Abstract
Objective
A physiologic increase of reactive oxygen species (ROS) is observed through pregnancy. ROS-induced damage to major cellular elements, specifically protein peroxidation, can lead to fetal and placental tissue senescence and inflammation often associated with normal parturition. The purpose of this study was to examine the effects of oxidative stress (OS) in inducing changes in proteins, senescence, and sterile inflammation in pregnant mice.
Methods
CD-1 mice (n=5/group) on day 14 of gestation were subjected to minilaparotomy and the uterine horn between gestational sacs was injected with the following: saline (control), cigarette smoke extract (CSE) CSE diluted in saline and CSE + SB 203580 (SB) (a p38 mitogen-activated protein kinase (MAPK) inhibitor). Mice were sacrificed on day 18, and amniotic sacs, placentas and amniotic fluid (AF) were collected. Protein damage was evaluated by immunostaining for 3-Nitrotyrosine modified proteins (3-NT). Activation of prosenescence p38MAPK was evaluated by western blot. Senescence features, β-galactosidase (SA-β–Gal) and AF inflammatory cytokines were analyzed by immunostaining and multiplex luminex-based immunoassays, respectively. The data were analyzed by ANOVA and Tukey’s test, p < 0.05 was used for significance.
Results
Amniotic sac from CSE-treated animals showed significant protein peroxidation compared to control as indicated by 3-NT staining. CSE activated p38MAPK phosphorylation in amniotic sac but not in placenta. Membrane p38MAPK activation was reduced after treatment with SB. CSE increased fetal membrane senescence (staining for SA-β–Gal) and increased AF concentrations of all evaluated cytokines. High inflammation correlated with pup loss and a decrease in placental weight. Treatment with p38MAPK inhibitor (SB) minimized damages, senescence and sterile inflammation.
Conclusion
OS induction by cigarette smoke extract cause fetal tissue protein damage, p38MAPK activation, senescence and sterile inflammation in the amniotic cavity of mouse. Prevention of p38MAPK activation can be a novel approach to prevention of adverse pregnancy outcomes related to OS induced premature senescence.
Keywords: fetal membranes, protein damage, p38MAPK, Labor, Inflammation, Murine Model, cigarette smoke
Introduction
Oxidative stress and generation of reactive oxygen species (ROS) in the fetal compartments may contribute to a variety of a physiological processes during pregnancy [1]. ROS generated in gestational tissues is required for fetal, placental, and fetal membrane growth and tissue remodeling required to maintain normal pregnancy. ROS in tandem with inflammatory mediators such as cytokines matrix metalloproteinases, and prostaglandins [2, 3] are one of the major components that maintain pregnancy homeostasis. At term, ROS substantially increases in fetal compartments mainly due to increased metabolic demands, reduction in substrate supply, depletion of antioxidant reserves and elevated tissue oxygen requirements. Increase in ROS causes fetal tissue damage beyond repair and enhances inflammation at term, which promotes labor-associated changes in maternal compartments [4–6]. Inflammation, the main labor-inducer [7], is commonly associated with infection, however, non-infection factors such as maternal bleeding, deteriorating of the feto-maternal interface, physical stretch and the presence of Damage-associated molecular patterns (DAMPs) can also lead to intrauterine inflammation. In especial highlight, increases in ROS seen to induce a cycle of events that propels delivery [8], once in vitro studies have shown that treatment of chorioamnion explants with antioxidant reduces the release of prostaglandins, cytokines and metalloproteinases activity induced by lipopolysaccharide [9]. However, these in vitro data do not explain specific fetal signals that generate such an inflammatory overload at term in the absence of microbial invasion and intrauterine infection, a condition associated with term births and majority of preterm births [10].
