Abstract
Engineering artificial cells to mimic one or multiple fundamental cell biological functions is an emerging area of synthetic biology. Reconstituting functional modules from biological components in vitro is a challenging yet an important essence of bottom-up synthetic biology. Here we describe the concept of building artificial platelets using bottom-up synthetic biology and the four functional modules that together could enable such an ambitious effort.
Bottom-up synthetic biology is a fascinating yet challenging way of unravelling the complexities of biological systems. In such an approach, one attempts to assemble molecules in a well-defined fashion with predictable characteristics to obtain specific biological output. A major area of application that has emerged over the years is the construction of artificial cells. Artificial cells have been conceptualized as cell-like compartments containing the basic molecules of life which ideally can function similarly to a real cell in a given environment (1–3). However, given the sheer biological complexity of even the simplest single-celled organisms, the scientific aim of building an artificial cell can be restricted to recapitulating at least one major biological function. In this perspective article, we describe a conceptual framework of making artificial cells that recapitulate a function of natural platelets. We call these structures artificial platelets since they would have the capability to functionally assist and possibly replace natural platelets in the human body. We illustrate some proof of concept results and propose experimental techniques to achieve such a design in light of the current trends in synthetic biology and microfluidic technology.
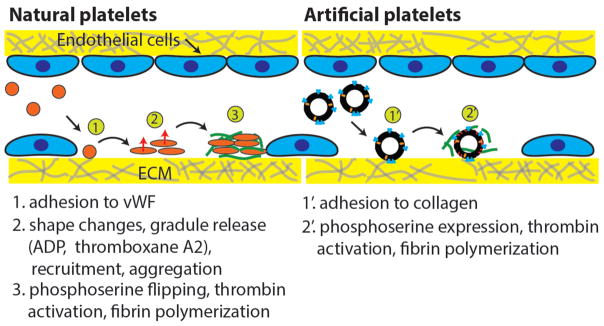
Hemostasis is a key biological process during which loss of blood in damaged blood vessels is prevented in the body. The importance of platelets in maintaining hemostasis in the circulatory system is well known and has been studied extensively in the past. Platelets are derived from fragments of megakaryocytes and flow along the walls of blood vessels to initiate hemostatic response upon detection of injury or damage (4). Platelets in their native state are inactive and are only activated in the presence of specific factors (e.g. von-Willebrand factors) from the surrounding endothelial cells at the site of vascular injury and also molecules such as ADP and thromboxane released by nearby activated platelets. Once activated, platelets serve two functions. In primary hemostasis, they form a plug at the site of injury by adhering to other activated cells using fibrinogen molecules and their specific receptors (e.g. GPIIb/IIa). Simultaneously, they also allow phosphatidylserine scrambling to the outer leaflet of their plasma membrane which catalyzes the conversion of prothrombin to thrombin by the formation of the ‘factor V, factor X’ complex (5). Thrombin plays a major role in the formation of a fibrin mesh on top of the plug of activated platelets which leads to secondary hemostasis. Intermediate steps involve collagen exposure and activation of other factors (e.g. factor XIII) which catalyze several reactions within the coagulation cascade. Although many molecules are involved in this entire process, there are only a few that initiate the chain of reactions. Therefore, it is possible to take inspiration from bottom-up synthetic biology to build some of these characteristics into an artificial cell to mimic platelet functionalities (Figure 1). While natural platelets undergo complex responses during endothelial damage and are involved in both primary and secondary hemostasis, the proposed artificial platelets based on lipid bilayer vesicles would only recapitulate secondary hemostasis.
Figure 1.
Comparison of natural platelet and artificial platelet functions.
Several diseases manifest from an impairment of the normal functioning of platelets. Patients suffering from different kinds of thrombocytopenia have low platelet production or increased platelet destruction resulting in a very low concentration of platelets in blood. In some autoimmune diseases like autoimmune lymphoproliferative syndrome (ALPS), a large number of white blood cells causes reduction in the number of platelets and red blood cells (6). Chemotherapy-induced bone marrow suppression as a side effect also leads to decreased platelet production. In all these cases, any minor injury or trauma can result in excessive blood loss due to longer clotting times, making such situations life threatening to the patients.
