Abstract
Cancer evolution is a step-wise non-linear process that may start early in life or later in adulthood, and includes pre-malignant (indolent) and malignant phases. Early somatic changes may not be detectable or are found by chance in apparently healthy individuals. The same lesions may be detected in pre-malignant clonal conditions. In some patients, these lesions may never become relevant clinically whereas in others, they act together with additional pro-oncogenic hits and thereby contribute to the formation of an overt malignancy. Although some pre-malignant stages of a malignancy have been characterized, no global system to define and to classify these conditions is available. To discuss open issues related to pre-malignant phases of neoplastic disorders, a working conference was organized in Vienna in August 2015. The outcomes of this conference are summarized herein and include a basic proposal for a nomenclature and classification of pre-malignant conditions. This proposal should assist in the communication among patients, physicians and scientists, which is critical as genome-sequencing will soon be offered widely for early cancer-detection.
Keywords: Pre-malignant states, Neoplastic stem cells, Clonal evolution, Cancer
Highlights
-
•
Premalignant neoplastic conditions are characterized by early somatic events without evidence of an overt neoplasm.
-
•
Overt neoplasms can be divided into premalignant (indolent) neoplasms and malignant (aggressive) neoplasms.
-
•
An updated nomenclature and classification of clonal conditions seems of utmost importance in the genome era of medicine.
1. Introduction
Cancer evolution is a step-wise process that involves multiple molecular defects, and is characterized by clonal diversification, sub-clone selection, and clonal expansion (Cahill et al., 1999, Greaves and Maley, 2012, Nowell, 1976, Vogelstein and Kinzler, 1993, Yates and Campbell, 2012). Inherited (germline) lesions, mutagenic factors, epigenetic events, and the (deregulated) immune system may contribute to cancer evolution, and all these factors may act together to drive carcinogenesis (Alexandrov et al., 2013, Baylin and Jones, 2011, Bodian et al., 2014, Negrini et al., 2010, Pan et al., 2014). In many disease-models, cancer evolution is a long-lasting process that involves multiple phases. Early stages are often silent clinically and either not detectable or detected by chance in apparently healthy individuals (Busque et al., 2012, Genovese et al., 2014, Laurie et al., 2012, Martincorena and Campbell, 2015). Other early stages may be identified through a clinically relevant (triggering) mediator, such as a vasoactive peptide or amine, produced by clonal cells (Akin et al., 2007, Gülen et al., 2014, Pelosof and Gerber, 2010).
In this early phase of cancer evolution, pre-malignant, self-renewing neoplastic stem cells are slowly cycling or dormant, and the resulting sub-clones are usually too small to be detected histologically (Corces-Zimmerman and Majeti, 2014, Pandolfi et al., 2013, Valent et al., 2013). In the next phase, the clonal condition forms slowly expanding, ‘indolent’ lesions that may still be overlooked clinically, but are usually detected by histology when the affected organ is examined in sufficient detail. Over time, one or more sub-clone(s) acquire(s) additional molecular lesions, and once a sufficient number of (driver) lesions have accumulated, the affected sub-clone(s) will expand and lead to a histologically visible neoplasm. Remarkably, such overt (large-sized) neoplasms may still exhibit an indolent course for a certain time period (months to years) before additional somatic lesions convert the disease into an aggressive malignancy (Ashkenazi et al., 2008, Baylin and Jones, 2011, Brosnan and Iacobuzio-Donahue, 2012, Gerlinger et al., 2014, Marusyk et al., 2012, Vogelstein and Kinzler, 1993).
Over the past few years, a number of projects have been initiated to explore whole genome profiles in healthy people or individuals at risk for development of cancer or other diseases (Altman, 2013, Jaiswal et al., 2014, Jaiswal et al., 2017, Khoury et al., 2003, Vassy et al., 2014, Yang et al., 2013). In most instances, blood cells are tested and are the subject of intensive research in this field. Indeed, it has been described that several mutations in critical target-genes, such as DNMT3A or TET2, are detectable in leukocytes in a group of healthy donors or those with ‘skewed’ hematopoiesis without evidence of an overt bone marrow neoplasm (Busque et al., 2012, Genovese et al., 2014, Jaiswal et al., 2014, Jaiswal et al., 2017, Laurie et al., 2012, Martincorena and Campbell, 2015). Although these conditions bear a certain risk for the development of a myeloid neoplasm and to develop cardiovascular disorders, the outcome in individual subjects is unpredictable and it thus remains uncertain how to classify and manage these conditions.
Based on these developments it is important and necessary to prepare sufficient screening programs, to standardize technologies, and to develop diagnostic algorithms and predictive scores that will support the management of ‘healthy’ individuals exhibiting somatic lesions. However, before such algorithms and management recommendations can be proposed, it is imperative to establish a basic nomenclature and common language through which these conditions are classified and explained to affected persons and physicians. To date, no global terminology and no generally accepted proposal to standardize evaluations and management strategies in affected individuals is available.
On the occasion of commemorating the 100th anniversary of the death of Paul Ehrlich, the Vienna Cancer Stem Cell Club and the Medical University of Vienna organized a working conference to discuss open issues related to pre-malignant neoplastic states in August 2015. The outcomes of this discussion are summarized herein together with a basic proposal to classify pre-malignant stages of cancer evolution. A detailed description of the consensus discussion and the consensus-making process are provided in the supplemental appendix. In the future, our proposal may be revised and adjusted to various disease models and organ systems, and may provide a useful communication-platform for physicians, scientists, and affected individuals.
2. Historical Overview
A detailed historical overview is provided in the supplement. In the second half of the 20th century, a robust multi-hit theory of cancer evolution was established. Later, a nomenclature of premalignant (indolent) and aggressive (malignant) conditions was proposed and was adopted by the World Health Organization (WHO). An example for an early premalignant condition defined by a molecular abnormality is monoclonal gammopathy of uncertain (unknown) significance (MGUS) (Kyle, 1995, Davies et al., 2003). More recently, similar conditions have been proposed for early pre-phases of myeloid neoplasms defined by certain somatic mutations (Jaiswal et al., 2014, Steensma et al., 2015). These cases highlight the fact that even the earliest molecular events that occur during cancer evolution may be detected by sensitive testing for somatic mutations.
