Abstract
There are different types of nutritionally mediated oxidative stress sources that trigger inflammation. Much information indicates that high intakes of macronutrients can promote oxidative stress and subsequently contribute to inflammation via nuclear factor-kappa B- (NF-κB-) mediated cell signaling pathways. Dietary carbohydrates, animal-based proteins, and fats are important to highlight here because they may contribute to the long-term consequences of nutritionally mediated inflammation. Oxidative stress is a central player of metabolic ailments associated with high-carbohydrate and animal-based protein diets and excessive fat consumption. Obesity has become an epidemic and represents the major risk factor for several chronic diseases, including diabetes, cardiovascular disease (CVD), and cancer. However, the molecular mechanisms of nutritionally mediated oxidative stress are complex and poorly understood. Therefore, this review aimed to explore how dietary choices exacerbate or dampen the oxidative stress and inflammation. We also discussed the implications of oxidative stress in the adipocyte and glucose metabolism and obesity-associated noncommunicable diseases (NCDs). Taken together, a better understanding of the role of oxidative stress in obesity and the development of obesity-related NCDs would provide a useful approach. This is because oxidative stress can be mediated by both extrinsic and intrinsic factors, hence providing a plausible means for the prevention of metabolic disorders.
1. Introduction
There are different types of nutritionally mediated oxidative stress sources that trigger inflammation. Oxidative stress plays a crucial role in the development of numerous human diseases [1]. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced continuously in the body via oxidative metabolism, mitochondrial bioenergetics, and immune function [2]. The most frequent forms of ROS include superoxide anion, hyphochlorous acid, hydrogen peroxide, singlet oxygen, hypochlorite, hydroxyl radical, and lipid peroxides, which are involved in the progression, growth, death, and differentiation of cells. They can bind with nucleic acids, enzymes, membrane lipids, proteins, and other small molecules [1]. Short-term postprandial mitochondrial oxidative stress causes inflammation, which is mainly mediated by nuclear factor-kappa B (NF-κB) [3]. Conversely, long-term chronic overconsumption contributes to obesity, which induces permanent states of inflammation via the generation of white adipose tissue which secretes proinflammatory factors [4]. Extensive research has shown that high-glucose and a high-fat diet mediate inflammation, which suggests that oxidative stress may alter cellular physiological processes [5, 6].
Substantial evidence highlights the detrimental impact of diets high in refined carbohydrates and saturated fat [7]. Cardiovascular disease (CVD), obesity, type 2 diabetes, and nonalcoholic fatty liver disease are attributed to the overconsumption of foods high in carbohydrates and saturated fats, the saturation of nutrient storage, and sedentary lifestyles [8, 9]. Studies exploring the influence of a Westernized dietary pattern on inflammatory diseases, such as colorectal cancer [10], have consistently shown a similar trend. Such findings highlight the fundamental idea that diet quality can impact immune function and systematic inflammation. In a study by Song et al. [11] focusing on carbohydrate and refined-grain intake and metabolic syndrome outcome in Korean men and women, women were shown to have a greater likelihood of metabolic syndrome with refined-grain consumption compared to the men, suggesting that refined-grain intakes are linked to a high level of inflammation.
The prevalence of obesity has doubled from 1980 to 2008 worldwide. In 2008, more than 50% of men and women in the WHO European Region were overweight, and nearly 20% of men and 23% of women were obese [12]. Nearly 1.5 billion people worldwide are obese or overweight which increases their risk of developing inflammatory disturbances, CVD, nonalcoholic fatty liver disease, coronary heart disease, and type 2 diabetes [13, 14].
The effects of oxidative stress are related to the type of macronutrients consumed and their absolute quantity [15]; both of these aspects contribute to oxidative stress and may favor the development of obesity and obesity-related noncommunicable diseases (NCDs) [16]. However, the molecular mechanisms of nutritionally mediated oxidative stress are complex and poorly understood. Therefore, this review aimed to explore how dietary choices exacerbate or dampen oxidative stress and inflammation. We also discussed the implications of oxidative stress in the adipocyte and glucose metabolism and obesity-associated NCDs. A better understanding of the role of oxidative stress in obesity and the development of obesity-related NCDs would provide a useful approach. This is because oxidative stress can be mediated by both extrinsic and intrinsic factors, hence providing a plausible means for the prevention of metabolic disorders.
2. Oxidative Stress
The harmful effects of free RNS and ROS radicals cause a potential biological damage, namely, nitrosative stress and oxidative stress, respectively [17]. ROS are generated in normal aerobic metabolism as a by-product; however, when the level is increased under stress, it may cause basic health hazard [18]. The mitochondrion is the predominant cell organelle in ROS production [19]. It generates adenosine triphosphate (ATP) via a series of oxidative phosphorylation processes [19]. During this process, one or two electron reductions instead of four electron reductions of oxygen have occurred, which subsequently leads to the formation of H2O2 or O2∙, and convert to other ROS [19]. The major form of RNS includes nitric oxide (NO) and peroxynitrite (ONOO−) [17]. When excess NO is present, this reaction leads to the formation of nitrogen dioxide radical [17]. Higher NO concentration leads to the formation of N2O3 and this usually results in nitrosation [17].
Oxygen free radicals, including alkyl peroxyl radical (∙OOCR), hydroxyl radical (OH∙−), and superoxide anion radical (O2∙−), are potent initiators in lipid peroxidation, the role of which is well-established in the pathogenesis of diseases [18]. Once lipid peroxidation is initiated, a propagation of chain reactions will take place until termination products are produced [18]. Thus, end products of lipid peroxidation, for example, F2-isoprostanes, 4-hydroxy-2-nonenol (4-HNE), and malondialdehyde (MDA), are accumulated in biological systems [18]. DNA bases are very susceptible to ROS oxidation, and the major detectable oxidation product of DNA bases is 8-hydroxy-2-deoxyguanosine [18]. Oxidation of DNA bases can cause mutations and deletions in both nuclear and mitochondrial DNA. Mitochondrial DNA is relatively prone to oxidative damage due to its proximity to ROS and its deficient repair capacity compared to that of the nuclear DNA [18]. These oxidative modifications cause functional changes in structural and enzymatic proteins, which may lead to substantial physiological impact [18]. In addition, redox modulations of transcription factors also increase or decrease their specific DNA binding activities and thereby altering gene expression [18].
3. Nutritionally Mediated Oxidative Stress
3.1. High Carbohydrates
Much information indicates that high intakes of macronutrients can promote oxidative stress and subsequently contribute to inflammation via NF-κB-mediated cell signaling pathways [20]. Dietary carbohydrates are important to highlight here because they may contribute to the long-term consequences of nutritionally mediated inflammation [21]. Dietary carbohydrate intake has gained attention among researchers because of the associations between a high glycemic index (GI) or glycemic load (GL) diet with diabetes, obesity, cancer, and coronary heart disease [22, 23]. High GL diets have been characterized as a common feature of Western culture; they are heavy in added sugars and refined carbohydrates [24]. By contrast, low GI foods were found to decrease postprandial glycemia in overweight/obese [23] and type 2 diabetes patients [25]. Consistent relationships between high GI and diabetes have been demonstrated in observational and cohort studies [26–28].
The high GI of white rice may lead to high oxidative stress [29]. Most Asian populations consume large amounts of rice as a staple food; thus, dietary carbohydrate intake plays a substantial role in the development of metabolic diseases in Asian populations. In support of this, a positive relationship between rice intake or total carbohydrates and diabetes has been demonstrated in Japanese women [30, 31]. In addition to diabetes outcome, a high intake of refined-grain was also positively linked to fasting blood glucose and triglyceride levels and negatively associated with high-density lipoprotein (HDL) cholesterol in Asian Indian and Korean populations [11, 32], indicating that a high GI diet may negatively impact health.
The elevation of oxidative stress is linked to chronic inflammation [33]; other sources may also further increase the accumulation of proinflammatory cytokines in a “vicious cycle” [34]. In cultured adipocytes, ROS promotes the production of cytokine interleukin-6 (IL-6) and proinflammatory monocyte chemotactic protein-1 (MCP-1) expression [35, 36]. In the adipose tissue, this can activate macrophage infiltration and subsequently result in a proinflammatory environment [37, 38]. ROS can also stimulate signal transduction pathways (mainly via NF-κB), which activates the production of tumor necrosis factor-α (TNF-α) and IL-6 [35, 39, 40]. Further, oxidative stress can also promote cells into cellular senescence, particularly adipocyte senescence, partly via cellular oxidation damage [41, 42]. Adipocyte senescence may recruit macrophages and elevates the production of proinflammatory cytokines [42, 43].
Excessive high caloric intake from either a high-carbohydrate or high-fat diet will cause more substrates to enter into mitochondrial respiration [44]. Subsequently, the number of electrons donated to the electron transport chain may increase [45]. Upon reaching a threshold voltage, extra electrons might back up at complex III with further donations to molecular oxygen, which produces high levels of superoxide [45].
Intriguingly, extremely high amounts of carbohydrates may lead to the reduction of insulin binding and the downregulated transcription of insulin receptor expression in the skeletal muscle [46]. High insulin and glucose levels may decrease insulin binding to the insulin receptor in adipocytes [47], negatively affecting Akt activity. The accumulation of ROS/RNS or a reduction of antioxidant capacity due to increased carbohydrate metabolism in insulin target tissues may change the phosphorylation status of these signaling pathways, subsequently resulting in deactivation. Indeed, exposure to hydrogen peroxide (H2O2) promotes a significant loss in distal and proximal insulin signaling and decreased glucose transport in muscles and adipocytes in vitro [48].
Evidence from an epidemiological study demonstrated that the consumption of refined carbohydrates, such as fructose-rich syrups, potentially leads to the epidemics of type 2 diabetes and obesity [49, 50]. Indeed, fructose-rich syrups may potentially pose a risk of diabetes and CVD [49]. Animal model studies further demonstrate that feeding normal rats fructose-rich diets may induce several endocrine and metabolic derangements, interfering with many organs and tissues [51, 52]. Because the liver is predominantly responsible for fructose metabolism and uptake, several studies are focusing on hepatic glucose metabolism [53]. Although the molecular link underlying fructose detrimental effects and carbohydrate metabolism requires further elucidation, most of the experimental studies indicate that oxidative stress could play a central role [54, 55]. In this regard, a key mode of action to explain this relationship is via fructose-induced oxidative stress which subsequently leads to impaired carbohydrate metabolism. Data from animal experiments have shown a greater likelihood of inflammation after the administration of fructose [51]. Such findings highlight the association of insulin resistance and fructose and its role in hepatic metabolism and carbohydrate metabolism against the anabolic pathway and impaired glucose tolerance [52, 55, 56]. Castro et al. [53] further demonstrated that fructose may modulate the liver glucokinase activity via the production of ROS. These data imply that numerous metabolic changes induced by fructose in the liver are more likely initiated by an increase of fructose phosphorylation by fructokinase, followed by adaptive changes that attempt to switch the substrate flow from mitochondrial metabolism to energy storage [53].
3.2. High Animal-Based Proteins
In developed countries, meat composes a significant proportion of the normal diet and consists of 15% of the daily energy intake, 40% of daily protein, and 20% of daily fat [57]. Meat is high in dietary protein and saturated fatty acids (SFAs). Fermentation of the excessive proteins in the gut produces metabolites such as ammonia (NH3) and hydrogen sulfide (H2S), which are compounds known to trigger the toxicity of the mucosa [58]. Meat can be marketed fresh or processed, the latter of which includes curing, salting, stuffing, smoking, drying, and fermentation [59]. Although meat contains high amounts of dietary protein, it can also be a source of mutagens due to the presence of N-nitroso compounds (NOC) in processed meats and heterocyclic amines (HCA) and polycyclic aromatic hydrocarbons (PAH) during high-temperature cooking and grilling [60].
Research has shown an association between the intake of well-done red meat and colorectal cancer, which could be partially explained by the formation of carcinogenic HCA and PAH. Although meat is high in SFAs, a study evaluating the mechanisms behind this finding suggests that these associations are more likely caused by something other than SFA content. However, the formation of cyto and genotoxic lipid oxidation products, such as malondialdehyde (MDA), 4-hydroxy-2- nonenal (4-HNE), and N-nitroso compounds (NOC) catalyzed by heme-Fe during digestion, is regarded as the most plausible determinant that contributes to the increased risk of colorectal cancer [61, 62]. A high intake of red meat has been demonstrated to increase NOC formation in humans, which is related to the colonic development of the NOC-specific DNA adduct O6-carboxymethylguanine (O6-C-MeG) [63].
Free Fe2+ markedly increases during the cooking of uncured meats [63]. Conversely, nitrite curing prevents the degradation of heme-Fe through the stabilization of the porphyrin ring [63]. Heat treatment also causes a reduction of antioxidant enzymes, such as glutathione peroxidase [64, 65], and generates oxygen from oxymyoglobin, which contributes to the production of H2O2 [66]. Further, free Fe2+ catalyzes the Fenton reaction when oxidative processes are initiated [67]. Through this reactive nature, ROS results in oxidative damage to meat proteins, which further explains the high formation of 4-HNE and MDA when uncured pork is heated [68]. Compared to cooked meat, a slightly lower concentration of simple aldehydes was observed in overcooked uncured pork. This could be explained by the evaporation of aldehydes caused by the reduction of the prooxidant effect of oxymyoglobin when heated to above 75 °C or intense heating [69]. Rather, when meats are nitrite-cured, less degradation of the heat-stable NO-heme may contribute to a reduced release of Fe2+ to initiate oxidation processes, which subsequently results in a reduction of lipid oxidation. Because the Fenton reaction is a chain reaction, a higher dosage of oxidation products after digestion was expected [70]. A further study reported by Van Hecke et al. [63] showed that the antioxidant effect of nitrite-curing during digestion was significantly reduced in overcooked nitrite-cured pork. Consistent with the study reported by Van Hecke et al. [63], Okayama et al. [71] also found that a prolonged cooking time or a temperature reaching 80 °C increased the decomposition of nitrite. A 1 : 1 ratio of nitric oxide (∙NO) to ROS activates lipid oxidation whereas ∙NO > ROS suppresses this process [72]. Accordingly, low residual nitrite caused by intense heating is more likely to alter the ∙NO : ROS ratio; thus, nitrite could change from an antioxidant to prooxidant behavior, which might explain the increased formation of oxidation products in overcooked nitrite-cured meats. In an earlier study by Ayala et al. [73], MDA was shown to be absorbed in the bloodstream and produce lipid oxidation products that could reach tissues and cause DNA damage. Low lipid oxidation product levels in colonic digests are attributed to Schiff base formation with proteins, which thus binds with bacterial DNA [74] or is oxidized by bacterial aldehyde dehydrogenase activity. Collectively, the effect of nitrite curing of meat in the colonic step was predominant since it was linked to a low level of MDA but proportionally increased 4-HNE levels and doubled heptanal amounts in the overcooked and cooked meats [63].
In addition, the nitrite-curing of beef and pork also caused a twofold difference in heptanal levels in stimulated colonic digests compared to their counterparts [70]. Lipid aldehydes, such as 4-HNE and MDA, react with protein chains leading to protein aggregation, causing the protein to be less susceptible to pepsin activity [70]. Overcooked nitrite-cured pork has low concentrations of protein carbonyl compounds and lipid oxidation products before digestion; this likely occurs because the meat proteins are initially well-digested in the stomach, after which the low levels of residual protein bind with 4-HNE and MDA, which subsequently form in a later phase of digestion [70]. The rate of protein digestibility is vitally important in association to colorectal cancer because higher levels of residual protein reaching the colon could result in the formation of potentially harmful protein fermentation products, such as p-cresol, ammonia, indole, and phenol [75]. NOC can be stimulated either enzymatically or nonenzymatically via oxidation [76, 77]. This nonenzymatic stimulation of NOC can be generated by a hydroxyl radical-generating system containing H2O2, Cu2+, Fe2+, and ascorbic acid. All these compounds are present in meat. When H2O2, Cu2+, or Fe2+ was eliminated from a reaction mixture with N-nitroso-N-methylpentylamine, the mutagenicity of these mixtures was reduced [76].
3.3. Excessive Consumption of Fats
An extensive body of systematic reviews of randomized trials [78, 79] and prospective cohort studies [78, 80] has urged for a reevaluation of dietary guidelines for consumption and a reappraisal of the impact of SFAs on health. Although research has demonstrated an association between SFAs and CVD [81], not all data demonstrated such a link. De Souza et al. [82] did not identify an association of SFA intake and CVD, coronary heart disease, ischemic stroke, or type 2 diabetes. Interestingly, a study has reported that the total fat and types of fat were inversely associated with total mortality [83]. Additionally, no association was reported between the total fat and types of fat with CVD mortality and myocardial infarction [83].
Substantial evidence has suggested that SFAs can boost proinflammatory signaling. The lengths of SFA chains can produce different physiological responses [84, 85], but many mechanisms are still debated. Long-chain SFAs including palmitate and myristate acids are typically known for their harmful effects against endothelial cells, which can induce apoptosis through the induction of NF-κB in human coronary artery endothelial cells (HCAECs) [86, 87]. Harvey et al. [86] showed that long-chain SFAs can promote proinflammatory endothelial cell phenotypes through the incorporation into endothelial cell lipids. Conversely, short- and medium-chain SFAs do not incorporate or contribute to lipotoxicity. Particularly, stearic acid stimulates the upregulation of ICAM-1 human aortic endothelial cells (HAECs) via an NF-κB dependent manner [86].