Multiple enzymatic and non-enzymatic processes can generate ROS in fetal cells [10], and the exposition of intrauterine cavity to abnormal environment and chemicals substances can generate ROS [11, 12]. As mentioned above, the main concern about the excessive ROS is the toxic effects in damaging cellular components such as proteins, lipids, carbohydrates and DNA [13–15]. Cellular damage-associated processes can induce the arrest of cellular growth resulting in senescence, a mechanism associated with aging, defined as the irreversible loss of replicative capacity in somatic cells [16]. Although senescence is a normal biological process, it can be either beneficial or deleterious, since contributes for tumor suppression and is also linked to the aging related diseases [17]. Senescent cells acquire many changes in expressions, altered mRNA and proteins, including a wide range of growth factors, proteases, chemokines and cytokines [18]. This is referred as senescence-associated secretory phenotype (SASP), contributing to ‘sterile inflammation’ associated with term parturition [17, 18]. The regulation of the SASP complex is still not well established; however, its main consequence is the influence on tissue microenvironment through the induction of a local proinflammatory response [19]. Senescent cells and SASP were recently highlighted in human fetal membranes at term labor [4]. At term, ROS contributes to senescence of fetal cells and sterile inflammation; however, the mechanism by which senescent cells could lead to labor is still unclear.
One important feature of ROS-induced senescence is the telomere shortening that leads to loss of cell functions when it reaches critical lengths. Cell recognizes this change as a signal to exit the cell cycle and activates apoptotic or senescent pathways. We have previously demonstrated human amnion cells treated with ROS inducers (cigarette smoking extract [CSE]) show reduction of telomere length, senescence morphology and biochemical changes mediated through p38 mitogen activated protein kinase (p38MAPK) pathway. Inflammation in these cells were reduced by p38MAPK inhibitor confirming stress signal associated development of inflammation in the absence of infection [6, 20].
ROS generated by CSE includes hydrogen peroxide superoxide, hydroxyl radical, nitric oxide, which are potent oxidizing and nitrating compound that have been linked to a variety of pathological conditions [21]. To further expand our knowledge and test the validity of our in vitro model of ROS induced senescence and sterile inflammation [6, 22], we tested the effects of CSE induced OS on pregnant mice. Specifically, how OS effects the amniotic sac (fetal membranes, placental membranes, amniochorion), placenta, and pregnancy outcomes.
Material and Methods
Water soluble cigarette smoking extract (CSE) stimulation
Water soluble cigarette smoking extract (CSE) preparation was adapted as previously reported [6, 23], by bubbling smoke that is drawn from a single lit commercial cigarette that represented high tar (unfiltered Camel; R.J.Reynolds Tobacco Co, Winston-Salem, NC) through 25 mL of saline. The treated saline was sterilized by using a filter 0,2µm (Corning, New York, USA). Our previous results showed amnion cells are not viable under concentrate CSE treatment [6], therefore, the 1:10 dilution was used for injecting into intrauterine cavity.
Animals
The Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch at Galveston approved the study protocol. CD-1 pregnant mice were purchased from Charles River Laboratories (Wilmington, MA). Animals were shipped on day 10 of gestation and acclimated in a temperature- and humidity-controlled facility with automatically controlled 12:12 hour light and dark cycles. Mice were allowed to consume regular chow and drinking solution ad libitum.
At day 14th of pregnancy, the pregnant CD-1 mice (n=5/group) were weighed and subjected to minilaparotomy in the lower abdomen and injection of 150uL of the treatment into uteri in between 2–3 gestational sacs according to the following experimental groups: 1) cigarette smoke extract (CSE) diluted in saline; 2) CSE in combination with SB203580 (p38MAPK inhibitor) and 3) saline (control). CSE concentration was validated in an amnion cells model and in vivo prior to these experiments to rule out toxicity [24, 25]. After sacrificing the animals by using carbon dioxide inhalation according to the IACUC and American Veterinary Medical Association guidelines on day 18, maternal, fetal, and placental weight were documented and pup loss/reabsorption was counted. Amniotic sacs and placentae were collected in either formalin 10%or fresh frozen in liquid nitrogen and stored at −80°C until further analysis.
