Abstract
Primary liver cancer (PLC) is the sixth most common cancer worldwide and the second most common cause of cancer death. Future predictions can inform health planners and raise awareness of the need for cancer control action. We predicted the future burden of PLC in 30 countries around 2030. Incident cases of PLC (ICD-10 C22) were obtained from 30 countries for 1993–2007. We projected new PLC cases through to 2030 using age-period-cohort models (NORDPRED). Age-standardized incidence rates per 100,000 person-years were calculated by country and sex. Increases in new cases and rates of PLC are projected in both sexes. Among men, the largest increases in rates are in Norway (2.9% per annum), US whites (2.6%), and Canada (2.4%), and among women in the US (blacks 4.0%), Switzerland (3.4%), and Germany (3.0%). The projected declines are in China, Japan, Singapore, and parts of Europe (e.g. in Estonia, Czech Republic, Slovakia). A 35% increase in the number of new cases annually is expected compared to 2005. This increasing burden reflects both increasing rates (and the underlying prevalence of risk factors) and demographic changes. Japan is the only country with a predicted decline in the net number of cases and annual rates by 2030.
Conclusion
Our reporting of a projected increase in PLC incidence to 2030 in 30 countries serves as a baseline for anticipated declines in the longer-term via the control of HBV and HCV infections through vaccination and treatment. However, the prospects that rising levels of obesity and its metabolic complications may lead to an increased increasing risk of PLC that potentially offset these gains, is a concern.
Keywords: liver, cancer, incidence, projections, worldwide
Primary liver cancer (PLC) is the sixth most common cancer worldwide and the second most common cause of cancer death. In 2012, 782,000 new cases and nearly 746,000 liver cancer deaths were estimated to have occurred globally.(1) Recent data from the International Agency for Research on Cancer (IARC) shows that 83% of PLC cases occur in countries in economic transition,(2) with China accounting for over 50% of the world’s burden.(1) The highest incidence rates in the world occur in Mongolia, with the disease contributing almost two-fifths of the total cancer burden in the country.(2, 3)
Hepatocellular carcinoma (HCC) accounts for 75–85% of PLC cases, intrahepatic cholangiocarcinoma (ICC) for 10–15%, while the residual cases include other rare types.(4–6) An accurate estimate of the incidence of PLC by subtype is obscured by difficulties in detecting and definitively diagnosing PLC.(7) A lack of reliable data and non-standardized incidence reporting methods in many countries make comparative assessments challenging. As a further complication, liver is a common site of cancer metastasis from other organs, making a diagnostic distinction between primary and metastatic liver cancer difficult in the absence of histologic examination.
The main risk factors for PLC are chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV),(5, 6, 8) aflatoxin B1 contaminated foodstuffs, heavy alcohol intake, obesity, and type 2 diabetes mellitus.(6, 9, 10) With non-alcoholic fatty liver disease (NAFLD) being closely associated with diabetes and obesity, the increasing prevalence of obesity is likely to be an important contributing factor for the rising incidence of HCC.(10)
The major risk factors for PLC vary by region. In most high-risk HCC areas (e.g. China, Africa), the key determinants are chronic HBV infection and aflatoxin exposure, whereas in Japan and Egypt, it is HCV infection.(11–13) In low-risk HCC areas, metabolic disorders including obesity and diabetes are important.(14) In the U.S., HCV is an important factor although metabolic disorders are associated with a larger relative proportion of the total HCC burden.(15) ICC is a common malignancy in regions of South Eastern Asia (e.g. Thailand, Laos), where high rates of infestation with bile duct flukes Opisthorcis viverrine and Clonorchis sinesis are the major risk factor.(9, 16)
Quantitative projections of the future burden of PLC can inform prevention strategies aimed at reducing PLC occurrence. With a vaccine that is 95% effective against HBV infection available since 1982,(17) and an increasing uptake of universal vaccination in many high HBV prevalence countries, our reporting of projected PLC incidence to 2030 in 30 countries serves as a baseline for future anticipated declines. We discuss geographical and temporal variability of PLC rates in the context of the main risk factors.
Methods
Newly diagnosed cases of PLC (ICD-10 topography code C22 or ICD-9 topography code 155.0, all histological types) were available from high-quality national (n=16) and regional (n=53; see list of countries in Tables 1 and 2) population-based cancer registries in 30 countries (31 populations: US for two races) over the period 1993–2007 by age group (ages 0–4, 5–9,.., 80 and over) following extraction from the IARC’s Cancer Incidence in Five Continents series (CI5).(18) The CI5 database contains annual incidence rates, number of cancer cases by period, age group, sex, cancer site and the corresponding population at risk for selected populations for which good quality data are available.(18) Data covered 3.4% of the world population (Africa: 0%, Asia: 0.78%, Europe: 18.63%, Latin and Central America: 0.36%, North America: 8.46%, and Oceania: 73.13%). Registries included here had consistently high quality data over time. Multiple regional registries in a given country were aggregated as proxies of national data.
Table 1.
