Abstract
The nucleus pulposus (NP) of intervertebral discs (IVD) undergoes dramatic changes with aging including loss of its gelatinous structure and large, vacuolated notochordal cells (NCs) in favor of a matrix-rich structure populated by small NP cells (sNPCs). NP maturation also involves a loading-pattern shift from pressurization to matrix deformations, and these events are thought to predispose to degeneration. Little is known of the triggering events and cellular alterations involved with NP maturation, which remains a fundamental open spinal mechanobiology question. A mouse IVD organ culture model was used to test the hypotheses that hyperosmotic overloading will induce NP maturation with transition of NCs to sNPCs while also increasing matrix accumulation and altering osmoregulatory and mechanotransductive proteins. Results indicated that static hyperosmolarity, as might occur during growth, caused maturation of NCs to sNPCs and involved a cellular differentiation process since known NC markers (cytokeratin-8, -19 and sonic hedgehog) persisted without increased cell apoptosis. Osmosensitive channels Aquaporin 3 (Aqp3) and transient receptor potential vanilloid-4 (TRPV4) expression were both modified with altered osmolarity, but increased Aqp3 with hyperosmolarity was associated with NC to sNPC differentiation. NC to sNPC differentiation was accompanied by a shift in cellular mechanotransduction proteins with decreased N-cadherin adhesions and increased Connexin 43 connexons. We conclude that hyperosmotic overloading can promote NC differentiation into sNPCs. This study identified osmolarity as a triggering mechanism for notochordal cell differentiation with associated shifts in osmoregulatory and mechanotransductive proteins that are likely to play important roles in intervertebral disc aging.
Keywords: Intervertebral disc, Nucleus pulposus, Notochordal cell, Osmolarity, Mechanobiology
INTRODUCTION
Low back pain is the leading cause of global disability and is commonly associated with intervertebral disc (IVD) degeneration1; 2. The transition of the nucleus pulposus (NP) from a gelatinous structure populated with large vacuolated notochordal cells (NCs) to a more fibrous matrix-rich structure populated with small NP cells (sNPCs) is among the earliest changes in human IVDs3; 4. The young, healthy NP mainly contains a continuous and rich network of large, vacuolated NCs with little extracellular matrix material5, with lineage tracing studies indicating that these cells are remnants of the embryonic notochord6; 7. The mature NP is predominantly comprised of small, non-vacuolated sNPCs4; 8 that exist as discrete cells or cell clusters surrounded by a denser matrix5. The loss of NCs has long been thought to predispose the IVD to degeneration since it was observed that chondrodystrophic dogs (which lose NCs early in life) are afflicted by age-related IVD degeneration while non-chondrodystrophic dogs (which retain their NCs) rarely develop IVD degeneration4; 9.
There is relatively little known of the mechanobiological triggering mechanisms involved in this transition from NC-rich NP with gelatinous structure to a sNPC-rich NP with denser matrix. While the physicochemical causes for NC to sNPC transition are unknown, reduced nutrient supply and mechanical overloading are implicated10–12. Mechanical loading remains a more likely candidate since porcine NP explants and rabbit IVDs with ample nutrient supply underwent NC to sNPC transition with glycosaminoglycan (GAG) accumulation following exposure to high magnitudes of hydrostatic pressure or mechanical compression11; 12. Osmotic loading is likely to be important in NC to sNPC transition since osmolarity is altered under both mechanical overloading conditions and from GAG accumulation. NP cells experience varying osmotic conditions as loading changes during diurnal cycles, exercise, and aging. Altered IVD osmolarity in mature human, bovine and rabbit IVDs and IVD cells also induced temporal changes in matrix molecule gene expression and affected the cellular response to mechanical stimuli13; 14. Additionally, nuclear transcription factor tonicity enhancer-binding protein (TonEBP), an essential osmoregulatory factor, is expressed in IVD cells further suggesting osmosensitivity of NP cells15. While osmolarity can influence NP cell behavior, it is unknown if alterations in osmolarity can induce a transition of NCs to sNPCs, and whether this transition can affect osmoregulatory proteins and cell communication patterns.
Osmotically-regulated proteins likely to be important in the IVD include calcium channel transient receptor potential vanilloid-4 (TRPV4) and water channels Aquaporins 1 and 3 (Aqp 1 and 3). TRPV4 is a nonselective calcium- (Ca2+) permeable ion channel that triggers K+ and Cl− channel activation and Ca2+-dependent Ca2+ release from intracellular stores. TRPV4 has been postulated as the osmosensor, playing a significant role in the cell volume regulation since it is expressed in tissues that experience large shifts in osmolarity like the kidney, cochlea, skin, and brain16,17. TRPV4 activation has also been associated with upregulation of metabolic processes and the suppression of catabolic processes in chondrocytes18; 19, suggesting that TRPV4 could play an important role in NP cell differentiation. Aqps are a family of osmosensitive transmembrane proteins ubiquitously expressed throughout the body, including the IVD, and can control intracellular water equilibrium and cell volume under hyperosmolar and hypoosmolar conditions by selectively shuttling water and certain solutes in and out of cells20–22. Aqp1 and 3 expressions have been shown to decrease with IVD degeneration and aging23–25. The loss of the juvenile NC phenotype was recently reported to involve a loss of n-cadherin expression (N-cad)26; 27, suggesting that mechanotransduction by direct cell-to-cell connectivity is an essential feature in the NC phenotype. NCs also stain positively for the transmembrane protein Connexin-43 (Cx-43)27; 28, indicating that mechanotransduction and intercellular communication via functional gap junctions is an important functional phenotype of NC cells.
The purpose of this study was to investigate osmosensitive processes involved in the maturation of the NP and transition of NCs to sNPCs. Identifying the triggering mechanisms of NP cell maturation and the associated regulatory mechanisms will add to the fundamental understanding of IVD biology and may reveal therapeutic targets to slow IVD aging or prevent degeneration. A mouse IVD organ culture model was used to investigate how osmotic conditions and durations affected: 1) NC to sNPC transition; 2) NP matrix content; 3) NC markers and apoptosis; 4) osmosensitive protein expression for TRPV4, Aqp1 & Aqp3; and 5) mechanotransduction protein expression of N-cad and Cx-43. Hypothesis one was that excessive hyperosmotic conditions would result in NC to sNPC differentiation with reduced cell size and sustained NC marker expression without increased apoptosis. Hypothesis two was that NC to sNPC differentiation would be associated with alterations in osmosensitive protein expression for TRPV4, Apq1 & Aqp3. Hypothesis three was that osmolarity-dependent NC to sNPC differentiation would also involve altered mechanotransduction mechanisms with a loss of N-cad expression and an increase in Cx-43 expression. A juvenile human IVD was analyzed histologically to aid in contextualization of mouse studies to the human condition.
MATERIALS AND METHODS
Ethical Statement
Tissue samples were obtained via an Institutional Animal Care and Use Committee approved protocol. Mouse-tail IVD organ culture specimens did not require use of live animal testing.
Subjects and Tissue Collection
A total of 96 Coccygeal vertebra-IVD-vertebra motion segments from sixteen normally-developing and healthy 12-week old female C57BL/6 mice were dissected and cultured for a 14-day osmotic loading organ culture experiment (Fig. 1). Vertebra-IVD-vertebra segments (i.e., 6 IVD units caudal 2–3 through caudal 7–8) were dissected within 10 minutes of death, immediately sterilized, and randomly placed in isosmotic media (330mOsm/L) for a 3-day stabilization period before being subjected to multiple osmotic loading conditions for an 11-day osmotic application period. Motion segments were cultured under free swelling conditions without mechanical loading.
Figure 1. Study Design.