Murumalla and Gunasekaran [88] reported that SFAs (lauric acid and palmitic acid) did not stimulate Toll-like receptors 4 (TLR4) or 2 (TLR2) in HEK-Blue cells transfected with TLR2 and TLR4. Despite the inverse association between SFAs and TLR4 or TLR2, not all studies agreed. Huang et al. [84] found that palmitic acid and lauric acid activated TLR2 and TLR4 in RAW264.7 macrophages and transiently transfected human monocytic (THP-1) monocytes. Data from human studies exploring the impact of SFAs on gene expression are limited, but evidence from epidemiological studies indicates the association between SFA consumption and CVD. Nonetheless, the meta-analyses of prospective studies exploring the relationship between CVD and SFA showed a consistent poor association. From the study reviewed, metaregressions conducted in randomized trials demonstrated that polyunsaturated fatty acids (PUFAs) replacing SFAs did not lead to any changes in CVD risk [89]. Inconclusive findings suggest that SFAs are generally grouped together although medium-chain SFAs may provide beneficial health effects such as preventing obesity and the inhibition of body fat accumulation [90]. The impact of high-SFA diet on gene expression in adipose tissue was also presented by Youseef-Elabd et al. [91]. In particular, an SFA diet led to an upregulation of genes such as integrin beta 2 (ITGB2), cathepsin S (CTSS), and interleukin-8 (IL-8) in moderately overweight individuals, suggesting that these changes were linked to diet-induced changes rather than obesity.
A high-fat diet (HFD) was demonstrated to be a significant risk factor for health. Animals feeding a long-term HFD show increased oxidative stress and dysfunctional mitochondria in several organs [92–94]. Several research studies have also indicated that high-fat consumption causes a significant reduction in auditory function [95, 96]. This study demonstrated that long-term HFD reduced auditory function and promoted age-related hearing loss [97]. From the study reviewed, feeding rats with a HFD for a period of 12 months may increase plasma triglycerides, total cholesterol, and nonesterified fatty acid levels, causing an increase in blood oxidative stress parameters. A HFD was shown to not only aggravate the lipid profile it also further enhanced ROS accumulation and triggered mitochondrial damage in the inner ear [97], suggesting enormous detrimental impacts of a HFD on health.
Several studies have corroborated this finding and found that increased caloric intake or obesity is associated with increased mitochondrial superoxide production. Data reported by Anderson et al. [98] have shown that feeding a HFD to both mice and humans causes a significant elevation of H2O2 from the mitochondria isolated from the skeletal muscle. From the study reviewed, H2O2 emission was used as a surrogate of superoxide emission as mitochondrial superoxide and is converted to H2O2 by superoxide dismutase 2 (SOD 2). Further, ROS accumulation has also been found in mitochondria isolated from adipose [99], liver [100], and kidney [101] tissue in high-fat or obese-treated animals. In another study, Valenzuela et al. [102] found that liver enzyme activity such as superoxide dismutase (SOD), catalase, glutathione peroxidase, and glutathione reductase was significantly reduced by a HFD diet-fed mice.
Additionally, an adipogenic diet and the accumulation of adipose tissue can trigger oxidative stress in mammalian tissues. Some studies supported the hypothesis that HFD promotes inflammation in the intestine, particularly in the small intestine. This observation may represent an early event that precedes and predisposes the individual to insulin resistance and obesity [103]. de La Serre et al. [104] reported that HFD activates myeloperoxidase activity, an inflammation marker, in the ileum of obesity-prone Sprague-Dawley rats. A study by de Wit et al. [105] further supported that HFD activates macrophage migration inhibitory factor expression in the ileum of obesity-prone C57BL/6J mice. Consistent with studies reported by de La Serre et al. [104] and de Wit et al. [105], Ding et al. [106] and Cortez et al. [107] also found that TNF-α expression was activated after 2 to 6 weeks of HFD administration and led to weight gain and an increased body fat mass. High-fat consumption also stimulates Kupffer cells (the resident macrophages of the liver) in mice and causes an elevation of the M1-polarized population, which is linked to the pathogenesis of obesity-induced fatty liver disease and insulin resistance [108]. Consequently, obesity is associated with a marked increase in oxidative damage to all cellular macromolecules [14, 109, 110].
The mechanisms underlying the elevation of oxidative stress in metabolic disorders are not fully understood, but it is hypothesized that mitochondrial dysfunction [16], augmented by NADPH oxidase activity [111], and increased fatty acid oxidation [112] contribute to these phenomena. Most of the studies so far addressed abnormal gene expression in the adipose tissues and liver, accompanied by upregulated NADPH oxidase expression and downregulated antioxidative enzyme expression [113, 114]. HFD promotes dyslipidemia, which is associated with oxidative stress, an accumulation of some transition metals and elevated free radicals [115]. Fat accumulation has also been linked to systemic oxidative stress in mice and humans via the increased accumulation of ROS, accompanied by the improved expression of NADPH oxidase and the decreased expression of antioxidative enzymes [114]. Moreover, HFD provokes lipid peroxidation and oxidative stress, whereas NADPH oxidase activation deregulates the production of redox-sensitive transcription mRNA such as NF-κB and adipocytokines (fat-derived hormones) including plasminogen activator inhibitor-1, monocyte chemotactic protein-1 (MCP-1), IL-6, adiponectin, and other inflammatory cytokines form different metabolic tissues [116].
Additionally, HFD raises the level of chylomicrons in the intestine. These chylomicrons enter circulation and cause the generation of free fatty acids (FFAs), which are taken up by the liver. These hepatic FFAs may either enter the mitochondria for β-oxidation or be esterified into triglycerides [117, 118]. Triglycerides are either accumulated in hepatocytes as small droplets or generate very low-density lipoprotein (VLDL) which is thereby converted into low-density lipoprotein (LDL) [118]. An excessive LDL burden in the blood due to its excessive accumulation or lack of LDL-receptors in hepatocytes may form oxidized-LDL (Ox-LDL), which in turn is engulfed by macrophages to become foam cells. Subsequently, foam cells accumulate in the arterial endothelium to form plaque. Ultimately, these lead to cardiovascular and circulatory disorders such as thromboembolism, hypertension, atherosclerosis, and heart block [119–121]. Subsequently, the mitochondrial β-oxidation of FFAs is linked to the conversion of oxidized cofactors (NAD+ and FAD) into reduced cofactors NADH and FADH2 and is thereby reoxidized and restored back into NAD+ and FAD by the mitochondrial respiratory chain. During reoxidation, NADH and FADH2 transfer electrons to the first complexes of the respiratory chain. Most of these electrons then migrate up to cytochrome-c oxidase and thereby combine with protons and oxygen to form water. These intermediates may interact with oxygen and produce more and more superoxide anion radicals and other ROS [122–125]. Therefore, the high consumption of fat-rich diets promotes mitochondrial β-oxidation of FFAs and subsequently leads to an excess electron flow using cytochrome-c oxidase, which elevates the accumulation of ROS. Mitochondria are a vitally important cellular source of ROS; they oxidize the unsaturated lipids of fat deposits to cause lipid peroxidation. ROS and lipid peroxidation can consume vitamins and antioxidant enzymes [125, 126]. The depletion of these protective substances may hamper ROS inactivation and promote ROS-mediated damage and lipid peroxidation [114]. This HFD-induced ROS may stimulate the proinflammatory state and thereby activate the NF-κB transcription factor. Further, HFD also may trigger ROS or NF-κB, which induces NF-κB-dependent proinflammatory agents such as TNF-α, inducible nitric oxide synthase (iNOS), and interferon-γ (IFN-γ) [101, 127, 128]. These data converge to provide evidence supporting the role of oxidative stress induced by HFD in metabolic disorders. Surprisingly, an in vitro study showed that free fatty acids increased ROS accumulation, indicating that increased fatty acids in obesity may provide an extra source of additional electron transport chain substrates via the oxidation of fatty acids [111, 129]. In addition to the generation of ROS, the overproduction of nitric oxide (NO) through the activation of iNOS also causes an accumulation of RNS [130, 131]. Taken together, chronic consumption of high GI foods may cause oxidative stress via the formation of free radicals that are capable in destroying biological molecules and initiate abnormal cell growth through gene mutation [132]. Further, HCA formed during high-temperature cooking and grilling of meat may cause oxidation of proteins and lipids, thereby resulting in oxidative stress and may subsequently increase the risk of chronic diseases [133], while the HFD may serve as a stimulus to elevate the systemic inflammatory response in the development of obesity, CVD, diabetes, and cancers [134–137]. Overall, these data imply that high-carbohydrate/high-calorie/high-fat diets stimulate oxidative stress by augmenting the inflammatory response and elevating inflammatory markers.
4. Molecular Connectivity of Oxidative Stress-Induced Diseases
4.1. Obesity and Adipocyte Dysfunction
Obesity has been recognized as a heritable disorder in recent decades [138]. It has become increasingly clear that sedentary lifestyles and an increased availability of inexpensive calorie-dense foods have played a pivotal role in creating an obesogenic environment, which has contributed to the obesity epidemic [139–141]. The individual heritability of obesity susceptibility genes and interaction of the nutrients in the obesogenic environment, particularly dietary macronutrients, including refined carbohydrates and saturated fats, are linked to weight gain and may subsequently contribute to obesity [142, 143]. Thus, the functions of obesity susceptibility genes may be associated with this major health concern.
Obesity is considered a chronic low-grade inflammatory stress condition modulated by immune cells via the infiltration of adipose tissue, along with metabolic stress when oversupplied with glucose and lipids in adipocytes [144, 145]. Inflammatory cytokines have been observed in many fat cells; they are involved in fat metabolism and are associated with all indices of obesity, particularly abdominal obesity [146]. The alterations of leptin and hypothalamic pituitary adrenal (HPA) axis dysfunction, adipocyte function, and fatty acid levels and oxidative stress have been suggested to play a vitally important role in obesity-associated inflammation [146]. In general, the association between excessive nutrient uptake (sugars, lipids, and fatty acids) and metabolic disturbances is modulated by several types of cells, such as adipocytes and resident or infiltrating immune cells including monocytes, T cells, mast cells, and macrophages, which indirectly modify adipocyte function and dysfunction [147, 148]. A study by Lim et al. [149] found that dietary fatty acids activate protease-activated receptor 2 (PAR2) expression, which is a new biomarker for obesity and a substantial contributor in metabolic dysfunction and inflammation.
Studies have shown that ROS is generated from hypertrophic adipocytes induced by a HFD. The expansion of fat mass occurs via two concomitant processes in white adipose tissue expansion: hyperplasia (increased numbers of fat cells associated with the differentiation of adipocyte precursors) and hypertrophy (increased size of fat cells) [150–152]. Several studies have shown a close relationship between ROS and fat mass expansion [153, 154]. Fat accumulation parallels with ROS, as demonstrated by an increase of ROS accumulation during adipocyte 3T3-L1 alteration [155, 156]. Leptin, a white adipose tissue-derived hormone, has been reported to promote the elevation of ROS accumulation in endothelial cells [157, 158]. NF-κB can be stimulated by leptin in an oxidant-dependent manner. This finding is linked with an increased expression of monocyte chemoattractant protein-1 (MCP-1), which enhances atherosclerosis by supporting the relocation of inflammatory cells [152, 159]. Further, leptin also activates ROS in vascular smooth muscle cells via the protein kinase C-dependent activation of NAD(P)H oxidase [160]. Leptin promotes the release of active macrophage lipoprotein lipase via an oxidative stress-dependent pathway, signifying a proatherogenic effect of leptin on macrophages in diabetes [157]. By contrast, the exposition of adipocytes to high ROS levels suppresses the secretion and expression of adiponectin [161], an adipokine that shows anti-inflammatory, antiatherogenic, and insulin-sensitizing properties [162]. Collectively, systemic oxidative stress-associated HFD and obesity may lead to insulin sensitivity of metabolic organs, which thus promotes the inflammatory response [163].
Obesity has been demonstrated to drive the development of insulin resistance. However, not all obese individuals develop type 2 diabetes mellitus or insulin resistance, indicating that the biological mechanism underlying the association between obesity and insulin resistance must be well-controlled under certain circumstances [164]. Obesity has become an epidemic and represents the major risk factor for several chronic diseases, including diabetes, CVD, and cancer [165]. Therefore, the present study focused on the detrimental impact of oxidative stress on diabetes, CVD, and cancer outcomes.
4.2. Diabetes
Type 2 diabetes is the most common metabolic disorder, affecting 422 million people worldwide in 2014 [166], with nearly half of all deaths attributable to high blood glucose [166]. Type 2 diabetes is currently the most common form of the disease, representing nearly 90–95% of diabetes mellitus cases. Diabetes mellitus is a complex and progressive disease that is accompanied by several complications such as nephropathy, retinopathy, neuropathy, and micro- and macrovascular damage [167].
Oxidative stress has been identified as a major risk factor in the development of diabetes [168]. Numerous risk factors including increased age, unhealthy dietary intake, and obesity all lead to an oxidative environment that may modify insulin sensitivity either via the elevation of insulin resistance or the impairment of glucose tolerance [169]. The mechanisms that implicate these diseases are complex and involve several cell signaling pathways [170]. Hyperglycemia is linked to diabetes and subsequently contributes to its progression and an overall oxidative environment [171]. Macro- and microvascular complications contribute to the morbidity and mortality of diabetic patients, and all these factors are associated with oxidative stress [172].
The derangement of molecular and cellular processes is common in type 2 diabetes, particularly in β cells. Pathophysiologically, ROS and RNS, such as H2O2, superoxide anion (O2∙−), NO, peroxynitrite (ONOO−), and hydroxyl radical (OH∙), all contribute to primary physiologic and metabolic processes. Mitochondrial function impairment leads to a reduction in ATP generation capacity, which in turn leads to β cell glucose-stimulated insulin secretion (GSIS), the NADPH complex, and Ca2+ signaling related to neurotransmission [173, 174].
Insulin resistance plays a predominant role in the development and progression of metabolic dysfunction associated with obesity. Insulin resistance refers to the impairment of the cellular response in insulin-sensitive tissues such as skeletal muscle, adipose, liver, and brain tissues [175–177]. Subsequently, this may lead to a reduction of glucose uptake, accompanied by the elevation of hepatic glucose output, and thereby contribute to plasma glucose concentrations [178]. The subsequent changes of glucose homeostasis may place a burden on pancreatic β cells to secrete and produce more insulin to restore normal blood carbohydrate levels [179]. Nonetheless, this compensatory mechanism may ameliorate glucose levels in an early or prediabetes condition, characterized by continuous insulin resistance and high exposure of β cells to blood glucose and lipids [180]. This may boost β cell failure and dysfunction and culminate in overt diabetes [176].
Pancreatic islets are highly vascularized and specialized structures that control the nutrient contents in the bloodstream and are mainly comprised of five cell types: α cells, β cells, δ cells, ghrelin cells (γ cells), and pancreatic peptide- (PP-) secreting cells [181]. Islets generate blood from the splenic branches and pancreaticoduodenal arteries and interact to increase dietary nutrients to secrete insulin from α and β cells into glucagon and the bloodstream, respectively (during nutrient-deprived conditions such as starvation and fasting) [175]. The pancreatic β cell response to glucose depends on the acute regulation of intracellular or extracellular ROS and RNS [173, 174]. The elevation of glycolytic flux promotes ATP production and oxidative phosphorylation, which subsequently results in the formation of O2∙− released from the electron transport chain [182]. Additionally, an initial adaptive response is modulated through the pentose phosphate pathway in which surfeit glucose is converted to pentose and glucose carbon is deviated away from excessive oxidative and glycolysis phosphorylation. However, shuttling glucose in this direction may also increase NADPH oxidase (NOX) activity and subsequently lead to increased O2∙− synthesis. Indeed, high glucose levels may increase ROS through other possible mechanisms, such as the generation of advanced glycation end products (AGEs) and glucose autoxidation [183].
Once insulin is released into the blood circulation by β cells in response to increased blood glucose levels, insulin exhibits its anabolic effects through the transmembrane insulin receptor (IR) in target tissues. Interaction with insulin fosters the autophosphorylation of the receptor with the phosphorylation and recruitment of insulin receptor substrate (IRS) proteins and the stimulation of other related downstream signaling cascades, such as protein kinase B (Akt) and phosphatidylinositol-3-kinase (PI3K) [184]. Akt has been identified as a primary regulator in vesicle translocation of glucose transporter type 4 (GLUT-4) to the plasma membrane, which is crucial in the intracellular uptake of free glucose in insulin-sensitive tissues [48].
Numerous studies have indicated that there is an association between increased nitrosylation and carbonylation of proteins in obese- or insulin-resistant phenotypes and insulin-sensitive tissues [110, 185–187]. This suggests that an insulin-resistant phenotype may promote the reduction of insulin receptor expression. Thus, prolonged hyperinsulinaemia and chronic hyperglycemia, along with increased ROS and RNS levels, are hypothesized to influence insulin receptor gene expression through the derangement of key transcription factors such as high mobility group AT-hook 1 (HMGA-1) [188]; they may also increase insulin receptor-desensitization, which under normal circumstances is a process under the negative-feedback control [189, 190]. Taken together, the development and progression of diabetes mellitus is associated with β cell dysfunction and insulin resistance, and this phenomenon is normally related to obesity [175].
4.3. Cardiovascular Disease
Oxidative stress is implicated in the progression and development of cardiovascular disease (CVD) [191]. Its burden is attributable to lifestyle factors, particularly smoking, alcohol consumption, sedentary lifestyles, and dietary intake [192]. In Malaysia, western dietary habits that are high in fat and low in dietary fiber lead to the increase in CVD incidence [193]. Chronic and low-grade inflammation has been suggested as a major pathophysiology in obesity and its associated diseases such as CVD [194]. C-reactive protein (CRP) has been shown to be an independent risk factor for the development of CVD [195, 196]. The elevation of CRP in obesity could be attributed to macrophage infiltration into the expanded adipose tissue and subsequently leads to the production and release of macrophage-derived proinflammatory cytokines such as IL-6 and TNF-α [197, 198].
One common feature of CVD is increased oxidative stress in the heart [199]. Specifically, systemic oxidative damage in patients with CVD was due to ROS accumulation and reduced antioxidant defense [200]. A HFD increased ROS accumulation and reduced antioxidant capacity, thus causing a variety of disorders including endothelial dysfunction, which is characterized by a decreased bioavailability of vasodilators, namely, NO, and promotes endothelium-derived contractile factors causing atherosclerotic disease [201]. One potential biological mechanism linking cardiac oxidative stress has been described by Ilkun and Boudina [201] and includes mitochondrial dysfunction, increased fatty acid oxidation, and increased NADPH oxidase activity. Ilkun and Boudina [201] demonstrated that the modes of action underlying cardiac pathology are complex and might include altered calcium homeostasis, lipid accumulation, abnormal autophagy, increased fibrosis and stiffness, increased oxidative stress, and mitochondrial dysfunction. Collectively, mitochondrial and extramitochondrial sources of ROS and a reduction of antioxidant defense mechanisms have occurred in the myocardium of human and animals [201].