Current treatment methods for platelet associated diseases include high dosage of corticosteroids, spleen removal surgery, rituximab, immunoglobulin, immunosuppressant or androgen therapies (7–9). All of these interventions have serious side effects and in most cases do not result in complete remission. An alternative strategy to treat low platelet count is through platelet transfusion (10). Although the treatment has high efficacy when successful, there are several limitations and risks associated with platelet transfusion. Disease transmission by virus or malignant cells is an unavoidable risk even though many screening tests have been developed over the years. Furthermore, platelet storage and handling is a major issue as they are prone to activation outside the body and even at low temperatures. They can only be stored at room temperature for about 5 to 7 days without a significant decrease in cell viability. Storage at room temperature also increases the chances of bacterial contamination which can lead to bacterial sepsis in patients. Ideally, our artificial platelet concept would allow on demand synthesis (with some lag time) and avoid complications with storage. Finally, inflammation due to an immune response is a major concern as it may result in sepsis-like symptoms and possible respiratory failure (11). Hence, given the importance of platelets and the associated complexities that arise due to the abovementioned disorders, it presents an important challenge that an alternative to natural platelets is developed.
Current state of artificial platelets
Until a few years back, most studies involving augmentation of hemostatic pathways were aimed at enhancing platelet adhesion and hemostatic plug formation. One such approach is that of poly(lactic-co-glycolic) acid-poly(L-lysine) (PLGA-PLL) nanoparticles with attached polyethylene glycol (PEG) chains conjugated with arginylglycylaspartic acid (RGD) peptides (12). These microspheres were shown to function as substrates for platelet activation and aggregation in the presence of ADP with minimum non-specific binding. They were successful in significantly reducing clotting times in rats and were also removed from the circulatory system within a week after injection. However, these nanoparticles do not function as platelets but merely augment one aspect of the complex chain of events leading to hemostasis. In recent years, attempts have been made at targeting more complex platelet functions such as clot contraction. Platelet-like particles (PLPs) were developed from polyacrylamide-based microgels with motifs having high selectivity for nascent fibrin protofibrils and minimal affinity for fibrinogen (13). These platelet-sized particles were made from a crosslinker-free, precipitation polymerization method that imparted high deformability matching that of natural platelets. These resulted in clot-specific binding of these microspheres to fibrin and subsequent induction of clot collapse. In another study, nanoparticles were enclosed in plasma membrane derived from human platelets (14). The aim was to retain important surface characteristics of natural platelets. These platelet membrane-cloaked nanoparticles (PNPs) could bind to type IV collagen and had reduced uptake by immune cells in circulation. Furthermore, targeted drug unloading capabilities were demonstrated with efficient adhesion at injured vasculature sites. Membrane cloaking of nanoparticles with lipid bilayer was also leveraged in a different study to structurally and functionally imitate platelet granules (15). These artificial platelet models represent a different focus from using synthetic compounds to reproduce certain biochemical pathways towards emulating processes present in natural platelets. Therefore, a logical progression would be to build a minimal system from biological constituents that could carry out some of the essential steps in the hemostatic process. This is where we believe bottom-up synthetic biology provides some unconventional ideas.
Inspiration from synthetic biology on building artificial cell
Over the last decade, numerous tools have been developed in the field of bottom-up synthetic biology which have expanded the range of what we can engineer with biomolecules. With the advent of cell-free systems, studies of protein production and regulation of transcription and translation with specific genetic models have become robust and efficient (16–18). Furthermore, interactions between different molecules (e.g. lipids, nucleic acids, membrane proteins, etc.) under a variety of well-defined conditions can be reconstituted to recapitulate biological processes and be used to effectively optimize desired biomimetic effects (19–21). Given the similarity of a cell-free solution to that of the cytoplasm of a living cell in terms of protein production from genes, it is possible to reverse engineer an artificial cell by encapsulating cell-free systems within a membrane-bound compartment that simulates the plasma membrane of real cells. Recent developments in the use of microfluidic technology to generate lipid vesicles have provided multiple ways of encapsulating multi-constituent solutions inside them (22–24). Using cell-free systems and such microfluidic setups, we and others have previously reconstituted functionally active proteins in liposomes with real time production of proteins from programmed DNA as an early prototype of an artificial cell (25–28). Three important elements of an artificially constructed cell have been identified, namely an information module, a metabolism system, and an enclosure which can interact with the surrounding environment (1, 29). Towards our goal of creating an artificial platelet, it is possible to use phospholipid vesicles (which would form an interacting membrane similar to the plasma membrane of real cells) that enclose a cell-free solution (which would provide the metabolism system) containing DNA encoding genes for the production of essential proteins (which would serve as the information module).