Immunohistochemistry
Amniotic sac tissue sections were fixed in 10% formalin for 48 hours and embedded in paraffin. Sections were cut at 5-µm thickness and adhered to a positively charged slide and attached by keeping them at 57 °C for 45 min. Slides were deparaffinized using Xylene and rehydrated with 100% alcohol, 95% alcohol, and normal saline (pH 7.4) and stained using oxidative stress marker 3-Nitrotyrosine modified proteins (3-NT). Staining of 3-NT reveals oxidative stress-induced damage at the protein level. Five images for each category were taken at 40X magnification. Images were processed with ImageJ and staining intensity was measured in a uniform manor.
Western Blot
Amniotic sac and placenta samples were lysed in a RIPA lysis buffer with freshly added protease and phosphatase inhibitors (0.01%). The insoluble material was removed by centrifugation at 10,000 rpm for 20 min at 4°C. The concentration of protein in each tissue lysate was determined by using the BCA protein assay kit (Pierce BCA Protein Assay Kit, Thermo Scientific). The same amount of protein (30 µg) from each sample was loaded onto a 10% SDS-PAGE gel and electrophoresed at 120 V. The resolved proteins were transferred to a PVDF membrane using the iBlot transfer apparatus (Bio-Rad Laboratories). The membranes were blocked in Tris-Buffered Saline (TBS) containing 0.1% Tween 20 (TBS-T) and 5% skim milk for 2h at room temperature. Blots were incubated separately with total p38MAPK (Cell Signaling, Danvers, MA, USA, #9212), phosphorylated (P)-p38MAPK (Cell Signaling, #9211S), or β-actin (Sigma-Aldrich, #A5441) specific primary antibody at 4°C and shaken overnight. Blots were washed three times with TBS-T and incubated with appropriate peroxidase-conjugated IgG secondary antibody for 1h at RT. All blots were developed using chemiluminescence reagents ECL Western Blotting Detection System (Amersham Piscatawya, NJ, USA), in accordance with the manufacturer’s recommendations, followed by autoradiography.
Senescence associated β-galactosidase assay (SA-β–Gal)
The SA-β–Gal, a senescence cellular marker, was evaluated by using a commercial histochemical staining assay, following the manufactory’s instructions (Senescence cells Histochemical Staining Kit; Sigma–Aldrich). Briefly, paraffin amnion sac and placental samples were fixed for 6–7 min using the provided Fixation Buffer, washed in PBS and incubated for 1h at 37°C with fresh β-gal solution. Following incubation, tissues were evaluated using a standard light microscope for SA-β–Gal blue staining (dark grey in black and white images).
Luminex assay for inflammatory cytokines
Multiplex luminex-based immunoassay was performed for the cytokines IL-1β, IL-6, IL-8 and TNF-α using antibody-coated beads (Biosource International, Camarillo, CA, Luminex Corporation, Austin, TX) as indicators of SASP profile in amniotic fluid and maternal serum. Standard curves were developed with duplicate samples of known quantities of recombinant proteins that were provided by the manufacturer. Sample concentrations were determined by relating the absorbance that was obtained to the standard curve by linear regression analysis.
Statistical Analysis
Significant differences were analyzed by GraphPad Prism (version 7) software One-Way ANOVA followed by Tukey’s Multiple Comparison post-hoc test was used for comparison among the studied groups. A P value < 0.05 was considered significant.
Results
Oxidative stress induces protein oxidative damage and p38MAPK activation in the amniotic sac
OS produces highly reactive peroxynitrite radicals that react with tyrosine residues on proteins, resulting in a stable polypeptide-bound 3-NT [5]. 3-NT is therefore a marker for protein peroxidation and oxidative stress. Intensity of staining in amniotic sac, specifically in amnion epithelial cells (red arrow) (Fig. 1A), from CSE-treated animals were significantly higher (P<0.0001) compared to amniotic sacs from saline controls. Protein damage was significantly inhibited (P=0.0007) by the addition of p38MAPK inhibitor SB203580 (SB) (Fig. 1A) compared to CSE injections alone. Our previous data indicated that amnion cells under oxidative stress develop senescence features primarily through p38MAPK activation [6, 26]. Here we reproduce these data in vivo in murine models of pregnancy. As shown in Figure 1 (B), CSE treatment induced higher P-p38MAPK than unstimulated controls (P=0.03). Co-treatment of animals with CSE and p38MAPK inhibitor, decreased p38MAPK activation in the amniotic sac. A similar trend was seen between CSE treated and saline treated groups in placental samples regarding p38MAPK activation (Fig. 1C).
