Abstract
Background
Sleep disruptions are an important consequence of alcohol use disorders. There is a dearth of preclinical studies examining sex differences in sleep patterns associated with ethanol dependence despite documented sex differences in alcohol related behaviors and withdrawal symptoms. The purpose of this study was to investigate the effects of chronic intermittent ethanol (EtOH) on sleep characteristics in female and male mice.
Methods
Female and male C57BL6/J mice had access to EtOH/water two bottle choice (2BC) 2hr/day for 3 weeks followed by exposure to EtOH vapor (vapor-2BC) or air (control-2BC) for 5 cycles of 4 days. An additional group never experienced EtOH (naïve). Mice were implanted with EEG electrodes and vigilance states were recorded across 24hr on the fourth day of withdrawal. The amounts of wakefulness (W), slow-wave sleep (SWS), and rapid eye movement (REM) sleep were calculated and spectral analysis was performed by fast Fourier transformation.
Results
Overall, vapor-2BC mice showed a decrease in the amount of SWS four days into withdrawal as well as a decrease in the power density of slow waves, indicating disruptions in both the amount and quality of sleep in EtOH dependent mice. This was associated with a decrease in duration and an increase in number of SWS episodes in males and an increase in latency to sleep in females.
Conclusions
Our results revealed overall deficits in sleep regulation in EtOH dependent mice of both sexes. Female mice appeared to be more affected with regard to the triggering of sleep, while male mice appeared more sensitive to disruptions in the maintenance of sleep.
Keywords: Alcohol or ethanol withdrawal, chronic intermittent ethanol, sleep and EEG, sex differences, mouse
Introduction
Alcohol use disorder is a psychiatric disease characterized by a loss of control over the ingestion of alcohol, compulsive use of alcohol, and the presence of withdrawal symptoms when the ingestion of alcohol is stopped (https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-use-disorders). Some of the most frequent disturbances reported during alcohol withdrawal include irritability, sweating, tremors and anxiety (Sachdeva et al., 2015). In addition to the aforementioned disturbances, sleep abnormalities during acute and chronic alcohol intoxication have been reported, as well as during alcohol withdrawal. Sleep impairments include difficulty falling asleep, frequent awakening, daytime sleepiness, abnormal sleep quality and insomnia (Brower 2001; Chaudhary et al., 2015; Colrain et al., 2014; Ebrahim et al., 2013). These sleep alterations negatively affect many aspects of life for the alcoholic, including deficits in learning and memory process, emotional stability, and job performance (Chakravorty et al., 2014; Chaudhary et al., 2015). Importantly, sleep disturbances associated with alcohol withdrawal are considered important predictors of relapse (Brower et al 1998; Brower et al., 2001). Indeed, Gillin and colleagues reported that increased levels of rapid eye movement (REM sleep), and reduced REM sleep latency in alcoholics predicted relapse to drinking within the first months of abstinence (Gillin et al., 1994), while Brower and colleagues reported that insomnia may delay recovery from alcohol addiction and precipitate relapse within the first stages of recovery (Brower et al., 2001). Thus, sleep improvement is considered an important target for treatments for alcoholism (Brower 2015; Kolla et al., 2011).
Sex differences in humans have been reported in patterns of EtOH drinking, abuse, physiological responses (Nolen-Hoeksema 2004; Wilsnack et al 2000), withdrawal (Devaud et al., 2006), and relapse (Boykoff et al., 2015) and women are considered more sensitive to EtOH- or EtOH withdrawal-induced harm than men (Hommer, 2003). Effects of sex on alcohol-related sleep disturbances have been investigated; however, larger sample sizes of female and male alcoholics are needed for a more complete understanding of potential sex differences (Colrain et al., 2014). For example, while no sex differences were noted in a study of insomnia in treatment seeking alcoholics, only 26 of the 172 subjects were female (Brower et al., 2001). In a more balanced study (59 women, 34 men), albeit using healthy non-alcoholic subjects, it was shown that equal blood alcohol levels were associated with greater disruptions in sleep in the women (Arnedt et al., 2011). Finally, Colrain and colleagues (2009) studied recovering alcoholics (15 women, 27 men) and found no significant sex differences, although alcoholic men seemed to show a larger reduction in delta activity during non-REM sleep relative to male controls than female alcoholics did compared to female controls. Male and female subjects had different alcohol histories and lengths of sobriety arguing for the need for larger, more balanced studies.
While no animal model of ethanol (EtOH) dependence and withdrawal fully emulates the human condition, several behavioral and neurochemical impairments observed in alcoholics can be satisfactorily produced in animal models. Indeed it has been reported that administration of EtOH through several routes, including vapor chambers, intragastric dosing, drinking and liquid diets, causes disruptions in sleep in rats. These alterations include increases in the amount of wakefulness, reductions in the amount of sleep, changes in REM sleep, and modifications in the electroencephalographic (EEG) power spectrum during EtOH withdrawal (Gitlow et al., 1973; Kubota et al., 2002; Mendelson et al., 1978; Mukherjee et al., 2008; Mukherjee and Simasko 2009; Rouhani et al., 1998; Thakkar et al., 2015). For example, while Ehlers and Slawecki (2000) reported no changes in the amount of slow wave sleep (SWS) immediately following EtOH exposure and 5 weeks after EtOH withdrawal, they did find a significant reduction in the EEG power spectrum of delta, theta and beta bands, suggesting disruptions in sleep quality. Like rats, mice also show disturbances in both the amount and architecture of sleep during repeated chronic EtOH exposure and withdrawal episodes (Veatch 2006). In this case increases in REM sleep and decreases in non-REM sleep were observed with the latter normalizing by the fourth day of withdrawal.
Although the above-mentioned studies provide important information about EtOH withdrawal sleep alterations in rodents, all of them have been performed exclusively in males. The lack of information regarding EtOH withdrawal related sleep alterations in females is surprising as there are important sex differences in EtOH-related behaviors in general and withdrawal specifically (Becker & Koob, 2016; Finn et al., 2010). In fact, it has been shown that male rodents generally have both a greater withdrawal response and a slower recovery from EtOH withdrawal than female rodents. For example, Reilly and colleagues (2009) reported that male rats showed a significant increase in startle reflex during EtOH withdrawal, while females and ovariectomized female rats showed small to no changes compared to controls. Likewise, it has been shown that male rats (Devaud and Chadda 2001) and mice (Veatch et al., 2007) had greater seizure susceptibility than females during EtOH withdrawal. Additionally it has been reported that male rats had a greater anxiety-like response, measured by the suppression of social interaction, than female rats during withdrawal (Varlinskaya and Spear 2004). Male rodents aren’t necessarily more sensitive than females in all cases. For example, female rodents drink more EtOH than males (e.g., Belknap et al., 1993; Chester et al., 2008; Finn et al., 2004; Lancaster et al., 1996; Torres et al., 2014; Yoneyama et al., 2008), show a bigger increase in both corticosterone and adrenocorticotropic hormone levels following acute EtOH (Rivier, 1993), and greater EtOH-induced hypothermia (Webb et al., 2002) relative to males.
Given the documented sex differences in EtOH-related, and especially EtOH withdrawal-related behaviors and the importance of sleep disturbances in increasing the negative reinforcing aspects of EtOH, it is of critical importance to examine the effects of chronic EtOH exposure on sleep in both males and females. The chronic intermittent EtOH (CIE) model is used which allows for the examination of sleep in mice that have a history of EtOH drinking combined, in the dependent group, with passive EtOH vapor exposure. We have focused on four days into withdrawal, after acute withdrawal signs have subsided, but when dependent mice show increased EtOH drinking (Becker & Lopez, 2004).
Materials and Methods
Animals
Adult female and male C57BL/6J mice (Jackson Laboratories, ME) were used in this study. Body weights averaged 22.20 g (females) and 28.42 g (males) at the start of the experiment. Mice were housed four per cage (except during the 2-hr drinking sessions) separated by sex in standard plastic cages on a reversed 12-h light/dark period (lights on at 8:00 PM), with food and water available ad libitum. All procedures were IACUC approved and met the guidelines of the National Institute of Health detailed in the Guide for the Care and Use of Laboratory Animals.