However, several questions remain. For example, it remains unknown whether a clonal pre-malignant process has the ability to progress in the absence of any detectable somatic mutation. Is every somatically mutated cell capable of producing a persistent lesion? Based on the model of cancer stem cells, only cells with self-renewal capacity should have the ability to produce a permanent lesion (neoplasm). However, some of the somatic lesions that are acquired may ‘reprogram’ more mature target cells into real ‘neoplastic stem cells’ with unlimited growth capabilities. In some instances, the lesion may be too small to follow or may seemingly disappear (but in fact persists in small clones).
Despite these uncertainties the faculty agreed that a persistent somatic mutation is highly indicative of a stable clonal process (derived from neoplastic stem cells) that bears a theoretical potential to develop into a neoplastic condition (provided that the lesion persists in the follow up). Based on this assumption, current knowledge about cancer biology, and outcomes of our conference, the following definitions are proposed.
3. Basic Definitions: Normal, Reactive, Clonal, Neoplastic, Malignant
In healthy tissues, normal stem cells undergo proliferation, self-renewal, and differentiation with subsequent maturation. These three processes are tightly regulated and are adjusted precisely to the demand to replenish mature end cells in a given organ (Knoblich, 2008, Manz and Boettcher, 2014, Wilson et al., 2009). In regenerative and reactive states, the turnover rate may increase to guarantee the production of a sufficient number (often transient excess) of terminally differentiated cells (Manz and Boettcher, 2014). In such states, the overall response is considered polyclonal and physiologic, even if somatically mutated clones (if present) may also expand transiently.
As assessed by morphology and/or histology alone, it may be difficult to distinguish between ‘pure reactive’ and ‘early neoplastic’ conditions, especially when no definitive cell atypia is present. In these cases, molecular markers and phenotypic studies are often employed and may assist in the final diagnosis. However, even in the presence of a molecular lesion, the nature and prognosis of the condition may not always be clear, especially when only a few cells appear to be mutated and no follow up is available. In this regard it is also noteworthy that in some neoplastic states, like Hodgkin lymphoma, the malignant cells represent only a minor fraction of the total expanded lymphoma mass.
Based on our current understanding of how a neoplasm develops, any somatic (molecular or cytogenetic) lesion that is detected must be regarded as an indication of a clonal process with certain neoplastic potential, provided that it i) persists (demonstrable at repeated time points), ii) has a pro-oncogenic potential, and iii) is expressed in a relevant type and number of cells. For example, based on available guidelines, demonstration of (only) one single abnormal metaphase is insufficient to define a (relevant) clone in cytogenetic studies (Simons et al., 2013). The following definitions should delineate between normal, reactive, neoplastic, neoplastic-pre-malignant, and malignant (aggressive):
Physiologic cells are normal or reactive cells defined as normal-appearing and normal-behaving cells that contain a genome that is not different from the genetic background (germline) of the individual examined by conventional sequencing analysis and/or karyotyping. ‘Reactive’ cells are defined by i) a normal or slightly abnormal morphology (no major cell atypia) with or without abnormal cell composition (e.g. an increase in immature cells in reactive states: e.g. left shifted reactive myelopoiesis, hyper-regenerative states), by ii) being related to a triggering (reversible) event, and by iii) possessing a genome that is not different from the genetic background by conventional sequencing analysis and karyotyping.
‘Clonal’ (‘monoclonal’) cells are defined by the presence of at least one somatic mutation (lesion) that is detectable by conventional sequencing or karyotyping in affected cells (sometimes with clonal viral integration into the genome, e.g. EBV), but is not detectable in the germline of the affected individual. If no such lesion is found, the cells may still be clonal and pathologic (abnormal) in nature which may be documented i) by histomorphologic criteria or ii) indirectly by phenotypic or functional studies. However, with increasing sensitivity, sequencing studies are expected to reveal somatic aberrations in most if not all clonal states within the near future. Depending on the involvement of (neoplastic) stem cells and their ability to escape immune surveillance as well as the presence of triggering factors (integrating viruses, bacteria, inflammation, etc.) such monoclonal cells may or may not persist. In some instances, the size of the clone may be small and thus only be detected transiently during a reactive process and then may (seemingly) ‘disappear’.
Only persistent clonal cells should be regarded as neoplastic because only these cells have a certain potential to produce an overt neoplasm. As a result, ‘neoplastic’ is defined by the presence of at least one relevant somatic lesion in (a relevant number of) abnormal cells that persist. Neoplastic cells always contain a stem cell compartment and may or may not be (or become) clinically relevant. Clinically relevant neoplastic cells may produce i) a paraneoplastic condition (only), ii) an indolent (pre-malignant) neoplasm, or iii) an overt malignancy (aggressive neoplasm).
Neoplastic cells (and their stem cells) can be divided into ‘pre-malignant’ cells and ‘malignant’ (neoplastic) cells. Similarly, clinically detectable neoplasms should be divided into pre-malignant (indolent or non-aggressive) neoplasms and malignant (aggressive) neoplasms, general concepts also propagated by the WHO. As long as no aggressive neoplasm can be diagnosed, the cells and the related condition (neoplasm) should be termed pre-malignant or indolent, and the neoplastic cells that form these lesions should be termed pre-malignant neoplastic cells. Examples of pre-malignant neoplastic conditions are adenomas, indolent lymphomas, indolent systemic mastocytosis, or early stages of myeloproliferative neoplasms (MPN). However, pre-malignant (stem) cells have a certain potential to transform into fully malignant (stem) cells after a variable latency period.
Malignant neoplastic cells (cancer cells) are defined by their ability to produce an overt malignancy (cancer). Clinically, the term ‘aggressive’ or ‘malignant’ is usually associated with overt (and often rapid) organ damage. As a result, aggressive (malignant) neoplasms are defined by the potential lethality in their natural clinical course (without therapy). However, it is worth noting that rarely also indolent (premalignant) neoplasms can produce a lethal condition, for example, a benign meningioma that results in death because of its growth in a critical region of the brain or a myelodysplastic syndrome (MDS) with severe cytopenia resulting in septicemia. A summary of axiom definitions is provided in Table 1.
Table 1.
