Abstract
Background/Aims
The efficacy of standard triple therapy (STT) in treating Helicobacter pylori infection has decreased. Many investigators have attempted to increase the eradication rate. We investigated the outcomes of concomitant therapy (CT) and STT combined with probiotics (STP) as a first-line treatment for H. pylori infection.
Methods
We reviewed the medical records of 361 patients who received either STP (n=286) or CT (n=75). The STP group received STT combined with a probiotic preparation for 1 week. The CT group received STT and metronidazole for 1 week.
Results
The intention-to-treat and per-protocol eradication rates were 83.6% (95% confidence interval [CI], 79.0 to 87.7) and 87.1% (95% CI, 81.2 to 89.7) in the STP group and 86.7% (95% CI, 78.7 to 93.3) and 91.4% (95% CI, 83.6 to 97.1) in the CT group (p=0.512 and p=0.324), respectively. The frequency of adverse effects was higher in the CT group (28.2%) than in the STP group (12.8%) (p=0.002).
Conclusions
STP and CT are encouragingly efficacious as first-line treatments for H. pylori infection. Therefore, adding probiotics to STT may be a feasible option to avoid side effects.
Keywords: Helicobacter pylori, Probiotics, Metronidazole
INTRODUCTION
Helicobacter pylori is a gram-negative, flagellated bacterium that is often found on the luminal surface of the gastric epithelium. It is well known as the main cause of gastritis, gastroduodenal ulcer, gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancer.1 In South Korea, the rate of H. pylori infection is 59.6%, which is higher than that in Western countries.2 Furthermore, gastric cancer is one of the most common cancers in South Korea, in contrast with other countries.2,3 To prevent gastric cancer, it is useful to eradicate H. pylori infection. In this regard, standard triple therapy (STT), a combination treatment that involves a proton-pump inhibitor (PPI), amoxicillin, and clarithromycin, is the most commonly recommended first-line therapy.4,5
In recent years, the efficacy of STT for H. pylori eradication has decreased. The most important cause of this decrease may be resistance to antibiotics, especially to clarithromycin.6 To circumvent this decrease in the eradication rate of STT, various methods have been considered; for instance, some clinicians have added probiotics or metronidazole to the STT.7,8
Most specifically, concomitant therapy (CT) is a non-bismuth quadruple-based therapy wherein patients are treated using omeprazole, metronidazole, amoxicillin, and clarithromycin for 5 to 7 days. Previous studies have demonstrated that CT has better results than STT.9–11 In South Korea, it is standard practice to use CT for 7 or 14 days.12 However, the country is known to have a high rate of antibiotic resistance. Specifically, resistance rates against clarithromycin, amoxicillin, and metronidazole in 2011 to 2012 isolates were 16.0%, 2.1%, and 56.3%, respectively, and antibiotic resistance is increasing rapidly.13 In addition, eradication rates are affected by patient compliance as well as antibiotic resistance. Metronidazole induces several side effects, particularly gastrointestinal symptoms. Patients receiving metronidazole have complained of nausea, vomiting, headache, and metallic taste.14 Such serious side effects can cause low patient compliance. For this reason, to avoid the side effects associated with CT for H. pylori infection, supplementation of probiotics to STT has been more recently used as an alternative. However, it is unclear whether probiotic supplementation is efficacious in H. pylori treatment. In the present study, we aimed to compare eradication rates and adverse events between CT and STT plus probiotic treatment for first-line H. pylori eradication. In addition, several types of probiotics are used recently. Subgroup analysis was performed in the STT combined with a probiotic (STP) group owing to the possibility of differences in outcomes depending on the type of probiotics.
We compared CT including metronidazole which has an increased antibiotic resistance rate and more side effects, with STP which is generally known to be safe and beneficial.
MATERIALS AND METHODS
1. Study population
We reviewed the medical records of patients who had been prescribed either STP or CT for H. pylori eradication at Incheon Sarang Hospital between March 2013 and February 2014. All patients were at least 18 years old, and none had ever received treatment for H. pylori infection. This study was performed in accordance with the principles of the Declaration of Helsinki and approved by the ethics committee of Incheon Sarang Hospital (reg. no. 2015-02). The informed consent was waived.
The exclusion criteria were as follows: (1) the use of an H2 receptor antagonist; (2) prescription of PPI or antibiotics in the previous 4 weeks; (3) use of a nonsteroidal anti-inflammatory drug in the 2 weeks before the 13C-urea breath test; (4) advanced gastric cancer; (5) previous gastric surgery; (6) systemic illness, such as chronic renal disease, or liver cirrhosis; (7) pregnancy; (8) age <18 years; or (9) insufficient data.