Chronic intermittent ethanol-two bottle choice consumption (CIE-2BC)
In the CIE-2BC drinking procedure used in this study, animals exhibit increased alcohol intake in a limited-access EtOH/water choice after chronic passive exposure to EtOH vapor. This procedure has been successfully used in rats (Vendruscolo and Roberts, 2014) and C57BL/6J mice (Becker and Lopez, 2004; Finn et al., 2007; Griffin et al., 2009) to model the motivational aspects of EtOH dependence and excessive EtOH drinking associated with the addicted state. In brief, for the first 15 days of testing (5 days per week for 3 weeks), 30 min before the lights went off (7:30 a.m.), mice were individually housed for 2 h with access to 2 drinking tubes, one containing 15% EtOH (v/v, prepared with Ethyl alcohol 200 proof. Pharmco-AAPER, Brookfield, CT, USA) and the other containing water. EtOH and water consumption during the 2 h period was recorded. Following this baseline period of drinking, mice were divided by sex, based on equal EtOH and water consumption, into 2 balanced treatment groups that were exposed to intermittent EtOH vapor (17 male and 14 female mice, the vapor-2BC group) or air in identical chambers (20 male and 16 female mice, the control-2BC group). Chambers consisted of standard plastic mouse-sized shoebox cages containing up to 4 mice per chamber (La Jolla Alcohol Research Inc., La Jolla, CA, USA). EtOH vapor was created by pumping 95% EtOH into a 2L Erlenmeyer vacuum flask kept at 50 °C on a warming tray. Air was blown over the bottom of the flask at a rate of 11 L/min. Concentrations of EtOH vapor were adjusted by varying the rate at which the EtOH was pumped into the flask which, in turn, was based on the blood alcohol levels of the mice. Vapor chamber concentrations were adjusted to match blood alcohol levels of females and males and were 2.47 +/− 0.01% for female, and 2.87 +/− 0.01% for males. The EtOH vapor-2BC group was injected with 1.75 g/kg EtOH and 68.1 mg/kg pyrazole (alcohol dehydrogenase inhibitor, Sigma-Aldrich) and placed in the chambers to receive vapor for 16 hr (with 8 hr off). Following the fourth day of exposure, mice were allowed 72 h of undisturbed time in their home cages. The mice were then given 5 days of access to 2 bottles containing 15% EtOH or water for 2 h to measure EtOH drinking. The 4 days of vapor or air exposure and 5 days of 2 bottle choice testing were repeated for a total of 4 cycles plus another cycle of only the vapor/air following surgery and recovery (see below). The control-2BC group was injected with 68.1 mg/kg pyrazole in 0.9% saline and placed in chambers delivering air for the same periods as the EtOH vapor group and received 2BC testing at the same time as the vapor group. Two additional groups of naïve mice (20 male and 8 female mice) never were treated with EtOH or pyrazole. Following each 16 h bout of EtOH vapor exposure, mice were removed and on the 3rd day of each cycle, tail blood was sampled for blood alcohol levels. Blood was collected in capillary tubes and emptied into 1.5 ml centrifuge tubes containing evaporated heparin and kept on ice. Samples were centrifuged and serum was decanted into fresh 1.5 ml centrifuge tubes. The serum was injected into an oxygen-rate alcohol analyzer (Analox Instruments, Lunenburg, MA, USA) for blood alcohol (BAL) determination. Five pairs of EtOH standards (0.5–3.0 mg/ml) were analyzed prior to the samples. Target blood alcohol levels were 200–250 mg%. After the 4th cycle, EEG recording electrodes were implanted in all groups and, following recovery, mice in the vapor-2BC group received a fifth and final cycle of vapor exposure before recordings. The testing timeline is illustrated in Figure 1.
Figure 1.
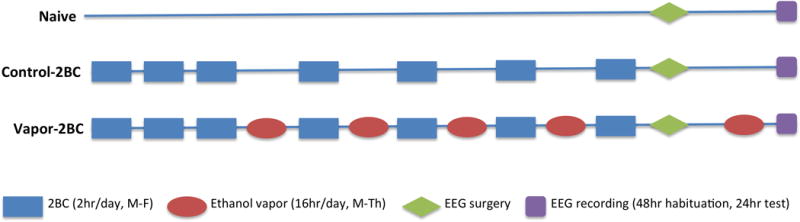
Experimental timeline.
Surgical procedure
All mice used in this study were implanted under isoflurane anesthesia (1%–2%), with a standard set of stainless-steel screw electrodes for chronic sleep recordings. The EEG was recorded from electrodes placed in the frontal and parietal bone over the hippocampus (P = −2.06; L = 1.5; H = 1.0 to bregma according to The Mouse Brain in Stereotaxic Coordinates (Franklin and Paxinos, 2001). A third EEG electrode was placed in the skull over the cerebellum and served to ground the animal to reduce signal artifacts. Two wire electrodes inserted in the neck musculature were used to record postural tone through electromyography (EMG) activity. Insulated leads from the EEG and EMG electrodes were then soldered to a miniconnector that was cemented to the skull with dental acrylic. During anesthetic recovery, mice were monitored for resumption of normal righting capability and locomotion. All mice were allowed a 1-week recovery. A total of 57 male and 38 female mice were implanted for electrophysiological recordings. However, one male mouse from the vapor-2BC group, two male mice from the control-2BC group and two male mice from the naïve group died after surgery, whereas the EEG recording electrodes of two male mice from the control-2BC group and three mice from the naïve group damaged their electrodes and they were not used in the study. Likewise, one female mouse from the naïve group and one female mouse from the vapor-2BC group damaged their EEG electrodes. Therefore a total of 47 males and 36 female mice were used.
Experimental Protocol and Data Acquisition
Two days after the last round of vapor (withdrawal) or control conditions, mice were connected to commutators with flexible cables allowing their unrestricted movement within the cage and were habituated to the recording cages for 48 hours. Without disturbing the animals recordings began at the 4 day withdrawal point, after light onset (ZT-0; ZT = zeitgeber time), and the signals were continuously recorded from EEG and EMG electrodes across 24 hours. The EEG and EMG signals were amplified by a Grass Model 7D polygraph (Astro-Med, Icn Product Group. West Warwick, RI. USA) in a frequency range of 0.30 to 70 Hz and sampled at 256 Hz. The EEG and EMG were displayed on a computer monitor and stored with a resolution of 128 Hz in the hard drive of a computer for the off-line analysis of the sleep-wake states and spectral analysis using software supplied by Kissei-Comtec (Irvine, CA. USA).
EEG Analysis
The polygraphic results were analyzed semiautomatically in 15-second epochs and classified according to the following stages of vigilance: wakefulness (W), SWS, and REM sleep, as described previously (Bourgin et al., 2007; Huitron-Resendiz et al., 2004). Electrophysiologic criteria were used to define the sleep-wake stages, as follows: W was characterized by desynchronization of the EEG and high theta activity and the presence of muscle tone; SWS by high-voltage waves, high delta activity, and decreased voltage in the EMG; and REM sleep by desynchronization of the EEG, high theta activity, and absence of voltage in the EMG. Total time spent in W, SWS, and REM sleep in periods of 12 hours was calculated. The frequency, duration and latency of the individual SWS and REM-sleep episodes were evaluated. In addition to standard sleep analysis, EEG spectral analyses in the different sleep-wake stages were performed by Fourier fast transformer analysis using 4-second epochs, giving 0.25-Hz bins from 0 to 70 Hz. Each bin was named after its lower limit. Epochs with artifacts were excluded by software-visual review recognition of the polygraph records. In W, SWS, and REM sleep, only epochs that were both preceded and followed by the same stage were included in the analysis.
