Abstract
Background
Probiotics are commonly used for the prevention of antibiotic-associated diarrhea (AAD). However, the optimum regimen remains controversial.
Objective
The objective of this article is to compare and rank the relative efficacy and tolerability among all available probiotic agents for AAD through a network meta-analysis.
Methods
Eligible studies were identified by searching PubMed, Embase, Medline, Cochrane library and Web of Science for randomized controlled trials (RCTs) that examined the efficacy of probiotic therapy for AAD. A random-effects model was applied within a frequentist framework. Quality of evidence was performed by the GRADE approach. The project was prospectively registered with PROSPERO (CRD 42016050776).
Results
Fifty-one articles (60 comparisons, 9569 participants), including 10 probiotic interventions, were identified. Lactobacillus rhamnosus GG (LGG) had the highest probability of being ranked best both in effectiveness (odds ratio (OR), 95% confidence interval (CI) = 0.28 (0.17, 0.47)) and tolerance (0.44 (0.23, 0.84)) on prevention of AAD. With regard to reducing Clostridium difficile infection rate, Lactobacillus casei (L. casei) was considered better efficacy (0.04 (0.00, 0.77)) and medium tolerance (0.56 (0.19, 1.66)). Strain combination reported no superiority over single strain in either efficacy or tolerability.
Conclusions
LGG is probably the best option to consider when AAD is indicated. L. casei appears to be the most efficacious choice when associated with severe C. difficile-related cases.
Keywords: Probiotic, antibiotic-associated diarrhea, Clostridium difficile infection, network meta-analysis
KEY SUMMARY
Summarize the established knowledge on this subject
The prevalence of antibiotic-associated diarrhea (AAD) has been increasing over the last decades owing to the widespread use of antibiotics.
Several probiotic regimens have been utilized in clinical practice for the management of AAD; however, the optimum regimen remains controversial.
What are the significant and/or new findings of this study?
Lactobacillus rhamnosus GG is probably the best option to consider when AAD is indicated.
Lactobacillus casei appears to be the most efficacious choice when associated with severe Clostridium difficile-related cases.
Strain combination reported no superiority over single strain alone in either efficacy or tolerability for patients with AAD.
Introduction
Antibiotic-associated diarrhea (AAD), emerging as a common complication of antibiotic use, is defined as unexplained diarrhea that occurs in association with antibiotics.1 The prevalence of AAD ranges from 5% to 49%, occurring at any point from the initiation of therapy to two months after antibiotic exposure.2 Disruptions of indigenous gastrointestinal microbiota and mucosal integrity, overgrowth of pathogens, and metabolic imbalances have been considered as major mechanisms involved in the pathogenesis of AAD.3,4 Typically, AAD is brief and self-limiting with no specific pathogenic agent identified; however, Clostridium difficile is responsible for the most severe cases in 10%∼25% of all episodes of AAD, which may lead to electrolyte disturbances, pseudo-membranous colitis, toxic megacolon and, rarely, death.5,6 Risk factors such as prolonged use of broad-spectrum antibiotics, old age, extended hospitalization, oral administration, and comorbidities are all proposed to influence the incidence and development of AAD.5,7,8 Antibiotics such as broad-spectrum β-lactams, glycopeptides and fluoroquinolones that act on anaerobes are most commonly linked to AAD.9 Current standard treatments for AAD have limitations. Mild cases have no established therapy, and are customarily treated with discontinuation of the antibiotic, supportive care and dietary changes. However, serious cases often require bed rest, intravenous fluids, and additional antibiotics such as metronidazole or vancomycin, and may relapse in almost 25% patients.10
Probiotics, defined as non-pathogenic living microorganisms, when ingested in adequate amounts, may colonize the intestinal tract and promote microbiota restoration, conferring a health benefit on the host.11 The rationale underlying probiotic administration is derived from normalization of unbalanced commensal gut microbiota, modulation of mucosal function and metabolic process, stimulating systemic immune response and suppressing pathogenic bacteria colonization, using specific probiotic strains.12 Based on this, a variety of organisms, such as Lactobacillus, Saccharomyces and Bifidobacterium, have been recommended empirically in recent epidemiological studies, both during and after antibiotic intake for the management of AAD, with encouraging results.1,6 However, it remains controversial which certain probiotic strains are more effective and also tolerable, or whether a multi-strain combination is superior to a single-strain probiotic preparation alone.
Previous traditional pairwise meta-analyses have generally estimated direct comparisons between probiotics, which undermined findings about relative efficacy and tolerance of interventions, because they can answer only questions about pairs.13–15 Network meta-analysis is an extension of head-to-head meta-analysis that aims to combine direct and indirect evidence into a single effect size and rank all available treatments, calculating estimates for interventions even if they have not been compared directly in current research, provided that a common comparator exists.16 In this study, therefore, we performed a systematic review and network meta-analysis of randomized controlled trials (RCTs) in order to comprehensively compare and rank the relative efficacy and tolerability among all available probiotic preparations for the prevention of AAD. Incidence of diarrhea and C. difficile infection rate were summarized to estimate treatment efficacy, while total occurrence of adverse events and occurrence of adverse event subtypes were adopted for tolerability assessment.