Figure 1. Oxidative Stress Induced Protein Oxidation Damage and p38MAPK Activation in the Amniotic Sac.
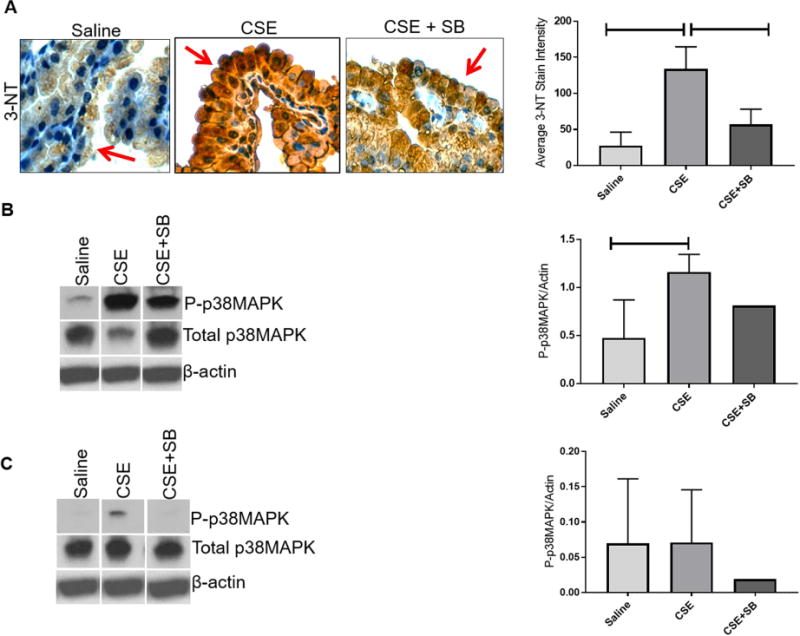
A) CSE injections induced 3-Nitrotyrosine (3-NT) in the amniotic sac (P<0.0001) (brown Stain) compared to controls, which was reduced by SB203580 (SB), an inhibitor of p38MAPK (P=0.0007) compared to controls. Images were taken at a 40X magnification. CSE injections led to the phosphorylation and activation of p38MAPK (P=0.0455) in the amniotic sac (B) and placenta (C) compared to controls. SB203580 reduced p38MAPK activation compared to CSE alone.
CSE induced senescence, sterile inflammation, and adverse pregnancy outcomes
SA-β–Gal, identified by blue staining (dark grey in black and white images), was induced by CSE injections and minimized by co-injection of CSE with SB (Fig. 2A). CSE induced sterile inflammation was also examined by measuring mouse amniotic fluid cytokine levels. Although not statistically significant, amniotic fluid from animals injected with CSE increased concentrations of SASP and inflammation mediators; specifically, IL-1β, IL-6, IL-8, and TNF-α, and the co-treatment with CSE and SB decreased these productions (Fig. 2B–D).
Figure 2. CSE Injections Induce Senescence and Inflammation in Mice.
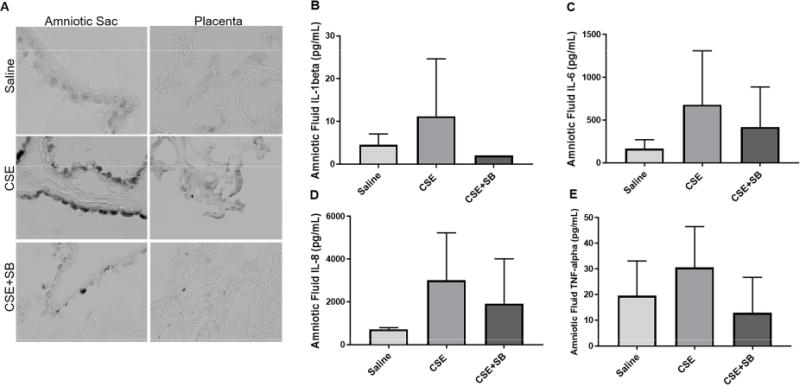
A) CSE injections induced senescence associated β-galactosidase assay (SA-β-Gal) in the amniotic sac and placenta (dark grey). This was prevented by the inhibition of p38MAPK with SB203580 (SB). Images were taken at a 40X magnification. Amniotic fluid from CSE injected CD-1 mice showed an increasing trend of inflammatory markers IL-1β (B), IL-6 (C), IL-8 (D), and TNF-α (E) Increase in cytokine response was minimized by co-treatment with SB.