2. Study design
We reviewed the medical records of 361 patients who received either STP (n=286) or CT (n=75) for H. pylori eradication. The STP group received standard-dose PPI (rabeprazole, 20 mg), clarithromycin (500 mg), amoxicillin (1 g), and a probiotic preparation twice daily for 1 week. The probiotic bacterial culture used were either Bacillus subtilis combined with Streptococcus faecium (STP-I) or freeze-dried Lactobacillus casei var. rhamnosus (STP-II). The CT group received a standard-dose PPI (rabeprazole, 20 mg), clarithromycin (500 mg), amoxicillin (1 g), and metronidazole (500 mg) twice daily for 1 week. Four weeks after the treatment period, the patients underwent a 13C-urea breath test to confirm H. pylori eradication.
The primary outcome of this study was the H. pylori eradication rate using either STP or CT. Treatment compliance and side effects were also assessed.
3. Diagnosis of H. pylori infection
H. pylori infection was defined as a positive result on histologic evaluation or the rapid urease test. Specimens were obtained through endoscopic biopsy, fixed with formalin, and confirmed with Giemsa staining. The rapid urease test was interpreted to be positive if the color of the gel changed to pink or red after 24 hours at room temperature.
4. 13C-urea breath test
An initial breath sample was obtained after a 4-hour fast, wherein 100 mg of 13C-urea powder (UBiTkit; Otsuka Pharmaceutical, Tokyo, Japan) dissolved in 100 mL of water was administered orally. The second breath sample was obtained 20 minutes later, and the cutoff value was 2.6‰. The collected samples were analyzed using an isotope ratio mass spectrometer (UBiT-IR300; Otsuka Pharmaceutical).
5. Statistical analysis
Eradication rates were evaluated using intention-to-treat (ITT) and per-protocol (PP) analyses. The ITT analysis included all the assigned patients; patients with an unknown infection status were considered having eradication failure. The PP analysis excluded patients with an unknown H. pylori status after therapy, those who took less than 90% of the treatment dose, and those lost to follow-up or with missing data.
The outcomes were analyzed using the Student t-test, the chi-square test and, Fisher exact tests. Independent factors influencing treatment efficacy were evaluated by univariate analysis using the chi-square and Fisher exact tests; p-values <0.05 were considered statistically significant. Multivariate analysis was also performed to identify factors influencing the eradication rate. The analysis was conducted using PASW Statistics for Windows version 19.0 (IBM Corp., Armonk, NY, USA). Statistical power was calculated by using G power version 3.1 (Universität Düsseldorf, Düsseldorf, Germany), on the basis of a non-inferiority margin of 15% and an expected level for the primary outcome of 85% with a power of 0.80 and α-value of 0.05.
RESULTS
1. Study group characteristics
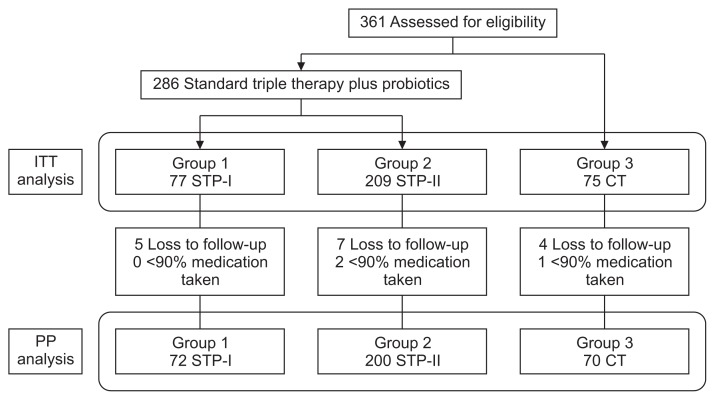
Fig. 1 shows the flow chart of patients who received each treatment regimen for H. pylori infection during the study period. A total of 361 patients were analyzed in the ITT population. Among them, 286 patients received STP: 77 were treated using STP-I and 209 using STP-II. Meanwhile, 75 patients received CT. Of all 361 patients, 272 from the STP group, and 70 from the CT group were analyzed using PP. Table 1 shows the baseline characteristics, which did not differ between groups.
Fig. 1.
Study protocol flow chart.
ITT, intention-to-treat; PP, per-protocol; STP, standard triple therapy combined with probiotics; STP-I, Bacillus subtilis and Streptococcus faecium culture; STP-II, Lactobacillus casei var. rhamnosus culture; CT, concomitant therapy.
Table 1.