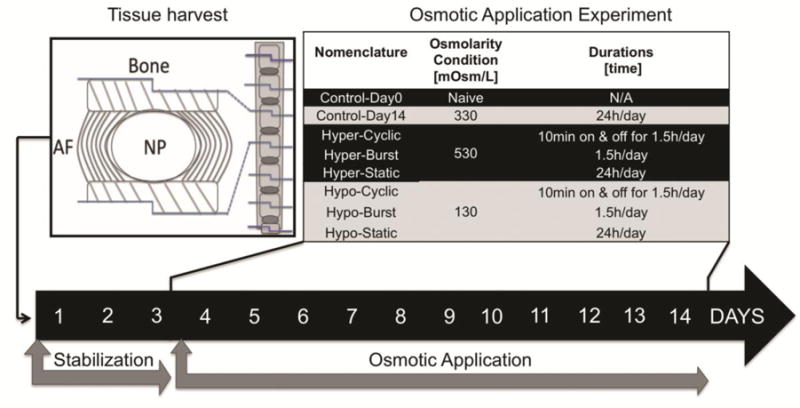
Six coccygeal IVD motion segments from 12-week-old mice were harvested and used for a 14-day organ culture experiment assessing the effects of osmolarity on the NP phenotype. An initial 3-day stabilization step was implemented for all samples to reach a steady-state condition after dissection, ensuring equal initial conditions. The following 11-day osmotic application step involved two osmolarity conditions: hyperosmolarity (Hyper) and hypoosmolarity (Hypo); and three durations: Cyclic, Burst and Static. Day 0 (Control-Day0) and time-matched (Control-Day14) controls were used. Culturing conditions included daily media changes for similar nutrition levels per group.
Organ Culture Model
The organ culture system consisted of a 48-well plate incubated at 37 C° with 5% CO2, and 21% O2. 21% O2 was used to provide NP cells with ample O2 to avoid confounding the effects of media osmolarity with hypoxia during culture since NCs are known to consume more O2 than sNPCs10 and since O2 levels in IVD tissues are known to be substantially lower than levels in the media (or vasculature) while in organ culture. Two control groups included a naïve control harvested prior to the start of the culture experiment (Control-Day0, n=8 IVDs), and a time-matched control culture in isoosmolar conditions for 14-days (Control-Day14, n=8 IVDs) (Fig. 1). All other motion segments were stabilized for 3 days in isoosmolar control media (DMEM+F-12K (50:50), 10% FBS, 1% pen-strep and 0.2% AA; 330 mOsm/L), and then cultured for another 11-days under hypoosmotic (Hypo, 130 mOsm/L) or hyperosmotic (Hyper, 530 mOsm/L) conditions using three different durations: Cyclic (10 minutes on and off for 1.5h/day, Hyper-Cyclic, and Hypo-Cyclic, n=8–12 IVDs/group), Burst (1.5h/day, Hyper-Burst and Hypo-Burst, n=8–12 IVDs/group) and Static (24h/day, Hyper-Static, and Hypo-Static, n=8–12 IVDs/group) for a total of 14 days in culture (Fig. 1). Media were quickly changed using pipets; static media were also changed daily to ensure nutrient conservation between groups and to account for any effect of perturbing the motion segments with the pipet. Hypoosmolar media was generated by dilution of isoosmolar media with sterile deionized water. Hyperosmolar media was generated by the addition of sucrose (S6-500, Fisher Scientific, PA, USA) to the hyposmolar media, allowing for nutrient conservation between groups. Media osmolality was assessed by freezing point osmometry and converted to osmolarity for further analysis.
Histology and Immunohistochemistry
After culture, vertebra-IVD-vertebra segments were fixed in buffered zinc formalin, decalcified in buffered formic acid, and embedded in paraffin wax. 5μm thick sections were cut (RM2165, Leica Biosystems, IL, USA) and stained with hematoxylin and eosin (H&E) for assessment of cell morphology and Saf-O/LG for assessment of GAG and COL content. An 8-month-old human L4/5 IVD section was also stained with Saf-O/LG to compare morphology between human and mouse. Separate experimental mouse sections were treated with Proteinase-K for antigen retrieval (S3020, Agilent, CA, USA) then immunostained with TRPV4 (1:500, ab39260, Abcam, MA, USA), Aqp1 (1:500, ab9566, Abcam, MA, USA), and Aqp3 (1ug/ml, ab125219, Abcam, MA, USA) primary antibodies. Sections undergoing the largest differences (Hyper-Static, Hypo-Static groups, and both controls) were further evaluated with immunostaining for notochordal markers and mechanotransduction proteins. Notochordal makers included cytokeratin 8 (Ck8, 1:1000, ab53280, Abcam, MA, USA), cytokeratin 19 (Ck19, 1:200, ab52625, Abcam, MA, USA), and sonic hedgehog (Shh 1:100, NBP2-22139, Novus Biologicals, CO, USA). Mechanotransduction makers included N-cadherin (N-cad, 1:600, ab76011, Abcam, MA, USA) cell-cell adhesions and Connexin-43 gap junctions (Cx-43, 1:2000, ab11370, Abcam, MA, USA). Anti-rabbit Ig (MP-7401, Vector, CA, USA) secondary antibody was used with all primary antibodies. Immunostaining protocols used positive control tissue for adequate protein expression. The omission of the primary antibody was used as a negative control, and toluidine blue was used as a counterstain.
Imaging, and Matrix and Protein Expression Quantification
Mosaic images of the whole NP were captured at 40× magnification, with identical brightness intensity and exposure times, using a computerized upright bright-field microscope (ImagerZ1, Zeiss, Germany). Image analysis was performed using a custom ImageJ macro implementing the Univectorselection and Color Deconvolution plugin functions (V1.5, NIH, USA) to assess matrix composition and expression levels of Saf-O/LG and all antibodies. Precise color channel absorption vectors were determined from single-colored stain sections. The deconvolution vectors representing the colors of interest were imported into Cell Profiler software (V2.1.1, NIH, NSF and Broad Institute, USA) for automated analysis. A custom pipe-line was implemented in Cell Profiler, which segmented, cleaned and then quantified mean intensity levels of each stain.
NP maturation, NC to sNPC transition Scale and Matrix Content
A semi-quantitative grading scale from 1 to 5 was developed to define the relative proportion of NCs and sNPCs, with 1 representing all sNPC and 5 representing all NC cells (Supplemental Fig. 1). H&E, and Safranin-O/Light Green stains were used to characterize NC to sNPC transition by assessment of cell morphology and matrix characteristics. NCs are large, vacuolated cells with continuous cell-cell connectivity. sNPCs are small cells without vacuoles surrounded by matrix in discrete form or cell clusters4; 5; 9; 29. NP tissue that was predominantly cellular with low matrix material was indicative of NC phenotype while substantial matrix accumulation was more indicative of sNPC phenotype.
Apoptosis and characterization of notochordal cell lineage
To identify whether NCs and sNPCs were derived from similar notochord lineage, sections were stained with notochordal markers Ck8, Ck19 and Shh. Expression of NC markers was evaluated qualitatively for Hypo-Static and Hyper-Static groups which exhibited largest effects, and for both control groups. TUNEL assay (DeadEnd, TB235, Promega, USA) was also performed to evaluate apoptosis across groups. Positive control sections were created using DNase enzyme to adequately assess presence of apoptosis by DNA fragmentation. Fluorescein intensity (marker of DNA fragmentation level) above 20% of positive control intensity indicated apoptosis on the experimental groups. Continuous expression of NC markers Ck8, Ck19, and Shh in sNPC without evidence for apoptosis was considered as preservation of the NC lineage.
Statistics
Gaussian distribution was assessed via a D’Agostino-Pearson omnibus normality test (Prism6, GraphPad, CA, USA). A Two-way ANOVA with p<0.05 was used to assess the effects of duration and condition with possible interactions. For mechanotransduction proteins, One-way ANOVA was used. Tukey post-hoc test was used to assess individual variances against naïve Control-Day0 (baseline) and Control-Day14 (time matched culture control).