4.4. Cancer
Research has demonstrated that high oxidative stress leads to cancer, including colorectal cancer [202]. Oxidative stress is hypothesized to be associated with obesity and cancer. A study in an animal obese model of nonalcoholic steatohepatitis supports these hypotheses, suggesting that the absence of adiponectin promotes hepatic tumor formation and elevates oxidative stress [203]. Indeed, ROS plays a crucial role in cancer development [204, 205]. The elevation of ROS leads to increased mutation rates or susceptibility to mutagenic agents and thus contributes to DNA damage during the early stages of carcinogenesis [205]. The elevation of ROS has also been demonstrated in tumor proliferation via the ligand-independent transactivation of receptor tyrosinekinase [204], which can promote metastasis and the invasion of cancer cells [206]. Semenza [207] observed that ROS can promote the stabilization of hypoxia-inducible factor 1, a transcription factor of vascular endothelial growth factor, which facilitates tumor angiogenesis.
Intriguingly, data from a previous study have shown that insulin is a proliferation factor for prostate cancer; thus, the reduction of carbohydrates may subsequently decrease serum insulin and slow down prostate cancer proliferation [208]. Epidemiological studies have shown that patients with type 2 diabetes and obesity have a greater likelihood of having liver, colorectal, breast, and pancreatic cancers [209, 210]. These findings suggest that leptin [203, 211], insulin/insulin-like growth factor-1 [212, 213], adiponectin [203, 211], and inflammation [214, 215] are additive between type 2 diabetes or obesity and cancers. Fat accumulation is often linked with systemic oxidative stress via elevation of ROS [114]. A previous study stated that increased oxidative stress can lead to chronic inflammation, which in turn could modulate chronic diseases such as cancer [216]. Oxidative stress can trigger a wide range of transcription factors such as Wnt/β-catenin, NF-κB, and nuclear factor E2-related factor 2 (Nrf2) and thereby activates inflammatory pathways [216]. Taken together, these findings suggest that increased circulating or local ROS levels derived from the expansion of the adipose tissue in a tumor environment provoke oxidative stress within tumor cells and thereby lead to an increased risk for cancer progression in patients with type 2 diabetes or obesity.
5. Diet Ameliorates Oxidative Stress-Induced Diseases
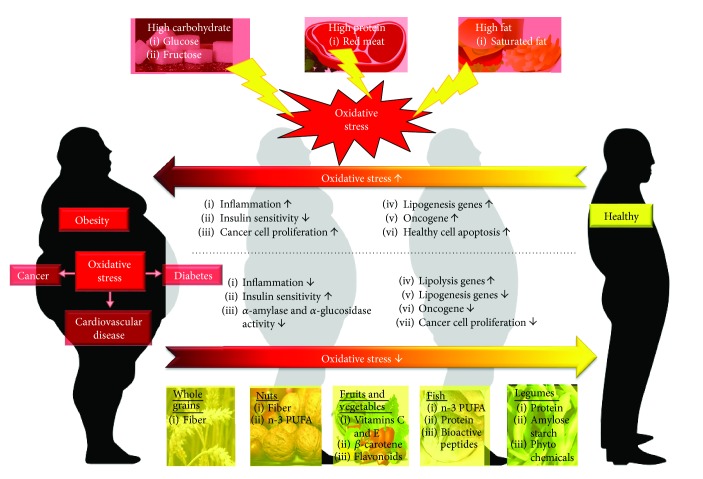
Oxidative stress is increased in diabetic patients and cancer cells [217, 218]. Higher intracellular glucose concentrations can generate ROS via several pathways [219], and the progression and development of these diseases could be prevented by changing dietary habits [220]. It was evident that high-glucose and an animal-based protein diet and excessive fat consumption can promote oxidative stress [221], for example, excessive omega-6 stimulates inflammation [222]; however, there are other dietary choices (the Mediterranean and Okinawan diets of the Greek and Japanese populations) that can reduce inflammation [223]. Figure 1 summarizes the dietary intake pattern in relation to human health.
Figure 1.
Dietary intake patterns affect human health state. High-carbohydrate and an animal-based protein diet and excessive fat consumption will eventually lead to obesity as well as other obesity-related diseases such as cardiovascular diseases (CVD), diabetes, and cancer. The key pathway involved in the pathogenesis is via the elevation of oxidative stress. Subsequently, inflammation occurs resulting in the reduction of insulin sensitivity, increased cancer cell proliferation, involvement of gene in lipogenesis, and cancer development of which is activated and accompanied by apoptosis of healthy cells. To revert these unhealthy conditions, consumption of healthy diet is essential. Healthy diet includes whole grains, nuts, fruits and vegetables, fish, and legumes. In general, a healthy diet contains dietary fiber, unsaturated fatty acids like monounsaturated fatty acid (MUFA) and n-3 polyunsaturated fatty acid (n-3 PUFA), protein, vitamins, minerals, and others health-promoting components. All these components exhibit antioxidant ability thereby reduce oxidative stress. The healthy diet could reduce inflammation, cancer development, and lipogenesis transcriptional expression. It also increases insulin sensitivity accompanied by the reduction of α-amylase and α-glucosidase activity. A healthy dietary pattern is crucial for maintaining good health.
5.1. Whole Grains
Numerous components of the diet may promote inflammation. Whole grains comprised of germ, endosperm, and bran are rich in vitamins, fibers, minerals, and phytochemicals such as carotenoids, lignans, vitamin E, inulin, β-glucan, sterols, and resistant starch [224]. As an example, the fiber found in whole grain foods appears to play a role in immune-modulating functions [225, 226]. Fiber affects microbiota in the gut [227], which affects immune function [228]. In support of this, the intake of whole grains such as sorghum benefits the gut microbiota and indices associated with oxidative stress, obesity, inflammation, hypertension, and cancer [229]. Whole grain foods are rich in phytochemicals and provide protection against oxidative stress, which can result in inflammation. Polyphenol compounds present in wheat sprouts may benefit a certain group of the population because they appear to combat oxidative stress associated with obesity [230] and enhance glucose metabolism [231].
Data from a meta-analysis have demonstrated that a high intake of whole grain products is associated with a reduction of total cancer risk [232]. In a Scandinavian HELGA cohort study, intakes of whole grains were found to be inversely associated with colorectal cancer incidence [233]. A study by Tan et al. [202] and Tan et al. [234] further supported the role of a unique complex of bioactive constituents in brewers' rice, which is a rice by-product in the rice industry that exerts significant nutritional value to combat colon carcinogenesis. Anti-inflammatory effects of brewers' rice protect against oxidative stress and free radical damage by improved antioxidant enzymes such as MDA, SOD, and NO. They also inhibit DNA damage caused by ROS via the upregulation of the Nrf2 signaling pathway. Several studies have also reached a similar finding, in which rice by-products have an antiproliferative activity against cancer [235, 236]. Strikingly, feeding with brewers' rice not only reduced the number of aberrant crypt foci (ACF) [237]; in fact, the relative proportions of natural antioxidant compounds in brewers' rice have also been reported to attenuate liver and kidney damage in azoxymethane-induced oxidative stress in rats, as reported by Tan et al. [238], suggesting that bioactive constituents present in whole grains may ameliorate oxidative stress.
In addition to the effects observed on cancer, germinated brown rice has been extensively studied in the past few decades. Germinated brown rice has a significant nutritional value. In addition to containing high amounts of minerals, vitamins, and fiber, germinated brown rice is also rich in a variety of bioactive compounds and has drawn a great deal of interest in the prevention of CVD risk. These bioactive compounds were demonstrated to have antioxidant activities that are suggested to alleviate CVD risk via the modulation of hepatic cholesterol metabolism and oxidative stress [239, 240]. Accordingly, germinated brown rice modulates lipid metabolism via the transcriptional regulation of peroxisome proliferator-activated receptor gamma (PPARγ), hepatic lipoprotein lipase (LPL), ATP-binding cassette, subfamily A (ABCA), v-akt murine thymoma viral oncogene homologue 1 and homologue 3 (AKT1 and AKT3), and adiponectin [239]. In this regard, natural components present in whole grain such as polyphenolic compounds have the potential to suppress proinflammatory immune signaling and subsequently improve lipid metabolism and inhibit cancer development [241].
Notably, the nutritional values of fiber components such as arabinoxylans and β-glucans are also found in whole grains. Studies have revealed a positive association between wheat and rye arabinoxylans and water-soluble maize on caecal fermentation, the reduction of serum cholesterol, and the production of short-chain fatty acids [242, 243]. Dietary fibers present in whole grain also play a central role to enhance immune function through the production of short-chain fatty acids, suggesting that increasing the intake of fermentable dietary fiber may be vitally important in reducing inflammation [244, 245]. Short-chain fatty acids may promote T helper cells, neutrophils, macrophages, and cytotoxic activity in natural killer cells [246]. Further, the fermentation of dietary fiber in the colon and changes in gut microbiota are associated with impaired gastrointestinal tolerance [247]. Together with the gut immune system, mucosal and colonic microflora prevent pathogenic bacteria from invading the gastrointestinal tract [248]. The intestinal flora salvages energy via the fermentation of undigested carbohydrates in the upper gut [246]. The predominant substrates are dietary carbohydrates and mucus, which escape digestion in the upper gastrointestinal tract [246]. These include nonstarch polysaccharides (such as hemicelluloses, celluloses, gums, and pectins), resistant starch, sugar alcohols, and nondigestible oligosaccharides [246]. The primary fermentation pathway produces pyruvate from hexoses in undigested carbohydrates [246]. Colonic bacteria use a wide range of carbohydrates to hydrolyze enzymes and produce methane, hydrogen, short-chain fatty acids (primarily butyrate, propionate, and acetate), carbon dioxide, and lactate [249]. In this regard, these components activate fermentation, increase bacterial and fecal mass, and ultimately lead to a stool bulking effect [246]. Overall, this suggests that the protective effect of whole grains on oxidative stress may be mediated partly via the synergistic/additive effects of these bioactive components.
5.2. Nuts
When a landmark epidemiological study found that a high frequency of nut consumption was related to a reduction of CVD [250], nuts were brought from obscurity to prominence as a crucial health food. In the last 15 years since this first epidemiological study, scientific research on the health effects of nuts has not only focused on the area of coronary heart disease and its risk factors but has also extended to other areas of health. In addition, clinical trials have found that diets enriched with nuts reduce oxidative stress and inflammation [251] and alleviate endothelial dysfunction or insulin resistance [252]. Another clinical study consistently reported a hypocholesterolemic potential of regular nut consumption, which partly explains how walnuts reduce the risk of CVD [253].
Nuts are not only a high-fat and energy-dense food but they are also rich in bioactive constituents [254] that are believed to have anti-inflammatory and anticarcinogenic properties including folic acids and several phytochemicals [255, 256]. Notably, collective findings suggest that a protective role of nuts on colorectal and endometrial cancer prevention is possible [257–259].
A crucial underlying mechanism of action that has been proposed to explain an inverse relationship between the frequency of nut-enriched consumption and risk of obesity is unsaturated fatty acids. Healthy fats (unsaturated fatty acids) in nuts contribute to the prevention of diabetes and CVD risk. By contrast, nuts are complex food matrices that are also a source of other bioactive constituents, namely, tocopherols and phenolic compounds [260]. Compelling evidence suggests that monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) are more readily oxidized [261] and have a greater thermogenic effect [262] than do saturated fatty acids, which might contribute to less fat accumulation. Due to their unique fat and nonfat composition, nuts are more likely to mediate inflammation and oxidative stress.
Because nuts contain the abundance of unsaturated fatty acids, protein, and fiber, they are a highly satiating food [263]. Thereby, after consuming nuts, hunger is reduced and subsequent food intake is curtailed [264]. The physical structure of nuts may also lead to their satiety effect because they must be masticated, small enough for swallowing. Mastication stimulates nutrient, mechanical, and sensory signaling systems that may alter appetitive sensations [265]. Additionally, a small degree of fat absorption may occur after nut consumption because fat is found within the wall cellular structures that are not fully digested in the gut [266], which could be compounded by incomplete mastication [267]. Data from population-based studies indicate an inverse relationship between nut intake, such as almonds and CRP [268, 269]. Plasma IL-6 levels were reduced after a Mediterranean diet with nuts compared to a control diet [270, 271]. Similarly, previous studies reported by Zhao et al. [272] and Zhao et al. [273] also evaluated walnuts rich in PUFAs and, in particular, alpha-linolenic acid (ALA), in relation to proinflammatory cytokine production [273] and inflammatory markers [272] by blood mononuclear cells. The data showed that compared to the average American diet, the CRP levels were reduced by 75% in subjects consuming an ALA diet; conversely, levels in subjects consuming the linoleic acid (LA) diet decreased by 45% [272]. Indeed, reductions in multiple inflammatory markers such as IL-6, IL-1β, and TNF-α produced by cultured mononuclear cells were observed from subjects who consumed an ALA-enriched diet [273].
Based on the findings for marine-derived omega-3 PUFA, ALA would be expected to have anti-inflammatory properties. This was evaluated in a clinical study with a relatively small observed effect [274]. However, an in vitro study in which THP-1 cells were supplemented with LA, ALA, docosahexaenoic acid (DHA), and palmitic acid in the presence of lipopolysaccharide [275] showed a significant reduction in TNF-α, IL-1β, and IL-6 after treatment with DHA, ALA, and LA compared to palmitic acid, indicating that ALA present in walnuts elicits an anti-inflammatory response. Notably, cellular adhesion molecules are biochemical markers of endothelial dysfunction concomitantly with inflammation. In a further study focused on CVD outcomes, Zhao et al. [273] compared hypercholesterolemic subjects who consumed a diet high in ALA, a diet high in LA, and an American diet, respectively. The data showed that participants who consumed 15 g of walnut oil along with 37 g of walnuts/daily for 6 weeks demonstrated a reduction in CRP, cellular adhesion molecule soluble intercellular adhesion molecule (sICAM) 1, and E-selectin. Importantly, some research has emerged to suggest that CVD risk factors negatively affect endothelial function and are involved in the modulation of LDL cholesterol [276, 277]. In support of this, the acute consumption of walnuts oils is favorably affected and shows a better endothelial function [278]. Further, walnuts and walnut oil may influence inflammation, at least in part, via the elevation of cholesterol efflux, which is a reverse cholesterol transport that is crucial for the removal of cholesterol from peripheral tissues and indicates cardioprotective effects [253]. Taken together, nuts seem a good dietary choice for providing nutrients and preventing obesity and other chronic diseases. However, the bioactive components responsible for the effects that we stated above require further elucidation.
5.3. Fruits and Vegetables
Fruits and vegetables are rich in minerals, vitamins, and dietary fiber. High intakes of fruits and vegetables are inversely associated with mortality and the incidence of obesity-related diseases such as CVD, type 2 diabetes, and cancer [279]. Such protection has been accredited to antioxidant vitamins such as β-carotene, vitamin E, and vitamin C [280]. In general, more than 85% of the total antioxidants in fruits and vegetables are hydrophilic antioxidants [281]. Beta-carotene and vitamins E and C are vitally important for the proper regulation of physiological function [282]. The essential role of vitamin E in maintaining the oxidative-antioxidant balance is well-recognized, yet vitamin C can enhance the antioxidant protection [282]. Beta-carotene is usually found in bright-colored fruits and vegetables [283]. It has been demonstrated to maintain the immune system and exert an ability to decrease LDL-cholesterol oxidation through the modulation of antioxidant enzymes [283]. In addition to the vitamin antioxidants stated above, other dietary components such as flavonoids may protect against oxidative stress. Flavonoids are plant polyphenolic compounds ubiquitous in fruits and vegetables. Flavonoids exert multiple biological activities such as antitumor effects, anti-inflammatory activity, antioxidant activity, and antimicrobial action, and they suppress platelet aggregation [284].
An animal study has demonstrated that a diet supplemented with β-carotene from fruit significantly downregulated the expression of fatty acid synthase, acetyl-CoA carboxylase, and fat synthesis-related genes [285]. Findings from a population-based study mirror some of those from preclinical data obtained from an in vivo study. Data from a population-based study reported that high intakes of fruits and vegetables significantly decreased energy consumption, waist circumference, body weight, and sagittal abdominal diameter in overweight and obese men and women [286].
Compelling epidemiological studies have revealed that intakes of fruits and vegetables induce protective cardiometabolic effects. A study showed that encapsulated fruit and vegetable-concentrated juice decreased total cholesterol, LDL-cholesterol, plasma TNF-α, and systolic blood pressure, in addition to increasing total lean mass [287]. The improvements in these indices could be attributed to the alteration of gene expression via several signaling pathways such as AMP-activated protein kinase (AMPK) and NF-κB associated genes [287]. Body composition, blood lipids, and systemic inflammation were improved in obese subjects after consuming fruits and vegetables and thus provide a useful approach for reducing the obesity-induced chronic diseases risk [288]. Further, fruits and vegetables can also prevent CVDs or assist with the restoration of function and morphology of vessels and the heart after injury. Fruits and vegetables are thought to protect against CVD by regulating lipid metabolism, protecting vascular endothelial function, suppressing platelet function, modulating blood pressure, inhibiting thrombosis, attenuating inflammation, alleviating ischemia/reperfusion injury, and reducing oxidative stress [289, 290].
In addition to the effects observed in obesity and CVD, a beneficial effect of fruit and vegetable consumption in human has also been reported on the incidence of type 2 diabetes. Data from a meta-analysis included a study from 1966 to 2014 that demonstrated that a high intake of fruit, particularly berries, and yellow, cruciferous, green leafy vegetables or their fibers, is negatively linked to type 2 diabetes [291]. As an example, lactucaxanthin (Lxn), a carotenoid in lettuce (Lactuca sativa), suppresses α-amylase and α-glucosidase activity both in vitro and in diabetic rats [292]. Such findings highlight the role of unique complexes of bioactive components in fruits and vegetables.