Statistical analysis
We based group sizes on power analysis (https://www.dssresearch.com/KnowledgeCenter/toolkitcalculators/statisticalpowercalculators.aspx) using previous data collected in our laboratory. Previous CIE studies involving control-2BC and vapor-2BC groups had powers of 0.75 with an N = 8, 0.83 with an N = 9, and 0.94 with an N = 12. For pilot studies looking at the effects of EtOH withdrawal on EEG we started with an N = 4, and using these data to calculate sample size, found that an N of 6 would be sufficient to achieve a power of 0.90 for EtOH/control comparisons. Based on these calculations we started with larger N’s for the ethanol drinking groups and somewhat smaller N’s for the naïve groups that were only being used for the EEG comparisons and made sure we accounted for loss due to EtOH vapor chamber or surgical problems (described above). Final group sizes for the EtOH naïve groups were 7 females and 15 males, 16 females and 16 males for the control-2BC groups, and 13 females and 16 males for the vapor-2BC groups.
We followed recommendations provided by McCarthy (2015) and Guizzetti et al., (2016) to design and analyze experiments addressing sex as a biological variable and did not monitor the estrus cycle or perform gonadectomies in this initial study. Comparing and contrasting males and females may lead to the discovery of novel mechanisms related to EtOH withdrawal and sleep and the present findings could be used as a tool to interrogate sex differences in these processes.
Two-way repeated measures analysis of variance (ANOVA) with the between-subject factor sex and the within-subject factor treatment (naïve, control-2BC and vapor-2BC) was used to assess differences in EtOH intake, vigilance states, and EEG power between groups during EtOH withdrawal. We examined the light and dark phases separately for sleep and EEG power as phase differences were expected and we wanted to focus on group and sex differences. The Fisher’s PLSD test was used for specific comparisons when indicated by the ANOVA. In addition, separate one-way ANOVAs were performed to compare treatment effects in female and male mice. Significant main effects were further analyzed with post hoc analyses (Fisher’s PLSD test) to compare sex differences. Results were considered significant with P < 0.05 for significance.
Results
Our results showed that, overall, female mice drank more EtOH than males (Fig. 2A: F(1,57) = 34.65, p < 0.0001) and there was an overall significant effect of group (F(1,57) = 20.64, p < 0.0001); however, chronic intermittent EtOH vapor exposure resulted in similar escalation of EtOH drinking in both sexes (i.e. no significant sex × group interaction, F(1,228) = 1.55, p = 0.11). In fact, each of the CIE tests resulted in higher ethanol drinking than observed during baseline (p < 0.001). Blood alcohol levels associated with EtOH vapor exposure were similar in females and males, indicating that our adjustments in vapor concentrations were successful (Fig. 2B).
Figure 2.
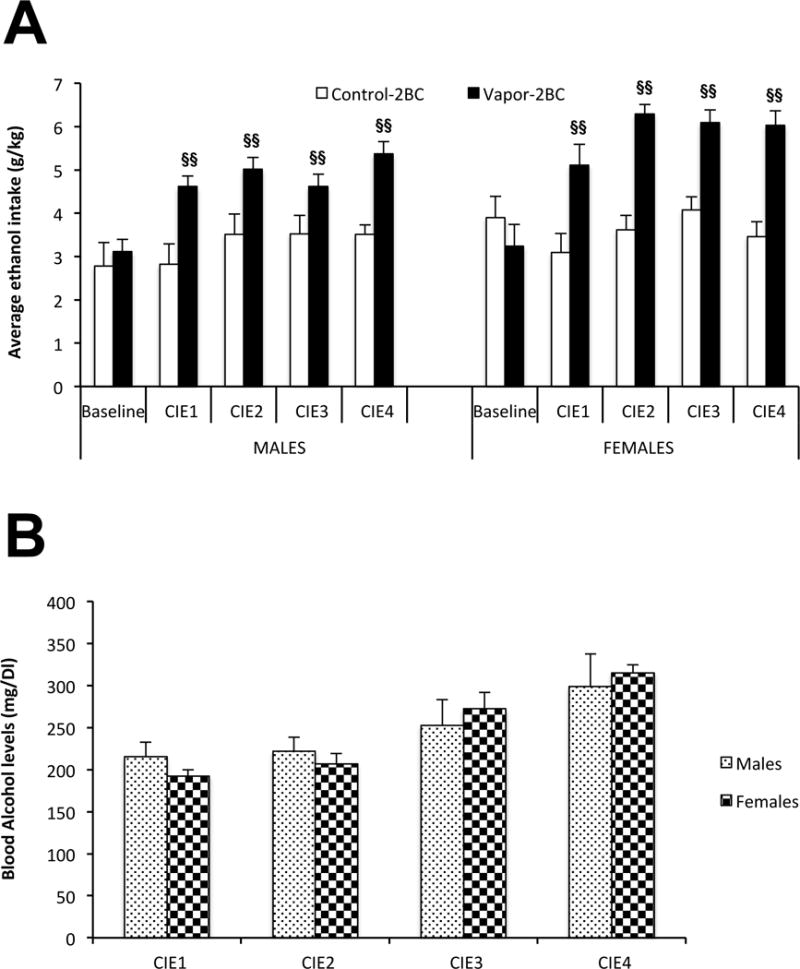
Escalated EtOH drinking following chronic intermittent EtOH (CIE) [A], and blood alcohol levels during vapor exposure (B). Values (Mean +/− SEM) were compared using a two way ANOVA and Fisher’s PLSD tests. Data are presented separately for males and females; however the post-hoc statistics were performed with sexes combined since there were no significant effects involving sex. Overall, ethanol intake was higher in each CIE test relative to baseline in the vapor-2BC group, with no change over tests in control-2BC mice. §§ p < 0.05 vs. control-2BC with sexes combined.
Alterations in vigilance states at four days of EtOH withdrawal
Each group of mice (naïve, control-2BC and vapor-2BC) used in the study showed expected increases in sleep during lights on and increases in wakefulness during lights off. However, statistical analysis showed a significant effect of group in the amount of W (F(2,77) = 19.28; p < 0.0001) during the light period, with no significant effect of sex (F(1,77) = 0.6, p = 0.44) or group × sex (F(2,77) = 1.31, p = 0.28). Post hoc analysis revealed that the amount of W in the vapor-2BC mice was significantly higher than that of the naïve and control-2BC groups (p = 0.0005 and p < 0.0001, respectively: Fig. 3A). There was a concomitant group difference in SWS (F(2,77) = 17.47; p < 0.0001), again with no effects involving sex. Post hoc analysis revealed that the amount of SWS in the light period for vapor-2BC groups was significantly lower than that of the naïve and control-2BC groups (p = 0.0004 and p < 0.0001, respectively: Fig. 3B). No significant group differences were observed in the amount of W during the dark period (F(2,77) = 2.97, p = 0.06; Fig. 3A), but there was a significant effect of group on SWS during this phase (F(2,77) = 3.18, p = 0.047). Post hoc analysis showed a significant decrease in SWS in vapor-2BC relative to naïve mice (p = 0.02). However, again, there were no significant effects involving sex on W and SWS during the dark phase. These results suggested that a history of EtOH vapor decreases sleep quantity to a similar degree in female and male mice.
Figure 3.
