Abstract
Sigma-1 receptor (σ1R) knockout (KO) CD1 mice, generated by homologous recombination, and separate pharmacological studies in wild type (WT) mice were done to investigate the role of this receptor in the development of pain-related behaviours (thermal hyperalgesia and mechanical allodynia) in mice after spinal cord contusion injury (SCI) – a model of central neuropathic pain. The modulatory effect of σ1R KO on extracellular mediators and signalling pathways in the spinal cord was also investigated. In particular, changes in the expression of inflammatory cytokines (tumour necrosis factor TNF-α, interleukin IL-1β) and both the expression and activation (phosphorylation) of the N-methyl-D-aspartate receptor subunit 2B (NR2B-NMDA) and extracellular signal-regulated kinases (ERK1/2) were analysed. Compared with WT mice, both mechanical and thermal hypersensitivity were attenuated in σ1R KO mice following SCI. Accordingly, treatment of WT mice with the σ1R antagonist MR309 (previously developed as E-52862; S1RA) after SCI exerted antinociceptive effects (i.e. reduced mechanical allodynia and thermal hyperalgesia). Attenuated nociceptive responses in σ1R KO were accompanied by reduced expression of TNF- α and IL-1β as well as decreased activation/phosphorylation of NR2B-NMDA receptors and ERK1/2. These findings suggest that σ1R may modulate central neuropathic pain and point to regulation of sensitization-related phenomena as a possible mechanism.
Introduction
Spinal cord injury (SCI) may trigger central neuropathic pain, which is defined as the pain caused by a lesion or disease of the central somatosensory nervous system1. Prevalence rates for SCI-related neuropathic pain are over 50% and such pain states are in general refractory to treatment and have a poor prognosis2. Central neuropathic pain is a serious health condition, with considerable impact on patients’ quality of life and high individual and societal economic burden3. It is thus imperative to focus research efforts on identifying new therapeutic approaches.
Among possible new pharmacological strategies, we assessed here the modulation of the sigma-1 receptor (σ1R), a neuromodulatory, ligand-regulated membrane protein chaperone that exerts its function through multiprotein complex assembly4–7. σ1R is expressed in important pain control areas in the central nervous system8–10, where it regulates neuronal plasticity and activity-dependent sensitization11–14. Remarkably, findings at the electrophysiological (spinal wind-up recordings), neurochemical (spinal release of neurotransmitters) and molecular (NMDAR function) level support a role for σ1R antagonists in inhibiting the amplified response of spinal cord neurons to sustained afferent input14. Electrophysiologically, σ1R antagonists inhibited the spinal wind-up amplification phenomenon when trains of nociceptive stimuli were applied to isolated spinal cords13,15, and spinal wind-up amplification was reduced in spinal cords from σ1R KO compared to WT mice12. Accordingly, in neurochemical studies (i.e., dorsal horn microdialysis), σ1R antagonist (MR309; S1RA)–mediated antinociception was shown to promote plastic descending inhibitory pathways (enhanced noradrenaline levels) and stopped down plastic excitatory synaptic strengthening (attenuated glutamate release) in the dorsal horn. Sustained glutamate release in the dorsal horn is known to promote plastic changes leading to central (spinal) sensitization phenomena and, among glutamate receptors, the NMDAR is key for such activity-dependent plasticity underlying pain sensitization/hypersensitivity16–19. As shown at the molecular level, the σ1R interacts with and is functionally coupled to NMDAR20–22 and this interaction accounts for its modulatory effects: σ1R antagonists decreased glutamate NMDAR currents, and NMDA current was shown to be reduced in σ1R KO respect to WT mice23. Interestingly, σ1R is reported to play a role in regulating stress-response signalling and ultimately in cell survival4,7.
The present study comprised two independent sets of experiments to investigate the role of this chaperone protein in the development of central neuropathic pain-related behaviours after mild spinal cord contusion injury in mice. In the first set of experiments (Part 1), we used homozygous σ1R KO (σ1R−/−) mice generated by homologous recombination15, and in the second set of studies (Part 2) we utilized pharmacological blockade.
In previous studies, σ1R KO animals have been used to investigate the role of σ1R in animal models where pain was primarily induced at the periphery, such as paw capsaicin and formalin injection24,25, inflammatory pain models26,27, and peripheral neuropathic models, including sciatic constriction injury12 and paclitaxel-induced neuropathy28. In all these models, pain-related behaviours (e.g., formalin-induced paw licking/biting, capsaicin-induced mechanical allodynia or nerve injury-induced mechanical allodynia) were absent or attenuated in σ1R KO mice compared to WT mice. However, the phenotype of σ1R KO mice when exposed to a central nervous system injury, and spinal cord contusion in particular, remains unknown. To this end, in the present study WT and σ1R KO mice were subjected to spinal cord contusion29 and their responses to mechanical and thermal stimulation recorded up to 4 weeks after injury.
As a mechanistic correlate, given the role of σ1R in modulating central sensitization phenomena14,30, we also investigated phosphorylation in the spinal cord of the glutamate N-methyl-D-aspartate (NMDA) receptor and the extracellular signal-regulated kinase (ERK1/2); both of which are reportedly involved in central sensitization19 and hypersensitivity in neuropathic pain conditions31,32. Moreover, considering the role of pro-inflammatory cytokines in the pathophysiology of neuropathic pain after SCI, the expression of TNF-α and IL-1β33–35 was investigated.
Finally, in order to study the role of σ1R in the expression of SCI-induced neuropathic pain (i.e., once pain had developed), data from σ1R KO mice were complemented with pharmacological data obtained from separate studies in which the σ1R antagonist MR309 (S1RA) was administered to SCI WT mice. MR309 is a selective σ1R antagonist that has demonstrated efficacy in multiple translationally-driven peripheral neuropathic pain models36, but no information is available as it regards to its potential antinociceptive effect in central neuropathic pain models (i.e., spinal cord contusion). Thus, the main objectives of the present work were to study the involvement of σ1R in mild SCI-induced central neuropathic pain through genetic (σ1R KO mice) and pharmacological (MR309 administration, dose-response study in WT mice) approaches.
Results
General observations and mice genotyping
Following a protocol animal welfare supervision based on Morton D.B and Griffiths P.H. guidelines (1985)37, changes in coat and skin, vibrissae of nose, nasal secretions, signs of autotomy of hindpaw and/or forepaw, or aggressiveness were not detected neither in WT nor σ1R KO mice after SCI at any time of the experimental period. Animals showed no significant weight loss throughout the experiment.
Locomotor disturbance in σ1R KO and WT mice after mild SCI
In preclinical studies, SCI may be classified as mild, moderate or severe according to the motor dysfunction displayed by the injured animals38,39, which it is usually evaluated by means of Basso Mouse Scale for locomotion (BMS)40, among other available locomotor functional tests. Thus, SCI may be classified as mild (BMS-scores >6), moderate (BMS-scores 4–5) or severe (BMS-scores <4) according to the motor dysfunction following injury. In previous studies, 2g-weight contusion was shown to result in mild locomotor disturbances without paralysis in WT animals41. Thus, we first evaluated whether the same contusion procedure produces the same effect in σ1R KO mice. To this end, a BMS test40 was used to compare locomotor function up to 4 weeks after SCI. Multivariate analysis of variance (MANOVA) revealed significant effects on day (F(3,48) = 180.22, p < 0.001), surgery (F(2,50) = 170.73, p < 0.001) and genotype (F(1,50) = 5.49, p = 0.05) factors and significant interactions for day × surgery (F(6,96) = 32.803, p < 0.001) and day × genotype (F(3,48) = 2.82, p < 0.05). Moreover, significant group differences were detected by analysis of variance (ANOVA) analysis in BMS scores at 7, 14 and 28 (all p values < 0.001) days post-injury (dpi) (Fig. 1). While at 7 dpi both sham and contusioned experimental groups showed a deficit in coordination compared to naïve groups from both genotypes, at 28 dpi only contusioned WT and σ1R KO mice showed significant lower BMS scores with respect to all other (naïve and sham) groups (Fig. 1). No remarkable differences were found when compared WT and σ1R KO mice except for slightly higher locomotor impairment transiently seen in sham KO versus sham WT mice at 7 and 14 dpi, but not at 28 dpi, and in SCI KO versus SCI WT at 28 dpi. By the last day of evaluation (28 dpi), impairment remained significant in WT and σ1R KO mice subjected to SCI (but not in sham groups). According to BMS scale, the rating scores (mean ± SEM) of these groups (WT = 7.4 ± 0.40; σ1R KO = 6.25 ± 0.44) denoted an impairment slightly higher in KO mice subjected to SCI, but only altered paw position and no altered horizontal locomotion, indicating no major impairment in coordination and locomotor function, was scored in both genotypes. In summary, values above 6 in the BMS test were obtained in mice of both genotypes subjected to SCI, indicating only mild locomotor dysfunction without major impairments. Overall, neither sham surgeries nor spinal cord contusion resulted in either paralysis or major locomotor dysfunction at 28 dpi, at any experimental group.