CSE injection results in adverse pregnancy outcome
Injections of CSE also induced adverse pregnancy outcomes by increasing pup reabsorption/pup loss and lowering placental weight. These trends were reversed with the addition of SB (Fig. 3A–B). Maternal weight was not affected regardless of treatment (Fig. 3C). Additionally, treatments of SB only did not result in any changes from saline injected mice.
Figure 3. CSE Injections Induce Pup Loss and a Decrease in Placental Weight.
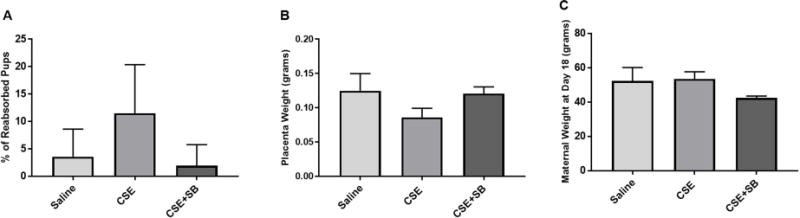
A) CSE induced pup reabsorption or loss was higher than saline injected mice. SB203580 (SB), a p38MAPK inhibitor, prevented pup loss compared to CSE alone. B) CSE injections decreased placental weight compared to saline. Co-treatment with CSE+SB prevented this decrease in weight. C) Co-treatment with CSE+SB decreased maternal weight, which was reversed with CSE.
Discussion
OS and inflammation are physiologic components of pregnancy. Parturition is associated with enhancement of these physiologic factors to a pathologic level to help induction of labor and promoting delivery. Recent reports has indicated OS induced damages, activation of stress associated signaling pathways, development of senescence and sterile inflammation in term human parturition [26, 27]. This was recapitulated in vitro using primary amnion epithelial cells exposed to OS inducer. OS inducer, CSE treatment of cells and tissues have been shown to mimic OS effects seen at term and preterm parturition [6]. In this study, we investigated the impact of OS in mouse amniotic sac in promoting changes as observed in vitro and in clinical setting during parturition. They key findings from this study are 1) CSE induced OS in murine amniotic sac leading to protein peroxidation 2) OS lead to p38MAPK mediated senescence activation, senescence, and sterile inflammation, and 3) CSE treatment induced pup reabsorption/loss and a decrease in placental weight. 4) OS induced damage leading to senescence and adverse pregnancy outcomes were reversed with co-treatment of SB20358. Highlighting the importance of p38MAPK activation in the initiation of labor. OS induced changes were minimal in placenta compared to amniotic sac. This could be due to the site of administration of CSE or more pronounced response by amniotic membranes than placenta. Alternate hypothesis is that placenta, as the major organ required for fetal growth and survival, has minimal contributions in parturition as it is required to support fetal life until delivery. Though small, these changes show that preterm OS has the ability to induce senescence and adverse pregnancy out comes such as placental abnormalities (preeclampsia) and loss of pups (still birth) [28].