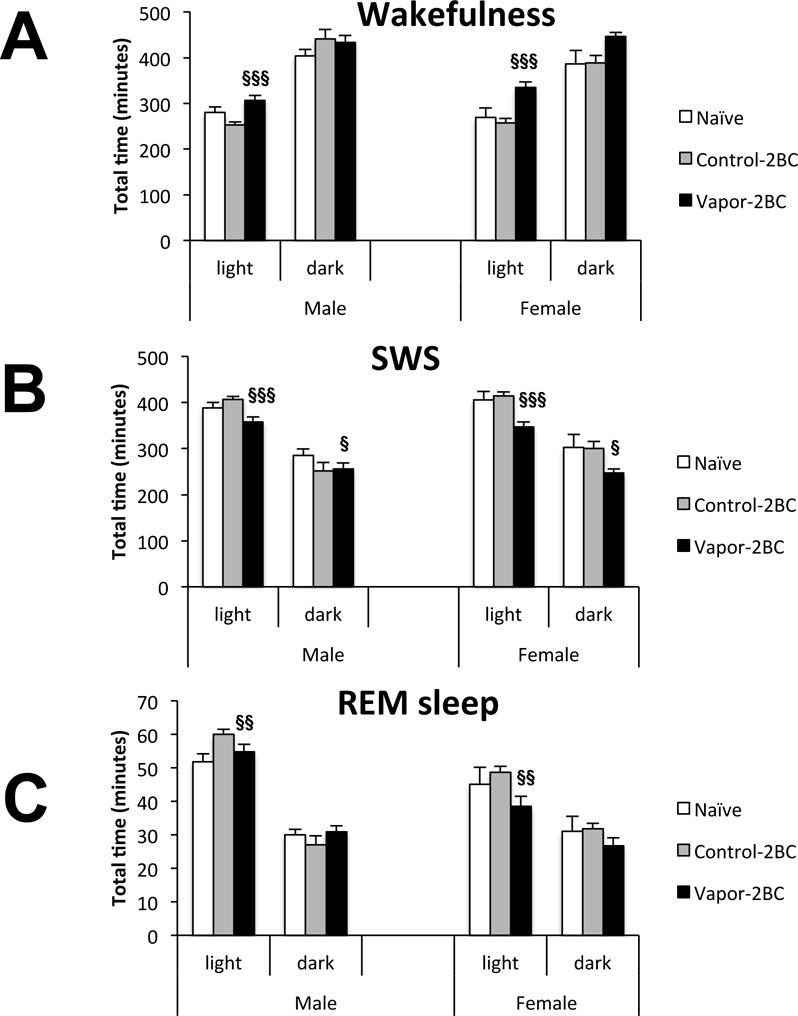
Total time of W (A), SWS (B) and REM sleep (C) 4 days after EtOH withdrawal. Each bar represents total amounts of these vigilance states across 12 hours of light and 12 hours of dark. Values (Mean +/− SEM) were compared using a two way ANOVA and Fisher’s PLSD tests. Data are presented separately for males and females; however the post-hoc statistics were performed with sexes combined since there were no significant effects involving sex. In the light phase, vapor-2BC mice had more time in W and less time in SWS than both control-2BC and naïve mice. REM was significantly reduced in vapor-2BC mice as compared to control-2BC mice in the light phase. In the dark phase, vapor-2BC mice spent less time in SWS relative to naïve mice. § p < 0.05 vs. naïve, §§ p < 0.05 vs. control-2BC, §§§ vs. both control-2BC & naïve, all with sexes combined.
Ethanol vapor exposure also resulted in alterations in REM sleep, but only during the light phase (F(2,77) = 5.91; p = 0.004). In this phase there was also an overall sex difference (F(1,77) = 30.66; p < 0.0001), with males spending more time in REM than females. Because there was not a significant group × sex interaction (F(2,77) = 1.67; p = 0.19), post hoc analysis was done on sexes combined and revealed that vapor-2BC mice had reduced REM sleep during the light period, compared to control-2BC mice (p = 0.004; Fig. 3C).
Alterations in overall sleep amounts could be due to changes in the duration and/or number of SWS episodes. Indeed there was a significant effect of group on the duration of SWS episodes in the light phase (Fig. 4A: F(2,77) = 4.21; p = 0.018), but also a significant group × sex interaction (F(2,77) = 3.96; p = 0.023). Looking at the sexes separately, the group effect was only significant in males (F(2,44) = 7.02; p = 0.002), but not in females (F(2,44) = 2.58; p = 0.09) Post hoc analysis showed that vapor-2BC male mice had significantly shorter episodes than naïve and 2-BC mice (p = 0.0005 and p = 0.041, respectively). A significant effect of group on duration of SWS episodes during the dark period was also observed (F(2,77) = 4.54; p = 0.014), as was a significant overall effect of sex (F(1,77) = 7.49; p = 0.0077). Because there was not a significant group × sex interaction (F(2,77) = 0.33; p = 0.72), Post hoc analysis was done on sexes combined and revealed that vapor-2BC mice had significantly shorter sleep episodes than naïve and 2-BC mice (p = 0.009 and p = 0.011, respectively).
Figure 4.
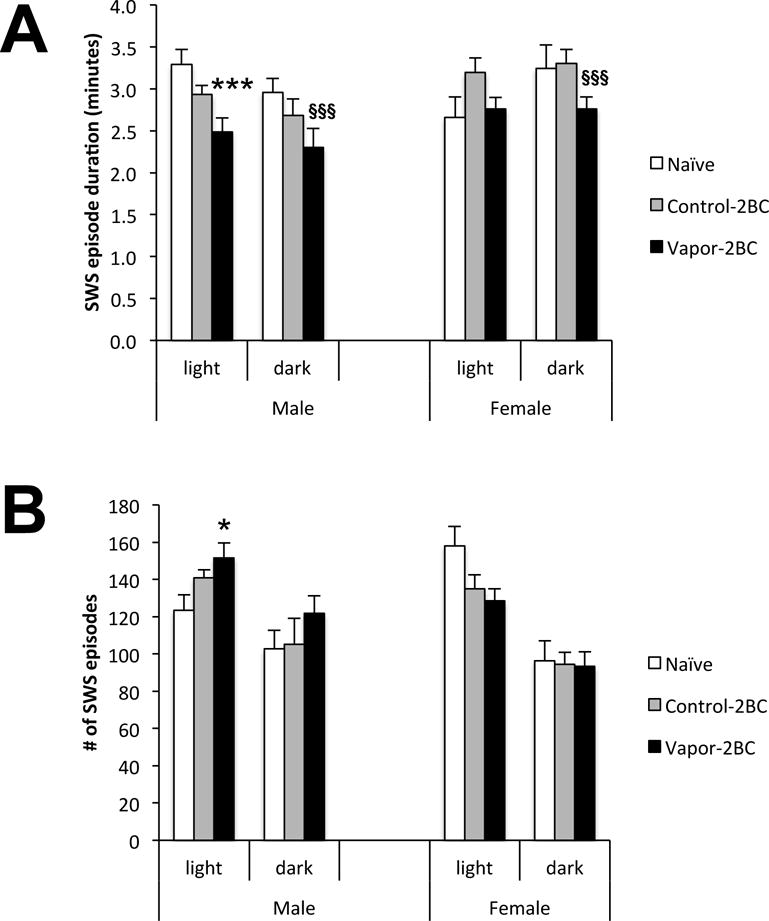
SWS episode duration (A) and number of SWS episodes (B) 4 days into EtOH withdrawal. Values (Mean +/− SEM) were compared using a two way ANOVA and Fisher’s PLSD tests. Because there was a group × sex interaction in episode duration, sexes were examined separately, revealing that vapor-2BC males had significantly shorter episodes than control-2BC and naïve males. There was a group difference in episode duration in the light phase, but no sex difference. Overall, vapor-2BC mice had shorter SWS episodes than both control groups. In the light phase there was a group × sex interaction, with vapor-2BC males having more SWS episodes relative to naïve males. * p < 0.05 vs. naïve, *** p < 0.05 vs control-2BC & naïve, sexes analyzed separately, §§§ p < 0.05 vs. control-2BC & naïve, sexes combined.
The only significant effect on the number of SWS episodes was a group × sex interaction in the light phase (Fig. 4B: F(2,77) = 6.46; p = 0.003). Only males had a significant group difference (F(2,44) = 4.07; p = 0.023), with post hoc analysis revealing an increase in number of SWS episodes in vapor-2BC vs. naïve mice (p = 0.007). The increase in the number of SWS episodes and the reduction in the duration of SWS episodes could be considered a fragmentation of sleep, and because this was significant only in males during the light phase, these results suggest that chronic passive exposure to EtOH vapor and limited-access to EtOH/water choice altered mechanisms associated with the maintenance of SWS to a greater degree in males.
Analysis of REM episode duration (Fig. 5A) and number (Fig. 5B) revealed only trends involving group, with the only statistically significant effect being an overall sex difference in number of REM episodes during the light phase (F(1,77) = 7.39; p = 0.008).
Figure 5.