Definitions and proposed terminology of pre-malignant and malignant cells.
| Operative term | Definition and criteria |
|---|---|
| Normal cellsa | Cells with normal gene composition, normal morphology, and normal function; no somatic lesions are found and the germline background is normal or it is abnormal but does not interfere with morphology or function of these cells [even if being disease-triggering in other organsb]. |
| Reactive cellsa | The same definition and criteria apply as for normal cells, but cell composition within an organ may be altered and/or slight deviations in morphology and/or numbers of cells are detected. In most cases a triggering reactive process is found. |
| Clonal cells (Monoclonal cells) | Persistent or non-persistent cells that harbor one or more somatic lesions (mutations) that are/is not detected in the germline in the same patient. In the absence of any visible mutation, the clonal nature of cells may still be confirmed or at least proposed by other established assays, such as immunofixation (to detect a paraprotein), PCR detection of IGH, IGL, or TCR gene rearrangements in case of lymphoid cells and clonal virus integration in the genome in viral-associated neoplasms; or by immunophenotyping in certain hematologic neoplasmsc. |
| Neoplastic cells | Monoclonal cells (same definition as above) that persist and are thus detectable in repeat testing in follow up.dNeoplastic cells always contain a (neoplastic) stem cell compartment. Neoplastic (stem) cells either form an overt neoplasm or have a (variable) potential to develop a neoplasm over time. Neoplastic cells can be divided into pre-malignant and malignant neoplastic cells |
| Pre-malignant Neoplastic cells | Neoplastic cells (definitions/criteria as above) that form no visible (sometimes an occult) or a visible neoplastic condition that behaves as an indolent (= pre-malignant) disease. Most pre-malignant neoplastic conditions have a variable (often unpredictable) potential to transform into a fully malignant/aggressive neoplasm (cancer). |
| Malignant cells (= Cancer cells) | Neoplastic cells that comprise a clinically overt malignancy (cancer, acute leukemia, aggressive lymphoma, etc.). In many instances, these cells exhibit a highly abnormal morphology, multiple molecular lesions, and a relatively high proliferation rate. In solid cancers, malignant cells often grow in an invasive manner. In each instance, the expansive growth is usually associated with overt organ damage. |
Normal and reactive cells can be collectively referred to as ‘physiologic cells.
Example: HFE gene mutated leukocytes.
Examples are expression of CD5 on B cells in B chronic lymphocytic leukemia, or expression of CD25 on mast cells in systemic mastocytosis.
Detectable for at least 3 months.
4. Defined Phases of Cancer Evolution
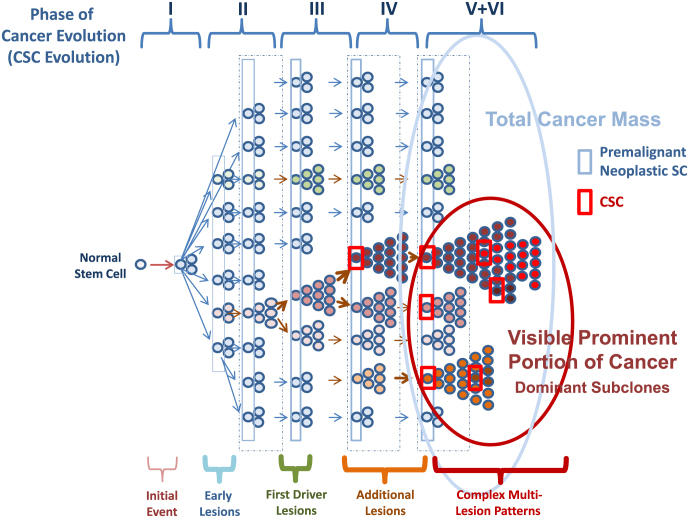
For a number of cancer/leukemia types defined by a specific driver mutation, such as BCR-ABL1 + chronic myeloid leukemia (CML), six phases of cancer evolution have been postulated (Valent, 2011, Valent et al., 2012b) (Fig. 1). The genetic background (germline) can be regarded as Phase “0” and as such can contribute to a (familial) predisposition for cancer evolution, either by lack of a tumor suppressor or by pro-oncogenic gain-of-function mutations in critical target genes (Astuti et al., 2012, Brugieres et al., 2012, Dessars et al., 2009, Gustafson et al., 2015, Hinds et al., 2016, Lapunzina et al., 2014, Spinner et al., 2014). Phase 1 is a pre-malignant phase where a persistent clonal (somatic) lesion is present in small-sized clones, but no disease-specific aberration and no overt neoplasm is found. Examples are healthy individuals with DNMT3A or TET2 mutations (at low variant allele frequency, VAF) or other mutations (at low VAF) that are (per se) not able to create a malignancy. This phase is usually overlooked with current screening assays, but is expected to be detected in many individuals in the future through the broad application of large-scale (and high sensitive) genome sequencing studies.
Fig. 1.
Model of cancer evolution
an initial transforming event (Initial Event) converts a normal stem/progenitor cell into a somatically mutated clonal stem cell. In case the stem cell can survive and retains self-renewal capacity, it has become a neoplastic premalignant stem cell (Early Lesions). Usually, these cells are slowly cycling cells or dormant cells and contain passenger mutations. After several years or decades, the resulting clone has acquired first driver lesions and expands and may have replaced some or most of the polyclonal cells in the normal organ (First Driver Lesions). At that time, neoplastic cells and normal cells are often indistinguishable by morphology or in functional terms. In a next step, one or more sub-clones acquire additional driver-lesions (Additional Lesions). Depending on the type of lesion and the type of preformed clones and their passenger signature, the resulting new subclones may either expand immediately, or may again reside in a slowly cycling or even dormant stage. In these patients, the driver mutation may or may not be detectable depending on the size of the affected sub-clones. However, as soon as additional driver lesions have been acquired, the resulting sub-clones can finally expand and replace the normal organ (Complex Multi-Lesion Patterns). In many cases, the created neoplasm may still behave as an indolent driver-positive neoplasms for some time. However, unless treated, many of these conditions will finally progress to an aggressive malignancy.
An important aspect is that clonal hematopoiesis increases with age (Busque et al., 2012, Jaiswal et al., 2014, Jaiswal et al., 2017). Therefore, this condition has also been referred to as age-related clonal hematopoiesis (ARCH) (Jaiswal et al., 2014). However, it remains unknown whether this early potential pre-phase of a neoplasm is associated with the natural process of aging. Indeed, during the process of aging, our cells may gradually shift from a polyclonal to a (more and more) restricted, oligo-clonal and subsequently a mono-clonal (somatic) state in various organs.
Another interesting aspect is that this early phase of cancer evolution may become clinically relevant and visible many years after an overt malignancy (with a distinct driver mutation) was detected, for example, when the dominant (driver +) sub-clone(s) had been eliminated by therapy and a relapse with a new driver lesion occurs. Examples are JAK2 V617F-negative secondary acute myeloid leukemia (AML) in patients with JAK2 V617F + MPN or a BCR-ABL1-negative blast phase in BCR-ABL1 + CML. Finally, such early lesions may trigger clinically relevant non-neoplastic disease processes such as cardiovascular disorders (Jaiswal et al., 2014, Jaiswal et al., 2017).
