Abstract
The effect of redox metals such as iron and copper on multiple sclerosis and amyotrophic lateral sclerosis has been intensively studied. However, the origin of these disorders remains uncertain. This review article critically describes the physiology of redox metals that produce oxidative stress, which in turn leads to cascades of immunomodulatory alteration of neurons in multiple sclerosis and amyotrophic lateral sclerosis. Iron and copper overload has been well established in motor neurons of these diseases’ lesions. On the other hand, the role of other metals like cadmium participating indirectly in the redox cascade of neurobiological mechanism is less studied. In the second part of this review, we focus on this less conspicuous correlation between cadmium as an inactive-redox metal and multiple sclerosis and amyotrophic lateral sclerosis, providing novel treatment modalities and approaches as future prospects.
Facts
Essential metals (e.g., iron and copper) regulate gene expression, maintain cell structure, conduct neurotransmission, and are involved in homeostasis of antioxidant response.
Transmembrane proteins, including Ctr 1, DMT1, ATPases (ATP7A and ATP7B) play crucial roles in the intracellular copper regulation related to ALS pathophysiology.
Cadmium is known to influence multiple sclerosis-related motor speed, attention, memory and its turnover influences Polyneuropathy.
Open questions
What is the correlation between metalomics and MS/ALS?
What is the impact of crosstalk between molecular machinery regulating lesser known trace reactive metals into the MS/ALS metalomics profile?
How does CCSVI-related extra-cranial venous strictures in MS patients influence the homeostasis of the reactive metal via local related arterial and venous circulation?
Introduction
Multiple sclerosis (MS) is a demyelinating disease of the central nervous system (CNS). Its resultant inflammation causes oligodendrocyte degeneration, myelin sheath destruction, and neurodegeneration1–4. Although the origin of MS is still unknown, genetic predisposition and environmental toxicity activate the immune system against neural cells5,6. The onset of MS is often in young people aged between 20 and 40 years7,8. Caucasians race, particularly those of northern European descent and white women living in cold and humid areas are more affected by MS9.
Amyotrophic lateral sclerosis (ALS) is an adult onset, fatal, and quick destructive CNS disease, one of the most common neurodegenerative disorders with incidence around 1/100,000 with growing population in many countries10,11. 90% of ALS cases are sporadic and the rest are linked to genetics (familial), but their manifestations and pathological mechanisms are similar. ALS is clinically categorized as a heterogeneous disease, where the onset age, area and initial of symptoms, and speed of progression are varied among patients. Upper and lower motor neurons in the brain stem, cerebral cortex, and spinal cord are the most regions attacked by ALS. Considering clinical heterogeneity, ALS often manifests with progressive muscles atrophy, causing paralysis and death in 2–5 years after symptom onset in most patients. Respiratory failure resulted from neuronal and skeletal injury is typically identified as the primary cause of death. Despite the unknown and complex origin of ALS, numerous reasons, including redox metals dys-homeostasis, overproduced oxidative stress, mitochondrial dysfunction, neuro-inflammation, and glutamate excitotoxicity are responsible for motor neuron loss10–13.
Metals are essential cofactors for enzymes and structural elements for stabilizing static biomolecules14. They also participate in principal biological metabolisms of the brain, including neurotransmitter synthesis, nerve transition, and oxygen transport15. Recently, notable attention has been paid to redox-active metals such as iron (Fe) and copper (Cu), and redox-inactive metals like cadmium (Cd) due to their inevitable capacity in neurodegeneration. Oxidative stress (overproduction of reactive oxygen species (ROS) and reactive nitrogen species (RNS)) is produced by either metals dys-homeostasis or an imbalance between the formation of free radicals and their destruction by antioxidants, leading to cellular damage, aging, and apoptosis through oxidation of principal cellular components (i.e., lipids, proteins, and DNA). It is obscure that metals interaction is initial or secondary factors, or outcome of the neurodegeneration11,15–19. Mitochondria are the main site of ROS production and cells apoptosis (see Fig. 1 for details). They are vulnerable to ROS and it has been confirmed that mitochondrial injury intensifies ROS and oxidative damage in MS20,21.
Fig. 1. ROS and anti-ROS cellular machinery involved intracellular homeostasis of protein/lipid/DNA.
. ROS is formed by complex I and III in the electron transport chain in the inner layer of mitochondria through oxidative phosphorylation process, consuming oxidation of NADH or FADH to generate potential energy for protons19,211,212.
Herein, we review the role of redox-active metals (iron and copper) and redox-inactive metal (cadmium) in MS/ALS. First, we briefly explain the iron and copper homeostasis in the human body and the cell biology of these metals in MS/ALS. Second, we summarize recent progress on the role of iron and copper in MS/ALS. Third, we emphasize the effect of cadmium on these diseases. The last section provides our future perspectives and conclusions.
Iron homeostasis
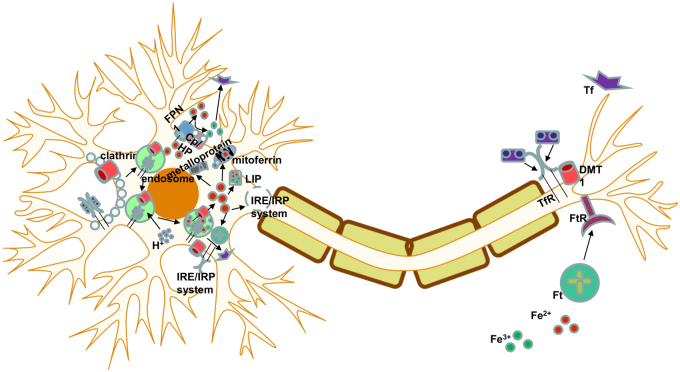
Iron is a redox-active metal circulating between Fe2+ and Fe3+ ionic states. Cellular iron homeostasis is tuned by iron-responsive/regulatory element proteins (IRE and IRP), adjusting the process of iron uptake and storage to maintain iron balance in different cells17. Brain cells synthesize several receptors (e.g., transferrin receptor (TfR), divalent metal transporter 1 (DMT1), amyloid precursor protein (APP), ferroportin 1 (FPN1), ceruloplasmin (CP), and ferritin) and handle iron trafficking by many ways depending on functions and requirements (see Figs. 2 and 3 for details)22,23.
Fig. 2. Iron metabolism in the brain. Astrocytes express CP to oxidize Fe2+.
. Oligodendrocytes, a primary target in inflammatory attack, and synthesize Tf that controls intracellular iron transport. Microglia represent DMT1, APP, and ferritin, assisting neurons to maintain iron hemostasis in the brain environment. They also protect normal neuron function by iron regulation. The ferric iron (Fe3+) derived from diet, excreted enterocytes, and reticulocytes binds to transferrin (Tf). This combination uptake in the endothelial surfaces in the BBB is mediated by TfR. Fe3+ releases from Tf-TFR complex in the endosome and is catalyzed to ferrous iron (Fe2+). Thereby, TfR is recycled to bind to the iron Tf complex in the plasma. Alternatively, Fe2+ is transported to cytosol of endothelial cells and extracellular fluid by DMT1 and FPN1, respectively. In addition, released Fe2+ is quickly converted to Fe3+ by CP, expressed by both astrocytes and endothelial cells followed by bonding to Tf or low molecular weight molecules (e.g., citrate and ATP). Non-Tf-bound iron (NTBI) synthesized in the cytosol is the iron source for oligodendrocytes and astrocytes where Tf is highly saturated by iron22,23
Fig. 3.
Neuronal iron homeostasis
Cell biology of Iron and its role in MS/ALS
Iron is accommodated in different parts of the human body. Sixty-five percent of iron exists in hemoglobin, 25% of the total iron is bound to storage proteins (ferritin and hemosiderin), and 10% of the iron concentration participates in the structure of myoglobin, cytochromes, and enzymes. Only 0.1% of iron binds to Tf and circulates in plasma17,24. In the brain, the majority of iron components is stored as non-heme iron in oligodendrocytes and myelin. The accumulation of iron is elevated by increase in age in the normal human brain, particularly after 40–50, which is the time of onset of two forms of MS known as primary progressive MS (PPMS) and secondary progressive MS (SPMS)1. Basal ganglia are known as the high iron content region in the brain25, and the elevation of iron deposition in basal ganglia is related to the normal aging process26.
Iron is a cofactor in the catalytic center of various enzymes for normal brain metabolism, including oxidative phosphorylation, myelination, neurotransmitter formation, etc. Moreover, iron participates in normal physiological processes within oligodendrocytes for construction of myelin where enzymes machinery utilizes iron1. Tight homeostasis of cellular iron is required to maintain the normal level of iron, since its excessive concentration can become deleterious for cells function27–30. Iron mismanagement can cause microglia activation, induction of mitochondria dysfunction, generation of free radicals in the brain22,31–34. Indeed, the redox capacity of free iron to carry out one-electron reactions, catalyzing the formation of ROS, is proposed as the key factor in MS/ALS1. It remains unclear that iron deposition is an epiphenomenon or an initializer in the MS development32.
Superoxide dismutase (SOD1) mutation gene is the most frequent mutation in ALS35, detected in 20% of familial cases and in about 2% of cases overall. SOD1 mutation participates in pathogenesis of ALS through generation of oxidative stress, cytoskeletal abnormality, glutamate toxicity, mitochondrial dysfunction, and extracellular toxicity. It is unclear whether an altered enzyme activity or indirectly a disturbance in transition metal homeostasis is involved in the disease pathogenesis36,37. Under the normal condition, mitochondria convert 1–3% of oxygen molecules to superoxide radicals, which are later removed by SOD1. In the absence of SOD1, slow dysmutation process causes oxidative stress. In fact, superoxide radicals release iron from iron-containing proteins (ferritin) in vivo stress conditions. They also partake in Haber–Weiss reaction, the combination of Fenton reaction and the reduction of Fe3+ by superoxide and formation of Fe2+. The liberated iron, Fe2+, participates in a Fenton reaction and produces free radicals (e.g., reactive hydroxyl radicals) that damage cells24,36.
Anomalous iron handling may be exacerbated by genetic predisposition due to particular gene variants in different iron homeostasis genes27,38–41. The mutation of Hfe is proposed as a risk factor of MS40 and ALS36,42–44. It leads to Hemochromatosis and decreases the expression of Cu/Zn SOD136,42–44.
Iron and MS
As mentioned above, iron dys-homeostasis can lead to neurodegeneration, which is relevant to MS pathology. The relationship between abnormal iron content and an abundance of oxidative damage is reported in MS1,45–47. Hametner et al. observed the elevation of iron accumulation with age increase in the white matter (WM)1. Iron-rich oligodendrocytes, myelin, and microglia were also destroyed in the active MS lesion, increasing the liberated iron, thereby intensifying oxidative damage and neurodegeneration4,48,49. Conversely, considerable reduction of iron and elevation of oxidative stress are found in MS patients16,50.
Numerous experimental animal models have been employed to investigate mechanisms of MS pathology. Experimental allergic encephalomyelitis (EAE) is one of the most commonly used models. Iron accumulation, which presumably comes from myelin, oligodendrocytes, and the breakdown of the blood–brain barrier (BBB), was found during active and recovery phases of EAE47.