Methods
Our study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Cochrane Collaboration recommendations.17,18 The project was prospectively registered with the PROSPERO database of systematic reviews (CRD 42016050776).19
Search strategy
For this network meta-analysis, an experienced medical investigator (ZCY) searched PubMed, Embase, Medline, Cochrane library and Web of Science for RCTs published from January 1, 1996 to December 31, 2016 that examined the efficacy of probiotic therapy for AAD. Publications with the following search terms and Medical Subject Headings (MeSH) headings were included: ((probiotic OR Lactobacillus) OR Bifidobacterium) OR Saccharomyces) OR yogurt) AND antibiotic AND diarrhea. No language restriction was imposed. Major scientific websites and conference abstracts or presentations were also considered. Reference lists of the obtained papers, relevant reviews and meta-analyses were retrieved manually to identify articles not included in the preliminary searches.
Selection criteria
The following studies were eligible for inclusion: RCTs irrespective of language, in which an identified probiotic agent was compared to active or placebo control; participants were individuals who received oral antibiotic therapy for any reason; the intervention was oral probiotics intake in either supplements (e.g. capsule, sachet) or foods (e.g. yogurt) during or after oral antibiotic administration, with any duration and dose; the incidence of diarrhea, as defined by the individual studies, was listed as one of the major outcomes; articles were published in the last 20 years.
Data extraction and quality assessment
Three reviewers (CJY, ZCY and ZYQ) independently screened citations for eligibility by title, abstract and full text if necessary, evaluated methodological quality, and extracted the relevant data from the enrolled studies with a predesigned Excel spreadsheet, including general details of the study, participants’ information, characteristics of the treatments and main outcome measures. Risk of bias of individual studies was assessed with the Cochrane Collaboration tool in seven specified domains.18 Each domain was classified as “low risk,” “unclear” or “high risk.” Discrepancies among investigators during screening, data extraction and quality assessment process were resolved by joint review.
Statistical analyses
Direct comparisons of therapies were estimated by traditional approach. Since all data were expressed as dichotomous endpoints, we summarized the estimates as odds ratio (OR), with 95% confidence intervals (CIs).20, 21 To provide more conservative pooled effects, a random-effects model was performed. Heterogeneity among studies was estimated by the I2 statistic and Cochran Q test.22, 23 A p value less than 0.10 for the Q test was considered as significant.18 The restricted maximum likelihood (REML) method was applied to estimate the heterogeneity variance both in pairwise and network meta-analysis.24 Publication bias was assessed examining comparison-adjusted funnel plots asymmetry25 and Begg’s and Egger’s tests, where appropriate.26
In addition to the direct comparisons, we analyzed pooled data for probiotic treatments using random-effects models within a frequentist network meta-analysis. Pooled effect sizes were summarized as ORs, with 95% CIs. To summarize the efficacy and tolerability of each probiotic therapy, we estimated the absolute rates of various probiotic preparations as well. Relative ranks were accessed based on surface under the cumulative ranking curve (SUCRA),27 and the resultant rankings were presented graphically.25 Network inconsistency was appraised by comparing the indirect and direct evidence using the design-by-treatment interaction model (Higgins model),28 and the node-splitting approach for major outcomes.29 Meta-regression analyses were applied to verify similarities among studies by adding another covariate, which enabled confounders to be taken into account and allowed estimation of the summary attributable fraction for a set of exposures. A p value less than 0.05 was considered as significant.30,31
Quality of evidence contributing to each estimate was assessed according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria. For each comparison, we rated the quality of evidence as “high,” “moderate,” “low” or “very low.”32
Data synthesis was conducted using STATA software (version 14.0), Cochrane Collaboration review manager software (RevMan, version 5.3) and GRADEprofiler (version 3.5).
Results
Study characteristics and methodological quality
The literature search process is shown in Supplementary Material 1: Figure S1. From 3265 unique publications, we identified 51 articles (60 comparisons, 9569 participants, see Supplementary Material 2) meeting the inclusion criteria, which covered 10 different probiotic therapies listed in Table 1. Overall, 4819 participants were allocated to an active intervention and 4750 to placebo. Across trials, the average age of the participants was 43.2 years, the percentage of male patients was approximately 59.5% and mean sample size of each comparison arm was 205. The baseline characteristics of the enrolled records are presented in Supplementary Material 1: Table S1. A graph and summary of methodological quality assessment for individual studies are summarized in Supplementary Material 3: Figure S3 and Table S3.
Table 1.
General characteristics of probiotic therapies on prevention of antibiotic-associated diarrhea.