Phase 2 is defined by (a) persistent disease-related somatic driver lesion(s) in small-sized clones. Examples are minimal amounts of BCR-ABL1 or JAK2 V617F in apparently healthy individuals (Biernaux et al., 1995, Passamonti et al., 2007). These clones may be detected by chance or remain undetected for some time until the clone expands in size. In other cases, the clone-size remains small over years or even decades, especially when no or only a few additional mutations are acquired. In other words, driver lesions are not necessarily capable of producing an overt neoplasm unless additional lesions are acquired by the driver-expressing sub-clones. In this regard it should be mentioned that many driver lesions, such as KIT D816V, induce survival and differentiation rather than proliferation in stem cells (Mayerhofer et al., 2008). However, after a certain latency period, one or more sub-clones acquire additional lesions and hits, and then expand.
In Phase 3, the clonal process comprising one or more lesion(s) has replaced some or most of the normal organ, and sometimes even mimics the organ, such as in very early chronic phase CML (normal leukocyte counts), early stages of a follicular lymphoma, very early stages of JAK2 V617F + neoplasms, or early stages of adenomas. As assessed by morphology, the mutated cells may or may not show atypia in cytological and/or histomorphologic analyses. In Phase 4, an overt but pre-malignant neoplasm is detected by clinical, laboratory, and histomorphologic (WHO) criteria. Examples are non-invasive local (in situ) carcinomas, overt chronic phase CML, or early stage indolent lymphomas.
Phase 5 is characterized by a more diffuse or invasive growth and/or clinical and laboratory signs of acceleration/progression (example: accelerated phase CML, aggressive lymphomas, invasive carcinoma). Phase 6 is a terminal stage of an advanced malignancy that usually consists of mainly immature cells (often with high proliferation rate) and is usually resistant to conventional therapies (examples: secondary AML, blast phase of CML, metastatic carcinoma, aggressive therapy-resistant lymphomas, mast cell leukemia and others). It should be noted, however, that sometimes, earlier phases of a malignancy may also cause life-threatening conditions (examples: unresectable cerebral tumor or MDS). The six proposed phases of cancer evolution are shown in Fig. 1 and Table 2.
Table 2.
The six phases of cancer evolution and clinical correlates.
| Phase | Biologic status | Clinical correlates (examples) |
|---|---|---|
| 0 | Genetic Background (SNPs, mutations, absent TSGs) | Familial predispositions and familial clustering of cancer |
| I | Somatic evolution: step-wise acquisition of somatic passenger lesions over time (during aging) | Passenger mutations found in healthy individuals; e.g. CHIP with DNMT3A mutation; or clonal IGH/IGL or TCR gene rearrangements (but without a morphologic or phenotypic correlate) |
| II | Acquisition of driver lesions in small-sized sub-clones | Low levels of JAK2 V617F or BCR-ABL1 in apparently healthy individuals; or clonal persistent BCL2 rearrangements in healthy individuals (CHOP); microadenomas, BRAF-mutated pigment-lesions in the skin, small-sized T cell or B cell clones with aberrant specific immunophenotype |
| III | Expansion of sub-clones carrying driver lesions – until replacement of the normal organ system, but no evidence of invasive or aggressive expansion; in many instances, neoplastic cells replace the normal tissue/organ | Indolent neoplasms, pre-invasive carcinomas, early stages of indolent NHL, in situ indolent NHL; LR-MDS; early chronic phase CML, early stages of JAK2-mutated MPN; indolent systemic mastocytosis |
| IV | Overt expansion beyond the affected organ system; but may still be more or less indolent | Chronic phase CML; overt MPN; MDS, minimal invasive tumors; overt indolent NHL; smoldering systemic mastocytosis |
| V | Aggressive/advanced malignancy⁎ | Accelerated CML or MPN; local solid tumors/carcinoma; grade IIIa FL, myeloma, aggressive systemic mastocytosis |
| VI | Progressive malignancya Metastatic cancer | Blast phase of CML, (secondary) AML, grade IIIb FL, aggressive NHL; mast cell sarcoma, mast cell leukemia; metastatic solid tumor/carcinoma |
In some phase V/VI malignancies such as aggressive NHL or mast cell leukemia, the previous (preceding) phases of cancer evolution remain undetected in most patients. Abbreviations: CHIP, clonal hematopoiesis of indeterminate potential; CHOP, clonal hematopoiesis with substantial oncogenic potential; NHL, Non Hodgkin lymphoma; LR-MDS, low-risk myelodysplastic syndrome; CML, chronic myeloid leukemia; MPN, myeloproliferative neoplasm; AML, acute myeloid leukemia; FL, follicular lymphoma.
5. Proposal for the classification of pre-malignant conditions
Whereas axiom definitions and related theories about cancer evolution may be essential to establish a theoretical model as a basis for our understanding of how cancer evolution can be evaluated in the context of genome-wide aberration-profiles, it is equally important to provide an appropriate communication-system and solid nomenclature to physicians and scientists who (will) have to deal with a rapidly increasing number of referrals of clonal pre-malignant conditions in daily practice as whole (or large scale) genome sequencing will soon be implemented in health care systems. A key to the development of such a nomenclature is our knowledge about the likelihood a clonal condition will progress to an overt malignancy after a certain time-interval. Unfortunately, in many (if not most) instances the actual risk is not known for individual somatic mutations and various combinations of lesions. However, there are certain mutations in critical driver genes that have been associated with a quite substantial risk of cancer evolution. Good examples are BCR-ABL1 and JAK2 V617F in myeloid neoplasms, RAS mutations in colon and pancreatic cancer, BRAF mutations in thyroid cancer, melanomas, and hairy cell leukemia, or BRCA1 and BRCA2 mutations in ovarian-, breast-, and prostatic carcinomas.