Primary liver cancer incidence among men in 2005 and in 2030 in countries with corresponding percentage changes in incidence
| Country | Coverage (%) |
Annual Population (Million) |
Number of new cases (Annual) |
Age standardized rate (ASR) |
Change in incidence (%) |
Change in incidence due to population (%) |
Change in incidence due to risk (%) |
ASR annual change (%) |
ASR change (%) |
Cumulative risk (0–74 years) |
Cumulative risk rank |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| 2005 | 2030 | 2005 | 2030 | 2005 | 2030 | 2005 | 2030 | 2005 | 2030 | |||||||
| Americas | ||||||||||||||||
| Canada* | 10 | 16.0 | 20.1 | 871 | 3313 | 3.6 | 6.5 | 280.4 | 93.9 | 186.5 | 2.4 | 83.0 | 0.43 | 0.76 | 27 | 20 |
| Colombia* | 5 | 21.4 | 26.1 | 647 | 2685 | 4.2 | 6.7 | 315.2 | 156.5 | 158.7 | 1.9 | 61.6 | 0.49 | 0.87 | 24 | 16 |
| USA: Black* | 9 | 18.7 | 23.7 | 2216 | 7068 | 11.6 | 19.5 | 219.0 | 69.5 | 149.5 | 2.1 | 68.2 | 1.36 | 2.36 | 6 | 1 |
| USA: White* | 9 | 117.7 | 133.4 | 10292 | 30506 | 5.9 | 11.2 | 196.4 | 49.6 | 146.8 | 2.6 | 89.2 | 0.69 | 1.35 | 15 | 7 |
| Asia | ||||||||||||||||
| China* | 1 | 671.4 | 729.1 | 171408 | 204076 | 24.1 | 14.3 | 19.1 | 91.4 | −72.4 | −2.1 | −40.7 | 2.74 | 1.72 | 2 | 4 |
| Japan* | 10 | 61.9 | 58.2 | 31805 | 16498 | 23.6 | 10.8 | −48.1 | 35.8 | −83.9 | −3.1 | −54.2 | 2.97 | 1.27 | 1 | 8 |
| Singapore* | 77 | 2.2 | 3.2 | 452 | 1030 | 17.4 | 11.6 | 127.7 | 211.6 | −83.9 | −1.6 | −33.3 | 2.06 | 1.26 | 3 | 9 |
| Oceania | ||||||||||||||||
| Australia | 100 | 9.9 | 14.2 | 754 | 2457 | 5.1 | 8.5 | 225.7 | 88.1 | 137.6 | 2.1 | 67.7 | 0.60 | 1.10 | 19 | 12 |
| New Zealand | 100 | 2.0 | 2.5 | 148 | 393 | 5.2 | 6.5 | 166.2 | 75.9 | 90.4 | 0.9 | 26.4 | 0.60 | 0.83 | 18 | 19 |
| Northern Europe | ||||||||||||||||
| Denmark | 100 | 2.7 | 3.0 | 189 | 353 | 4.1 | 5.5 | 86.2 | 51.4 | 34.8 | 1.1 | 33.1 | 0.51 | 0.62 | 23 | 24 |
| Estonia | 100 | 0.6 | 0.6 | 34 | 35 | 3.7 | 3.1 | 2.1 | 36.8 | −34.7 | −0.6 | −14.2 | 0.43 | 0.37 | 26 | 30 |
| Finland | 100 | 2.6 | 2.8 | 238 | 624 | 5.2 | 6.6 | 161.7 | 77.0 | 84.7 | 0.9 | 25.7 | 0.59 | 0.73 | 20 | 21 |
| Latvia | 100 | 1.1 | 0.8 | 82 | 148 | 5.3 | 8.9 | 79.4 | 9.7 | 69.8 | 2.1 | 67.3 | 0.63 | 1.11 | 17 | 11 |
| Lithuania | 100 | 1.6 | 1.2 | 94 | 118 | 4.1 | 4.6 | 25.5 | 3.3 | 22.2 | 0.5 | 12.3 | 0.52 | 0.57 | 22 | 26 |
| Norway | 100 | 2.3 | 3.0 | 84 | 241 | 2.2 | 4.6 | 186.4 | 72.5 | 113.9 | 2.9 | 105.1 | 0.24 | 0.50 | 31 | 29 |
| Sweden | 100 | 4.5 | 5.4 | 293 | 639 | 3.4 | 5.2 | 118.0 | 51.3 | 66.8 | 1.7 | 51.6 | 0.40 | 0.64 | 28 | 22 |
| United Kingdom* | 67 | 29.5 | 34.8 | 1988 | 4811 | 3.8 | 5.6 | 142.0 | 57.2 | 84.8 | 1.6 | 47.3 | 0.44 | 0.64 | 25 | 23 |
| Western Europe | ||||||||||||||||
| Austria* | 13 | 4.0 | 4.4 | 480 | 882 | 7.1 | 8.1 | 83.8 | 69.0 | 14.8 | 0.5 | 12.7 | 0.83 | 1.03 | 12 | 14 |
| France* | 10 | 29.9 | 33.2 | 5815 | 9894 | 11.0 | 13.1 | 70.1 | 52.0 | 18.1 | 0.7 | 19.5 | 1.40 | 1.70 | 5 | 5 |
| Germany* | 1 | 39.8 | 39.2 | 5680 | 10867 | 7.6 | 9.3 | 91.3 | 48.0 | 43.3 | 0.8 | 22.5 | 0.95 | 1.15 | 8 | 10 |
| Netherlands | 100 | 8.1 | 8.8 | 260 | 536 | 2.1 | 2.5 | 106.2 | 66.0 | 40.3 | 0.7 | 20.4 | 0.24 | 0.30 | 30 | 31 |
| Switzerland* | 31 | 3.6 | 4.6 | 637 | 1659 | 10.3 | 14.3 | 160.4 | 80.5 | 79.9 | 1.3 | 39.6 | 1.28 | 1.73 | 7 | 3 |
| Southern Europe | ||||||||||||||||
| Croatia | 100 | 2.1 | 1.9 | 274 | 422 | 7.7 | 8.9 | 54.0 | 30.9 | 23.1 | 0.6 | 16.3 | 0.90 | 1.06 | 10 | 13 |
| Italy* | 9 | 28.5 | 28.9 | 7036 | 10642 | 12.3 | 16.4 | 51.2 | 47.9 | 3.4 | 1.1 | 32.6 | 1.51 | 1.93 | 4 | 2 |
| Malta | 100 | 0.2 | 0.2 | 8 | 24 | 2.7 | 4.2 | 211.3 | 78.4 | 132.9 | 1.8 | 57.7 | 0.31 | 0.52 | 29 | 28 |
| Slovenia | 100 | 1.0 | 1.0 | 105 | 228 | 6.8 | 6.8 | 116.6 | 71.4 | 45.2 | 0.1 | 1.3 | 0.87 | 0.86 | 11 | 17 |
| Spain* | 11 | 21.6 | 22.6 | 2980 | 6468 | 8.1 | 13.5 | 117.0 | 60.5 | 56.6 | 2.1 | 66.7 | 0.94 | 1.57 | 9 | 6 |
| Eastern Europe | ||||||||||||||||
| Czech Republic | 100 | 5.0 | 5.2 | 542 | 691 | 6.8 | 5.3 | 27.5 | 57.6 | −30.1 | −1.0 | −22.5 | 0.82 | 0.62 | 13 | 25 |
| Poland* | 2 | 18.6 | 18.0 | 1224 | 3248 | 4.8 | 7.5 | 165.4 | 59.1 | 106.3 | 1.8 | 56.7 | 0.63 | 0.96 | 16 | 15 |
| Russian Federation* | 3 | 66.8 | 64.2 | 3845 | 7937 | 4.6 | 8.0 | 106.4 | 29.2 | 77.2 | 2.2 | 73.0 | 0.54 | 0.84 | 21 | 18 |
| Slovakia | 100 | 2.6 | 2.6 | 210 | 244 | 6.4 | 4.5 | 16.0 | 61.7 | −45.8 | −1.4 | −29.6 | 0.81 | 0.53 | 14 | 27 |
Regional registries: Austria (Tyrol and Vorarlberg), Canada (Manitoba, Nova Scotia and Saskatchewan), China (Shanghai and Hong Kong), Colombia (Cali), France (Bas-Rhin, Isere, Somme, Tarn and Herault), Germany (Saarland), Italy (Ferrara, Modena, Parma, Ragusa, Romagna, Sassari, Torino and Varese), Japan (Miyagi, Nagasaki and Osaka), Poland (Cracow city), Russian Federation (St Petersburg), Singapore (Resident), Spain (Albacete, Cuenca, Girona, Granada, Murcia, Navarra and Tarragona), Switzerland (Geneva, Graubunden and Glarus, Neuchatel, St Gall-Appenzell, Vaud and Valais), United Kingdom (Northern Ireland, England and Scotland), USA (9 SEER with coverage since 1993).