| Intervention abbreviations | General characteristics |
|---|---|
| Multi-genera II | Combinations of two types of genera (Lactobacillus + Bifidobacterium, Lactobacillus + Streptococcus, Bifidobacterium + Streptococcus, Bifidobacterium + Clostridium) |
| Multi-genera III | Combinations of three or more types of genera (Lactobacillus + Bifidobacterium + Streptococcus, Lactobacillus + Bifidobacterium + Enterococcus, Lactobacillus + Bifidobacterium + Lactococcus + Saccharomyces + Leuconostoc, Lactobacillus + Bifidobacterium + Propionibacterium) |
| LGG | Lactobacillus rhamnosus GG |
| L. rhamnosus | Lactobacillus rhamnosus species except for Lactobacillus rhamnosus GG |
| L. casei | Lactobacillus casei species |
| L. acidophilus | Lactobacillus acidophilus species |
| L. reuteri | Lactobacillus reuteri species |
| L. plantarum | Lactobacillus plantarum species |
| B. clausii | Bacillus clausii species |
| S. boulardii | Saccharomyces boulardii species |
Treatment efficacy
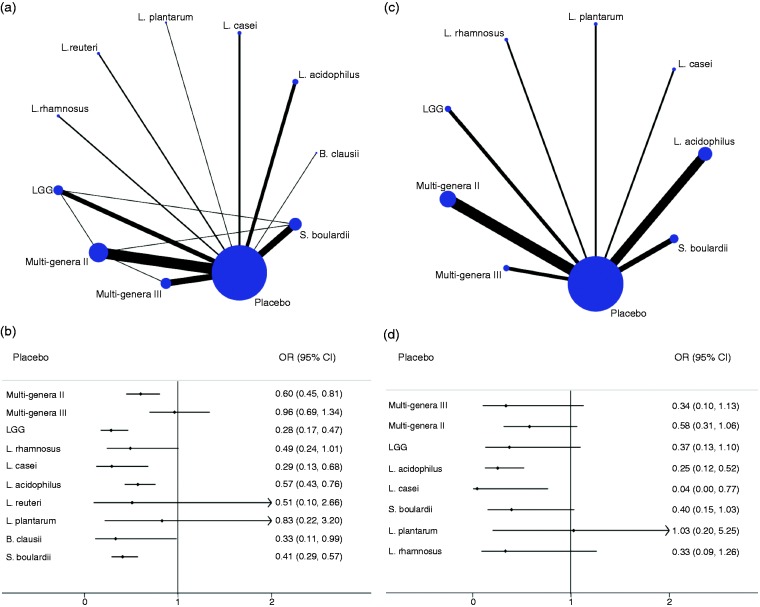
In terms of the primary outcome, the incidence of diarrhea, and the efficacy of probiotics on AAD was assessed for 10 interventions, presented in 51 publications (60 comparisons, 9569 participants), and each probiotic therapy was estimated in at least one RCT. Of 55 possible comparisons between probiotic treatments, 14 (25.5%) were compared in the studies directly (Figure 1(a)). All the regimens reduced the incidence of diarrhea; however, only six probiotic therapies showed superior efficacy compared with drug placebo in the network meta-analysis, those being LGG (0.28 (0.17, 0.47)), L. casei (0.29 (0.13, 0.68)), B. clausii (0.33 (0.11, 0.99)), S. boulardii (0.41 (0.29, 0.57)), L. acidophilus (0.57 (0.43, 0.76)) and Multi-genera II (0.60 (0.45, 0.81)), respectively (Figure 1(b)). Table 2 presents the efficacy results of each intervention compared with placebo. The incidence of diarrhea of any regimen ranged from 5% to 36% for all options. Ranking on the incidence of diarrhea, LGG was the highest one, followed by L. casei, L. plantarum, S. boulardii, L. acidophilus, L. rhamnosus, B. clausii, L. reuteri, Multi-genera II, Multi-genera III and placebo (Table 2). Despite L. plantarum therapy ranking in third place, information on the intervention was offered by only one report. The GRADE summary of findings for rating for all comparisons on incidence of diarrhea is summarized in Table 2 and Supplementary Material 4: Table S4.1; the quality of evidence was rated as moderate. Heterogeneity in the traditional meta-analysis was generally moderate (see Supplementary Material 4: Table S4.2 and Table S4.3).
Figure 1.
Network diagram and forest plot for treatment efficacy. (a) Network diagram of eligible comparisons for efficacy analysis of the incidence of diarrhea. The width of lines corresponds to the number of trials comparing every pair of interventions, and the size of nodes is proportional to the number of randomly assigned participants (indicates the sample size). (b) Forest plot of network meta-analysis for the incidence of diarrhea compared with placebo arm. (c) Network diagram of eligible comparisons for efficacy analysis of Clostridium difficile infection rate. The width of lines corresponds to the number of trials comparing every pair of interventions, and the size of nodes is proportional to number of randomly assigned participants (indicates the sample size). (d) Forest plot of network meta-analysis for C. difficile infection rate compared with placebo arm. LGG: Lactobacillus rhamnosus GG.
Table 2.
Quality of evidence, SUCRA values and effectiveness ranking for treatment efficacy.