Fruits and vegetables not only reduce obesity, CVD, and diabetes but they also inhibit several cancers, demonstrating the numerous functional potentials of fruits and vegetables. Epidemiological studies have shown an inverse relationship between fruit and vegetable intakes and cancer risks such as colon, breast, and prostate cancers. This suppressive effect was mainly observed in cruciferous and green-yellow vegetables [293] via the modulation of genes involved in proliferation and glucose metabolism and the induction of several antioxidant genes [294]. Notably, dietary fiber in fruits and vegetables will undergo fermentation by gut microbiota, which may lead to the production of short-chain fatty acids. Short-chain fatty acids such as acetate [295], butyrate [296], and propionic acids [297] may have protective effects against cancers. It is possible that only certain types of fruits and vegetables confer protection against oxidative stress [283]. Since some bioactive compounds regulate the same gene expression and pathways targeted by drugs, diets high in fruits and vegetables in combination with medical therapies are being considered as a novel treatment strategy [298]. Overall, bioactive constituents in fruits and vegetables might be promising tools for the alleviation of a wide range of diseases [299].
5.4. Fish
Fish is an essential source of dietary protein, omega-3 fatty acids, and minerals. Nakamura et al. [300] demonstrated that individuals who consume fish daily were inversely associated with obesity compared to those with normal weight or underweight. The intake of fish has been linked to a reduced risk of obesity [301], yet the composition of fish often includes representative PUFA amounts, such as n-3 fatty acids, whose chemical structure makes them prone to peroxidation and are found abundantly in fatty fish. Therefore, our body becomes more susceptible to oxidative stress and subsequently activates the lipid peroxidation process [302]. Undoubtedly, PUFA intake is essential as they have well-established health benefits especially in preventing heart disease [303]. However, it is recommended to have an adequate vitamin E to match the increased of PUFA intake [304]. This is because lipophilic antioxidant vitamin E plays a vital role in protecting PUFA [305]. In addition, an animal study has shown that the vitamin E requirement is increased almost proportionally with the degree of unsaturation of the PUFA [304].
The consumption of lean fish has a beneficial impact on insulin sensitivity, glucose homeostasis, and lipid metabolism [306, 307]. Aadland et al. [306] further demonstrated that intakes of lean fish for 4 weeks reduced the ratio of total to HDL cholesterol in serum, decreased the VLDL concentration, and reduced fasting and postprandial triacylglycerol (TAG) compared to those with a nonseafood diet, suggesting the cardioprotective potential of lean-seafood intake. A similar dietary intake was also found to reduce the urinary excretion of metabolites involved in mitochondrial lipid and energy metabolism, possibly facilitating a higher lipid catabolism [308]. Intriguingly, lean fish contains relatively low amounts of marine n-3 fatty acids, and thereby the beneficial effects of fish are not solely ascribed to the lipid composition.
Dietary protein has been suggested as the most effective food macronutrient to provide a satiating effect. Therefore, protein-rich foods can facilitate in the modulation of food intake, promoting body weight loss and maintaining body weight thereafter. Glucagon-like peptide-1 (GLP-1) release stimulated by a high-protein meal is evoked by carbohydrate content. Indeed, cholecystokinin (CCK) and peptide YY (PYY) release is activated by a high-protein meal [309].
Fish not only contains macronutrients but also has a substantial antioxidant source due to its composition and offers a relatively low level of saturated fat compared to other food items. Taurine, an amino acid that is abundantly found in fish, is a vital antioxidant source. Studies have shown that taurine can effectively combat metabolic syndrome by regulating glucose metabolism, reducing triglycerides to prevent obesity, regulating the renin-angiotensin-aldosterone and kallikrein-kinin systems to decrease blood pressure, and lowering cholesterol (particularly reducing VLDL + LDL cholesterol and promoting HDL cholesterol) to prevent diet-induced hypercholesterolemia [310].
Notably, the production of fish protein peptides (hydrolysates) maximizes the usage of fish protein because peptides have a health-promoting potential [311]. Techniques such as autolysis, thermal hydrolysis, and enzymatic hydrolysis have been developed to produce fish hydrolysates. The antiviral-, cardioprotective- (antihypertensive, antiatherosclerotic, and anticoagulant), analgesic-, antimicrobial-, antioxidative-, antitumor-, immunomodulatory-, neuroprotective, and appetite-suppressing activities have drawn attention from the pharmaceutical industry, which attempts to design the treatment and prevention of certain diseases [312]. Lassoued et al. [313] and Razali et al. [314] reported that peptides derived from fish proteins exhibit significant antioxidative activity in oxidative systems. The dietary intake of antioxidant compounds can strengthen the body's oxidant status and facilitate a balanced condition in terms of oxidants/antioxidants in the body.
In addition to fish and its protein peptides, neovastat (AE-941), a liquid extract derived from the cartilage of sharks, exerts antiangiogenic, anti-inflammatory, and antitumor properties both in vitro and in vivo [315]. These favorable effects are mediated via the suppression of matrix metalloproteinases (MMP)-2, MMP-9, and MMP-12 and the activation of tissue plasminogen activator enzymatic activities [315].
Another metabolic disorder is hypertension, which occurs when renin produces angiotensin I from angiotensinogen. The angiotensin I-converting enzyme (ACE) cleaves angiotensin I to angiotensin II, which is a potent vasoconstrictor [316]. Accordingly, Balti et al. [317] have sourced bioactive constituents from different types of fish in ACE-inhibitor activity studies with molecular weights of <10 kDa. From a review study, Balti et al. [317] found that bioactive peptides are suitable competitive inhibitors that can bind to the active site of ACE and thereby block its activity. Collectively, it remains unknown whether PUFA content or its antioxidant is responsible for its beneficial effects; thus, further study is necessary to conclusively resolve the question behind the anti-inflammatory effects of fish.
5.5. Legumes
Legumes are a primary component of the Mediterranean diet. They are rich in fiber and protein, which can facilitate in lowering energy density and reducing the glycemic response [318]. Legumes also contain B vitamins and minerals, such as potassium, calcium, and iron. Most of the nutritional value in legumes is contributed by their relative proportions of protein, fibers [319], and phytochemicals such as isoflavones, phytoestrogens, saponins, oligosaccharides, lectins, and phenolic compounds [320]. Due to their high nutritional values, legume intake has been demonstrated to have beneficial effects in the prevention of obesity and other related disorders [321].
Compared to those who rarely or never consume legumes, adults who consume legumes have a significantly lower body mass index and waist circumference. Children who consume legumes had smaller waist circumferences compared to those who never consume legumes [322]. Shinohara et al. [323] further demonstrated that ethanol extracts of chickpeas improved total lipid indices and gene expression associated with fatty acid metabolism in adipocytes. Studies have shown that enzymes involved in lipogenesis such as AMPK, acetyl-CoA carboxylase (ACC), and liver kinase B1 (LKB1) were inactivated by phosphorylation. Further, lipolysis was increased by the extract through the stimulation of palmitoyltransferase 1 (CPT1) and uncoupling protein 2 (UCP2), which has been reported as a crucial protein in fatty acid oxidation [323].
Starch digestibility and composition influence glycemic response. Legumes are high in amylose starch. Nonetheless, the digestion of high amylose starch is significantly lower compared to that of high amylopectin starch [324]. Yang et al. [325] reported a more sustainable plasma glucose level after a high-amylose meal compared to a high-amylopectin meal [325]. Furthermore, legumes have a high protein content; thus, the interaction of protein-starch may further hamper digestibility [326]. Moreover, high amounts of dietary fiber markedly reduced the extent and rate of legume starch digestibility. A high intake of fiber may promote satiety, enhance insulin resistance, and decrease the glycemic response [327]. Evidence from epidemiological studies shows that legume intake is negatively associated with fasting glucose levels [328].
Notably, data from large-scale epidemiological studies found that legume consumption is negatively associated with CVD mortality. Compared to the highest and lowest legume consumption, high legume consumption showed a 6% decreased risk of CVD [329]. Isoflavones are believed to have hypolipidemic activity by binding with estrogen receptors when circulating estrogen is low and thereby translocating to the nucleus, which interacts with a DNA sequence near the promoter region of target genes and results in DNA transcription [330]. Through this mechanism, isoflavone may act as a ligand for lipid-regulating proteins including PPAR, farnesoid X receptor, and liver X receptor, which facilitates cholesterol reabsorption, bile acid synthesis, and hepatic lipid synthesis [331].
In addition to the effects mentioned above, legumes have the potential to protect against cancers. For example, soy food protects against estrogen receptor-negative breast cancer [332]. A study reported by Guo et al. [332] demonstrated that in women with high soy intakes, tumor suppressor genes were upregulated (miR-29a-3p and IGF1R), and oncogenes were downregulated (KRAS and FGFR4). Consistent with the study reported by Guo et al. [332], green pea- (Pisum sativum) extracted lectin has also been reported to have antiproliferative activity against liver cancer cell lines [333]. Despite the limited available evidence to draw a firm conclusion, some studies suggest that legumes may be potentially beneficial to some population segments. Collectively, future studies may elucidate the role of legumes in human health, yet their use within a balanced diet should be considered in the absence of clear contraindications.
6. Summary and Future Prospects
This review has provided clear evidence of the identification of known sources of nutritionally mediated oxidative stress as a mediating pathway for both risks of obesity and other obesity-associated diseases. Oxidative stress is a central player of metabolic ailments associated with high-carbohydrate and animal-based protein diets and excessive fat consumption. There is inconsistent research supporting the clinical use of antioxidant agents in preventing or delaying the onset and progression of metabolic disorders such as diabetic complications and cancer [334–337], and most clinical studies are limited in their sample size and duration of the study. Despite this, preclinical studies in vitro and animal experiments have provided in-depth insight into the modulation of these diseases. Several anti-inflammatory dietary sources such as whole grains, nuts, fruits and vegetables, and others can delay the onset of insulin resistance, prevent adipocyte and endothelial dysfunctions, and prevent tumor proliferation by reacting with oxidizing free radicals and inhibiting the inflammatory response. Therefore, more randomized clinical trials are warranted to evaluate the overall long-term effects of dietary intervention.
7. Conclusions
The available research strongly supports that a diet high in carbohydrates and animal proteins and excessive fat consumption produces ROS and subsequently leads to oxidative stress. The best dietary advice for the prevention and management of obesity and other metabolic disorders includes replacing refined carbohydrates with whole grains, increasing fruits and vegetables, substituting total and saturated fat with MUFAs, and consuming a moderate amount of calories with an ultimate goal of maintaining an ideal body weight. Overall, further studies are warranted to gain a better understanding of the types and the degree of ROS generation in relation to diet-induced metabolic disorders.
Conflicts of Interest
The authors declare that there are no conflicts of interests regarding the publication of this article.
References
- 1.Rajendran P., Nandakumar N., Rengarajan T., et al. Antioxidants and human diseases. Clinica Chimica Acta. 2014;436:332–347. doi: 10.1016/j.cca.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Roleira F. M. F., Tavares-da-Silva E. J., Varela C. L., et al. Plant derived and dietary phenolic antioxidants: anticancer properties. Food Chemistry. 2015;183:235–258. doi: 10.1016/j.foodchem.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Jung K. J., Lee E. K., Kim J. Y., et al. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflammation Research. 2009;58(3):143–150. doi: 10.1007/s00011-008-7227-2. [DOI] [PubMed] [Google Scholar]
- 4.Fernández-Sánchez A., Madrigal-Santillán E., Bautista M., et al. Inflammation, oxidative stress, and obesity. International Journal of Molecular Sciences. 2011;12(5):3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herieka M., Erridge C. High-fat meal induced postprandial inflammation. Molecular Nutrition and Food Research. 2014;58(1):136–146. doi: 10.1002/mnfr.201300104. [DOI] [PubMed] [Google Scholar]
- 6.Jansen F., Yang X., Franklin B. S., et al. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovascular Research. 2013;98(1):94–106. doi: 10.1093/cvr/cvt013. [DOI] [PubMed] [Google Scholar]
- 7.DiNicolantonio C. J., Lucan S. C., O’Keefe J. H. The evidence for saturated fat and sugar related to coronary heart disease. Progress in Cardiovascular Diseases. 2016;58(5):464–472. doi: 10.1016/j.pcad.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer A., Fairlie D. P., Prins J. B., Hammock B. D., Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nature Reviews Endocrinology. 2010;6(2):71–82. doi: 10.1038/nrendo.2009.264. [DOI] [PubMed] [Google Scholar]
- 9.Vos M. B., Lavine J. E. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. 2013;57(6):2525–2531. doi: 10.1002/hep.26299. [DOI] [PubMed] [Google Scholar]
- 10.Park Y., Lee J., Oh J. H., Shin A., Kim J. Dietary patterns and colorectal cancer risk in a Korean population: a case-control study. Medicine. 2016;95(25, article e3759) doi: 10.1097/MD.0000000000003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song S., Lee J. E., Song W. O., Paik H.-Y., Song Y. J. Carbohydrate intake and refined-grain consumption are associated with metabolic syndrome in the Korean adult population. Journal of the Academy of Nutrition and Dietetics. 2014;114(1):54–62. doi: 10.1016/j.jand.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Data and Statistics. The Challenge of Obesity-Quick Statistics. 2017. http://www.euro.who.int/en/health-topics/noncommunicable-diseases/obesity/data-and-statistics.