Magnetic Resonance Imaging (MRI) phase have widely been used to illustrate the iron accumulation in MS lesions. Moreover, increased local field in basal ganglia can be interpreted as pathological iron accumulation and peripheral phase rings display iron-positive macrophages at the edge of lesions51,52. Mehta et al. showed the distribution of iron in non-phagocytosing microglia at the periphery of demyelinated sections with Perls’ stain and immunohistochemistry53. Iron-containing macrophages represented markers of proinflammatory (M1) contrast. Likewise, iron was preferentially taken up by non-phagocytosing, M1-polarized macrophages, and induced M1 (super) polarization in human macrophage cultures. Bagnato et al. also observed oligodendrocytes in normal WM and microglia at the edges of WM lesions as iron origins in gradient echo MRI54. In another example, susceptibility weighted imaging-filtered phase images demonstrated high iron content in phase MS lesions55. Interestingly, Adams et al. found additional source of iron in the CNS and showed that chronic inflammation and vein walls damage lead to the deposition of hemosiderin within and outside of lesions56. The histologic evidence of red blood cells extravasation through the BBB is currently mirrored by the frequent MRI of micro-bleedings around the brain venules54,56,57. Moreover, differently from the other neurodegenerative disorders, each MS lesion is crossed by a central venule, which may exhibit increased pressure as a consequence of restricted outflow in the jugular system. Therefore, chronic cerebrospinal venous insufficiency (CCSVI) may favorite the BBB leakage and iron loading of heme origin58.
Iron deposition can be represented by T2 hypointensity or black T2 in MRI. Gray matter (GM) T2 hypointensity in MS patients is related to physical disability, brain atrophy, and disease course59,60. Bakshi et al. reported black T2 in subcortical nuclei in wheelchair-bound and SPMS patients prominently correlated to longer disease course and ambulatory disorders59. Additionally, the role of iron is emphasized in cognitive disorders of MS patients61,62. A study by Brass et al. revealed the relationship between T2 hypointensity, cognitive impairments, and the effect of iron content in basal ganglia in neuropsychological disorders61.
The demyelination of WM has long been considered as a significant sign of MS, though, the importance of the GM demyelination is emphasized63,64. Indeed, high iron deposition in deep GM is shown in MS cases34,65,66. It has been proposed that WM damage disturbs the axonal iron transition and elevates the iron deposition in deep GM34. Haider et al. suggested the correlation between high iron bulk, deep GM demyelination, and clinical disability65.
Iron and ALS
Since iron is known to promote the motor neuron degeneration, some trials have examined the iron state in ALS patients. It has been demonstrated that the excess ferritin level can worsen muscle degeneration and shorten patients’ survival10,67–69. High iron concentrations is also reported in the spinal cord of ALS patients70–73. Hozumi et al. reported an increase of the iron content in the cerebrospinal fluid of ALS cases74. Interestingly, Mizuno et al. noticed the presence of Tf (an iron regulator) in Bunina bodies and some of basophilic inclusions, where the pathogenesis of ALS occurs75.
Similar to MS, T2 shortening reveals iron deposition in the brains of ALS patients and iron is considered as a biomarker for ALS76–78. Nevertheless, Hecht et al. declared that hypointensities are not due to iron accumulation in ALS patients and offered alternative sources like free oxygen radicals79.
Animal models have been adopted to demonstrate the association between iron and ALS. The disturbance of iron homeostasis was observed in a murine model and it has since been suggested that the inhibition of axonal transport iron, perturbation of proteins adjusting the iron influx, and elevation of mitochondrial iron storage in neurons and glia lead to iron accumulation in SOD1 transgenic mice37. The blood accumulation in damaged blood vessels and iron deposition created motor neuron degeneration in SOD1 transgenic mice80. .Also, the elevation of iron-related mRNA expression increased iron and subsequent oxidative damage in SOD1-G93A mice12. In some trials, iron chelator therapy has been administered in a G93A-SOD1 murine model of ALS, resulting in neuroprotective effects and increased lifespan37,81,82.
Copper homeostasis
The majority of dietary copper is absorbed by the small intestine and stored in the liver. The biliary pathway is responsible for 80% of copper excretion through the liver24. In the blood stream, around 65–90% of serum copper is attached to Ceruloplasmin, while remaining is bound to serum albumin, transcurein, and amino acids for delivery to tissues24,83. Only unbound copper ions can pass through the BBB84. The basal ganglia, cerebellar granular neurons, neuropil of the cerebral cortex, astrocytes, and hippocampus can accommodate high concentrations of copper15. Astrocytes are the primary cells regulate extracellular ions in the brain85,86. Moreover, astrocytes are considered as major contributors to copper homeostasis and high storage region of copper (see Fig. 4 for details)86,87.
Fig. 4. Copper homeostasis in the brain.
A group of transmembrane proteins including Ctr 1, DMT1, ATPases (ATP7A and ATP7B) play crucial roles for intracellular copper regulation. Ctr1 is an essential copper transporter expressed in intestinal and brain cells to handle copper influx. DMT1 is also expressed in brain tissues and may contribute to copper uptake86,88. ATP7a acts as a critical source of brain copper and mediates copper movement across the basolateral membrane into the extra-vascular space of the brain. It also exports copper for subsequent incorporation into Cu-dependent enzymes15,86,96. ATP7b also transfers copper across membranes, however, the function of ATP7b is less clear compared to ATP7a86,88. Copper chaperone proteins control copper traffic and delivery into specific cellular targets. Moreover, chaperone for SOD1 (CCS), chaperone for cytochrome C oxygenase (Cox17), anti-oxidant protein 1 (Atox 1) deliver copper to SOD1, cytochrome oxidase, and ATP7a, respectively15,86,88. MTs are low molecular weight proteins with neuroprotective roles and a high number of cysteine residues for metal binding such as copper and zinc86,88,154. There are four types of MTs in mammals. MT1 and MT2 are expressed in all tissues, MT3 exists in CNS, and MT4 is found in the stratified squamous epithelia. MTs are known as copper buffers in the glutamatergic synapse where excess copper induces a high level of MTs86,88.
Cell biology of copper and its role in MS/ALS
Copper participates in the structure and function of several brain enzymes, regulating neurotransmitters synthesis, iron metabolism, oxidative defense, etc. Since most of the copper is presented as cuprous (Cu+) and cupric (Cu2+) ions in biological systems, it mediates electron transfer in redox reactions88. Therefore, cellular copper availability should be precisely adjusted for essential enzyme activity and prevention of oxidative damage15,24,83,89–96. The measurements showed abnormal copper level in ALS patients97,98. The high concentration of copper generates oxidative stress through two mechanisms, that is, catalyzing the production of hydroxyl radicals in a Fenton-like reaction as redox-active form and reducing the GSH (an antioxidant that removes ROS).
Copper and MS
The role of copper in MS pathology is proposed to be via excessive copper and subsequent oxidative damage9,99–102. The injury of mitochondrial electron transport system, cytochrome oxidase, and activated glia increase copper contents103. However, conflicting findings have also been reported104–111.
Cuprizone drug administration (a copper chelator) in animals is a method to model toxic de/remyelination of the CNS. This model supports the idea that copper dys-homeostasis can mediate ROS. Furthermore, Cuprizone carries copper into the CNS and induces prominent demyelination lesions through oxidative stress and oligodendrocytes toxicity112–123. Animal studies have also demonstrated the positive effect of Clioquinol (CQ) as another metal chelator on suppression of different neurodegenerative diseases (i.e., Parkinson’s and Alzheimer’s)124,125. Choi et al. found that CQ can diminish the activation of microglia in the spinal cord of EAE and improve clinical symptoms126.
Copper and ALS
SOD1 genetic mutation has been extensively investigated in animal models of ALS that express human mutant SOD1 protein. SOD1 is a major copper-binding protein with the highest affinity to copper and scavenger function of free radicals127. In addition to redox homeostasis, SOD1 plays a critical role on intracellular copper buffering128. Evidence from clinical trials confirmed that the elevation of SOD1 (D90A and G93A) levels lead to the disruption of copper metabolism and its accumulation in the spinal cord of mice, the most common region injured by ALS129–133. Although wild-type SOD1 is considered non-pathogenic, the overexpression of wild-type human SOD1 enhances the amount of copper in an age-related manner130, and causes neurotoxicity in the spinal cord134. Interestingly, Tokuda et al. observed high copper content in the outside SOD1 active region (non-SOD1 Cu level), which promotes disease progression in transgenic mice130. Regardless of SOD1 copper-binding ability, copper-regulating proteins are also affected by SOD1 mutant expression, leading to abnormal copper accumulation. Indeed, mutant SOD1 alters the expression of CTR1 and ATP7a as copper importer and exporter, respectively129,130. Additional trials on the spinal cord of transgenic mice revealed low copper concentration in mutant SOD1133,135,136, and a correlation between the degree of low copper content and ALS manifestations137.
There are three forms of SOD1, varying by the metal content, which are as follows: (a) fully metalated form (Holo) SOD1 that bind to one copper and one zinc, (b) demetalated (apo) SOD1, and (c) metal-deficient SOD1 that bind to one metal, copper or zinc. The fully metalated form has high stability and half-life time138. Enormous ALS-associated SOD1 mutations alter metal binding affinity and protein structure. Copper deficiency leads to improper hydrophobicity in wild and mutant types of SOD1, while adding copper can improve this defect139,140. Moreover, copper deficiency has been shown in recombinant SOD1 mutant and >50% of SOD1–G37R proteins in the spinal cord of transgenic mice141–144. The copper treatment could increase the concentration of the fully metalated form144. Nevertheless, a study claimed that a G37R mutation is inert to the SOD1 structure in its metals binding region145.
A trial in mice with genetically modified copper-regulating enzymes demonstrated the role of copper homeostasis in SOD1 toxicity. The CCS overexpression in SOD1-G93A mice can worsen the disease progression, though, it promotes the holo-SOD1 form146. This defect can be treated by copper delivery with a drug known as CuATSM147. Also, the deletion of CCS in SOD1 transgenic mice caused reduction of copper loaded SOD1. Interestingly, other copper-dependent enzymes and the disease progression are not affected by this procedure148. These suggest that copper trafficking by CCS is not related to SOD1 toxicity149.
Metallothioneins (MTs) are also crucial for balancing the intracellular copper content150, and provide copper for SOD1 and other enzymes151,152. The use of MTs in copper dys-homeostasis confirmed the important role of MTs in pathological signs without altering the SOD1 function153–157. The delivery of MT-III improved motor neurons loss155, whereas the reduction of MTs expression prominently enhanced disease onset and progression in SOD1 mice156. The diminution of MT-III mRNA was confirmed in the sporadic ALS158. Additionally, the effect of Dexamethasone on the elevation of MTs and decrease of the disease progression was proved in G93A-SOD1 mice153,159. Conversely, the elevation of astrocytic MTs immunoreactivity was observed in the spinal cord of ALS patients160.
Copper delivery therapy with CuATSM that releases copper into oxidative tissues could promote the survival of animal ALS models144,147,161–163. Utilization of CuATSM in SOD & CCS mice reduced mortality and motor neuron deficit in symptomatic cases147. Also, CuATSM is suggested to help familial SOD1 ALS patients, representing human CCS. On the other hand, various copper chelators, including Ammonium tetrathiomolybdate (TTM)130,164, d-penicillamine165, Trientine166–168, and lipophilic metal chelator were examined in SOD1 mice169,170. They could remove copper accumulation, inhibit peroxidase action of SOD1, and postpone disease progression130,165–171.
Pyrrolidine dithiocarbamate (PDTC) (an antioxidant drug) regulates proinflammatory and apoptosis genes. Despite the beneficial effect of PDTC in animals models of diseases like Alzheimer’s172, it decreased the survival of G93A-SOD1 ALS rat models173. Additionally, the copper distribution was also elevated in the spinal cord of these rats, suggesting that the excess copper may increase neurotoxicity of mutant SOD1. Similarly, oral administration of PDTC enhanced the copper concentration and the level of lipid peroxidation products due to the oxidative stress in the rat peripheral nerve174.