| Interventions | Number of trials | Network meta-analysis: OR (95% CI) | Quality of evidence | Pairwise meta-analysis: OR (95% CI) | Quality of evidence | Absolute rates | SUCRA value | Mean ranka |
|---|---|---|---|---|---|---|---|---|
| (a) Incidence of diarrhea | ||||||||
| Placebo | 56 | Reference | Reference | Reference | Reference | 0.18 | 5.0 | 10.5 |
| Multi-genera II | 9 | 0.66 (0.45, 0.81) | Lowb,c | 0.71 (0.60, 0.83) | Lowb,c | 0.14 | 31.0 | 7.9 |
| Multi-genera III | 16 | 0.96 (0.69, 1.34) | Moderateb | 0.94 (0.68, 1.31) | Moderatec | 0.11 | 15.8 | 9.4 |
| LGG | 6 | 0.28 (0.17, 0.47) | Moderateb,e,g | 0.26 (0.17, 0.40) | Moderateb,g | 0.05 | 83.1 | 2.7 |
| L. rhamnosus | 2 | 0.49 (0.24, 1.01) | Moderateb | 0.46 (0.24, 0.92) | Moderateb,g | 0.06 | 47.2 | 6.3 |
| L. casei | 3 | 0.29 (0.13, 0.68) | Moderateb,g | 0.27 (0.16, 0.46) | Moderateb,g | 0.06 | 80.8 | 2.9 |
| L. acidophilus | 5 | 0.57 (0.43, 0.76) | Moderateb,e,g | 0.46 (0.34, 0.61) | Moderateb,g | 0.21 | 55.7 | 5.4 |
| L. reuteri | 2 | 0.51 (0.10, 2.66) | Moderateb,c,g | 0.45 (0.16, 1.26) | Lowb,c,g | 0.36 | 44.5 | 6.5 |
| L. plantarum | 1 | 0.83 (0.22, 3.20) | Lowb,e | 0.82 (0.22, 3.14) | Moderateb | 0.05 | 76.2 | 3.4 |
| B. clausii | 1 | 0.33 (0.11, 0.99) | Moderateb,g | 0.29 (0.11, 0.77) | Moderateb,g | 0.10 | 45.8 | 6.4 |
| S. boulardii | 11 | 0.41 (0.29, 0.57) | Moderateb,c,g | 0.36 (0.28, 0.46) | Lowb,c | 0.08 | 64.9 | 4.5 |
| (b) C. difficile infection rate | ||||||||
| Placebo | 21 | Reference | Reference | Reference | Reference | 0.06 | 5.6 | 8.5 |
| Multi-genera II | 6 | 0.34 (0.10, 1.13) | Lowb,f | 0.54 (0.35, 0.85) | Lowb,f | 0.02 | 29.5 | 6.6 |
| Multi-genera III | 2 | 0.58 (0.31, 1.06) | Moderateb,g | 0.30 (0.10, 0.89) | Moderateb,g | 0.08 | 64.4 | 3.9 |
| LGG | 2 | 0.35 (0.10, 1.26) | Moderateb,g | 0.36 (0.14, 0.92) | Moderateb,g | 0.04 | 55.0 | 4.6 |
| L. rhamnosus | 1 | 0.32 (0.07, 1.49) | Moderateb,g | 0.35 (0.11, 1.12) | Lowb,e,g | 0.02 | 56.8 | 4.5 |
| L. casei | 1 | 0.04 (0.00, 0.77) | Lowb,e,g | 0.16 (0.06, 0.47) | Moderateb,g | 0.00 | 79.0 | 2.7 |
| L. acidophilus | 5 | 0.20 (0.08, 0.48) | Moderatea,g | 0.22 (0.13, 0.38) | Moderateb,g | 0.02 | 76.2 | 2.9 |
| L. plantarum | 1 | 1.03 (0.17, 6.32) | Lowb,e | 1.03 (0.20, 5.24) | Lowb,e | 0.04 | 29.3 | 6.7 |
| S. boulardii | 3 | 0.35 (0.15, 0.85) | Moderatec,g | 0.35 (0.19, 0.63) | Moderatec,g | 0.03 | 54.2 | 4.7 |
SUCRA: surface under the cumulative ranking curve; LGG: Lactobacillus rhamnosus GG; CI: confidence interval; OR: odds ratio.
Rank was derived from the incidence of diarrhea values or C. difficile infection rate, 1 = best efficacy.
Limitations in study design or execution (risk of bias). cInconsistency in results. eImprecision of results. fPublication bias. gMagnitude of effect.
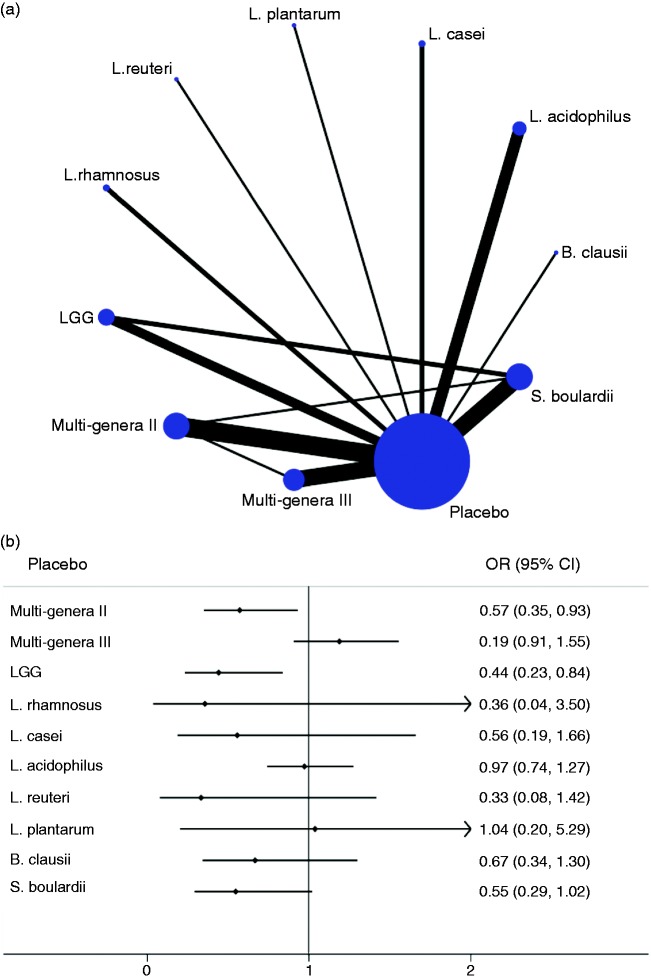
Secondary outcomes comprised three indicators: C. difficile infection rate, fever rate and dehydration rate. In terms of C. difficile infection rate, 21 publications (21 comparisons, 6623 participants) and eight probiotic regimens were analyzed. Of 36 possible comparisons between interventions, eight (22.2%) were compared directly in the reports (Figure 1(c)). Apart from L. plantarum, all probiotics indicated superior efficacy, and significant reductions were observed in two probiotic therapies (L. casei (0.04 (0.00, 0.77)) and L. acidophilus (0.25 (0.12, 0.52))), when compared with the placebo arm (Figure 1(d)). Ranking C. difficile infection rate, L. casei was the highest one, followed by L. acidophilus, Multi-genera III, L. rhamnosus, LGG, S. boulardii, Multi-genera II, L. plantarum and placebo (Table 2). Quality of evidence was rated as moderate for C. difficile infection rate (Table 2). Significant reduction was observed in fever rate (0.57 (0.35, 0.90)) and dehydration rate (0.31 (0.15, 0.67)) when probiotic therapy was applied for AAD (see Supplementary Material 4: Figure S4.1).