- 13.Wang Y. C., McPherson K., Marsh T., Gortmaker S. L., Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. The Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda M., Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obesity Research and Clinical Practice. 2013;7(5):e330–e341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Kitabchi A. E., McDaniel K. A., Wan J. Y., et al. Effects of high-protein versus high-carbohydrate diets on markers of β-cell function, oxidative stress, lipid peroxidation, proinflammatory cytokines, and adipokines in obese, premenopausal women without diabetes: a randomized control trial. Diabetes Care. 2013;36(7):1919–1925. doi: 10.2337/dc12-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuzefovych L. V., Musiyenko S. I., Wilson G. L., Rachek L. I. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 2013;8(1, article e54059) doi: 10.1371/journal.pone.0054059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graves D. B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. Journal of Physics D: Applied Physics. 2012;45(26, article 263001):1–42. doi: 10.1088/0022-3727/45/26/263001. [DOI] [Google Scholar]
- 18.Rahal A., Kumar A., Singh V., et al. Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Research International. 2014;2014:19. doi: 10.1155/2014/761264.761264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue M., Sato E. F., Nishikawa M., et al. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Current Medicinal Chemistry. 2003;10(23):2495–2505. doi: 10.2174/0929867033456477. [DOI] [PubMed] [Google Scholar]
- 20.Jia Q.-Q., Wang J.-C., Long J., et al. Sesquiterpene lactones and their derivatives inhibit high glucose-induced NF-κB activation and MCP-1 and TGF-β1 expression in rat mesangial cells. Molecules. 2013;18(10):13061–13077. doi: 10.3390/molecules181013061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piya M. K., McTernan P. G., Kumar S. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. Journal of Endocrinology. 2013;216(1):T1–T15. doi: 10.1530/JOE-12-0498. [DOI] [PubMed] [Google Scholar]
- 22.Turati F., Galeone C., Gandini S., et al. High glycemic index and glycemic load are associated with moderately increased cancer risk. Molecular Nutrition Food Research. 2015;59(7):1384–1394. doi: 10.1002/mnfr.201400594. [DOI] [PubMed] [Google Scholar]
- 23.Schwingshackl L., Hoffmann G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutrition, Metabolism and Cardiovascular Diseases. 2013;23(8):699–706. doi: 10.1016/j.numecd.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Schulte E. M., Avena N. M., Gearhardt A. N. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS One. 2015;10(2, article e0117959) doi: 10.1371/journal.pone.0117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolever T. M., Gibbs A. L., Chiasson J. L., et al. Altering source or amount of dietary carbohydrate has acute and chronic effects on postprandial glucose and triglycerides in type 2 diabetes: Canadian Trial of Carbohydrates in Diabetes (CCD) Nutrition, Metabolism, and Cardiovascular Diseases. 2013;23(3):227–234. doi: 10.1016/j.numecd.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Bhupathiraju S. N., Tobias D. K., Malik V. S., et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. The American Journal of Clinical Nutrition. 2014;100(1):218–232. doi: 10.3945/ajcn.113.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong J. Y., Zhang L., Zhang Y. H., Qin L. Q. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. The British Journal of Nutrition. 2011;106(11):1649–1654. doi: 10.1017/S000711451100540X. [DOI] [PubMed] [Google Scholar]
- 28.Ma X. Y., Liu J. P., Song Z. Y. Glycemic load, glycemic index and risk of cardiovascular diseases: meta-analyses of prospective studies. Atherosclerosis. 2012;223(2):491–496. doi: 10.1016/j.atherosclerosis.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 29.Miller J. B., Pang E., Bramall L. Rice: a high or low glycemic index food? The American Journal of Clinical Nutrition. 1992;56(6):1034–1036. doi: 10.1093/ajcn/56.6.1034. [DOI] [PubMed] [Google Scholar]
- 30.Hu E. A., Pan A., Malik V., Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ. 2012;344(mar15 3, article e1454) doi: 10.1136/bmj.e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanri A., Mizoue T., Noda M., et al. Rice intake and type 2 diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. American Journal of Clinical Nutrition. 2010;92(6):1468–1477. doi: 10.3945/ajcn.2010.29512. [DOI] [PubMed] [Google Scholar]
- 32.Radhika G., Van Dam R. M., Sudha V., Ganesan A., Mohan V. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57) Metabolism. 2009;58(5):675–681. doi: 10.1016/j.metabol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Wiegman C. H., Michaeloudes C., Haji G., et al. Oxidative stress–induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. Journal of Allergy and Clinical Immunology. 2015;136(3):769–780. doi: 10.1016/j.jaci.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.di Penta A., Moreno B., Reix S., et al. Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation. PLoS One. 2013;8(2, article e54722) doi: 10.1371/journal.pone.0054722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elmarakby A. A., Sullivan J. C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovascular Therapeutics. 2012;30(1):49–59. doi: 10.1111/j.1755-5922.2010.00218.x. [DOI] [PubMed] [Google Scholar]
- 36.Sung F. L., Zhu T. Y., Au-Yeung K. K. W., Siow Y. L., Karmin O. Enhanced MCP-1 expression during ischemia/reperfusion injury is mediated by oxidative stress and NF-κB. Kidney International. 2002;62(4):1160–1170. doi: 10.1111/j.1523-1755.2002.kid577.x. [DOI] [PubMed] [Google Scholar]
- 37.Murphy M. P. How mitochondria produce reactive oxygen species. Biochemical Journal. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112(12):1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park J., Choe S. S., Choi A. H., et al. Increase in glucose-6-phosphate dehydrogenase in adipocytes stimulates oxidative stress and inflammatory signals. Diabetes. 2006;55(11):2939–2949. doi: 10.2337/db05-1570. [DOI] [PubMed] [Google Scholar]
- 40.Rahman I., Gilmour P. S., Jimenez L. A., MacNee W. Oxidative stress and TNF-α induce histone acetylation and NF-ĸB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Molecular and Cellular Biochemistry. 2002;234/235(1):239–248. doi: 10.1023/A:1015905010086. [DOI] [PubMed] [Google Scholar]
- 41.MacKellar J., Cushman S. W., Periwal V. Waves of adipose tissue growth in the genetically obese Zucker fatty rat. PLoS One. 2010;5(1, article e8197) doi: 10.1371/journal.pone.0008197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minamino T., Orimo M., Shimizu I., et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nature Medicine. 2009;15(9):1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 43.Lafontan M. Adipose tissue and adipocyte dysregulation. Diabetes and Metabolism. 2014;40(1):16–28. doi: 10.1016/j.diabet.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Teodoro J. S., Duarte F. V., Gomes A. P., et al. Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: a possible role for SirT3 activation. Mitochondrion. 2013;13(6):637–646. doi: 10.1016/j.mito.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 46.Catena C., Cavarape A., Novello M., Giacchetti G., Sechi L. A. Insulin receptors and renal sodium handling in hypertensive fructose-fed rats. Kidney International. 2003;64(6):2163–2171. doi: 10.1046/j.1523-1755.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- 47.Burén J., Liu H.-X., Lauritz J., Eriksson J. W. High glucose and insulin in combination cause insulin receptor substrate-1 and -2 depletion and protein kinase B desensitisation in primary cultured rat adipocytes: possible implications for insulin resistance in type 2 diabetes. European Journal of Endocrinology. 2003;148(1):157–167. doi: 10.1530/eje.0.1480157. [DOI] [PubMed] [Google Scholar]
- 48.Henriksen E. J., Diamond-Stanic M. K., Marchionne E. M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radical Biology & Medicine. 2011;51(5):993–999. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DiNicolantonio J. J., O’Keefe J. H., Lucan S. C. Added fructose: a principle driver of type 2 diabetes mellitus and its consequences. Mayo Clinic Proceedings. 2015;90(3):372–381. doi: 10.1016/j.mayocp.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 50.Hu F. B., Malik V. S. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiology and Behavior. 2010;100(1):47–54. doi: 10.1016/j.physbeh.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alzamendi A., Giovambattista A., Raschia A., et al. Fructose-rich diet-induced abdominal adipose tissue endocrine dysfunction in normal male rats. Endocrine. 2009;35(2):227–232. doi: 10.1007/s12020-008-9143-1. [DOI] [PubMed] [Google Scholar]
- 52.Francini F., Castro M. C., Schinella G., et al. Changes induced by a fructose-rich diet on hepatic metabolism and the antioxidant system. Life Sciences. 2010;86(25-26):965–971. doi: 10.1016/j.lfs.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Castro M. C., Francini F., Gagliardino J. J., Massa M. L. Lipoic acid prevents fructose-induced changes in liver carbohydrate metabolism: role of oxidative stress. Biochimica et Biophysica Acta. 2014;1840(3):1145–1151. doi: 10.1016/j.bbagen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Castro M. C., Massa M. L., Arbeláez L. G., Schinella G., Gagliardino J. J., Francini F. Fructose-induced inflammation, insulin resistance and oxidative stress: a liver pathological triad effectively disrupted by lipoic acid. Life Sciences. 2015;137:1–6. doi: 10.1016/j.lfs.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Castro M. C., Francini F., Schinella G., et al. Apocynin administration prevents the changes induced by a fructose-rich diet on rat liver metabolism and the antioxidant system. Clinical Science. 2012;123(12):681–692. doi: 10.1042/CS20110665. [DOI] [PubMed] [Google Scholar]
- 56.Castro M. C., Massa M. L., Del Zotto H., Gagliardino J. J., Francini F. Rat liver uncoupling protein 2: changes induced by a fructose-rich diet. Life Sciences. 2011;89(17-18):609–614. doi: 10.1016/j.lfs.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 57.Food and Agriculture Organization of the United Nations Statistics Division. Food Balance Sheets Millennium Issue 1999–2001 Special Charts. 2010. http://www.fao.org/economic/ess/food-balance-sheets-millennium-issue-1999-2001-special-charts/en/
- 58.Scott K. P., Gratz S. W., Sheridan P. O., Flint H. J., Duncan S. H. The influence of diet on the gut microbiota. Pharmacological Research. 2013;69(1):52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Food and Agriculture Organization of the United Nations (FAOSTAT) 2015. http://faostat3.fao.org/download/Q/QL/E.
- 60.Ledesma E., Rendueles M., Díaz M. Contamination of meat products during smoking by polycyclic aromatic hydrocarbons: processes and prevention. Food Control. 2016;60:64–87. doi: 10.1016/j.foodcont.2015.07.016. [DOI] [Google Scholar]
- 61.Demeyer D., Mertens B., De Smet S., Ulens M. Mechanisms linking colorectal cancer to the consumption of (processed) red meat. A review. Critical Reviews in Food Science and Nutrition. 2015;56(16):2747–2766. doi: 10.1080/10408398.2013.873886. [DOI] [PubMed] [Google Scholar]
- 62.Corpet D. E. Red meat and colon cancer: should we become vegetarians, or can we make meat safer? Meat Science. 2011;89(3):310–316. doi: 10.1016/j.meatsci.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 63.Van Hecke T., Vossen E., Hemeryck L. Y., vanden. Bussche J., Vanhaecke L., De Smet S. Increased oxidative and nitrosative reactions during digestion could contribute to the association between well-done red meat consumption and colorectal cancer. Food Chemistry. 2015;187:29–36. doi: 10.1016/j.foodchem.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 64.Serpen A., Gökmen V., Fogliano V. Total antioxidant capacities of raw and cooked meats. Meat Science. 2012;90(1):60–65. doi: 10.1016/j.meatsci.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 65.Hoac T., Daun C., Trafikowska U., Zackrisson J., Akesson B. Influence of heat treatment on lipid oxidation and glutathione peroxidase activity in chicken and duck meat. Innovative Food Science and Emerging Technologies. 2006;7(1-2):88–93. doi: 10.1016/j.ifset.2005.10.001. [DOI] [Google Scholar]
- 66.Ishikawa S., Tamaki S., Ohata M., Arihara K., Itoh M. Heme induces DNA damage and hyperproliferation of colonic epithelial cells via hydrogen peroxide produced by heme oxygenase: a possible mechanism of heme-induced colon cancer. Molecular Nutrition Food Research. 2010;54(8):1182–1191. doi: 10.1002/mnfr.200900348. [DOI] [PubMed] [Google Scholar]
- 67.Bokare A. D., Choi W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. Journal of Hazardous Materials. 2014;275:121–135. doi: 10.1016/j.jhazmat.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 68.Steppeler C., Haugen J.-E., Rødbotten R., Kirkhus B. Formation of malondialdehyde, 4-hydroxynonenal, and 4-hydroxyhexenal during in vitro digestion of cooked beef, pork, chicken, and salmon. Journal of Agricultural and Food Chemistry. 2016;64(2):487–496. doi: 10.1021/acs.jafc.5b04201. [DOI] [PubMed] [Google Scholar]
- 69.Bou R., Guardiola F., Codony R., Faustman C., Elias R. J., Decker E. A. Effect of heating oxymyoglobin and metmyoglobin on the oxidation of muscle microsomes. Journal of Agricultural and Food Chemistry. 2008;56(20):9612–9620. doi: 10.1021/jf8009848. [DOI] [PubMed] [Google Scholar]
- 70.Van Hecke T., Vanden Bussche J., Vanhaecke L., Vossen E., Van Camp J., De Smet S. Nitrite curing of chicken, pork, and beef inhibits oxidation but does not affect N-nitroso compound (NOC)-specific DNA adduct formation during in vitro digestion. Journal of Agricultural and Food Chemistry. 2014;62(8):1980–1988. doi: 10.1021/jf4057583. [DOI] [PubMed] [Google Scholar]
- 71.Okayama T., Fujii M., Yamanoue M. Effect of cooking temperature on the percentage colour formation, nitrite decomposition and sarcoplasmic protein denaturation in processed meat products. Meat Science. 1991;30(1):49–57. doi: 10.1016/0309-1740(91)90034-N. [DOI] [PubMed] [Google Scholar]
- 72.Darley-Usmar V., Wiseman H., Halliwell B. Nitric oxide and oxygen radicals: a question of balance. FEBS Letters. 1995;369(2-3):131–135. doi: 10.1016/0014-5793(95)00764-Z. [DOI] [PubMed] [Google Scholar]
- 73.Ayala A., Muñoz M. F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity. 2014;2014:31. doi: 10.1155/2014/360438.360438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nair U., Bartsch H., Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radical Biology & Medicine. 2007;43(8):1109–1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 75.Windey K., De Preter V., Verbeke K. Relevance of protein fermentation to gut health. Molecular Nutrition and Food Research. 2012;56(1):184–196. doi: 10.1002/mnfr.201100542. [DOI] [PubMed] [Google Scholar]
- 76.Miura M., Inami K., Yoshida M., Yamaguchi K., Mashino T., Mochizuki M. Isolation and structural identification of a direct-acting mutagen derived from N-nitroso-N-methylpentylamine and Fenton’s reagent with copper ion. Bioorganic and Medicinal Chemistry. 2011;19(18):5693–5697. doi: 10.1016/j.bmc.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 77.Tsutsumi N., Inami K., Mochizuki M. Activation mechanism for N-nitroso-N-methylbutylamine mutagenicity by radical species. Bioorganic and Medicinal Chemistry. 2010;18(23):8284–8288. doi: 10.1016/j.bmc.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 78.Chowdhury R., Warnakula S., Kunutsor S., et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Annals of Internal Medicine. 2014;160(6):398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 79.Harcombe Z., Baker J. S., Cooper S. M., et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Heart. 2015;2(1, article e000196) doi: 10.1136/openhrt-2014-000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siri-Tarino P. W., Sun Q., Hu F. B., Krauss R. M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. American Journal of Clinical Nutrition. 2010;91(3):535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hooper L., Martin N., Abdelhamid A., Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database of Systematic Reviews. 2015;(6, article CD011737) doi: 10.1002/14651858.cd011737. [DOI] [PubMed] [Google Scholar]
- 82.De Souza R. J., Mente A., Maroleanu A., et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:p. h3978. doi: 10.1136/bmj.h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dehghan M., Mente A., Zhang X., et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. The Lancet. 2017;390(10107):2050–2062. doi: 10.1016/S0140-6736(17)32252-3. [DOI] [PubMed] [Google Scholar]
- 84.Huang S., Rutkowsky J. M., Snodgrass R. G., et al. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. Journal of Lipid Research. 2012;53(9):2002–2013. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rioux V., Legrand P. Saturated fatty acids: simple molecular structures with complex cellular functions. Current Opinion in Clinical Nutrition and Metabolic Care. 2007;10(6):752–758. doi: 10.1097/MCO.0b013e3282f01a75. [DOI] [PubMed] [Google Scholar]
- 86.Harvey K. A., Walker C. L., Pavlina T. M., Xu Z., Zaloga G. P., Siddiqui R. A. Long-chain saturated fatty acids induce proinflammatory responses and impact endothelial cell growth. Clinical Nutrition. 2010;29(4):492–500. doi: 10.1016/j.clnu.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 87.Staiger K., Staiger H., Weigert C., Haas C., Häring H.-U., Kellerer M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-κB activation. Diabetes. 2006;55(11):3121–3126. doi: 10.2337/db06-0188. [DOI] [PubMed] [Google Scholar]
- 88.Murumalla R. K., Gunasekaran M. K. Fatty acids do not pay the toll: effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids in Health and Disease. 2012;11(1):p. 175. doi: 10.1186/1476-511X-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoenselaar R. Saturated fat and cardiovascular disease: the discrepancy between the scientific literature and dietary advice. Nutrition. 2012;28(2):118–123. doi: 10.1016/j.nut.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 90.Nagao K., Yanagita T. Medium-chain fatty acids: functional lipids for the prevention and treatment of the metabolic syndrome. Pharmacological Research. 2010;61(3):208–212. doi: 10.1016/j.phrs.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 91.Youseef-Elabd E. M., McGee K. C., Tripathi G., et al. Acute and chronic saturated fatty acid treatment as a key investigator of the TLR-mediated inflammatory response in human adipose tissue, in vitro. Journal of Nutritional Biochemistry. 2012;23(1):39–50. doi: 10.1016/j.jnutbio.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bruce K. D., Cagampang F. R., Argenton M., et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50(6):1796–1808. doi: 10.1002/hep.23205. [DOI] [PubMed] [Google Scholar]
- 93.Yokota T., Kinugawa S., Hirabayashi K., et al. Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. The American Journal of Physiology - Heart and Circulatory Physiology. 2009;297(3):H1069–H1077. doi: 10.1152/ajpheart.00267.2009. [DOI] [PubMed] [Google Scholar]
- 94.Ballal K., Wilson C. R., Harmancey R., Taegtmeyer H. Obesogenic high fat western diet induces oxidative stress and apoptosis in rat heart. Molecular and Cellular Biochemistry. 2010;344(1-2):221–230. doi: 10.1007/s11010-010-0546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gopinath B., Flood V. M., Teber E., McMahon C. M., Mitchell P. Dietary intake of cholesterol is positively associated and use of cholesterol-lowering medication is negatively associated with prevalent age-related hearing loss. Journal of Nutrition. 2011;141(7):1355–1361. doi: 10.3945/jn.111.138610. [DOI] [PubMed] [Google Scholar]