Cadmium
Cadmium is categorized as a redox-inactive metal that cannot generate free radicals directly. It is rapidly absorbed by vegetables and grains, the main sources of dietary cadmium. Cadmium oxide produced during smoking is mainly responsible for cadmium exposure and deposition in lungs or other organs17. Cadmium may not behave like a metal ion, which participates in routine metabolism. Thus, human physiology is not evolved to handle cadmium metal tolerance and resistance (Table 1)175. Several decades ago, the toxic effect of cadmium on industrial workers was confirmed176,177. Its accumulation in tissues creates severe organ damage in the brain, kidney, lung, and testis24. . A combination of Cd and MT generates Cd–MT complex in the human body as reported in other divalent metal ions6,178. Importantly, cadmium influences the intra–extra neuronal homeostasis (see Fig. 5 for details).
Table 1.
Literature review on Cd neurotoxicity in humans and rats
| Year | Study design | Age group | E/C (n) | Exposure to Cd | Expose pathways | Effects | References |
|---|---|---|---|---|---|---|---|
| 1961 | Cross-sectional | Male worker | 106E/84C | — | Occupational exposure |
Anosmia | 216 |
| 1977 | Cross-sectional | Children | 31E/22C | CdH | Daily life | Neurological disorders, such as learning disabilities and hyperactivity |
217 |
| 1981 | Cross-sectional | Children | 73E/44C | CdH | Daily life | Dyslexic, learning disorder | 218 |
| 1981 | Cross-sectional | Workers | 49E | CdU | Occupational exposure |
Polyneuropathy | 219 |
| 1982 | Cross-sectional | Children | 149 | CdH | Daily life | Effect on verbal I.Q | 220 |
| 1985 | Case-control | Young men | 40 | CdH | Daily life | Behavioral difficulty | 221 |
| 1985 | Cross-sectional | Children | 69 | CdH | Daily life | Nonadaptive classroom behavior, affected behavioral development visuomotor skills ↓ |
222 |
| 1989 | Cross-sectional | Male workers | 31E | CdU | Occupational exposure |
↓ Attention, memory, and psychomotor speed |
223 |
| 1992 | Cross-sectional | Worker | 38E | CdU | Occupational exposure |
90% headache; 42% dizzy spells 21% weakness; 16% brain atrophy |
224 |
| 1992 | Cross-sectional | Worker | 55E/16C | CdU | Occupational exposure |
Hyposmia | 225 |
| 1997 | Case report | Old man | 1 | Multiple organ failure | Occupational exposure, acute |
Parkinsonism | 226 |
| 1999 | Cross-sectional | Worker | 13E/19C | CdU | Occupational exposure |
Polyneuropathy | 227 |
| 2000 | Cross-sectional | Adult worker | 42E/47C | CdU | Occupational exposure |
↓ Motor speed, attention, memory ↑ equilibrium, PNP, and concentration complaints |
228 |
| 2006 | Case report | Adult worker | 1 | CdU | Inhale the fumes | Peripheral neuropathy | 229 |
| 2009 | Cross-sectional | Children | 549 | CdH | Daily life | Withdrawal, social problems and attention problems associated |
230 |
| 2012 | Wister rats | Male | 20E/20C | Intratracheal instilletion | Experiment exposure | Dose- and time-dependent shif from slower to faster waves |
231 |
E exposed subjects, C control subjects; CdU urinary cadmium concentration; CdH concentration of cadmium in hair, IQ Intelligence Quotient
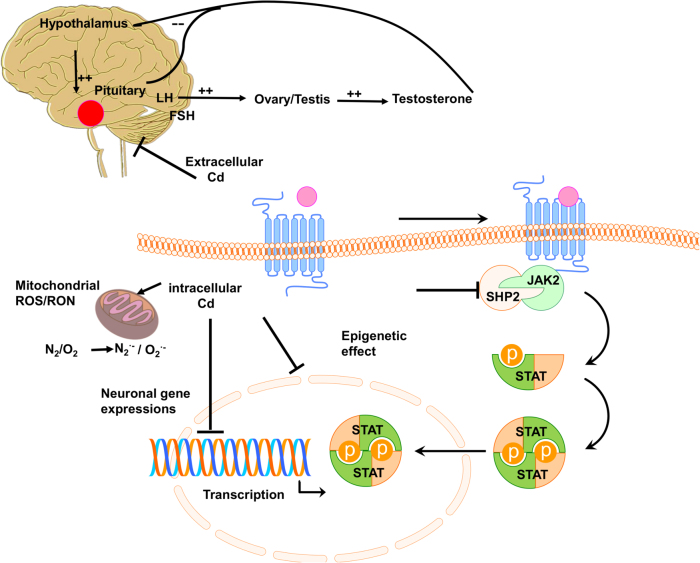
Fig. 5. Cadmium influence on intra- and extracellular neuronal homeostasis, contributing to CNS pathophysiology.
Extracellular cadmium has an estrogen-like effect, disturbing hormonal balance via the hypothalamic-pituitary-gonadal pathway. Intracellular cadmium disturbs neurogenesis and leads to neuronal apoptosis and ROS by impairing mitochondria signaling and inhibition of Jak/Stat signaling. The cadmium accumulation in the brain alters gene expression and causes epigenetic effects through DNA binding175. Moreover, it leads to oxidative stress via inhibition of antioxidant enzymes, depletion of antioxidants, dislocation of redox active metals, and suppression of the mitochondrial electron transport chain17,212. The replacement of iron and copper by cadmium, and thereby the increase of free iron and copper content, generate hydroxyl radicals and promote oxidative stress via Fenton’s reaction6,17,24,212. Additionally, the activity of different antioxidant enzymes, including Cu/Zn SOD1, glutathione peroxidase, glutathione reductase, and catalase is altered by cadmium intoxication213. Cadmium-induced selenium deficiency causes depletion of glutathione peroxidase17. Also, cellular antioxidant GSH is disrupted by cadmium and results in the elevation of ROS214. The excess of intracellular ROS inhibits the neural janus kinase (Jak) and tyrosine kinase, and leads to disruption of neural mitochondria215
Some studies demonstrated the direct relationship between cadmium and MS/ALS. An earlier study proved that the cadmium exposure can lead to retrograde axonal transport and neurotoxicity in rat motor neurons179. In other reports, cadmium injected to animals produced ROS, which in turn changed membrane fluidity, intracellular calcium levels180, lipid peroxidation, and protein carbonylation181. Also, cadmium induced neurotoxicity through decrease of GSH level in hippocampus and midbrain182, increase of free radicals, mitochondrial membrane dysfunction, and cell apoptosis in brain tissues183.
Cadmium and MS
Compared to iron and copper, few studies are available about cadmium toxicity in neurodegenerative disease like MS. Some reports showed high levels of cadmium in MS patients5,6,184. Aliomrani et al. found high concentration of cadmium in MS patients and its relationship with glutation-S-transferase (i.e., an oxidative stress correlated gene and metals biotransformation regulator)184.
Environmental factors are the key reasons for increasing the cadmium level in the human body. Excess cadmium contents are found either in MS patients living in industrial areas or in foods such as corn, rice, and wheat in industrial areas185. Also, the long-term exposure to air pollutions consisting of cadmium has potential roles in pathogenesis of MS186.
Cadmium and ALS
Cadmium mediates neurotoxicity and motor neuron disease by reducing Cu/Zn SOD1 enzyme, disrupting the BBB, and glutamate toxicity via upregulation of glutamate dehydrogenase and downregulation of glutamate uptake in glial cells (Fig. 5). These effects support the hypothesis about the relationship between cadmium and ALS pathogenesis97,187–190. The high concentration of cadmium is reported in CNS, blood, WM, and GM of the brain in ALS patients97,189,190. Wu-Tao et al. showed disruption of motor neuron conduction, Cu/Zn SOD1 activity, and spinal motor-neurons function in the cadmium-treated rat188. Interestingly, symptoms of disease and lab data from electromyography in battery factory workers showed that ALS onset was due to cadmium neurotoxicity through the reduction of SOD1 activity. Also, high levels of MTs and MT-bound cadmium in the liver and kidney, a sign of exposure to heavy metals, was found in patients187,191. Similarly, experiment on Escherichia coli showed the effect of cadmium on the reduction of SOD1 activity by misfolding of Cu/Zn SOD1 protein and elevation of MTs expression192. Conversely, another study reported no relationship between cadmium and ALS193.
Outlook and future prospects
New candidate metals (e.g., cadmium, arsenic, and nickel), which are indirectly involved in the ROS production, generally have long biological half-life due to the lack of a bodily recognition system. Therefore, these metals need extensive screening, particularly due to recent evidence of physiological correlations to cardiovascular diseases and CCSVI-MS. Recent developments in imaging techniques for metal detection may contribute significantly to elucidate the role of iron in MS/ALS pathology, and to develop metal-based disease biomarkers. Advanced MRI techniques and susceptibility weighted neuroimaging can detect metal deposition in the brain with high sensitivity194–196. Ultra-small superparamagnetic iron oxide (USPIO) nanoparticles can visualize pluriformity and cellular infiltration in MS precisely. Therefore, USPIO-enhanced MRI can be a new marker for observing WM inflammation, which cannot be visualized by routine techniques197. One important consideration is early exposure of these metals during pregnancy and childhood, and their late onset and association with MS/ALS. In this context, heritable changes without substantial DNA changes and their epigenetic correlations could shed new evidences about metal-induced biomolecular alterations in MS/ALS. Unlike mutations, epigenetic changes can be reversible and responsive to environmental influences, but can also have a profound impact on genetic expression. Formation of 5-methylcytosine through DNA methylation at the 5′ position of the cytosine ring of CpG Island to could be an epigenetic marker that regulates gene silencing/activation, as shown in few reports in response to metal exposure to biological systems198. ROS/RNS-mediated DNA damage can cause an imbalance in normal methyltransferase activity, leading to dysregulation. Aberrant gene expression due to gene-specific hypo/hyper-DNA methylation may lead to diminished glutathione activity, making neuronal systems prone to oxidative stress.
Although the involvement of iron dysregulation in MS seems apparent, the disease mechanism has yet to be clearly delineated. Many clinicians argue that there is inadequate evidence to support the iron-related hypothesis in MS/ALS. The limited efficacy of current therapies in the prevention of relapse/disability warrants exploration of alternative possibilities. MS patients differ widely in clinical outcome and symptoms as evident from plethora of literature, except those clinically definitive MS diagnosed patients. These uncertainties make MS disease pathogenesis worse. Integrating iron homeostatic biochemical markers with genetic screening could be used as novel MS treatment. It could tremendously help to identify MS cohorts requiring nontraditional treatment plans, to target MS pathogenesis aggravated by iron deposition in the brain. It may also help such patients to regulate their dietary iron supplement to avoid uncertainty related with iron in MS/ALS. If disease-related peripheral blood iron level and molecular regulators such as ferritin, transferrin saturation are known among such subgroup, clinical improvement can be achieved via dietary iron supplementation. Several clinical trials are underway to create a systematic approach to identify individualized therapy (Table 2). An interesting future approach to MS diagnostics could include correlating metal concentrations in blood with positive MRI-based diagnoses of MS. It could lead to earlier and more routine detection of MS. This may fill the gap created due to the lack of satisfactory treatments and will put forth a better prospect of obtaining definitive clinical evidence for efficacy/fail-safe therapeutic design. Last but not the least, the use of untethered, wirelessly controlled, mobile, milli/microrobots as unconventional approaches can be utilized to enhance the quality of life in post-treatment of MS/ALS while reducing emotional load, recovery time, and cost, which in recent times became a topic of choice among clinicians for future prospects199–204. Collaborative efforts between robotic researchers, clinicians, biomedical engineers, materials scientists and chemists have led to superior biomaterial-based design of daily need utilities to reduce the toxic metals exposure199–201,206–208. Plaques and implants could be future blockbuster therapeutic procedure to cure MS/ALS209.