Treatment tolerability
Total occurrence of adverse events
Thirty-two trials were enrolled in contrast with total occurrence rates of adverse reactions among 10 probiotic therapies. Of 55 possible comparisons between interventions, 13 (23.6%) were compared directly in the reports (Figure 2(a)). In terms of all adverse reactions, only LGG (0.44 (0.23, 0.84)) and Multi-genera II (0.57 (0.35, 0.93)) were significantly better tolerated than drug placebo, while most probiotic treatments did not report superiority over the placebo arm (Figure 2(b)). Table 3 indicates the comparisons of all adverse effects for each probiotic regimen with placebo. Ranking total tolerance, L. reuteri was the highest one, followed by L. plantarum, LGG, S. boulardii, L. casei, L. rhamnosus, Multi-genera II, L. acidophilus, B. clausii, placebo and Multi-genera III (Table 3). The incidence rate of any adverse reactions of each regimen ranged from 1% to 37% for all interventions. Quality of evidence seemed to be low to moderate for total occurrence of adverse events (Table 3 and Supplementary Material 5: Table S5.1). All other comparisons and the data on traditional meta-analyses are available in Supplementary Material 5: Table S5.1 and Table S5.2.
Figure 2.
Network diagram and forest plot for treatment tolerability. (a) Network diagram of eligible comparisons for tolerability analysis of total occurrence of adverse events. The width of the lines corresponds to the number of trials comparing every pair of interventions, and the size of nodes is proportional to the number of randomly assigned participants (indicates the sample size). (b) Forest plot of network meta-analysis for total occurrence of adverse events compared with placebo arm. LGG: Lactobacillus rhamnosus GG.
Table 3.
Quality of evidence, SUCRA and effectiveness ranking for total occurrence of adverse events.
| Interventions | Number of trials | Adverse event |
Mean occurrence rate of adverse events | SUCRA value | Mean ranka | |||
|---|---|---|---|---|---|---|---|---|
| Network meta-analysis: OR (95% CI) | Quality of evidence | Pairwise meta-analysis: OR (95% CI) | Quality of evidence | |||||
| Placebo | 37 | Reference | Reference | Reference | Reference | 0.22 | 20.2 | 9.0 |
| Multi-genera II | 8 | 0.57 (0.35, 0.93) | Lowb,c,f,g | 0.80 (0.68, 0.94) | Lowb,c,f,g | 0.16 | 46.0 | 6.4 |
| Multi-genera III | 6 | 1.19 (0.91, 1.55) | Moderateb | 1.33 (0.98, 1.80) | Moderateb | 0.29 | 6.4 | 10.4 |
| LGG | 5 | 0.44 (0.23, 0.84) | Moderateb,g | 0.27 (0.14, 0.52) | Moderateb,g | 0.12 | 69.3 | 4.1 |
| L. rhamnosus | 1 | 0.36 (0.04, 3.50) | Lowc,e,g | 0.39 (0.05, 2.80) | Lowc,e,g | 0.01 | 54.7 | 5.5 |
| L. casei | 2 | 0.56 (0.19, 1.66) | Moderatec,g | 0.48 (0.22, 1.04) | Moderatee,g | 0.15 | 59.3 | 5.1 |
| L. acidophilus | 5 | 0.97 (0.74, 1.27) | Moderateb | 0.95 (0.67, 1.34) | Moderateb | 0.37 | 41.5 | 6.9 |
| L. reuteri | 1 | 0.33 (0.08, 1.42) | Lowb,c,g | 0.25 (0.06, 0.94) | Lowb,c,g | 0.15 | 77.8 | 3.2 |
| L. plantarum | 1 | 1.04 (0.20, 5.29) | Lowb,e | 1.04 (0.20, 5.28) | Moderateb | 0.04 | 73.8 | 3.6 |
| B. clausii | 1 | 0.67 (0.34, 1.30) | Moderateb,g | 0.42 (0.19, 0.91) | Moderateb,g | 0.44 | 37.9 | 7.2 |
| S. boulardii | 7 | 0.55 (0.29, 1.02) | Moderatec,g | 0.51 (0.36, 0.74) | Moderatec,g | 0.14 | 63.2 | 4.7 |
SUCRA: surface under the cumulative ranking curve; LGG: Lactobacillus rhamnosus GG; CI: confidence interval; OR: odds ratio.
Rank was derived from occurrence rate values of adverse events for all studies, 1 = best tolerance.
Limitations in study design or execution (risk of bias). cInconsistency in results. eImprecision of results. fPublication bias. gMagnitude of effect.