Figure 1.
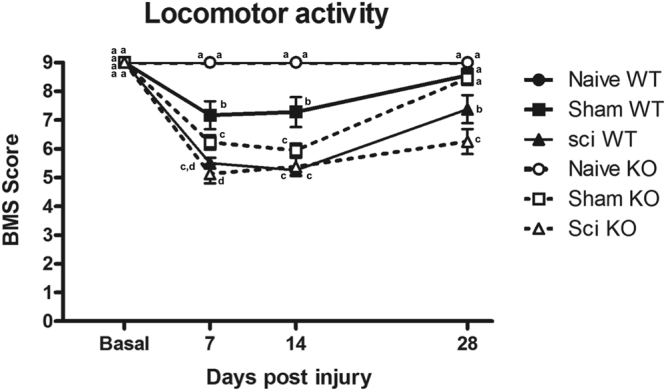
Locomotor recovery assessment using Basso Mouse Scale (BMS) after spinal cord injury (SCI) in wild type (WT) and sigma-1 receptor (σ1R) knockout (KO) mice. Each point and vertical line represents the mean ± standard error of the mean (n = 9–12 per group). a–c: groups not sharing a letter are significantly different, p < 0.05. Results reveal mild BMS alteration associated with SCI in both WT and σ1R KO mice, referring to altered paw position but not to altered horizontal locomotion.
Attenuation of mechanical allodynia and thermal hyperalgesia in σ1R KO mice after SCI
σ1R is known to be involved in the modulation of neuropathic pain after painful peripheral nerve injury5,10,42. In this study we evaluated and compared the response to mechanical and thermal stimulation of σ1R KO and WT in the SCI model up to 4 weeks after SCI.
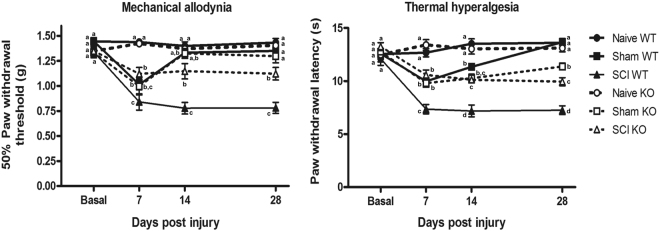
Mechanical allodynia was assessed via measurement of hind paw withdrawal threshold in response to von Frey filament stimulation43. The MANOVA analysis indicated significant effects on day (F(3,48) = 22.814, p < 0.001), surgery (F(2,50) = 78.85, p < 0.001) and genotype (F(1,50) = 5.49, p = 0.023) factors and significant interactions for day × surgery (F(6,96) = 13.927, p < 0.001) and day × genotype (F(3,48) = 4.536, p < 0.001). On further ANOVA analysis, significant group differences were found on post-injury days 7, 14 and 28 (all p values < 0.001) (Fig. 2A). Naïve animals from both genotypes did not show mechanical allodynia throughout the experimental period, and no differences in mechanical sensitivity were found when compared naïve mice from both genotypes. Similarly, no differences were found when comparing sham mice of both genotypes. Sham-operated mice showed a significant decrease (p value < 0.05, Duncan test) in mechanical paw withdrawal thresholds at 7 dpi when compared with naïve mice, but mechanical allodynia was markedly attenuated at 14 dpi and was absent at 28 dpi in sham mice from both genotypes. Mechanical allodynia developed following SCI in both σ1R KO and WT, but the time course and severity were different when both genotypes were compared. By 7 dpi mechanical allodynia clearly developed in SCI mice (similar to sham mice), but it was attenuated in SCI σ1R KO when compared with SCI WT mice. At 14 dpi mechanical allodynia was apparent in SCI (but not in sham-operated) mice and reduced in SCI σ1R KO compared with SCI WT mice. Finally, at 28 dpi, mechanical allodynia was markedly reduced in SCI σ1R KO compared with SCI WT. Indeed, σ1R KO mice subjected to a SCI showed an average 54% reduction in mechanical allodynia at 7, 14, and 28 dpi when compared to WT SCI mice.
Figure 2.
Time course of spinal cord injury (SCI)-induced mechanical allodynia and thermal hyperalgesia in wild type (WT) and sigma-1 receptor (σ1R) knockout (KO) mice. Each point and vertical line represents the mean ± standard error of the mean (n = 9–12 per group). a–c: groups not sharing a letter are significantly different, p < 0.05. (A) Mechanical allodynia and (B) thermal hyperalgesia was clearly evidenced on all measurement days in SCI WT mice. The hypersensitivity was attenuated in homozygous σ1R KO mice on days 7, 14 and 28 after SCI.
Thermal hyperalgesia was assessed by measuring hind paw withdrawal latency in response to thermal stimulation (radiant heat) in the plantar test44. The MANOVA analysis indicated significant effects on day (F(3,48) = 28.853, p < 0.001), surgery (F(2,50) = 123.64, p < 0.001) and genotype (F(1,50) = 15.1, p = 0.04) factors and significant interactions for day × surgery (F(6,96) = 21.07, p < 0.001) and day × genotype (F(3,48) = 2.703, p < 0.05). On further ANOVA analysis, significant group differences were found on post-injury days 7, 14 and 28 (all p values < 0.001) (Fig. 2B). Similar to mechanical allodynia, thermal hyperalgesia did not develop throughout the experimental period in naïve animals, and no differences in thermal sensitivity were found when compared naïve mice from both genotypes. A significant decrease in paw withdrawal latency (i.e. thermal hyperalgesia) was found in sham mice at 7 and 14 dpi (p values < 0.05, Duncan test) when compared with naïve mice. At the end of the experimental period (day 28), thermal hyperalgesia was absent in WT subjected to sham surgery, but a slight hyperalgesia still remained in sham σ1R KO mice. SCI induced a marked and long- lasting thermal hyperalgesia in WT mice, already remarkable at day 7 (significantly higher than in sham groups; p values < 0.05, Duncan test) and maintained throughout the experimental period. Thermal hyperalgesia was markedly attenuated in SCI σ1R KO respect to SCI WT mice at all time points. Indeed, σ1R KO mice subjected to SCI showed an average 51% reduction in thermal hyperalgesia at 7, 14, and 28 dpi when compared with WT SCI mice.
Altogether, although baseline perception of sensory mechanical and thermal stimuli was similar in σ1R KO and WT mice, as evidenced by indistinguishable mechanical thresholds and thermal latencies for paw withdrawal in naïve mice of both genotypes, mechanical and thermal hypersensitivity induced by a spinal cord contusion were significantly lower (p values < 0.05, Duncan test) in σ1R KO animals compared with WT mice.
Differences on central sensitization-related molecular biomarkers between σ1R KO and WT mice after SCI
In order to gain mechanistic insights, the spinal expression and phosphorylation of extracellular signal-regulated kinases (ERK1/2) and NMDA receptor NR2B subunit, which have been reported to be involved in central sensitization in neuropathic pain states31,32,45,46, were investigated 28 days after injury.
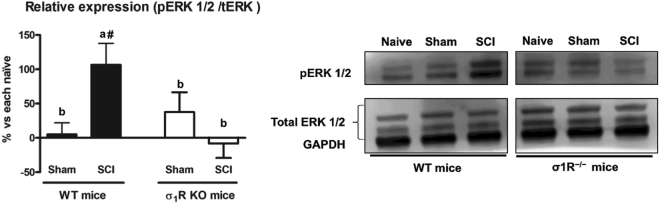
Significant group differences were detected by ANOVA analysis in ERK1/2 phosphorylation (pERK1/2). As expected, a significant increase of pERK1/2 (p < 0.05) was found in spinal cords of contusioned WT mice compared to WT naïve and WT sham. However, no significant differences (p > 0.05) were found between σ1R KO experimental groups, indicating that activation/phosphorylation of ERK did not significantly occur in lesioned σ1R KO mice (Fig. 3A). Accordingly, the pERK1/2 increase relative to corresponding naïves was significantly higher (p values < 0.05, Duncan test) in contusioned WT mice compared to both sham and contusioned σ1R KO mice. No significant changes in total ERK protein in both genotypes were found when the SCI groups were compared with their respective naïve or sham-operated groups.