In the next sections, we systematically lay out possible ways of using bottom-up synthetic biology as a platform for creating a biological system that has the potential to serve as an artificial platelet.
Concept for building artificial platelets
The primary platelet function to recapitulate is coagulation (5). Natural platelets bind to collagen when the endothelium is breached. During primary hemostasis, activation of platelets triggers a complex set of events including the release of granule contents, remodeling of the cytoskeleton, and aggregation with adjacent platelets. Secondary hemostasis is the classic blood coagulation pathway that consists of three pathways: intrinsic, extrinsic, and common. The intrinsic (contact activation) and extrinsic (tissue factor) pathways both lead to the activation of thrombin from prothrombin via activated tissue factors. The burst of thrombin converts fibrinogen to fibrin which forms a cross-linked clot.
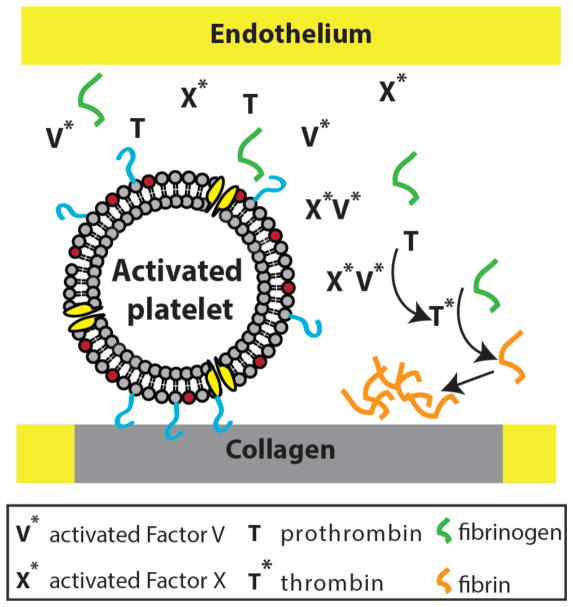
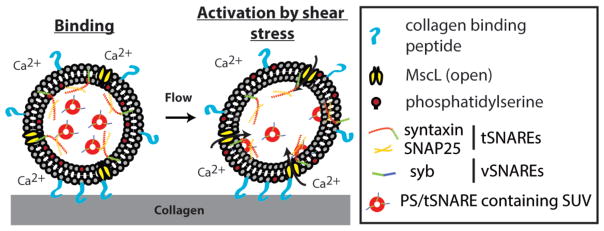
In addition to intrinsic and extrinsic coagulation pathway, a hallmark of an activated platelet is the surface expression of negatively charged phosphatidylserine (PS). PS has been shown to regulate blood coagulation (5), and unpublished work from our group suggests that PS-containing membrane is essential for thrombin generation. Taken this and considering the biology of coagulation pathways, the following picture emerges (Figure 2): Collagen-bound artificial platelets with exposed PS recruit activated factors V and X (a result of activated natural platelets). This assembly promotes the conversion of prothrombin to thrombin, which proteolytically cleaves fibrinogen to fibrin forming a fibrin network. Although this seems feasible diagrammatically, how to couple collagen binding to the surface presentation of PS underlines a central challenge to this concept of an artificial platelet. In the next section, we present possible ideas to engineer an artificial platelet wherein the presentation of PS, through shear stress dependent calcium-induced membrane fusion, is coupled to its binding to extracellular collagen (Figure 3).
Figure 2.
‘Smart’ lipid bilayer vesicles as artificial platelets for clot initiation. Artificial platelets can mediate fibrin clot formation via activated Factor V and X that converts prothrombin to thrombin. The assembly of activated blood clotting factors depends on PS.
Figure 3.
Shear stress-induced activation of artificial platelets. Binding of artificial platelets to exposed collagen leads to increased membrane tension due to shear stress that subsequently opens a mechanosensitive channel. Influx of ‘extracellular’ calcium triggers SNARE-mediated membrane fusion of PS-containing SUVs to deliver PS to the surface of artificial platelets.
Although the biology of platelet function is more complex than what we have described and a significant amount of platelet biology is known, it is perhaps a sufficient starting point to ask whether we could create a scheme whereby a localized burst of thrombin mediated by an externally injected agent, at the site of endothelium injury, can elicit fibrin clot formation as a way to augment natural platelets in acute bleeding. ‘Smart’ lipid vesicles capable of expressing proteins of interests as artificial platelets is an intriguing possibility. As stated before, the focus is to recapitulate function rather than trying to reconstitute the essential molecular components of natural platelets.