Correlations and Regressions
To identify the most significant and influential matrix and osmosensitive markers defining NC to sNPC transition, a quadratic step-wise regression analysis was used based on variance criteria (inclusion, p<0.05, and exclusion p>0.05) using Matlab software (R2015a, Mathworks, MA, USA). Linear correlations were then assessed via the least-square method to identify possible associations of NC to sNPC transition with matrix content (GAG and COL stain intensity), osmosensitive markers expression (TRPV4 and Aqp1and3) and mechanotransduction proteins (N-cad and Cx-43), while considering the combined effects of both condition and duration, and individual effects of condition or duration (Prism 6, GraphPad, CA, USA). Assessment of condition or duration was accomplished by averaging the effects of one variable while keeping the other constant.
RESULTS
Hyperosmolarity-Dependent Cell (NC to sNPC transition) and Matrix Maturation
H&E and Saf-O/LG staining demonstrated most cells maintained NC characteristics in all experimental groups except for the Hyper-Static group. The Hyper-Static group demonstrated rich sNPCs characteristics (small, non-vacuolated cells, separated by matrix to form discrete cells and cell-clusters; Fig. 2A, 2C, and Supplemental Fig. 2) and was similar morphologically to the juvenile human NP (Fig. 2D). Two-way ANOVA analysis indicated that interactions between duration and conditions were significant sources of variation for NC to sNPC transition (p<0.03). Hyper-Static and Hyper-Burst conditions increased GAG content, while GAG stain intensity was unchanged from baseline in all other conditions (Fig. 2B). The NP matrix content (GAG and COL) did not change due to culture conditions (p>0.05, Control-Day0 and Control-Day14), and COL stain intensity was not affected by changes to osmolarity condition or duration (data not shown). Two-way ANOVA analysis indicated that condition, and not duration, was a significant source of variation for GAG content (p<0.001) without significant interactions (p>0.05). Accordingly, the Hyper-Static group induced NC to sNPC transition (p<0.05, compared to Control-Day0, Fig. 2A and Supplemental Fig. 1) while all other groups maintained predominantly NC characteristics similar to both control groups (p>0.05, against Control-Day0 and against Control-Day14). Linear correlations indicate that GAG stain intensity was moderately related to NC to sNPC transition when considering the combined effects of condition and duration (Table 1, R2=0.43; p=0.08). Similarly, stepwise regression analysis indicated that GAG alone was the most and only significant factor associated with NC to sNPC transition (Table 1, p<0.05).
Figure 2. Hyper-Static osmolarity induced NC to sNPC transition and increased GAG staining.
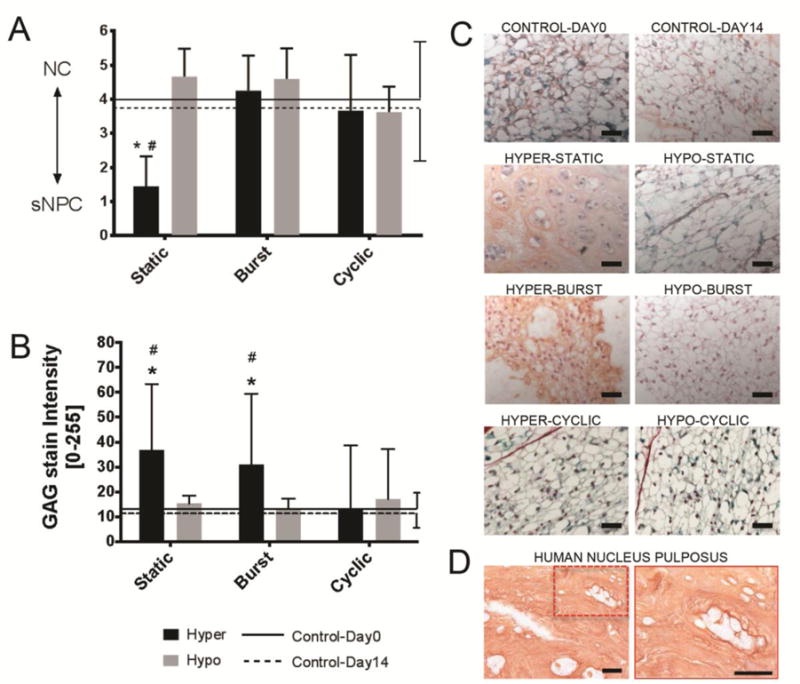
Bar plots (mean ± SD) indicating A) NC to sNPC transition and B) GAG (Saf-O) matrix staining intensity as a function of osmolarity condition and duration with comparisons against Day0 and Day14 control groups. Lower y-axis values indicate a higher proportion of sNPCs. Two-way ANOVA analysis indicated that neither duration nor conditions were significant sources of variation for NC to sNPC transition; moreover, a significant interaction was present between variables (p<0.03). Also, Two-way ANOVA analysis indicated that condition, and not duration, was a significant source of variation for GAG content (p<0.001) without significant interactions. Tukey multiple comparison posthoc test. * = p<0.05 against Control-Day0 and # = p<0.05 against Control-Day14. Presented images are Safranin-O/Light Green (Saf-O/LG) staining depicting the C) mouse and D) human nucleus pulposus region of interest. NP cell morphology was identified with high magnification (40×) images of collagen and GAG staining. NC morphology involved large cell size and presence of vacuoles while sNPC morphology involved small cells size, lack of vacuoles and accumulation of matrix between discrete cells or cell-clusters. Hyper-Static induced NC to sNPC transition with reduced cell size, loss of vacuoles and accumulation of matrix while although all other conditions retained the NC phenotype. Scale bar 50um.
Table 1.
Correlations analysis.
| GAG | COL | ||
|---|---|---|---|
| Effects of condition and duration | |||
| NC➔sNPC | 0.43 (0.08) | 0.01 (0.85) | |
| Effects of condition only | |||
| NC➔sNPC | 0.66 (0.19) | 0.08 (0.73) | |
| Effects of duration only | |||
| NC➔sNPC | 0.11 (0.60) | 0.12 (0.58) | |
| Step-Wise | NC to sNPC =4.3-0.03*GAG, R2=0.1; p<0.05 | ||
R2 values between cell phenotype transition with and matrix and phenotypic markers (p-value). Bold = p<0.10
Osmolarity-Dependent Differentiation and Apoptosis
All three measured notochordal markers (Ck8, Ck19, and SHH) were expressed in both Hyper-Static and Hypo-Static groups and in both Controls, indicating preservation of notochordal lineage (Fig. 3). Additionally, there was no evidence for increased apoptosis compared to baseline with TUNEL staining in any of the groups (Supplemental Fig. 3).
Figure 3. Immunostaining for NC phenotypic markers persisted in all NC and sNPCs across loading groups.
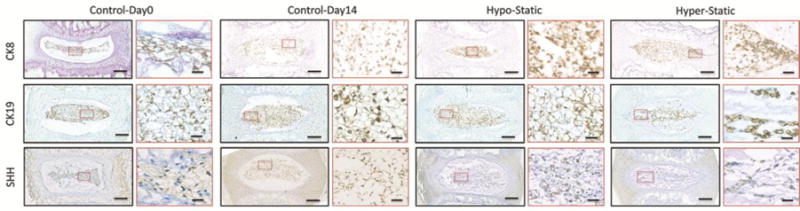
The Hyper-Static samples, which had predominantly NCs are compared with Control-Day0, Control-Day14 and Hypo-Static groups which had predominantly sNPCs. Expression for known NC-phenotypic markers cytokeratin 8 (Ck8), cytokeratin 19 (Ck19), and sonic hedgehog (Shh) persisted in all NP cells. Images shown for groups exhibiting largest cell morphological changes to NP cells. Scale bar 100um and 20um.