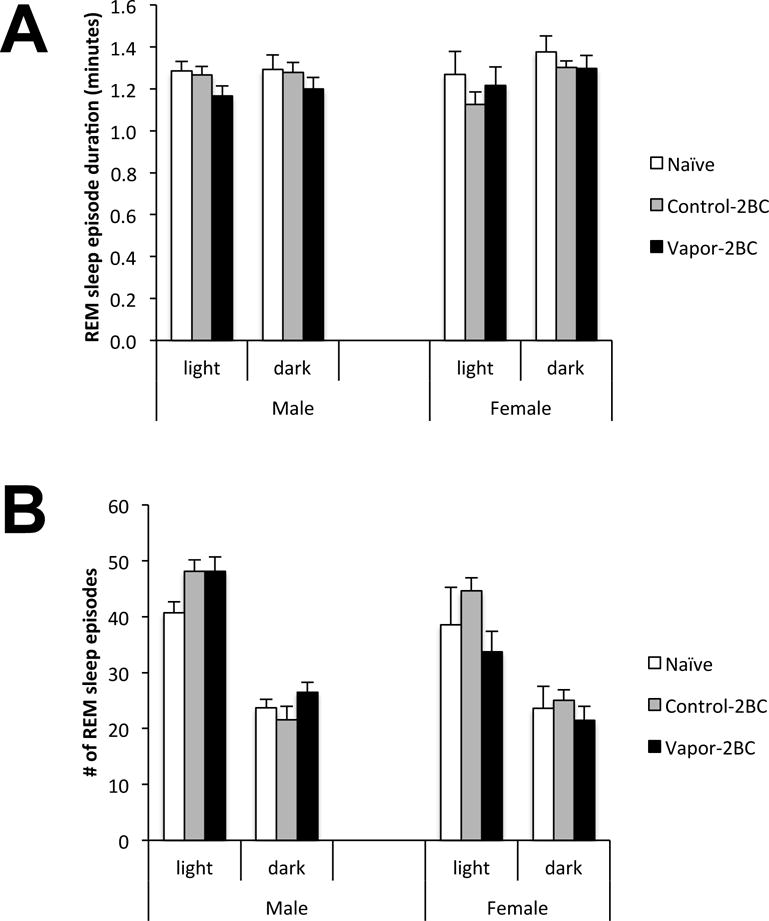
REM episode duration (A) and number of SWS episodes (B) 4 days into EtOH withdrawal. Values (Mean +/− SEM) were compared using a two way ANOVA. There were no significant effects of group on these measures.
Because we found group differences in SWS, we were interested in examining the latency to fall asleep following lights on. Statistical analysis showed a significant effect of sex on the latency to the first SWS episode (F(2,77) = 5.508; p < 0.0215). Post hoc analysis revealed that SWS latency was significantly increased in vapor-2BC females compared to the naïve group (p = 0.0155). No significant differences were observed in males (Fig. 6A). Likewise, statistical analysis showed a significant effect of sex on the latency for the first REM sleep episode (F(2,77) = 7.699; p < 0.0069). Post hoc analysis revealed that vapor-2BC female mice had a significant increase in the latency to REM compared to the naïve and control-2BC groups (p = 0.046 and p = 0.043, respectively), whereas no significant differences were observed between groups in male mice (Fig. 6B).
Figure 6.
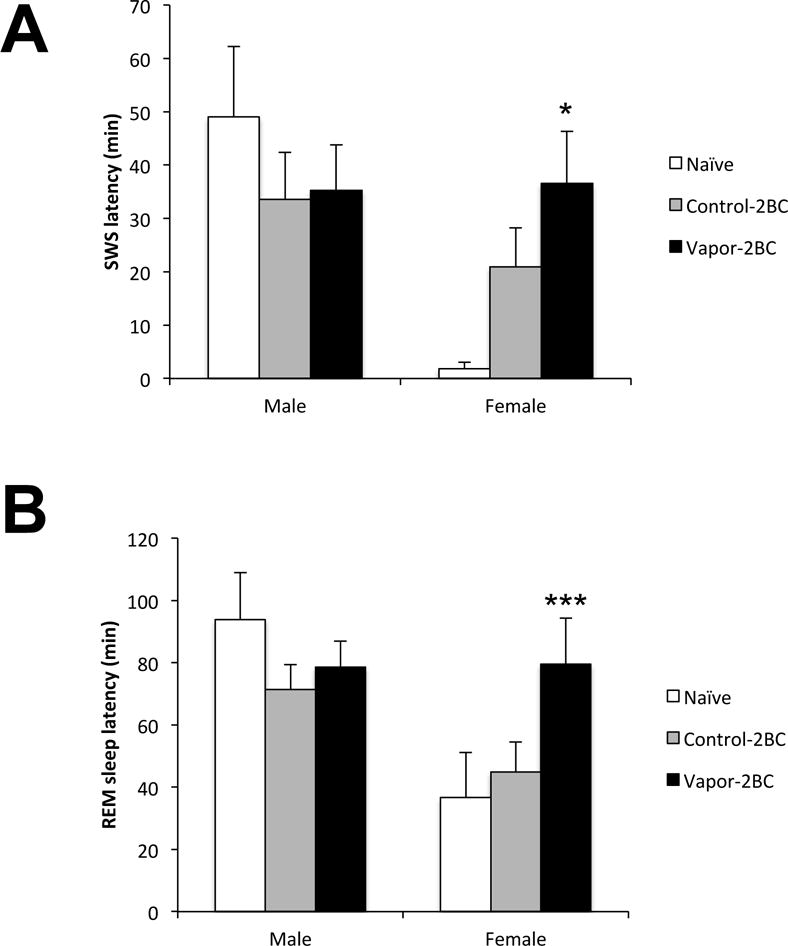
Latencies to the first episode of SWS (A) and REM sleep (B) are shown. Values (Mean +/− SEM) were compared using a two way ANOVA and Fisher’s PLSD tests. There was an overall sex difference and SWS latency of Vapor-2BC females was significantly greater than that of naïve mice and REM latency of vapor-2BC females was significantly greater than both control groups. * p < 0.05 vs. naïve, *** p < 0.05 vs. control-2BC & naïve, sexes analyzed separately.
Taken together, these results strongly indicate that the chronic passive exposure to intermittent EtOH vapor plus limited-access EtOH/water 2BC causes important abnormalities in the regulation of SWS at four days of withdrawal, compared to naïve and 2BC EtOH control mice. While the vapor-2BC group had decreased SWS independent of sex, males had decreased SWS episode duration and increased number of episodes and females had a decreased latency to SWS. This suggests that the underlying mechanisms for sleep disruption may be sex dependent.
The time course analysis of SWS is shown (Fig. 7) in order to illustrate hourly sleep across the groups. Overall, there were significant effects of group (F(2,77) = 9.4, p = 0.0002), hour (F(23,1771) = 26.1, p < 0.0001) and sex × hour (F(23,1771) = 1.6, p = 0.03), with vapor-2BC mice having significantly less SWS than control-2BC (p = 0.0003) and naïve (p = 0.0011) mice. The group × hour interaction was not significant; therefore, individual time points were not compared between groups.
Figure 7.