Table 2.
Primary liver cancer incidence among women in 2005 and in 2030 in countries with corresponding percentage changes in incidence
| Country | Coverage (%) |
Annual Population (Million) |
Number of new cases (Annual) |
Age standardized rate (ASR) |
Change in incidence (%) |
Change in incidence due to population (%) |
Change in incidence due to risk (%) |
ASR annual change (%) |
ASR change (%) |
Cumulative risk (0–74 years) |
Cumulative risk rank |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| 2005 | 2030 | 2005 | 2030 | 2005 | 2030 | 2005 | 2030 | 2005 | 2030 | |||||||
| Americas | ||||||||||||||||
| Canada* | 10 | 16.2 | 20.3 | 404 | 861 | 1.4 | 1.5 | 113.3 | 85.1 | 28.2 | 0.4 | 10.2 | 0.15 | 0.15 | 28 | 26 |
| Colombia* | 5 | 21.9 | 27.1 | 594 | 1536 | 3.1 | 3.0 | 158.4 | 154.3 | 4.0 | −0.2 | −5.7 | 0.35 | 0.31 | 6 | 14 |
| USA: Black* | 9 | 20.6 | 25.5 | 804 | 3751 | 3.1 | 8.3 | 366.5 | 80.4 | 286.1 | 4.0 | 167.2 | 0.37 | 1.03 | 5 | 1 |
| USA: White* | 9 | 120.5 | 134.2 | 4139 | 8324 | 1.9 | 2.5 | 101.1 | 50.5 | 50.6 | 1.1 | 31.7 | 0.21 | 0.28 | 19 | 16 |
| Asia | ||||||||||||||||
| China* | 1 | 634.1 | 686.5 | 50348 | 75999 | 7.0 | 4.5 | 50.9 | 121.7 | −70.7 | −1.7 | −35.5 | 0.82 | 0.53 | 2 | 3 |
| Japan* | 10 | 64.9 | 61.9 | 15493 | 11412 | 7.9 | 4.4 | −26.3 | 47.3 | −73.6 | −2.3 | −44.4 | 0.97 | 0.53 | 1 | 4 |
| Singapore* | 77 | 2.3 | 3.3 | 141 | 355 | 4.6 | 3.4 | 151.2 | 251.1 | −99.9 | −1.3 | −27.1 | 0.50 | 0.33 | 3 | 10 |
| Oceania | ||||||||||||||||
| Australia | 100 | 10.0 | 14.3 | 322 | 956 | 1.7 | 2.6 | 196.8 | 85.7 | 111.2 | 1.6 | 48.7 | 0.19 | 0.28 | 23 | 15 |
| New Zealand | 100 | 2.1 | 2.6 | 66 | 176 | 1.9 | 2.7 | 168.6 | 80.3 | 88.3 | 1.4 | 42.3 | 0.23 | 0.35 | 16 | 9 |
| Northern Europe | ||||||||||||||||
| Denmark | 100 | 2.7 | 3.0 | 87 | 57 | 1.6 | 1.0 | −34.7 | 40.5 | −75.2 | −1.8 | −36.6 | 0.17 | 0.10 | 26 | 29 |
| Estonia | 100 | 0.7 | 0.7 | 29 | 28 | 1.8 | 1.7 | −1.3 | 18.7 | −20.0 | −0.3 | −7.1 | 0.19 | 0.19 | 24 | 24 |
| Finland | 100 | 2.7 | 2.9 | 140 | 192 | 2.1 | 1.8 | 37.1 | 53.3 | −16.3 | −0.5 | −11.9 | 0.22 | 0.21 | 18 | 21 |
| Latvia | 100 | 1.2 | 1.0 | 55 | 55 | 1.8 | 2.1 | −0.0 | 3.6 | −3.6 | 0.6 | 15.3 | 0.21 | 0.27 | 20 | 17 |
| Lithuania | 100 | 1.8 | 1.4 | 62 | 42 | 1.4 | 0.9 | −32.1 | 10.2 | −42.3 | −1.7 | −34.4 | 0.17 | 0.10 | 27 | 28 |
| Norway | 100 | 2.3 | 2.9 | 48 | 62 | 1.0 | 0.7 | 30.1 | 49.3 | −19.2 | −1.5 | −30.7 | 0.11 | 0.08 | 29 | 31 |
| Sweden | 100 | 4.6 | 5.4 | 172 | 241 | 1.7 | 1.9 | 40.1 | 33.3 | 6.9 | 0.5 | 13.7 | 0.20 | 0.21 | 21 | 20 |
| United Kingdom* | 67 | 30.7 | 35.3 | 1201 | 1922 | 1.8 | 1.8 | 60.0 | 41.5 | 18.5 | 0.0 | 0.1 | 0.20 | 0.19 | 22 | 23 |
| Western Europe | ||||||||||||||||
| Austria* | 13 | 4.2 | 4.5 | 265 | 556 | 2.5 | 3.2 | 110.3 | 40.9 | 69.3 | 1.0 | 28.9 | 0.28 | 0.32 | 11 | 12 |
| France* | 10 | 31.3 | 34.8 | 1531 | 2297 | 2.2 | 2.1 | 50.0 | 45.8 | 4.2 | −0.1 | −2.8 | 0.25 | 0.26 | 15 | 18 |
| Germany* | 1 | 41.4 | 40.1 | 2935 | 7879 | 2.9 | 6.0 | 168.4 | 27.5 | 141.0 | 3.0 | 107.8 | 0.33 | 0.69 | 7 | 2 |
| Netherlands | 100 | 8.2 | 8.8 | 113 | 204 | 0.8 | 1.1 | 79.6 | 47.0 | 32.6 | 1.5 | 44.3 | 0.09 | 0.13 | 31 | 27 |
| Switzerland* | 31 | 3.8 | 4.6 | 175 | 439 | 2.3 | 5.3 | 151.0 | 57.0 | 94.0 | 3.4 | 129.8 | 0.27 | 0.50 | 12 | 5 |
| Southern Europe | ||||||||||||||||
| Croatia | 100 | 2.3 | 2.0 | 150 | 108 | 2.7 | 1.8 | −28.1 | 26.7 | −54.8 | −1.7 | −35.5 | 0.30 | 0.22 | 9 | 19 |
| Italy* | 9 | 30.1 | 30.2 | 3474 | 5346 | 4.0 | 4.0 | 53.9 | 43.5 | 10.4 | 0.1 | 1.7 | 0.47 | 0.49 | 4 | 6 |
| Malta | 100 | 0.2 | 0.2 | 4 | 7 | 0.9 | 0.9 | 52.9 | 71.9 | −19.0 | −0.2 | −4.9 | 0.10 | 0.09 | 30 | 30 |
| Slovenia | 100 | 1.0 | 1.0 | 47 | 117 | 2.1 | 3.0 | 150.5 | 47.3 | 103.3 | 1.5 | 46.1 | 0.22 | 0.38 | 17 | 8 |
| Spain* | 11 | 22.2 | 23.4 | 1310 | 2542 | 2.4 | 3.4 | 94.1 | 53.4 | 40.7 | 1.4 | 40.5 | 0.28 | 0.41 | 10 | 7 |