Figure 3.
Spinal ERK1/2 phosphorylation (pERK) expression at day 28 after spinal cord injury (SCI) in wild type (WT) and sigma-1 receptor (σ1R) knockout (KO) mice. Quantification and representative immunoblots of total ERK, pERK and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Protein expressions were normalized to GAPDH and data is presented as a percentage respect to WT naïve or KO naïve mice (mean ± standard error of the mean; n = 5–6). a–b: groups not sharing a letter are significantly different, p < 0.05; #significant differences vs. naïve (p < 0.05). σ1R KO mice subjected to a spinal cord contusion did not show significant upregulation of pERK in contrast to WT SCI mice. Full-length blots are presented in Supplementary Figure S1.
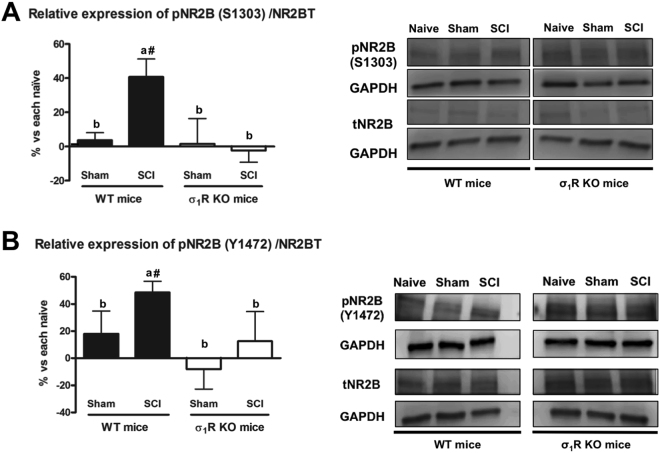
Similarly, ANOVA analysis revealed significant group differences in Ser1303 and Tyr1472 phosphorylation of NMDA receptor NR2B subunit (p < 0.05). WT mice subjected to SCI (but not sham-operated animals) showed a significant increase in phosphorylation at Ser1303 in the NR2B subunit compared with the WT naïve group. In contrast to WT mice, levels of Ser1303 phosphorylation in NR2B were not significantly modified in σ1R KO mice subjected to either SCI or sham operation (Fig. 4A). Likewise, Tyr1472 phosphorylation in the NR2B subunit was significantly increased in SCI WT animals (p < 0.05) when compared with WT naïve animals, whereas no changes were found following SCI or sham operation in σ1R KO mice (Fig. 4B). No significant changes in the total levels of NMDAR-NR2B were observed in any of the experimental groups.
Figure 4.
Spinal Tyr1472 and Ser1303 phosphorylation of N-methyl-D-aspartate (NMDA) receptor NR2B subunit at day 28 after spinal cord injury (SCI) in wild type (WT) and sigma-1 receptor (σ1R) knockout (KO) mice. (A) Quantification and representative immunoblots of total NR2B, pS1303NR2B and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (B) Quantification and representative immunoblots of total NR2B, pY1472NR2B and GAPDH. a–b: groups not sharing a letter are significantly different, p < 0.05; #significant differences vs. naïve (p < 0.05). Results were presented as the mean ± standard error of the mean (n = 5–6). Protein expressions were normalized to GAPDH. Data was presented as a percentage respect to WT naïve or KO naïve mice. After SCI, increased levels of Tyr1472 and Ser1303 phosphorylation of NMDA receptor NR2B subunit were evidenced in WT mice, whereas no changes were detected in KO mice. Full-length blots are presented in Supplementary Figure S2.
Overall, data point to prevention of pERK1/2 and pNR2B upregulation in the spinal cord as a molecular, mechanistic readout for the attenuation of SCI-induced pain-related behaviours in σ1R KO mice.
Pro-inflammatory cytokines TNF-α and IL-1β are not upregulated in σ1R KO mice after SCI
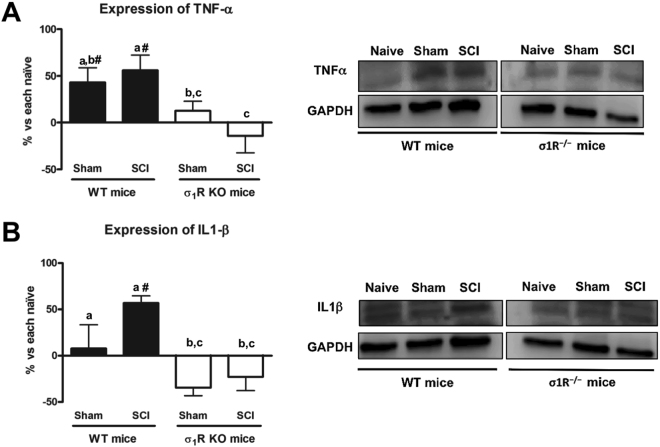
Since the pro-inflammatory cytokines TNF-α and IL-1β have been associated with central neuropathic pain after spinal cord injury33–35, we also assessed whether their expression in spinal cord is attenuated in σ1R KO mice 28 days after injury. The ANOVA analysis revealed significant differences between groups in both TNF-α and IL-1β expression (p < 0.05). While contusioned WT mice (and WT sham-operated mice in the case of TNF-α) showed a significant upregulation of TNF-α (p < 0.05) (Fig. 5A) and IL-1β (Fig. 5B) in comparison to WT naïve animals, no differences (p > 0.05) were found between σ1R KO mice experimental groups, indicating that the expression of these pro-inflammatory cytokines in the spinal cord was not upregulated at 28 dpi in σ1R KO mice, subjected to either SCI or to sham operation.
Figure 5.
Spinal inflammatory cytokines (tumour necrosis factor [TNF]-α and interleukin[IL]-1β) expression at day 28 after spinal cord injury (SCI) in wild type (WT) and sigma-1 receptor knockout (KO). (A) Quantification and representative immunoblots of TNF-α and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). a–b: groups not sharing a letter are significantly different, p < 0.05. (B) Quantification and representative immunoblots of IL-1β and GAPDH. a-c: groups not sharing a letter are significantly different, p < 0.05; #significant differences vs. naïve (p < 0.05). Results were presented as the mean ± standard error of the mean (n = 5–6). Protein expressions were normalized to GAPDH. Data was presented as a percentage respect to WT naïve or KO naïve mice. After SCI, an increase in TNF-α and IL-1β protein levels in spinal cord was evidenced, whereas the deficiency of σ1R prevented its upregulation. Full-length blots are presented in Supplementary Figure S3.
Acute σ1R receptor antagonist (MR309) administration reverses mechanical allodynia and thermal hyperalgesia in WT mice at day 28 after SCI
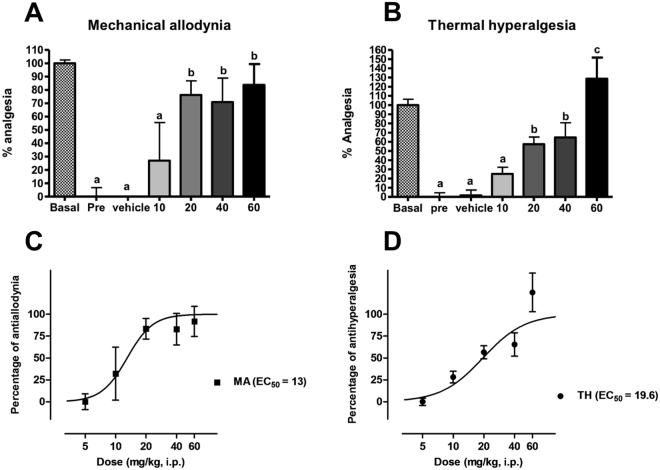
Data coming from previous experiments using genetically modified mice suggest that σ1R plays a role in central neuropathic pain development after spinal cord contusion and that the absence of this protein in KO mice results in attenuated neuropathic pain. In order to discard interfering adaptive changes in gene expression in the KO mice we investigated the effect of treatment with the selective σ1R antagonist MR309 (S1RA)13 on the nociceptive behaviour of spinal cord-injured WT mice. That is, does the absence of the protein in KO mice mimic the modulatory effect of pharmacologic antagonism when the protein is present in WT mice? To this end, we administered MR309, at doses that do not interfere motor coordination13, in an acute dose–response study (10, 20, 40 and 60 mg/kg) to WT mice at 28 dpi, and mechanical allodynia and thermal hyperalgesia were recorded 30 min after i.p. administration.
