Key Points
Question
Does weaning to an extensively hydrolyzed formula decrease the cumulative incidence of type 1 diabetes in children at risk?
Findings
In this randomized clinical trial that included 2159 children with human leukocyte antigen–conferred susceptibility to type 1 diabetes and at least 1 affected family member, weaning to a hydrolyzed formula compared with a conventional formula did not significantly decrease the cumulative incidence of type 1 diabetes after a median of 11.5 years (8.4% vs 7.6%).
Meaning
Weaning to a hydrolyzed formula did not reduce the risk of type 1 diabetes in children with an increased disease risk.
Abstract
Importance
Early exposure to complex dietary proteins may increase the risk of type 1 diabetes in children with genetic disease susceptibility. There are no intact proteins in extensively hydrolyzed formulas.
Objective
To test the hypothesis that weaning to an extensively hydrolyzed formula decreases the cumulative incidence of type 1 diabetes in young children.
Design, Setting, and Participants
An international double-blind randomized clinical trial of 2159 infants with human leukocyte antigen–conferred disease susceptibility and a first-degree relative with type 1 diabetes recruited from May 2002 to January 2007 in 78 study centers in 15 countries; 1081 were randomized to be weaned to the extensively hydrolyzed casein formula and 1078 to a conventional formula. The follow-up of the participants ended on February 28, 2017.
Interventions
The participants received either a casein hydrolysate or a conventional adapted cow’s milk formula supplemented with 20% of the casein hydrolysate. The minimum duration of study formula exposure was 60 days by 6 to 8 months of age.
Main Outcomes and Measures
Primary outcome was type 1 diabetes diagnosed according to World Health Organization criteria. Secondary outcomes included age at diabetes diagnosis and safety (adverse events).
Results
Among 2159 newborn infants (1021 female [47.3%]) who were randomized, 1744 (80.8%) completed the trial. The participants were observed for a median of 11.5 years (quartile [Q] 1-Q3, 10.2-12.8). The absolute risk of type 1 diabetes was 8.4% among those randomized to the casein hydrolysate (n = 91) vs 7.6% among those randomized to the conventional formula (n = 82) (difference, 0.8% [95% CI, −1.6% to 3.2%]). The hazard ratio for type 1 diabetes adjusted for human leukocyte antigen risk group, duration of breastfeeding, duration of study formula consumption, sex, and region while treating study center as a random effect was 1.1 (95% CI, 0.8 to 1.5; P = .46). The median age at diagnosis of type 1 diabetes was similar in the 2 groups (6.0 years [Q1-Q3, 3.1-8.9] vs 5.8 years [Q1-Q3, 2.6-9.1]; difference, 0.2 years [95% CI, −0.9 to 1.2]). Upper respiratory infections were the most common adverse event reported (frequency, 0.48 events/year in the hydrolysate group and 0.50 events/year in the control group).
Conclusions and Relevance
Among infants at risk for type 1 diabetes, weaning to a hydrolyzed formula compared with a conventional formula did not reduce the cumulative incidence of type 1 diabetes after median follow-up for 11.5 years. These findings do not support a need to revise the dietary recommendations for infants at risk for type 1 diabetes.
Trial Registration
clinicaltrials.gov Identifier: NCT00179777
This randomized clinical trial tested the hypothesis that weaning to an extensively hydrolyzed formula decreases the cumulative incidence of type 1 diabetes in young children.
Introduction
Type 1 diabetes is considered to be a chronic immune-mediated disease characterized by selective loss of insulin-producing β cells in the pancreatic islets in genetically susceptible individuals. Overt clinical disease is preceded by an asymptomatic period of highly variable duration during which diabetes-associated autoantibodies appear in the peripheral circulation as markers of emerging β-cell autoimmunity. Several disease-related autoantibodies predict clinical type 1 diabetes, including classic islet cell antibodies (ICAs), insulin autoantibodies (IAAs), and autoantibodies to glutamic acid decarboxylase (GAD); the tyrosine phosphatase-related insulinoma-associated 2 molecule (IA-2); and zinc transporter 8. In natural history studies from infancy, positivity for 2 or more autoantibodies signals a risk of approximately 70% for the development of clinical diabetes over the subsequent 10 years.