| Eastern Europe | ||||||||||||||||
| Czech Republic | 100 | 5.2 | 5.3 | 313 | 457 | 2.7 | 2.7 | 45.8 | 45.5 | 0.3 | −0.0 | −1.1 | 0.31 | 0.33 | 8 | 11 |
| Poland* | 2 | 19.8 | 19.2 | 809 | 1105 | 2.4 | 2.4 | 36.6 | 43.0 | −6.4 | 0.1 | 2.7 | 0.25 | 0.31 | 14 | 13 |
| Russian Federation* | 3 | 76.7 | 74.5 | 2843 | 2727 | 1.8 | 1.5 | −4.1 | 24.8 | −28.9 | −0.6 | −14.5 | 0.18 | 0.17 | 25 | 25 |
| Slovakia | 100 | 2.8 | 2.8 | 124 | 130 | 2.4 | 1.6 | 4.8 | 52.5 | −47.6 | −1.6 | −32.9 | 0.27 | 0.20 | 13 | 22 |
Regional registries: Austria (Tyrol and Vorarlberg), Canada (Manitoba, Nova Scotia and Saskatchewan), China (Shanghai and Hong Kong), Colombia (Cali), France (Bas-Rhin, Isere, Somme, Tarn and Herault), Germany (Saarland), Italy (Ferrara, Modena, Parma, Ragusa, Romagna, Sassari, Torino and Varese), Japan (Miyagi, Nagasaki and Osaka), Poland (Cracow city), Russian Federation (St Petersburg), Singapore (Resident), Spain (Albacete, Cuenca, Girona, Granada, Murcia, Navarra and Tarragona), Switzerland (Geneva, Graubunden and Glarus, Neuchatel, St Gall-Appenzell, Vaud and Valais), United Kingdom (Northern Ireland, England and Scotland), USA (9 SEER with coverage since 1993).
Incidence rates were calculated by 5-year period of diagnosis and 5-year age group based on the annual number of PLC incidence cases from each population-based cancer registry by sex. Age-standardized incidence rates (ASR) per 100,000 person-years were computed at the national level by sex using the world standard population of Doll et al. (1966).(19) Cumulative risks were computed for ages 0–74 and expressed as percentages, assuming an absence of competing causes of death.
To predict the numbers of new PLC cases and rates in 2030, we fitted an age-period-cohort model to recent trends in incidence rates, assuming such temporal patterns serve as proxies for the changing prevalence and distribution of risk factors over time. The NORDPRED software package, developed and implemented in R, has been shown to perform well empirically in projecting recent trends into the future.(20) The three or four most recent 5-year observed periods (depending on national, or regional registry availability, where applicable) were extrapolated using a power function to level off the growth, with a projection of the recent linear trend for the last ten years that was attenuated by 25%, 50% in the second and third prediction periods, respectively, and 75% for both fourth and fifth prediction periods. Mean annual differences in the numbers of predicted PLC cases in 2030 relative to 2005 are partitioned into changes in risk (rates), and changes in demographics (population growth and ageing). The numbers of new cases were predicted in the year 2030 by taking a weighted average of the projected incidence rates in the last two future prediction periods, centering on 2030, and then applying the UN national population forecasts available for each country for that year.
Stata (v13, StataCorp LP, College Station, TX) was used for data management and plotting the observed and modelled trends. The modelling analyses were performed using R (v2.15, R Foundation for Statistical Computing, Vienna) and the functions available in the Epi package (version 1.1.36), R Studio and NORDPRED software package (http://www.kreftregisteret.no/en/Research/Projects/Nordpred/Nordpred-software/).(21)
Results
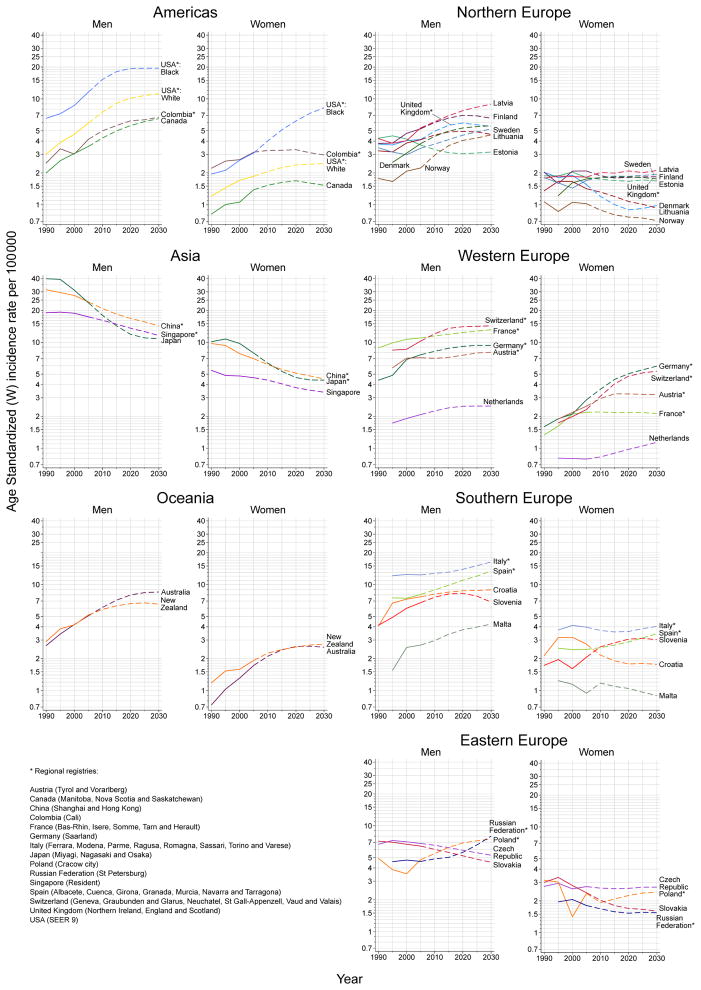
The observed and predicted trends in PLC rates among men and women are presented in Figure 1. Tables 1 and 2 show the observed number of cases and rates in 2005 for both men and women, respectively, alongside the respective predicted values for 2030, and the overall percentage change over the 25-year period, partitioned into demographic and risk components.
Figure 1.