The administration of the σ1R antagonist elicited significant effects on mechanical allodynia for time-point (basal [i.e. pre-injury], pre-treatment and 30 min after treatment) (F(2,25) = 135.055, p < 0.001) and dose (F(4,26) = 2.642, p < 0.05) factors, and significant interaction for time-point × dose (F(2,25) = 4.971, p < 0.001). Likewise, the MANOVA analysis of thermal hyperalgesia indicated significant effects for time-point (F(2,25) = 98.154, p < 0.001) and dose (F(4,26) = 4.394, p < 0.005) factors and significant interaction for time-point × dose (F(2,25) = 5.566, p < 0.001). Thus, the administration of MR309 reversed both mechanical allodynia (Fig. 6A) and thermal hyperalgesia (Fig. 6B) in SCI WT mice with similar ED50s (mechanical allodynia ED50 = 13 ± 3.14 mg/kg, Fig. 6C; and thermal hyperalgesia ED50 = 19.6 ± 2.54 mg/kg, Fig. 6D). ANOVA analysis of mechanical allodynia revealed that doses from 20 to 60 mg/kg (but not 10 mg/kg) exerted a similar and significant antiallodynic effect (p < 0.05) when compared to vehicle treatment. In the case of thermal hyperalgesia, the antinociceptive effect was significant from 20 to 60 mg/kg (p values < 0.05; but not at 10 mg/kg, p values > 0.05, Duncan test) and maximal at 60 mg/kg. Altogether, pharmacological results reinforce the findings in σ1R KO mice, without any apparent side effect observed.
Figure 6.
Dose–response effect of MR309 treatment on spinal cord injury (SCI)-induced mechanical allodynia and thermal hyperalgesia in wild type (WT) mice. Analgesic effects on (A,C) mechanical allodynia and (B,D) thermal hyperalgesia of the sigma-1 receptor antagonist MR309 (S1RA) 30 min after administration at 28 days after SCI. Each bar and vertical line represents the mean ± standard error of the mean (n = 4–7 per group). a–c: groups not sharing a letter are significantly different, p < 0.05. MR309 treatment dose-dependently reversed both mechanical and thermal hypersensitivity.
Discussion
In this study we used σ1R KO mice to determine the role of σ1R in central neuropathic pain-related behaviours triggered by spinal cord contusion. Our findings indicate that mechanical allodynia and thermal hyperalgesia found after SCI in WT mice are significantly attenuated in mice lacking σ1R, up to four weeks after injury. Moreover, our results indicate that attenuation of neuropathic pain-related behaviours in σ1R KO animals occurs concomitantly with a substantial reduction in the spinal cord of both ERK1/2 and NMDA-NR2B phosphorylation as well as with a reduced spinal expression of the pro-inflammatory cytokines TNF-α and IL-1β.
Normal sensory mechanical and thermal thresholds were not modified in naïve σ1R KO compared with WT mice, suggesting that σ1R KO perceive mechanical and thermal stimuli normally in the absence of lesion. However, when the nervous system becomes injured following SCI, nociceptive behaviours in response to mechanical and thermal stimuli were attenuated in σ1R KO versus WT mice. Similar results were obtained in previous studies where pain was induced at the periphery, with normal perception at baseline but increased nociceptive thresholds/latencies in σ1R KO mice following nerve injury12,28 or paw injection of chemical irritants24–27. In this study, mechanical allodynia was reduced an average of 54% and thermal hyperalgesia was reduced an average of 51% in σ1R KO versus WT mice. This agrees with findings supporting that σ1R plays a major role in the mechanisms underlying sensitization/hypersensitivity of pain pathways14. Accordingly, the spinal wind-up/amplification response to sustained stimulation of C- fibres is attenuated in isolated spinal cords from σ1R KO respect to WT mice12. It is also worth to mention that attenuated pain-related behaviours in σ1R KO were accompanied by slight but significantly lower BMS scores compared to WT mice. For the purpose of this study, not aimed at studying motor dysfunction after SCI, since differences are mild and BMS scores exceeded 6 points at day 28 in both genotypes, it may be stated that no major locomotor dysfunction occurs40 interfering the assessment and comparison of nociceptive readouts.
Observations in σ1R-deficient mice are supported by a pharmacological approach. The antiallodynic and antihyperalgesic effects exerted by the σ1R antagonist MR3069 on WT SCI mice here is in agreement with the antinociceptive effects of MR309 reported in a multitude of other models involving sensitization of pain pathways, such as formalin and capsaicin sensitization13, peripheral somatic or cephalic nerve injury14,42, chemotherapy-induced neuropathy28,42, diabetic-induced neuropathy42 or inflammation26,27. Behavioural findings (in animal pain models), as well as electrophysiological evaluations (spinal wind-up recordings) and neurochemical studies (spinal release of neurotransmitters) levels, provide evidence to suggest that σ1R antagonists inhibits hyperexcitability in sensitizing conditions: MR309 reduced the wind-up/amplification response to sustained stimulation of C-fibres in isolated WT spinal cords13,15 and inhibited formalin-evoked glutamate but enhanced noradrenaline release in the dorsal horn of the spinal cord47. This is in agreement with a modulatory effect on activity-dependent plastic changes, as a result of stimulating inhibitory pathways and dampening plastic excitatory changes in the dorsal horn. It thus seems that σ1R plays a major role in the mechanisms underlying activity-dependent plasticity/sensitization and ultimately pain hypersensitivity, perhaps irrespective of the primary lesion site and aetiology, and that the absence/blockade of σ1R inhibits such sensitization-related phenomena14,48.
We thus focused on some key central sensitization-related changes to reveal potential molecular pathways (either causative or consequential). We analysed the expression and activation (phosphorylation) of ERK1/2 which are known, together with other protein kinases, to be involved in central/spinal sensitization. ERK, a mitogen-activated protein kinase (MAPK), mediates intracellular signal transduction as a result of activation by a range of different stimuli. Phosphorylation of ERK in the dorsal horn in nociceptive neurons49–52 and/or reactive astrocytes51–55 has been described to play a major role (based on studies with ERK inhibitors) in hypersensitivity after peripheral nerve injuries. Spinal pERK levels can be increased due to traumatic injuries, particularly spinal injuries such as contusion, excitotoxic injury or chronic complete transection56,57. Our results in WT mice subjected to SCI agree with previous literature. Interestingly, in σ1R KO mice, spinal cord pERK1/2 remains unchanged following SCI, which would agree with the reduced mechanical and thermal hypersensitivity in these mice lacking σ1R. Previous studies also reported no changes in spinal pERK1/2 expression in peripheral nerve-induced or inflammatory pain models in σ1R KO mice: phosphorylation of ERK was increased in the ipsilateral spinal hemicord of WT mice but not in σ1R KO mice after partial sciatic nerve ligation12; and paclitaxel treatment induced peripheral neuropathy associated with pERK increase in WT but not in σ1R KO mice28. These findings suggest that σ1R facilitates ERK activation in the spinal cord in peripheral neuropathic pain animal models. In the present study, we provide evidence of a similar pattern in a spinal cord contusion model of central neuropathic pain, with complexities far beyond pain including outstanding neurodegenerative processes.
Activation of spinal dorsal horn NMDA receptors is essential for the initiation of central sensitization. NMDA receptors are formed by several subunits, with the NMDA-NR2B subunits reported to contribute to the development of central sensitization and neuropathic pain58. Specifically, it has been reported that NR2B phosphorylation of dorsal horn neurons is enhanced in neuropathic59 and post-inflammatory60 pain models, and the use of NR2B antagonists has been shown to reduce inflammatory61,62, peripheral61,63 and spinal cord injury-induced neuropathic pain64. Furthermore, σ1R activation has been associated with NMDA-NR2 phosphorylation in the hippocampus65 and this phenomenon was reduced in σ1R KO mice23. Glutamate is a key neurotransmitter and the NMDA receptor is a key channel for synaptic plasticity, mediates nociceptive transmission from afferent nociceptive fibres to dorsal horn spinal cord neurons, and promotes calcium entry and subsequent intracellular calcium-dependent signalling in dorsal horn neurons. Phosphorylation of NMDA receptor subunits mainly results in increased postsynaptic glutamate signalling (increased calcium permeability) and thus hyperexcitability/sensitization of spinal cord neurons. In particular, phosphorylation of NR2B at S1303, probably by protein kinase C (PKC), has been shown to potentiate NMDA receptor currents whereas phosphorylation at Y1472, probably by the protein tyrosine kinase Fyn, stabilizes active signalling NMDA receptors on the plasma membrane66. As expected, phosphorylation of NMDA-NR2B, presumably at PKC and Fyn sites, was increased in WT mice subjected to SCI compared to both naïve and sham WT animals. In contrast, phosphorylation of NMDA-NR2B at these sites (S1303 and Y1472) did not occur in σ1R KO mice following SCI. These findings suggest that σ1R facilitates NMDA receptor sensitization in the spinal cord in this SCI model of neuropathic pain. Accordingly, inhibition of NMDA receptor phosphorylation in mice lacking σ1R could contribute, at least in part, to the attenuated hypersensitivity of σ1R KO mice to mechanical and thermal stimuli after SCI. A role for σ1R in NMDA receptor sensitization in the dorsal horn is also supported by the enhancement by the σ1R agonists PRE-084 or carbetapentane and inhibition by the σ1R antagonist BD-1047 of the phosphorylation of NMDA-NR1 subunits at PKC and PKA sites in peripheral injury models11,67. At the molecular level, it has been reported that σ1R binds to NMDA-NR1 subunit and modulates NMDA receptors, and that σ1R antagonists (i.e., MR309) inhibit NMDA receptor function by dissociating σ1R from the NMDA receptor, thus allowing the interaction of the NMDA-NR1 receptor with calcium-calmodulin, a negative feedback regulator of NMDA function22.