Demographic Characteristics of the Study Population
| Characteristic | STP | CT | p-value | |
|---|---|---|---|---|
|
| ||||
| STP-I | STP-II | |||
| No. of patients | 77 | 209 | 75 | |
| Age, mean±SD, yr | 51.6±14.6 | 56.3±12.0 | 52.4±13.2 | 0.116 |
| Sex (male/female) | 42/35 | 114/95 | 38/37 | 0.549 |
| Comorbidity, % | ||||
| Hypertension | 13.0 | 17.2 | 25.3 | 0.064 |
| Diabetes | 7.8 | 6.7 | 8.0 | 0.764 |
| Current smoking, % | 29.9 | 23.0 | 24.7 | 0.883 |
| Alcohol intake, % | 36.4 | 34.4 | 26.7 | 0.175 |
| Endoscopic diagnosis, % | ||||
| Presence of ulcer | 83.1 | 62.7 | 76.0 | 0.189 |
| Atrophic change* | 35.1 | 60.3 | 52.0 | 0.817 |
| Internal metaplasia, % | 35.1 | 40.7 | 30.7 | 0.176 |
STP, standard triple therapy combined with probiotics; STP-I, Bacillus subtilis and Streptococcus faecium culture; STP-II, Lactobacillus casei var. rhamnosus culture; CT, concomitant therapy.
Endoscopic finding of atrophic border consisting of visible transparent vessels or mucosal thinning.
2. Outcomes of CT and STP
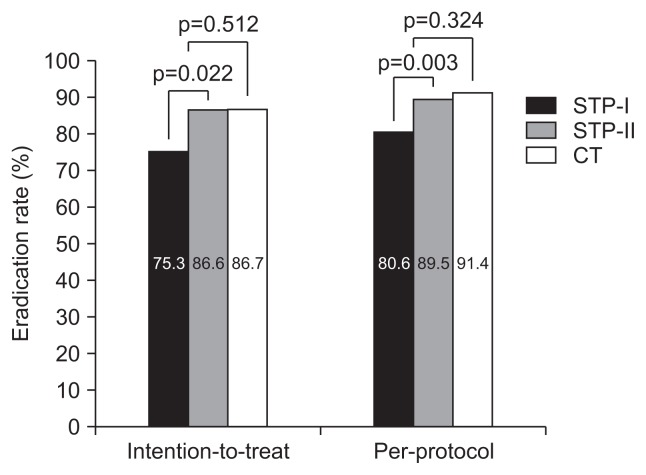
H. pylori eradication rates, compliance, and side effects are listed in Tables 2 and 3. In the ITT analysis, the eradication rates were 83.6% (95% confidence interval [CI], 79.0 to 87.7) in the STP group and 86.7% (95% CI, 78.7 to 93.3) in the CT group. The eradication rates in the PP analysis were 87.1% (95% CI, 81.2 to 89.7) in the STP group and 91.4% (95% CI, 83.6 to 97.1) in the CT group. There was no significant difference in eradication rates between the STP and CT groups. However, in both the ITT and the PP analyses, there was a significant difference in the eradication rate between the STP-I and STP-II groups (p=0.022 and p=0.003, respectively). Fig. 2 shows the eradication rates in the STP and CT groups. Both the STP and CT groups showed good compliance (95.1% and 93.3%, respectively), and there was no significant difference in compliance between the two groups. There was also no significant difference in compliance between the STP-I and STP groups.
Table 2.
Eradication and Compliance rates in CT and STP Groups
| STP | CT | p-value | STP-I | STP-II | p-value | |
|---|---|---|---|---|---|---|
| H. pylori eradication | ||||||
| Intention-to-treat | 239/286 (83.6) | 65/75 (86.7) | 0.512 | 58/77 (75.3) | 181/209 (86.6) | 0.022 |
| Per-protocol | 237/272 (87.1) | 64/70 (91.4) | 0.324 | 58/72 (80.6) | 179/200 (89.5) | 0.003 |
| Compliance | 272/286 (95.1) | 70/75 (93.3) | 0.541 | 72/77 (93.5) | 200/209 (95.7) | 0.447 |
Data are presented as number/number (%).
CT, concomitant therapy; STP, standard triple therapy combined with probiotics; STP-I, Bacillus subtilis and Streptococcus faecium culture; STP-II, Lactobacillus casei var. rhamnosus culture; H. pylori, Helicobacter pylori.
Table 3.