Trends in primary liver cancer incidence rates in 30 countries 1990–2030: observed age-standardised rates per 100,000 (solid lines) versus predicted rates (dashed lines), by region and by sex (age-standardised rates per 100,000 men/women)
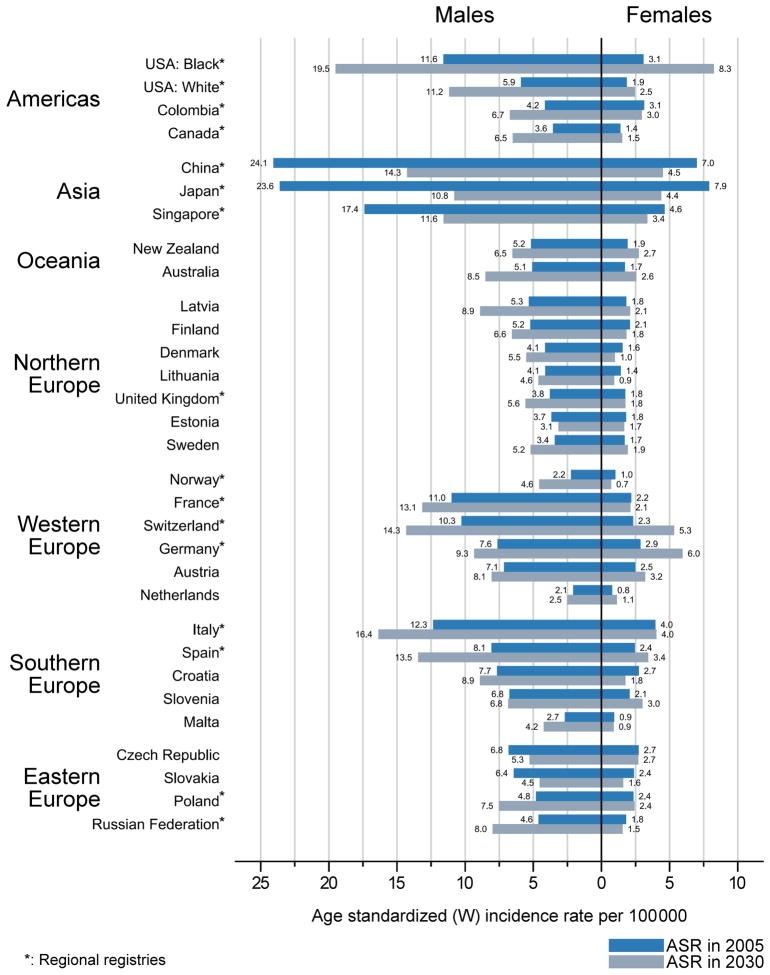
Changes in PLC incidence rates 2005–30
The rates circa 2005 and 2030 are compared in Figure 2. The percentage change in ASR over the 25-year period is predicted to be greater than 30% among men in 15 countries, and among women in eight countries. Average increases in PLC (≥1% per annum) are predicted among men in approximately half of the study populations (n=15, 50%). Some of the largest rate increases among men are predicted in Norway (2.9% per annum), US whites (2.6%), Canada (2.4%), Russian Federation (2.2%), and in US blacks, Latvia, Spain and Australia (2.1%). Equivalent increases in PLC among women are predicted in fewer (n=10) populations (32% of those studied), with the greatest increases expected among US blacks (4.0%), Switzerland (3.4%), Germany (3.0%), and Australia (1.6%).
Figure 2.
Primary liver cancer age-standardised incidence rates per 100,000 in men and women in 2005 and predicted rates circa 2030 in 30 countries
Average decreases in PLC among men are predicted in six countries: Japan (−3.1%), China (−2.1%), Singapore (−1.6%), Slovakia (−1.4%), Czech Republic (−1.0%), and Estonia (−0.6%). Decreases in PLC among women are predicted in 14 countries (47% of selected countries). The largest decreases are projected in Japan (−2.3%), Denmark (−1.8%), and China, Lithuania and Croatia (each −1.7%).
Changes in numbers of new cases of PLC 2005–30
We predicted that in the selected countries by 2030, there will be a 35% increase in the future number of new cases of PLC annually, from 339,000 in 2005 to over 459,000 in 2030 (329,000 among men and 130,000 among women). Despite a predicted demographic increase due to population growth and ageing in most countries (n=23, 77%; exceptions are Switzerland, Italy and Spain), changes in risk are the main drivers of the rising number of new cases annually in future years. Japan is the only country where a declining number of new cases is predicted by 2030 (a 48% reduction among men, 26% among women); this is due in part to rapidly declining rates, as well as in relative terms, a more modest demographic transition over the next decades.
Discussion
This study is the first to our knowledge to predict the future burden of PLC from a global context. In the next 15 years, we project that both the number of new cases and the incidence rates of PLC will increase in most countries under study. There are exceptions: in several Asian countries (China, Japan, Singapore) and some European countries (Estonia, Czech Republic, and Slovakia), there will be declines in rates in both sexes. However, increases in the absolute number of PLC cases can be expected in the vast majority of countries by 2030 due to continuations in population ageing and growth. Japan is one exception, where a 41% reduction in new cases of PLC by 2030 largely due to changing risk patterns, with the projected declines in rates resulting from diminishing HCV-related HCC from around 2000.(22) In contrast, the declining rates of PLC in China have been preceded by declining aflatoxin B1 levels in the population. (13, 23) It is unclear why rates are decreasing in Estonia, Czech Republic, and Slovakia. Changing distributions of risk factors, especially HBV, HCV, alcohol consumption, and obesity, could alter future trends and projections.
Data on the prevalence of obesity and alcohol per capita consumption obtained from the WHO Indicator and Measurement Registry indicate major geographic and temporal variations.(24) The global prevalence of obesity in 2010 was 13.7% among women and 9.3% among men. In about two-thirds of the countries under study, the prevalence of obesity is now 20% or greater (Annex Figure 1). Despite heterogeneity in trends in alcohol consumption per capita of pure alcohol over time (Annex Figure 2), an increasing consumption of alcohol is observed 1995–2015 and predicted to further rise by 2025 in most countries (n=16, 55%). Annex Figure 3 demonstrates a correlation between PLC rates and national levels of alcohol consumption.
