able to reduce the bleeding time as well as blood volume loss in an ear-punch bleeding model and abdominal surgical incision model in thrombocytopenic rabbits [419]. In vitro platelet-aggregating capacity of the fibrinogen-coated albumin particles under shear flow conditions was also reported in early 2000 by the research group from Waseda University and Keio University in Japan [420]. Another similar design of fibrinogen-coated albumin particles was reported under the product name Fibrinoplate™ by a company called Advanced Therapeutics & Co, but further information about this product was not available after 2011. Figure 12 shows selected results from studies carried out with various fibrinogen-coated particle designs. Figure 12A shows the effect of administering fibrinogen-coated albumin microcapsules (Synthocyte™) in reducing ear punch bleeding time in thrombocytopenic rabbits, where induction of thrombocytopenia (TCP) significantly increased bleeding time from normal and administration of Synthocyte™ particles (SC) could significantly reduce bleeding time in these animals, compared to administration of control particles (CP) or saline. Figure 12B shows scanning electron micrograph of the Synthocyte™ particles incorporated with platelets and fibrin in hemostatic clots. Figure 12C shows the ability of fibrinogen-coated albumin particles to bind to platelet-immobilized surfaces in absence versus presence of Ca++ ions, compared to binding of unmodified albumin particles, while Figure 12D shows representative fluorescence microscopy images of these binding studies. These results establish that fibrinogen-coated particles can interact and bind with active (e.g. Ca++ activated) platelets under flow environment, possibly via interaction with platelet surface integrin GPIIb-IIIa. Thus, these technologies seem to efficiently leverage and amplify the active platelet aggregation component of hemostasis by means of a ‘super-fibrinogen’ design. Off-target systemic thromboembolic risks can be a possible issue with such designs, and therefore rigorous in vivo pre-clinical studies are needed to establish safety and clearance.
Figure 12.
Selected results from studies carried out with various fibrinogen-coated particle designs. 12A shows the effect of administering fibrinogen-coated albumin microcapsules (Synthocyte™) in reducing ear punch bleeding time in thrombocytopenic rabbits, where induction of thrombocytopenia (TCP) significantly increased bleeding time from normal levels and administration of Synthocyte™ particles (SC) could significantly reduce bleeding time in these animals, compared to administration of control particles (CP) or saline; 12B shows scanning electron micrograph of the Synthocyte™ particles incorporated with platelets and fibrin in hemostatic clots; 12C shows the ability of fibrinogen-coated albumin particles to bind to platelet-immobilized surfaces in absence versus presence of Ca++ ions, compared to binding of unmodified albumin particles and 12D shows representative fluorescence microscopy images of these binding studies, confirming high binding of fibrinogen-coated particles and establishing that these particles can interact with active platelets under flow environment, possibly via interaction with platelet surface integrin GPIIb-IIIa, thereby mimicking and amplifying the active platelet aggregation component of hemostasis. Figure components adapted and reproduced with permission.[419,420] Copyright 1999, Macmillan Publishers Ltd and 2001, American Chemical Society.
4.6. Synthetic biomaterials-based platelet mimics and substitutes
Creating platelet-mimetic designs by utilizing isolated platelet membranes, fibrinogen coatings and platelet surface-relevant recombinant protein fragments can present issues of batch-to-batch variation in bioactivity, protein stability and solubility, potential immunogenicity and high cost. Therefore, a significant research interest has been focused on utilization of synthetic ligand systems (e.g. peptides) decorated on semi-synthetic or synthetic particle platforms that can render hemostatic mechanisms of platelets while providing advantages of controllable and scalable manufacture, consistent bioactivity, long shelf-life and reduced biological/immunological side effects. To this end, the majority of research approaches has been focused in the area of mimicking fibrinogen-binding to active platelet surface integrin GPIIb-IIIa by decorating particle surfaces with fibrinogen-relevant synthetic peptide ligands, in essence once again rendering a ‘super-fibrinogen’ type design. Fibrinogen is a hepatic protein, approximately 340,000 in molecular weight and present in the blood at concentration ranges of 200–400 mg/dL. Fibrinogen has an approximately 45 nm long macromolecular structure consisting of two outer D-domains, each connected by a coiled-coil segment to its central E domain. The molecule contains two sets of three polypeptide chains, termed Aα, Bβ and γ, which are joined together in the N-terminal E domain by five symmetrical disulfide bridges [383, 421]. Upon activation of platelets in a hemostatic (or thrombotic) environment, the surface integrin GPIIb/IIIa assumes a ligand-binding conformation that can bind to fibrinogen via the Aα chain tetrapeptide Arg-Gly-Asp-Ser (RGDS) and the γ chain 12-residue peptide His-Leu-Gly-Gly-Ala-Lys-Gln-Ala-Gly-Asp-Val (HLGGAKQAGDV) [422, 423]. Since these peptide sequences are present on both termini of fibrinogen, the fibrinogen molecule can act as a bridging system between active platelets (schematic shown previously in Figure 9), resulting in aggregation of these platelets to form the primary hemostatic plug. Based on these mechanisms, one of the earliest semi-synthetic designs was that of ‘Thromboerythrocytes’ reported by Coller et al, where RGD sequence Ac-CGGRGDF-NH2 was covalently conjugated to the surface amino group of RBCs using a heterobifunctional crosslinking reagent (N-maleimido-6-aminocaproyl ester of 1-hydroxy-2-nitrobenzene4-sulfonic acid) [424]. These RGD-decorated RBCs showed binding to active platelet integrin GPIIb-IIIa, were capable of co-aggregating with activated platelets and could bind to active platelets on collagen-coated surfaces. Decoration of synthetic particle platforms (latex beads, poly-lactic acid based polymeric particles etc.) with RGD sequences (e.g. CGGRGDF on latex beads and GRGDS on poly-lactic acid based particles) have been reported by Takeoka and colleagues [425] as well as Lavik and colleagues [426, 427], in the context of mimicking and amplifying the fibrinogen-mediated interactions with integrin GPIIb-IIIa on activated platelets. Interestingly, in the studies conducted by Takeoka et al with CGGRGDF-decorated particle systems, it was found that these particles could promote activation (and subsequent aggregation) of inactive platelets [425]. Similar effects of linear RGDS-containing peptides have also been reported by Du et al and Beer et al [422, 428]. Furthermore, the RGD tri-peptide sequence is known to have ubiquitous cell- and matrix-recognition capabilities [429, 430]. In fact, this sequence has been reported in a variety of research including targeting to tumor cells, endothelial cells, bone cells etc. beyond its interaction with platelets. Therefore, utilizing CGGRGDF or GRGDS type of linear ubiquitous RGD peptides to mimic hemostasis-relevant fibrinogen-mimetic platelet aggregation mechanisms on synthetic platelet designs may pose major systemic thromboembolic risks (due to activation of circulating resting platelets) as well as render cross-reactivity with other cellular entities in vivo that may affect clinical translation. Because of the issues associated with these linear ubiquitous RGD peptides, Takeoka and colleagues have also looked into utilizing fibrinogen γ-chain relevant peptides, specifically the sequence HLGGAKQAGDV otherwise known as the H12 peptide, to decorate particle platforms. This peptide was used to modify the surface of latex beads, albumin particles and liposomes, and the resultant systems were evaluated for their platelet-mimetic pro-aggregatory hemostatic activities [431–435]. In vitro, the H12-decorated latex beads showed minimal aggregation effect on inactive platelets, suggesting that this peptide may confer improved systemic safety compared to RGDS peptides. Albumin microparticles surface-decorated with H12 peptides were able to enhance platelet aggregation on collagen-immobilized surfaces in vitro and improve ear punch bleeding time in thrombocytopenic rabbits and tail bleeding time in thrombocytopenic rats, both by approximately 50%. Liposomes surface-decorated with H12 peptides showed similar hemostatic capability, and encapsulating ADP in such liposomes enabled augmentation of platelet activation and aggregation as well as correction of ear bleeding time in thrombocytopenic rabbits and tail bleeding time in thrombocytopenic rats. These studies however did not provide any information regarding the overall biodstribution of the particles.
Figure 13 shows selected results from in vitro and in vivo studies that have been carried out using particle designs surface-decorated with fibrinogen-relevant RGD or H12 peptides to mimic and amplify fibrinogen-mediated platelet aggregation. Figure 13A shows platelet aggregometry studies with RGD peptide-decorated RBCs (Thromboerythrocytes), where unmodified control erythrocytes were unable to enhance aggregation of ADP-activated platelets and Thromboerythrocytes were unable to enhance aggregation of inactive platelets or integrin GPIIb-IIIa-blocked platelets, but Thromboerythrocytes were able to significantly enhance the aggregation of ADP-activated platelets. Figure 13B shows representative fluorescent images as well as quantitative surface-coverage data of H12 peptide-decorated, or RGD peptide-decorated or undecorated fluorescently-labeled latex beads suspended in reconstituted blood binding to platelet-immobilized surfaces, indicating the enhanced binding capability of H12-decorated and RGD-decorated beads to platelets. Figure 13C shows the capability of H12 peptide-decorated PEGylated albumin particles (H12-PEG-Alb) to significantly reduce ear punch bleeding time upon intravenous administration in thrombocytopenic rabbits, compared to administration of control undecorated PEG-albumin (PEG-Alb) particles. Figure 13D shows the capability of H12 peptide-decorated PEGylated liposomal vesicles (H12-PEG-vesicles) to significantly reduce tail bleeding time in thrombocytopenic rats, compared to administration of control undecorated PEG-vesicles. Figure 13E shows quantitative bleeding time reduction data and representative fluorescence image of hemostasized vessel, where administration of RGD-decorated polymeric nanoparticles (GRGDS-PLGA-PLL particles) significantly reduced bleeding time in a rat femoral artery injury model, compared to administration of undecorated particles or saline.
Figure 13.
Selected results from studies carried out with various particle platforms surface-decorated with fibrinogen-relevant RGD or H12 peptides to mimic platelet aggregation mechanism. 13A shows platelet aggregometry studies with RGD-decorated RBCs (Thromboerythrocytes), where unmodified control erythrocytes were unable to enhance aggregation of ADP-activated platelets and Thromboerythrocytes were unable to enhance aggregation of inactive platelets or integrin GPIIb-IIIa-blocked platelets, but they could significantly enhance the aggregation of ADP-activated platelets, thereby mimicking fibrinogen and GPIIb-IIIa mediated platelet aggregation mechanism; 13B shows representative fluorescent images as well as quantitative surface-coverage data of H12 peptide-decorated, or RGD peptide-decorated or undecorated fluorescently-labeled latex beads suspended in reconstituted blood and flowed over platelet-immobilized surfaces, indicating the enhanced GPIIb-IIIa-binding capability of H12-decorated and RGD-decorated beads to platelets; 13C shows the capability of H12-decorated PEGylated albumin particles (H12-PEG-Alb) to significantly reduce ear punch bleeding time upon intravenous administration in thrombocytopenic rabbits, compared to administration of control undecorated PEG-albumin (PEG-Alb) particles; 13D shows the capability of H12-decorated PEGylated liposomal vesicles (H12-PEG-vesicles) to significantly reduce tail bleeding time in thrombocytopenic rats, compared to administration of control undecorated PEG-vesicles; 13E shows quantitative bleeding time reduction data and representative fluorescence image of hemostasized vessel, where administration of RGD-decorated polymeric nanoparticles (GRGDS-PLGA-PLL particles) significantly reduced bleeding time in a rat femoral artery injury model, compared to undecorated particles or saline. Figure components adapted and reproduced with permission.[424,425,426,431,432] Copyright 1992, American Society for Clinical Investigation; 2003, Elsevier; 2008, John Wiley & Sons Inc.; 2005, American Chemical Society and 2009, AAAS.
Regarding use of peptides to mimic platelet’s adhesion mechanisms, an early demonstration is found in reports from Gyongyossy-Issa and colleagues involving the development of platelet GPIbα-relevant 15-mer peptides that have binding capability to vWF [436]. These peptides were tethered onto liposomal surfaces stabilized via cross-linking, and the resultant liposomal constructs, 150–200 nm in diameter, were tested as ‘synthetic platelet substitutes’ [437]. Although the biochemical characterizations of these constructs have been reported, the actual hemostatic efficacy evaluation of these constructs in vivo have not been reported in any pre-clinical animal model. Takeoka and colleagues have demonstrated the possibility of combining the platelet-mimetic pro-adhesive function with pro-aggregatory function by either co-conjugation of rGPIbα and H12 peptide on the same latex bead, or mixing rGPIbα-decorated latex beads with H12 peptide-decorated latex beads [438]. When the two motifs were conjugated onto the surface of the same bead, the large size of the rGPIbα motif sterically masked the action of the smaller H12 peptide, and therefore the effect seen was mostly of platelet-mimetic adhesion but no aggregation. In contrast, when each motif was conjugated to separate beads and the beads were mixed together, the resultant mixture significantly enhanced the aggregation and surface-coverage of platelets on collagen-coated surfaces in presence of soluble VWF under shear flow conditions, when compared to H12 peptide-decorated beads only. These studies suggested that if platelet-relevant pro-adhesive and pro-aggregatory functionalities could be combined on a single particle platform without the issue of mutual steric interference, the resultant design would have superior hemostatic capacity compared to designs that bear only pro-adhesive or only pro-aggregatory function. Rationalizing from this idea, Sen Gupta and colleagues have utilized the technique of heteromultivalent surface-modification to decorate synthetic particle surfaces simultaneously with VWF-binding peptides (VBP), collagen-binding peptides (CBP) and active platelet GPIIb-IIIa-binding fibrinogen-mimetic peptides (FMP), to create a unique synthetic platelet design, the SynthoPlate™ [439, 440]. The VBP, Thr-Arg-Tyr-Leu-Arg-Ile-His-Pro-Glu-Ser-Trp-Val-His-Glu-Ile (TRYLRIHPQSWVHQI) was derived from the C2 domain (residues 2303–2332) of the coagulation factor FVIII, based on reports of FVIII binding domains on VWF [441]. The CBP sequence was a single chain 7-mer repeat of the Glycine(G)-Proline(P)-Hydroxyproline(O) tri-peptide (i.e. -[GPO]7-) that has helicogenic affinity to fibrillar collagen but minimal ability to activate platelets via platelet collagen receptors GPIa/IIa and GPVI (hence no systemic thrombotic risk) [442, 443]. The FMP sequence was the cyclic RGD peptide cyclo-CNPRGDY[-OEt]RC, which was reported to have high affinity and selectivity for active platelet GPIIb-IIIa but minimal affinity for resting GPIIb-IIIa (hence reduced systemic thromboembolic risk) as well as minimal selectivity for other common cell integrins (hence reduced cross-reactivity in vivo) [444]. Each peptide was conjugated to a poly(ethylene glycol)-terminated lipid (i.e. PEG-lipid) molecule via amide, thioether or ‘click’ chemistry to form lipid-PEG-peptide conjugates [440]. Resultant conjugates could be self-assembled and extruded into unilamellar liposomal vesicles such that the vesicle’s outer leaflet had heteromultivalent decorations of VBP, CBP and FMP motifs. Due to the small size of the peptides, they did not sterically interfere with respective bioactivities. This integrative synthetic platelet design (bearing VBP + CBP + FMP peptides together) was able to demonstrate platelet-mimetic hemostasis-relevant bioactivities in vitro, and significantly reduced tail transection bleeding time in normal as well as thrombocytopenic mice at superior levels compared to particles decorated with pro-adhesive (VBP + CBP) peptides only or pro-aggregatory (FMP) peptides only [445, 446, 448]. In thrombocytopenic mice, the hemostatic efficacy of these particles were found to be dose-dependent, with doses comparable to normal murine platelet count (800–1000 particles per nL) improving bleeding time in thrombocytopenic mice to levels of normal mice [440]. Immunofluorescence analysis showed that the SynthoPlate™ particles could co-localize with injured endothelium and locally available active platelets to enhance primary hemostasis (platelet recruitment and aggregation) and could in effect also enhance secondary hemostasis (fibrin generation). In addition, it was demonstrated that SynthoPlate™ was systemically safe (no systemic pro-thrombotic or pro-coagulant risk) and could be eliminated mostly via liver over time [440]. The size, shape and stiffness aspects of platelets influence their vascular margination towards the wall under a hemodynamic flow environment [449, 450]. Based upon such observations, the heteromultivalent surface-modification strategy used for SynthoPlate™ particles, could also be adapted to other particle platforms (e.g. discoid albumin particles) to integrate platelet-relevant hemostatic surface chemistry with platelet’s fluid dynamic margination-influencing biophysical (e.g. geometric) properties [451]. Figure 14 shows representative results from hemostatic studies carried out with platelet-inspired ‘integrative’ particle technologies that combine platelet-relevant adhesion and aggregation mechanisms on a single particle platform. Figure 14A shows platelet aggregometry studies where undecorated latex beads, or H12 peptide-decorated latex beads, or latex beads co-decorated with H12 peptide and rGPIbα fragments were added to ADP-activated platelets and the results showed that the co-decorated beads had no additional aggregation enhancement effects, possibly due to the masking of small H12 peptides by the large rGPIbα fragments. Figure 14B shows that intravenous administration of liposome-based ‘synthetic platelet’ particles decorated with VBP, CBP and FMP peptides (SynthoPlate™) resulted in significant reduction in tail bleeding time in normal mice, compared to administration of liposomes bearing only VBP + CBP peptides (‘adhesion only’ particles) or liposomes bearing only FMP peptides (‘aggregation only’ particles). These same particles could also dose-dependently reduce tail-bleeding time in severely thrombocytopenic mice, compared to administration of saline or control particles (Figure 14D). Such heteromultivalent decoration could also be adapted onto other particle platforms, as shown in the example fluorescent images in Figure 14C where discoid albumin particles surface-decorated heteromultivalently with all three peptides showed higher co-localization with active platelets to form visible aggregates in collagen-coated microfluidic channels. While such heteromultivalent integrative designs demonstrate combinatorial functional advantages in mimicking platelet mechanisms, in advancement and translation of such technologies, there may be potential challenges regarding large-scale manufacturing of such multi-component designs especially in the context of Good Manufacturing Practice (GMP) scale process development [452]. Also, these liposome-based and albumin-based particle designs as well as polymeric nanoparticle based designs described previously, should be extensively studied for dose limitations in terms of toxicity (i.e. maximum tolerance), systemic risks, and possible immune response risks. In order to utilize such designs as intravenous hemostat technologies, it would be critical to carry out dose escalation studies in appropriate in vivo models, along with systemic monitoring of hemodynamics and vitals. For example, it has been demonstrated in the field of nanoparticle-based drug delivery research that certain liposome-based formulations can result in complement-activation related pseudoallergy (CARPA) reactions [453, 454] depending on dose, particle size and surface-charge and administration speed. Further studies are therefore warranted to characterize such risks for ‘synthetic platelet surrogate’ designs and establish mechanistic understanding as well as mitigation steps if such risks are present.