Side Effects in CT and STP Groups
| STP (n=274) | CT (n=71) | p-value | STP-I (n=72) | STP-II (n=202) | p-value | |
|---|---|---|---|---|---|---|
| Side effect | 35 (12.8) | 20 (28.2) | 0.002 | 8 (11.1) | 27 (13.4) | 0.623 |
| Dyspepsia | 12 (4.4) | 6 (8.5) | 3 (4.2) | 9 (4.5) | ||
| Nausea/vomiting | 8 (2.9) | 5 (7.0) | 1 (1.4) | 7 (3.5) | ||
| Dry mouth | 7 (2.6) | 4 (5.6) | 1 (1.4) | 6 (3.0) | ||
| Diarrhea | 4 (1.5) | 3 (4.2) | 1 (1.4) | 3 (1.5) | ||
| Abdominal pain | 3 (1.1) | 1 (1.4) | 1 (1.4) | 2 (1.0) | ||
| Other | 1 (0.4) | 1 (1.4) | 1 (1.4) | 0 |
Data are presented as number (%).
Since side effects were the cause of discontinuation of the medication, patients who did not take more than 90% of the medication were included in the analysis.
CT, concomitant therapy; STP, standard triple therapy combined with probiotics; STP-I, Bacillus subtilis and Streptococcus faecium culture; STP-II, Lactobacillus casei var. rhamnosus culture.
Fig. 2.
Overall Helicobacter pylori eradication rates using standard triple therapy (STT) plus Bacillus subtilis and Streptococcus faecium culture (STP-I), STT plus freeze-dried Lactobacillus casei var. rhamnosus culture (STP-II), or concomitant therapy (CT).
The CT group showed more serious adverse events than the STP groups. Common adverse events were dyspepsia and nausea/vomiting. Other side effects reported were dry mouth, diarrhea, and abdominal pain, among others. There was no significant difference in side effects between STP-I and STP-II (Table 3).
3. Clinical factors affecting the efficacy of CT and STP
Univariate analysis was performed to identify clinical factors that affected the efficacy of STP and CT for H. pylori infection (Table 4). In the CT group, the eradication rate was significantly higher in patients with peptic ulcer than in those with only gastritis (p=0.016). Type 2 diabetes mellitus (DM) also affected the eradication rate in the CT group; specifically, patients with type 2 DM had a lower eradication rate than those without DM (p=0.006). In the STP group, the eradication rate differed depending on the kind of probiotics used. Specifically, the eradication rate in the STP-II group was higher than that in the STP-I group (p=0.048). No other factors in the STP group or CT groups affected the eradication response.
Table 4.
Univariate Analysis of Clinical Factors Affecting the Eradication Rate in Each Group
| Clinical factor | Standard triple therapy plus probiotics | Concomitant therapy | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| No. of patients | Eradication rate, % | p-value | No. of patients | Eradication rate, % | p-value | |
| Sex | 0.143 | 0.374 | ||||
| Male | 125 | 89.9 | 36 | 94.4 | ||
| Female | 149 | 84.0 | 35 | 88.6 | ||
| Presence of ulcer | 0.796 | 0.016 | ||||
| (+) | 193 | 87.6 | 56 | 96.4 | ||
| (−) | 81 | 86.4 | 15 | 73.3 | ||
| Atrophic change | 0.191 | 0.107 | ||||
| (+) | 144 | 84.7 | 35 | 85.7 | ||
| (−) | 130 | 90.0 | 36 | 97.2 | ||
| Intestinal metaplasia | 0.726 | 0.658 | ||||
| (+) | 110 | 86.4 | 22 | 95.5 | ||
| (−) | 164 | 87.8 | 49 | 89.8 | ||
| HTN | 0.212 | 0.625 | ||||
| (+) | 43 | 81.4 | 17 | 88.2 | ||
| (−) | 231 | 88.3 | 54 | 92.6 | ||
| Type 2 DM | 1.000 | 0.006 | ||||
| (+) | 17 | 88.2 | 6 | 50.0 | ||
| (−) | 257 | 87.2 | 65 | 95.4 | ||
| Alcohol intake | 0.366 | 0.166 | ||||
| (+) | 97 | 89.7 | 18 | 83.3 | ||
| (−) | 177 | 85.9 | 53 | 94.3 | ||
| Current smoking | 0.545 | 0.611 | ||||
| (+) | 66 | 89.4 | 16 | 87.5 | ||
| (−) | 208 | 86.5 | 55 | 92.7 | ||
| Probiotics | 0.048 | |||||
| STP-I | 72 | 80.6 | ||||
| STP-II | 202 | 89.6 | ||||
Patients who did not take more than 90% of the patients were included in the analysis because they were eradicated.