Osmolarity-Dependent Expression of TRPV4 and Aqps 1&3
TRPV4 expression was maintained in all conditions and durations except for the Hyper-Cyclic group (Fig. 4A) where a significant reduction was found. Two-way ANOVA analysis indicated that condition and duration were significant sources of variation (p<0.05) without significant interaction present between variables (p<0.05). Aqp1 expression did not change with osmolarity condition or duration (Fig. 4B). Two-way ANOVA analysis indicated that neither condition nor duration were significant sources of variation for Aqp1 expression (p<0.001) with non-significant interactions between variables (p<0.05). On the other hand, Aqp3 expression was significantly increased in hyperosmolar conditions but maintained in hypoosmolar conditions, independent of duration. Moreover, Aqp3 decreased in Cyclic groups independent of condition (Fig. 4C). Two-way ANOVA analysis indicated that both condition and duration were significant sources of variation (p<0.004) with significant interactions between both variables (p<0.001). Linear regressions did not identify any significant associations between osmosensitive proteins and NC to sNPC transition (Table 2). However, step-wise regression analysis identified Aqp3 to be the most and only significant factor defining NC to sNPC transition (p<0.05). Further analysis indicated that Apq3 influence on cell phenotype was mainly driven by osmolarity condition and not duration (Table 2, R2=0.84; p<0.05) indicating the importance of overloading NC to sNPC transition. Linear regression and step-wise regression analysis indicated that GAG and COL expression were not significantly associated with osmosensitive proteins when considering any condition and duration (Table 3).
Figure 4. Aqp3 expression was most sensitive to changes in osmotic conditions.
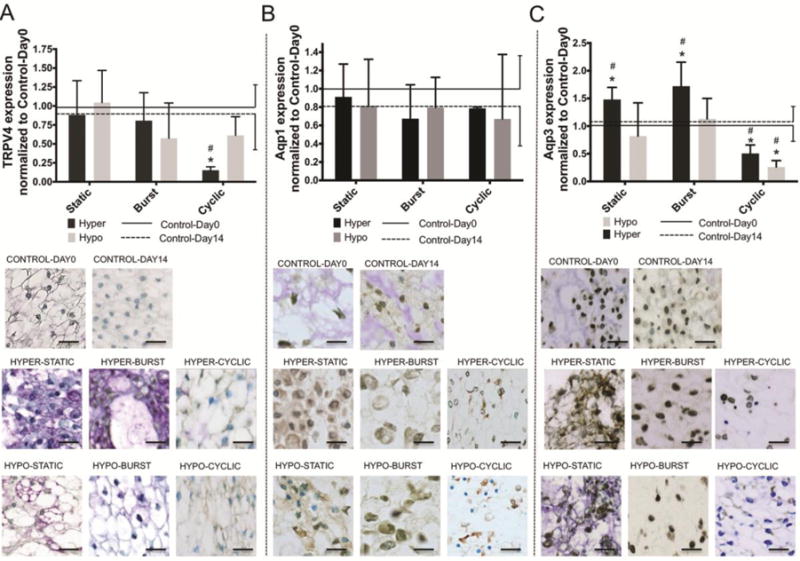
Bar plot (mean ± SD) of nucleus pulposus osmoregulatory proteins A) TRPV4, B) Aqp1 and C) Aqp3 normalized to Control-Day0 as a function of osmolarity condition and duration. Aqp3 expression increased under Static and Burst hyperosmolar conditions; TRPV4 and Aqp3 decreased under Cyclic conditions; however, Aqp1 was unaltered. TRPV4 Two-way ANOVA analysis indicated that condition and duration were significant sources of variation (p<0.05) without significant interaction present between variables. Also, Two-way ANOVA analysis indicated that neither condition nor duration were significant sources of variation for Aqp1 expression (p<0.001) without significant interactions. Finally, Aqp3 Two-way ANOVA analysis indicated that condition and duration were significant source of variation (p<0.004) with significant interactions between variable (p<0.001). Tukey multiple comparisons Posthoc test. * = p<0.05 against Control-Day0 and # = p<0.05 against Control-Day14. Scale bar 25um.
Table 2.
Correlations analysis.
| TRPV4 | Aqp1 | Aqp3 | |
|---|---|---|---|
| Effects of condition and duration | |||
| NC➔sNPC | 0.0001 (0.98) | 0.10 (0.45) | 0.02 (0.76) |
| Effects of condition only | |||
| NC➔sNPC | 0.33 (0.43) | 0.02 (0.84) | 0.84 (0.08) |
| Effects of duration only | |||
| NC➔sNPC | 0.03 (0.78) | 0.02 (0.80) | 0.14 (0.53) |
| Step-Wise | NC to sNP=4.99+1.68*Apq3+0.44*(Aqp3)2, R2=0.1; p<0.05 | ||
R2 values between cell phenotype and osmoregulatory protein expression (p-value). Bold = p<0.10
Table 3.
Correlation analysis.
| GAG | COL | |
|---|---|---|
| Effects of condition and duration | ||
| TRPV4 | 0.06 (0.57) | 0.01 (0.81) |
| Aqp1 | 0.003 (0.89) | 0.10 (0.45) |
| Aqp3 | 0.39 (0.10) | 0.47 (0.06) |
| Effects of condition only | ||
| TRPV4 | 0.69 (0.17) | 0.72 (0.15) |
| Aqp1 | 0.11 (0.66) | 0.99 (0.01) |
| Aqp3 | 0.28 (0.47) | 0.001 (0.99) |
| Effects of duration only | ||
| TRPV4 | 0.01 (0.85) | 0.001 (0.96) |
| Aqp1 | 0.03 (0.78) | 0.20 (0.46) |
| Aqp3 | 0.18 (0.48) | 0.20 (0.45) |
R2 between matrix phenotype and osmoregulatory proteins expression (p-value). Bold means p<0.10
Osmolarity-Dependent Changes in Mechanotransduction
N-cad and Cx-43 were expressed in both Control-Day0 and Control-Day14 groups and in both Hyper-Static and Hypo-Static groups. Under Static Hyperosmolar conditions, N-cad was significantly downregulated and Cx-43 was significantly upregulated (Fig. 5). Condition and duration were significant sources of variation for N-cad expression (p<0.05) with significant interaction present between variables (P<0.05). On the other hand, condition, not duration, trended toward significant sources of variation for Cx-43 expression (Table 4, p=0.09) with significant interaction present between variables (p<0.05).
Figure 5. NCs express greater N-cad while sNPCs expressed greater Cx-43.
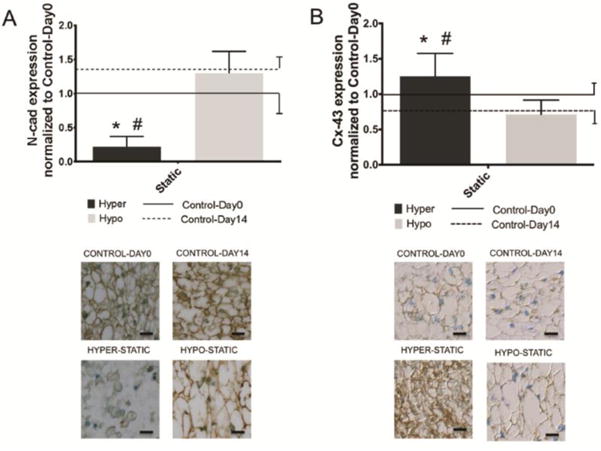
Bar plots (mean ± SD) for A) N-cad and B) Cx-43 normalized to Control-Day0 as a function of osmolarity condition and duration. Results analyzed for the osmotic loading groups with the largest changes in NP cell morphology. Also indicated are immunostaining representative images of N-cad and Cx-43. N-cad Two-way ANOVA analysis indicated that condition and duration were significant sources of variation (p<0.05) with significant interaction present between variables (P<0.05). Also, Cx-43 Two-way ANOVA analysis indicated that condition, not duration, was significant sources of variation (p<0.05) with significant interaction present between variables (p<0.05). Tukey Posthoc test. * = p<0.05 against Control-Day0 and # = p<0.05 against Control-Day14.