Figure 14.
Selected results from hemostatic studies carried out with platelet-inspired ‘integrative’ particle technologies that combine platelet-relevant adhesion and aggregation mechanisms on a single particle platform. 14A shows platelet aggregometry studies where undecorated latex beads, or H12 peptide-decorated latex beads, or latex beads co-decorated with H12 peptides and rGPIbα fragments were added to ADP-activated platelets and the results showed that the co-decorated beads had no additional aggregation enhancement effects, possibly due to the masking of small H12 peptides by the large rGPIbα fragments; This masking effect could be resolved by utilizing heteromultivalent decoration of small peptides, as shown in the example in 14B where intravenous administration of liposomes heteromultivalently decorated with vWF-binding, collagen-binding and fibrinogen-mimetic peptides (functionally integrated particles) resulted in significant reduction in tail bleeding time in normal mice, compared to administration of liposomes bearing only pro-adhesive peptides (‘adhesion only’ particles) or liposomes bearing only pro-aggregatory peptides (‘aggregation only’ particles); such heteromultivalent decoration could be adapted onto other particle platforms, as shown in the example in 14C where discoid albumin particles (red fluorescent) surface-decorated heteromultivalently with all three peptides showed higher co-localization with active platelets (green fluorescent) to form visible aggregates in collagen-coated microfluidic channels, compared to particles bearing proaggregatory peptides only (‘FMP only’) or pro-adhesive peptides only (‘VBP + CBP’ only); 14D shows representative results from tail bleeding studies in thrombocytopenic mice where intravenously administered synthetic platelet (SynthoPlate™) particles (liposomes surface-decorated heteromutivalently with VBP, CBP and FMP) could dose-dependently reduce tail bleeding time, compared to administration of saline or control particles. Figure components adapted and reproduced with permission.[438,440,448,451] Copyright 2006, Springer; 2013, Elsevier; 2014, American Chemical Society and 2017, International Society on Thrombosis and Haemostasis.
5. Discussion
Bleeding due to injuries, trauma, surgical procedures and congenital or drug-induced coagulation disorders, can lead to a variety of health risks. Therefore, bleeding risks and incidents need to be rapidly treated [6, 455]. In many bleeding injuries, the body’s inherent mechanisms become insufficient to rapidly staunch bleeding, and this necessitates the administration of certain materials and technologies to facilitate, augment, compensate or mimic the natural mechanisms of hemostasis. In the current article, we have comprehensively reviewed the various materials and technologies currently approved, as well as, under research for hemostatic applications. The bulk of hemostatic materials research has been directed towards technologies for management externally accessible bleeding scenarios, ranging from fibrin and thrombin glues, to zeolite powders, to bioactive dressings, foams, gels, tourniquets and self-expanding tamponades. In recent years, there has been a concerted effort to evaluate this large volume of technologies in standardized bleeding models, especially for heavy-bleeding injuries. These efforts have been mostly led by the US military’s medical research divisions, since improving military survival through rapid hemostasis on the battlefield has become of paramount importance due to continued wars and conflicts over the last decade. In this context, Kheirabadi has recently described the ideal characteristics of externally administered hemostatic dressings [456]. Arnaud et al reported on the comparative evaluation of ten clinically approved hemostatic dressings in a groin transection model in pigs, by monitoring mean arterial blood pressure, rate and time of survival, blood loss and post-treatment re-bleeding [266, 267]. In these studies, it was found that Quikclot® ACS (zeolite impregnated flexible absorbent sponge), Celox® (chitosan granule), WoundStat® (smectic mineral on flexible polymer) and X-sponge® (kaolin impregnated flexible gauze) all have comparable superior hemostatic capabilities relative to standard gauze or rigid hemostatic technologies like HemCon®. Rall et al carried out similar comparative evaluation of several such dressings in a pig arterial bleeding model, and found that Quikclot®, Celox® and HemCon® do not have statistically significant differences in their hemostatic capabilities, and they all meet the current established standards put forward by the Committee on Tactical Combat Casualty Care for point-of-care bleeding management [457]. Rall et al essentially concluded that the lack of clear superiority of any of these hemostatic technologies is an indication that current clinically approved hemostatic dressing technology has potentially reached a plateau for efficacy. A potential improvement of this scenario may be achieved by the latest volume-filling technologies like XSTAT®, Traumagel® etc., especially to manage complex junctional, intra-cavitary or open wound bleeding. Many of the current clinically approved or newer pre-clinically evaluated technologies contain bioactive materials for augmenting coagulation (minerals, collagen, chitosan, alginate, fibrinogen/fibrin etc.). Some of these may be biodegradable and resorbable, while some may require subsequent removal before surgical procedures. In this aspect, it is extremely important to study the biodegradation/resorption pathways, risk of residual materials post-removal and short and long term thrombo-inflammatory responses of resorbed or residual material. Almost all evaluations of the hemostatic materials and technologies so far have been in short term hemostatic capability and resultant survival, with limited focus on biodegradation /resorption /removal pathways and long term in vivo effects. Multi-disciplinary efforts involving biomedical and materials engineers, emergency medical and surgical personnel and physiology/pathology experts should be dedicated to such evaluations in the long term.
While externally administered and intra-cavitary hemostatic materials and technologies have significantly improved the treatment options for accessible injuries, emergency or prophylactic management of internal non-compressible hemorrhage is still heavily dependent on transfusion of whole blood or blood components (RBC, plasma and platelets). These transfusion methods and products are essentially meant to compensate for blood volume loss and to facilitate or augment physiological inherent mechanisms of primary and secondary hemostasis. Along with blood transfusion, there are a few pharmaceutical compounds like desmopressin, aprotinin and tranexamic acid (TXA) that have been either approved or under clinical trial for intravenous administration to augment hemostasis [458]. These pharmaceutical compounds act essentially as facilitators or stabilizers of hemostatic pathway components, but are not directly pro-hemostatic agents. For example, desmopressin acts by mimicking the function of natural vasopressin to systemically increase the plasma concentration of Factor VIII and VWF, and therefore can be used to treat mild hemophilia and VWF disease [459]. However, meta-analysis reports of controlled clinical trials with desmopressin have indicated that such systemic amplification of coagulation facilitators may pose a risk of off-target thrombosis [460, 461]. Aprotinin is a broad-spectrum inhibitor of serine proteases, and therefore its use in hemostasis is debatable since it can block pro-hemostatic factors (e.g. thrombin) as well as anti-coagulatory factors (e.g activated protein C) and fibrinolytic factors (e.g. plasmin) [462]. Nonetheless, the use of this agent has shown benefit in management of bleeding complications in several surgical procedures [463, 464]. As described previously, TXA does not promote clotting but rather maintains clot strength and stability by preventing its plasmin-induced breakdown [465]. This property has shown benefits in preventing bleeding related morbidities and mortalities [361]. Along with the use of these pharmaceutical agents, blood component (especially platelet) transfusion remains the primary intravascular strategy in rendering hemostasis, and the use of blood products present significant challenges due to limited availability, high cost of purification and storage, contamination risks, and immunological side-effects. While efficient blood-banking protocols and modern pathogen reduction technologies work relentlessly to resolve some of these challenges in locations with suitable medical facilities, the challenges persist in remote locations and austere battlefield conditions. This has triggered robust research endeavors in development of semi-synthetic or synthetic materials based systems that can provide the hemostatic functions of blood components while circumventing the issues associated with natural blood products. In the context of hemostasis, these research endeavors are currently directed towards materials that capture the biology or functions of platelets and the coagulation cascade components.
Regarding mimicry of platelet functions, one straightforward approach that several research groups have employed involves detergent-based extraction of natural platelet’s membrane and then coating synthetic particles with this membrane material. Since many of platelet’s hemostatic mechanisms are associated with its surface biology, this approach, theoretically, can capture and leverage this surface biology on synthetic platforms for hemostatic (and drug delivery) applications. Additionally, due to a significant history of research on the evaluation of infusible platelet membrane (IPM) vesicles as intravenous hemostats, the membrane-coated particle approaches can potentially streamline their design optimization. One concern in this approach is the loss of bioactivity of certain platelet surface motifs that is rendered by the various membrane extraction and purification steps, which may in turn lead to capturing only a part of hemostatic mechanisms or presenting batch-to-batch inconsistency in hemostatic function. Another concern is that the limited availability of platelet concentrates may in turn affect the availability of extractable platelet membranes and this can become a logistical barrier to translation. Such issues can be potentially resolved by utilizing recombinant protein fragments or small peptide ligands that can mimic the functions of platelet’s hemostasis-relevant surface components without having to mimic the complete surface membrane and complex biology. Examples of this approach are seen in designs that utilize recombinant GPIbα, GPIa-IIa and VWF-A1 protein fragments, as well as, in strategies that employ VWF-binding, collagen-binding and integrin GPIIb-IIIa-binding peptides to decorate synthetic particle platforms. It is important to note here that platelet’s hemostasis-relevant surface mechanisms are driven by multiple interactions occurring in tandem, and therefore the design of a synthetic platelet mimic can significantly benefit from incorporating such heterotypic interactions via materials engineering. This aspect was first emphasized in studies where multiple recombinant protein fragments (e.g. rGPIbα that binds VWF and rGPIa/IIa that binds collagen) were combined on latex beads or albumin particles. The recombinant technology usually involves high cost, and the large size of these recombinant protein fragments often poses mutual steric interference to their respective bioactivity when conjugated together on micro- or nano-scale particles. Protein fragments may also pose risks of immunogenicity over time. These issues can be potentially resolved by identifying and utilizing small peptides instead of protein fragments that can perform the same platelet-mimetic functions. It was demonstrated in our recent reports that platelet’s hemostatically relevant adhesive and aggregatory functions could be efficiently captured via heteromultivalent modification of lipidic and polymeric particles with multiple types of small peptides. Such approaches may allow easy manufacture and scale up by leveraging modern technologies of automated peptide synthesis, purification and high throughput particle manufacture methods. These strategies can also incorporate biophysical components of particle design (e.g. shape, size, flexibility etc.) that mimic the fluid dynamic behavior of platelets regarding their interaction with the RBC volume and efficient margination to vessel wall. Exploiting such biophysical parameters for particle design can open new doors not only for synthetic platelet technologies, but also for efficient platforms in vascular drug delivery [466]. A variety of particle fabrication technologies to modulate physico-mechanical and geometric parameters have been established in recent years, and this is expected to benefit the incorporation of such parameters in refining the materials and technologies for synthetic platelet research. Upon adhesion and activation at the injury site due to physical (shear) and biochemical (receptor-based signaling cascades) triggers, platelets transform from the ‘resting’ biconvex discoid morphology to an ‘activated’ stellate pseudopodal morphology with significant reduction in modulus and remain irreversibly adhered at the injury site for hemostasis. This phenomenon can inspire the development of materials with environment-responsive physico-chemical and mechanical properties for development of platelet-mimetic hemostats. Regarding utilization of semi-synthetic and synthetic particle based systems for designing platelet surrogates, it is critical to study and mitigate systemic risks associated with the particle material itself, as well as, with the surface chemistries and ligands utilized for particle surface decoration. In previous sections, the potential risks associated with using ubiquitous cross-reactive ligands (e.g. RGDS) have been discussed. In the same note, every ligand motif should be studied both in free form as well as particle-tethered form for systemic effects on platelets (e.g. by aggregometry) and plasma (e.g. by thrombin generation assays). In addition, the base materials used for particle construction should be chosen carefully to ensure minimal toxicity and high biocompatibility. It is also important to determine the spatiotemporal fate of these particles and materials once administered in vivo, in the context of biodegradation, clearance mechanisms and elimination pathways. Maintaining systemic safety while rendering targeted hemostatic action at the bleeding site within an appropriate time window and undergoing effective clearance, will be the key to success for any of these technologies.