The incidence of type 1 diabetes is increasing at an accelerating rate among children in North America and in most European countries. Accumulating evidence suggests that β-cell autoimmunity emerges early in life. Accordingly, any measure aimed at primary prevention of type 1 diabetes (ie, prevention of the initiation of the diabetic disease process) has to be initiated in infancy. In addition, there is a growing body of data suggesting that factors affecting the emergence of autoimmunity may be different from those associated with progression from autoimmunity to diabetes.
Some epidemiological and immunological studies suggest that exposure to complex foreign proteins in early infancy may increase the risk of β-cell autoimmunity and type 1 diabetes in genetically susceptible individuals, although others do not. In our previous study, weaning to an extensively hydrolyzed casein formula did not decrease the cumulative incidence of diabetes-associated autoantibodies by 7 years of age in at-risk children. This article reports on the intervention effect on diabetes incidence by 11.5 years of age in the TRIGR (Trial to Reduce Insulin-Dependent Diabetes Mellitus in the Genetically at Risk) Study.
Methods
Study Design
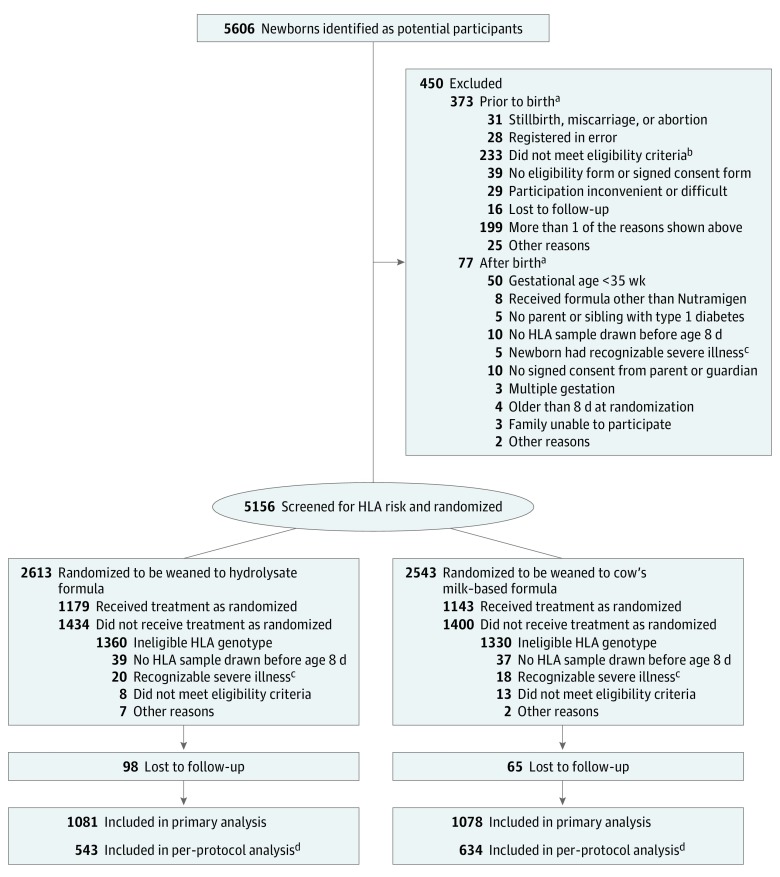
A randomized, double-blind study was conducted in 78 study centers from 15 countries as previously described. The study protocol is available in Supplement 1. Newborn infants who had a first-degree relative with type 1 diabetes and defined human leukocyte antigen (HLA) genotypes were recruited between May 2002 and January 2007 and followed up until the youngest participant reached 10 years of age in February 2017. Randomization of the infants who met the inclusion criteria took place before birth or immediately after birth (Figure 1). Randomization was stratified by study center, with a block size of 4. Written informed consent was obtained from the family before enrollment. The study was approved by the ethics committees of all participating centers.