Given the increasing rates of obesity(14) and recent or predicted declines in the prevalence of HBV and HCV,(8, 25) the relevant importance of non-viral risk factors for HCC is expected to increase in the future. In recent decades, men and women in most countries have gained weight,(26) with the prevalence of adult obesity nearly doubling from 1980 to 2008.(24) Obesity is associated with diabetes, NAFLD, and NAFLD’s most severe form, non-alcoholic steatohepatitis (NASH).(27–29) In the setting of NASH/NAFLD with chronic HBV or HCV infection, the odds of HCC increases.(30) With shifts in adult obesity occurring worldwide, and with many lower- and middle-income countries in Africa(31, 32) and Asia(30) undergoing nutrition transition,(33) increasing rates of obesity are likely contributing to an increase in obesity-related HCC.(4) Rates of obesity classified as body mass index (BMI) ≥30 kg/m2 are <10% in Asian countries. However, the proportion of people in these countries at high risk of diabetes and NASH/NAFLD is significant, as these conditions occurs at BMI levels lower than the existing WHO cut-off points for overweight/obesity.(34)
Over two decades ago, HBV and HCV infections were shown to increase the risk of HCC in humans.(35) A subsequent focus on prevention, with recent WHO data on HBV immunization(24) indicating at least 90% HBV vaccine coverage among 1-year-olds in most countries included in the current report (n=18), is likely to result in further reductions of infection-related HCC in the future. For example, over 90% coverage has been reported for Singapore since 1993, for Italy since 1991. While individuals vaccinated against HBV are too young to have a substantial effect on the rates projected in the current study, neonatal HBV vaccination is likely to result in further reductions of HBV-related HCC in the future.
The prevalence of HCV infection varies by region, with lower rates in the Americas, South-East Asia and high-income Asia Pacific (1.2–2.0%), and higher rates in countries in the Central, East, and South Asia (3.4%–3.8%), and North Africa/Middle East (3.6%).(36) In 2005 over 185 million people (2.8%) were infected with HCV.(36) In the US, increasing HCC rates have been affected by ageing of persons chronically infected with HCV, who acquired infections during the 1960–1970s.(4) In Japan, HCV infection is present as a risk factor in 70% of cases, but rates of HCC are declining as infections were mostly acquired in the 1940–1950s.(22) The advent of HCV RNA tests and their application in screening of blood donors, significantly improved the safety of blood products in many countries.(37) Education and increased awareness of HCV in the general population, targeted HCV screening of at-risk groups, and needle and syringe exchange programmes are some of the strategies that have halted disease transmission.(37) In countries where new second-generation direct-acting antivirals (DAAs) for HCV are available, PLC rates are unlikely to be affected unless major efforts are undertaken to expand treatment to underserved sub-populations in the community.(38, 39) Nevertheless, some countries are attempting to make these therapies widely available. For example, the Australian government has funded new therapies for HCV at trivial or no cost to all patients with HCV infection, irrespective of cirrhosis status.(38) The underlying premise of such universal HCV treatment is to prevent cirrhosis, which is precursor lesion for HCC. However, time will tell whether compliance issues, reinfection, immigration and treatment uptake will have an effect on HCV infection complications in the long-term.(38, 39) Early case-series data have been conflicting regarding the risk of HCC after achievement of cure (sustained viral response) after DAA treatment. Some recent studies suggest occurrence and recurrence of HCC are high among patients with HCV-related cirrhosis after sustained viral response, hypothesising viral-initiated liver inflammation may serve a tumour surveillance role controlling HCC growth.(40–42) Other studies have reported lower HCC recurrence among patients treated with DAA compared with untreated patients.(43) These data suffer from bias inherent in observational studies, and large-scale, well controlled prospective cohort studies are needed to clarify these risks. Irrespective, international society Guidelines advocate for HCC surveillance for all cirrhotic patients during and after antiviral therapy irrespective of sustained viral response.(7)
Exposure to aflatoxin B1, a carcinogen produced by Aspergillus fungi,(44) is a significant environmental co-factor in liver cancer globally,(9) with a population attributable risk of 23% in high-risk regions (e.g. Africa, East Asia).(16) Aflatoxins contaminate many foodstuffs (e.g. cereals, oilseeds, ground nuts),(11) with dietary exposure in countries in Africa and Asia being up to 400 times higher than exposure levels in North America, Europe and Australia.(45, 46) Most HCC cases occur in low-income countries, where both aflatoxin exposure and chronic HBV infection are common, and where the relative attributable fraction of HBV and aflatoxin is difficult to distil.(46) Due to a synergistic interaction between aflatoxin exposure and HBV, co-exposure increases the odds of HCC up to 30 times compared to exposure to one of these factors alone.(47) HBV-positive individuals contribute a substantial portion of the total global aflatoxin-induced HCC burden in China, Southeast Asia, and sub-Saharan Africa.(9) While aflatoxin regulatory programs are in place in most countries, in the most affected areas these are often not enforced.(48) However, notably where reduction in aflatoxin exposure has been achieved, it has preceded decreases in PLC incidence.(23) The control of both HBV and aflatoxin risk factors in high-risk countries should be a priority. Whether aflatoxin has a synergistic effect on HCV-induced liver cancer has not been extensively studied.(49)
Heavy episodic alcohol drinking contributes 15–34% of HCC cases in many developed countries.(15, 50) WHO projects alcohol consumption will increases in about half of the countries included here in the next decade, thus one may expect the proportion of HCC attributable to alcohol, as the sole or a contributing factor, in these countries will also increase in the future. Hence, public health policies discouraging harmful alcohol consumption are critical (e.g. education, taxation, regulations on selling alcoholic beverages). However, education alone is unlikely to bring change. While alcohol control policies have been in place in many countries for decades, some countries (e.g. in Northern Europe) have experienced an increase in alcohol consumption while others (e.g. Italy and France) have seen a decrease.(51) Socio-demographic changes (e.g. changes in the role of women in society, increase in health awareness), economic shifts (e.g. industrialization, increased income), and migration (e.g. urbanization) during the 1970–2000s are believed to be the key factors contributing to the decrease in alcohol consumption in these countries.(51, 52)
As noted in the current report, PLC rates are higher among men compared to women worldwide. These sex differences in PLC rates are hypothesized to be due to differences in the prevalence of known risk factors, sex steroid hormones, immune response, and/or epigenetics between men and women.(53) However, it is unclear what risk factors are driving the rate change discrepancies between sexes noted in certain populations. Many additional factors have influenced the historical trends in PLC incidence rates, and consequently also have the potential to affect projections. The quality of reporting of PLC, demographic changes, enhanced diagnostic practices, the introduction of specific initiatives (e.g. vaccination, donor screening), and in exposure to other risk factors are likely to have an impact.
Petrick et al(4) has examined international trends of PLC by histologic subtype during 2003–2007. HCC accounted for the majority of all histologically verified tumours and incidence rates of HCC were higher than incidence rates of ICC in 38 countries worldwide (30 of which were included here). The only exception was Thailand, where the rates of ICC and HCC were mostly similar.(4) Rates of ICC in Thailand have been rising with successive birth cohorts, while ICC rates have plateaued in other countries among recent generations.(4) One major limitation in all such studies, including the present one, is that the vast majority of liver cancer is never confirmed histopathologically. Health education and promotion programmes to enhance knowledge of the risk of infestation with bile duct flukes Opisthorcis viverrine and Clonorchis sinesis should be of upmost priority to halt the increase of preventable new ICC cases in high-risk countries of South Eastern Asia.(9, 16)
Strengths and limitations
Future projections depend on many assumptions, the key factor being whether past trends in rates will continue into the future. The predicted numbers of cases are also reliant on population projections which are themselves future estimates based on assumptions on birth and death rates, immigration and emigration. The extent to which registry coverage is representative of the national profile of PLC is a possible limitation, with projections in thirteen countries based on regional registries covering less than one-third of their respective national populations. The rates in these countries were used a proxy of the (unknown) national profile and therefore comparisons should be interpreted with some caution. On the other hand, the CI5 process focuses on the completeness, quality and comparability of the data (e.g., through the definition of an incident case of cancer, the stability of incidence rates over time, and the proportion of cases microscopically verified).(18) Registries included here were population-based and considered by the CI5 editors as of consistently high quality, having been included in successive volumes. Another limitation is the potential misclassification of cancer type (e.g. cancers arising in the pancreas and bile ducts, metastasis of cancer from other organs).