Regarding utilization of coagulation cascade components for intravascular hemostasis, the direct use of recombinant coagulation factors is clinically well established in selective groups of patients. The bulk of these approaches are essentially directed at amplifying the generation of thrombin through tissue-factor pathway or common pathway of coagulation, such that the thrombin can convert fibrinogen to fibrin to facilitate clotting. It is important to note that the generation of thrombin is significantly enhanced in presence of active platelet membrane via formation of tenase complex (FVIIIa + FIXa) and prothrombinase complex (FXa + FVa) on the phosphatidyl serine-rich membrane surface. Therefore, use of coagulation factors in patients with platelet deficiency or dysfunction may have only limited benefit towards hemostasis. In comparison, use of materials and technologies that can promote activation of certain coagulation cascade components independent of platelet presence may show some benefit towards subsequently amplifying hemostasis. One such interesting material is the PolyP coating on particles that attempts to mimic the action of polyphosphates secreted from active platelets by activating FXI (to FXIa) and FIX (to FIXa) en route to activation of FX and subsequently amplifying thrombin generation through the tenase and prothrombinase complexes on active platelet surface. While this mechanism and technology have generated substantial interest in recent hemostasis research, it would be important to limit the action of PolyP-coated particles at the bleeding site, to avoid systemic hypergeneration of thrombin and fibrin. One potential approach to shielding of the PolyP coating can be the use of a mask that may be cleaved site-selectively by bleeding site relevant enzyme action, thereby exposing the PolyP only locally to amplify coagulation cascade activation and propagation. Precise regulation of these mechanisms will be important to avoid systemic effects. The same rationale holds true for technologies that may attempt to coat synthetic or semi-synthetic particle platforms with tissue factor or negatively charged phospholipids. Other pro-coagulant technologies that strengthen the fibrin clot (e.g. fibrin-crosslinking microgel particles or FXIIIa action mimicking polymers) may have to depend upon sufficient prior generation of fibrin at the bleeding site to be able to significantly benefit in vivo from their mechanism of fibrin-strengthening. This in turn would require prior sufficient generation of thrombin and resultant conversion of fibrinogen in a site-selective manner at the bleeding site, which in turn requires the involvement of tissue factor-dependent thrombin generation and platelet-dependent thrombin amplification pathways at the site. Therefore, future research should be directed at modularly combining the hemostasis mimicking and enhancing mechanisms of the different materials and technologies to find an optimum system that (i) promotes and enhances active platelet or platelet-mimetic aggregation at the bleeding site, (ii) augments thrombin generation and fibrin formation selectively at that site and (iii) strengthens the formed fibrin biopolymeric network to reduce clot lysis and amplify hemostasis. While individual components of such systems are currently under pre-clinical research, these and potential integrated systems need to be rigorously studied for biodistribution, systemic safety and site-selective hemostatic action in multiple well-characterized bleeding models in vivo. Through robust interdisciplinary research between materials scientists, chemical and biomedical engineers, molecular biologists, biochemists and clinicians, future advancement of hemostatic materials and technologies can be envisioned to revolutionize bleeding treatment and transfusion medicine approaches.
Supplementary Material
Acknowledgments
This work was supported by the National Heart Lung and Blood Institute (NHLBI) of the National Institutes of Health under award numbers R01 HL121212 (PI: Sen Gupta). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest. ASG and CLP are inventors on patents related to SynthoPlate™ technology, US 9107845 and heteromultivalent nanoparticle compositions, US 9107963.
References
- 1.Kauvar DS, Lefering R, Wade CE. J Trauma. 2006;60:S3. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Krug EG, Sharma GK, Lozano R. Am. J. Public Health. 2000;90:523. doi: 10.2105/ajph.90.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champion HR, Bellamy RF, Roberts CP, Leppaniemi A. J Trauma. 2003;54:S13. doi: 10.1097/01.TA.0000057151.02906.27. [DOI] [PubMed] [Google Scholar]
- 4.Butler FK, Jr, Blackbourne LH. J Trauma Acute Care Surg. 2012;73:S395. doi: 10.1097/TA.0b013e3182754850. [DOI] [PubMed] [Google Scholar]
- 5.Holcomb JB. Hematology. 2010:465. doi: 10.1182/asheducation-2010.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons JW, Pittet J-F, Pierce B. Curr Anesthesiol Rep. 2014;4:189. doi: 10.1007/s40140-014-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furie B, Cassileth P. Clinical hematology and oncology: presentation, diagnosis, and treatment. London: Churchill Livingstone; 2003. [Google Scholar]
- 8.Blajchman MA. J Thromb Haemost. 2003;1:1637. doi: 10.1046/j.1538-7836.2003.00332.x. [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD. Semin Hematol. 2000;37:11. doi: 10.1016/s0037-1963(00)90048-9. [DOI] [PubMed] [Google Scholar]
- 10.Clark HS. The history of hemostasis. Paul B. Hoeber, Inc.; New York: 1929. [Google Scholar]
- 11.Morawitz P. Ergebn Physiol. 1905;4:307. [Google Scholar]
- 12.Boulton F. Transfusion Medicine. 2006;16:1. doi: 10.1111/j.1365-3148.2006.00643.x. [DOI] [PubMed] [Google Scholar]
- 13.Macfarlane RG. Nature. 1964;202:498. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 14.Davie EW, Ratnoff OD. Science. 1964;145:1310. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 15.Ruggeri ZM, Mendolicchio GL. Circ Res. 2007;100:1673. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 16.Varga-Szabo D, Pleines I, Nieswandt B. Arterioscler Thromb Vasc Biol. 2008;28:403. doi: 10.1161/ATVBAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 17.Versteeg HH, Heemskerk JWM, Levi M, Reitsma PH. Physiol Rev. 2013;93:327. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 18.Eyre L, Gamlin F. Anaesthesia and Intensive Care Med. 2010;11:6. [Google Scholar]
- 19.Roberts HR, Hoffman M, Monroe DM. Semin Thromb Hemost. 2006;32:32. doi: 10.1055/s-2006-939552. [DOI] [PubMed] [Google Scholar]
- 20.Gale AJ. Toxicol Pathol. 2011;39:273. doi: 10.1177/0192623310389474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman M. J Thromb Thrombolysis. 2003;16:17. doi: 10.1023/B:THRO.0000014588.95061.28. [DOI] [PubMed] [Google Scholar]
- 22.Heemskerk JWM, Bevers EM, Lindhout T. Thromb Haemost. 2002;88:186. [PubMed] [Google Scholar]
- 23.Becker RC. J Thromb Thrombolysis. 2005;20:65. doi: 10.1007/s11239-005-3118-3. [DOI] [PubMed] [Google Scholar]
- 24.Yamaji-Hasegawa A, Tsujimoto M. Biol Pharm Bull. 2006;29:1547. doi: 10.1248/bpb.29.1547. [DOI] [PubMed] [Google Scholar]
- 25.Zwaal RF, Comfurius P, Bevers EM. Cell Mol Life Sci. 2005;62:971. doi: 10.1007/s00018-005-4527-3. [DOI] [PubMed] [Google Scholar]
- 26.Lentz BR. Pogress in Lipid Res. 2003;42:423. doi: 10.1016/s0163-7827(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 27.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Proc Natl Acad Sci USA. 2006;103:903. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz FA, Lea CR, Oldfield E, Docampo R. J Biol. Chem. 2004;279:44250. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- 29.Morrissey JH, Choi SH, Smith SA. Blood. 2012;119:5972. doi: 10.1182/blood-2012-03-306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrissey JH. Int J Hematol. 2012;95:346. doi: 10.1007/s12185-012-1054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É. Physiol Rev. 2011;91:931. doi: 10.1152/physrev.00016.2010. [DOI] [PubMed] [Google Scholar]
- 32.Ariēns RAS, Lai T-S, Weisel JW, Greenberg CS, Grant PJ. Blood. 2002;100:743. doi: 10.1182/blood.v100.3.743. [DOI] [PubMed] [Google Scholar]
- 33.Jen CJ, McIntire LV. Cell. Motil. 1982;2:445. doi: 10.1002/cm.970020504. [DOI] [PubMed] [Google Scholar]
- 34.Weisel JW. Biophys. Chem. 2004;112:267. doi: 10.1016/j.bpc.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 35.Lam WA, Chaudhuri O, Crow A, Webster KD, Li T-D, Kita A, Huang J, Fletcher DA. Nature Mat. 2011;10:61. doi: 10.1038/nmat2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapin JC, Hajjar KA. Blood Rev. 2015;9:17. doi: 10.1016/j.blre.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Travis J, Salvesen GS. Annu. Rev. Biochem. 1983;52:655. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- 38.Broze GJ, Higuchi DA. Blood. 1996;88:3815. [PubMed] [Google Scholar]
- 39.Mosnier OL, Lisman T, van den Berg HM, Nieuwenhuis HK, Meijers JC, Bouma BN. Thromb. Haemost. 2001;86:1035. [PubMed] [Google Scholar]
- 40.Hoylaerts M, Rijken DC, Lijnen HR, Collen D. J Biol. Chem. 1982;257:2912. [PubMed] [Google Scholar]
- 41.Plow EF, Collen D. Blood. 1981;58:1069. [PubMed] [Google Scholar]
- 42.Peng T. Trends Biomater Artif Organs. 2010;24:27. [Google Scholar]
- 43.di Lena F. J Mater Chem B. 2014;2:3567. doi: 10.1039/c3tb21739f. [DOI] [PubMed] [Google Scholar]
- 44.Alam HB, Burris D, Dacorta JA, Rhee P. Military Medicine. 2005;170:63. doi: 10.7205/milmed.170.1.63. [DOI] [PubMed] [Google Scholar]
- 45.Pusateri AE, Holcomb JB, Kheirabadi BS, Alam HB, Wade CE, Ryan KL. J Trauma. 2006;60:674. doi: 10.1097/01.ta.0000196672.47783.fd. [DOI] [PubMed] [Google Scholar]
- 46.Annabi N, Tamayol A, Shin SR, Ghaemmaghami AM, Peppas NA, Khademhosseini A. Nano Today. 2014;9:574. doi: 10.1016/j.nantod.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabay M, Boucher BA. Pharmacotherapy. 2013;33:935. doi: 10.1002/phar.1291. [DOI] [PubMed] [Google Scholar]
- 48.Blackbourne LH, Baer DG, Eastridge BJ, Kheirabadi B, Kragh JF, Cap AP, Dubick MA, Morrison JJ, Midwinter MJ, Butler FK, Kotwal RS, Holcomb JB. J Trauma Acute Care Surg. 2012;73:S372. doi: 10.1097/TA.0b013e3182755662. [DOI] [PubMed] [Google Scholar]
- 49.Chandler MH, Roberts M, Sawyer M, Myers G. Seminars in Cardiothoracic and Vascular Anesthesia. 2012;16:153. doi: 10.1177/1089253212452344. [DOI] [PubMed] [Google Scholar]
- 50.Kauvar DS, Holcomb JB, Norris GC, Hess JR. J Trauma. 2006;61:181. doi: 10.1097/01.ta.0000222671.84335.64. [DOI] [PubMed] [Google Scholar]
- 51.Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Azarow K, Holcomb JB. Crit Care Med. 2007;35:2576. doi: 10.1097/01.CCM.0000285996.65226.A9. [DOI] [PubMed] [Google Scholar]
- 52.Spitalnik SL, Triulzi D, Devine DV, Dzik WH, Eder AF, Gernsheimer T, Josephson CD, Kor DJ, Luban NLC, Roubinian NH, Mondoro T, Wolniak LA, Zhou S, Glynn S. Transfusion. 2015;55:2282. doi: 10.1111/trf.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, Cipolle MD, Cohn CS, Fung MK, Grossman BJ, Mintz PD, O’Malley BA, Sesok-Pizzini DA, Shander A, Stack GE, Webert KE, Weinstein R, Welch BG, Whitman GJ, Wong EC, Tobian AAR. Ann Intern Med. 2015;162:205. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 54.Etchill EW, Myers SP, Raval JS, Hassoune A, Sen Gupta A, Neal MD. Shock. 2017;47:537. doi: 10.1097/SHK.0000000000000794. [DOI] [PubMed] [Google Scholar]
- 55.Roberts HR. Semin Hematol. 2001;38:48. doi: 10.1016/s0037-1963(01)90148-9. [DOI] [PubMed] [Google Scholar]
- 56.Berrettini M, Mariani G, Schiavoni M, Rocino A, Di Paolantonio T, Longo G, Morfini M. Haematologica. 2001;86:640. [PubMed] [Google Scholar]
- 57.Fries D, Martini WZ. Br J Anaesthesia. 2010;105:116. doi: 10.1093/bja/aeq161. [DOI] [PubMed] [Google Scholar]
- 58.Rahe-Meyer N, Sørensen B. J Thromb Haemost. 2011;9:1. doi: 10.1111/j.1538-7836.2010.04099.x. [DOI] [PubMed] [Google Scholar]
- 59.Levy JH, Goodnough LT. Blood. 2015;125:1387. doi: 10.1182/blood-2014-08-552000. [DOI] [PubMed] [Google Scholar]
- 60.Corash L. Transfus Med Rev. 1999;13:18. doi: 10.1016/s0887-7963(99)80085-6. [DOI] [PubMed] [Google Scholar]
- 61.Heal JM, Blumberg N. Blood Reviews. 2004;18:149. doi: 10.1016/S0268-960X(03)00057-2. [DOI] [PubMed] [Google Scholar]
- 62.Mohanty D. Asian J Transfus Sci. 2009;3:18. doi: 10.4103/0973-6247.45257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee D, Blajchman MA. Br J Haematol. 2001;114:496. doi: 10.1046/j.1365-2141.2001.03004.x. [DOI] [PubMed] [Google Scholar]
- 64.Blajchman MA. Transfus Clin Biol. 2001;8:267. doi: 10.1016/s1246-7820(01)00127-6. [DOI] [PubMed] [Google Scholar]
- 65.Epstein JS, Vostal JG. Transfusion. 2003;43:1347. doi: 10.1046/j.1537-2995.2003.00584.x. [DOI] [PubMed] [Google Scholar]