Figure 1. Screening, Randomization, and Follow-up.
HLA indicates human leukocyte antigen.
aThe sum of the individual reasons is higher than the total because a participant may have had more than 1 reason.
bA total of 134 for gestational age greater than 35 weeks; 30 received formula other than Nutramigen, 6 with no parent or sibling with type 1 diabetes, 24 with no HLA sample drawn before age 8 days, 21 newborns had recognizable severe illness, 16 had no signed consent from parent or guardian, 5 with multiple gestation, 21 older than 8 days at randomization, 6 with families unable to participate, and 3 with possibility of random assignment. Note: the sum of individual reasons is higher than the total because a participant may have had more than 1 reason.
cRecognizable severe illness within 7 days of birth.
dPer-protocol analysis included participants with exposure to the study formula for 60 days or longer and no exposure to nonallowed foods.
Dietary Intervention
Infants were randomly assigned weaning to either the intervention or control formulas, which were produced specifically for this study. Randomization was carried out in each strata within 4 blocks. The intervention formula was an extensively hydrolyzed casein-based formula, while the control formula was composed of 80% intact cow’s milk protein and 20% hydrolyzed milk protein and formulated so that the taste and smell would be indistinguishable from the intervention formula. Study formulas were prepared and coded with the use of 4 colors by Mead Johnson Nutritional and were blinded to all investigators except the data management unit. Newborn infants requiring supplemental feeding before randomization (eg, infants born at night or on weekends) received banked breast milk or Nutramigen, an extensively hydrolyzed casein-based formula.
Breastfeeding was practiced at the discretion of the participating mothers, and maternal diets were unmodified. Breastfeeding was encouraged and exceeded national averages in both groups. The dietary intervention period lasted until the infant was at least 6 months of age and, if by that time the child had not received the study formula for at least 60 days, study formula feeding was continued until 60 days of study formula exposure was reached, but not beyond 8 months of age. Parents were asked not to feed the children any commercial or other baby foods containing bovine protein during the intervention period. Adherence to the protocol was monitored by means of regular family nutrition interviews (at the age of 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 months) and by the analysis of cow’s milk antibodies in serum samples.
HLA Genotyping
Cord blood or a heel stick blood sample collected on filter paper shortly after birth was immediately sent to the Turku (Europe and Australia) or Pittsburgh (North America) laboratories for HLA genotyping. HLA genotyping for the selected DQB1 and DQA1 alleles was performed using sequence-specific oligonucleotide hybridization, with quality control between the 2 laboratories carefully maintained. The following genotypes were regarded as eligible: (1) HLA DQB1*02/DQB1*03:02 (high risk); (2) HLA DQB1*03:02/x (x not DQB1*02, DQB1*03:01, or DQB1*06:02) (moderate risk); (3) HLA DQA1*05-DQB1*02/y (y not DQA1*02:01-DQB1*02, DQB1*03:01, DQB1*06:02, or DQB1*06:03) (mild risk); and (4) HLA DQA1*03-DQB1*02/y (y not DQA1*02:01-DQB1*02, DQB1*03:01, DQB1*06:02, or DQB1*06:03) (rare mild risk).
β-Cell Autoimmunity
ICAs were detected using indirect immunofluorescence. The other 3 autoantibodies were quantified with the use of specific radiobinding assays in the Scientific Laboratory, Children’s Hospital, University of Helsinki, Helsinki, Finland, with cutoff limits for positivity of 2.5 JDF units for ICAs, 2.80 relative units (RU) for IAA, 5.36 RU for GAD autoantibodies, and 0.77 RU for IA-2 autoantibodies. The disease sensitivity and specificity of the ICA assay were 100% and 98%, respectively, in the fourth round of the international workshops on standardization of the ICA assay. According to the Diabetes Autoantibody Standardization Program and the International Autoantibody Standardization Program workshop results in 2002-2016, the disease sensitivities of the IAA, GAD autoantibody, and IA-2 autoantibody radiobinding assays were 42% to 62%, 70% to 92%, and 62% to 80%, respectively. The corresponding disease specificities were 93% to 99%, 90% to 98%, and 93% to 100%, respectively.