- 96.Spankovich C., Hood L. J., Silver H. J., Lambert W., Flood V. M., Mitchell P. Associations between diet and both high and low pure tone averages and transient evoked otoacoustic emissions in an older adult population-based study. Journal of the American Academy of Audiology. 2011;22(1):49–58. doi: 10.3766/jaaa.22.1.6. [DOI] [PubMed] [Google Scholar]
- 97.Du Z., Yang Y., Hu Y., et al. A long-term high-fat diet increases oxidative stress, mitochondrial damage and apoptosis in the inner ear of d-galactose-induced aging rats. Hearing Research. 2012;287(1-2):15–24. doi: 10.1016/j.heares.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 98.Anderson E. J., Lustig M. E., Boyle K. E., et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. Journal of Clinical Investigation. 2009;119(3):573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Curtis J. M., Grimsrud P. A., Wright W. S., et al. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59(5):1132–1142. doi: 10.2337/db09-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raffaella C., Francesca B., Italia F., Marina P., Giovanna L., Susanna I. Alterations in hepatic mitochondrial compartment in a model of obesity and insulin resistance. Obesity. 2008;16(5):958–964. doi: 10.1038/oby.2008.10. [DOI] [PubMed] [Google Scholar]
- 101.Ruggiero C., Ehrenshaft M., Cleland E., Stadler K. High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. American Journal of Physiology Endocrinology and Metabolism. 2011;300(6):E1047–E1058. doi: 10.1152/ajpendo.00666.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Valenzuela R., Echeverria F., Ortiz M., et al. Hydroxytyrosol prevents reduction in liver activity of Δ-5 and Δ-6 desaturases, oxidative stress, and depletion in long chain polyunsaturated fatty acid content in different tissues of high-fat diet fed mice. Lipids in Health and Disease. 2017;16(1):p. 64. doi: 10.1186/s12944-017-0450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun J., Qiao Y., Qi C., et al. High-fat-diet–induced obesity is associated with decreased antiinflammatory Lactobacillus reuteri sensitive to oxidative stress in mouse Peyer’s patches. Nutrition. 2016;32(2):265–272. doi: 10.1016/j.nut.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 104.de La Serre C. B., Ellis C. L., Lee J., Hartman A. L., Rutledge J. C., Raybould H. E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. American Journal of Physiology Gastrointestinal and Liver Physiology. 2010;299(2):G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Wit N. J., Bosch-Vermeulen H., de Groot P. J., et al. The role of the small intestime in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Medical Genomics. 2008;1(1):p. 14. doi: 10.1186/1755-8794-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ding S., Chi M. M., Scull B. P., et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5(8, article e12191) doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cortez M., Carmo L. S., Rogero M. M., Borelli P., Fock R. A. A high-fat diet increases IL-1, IL-6, and TNF-α production by increasing NF-κB and attenuating PPAR-γ expression in bone marrow mesenchymal stem cells. Inflammation. 2013;36(2):379–386. doi: 10.1007/s10753-012-9557-z. [DOI] [PubMed] [Google Scholar]
- 108.Gao D., Nong S., Huang X., et al. The effects of palmitate on hepatic insulin resistance are mediated by NADPH oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. Journal of Biological Chemistry. 2010;285(39):29965–29973. doi: 10.1074/jbc.M110.128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grimsrud P. A., Picklo M. J., Griffin T. J., Bernlohr D. A. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Molecular & Cellular Proteomics. 2007;6(4):624–637. doi: 10.1074/mcp.M600120-MCP200. [DOI] [PubMed] [Google Scholar]
- 110.Grimsrud P. A., Xie H., Griffin T. J., Bernlohr D. A. Oxidative stress and covalent modification of protein with bioactive aldehydes. The Journal of Biological Chemistry. 2008;283(32):21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Furukawa S., Fujita T., Shimabukuro M., et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. Journal of Clinical Investigation. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee J., Ellis J. M., Wolfgang M. J. Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation. Cell Reports. 2015;10(2):266–279. doi: 10.1016/j.celrep.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee C. Y. The effect of high-fat diet-induced pathophysiological changes in the gut on obesity: what should be the ideal treatment? Clinical and Translational Gastroenterology. 2013;4(7, article e39) doi: 10.1038/ctg.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Le Lay S., Simard G., Martinez M. C., Andriantsitohaina R. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxidative Medicine and Cellular Longevity. 2014;2014:18. doi: 10.1155/2014/908539.908539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Charradi K., Elkahoui S., Limam F., Aouani E. High-fat diet induced an oxidative stress in white adipose tissue and disturbed plasma transition metals in rat: prevention by grape seed and skin extract. The Journal of Physiological Sciences. 2013;63(6):445–455. doi: 10.1007/s12576-013-0283-6. [DOI] [PubMed] [Google Scholar]
- 116.Kaur J. A comprehensive review on metabolic syndrome. Cardiology Research and Practice. 2014;2014:21. doi: 10.1155/2014/943162.943162 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Cole M. A., Murray A. J., Cochlin L. E., et al. A high fat diet increases mitochondrial fatty acid oxidation and uncoupling to decrease efficiency in rat heart. Basic Research in Cardiology. 2011;106(3):447–457. doi: 10.1007/s00395-011-0156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pessayre D., Berson A., Fromenty B., Mansouri A. Mitochondria in steatohepatitis. Seminars in Liver Disease. 2001;21(1):057–070. doi: 10.1055/s-2001-12929. [DOI] [PubMed] [Google Scholar]
- 119.Pirillo A., Norata G. D., Catapano A. L. LOX-1, OxLDL, and atherosclerosis. Mediators of inflammation. 2013;2013:12. doi: 10.1155/2013/152786.152786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mitra S., Goyal T., Mehta J. L. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovascular Drugs and Therapy. 2011;25(5):419–429. doi: 10.1007/s10557-011-6341-5. [DOI] [PubMed] [Google Scholar]
- 121.Horton J. D., Goldstein J. L., Brown M. S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. Journal of Clinical Investigation. 2002;109(9):1125–1131. doi: 10.1172/JCI0215593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hamanaka R. B., Chandel N. S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends in Biochemical Sciences. 2010;35(9):505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brand M. D. The sites and topology of mitochondrial superoxide production. Experimental Gerontology. 2010;45(7-8):466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matsuzawa-Nagata N., Takamura T., Ando H., et al. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57(8):1071–1077. doi: 10.1016/j.metabol.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 125.Pessayre D., Mansouri A., Fromenty B. Nonalcoholic steatosis and steatohepatitis. V. Mitochondrial dysfunction in steatohepatitis. American Journal of Physiology Gastrointestinal and Liver Physiology. 2002;282(2):G193–G199. doi: 10.1152/ajpgi.00426.2001. [DOI] [PubMed] [Google Scholar]
- 126.Jain S. K., Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochemical and Biophysical Research Communications. 2013;437(1):7–11. doi: 10.1016/j.bbrc.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vial G., Dubouchaud H., Couturier K., et al. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. Journal of Hepatology. 2011;54(2):348–356. doi: 10.1016/j.jhep.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 128.Weisberg S. P., Leibel R., Tortoriello D. V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149(7):3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kawasaki N., Asada R., Saito A., Kanemoto S., Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Scientific Reports. 2012;2(1):p. 799. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Savini I., Catani M. V., Evangelista D., Gasperi V., Avigliano L. Obesity-associated oxidative stress: Strategies finalized to improve redox state. International Journal of Molecular Sciences. 2013;14(5):10497–10538. doi: 10.3390/ijms140510497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rahman M. M., Varghese Z., Moorhead J. F. Paradoxical increase in nitric oxide synthase activity in hypercholesterolaemic rats with impaired renal function and decreased activity of nitric oxide. Nephrology Dialysis Transplantation. 2001;16(2):262–268. doi: 10.1093/ndt/16.2.262. [DOI] [PubMed] [Google Scholar]
- 132.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 133.Carvalho A. M., Miranda A. M., Santos F. A., Loureiro A. P. M., Fisberg R. M., Marchioni D. M. High intake of heterocyclic amines from meat is associated with oxidative stress. British Journal of Nutrition. 2015;113(08):1301–1307. doi: 10.1017/S0007114515000628. [DOI] [PubMed] [Google Scholar]
- 134.Fedele S., Sabbah W., Donos N., Porter S., D’Aiuto F. Common oral mucosal diseases, systemic inflammation, and cardiovascular diseases in a large cross-sectional US survey. American Heart Journal. 2011;161(2):344–350. doi: 10.1016/j.ahj.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 135.Grange J. M., Krone B., Mastrangelo G. Infection, inflammation and cancer. International Journal of Cancer. 2011;128(9):2240–2241. doi: 10.1002/ijc.25533. [DOI] [PubMed] [Google Scholar]
- 136.Wijnstok N. J., Twisk J. W., Young I. S., et al. Inflammation markers are associated with cardiovascular diseases risk in adolescents: the Young Hearts project 2000. Journal of Adolescent Health. 2010;47(4):346–351. doi: 10.1016/j.jadohealth.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grivennikov S. I., Greten F. R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Conn C. A., Vaughan R. A., Garver W. S. Nutritional genetics and energy metabolism in human obesity. Current Nutrition Reports. 2013;2(3):142–150. doi: 10.1007/s13668-013-0046-2. [DOI] [Google Scholar]
- 139.Chaput J. P., Klingenberg L., Astrup A., Sjödin A. M. Modern sedentary activities promote overconsumption of food in our current obesogenic environment. Obesity Reviews. 2011;12(5):e12–e20. doi: 10.1111/j.1467-789X.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- 140.Ferdinand A. O., Sen B., Rahurkar S., Engler S., Menachemi N. The relationship between built environments and physical activity: a systematic review. American Journal of Public Health. 2012;102(10):e7–e13. doi: 10.2105/AJPH.2012.300740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yousefian A., Leighton A., Fox K., Hartley D. Understanding the rural food environment - perspectives of low-income parents. Rural Remote Health. 2011;11(2):p. 1631. [PubMed] [Google Scholar]
- 142.Spadaro P. A., Naug H. L., Du Toit E. F., Donner D., Colson N. J. A refined high carbohydrate diet is associated with changes in the serotonin pathway and visceral obesity. Genetics Research. 2015;97, article e23 doi: 10.1017/S0016672315000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bouchard C. Gene-environment interactions in the etiology of obesity: defining the fundamentals. Obesity. 2008;16(3):S5–S10. doi: 10.1038/oby.2008.528. [DOI] [PubMed] [Google Scholar]
- 144.Oakes N. D., Kjellstedt A., Thalén P., Ljung B., Turner N. Roles of fatty acid oversupply and impaired oxidation in lipid accumulation in tissues of obese rats. Journal of Lipids. 2013;2013:12. doi: 10.1155/2013/420754.420754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hotamisligil G. S. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 146.de Heredia F. P., Gómez-Martinez S., Marcos A. Obesity, inflammation and the immune system. The Proceedings of the Nutrition Society. 2012;71(02):332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 147.Iyer A., Lim J., Poudyal H., et al. An inhibitor of phospholipase A2 group IIA modulates adipocyte signaling and protects against diet-induced metabolic syndrome in rats. Diabetes. 2012;61(9):2320–2329. doi: 10.2337/db11-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lim J., Iyer A., Suen J. Y., et al. C5aR and C3aR antagonists each inhibit diet-induced obesity, metabolic dysfunction, and adipocyte and macrophage signaling. The FASEB Journal. 2013;27(2):822–831. doi: 10.1096/fj.12-220582. [DOI] [PubMed] [Google Scholar]
- 149.Lim J., Iyer A., Liu L., et al. Diet-induced obesity, adipose inflammation, and metabolic dysfunction correlating with PAR2 expression are attenuated by PAR2 antagonism. The FASEB Journal. 2013;27(12):4757–4767. doi: 10.1096/fj.13-232702. [DOI] [PubMed] [Google Scholar]
- 150.Massiera F., Barbry P., Guesnet P., et al. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. Journal of Lipid Research. 2010;51(8):2352–2361. doi: 10.1194/jlr.M006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zeyda M., Stulnig T. M. Obesity, inflammation, and insulin resistance – a mini-review. Gerontology. 2009;55(4):379–386. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- 152.Shoelson S. E., Herrero L., Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132(6):2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 153.Zhang Y., Fischer K. E., Soto V., et al. Obesity-induced oxidative stress, accelerated functional decline with age and increased mortality in mice. Archives of Biochemistry and Biophysics. 2015;576:39–48. doi: 10.1016/j.abb.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goossens G. H., Blaak E. E. Adipose tissue dysfunction and impaired metabolic health in human obesity: a matter of oxygen? Frontiers in Endocrinology. 2015;6:p. 55. doi: 10.3389/fendo.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gao C.-L., Zhu C., Zhao Y.-P., et al. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Molecular and Cellular Endocrinology. 2010;320(1-2):25–33. doi: 10.1016/j.mce.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 156.Lee H., Lee Y. J., Choi H., Ko E. H., Kim J. W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. The Journal of Biological Chemistry. 2009;284(16):10601–10609. doi: 10.1074/jbc.M808742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Maingrette F., Renier G. Leptin increases lipoprotein lipase secretion by macrophages: involvement of oxidative stress and protein kinase C. Diabetes. 2003;52(8):2121–2128. doi: 10.2337/diabetes.52.8.2121. [DOI] [PubMed] [Google Scholar]
- 158.Yamagishi S. I., Edelstein D., Du X. L., Kaneda Y., Guzmán M., Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. The Journal of Biological Chemistry. 2001;276(27):25096–25100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 159.Bouloumie A., Marumo T., Lafontan M., Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB Journal. 1999;13(10):1231–1238. [PubMed] [Google Scholar]
- 160.Li L., Mamputu J. C., Wiernsperger N., Renier G. Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: Inhibitory effect of metformin. Diabetes. 2005;54(7):2227–2234. doi: 10.2337/diabetes.54.7.2227. [DOI] [PubMed] [Google Scholar]
- 161.Wang C. H., Wang C. C., Huang H. C., Wei Y. H. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS Journal. 2013;280(4):1039–1050. doi: 10.1111/febs.12096. [DOI] [PubMed] [Google Scholar]
- 162.Ye R., Scherer P. E. Adiponectin, driver or passenger on the road to insulin sensitivity? Molecular Metabolism. 2013;2(3):133–141. doi: 10.1016/j.molmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Anhê F. F., Roy D., Pilon G., et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2014;64(6):872–883. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 164.Styskal J., Remmen H. V., Richardson A., Salmon A. B. Oxidative stress and diabetes: what can we learn about insulin resistance from antioxidant mutant mouse models? Free Radical Biology & Medicine. 2012;52(1):46–58. doi: 10.1016/j.freeradbiomed.2011.10.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.World Health Organization. Obesity. 2016. http://www.who.int/topics/obesity/en/
- 166.World Health Organization. Global Report on Diabetes. Geneva: World Health Organization; 2016. [Google Scholar]
- 167.Fowler M. J. Microvascular and macrovascular complications of diabetes. Clinical Diabetes. 2011;29(3):116–122. doi: 10.2337/diaclin.29.3.116. [DOI] [Google Scholar]
- 168.Maiese K. New insights for oxidative stress and diabetes mellitus. Oxidative Medicine and Cellular Longevity. 2015;2015:17. doi: 10.1155/2015/875961.875961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang J., Light K., Henderson M., et al. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. The Journal of Nutrition. 2013;144(1):81–86. doi: 10.3945/jn.113.182519. [DOI] [PubMed] [Google Scholar]
- 170.Mackenzie R. W. A., Elliott B. T. Akt/PKB activation and insulin signaling: a novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes, Metabolic Syndrome and Obesity. 2014;7:55–64. doi: 10.2147/dmso.s48260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yan L.-J. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. Journal of Diabetes Research. 2014;2014:11. doi: 10.1155/2014/137919.137919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ullah A., Khan A., Khan I. Diabetes mellitus and oxidative stress—a concise review. Saudi Pharmaceutical Journal. 2016;24(5):547–553. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Newsholme P., Rebelato E., Abdulkader F., Krause M., Carpinelli A., Curi R. Reactive oxygen and nitrogen species generation, antioxidant defenses, and β-cell function: a critical role for amino acids. Journal of Endocrinology. 2012;214(1):11–20. doi: 10.1530/JOE-12-0072. [DOI] [PubMed] [Google Scholar]
- 174.Gray J. P., Heart E. Usurping the mitochondrial supremacy: extra mitochondrial sources of reactive oxygen intermediates and their role in beta cell metabolism and insulin secretion. Toxicology Mechanisms and Methods. 2010;20(4):167–174. doi: 10.3109/15376511003695181. [DOI] [PubMed] [Google Scholar]
- 175.Newsholme P., Cruzat V., Arfuso F., Keane K. Nutrient regulation of insulin secretion and action. The Journal of Endocrinology. 2014;221(3):R105–R120. doi: 10.1530/JOE-13-0616. [DOI] [PubMed] [Google Scholar]
- 176.Talbot K., Wang H. Y., Kazi H., et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. Journal of Clinical Investigation. 2012;122(4):1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bomfim T. R., Forny-Germano L., Sathler L. B., et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Aβ oligomers. The Journal of Clinical Investigation. 2012;122(4):1339–1353. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lamming D. W., Ye L., Katajisto P., et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Takamoto I., Kubota N., Nakaya K., et al. TCF7L2 in mouse pancreatic beta cells plays a crucial role in glucose homeostasis by regulating beta cell mass. Diabetologia. 2014;57(3):542–553. doi: 10.1007/s00125-013-3131-6. [DOI] [PubMed] [Google Scholar]
- 180.Kim J. Y., Park K. J., Kim G. H., et al. In vivo activating transcription factor 3 silencing ameliorates the AMPK compensatory effects for ER stress-mediated β-cell dysfunction during the progression of type-2 diabetes. Cellular Signalling. 2013;25(12):2348–2361. doi: 10.1016/j.cellsig.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 181.Wierup N., Sundler F., Heller R. S. The islet ghrelin cell. Journal of Molecular Endocrinology. 2014;52(1):R35–R49. doi: 10.1530/JME-13-0122. [DOI] [PubMed] [Google Scholar]