- 66.Galan AM, Lozano M, Molina P, Navalon F, Marschner S, Goodrich R, Escolar G. Transfusion. 2011;51:808. doi: 10.1111/j.1537-2995.2010.02914.x. [DOI] [PubMed] [Google Scholar]
- 67.Lambert MP, Sullivan SK, Fuentes R, French DL, Poncz M. Blood. 2013;121:3319. doi: 10.1182/blood-2012-09-455428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Solheim BG. Transfus Apher Sci. 2008;39:75. doi: 10.1016/j.transci.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Seghatchian J, de Sousa G. Transfus Apher Sci. 2006;35:189. doi: 10.1016/j.transci.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Janetzko K, Hinz K, Marschner S, Goodrich R, Klüter H. Vox Sanguinis. 2009;97:234. doi: 10.1111/j.1423-0410.2009.01193.x. [DOI] [PubMed] [Google Scholar]
- 71.Pidcoke HF, McFaul SJ, Ramasubramanian AK, Parida BK, Mora AG, Fedyk CG, Valdez-Delgado KK, Montgomery RK, Reddoch KM, Rodriguez AC, Aden JK, Jones JA, Bryant RS, Scherer MR, Reddy HL, Goodrich RP, Cap AP. Transfusion. 2013;53:137S. doi: 10.1111/trf.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Avanzi MP, Mitchell WB. Br J Haematol. 2014;165:237. doi: 10.1111/bjh.12764. [DOI] [PubMed] [Google Scholar]
- 73.Nakamura S, Takayama N, Hirata S, Seo H, Endo H, Ochi K, Fujita K, Koike T, Harimoto K, Dohda T, Watanabe A, Okita K, Takahashi N, Sawaguchi A, Yamanaka S, Nakauchi H, Nishimura S, Eto K. Cell Stem Cell. 2014;14:535. doi: 10.1016/j.stem.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 74.Thon JN, Mazutis L, Wu S, Sylman JL, Ehrlicher A, Machlus KR, Feng Q, Lu S, Lanza R, Neeves KB, Weitz DA, Italiano JE., Jr Blood. 2014;124:1857. doi: 10.1182/blood-2014-05-574913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kragh JF, Jr, Swan KG, Smith DC, Mabry RL, Blackbourne LH LH. Am J Surgery. 2012;203:242. doi: 10.1016/j.amjsurg.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 76.Pilcher LS. Treatment of Wounds: Its Principles and Practice, General and Special. 2. William Wood; New York: 1899. [Google Scholar]
- 77.Lakstein D, Blumenfeld A, Sokolov T, Lin G, Bssorai R, Lynn M, Ben-Abraham MR. J Trauma. 2003;54:S221. doi: 10.1097/01.TA.0000047227.33395.49. [DOI] [PubMed] [Google Scholar]
- 78.Kragh JF, Jr, Walters TJ, Baer DG, Fox CJ, Wade CE, Salinas J, Holcomb JB. Annals of Surgery. 2009;249:1. doi: 10.1097/SLA.0b013e31818842ba. [DOI] [PubMed] [Google Scholar]
- 79.Walters TJ, Wenke JC, Kauvar DS, McManus JG, Holcomb JB, Baer DG. Prehospital Emergency Care. 2005;9:416. doi: 10.1080/10903120500255123. [DOI] [PubMed] [Google Scholar]
- 80.Moore FA. Annals of Surgery. 2009;249:8. doi: 10.1097/SLA.0b013e3181932329. [DOI] [PubMed] [Google Scholar]
- 81.Cain JS. J Trauma: War Trauma. 2008;3:16. [Google Scholar]
- 82.Ivanova EP, Bazaka K, Crawford R. New Functional Biomaterials for Medicine and Healthcare. Elsevier Science; 2014. book; p. 32. [Google Scholar]
- 83.Pierce AM, Wiebkin OW, Wilson DF. J Oral Pathol. 1984;13:661. doi: 10.1111/j.1600-0714.1984.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 84.Lewis KM, Spazierer D, Urban MD, Lin L, Redl H, Goppelt A. Eur Surg. 2013;45:213. doi: 10.1007/s10353-013-0222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Edwards JV, Prevost N. J Funct Biomater. 2011;2:391. doi: 10.3390/jfb2040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Edwards JV, Graves E, Bopp A, Prevost N, Santiago M, Condon B. J Funct Biomater. 2014;5:273. doi: 10.3390/jfb5040273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Calvini P, Conio G, Lorenzoni M, Pedemonte E. Cellulose. 2004;11:99. [Google Scholar]
- 88.Calvini P, Conio G, Princi E, Vicini S, Pedemonte E. Cellulose. 2006;13:571. [Google Scholar]
- 89.Zimnitsky DS, Yurkshtovich TL, Bychkovsky PM. J Polym Sci. Part A: Polym Chem. 2004;42:4785. [Google Scholar]
- 90.Miller JM, Jackson DA, Collier CS. Med Surgery. 1961;19:196. [PubMed] [Google Scholar]
- 91.Bajerová M, Krejčová K, Rabišková M, Gajdziok J, Masteiková R. Adv Polym Technol. 2009;28:199. [Google Scholar]
- 92.Frantz VK, Lattes R. J Am Med Assoc. 1945;129:798. [Google Scholar]
- 93.Yackel EC, Kenyon WO. J Am Chem Soc. 1941;64:121. [Google Scholar]
- 94.Frantz V. Ann Surg. 1943;118:116. doi: 10.1097/00000658-194311810-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu Y, He J, Cheng W, Gu H, Guo Z, Gao S, Huang Y. Carbohydrate Polymers. 2012;88:1023. [Google Scholar]
- 96.Keshavarzi S, Macdougall M, Lulic D, Kasasbeh A, Levy M. Wounds. 2013;25:160. [PubMed] [Google Scholar]
- 97.Christenson LJ, Otley CC, Roenigk RK. Dermatol Surg. 2004;30:1593. doi: 10.1111/j.1524-4725.2004.30574.x. [DOI] [PubMed] [Google Scholar]
- 98.Mair H, Kaczmarek I, Oberhoffer M, Groetzner J, Daebritz S, Reichart B. J Thorac Cardiovasc Surg. 2005;130:605. doi: 10.1016/j.jtcvs.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 99.Arat YO, Dorotheo EU, Tang RA, Boniuk M, Schiffman JS. Ophthalmology. 2006;113:333. doi: 10.1016/j.ophtha.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 100.Laurence S, Bareille R, Baquey C, Fricain JC. J Biomed Mater Res – Part A. 2005;73:422. doi: 10.1002/jbm.a.30280. [DOI] [PubMed] [Google Scholar]
- 101.Dimitrijevich SD, Tatarko M, Gracy RW. Carbohydrate Research. 1990;195:247. doi: 10.1016/0008-6215(90)84169-u. [DOI] [PubMed] [Google Scholar]
- 102.Dimitrijevich SD, Tatarko M, Gracy RW, Wise GE. Carbohydrate Research. 1990;198:331. doi: 10.1016/0008-6215(90)84303-c. [DOI] [PubMed] [Google Scholar]
- 103.Muench TR, Kong W, Harmon AM. Biomaterials. 2010;31:3649. doi: 10.1016/j.biomaterials.2010.01.070. [DOI] [PubMed] [Google Scholar]
- 104.Hoekstra MJ, Hermans MH, Richters CD, Dutrieux RP. J Wound Care. 2002;11:113. doi: 10.12968/jowc.2002.11.3.26384. [DOI] [PubMed] [Google Scholar]
- 105.Morgan DA. Pharm J. 1999;263:820. [Google Scholar]
- 106.Moody A. Br J Comm Nurs. 2006;11:S12. doi: 10.12968/bjcn.2006.11.Sup3.21212. [DOI] [PubMed] [Google Scholar]
- 107.Bennett-Marsden M. Clinical Pharmacist. 2010;2:363. [Google Scholar]
- 108.Gray E. Surg Gynecol Obstet. 1915;21:452. [Google Scholar]
- 109.Harvey S. Boston Med Surg. 1916;174:658. [Google Scholar]
- 110.Spotnitz WD. World J Surg. 2010;34:632. doi: 10.1007/s00268-009-0252-7. [DOI] [PubMed] [Google Scholar]
- 111.Albala D. Cardiovasc Surg. 2003;11:S5. doi: 10.1016/S0967-2109(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 112.Sierra DH. J Biomater Appl. 1993;7:309. doi: 10.1177/088532829300700402. [DOI] [PubMed] [Google Scholar]
- 113.Borst HG, Haverich A, Walterbusch G, Maatz W. J Thorac Cardiovasc Surg. 1982;84:548. [PubMed] [Google Scholar]
- 114.Sawamura Y, Asaoka K, Terasaka S, Tada M, Uchida T. Neurosurgery. 1999;44:332. doi: 10.1097/00006123-199902000-00048. [DOI] [PubMed] [Google Scholar]
- 115.Mandell SP, Gibran NS. Expert Opin Biol Ther. 2014;14:821. doi: 10.1517/14712598.2014.897323. [DOI] [PubMed] [Google Scholar]
- 116.Cronkite EP, Lozner EL, Deaver JM. J Am Med Assoc. 1944;124:976. [Google Scholar]
- 117.Tedrick RT, Warner ED. Surgery. 1944;15:90. [Google Scholar]
- 118.Erdogan D, Van Gulik TM. J Biomed Mater Res - Part B: Applied Biomaterials. 2008;85:272. doi: 10.1002/jbm.b.30916. [DOI] [PubMed] [Google Scholar]
- 119.Scheyer M, Zimmermann G. Surg Endosc. 1996;10:501. doi: 10.1007/BF00188394. [DOI] [PubMed] [Google Scholar]
- 120.Schiele U, Kuntz G, Riegler A. Clin Mater. 1992;9:169. doi: 10.1016/0267-6605(92)90097-d. [DOI] [PubMed] [Google Scholar]
- 121.Larson MJ, Bowersox JC, Lim RC, Jr, Hess JR. Arch Surg. 1995;130:420. doi: 10.1001/archsurg.1995.01430040082018. [DOI] [PubMed] [Google Scholar]
- 122.Jackson MR, Friedman SA, Carter AJ, Bayer V, Burge JR, MacPhee MJ, Drohan WN, Alving BM. J Vasc Surg. 1997;26:274. doi: 10.1016/s0741-5214(97)70189-7. [DOI] [PubMed] [Google Scholar]
- 123.Pusateri AE, Kheirabadi BS, Delgado AV, Doyle JW, Kanellos J, Uscilowicz JM, Martinez RS, Holcomb JB, Modrow HL. J Biomed Mater Res Part B: Applied Biomaterials. 2004;70:114. doi: 10.1002/jbm.b.30031. [DOI] [PubMed] [Google Scholar]
- 124.Holcomb J, MacPhee M, Hetz S, Harris R, Pusateri A, Hess J. Arch Surg. 1998;133:32. doi: 10.1001/archsurg.133.1.32. [DOI] [PubMed] [Google Scholar]
- 125.Holcomb J, Pusateri A, Harris R. J Trauma. 1999;46:49. doi: 10.1097/00005373-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 126.Jackson M, Taher M, Burge J, Krishnamurti C, Reid T, Alving B. J Trauma. 1998;45:662. doi: 10.1097/00005373-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 127.Kheirabadi BS, Acheson EM, Deguzman R, Crissey JM, Delgado AV, Estep SJ, Holcomb JB. Infection and Critical Care. 2007;62:94. doi: 10.1097/01.ta.0000251595.45451.d0. [DOI] [PubMed] [Google Scholar]
- 128.Pusateri AE, Holcomb JB, Harris RA, MacPhee MJ, Charles NC, Beall LD, Hess JR. Military Med. 2001;166:217. [PubMed] [Google Scholar]
- 129.Morey AF, Anema JG, Harris R, Gresham V, Daniels R, Knight RW, Beall D, Macphee M, Cornum RL. J Urol. 2001;165:955. [PubMed] [Google Scholar]
- 130.Anema JG, Morey AF, Harris R, MacPhee M, Cornum RL. World J Surg. 2001;25:1573. doi: 10.1007/s00268-001-0152-y. [DOI] [PubMed] [Google Scholar]
- 131.Cornum R, Bell J, Gresham V, Brinkley W, Beall D, Macphee M. J Urol. 1999;162:1817. [PubMed] [Google Scholar]
- 132.Delgado AV, Kheirabadi BS, Fruchterman TM, Scherer M, Cortez D, Wade CE, Dubick MA, Holcomb J. J Trauma. 2008;64:75. doi: 10.1097/TA.0b013e31815b843c. [DOI] [PubMed] [Google Scholar]
- 133.Lundblad RL, Bradshaw RA, Gabriel D, Ortel TL, Lawson J, Mann KG. Thromb Haemost. 2004;91:851. doi: 10.1160/TH03-12-0792. [DOI] [PubMed] [Google Scholar]
- 134.Jackson MR. Am J Surg. 2001;182:S1. [Google Scholar]
- 135.Kjaergard H, Weis-Fogh U. Eur Surg Res. 1994;26:273. doi: 10.1159/000129346. [DOI] [PubMed] [Google Scholar]
- 136.Saltz R, Sierra D, Feldman D, Saltz MB, Dimick A, Vasconez LO. Plast Reconstr Surg. 1991;88:1005. [PubMed] [Google Scholar]
- 137.Kheirabadi BS, Pearson R, Rudnicka K, Somwaru L, MacPhee M, Drohan W, Tuthill D. J Surg Res. 2001;100:84. doi: 10.1006/jsre.2001.6226. [DOI] [PubMed] [Google Scholar]
- 138.Joch C. Cardiovasc Surg. 2003;11:23. doi: 10.1016/S0967-2109(03)00068-1. [DOI] [PubMed] [Google Scholar]
- 139.Izumi Y, Gika M, Shinya N, Miyabashira S, Imamura T, Nozaki C, Kawamura M, Kobayashi K. J Trauma. 2007;63:783. doi: 10.1097/TA.0b013e318151ffdc. [DOI] [PubMed] [Google Scholar]
- 140.Rothwell SW, Fudge JM, Reid TJ, Krishnamurti C. Thromb Res. 2002;108:341. doi: 10.1016/s0049-3848(03)00111-7. [DOI] [PubMed] [Google Scholar]
- 141.Phillips M, Dickneite G, Metzner H. Cardiovasc Surg. 2003;11:13. doi: 10.1016/S0967-2109(03)00066-8. [DOI] [PubMed] [Google Scholar]
- 142.Busuttil RW. J Am Coll Surg. 2003;197:1021. doi: 10.1016/j.jamcollsurg.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 143.Kheirabadi BS, Pearson R, Tuthill D, Rudnicka K, Holcomb JB, Drohan W, MacPhee MJ. J Trauma - Injury, Infection and Critical Care. 2002;52:1107. doi: 10.1097/00005373-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 144.Ortel TL, Mercer MC, Thames EH, Moore KD, Lawson JH. Ann Surg. 2001;233:88. doi: 10.1097/00000658-200101000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kheirabadi BS, Pusateri AE, Sondeen JL, Delgado AV, Modrow HE, Hess JR, Holcomb JB. NATO Science and Technology Organization, Symposium Paper RTO-MPHFM-109. 2004:P34. [Google Scholar]
- 146.Krishnan LK, Mohanty M, Umashankar PR, Lal AV. Biomaterials. 2004;25:5557. doi: 10.1016/j.biomaterials.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 147.Wang LZ, Gorlin J, Michaud SE, Janmey PA, Goddeau RP, Kuuse R, Uibo R, Adams D, Sawyer ES. Thromb Res. 2000;100:537. doi: 10.1016/s0049-3848(00)00362-5. [DOI] [PubMed] [Google Scholar]
- 148.Michaud SE, Wang LZ, Korde N, Bucki R, Randhawa PK, Pastore JJ, Falet H, Hoffmeister K, Kuuse R, Uibo R, Herod J, Sawyer E, Janmey PA. Thromb Res. 2002;107:245. doi: 10.1016/s0049-3848(02)00333-x. [DOI] [PubMed] [Google Scholar]
- 149.Rothwell SW, Reid TJ, Dorsey J, Flournoy WS, Bodo M, Janmey PA, Sawyer E. J Trauma - Injury, Infection and Critical Care. 2005;59:143. doi: 10.1097/01.ta.0000171528.43746.53. [DOI] [PubMed] [Google Scholar]
- 150.Laidmäe I, McCormick ME, Herod JL, Pastore JJ, Salum T, Sawyer ES, Janmey PA, Uibo R. Biomaterials. 2006;27:5771. doi: 10.1016/j.biomaterials.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 151.Rothwell SW, Settle T, Wallace S, Dorsey J, Simpson D, Bowman JR, Janmey P, Sawyer E. Biologicals. 2010;38:619. doi: 10.1016/j.biologicals.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mullin JL, Gorkun OV, Binnie CG, Lord ST. J Biol Chem. 2000;275:25239. doi: 10.1074/jbc.M004142200. [DOI] [PubMed] [Google Scholar]