Table 2.
Clinical Trials in Iron and copper related with MS and ALS pathology (Data from clinicaltrials.gov)
| Interventions | Study phase | Condition | Location |
|---|---|---|---|
| Drug (Rebif) | 4 | MS | EMD Serono, Inc.Rockland, Massachusetts, USA |
| Injection (autologous stem cells) | 2 | MS | NA |
| Drug (dexrazoxane plus Mitoxantrone and Placebo plus Mitoxantrone) | 2 | MS | Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany |
| Drug (ferumoxytol) | Early phase 1 | MS | National Institutes of Health Clinical Center, 9000 Rockville Pike, Bethesda, Maryland, USA |
| Drug (deferiprone and Placebo oral table) | 2 and 3 | ALS | NA |
| Drug (deferiprone) | 2 | ALS | Hôpital Roger Salengro, CHRU de Lille, Lille, France |
| Drug (copper) | 2 | ALS | Phoenix Neurological Associates, Phoenix, Arizona, USA |
| Drug (Cu(II)ATSM) | 2 | ALS | Macquarie University, Sydney, New South Wales, Australia |
Conclusion
We highlighted the recent progress in the role of redox metals in MS/ALS. The redox capacity of iron and copper, their contribution to Fenton reactions, and production of oxidative stress are identified as prominent factors for neurodegeneration in MS/ALS. Moreover, metals dys-homeostasis is reported to cause oxidative damage. The role of SOD1 mutation on copper dys-regulation in neurodegenerative disorders, particularly in ALS has been noticed by some researchers. In addition, metal chelator therapy in animal models of these disorders rescue neuronal degeneration and increased survival. On the other hand, there have been few studies on the effect of cadmium on MS/ALS; its neurotoxicity has proved through oxidative stress formation, reduction of antioxidant enzymes activity, and cellular antioxidants. Despite extensive studies to date, the main cause of these neurodegenerative disorders is still unknown and requires further investigation.
Acknowledgements
We thank Morteza Amjadi for proofreading, Yang Xiao and Shuo Wang for their assistance with the graphic designs. A.V.S. thanks Max Planck Society for the grass root project grant 2017 (M10335) and 2018 (M10338).
Competing interests
The authors declare no competing financial interests.
Footnotes
Edited by A. Verkhratsky
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Paolo Zamboni, Email: zambo@unife.it.
Ajay Vikram Singh, Email: avsingh@is.mpg.de.
References
- 1.Hametner S, et al. Iron and neurodegeneration in the multiple sclerosis brain. Ann. Neurol. 2013;74:848–861. doi: 10.1002/ana.23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh AV, Zamboni P. Anomalous venous blood flow and iron deposition in multiple sclerosis. J. Cereb. Blood. Flow. Metab. 2009;29:1867–1878. doi: 10.1038/jcbfm.2009.180. [DOI] [PubMed] [Google Scholar]
- 3.Singh AV. Multiple sclerosis takes venous route: CCSVI and liberation therapy. Indian. J. Med. Sci. 2010;64:337–340. doi: 10.4103/0019-5359.99879. [DOI] [PubMed] [Google Scholar]
- 4.Raymond MJ, et al. Multiaxial polarity determines individual cellular and nuclear chirality. Cell. Mol. Bioeng. 2017;10:63–74. doi: 10.1007/s12195-016-0467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghoreishi A, Mohseni M, Amraei R, Alizadeh A, Mazloomzadeh S. Investigation the amount of copper, lead, zinc and cadmium levels in serum of Iranian multiple sclerosis patients. J. Chem. Pharm. Sci. 2015;8:40–45. [Google Scholar]
- 6.Aliomrani M, et al. Blood concentrations of cadmium and lead in multiple sclerosis patients from Iran. Iran. J. Pharm. Res. 2016;15:825. [PMC free article] [PubMed] [Google Scholar]
- 7.Ibrahim SM, Gold R. Genomics, proteomics, metabolomics: what is in a word for multiple sclerosis? Curr. Opin. Neurol. 2005;18:231–235. doi: 10.1097/01.wco.0000169738.06664.3b. [DOI] [PubMed] [Google Scholar]
- 8.Reiber H, Teut M, Pohl D, Rostasy K, Hanefeld F. Paediatric and adult multiple sclerosis: age-related differences and time course of the neuroimmunological response in cerebrospinal fluid. Mult. Scler. 2009;15:1466–1480. doi: 10.1177/1352458509348418. [DOI] [PubMed] [Google Scholar]
- 9.Johnson S. The possible role of gradual accumulation of copper, cadmium, lead and iron and gradual depletion of zinc, magnesium, selenium, vitamins B2, B6, D, and E and essential fatty acids in multiple sclerosis. Med. Hypotheses. 2000;55:239–241. doi: 10.1054/mehy.2000.1051. [DOI] [PubMed] [Google Scholar]
- 10.Goodall EF, Haque MS, Morrison KE. Increased serum ferritin levels in amyotrophic lateral sclerosis (ALS) patients. J. Neurol. 2008;255:1652–1656. doi: 10.1007/s00415-008-0945-0. [DOI] [PubMed] [Google Scholar]
- 11.Carrì MT, Ferri A, Cozzolino M, Calabrese L, Rotilio G. Neurodegeneration in amyotrophic lateral sclerosis: the role of oxidative stress and altered homeostasis of metals. Brain Res. Bull. 2003;61:365–374. doi: 10.1016/S0361-9230(03)00179-5. [DOI] [PubMed] [Google Scholar]
- 12.Hadzhieva M, et al. Dysregulation of iron protein expression in the G93A model of amyotrophic lateral sclerosis. Neuroscience. 2013;230:94–101. doi: 10.1016/j.neuroscience.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Tokuda E, Furukawa Y. Copper homeostasis as a therapeutic target in amyotrophic lateral sclerosis with SOD1 mutations. Int. J. Mol. Sci. 2016;17:636. doi: 10.3390/ijms17050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Que EL, Domaille DW, Chang CJ. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 2008;108:1517–1549. doi: 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]
- 15.Crichton RR, Dexter D, Ward RJ. Metal based neurodegenerative diseases—from molecular mechanisms to therapeutic strategies. Coord. Chem. Rev. 2008;252:1189–1199. doi: 10.1016/j.ccr.2007.10.019. [DOI] [Google Scholar]
- 16.Visconti A, et al. Concentration of elements in serum of patients affected by multiple sclerosis with first demyelinating episode: a six-month longitudinal follow-up study. Ann. Ist. Super. Sanita. 2004;41:217–222. [PubMed] [Google Scholar]
- 17.Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K. Redox-and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016;90:1–37. doi: 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- 18.Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40:959–975. doi: 10.1016/S0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria central role of complex III. J. Biol. Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 20.Mahad D, Ziabreva I, Lassmann H, Turnbull D. Mitochondrial defects in acute multiple sclerosis lesions. Brain. 2008;131:1722–1735. doi: 10.1093/brain/awn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell GR, et al. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann. Neurol. 2011;69:481–492. doi: 10.1002/ana.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oshiro S, Morioka MS, Kikuchi M. Dysregulation of iron metabolism in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Adv. Pharmacol. Sci. 2011;2011:378278. doi: 10.1155/2011/378278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biasiotto G, Di Lorenzo D, Archetti S, Zanella I. Iron and neurodegeneration: is ferritinophagy the link? Mol. Neurobiol. 2016;53:5542–5574. doi: 10.1007/s12035-015-9473-y. [DOI] [PubMed] [Google Scholar]
- 24.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Hallgren B, Sourander P. The effect of age on the non‐haemin iron in the human brain. J. Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- 26.Aquino D, et al. Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects 1. Radiology. 2009;252:165–172. doi: 10.1148/radiol.2522081399. [DOI] [PubMed] [Google Scholar]
- 27.Zamboni P, et al. Serum iron and matrix metalloproteinase‐9 variations in limbs affected by chronic venous disease and venous leg ulcers. Dermatol. Surg. 2005;31:644–649. doi: 10.1097/00042728-200506000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Singh AV, et al. Investigation of in vitro cytotoxicity of the redox state of ionic iron in neuroblastoma cells. J. Neurosci. Rural Pract. 2012;3:301. doi: 10.4103/0976-3147.102611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zamboni P, et al. The overlapping of local iron overload and HFE mutation in venous leg ulcer pathogenesis. Free. Radic. Biol. Med. 2006;40:1869–1873. doi: 10.1016/j.freeradbiomed.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 30.Zamboni P. The big idea: iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis. J. R. Soc. Med. 2006;99:589–593. doi: 10.1177/014107680609901122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haacke EM, et al. Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. J. Magn. Reson. Imaging. 2009;29:537–544. doi: 10.1002/jmri.21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stankiewicz J, et al. Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics. 2007;4:371–386. doi: 10.1016/j.nurt.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeVine SM, Chakrabarty A. The role of iron in the pathogenesis of experimental allergic encephalomyelitis and multiple sclerosis. Ann. N. Y. Acad. Sci. 2004;1012:252–266. doi: 10.1196/annals.1306.021. [DOI] [PubMed] [Google Scholar]
- 34.Bergsland N, et al. White matter tract injury is associated with deep gray matter iron deposition in multiple sclerosis. J. Neuroimaging. 2017;27:107–113. doi: 10.1111/jon.12364. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida M, et al. A mutation database for amyotrophic lateral sclerosis. Hum. Mutat. 2010;31:1003–1010. doi: 10.1002/humu.21306. [DOI] [PubMed] [Google Scholar]
- 36.Wang XS, et al. Increased incidence of the Hfe mutation in amyotrophic lateral sclerosis and related cellular consequences. J. Neurol. Sci. 2004;227:27–33. doi: 10.1016/j.jns.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Jeong SY, et al. Dysregulation of iron homeostasis in the CNS contributes to disease progression in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2009;29:610–619. doi: 10.1523/JNEUROSCI.5443-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh AV, Subhashree L, Milani P, Gemmati D, Zamboni P. Interplay of iron metallobiology, metalloproteinases, and FXIII, and role of their gene variants in venous leg ulcer. Int. J. Low. Extrem. Wounds. 2010;9:166–179. doi: 10.1177/1534734610384653. [DOI] [PubMed] [Google Scholar]
- 39.Gemmati D, et al. Influence of gene polymorphisms in ulcer healing process after superficial venous surgery. J. Vasc. Surg. 2006;44:554–562. doi: 10.1016/j.jvs.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Gemmati D, et al. Polymorphisms in the genes coding for iron binding and transporting proteins are associated with disability, severity, and early progression in multiple sclerosis. BMC Med. Genet. 2012;13:70. doi: 10.1186/1471-2350-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamboni P, et al. Hemochromatosis C282Y gene mutation increases the risk of venous leg ulceration. J. Vasc. Surg. 2005;42:309–314. doi: 10.1016/j.jvs.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Restagno G, et al. HFE H63D polymorphism is increased in patients with amyotrophic lateral sclerosis of Italian origin. J. Neurol. Neurosurg. Psychiatry. 2007;78:327–327. doi: 10.1136/jnnp.2006.092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodall E, et al. Association of the H63D polymorphism in the hemochromatosis gene with sporadic ALS. Neurology. 2005;65:934–937. doi: 10.1212/01.wnl.0000176032.94434.d4. [DOI] [PubMed] [Google Scholar]
- 44.Sutedja NA, et al. The association between H63D mutations in HFE and amyotrophic lateral sclerosis in a Dutch population. Arch. Neurol. 2007;64:63–67. doi: 10.1001/archneur.64.1.63. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira KPZ, et al. Disease progression and oxidative stress are associated with higher serum ferritin levels in patients with multiple sclerosis. J. Neurol. Sci. 2017;373:236–241. doi: 10.1016/j.jns.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 46.Iranmanesh M, Iranmanesh F, Sadeghi H. Serum level of iron, zinc and copper in patients with multiple sclerosis. J. Jahr. Uni. Med. Sci. 2013;10:1–5. [Google Scholar]
- 47.Forge JK, Pedchenko TV, Le Vine SM. Iron deposits in the central nervous system of SJL mice with experimental allergic encephalomyelitis. Life. Sci. 1998;63:2271–2284. doi: 10.1016/S0024-3205(98)00512-8. [DOI] [PubMed] [Google Scholar]
- 48.Khare M, Singh A, Zamboni P. Prospect of brain machine interface in motor disabilities: the future support for multiple sclerosis patient to improve quality of life. Ann. Med. Health Sci. Res. 2014;4:305–312. doi: 10.4103/2141-9248.133447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uttara B, Singh AV, Zamboni P, Mahajan R. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alimonti A, et al. Serum chemical elements and oxidative status in Alzheimer’s disease, Parkinson disease and multiple sclerosis. Neurotoxicology. 2007;28:450–456. doi: 10.1016/j.neuro.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Hammond KE, et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann. Neurol. 2008;64:707–713. doi: 10.1002/ana.21582. [DOI] [PubMed] [Google Scholar]
- 52.Bian W, et al. A serial in vivo 7T magnetic resonance phase imaging study of white matter lesions in multiple sclerosis. Mult. Scler. 2013;19:69–75. doi: 10.1177/1352458512447870. [DOI] [PubMed] [Google Scholar]
- 53.Mehta V, et al. Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions. PLoS ONE. 2013;8:e57573. doi: 10.1371/journal.pone.0057573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bagnato F, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602–3615. doi: 10.1093/brain/awr278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hagemeier J, et al. Iron deposition in multiple sclerosis lesions measured by susceptibility‐weighted imaging filtered phase: A case control study. J. Magn. Reson. Imaging. 2012;36:73–83. doi: 10.1002/jmri.23603. [DOI] [PubMed] [Google Scholar]
- 56.Adams C. Perivascular iron deposition and other vascular damage in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 1988;51:260–265. doi: 10.1136/jnnp.51.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zivadinov R, et al. Cerebral microbleeds in multiple sclerosis evaluated on susceptibility-weighted images and quantitative susceptibility maps: a case-control study. Radiology. 2016;281:884–895. doi: 10.1148/radiol.2016160060. [DOI] [PubMed] [Google Scholar]
- 58.Zamboni, P. The contribution of extra cranial venous drainage to neuro-inflammation in multiple sclerosis. Neuroinflammation (2018).