Modular design of artificial platelets
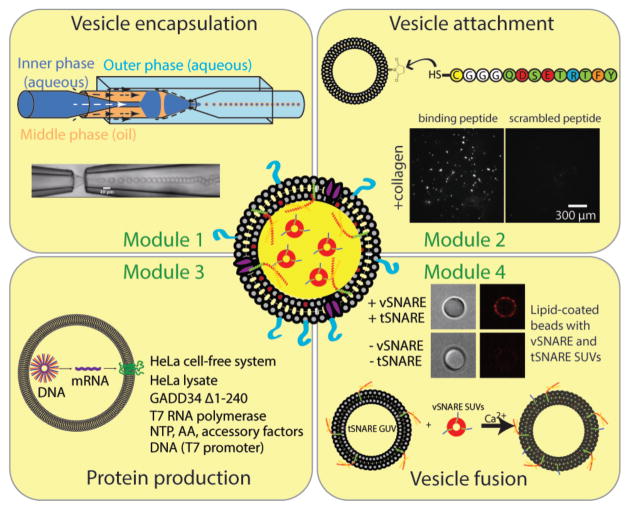
In order to realize artificial platelet engineering, we look at the process of coagulation in a step-by-step manner. If one can delineate the complete process into individual modules that can be engineered separately, it might be possible to combine them together to achieve the desired functionality. For example, one can envision the attachment of these artificial platelets to the extracellular matrix containing collagen and other binding factors such as the von Willebrand factor (vWF). This step would require the complementary binding molecules to be reconstituted in the artificial platelets first. Thereafter, some inherent process within the artificial platelet would lead to the exposure of PS lipids on the outer leaflet of the platelet membrane and thus mediate the assembly of the prothrombinase complex. Additionally, in order to achieve temporal control over PS exposure, dynamics of vesicle fusion can be coupled with chemical signaling such as the influx of calcium ions. Since attached artificial platelets would experience shear stress from blood flow, it is possible to activate them mechanically by induced shear forces on their lipid bilayer membranes. Mechanosensitive channels can be used to mediate the influx of chemical species upon shear force-induced activation. Thus, these important considerations can be organized into four major functional modules, the construction of which will set a foundation for the assembly of the proposed artificial platelet. A bottom-up synthetic biology approach to engineer artificial platelets is challenging, especially given the complexity of biological systems. However, modular design enables complex engineering and makes the challenge more tractable. Moreover, modules can be developed independently and swapped out with other functional modules that serve the same purposes. Below, we will describe the four functional modules that would make our artificial platelet design possible and present evidence and ideas on how each module can be put together.
Vesicle encapsulation (Figure 4, top left)
Figure 4.
Modular design of artificial platelets consists of four functional modules: 1) vesicle encapsulation; 2) vesicle attachment; 3) protein production; 4) vesicle fusion.
A hallmark of any cellular life is that a cell is bound by a membrane. In this case, we will use lipid bilayer vesicles to model artificial cells since a lipid bilayer serves as a natural environment for membrane proteins. Proteins encapsulated in a vesicle can compartmentalize enzymatic reactions and/or perform work. While there are numerous approaches to make lipid bilayer vesicles, controlled vesicle encapsulation is more challenging. Here we propose to use double emulsion templated vesicles generated by capillary microfluidics as a means to obtain monodispersed vesicles enclosing solutions of interests (22, 27, 31). While this double emulsion technique uses an organic phase and leftover solvents may be undesirable, there has been a recent report where solvent de-wetting occurs rapidly by dissolving phospholipids in octanol (24). Another work demonstrated the use of a diblock copolymer to preferentially steer solvent de-wetting in these double emulsions (32). Furthermore, we have functionally reconstituted mechanosensitive channels in lipid bilayer vesicles made by capillary microfluidics (28). With this approach, vesicle sizes, membrane compositions, and encapsulated materials can be readily controlled. As alternatives, other microfluidic approaches can be used that would achieve a similar goal (23, 24). Current microfluidic vesicle encapsulation strategies typically produce vesicles with sizes on the order of tens of micrometers, which are larger than platelet sizes. While this remains a technological challenge yet to be overcome, one could envision optimizing microfluidic approaches for generating smaller, platelet-sized vesicles or adopting droplet splitting microfluidics for making smaller vesicles from larger vesicles (33). Droplet splitting is possible with emulsion droplets but this has not been demonstrated or shown to work with lipid vesicles. Artificial platelets that are 3–5 μm would be ideal sizes. Even though this size range is not possible at present, artificial platelet construction and testing can be done at a larger size for demonstrating key concepts and features.