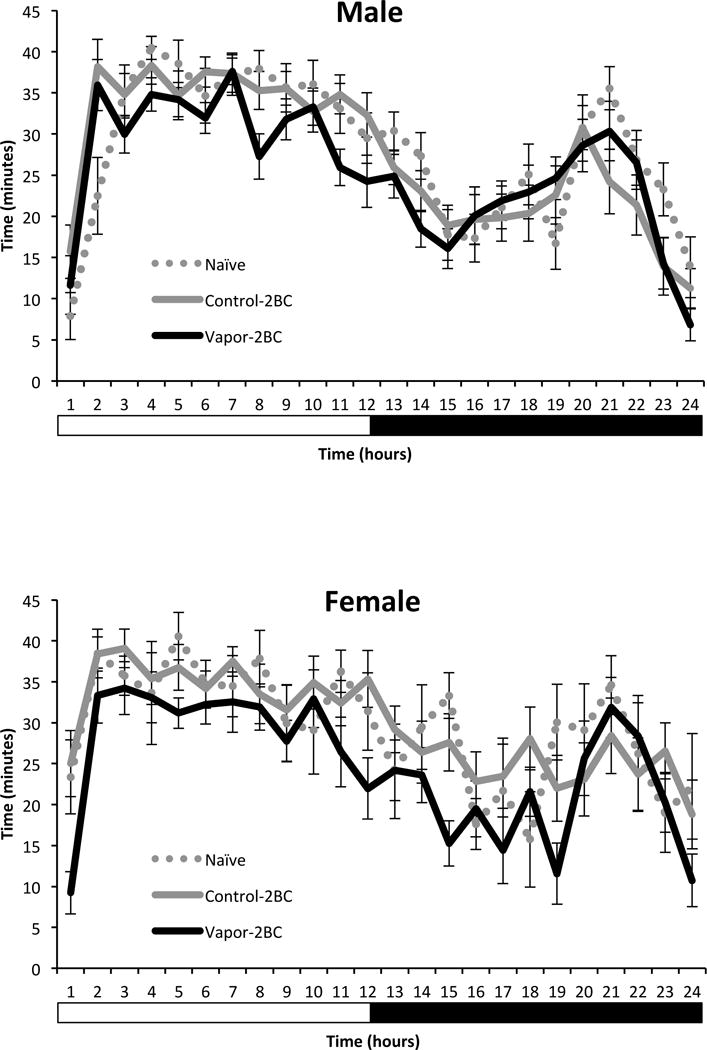
Hourly SWS in naïve, control-2BC and vapor-2BC mice (data from Fig. 3B in more detail). Overall, vapor-2BC mice slept less than both non-dependent groups.
Alterations in EEG spectral power at four days of EtOH withdrawal
Slow waves between 0.5-4.0 Hz (delta band) are predominant in SWS; waves between 6.0-12.0 Hz (theta band), 8.0-12.0 Hz (alpha band), 15.0-30.0 Hz (beta band), and 30.0-60 Hz (gamma band) are more consistently observed during W; and theta band between 4.0-9.0 Hz is more predominant during REM sleep (Borbely et al. 1984; Huitron-Resendiz et al 2004). Therefore, we analyzed and compared the mean EEG spectral power for specific frequency bands during W, SWS and REM sleep. Results showed a significant effect of treatment on delta power during SWS at four days of withdrawal in the light phase (Fig. 8: F(2,77) = 3.212; p = 0.0457). Post hoc analysis revealed that delta power was reduced in vapor-2BC mice (P = 0.012), compared to naïve mice. The group effect was not significant in the dark phase (F(2,77) = 2.9, p = 0.06). Example traces illustrate this finding (Fig. 9). No differences were observed in the power spectra of theta, alpha, beta and gamma bands during W, and in the theta bands in REM sleep. In addition there were no significant effects involving sex on these measures.
Figure 8.
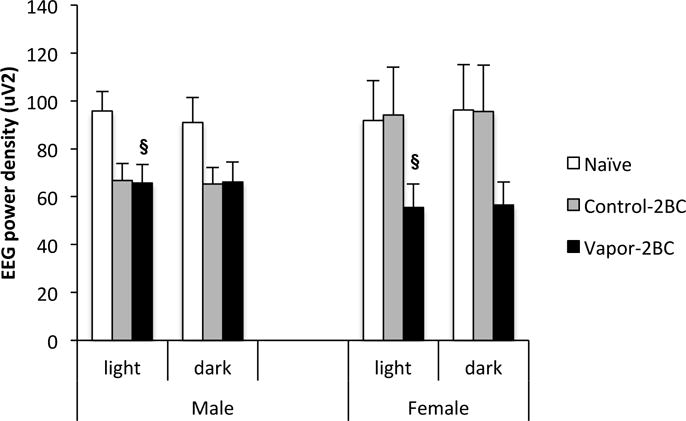
Delta (0.5-4.0 Hz) power values obtained during SWS across 12 hour light and dark periods are shown. Values (Mean +/− SEM) were compared using a two way ANOVA and Fisher’s PLSD tests. Vapor-2BC mice had decreased delta power relative to naïve mice during the light phase. § p < 0.05 vs. naïve, sexes combined.
Figure 9.
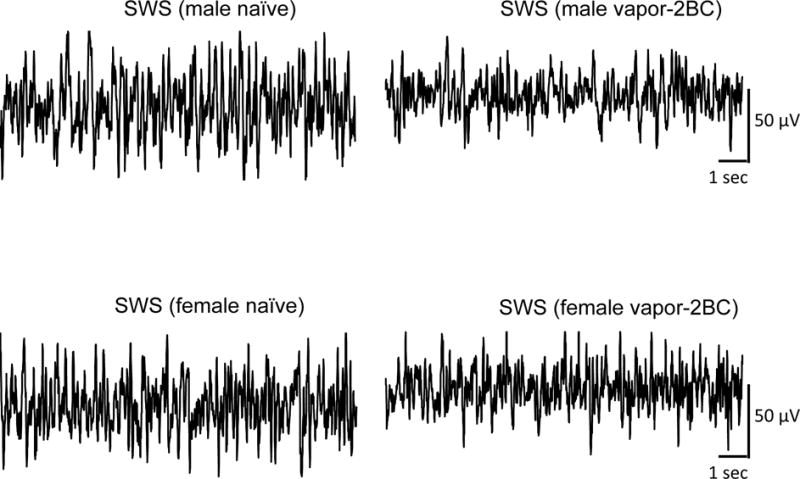
Representative tracings of SWS recorded in two naïve mice (a male and a female), and two vapor-2BC treated animals (a male and a female). Note that independent of sex, vapor-2BC treatment was associated with a reduction in the slow waves characteristic of SWS compared to naïve animals. EEG traces represent 15 seconds of recording.
Discussion
First of all, these results indicated that, although they drink more ethanol overall, female C57BL/6J mice develop escalated EtOH drinking with CIE exposure to an equal degree as males. This is consistent with our previous findings (Bray et al., 2017) as well as others (Bergeson et al., 2016; Crabbe et al., 2012). Another recent study showed escalated EtOH drinking in male C57BL/6J mice, but not in females (Jury et al., 2016); however these results were based on 24hr EtOH drinking as opposed to the limited access model most commonly associated with CIE models. It is possible that the patterns of intake across the 24hr periods were altered by CIE or that there was some sort of ceiling effect in the females.
Findings from the present study showed that chronic passive exposure to EtOH vapor, together with the limited-access EtOH/water choice procedure, causes alterations in the states of sleep in mice four days following withdrawal. For the most part the control-2BC and naïve groups looked quite similar and this was born out statistically. However, in the cases of latencies to SWS (Fig. 6A) and EEG power density (Fig. 8) it appeared that non-dependent limited access ethanol drinking had subtle effects. For example, the latencies to SWS of control-2BC mice appeared more similar to those of vapor-2BC mice than naïve mice. In addition, EEG power densities of male control-2BC mice appeared identical to vapor-2BC mice. We were intentionally focusing on protracted abstinence in dependent mice for this experiment and therefore tested the mice 4 days post-vapor, meaning that the control-2BC mice had been EtOH free for just over 3 weeks. While it is surprising that this group would show effects on sleep at this time point, it is true that they did drink quite a bit of EtOH throughout the experiment, in many cases consistent with binge-like levels. We are currently studying the effects of binge-like ethanol consumption on sleep parameters in female and male mice so should be able to shed more light on this interesting finding.
We observed subtle, but significant sex differences in the effects of EtOH vapor plus drinking that suggest at least partially differential mechanisms to this end. Our results showed that the reduction in the total amount of SWS in males was associated with a reduction in the mean duration and an increase in the number of the SWS episodes, suggesting abnormalities in the mechanisms of maintenance of sleep. In contrast, the disruptions in the amount of SWS in vapor-2BC female mice were associated with an increased latency to sleep, suggesting abnormalities in the mechanisms of triggering of sleep. These findings suggest possible impairments in maintenance of sleep in vapor-2BC male mice and triggering of sleep in vapor-2BC female mice.