Occurrence of adverse event subtypes
We summarized seven kinds of side effects for probiotics on prevention of AAD (Supplementary Material 5: Figure S5.1), and five of them were processed by further network meta-analysis owing to limited sample size. Quality of evidence, relative ranks and occurrence rates of probiotic therapies in regard to subtypes of adverse reactions are listed in Supplementary Material 5: Table S5.3. In terms of constipation or vomiting, Multi-genera II might be a relatively optimal choice, although participants reported experiencing nausea more often. The lowest rate of abdominal/epigastric pain was reported with L. reuteri, while undergoing flatulence more often. Nausea was experienced less frequently with LGG, while patients experienced abdominal/epigastric pain more often. Other results of network and pairwise meta-analysis are presented in Supplementary Material 5. A summary table of all endpoints for efficacy and tolerability is available in Supplementary Material 5: Table S5.4.
Benefits versus harms
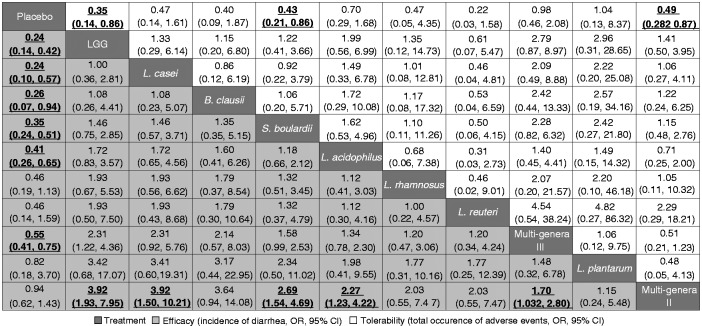
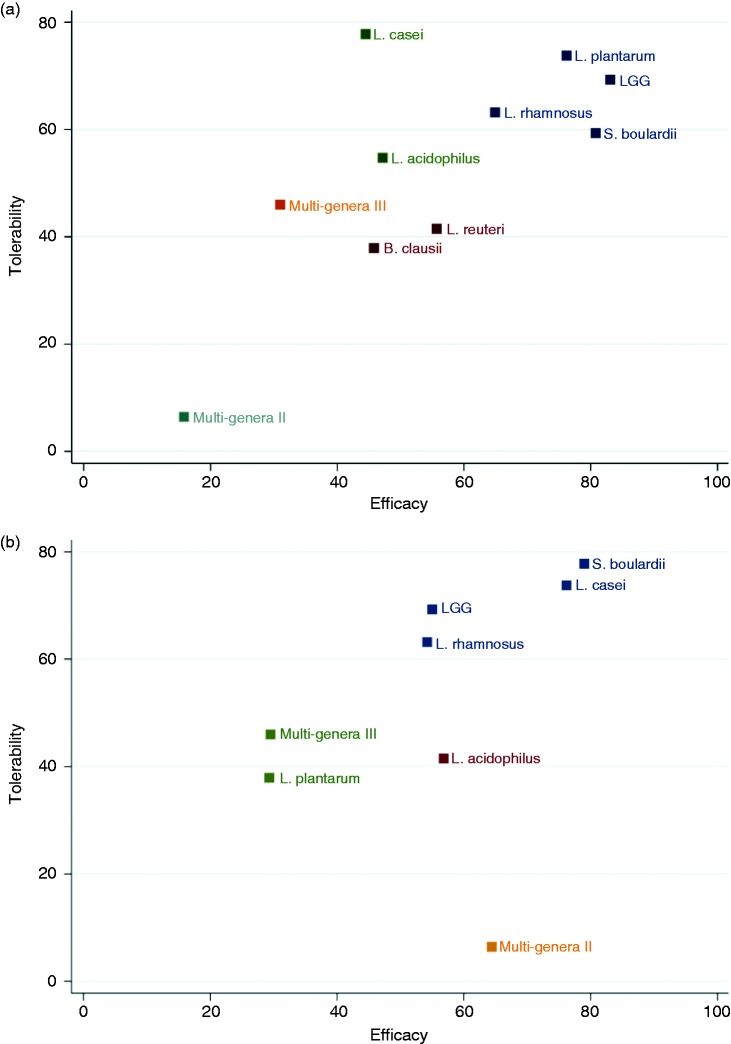
Figure 3 represented all probiotic therapies in order of relative efficacy ranks, indicating the individual contributions to the holistic results of effectiveness and tolerability. LGG, L. casei, B. clausii, S. boulardii and L. acidophilus were among the most effective interventions, whereas LGG performed better in tolerance. Each probiotic preparation was ranked according to both dimensions of efficacy and tolerability in Figure 4. With regards to the incidence of diarrhea, LGG, S. Boulardii, L. rhamnosus and L. plantarum were better in either efficacy or tolerability, as located in the upper right corner (Figure 4(a)). In terms of C. difficile infection rate, L. casei and S. Boulardii showed better efficacy and tolerance, as shown in the upper right (Figure 4(b)).
Figure 3.
Network meta-analysis of major efficacy and tolerability. Probiotics were reported in order of efficacy ranking according to odds ratios (ORs). The column-defining treatment was compared with the row-defining treatment. For efficacy, an OR value below 1 favors the column-defining treatment; for tolerability, an OR value below 1 favors the row-defining treatment. Statistically significant results are shown in bold and underlined. LGG: Lactobacillus rhamnosus GG; CI: confidence interval.
Figure 4.
Ranking for efficacy and tolerability of probiotic therapies in network meta-analysis. (a) The incidence of diarrhea versus total occurrence of adverse events. (b) Clostridium difficile infection rate versus total occurrence of adverse events. LGG: Lactobacillus rhamnosus GG.