Table 4.
Correlations analysis.
| N-cad | Cx-43 | |
|---|---|---|
| Effects of condition and duration | ||
| NC➔sNPC | 0.85 (0.01) | 0.56 (0.09) |
| Effects of condition only | ||
| NC➔sNPC | 0.65 (0.20) | 0.65 (0.20) |
| Effects of duration only | ||
| NC➔sNPC | 0.43 (0.34) | 0.45 (0.33) |
R2 values between cell phenotype and osmoregulatory protein expression (p-value). Bold = p<0.10
DISCUSSION
The loss of the highly gelatinous NC-rich NP has long been considered a key change in IVD biology. This open mechanobiology question is critically important because NCs are considered signaling centers that orchestrate IVD growth and maintenance and a source of NP progenitor cells30–33. NCs and their soluble factors may also prevent painful IVD degeneration by stimulating GAG production in mature sNPCs and mesenchymal stem cells32; 34–37, by preventing apoptosis, and/or by inhibiting neurite cell growth and angiogenesis36; 38–40. This work used a mouse whole IVD organ culture model and determined that static hyperosmotic loading was capable of inducing a transition of NCs to sNPCs and identified important osmoregulatory and mechanotransduction proteins involved in this process (Fig. 6). The transition of NCs to sNPCs occurred via differentiation and not by replacement with an external cell source since notochordal markers persisted in sNPCs and there was no evidence for increased apoptosis in any group. No changes in apoptosis were evident, which also suggests that the culture conditions did not have a significant negative effect on cell viability. Aqp3 and TRPV4 were both affected by osmolarity changes indicating their role in NP cell osmoregulation, but only Aqp3 expression was significantly associated with NC to sNPC transition, indicating a potential osmoregulatory role for Aqp3 in this differentiation process. N-cad and Cx-43 were also affected by osmolarity. The Hyper-Static group which had increased sNPCs had increased expression of Cx-43 connexons while other groups which retained NCs had increased expression of N-cad.
Figure 6. Conceptual model detailing osmolarity-dependent NC to sNPC differentiation.
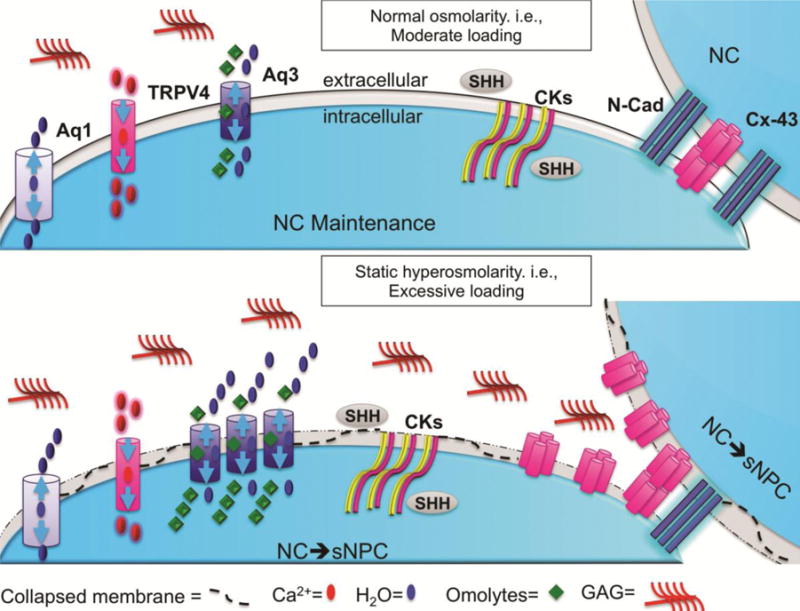
Under normal conditions, cells can maintain a healthy cell volume by adjusting expression (number of channels) and activation (how long channel is open) of ion and water channels. TRPV4 adjusts intracellular Ca2+ while Aqp 1 and 3 adjusts intracellular water/osmolytes to maintain cell volume in response to osmotic gradients. Under long-term static hyperosmolarity conditions, resulted in differentiation of NCs to sNPCs with increased extracellular matrix GAG accumulation. During this transition of NCs to sNPCs, Aqp3 expression was increased, while Aqp 1 and TRPV4 remained constant. Under all osmolarity conditions, all NP cells expressed sustained expression of notochordal markers CK8, Ck19 and Shh, suggesting the transition of NCs to sNPCs was a differentiation process. Also shown are the changes in the NP mechanotransduction proteins with NP cell differentiation with NCs having greater expression of N-cad and sNPC having greater expression of Cx-43.
Static hyperosmotic conditions in this study induced transition from NCs to sNPCs and caused GAG accumulation implicating osmotic overloading as a key factor in NC differentiation. The literature demonstrates that multiple physicochemical changes can result in the loss of NCs in IVD tissue including nutritional deprivation caused by endplate sclerosis10, mechanical loading or overloading11; 12 and injury-induced sequelae culminating in a fibrocartilage phenotype41. These complex and multi-factorial processes that induce shifts from NCs to sNPCs can all influence GAG content or water content, so that osmolarity is also affected. NP vacuoles, a hallmark of NCs, have been proposed to act as osmometers themselves, as a bolus of hyperosmolar (550 mOsm/L) media reduced and hypoosmolar (210 mOsm/L) media increased canine NP cell vacuole volume42. Our results are consistent insofar as hyperosmotic conditions resulted in a reduction of NCs and their vacuoles. One prior study suggested hyperosmotic culture conditions (400 mOsm/L) preserved NC phenotype43, which contrasts with this study. These differences may be attributed to our greater hyperosmotic conditions (550 vs. 400 mOsm/L) or other differences in culture conditions (organ vs. cell culture, DMEM vs. α-MEM) which prevent a direct comparison.
This study precisely controlled osmolarity and osmotic load durations using a mouse organ culture model. A mouse organ culture model was used since mice retain NCs throughout their lifetime8 so that observed shifts in NP cells can be attributed to the applied osmolarity changes rather than aging. Osmotic conditions vary within the NP, as loading changes over aging, exercise, and the diurnal cycle. The loading durations were chosen to approximately represent different conditions experienced by humans. Static (24 hours/day) introduced sustained changes in IVD loading, intradiscal pressure, and intradiscal osmolarity, similar to what is experienced in growth, development, and aging. Burst (90 minutes/day) subjects the IVD to a brief daily period of sustained IVD loading to represent exercise. Cyclic (10 minutes on/off for 90 minutes/day) represents several brief periods of exertion akin to normal daily movement. Hyper-Static conditions, which subjected the IVD to a sustained hyperosmolar environment, induced NP cell differentiation. In humans, NCs disappear and are replaced by sNPCs within the first decade of life8, which is a period of rapid growth associated with increasing osmolarity due to increased GAG accumulation and muscle loading. As such, this NP cell differentiation is likely to be a healthy process associated with IVD during growth rather than a pathologic degenerative change. The mouse NP cells and tissues under static hyperosmolar conditions were similar to cell and tissue morphology observed for a young human NP. In both species, some NP cells still had vacuoles and the NP was still hyper cellular, which is likely associated with the young ages, the short-term mouse organ culture duration, and the lack of steady-state conditions in the growing human. In contrast, NP cells did not differentiate from NCs to sNPCs under Burst or Cyclic osmotic durations, which are more representative of diurnal or short-term loading cycles, suggesting NCs were able to maintain osmotic homeostasis under short-term changes in loading conditions. This is a simplified model system to highlight the importance of osmolarity for NP cell differentiation, and while changes were similar to a single human IVD, it is clear that human NP cells undergo complex processes during growth and aging spanning long time periods, and likely involve interactions with additional cell types in vivo.