- 59.Bakshi R, Shaikh ZA, Janardhan V. MRI T2 shortening (‘black T2’) in multiple sclerosis: frequency, location, and clinical correlation. Neuroreport. 2000;11:15–21. doi: 10.1097/00001756-200001170-00004. [DOI] [PubMed] [Google Scholar]
- 60.Tjoa C, Benedict R, Weinstock-Guttman B, Fabiano A, Bakshi R. MRI T2 hypointensity of the dentate nucleus is related to ambulatory impairment in multiple sclerosis. J. Neurol. Sci. 2005;234:17–24. doi: 10.1016/j.jns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Brass SD, Benedict RH, Weinstock-Guttman B, Munschauer F, Bakshi R. Cognitive impairment is associated with subcortical magnetic resonance imaging grey matter T2 hypointensity in multiple sclerosis. Mult. Scler. 2006;12:437–444. doi: 10.1191/135248506ms1301oa. [DOI] [PubMed] [Google Scholar]
- 62.Modica C, et al. Iron and volume in the deep gray matter: association with cognitive impairment in multiple sclerosis. Am. J. Neuroradiol. 2015;36:57–62. doi: 10.3174/ajnr.A3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bø L, Vedeler CA, Nyland H, Trapp BD, Mørk SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult. Scler. 2003;9:323–331. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- 64.Peterson JW, Bö L, Mörk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann. Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 65.Haider L, et al. Multiple sclerosis deep grey matter: the relation between demyelination, neurodegeneration, inflammation and iron. J. Neurol. Neurosurg. Psychiatry. 2014;85:1386–1395. doi: 10.1136/jnnp-2014-307712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ge Y, et al. Quantitative assessment of iron accumulation in the deep gray matter of multiple sclerosis by magnetic field correlation imaging. Am. J. Neuroradiol. 2007;28:1639–1644. doi: 10.3174/ajnr.A0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veyrat-Durebex C, et al. Iron metabolism disturbance in a French cohort of ALS patients. BioMed. Res. Int. 2014;2014:485723. doi: 10.1155/2014/485723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nadjar Y, et al. Elevated serum ferritin is associated with reduced survival in amyotrophic lateral sclerosis. PLoS ONE. 2012;7:e45034. doi: 10.1371/journal.pone.0045034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikeda K, Hirayama T, Takazawa T, Kawabe K, Iwasaki Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern. Med. 2012;51:1501–1508. doi: 10.2169/internalmedicine.51.7465. [DOI] [PubMed] [Google Scholar]
- 70.Ince P, et al. Iron, selenium and glutathione peroxidase activity are elevated in sporadic motor neuron disease. Neurosci. Lett. 1994;182:87–90. doi: 10.1016/0304-3940(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 71.Kasarskis EJ, Tandon L, Lovell MA, Ehmann WD. Aluminum, calcium, and iron in the spinal cord of patients with sporadic amyotrophic lateral sclerosis using laser microprobe mass spectroscopy: a preliminary study. J. Neurol. Sci. 1995;130:203–208. doi: 10.1016/0022-510X(95)00037-3. [DOI] [PubMed] [Google Scholar]
- 72.Markesbery WR, et al. Neutron activation analysis of trace elements in motor neuron disease spinal cord. Neurodegeneration. 1995;4:383–390. doi: 10.1006/neur.1995.0046. [DOI] [PubMed] [Google Scholar]
- 73.Yasui M, Ota K, Garruto RM. Concentrations of zinc and iron in the brains of Guamanian patients with amyotrophic lateral sclerosis and parkinsonism-dementia. Neurotoxicology. 1993;14:445–450. [PubMed] [Google Scholar]
- 74.Hozumi I, et al. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J. Neurol. Sci. 2011;303:95–99. doi: 10.1016/j.jns.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 75.Mizuno Y, et al. Transferrin localizes in Bunina bodies in amyotrophic lateral sclerosis. Acta Neuropathol. 2006;112:597–603. doi: 10.1007/s00401-006-0122-4. [DOI] [PubMed] [Google Scholar]
- 76.Ignjatović A, Stević Z, Lavrnić S, Daković M, Bačić G. Brain iron MRI: a biomarker for amyotrophic lateral sclerosis. J. Magn. Reson. Imaging. 2013;38:1472–1479. doi: 10.1002/jmri.24121. [DOI] [PubMed] [Google Scholar]
- 77.Oba H, et al. Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology. 1993;189:843–846. doi: 10.1148/radiology.189.3.8234713. [DOI] [PubMed] [Google Scholar]
- 78.Imon Y, et al. A decrease in cerebral cortex intensity on T2-weighted with ageing images of normal subjects. Neuroradiology. 1998;40:76–80. doi: 10.1007/s002340050544. [DOI] [PubMed] [Google Scholar]
- 79.Hecht M, Fellner C, Schmid A, Neundörfer B, Fellner F. Cortical T2 signal shortening in amyotrophic lateral sclerosis is not due to iron deposits. Neuroradiology. 2005;47:805–808. doi: 10.1007/s00234-005-1421-5. [DOI] [PubMed] [Google Scholar]
- 80.Winkler EA, et al. Blood–spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc. Natl Acad. Sci. 2014;111:E1035–E1042. doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Q, et al. Prevention of motor neuron degeneration by novel iron chelators in SOD1G93A transgenic mice of amyotrophic lateral sclerosis. Neurodegener. Dis. 2011;8:310–321. doi: 10.1159/000323469. [DOI] [PubMed] [Google Scholar]
- 82.Kupershmidt L, et al. Neuroprotective and neuritogenic activities of novel multimodal iron-chelating drugs in motor-neuron-like NSC-34 cells and transgenic mouse model of amyotrophic lateral sclerosis. Faseb. J. 2009;23:3766–3779. doi: 10.1096/fj.09-130047. [DOI] [PubMed] [Google Scholar]
- 83.Zheng W, Monnot AD. Regulation of brain iron and copper homeostasis by brain barrier systems: implication in neurodegenerative diseases. Pharmacol. Ther. 2012;133:177–188. doi: 10.1016/j.pharmthera.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi BS, Zheng W. Copper transport to the brain by the blood–brain barrier and blood-CSF barrier. Brain Res. 2009;1248:14–21. doi: 10.1016/j.brainres.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scheiber IF, Dringen R. Astrocyte functions in the copper homeostasis of the brain. Neurochem. Int. 2013;62:556–565. doi: 10.1016/j.neuint.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 86.Scheiber IF, Mercer JF, Dringen R. Metabolism and functions of copper in brain. Prog. Neurobiol. 2014;116:33–57. doi: 10.1016/j.pneurobio.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 87.West AK, Hidalgo J, Eddins D, Levin ED, Aschner M. Metallothionein in the central nervous system: roles in protection, regeneration and cognition. Neurotoxicology. 2008;29:489–503. doi: 10.1016/j.neuro.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manto M. Abnormal copper homeostasis: mechanisms and roles in neurodegeneration. Toxics. 2014;2:327–345. doi: 10.3390/toxics2020327. [DOI] [Google Scholar]
- 89.Lutsenko S, Gupta A, Burkhead JL, Zuzel V. Cellular multitasking: the dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance. Arch. Biochem. Biophys. 2008;476:22–32. doi: 10.1016/j.abb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 91.Marikovsky M, Ziv V, Nevo N, Harris-Cerruti C, Mahler O. Cu/Zn superoxide dismutase plays important role in immune response. J. Immunol. 2003;170:2993–3001. doi: 10.4049/jimmunol.170.6.2993. [DOI] [PubMed] [Google Scholar]
- 92.Tapiero H, Townsend D, Tew K. Trace elements in human physiology and pathology. Copp. Biomed. Pharmacother. 2003;57:386–398. doi: 10.1016/S0753-3322(03)00012-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Konstantinova SG, Russanova IE, Russanov EM. Do the copper complexes of histamine, histidine and of two H2-antagonists react with O− 2? Free. Radic. Res. Commun. 1991;12:215–220. doi: 10.3109/10715769109145789. [DOI] [PubMed] [Google Scholar]
- 94.Socha K, et al. Dietary habits; concentration of copper, zinc, and Cu-to-Zn ratio in serum and ability status of patients with relapsing-remitting multiple sclerosis. Nutrition. 2017;39:76–81. doi: 10.1016/j.nut.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 95.Banci L, et al. Affinity gradients drive copper to cellular destinations. Nature. 2010;465:645. doi: 10.1038/nature09018. [DOI] [PubMed] [Google Scholar]
- 96.Dringen R, Scheiber IF, Mercer JF. Copper metabolism of astrocytes. Front. Aging Neurosci. 2013;5:9. doi: 10.3389/fnagi.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roos PM, Vesterberg O, Syversen T, Flaten TP, Nordberg M. Metal concentrations in cerebrospinal fluid and blood plasma from patients with amyotrophic lateral sclerosis. Biol. Trace Elem. Res. 2013;151:159–170. doi: 10.1007/s12011-012-9547-x. [DOI] [PubMed] [Google Scholar]
- 98.Ibrahimagić O, Sinanović O, Zonić L, Hudić J. Amyotrophic lateral sclerosis in younger age associated with abnormality of copper level. Med. Arh. 2006;60:108–109. [PubMed] [Google Scholar]
- 99.Van Horssen J, Witte ME, Schreibelt G, De Vries HE. Radical changes in multiple sclerosis pathogenesis. Biochim. Biophys. Acta. 2011;1812:141–150. doi: 10.1016/j.bbadis.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 100.Aspli KT, et al. Iron and copper in progressive demyelination–New lessons from Skogholt’s disease. J. Trace Elem. Med. Biol. 2015;31:183–187. doi: 10.1016/j.jtemb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 101.Ghazavi A, Kianbakht S, Ghasami K, Mosayebi G. High copper and low zinc serum levels in Iranian patients with multiple sclerosis: a case control study. Clin. Lab. 2012;58:161–164. [PubMed] [Google Scholar]
- 102.Sedighi B, Ebrahimi HA, Haghdoost AA, Abotorabi M. Comparison of serum levels of copper and zinc among multiple sclerosis patients and control group. Iran J. Neurol. 2013;12:125. [PMC free article] [PubMed] [Google Scholar]
- 103.Melø TM, et al. Manganese, copper, and zinc in cerebrospinal fluid from patients with multiple sclerosis. Biol. Trace Elem. Res. 2003;93:1–8. doi: 10.1385/BTER:93:1-3:1. [DOI] [PubMed] [Google Scholar]
- 104.Mandelbrote B, Stanier M, Thompson R, THBUSTON M. Studies on copper metabolism in demyelinating diseases of the central nervous system. Brain. 1948;71:212–228. doi: 10.1093/brain/71.2.212. [DOI] [PubMed] [Google Scholar]