These lesions may occur in apparently healthy individuals without any histological signs or clinical symptoms, but the risk of disease manifestation must be considered as substantial or even high. Therefore, these mutations, if somatic and persistent, should be regarded as associated with a high oncogenic potential, and should thus be separated from other (more passenger-type) lesions with apparently lower risk. For such other lesions with unknown or lower risk, the recommendation is to call the condition ‘clonal condition of undetermined (unknown) clinical significance’ or ‘clonal condition of indeterminate clinical potential’. An example would be clonal hematopoiesis of indeterminate potential (CHIP) (Steensma et al., 2015). However, in the case of a ‘higher risk mutation’, the nomenclature should change to a more definitive term, such as ‘clonal hematopoiesis with substantial oncogenic potential’ (CHOP) provided that the clonal cells expressing the mutation are persistent and the (higher) VAF is indicative of a relevant number of affected cells. Still, however, the actual risk of progression from clonal hematopoiesis to an overt neoplasm remains to be determined for various somatic mutations, for the VAF burden (in each type of lesion) and for various lesion-combinations, in larger studies. In addition, the clinical impact of each mutation (and mutation-pattern) has to be based on additional clinical and laboratory parameters, including mutagenic events, age, co-morbidities and the overall situation in each case. Examples of conditions that may fulfil CHIP or CHOP criteria are listed in Table 3.
Table 3.
Examples of molecular and cytogenetic lesions (hematology-context) detectable in apparently healthy individuals and correlation with proposed terminology.
| Term | Molecular correlate | Examples | at Risk for |
|---|---|---|---|
| CHIP | Early mutations and cytogenetic lesions that may be detected in healthy individuals (passenger lesions⁎) | TET2 mutation | Myeloid neoplasms |
| DNMT3A mutation | Myeloid neoplasms | ||
| GNAS mutations | Myeloid neoplasms | ||
| ASXL1 mutations | Myeloid neoplasms | ||
| SF3B1 mutations | Myeloid neoplasms | ||
| PPM1D mutations | Myeloid neoplasms | ||
| IGH-rearrangement | B cell neoplasms | ||
| TCR-rearrangement | T cell neoplasms | ||
| -Y | BM neoplasms | ||
| CHOP | Disease-determining mutations and related karyotype anomalies (driver lesions⁎) | BCR-ABL1 p210 | CML |
| JAK2 V617F | MPN | ||
| FIP1L1-PDGFRA, del CHIC2 | CEL/HES | ||
| KIT D816V | SM | ||
| RUNX1- RUNX1T1 | AML | ||
| CBFβ-MYH11 | AML | ||
| FLT3 ITD mutations | AML | ||
| KRAS, HRAS mutations | AML | ||
| BCL2-IGJ(H) | FL | ||
| IGH-CCND1 | MCL | ||
| − 7,+8,5q-, … | MDS/AML | ||
| t(8;21), inv16, … | AML | ||
| t(9;22) | CML | ||
| t(14;18) | FL | ||
| t(11;14) | MCL | ||
| t(8;14) | Burkitt NHL |
Depending on germline patterns, affected cells and organs, and additional lesions, so-called passenger lesions may become drivers of oncogenesis, and vice versa, some of the drivers may be detected over decades without visible signs of tumor formation. Therefore, the terms ‘driver’ and ‘passengers’ lesions (mutations) should be used with caution and always in the context of the overall situation in each patient. Abbreviations: CHIP, clonal hematopoiesis of indeterminate clinical potential; CHOP, clonal hematopoiesis with substantial oncogenic potential; CML, Ph + chronic myeloid leukemia; CEL, chronic eosinophilic leukemia; HES, hypereosinophilic syndrome; SM, systemic mastocytosis; AML, acute myeloid leukemia; FL, follicular lymphoma; MCL, mantle cell lymphoma; TCR, T cell receptor; IGH, immunoglobulin heavy chain gene.
In another group of patients, the morphology or numbers of affected cells may be abnormal, but definitive criteria for an overt neoplasm or a malignancy are not (yet) met, even if a somatic lesion is detected. Examples are an increase in (clonal) lymphocytes or plasma cells without histologic evidence or criteria qualifying for a lymphoproliferative disease, idiopathic cytopenia of undetermined significance (ICUS), or idiopathic eosinophilia of unknown significance (HE-US). Again, the final terminology may depend on the presence and type of molecular lesion(s) detected and the number and types of cell(s) involved.
For example, hypereosinophilia (HE) without any molecular aberration or marker and without organ damage may well be called HE-US (Valent et al., 2012a). However, even a slight eosinophilia with documented expression of FIP1L1-PDGFRA in neoplastic cells should not be called a condition of undetermined significance (US), even if no organ damage is seen, simply because the risk for such a patient to develop clinically significant organ damage (e.g. cardiac thrombosis/fibrosis) or blast phase over time is high (Gotlib and Cools, 2008, Valent et al., 2012a, Metzgeroth et al., 2013). In these patients, the primary diagnosis by WHO criteria is a myeloid or lymphoid neoplasm with rearranged PDGFR and further investigations define the underlying histopathologic diagnosis (Gotlib and Cools, 2008, Valent et al., 2012a). If no such diagnosis can be established and no marked eosinophilia is present, the condition should be termed clonal hematopoiesis (eosinophilia) with FIP1L1-PDGFRA and evident oncogenic potential (CHOP). This is of particular importance as early treatment with low-dose imatinib is usually sufficient to prevent eosinophil-induced organ damage in these patients (Gotlib and Cools, 2008, Metzgeroth et al., 2013). Examples for early clonal lesions and other related premalignant conditions as well as potential mechanisms underlying clonal stability, instability and progression from pre-malignant to malignant conditions are discussed in the supplement. In addition, the supplemental appendix contains a discussion of the new concept in the context of premalignant and malignant neoplastic stem cells. The role and impact of neoplastic stem cell classes in the evolution of premalignant and malignant conditions (cancer) is shown in Fig. 1.
6. Impact of the Proposal on Daily Practice and Health Care Strategies
In the past few years, major attempts have been made to develop large-scale genome sequencing technologies for use in daily practice in various disease models (Altman, 2013, Jaiswal et al., 2014, Khoury et al., 2008, Lynch et al., 2007, Malhotra, 2014, Vassy et al., 2014, Wang et al., 2015). Such advanced technologies will in the near future be used routinely in patients with suspected or overt malignancies. In addition, these techniques will soon be applied for early cancer detection in apparently healthy individuals. As a consequence, many healthy individuals will undergo large-scale genomic sequencing; and many will be informed that they are ‘carriers’ of one or more pro-oncogenic somatic mutations or other genetic deviations predisposing for the development of neoplastic conditions (Altman, 2013, Jaiswal et al., 2014, Steensma et al., 2015). Therefore, physicians are likely to be besieged with questions from patients and otherwise healthy individuals, and challenged by the need to manage referrals and cohorts of somatically altered individuals.