The literature presents two well-established models that explain NC disappearance and replacement by sNPCs. First, using NC markers (e.g., brachyury, sonic hedgehog, cytokeratins) and lineage tracing studies, NCs are thought to differentiate into sNPCs10; 11; 29; 44–46; 6; 31; 45; 47,7,48. The second is a repopulation event where NCs die and are replaced with mesenchymally-derived chondrocytes that migrate through the cartilage endplate to form a fibrocartilaginous NP structure10; 11; 49–51. In order to determine if sNPCs present in the Hyper-Static condition were derived from NCs, we performed immunohistochemistry for three markers (Ck8, Ck19, and Shh) of the notochordal cell lineage52. Both NC & sNPC cell types expressed all three notochordal markers (Fig. 3) without evidence for increased apoptosis (Supplemental Fig. 3), indicating that both NCs and sNPCs are of notochordal origin with the observed NC to sNPC transition resulting from NC differentiation. The evidence for NCs differentiation into sNPCs is further supported by this study since hyperosmolar loading in our organ culture model avoids tissue damage or systemic circulation which excludes exogenous cell sources. NC differentiation is also supported by findings showing a rapid loss of NC phenotype in 3D cell culture48. While both NCs and sNPCs are of notochordal origin, our study is limited in that we did not measure proliferation markers to identify if there was selective proliferation of a distinct sub-population of NCs during Hyper-Static conditions, which warrants future investigation. While the term “chondrocyte-like cell” is common in the literature, we use sNPC to describe the same cells because we believe it better connotes the growing evidence that these cells are of notochordal origin without suggesting they are of chondrocytic origin. We note that injury, repair, and degeneration in humans and in vivo animal models are complicated processes likely to involve migration of exogenous cells in addition to sNPCs that are differentiated from NCs.
NC differentiation to sNPC was hypothesized to involve changes in expression of the osmosensitive channels TRPV4, Apq1 & Apq3. These three channels have distinct roles in intracellular osmoregulation and expression and activity of one channel can involve compensatory regulation by the other channels. We measured changes in channel expression, which is indicative of changes in the number of channels, although changes in the amount of activation can also affect the transport of osmolytes and water53. When the activity of existing channels does not adequately regulate intracellular osmolarity, cells can adapt by expressing more (or fewer) channels. Channel levels were generally maintained in conditions with lesser osmotic demands (e.g., hypoosmolar conditions), suggesting that these cells were able to effectively adapt to changes in osmolarity by transporting sufficient osmolytes/water by activating existing numbers of channels. Interestingly, Apq3 and TRPV4 expression levels were decreased under Cyclic conditions, suggesting relatively rapid changes in osmotic conditions may require fewer channels for maintaining homeostasis.
In this study, NC to sNPC differentiation was strongly correlated with increased Aqp3 expression, and TRPV4 and Aqp3 were both affected by osmotic changes and played osmoregulatory roles. Hyperosmolarity can result in cell shrinkage, membrane collapse, and increased osmotic stresses (Fig. 6). Increased Aqp3 expression appeared to be sufficient to maintain intracellular osmolarity without NC phenotypic changes during acute shifts in osmolarity (e.g., Burst-Hyper conditions up-regulated Aqp3 without NC differentiation). While Aqp3 expression increased under Hyper-Static conditions, this was considered overloading since it did not appear to be sufficient to maintain intracellular osmolarity and cellular differentiation occurred from NCs to sNPCs. The associated increase in GAG accumulation under this condition may be another compensatory cellular response to balance increased external hyperosmotic overloading with increased extracellular matrix fixed charge density. Our results suggest Aqp3 and TRPV4 may be important osmoregulators in the IVD, and Aqp3 is a possible osmoregulatory channel regulating NC differentiation to sNPCs. Previous studies have shown Aqp3 expression decreases with IVD aging and degeneration23–25. Thus, Aqp3 may be important for osmoregulation in the healthy, highly-hydrated IVDs with vacuolated NCs, and its function and therefore expression may be less important in degenerated or aged IVDs. The decrease may be due to reduced need for osmoregulation due to the known age-associated decrease in IVD water content or because of direct cellular changes that occur with aging and degeneration. Future mechanistic studies would help confirm the regulatory roles of these channels in NP cell maturation.
We hypothesized that osmolarity-dependent differentiation of NCs to sNPCs would involve adaptation of mechanotransduction mechanisms, and this differentiation was found to be associated with significantly decreased N-cad and a trend of increased Cx-43 (Table 4). N-cad and Cx-43 are both expressed in the IVD and have both been shown to be involved in mechanotransduction in various other tissues54; 55. Most osmotic conditions retained large NC clusters in a highly gelatinous matrix and high amounts of N-cad staining, which is likely to be similar to the soft substrates shown by Hwang et al., to be necessary for NC cluster formation26. Since N-cad interactions with β-catenin was a proposed mechanism regulating NP cell phenotype27, our results suggest that the loss of N-cad and increased GAG accumulation together result in an increased cell-matrix stiffness that contribute to loss of the NC phenotype.
The reduced N-cad connections of sNPCs likely required alternative modes of cell-cell communication, and our results suggested this compensation involved increased Cx-43 expression. Connexins are a family of structurally related trans-membrane proteins that allow passage of small molecules from one cell to another either by assembling to create gap-junctions for direct cell-to-cell communication or by functioning as standalone transmembrane channels, or connexons, that allow intercellular communications through processes in the pericellular space54. NP cell-cell communications are known to occur with functional Cx-43 positive gap junctions28, and we infer that the trend of increased Cx-43 expression in static hyperosmolar conditions suggested a shift towards intercellular standalone transmembrane connexon channels for communication in sNPCs.
The osmolarity of the culture media was carefully controlled, yet the osmotic environment of the NP was not directly controlled or measured. The NP may have spatiotemporal osmotic gradients upon rapid changes to the culture media, and NP osmolarity may not have fully equilibrated for cyclic or burst groups. Additionally, it is possible that sucrose did not diffuse fully into the IVD, which may have resulted in an intradiscal osmotic environment different from the culture media. However, the largest NP cell maturation effects were seen for Hyper-Static conditions, demonstrating the NP experienced changes in extracellular osmolarity compared to controls. Osmotic and hydrostatic pressure conditions interact for confined tissues56, and the NP may have experienced changes in intradiscal pressurization when exposed to hyper- or hypoosmolar conditions due to changes in NP swelling within the IVD, yet these hydrostatic pressures are expected to be relatively small for the isolated IVD organs without rigid confinement or loading from external musculature. A direct measurement of intradiscal osmolarities and pressures may help quantify the dose response of the observed effects, yet are challenging for small mouse IVD tissues and we believe unlikely to change the main findings that osmolarity influences NP cell maturation.
In conclusion, NCs are very sensitive to microenvironmental stimuli with static hyperosmotic overloading leading to NC to sNPC differentiation and GAG accumulation. Differentiation was implicated since NCs and sNPCs both expressed notochordal-specific markers without evidence for increased apoptosis. Apq3 was highly associated with NP cell differentiation for static conditions, and TRPV4 was another osmoregulatory protein associated with transition to a sNPC cell population in the NP. Finally, functional mechanotransduction changes during NC maturation involved reduced direct cell-cell N-cad-positive adhesions and increased Cx-43-positive connexins. We conclude that NP cells can adapt to osmolarity changes by altering osmoregulatory proteins, mechanotransduction proteins, and GAG synthesis, and that osmotic overloading can induce substantial phenotypic changes including differentiation from NCs to sNPCs.