Vesicle attachment (Figure 4, top right)
For our artificial platelets to function properly, they will be circulating in the vasculature and will bind to exposed collagen when there is an endothelial injury. A candidate solution is to conjugate a collagen binding peptide harboring a free terminal cysteine to the surface of lipid vesicles using maleimide chemistry (30). Surface density of collagen peptide can be controlled by the fraction of reactive maleimide and specificity can be ascertained by scrambling the order of collagen binding peptide, as shown. As alternatives to using collagen binding peptide, one can conjugate Fab antibody fragment using a similar chemistry or reconstituting collagen-specific glycoprotein Ia/IIa and vWF which are the natural adhesion molecules used by platelets to bind to extracellular matrix (5, 30).
Protein production (Figure 4, bottom left)
In order for artificial platelets to function, protein machineries are indispensable. As indicated above, cell-free expression (CFE) systems can be used for synthesizing proteins of interest in an artificial cell. CFE systems that combines transcription and translation have a broad range of applications in both basic and applied science, and both bacterial and mammalian CFE have been used as the ‘cytosol’ of artificial cells (26–28, 34). As an alternative to CFE for protein production, conventional biochemical reconstitution using purified proteins can be considered. Working with purified proteins has advantages of its own where protein concentrations can be readily controlled. However, cell-free protein synthesis has several advantages over purified proteins. Reconstitution of purified membrane proteins in lipid bilayer vesicles is difficult since it requires detergent and this can lead to uncontrolled orientation in the bilayer. Depending on the protein of interest, protein purification can present its own challenges where special handling may be required. Mammalian CFE allows an efficient means to produce virtually any proteins of interest. A key advantage of producing proteins using CFE is that it offers tremendous flexibility to a researcher as DNA encoding proteins of interest can be readily designed. Although protein production in mammalian CFE is limited to ~6 hours, sufficient proteins would be made in artificial platelets to carry out their proposed functions. Mammalian cell-free lysates contain microsomes which are endoplasmic reticulum fragments that support the native expression, folding, and post-translational modification of membrane proteins. Different proteins can be expressed simultaneously for several hours at concentrations up to a few tens of μg/ml in bulk reaction (16). When encapsulated, protein production is expected to be faster due to a higher rate of gene expression in confined volumes (35). Extending protein production lifetime is an important current effort in CFE research, but we do not expect prolong protein expression to be necessary in the proposed artificial platelets.
Using microfluidic devices described in vesicle encapsulation (25, 27), one can encapsulate these CFE systems with added DNA containing the genes of interest inside phospholipid vesicles. In particular, the artificial platelet design relies on the expression of a mechanosensitive (MS) channel in addition to other required proteins. Mechanosensitive channel of large conductance, MscL, is a well characterized prototypical bacterial MS channel that gates purely from increased membrane tension (36, 37). Gating of MscL occurs at a membrane tension of ~10 mN/m (38). Theoretical modeling has pointed to the possibility that MscL can be gated open by shear flow (39, 40), at a dimensionless capillary number of Ca = 8. However, whether shear stress can activate MscL or not has never been investigated experimentally, to the best of our knowledge. In principle, shear stress can deform bilayer membrane and increase membrane tension. Functional expression of MscL, by increasing membrane tension due to osmotic pressure, has been demonstrated in a CFE-containing artificial cell system (28). Thus, CFE may be a suitable approach for protein production in artificial platelets.