A more complete account of the future trends of PLC incidence would be possible if comparable PLC incidence data were assembled from a more geographically diverse set of registries. The lack of data from Africa is a key limitation. Africa has high rates of PLC,(2) high rates of HBV and HCV infection,(36, 54) variable HBV vaccination coverage,(24) and aflatoxin abatement programs are scarce.(9) A greater availability and better reporting of PLC rates is anticipated through the ongoing activities of the Global Initiative for Cancer Registry Development (http://gicr.iarc.fr), a multi-partner initiative coordinated by IARC that aims to provide measurable improvements in the coverage, quality and networking capacity of cancer registries in across low and middle income countries.
Lastly, BMI and alcohol consumption data discussed here are based on ecological data. Ecological studies are limited by an inability to infer that the observed associations apply to individuals, rather than to the groups under study. They provide a broad level association of risk and outcome, without inference of causality. Nevertheless, findings about the associations between BMI and alcohol consumption and HCC are well aligned with results from studies based on individual level data.(15, 30, 50)
Despite these limitations, projections presented here provide a necessary baseline for future planning of resources and cancer control. The projected number of cases estimated here was extrapolated from within recorded temporal data from population-based cancer registries that have been evaluated as being of consistently high quality over time through careful attention to the completeness, comparability and validity of the respective datasets by the CI5 editors in each volume.
Conclusions
An increase in the number of new cases of PLC each year is predicted to 2030 in most studied countries, as a result of both changes in risk factors as well as population ageing and growth. HBV and HCV infections being major risk factors for HCC worldwide, and with increasing control of HBV through vaccination and improved access to treatment, viral hepatitis-related PLC rates may decrease in the future. Unfortunately, decreased in PLC rates may be offset by increasing levels of obesity and its metabolic complications and increasing alcohol consumption. Continued control of aflatoxin exposure, the complete roll-out of the HBV vaccination, discouragement of heavy alcohol intake as well as new primary prevention strategies for obesity should be core components of programmes aimed to reduce the future burden of PLC in short- and long-term future.
Supplementary Material
Acknowledgments
PC Valery was supported by a NHMRC Career Development Fellowship (#1083090). KA McGlynn and JL Petrick were supported by the NIH Intramural Research Program, National Cancer Institute.
List of Abbreviations
- PLC
Primary liver cancer
- IARC
International Agency for Research on Cancer
- ICC
intrahepatic cholangiocarcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- NAFLD
non-alcoholic fatty liver disease
- DAAs
second-generation direct-acting antivirals
Footnotes
Conflict of interest: There are no financial disclosures.
Financial disclosure: There are no financial disclosures.
Contributors: PCV and FB contributed to the conception and design of the study. ML performed the data analysis and takes responsibility for the integrity and the accuracy of the data. PCV drafted the report. All authors contributed the interpretation of data, revising draft critically for important intellectual content, and approved the final version.
Data on HBV immunization coverage among 1-year-olds, prevalence of obesity (percentage of adult population with a body mass index (BMI) ≥30 kg/m2), and alcohol per capita (APC) consumption were obtained from the WHO Indicator and Measurement Registry.1 APC consumption (total amount of alcohol consumed per adult (15+ years) over a calendar year in litres of pure alcohol) was available yearly by country from 1980-2014, and projections available for 2015, 2020 and 2025.
Contributor Information
Patricia C. Valery, Email: Patricia.Valery@qimrberghofer.edu.au.
Mathieu Laversanne, Email: LaversanneM@iarc.fr.
Paul J. Clark, Email: drpjclark@gmail.com.
Jessica L. Petrick, Email: jessica.petrick@nih.gov.
Katherine A McGlynn, Email: mcglynnk@mail.nih.gov.
Freddie Bray, Email: brayf@iarc.fr.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer Incidence and Mortality Worldwide: IARC CancerBase No.11. Lyon, France: IARC; 2012. [Google Scholar]
- 2.International Agency for Research on Cancer (IARC) World Cancer Report 2014. Lyon, France: 2014. [Google Scholar]
- 3.Chimed T, Sandagdorj T, Znaor A, Laversanne M, Tseveen B, Genden P, Bray F. Cancer incidence and cancer control in Mongolia: Results from the National Cancer Registry 2008–12. Int J Cancer. 2017;140:302–309. doi: 10.1002/ijc.30463. [DOI] [PubMed] [Google Scholar]
- 4.Petrick JL, Braunlin M, Laversanne M, Valery P, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978–2007. Int J Cancer. 2016;139:1534–1545. doi: 10.1002/ijc.30211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterol. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 6.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–243. vii–x. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European association for the study of the Liver, European organisation for research and treament of cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Razavi H, Waked I, Sarrazin C, Myers RP, Idilman R, Calinas F, Vogel W, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepatitis. 2014;21:34–59. doi: 10.1111/jvh.12248. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. 2010;118:818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marengo A, Rosso C, Bugianesi E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu Rev Med. 2016;67:103–117. doi: 10.1146/annurev-med-090514-013832. [DOI] [PubMed] [Google Scholar]
- 11.Magnussen A, Parsi MA. Aflatoxins, hepatocellular carcinoma and public health. World J Gastroenterol. 2013;19:1508–1512. doi: 10.3748/wjg.v19.i10.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung H, Ueda T, Kudo M. Changing trends in hepatitis C infection over the past 50 years in Japan. Intervirol. 2010;53:39–43. doi: 10.1159/000252782. [DOI] [PubMed] [Google Scholar]
- 13.Sun Z, Chen T, Thorgeirsson SS, Zhan Q, Chen J, Park JH, Lu P, et al. Dramatic reduction of liver cancer incidence in young adults: 28 year follow-up of etiological interventions in an endemic area of China. Carcinogenesis. 2013;34:1800–1805. doi: 10.1093/carcin/bgt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatol. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 15.Makarova-Rusher OV, Altekruse SF, McNeel TS, Ulahannan S, Duffy AG, Graubard BI, Greten TF, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122:1757–1765. doi: 10.1002/cncr.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Chang CC, Marsh GM, Wu F. Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. Eur J Cancer. 2012;48:2125–2136. doi: 10.1016/j.ejca.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization; WHO, editor. Hepatitis B Fact sheet. 2016 (Updated July 2016) http://www.who.int/mediacentre/factsheets/fs204/en/