Supplementary Material
Images of NP regions using H&E and Saf-O/LG were used to define the cell morphology and transition from NCs to sNPCs (NC➔sNPC) using a grading scale ranging from 1 to 5. Characteristic Grade 1, 3 & 5 images are provided with H&E and Saf-O/LG staining. Grade 5 samples were nearly all NCs with yellow arrows pointing at representative NC traits like large, vacuolated cells with continuous cell-cell-contact. Grade 1 samples were nearly all sNPCs with black arrows pointing at representative sNPC traits like small cells, surrounded by GAG-rich matrix as distinct cells or discrete cell-clusters. Grade 3 samples have mixed population of NCs and sNPCs. GAG accumulation was most obvious on Saf-O/LG staining since matrix on H&E was only lightly stained.
Hematoxylin and eosin (H&E) staining depicting the whole IVD with nucleus pulposus (box) region of interest. NP cell morphology was identified with high magnification images of H&E. NC phenotype was characterized by large cells and presence of vacuoles, and sNPC phenotype involved small cells in discrete cells or cell-clusters with lack of vacuoles and high accumulation of matrix. Hyper-Static induced NC to sNPC transition with all other conditions retaining NC phenotype. Scale bar 100um and 20um. * Indicates representative vacuoles.
TUNEL demonstrated no changes in number of cells undergoing apoptosis with changes in osmolarity over the study period of 14 days compared to Control-Day0 and Control-Day 14 groups. Quantification of Tunnel positive cells was carried out on Hyper- and Hypo-Static osmolarity groups for completion. Positive control involved DNase enzyme treated sections. Intense fluorescence indicated increased DNA fragmentation. Fluorescein intensity above 20% of the positive control intensity indicated apoptosis in the experimental groups. Representative images showing Dapi (Blue), Fluorescein (Red) and merged images for all groups.
Acknowledgments
We gratefully acknowledge Dr. Alice Huang and Kristen Howell for their mouse tissue-sharing program. Authors gratefully acknowledge the technical assistance of Dr. Allison Bean, Noelle Aly, Faraz Ahmed, and Marina Tadros for their support with acquisition and analysis of images and for editing the manuscript. This study was funded by NIH grants R01AR064157 and R01AR069315.
Footnotes
Author Contributions:
PP contributed to research design, acquisition, analysis and interpretation of data, and drafted the paper. TE contributed to acquisition, analysis and interpretation of data, and critical revisions. DML contributed to acquisition, analysis and interpretation of data. DP contributed to research design, analysis and interpretation of data, and critical revisions. JCI contributed to research design, analysis and interpretation of data, and critical revisions. All authors read and approved the final manuscript.
References
- 1.Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung KM, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 2009;34:934–940. doi: 10.1097/BRS.0b013e3181a01b3f. [DOI] [PubMed] [Google Scholar]
- 3.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 4.Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003;9:667–677. doi: 10.1089/107632703768247368. [DOI] [PubMed] [Google Scholar]
- 5.Hunter CJ, Matyas JR, Duncan NA. The three-dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. J Anat. 2003;202:279–291. doi: 10.1046/j.1469-7580.2003.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237:3953–3958. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCann MR, Tamplin OJ, Rossant J, et al. Tracing notochord-derived cells using a Noto-cre mouse: implications for intervertebral disc development. Dis Model Mech. 2012;5:73–82. doi: 10.1242/dmm.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205:357–362. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maldonado BA, Oegema TR., Jr Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992;10:677–690. doi: 10.1002/jor.1100100510. [DOI] [PubMed] [Google Scholar]
- 10.Guehring T, Wilde G, Sumner M, et al. Notochordal intervertebral disc cells: sensitivity to nutrient deprivation. Arthritis Rheum. 2009;60:1026–1034. doi: 10.1002/art.24407. [DOI] [PubMed] [Google Scholar]
- 11.Guehring T, Nerlich A, Kroeber M, et al. Sensitivity of notochordal disc cells to mechanical loading: an experimental animal study. Eur Spine J. 2010;19:113–121. doi: 10.1007/s00586-009-1217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purmessur D, Guterl CC, Cho SK, et al. Dynamic pressurization induces transition of notochordal cells to a mature phenotype while retaining production of important patterning ligands from development. Arthritis Res Ther. 2013;15:R122. doi: 10.1186/ar4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haschtmann D, Stoyanov JV, Ferguson SJ. Influence of diurnal hyperosmotic loading on the metabolism and matrix gene expression of a whole-organ intervertebral disc model. J Orthop Res. 2006;24:1957–1966. doi: 10.1002/jor.20243. [DOI] [PubMed] [Google Scholar]
- 14.Wuertz K, Urban JP, Klasen J, et al. Influence of extracellular osmolarity and mechanical stimulation on gene expression of intervertebral disc cells. J Orthop Res. 2007;25:1513–1522. doi: 10.1002/jor.20436. [DOI] [PubMed] [Google Scholar]
- 15.Tsai TT, Danielson KG, Guttapalli A, et al. TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J Biol Chem. 2006;281:25416–25424. doi: 10.1074/jbc.M601969200. [DOI] [PubMed] [Google Scholar]
- 16.Liedtke W, Choe Y, Marti-Renom MA, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilius B, Vriens J, Prenen J, et al. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol. 2004;286:C195–205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 18.O’Conor CJ, Leddy HA, Benefield HC, et al. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A. 2014;111:1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phan MN, Leddy HA, Votta BJ, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borgnia M, Nielsen S, Engel A, et al. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- 21.Richardson SM, Knowles R, Marples D, et al. Aquaporin expression in the human intervertebral disc. J Mol Histol. 2008;39:303–309. doi: 10.1007/s10735-008-9166-1. [DOI] [PubMed] [Google Scholar]
- 22.Day RE, Kitchen P, Owen DS, et al. Human aquaporins: regulators of transcellular water flow. Biochim Biophys Acta. 2014;1840:1492–1506. doi: 10.1016/j.bbagen.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 23.Li SB, Yang KS, Zhang YT. Expression of aquaporins 1 and 3 in degenerative tissue of the lumbar intervertebral disc. Genetics and molecular research : GMR. 2014;13:8225–8233. doi: 10.4238/2014.October.8.4. [DOI] [PubMed] [Google Scholar]
- 24.Johnson ZI, Gogate SS, Day R, et al. Aquaporin 1 and 5 expression decreases during human intervertebral disc degeneration: Novel HIF-1-mediated regulation of aquaporins in NP cells. Oncotarget. 2015;6:11945–11958. doi: 10.18632/oncotarget.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tas U, Cayli S, Inanir A, et al. Aquaporin-1 and aquaporin-3 expressions in the intervertebral disc of rats with aging. Balkan medical journal. 2012;29:349–353. doi: 10.5152/balkanmedj.2012.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang PY, Jing L, Chen J, et al. N-cadherin is Key to Expression of the Nucleus Pulposus Cell Phenotype under Selective Substrate Culture Conditions. Sci Rep. 2016;6:28038. doi: 10.1038/srep28038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang PY, Jing L, Michael KW, et al. N-Cadherin-Mediated Signaling Regulates Cell Phenotype for Nucleus Pulposus Cells of the Intervertebral Disc. Cell Mol Bioeng. 2015;8:51–62. doi: 10.1007/s12195-014-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter CJ, Matyas JR, Duncan NA. The functional significance of cell clusters in the notochordal nucleus pulposus: survival and signaling in the canine intervertebral disc. Spine (Phila Pa 1976) 2004;29:1099–1104. doi: 10.1097/00007632-200405150-00010. [DOI] [PubMed] [Google Scholar]