Vesicle fusion (Figure 4, bottom right)
The final module of our artificial platelet design is delivery of PS by vesicle fusion which can be coupled to the influx of calcium ions. Calcium is an important secondary messenger in cell signaling and its influx can be used to elicit a biochemical response. Soluble N-ethylmaleimide-sensitive-factor attachment protein receptor (SNARE) proteins mediate membrane fusion between two opposing membranes containing v-SNARES (for vesicle) and t-SNARES (for target) (41). SNAP-25 and syntaxin can function as t-SNAREs and synaptobrevin can function as v-SNAREs (shown in Figure 3) that together form a thermodynamically stable complex that drives membrane fusion in a calcium dependent manner as shown by us and others (42). One way to realize such fusion is to encapsulate small unilamellar vesicles (SUVs) containing PS with biochemically reconstituted t-SNAREs (using purified proteins) within the larger vesicles devoid of PS, along with the CFE system expressing v-SNAREs. Importantly, generation of PS-containing SUVs and vesicle encapsulation are two separate and independent processes so the larger vesicles do not contain PS prior to membrane fusion. Due to a concentration difference of membrane proteins between the SUVs and the larger vesicle bilayer, preferential insertion of v-SNAREs in the larger vesicle membrane will result in differential SNARE protein localization as conceived above. Most vesicle generation techniques cannot create asymmetric lipid bilayers. However, fusion of PS-containing SUVs with the larger vesicle is expected to deliver PS to both the inner and outer leaflets. Since our artificial platelet activation mechanism is to present PS to the vesicle surface, one can envision other functional modules that can achieve a similar outcome. For instance, platelets are known to use a phospholipid scramblase TMEM16F to expose PS to the outer leaflet whose activity is dependent on calcium ions (43). While reconstituting TMEM16F activity may seem attractive, one would also need to start with an asymmetric membrane composition (PS only in the inner leaflet) as an initial condition. This may be accomplished by expressing another enzyme phosphatidylserine synthase that converts phosphatidylcholine to phosphatidylserine. While one might prefer the artificial platelets to have a lipid composition and leaflet asymmetry similar to those found in natural platelets, this artificial platelet design is aimed at reconstituting the primary function of thrombin activation and the subsequent formation of fibrin clots. Therefore, differences in membrane composition of these artificial platelets should not affect the reactions mediated by the exposure of PS. Another possibility is that it may be the negative charge of PS that is required for the subsequent coagulation cascade. If this is the case, it could open up other approaches towards engineering an artificial platelet that displays negative charges upon binding to collagen.
Thus, the final assembly would contain the functional aspects of the above mentioned modules. The mechanism of the formation of a fibrin clot according to our proposed scheme can be summarized as follows: A collagen-bound vesicle simulating an artificial platelet will experience shear flow in the blood which increases its membrane tension. The change in mechanical environment serves as an external signal to trigger artificial platelet activation. The artificial platelets bear mechanosensitive channels that open with increased membrane tension. Subsequently, the opening of these mechanosensitive channels lead to the influx of extracellular calcium. Calcium ions can trigger membrane fusion of PS-containing SUVs within artificial platelets via membrane fusion machineries such as the SNARE proteins. This in turn will cause the delivery of PS to the outer leaflet of the platelet membrane. As mentioned before, PS exposure catalyzes the formation of thrombin leading to the generation of a fibrin clot at the site of vesicle attachment.
Challenges and future outlook
The four functional modules described above, when combined, should constitute working artificial platelets based on our initial platelet activation design – though demonstrating this remains a challenge. What does the future hold for artificial cell research? While modular approaches have been transformative in modern technology, such as in the assembly of computers and cars from discrete functional components; the adaptation of this in bottom-up synthetic biology has been more challenging. Fundamental limitations include the unpredictability of the system native to biology and establishing well-defined interfaces between modules. Although each module can function independently, how modules can be coupled together without a loss of function is a current challenge as well as opportunity. We have recently demonstrated an artificial cell that senses a mechanical (osmotic pressure) input and a chemical (external calcium) input (28). Coupling environmental sensing with functional actuation is the next frontier. By engineering a system that recapitulates cell functions, it may be possible one day to create artificial cells that supplement the functions of real cells.
Acknowledgments
This work is supported by the National Science Foundation MCB-1612917 and the NIH Director’s New Innovator Award DP2 HL117748-01 to A.P.L. The authors wish to thank past and current members of the lab including Victoria Murray, Lap Man Lee, Jonathan Muncie, Christopher Coyne, Jin Woo Lee, and Kenneth Ho. The authors also thank Dan Fletcher and Wilbur Lam for helpful discussion. The perspective is not meant to exhaustively review work in bottom-up synthetic biology in building artificial cells. We apologize for the omission of relevant literature.