HTN, hypertension; DM, diabetes mellitus; STP, standard triple therapy plus probiotics; STP-I, Bacillus subtilis and Streptococcus faecium culture; STP-II, Lactobacillus casei var. rhamnosus culture.
Multivariate analysis revealed that the eradication rate was inversely correlated with age in the STP group (p=0.011). The type of probiotic used also affected the eradication rate. In the CT group, type 2 DM and alcohol consumption lowered the eradication rate (p=0.002 and p=0.011, respectively). Table 5 shows the multivariate analysis of clinical factors related to the eradication failure in each group.
Table 5.
Multivariate Analysis of Clinical Factors Related to Eradication Failure in Each Group
| Clinical factor | Coefficient | SE | OR (95% CI) | p-value |
|---|---|---|---|---|
| Standard triple therapy plus probiotics | ||||
| Age | 0.04 | 0.02 | 1.04 (1.01–1.07) | 0.011 |
| Type of probiotic (STP-I vs STP-II) | 0.92 | 0.39 | 2.50 (1.17–5.37) | 0.019 |
| Concomitant therapy | ||||
| Type 2 DM | 5.02 | 1.62 | 152.12 (6.34–3,648.4) | 0.002 |
| Alcohol | 3.56 | 1.40 | 35.08 (2.27–541.97) | 0.011 |
SE, standard error; OR, odds ratio; CI, confidence interval; STP, standard triple therapy plus probiotics; STP-I, Bacillus subtilis and Streptococcus faecium culture; STP-II, Lactobacillus casei var. rhamnosus culture; DM, diabetes mellitus.
DISCUSSION
Previous studies have suggested that CT is more effective than STT for H. pylori eradication in areas where antibiotic resistance is high.9,15 In some countries, including South Korea, many clinicians prescribe CT for H. pylori eradication. However, CT has more side effects than STT, and some studies showed that probiotics were effective for the eradication of H. pylori in the Asian population including Korea. For this reason, some investigators have considered adding a probiotic regimen to STT instead.16–18
In the present study, we investigated the effects of probiotics on H. pylori eradication. Probiotics have been shown to exert in vitro and in vivo bactericidal effects against H. pylori, suggesting that they interfere with H. pylori colonization.19 Even though a meta-analysis showed that probiotics with STT plus a 14-day course of treatment did not improve the eradication of H. pylori infection, some meta-analyses showed that certain single-strain probiotics or multi-strain probiotics as adjunct therapy improve H. pylori eradication and decrease the adverse effect of antibiotics.7,20–22 Furthermore, a meta-analysis suggested that probiotic preparations during STT improved the eradication rate, particularly in Asian patients.23 It is widely believed that probiotics can improve H. pylori eradication. In the ITT analysis, the overall eradication rates in the STP and CT groups were 83.6% and 86.7%, respectively, with no significant between-group difference (p=0.512). The eradication rates in both groups were higher than those reported in previous studies in Korea. This result may be due to the high compliance of the participants in this study, the different types of probiotics, and the relatively high presence of ulcers in this study.18,24–26 In our study, Lactobacillus strain was used predominantly, which seems to be more effective than other probiotics. In addition, the eradication rate was higher in the presence of ulcer; the relatively high proportion of ulcer in our study was due to the high eradication rate.
STP was not inferior to CT in terms of H. pylori eradication. Likewise, in our PP analysis, the overall eradication rate did not differ between the STP and CT groups. Because the resistance rates against metronidazole in Korea were relatively high compared with those in other countries, STP may be effective in populations with high resistance rates.
In the current study, STP showed a lower rate of side effects than CT. The most common side effect was dyspepsia; others were nausea, vomiting, and dry mouth, among others. Indeed, such side effects are one of the known limitations of CT. Conversely, probiotics exert a health benefit on the host beyond inherent basic nutrition.27 Many studies have shown that probiotics are useful to prevent and treat not only gastrointestinal diseases such as necrotizing enterocolitis, antibiotic-associated diarrhea, and inflammatory bowel disease, but also upper respiratory tract infections, atopic eczema in cow’s milk allergy, and infectious disease.28–32 In addition, probiotics are non-pathogenic microorganisms that rarely induce side effects.