- 182.Schoonbroodt S., Piette J. Oxidative stress interference with the nuclear factor-κB activation pathways. Biochemical Pharmacology. 2000;60(8):1075–1083. doi: 10.1016/S0006-2952(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 183.Verdile G., Keane K. N., Cruzat V. F., et al. Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediators of Inflammation. 2015;2015:17. doi: 10.1155/2015/105828.105828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.White M. F. Insulin signaling in health and disease. Science. 2003;302(5651):1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 185.Ovadia H., Haim Y., Nov O., et al. Increased adipocyte-S-nitrosylation targets anti-lipolytic action of insulin relevance to adipose tissue dysfunction in obesity. Journal of Biological Chemistry. 2011;286(35):30433–30443. doi: 10.1074/jbc.M111.235945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Muellenbach E. M., Diehl C. J., Teachey M. K., et al. Metabolic interactions of AGE inhibitor pyridoxamine and antioxidant α-lipoic acid following 22 weeks of treatment in obese Zucker rats. Life Sciences. 2009;84(15-16):563–568. doi: 10.1016/j.lfs.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Muellenbach E. A., Diehl C. J., Teachey M. K., et al. Interactions of the advanced glycation end product inhibitor pyridoxamine and the antioxidant alpha-lipoic acid on insulin resistance in the obese Zucker rat. Metabolism. 2008;57(10):1465–1472. doi: 10.1016/j.metabol.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Foti D., Chiefari E., Fedele M., et al. Lack of the architectural factor HMGA1 causes insulin resistance and diabetes in humans and mice. Nature Medicine. 2005;11(7):765–773. doi: 10.1038/nm1254. [DOI] [PubMed] [Google Scholar]
- 189.Saltiel A. R. Insulin resistance in the defense against obesity. Cell Metabolism. 2012;15(6):798–804. doi: 10.1016/j.cmet.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 190.Garvey W. T., Olefsky J. M., Marshall S. Insulin induces progressive insulin resistance in cultured rat adipocytes: sequential effects at receptor and multiple postreceptor sites. Diabetes. 1986;35(3):258–267. doi: 10.2337/diab.35.3.258. [DOI] [PubMed] [Google Scholar]
- 191.Siti H. N., Kamisah Y., Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vascular Pharmacology. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 192.Lv J., Yu C., Guo Y., et al. Adherence to healthy lifestyle and cardiovascular diseases in the Chinese population. Journal of the American College of Cardiology. 2017;69(9):1116–1125. doi: 10.1016/j.jacc.2016.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mohd Ghazali S., Seman Z., Cheong K. C., et al. Sociodemographic factors associated with multiple cardiovascular risk factors among Malaysian adults. BMC Public Health. 2015;15(1):p. 68. doi: 10.1186/s12889-015-1432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pereira S. S., Alvarez-Leite J. I. Low-grade inflammation, obesity, and diabetes. Current Obesity Reports. 2014;3(4):422–431. doi: 10.1007/s13679-014-0124-9. [DOI] [PubMed] [Google Scholar]
- 195.Bastien M., Poirier P., Lemieux I., Després J.-P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Progress in Cardiovascular Diseases. 2014;56(4):369–381. doi: 10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 196.Devaraj S., Singh U., Jialal I. Human C-reactive protein and the metabolic syndrome. Current Opinion in Lipidology. 2009;20(3):182–189. doi: 10.1097/MOL.0b013e32832ac03e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Scarpellini E., Tack J. Obesity and metabolic syndrome: an inflammatory condition. Digestive Diseases. 2012;30(2):148–153. doi: 10.1159/000336664. [DOI] [PubMed] [Google Scholar]
- 198.Lee D. E., Kehlenbrink S., Lee H., Hawkins M., Yudkin J. S. Getting the message across: mechanisms of physiological cross talk by adipose tissue. American Journal of Physiology Endocrinology and Metabolism. 2009;296(6):E1210–E1229. doi: 10.1152/ajpendo.00015.2009. [DOI] [PubMed] [Google Scholar]
- 199.Bugger H., Abel E. D. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clinical Science. 2008;114(3):195–210. doi: 10.1042/CS20070166. [DOI] [PubMed] [Google Scholar]
- 200.Lee R., Margaritis M., Channon M. K., Antoniades C. Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Current Medicinal Chemistry. 2012;19(16):2504–2520. doi: 10.2174/092986712800493057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ilkun O., Boudina S. Cardiac dysfunction and oxidative stress in the metabolic syndrome: an update on antioxidant therapies. Current Pharmaceutical Design. 2013;19(27):4806–4817. doi: 10.2174/1381612811319270003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tan B. L., Norhaizan M. E., Huynh K., Yeap S. K., Hazilawati H., Roselina K. Brewers’ rice modulates oxidative stress in azoxymethane-mediated colon carcinogenesis in rats. World Journal of Gastroenterology. 2015;21(29):8826–8835. doi: 10.3748/wjg.v21.i29.8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.VanSaun M. N. Molecular pathways: adiponentin and leptin signaling in cancer. Clinical Cancer Research. 2013;19(8):1926–1932. doi: 10.1158/1078-0432.CCR-12-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fiaschi T., Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. International Journal of Cell Biology. 2012;2012:8. doi: 10.1155/2012/762825.762825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hanahan D., Weinberg R. A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 206.Gupta S. C., Hevia D., Patchva S., Park B., Koh W., Aggarwal B. B. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxidants & Redox Signaling. 2012;16(11):1295–1322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Semenza G. L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29(5):625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lubik A. A., Gunter J. H., Hendy S. C., et al. Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Research. 2011;71(17):5754–5764. doi: 10.1158/0008-5472.CAN-10-2470. [DOI] [PubMed] [Google Scholar]
- 209.Yang X.-L., Chan J. C. N. Diabetes, insulin and cancer risk. World Journal of Diabetes. 2012;3(4):60–64. doi: 10.4239/wjd.v3.i4.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shi Y., Hu F. B. The global implications of diabetes and cancer. The Lancet. 2014;383(9933):1947–1948. doi: 10.1016/s0140-6736(14)60886-2. [DOI] [PubMed] [Google Scholar]
- 211.Khandekar M. J., Cohen P., Spiegelman B. M. Molecular mechanisms of cancer development in obesity. Nature Reviews Cancer. 2011;11(12):886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 212.Gallagher E. J., LeRoith D. Minireview: IGF, insulin, and cancer. Endocrinology. 2011;152(7):2546–2551. doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
- 213.Vucenik I., Stains J. P. Obesity and cancer risk: evidence, mechanisms, and recommendations. Annals of the New York Academy of Sciences. 2012;1271(1):37–43. doi: 10.1111/j.1749-6632.2012.06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Harvey A. E., Lashinger L. M., Hursting S. D. The growing challenge of obesity and cancer: an inflammatory issue. Annals of the New York Academy of Sciences. 2011;1229(1):45–52. doi: 10.1111/j.1749-6632.2011.06096.x. [DOI] [PubMed] [Google Scholar]
- 215.Hursting S. D., Berger N. A. Energy balance, host-related factors, and cancer progression. Journal of Clinical Oncology. 2010;28(26):4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Reuter S., Gupta S. C., Chaturvedi M. M., Aggarwal B. B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biology & Medicine. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nourazarian A. R., Kangari P., Salmaninejad A. Roles of oxidative stress in the development and progression of breast cancer. Asian Pacific Journal of Cancer Prevention. 2014;15(12):4745–4751. doi: 10.7314/APJCP.2014.15.12.4745. [DOI] [PubMed] [Google Scholar]
- 218.Sandireddy R., Yerra V. G., Areti A., Komirishetty P., Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. International Journal of Endocrinology. 2014;2014:10. doi: 10.1155/2014/674987.674987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Campos J., Schmeda-Hirschmann G., Leiva E., et al. Lemon grass (Cymbopogon citratus (D.C) Stapf) polyphenols protect human umbilical vein endothelial cell (HUVECs) from oxidative damage induced by high glucose, hydrogen peroxide and oxidised low-density lipoprotein. Food Chemistry. 2014;151:175–181. doi: 10.1016/j.foodchem.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 220.Afshin A., Micha R., Khatibzadeh S., et al. The impact of dietary habits and metabolic risk factors on cardiovascular and diabetes mortality in countries of the Middle East and North Africa in 2010: a comparative risk assessment analysis. BMJ Open. 2015;5(5, article e006385) doi: 10.1136/bmjopen-2014-006385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Guo R., Li W., Liu B., Li S., Zhang B., Xu Y. Resveratrol protects vascular smooth muscle cells against high glucose-induced oxidative stress and cell proliferation in vitro. Medical Science Monitor Basic Research. 2014;20:82–92. doi: 10.12659/MSMBR.890858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wendell S. G., Baffi C., Holguin F. Fatty acids, inflammation, and asthma. Journal of Allergy and Clinical Immunology. 2014;133(5):1255–1264. doi: 10.1016/j.jaci.2013.12.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Casas R., Sacanella E., Urpί-Sardà M., et al. The effects of the Mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS ONE. 2014;9(6, article e100084) doi: 10.1371/journal.pone.0100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Donkor O. N., Stojanovska L., Ginn P., Ashton J., Vasilijevic T. Germinated grains-sources of bioactive compounds. Food Chemistry. 2012;135(3):950–959. doi: 10.1016/j.foodchem.2012.05.058. [DOI] [PubMed] [Google Scholar]
- 225.Dawood M. A. O., Koshio S., Ishikawa M., et al. Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquaculture Nutrition. 2017;23(1):148–159. doi: 10.1111/anu.12376. [DOI] [Google Scholar]
- 226.Daou C., Zhang H. Oat beta-glucan: its role in health promotion and prevention of diseases. Comprehensive Reviews in Food Science and Foods Safety. 2012;11(4):355–365. doi: 10.1111/j.1541-4337.2012.00189.x. [DOI] [Google Scholar]
- 227.Tachon S., Zhou J., Keenan M., Martin R., Marco M. L. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiology Ecology. 2013;83(2):299–309. doi: 10.1111/j.1574-6941.2012.01475.x. [DOI] [PubMed] [Google Scholar]
- 228.Parikh S., Pollock N. K., Bhagatwala J., et al. Adolescent fiber consumption is associated with visceral fat and inflammatory markers. The Journal of Clinical Endocrinology and Metabolism. 2012;97(8):E1451–E1457. doi: 10.1210/jc.2012-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.De Morais Cardoso L., Pinheiro S. S., Martino H. S. D., Pinheiro-Sant’Ana H. M. Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Critical Reviews in Food Science and Nutrition. 2017;57(2):372–390. doi: 10.1080/10408398.2014.887057. [DOI] [PubMed] [Google Scholar]
- 230.Detopoulou P., Panagiotakos D. B., Chrysohoou C., et al. Dietary antioxidant capacity and concentration of adiponectin in apparently healthy adults: The ATTICA study. European Journal of Clinical Nutrition. 2010;64(2):161–168. doi: 10.1038/ejcn.2009.130. [DOI] [PubMed] [Google Scholar]
- 231.Hanhineva K., Törrönen R., Bondia-pons I., et al. Impact of dietary polyphenols on carbohydrate metabolism. International Journal of Molecular Sciences. 2010;11(4):1365–1402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Aune D., Keum N., Giovannucci E., et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353, article i2716 doi: 10.1136/bmj.i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kyrø C., Skeie G., Loft S., et al. Intake of whole grains from different cereal and food sources and incidence of colorectal cancer in the Scandinavian HELGA cohort. Cancer Causes Control. 2013;24(7):1363–1374. doi: 10.1007/s10552-013-0215-z. [DOI] [PubMed] [Google Scholar]
- 234.Tan B. L., Norhaizan M. E., Huynh K., et al. Water extract of brewers’ rice induces apoptosis in human colorectal cancer cells via activation of caspase-3 and caspase-8 and downregulates the Wnt/β-catenin downstream signaling pathway in brewers’ rice-treated rats with azoxymethane-induced colon carcinogenesis. BMC Complementary and Alternative Medicine. 2015;15(1):p. 205. doi: 10.1186/s12906-015-0730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tan B. L., Norhaizan M. E. Scientific evidence of rice by-products for cancer prevention: chemopreventive properties of waste products from rice milling on carcinogenesis in vitro and in vivo. BioMed Research International. 2017;2017:18. doi: 10.1155/2017/9017902.9017902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Esa N. M., Ling T. B., Peng L. S. By-products of rice processing: an overview of health benefits and applications. Journal of Rice Research. 2013;1(1):p. 107. doi: 10.4172/jrr.1000107. [DOI] [Google Scholar]
- 237.Tan B. L., Norhaizan M. E., Pandurangan A. K., Hazilawati H., Roselina K. Brewers’ rice attenuated aberrant crypt foci developing in colon of azoxymethane-treated rats. Pakistan Journal of Pharmaceutical Sciences. 2016;29(1):205–212. [PubMed] [Google Scholar]
- 238.Tan B. L., Norhaizan M. E., Hairuszah I., Hazilawati H., Roselina K. Brewers’ rice: a by-product from rice processing provides natural hepatorenal protection in azoxymethane-induced oxidative stress in rats. Oxidative Medicine and Cellular Longevity. 2015;2015:10. doi: 10.1155/2015/539798.539798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Imam M. U., Ishaka A., Ooi D.-J., et al. Germinated brown rice regulates hepatic cholesterol metabolism and cardiovascular disease risk in hypercholesterolaemic rats. Journal of Functional Foods. 2014;8:193–203. doi: 10.1016/j.jff.2014.03.013. [DOI] [Google Scholar]
- 240.Esa N. M., Abdul Kadir K.-K., Amom Z., Azlan A. Antioxidant activity of white rice, brown rice and germinated brown rice (in vivo and in vitro) and the effects on lipid peroxidation and liver enzymes in hyperlipidaemic rabbits. Food Chemistry. 2013;141(2):1306–1312. doi: 10.1016/j.foodchem.2013.03.086. [DOI] [PubMed] [Google Scholar]
- 241.Wang S., Moustaid-Moussa N., Chen L., et al. Novel insights of dietary polyphenols and obesity. The Journal of Nutritional Biochemistry. 2014;25(1):1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Masisi K., Le K., Ghazzawi N. Dietary corn fractions reduce atherogenesis in low-density lipoprotein receptor knockout mice. Nutrition Research. 2017;37:87–96. doi: 10.1016/j.nutres.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 243.Hopkins M. J., Englyst H. N., Macfarlane S., Furrie E., Macfarlane G. T., McBain A. J. Degradation of cross-linked and non-cross-linked arabinoxylans by the intestinal microbiota in children. Applied and Environmental Microbiology. 2003;69(11):6354–6360. doi: 10.1128/AEM.69.11.6354-6360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Silveira A. L. M., Ferreira A. V. M., de Oliveira M. C., et al. Preventive rather than therapeutic treatment with high fiber diet attenuates clinical and inflammatory markers of acute and chronic DSS-induced colitis in mice. European Journal of Nutrition. 2017;56(1):179–191. doi: 10.1007/s00394-015-1068-x. [DOI] [PubMed] [Google Scholar]
- 245.Maslowski K. M., Vieira A. T., Ng A., et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hur K. Y., Lee M.-S. Gut microbiota and metabolic disorders. Diabetes and Metabolism Journal. 2015;39(3):198–203. doi: 10.4093/dmj.2015.39.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kamada N., Núñez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology. 2014;146(6):1477–1488. doi: 10.1053/j.gastro.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Vogt S. L., Pena-Diaz J., Finlay B. B. Chemical communication in the gut: effects of microbiota-generated metabolites on gastrointestinal bacterial pathogens. Anaerobe. 2015;34:106–115. doi: 10.1016/j.anaerobe.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 250.Grosso G., Yang J., Marventano S., Micek A., Galvano F., Kales S. N. Nut consumption on all-cause, cardiovascular, and cancer mortality risk: a systematic review and meta-analysis of epidemiologic studies. The American Journal of Clinical Nutrition. 2015;101(4):783–793. doi: 10.3945/ajcn.114.099515. [DOI] [PubMed] [Google Scholar]
- 251.López-Uriarte P., Bulló M., Casas-Agustench P., Babio N., Salas-Salvadó J. Nuts and oxidation: a systematic review. Nutrition Reviews. 2009;67(9):497–508. doi: 10.1111/j.1753-4887.2009.00223.x. [DOI] [PubMed] [Google Scholar]
- 252.Kendall C. W., Josse A. R., Esfahani A., Jenkins D. J. Nuts, metabolic syndrome and diabetes. British Journal of Nutrition. 2010;104(04):465–473. doi: 10.1017/S0007114510001546. [DOI] [PubMed] [Google Scholar]
- 253.Kris-Etherton P. M. Walnuts decrease risk of cardiovascular disease: a summary of efficacy and biologic mechanisms. Journal of Nutrition. 2014;144(4):547S–554S. doi: 10.3945/jn.113.182907. [DOI] [PubMed] [Google Scholar]
- 254.Ros E., Tapsell L. C., Sabaté J. Nuts and berries for heart health. Current Atherosclerosis Reports. 2010;12(6):397–406. doi: 10.1007/s11883-010-0132-5. [DOI] [PubMed] [Google Scholar]
- 255.Bao Y., Han J., Hu F. B., et al. Association of nut consumption with total and cause-specific mortality. The New England Journal of Medicine. 2013;369(21):2001–2011. doi: 10.1056/NEJMoa1307352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Blomhoff R., Carlsen M. H., Andersen L. F., Jacobs D. R. Health benefits of nuts: potential role of antioxidants. British Journal of Nutrition. 2006;96(S2):S52–S60. doi: 10.1017/BJN20061864. [DOI] [PubMed] [Google Scholar]
- 257.Nagel J. M., Brinkoetter M., Magkos F., et al. Dietary walnuts inhibit colorectal cancer growth in mice by suppressing angiogenesis. Nutrition. 2012;28(1):67–75. doi: 10.1016/j.nut.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lux S., Scharlau D., Schlörmann W., Birringer M., Glei M. In vitro fermented nuts exhibit chemopreventive effects in HT29 colon cancer cells. British Journal of Nutrition. 2012;108(07):1177–1186. doi: 10.1017/S0007114511006647. [DOI] [PubMed] [Google Scholar]
- 259.Yeh C.-C., You S.-L., Chen C.-J., Sung F.-C. Peanut consumption and reduced risk of colorectal cancer in women: a prospective study in Taiwan. World Journal of Gastroenterology. 2006;12(2):222–227. doi: 10.3748/wjg.v12.i2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Alasalvar C., Bolling B. W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. British Journal of Nutrition. 2015;113(S2):S68–S78. doi: 10.1017/S0007114514003729. [DOI] [PubMed] [Google Scholar]
- 261.Piers L. S., Walker K. Z., Stoney R. M., Soares M. J., O’Dea K. The influence of the type of dietary fat on postprandial fat oxidation rates: monounsaturated (olive oil) vs saturated fat (cream) International Journal of Obesity and Related Metabolic Disorders. 2002;26(6):814–821. doi: 10.1038/sj.ijo.0801993. [DOI] [PubMed] [Google Scholar]
- 262.Casas-Agustench P., López-Uriarte P., Bullo M., Ros E., Gomez-Flores A., Salas-Salvado J. Acute effects of three high-fat meals with different fat saturations on energy expenditure, substrate oxidation and satiety. Clinical Nutrition. 2009;28(1):39–45. doi: 10.1016/j.clnu.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 263.Ros E. Health benefits of nut consumption. Nutrients. 2010;2(7):652–682. doi: 10.3390/nu2070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jaceldo-Siegl K., Sabaté J., Rajaram S., Fraser G. E. Long-term almond supplementation without advice on food replacement induces favourable nutrient modifications to the habitual diets of free-living individuals. British Journal of Nutrition. 2004;92(03):533–540. doi: 10.1079/BJN20041223. [DOI] [PubMed] [Google Scholar]