- 105.Plum CM, Hansen SE. Studies on variations in serum copper and serum copper oxidase activity, together with studies on the copper content of the cerebrospinal fluid, with particular reference to the variations in multiple sclerosis. Acta Psychiatr. Scand. 1960;35:41–78. doi: 10.1111/j.1600-0447.1960.tb08680.x. [DOI] [PubMed] [Google Scholar]
- 106.Wikström J, Westermarcik T, Palo J. Selenium, vitamin E and copper in multiple sclerosis. Acta Neurol. Scand. 1976;54:287–290. doi: 10.1111/j.1600-0404.1976.tb04806.x. [DOI] [PubMed] [Google Scholar]
- 107.Bammer H. Caeruloplasmin-und kupferstoffwechsel bei multipler sklerose. J. Neurol. 1966;189:312–329. doi: 10.1007/BF00244226. [DOI] [PubMed] [Google Scholar]
- 108.Giacoppo S, et al. Heavy metals and neurodegenerative diseases: an observational study. Biol. Trace Elem. Res. 2014;161:151–160. doi: 10.1007/s12011-014-0094-5. [DOI] [PubMed] [Google Scholar]
- 109.Tamburo E, Varrica D, Dongarrà G, Grimaldi LME. Trace elements in scalp hair samples from patients with relapsing-remitting multiple sclerosis. PLoS ONE. 2015;10:e0122142. doi: 10.1371/journal.pone.0122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Palm R, Hallmans G. Zinc and copper in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 1982;45:691–698. doi: 10.1136/jnnp.45.8.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kapaki E, Segditsa J, Papageorgiou C. Zinc, copper and magnesium concentration in serum and CSF of patients with neurological disorders. Acta Neurol. Scand. 1989;79:373–378. doi: 10.1111/j.1600-0404.1989.tb03803.x. [DOI] [PubMed] [Google Scholar]
- 112.Norkute A, et al. Cuprizone treatment induces demyelination and astrocytosis in the mouse hippocampus. J. Neurosci. Res. 2009;87:1343–1355. doi: 10.1002/jnr.21946. [DOI] [PubMed] [Google Scholar]
- 113.Torkildsen Oslash, Brunborg L, Myhr KM, Bø L. The cuprizone model for demyelination. Acta Neurol. Scand. 2008;117:72–76. doi: 10.1111/j.1600-0404.2008.01036.x. [DOI] [PubMed] [Google Scholar]
- 114.Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herring NR, Konradi C. Myelin, copper, and the cuprizone model of schizophrenia. Front. Biosci. (Sch. Ed.) 2011;3:23. doi: 10.2741/s129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wergeland S, Torkildsen Oslash, Myhr KM, Mørk SJ, Bø L. The cuprizone model: regional heterogeneity of pathology. APMIS. 2012;120:648–657. doi: 10.1111/j.1600-0463.2012.02882.x. [DOI] [PubMed] [Google Scholar]
- 117.Zatta P, et al. Copper and zinc dismetabolism in the mouse brain upon chronic cuprizone treatment. Cell. Mol. Life. Sci. 2005;62:1502–1513. doi: 10.1007/s00018-005-5073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Groebe A, et al. Cuprizone treatment induces distinct demyelination, astrocytosis, and microglia cell invasion or proliferation in the mouse cerebellum. Cerebellum. 2009;8:163–174. doi: 10.1007/s12311-009-0099-3. [DOI] [PubMed] [Google Scholar]
- 119.Silvestroff L, et al. Cuprizone‐induced demyelination in CNP:: GFP transgenic mice. J. Comp. Neurol. 2010;518:2261–2283. doi: 10.1002/cne.22330. [DOI] [PubMed] [Google Scholar]
- 120.Hoffmann K, Lindner M, Gröticke I, Stangel M, Löscher W. Epileptic seizures and hippocampal damage after cuprizone-induced demyelination in C57BL/6 mice. Exp. Neurol. 2008;210:308–321. doi: 10.1016/j.expneurol.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 121.Pott F, et al. Cuprizone effect on myelination, astrogliosis and microglia attraction in the mouse basal ganglia. Brain Res. 2009;1305:137–149. doi: 10.1016/j.brainres.2009.09.084. [DOI] [PubMed] [Google Scholar]
- 122.Skripuletz T, et al. Cortical demyelination is prominent in the murine cuprizone model and is strain-dependent. Am. J. Pathol. 2008;172:1053–1061. doi: 10.2353/ajpath.2008.070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koutsoudaki PN, et al. Demyelination of the hippocampus is prominent in the cuprizone model. Neurosci. Lett. 2009;451:83–88. doi: 10.1016/j.neulet.2008.11.058. [DOI] [PubMed] [Google Scholar]
- 124.Kaur D, et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson’s disease. Neuron. 2003;37:899–909. doi: 10.1016/S0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 125.Cherny RA, et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/S0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 126.Choi BY, et al. Copper/zinc chelation by clioquinol reduces spinal cord white matter damage and behavioral deficits in a murine MOG-induced multiple sclerosis model. Neurobiol. Dis. 2013;54:382–391. doi: 10.1016/j.nbd.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 127.Gil-Bea FJ, Aldanondo G, Lasa-Fernández H, de Munain AL, Vallejo-Illarramendi A. Insights into the mechanisms of copper dyshomeostasis in amyotrophic lateral sclerosis. Expert. Rev. Mol. Med. 2017;19:e7. doi: 10.1017/erm.2017.9. [DOI] [PubMed] [Google Scholar]
- 128.Culotta VC, Joh HD, Lin SJ, Slekar KH, Strain J. A physiological role for Saccharomyces cerevisiae copper/zinc superoxide dismutase in copper buffering. J. Biol. Chem. 1995;270:29991–29997. doi: 10.1074/jbc.270.50.29991. [DOI] [PubMed] [Google Scholar]
- 129.Tokuda E, Okawa E, Ono Si. Dysregulation of intracellular copper trafficking pathway in a mouse model of mutant copper/zinc superoxide dismutase‐linked familial amyotrophic lateral sclerosis. J. Neurochem. 2009;111:181–191. doi: 10.1111/j.1471-4159.2009.06310.x. [DOI] [PubMed] [Google Scholar]
- 130.Tokuda E, Okawa E, Watanabe S, Ono Si, Marklund SL. Dysregulation of intracellular copper homeostasis is common to transgenic mice expressing human mutant superoxide dismutase-1s regardless of their copper-binding abilities. Neurobiol. Dis. 2013;54:308–319. doi: 10.1016/j.nbd.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 131.Tokuda E, et al. Metallothionein proteins expression, copper and zinc concentrations, and lipid peroxidation level in a rodent model for amyotrophic lateral sclerosis. Toxicology. 2007;229:33–41. doi: 10.1016/j.tox.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 132.Li QX, et al. Overexpression of Aβ is associated with acceleration of onset of motor impairment and superoxide dismutase 1 aggregation in an amyotrophic lateral sclerosis mouse model. Aging Cell. 2006;5:153–165. doi: 10.1111/j.1474-9726.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 133.Lelie HL, et al. Copper and zinc metallation status of copper-zinc superoxide dismutase from amyotrophic lateral sclerosis transgenic mice. J. Biol. Chem. 2011;286:2795–2806. doi: 10.1074/jbc.M110.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jonsson PA, et al. Motor neuron disease in mice expressing the wild type-like D90A mutant superoxide dismutase-1. J. Neuropathol. Exp. Neurol. 2006;65:1126–1136. doi: 10.1097/01.jnen.0000248545.36046.3c. [DOI] [PubMed] [Google Scholar]
- 135.Zetterström P, et al. Soluble misfolded subfractions of mutant superoxide dismutase-1s are enriched in spinal cords throughout life in murine ALS models. Proc. Natl Acad. Sci. 2007;104:14157–14162. doi: 10.1073/pnas.0700477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bourassa MW, Brown HH, Borchelt DR, Vogt S, Miller LM. Metal-deficient aggregates and diminished copper found in cells expressing SOD1 mutations that cause ALS. Front. Aging Neurosci. 2014;6:110. doi: 10.3389/fnagi.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pratt AJ, et al. Aggregation propensities of superoxide dismutase G93 hotspot mutants mirror ALS clinical phenotypes. Proc. Natl Acad. Sci. 2014;111:E4568–E4576. doi: 10.1073/pnas.1308531111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rodriguez JA, et al. Familial amyotrophic lateral sclerosis-associated mutations decrease the thermal stability of distinctly metallated species of human copper/zinc superoxide dismutase. J. Biol. Chem. 2002;277:15932–15937. doi: 10.1074/jbc.M112088200. [DOI] [PubMed] [Google Scholar]
- 139.Lynch SM, Colón W. Dominant role of copper in the kinetic stability of Cu/Zn superoxide dismutase. Biochem. Biophys. Res. Commun. 2006;340:457–461. doi: 10.1016/j.bbrc.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 140.Tiwari A, et al. Metal deficiency increases aberrant hydrophobicity of mutant superoxide dismutases that cause amyotrophic lateral sclerosis. J. Biol. Chem. 2009;284:27746–27758. doi: 10.1074/jbc.M109.043729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Corson LB, Strain JJ, Culotta VC, Cleveland DW. Chaperone-facilitated copper binding is a property common to several classes of familial amyotrophic lateral sclerosis-linked superoxide dismutase mutants. Proc. Natl Acad. Sci. 1998;95:6361–6366. doi: 10.1073/pnas.95.11.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hayward LJ, et al. Decreased metallation and activity in subsets of mutant superoxide dismutases associated with familial amyotrophic lateral sclerosis. J. Biol. Chem. 2002;277:15923–15931. doi: 10.1074/jbc.M112087200. [DOI] [PubMed] [Google Scholar]
- 143.Ratovitski T, et al. Variation in the biochemical/biophysical properties of mutant superoxide dismutase 1 enzymes and the rate of disease progression in familial amyotrophic lateral sclerosis kindreds. Hum. Mol. Genet. 1999;8:1451–1460. doi: 10.1093/hmg/8.8.1451. [DOI] [PubMed] [Google Scholar]
- 144.Roberts BR, et al. Oral treatment with CuII (atsm) increases mutant SOD1 in vivo but protects motor neurons and improves the phenotype of a transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2014;34:8021–8031. doi: 10.1523/JNEUROSCI.4196-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Banci L, et al. Metalation of the amyotrophic lateral sclerosis mutant glycine 37 to arginine superoxide dismutase (SOD1) apoprotein restores its structural and dynamical properties in solution to those of metalated wild-type SOD1. Biochemistry. 2007;46:9953–9962. doi: 10.1021/bi700620r. [DOI] [PubMed] [Google Scholar]