In order to address this challenge, it is of utmost importance to develop a basic nomenclature and classification for the somatic lesions and combinations of lesions that may be detected in apparently healthy individuals, and to initiate observational trials in order to define the prognostic impact and estimate the related risk that each individual lesion (mutation) and each combination of somatic and/or germ line mutations imparts. Our proposal may be a first step in creating a basic terminology and a related classification of pre-malignant neoplastic conditions and may thus support the community with a basic communication system.
However, a number of questions and issues have to be addressed. First, the techniques applied need to have sufficient accuracy, reproducibility, sensitivity, and specificity, and will need to be standardized and validated. Another open issue is the optimal germline control. In hematopoietic neoplasms buccal swabs, hair follicles, nails, sorted T cells, and cultured fibroblasts have been proposed. However, these sources may also contain blood-derived cells. The faculty is of the opinion that reports providing information about genomic (or exome) profiles must include exact information regarding the method applied (basic system and bioinformatics) as well as information concerning the germline control. Whenever possible, the allelic burden (VAF) should be defined.
One proposal offers a mutant allele fraction of ≥ 2% in blood leukocytes as a work-definition for CHIP (Steensma et al., 2015). This cut-off seems feasible for currently applied sequencing approaches. However, the faculty is of the opinion that the limit of detection of the applied method has to be reported. In the case of a germline pattern (all cells affected), the interpretation of what is a somatic mutation and what is ‘germline’ may sometimes be difficult to assess. Whenever possible germline deviations should be discriminated from somatic mutations and classified according to current guidelines (Richards et al., 2015). Perhaps the most important question will be how to manage cases with predisposing genetic lesions, clonal hematopoiesis or other similar conditions in otherwise healthy individuals in daily practice. So far, no generally accepted diagnostic algorithm or management recommendation exists. A proposal for the management of CHIP (ARCH) and CHOP is shown in Supplemental Fig. S1.
However, other questions also remain. For example, the clinical (prognostic) impact of the allelic burden (and of changes in allelic burden, VAF) of various target genes in various clinical conditions remains largely unknown. What are driver mutations and what are passenger mutations, and can they all be related to CHIP/ARCH or CHOP with certainty (Illingworth and Mustonen, 2011, Jaiswal et al., 2014, Steensma et al., 2015)? Sometimes it may be difficult or even impossible to differentiate between CHIP and CHOP – especially in multi-mutated cases – and the clinical impact of such mutations may depend on additional factors (apart from the type of mutation) like age or co-morbidities. Should all individuals with CHIP undergo a BM examination? Should management guidelines include recommendations aimed at avoiding potential mutagenic events (e.g. tobacco smoking, radiation, repeated CT scanning) in these cases? These questions should be addressed in forthcoming observational studies.
Acknowledgments
Acknowledgements
We would like to thank all involved members of the Vienna Cancer Stem Cell Club (VCSCC), Ludwig Boltzmann Cluster Oncology (LBC ONC), and Medical University of Vienna for their help and support in organizing the Working Conference on Pre-malignant Clonal Conditions in Vienna in August 2015. This study was supported by the Austrian Science Fund (FWF), SFB projects F4701-B20 and F4704-B20 and by a Stem Cell Grant of the Medical University of Vienna. DDM is supported by the Division of Intramural Research, NIAID, NIH (USA). We would like to thank Julia Neusiedler-Nicolas for the critically reading the manuscript.
Disclosures
Peter Valent: has nothing to disclose. Cem Akin: reports personal fees from Novartis, personal fees from Deciphera, personal fees from Patahra Pharma, outside the submitted work. Michel Arock: has nothing to disclose. Chistoph Bock: has nothing to disclose. Tracy I. George: reports grants from Allakos, Inc., personal fees from Novartis, Inc., personal fees from Blueprint Medicine, outside the submitted work. Stephen J. Galli: has nothing to disclose. Jason Gotlib: has nothing to disclose. Torsten Haferlach: has nothing to disclose. Gregor Hoermann: reports grants form Gilead, personal fees form Novartis, personal fees form Amgen, personal fees form Ariad, outside the submitted work. Olivier Hermine: reports grants and other form AB science, grants from Inatherys, grants form Celgene, grants form Novartis, from null, outside the submitted work. Ulrich Jäger: has nothing to disclose. Lukas Kenner: has nothing to disclose. Hans Kreipe: has nothing to disclose. Ravindra Majeti: reports personal fees from Forty Seven Inc., outside submitted work. Dean D. Metcalfe: has nothing to disclose. Alberto Orfao: has nothing to disclose. Andreas Reiter: reports personal fees from Novartis Pharma, personal fees from BMS, outside the submitted work. Wolfgang R. Sperr: has nothing to disclose. Philipp B. Staber: has nothing to disclose. Karl Sotlar: has nothing to disclose. Charles Schiffer: has nothing to disclose. Giulio Superti-Furga: has nothing to disclose. Hans-Peter Horny: has nothing to disclose.
Authors’ contributions
All authors of this article contributed equally by participating in pre-conference and post-conference discussion-rounds, by participating actively in the Working Conferences, by formulating consensus statements, by writing part of the draft, and by reading and reviewing the final manuscript. All authors approved the final version of the document.
Footnotes
We dedicate this article to the visionary concepts established by Paul Ehrlich and Theodor Boveri
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2017.11.024.