Pro-inflammatory cytokines play a major role in neuron–glia cross-talk in neuroinflammatory processes but they also play a role in pain and central sensitization phenomena in the context of neuropathic pain33–35,45,68–77. In particular, spinal TNF-α and IL-1β are involved in mechanisms underlying the development and maintenance of neuropathic pain by promoting synaptic plasticity and central sensitization. In fact, intrathecal injection of TNF-α in naïve mice induces hind paw mechanical hypersensitivity, as observed in sciatic nerve-injured mice, and increased glutamatergic neurotransmission (a hallmark of spinal synaptic plasticity and sensitization), by decreasing astrocytic glutamate clearance from the synaptic cleft78. TNF-α also evokes a dramatic increase in the frequency of spontaneous excitatory postsynaptic currents (sEPSC) and NMDA receptor-mediated currents in lamina II neurons79. Accordingly, hyperalgesia elicited by intrathecal TNF-α is prevented by intrathecal injection of the NMDA receptor antagonist MK-801, thus providing further evidence of the role that TNF-α plays in regulating NMDA receptor-mediated synaptic plasticity and sensitization in the spinal cord70,79. A similar effect, leading to increased excitability of dorsal horn spinal neurons and facilitation of nociceptive transmission, is reported for IL-1β, including increased sEPSCs and decreased spontaneous inhibitory postsynaptic currents (sIPSC)80. In this study, the expression of both TNF-α and IL-1β was increased in WT but not in σ1R KO mice subjected to SCI 28 dpi, thus suggesting that inhibition of pro-inflammatory cytokine expression in mice lacking σ1R may contribute, at least in part, to the attenuated hypersensitivity to mechanical and thermal stimuli after SCI. Altogether, mechanistic correlates, including expression of extracellular mediators (i.e., TNF-α and IL-1β), signalling through excitatory membrane receptors/channels (i.e., NMDA receptors) and intracellular signalling cascades (i.e., ERK/pERK), converge to central sensitization-related phenomena as underlying substrates for the σ1R-mediated modulatory effect, which is consistent with previous literature supporting a central inhibitory effect of σ1R antagonism on other pain conditions involving spinal plasticity/sensitization and ultimately pain hypersensitivity12–14. Neuropathic pain is known to be induced when the mechanisms mentioned above are engaged in the spinal cord, and inhibition of the mechanisms mentioned above has been found to reduce neuropathic pain. However, it is unclear if these changes are causal in nature or just a consequence of changes upstream. Protection against excitotoxic damage via inhibition of glutamatergic NMDA signalling, as expected by σ1R antagonism based on the reported σ1R-NMDAR interaction21, could result in reduced neuronal stress and degeneration, and thus in reduced activation of microglia and astrocytes and reduced release of neuroinflammatory mediators. However, we don’t know if neuroprotection actually occurs and if it is upstream and plays thus a causative role. In fact, neuroprotection could also be a consequence of reduced glial activation and secretion of neuroinflammatory mediators.
σ1R expression was found to be increased in dorsal horn astrocytes following thoracic spinal cord hemisection, and administration of the σ1R antagonist BD-1047 on postoperative days 0–7 was found to inhibit expression of the astrocytic gap junction protein connexin 43, astrocyte activation in the lumbar dorsal horn, and ultimately mechanical allodynia81. These findings regarding allodynia modulation, although in a different model (hemisection versus traumatic contusion injury), further support a role for σ1R in central neuropathic pain following spinal cord injury. In the present work we used the contusion model as most of human injuries involve tissue damage due to impact against the spinal cord. Indeed, more than 500,000 people suffer acute traumatic SCI worldwide every year, and global prevalence is expected to increase82. We focused on three central sensitization-related mechanistic correlates, including extracellular mediators (i.e., TNF-α and IL-1β), membrane receptors/channels (i.e., NMDA receptors) and intracellular signalling cascades (i.e., ERK/pERK), and we first describe that increased spinal activity/expression of ERK, NMDA-NR2 and TNF-α and IL-1β after SCI did not occur in mice lacking σ1Rs, concomitant with attenuation of nociceptive behaviours. Functional activity/expression of all three central sensitization markers may require and/or engage astrocytes and perhaps microglia as well, and ultimately affect neuronal activity. It is clear that their functional activity/expression connects with synaptic plasticity, central sensitization and neuropathic pain. It is unclear, however, how σ1R modulates complex changes accounting for central sensitization in neuropathic pain and other pain sensitizing conditions, not only after peripheral injuries but also, specially, when the primary lesion occurs in the CNS.
Methods
Animals
Wild-type (WT) adult female CD1 mice that weighted a median of 22gr (19–26 gr) were obtained from Charles River Laboratories (France). In parallel, homozygous σ1R knockout (σ1R−/−; σ1R KO) mice with a median weight of 23gr (20–26 gr) were generated and characterized as described previously83, backcrossed onto the CD-1 background with selection for the mutant σ1R gene at each generation. After 10 generations of successive backcrosses with pure CD-1, mice harbouring the mutation were then bred to homozygosity and used in this study. Both WT and KO mice were 5-week–old (4–5 weeks old).
Mice were housed in a colony room at 21 ± 1 °C and 40–60% humidity, with a 12:12 hours light/dark cycle and access to food and water ad libitum, in groups of five in 331 × 159 × 132 cm plexiglass cages with a wood-shaving bedding. Cages were changed twice weekly. Behavioural testing was carried out in a soundproof experimental room. All mice were allowed to acclimatise to the facility rooms before commencing any behavioural or surgical procedures, which were all conducted during the light cycle. Sentinel mice were routinely tested for pathogens and facilities remained pathogen free during the whole experimental period.
All experimental procedures and animal husbandry were conducted following the ARRIVE guidelines and according to the ethical principles of the I.A.S.P. for the evaluation of pain in conscious animals84 and the European Parliament and the Council Directive of 22 September 2010 (2010/63/EU), and were approved by the Animal Ethics Committee of the Parc Científic of Barcelona.
Experimental design and dosing
Two independent sets of experiments were conducted. Experiments in Part 1 were designed to study whether the lack σ1R may result in attenuation of pain-related behaviours in mice after central nervous system injury. To this end, CD-1 WT and σ1R KO female mice were subjected to SCI and their functional responses were assessed at 7, 14 and 28 dpi, including locomotor and nociceptive behaviours (mechanical allodynia and thermal hyperalgesia), comparing them with naïve and sham animals. Locomotor recovery was assessed first, using BMS scale in an open field maze, afterwards mechanical allodynia was measured by the Von Frey filament tests and finally, the thermal hyperalgesia was assessed by the plantar test. The behavioural tests were performed in this specific order and with an interval of 20 min between each test. Moreover, biochemical assays were performed to relate mechanical allodynia and thermal hyperalgesia findings to neuropathic pain biomarkers. All of these procedures are described below.
A second set of experiments (Part 2) was designed once results suggested significant attenuation of pain-related behaviours in σ1R KO mice after SCI. The objective of these experiments was to elucidate whether the σ1R antagonist MR309 (S1RA) decreased mechanical allodynia and thermal hyperalgesia in SCI WT mice. Dose–response studies (0, 20, 40, and 60 mg/kg i.p.; vehicle: hydroxypropyl methylcellulose) were performed at 28 days post-injury evaluating mechanical allodynia and thermal hyperalgesia 30 min after dosing, obtaining afterwards the percentage of analgesia.