- 146.Son M, et al. Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc. Natl Acad. Sci. 2007;104:6072–6077. doi: 10.1073/pnas.0610923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Williams JR, et al. Copper delivery to the CNS by CuATSM effectively treats motor neuron disease in SOD G93A mice co-expressing the Copper-Chaperone-for-SOD. Neurobiol. Dis. 2016;89:1–9. doi: 10.1016/j.nbd.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Subramaniam JR, et al. Mutant SOD1 causes motor neuron disease independent of copper chaperone-mediated copper loading. Nat. Neurosci. 2002;5:301. doi: 10.1038/nn823. [DOI] [PubMed] [Google Scholar]
- 149.Beckman JS, Estvez AG, Barbeito L, Crow JP. CCS knockout mice establish an alternative source of copper for SOD in ALS. Free. Radic. Biol. Med. 2002;33:1433–1435. doi: 10.1016/S0891-5849(02)01092-4. [DOI] [PubMed] [Google Scholar]
- 150.Tapia L, et al. Metallothionein is crucial for safe intracellular copper storage and cell survival at normal and supra-physiological exposure levels. Biochem. J. 2004;378:617–624. doi: 10.1042/bj20031174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu SX, et al. Reconstitution of apo-superoxide dismutase by nitric oxide-induced copper transfer from metallothioneins. Chem. Res. Toxicol. 2000;13:922–931. doi: 10.1021/tx0000623. [DOI] [PubMed] [Google Scholar]
- 152.Seagrave J, Hanners JL, Taylor W, O’Brien HA. Transfer of copper from metallothionein to nonmetallothionein proteins in cultured cells. Biol. Trace Elem. Res. 1986;10:163. doi: 10.1007/BF02795615. [DOI] [PubMed] [Google Scholar]
- 153.Tokuda E, Watanabe S, Okawa E, Ono Si. Regulation of intracellular copper by induction of endogenous metallothioneins improves the disease course in a mouse model of amyotrophic lateral sclerosis. Neurotherapeutics. 2015;12:461–476. doi: 10.1007/s13311-015-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hozumi I, et al. The expression of metallothioneins is diminished in the spinal cords of patients with sporadic ALS. Amyotroph. Lateral Scler. 2008;9:294–298. doi: 10.1080/17482960801934312. [DOI] [PubMed] [Google Scholar]
- 155.Hashimoto K, Hayashi Y, Watabe K, Inuzuka T, Hozumi I. Metallothionein-III prevents neuronal death and prolongs life span in amyotrophic lateral sclerosis model mice. Neuroscience. 2011;189:293–298. doi: 10.1016/j.neuroscience.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 156.Nagano S, et al. Reduction of metallothioneins promotes the disease expression of familial amyotrophic lateral sclerosis mice in a dose‐dependent manner. Eur. J. Neurosci. 2001;13:1363–1370. doi: 10.1046/j.0953-816x.2001.01512.x. [DOI] [PubMed] [Google Scholar]
- 157.Tokuda E, Okawa E, Watanabe S, Ono Si. Overexpression of metallothionein-I, a copper-regulating protein, attenuates intracellular copper dyshomeostasis and extends lifespan in a mouse model of amyotrophic lateral sclerosis caused by mutant superoxide dismutase-1. Hum. Mol. Genet. 2013;23:1271–1285. doi: 10.1093/hmg/ddt517. [DOI] [PubMed] [Google Scholar]
- 158.Ishigaki S, et al. Differentially expressed genes in sporadic amyotrophic lateral sclerosis spinal cords–screening by molecular indexing and subsequent cDNA microarray analysis. FEBS Lett. 2002;531:354–358. doi: 10.1016/S0014-5793(02)03546-9. [DOI] [PubMed] [Google Scholar]
- 159.Kelly EJ, Sandgren EP, Brinster RL, Palmiter RD. A pair of adjacent glucocorticoid response elements regulate expression of two mouse metallothionein genes. Proc. Natl Acad. Sci. 1997;94:10045–10050. doi: 10.1073/pnas.94.19.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Smitt PS, Blaauwgeers H, Troost D, de Jong JV. Metallothionein immunoreactivity is increased in the spinal cord of patients with amyotrophic lateral sclerosis. Neurosci. Lett. 1992;144:107–110. doi: 10.1016/0304-3940(92)90727-O. [DOI] [PubMed] [Google Scholar]
- 161.Soon CP, et al. Diacetylbis (N (4)-methylthiosemicarbazonato) copper (II)(CuII (atsm)) protects against peroxynitrite-induced nitrosative damage and prolongs survival in amyotrophic lateral sclerosis mouse model. J. Biol. Chem. 2011;286:44035–44044. doi: 10.1074/jbc.M111.274407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McAllum EJ, et al. Therapeutic effects of CuII (atsm) in the SOD1-G37R mouse model of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2013;14:586–590. doi: 10.3109/21678421.2013.824000. [DOI] [PubMed] [Google Scholar]
- 163.Hilton JB, et al. CuII (atsm) improves the neurological phenotype and survival of SOD1G93A mice and selectively increases enzymatically active SOD1 in the spinal cord. Sci. Rep. 2017;7:42292. doi: 10.1038/srep42292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tokuda E, et al. Ammonium tetrathiomolybdate delays onset, prolongs survival, and slows progression of disease in a mouse model for amyotrophic lateral sclerosis. Exp. Neurol. 2008;213:122–128. doi: 10.1016/j.expneurol.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 165.Hottinger AF, Fine EG, Gurney ME, Zurn AD, Aebischer P. The copper chelator D‐penicillamine delays onset of disease and extends survival in a transgenic mouse model of familial amyotrophic lateral sclerosis. Eur. J. Neurosci. 1997;9:1548–1551. doi: 10.1111/j.1460-9568.1997.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 166.Nagano S, Ogawa Y, Yanagihara T, Sakoda S. Benefit of a combined treatment with trientine and ascorbate in familial amyotrophic lateral sclerosis model mice. Neurosci. Lett. 1999;265:159–162. doi: 10.1016/S0304-3940(99)00227-X. [DOI] [PubMed] [Google Scholar]
- 167.Andreassen OA, et al. Effects of an inhibitor of poly (ADP-ribose) polymerase, desmethylselegiline, trientine, and lipoic acid in transgenic ALS mice. Exp. Neurol. 2001;168:419–424. doi: 10.1006/exnr.2001.7633. [DOI] [PubMed] [Google Scholar]
- 168.Nagano S, et al. The efficacy of trientine or ascorbate alone compared to that of the combined treatment with these two agents in familial amyotrophic lateral sclerosis model mice. Exp. Neurol. 2003;179:176–180. doi: 10.1016/S0014-4886(02)00014-6. [DOI] [PubMed] [Google Scholar]
- 169.Petri S, et al. The lipophilic metal chelators DP‐109 and DP‐460 are neuroprotective in a transgenic mouse model of amyotrophic lateral sclerosis. J. Neurochem. 2007;102:991–1000. doi: 10.1111/j.1471-4159.2007.04604.x. [DOI] [PubMed] [Google Scholar]
- 170.Azzouz M, et al. Prevention of mutant SOD1 motoneuron degeneration by copper chelators in vitro. J. Neurobiol. 2000;42:49–55. doi: 10.1002/(SICI)1097-4695(200001)42:1<49::AID-NEU5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 171.Xu S, et al. Ammonium tetrathiomolybdate as a water-soluble and slow-release hydrogen sulfide donor. Bioorg. Med. Chem. Lett. 2016;26:1585–1588. doi: 10.1016/j.bmcl.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cheng G, Whitehead SN, Hachinski V, Cechetto DF. Effects of pyrrolidine dithiocarbamate on beta-amyloid (25–35)-induced inflammatory responses and memory deficits in the rat. Neurobiol. Dis. 2006;23:140–151. doi: 10.1016/j.nbd.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 173.Ahtoniemi T, et al. Pyrrolidine dithiocarbamate inhibits induction of immunoproteasome and decreases survival in a rat model of amyotrophic lateral sclerosis. Mol. Pharmacol. 2007;71:30–37. doi: 10.1124/mol.106.028415. [DOI] [PubMed] [Google Scholar]
- 174.Calviello G, et al. Repeated exposure to pyrrolidine-dithiocarbamate induces peripheral nerve alterations in rats. Toxicol. Lett. 2005;158:61–71. doi: 10.1016/j.toxlet.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 175.Wang B, Du Y. Cadmium and its neurotoxic effects. Oxid. Med. Cell. Longev. 2013;2013:898034. doi: 10.1155/2013/898034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bulmer F, Rothwell H, Frankish E. Industrial cadmium poisoning: a report of fifteen gases, including two deaths. Can. Public Health J. 1938;29:19–26. [Google Scholar]
- 177.Friberg L. Health Hazards in the Manufacture of Alkaline Accumulators with special reference to chronic cadmium poisoning. a clinical and experimental study. Acta Med. Scand. 1950;138:124. [PubMed] [Google Scholar]
- 178.Singh A, Patil R, Kasture M, Gade W, Prasad B. Synthesis of Ag–Pt alloy nanoparticles in aqueous bovine serum albumin foam and their cytocompatibility against human gingival fibroblasts. Colloids Surf. B. Biointerfaces. 2009;69:239–245. doi: 10.1016/j.colsurfb.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 179.Arvidson B. Retrograde axonal transport of cadmium in the rat hypoglossal nerve. Neurosci. Lett. 1985;62:45–49. doi: 10.1016/0304-3940(85)90282-4. [DOI] [PubMed] [Google Scholar]
- 180.Kumar R, Agarwal AK, Seth PK. Oxidative stress-mediated neurotoxicity of cadmium. Toxicol. Lett. 1996;89:65–69. doi: 10.1016/S0378-4274(96)03780-0. [DOI] [PubMed] [Google Scholar]
- 181.Sinha M, Manna P, Sil PC. Cadmium‐induced neurological disorders: prophylactic role of taurine. J. Appl. Toxicol. 2008;28:974–986. doi: 10.1002/jat.1363. [DOI] [PubMed] [Google Scholar]
- 182.Shukla GS, Srivastava R, Chandra S. Glutathione status and cadmium neurotoxicity: studies in discrete brain regions of growing rats. Fundam. Appl. Toxicol. 1988;11:229–235. doi: 10.1016/0272-0590(88)90147-9. [DOI] [PubMed] [Google Scholar]
- 183.Lopez E, Arce C, Oset-Gasque M, Canadas S, Gonzalez M. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free. Radic. Biol. Med. 2006;40:940–951. doi: 10.1016/j.freeradbiomed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 184.Aliomrani M, et al. Correlation between heavy metal exposure and GSTM1 polymorphism in Iranian multiple sclerosis patients. Neurol. Sci. 2017;38:1271–1278. doi: 10.1007/s10072-017-2934-5. [DOI] [PubMed] [Google Scholar]
- 185.Moradi A, Honarjoo N, Etemadifar M, Fallahzade J. Bio-accumulation of some heavy metals in blood serum of residents in Isfahan and Shiraz, Iran. Environ. Monit. Assess. 2016;188:269. doi: 10.1007/s10661-016-5217-3. [DOI] [PubMed] [Google Scholar]