- 153.Ham SW, Lew WK, Weaver FA. J Blood Med. 2010;1:135. doi: 10.2147/JBM.S6622. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 154.Cheng CM, Meyer-Massetti C, Kayser SR. Clin Therapeutics. 2009;31:32. doi: 10.1016/j.clinthera.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 155.Bishop P, Lawson J. Nature Reviews Drug Discovery. 2004;3:684. doi: 10.1038/nrd1443. [DOI] [PubMed] [Google Scholar]
- 156.Houdebine LM. Comp Immun Microbiol Inf Dis. 2009;32:107. doi: 10.1016/j.cimid.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Spotnitz WD. ISRN Surgery. 2014 doi: 10.1155/2014/203943. Article ID 203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Buchta C, Dettke M, Funovics PT, Höcker P, Knöbl P, Macher M, Quehenberger P, Treitl C, Worel N. Vox Sang. 2004;86:257. doi: 10.1111/j.0042-9007.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 159.Davidson BR, Burnett S, Javed MS, Seifalian A, Moore D, Doctor N. Br J Surg. 2000;87:790. doi: 10.1046/j.1365-2168.2000.01427.x. [DOI] [PubMed] [Google Scholar]
- 160.Kjaergard HK, Trumbull HR. Ann Thorac Surg. 2000;69:1173. doi: 10.1016/s0003-4975(99)01560-x. [DOI] [PubMed] [Google Scholar]
- 161.Drake DB, Wong LG. Ann Plast Surg. 2003;50:367. doi: 10.1097/01.SAP.0000041484.22953.6D. [DOI] [PubMed] [Google Scholar]
- 162.Fukunaga N, Uchida T, Kaetsu H, Kawakami K, Ishihara Y, Funatsu A. Int J Adhesion and Adhesives. 1998;18:345. [Google Scholar]
- 163.Holcomb JB, McClain JM, Pusateri AE, Beall D, Macaitis JM, Harris RA, MacPhee MJ, Hess JR. J Trauma. 2000;49:246. doi: 10.1097/00005373-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 164.Kheirabadi BS, Sieber J, Bukhari T, Rudnicka K, Murcin LA, Tuthill D. J Surg Res. 2008;144:145. doi: 10.1016/j.jss.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 165.Finley DS, Lee DI, Eichel L, Uribe CA, McDougall EM, Clayman RV. J Urol. 2005;173:1477. doi: 10.1097/01.ju.0000154165.12738.7f. [DOI] [PubMed] [Google Scholar]
- 166.Friess W. Eur J Pharm Biopharm. 1998;45:113. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 167.Gelse K, Pöschl E, Aigner T. Adv Drug Del Rev. 2003;55:1531. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 168.Savage B, Almus-Jacobs F, Ruggeri ZM. Cell. 1998;94:657. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 169.Chen J, Lopez J. Microcirculation. 2005;12:235. doi: 10.1080/10739680590925484. [DOI] [PubMed] [Google Scholar]
- 170.Farndale RW, Sixma JJ, Barnes MJ, De Groot PG. J Thromb Haemost. 2004;2:561. doi: 10.1111/j.1538-7836.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 171.Hait MR, Battista OA, Stark RB, McCord CW. Surg Forum. 1969;20:51. [PubMed] [Google Scholar]
- 172.Hait MR. Am J Surg. 1970;120:330. [PubMed] [Google Scholar]
- 173.Hait MR, Robb CA, Baxter CR, Borgmann AR, Tippett LO. Am J Surg. 1973;125:284. doi: 10.1016/0002-9610(73)90042-1. [DOI] [PubMed] [Google Scholar]
- 174.Silverstein ME, Keown K, Owen JA, Chvapil M. J Trauma. 1980;20:688. doi: 10.1097/00005373-198008000-00010. [DOI] [PubMed] [Google Scholar]
- 175.Wagner WR, Pachence JM, Ristich J, Johnson PC. J Surg Res. 1996;66:100. doi: 10.1006/jsre.1996.0379. [DOI] [PubMed] [Google Scholar]
- 176.Zwischenberger JB, Brunston RL, Jr, Swann JR, Conti VR. J Invest Surg. 1999;12:101. doi: 10.1080/089419399272656. [DOI] [PubMed] [Google Scholar]
- 177.Yang C, Hillas P, Tang J, Balan J, Notbohm H, Polarek J. J Biomed Mater Res - Part B Applied Biomaterials. 2004;69:18–24. doi: 10.1002/jbm.b.20030. [DOI] [PubMed] [Google Scholar]
- 178.Browder IW, Litwin MS. Am Surg. 1986;52:492. [PubMed] [Google Scholar]
- 179.Silverstein ME, Chvapil M. J Trauma. 1981;21:388. doi: 10.1097/00005373-198105000-00011. [DOI] [PubMed] [Google Scholar]
- 180.Prior JJ, Wallace DG, Harner A, Powers N. Ann Thorac Surg. 1999;68:479. doi: 10.1016/s0003-4975(99)00552-4. [DOI] [PubMed] [Google Scholar]
- 181.Nelson PA, Nol Powers J, Estridge TD, Elder EA, Alea AD, Sidhu PK, Sehl LC, Delustro FA. J Biomed Mater Res. 2001;58:710. doi: 10.1002/jbm.10025. [DOI] [PubMed] [Google Scholar]
- 182.Chapman WC, Sherman R, Boyce S, Malawer M, Hill A, Buncke G, Block JE, Fung JJ, Clavien P, Lee KF, Lebovic GS, Wren SM, Diethrich E, Goldsein R. Surgery. 2001;129:445. [Google Scholar]
- 183.Bochicchio G, Dunne J, Bochicchio K, Scalea T. J Trauma - Injury, Infection and Critical Care. 2004;56:76. doi: 10.1097/01.TA.0000103985.26884.D2. [DOI] [PubMed] [Google Scholar]
- 184.Bigi A, Cojazzi G, Panzavolta S, Roveri N, Rubini K. Biomaterials. 2002;23:4827. doi: 10.1016/s0142-9612(02)00235-1. [DOI] [PubMed] [Google Scholar]
- 185.Bertoni F, Barbani N, Giusti P, Giardelli G. Biotechnology Letters. 2006;28:697. doi: 10.1007/s10529-006-9046-2. [DOI] [PubMed] [Google Scholar]
- 186.Kabiri M, Emami SH, Rafinia M, Tahriri M. Curr Appl Phys. 2011;11:457. [Google Scholar]
- 187.Traver MA, Assimos DG. Rev Urol. 2006;8:104. [PMC free article] [PubMed] [Google Scholar]
- 188.Xie X, Tian J, Lv F, Wu R, Tang W, Luo Y, Huang Y, Tang J. Act Pharmacol Sin. 2013;34:983. doi: 10.1038/aps.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Eastoe JE, Leach AA, editors. Chemical Constitution of Gelatin. Academic Press; London, UK: 1977. [Google Scholar]
- 190.Jenkins HP, Senz EH, Owen HW, Jampolis RW. J Am Med Assoc. 1946;132:614. doi: 10.1001/jama.1946.02870460004002. [DOI] [PubMed] [Google Scholar]
- 191.Fairbairn JW, Whittet TD. Pharm J. 1948;160:149. [PubMed] [Google Scholar]
- 192.Permpongkosol S, Nicol TL, Bagga HS, Kohanim S, Kavoussi L, Solomon SB. J Vasc Interv Radiol. 2006;17:1505. doi: 10.1097/01.RVI.0000235773.23394.51. [DOI] [PubMed] [Google Scholar]
- 193.Griffith BC, Morey AF, Rozanski TA, Harris R, Dalton SR, Torgerson SJ, Partyka SR. J Urol. 2004;171:445. doi: 10.1097/01.ju.0000101000.08371.79. [DOI] [PubMed] [Google Scholar]
- 194.Lewis KM, Atlee HD, Mannone AJ, Dwyer J, Lin L, Goppelt A. J Invest Surg. 2013;26:141. doi: 10.3109/08941939.2012.724519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.L’Esperance JO, Sung JC, Marguet CG, Maloney ME, Springhart WP, Preminger GM, Albala DM. J Endourol. 2005;19:1114. doi: 10.1089/end.2005.19.1114. [DOI] [PubMed] [Google Scholar]
- 196.Bak JB, Singh A, Shekarriz B. J Urol. 2004;171:780. doi: 10.1097/01.ju.0000104800.97009.c6. [DOI] [PubMed] [Google Scholar]
- 197.Weaver FA, Hood DB, Zatina M, Messina L, Badduke B. Ann Vasc Surg. 2002;16:286. doi: 10.1007/s10016-001-0073-0. [DOI] [PubMed] [Google Scholar]
- 198.Oz MC, Rondinone JF, Shargill NS. J Card Surg. 2003;18:486. doi: 10.1046/j.0886-0440.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 199.Leixnering M, Reichetseder J, Schultz A, Figl M, Wassermann E, Thurnher M, Redl H. J Trauma. 2008;64:456. doi: 10.1097/TA.0b013e3180340de1. [DOI] [PubMed] [Google Scholar]
- 200.Brachet J, Goudot B, Dreyfus G, Banfi C, Ayle NA, Aota M, Brodaty D, Dubois C, Delentdecker P, Guilmet D. J Card Surg. 1997;12:243. [PubMed] [Google Scholar]
- 201.Kodama K, Doi O, Higashiyama M, Yokouchi H. Eur J Cardiothorac Surg. 1997;11:333. doi: 10.1016/s1010-7940(96)01006-8. [DOI] [PubMed] [Google Scholar]
- 202.Kunihara T, Shiiya N, Matsuzaki K, Murashita T, Matsui Y. Ann Thorac Cardiovasc Surg. 2008;14:88. [PubMed] [Google Scholar]
- 203.Yang C, Hillas P, Tang J, Balan J, Notbohm H, Polarek J. J Biomed Mater Res. 2004;69B:18. doi: 10.1002/jbm.b.20030. [DOI] [PubMed] [Google Scholar]
- 204.Yang C, Hillas PJ, Baez JA, Nokelainen M, Balan J, Tang J, Spiro R, Polarek JW. BioDrugs. 2004;18:103. doi: 10.2165/00063030-200418020-00004. [DOI] [PubMed] [Google Scholar]
- 205.Olsen D, Yang C, Bodo M, Chang R, Leigh S, Baez J, Carmichael D, Perala M, Hamalainen ER, Jarvinen M, Polarek J. Adv Drug Del Rev. 2003;55:1547. doi: 10.1016/j.addr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 206.Rathinamoorthy R, Sasikala L. Int J Pharmacy and Pharm Sci. 2011;3:38. [Google Scholar]
- 207.Lee KY, Mooney DJ. Prog Polym Sci. 2012;37:106. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Knill CJ, Kennedy JF, Mistry J, Miraftab M, Smart G, Groocock MR, Williams HJ. Carbohydrate Polym. 2004;55:65. [Google Scholar]
- 209.Thomas S. J Wound Care. 2000;9:56. doi: 10.12968/jowc.2000.9.2.26338. [DOI] [PubMed] [Google Scholar]
- 210.Segal HC, Hunt BJ, Gilding K. J Biomater Appl. 1998;12:249. doi: 10.1177/088532829801200305. [DOI] [PubMed] [Google Scholar]
- 211.Matthew IR, Browne RM, Frame JW, Millar BG. Oral Med Oral Pathol. 1994;77:456. doi: 10.1016/0030-4220(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 212.Matthew IR, Browne RM, Frame JW, Millar BG. Br J Oral Maxillofac Surg. 1993;31:165. doi: 10.1016/0266-4356(93)90117-f. [DOI] [PubMed] [Google Scholar]
- 213.Paul W, Sharma CP. Trends Biomater Artif Organs. 2004;18:18. [Google Scholar]
- 214.Li D, Li P, Zang J, Liu J. J Biomed Biotechnol. 2012;2012:981321. doi: 10.1155/2012/981321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rong J, Liang M, Xuan F, Sun J, Zhao L, Zhen H, Tian X, Liu D, Zhang Q, Peng C, Yao T, Li F, Wang X, Han Y, Yu W. Int J Biol Macromol. 2015;75:479. doi: 10.1016/j.ijbiomac.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 216.Qin Y. Polym Int. 2008;57:171. [Google Scholar]
- 217.Thu HE, Zulfakar MH, Ng SF. Int J Pharm. 2012;434:375. doi: 10.1016/j.ijpharm.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 218.Mogoşanu GD, Grumezescu AM. Int J Pharm. 2014;463:127. doi: 10.1016/j.ijpharm.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 219.Balakrishnan B, Mohanty M, Umashankar PR, Jayakrishnan A. Biomaterials. 2005;26:6335. doi: 10.1016/j.biomaterials.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 220.Dutta PK, Dutta J, Tripathi VS. J Sci Industrial Res. 2004;63:20. [Google Scholar]
- 221.Malette WG, Quigley HJ, Gaines RD, Johnson ND, Rainer WG. Ann. Thorac. Surg. 1983;36:55. doi: 10.1016/s0003-4975(10)60649-2. [DOI] [PubMed] [Google Scholar]
- 222.Bennett BL, Littlejohn L. Mil. Med. 2014;179:497. doi: 10.7205/MILMED-D-13-00199. [DOI] [PubMed] [Google Scholar]
- 223.Chan LW, Kim CH, Wang X, Pun SH, White NJ, Kim TH. Acta Biomater. 2016;31:178. doi: 10.1016/j.actbio.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hattori H, Amano Y, Nogami Y, Takase B, Ishihara M. Annals Biomed. Eng. 2010;38:3724. doi: 10.1007/s10439-010-0121-4. [DOI] [PubMed] [Google Scholar]
- 225.Whang HS, Kirsch W, Zhu YH, Yang CZ, Hudson SM. J. Macromol. Sci.: Part C, Polym. Rev. 2005;45:309. [Google Scholar]
- 226.Okamoto Y, Yano R, Miyatake K, Tomohiro I, Shigemasa Y, Minami S. Carbohydrate Polym. 2003;53:337. [Google Scholar]
- 227.Chou T-C, Fu E, Wu C-J, Yeh J-H. Biochem. Biophys. Res. Comm. 2003;302:480. doi: 10.1016/s0006-291x(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 228.Benesch J, Tengvall P. Biomaterials. 2002;23:2561. doi: 10.1016/s0142-9612(01)00391-x. [DOI] [PubMed] [Google Scholar]
- 229.Pusateri AE, McCarthy SJ, Gregory KW, Harris RA, Cardenas L, McManus AT, Goodwin CW., Jr J. Trauma. 2003;54:177. doi: 10.1097/00005373-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 230.Wedmore I, McManus JG, Psateri AE, Holcomb JB. J. Trauma. 2006;60:655. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 231.Brown MA, Daya MR, Worley JA. J. Emerg. Med. 2009;37:1. doi: 10.1016/j.jemermed.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 232.Sohn VY, Eckert MJ, Martin MJ, Arthurs ZM, Perry JR, Beekley A, Rubel EJ, Adams RP, Bickett GL, Rush RM., Jr J. Surg. Res. 2009;154:258. doi: 10.1016/j.jss.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 233.Kheirabadi BS, Edens JW, Terrazas IB, Estep JS, Klemcke HG, Dubick MA, Holcomb JB. J. Trauma. 2009;66:316. doi: 10.1097/TA.0b013e31819634a1. [DOI] [PubMed] [Google Scholar]
- 234.Gustafson SB, Fulkerson P, Bildfell R, Aguilera L, Hazzard TM. Prehosp. Emerg. Care. 2007;11:172. doi: 10.1080/10903120701205893. [DOI] [PubMed] [Google Scholar]
- 235.Millner RWJ, Lockhart AS, Bird H, Alexiou C. Ann. Thorac. Surg. 2009;87:e13. doi: 10.1016/j.athoracsur.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 236.Pozza M, Millner RWJ. Eur. J. Emerg. Med. 2011;18:31. doi: 10.1097/MEJ.0b013e32833a5ee4. [DOI] [PubMed] [Google Scholar]
- 237.Quayle JM, Thomas GOR. J. Royal Army Med. Corps. 2011;57:419. doi: 10.1136/jramc-157-04-17. [DOI] [PubMed] [Google Scholar]
- 238.Nie W, Yuan X, Zhao J, Zhou Y, Bao H. Carbohydrate Polym. 2013;96:342. doi: 10.1016/j.carbpol.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 239.Dowling MB, Smith W, Balogh P, Duggan MJ, MacIntire IC, Harris E, Mesar T, Raghavan SR, King DR. J. Surgical Res. 2015;193:316. doi: 10.1016/j.jss.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 240.Mathias J-D, Tessier-Doyen N, Michaud P. Int. J. Mol. Sci. 2011;12:1175. doi: 10.3390/ijms12021175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Testauri A, Honorez C, Barillec A, Langevin D, Drenckhan W. Macromolecules. 2010;43:6166. [Google Scholar]
- 242.Vournakis JN, Demcheva M, Whitson A, Guirca R, Pariser ER. J. Trauma. 2004;57:S2. doi: 10.1097/01.ta.0000136741.66698.9d. [DOI] [PubMed] [Google Scholar]