Appendix A. Supplementary data
Supplementary material
References
- Akin C., Scott L.M., Kocabas C.N., Kushnir-Sukhov N., Brittain E., Noel P., Metcalfe D.D. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with “idiopathic” anaphylaxis. Blood. 2007;110:2331–2333. doi: 10.1182/blood-2006-06-028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Børresen-Dale A.L. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman R.B. Personal genomic measurements: the opportunity for information integration. Clin. Pharmacol. Ther. 2013;93:21–23. doi: 10.1038/clpt.2012.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi R., Gentry S.N., Jackson T.L. Pathways to tumorigenesis—modeling mutation acquisition in stem cells and their progeny. Neoplasia. 2008;10:1170–1182. doi: 10.1593/neo.08572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti D., Morris M.R., Cooper W.N., Staals R.H., Wake N.C., Fews G.A., Gill H., Gentle D., Shuib S., Ricketts C.J. Germline mutations in DIS3L2 cause the Perlman syndrome of overgrowth and Wilms tumor susceptibility. Nat. Genet. 2012;44:277–284. doi: 10.1038/ng.1071. [DOI] [PubMed] [Google Scholar]
- Baylin S.B., Jones P.A. A decade of exploring the cancer epigenome - biological and translational implications. Nat. Rev. Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaux C., Loos M., Sels A., Huez G., Stryckmans P. Detection of major bcr-abl gene expression at a very low level in blood cells of some healthy individuals. Blood. 1995;86:3118–3122. [PubMed] [Google Scholar]
- Bodian D.L., McCutcheon J.N., Kothiyal P., Huddleston K.C., Iyer R.K., Vockley J.G., Niederhuber J.E. Germline variation in cancer-susceptibility genes in a healthy, ancestrally diverse cohort: implications for individual genome sequencing. PLoS One. 2014;9:e94554. doi: 10.1371/journal.pone.0094554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan J.A., Iacobuzio-Donahue C.A. A new branch on the tree: next-generation sequencing in the study of cancer evolution. Semin. Cell Dev. Biol. 2012;23:237–242. doi: 10.1016/j.semcdb.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugieres L., Remenieras A., Pierron G., Varlet P., Forget S., Byrde V., Bombled J., Puget S., Caron O., Dufour C. High frequency of germline SUFU mutations in children with desmoplastic/nodular medulloblastoma younger than 3 years of age. J. Clin. Oncol. 2012;30:2087–2093. doi: 10.1200/JCO.2011.38.7258. [DOI] [PubMed] [Google Scholar]
- Busque L., Patel J.P., Figueroa M.E., Vasanthakumar A., Provost S., Hamilou Z., Mollica L., Li J., Viale A., Heguy A. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat. Genet. 2012;44:1179–1181. doi: 10.1038/ng.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill D.P., Kinzler K.W., Vogelstein B., Lengauer C. Genetic instability and darwinian selection in tumours. Trends Cell Biol. 1999;9:57–60. [PubMed] [Google Scholar]
- Corces-Zimmerman M.R., Majeti R. Pre-leukemic evolution of hematopoietic stem cells: the importance of early mutations in leukemogenesis. Leukemia. 2014;28:2276–2282. doi: 10.1038/leu.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies F.E., Dring A.M., Li C., Rawstron A.C., Shammas M.A., O'Connor S.M., Fenton J.A., Hideshima T., Chauhan D., Tai I.T. Insights into the multistep transformation of MGUS to myeloma using microarray expression analysis. Blood. 2003;102:4504–4511. doi: 10.1182/blood-2003-01-0016. [DOI] [PubMed] [Google Scholar]
- Dessars B., De Raeve L.E., Morandini R., Lefort A., El Housni H., Ghanem G.E. Genotypic and gene expression studies in congenital melanocytic nevi: insight into initial steps of melanotumorigenesis. J. Invest. Dermatol. 2009;129:139–147. doi: 10.1038/jid.2008.203. [DOI] [PubMed] [Google Scholar]
- Genovese G., Kähler A.K., Handsaker R.E., Lindberg J., Rose S.A., Bakhoum S.F., Chambert K., Mick E., Neale B.M., Fromer M. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlinger M., McGranahan N., Dewhurst S.M., Burrell R.A., Tomlinson I., Swanton C. Cancer: evolution within a lifetime. Annu. Rev. Genet. 2014;48:215–236. doi: 10.1146/annurev-genet-120213-092314. [DOI] [PubMed] [Google Scholar]
- Gotlib J., Cools J. Five years since the discovery of FIP1L1-PDGFRA: what we have learned about the fusion and other molecularly defined eosinophilias. Leukemia. 2008;22:1999–2010. doi: 10.1038/leu.2008.287. [DOI] [PubMed] [Google Scholar]
- Greaves M., Maley C.C. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülen T., Hägglund H., Sander B., Dahlén B., Nilsson G. The presence of mast cell clonality in patients with unexplained anaphylaxis. Clin. Exp. Allergy. 2014;44:1179–1187. doi: 10.1111/cea.12369. [DOI] [PubMed] [Google Scholar]
- Gustafson S.L., Raymond V.M., Marvin M.L., Else T., Koeppe E., Stoffel E.M., Everett J.N. Outcomes of genetic evaluation for hereditary cancer syndromes in unaffected individuals. Familial Cancer. 2015;14:167–174. doi: 10.1007/s10689-014-9756-x. [DOI] [PubMed] [Google Scholar]
- Hinds D.A., Barnholt K.E., Mesa R.A., Kiefer A.K., Do C.B., Eriksson N., Mountain J.L., Francke U., Tung J.Y., Nguyen H.M., Zhang H., Gojenola L., Zehnder J.L., Gotlib J. Germ line variants predispose to both JAK2 V617F clonal hematopoiesis and myeloproliferative neoplasms. Blood. 2016;128:1121–1128. doi: 10.1182/blood-2015-06-652941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth C.J., Mustonen V. Distinguishing driver and passenger mutations in an evolutionary history categorized by interference. Genetics. 2011;189:989–1000. doi: 10.1534/genetics.111.133975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S., Fontanillas P., Flannick J., Manning A., Grauman P.V., Mar B.G., Lindsley R.C., Mermel C.H., Burtt N., Chavez A. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S., Natarajan P., Silver A.J., Gibson C.J., Bick A.G., Shvartz E., McConkey M., Gupta N., Gabriel S., Ardissino D. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury M.J., McCabe L.L., McCabe E.R. Population screening in the age of genomic medicine. N. Engl. J. Med. 2003;348:50–58. doi: 10.1056/NEJMra013182. [DOI] [PubMed] [Google Scholar]
- Khoury M., Berg A., Coates R., Evans J., Teutsch S., Bradley L. The evidence dilemma in genomic medicine. Health Aff. 2008;27:1600–1611. doi: 10.1377/hlthaff.27.6.1600. [DOI] [PubMed] [Google Scholar]
- Knoblich J.A. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kyle R.A. Monoclonal gammopathy of undetermined significance (MGUS) Baillieres Clin. Haematol. 1995;8:761–781. doi: 10.1016/s0950-3536(05)80258-6. [DOI] [PubMed] [Google Scholar]