Meta-regression, modeling assumptions, and publication bias
Meta-regression with the primary outcome indicated no significant differences in mean age (p = 0.17), sex ratio (p = 0.76), recruiting area (p = 0.45), antibiotic in use (p = 0.07), indication (p = 0.61), dosage (p = 0.45) and duration (p = 0.97) of the probiotic therapy, which did not cause extensive changes in the results. Data on meta-regression are available in Supplementary Material 4: Table S4.4.
A design-by-treatment interaction model demonstrated no evidence for inconsistency in incidence of diarrhea (p = 0.72) and total occurrence of adverse events (p = 0.35). Similarly, no major inconsistency was identified by the node-splitting approach for major endpoints. As the sample size of trials for each group was limited, subgroup analysis was not further conducted.
Visual inspection of comparison-adjusted funnel plots for the major endpoints did not exhibit prominent asymmetry, and indicated no significant evidence of publication bias in the current meta-analysis (see Supplementary Material 4: Figure S4.2 and Supplementary Material 5: Figure S5.2).
Discussion
In this network meta-analysis, we combined direct and indirect evidence from 51 RCTs (60 trials) involving 9569 participants with AAD to estimate the relative efficacy and tolerability of all currently available probiotic strategies. Overall, most probiotic treatments presented sufficient effect and good tolerance compared with placebo. We made several key observations: (a) LGG had the highest probability of being ranked best in both effectiveness and tolerance for reducing the incidence of diarrhea in prevention of AAD, and significant benefits were noticed compared with placebo drug based on moderate quality of evidence; (b) with regard to reducing C. difficile infection rate, L. casei was considered to have better efficacy and tolerance based on moderate confidence in estimates; (c) strain combination reported no superiority over single strain alone in either efficacy or tolerability; and (d) no dose correlation of treatment efficacy was indicated based on network model and meta-regression.
Most of our findings shared a similar trend with previous pairwise meta-analyses and clinical trials. A subgroup analysis was conducted by Jafarnejad et al.33 to estimate comparisons among different genera, which illustrated prevention or treatment of AAD consists primarily of Lactobacillus and S. boulardii. Moreover, results from Beausoleil and colleagues34 and Dietrich et al.2 coincided with the results of L. casei in decreasing the incidence of C. difficile infection.
Our study was the first network meta-analysis of probiotic preparations for management of AAD. By means of a network approach, we were able to systematically evaluate multiple interventions and to offer a ranking order for each regimen grounded in its capacity to improve clinical efficacy and the probability to cause adverse effects. The integration of indirect and direct evidence leads to a gain of statistical precision compared with previous studies, which also facilitates interpretation because it makes comparisons between therapies explicit.13,14,33 The design-by-treatment interaction model and node-splitting approach were applied to address concerns regarding potential inconsistency. The GRADE approach was performed to estimate the quality of evidence derived from network and pairwise meta-analysis, which allowed for separate quality assessment for various outcomes. In addition, rigorous screening and large sample size enabled us to provide a precise estimation of major clinical outcomes. Confidence in results is strengthened by the magnitude of effect estimates, stable sensitivity and high consistency between pairwise and network analysis.
The findings of this study might be interpreted considering the following limitations. First, our meta-analysis shares the limitations of the enrolled individual studies. Potential selective reporting, incomplete data, suboptimal allocation concealment and random sequence generation within some individual trials should be considered when interpreting our research. Second, baseline differences in participant-related, intervention-related and disease-related characteristics limited the comparability of trials. To seek heterogeneity, we performed meta-regression to account for the differentials across trials and no major difference was presented, suggesting no significant causes of concern. Moreover, the clinical interpretation of our study is limited owing to the small sample size of some individual studies and the limited number of trials in some nodes. Some of the studies enrolled focused on efficacy outcomes, with limited estimates reported on treatment-related adverse events; hence, we could not perform a thorough assessment of risk-benefit profile in detail. Since trials have been undertaken over 20 years, diagnostic and treatment techniques may have gradually improved. Finally, the extent to which indirect evidence is considered can affect the clinical interpretation of a network meta-analysis. For the incidence of diarrhea, major network inconsistency was not observed in most comparisons, which indicated that results from the indirect estimates were similar to direct evidence. Although node-splitting is limited to closed loops, the most effective or tolerable regimens in our meta-analysis were all part of the closed loops. Confirmation of superiority of these interventions requires direct comparisons, and our rankings should be interpreted modestly as a majority of comparisons across interventions did not obtain statistical significance.
In summary, our network meta-analysis suggests that LGG may be superior to other probiotic treatments for AAD both in efficacy and tolerance. Furthermore, in terms of secondary endpoints, C. difficile infection rate, L. casei appears to be the most efficacious choice when associated with severe C. difficile-related cases. In the future, large, well-designed and multicenter RCTs comparing different probiotic regimens and sensible new options are warranted to achieve a crucial conclusion. More high-quality meta-analyses focused on probiotic dosage, duration and administration timing for different populations should be considered in future studies.