- 243.Fischer T, Bode A, Demcheva M, Vournakis J. J. Biomed. Mater. Res. 2007;80A:167. doi: 10.1002/jbm.a.30877. [DOI] [PubMed] [Google Scholar]
- 244.Connolly RJ. J. Trauma. 2004;57:S26. doi: 10.1097/01.ta.0000136746.97192.b2. [DOI] [PubMed] [Google Scholar]
- 245.Jewelewicz DD, Cohn SM, Crookes BA, Proctor KG. J. Trauma. 2003;55:275. doi: 10.1097/01.TA.0000079375.69610.89. [DOI] [PubMed] [Google Scholar]
- 246.King DR, Cohn SM, Proctor KG Miami Clinical Trials Group. J. Trauma. 2004;57:756. doi: 10.1097/01.ta.0000147501.64610.afs. [DOI] [PubMed] [Google Scholar]
- 247.Ersoy G, Kaynak MF, Yilmaz O, Rodoplu U, Maltepe F, Gokmen N. Adv. Ther. 2007;24:485. doi: 10.1007/BF02848770. [DOI] [PubMed] [Google Scholar]
- 248.Burgert JM, Gegel BT, Austin R, III, Davila A, Deeds J, Hodges L, Hover A, Lockhart C, Simpson G, Weaver S, Wolfe W, Johnson D. Amer. Assoc. Nurse Anesth. 2010;78:230. [PubMed] [Google Scholar]
- 249.Gegel BT, Burgert JM, Lockhart MC, Austin R, III, Davila A, Deeds J, Hodges L, Hover A, Roy J, Simpson G, Weaver S, Wolfe W, Johnson D. Amer. Assoc. Nurse Anesth. 2010;78:115. [PubMed] [Google Scholar]
- 250.Alam HB, Chen Z, Jaskille A, Querol RI, Koustova E, Inocencio R, Conran R, Seufert A, Ariaban N, Toruno K, Rhee P. J. Trauma. 2004;56:974. doi: 10.1097/01.ta.0000127763.90890.31. [DOI] [PubMed] [Google Scholar]
- 251.Pusateri AE, Delgado AV, Dick EJ, Jr, Martinez RS, Holcomb JB, Ryan KL. J. Trauma. 2004;57:555. doi: 10.1097/01.ta.0000136155.97758.cd. [DOI] [PubMed] [Google Scholar]
- 252.Inaba K, Rhee P, Teixeira PG, Barmparas G, Putty B, Branco BC, Cohn S, Demetriades D. J. Trauma. 2011;71:131. doi: 10.1097/TA.0b013e31821cb7cd. [DOI] [PubMed] [Google Scholar]
- 253.Li J, Cao W, Lv X, Jiang L, Li Y, Li W, Chen S, Li X. Act. Pharamcol. Sin. 2013;34:367. doi: 10.1038/aps.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Rhee P, Brown C, Martin M, Salim A, Plurad D, Green D, Chambers L, Demetriades D, Velmahos G, Alam H. J. Trauma. 2008;64:1093. doi: 10.1097/TA.0b013e31812f6dbc. [DOI] [PubMed] [Google Scholar]
- 255.Gegel BT, Austin PN, Johnson AD. Amer. Assoc. Nurse Anesth. 2013;81:453. [PubMed] [Google Scholar]
- 256.Margulis V, Matsumoto ED, Svatek R, Kabbani W, Cadeddu JA, Lotan Y. J. Urol. 2005;174:761. doi: 10.1097/01.ju.0000164727.53999.fe. [DOI] [PubMed] [Google Scholar]
- 257.Johnson D, Bates S, Nukalo S, Staub A, Hines A, Leishman T, Michel J, Sikes D, Gegel B, Burgert J. Ann. Med. Surg. 2014;3:21. doi: 10.1016/j.amsu.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wright JK, Kalns J, Wolf EA, Traweek F, Schwarz S, Loeffler CK CK, Snyder W, Yantis LD, Jr, Eggers J. J. Trauma. 2004;57:224. doi: 10.1097/01.ta.0000105916.30158.06. [DOI] [PubMed] [Google Scholar]
- 259.McManus J, Hurtado T, Pusateri A, Knoop KJ. Prehospital Emerg. Care. 2007;11:67. doi: 10.1080/10903120601021176. [DOI] [PubMed] [Google Scholar]
- 260.Ostomel TA, Stoimenov PK, Holden PA, Alam HB, Stucky GD. J. Thromb. Thrombolysis. 2006;22:55. doi: 10.1007/s11239-006-7658-y. [DOI] [PubMed] [Google Scholar]
- 261.Li Y, Li H, Xiao L, Zhou L, Shentu J, Zhang X, Fan J. Materials. 2012;5:2586. [Google Scholar]
- 262.Ostomel TA, Shi Q, Stoimenov PK, Stucky GD. Langmuir. 2007;23:11233. doi: 10.1021/la701281t. [DOI] [PubMed] [Google Scholar]
- 263.Arnaud F, Tomori T, Carr W, Mckeague A, Teranishi K, Prusaczyk K, Mccarron R. Ann. Biomed. Eng. 2008;36:1708. doi: 10.1007/s10439-008-9543-7. [DOI] [PubMed] [Google Scholar]
- 264.Gegel B, Burgert J, Gasko J, Campbell C, Martens M, Keck J, Reynolds H, Loughren M, Johnson D. Mil. Med. 2012;177:1543. doi: 10.7205/milmed-d-12-00165. [DOI] [PubMed] [Google Scholar]
- 265.Kheirabadi BS, Scherer MR, Estep JS, Dubick MA, Holcomb JB. J. Trauma. 2009;67:450. doi: 10.1097/TA.0b013e3181ac0c99. [DOI] [PubMed] [Google Scholar]
- 266.Arnaud F, Teranishi K, Okada T, Parreno-Sadalan D, Hupalo D, McNamee G, Carr W, Burris D, McCarron R. J. Surg. Res. 2011;169:92. doi: 10.1016/j.jss.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 267.Arnaud F, Tomori T, Saito R, McKeague A, Prusaczyk WK, McCarron RM. J. Trauma. 2007;63:775. doi: 10.1097/TA.0b013e31805f7023. [DOI] [PubMed] [Google Scholar]
- 268.Ward KR, Tiba MH, Holbert WH, Blocher CR, Draucker GT, Proffitt EK, Bowlin GL, Ivatury RR, Diegelmann RF. J. Trauma. 2007;63:276. doi: 10.1097/TA.0b013e3180eea8a5. [DOI] [PubMed] [Google Scholar]
- 269.Carraway JW, Kent D, Young K, Cole A, Friedman R, Ward KR. Resuscitation. 2008;78:230. doi: 10.1016/j.resuscitation.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 270.Arnaud F, Teranishi K, Tomori T, Carr W, McCarron R. J. Vasc. Surg. 2009;50:632. doi: 10.1016/j.jvs.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 271.Kheirabadi BS, Mace JE, Terrazas IB, Fedyk CG, Estep JS, Dubick MA, Blackbourne LH. J. Trauma. 2010;68:269. doi: 10.1097/TA.0b013e3181c97ef1. [DOI] [PubMed] [Google Scholar]
- 272.Ginsberg I, de Vries A, Katchalski E. Science. 1952;116:15. doi: 10.1126/science.116.3001.15. [DOI] [PubMed] [Google Scholar]
- 273.Nie W, Yuan X, Zhao J, Zhou Y, Bao H. Carbohydrate Polym. 2013;96:342. doi: 10.1016/j.carbpol.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 274.Wang R, Zhou B, Liu W, Feng X, Li S, Yu D, Chang J, Chi B, Xu H. J. Biomat. Appl. 2015;29:1167. doi: 10.1177/0885328214553960. [DOI] [PubMed] [Google Scholar]
- 275.Pedersen AH, Lund-Hansen T, Bisgaard-Frantzen H, Olsen F, Petersen LC. Biochemistry. 1989;28:9331. doi: 10.1021/bi00450a013. [DOI] [PubMed] [Google Scholar]
- 276.Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. Biomaterials. 2003;24:1121. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 277.Wang MY, Armstrong JK, Fisher TC, Meiselman HJ, McComb GJ, Levy ML. Neurosurgery. 2001;49:962. doi: 10.1097/00006123-200110000-00031. [DOI] [PubMed] [Google Scholar]
- 278.Wellisz T, Armstrong JK, Cambridge J, Fisher TC. J. Craniofac. Surg. 2006;17:420. doi: 10.1097/00001665-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 279.Henderson PW, Kadouch DJM, Singh SP, Zawaneh PN, Weiser J, Yazdi S, Weinstein A, Krotscheck U, Wechsler B, Putnam D, Spector JA. J. Biomed. Mater. Res. Part A. 2010;93:776. doi: 10.1002/jbm.a.32586. [DOI] [PubMed] [Google Scholar]
- 280.Wallace DG, Cruise GM, Rhee WM, Schroeder JA, Prior JJ, Ju J, Maroney M, Duronio J, Ngo MH, Estridge T, Coker GC. J. Biomed. Mater. Res. (Appl. Biomater.) 2001;58:545. doi: 10.1002/jbm.1053. [DOI] [PubMed] [Google Scholar]
- 281.Hill A, Estridge TD, Maroney M, Monnet E, Egbert B, Cruise G, Coker GC. J. Biomed. Mater. Res. 2001;58:308. doi: 10.1002/1097-4636(2001)58:3<308::aid-jbm1022>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 282.Buskens E, Meijboom MJ, Kooijman H, Van Hout BA. J. Cardiovasc. Surg. 2006;47:161. [PubMed] [Google Scholar]
- 283.Traver MA, Assimos DG. Rev. Urol. 2006;8:104. [PMC free article] [PubMed] [Google Scholar]
- 284.Seifman BD, Rubin MA, Williams AL, Wolf JS. J. Urol. 2002;167:1872. [PubMed] [Google Scholar]
- 285.Montanaro L, Arciola CR, Cenni E, Ciapetti G, Savioli F, Filippini F, Barsanti LA. Biomaterials. 2001;22:59. doi: 10.1016/s0142-9612(00)00163-0. [DOI] [PubMed] [Google Scholar]
- 286.Al-Belasy FA, Amer MZ. J. Oral Maxillofac. Surg. 2003;61:1405. doi: 10.1016/j.joms.2002.12.001. [DOI] [PubMed] [Google Scholar]
- 287.Mehdizadeh M, Yang J. Macromol. Biosci. 2013;13:271. doi: 10.1002/mabi.201200332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Singer AJ, McClain SA, Katz A. Otolaryngol. Head Neck Surg. 2004;130:553. doi: 10.1016/j.otohns.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 289.Fan Y, Sun H, Pei G, Ruan C. Injury. 2008;39:61. doi: 10.1016/j.injury.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 290.Golling M, Schaudt A, Mehrabi A, Mood ZA, Bechstein WO. Surg. Today. 2008;38:188. doi: 10.1007/s00595-007-3587-4. [DOI] [PubMed] [Google Scholar]
- 291.Horák D, Svec F, Isakov Y, Polyaev Y, Adamyan A, Konstantinov K, Shafranov V, Voronkova O, Nikanorov A, Trostenyuk N. Clin. Mater. 1992;9:43. doi: 10.1016/0267-6605(92)90009-i. [DOI] [PubMed] [Google Scholar]
- 292.Antonik LM, Khabibulina G, Fadeeva TV, Kogan AS, Malyshev AV. Pharm. Chem. J. 2007;41:278. [Google Scholar]
- 293.Carr M, Kenawy E, Layman L, Wnek G, Ward K, Barbee W, Tiba M. Shock. 2002;17:54. [Google Scholar]
- 294.Casey BJ, Behrens AM, Hess JR, Wu ZJ, Griffith BP, Kofinas P. Biomacromolecules. 2010;11:3248. doi: 10.1021/bm101147w. [DOI] [PubMed] [Google Scholar]
- 295.Pasic M, Unbehaun A, Drews T, Hetzer R. Interact. CardioVasc. Thorac. Surg. 2011;13:532. doi: 10.1510/icvts.2011.276360. [DOI] [PubMed] [Google Scholar]
- 296.Mehdizadeh MR, Weng H, Gyawali D, Tang L, Yang J. Biomaterials. 2012;33:7972. doi: 10.1016/j.biomaterials.2012.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ji Y, Liang K, Zhu L. J. Mater. Sci. Mater. Med. 2016;27:30. doi: 10.1007/s10856-015-5649-2. [DOI] [PubMed] [Google Scholar]
- 298.Zhang H, Bré LP, Zhao T, Zheng Y, Newland B, Wang W. Biomaterials. 2014;35:711. doi: 10.1016/j.biomaterials.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 299.Ye Z, Zhang H, Luo H, Wang S, Zhou Q, Du X, Tang C, Chen L, Liu J, Shi Y-K, Zhang EY, Ellis-Behnke R, Zhao X. J. Pept. Sci. 2008;14:152. doi: 10.1002/psc.988. [DOI] [PubMed] [Google Scholar]
- 300.Wang T, Zhong X, Wang S, Lv F, Zhao X. Int. J. Mol. Sci. 2012;13:15279. doi: 10.3390/ijms131115279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Cormier AR, Pang X, Zimmerman MI, Zhou H-X, Paravastu AK. ACS Nano. 2013;7:7562. doi: 10.1021/nn401562f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hsu BB, Conway W, Tschabrunn CM, Mehta M, Perez-Cuevas MB, Zhang S, Hammond PT. ACS Nano. 2015;9:9394. doi: 10.1021/acsnano.5b02374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Tyan YC, Liao JD, Lin SP. J. Mater. Sci.: Mater. Med. 2003;14:775. doi: 10.1023/a:1025036421604. [DOI] [PubMed] [Google Scholar]
- 304.Lin WC, Tseng CH, Yang MC. Macromol. Biosci. 2005;5:1013. doi: 10.1002/mabi.200500077. [DOI] [PubMed] [Google Scholar]
- 305.Zahedi P, Rezaeian I, Ranaei-Siadat S-O, Jafari S-H, Supaphol P. Polym. Adv. Tech. 2010;21:77. [Google Scholar]
- 306.Naimer SA. Health. 2014;6:479. [Google Scholar]
- 307.Naimer SA, Neville A. Injury. 2004;35:974. doi: 10.1016/S0020-1383(03)00316-4. [DOI] [PubMed] [Google Scholar]
- 308.Shipman N, Lessard CS. Military Med. 2009;174:86. doi: 10.7205/milmed-d-00-9908. [DOI] [PubMed] [Google Scholar]
- 309.Naimer SA, Chemla F. Am. J. Emerg. Med. 2000;18:816. doi: 10.1053/ajem.2000.18126. [DOI] [PubMed] [Google Scholar]
- 310.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, Hoyt DB, Bouillon B. J. Trauma. 2008;65:748. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 311.Lashoff-Sullivan M, Shoffstall A, Lavik E. Nanoscale. 2013;5:10719. doi: 10.1039/c3nr03595f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Lomas-Niera JL, Perl M, Chung CS, Ayala A. Shock. 2005;24:33. doi: 10.1097/01.shk.0000191411.48719.ab. [DOI] [PubMed] [Google Scholar]
- 313.Satterly S, Nelson D, Zwintscher N, Oguntoye M, Causey W, Theis B, Huang R, Haque M, Martin M, Bickett G, Rush RM., Jr J. Surg. Educ. 2013;70:206. doi: 10.1016/j.jsurg.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 314.Englehart MS, Cho SD, Tieu BH, Morris MS, Underwood SJ, Karahan A, Muller PJ, Differding JA, Farrell DH, Schreiber MA. J. Trauma. 2008;65:884. doi: 10.1097/TA.0b013e318187800b. [DOI] [PubMed] [Google Scholar]
- 315.Sambasivan CN, Cho SD, Zink KA, Differding JA, Schreiber MA. Am. J. Surg. 2009;197:576. doi: 10.1016/j.amjsurg.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 316.Kheirabadi BS, Klemcke HG. NATO Science and Technology Organization, Symposium Paper RTO-MP-HFM-109. 2004:P20. [Google Scholar]
- 317.Mueller GR, Pineda TJ, Xie HX, Teach JS, Barofsky AD, Schmid JR, Gregory KW. J. Trauma Acute Care Surg. 2012;73:S134. doi: 10.1097/TA.0b013e3182617c3c. [DOI] [PubMed] [Google Scholar]
- 318.Kragh JK, Jr, Aden JK, Steinbaugh J, Bullard M, Dubick MA. Am. J. Emerg. Med. 2015;33:974. doi: 10.1016/j.ajem.2015.03.048. [DOI] [PubMed] [Google Scholar]
- 319.Duggan M, Rago A, Sharma U, Zugates G, Freyman T, Busold R, Caulkins J, Pham Q, Chang Y, Mejaddam A, Beagle J, Velmahos G, deMoya M, Zukerberg L, Ng TF, King DR. J. Trauma Acute Care Surg. 2013;74:1462. doi: 10.1097/TA.0b013e31828da937. [DOI] [PubMed] [Google Scholar]
- 320.Peev MP, Rago A, Hwabejire JO, Duggan MJ, Beagle J, Marini J, Zugates G, Busold R, Freyman T, Velmahos GS, deMoya MA, Yeh DD, Fagenholz PJ, Sharma U, King DR. J. Trauma Acute Care Surg. 2014;76:619. doi: 10.1097/TA.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 321. [Accessed on June 13, 2017];Source. http://cresilon.com.