- Lapunzina P., López R.O., Rodríguez-Laguna L., García-Miguel P., Martínez A.R., Martínez-Glez V. Impact of NGS in the medical sciences: genetic syndromes with an increased risk of developing cancer as an example of the use of new technologies. Genet. Mol. Biol. 2014;37(S1):241–249. doi: 10.1590/s1415-47572014000200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie C.C., Laurie C.A., Rice K., Doheny K.F., Zelnick L.R., McHugh C.P., Ling H., Hetrick K.N., Pugh E.W., Amos C. Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nat. Genet. 2012;44:642–650. doi: 10.1038/ng.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch H.T., Fusaro R.M., Lynch J.F. Hereditary cancer syndrome diagnosis: molecular genetic clues and cancer control. Future Oncol. 2007;3:169–181. doi: 10.2217/14796694.3.2.169. [DOI] [PubMed] [Google Scholar]
- Malhotra J. Molecular and genetic epidemiology of cancer in low- and medium-income countries. Ann. Glob. Health. 2014;80:418–425. doi: 10.1016/j.aogh.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Manz M.G., Boettcher S. Emergency granulopoiesis. Nat. Rev. Immunol. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- Martincorena I., Campbell P.J. Somatic mutation in cancer and normal cells. Science. 2015;349:1483–1489. doi: 10.1126/science.aab4082. [DOI] [PubMed] [Google Scholar]
- Marusyk A., Almendro V., Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat. Rev. Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- Mayerhofer M., Gleixner K.V., Hoelbl A., Florian S., Hoermann G., Aichberger K.J., Bilban M., Esterbauer H., Krauth M.T., Sperr W.R. Unique effects of KIT D816V in BaF3 cells: induction of cluster formation, histamine synthesis, and early mast cell differentiation antigens. J. Immunol. 2008;180:5466–5476. doi: 10.4049/jimmunol.180.8.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzgeroth G., Schwaab J., Gosenca D., Fabarius A., Haferlach C., Hochhaus A., Cross N.C., Hofmann W.K., Reiter A. Long-term follow-up of treatment with imatinib in eosinophilia-associated myeloid/lymphoid neoplasms with PDGFR rearrangements in blast phase. Leukemia. 2013;27:2254–2256. doi: 10.1038/leu.2013.129. [DOI] [PubMed] [Google Scholar]
- Negrini S., Gorgoulis V.G., Halazonetis T.D. Genomic instability—an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- Nowell P.C. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Pan Y., Karagiannis K., Zhang H., Dingerdissen H., Shamsaddini A., Wan Q., Simonyan V., Mazumder R. Human germline and pan-cancer variomes and their distinct functional profiles. Nucleic Acids Res. 2014;42:11570–11588. doi: 10.1093/nar/gku772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfi A., Barreyro L., Steidl U. Concise review: preleukemic stem cells: molecular biology and clinical implications of the precursors to leukemia stem cells. Stem Cells Transl. Med. 2013;2:143–150. doi: 10.5966/sctm.2012-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti F., Rumi E., Pietra D., Lazzarino M., Cazzola M. JAK2 (V617F) mutation in healthy individuals. Br. J. Haematol. 2007;136:678–679. doi: 10.1111/j.1365-2141.2006.06483.x. [DOI] [PubMed] [Google Scholar]
- Pelosof L.C., Gerber D.E. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin. Proc. 2010;85:838–854. doi: 10.4065/mcp.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons A., Shaffer L.G., Hastings R.J. Cytogenetic nomenclature: changes in the ISCN 2013 compared to the 2009 edition. Cytogenet. Genome Res. 2013;141:1–6. doi: 10.1159/000353118. [DOI] [PubMed] [Google Scholar]
- Spinner M.A., Sanchez L.A., Hsu A.P., Shaw P.A., Zerbe C.S., Calvo K.R., Arthur D.C., Gu W., Gould C.M., Brewer C.C. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensma D.P., Bejar R., Jaiswal S., Lindsley R.C., Sekeres M.A., Hasserjian R.P., Ebert B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valent P. Targeting of leukemia-initiating cells to develop curative drug therapies: straightforward but nontrivial concept. Curr. Cancer Drug Targets. 2011;11:56–71. doi: 10.2174/156800911793743655. [DOI] [PubMed] [Google Scholar]
- Valent P., Klion A.D., Horny H.P., Roufosse F., Gotlib J., Weller P.F., Hellmann A., Metzgeroth G., Leiferman K.M., Arock M. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J. Allergy Clin. Immunol. 2012;130:607–612.e9. doi: 10.1016/j.jaci.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valent P., Bonnet D., De Maria R., Lapidot T., Copland M., Melo J.V., Chomienne C., Ishikawa F., Schuringa J.J., Stassi G. Cancer stem cell definitions and terminology: the devil is in the details. Nat. Rev. Cancer. 2012;12:767–775. doi: 10.1038/nrc3368. [DOI] [PubMed] [Google Scholar]
- Valent P., Bonnet D., Wöhrer S., Andreeff M., Copland M., Chomienne C., Eaves C. Heterogeneity of neoplastic stem cells: theoretical, functional, and clinical implications. Cancer Res. 2013;73:1037–1045. doi: 10.1158/0008-5472.CAN-12-3678. [DOI] [PubMed] [Google Scholar]
- Vassy J.L., Lautenbach D.M., McLaughlin H.M., Kong S.W., Christensen K.D., Krier J., Kohane I.S., Feuerman L.Z., Blumenthal-Barby J., Roberts J.S. MedSeq Project. The MedSeq Project: a randomized trial of integrating whole genome sequencing into clinical medicine. Trials. 2014;15:85. doi: 10.1186/1745-6215-15-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K.W. The multistep nature of cancer. Trends Genet. 1993;9:138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- Wang E., Zaman N., Mcgee S., Milanese J.S., Masoudi-Nejad A., O'Connor-McCourt M. Predictive genomics: a cancer hallmark network framework for predicting tumor clinical phenotypes using genome sequencing data. Semin. Cancer Biol. 2015;30:4–12. doi: 10.1016/j.semcancer.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Wilson A., Laurenti E., Trumpp A. Balancing dormant and self-renewing hematopoietic stem cells. Curr. Opin. Genet. Dev. 2009;19:461–468. doi: 10.1016/j.gde.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Yang Y., Muzny D.M., Reid J.G., Bainbridge M.N., Willis A., Ward P.A., Braxton A., Beuten J., Xia F., Niu Z. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N. Engl. J. Med. 2013;369:1502–1511. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates L.R., Campbell P.J. Evolution of the cancer genome. Nat. Rev. Genet. 2012;13:795–806. doi: 10.1038/nrg3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material