Importantly, we observed an overall reduction in delta power in vapor-2BC mice, suggesting that in addition to the effects of EtOH on the mechanism that regulate sleep, the quality of sleep at four days of EtOH withdrawal is affected. Slow wave activity, also known as SWA (EEG frequency band between 0.75-4.0 Hz), increases with lengthening wake duration and dissipates during SWS, and has been generally accepted as an index of sleep homeostasis (Borbely et al., 1984). Therefore, a significant decrease in sleep quality is characterized by increases in sleep fragmentation and decreased SWA, both observed in vapor-2BC mice. These results suggest that EtOH dependence disrupts sleep quality in addition to sleep quantity. These findings are consistent with previous studies carried out in male rats in which reductions in the amount of sleep and the alterations in delta power have been observed during EtOH withdrawal (Criado et al., 2008; Ehlers and Slawecki 2000).
Several studies have shown that male rats have enhanced seizure susceptibility, more anxiety-like responses in the social interaction test, bigger increases in acoustic startle responses, longer recovery to normal EtOH-induced loss of righting reflex times, and more impaired body temperature rhythms than females during EtOH withdrawal. Based on these studies it has been proposed that male rodents have both slower recovery and greater severity of withdrawal (Becker and Koob 2016; Veatch et al., 2007; Walls et al., 2012). In contrast, Bekman and collaborators (2013) and Hommer (2003) reported that women have greater vulnerability toward EtOH withdrawal-induced harm than men, and that high-dose drinking is associated with more severe negative effects that take longer to resolve in women following heavy drinking episodes, and lead to more rapid development of neurotoxicity and alcoholic liver disease (Hommer, 2003). Interestingly, our electroencephalographic recordings of sleep showed that at four days of withdrawal vapor-2BC mice had lower amounts of SWS than control mice during the light and dark phases, with no overall sex differences. However, it is possible that different mechanisms underlie this difference, as males had increased numbers of sleep episodes with decreased episode duration and females showed increased latency to sleep. The male finding suggests fragmented sleep whereas the female finding suggests possible characteristics of insomnia.
It is well known that an intricate neurocircuitry consisting of neurochemicals in distinct nuclei residing in the basal forebrain, preoptic area, lateral hypothalamus and brain stem is responsible for the induction and maintenance of arousal and SWS sleep (Jones, 2005; Siegel, 2011; Szymusiak and McGinty, 2008). Some reports suggest that there are sex differences in the hormonal modulation of both the activity of sleep nuclei and the expression of the sleep neuro-related substances. For example, Hadjimarkou and colleagues (2008) reported that female rats treated with estradiol showed a significant reduction in neuronal activity of ventrolateral preoptic area neurons (VLPO) in the hypothalamus. During SWS the sleep-active neurons in the VLPO cluster inhibit the activity of the neurons in the tuberomammillary, dorsal raphe and locus coeruleus nuclei by releasing galanin and GABA, thus maintaining SWS (Saper et al., 2010). Interestingly, an increase in progesterone, estrogen and estradiol levels in female rats and guinea pigs correlates with a reduction in the amount of SWS and REM sleep, as well as with a reduction in the slow-wave activity (0.4-4 Hz) during SWS (Deurveilher et al., 2011). Furthermore, there is evidence that EtOH intoxication and EtOH withdrawal elicit a rapid rise in the levels of plasma corticosterone in male, but not female rodents (Janis et al., 1998), whereas levels of both progesterone and the neuroactive steroid allopregnanolone, are significantly higher in the brains of female rats relative to male rats (Tanchuck-Nipper et al., 2015). Therefore, in view of these studies, it is possible that hormonal differences between sexes play a role in the differential sleep disturbances observed in this study.
Conclusions
Sleep quantity and quality were disrupted four days into EtOH withdrawal in both female and male mice; however, there were sex differences in the type of disruption. Female mice appeared to be more affected with regard to latency to sleep, while male mice appeared more sensitive to sleep episode characteristics. Because sleep disturbances are considered so important in continued EtOH drinking and relapse, this model will be of great importance in examining possible therapeutics. Future work will examine the effects of target compounds on sleep and drinking and further explore the relationship between these sleep disturbances and escalated EtOH drinking associated with dependence. In addition, this model can be used to further explore the mechanisms underlying sex differences in the sleep disturbances associated with EtOH dependence and withdrawal.
Acknowledgments
This study was supported by the Integrated Neuroscience Initiative on Alcoholism (INIA)– West AA020893 and AA013498 and The Scripps Research Institute’s Mouse Behavioral Assessment Core. This is manuscript # 29559 from The Scripps Research Institute.
Footnotes
DR. AMANDA J. ROBERTS (Orcid ID : 0000-0002-4044-8884)
Conflicts of Interest
The authors declare they have no conflicts of interest.
References
- Arnedt JT, Rohsenow DJ, Almeida AB, Hunt SK, Gokhale M, Gottlieb D, Howland J. Sleep following alcohol intoxication in healthy, young adults: effects of sex and family history of alcoholism. Alcohol Clin Exp Res. 2011;35(5):870–878. doi: 10.1111/j.1530-0277.2010.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28(12):1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev. 2016;68(2):242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekman NM, Winward JL, Lau LL, Wagner CC, Brown SA. The impact of adolescent binge drinking and sustained abstinence on affective state. Alcohol Clin Exp Res. 2013;37(8):1432–1439. doi: 10.1111/acer.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112(4):503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bergeson SE, Nipper MA, Jensen J, Helms ML, Finn DA. Tigecycline reduces ethanol intake in dependent and nondependent male and female C57BL/6J mice. Alcohol Clin Exp Res. 2016;40(12):2491–2498. doi: 10.1111/acer.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA, Tobler I, Hanagasioglu M. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res. 1984;14(3):171–182. doi: 10.1016/0166-4328(84)90186-4. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Fabre V, Huitron-Resendiz S, Henriksen SJ, Prospero-Garcia O, Criado JR, de Lecea L. Cortistatin promotes and negatively correlates with slow-wave sleep. Eur J Neurosci. 2007;26(3):729–738. doi: 10.1111/j.1460-9568.2007.05696.x. [DOI] [PubMed] [Google Scholar]
- Boykoff N, Schneekloth TD, Hall-Flavin D, Loukianova L, Karpyak VM, Stevens SR, Biernacka JM, Mrazek DA, Frye MA. Gender differences in the relationship between depressive symptoms and cravings in alcoholism. Am J Addict. 2010;19(4):352–356. doi: 10.1111/j.1521-0391.2010.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray JG, Roberts AJ, Gruol DL. Transgenic mice with increased astrocyte expression of CCL2 show altered behavioral effects of alcohol. Neuroscience. 2017;354:88–100. doi: 10.1016/j.neuroscience.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res. 1998;22(8):1864–1871. [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health. 2001;25(2):110–125. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158(3):399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Assessment and treatment of insomnia in adult patients with alcohol use disorders. Alcohol. 2015;49(4):417–427. doi: 10.1016/j.alcohol.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Chakravorty S, Jackson N, Chaudhary N, Kozak PJ, Perlis ML, Shue HR, Grandner MA. Daytime sleepiness: associations with alcohol use and sleep duration in americans. Sleep Disord. 2014;2014:959152. doi: 10.1155/2014/959152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary NS, Kampman KM, Kranzler HR, Grandner MA, Debbarma S, Chakravorty S. Insomnia in alcohol dependent subjects is associated with greater psychosocial problem severity. Addict Behav. 2015;50:165–172. doi: 10.1016/j.addbeh.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD, Hughes ML, Keuneke KJ. Age- and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference. Alcohol Clin Exp Res. 2008;32(10):1782–1794. doi: 10.1111/j.1530-0277.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Nicholas CL, Baker FC. Alcohol and the sleeping brain. Handb Clin Neurol. 2014;125:415–31. doi: 10.1016/B978-0-444-62619-6.00024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Turlington S, Baker FC. Impact of Alcoholism on Sleep Architecture and EEG Power Spectra in Men and Women. Sleep. 2009;32(10):1341–1352. doi: 10.1093/sleep/32.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Huang LC, Schlumbohm JP, Spence SE, Barkley-Levenson AM, Finn DA, Rhodes JS, Cameron AJ. Ethanol Withdrawal-Associated Drinking and Drinking in the Dark: Common and Discrete Genetic Contributions. Addict Genet. 2012;1:3–11. doi: 10.2478/addge-2012-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Effects of adolescent ethanol exposure on sleep in adult rats. Alcohol. 2008;42(8):631–639. doi: 10.1016/j.alcohol.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurveilher S, Rusak B, Semba K. Female reproductive hormones alter sleep architecture in ovariectomized rats. Sleep. 2011;34(4):519–530. doi: 10.1093/sleep/34.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol Clin Exp Res. 2001;25(11):1689–1696. [PubMed] [Google Scholar]