- 322.Cotton BA, Gunter OL, Isbell J, Au BK, Robertson AM, Morris JA, Jr, St Jacques P, Young PP. J. Trauma. 2008;64:1177. doi: 10.1097/TA.0b013e31816c5c80. [DOI] [PubMed] [Google Scholar]
- 323.Briggs A, Askari R. Int. J. Surg. 2016;33:218. doi: 10.1016/j.ijsu.2016.03.064. [DOI] [PubMed] [Google Scholar]
- 324.Pohlman TH, Walsh M, Aversa J, Hutchison EM, Olsen KP, Reed RL. Blood Reviews. 2015;29:251. doi: 10.1016/j.blre.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 325.Curry N, Rourke C, Davenport R, Stanworth S, Brohi K. Scandinavian J. Trauma Resusc. Emerg. 2014;22:A5. [Google Scholar]
- 326.Evatt BL, Farrugia A, Shapiro A, Wilde JT. Haemophilia. 2002;8:221. doi: 10.1046/j.1365-2516.2002.00612.x. [DOI] [PubMed] [Google Scholar]
- 327.Liumbruno G, Bennardello F, Lattanzio A, Piccoli P, Rossetti G. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party. Blood Transfus. 2009;7:132. [Google Scholar]
- 328.Yang L, Stansworth S, Hopewell S, Doree C, Murphy M. Transfusion. 2012;52:1673. doi: 10.1111/j.1537-2995.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 329.Arya RC, Wander GS, Gupta P. J. Anaesthesiol. Clin. Pharmacol. 2011;27:278. doi: 10.4103/0970-9185.81849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Klein HG. Am. J. Surg. 1995;170:S21. doi: 10.1016/s0002-9610(99)80054-3. [DOI] [PubMed] [Google Scholar]
- 331.Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. N. Engl. J. Med. 1999;340:438. doi: 10.1056/NEJM199902113400606. [DOI] [PubMed] [Google Scholar]
- 332.Midathada MV, Mehta P, Waner M, Fink LM. Am J Clin Pathol. 2004;121:124. doi: 10.1309/D0G0-C96V-05CJ-5B4J. [DOI] [PubMed] [Google Scholar]
- 333.Gerotziafas GT, Zervas C, Gavrielidis G, Tokmaktsis A, Hatjiharissi E, Papaioannou M, Lazaridou A, Constantinou N, Samama MM, Chirstakis J. Am J Hematol. 2002;69:219. doi: 10.1002/ajh.10056. [DOI] [PubMed] [Google Scholar]
- 334.Pipe SW. Thromb Haemost. 2008;99:840. doi: 10.1160/TH07-10-0593. [DOI] [PubMed] [Google Scholar]
- 335.Ofosu FA, Freedman J, Semple JW. Thromb Haemost. 2008;99:851. doi: 10.1160/TH07-10-0592. [DOI] [PubMed] [Google Scholar]
- 336.Pipe SW. J Thromb Haemost. 2005;3:1692. doi: 10.1111/j.1538-7836.2005.01367.x. [DOI] [PubMed] [Google Scholar]
- 337.Toole JJ, Knopf JL, Wozney JM, Sultzman LA, Buecker JL, Pittman DD, Kaufman RJ, Brown E, Shoemaker C, Orr EC, Amphlett GW, Foster WB, Coe ML, Knutson GJ, Fass DN, Hewick RM. Nature. 1984;312:342. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- 338.Lusher JM, Arkin S, Abildgaard CF, Schwartz RS. N. Engl. J. Med. 1993;328:453. doi: 10.1056/NEJM199302183280701. [DOI] [PubMed] [Google Scholar]
- 339.Kurachi K, Davie EW. Proc. Natl Acad. Sci. USA. 1982;79:6461. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kaufman RJ, Wasley LC, Furie BC, Furie B, Shoemaker CB. J. Biol. Chem. 1986;261:9622. [PubMed] [Google Scholar]
- 341.White GC, Beebe A, Nielsen B B. Thromb. Haemost. 1997;78:261. [PubMed] [Google Scholar]
- 342.Butenas S, Brummel KE, Branda RF, Paradis SG, Mann KG. Blood. 2002;99:923. doi: 10.1182/blood.v99.3.923. [DOI] [PubMed] [Google Scholar]
- 343.Allen G, Monroe DM, Roberts HR, Hoffman M. Blood Coagulation and Fibrinolysis. 2000;11:3. doi: 10.1097/00001721-200004001-00002. [DOI] [PubMed] [Google Scholar]
- 344.Hagen FS, Gray CL, O’Hara P, Grant FJ, Saari GC, Woodbury RG, Hart CE, Insley M, Kisiel W, Kurachi K. Proc. Natl Acad. Sci. USA. 1986;83:2412. doi: 10.1073/pnas.83.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.O’Hara PJ, Grant FJ, Halderman BA, Gray CL, Insley MY, Hagen FS, Murray MJ. Proc. Natl Acad. Sci. USA. 1987;84:5158. doi: 10.1073/pnas.84.15.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Hedner U. Semin. Hematol. 2004;41:35. doi: 10.1053/j.seminhematol.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 347.Hedner U, Lee CA. Haemophilia. 2011;17:e172. doi: 10.1111/j.1365-2516.2010.02352.x. [DOI] [PubMed] [Google Scholar]
- 348.Levi M, Levy JH, Andersen HF, Truloff D. New Engl. J. Med. 2010;363:1791. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- 349.Björses K, Holst J. Eur. J. Vasc. Endovasc. Surg. 2007;33:363. doi: 10.1016/j.ejvs.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 350.Björses K, Holst J. J. Trauma. 2009;66:602. doi: 10.1097/TA.0b013e3181823533. [DOI] [PubMed] [Google Scholar]
- 351.Dutton RP, Parr M, Tortella BJ, Champion HR, Bernard GR, Boffard K, Bouillon B, Croce MA, Dimsits J, Holcomb JB, Leppaniemi A, Vincent J-L, Hauser CJ for the CONTROL Study Group. J. Trauma. 2011;71:12. doi: 10.1097/TA.0b013e31821a42cf. [DOI] [PubMed] [Google Scholar]
- 352.Mitra B, Cameron PA, Parr MJ, Philips L. Injury. 2012;43:1409. doi: 10.1016/j.injury.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 353.Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, Axelsen M, Kluger Y Novoseven Trauma Study Group. J. Trauma. 2005;59:8. doi: 10.1097/01.ta.0000171453.37949.b7. [DOI] [PubMed] [Google Scholar]
- 354.Mayo A, Misgav M, Kluger Y, Geenberg R, Pauzner D, Klausner J, Ben-Tal O. Vox Sanguinis. 2004;87:34. doi: 10.1111/j.1423-0410.2004.00533.x. [DOI] [PubMed] [Google Scholar]
- 355.Kudela D, Smith SA, May-Masnou A, Braun GB, Pallaoro A, Nguyen CK, Chuong TT, Nownes S, Allen R, Parker NR, Rashidi HH, Morrissey JH, Stucky GD. Angew. Chem. 2015;54:4018. doi: 10.1002/anie.201409639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Baylis JR, Yeon JH, Thomson MH, Kazerooni A, Wang X, St. John AE, Lim EB, Chien D, Lee A, Zhang JQ, Piret JM, Machan LS, Burke TF, White NJ, Kastrup CJ. Sci. Adv. 2015;1:e1500379. doi: 10.1126/sciadv.1500379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Walsh M, Shreve J, Thomas S, Moore E, Moore H, Hake D, Pohlman T, Davis P, Ploplis V, Piscoya A, Wegner J, Bryant J, Crepinsek A, Lantry J, Sheppard F, Castellino F. Semin. Thromb. Hemost. 2017;43:200. doi: 10.1055/s-0036-1597900. [DOI] [PubMed] [Google Scholar]
- 358.Okamoto S, Okamoto U. Keio J. Med. 1962;11:105. [Google Scholar]
- 359.Okamoto S, Hijikata-Okunomiya A, Wanaka K, Okada Y, Okamoto U. Semin. Thromb. Hemost. 1997;23:493. doi: 10.1055/s-2007-996127. [DOI] [PubMed] [Google Scholar]
- 360.The CRASH-2 Collaborators. Lancet. 2010;376:23. [Google Scholar]
- 361.Roberts I, Prieto-Merino D. J. Intensive Care. 2014;2:56. doi: 10.1186/s40560-014-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Arch. Surg. 2012;147:113. doi: 10.1001/archsurg.2011.287. [DOI] [PubMed] [Google Scholar]
- 363.Brown JB, Neal MD, Guyette FX, Peitzman AB, Billiar TR, Zuckerbraun BS, Sperry JL. Prehosp. Emerg. Care. 2015;19:79. doi: 10.3109/10903127.2014.936635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Jen CJ, McIntire LV. Cell Motil. 1982;2:445. doi: 10.1002/cm.970020504. [DOI] [PubMed] [Google Scholar]
- 365.Brown AC, Stabenfeldt SE, Ahn B, Hannan RT, Dhada KS, Herman ES, Stefanelli V, Guzzetta N, Alexeev A, Lam WA, Lyon LA, Barker TH. Nat. Mater. 2014;13:1108. doi: 10.1038/nmat4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Nikolajsen CL, Dyrlund TF, Poulsen ET, Enghild JJ, Scavenius C. J. Biol. Chem. 2014;289:6526. doi: 10.1074/jbc.M113.517904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Muszbek L, Bereczky C, Bagoly Z, Komáromi I, Katona É. Physiol. Rev. 2011;91:931. doi: 10.1152/physrev.00016.2010. [DOI] [PubMed] [Google Scholar]
- 368.Chan LW, Wang X, Wei H, Pozzo LD, White NJ, Pun SH. Sci. Trans. Med. 2015;7:277. doi: 10.1126/scitranslmed.3010383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Kolodziej AF, Nair SA, Graham P, McMurry TJ, Ladner RC, Wescott C, Sexton DJ, Caravan P. Bioconj. Chem. 2012;23:548. doi: 10.1021/bc200613e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Gillespie CJ, Sutherland AD, Mossop PJ, Woods RJ, Keck JO, Heriot AG. Dis. Colon Rectum. 2010;53:1258. doi: 10.1007/DCR.0b013e3181e10e90. [DOI] [PubMed] [Google Scholar]
- 371.Lubarsky M, Ray C, Funaki B. Semin. Intervent. Radiol. 2010;27:99. doi: 10.1055/s-0030-1247891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Vaidya S, Tozer KR, Chen J. Semin. Intervent. Radiol. 2008;25:204. doi: 10.1055/s-0028-1085930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Panagiotopoulos V, Gizewski E, Asgari S, Regel J, Forsting M, Wanke I. Am. J. Neuroradiol. 2009;30:99. doi: 10.3174/ajnr.A1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Thiex R, Williams A, Smith E, Scott RM, Orbach DB. Am. J. Neuroradiol. 2010;31:112. doi: 10.3174/ajnr.A1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Bakar B, Oruckaptan HH, Hazer BD, Saatci I, Atilla P, Kilic K, Muftuoglu SF. Neuroradiology. 2010;52:125. doi: 10.1007/s00234-009-0594-8. [DOI] [PubMed] [Google Scholar]
- 376.Gaharwar AK, Avery RK, Assmann A, Paul A, McKinley GH, Khademhosseini A, Olsen BD. ACS Nano. 2014;8:9833. doi: 10.1021/nn503719n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Avery RK, Albadawi H, Akbari M, Zhang YS, Duggan MJ, Sahani DV, Olsen BD, Khademhosseini A, Oklu R. Sci. Transl. Med. 2016;8:365ra156. doi: 10.1126/scitranslmed.aah5533. [DOI] [PubMed] [Google Scholar]
- 378.Fedel M, Endogan T, Hasirci N, Maniglio D, Morelli A, Chiellini F, Motta A. J. Bioactive Compat. Polym. 2012;27:295. [Google Scholar]
- 379.Gryschuk V, Galagan N. Biochem. Res. Int. 2016 doi: 10.1155/2016/2959414. Article ID 2959414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Andrews RK, Berndt MC. Thrombosis Research. 2004;114:447. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 381.Arnout J, Hoylaerts MF, Lijnen HR. Handb. Exp. Pharmacol. 2006;176:1. doi: 10.1007/3-540-36028-x_1. [DOI] [PubMed] [Google Scholar]
- 382.Bennett JS, Shattil SJ, Power JW, Gartner TK. J. Biol. Chem. 1988;263:12948. [PubMed] [Google Scholar]
- 383.Fuss C, Palmaz JC, Sprague EA. J. Vasc. Interv. Radiol. 2001;12:677. doi: 10.1016/s1051-0443(07)61437-7. [DOI] [PubMed] [Google Scholar]
- 384.Blann AD, Nadar SK, Lip GYH. Eur. Heart. J. 2003;24:2166. doi: 10.1016/j.ehj.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 385.André P. Brit. J. Haematol. 2004;126:298. doi: 10.1111/j.1365-2141.2004.05032.x. [DOI] [PubMed] [Google Scholar]
- 386.Boscarino C, Tien H, Acker J, Callum J, Hansen AL, Engels P, Glassberg E, Nathens A, Beckett A. Acute. Care. Surg. 2014;76:1013. doi: 10.1097/TA.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 387.Spinella PC, Dunne J, Beilman GJ, O'Connell RJ, Borgman MA, Cap AP, Rentas F F. Transfusion. 2012;52:1146. doi: 10.1111/j.1537-2995.2012.03594.x. [DOI] [PubMed] [Google Scholar]
- 388.Picker SM. J. Blood Disorders Transf. 2012;3:5. [Google Scholar]
- 389.Bode AP, Read MS. Transfusion. 2000;22:99. doi: 10.1016/s0955-3886(00)00028-x. [DOI] [PubMed] [Google Scholar]
- 390.Hoffmeister KM, Josefsson EC, Isaac NA, Clausen H, Hartwig JH, Stossel TP. Science. 2003;301:1531. doi: 10.1126/science.1085322. [DOI] [PubMed] [Google Scholar]
- 391.Jhang JS, Spitalnik SL. Curr. Hematol. Rep. 2005;4:483. [PubMed] [Google Scholar]