We used two probiotics; B. subtilis combined with S. faecium (STP-I) and freeze-dried Lactobacillus casei var. rhamnosus culture (STP-II). Lactobacillus, Streptococcus, and Bifidobacterium are representative strains of probiotics.33 Among them, Lactobacillus and Streptococcus were the most commonly used probiotic types, and these two strains were selected for the study. STP-I showed somewhat lower eradication rates than STP-II in this study. A meta-analysis showed that multi-strain probiotics improved H. pylori eradication rates, prevented any adverse reactions, and reduced antibiotic-associated diarrhea, especially probiotics including Lactobacillus and Bifidobacterium.7 Another meta-analysis showed that Lactobacillus-containing probiotic supplementation increased the H. pylori eradication rate.34 Although the study comparing the efficacy of Lactobacillus and other probiotics was not performed on a large population, Lactobacillus may be more effective for H. pylori eradication than other probiotics. A number of mechanisms have been proposed to explain the efficacy of probiotics in treating H. pylori. For example, probiotic bacteria may modulate H. pylori activity through both immunological and non-immunological mechanisms.35 Furthermore, they may reduce the cytokine reaction, reinforce the gastric defense mechanism, interact with the host’s innate immune system, and have direct bacteriostatic or bactericidal effects.36 Therefore, the differences in the eradication rate seen in the present study may have arisen because different probiotics inhibit H. pylori colonization through different mechanisms.
In addition, various clinical factors may have affected the eradication rate in each group. Patients who did not have ulcerous changes showed a lower eradication rate in the CT group by univariate analysis. That is, the present study indicated that gastric ulcer is a predictor of successful eradication. In a similar regard, previous studies have shown that the eradication rates of STT (according to endoscopic stage) were significantly different in patients with gastric ulcer.37 This may be because severe inflammatory reactions occur at an early stage after ulceration; this inflammation degrades the gastric mucus and epithelial layers, which may allow charged antibiotics in the gastric lumen to better penetrate the epithelium and alter vascular epithelial permeability, leading to better systemic delivery of drugs.38
Type 2 DM was associated with poor H. pylori eradication in the CT group by both univariate and multivariate analysis. Various studies have reported a higher prevalence of H. pylori infection and a lower eradication rate in patients with DM.39,40 Although the association of H. pylori infection with DM is still controversial, this study showed that STP may be a better treatment for H. pylori infection than CT in patients who have an ulcer or type 2 DM.41 Alcohol consumption was also associated with resistance to eradication in the CT group. Alcohol has been shown to affect the mucosal barrier and histology of the stomach, and the inflammatory changes occur that influence H. pylori.42
This study has several limitations. First, this was a retrospective study conducted at a single center. Second, the interview regarding side effects after treatment was not well organized, and was therefore insufficient. Third, the study population was too small. However, when calculating statistical power, the number of patients was considered sufficient. The numbers of patients and controls required to demonstrate non-inferiority were calculated. The calculated sample size was 69 for each intervention group to show the non-inferiority of STP, compared with CT. Nonetheless, this study provides valuable insight into the treatment of H. pylori infection because it is the first study to compare CT with STT plus probiotics. Finally, we did not test antibiotic susceptibility which may affect the clinical outcome of H. pylori eradication therapy.
In conclusion, STP and CT showed encouraging efficacies as first-line treatments for H. pylori infection. To avoid side effects, it may be feasible to add probiotics to STT. Specifically, freeze-dried Lactobacillus casei var. rhamnosus culture showed better efficacy than B. subtilis and S. faecium culture in the STP group. Ulcerous changes and type 2 DM were found to be important determinants of eradication in the CT group. Multivariate analysis revealed that, in the STP group, age and type of probiotic were independently predictive of eradication failure. In the CT group, the independent predictive factors were type 2 DM and alcohol intake.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.McColl KE. Clinical practice: Helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 2.Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J Gastroenterol Hepatol. 2014;29:1371–1386. doi: 10.1111/jgh.12607. [DOI] [PubMed] [Google Scholar]