- Devaud LL, Risinger FO, Selvage D. Impact of the hormonal milieu on the neurobiology of alcohol dependence and withdrawal. J Gen Psychol. 2006;133(4):337–356. doi: 10.3200/GENP.133.4.337-356. [DOI] [PubMed] [Google Scholar]
- Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res. 2013;37(4):539–549. doi: 10.1111/acer.12006. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20(2):173–179. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123(4):813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcohol Clin Exp Res. 2007;31(6):939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Beckley EH, Kaufman KR, Ford MM. Manipulation of GABAergic steroids: Sex differences in the effects on alcohol drinking- and withdrawal-related behaviors. Horm Behav. 2010;57(1):12–22. doi: 10.1016/j.yhbeh.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press Elsevier; 2001. [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Butters N, Demodena A, Schuckit M. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3-month follow-up. Arch Gen Psychiatry. 1994;51(3):189–197. doi: 10.1001/archpsyc.1994.03950030025003. [DOI] [PubMed] [Google Scholar]
- Gitlow SE, Bentkover SH, Dziedzic SW, Khazan N. Persistence of abnormal REM sleep response to ethanol as a result of previous ethanol ingestion. Psychopharmacologia (Berl) 1973;33(2):135–140. doi: 10.1007/BF00429083. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 2009;201(4):569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizzetti M, Davies DL, Egli M, Finn DA, Molina P, Regunathan S, Sohrabji F. SEX AND THE LAB: an alcohol-focused commentary on the NIH initiative to balance sex in cell and animal studies. Alcoholism, Clinical and Experimental Research. 2016;40(6):1182–1191. doi: 10.1111/acer.13072. http://doi.org/10.1111/acer.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci. 2008;27(7):1780–1792. doi: 10.1111/j.1460-9568.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- Hommer DW. Male and female sensitivity to alcohol-induced brain damage. Alcohol Res Health. 2003;27(2):181–185. [PMC free article] [PubMed] [Google Scholar]
- Huitron-Resendiz S, Sanchez-Alavez M, Wills DN, Cravatt BF, Henriksen SJ. Characterization of the sleep-wake patterns in mice lacking fatty acid amide hydrolase. Sleep. 2004;27(5):857–865. doi: 10.1093/sleep/27.5.857. [DOI] [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22(9):2055–2061. [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26(11):578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Jury NJ, DiBerto JF, Kash TL, Holmes A. Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol. 2016;58:53–60. doi: 10.1016/j.alcohol.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla BP, Mansukhani MP, Schneekloth T. Pharmacological treatment of insomnia in alcohol recovery: a systematic review. Alcohol Alcohol. 2011;46(5):578–585. doi: 10.1093/alcalc/agr073. [DOI] [PubMed] [Google Scholar]
- Kubota T, De A, Brown RA, Simasko SM, Krueger JM. Diurnal effects of acute and chronic administration of ethanol on sleep in rats. Alcohol Clin Exp Res. 2002;26(8):1153–1161. doi: 10.1097/01.ALC.0000024292.05785.03. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20(6):1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Incorporating sex as a variable in preclinical neuropsychiatric research. Schizophr Bull. 2015;41(5):1016–1020. doi: 10.1093/schbul/sbv077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson WB, Majchrowicz E, Mirmirani N, Dawson S, Gillin JC, Wyatt RJ. Sleep during chronic ethanol administration and withdrawal in rats. J Stud Alcohol. 1978;39(7):1213–1223. doi: 10.15288/jsa.1978.39.1213. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Kazerooni M, Simasko SM. Dose-response study of chronic alcohol induced changes in sleep patterns in rats. Brain Res. 2008;1208:120–127. doi: 10.1016/j.brainres.2008.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Simasko SM. Chronic alcohol treatment in rats alters sleep by fragmenting periods of vigilance cycling in the light period with extended wakenings. Behav Brain Res. 2009;198(1):113–124. doi: 10.1016/j.bbr.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clinical psychology review. 2004;24(8):981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Reilly W, Koirala B, Devaud LL. Sex differences in acoustic startle responses and seizure thresholds between ethanol-withdrawn male and female rats. Alcohol Alcohol. 2009;44(6):561–566. doi: 10.1093/alcalc/agp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res. 1993;17(4):854–859. doi: 10.1111/j.1530-0277.1993.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Rouhani S, Dall’Ava-Santucci J, Bajenaru O, Emmanouilidis E, Tran G, Manicom R, Dinh-Xuan AT, Poenaru S. Effects of muscimol or homotaurine on sleep-wake states in alcohol-dependent rats during withdrawal. Pharmacol Biochem Behav. 1998;59(4):955–960. doi: 10.1016/s0091-3057(97)00521-2. [DOI] [PubMed] [Google Scholar]
- Sachdeva A, Choudhary M, Chandra M. Alcohol Withdrawal Syndrome: Benzodiazepines and Beyond. J Clin Diagn Res. 2015;9(9):Ve01–ve07. doi: 10.7860/JCDR/2015/13407.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68(6):1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. REM sleep: a biological and psychological paradox. Sleep Med Rev. 2011;15(3):139–142. doi: 10.1016/j.smrv.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Hypothalamic regulation of sleep and arousal. Ann N Y Acad Sci. 2008;1129:275–286. doi: 10.1196/annals.1417.027. [DOI] [PubMed] [Google Scholar]
- Tanchuck-Nipper MA, Ford MM, Hertzberg A, Beadles-Bohling A, Cozzoli DK, Finn DA. Sex differences in ethanol’s anxiolytic effect and chronic ethanol withdrawal severity in mice with a null mutation of the 5α-reductase type 1 gene. Behav Genet. 2015;45(3):354–367. doi: 10.1007/s10519-014-9691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Sharma R, Sahota P. Alcohol disrupts sleep homeostasis. Alcohol. 2015;49(4):299–310. doi: 10.1016/j.alcohol.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, O’Dell LE. Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcohol Clin Exp Res. 2014;38(1):108–115. doi: 10.1111/acer.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28(1):40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Veatch LM. Disruptions in sleep time and sleep architecture in a mouse model of repeated ethanol withdrawal. Alcohol Clin Exp Res. 2006;30(7):1214–1222. doi: 10.1111/j.1530-0277.2006.00134.x. [DOI] [PubMed] [Google Scholar]
- Veatch LM, Wright TM, Randall CL. Only male mice show sensitization of handling-induced convulsions across repeated ethanol withdrawal cycles. Alcohol Clin Exp Res. 2007;31(3):477–485. doi: 10.1111/j.1530-0277.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48(3):277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls SA, Macklin ZL, Devaud LL. Ethanol-induced loss-of-righting response during ethanol withdrawal in male and female rats: associations with alterations in Arc labeling. Alcohol Clin Exp Res. 2012;36(2):234–241. doi: 10.1111/j.1530-0277.2011.01613.x. [DOI] [PubMed] [Google Scholar]
- Webb B, Burnett PW, Walker DW. Sex differences in ethanol-induced hypnosis and hypothermia in young Long-Evans rats. Alcohol Clin Exp Res. 2002;26(5):695–704. [PubMed] [Google Scholar]
- Wilsnack RW, Vogeltanz ND, Wilsnack SC, Harris TR. Gender differences in alcohol consumption and adverse drinking consequences: cross‐cultural patterns. Addiction. 2000;95(2):251–265. doi: 10.1046/j.1360-0443.2000.95225112.x. Fig. Legends. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42(3):149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