Recent findings suggest that senescence of the fetal membranes is a natural and physiological process that is initiated at the time of embryogenesis and placentation. Term labor can be considered as an end stage of life for the intra uterine tissues such as fetal membranes and placenta. This is documented by telomere shortening in fetal membrane cells, fetal leukocytes, as well as the placenta [29]. We have already reported that cell free fetal telomere fragments (cffTF) (cleaved portions of telomeres from fetal tissues) increase in term amniotic fluid [26] and they are capable of causing OS, senescence and preterm birth in mouse models, similar to the findings reported here. At term, increased cffTF, a damage associated molecular pattern marker (DAMP), leads to p38MAPK activation, senescence and SASP. Previous data as well as resent findings suggest that senescence induces cells to produce inflammatory cytokines [30], which, in turn, act in promoting labor and/or membrane weakening.
Mouse pregnancy is reported to be driven by a systemic progesterone withdraw followed by maternal signals to deliver the fetus [31]. Here we document that OS induced fetal tissue damage and senescence causing inflammatory signals, mainly from the chorioamnion that can lead to sterile inflammation and labor associated changes. These data suggest that besides maternal endocrine mediators (leuteolysis and systemic progesterone withdrawal) and paracrine, fetal signaling at term may be important for the initiation of labor in mice. Paracrine signaling may be mediated by sterile inflammatory markers produced in response to OS buildup in the intrauterine cavity. OS inducing risk factors can produce similar changes resulting in preterm parturition. Additionally, our study validates the use of mouse models to study p38MAPK activation and novel signaling cascaded which can be targeted to prevent adverse pregnancy outcomes related to premature senescence, especially considering new opportunities to study and understand its upstream regulators.
Highlights.
Uterine oxidative stress (OS) leads to fetal tissue protein oxidative damage.
OS leads to p38MAPK activation, senescence, and sterile inflammation.
OS treatment induced adverse pregnancy events.
OS induced damage, senescence, & adverse pregnancy outcomes were reversed with p38MAPK inhibitor.
p38MAPK plays an important role in the initiation of labor in a murine model.
Acknowledgments
Funding
This study is supported by 1R03HD086354-01 from NICHD to R Menon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peltier MR. Immunology of term and preterm labor. Reprod Biol Endocrinol. 2003;1:122. doi: 10.1186/1477-7827-1-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orsi NM, Tribe RM. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J Neuroendocrinol. 2008;20(4):462–9. doi: 10.1111/j.1365-2826.2008.01668.x. [DOI] [PubMed] [Google Scholar]
- 4.Menon R, Boldogh I, Hawkins HK, Woodson M, Polettini J, Syed TA, Fortunato SJ, Saade GR, Papaconstantinou J, Taylor RN. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. American Journal of Pathology. 2014;184(6):1740–51. doi: 10.1016/j.ajpath.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Dutta EH, Behnia F, Boldogh I, Saade GR, Taylor BD, Kacerovsky M, Menon R. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol Hum Reprod. 2016;22(2):143–57. doi: 10.1093/molehr/gav074. [DOI] [PubMed] [Google Scholar]
- 6.Menon R, Boldogh I, Urrabaz-Garza R, Polettini J, Syed TA, Saade GR, Papaconstantinou J, Taylor RN. Senescence of primary amniotic cells via oxidative DNA damage. PLoS ONE [Electronic Resource] 2013;8(12):e83416. doi: 10.1371/journal.pone.0083416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamel RM. The onset of human parturition. Arch Gynecol Obstet. 2010;281(6):975–82. doi: 10.1007/s00404-010-1365-9. [DOI] [PubMed] [Google Scholar]