- 392.Bode AP, Fischer TH. Artif. Cell Blood Substit. Biotechnol. 2007;35:125. doi: 10.1080/10731190600974962. [DOI] [PubMed] [Google Scholar]
- 393.Cap AP, Perkins JG. J. Trauma. 2011;70:S59. doi: 10.1097/TA.0b013e31821a606d. [DOI] [PubMed] [Google Scholar]
- 394.Lozano M. Blood. 2008;111:2951. [Google Scholar]
- 395.Reddoch KM, Pidcoke HF, Montgomery RK, Fedyk CG, Aden JK, Ramasubramanian AK, Cap AP. Shock. 2014;41:54. doi: 10.1097/SHK.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Nair PM, Pidcoke HF, Cap AP, Ramasubramanian AK. J. Trauma Acute Care Surg. 2014;77:S88. doi: 10.1097/TA.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 397.Nasiri S. Blood Transfus. 2013;11:337. doi: 10.2450/2013.0209-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Chao FC, Kim BK, Houranieh AM, Liang FH, Conrad MW, Swisher SN, Tullis JL. Transfusion. 1996;36:536. doi: 10.1046/j.1537-2995.1996.36696269513.x. [DOI] [PubMed] [Google Scholar]
- 399.Biró E, Akkerman JW, Hoek FJ, Gorter G, Pronk LM, Sturk A, Nieuwland R. J Thromb. Haemost. 2005;3:2754. doi: 10.1111/j.1538-7836.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- 400.Graham SS, Gonchoroff NJ, Miller JL. Am. J. Clin. Pathol. 2001;115:144. doi: 10.1309/CCDV-3BEP-XXKP-BKDM. [DOI] [PubMed] [Google Scholar]
- 401.Nasiri S, Heidari M, Rivandi M. Int. J Pharm. Sci. Res. 2012;3:4895. [Google Scholar]
- 402.Galán A-M, Bozzo J, Hernández M-R, Pino M, Reverter J-C, Mazzara R, Escolar G, Ordinas A. Transfusion. 2000;40:1074. doi: 10.1046/j.1537-2995.2000.40091074.x. [DOI] [PubMed] [Google Scholar]
- 403.Fitzpatrick GM, Cliff R, Tandon N. Transfusion. 2013;53:100S. doi: 10.1111/trf.12043. [DOI] [PubMed] [Google Scholar]
- 404.Sum R, Hager S, Pietramaggiori G, Orgill DP, Dee J, Rudolph A, Orser C, Fitzpatrick GM, Ho D. Transfusion. 2007;47:672. doi: 10.1111/j.1537-2995.2007.01170.x. [DOI] [PubMed] [Google Scholar]
- 405.Hu C-MJ, Fang RH, Wang K-C, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, Zhang L. Nature. 2015;526:118. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Hu Q, Sun W, Qian C, Wang C, Bomba HN, Gu Z. Adv. Mater. 2015;27:7043. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Li J, Ai Y, Wang L, Bu P, Sharkey CC, Wu Q, Wun B, Roy S, Shen X, King MR. Biomaterials. 2016;76:52. doi: 10.1016/j.biomaterials.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Modery-Pawlowski CL, Kuo HH, Baldwin WH, III, Sen Gupta A. Nanomedicine. 2013;8:1709. doi: 10.2217/nnm.13.113. [DOI] [PubMed] [Google Scholar]
- 409.Rybak MEM, Renzulli LA. Biomat. Artif. Cells Immob. Biotech. 1993;21:101. doi: 10.3109/10731199309117350. [DOI] [PubMed] [Google Scholar]
- 410.Takeoka S, Teramura Y, Okamura Y, Tsuchida E, Handa M, Ikeda Y. Biochem. Biophys. Res. Commun. 2002;296:765. doi: 10.1016/s0006-291x(02)00934-8. [DOI] [PubMed] [Google Scholar]
- 411.Nishiya T, Kainoh M, Murata M, Handa M, Ikeda Y. Blood. 2002;100:136. doi: 10.1182/blood.v100.1.136. [DOI] [PubMed] [Google Scholar]
- 412.Nishiya T, Kainoh M, Murata M, Handa M, Ikeda Y. Artif. Cells. Blood Substit. Immobil. Biotechnol. 2001;29:453. doi: 10.1081/bio-100108550. [DOI] [PubMed] [Google Scholar]
- 413.Takeoka S, Teramura Y, Ohkawa H, Ikeda Y, Tsuchida E. Biomacromolecules. 2000;1:290. doi: 10.1021/bm0055293. [DOI] [PubMed] [Google Scholar]
- 414.Doshi N, Orje JN, Molins B, Smith JW, Mitragotri S, Ruggeri ZM. Adv. Mater. 2012;24:3864. doi: 10.1002/adma.201200607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Coller BS. Blood. 1980;55:169. [PubMed] [Google Scholar]
- 416.Agam G, Livne AA. Eur. J. Clin. Invest. 1992;22:105. doi: 10.1111/j.1365-2362.1992.tb01943.x. [DOI] [PubMed] [Google Scholar]
- 417.Lee DH, Blajchman MA, Ho TWC, Yen RCK. Blood. 1995;86:902a. [Google Scholar]
- 418.Yen RCK, Ho TWC, Blajchman MA. Transfusion. 1995;35:41S. [Google Scholar]
- 419.Levi M, Friederich PW, Middleton S, de Groot PG, Wu YP, Harris R, Biemond BJ, Heijnen HF, Levin J, ten Cate JW. Nat. Med. 1999;5:107. doi: 10.1038/4795. [DOI] [PubMed] [Google Scholar]
- 420.Takeoka S, Teramura Y, Okamura Y, Handa M, Ikeda Y, Tsuchida E. Biomacromolecules. 2001;2:1192. doi: 10.1021/bm015554o. [DOI] [PubMed] [Google Scholar]
- 421.Mosesson MW. J. Thromb. Haemost. 2005;3:1894. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 422.Du X, Plow EF, Frelinger AL, III, O’Toole TE, Loftus JC, Ginsberg MH. Cell. 1991;65:409. doi: 10.1016/0092-8674(91)90458-b. [DOI] [PubMed] [Google Scholar]
- 423.Bennett JS. Trends Cardiovasc. Med. 1996;16:31. doi: 10.1016/1050-1738(95)00126-3. [DOI] [PubMed] [Google Scholar]
- 424.Coller BS, Springer KT, Beer JH, Mohandas N, Scudder LE, Norton KJ, West SM. J Clin. Invest. 1992;89:546. doi: 10.1172/JCI115619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Takeoka S, Okamura Y, Teramura Y, Watanabe N, Suzuki H, Tsuchida E, Handa M, Ikeda Y. Biochem. Biophys. Res. Comm. 2003;312:773. doi: 10.1016/j.bbrc.2003.10.184. [DOI] [PubMed] [Google Scholar]
- 426.Bertram JP, Williams CA, Robinson R, Segal SS, Flynn NT, Lavik EB. Sci. Transl. Med. 2009;1:11. doi: 10.1126/scitranslmed.3000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Lashoff-Sullivan MM, Shoffstall E, Atkins KT, Keane N, Bir C, VandeVord P, Lavik EB. Proc. Natl. Acad. Sci. USA. 2014;111:10293. doi: 10.1073/pnas.1406979111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Beer JH, Springer KT, Coller BS. Blood. 1992;79:117. [PubMed] [Google Scholar]
- 429.Ruoslahti E. Annual Rev. Cell. Dev. Biol. 1996;12:697. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 430.Humphries JD, Byron A, Humphries MJ. J. Cell Sci. 2006;119:3901. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Okamura Y, Takeoka S, Teramura Y, Maruyama H, Tsuchida E, Handa M, Ikeda Y. Transfusion. 2005;45:1221. doi: 10.1111/j.1537-2995.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 432.Okamura Y, Takeoka S, Eto K, Maekawa I, Fujie T, Maruyama H, Ikeda Y, Handa M. J. Thromb. Haemost. 2009;7:470. doi: 10.1111/j.1538-7836.2008.03269.x. [DOI] [PubMed] [Google Scholar]
- 433.Okamura Y, Maekawa I, Teramura Y, Fujie T, Maruyama H, Handa M, Ikeda Y, Takeoka S. Bioconj. Chem. 2005;16:1589. doi: 10.1021/bc050178g. [DOI] [PubMed] [Google Scholar]
- 434.Okamura Y, Fujie T, Nogawa M, Maruyama H, Fujie T, Maruyama H, Handa M, Ikeda Y, Takeoka S. Transfusion Med. 2008;18:158. doi: 10.1111/j.1365-3148.2008.00860.x. [DOI] [PubMed] [Google Scholar]
- 435.Okamura Y, Fujie T, Maruyama H, Handa M, Ikeda Y, Takeoka S. Transfusion. 2007;47:1254. doi: 10.1111/j.1537-2995.2007.01265.x. [DOI] [PubMed] [Google Scholar]
- 436.Del Carpio Munoz C, Campbell W, Constantinescu I, Gyongyossy-Issa MIC. J. Mol. Model. 2008;14:1191. doi: 10.1007/s00894-008-0375-z. [DOI] [PubMed] [Google Scholar]
- 437.Gyongyossy-Issa MIC, Kizhakkedathu J, Constantinescu I, Campbell W, del Carpio Munoz CA. 2008/0213369 A1. US Patent Application Publication US. 2008
- 438.Okamura Y, Handa M, Suzuki H, Ikeda Y, Takeoka S. J. Artif. Organs. 2006;9:251. doi: 10.1007/s10047-006-0345-0. [DOI] [PubMed] [Google Scholar]
- 439.Sen Gupta A, Ravikumar M, Modery-Pawlowski C. 9107845. US Patent. 2015
- 440.Shukla M, Sekhon UDS, Betapudi V, Li W, Hickman DA, Pawlowski CL, Dyer M, Neal MD, McCrae KR, Sen Gupta A. J. Tromb. Haemost. 2016 doi: 10.1111/jth.13579. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Nogami K, Shima M, Giddings JC, Takeyama M, Tanaka I, Yoshioka A. Int. J. Hematol. 2007;85:317. doi: 10.1532/IJH97.06192. [DOI] [PubMed] [Google Scholar]
- 442.Mo X, An Y, Yun C-S, Yu SM. Angew. Chem. Int. Ed. 2006;45:2267. doi: 10.1002/anie.200504529. [DOI] [PubMed] [Google Scholar]
- 443.Cejas MA, Chen C, Kinney WA, Maryanoff BE. Bioconj. Chem. 2007;18:1025. doi: 10.1021/bc070105s. [DOI] [PubMed] [Google Scholar]
- 444.Cheng S, Craig WS, Mullen D, Tschopp JS, Dixon D, Piersbacher MD. J. Med. Chem. 1994;37:1. doi: 10.1021/jm00027a001. [DOI] [PubMed] [Google Scholar]
- 445.Ravikumar M, Modery CL, Wong TL, Sen Gupta A. Bioconj. Chem. 2012;23:1266. doi: 10.1021/bc300086d. [DOI] [PubMed] [Google Scholar]
- 446.Ravikumar M, Modery CL, Wong TL, Sen Gupta A. Biomacromolecules. 2012;13:1495. doi: 10.1021/bm300192t. [DOI] [PubMed] [Google Scholar]
- 447.Haji-Valizadeh H, Modery-Pawlowski CL, Sen Gupta A. Nanoscale. 2014;6:4765. doi: 10.1039/c3nr06400j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Modery-Pawlowski CL, Tian LL, Ravikumar M, Wong TL, Sen Gupta A. Biomaterials. 2013;34:3031. doi: 10.1016/j.biomaterials.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 449.Al Momani T, Udaykumar HS, Marshall JS, Chandran KB. Ann Biomed Eng. 2008;36:905. doi: 10.1007/s10439-008-9478-z. [DOI] [PubMed] [Google Scholar]
- 450.Tokarev AA, Butylin AA, Ermakova EA, Shnol EE, Panasenko GP, Ataullakhanov FI. Biophys J. 2011;101:1835. doi: 10.1016/j.bpj.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Anselmo AC, Modery-Pawlowski CL, Menegatti S, Kumar S, Vogus DR, Tian LL, Chen M, Squires TM, Sen Gupta A, Mitragotri S. ACS Nano. 2014;8:11243. doi: 10.1021/nn503732m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Front. Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Szebani J, Baranyi L, Savay S, Bodo M, Morse DS, Basta M, Stahl GL, Bünger R, Alving CR. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H1319. doi: 10.1152/ajpheart.2000.279.3.H1319. [DOI] [PubMed] [Google Scholar]
- 454.Szebani J. Eur. J. Nanomed. 2012;1:33. [Google Scholar]
- 455.Cohen MJ. Thromb. Res. 2014;133:S25. doi: 10.1016/j.thromres.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 456.Kheirabadi B. US Army Med. Dep. J. 2011;2:25. [PubMed] [Google Scholar]
- 457.Rall JM, Cox JM, Songer AG, Cestero RF, Ross JD. J. Trauma. Acute Care Surg. 2013;75:S150. doi: 10.1097/TA.0b013e318299d909. [DOI] [PubMed] [Google Scholar]
- 458.Ryan KL, Cortez DS, Dick EJ, Jr, Pusateri AE. Resuscitation. 2006;70:133. doi: 10.1016/j.resuscitation.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 459.Fanchini M. Am. J. Hematol. 2007;82:731. doi: 10.1002/ajh.20940. [DOI] [PubMed] [Google Scholar]
- 460.Laupacis A, Fergusson D. Anesth. Analg. 1997;85:1258. doi: 10.1097/00000539-199712000-00014. [DOI] [PubMed] [Google Scholar]
- 461.Wells PS. Am. J. Ther. 2002;9:377. doi: 10.1097/00045391-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 462.Levy JH. Transfusion. 2004;44:58S. doi: 10.1111/j.0041-1132.2004.04173.x. [DOI] [PubMed] [Google Scholar]
- 463.Levi M, Cromheecke ME, de Jonge E, Prins MH, Briët E, Büller HR. Lancet. 1999;354:1940. doi: 10.1016/S0140-6736(99)01264-7. [DOI] [PubMed] [Google Scholar]
- 464.Capdevila X. Anesthesiology. 1998;88:50. doi: 10.1097/00000542-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 465.Roberts I, Prieto-Merino D, Manno D. Critical Care. 2014;18:685. doi: 10.1186/s13054-014-0685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Sen Gupta A. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016;8:255. doi: 10.1002/wnan.1362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.