- 29.Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn. 2010;239:2141–2148. doi: 10.1002/dvdy.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henriksson H, Thornemo M, Karlsson C, et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine (Phila Pa 1976) 2009;34:2278–2287. doi: 10.1097/BRS.0b013e3181a95ad2. [DOI] [PubMed] [Google Scholar]
- 31.Risbud MV, Shapiro IM. Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit Rev Eukaryot Gene Expr. 2011;21:29–41. doi: 10.1615/critreveukargeneexpr.v21.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:129–137. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- 33.Souter WA, Taylor TK. Sulphated acid mucopolysaccharide metabolism in the rabbit intervertebral disc. J Bone Joint Surg Br. 1970;52:371–384. [PubMed] [Google Scholar]
- 34.Korecki CL, Taboas JM, Tuan RS, et al. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1:18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Vries SA, Doeselaar MV, Meij BP, et al. The Stimulatory Effect of Notochordal Cell-Conditioned Medium in a Nucleus Pulposus Explant Culture. Tissue Eng Part A. 2015 doi: 10.1089/ten.TEA.2015.0121. [DOI] [PubMed] [Google Scholar]
- 36.Purmessur D, Cornejo MC, Cho SK, et al. Notochordal cell-derived therapeutic strategies for discogenic back pain. Global Spine J. 2013;3:201–218. doi: 10.1055/s-0033-1350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erwin WM, Ashman K, O’Donnel P, et al. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006;54:3859–3867. doi: 10.1002/art.22258. [DOI] [PubMed] [Google Scholar]
- 38.Cornejo MC, Cho SK, Giannarelli C, et al. Soluble factors from the notochordal-rich intervertebral disc inhibit endothelial cell invasion and vessel formation in the presence and absence of pro-inflammatory cytokines. Osteoarthritis Cartilage. 2015;23:487–496. doi: 10.1016/j.joca.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purmessur D, Cornejo MC, Cho SK, et al. Intact glycosaminoglycans from intervertebral disc-derived notochordal cell-conditioned media inhibit neurite growth while maintaining neuronal cell viability. Spine J. 2015;15:1060–1069. doi: 10.1016/j.spinee.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erwin WM, Islam D, Inman RD, et al. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R215. doi: 10.1186/ar3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang F, Leung VY, Luk KD, et al. Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J Pathol. 2009;218:113–121. doi: 10.1002/path.2519. [DOI] [PubMed] [Google Scholar]
- 42.Hunter CJ, Bianchi S, Cheng P, et al. Osmoregulatory function of large vacuoles found in notochordal cells of the intervertebral disc running title: an osmoregulatory vacuole. Molecular & cellular biomechanics : MCB. 2007;4:227–237. [PMC free article] [PubMed] [Google Scholar]
- 43.Spillekom S, Smolders LA, Grinwis GC, et al. Increased osmolarity and cell clustering preserve canine notochordal cell phenotype in culture. Tissue Eng Part C Methods. 2014;20:652–662. doi: 10.1089/ten.TEC.2013.0479. [DOI] [PubMed] [Google Scholar]
- 44.Minogue BM, Richardson SM, Zeef LA, et al. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010;12:R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang X, Jing L, Chen J. Changes in the molecular phenotype of nucleus pulposus cells with intervertebral disc aging. PLoS One. 2012;7:e52020. doi: 10.1371/journal.pone.0052020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lv F, Leung VY, Huang S, et al. In search of nucleus pulposus-specific molecular markers. Rheumatology (Oxford) 2014;53:600–610. doi: 10.1093/rheumatology/ket303. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Yan W, Setton LA. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J. 2006;15(Suppl 3):S303–311. doi: 10.1007/s00586-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omlor GW, Nerlich AG, Tirlapur UK, et al. Loss of notochordal cell phenotype in 3D-cell cultures: implications for disc physiology and disc repair. Arch Orthop Trauma Surg. 2014;134:1673–1681. doi: 10.1007/s00402-014-2097-2. [DOI] [PubMed] [Google Scholar]
- 49.Kim KW, Ha KY, Park JB, et al. Expressions of membrane-type I matrix metalloproteinase, Ki-67 protein, and type II collagen by chondrocytes migrating from cartilage endplate into nucleus pulposus in rat intervertebral discs: a cartilage endplate-fracture model using an intervertebral disc organ culture. Spine (Phila Pa 1976) 2005;30:1373–1378. doi: 10.1097/01.brs.0000166155.48168.0e. [DOI] [PubMed] [Google Scholar]
- 50.Kim KW, Kim YS, Ha KY, et al. An autocrine or paracrine Fas-mediated counterattack: a potential mechanism for apoptosis of notochordal cells in intact rat nucleus pulposus. Spine (Phila Pa 1976) 2005;30:1247–1251. doi: 10.1097/01.brs.0000164256.72241.75. [DOI] [PubMed] [Google Scholar]
- 51.Kim KW, Lim TH, Kim JG, et al. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine (Phila Pa 1976) 2003;28:982–990. doi: 10.1097/01.BRS.0000061986.03886.4F. [DOI] [PubMed] [Google Scholar]
- 52.Risbud MV, Schoepflin ZR, Mwale F, et al. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J Orthop Res. 2015;33:283–293. doi: 10.1002/jor.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walter BA, Purmessur D, Moon A, et al. Reduced tissue osmolarity increases TRPV4 expression and pro-inflammatory cytokines in intervertebral disc cells. Eur Cell Mater. 2016;32:123–136. doi: 10.22203/ecm.v032a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 55.Ganz A, Lambert M, Saez A, et al. Traction forces exerted through N-cadherin contacts. Biology of the cell. 2006;98:721–730. doi: 10.1042/BC20060039. [DOI] [PubMed] [Google Scholar]
- 56.Gu WY, Lai WM, Mow VC. A mixture theory for charged-hydrated soft tissues containing multi-electrolytes: passive transport and swelling behaviors. Journal of biomechanical engineering. 1998;120:169–180. doi: 10.1115/1.2798299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Images of NP regions using H&E and Saf-O/LG were used to define the cell morphology and transition from NCs to sNPCs (NC➔sNPC) using a grading scale ranging from 1 to 5. Characteristic Grade 1, 3 & 5 images are provided with H&E and Saf-O/LG staining. Grade 5 samples were nearly all NCs with yellow arrows pointing at representative NC traits like large, vacuolated cells with continuous cell-cell-contact. Grade 1 samples were nearly all sNPCs with black arrows pointing at representative sNPC traits like small cells, surrounded by GAG-rich matrix as distinct cells or discrete cell-clusters. Grade 3 samples have mixed population of NCs and sNPCs. GAG accumulation was most obvious on Saf-O/LG staining since matrix on H&E was only lightly stained.
Hematoxylin and eosin (H&E) staining depicting the whole IVD with nucleus pulposus (box) region of interest. NP cell morphology was identified with high magnification images of H&E. NC phenotype was characterized by large cells and presence of vacuoles, and sNPC phenotype involved small cells in discrete cells or cell-clusters with lack of vacuoles and high accumulation of matrix. Hyper-Static induced NC to sNPC transition with all other conditions retaining NC phenotype. Scale bar 100um and 20um. * Indicates representative vacuoles.
TUNEL demonstrated no changes in number of cells undergoing apoptosis with changes in osmolarity over the study period of 14 days compared to Control-Day0 and Control-Day 14 groups. Quantification of Tunnel positive cells was carried out on Hyper- and Hypo-Static osmolarity groups for completion. Positive control involved DNase enzyme treated sections. Intense fluorescence indicated increased DNA fragmentation. Fluorescein intensity above 20% of the positive control intensity indicated apoptosis in the experimental groups. Representative images showing Dapi (Blue), Fluorescein (Red) and merged images for all groups.