- 8.Chai M, Barker G, Menon R, Lappas M. Increased oxidative stress in human fetal membranes overlying the cervix from term non-labouring and post labour deliveries. Placenta. 2012;33(8):604–10. doi: 10.1016/j.placenta.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Lappas M, Permezel M, Rice GE. Mitogen-activated protein kinase proteins regulate LPS-stimulated release of pro-inflammatory cytokines and prostaglandins from human gestational tissues. Placenta. 2007;28(8–9):936–45. doi: 10.1016/j.placenta.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72(5):458–74. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polettini J, Silva MG, Kacerovsky M, Syed TA, Saade G, Menon R. Expression profiles of fetal membrane nicotinamide adenine dinucleotide phosphate oxidases (NOX) 2 and 3 differentiates spontaneous preterm birth and pPROM pathophysiologies. Placenta. 2014;35(3):188–94. doi: 10.1016/j.placenta.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Ilmonen P, Kotrschal A, Penn DJ. Telomere attrition due to infection. PLoS One. 2008;3(5):e2143. doi: 10.1371/journal.pone.0002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babizhayev MA, Yegorov YE. Smoking and health: association between telomere length and factors impacting on human disease, quality of life and life span in a large population-based cohort under the effect of smoking duration. Fundam Clin Pharmacol. 2011;25(4):425–42. doi: 10.1111/j.1472-8206.2010.00866.x. [DOI] [PubMed] [Google Scholar]
- 15.von Zglinicki T, Pilger R, Sitte N. Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radic Biol Med. 2000;28(1):64–74. doi: 10.1016/s0891-5849(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 16.Hayflick L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965;37:614–36. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 17.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192(4):547–56. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umbreit NT, Pellman D. Cancer biology: Genome jail-break triggers lockdown. Nature. 2017;550(7676):340–341. doi: 10.1038/nature24146. [DOI] [PubMed] [Google Scholar]
- 20.Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Frontiers in Immunology. 2014;5:567. doi: 10.3389/fimmu.2014.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasnis E, Bar-Shai M, Burbea Z, Reznick AZ. Mechanisms underlying cigarette smoke-induced NF-kappaB activation in human lymphocytes: the role of reactive nitrogen species. J Physiol Pharmacol. 2007;58(Suppl 5(Pt 1)):275–87. [PubMed] [Google Scholar]
- 22.Menon R, Fortunato SJ, Yu J, Milne GL, Sanchez S, Drobek CO, Lappas M, Taylor RN. Cigarette smoke induces oxidative stress and apoptosis in normal term fetal membranes. Placenta. 2011;32(4):317–22. doi: 10.1016/j.placenta.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Sheller S, Papaconstantinou J, Urrabaz-Garza R, Richardson L, Saade G, Salomon C, Menon R. Amnion-Epithelial-Cell-Derived Exosomes Demonstrate Physiologic State of Cell under Oxidative Stress. PLoS One. 2016;11(6):e0157614. doi: 10.1371/journal.pone.0157614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menon R, Boldogh I, Urrabaz-Garza R, Polettini J, Syed TA, Saade GR, Papaconstantinou J, Taylor RN. Senescence of Primary Amniotic Cells via Oxidative DNA Damage. Plos One. 2013;8(12) doi: 10.1371/journal.pone.0083416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon R, Boldogh I, Hawkins HK, Woodson M, Polettini J, Syed TA, Fortunato SJ, Saade GR, Papaconstantinou J, Taylor RN. Histological Evidence of Oxidative Stress and Premature Senescence in Preterm Premature Rupture of the Human Fetal Membranes Recapitulated in Vitro. American Journal of Pathology. 2014;184(6):1740–1751. doi: 10.1016/j.ajpath.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere Fragment Induced Amnion Cell Senescence: A Contributor to Parturition? PLoS ONE [Electronic Resource] 2015;10(9):e0137188. doi: 10.1371/journal.pone.0137188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bredeson S, Papaconstantinou J, Deford JH, Kechichian T, Syed TA, Saade GR, Menon R. HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes. PLoS ONE [Electronic Resource] 2014;9(12):e113799. doi: 10.1371/journal.pone.0113799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sultana Z, Maiti K, Dedman L, Smith R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am J Obstet Gynecol. 2017 doi: 10.1016/j.ajog.2017.11.567. [DOI] [PubMed] [Google Scholar]
- 29.Menon R, Yu J, Basanta-Henry P, Brou L, Berga SL, Fortunato SJ, Taylor RN. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One. 2012;7(2):e31136. doi: 10.1371/journal.pone.0031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ventura MT, Casciaro M, Gangemi S, Buquicchio R. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy. 2017;15:21. doi: 10.1186/s12948-017-0077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R525–45. doi: 10.1152/ajpregu.00153.2009. [DOI] [PubMed] [Google Scholar]