A set of 26 WT and 30 σ1R KO mice was used to compare behavioural endpoints and molecular markers in both genotypes after spinal cord contusion, and another set of 31 WT mice was used to assess the effect of σ1R antagonist (MR309) administration on behavioural responses. Animal sample size was calculated using GRANMO (Version 7.12 April 2012).
Surgical procedure
In order to obtain a mouse model of central neuropathic pain without locomotor paralysis, spinal cord contusion was conducted according to procedures explained elsewhere35,41. Briefly, animals were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and placed prone on a heating pad to maintain constant levels of body temperature. After back disinfection with povidone iodide, T8–T9 of the thoracic spinal cord was exposed via dorsal laminectomy, a metallic stage positioned over the exposed spinal cord and a 2 g weight then dropped onto the stage from a 25 mm height. Following this procedure, the wound was closed and animals allowed to recover in warmed cages with accessible food and water. After the surgical procedure, animals also received 0.5 mL saline solution (i.p.) to restore an eventual volemic deficit. In sham animals, the spinal cord was exposed as described above but not contusioned, and they underwent the same recovery procedures. Naïve mice did not receive any surgical manipulation. Mice were randomly allocated to experimental groups prior to surgical procedures.
Locomotor activity
Locomotor activity was evaluated by means of the BMS test40 and performed as described elsewhere35. Briefly, animals were placed individually into an open field (72 cm × 72 cm) and allowed to move freely for 5 min. During this time the hind limb movements of the animal were scored for locomotor function according to the BMS40, which ranges from 0 (no hind limb movement) to 9 (normal movement and coordinated gait).
Mechanical allodynia and thermal hyperalgesia tests
Mechanical allodynia was assessed via hind paw withdrawal from von Frey filament stimulation43. Mice were placed in test chambers with a metal mesh floor through which von Frey monofilaments (bending force range 0.04–2 g) were applied to the plantar surface. Paw withdrawal thresholds were measured using the up-down method paradigm. In this paradigm, the 0.4 g was applied first. The response to this filament determined which filament was applied next; a weaker filament was applied if the animal had responded to the previous filament, a stronger filament was applied if the animal did not respond to the previous filament. Clear paw withdrawal, shaking or licking were considered to be a response. This up-down procedure was limited to four assessments after the first response. Each filament was applied for 2 s, with inter-stimulus intervals of 5–10 s. Both hind paws were tested. The 50% paw withdrawal threshold was calculated using the Dixon formula: 50% paw withdrawal threshold (g) = [(10(Xf + κδ)/10 000)], where Xf is the value (in logarithmic units) of the final von Frey filament used, κ is a fixed tabular value for the pattern of positive/negative responses and δ is the mean difference (in log units) between stimuli. Both paws were evaluated since SCI model results in a bilateral injury and it is not possible to use contralateral paw as a natural intraindividual control.
Thermal hyperalgesia was assessed by determination of hind paw withdrawal latency in response to a thermal stimulus (radiant heat) administered via a plantar test analgesia meter (IITC, Life Science), according to the Hargreaves method44. Mice were placed into test enclosures, with the temperature-controlled (29 °C) glass surface of the plantar test device positioned directly underneath. Animals were then allowed to acclimatize for 45 min. The radiant heat source was then positioned under the plantar surface of the animal’s hind paw and activated. A light beam intensity that elicited baseline paw withdrawal latencies of 14–15 s was used. A maximum limit of 20 s was imposed in order to prevent tissue damage in the absence of a withdrawal response. The SCI model results in a bilateral injury, which does not allow the use of contralateral paw as a natural intraindividual control, so both paws were evaluated. The sum of the mean withdrawal latencies for both hindpaws were determined from the average of three separate trials, conducted at 5-min intervals.
Biochemical assays
Genotyping of σ1R KO mice
Genotyping was conducted using genomic DNA obtained from tail tips of WT and σ1R KO mice. DNeasy Blood & Tissue kits (QIAGEN, Madrid, Spain) were used to analyse samples, according to the manufacturer’s instructions. PCR amplifications were conducted using Eppendorf Hot-MasterMix (Eppendorf, Hamburg, Germany) and 0.5 µM of each primer (Invitrogen Ltd, Paisley, UK). PCR was conducted using a thermal controller (iCycler, Bio-Rad Laboratories, Hercules, CA) with initial template denaturation at 94 °C, followed by 35 cycles of 30 s at 94 °C, 45 s at 55 °C and 2 min at 70 °C. As a final extension step, a10-min cycle was run at 72 °C. The oligonucleotide primer (5′–3′) sequences specific for the genes examined were: 5′-AAT TTT GCT CCC CTC CTC-30 and 50-CGT TCA CAA ATA CCC ACT G-3′ for the WT allele and 5′-GGA ACC AGA TGA CCC ACA GGT GC-30 and 50-CGC CAT TCA GGC TGC GCA ACT GTT GGG-3′ for the mutant allele83. A range of molecular weight markers (EZ Load Molecular Rulers, Bio-Rad Laboratories, Hercules, CA, USA) were also used. Amplified products were analysed by electrophoresis on 2% agarose gel containing ethidium bromide. Gels were photographed using an ultraviolet (UV) transilluminator to visualize stained bands. All animals used in the present study had the genotype corresponding to their experimental group.
Western blotting
Twenty-eight days after surgery, WT and σ1R KO mice (n = 4–6 per group) were euthanized with sodium pentobarbital (100 mg/kg, i.p.) and spinal cord T8–T9 segments immediately removed, frozen in dry ice and stored at −80 °C.
Spinal cord tissue was homogenized by sonication in TRIS buffer (50 mM Tris, 150 nM NaCl, 1% NP-40, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, Triton X-100, 0.1% SDS, 1mMNa3VO4, 25mMNa), 5% protease inhibitor cocktail and 1% phosphatase inhibitor cocktail (all from Sigma-Aldrich Quimica S.A). The resultant homogenate was then centrifuged at 10,000 G at 4 °C for 10 min. The supernatant was decanted from the pellet, and the protein concentration from the obtained supernatant was measured using a Lowry assay. Samples were then stored at −80 °C until use.
Thirty microgram samples were fractionated using 10% (w/v) SDS–PAGE and transferred onto a polyvinylidene difluoride membrane, blocked either with 5% non-fat dry milk or bovine serum albumin (BSA) in tris–tween 20-buffered saline (T–TBS) for 1 hour at room temperature. Membranes were then incubated with primary antibodies overnight at 4 °C: rabbit anti-extracellular signal-regulated kinases (total ERK 1/2) (1:40000, M5670, Sigma-Aldrich), diphosphorylated ERKs (pERK1/2)(1/:800, 44680 G, invitrogen) were diluted in T–TBS containing 1% non-fat dry milk. Rabbit anti-pY1472-GluN2B (1/:1000, M2442, Sigma-Aldrich), anti-pS1303-GluN2B (1/:3000, ab81271, Abcam), anti-GluN2B (1:750, ab15557P, Merk Millipore), anti-TNF-α (1/500, ab6671, Abcam) and anti-IL-1β (1/500, ab9722, Abcam) were also used and diluted in T–TBS containing 1% BSA solution. To ensure equal protein loading, rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (1:40,000, Sigma-Aldrich Quimica S.A.) was used as a loading control. The blots were washed four times for 15 min with T–TBS and then incubated for 1 hour at room temperature with horseradish peroxidase–conjugated goat antirabbit IgG, purchased from Pierce Biotechnology Inc. (Rockford, IL, USA) and revealed by chemiluminescence (Immun-Star HRP Chemiluminescent Kit) from Bio-Rad. Chemiluminescence was detected with a C-DiGit® Blot Scanner (Li-cor).
The densiometric analysis of immunoreactive bands was done using the Image Studio Lite 5.2 (LI-COR Bioscience). pERK,Y1472-GluN2B and pS1302-GluN2B were normalized to total ERK and total gluN2B respectively, and in turn, normalized with respect to the intensity of the corresponding GAPDH immunreactivity. TNFα and IL1β were also normalized to the corresponding GAPDH intensity.
Statistical analysis
All functional and molecular biology measurements were performed in a blinded manner, using code numbers for both mice and samples. Results shown are mean ± SEM. Data were analysed using repeated measures MANOVA (Wilks’ criterion) and analysis of variance (ANOVA) followed by Duncan’s test, when applicable. In the pharmacological study, the percentage of antiallodynic or antihyperalgesic effect exerted by a treatment was calculated as follows: % effect = [(PWD − PWV)/(PWN − PWV)] × 100, where PWD and PWV are the paw withdrawal latency (s) or threshold (g) in drug-treated and pre-treated animals, respectively, and PWN is the paw withdrawal in naïve animals. A dose–response curve was plotted using nonlinear regression analysis, and the dose of drug that produced 50% of its maximal possible response (ED50) obtained. In all statistical analyses, the α level was set at 0.05. Statistical analyses were conducted using SPSS 23.0 for Windows.