- 3.Oh CM, Won YJ, Jung KW, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016;48:436–450. doi: 10.4143/crt.2016.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song ZQ, Zhou LY. Helicobacter pylori and gastric cancer: clinical aspects. Chin Med J (Engl) 2015;128:3101–3105. doi: 10.4103/0366-6999.169107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin WG, Lee SW, Baik GH, et al. Eradication rates of Helicobacter pylori in Korea over the past 10 years and correlation of the amount of antibiotics use: nationwide survey. Helicobacter. 2016;21:266–278. doi: 10.1111/hel.12279. [DOI] [PubMed] [Google Scholar]
- 6.Mégraud F. Failed eradication for Helicobacter pylori: what should be done? Dig Dis. 2016;34:505–509. doi: 10.1159/000445230. [DOI] [PubMed] [Google Scholar]
- 7.McFarland LV, Huang Y, Wang L, Malfertheiner P. Systematic review and meta-analysis: multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol J. 2016;4:546–561. doi: 10.1177/2050640615617358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puig I, Baylina M, Sánchez-Delgado J, et al. Systematic review and meta-analysis: triple therapy combining a proton-pump inhibitor, amoxicillin and metronidazole for Helicobacter pylori first-line treatment. J Antimicrob Chemother. 2016;71:2740–2753. doi: 10.1093/jac/dkw220. [DOI] [PubMed] [Google Scholar]
- 9.Jung SM, Cheung DY, Kim JI, Kim I, Seong H. Comparing the efficacy of concomitant therapy with sequential therapy as the first-line therapy of Helicobacter pylori eradication. Gastroenterol Res Pract. 2016;2016:1293649. doi: 10.1155/2016/1293649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HJ, Kim JI, Lee JS, et al. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J Gastroenterol. 2015;21:351–359. doi: 10.3748/wjg.v21.i1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SY, Park DK, Kwon KA, Kim KO, Kim YJ, Chung JW. Ten day concomitant therapy is superior to ten day sequential therapy for Helicobacter pylori eradication. Korean J Gastroenterol. 2014;64:260–267. doi: 10.4166/kjg.2014.64.5.260. [DOI] [PubMed] [Google Scholar]
- 12.Kim BJ, Kim HS, Song HJ, et al. Online registry for nationwide database of current trend of Helicobacter pylori eradication in Korea: interim analysis. J Korean Med Sci. 2016;31:1246–1253. doi: 10.3346/jkms.2016.31.8.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An B, Moon BS, Kim H, et al. Antibiotic resistance in Helicobacter pylori strains and its effect on H. pylori eradication rates in a single center in Korea. Ann Lab Med. 2013;33:415–419. doi: 10.3343/alm.2013.33.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zandbergen D, Slot DE, Niederman R, Van der Weijden FA. The concomitant administration of systemic amoxicillin and metronidazole compared to scaling and root planing alone in treating periodontitis: a systematic review. BMC Oral Health. 2016;16:27. doi: 10.1186/s12903-015-0123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kefeli A, Basyigit S, Yeniova AO, Kefeli TT, Aslan M, Tanas O. Comparison of three different regimens against Helicobacter pylori as a first-line treatment: a randomized clinical trial. Bosn J Basic Med Sci. 2016;16:52–57. doi: 10.17305/bjbms.2016.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinarong C, Siramolpiwat S, Wongcha-um A, Mahachai V, Vilaichone RK. Improved eradication rate of standard triple therapy by adding bismuth and probiotic supplement for Helicobacter pylori treatment in Thailand. Asian Pac J Cancer Prev. 2014;15:9909–9913. doi: 10.7314/APJCP.2014.15.22.9909. [DOI] [PubMed] [Google Scholar]
- 17.Du YQ, Su T, Fan JG, et al. Adjuvant probiotics improve the eradication effect of triple therapy for Helicobacter pylori infection. World J Gastroenterol. 2012;18:6302–6307. doi: 10.3748/wjg.v18.i43.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MN, Kim N, Lee SH, et al. The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter. 2008;13:261–268. doi: 10.1111/j.1523-5378.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 19.Gotteland M, Cruchet S. Suppressive effect of frequent ingestion of Lactobacillus johnsonii La1 on Helicobacter pylori colonization in asymptomatic volunteers. J Antimicrob Chemother. 2003;51:1317–1319. doi: 10.1093/jac/dkg227. [DOI] [PubMed] [Google Scholar]
- 20.Szajewska H, Horvath A, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2015;41:1237–1245. doi: 10.1111/apt.13214. [DOI] [PubMed] [Google Scholar]