References
- 1.Xu C, Hu S, Chen X. Artificial cells: from basic science to applications. Mater Today (Kidlington) 2016;19(9):516–32. doi: 10.1016/j.mattod.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwille P. Jump-starting life? Fundamental aspects of synthetic biology. J Cell Biol. 2015;210(5):687–90. doi: 10.1083/jcb.201506125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buddingh BC, van Hest JCM. Artificial Cells: Synthetic Compartments with Life-like Functionality and Adaptivity. Acc Chem Res. 2017;50(4):769–77. doi: 10.1021/acs.accounts.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen Gupta A. Bio-inspired nanomedicine strategies for artificial blood components. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017 doi: 10.1002/wnan.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lentz BR. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog Lipid Res. 2003;42(5):423–38. doi: 10.1016/s0163-7827(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 6.Sneller MC, Dale Janet K, Straus Stephen E. Autoimmune lymphoproliferative syndrome. Current opinion in rheumatology. 2003;15(4):417–21. doi: 10.1097/00002281-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Wattel E, Cambier N, Caulier MT, Sautiere D, Bauters F, Fenaux P. Androgen therapy in myelodysplastic syndromes with thrombocytopenia: a report on 20 cases. British journal of haematology. 1994;87(1):205–8. doi: 10.1111/j.1365-2141.1994.tb04895.x. [DOI] [PubMed] [Google Scholar]
- 8.Silverman GJ, Weisman Stuart. Rituximab therapy and autoimmune disorders: prospects for anti–B cell therapy. Arthritis & Rheumatology. 2003;48(6):1484–92. doi: 10.1002/art.10947. [DOI] [PubMed] [Google Scholar]
- 9.Berchtold P, McMillan R. Therapy of chronic idiopathic thrombocytopenic purpura in adults. Blood. 1989;74(7):2309–17. [PubMed] [Google Scholar]
- 10.Hillyer CD. Blood Banking and Transfusion Medicine: Basic Principles & Practice. Elsevier Health Sciences. 2007:308–10. [Google Scholar]
- 11.Connell N. Transfusion Medicine. Primary care. 2016;43(4):651–9. doi: 10.1016/j.pop.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Bertram JP, Williams CA, Robinson R, Segal SS, Flynn NT, Lavik EB. Intravenous hemostat: nanotechnology to halt bleeding. Sci Transl Med. 2009;1(11):11ra22. doi: 10.1126/scitranslmed.3000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown AC, Stabenfeldt SE, Ahn B, Hannan RT, Dhada KS, Herman ES, et al. Ultrasoft microgels displaying emergent platelet-like behaviours. Nat Mater. 2014;13(12):1108–14. doi: 10.1038/nmat4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu CM, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–21. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donovan AJ, Kalkowski J, Szymusiak M, Wang C, Smith SA, Klie RF, … Liu Y. Artificial dense granules: a procoagulant liposomal formulation modeled after platelet polyphosphate storage pools. Biomacromolecules. 2016;17(8):2572–81. doi: 10.1021/acs.biomac.6b00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson ED, Gan R, Hodgman CE, Jewett MC. Cell-free protein synthesis: applications come of age. Biotechnol Adv. 2012;30(5):1185–94. doi: 10.1016/j.biotechadv.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garamella J, Marshall R, Rustad M, Noireaux V. The All E. coli TX-TL Toolbox 2.0: A Platform for Cell-Free Synthetic Biology. ACS Synth Biol. 2016;5(4):344–55. doi: 10.1021/acssynbio.5b00296. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y. Cell-free synthetic biology: Engineering in an open world. Synthetic and Systems Biotechnology. 2017;2(1):23–7. doi: 10.1016/j.synbio.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu AP, Fletcher DA. Biology under construction: in vitro reconstitution of cellular function. Nat Rev Mol Cell Biol. 2009;10(9):644–50. doi: 10.1038/nrm2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwille P. Bottom-up synthetic biology: engineering in a tinkerer’s world. Science. 2011;333(6047):1252–4. doi: 10.1126/science.1211701. [DOI] [PubMed] [Google Scholar]
- 21.Nourian Z, Scott A, Danelon C. Toward the assembly of a minimal divisome. Syst Synth Biol. 2014;8(3):237–47. doi: 10.1007/s11693-014-9150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arriaga LR, Datta SS, Kim SH, Amstad E, Kodger TE, Monroy F, et al. Ultrathin shell double emulsion templated giant unilamellar lipid vesicles with controlled microdomain formation. Small. 2014;10(5):950–6. doi: 10.1002/smll.201301904. [DOI] [PubMed] [Google Scholar]