Supplementary Material
Acknowledgments
Author contributions are as follows: CJY and ZCY: study conception and design; acquisition, analysis, and interpretation of data; manuscript drafting; and study supervision. ZYQ: acquisition, analysis, and interpretation of data. DYJ: acquisition and interpretation of data; and manuscript drafting. ZMY: interpretation of data and study supervision. ZQC: analysis and interpretation of data; and study supervision. All authors reviewed and approved the final manuscript.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Pozzoni P, Riva A, Bellatorre AG, et al. Saccharomyces boulardii for the prevention of antibiotic-associated diarrhea in adult hospitalized patients: A single-center, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol 2012; 107: 922–931. [DOI] [PubMed] [Google Scholar]
- 2.Dietrich CG, Kottmann T, Alavi M. Commercially available probiotic drinks containing Lactobacillus casei DN-114001 reduce antibiotic-associated diarrhea. World J Gastroenterol 2014; 20: 15837–15844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkes GC, Sanderson JD, Whelan K. The mechanisms and efficacy of probiotics in the prevention of Clostridium difficile-associated diarrhoea. Lancet Infect Dis 2009; 9: 237–244. [DOI] [PubMed] [Google Scholar]
- 4.Korhonen R, Korpela R, Moilanen E. Signalling mechanisms involved in the induction of inducible nitric oxide synthase by Lactobacillus rhamnosus GG, endotoxin, and lipoteichoic acid. Inflammation 2002; 26: 207–214. [DOI] [PubMed] [Google Scholar]
- 5.Lönnermark E, Friman V, Lappas G, et al. Intake of Lactobacillus plantarum reduces certain gastrointestinal symptoms during treatment with antibiotics. J Clin Gastroenterol 2010; 44: 106–112. [DOI] [PubMed] [Google Scholar]
- 6.Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2013; 382: 1249–1257. [DOI] [PubMed] [Google Scholar]
- 7.Cimperman L, Bayless G, Best K, et al. A randomized, double-blind, placebo-controlled pilot study of Lactobacillus reuteri ATCC 55730 for the prevention of antibiotic-associated diarrhea in hospitalized adults. J Clin Gastroenterol 2011; 45: 785–789. [DOI] [PubMed] [Google Scholar]
- 8.Song HJ, Kim JY, Jung SA, et al. Effect of probiotic Lactobacillus (Lacidofil® Cap) for the prevention of antibiotic-associated diarrhea: A prospective, randomized, double-blind, multicenter study. J Korean Med Sci 2010; 25: 1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox MJ, Ahuja KD, Robertson IK, et al. Can probiotic yogurt prevent diarrhoea in children on antibiotics? A double-blind, randomised, placebo-controlled study. BMJ Open 2015; 5: e006474–e006474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol 2010; 105: 1636–1641. [DOI] [PubMed] [Google Scholar]
- 11.Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K + CL1285® in the reduction of antibiotic-associated diarrhea—a placebo controlled double-blind randomized, multi-center study. Arch Med Sci 2010; 6: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scahill SL. Probiotics. J Prim Health Care 2013; 5: 81–81. [PubMed] [Google Scholar]
- 13.Goldenberg JZ, Lytvyn L, Steurich J, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev 2015. CD004827. [DOI] [PubMed] [Google Scholar]
- 14.Videlock EJ, Cremonini F. Meta-analysis: Probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther 2012; 35: 1355–1369. [DOI] [PubMed] [Google Scholar]
- 15.Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea. JAMA 2012; 18: 1959–1969. [DOI] [PubMed] [Google Scholar]
- 16.Li BZ, Threapleton DE, Wang JY, et al. Comparative effectiveness and tolerance of treatments for Helicobacter pylori: Systematic review and network meta-analysis. BMJ 2015; 351: h4052–h4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009; 6: e1000100–e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J and Green S. Cochrane handbook for systematic reviews of interventions, v.5.1.0. Cochrane Collaboration, www.cochrane-handbook.org (2011, accessed 21 September 2016).
- 19.Centre for Reviews and Dissemination. Systematic Reviews: CRD s Guidance for Undertaking Reviews in Health Care (Internet). York, England: University of York, www.crd.york.ac.uk/PROSPERO. (2009, accessed 30 September 2016).
- 20.Engels EA, Schmid CH, Terrin N, et al. Heterogeneity and statistical significance in meta-analysis: An empirical study of 125 meta-analyses. Stat Med 2000; 19: 1707–1728. [DOI] [PubMed] [Google Scholar]
- 21.Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med 2002; 21: 1575–1600. [DOI] [PubMed] [Google Scholar]
- 22.Rücker G, Schwarzer G, Carpenter JR, et al. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Med Res Methodol 2008; 8: 79–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 24.Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 2016; 7: 55–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8: e76654–e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 27.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J Clin Epidemiol 2011; 64: 163–171. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res Synth Methods 2012; 3: 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010; 29: 932–944. [DOI] [PubMed] [Google Scholar]
- 30.Dias S, Sutton AJ, Welton NJ, et al. NICE DSU technical support document 3: heterogeneity: subgroups, meta- regression, bias and bias-adjustment. National Institute for Health and Care Excellence, www.nicedsu.org.uk. (2012, accessed 25 September 2016).
- 31.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003; 22: 2693–2710. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 2013; 66: 151–157. [DOI] [PubMed] [Google Scholar]
- 33.Jafarnejad S, Shab-Bidar S, Speakman JR, et al. Probiotics reduce the risk of antibiotic-associated diarrhea in adults (18–64 years) but not the elderly (>65 years): A meta-analysis. Nutr Clin Pract 2016; 31: 502–513. [DOI] [PubMed] [Google Scholar]
- 34.Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: A randomized, double-blind, placebo-controlled trial. Can J Gastroenterol 2007; 21: 732–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.