Data availability
All data generated or analysed during this study are included in this published article.
Electronic supplementary material
Acknowledgements
The present work was supported by the Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement de la Generalitat de Catalunya, Industrial Doctorates Plan, Government of Catalonia. Grant 2014-DI-026 ESTEVE – Universitat de Girona; and funded by ESTEVE and by the Vice-Chancellorship of Research of the University of Girona. Grant number MPCUdG2016/087. Editorial support (including copyediting and fact checking) was provided by Hannah Mace, MPharmacol at Aspire Scientific (Bollington, UK), and funded by Mundipharma Research GmBH & Co. KG (Limburg, Germany).
Author Contributions
All authors listed above have contributed sufficiently to be included as authors. P.B.-V., E.V. and J.M.V. conceived the experiments, contributed to the analysis and/or interpretation of data, the critical discussion of the results and the elaboration of the manuscript, and obtained funding for the study. S.C. has directly participated in the execution of the experimental work, both behavioural and biochemical assays, and has contributed to the critical discussion of the results and elaboration of the manuscript. G.G. gave support to S.C. for western blotting experiments. All the authors have revised the work critically for important intellectual content and approved the final version to be published. Also, agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing Interests
The authors José Miguel Vela and Georgia Gris are full-time employees of ESTEVE. The authors have no other relevant affiliation or financial involvement, have received no payment in preparation of this manuscript or have any conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. The rest of authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-22217-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Enrique Verdú, Email: enric.verdu@udg.edu.
Pere Boadas-Vaello, Email: pere.boadas@udg.edu.
References
- 1.Jensen TS, et al. A new definition of neuropathic pain. Pain. 2011;152:2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Burke D, Fullen BM, Stokes D, Lennon O. Neuropathic pain prevalence following spinal cord injury: A systematic review and meta-analysis. Eur J Pain. 2017;21:29–44. doi: 10.1002/ejp.905. [DOI] [PubMed] [Google Scholar]
- 3.Mann R, et al. Burden of spinal cord injury-related neuropathic pain in the United States: retrospective chart review and cross-sectional survey. Spinal Cord. 2013;51:564–570. doi: 10.1038/sc.2013.34. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 5.Zamanillo D, Romero L, Merlos M, Vela JM. Sigma 1 receptor: a new therapeutic target for pain. Eur J Pharmacol. 2013;716:78–93. doi: 10.1016/j.ejphar.2013.01.068. [DOI] [PubMed] [Google Scholar]
- 6.Mori T, Hayashi T, Hayashi E, Su TP. Sigma-1 receptor chaperone at the ER- mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One. 2013;8:e76941. doi: 10.1371/journal.pone.0076941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su TP, Su TC, Nakamura Y, Tsai SY. Sigma-1 receptor as a pluripotent modulator in the living system. Trends Pharmacol Sci. 2016;37:262–278. doi: 10.1016/j.tips.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso G, et al. Immunocytochemical localization of the sigma-(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/S0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 9.Kitaichi K, et al. Expression of the purported sigma(1) (sigma(1)) receptor in the mammalian brain and its possible relevance in deficits induced by antagonism of the NMDA receptor complex as revealed using an antisense strategy. J Chem Neuroanat. 2000;20:375–387. doi: 10.1016/S0891-0618(00)00106-X. [DOI] [PubMed] [Google Scholar]
- 10.Bangaru ML, et al. Sigma-1 receptor expression in sensory neurons and the effect of painful peripheral nerve injury. Mol Pain. 2013;9:47. doi: 10.1186/1744-8069-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HW, et al. Activation of the spinal sigma-1 receptor enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NR1 subunit in mice. Br J Pharmacol. 2008;154:1125–1134. doi: 10.1038/bjp.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Puente B, et al. Sigma-1 receptors regulate activity-induced spinal sensitization and neuropathic pain after peripheral nerve injury. Pain. 2009;145:294–303. doi: 10.1016/j.pain.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Romero L, et al. Pharmacological properties of S1RA, a new sigma-1 receptor antagonist that inhibits neuropathic pain and activity-induced spinal sensitization. Br J Pharmacol. 2012;166:2289–2306. doi: 10.1111/j.1476-5381.2012.01942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero L, Merlos M, Vela JM. Antinociception by sigma-1 receptor antagonists: central and peripheral effects. Adv Pharmacol. 2016;75:179–215. doi: 10.1016/bs.apha.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Mazo I, et al. Effects of centrally acting analgesics on spinal segmental reflexes and wind-up. Eur J Pain. 2015;19:1012–1020. doi: 10.1002/ejp.629. [DOI] [PubMed] [Google Scholar]
- 16.Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- 17.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–9. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 18.D’Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- 19.Latremoliere A, Woolf CJ. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balasuriya D, Stewart AP, Edwardson JM. The sigma-1 receptor interacts directly with GluN1 but not GluN2A in the GluN1/GluN2A NMDA receptor. J Neurosci. 2013;33:18219–18224. doi: 10.1523/JNEUROSCI.3360-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sánchez-Blázquez P, et al. The calcium-sensitive sigma-1 receptor prevents cannabinoids from provoking glutamate NMDA receptor hypofunction: Implications in antinociception and psychotic diseases. Int J Neuropsychopharmacol. 2014;17:1943–1955. doi: 10.1017/S1461145714000029. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-Muñoz M, et al. The σ1 receptor engages the redox-regulated HINT1 protein to bring opioid analgesia under NMDA receptor negative control. Antioxid Redox Signal. 2015;22(799):818. doi: 10.1089/ars.2014.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sha S, et al. Sigma-1 receptor knockout impairs neurogenesis in dentate gyrus of adult hippocampus via down-regulation of NMDA receptors. CNS Neurosci Ther. 2013;19:705–713. doi: 10.1111/cns.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cendán CM, Pujalte JM, Portillo-Salido E, Montoliu L, Baeyens JM. Formalin-induced pain is reduced in sigma(1) receptor knockout mice. Eur J Pharmacol. 2005;511:73–74. doi: 10.1016/j.ejphar.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 25.Entrena JM, et al. Sigma-1 receptors are essential for capsaicin-induced mechanical hypersensitivity: studies with selective sigma-1 ligands and sigma-1 knockout mice. Pain. 2009;143:252–261. doi: 10.1016/j.pain.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Tejada MA, et al. Sigma-1 receptor inhibition reverses acute inflammatory hyperalgesia in mice: role of peripheral sigma-1 receptors. Psychopharmacology (Berl). 2014;231:3855–3869. doi: 10.1007/s00213-014-3524-3. [DOI] [PubMed] [Google Scholar]
- 27.Gris G, Cobos EJ, Zamanillo D, Portillo-Salido E. Sigma-1 receptor and inflammatory pain. Inflamm Res. 2015;64:377–381. doi: 10.1007/s00011-015-0819-8. [DOI] [PubMed] [Google Scholar]
- 28.Nieto FR, et al. Role of sigma-1 receptors in paclitaxel-induced neuropathic pain in mice. J Pain. 2012;13:1107–1121. doi: 10.1016/j.jpain.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Cheriyan T, et al. Spinal cord injury models: a review. Spinal Cord. 2014;52:588–595. doi: 10.1038/sc.2014.91. [DOI] [PubMed] [Google Scholar]
- 30.Drews E, Zimmer A. Central sensitization needs sigma receptors. Pain. 2009;145:269–270. doi: 10.1016/j.pain.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Abe T, et al. Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci. 2005;22:1445–1454. doi: 10.1111/j.1460-9568.2005.04340.x. [DOI] [PubMed] [Google Scholar]
- 32.Wu LJ, Zhuo M. Targeting the NMDA receptor subunit NR2B for the treatment of neuropathic pain. Neurotherapeutics. 2009;6:693–702. doi: 10.1016/j.nurt.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paniagua-Torija B, Arevalo-Martin A, Molina-Holgado E, Molina-Holgado F, Garcia- Ovejero D. Spinal cord injury induces a long-lasting upregulation of interleukin-1β in astrocytes around the central canal. Neuroscience. 2015;284:283–289. doi: 10.1016/j.neuroscience.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci. 2016;10:98. doi: 10.3389/fncel.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Álvarez-Pérez B, et al. Epigallocatechin-3-gallate treatment reduces thermal hyperalgesia after spinal cord injury by down-regulating RhoA expression in mice. Eur J Pain. 2016;20:341–352. doi: 10.1002/ejp.722. [DOI] [PubMed] [Google Scholar]