- 23.Coyne CW, Patel K, Heureaux J, Stachowiak J, Fletcher DA, Liu AP. Lipid bilayer vesicle generation using microfluidic jetting. Journal of visualized experiments. 2014:e51510. doi: 10.3791/51510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshpande S, Caspi Y, Meijering AE, Dekker C. Octanol-assisted liposome assembly on chip. Nat Commun. 2016;7:10447. doi: 10.1038/ncomms10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noireaux V, Libchaber A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc Natl Acad Sci U S A. 2004;101(51):17669–74. doi: 10.1073/pnas.0408236101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caschera F, Lee JW, Ho KK, Liu AP, Jewett MC. Cell-free compartmentalized protein synthesis inside double emulsion templated liposomes with in vitro synthesized and assembled ribosomes. Chem Commun (Camb) 2016;52(31):5467–9. doi: 10.1039/c6cc00223d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho KK, Murray VL, Liu AP. Engineering artificial cells by combining HeLa-based cell-free expression and ultrathin double emulsion template. Methods Cell Biol. 2015;128:303–18. doi: 10.1016/bs.mcb.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majumder S, Garamella J, Wang YL, DeNies M, Noireaux V, Liu AP. Cell-sized mechanosensitive and biosensing compartment programmed with DNA. Chem Commun (Camb) 2017 doi: 10.1039/c7cc03455e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caschera F, Noireaux V. Integration of biological parts toward the synthesis of a minimal cell. Curr Opin Chem Biol. 2014;22:85–91. doi: 10.1016/j.cbpa.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Marques-Gallego P, de Kroon AI. Ligation strategies for targeting liposomal nanocarriers. Biomed Res Int. 2014;2014:129458. doi: 10.1155/2014/129458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shum HC, Lee D, Yoon I, Kodger T, Weitz DA. Double emulsion templated monodisperse phospholipid vesicles. Langmuir. 2008;24(15):7651–3. doi: 10.1021/la801833a. [DOI] [PubMed] [Google Scholar]
- 32.Deng N-N, Yelleswarapu Maaruthy, Huck Wilhelm TS. Monodisperse uni-and multicompartment liposomes. Journal of the American Chemical Society. 2016;138(24):7584–91. doi: 10.1021/jacs.6b02107. [DOI] [PubMed] [Google Scholar]
- 33.Yang YJ, Feng X, Xu N, Pang DW, Zhang ZL. Generation of sub-femtoliter droplet by T-junction splitting on microfluidic chips. Appl Phys Lett. 2013;102(12) [Google Scholar]
- 34.Ho KK, Lee JW, Durand G, Majumder S, Liu AP. Protein aggregation with poly(vinyl) alcohol surfactant reduces double emulsion-encapsulated mammalian cell-free expression. PLoS One. 2017;12(3):e0174689. doi: 10.1371/journal.pone.0174689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato A, Yanagisawa M, Sato YT, Fujiwara K, Yoshikawa K. Cell-sized confinement in microspheres accelerates the reaction of gene expression. Scientific reports. 2012:2. doi: 10.1038/srep00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teng J, Loukin S, Anishkin A, Kung C. The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflugers Arch. 2015;467(1):27–37. doi: 10.1007/s00424-014-1530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kocer A. Mechanisms of mechanosensing - mechanosensitive channels, function and re-engineering. Curr Opin Chem Biol. 2015;29:120–7. doi: 10.1016/j.cbpa.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Chiang CS, Anishkin A, Sukharev S. Gating of the large mechanosensitive channel in situ: estimation of the spatial scale of the transition from channel population responses. Biophys J. 2004;86(5):2846–61. doi: 10.1016/S0006-3495(04)74337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng Z, Pak OS, Feng Z, Liu AP, Young YN. On the gating of mechanosensitive channels by fluid shear stress. Acta Mechanica Sinica. 2016;32(6):1012–22. [Google Scholar]
- 40.Pak OS, Young YN, Marple GR, Veerapaneni S, Stone HA. Gating of a mechanosensitive channel due to cellular flows. Proc Natl Acad Sci U S A. 2015;112(32):9822–7. doi: 10.1073/pnas.1512152112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9(7):543–56. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 42.Hu K, Carroll J, Rickman C, Davletov B. Action of complexin on SNARE complex. J Biol Chem. 2002;277(44):41652–6. doi: 10.1074/jbc.M205044200. [DOI] [PubMed] [Google Scholar]
- 43.Yang H, Kim A, David T, Palmer D, Jin T, Tien J, et al. TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell. 2012;151(1):111–22. doi: 10.1016/j.cell.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]