- 186.Heydarpour P, et al. Potential impact of air pollution on multiple sclerosis in Tehran, Iran. Neuroepidemiology. 2014;43:233–238. doi: 10.1159/000368553. [DOI] [PubMed] [Google Scholar]
- 187.Bar-Sela S, Reingold S, Richter ED. Amyotrophic lateral sclerosis in a battery-factory worker exposed to cadmium. Int. J. Occup. Environ. Health. 2001;7:109–112. doi: 10.1179/oeh.2001.7.2.109. [DOI] [PubMed] [Google Scholar]
- 188.Wu-tao X. The study of amyotrophic lateral sclerosis like changes caused by chronic cadmium poisoning in rats [J] J. Chongqing Med. Uni. 2010;1:010. [Google Scholar]
- 189.Vinceti M, et al. Lead, cadmium, and selenium in the blood of patients with sporadic amyotrophic lateral sclerosis. Ital. J. Neurol. Sci. 1997;18:87–92. doi: 10.1007/BF01999568. [DOI] [PubMed] [Google Scholar]
- 190.Gellein K, Garruto RM, Syversen T, Sjøbakk TE, Flaten TP. Concentrations of Cd, Co, Cu, Fe, Mn, Rb, V, and Zn in formalin-fixed brain tissue in amyotrophic lateral sclerosis and Parkinsonism-dementia complex of Guam determined by High-resolution ICP-MS. Biol. Trace Elem. Res. 2003;96:39–60. doi: 10.1385/BTER:96:1-3:39. [DOI] [PubMed] [Google Scholar]
- 191.Smitt PAS, et al. Increased metallothionein in the liver and kidney of patients with amyotrophic lateral sclerosis. Arch. Neurol. 1992;49:721–724. doi: 10.1001/archneur.1992.00530310063013. [DOI] [PubMed] [Google Scholar]
- 192.Huang YH, et al. Effects of cadmium on structure and enzymatic activity of Cu, Zn‐SOD and oxidative status in neural cells. J. Cell. Biochem. 2006;98:577–589. doi: 10.1002/jcb.20772. [DOI] [PubMed] [Google Scholar]
- 193.Bergomi M, et al. Environmental exposure to trace elements and risk of amyotrophic lateral sclerosis: a population-based case–control study. Environ. Res. 2002;89:116–123. doi: 10.1006/enrs.2002.4361. [DOI] [PubMed] [Google Scholar]
- 194.Pascolo L, et al. Calcium micro-depositions in jugular truncular venous malformations revealed by Synchrotron-based XRF imaging. Sci. Rep. 2014;4:6540. doi: 10.1038/srep06540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Singh AV, Khare M, Gade W, Zamboni P. Theranostic implications of nanotechnology in multiple sclerosis: a future perspective. Autoimmune Dis. 2012;2012:160830. doi: 10.1155/2012/160830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zivadinov R, et al. Comparison of three different methods for measurement of cervical cord atrophy in multiple sclerosis. Am. J. Neuroradiol. 2008;29:319–325. doi: 10.3174/ajnr.A0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gidwani M, Singh AV. Nanoparticle enabled drug delivery across the blood brain barrier: in vivo and in vitro models, opportunities and challenges. Curr. Pharm. Biotechnol. 2013;14:1201–1212. doi: 10.2174/1389201015666140508122558. [DOI] [PubMed] [Google Scholar]
- 198.Takiguchi M, Achanzar WE, Qu W, Li G, Waalkes MP. Effects of cadmium on DNA-(Cytosine-5) methyltransferase activity and DNA methylation status during cadmium-induced cellular transformation. Exp. Cell. Res. 2003;286:355–365. doi: 10.1016/S0014-4827(03)00062-4. [DOI] [PubMed] [Google Scholar]
- 199.Vikram Singh A, Sitti M. Targeted drug delivery and imaging using mobile milli/microrobots: A promising future towards theranostic pharmaceutical design. Curr. Pharm. Des. 2016;22:1418–1428. doi: 10.2174/1381612822666151210124326. [DOI] [PubMed] [Google Scholar]
- 200.Singh AV, Hosseinidoust Z, Park BW, Yasa O, Sitti M. Microemulsion-based soft bacteria-driven microswimmers for active cargo delivery. ACS Nano. 2017;11:9759–9769. doi: 10.1021/acsnano.7b02082. [DOI] [PubMed] [Google Scholar]
- 201.Ajay, V. S. et al. Nanobiomaterials for vascular biology and wound management: A Review. Veins Lymph., (2017).
- 202.Singh AV, et al. Nanoengineering approaches to design advanced dental materials for clinical applications. J. Bionanosci. 2010;4:53–65. doi: 10.1166/jbns.2010.1026. [DOI] [Google Scholar]
- 203.Singh AV, Sitti M. Bacteria-driven particles: patterned and specific attachment of bacteria on biohybrid bacteria-driven microswimmers. Adv. Healthc. Mater. 2016;5:2306–2306. doi: 10.1002/adhm.201670097. [DOI] [PubMed] [Google Scholar]
- 204.Singh AV, et al. Quantitative characterization of the influence of the nanoscale morphology of nanostructured surfaces on bacterial adhesion and biofilm formation. PLoS ONE. 2011;6:e25029. doi: 10.1371/journal.pone.0025029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Singh SP, Rathee N, Gupta H, Zamboni P, Singh AV. Contactless and hassle free real time heart rate measurement with facial video. J. Card. Crit. Care. 2017;01:024–029. doi: 10.1055/s-0037-1604202. [DOI] [Google Scholar]
- 206.Singh AV, Baylan S, Park BW, Richter G, Sitti M. Hydrophobic pinning with copper nanowhiskers leads to bactericidal properties. PLoS ONE. 2017;12:e0175428. doi: 10.1371/journal.pone.0175428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Vikram, S. A.. et al. Three-dimensional patterning in biomedicine: Importance and applications in neuropharmacology. J. Biomed. Mater. Res. B Appl. Biomater. 10.1002/jbm.b.33922 (2017). [DOI] [PubMed]
- 208.Singh AV. Recent trends in nano-biotechnology reinforcing contemporary pharmaceutical design. Curr. Pharm. Des. 2016;22:1415–1417. doi: 10.2174/1381612822999160122121713. [DOI] [PubMed] [Google Scholar]
- 209.Dwivedi, C. et al. In vivo diabetic wound healing with nanofibrous scaffolds modified with gentamicin and recombinant human epidermal growth factor. J. Biomed. Mater. Res. A. 10.1002/jbm.a.36268 (2017). [DOI] [PubMed]
- 210.Lenaz G. Role of mitochondria in oxidative stress and ageing. Biochim. Biophys. Acta. 1998;1366:53–67. doi: 10.1016/S0005-2728(98)00120-0. [DOI] [PubMed] [Google Scholar]
- 211.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 212.Cuypers A, et al. Cadmium stress: an oxidative challenge. Biometals. 2010;23:927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- 213.Casalino E, Sblano C, Landriscina C. Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Arch. Biochem. Biophys. 1997;346:171–179. doi: 10.1006/abbi.1997.0197. [DOI] [PubMed] [Google Scholar]
- 214.Liu J, Qu W, Kadiiska MB. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009;238:209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fulgenzi A, Zanella SG, Mariani MM, Vietti D, Ferrero ME. A case of multiple sclerosis improvement following removal of heavy metal intoxication. Biometals. 2012;25:569–576. doi: 10.1007/s10534-012-9537-7. [DOI] [PubMed] [Google Scholar]
- 216.Adams R, Crabtree N. Anosmia in alkaline battery workers. Occup. Environ. Med. 1961;18:216–221. doi: 10.1136/oem.18.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pihl R, Parkes M. Hair element content in learning disabled children. Science. 1977;198:204–206. doi: 10.1126/science.905825. [DOI] [PubMed] [Google Scholar]
- 218.Capel ID, Pinnock MH, Dorrell H, Williams DC, Grant E. Comparison of concentrations of some trace, bulk, and toxic metals in the hair of normal and dyslexic children. Clin. Chem. 1981;27:879–881. [PubMed] [Google Scholar]
- 219.MNusio A, Szyrocka-Szwed K, Wojczuk J, Kudybka E. Evaluation of the neurological status and EEG findings in workers chronically exposed to cadmium. Wiadomosci Lek. 1981;34:1615–1620. [PubMed] [Google Scholar]
- 220.Thatcher R, Lester M, McAlaster R, Horst R. Effects of low levels of cadmium and lead on cognitive functioning in children. Arch. Environ. Occup. Health. 1982;37:159–166. doi: 10.1080/00039896.1982.10667557. [DOI] [PubMed] [Google Scholar]
- 221.Struempler RE, Larson GE, Rimland B. Hair mineral analysis and disruptive behavior in clinically normal young men. J. Learn. Disabil. 1985;18:609–612. doi: 10.1177/002221948501801009. [DOI] [PubMed] [Google Scholar]
- 222.Marlowe M, et al. Main and interaction effects of metallic toxins on classroom behavior. J. Abnorm. Child. Psychol. 1985;13:185–198. doi: 10.1007/BF00910641. [DOI] [PubMed] [Google Scholar]
- 223.Hart RP, Rose CS, Hamer RM. Neuropsychological effects of occupational exposure to cadmium. J. Clin. Exp. Neuropsychol. 1989;11:933–943. doi: 10.1080/01688638908400946. [DOI] [PubMed] [Google Scholar]
- 224.Bar-Sela S, Levy M, Westin J, Laster R, Richter E. Medical findings in nickel-cadmium battery workers. Isr. J. Med. Sci. 1992;28:578–583. [PubMed] [Google Scholar]
- 225.Rose CS, Heywood PG, Costanzo RM. Olfactory impairment after chronic occupational cadmium exposure. J. Occup. Environ. Med. 1992;34:600–605. [PubMed] [Google Scholar]
- 226.Okuda B, Iwamoto Y, Tachibana H, Sugita M. Parkinsonism after acute cadmium poisoning. Clin. Neurol. Neurosurg. 1997;99:263–265. doi: 10.1016/S0303-8467(97)00090-5. [DOI] [PubMed] [Google Scholar]
- 227.Viaene M, et al. Cadmium: a possible etiological factor in peripheral polyneuropathy. Neurotoxicology. 1999;20:7–16. [PubMed] [Google Scholar]
- 228.Viaene M, et al. Neurobehavioural effects of occupational exposure to cadmium: a cross sectional epidemiological study. Occup. Environ. Med. 2000;57:19–27. doi: 10.1136/oem.57.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sethi P, Khandelwal D, Sethi N. Cadmium exposure: health hazards of silver cottage industry in developing countries. J. Med. Toxicol. 2006;2:14–15. doi: 10.1007/BF03161007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bao QS, et al. Behavioural development of school-aged children who live around a multi-metal sulphide mine in Guangdong province, China: a cross-sectional study. BMC Public. Health. 2009;9:217. doi: 10.1186/1471-2458-9-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Papp A, et al. Consequences of subacute intratracheal exposure of rats to cadmium oxide nanoparticles: electrophysiological and toxicological effects. Toxicol. Ind. Health. 2012;28:933–941. doi: 10.1177/0748233711430973. [DOI] [PubMed] [Google Scholar]