- 36.Vela JM, Merlos M, Almansa C. Investigational sigma-1 receptor antagonists for the treatment of pain. Expert Opin Investig Drugs. 2015;24:883–896. doi: 10.1517/13543784.2015.1048334. [DOI] [PubMed] [Google Scholar]
- 37.Morton DB, Griffiths PH. Guidelines on the recognition of pain, distress and discomfort in experimental animals and a hypothesis for assessment. Vet Rec. 1985;116:431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- 38.Beare JE, et al. Gait analysis in normal and spinal contused mice using the TreadScan System. Journal of Neurotrauma. 2009;26:2045–2056. doi: 10.1089/neu.2009.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J. Endoplasmic reticulum stress and disrupted neurogenesis in the brain are associated with cognitive impairment and depressive-like behaviour after spinal cord injury. Journal of Neurotrauma. 2016;33:1919–1935. doi: 10.1089/neu.2015.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basso DM, et al. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 41.Kuhn PL, Wrathall JR. A mouse model of graded contusive spinal cord injury. J Neurotrauma. 1998;15:125–140. doi: 10.1089/neu.1998.15.125. [DOI] [PubMed] [Google Scholar]
- 42.Gris G, et al. The selective sigma-1 receptor antagonist E-52862 attenuates neuropathic pain of different aetiology in rats. Sci Rep. 2016;6:24591. doi: 10.1038/srep24591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 44.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 45.Ji RR, Gereau RW, 4th, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramesh G. Novel therapeutic targets in neuroinflammation and neuropathic pain. Inflamm Cell Signal. 2014;1:e111. doi: 10.14800/ics.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vidal-Torres A, et al. Effects of the selective sigma-1 receptor antagonist S1RA on formalin-induced pain behavior and neurotransmitter release in the spinal cord in rats. J Neurochem. 2014;129:484–494. doi: 10.1111/jnc.12648. [DOI] [PubMed] [Google Scholar]
- 48.Merlos, M., Romero, L., Zamanillo, D., Plata-Salamán, C. & Vela, J. M. Sigma-1 receptor and pain. Handb Exp Pharmacol. [Epub ahead of print] 10.1007/164_2017_9 (2017). [DOI] [PubMed]
- 49.Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004;74:2643–2653. doi: 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Ma W, Quirion R. The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Expert Opin Ther Targets. 2005;9:699–713. doi: 10.1517/14728222.9.4.699. [DOI] [PubMed] [Google Scholar]
- 51.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 52.Xu X, et al. Extracellular signal-regulated protein kinase activation in spinal cord contributes to pain hypersensitivity in a mouse model of type 2 diabetes. Neurosci Bull. 2014;30:53–66. doi: 10.1007/s12264-013-1387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ciruela A, et al. Identification of MEK1 as a novel target for the treatment of neuropathic pain. Br. J. Pharmacol. 2003;138:751–756. doi: 10.1038/sj.bjp.0705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma, W. & Quirion, R. Partial sciatic nerve ligation induces increase in the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) in astrocytes in the lumbar spinal dorsal horn and the gracile nucleus. Pain. 2002 99, 175–184 (2002). [DOI] [PubMed]
- 55.Crown ED, et al. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp. Neurol. 2006;199:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Cruz, C. D., McMahon, S. B. & Cruz, F. Spinal ERK activation contributes to the regulation of bladder function in spinal cord injured rats. Exp. Neurol. 2006, 200, 66–73 (2006). [DOI] [PubMed]
- 57.Yu CG, Yezierski RP. Activation of the ERK1/2 signaling cascade by excitotoxic spinal cord injury. Brain Res Mol Brain Res. 2005;138:244–255. doi: 10.1016/j.molbrainres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 58.Qu XX, et al. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp Neurol. 2009;215:298–307. doi: 10.1016/j.expneurol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 59.Katano T, et al. Involvement of spinal phosphorylation cascade of Tyr1472-NR2B, Thr286- CaMKII, and Ser831-GluR1 in neuropathic pain. Neuropharmacology. 2011;60:609–616. doi: 10.1016/j.neuropharm.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Slack S, Battaglia A, Cibert-Goton V, Gavazzi I. EphrinB2 induces tyrosine phosphorylation of NR2B via Src-family kinases during inflammatory hyperalgesia. Neuroscience. 2008;156:175–183. doi: 10.1016/j.neuroscience.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boyce S, et al. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology. 1999;38:611–623. doi: 10.1016/S0028-3908(98)00218-4. [DOI] [PubMed] [Google Scholar]
- 62.Park MK, et al. Peripheral administration of NR2 antagonists attenuates orofacial formalin- induced nociceptive behavior in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:982–986. doi: 10.1016/j.pnpbp.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 63.Jeon HJ, et al. Intracisternal administration of NR2 subunit antagonists attenuates the nociceptive behavior and p-p38 MAPK expression produced by compression of the trigeminal nerve root. Mol Pain. 2011;7:46. doi: 10.1186/1744-8069-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim Y, Cho HY, Ahn YJ, Kim J, Yoon YW. Effect of NMDA NR2B antagonist on neuropathic pain in two spinal cord injury models. Pain. 2012;153:1022–1029. doi: 10.1016/j.pain.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 65.Li Z, et al. Dehydroepiandrosterone sulfate prevents ischemia-induced impairment of long-term potentiation in rat hippocampal CA1 by up-regulating tyrosine phosphorylation of NMDA receptor. Neuropharmacology. 2006;51:9589–66. doi: 10.1016/j.neuropharm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoon SY, et al. An increase in spinal dehydroepiandrosterone sulfate (DHEAS) enhances NMDA-induced pain via phosphorylation of the NR1 subunit in mice: involvement of the sigma- 1 receptor. Neuropharmacology. 2010;59:460–467. doi: 10.1016/j.neuropharm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andrade P, et al. Role of TNF-alpha during central sensitization in preclinical studies. Neurol Sci. 2011;32:757–771. doi: 10.1007/s10072-011-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res. 2013;6:803–814. doi: 10.2147/JPR.S53660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meacham K, Shepherd A, Mohapatra DP, Haroutounian S. Neuropathic pain: Central vs. peripheral mechanisms. Curr Pain Headache Rep. 2017;21:28. doi: 10.1007/s11916-017-0629-5. [DOI] [PubMed] [Google Scholar]
- 73.Siddall, P. J. & Middleton, J. W. Spinal cord injury-induced pain: mechanisms and treatments. Pain Manag. 5, 493–507. [DOI] [PubMed]
- 74.Machelska H, Celik MÖ. Recent advances in understanding neuropathic pain: Glia, sex differences, and epigenetics. F1000Res. 2016;5:2743. doi: 10.12688/f1000research.9621.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kramer JL, et al. Neuropathic pain following traumatic spinal cord injury: Models, measurement, and mechanisms. J Neurosci Res. 2017;95:1295–1306. doi: 10.1002/jnr.23881. [DOI] [PubMed] [Google Scholar]
- 76.Tsuda M, Koga K, Chen T, Zhuo M. Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex. J Neurochem. 2017;141:486–498. doi: 10.1111/jnc.14001. [DOI] [PubMed] [Google Scholar]
- 77.Tsuda M. P2 receptors, microglial cytokines and chemokines, and neuropathic pain. J Neurosci Res. 2017;95:1319–1329. doi: 10.1002/jnr.23816. [DOI] [PubMed] [Google Scholar]
- 78.Morioka N, et al. Tumor necrosis factor-mediated downregulation of spinal astrocytic connexin43 leads to increased glutamatergic neurotransmission and neuropathic pain in mice. Brain Behav Immun. 2015;49:293–310. doi: 10.1016/j.bbi.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 79.Zhang L, et al. TNF-α contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. Pain. 2011;152:419–27. doi: 10.1016/j.pain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gustafson-Vickers SL, et al. Long-term actions of interleukin-1beta on delay and tonic firing neurons in rat superficial dorsal horn and their relevance to central sensitization. Mol Pain. 2008;4:63. doi: 10.1186/1744-8069-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi SR, et al. Astrocyte sigma-1 receptors modulate connexin 43 expression leading to the induction of below-level mechanical allodynia in spinal cord injured mice. Neuropharmacology. 2016;111:34–46. doi: 10.1016/j.neuropharm.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 82.Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. (2014). Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014;6:309–331. doi: 10.2147/CLEP.S68889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Langa F, et al. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur J Neurosci. 2003;18:2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- 84.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.