- 21.Zhang MM, Qian W, Qin YY, He J, Zhou YH. Probiotics in Helicobacter pylori eradication therapy: a systematic review and meta-analysis. World J Gastroenterol. 2015;21:4345–4357. doi: 10.3748/wjg.v21.i14.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu C, Sang J, He H, et al. Probiotic supplementation does not improve eradication rate of Helicobacter pylori infection compared to placebo based on standard therapy: a meta-analysis. Sci Rep. 2016;6:23522. doi: 10.1038/srep23522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu R, Chen K, Zheng YY, et al. Meta-analysis of the efficacy of probiotics in Helicobacter pylori eradication therapy. World J Gastroenterol. 2014;20:18013–18021. doi: 10.3748/wjg.v20.i47.18013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song MJ, Park DI, Park JH, et al. The effect of probiotics and mucoprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Helicobacter. 2010;15:206–213. doi: 10.1111/j.1523-5378.2010.00751.x. [DOI] [PubMed] [Google Scholar]
- 25.Chung JW, Han JP, Kim KO, et al. Ten-day empirical sequential or concomitant therapy is more effective than triple therapy for Helicobacter pylori eradication: a multicenter, prospective study. Dig Liver Dis. 2016;48:888–892. doi: 10.1016/j.dld.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Lim JH, Lee DH, Choi C, et al. Clinical outcomes of two-week sequential and concomitant therapies for Helicobacter pylori eradication: a randomized pilot study. Helicobacter. 2013;18:180–186. doi: 10.1111/hel.12034. [DOI] [PubMed] [Google Scholar]
- 27.Hill C, Guarner F, Reid G, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 28.Embleton ND, Zalewski S, Berrington JE. Probiotics for prevention of necrotizing enterocolitis and sepsis in preterm infants. Curr Opin Infect Dis. 2016;29:256–261. doi: 10.1097/QCO.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 29.Ehrhardt S, Guo N, Hinz R, et al. Saccharomyces boulardii to prevent antibiotic-associated diarrhea: a randomized, double-masked, placebo-controlled trial. Open Forum Infect Dis. 2016;3:ofw011. doi: 10.1093/ofid/ofw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lichtenstein L, Avni-Biron I, Ben-Bassat O. Probiotics and prebiotics in Crohn’s disease therapies. Best Pract Res Clin Gastroenterol. 2016;30:81–88. doi: 10.1016/j.bpg.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Kalima K, Lehtoranta L, He L, et al. Probiotics and respiratory and gastrointestinal tract infections in Finnish military conscripts: a randomised placebo-controlled double-blinded study. Benef Microbes. 2016;7:463–471. doi: 10.3920/BM2015.0172. [DOI] [PubMed] [Google Scholar]
- 32.Blattner CM, Crosby MS, Goedken M, Murase JE. Update: do probiotics prevent or treat pediatric atopic dermatitis? Pediatr Allergy Immunol. 2016;27:425–428. doi: 10.1111/pai.12539. [DOI] [PubMed] [Google Scholar]
- 33.Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014;11:4745–4767. doi: 10.3390/ijerph110504745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng X, Lyu L, Mei Z. Lactobacillus-containing probiotic supplementation increases Helicobacter pylori eradication rate: evidence from a meta-analysis. Rev Esp Enferm Dig. 2013;105:445–453. doi: 10.4321/S1130-01082013000800002. [DOI] [PubMed] [Google Scholar]
- 35.Safavi M, Sabourian R, Foroumadi A. Treatment of Helicobacter pylori infection: current and future insights. World J Clin Cases. 2016;4:5–19. doi: 10.12998/wjcc.v4.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boltin D. Probiotics in Helicobacter pylori-induced peptic ulcer disease. Best Pract Res Clin Gastroenterol. 2016;30:99–109. doi: 10.1016/j.bpg.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Seo SI, Kim SJ, Kim HS, et al. Is there any difference in the eradication rate of Helicobacter pylori infection according to the endoscopic stage of peptic ulcer disease? Helicobacter. 2015;20:424–430. doi: 10.1111/hel.12221. [DOI] [PubMed] [Google Scholar]
- 38.Gisbert JP, Marcos S, Gisbert JL, Pajares JM. Helicobacter pylori eradication therapy is more effective in peptic ulcer than in non-ulcer dyspepsia. Eur J Gastroenterol Hepatol. 2001;13:1303–1307. doi: 10.1097/00042737-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Ojetti V, Pellicano R, Fagoonee S, Migneco A, Berrutti M, Gasbarrini A. Helicobacter pylori infection and diabetes. Minerva Med. 2010;101:115–119. [PubMed] [Google Scholar]
- 40.Marietti M, Gasbarrini A, Saracco G, Pellicano R. Helicobacter pylori infection and diabetes mellitus: the 2013 state of art. Panminerva Med. 2013;55:277–281. [PubMed] [Google Scholar]
- 41.Dai YN, Yu WL, Zhu HT, Ding JX, Yu CH, Li YM. Is Helicobacter pylori infection associated with glycemic control in diabetics? World J Gastroenterol. 2015;21:5407–5416. doi: 10.3748/wjg.v21.i17.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tongtawee T, Dechsukhum C, Matrakool L, et al. High prevalence of Helicobacter pylori resistance to clarithromycin: a hospital-based cross-sectional study in Nakhon Ratchasima Province, Northeast of Thailand. Asian Pac J Cancer Prev. 2015;16:8281–8285. doi: 10.7314/APJCP.2015.16.18.8281. [DOI] [PubMed] [Google Scholar]