- 18.International Agency for Research on Cancer (IARC) Cancer incidence in five continents, volume X (electronic version) Lyon: IARC; 2013. [Google Scholar]
- 19.Doll R, Payne P, Waterhouse J. Cancer Incidence In Five Continents: A Technical Report. New York: 1966. [Google Scholar]
- 20.Moller B, Fekjaer H, Hakulinen T, Sigvaldason H, Storm HH, Talback M, Haldorsen T. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med. 2003;22:2751–2766. doi: 10.1002/sim.1481. [DOI] [PubMed] [Google Scholar]
- 21.Version R development Core team (2011) ed. Vienna, Austria: R Foundation for Statistical Computing; 2013. R: a language and environment for statistical computing [computer program] [Google Scholar]
- 22.Tanaka H, Imai Y, Hiramatsu N, Ito Y, Imanaka K, Oshita M, Hijioka T, et al. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med. 2008;148:820–826. doi: 10.7326/0003-4819-148-11-200806030-00004. [DOI] [PubMed] [Google Scholar]
- 23.Chen JG, Egner PA, Ng D, Jacobson LP, Munoz A, Zhu YR, Qian GS, et al. Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev Res (Phila) 2013;6:1038–1045. doi: 10.1158/1940-6207.CAPR-13-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. The World Health Organization Indicator and Measurement Registry. WHO; [Google Scholar]
- 25.Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, Bahalim AN, et al. National, regional, and global trends in adult overweight and obesity prevalences. Population Health Metrics. 2012:10. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:109–122. doi: 10.1002/dmrr.1291. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, Li G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130:1639–1648. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 29.Reeves HL, Zaki MY, Day CP. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. Dig Dis Sci. 2016;61:1234–1245. doi: 10.1007/s10620-016-4085-6. [DOI] [PubMed] [Google Scholar]
- 30.Mahady SE, George J. The future liver of the Asia pacific: fatter and firmer from more fructose and fortune? J Clin Exp Hepatol. 2013;3:106–113. doi: 10.1016/j.jceh.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziraba AK, Fotso JC, Ochako R. Overweight and obesity in urban Africa: A problem of the rich or the poor? BMC Public Health. 2009;9:465. doi: 10.1186/1471-2458-9-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kengne AP, Echouffo-Tcheugui JB, Sobngwi E, Mbanya JC. New insights on diabetes mellitus and obesity in Africa-part 1: prevalence, pathogenesis and comorbidities. Heart. 2013;99:979–983. doi: 10.1136/heartjnl-2012-303316. [DOI] [PubMed] [Google Scholar]
- 33.Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S2–9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- 34.Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 35.International Agency for Research on Cancer (IARC) Monographs on the evaluation of carinogenic risks to humans. Volume 59, Hepatitis viruses. Lyon: IARC; 1994. [Google Scholar]
- 36.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 37.Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48:148–162. doi: 10.1016/j.jhep.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 38.McCall C. Australia commits funds to curb hepatitis C epidemic. Lancet. 2016;387:p419. doi: 10.1016/S0140-6736(16)00212-9. [DOI] [PubMed] [Google Scholar]
- 39.Sievert W, Razavi H, Estes C, Thompson AJ, Zekry A, Roberts SK, Dore GJ. Enhanced antiviral treatment efficacy and uptake in preventing the rising burden of hepatitis C-related liver disease and costs in Australia. J Gastroenterol Hepatol. 2014;29(Suppl 1):1–9. doi: 10.1111/jgh.12677. [DOI] [PubMed] [Google Scholar]
- 40.Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727–733. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Foster GR, Irving WL, Cheung MC, Walker AJ, Hudson BE, Verma S, McLauchlan J, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224–1231. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 42.Ravi S, Axley P, Jones D, Kodali S, Simpson H, McGuire BM, Singal AK. Unusually High Rates of Hepatocellular Carcinoma After Treatment With Direct-Acting Antiviral Therapy for Hepatitis C Related Cirrhosis. Gastroenterology. 2017;152:911–912. doi: 10.1053/j.gastro.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Virlogeux V, Pradat P, Hartig-Lavie K, Bailly F, Maynard M, Ouziel G, Poinsot D, et al. Direct-acting antiviral therapy decreases hepatocellular carcinoma recurrence rate in cirrhotic patients with chronic hepatitis C. Liver Int Liver Int. 2017 Apr 19; doi: 10.1111/liv.13456. [Epub ahead of print] 2017. [DOI] [PubMed] [Google Scholar]
- 44.International Agency for Research on Cancer (IARC) “Aflatoxin” in Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. Lyon: IARC; 1993. [Google Scholar]
- 45.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 46.Palliyaguru DL, Wu F. Global geographical overlap of aflatoxin and hepatitis C: controlling risk factors for liver cancer worldwide. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30:534–540. doi: 10.1080/19440049.2012.751630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groopman JD, Kensler TW, Wild CP. Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Annu Rev Public Health. 2008;29:187–203. doi: 10.1146/annurev.publhealth.29.020907.090859. [DOI] [PubMed] [Google Scholar]
- 48.Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80:1106–1122. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- 49.Kuang SY, Lekawanvijit S, Maneekarn N, Thongsawat S, Brodovicz K, Nelson K, Groopman JD. Hepatitis B 1762T/1764A mutations, hepatitis C infection, and codon 249 p53 mutations in hepatocellular carcinomas from Thailand. Cancer Epidemiol Biomarkers Prev. 2005;14:380–384. doi: 10.1158/1055-9965.EPI-04-0380. [DOI] [PubMed] [Google Scholar]
- 50.Schutze M, Boeing H, Pischon T, Rehm J, Kehoe T, Gmel G, Olsen A, et al. Alcohol attributable burden of incidence of cancer in eight European countries based on results from prospective cohort study. BMJ. 2011;342:d1584. doi: 10.1136/bmj.d1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allamani A, Voller F, Decarli A, Casotto V, Pantzer K, Anderson P, Gual A, et al. Contextual determinants of alcohol consumption changes and preventive alcohol policies: a 12-country European study in progress. Subst Use Misuse. 2011;46:1288–1303. doi: 10.3109/10826084.2011.572942. [DOI] [PubMed] [Google Scholar]
- 52.Cogordan C, Kreft-Jais C, Guillemont J. Effects of alcoholic beverage control policies and contextual factors on alcohol consumption and its related harms in France from 1960 to 2000. Subst Use Misuse. 2014;49:1633–1645. doi: 10.3109/10826084.2014.913391. [DOI] [PubMed] [Google Scholar]
- 53.Dorak MT, Karpuzoglu E. Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet. 2012;3:268. doi: 10.3389/fgene.2012.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.