- 265.Mattes R. D., Dreher M. L. Nuts and healthy body weight maintenance mechanisms. Asia Pacific Journal of Clinical Nutrition. 2010;19(1):137–141. [PubMed] [Google Scholar]
- 266.Ellis P. R., Kendall C. W., Ren Y., et al. Role of cell walls in the bioaccessibility of lipids in almond seeds. The American Journal of Clinical Nutrition. 2004;80(3):604–613. doi: 10.1093/ajcn/80.3.604. [DOI] [PubMed] [Google Scholar]
- 267.Cassady B. A., Hollis J. H., Fulford A. D., Considine R. V., Mattes R. D. Mastication of almonds: effects of lipid bioaccessibility, appetite, and hormone response. American Journal of Clinical Nutrition. 2009;89(3):794–800. doi: 10.3945/ajcn.2008.26669. [DOI] [PubMed] [Google Scholar]
- 268.Sweazea K. L., Johnston C. S., Ricklefs K. D., Petersen K. N. Almond supplementation in the absence of dietary advice significantly reduces C-reactive protein in subjects with type 2 diabetes. Journal of Functional Foods. 2014;10:252–259. doi: 10.1016/j.jff.2014.06.024. [DOI] [Google Scholar]
- 269.Urpi-Sarda M., Casas R., Chiva-Blanch G., et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomarkers related to atherosclerosis. Pharmacological Research. 2012;65(6):577–583. doi: 10.1016/j.phrs.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 270.Colpo E., Vilanova C. D. D. A., Reetz L. G. B., et al. Brazilian nut consumption by healthy volunteers improves inflammatory parameters. Nutrition. 2014;30(4):459–465. doi: 10.1016/j.nut.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 271.Estruch R., Martínez-González M. A., Corella D., et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Annals of Internal Medicine. 2006;145(1):1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 272.Zhao G., Etherton T. D., Martin K. R., West S. G., Gillies P. J., Kris-Etherton P. M. Dietary α-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. The Journal of Nutrition. 2004;134(11):2991–2997. doi: 10.1093/jn/134.11.2991. [DOI] [PubMed] [Google Scholar]
- 273.Zhao G., Etherton T. D., Martin K. R., Gillies P. J., West S. G., Kris-Etherton P. M. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. American Journal of Clinical Nutrition. 2007;85(2):385–391. doi: 10.1093/ajcn/85.2.385. [DOI] [PubMed] [Google Scholar]
- 274.Rangel-Huerta O., Aguilera C. M., Mesa M. D., Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomarkers: a systematic review of randomised clinical trials. British Journal of Nutrition. 2012;107(S2):S159–S170. doi: 10.1017/S0007114512001559. [DOI] [PubMed] [Google Scholar]
- 275.Zhao G., Etherton T. D., Martin K. R., et al. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochemical and Biophysical Research Communications. 2005;336(3):909–917. doi: 10.1016/j.bbrc.2005.08.204. [DOI] [PubMed] [Google Scholar]
- 276.Lind L., Berglund L., Larsson A., Sundström J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation. 2011;123(14):1545–1551. doi: 10.1161/CIRCULATIONAHA.110.984047. [DOI] [PubMed] [Google Scholar]
- 277.Cortés B., Núñez I., Cofán M., et al. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. Journal of the American College of Cardiology. 2006;48(8):1666–1671. doi: 10.1016/j.jacc.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 278.Berryman C. E., Grieger J. A., West S. G., et al. Acute consumption of walnuts and walnut components differentially affect postprandial lipemia, endothelial function, oxidative stress, and cholesterol efflux in human with mild hypercholesterolemia. The Journal of Nutrition. 2013;143(6):788–794. doi: 10.3945/jn.112.170993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Saad B., Zaid H., Shanak S., Kadan S. Anti-diabetes and Anti-obesity Medicinal Plants and Phytochemicals. Switzerland: Springer; 2017. Prevention and treatment of obesity-related diseases by diet and medicinal plants; pp. 95–128. [DOI] [Google Scholar]
- 280.Leenders M., Leufkens A. M., Siersema P. D., et al. Plasma and dietary carotenoids and vitamins A, C and E and risk of colon and rectal cancer in the European Prospective Investigation into Cancer and Nutrition. International Journal of Cancer. 2014;135(12):2930–2939. doi: 10.1002/ijc.28938. [DOI] [PubMed] [Google Scholar]
- 281.Jędrejek D., Kontek B., Lis B., Stochmal A., Olas B. Evaluation of antioxidant activity of phenolic fractions from the leaves and petals of dandelion in human plasma treated with H2O2 and H2O2/Fe. Chemico-Biological Interactions. 2017;262:29–37. doi: 10.1016/j.cbi.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 282.Hecht F., Pessoa C. F., Gentile L. B., Rosenthal D., Carvalho D. P., Fortunato R. S. The role of oxidative stress on breast cancer development and therapy. Tumor Biology. 2016;37(4):4281–4291. doi: 10.1007/s13277-016-4873-9. [DOI] [PubMed] [Google Scholar]
- 283.Rodriguez-Casado A. The health potential of fruits and vegetables phytochemicals: notable examples. Critical Reviews in Food Science and Nutrition. 2016;56(7):1097–1107. doi: 10.1080/10408398.2012.755149. [DOI] [PubMed] [Google Scholar]
- 284.Priviero F. B., Gonçalves T. T., Lazaro C. M., et al. G-hesperidin supplementation impairs the beneficial effects of physical exercise on the body composition, biochemistry profile and oxidative stress in obese rats. The FASEB Journal. 2017;31(1):1013–1019. [Google Scholar]
- 285.Jin Q., Zhao H. B., Liu X. M., et al. Effect of β-carotene supplementation on the expression of lipid metabolism-related genes and the deposition of back fat in beef cattle. Animal Production Science. 2017;57(3):513–519. doi: 10.1071/AN15434. [DOI] [Google Scholar]
- 286.Järvi A., Karlström B., Vessby B., Becker W. Increased intake of fruits and vegetables in overweight subjects: effects on body weight, body composition, metabolic risk factors and dietary intake. British Journal of Nutrition. 2016;115(10):1760–1768. doi: 10.1017/S0007114516000970. [DOI] [PubMed] [Google Scholar]
- 287.Wood L. G., Williams E. J., Berthon B. S., Baines K. A. Effects of an encapsulated fruit and vegetable juice concentrate on obesity-induced systemic inflammation. The FASEB Journal. 2017;31(1):161–166. doi: 10.1016/j.jnim.2017.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Williams E. J., Baines K. J., Berthon B. S., Wood L. G. Effects of an encapsulated fruit and vegetable juice concentrate on obesity-induced systemic inflammation: a randomised controlled trial. Nutrients. 2017;9(2):p. 116. doi: 10.3390/nu9020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhao C.-N., Meng X., Li Y., et al. Fruits for prevention and treatment of cardiovascular diseases. Nutrients. 2017;9(6):p. 598. doi: 10.3390/nu9060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Farràs M., Basterra-Gortari F. J., Diez-Espino J., et al. Association between dietary fibre intake and fruit, vegetable or whole-grain consumption and the risk of CVD: results from the PREvención con DIeta MEDiterránea (PREDIMED) trial. British Journal of Nutrition. 2016;116(03):534–546. doi: 10.1017/s0007114516002099. [DOI] [PubMed] [Google Scholar]
- 291.Wang P. Y., Fang J. C., Gao Z. H., Zhang C., Xie S. Y. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: a meta-analysis. Journal of diabetes investigation. 2016;7(1):56–69. doi: 10.1111/jdi.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Gopal S. S., Lakshmi M. J., Sharavana G., Sathaiah G., Sreerama Y. N., Baskaran V. Lactucaxanthin – a potential anti-diabetic carotenoid from lettuce (Lactuca sativa) inhibits α-amylase and α-glucosidase activity in vitro and in diabetic rats. Food and Function. 2017;8(3):1124–1131. doi: 10.1039/C6FO01655C. [DOI] [PubMed] [Google Scholar]
- 293.Aune D., Giovannucci E., Boffetta P., et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. International Journal of Epidemiology. 2017;46(3):1029–1056. doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Huang Y., Su Z., Wu T., Saw C. L.-L., Kong A.-N. T. Mechanisms of prostate carcinogenesis and its prevention by a γ-tocopherol-rich mixture of tocopherols in TRAMP mice. Journal of Chinese Pharmaceutical Sciences. 2016;25(3):170–177. doi: 10.5246/jcps.2016.03.020. [DOI] [Google Scholar]
- 295.Schug Z. T., Voorde J. V., Gottlieb E. The metabolic fate of acetate in cancer. Nature Reviews Cancer. 2016;16(11):708–717. doi: 10.1038/nrc.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Han A., MacDonald A., Ahmed B., Whelan J., Donohoe D. Butyrate regulates its own metabolic fate as an HDAC inhibitor in colorectal cancer cells. The FASEB Journal. 2017;31(1):300–302. [Google Scholar]
- 297.Yang Y., Nirmagustina D. E., Kumrungsee T., Okazaki Y., Tomotake H., Kato N. Feeding of the water extract from Ganoderma lingzhi to rats modulates secondary bile acids, intestinal microflora, mucins, and propionate important to colon cancer. Bioscience, Biotechnology, and Biochemistry. 2017;81(9):1796–1804. doi: 10.1080/09168451.2017.1343117. [DOI] [PubMed] [Google Scholar]
- 298.Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nature Medicine. 2014;20(7):709–711. doi: 10.1038/nm.3624. [DOI] [PubMed] [Google Scholar]
- 299.Alissa E. M., Ferns G. A. Dietary fruits and vegetables and cardiovascular diseases risk. Critical Reviews in Food Science and Nutrition. 2017;57(9):1950–1962. doi: 10.1080/10408398.2015.1040487. [DOI] [PubMed] [Google Scholar]
- 300.Nakamura M., Ojima T., Nakade M., et al. Poor oral health and diet in relation to weight loss, stable underweight, and obesity in community-dwelling older adults: a cross-sectional study from the JAGES 2010 project. Journal of Epidemiology. 2016;26(6):322–329. doi: 10.2188/jea.JE20150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Rhee J. J., Kim E., Buring J. E., Kurth T. Fish consumption, omega-3 fatty acids, and risk of cardiovascular disease. American Journal of Preventive Medicine. 2017;52(1):10–19. doi: 10.1016/j.amepre.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Marventano S., Kolacz P., Castellano S., et al. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: does the ratio really matter? International Journal of Food Sciences and Nutrition. 2015;66(6):611–622. doi: 10.3109/09637486.2015.1077790. [DOI] [PubMed] [Google Scholar]
- 303.Bender N., Portmann M., Heg Z., Hofmann K., Zwahlen M., Egger M. Fish or n3-PUFA intake and body composition: a systematic review and meta-analysis. Obesity Reviews. 2014;15(8):657–665. doi: 10.1111/obr.12189. [DOI] [PubMed] [Google Scholar]
- 304.Raederstorff D., Wyss A., Calder P. C., Weber P., Eggersdorfer M. Vitamin E function and requirements in relation to PUFA. British Journal of Nutrition. 2015;114(08):1113–1122. doi: 10.1017/S000711451500272X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Niki E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radical Biology & Medicine. 2014;66:3–12. doi: 10.1016/j.freeradbiomed.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 306.Aadland E. K., Lavigne C., Graff I. E., et al. Lean-seafood intake reduces cardiovascular lipid risk factors in healthy subjects: results from a randomized controlled trial with a crossover design. The American Journal of Clinical Nutrition. 2015;102(3):582–592. doi: 10.3945/ajcn.115.112086. [DOI] [PubMed] [Google Scholar]
- 307.Rylander C., Sandanger T. M., Engeset D., Lund E. Consumption of lean fish reduces the risk of type 2 diabetes mellitus: a prospective population based cohort study of Norwegian women. PLoS One. 2014;9(2, article e89845):1–10. doi: 10.1371/journal.pone.0089845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Schmedes M., Aadland E. K., Sundekilde U. K., et al. Lean-seafood intake decreases urinary markers of mitochondrial lipid and energy metabolism in healthy subjects: metabolomics results from a randomized crossover intervention study. Molecular Nutrition and Food Research. 2016;60(7):1661–1672. doi: 10.1002/mnfr.201500785. [DOI] [PubMed] [Google Scholar]
- 309.Morell P., Fiszman S. Revisiting the role of protein-induced satiation and satiety. Food Hydrocolloids. 2017;68:199–210. doi: 10.1016/j.foodhyd.2016.08.003. [DOI] [Google Scholar]
- 310.Chen W., Guo J., Zhang Y., Zhang J. The beneficial effects of taurine in preventing metabolic syndrome. Food and Function. 2016;7(4):1849–1863. doi: 10.1039/C5FO01295C. [DOI] [PubMed] [Google Scholar]
- 311.Halim N. R. A., Yusof H. M., Sarbon N. M. Functional and bioactive properties of fish protein hydolysates and peptides: a comprehensive review. Trends in Food Science and Technology. 2016;51:24–33. doi: 10.1016/j.tifs.2016.02.007. [DOI] [Google Scholar]
- 312.Pangestuti R., Kim S.-K. Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Marine Drugs. 2017;15(3):p. 67. doi: 10.3390/md15030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lassoued I., Mora L., Barkia A., Aristoy M.-C., Nasri M., Toldrá F. Bioactive peptides identified in thornback ray skin's gelatin hydrolysates by proteases from Bacillus subtilis and Bacillus amyloliquefaciens. Journal of Proteomics. 2015;128:8–17. doi: 10.1016/j.jprot.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 314.Razali A., Amin A., Sarbon N. Antioxidant activity and functional properties of fractionated cobia skin gelatin hydrolysate at different molecular weight. International Food Research Journal. 2015;22(2):651–660. [Google Scholar]
- 315.Yadav L., Puri N., Rastogi V., Satpute P., Ahmad R., Kaur G. Matrix metalloproteinases and cancer-roles in threat and therapy. Asian Pacific Journal of Cancer Prevention. 2014;15(3):1085–1091. doi: 10.7314/APJCP.2014.15.3.1085. [DOI] [PubMed] [Google Scholar]
- 316.Ji L.-D., Li J.-Y., Yao B.-B., Cai X.-B., Shen Q.-J., Xu J. Are genetic polymorphisms in the renin–angiotensin–aldosterone system associated with essential hypertension? Evidence from genome-wide association studies. Journal of Human Hypertension. 2017;31(11):695–698. doi: 10.1038/jhh.2017.29. [DOI] [PubMed] [Google Scholar]
- 317.Balti R., Bougatef A., Sila A., Guillochon D., Dhulster P., Nedjar-Arroume N. Nine novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis) muscle protein hydrolysates and antihypertensive effect of the potent active peptide in spontaneously hypertensive rats. Food Chemistry. 2015;170:519–525. doi: 10.1016/j.foodchem.2013.03.091. [DOI] [PubMed] [Google Scholar]
- 318.Li J., Armstrong C. L., Campbell W. W. Effects of dietary protein source and quantity during weight loss on appetite, energy expenditure, and cardio-metabolic responses. Nutrients. 2016;8(2):p. 63. doi: 10.3390/nu8020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Abreu I., Reguera M., Bonilla A., Bolaños L., Bonilla I. Beneficial Plant-microbial Interactions: Ecology and Applications. Boca Raton, FL, USA: CRC Press; 2013. Mineral nutrition in the legume-rhizobia nitrogen fixing symbiosis; pp. 123–140. [DOI] [Google Scholar]
- 320.Zhu F., Du B., Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: a review. Critical Reviews in Food Science and Nutrition. 2017:1–11. doi: 10.1080/10408398.2016.1251390. [DOI] [PubMed] [Google Scholar]
- 321.Prieto M. S., Kales S. N. Dietary, lifestyle behaviors and obesity: towards modern science. Journal of Obesity & Eating Disorders. 2016;2(2):p. 21. doi: 10.21767/2471-8203.100021. [DOI] [Google Scholar]
- 322.Garcia-Bailo B., Jain N., Keeler C., Smith J. Legume consumption, diet quality and body weight: results from NHANES 2009–2012 and the food patterns equivalent database 2009–2012. The FASEB Journal. 2017;31(1):615–648. [Google Scholar]
- 323.Shinohara S., Gu Y., Yang Y., et al. Ethanol extracts of chickpeas alter the total lipid content and expression levels of genes related to fatty acid metabolism in mouse 3T3-L1 adipocytes. International Journal of Molecular Medicine. 2016;38(2):574–584. doi: 10.3892/ijmm.2016.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Miao M., Jiang B., Cui S. W., Zhang T., Jin Z. Slowly digestible starch-a review. Critical Reviews in Food Science and Nutrition. 2013;55(12):1642–1657. doi: 10.1080/10408398.2012.704434. [DOI] [PubMed] [Google Scholar]
- 325.Yang C., Chen D., Yu B., et al. Effect of dietary amylose/amylopectin ratio on growth performance, carcass traits, and meat quality in finishing pigs. Meat Science. 2015;108:55–60. doi: 10.1016/j.meatsci.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 326.Bhattarai R. R., Dhital S., Wu P., Chen X. D., Gidley M. J. Digestion of isolated legume cells in a stomach-duodenum model: three mechanisms limit starch and protein hydrolysis. Food and Function. 2017;8(7):2573–2582. doi: 10.1039/C7FO00086C. [DOI] [PubMed] [Google Scholar]
- 327.Grundy M. M.-L., Edwards C. H., Mackie A. R., Gidley M. J., Butterworth P. J., Ellis P. R. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. British Journal of Nutrition. 2016;116(05):816–833. doi: 10.1017/S0007114516002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Dhillon P. K., Bowen L., Kinra S., et al. Legume consumption and its association with fasting glucose, insulin resistance and type 2 diabetes in the Indian Migration Study. Public Health Nutrition. 2016;19(16):3017–3026. doi: 10.1017/S1368980016001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Marventano S., Pulido M. I., Sánchez-González C., et al. Legume consumption and CVD risk: a systematic review and meta-analysis. Public Health Nutrition. 2017;20(02):245–254. doi: 10.1017/S1368980016002299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ramdath D. D., Padhi E. M., Sarfaraz S., Renwick S., Duncan A. M. Beyond the cholesterol-lowering effect of soy protein: a review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients. 2017;9(4):p. 324. doi: 10.3390/nu9040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Xiao C. W., Wood C. M., Weber D., et al. Dietary supplementation with soy isoflavones or replacement with soy proteins prevents hepatic lipid droplet accumulation and alters expression of genes involved in lipid metabolism in rats. Genes and nutrition. 2014;9(1):p. 373. doi: 10.1007/s12263-013-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Guo X., Cai Q., Bao P., et al. Long-term soy consumption and tumor tissue microRNA and gene expression in triple-negative breast cancer. Cancer. 2016;122(16):2544–2551. doi: 10.1002/cncr.29981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.El-Aassar M., Hafez E. E., El-Deeb N. M., Fouda M. M. Microencapsulation of lectin anti-cancer agent and controlled release by alginate beads, biosafety approach. International Journal of Biological Macromolecules. 2014;69:88–94. doi: 10.1016/j.ijbiomac.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 334.Son S. M. Reactive oxygen and nitrogen species in pathogenesis of vascular complications of diabetes. Diabetes and Metabolism Journal. 2012;36(3):190–198. doi: 10.4093/dmj.2012.36.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Selvaraju V., Joshi M., Suresh S., Sanchez J. A., Maulik N., Maulik G. Diabetes, oxidative stress, molecular mechanism, and cardiovascular disease – an overview. Toxicology Mechanisms and Methods. 2012;22(5):330–335. doi: 10.3109/15376516.2012.666648. [DOI] [PubMed] [Google Scholar]
- 336.Guo Y., Jones D., Palmer J. L., et al. Oral alpha-lipoic acid to prevent chemotherapy-induced peripheral neuropathy: a randomized, double-blind, placebo-controlled trial. Support Care Cancer. 2014;22(5):1223–1231. doi: 10.1007/s00520-013-2075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Yasueda A., Urushima H., Ito T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: a systematic review. Integrative Cancer Therapies. 2016;15(1):17–39. doi: 10.1177/1534735415610427. [DOI] [PMC free article] [PubMed] [Google Scholar]