Outcomes
The primary end point was the diagnosis of diabetes according to World Health Organization criteria. According to those criteria, the diagnosis is based on (1) symptoms + a single random plasma glucose level of 200 mg/dL or greater (to convert to mmol/L, multiply by 0.0555) or (2) if no symptoms, the diagnosis requires a raised random plasma glucose reading of 200 mg/dL or greater on 2 occasions, a raised fasting plasma glucose reading of 126 mg/dL or greater, or a diabetic oral glucose tolerance test (OGTT, fasting venous plasma glucose ≥126 mg/dL and/or a 2-hour venous plasma glucose ≥200 mg/dL) on 2 occasions. OGTTs were performed by protocol on all study participants who had not been previously diagnosed at 6 and 10 years of age and at study end. Additional OGTTs were performed as clinically indicated. All diagnosed cases were centrally reviewed.
Adverse Events
Undesirable experiences occurring to a child during the trial, whether or not considered related to the investigational product, were reported as adverse events. Serious adverse events were reviewed centrally by the safety monitoring group for this study and were reported annually in tabular form to an external data safety and monitoring board, which reviewed each serious adverse event individually.
Statistical Analyses
The cumulative incidence of diabetes onset from the time of randomization within each group was estimated using a modified Kaplan-Meier diabetes-free survival function. The difference between groups in the cumulative incidence functions, and the associated hazard functions, was tested using the Mantel–log rank test on discrete time to type 1 diabetes (6-month intervals). The relative risk of diabetes onset between groups was estimated from the discrete Cox proportional hazard model. The proportionality assumption of the Cox proportional hazard model was tested. First, the Schoenfeld residuals were examined to determine whether there was an association with time. Second, the interaction of parameters of interest and time were included in the models and tested for significance. For treatment and the variables used in the adjusted models, the null hypothesis of proportionality failed to be rejected. The analyses were adjusted for HLA risk, duration of breastfeeding, duration of study formula consumption, sex, and region, while treating study center as a random effect. The critical value for the test statistic (P = .047) and confidence intervals in this primary analysis were adjusted for multiple looks, which took place during the trial and were based on the Lan and DeMets spending function. When comparing data between the 2 study groups, the t test was applied for normally distributed variables and the nonparametric Mann-Whitney U test for skewed variables.
The effects of weaning to the casein hydrolysate vs conventional formula were tested using the intention-to-treat principle including all HLA-eligible participants who were randomized to a treatment group. Tests of significance reported herein were 2-tailed. Statistical analyses were performed using SAS version 9.4 (SAS Institute). No imputation for missing values was performed; rather, observations with relevant missing values were excluded from respective analyses. The analysis of diabetes risk was also performed according to treatment received (per-protocol analysis). Participants were included in this analysis if they had exposure to the study formula for 60 days or longer and were not exposed to nonallowed foods. This study was designed such that given a confidence level of 95%, an estimated cumulative incidence of diabetes of 7.6% by the age of 10 years in the control group and an expected dropout rate of 20% by 10 years and a frequency of 10% of exclusive breastfeeding (up to age of 6 months), the study would have 80% power to detect a 40% change in the end point. As a post hoc analysis, the hazard ratio of the treatment groups was also calculated after adjusting for the age at which multiple autoantibodies appeared as an exploratory analysis.
Results
Altogether, 2159 newborn infants (1021 female [47.3%]) with an eligible HLA genotype (41.9% of the genotyped infants) were randomized to the intervention study. Five hundred sixteen infants (23.9%) carried the high-risk HLA genotype; 953 (44.1%), moderate-risk genotypes; 668 (31.0%), mild-risk genotypes; and 22 (1.0%), the rare mild-risk genotype. The first-degree relative with type 1 diabetes was the mother in 1052 infants (48.8%), the father in 722 (33.4%), and a sibling in 308 (14.3%), and 77 participants (3.5%) had multiple affected relatives. The median follow-up time for the diagnosis of diabetes was 11.5 years (Q1-Q3, 10.2-12.8 years; mean, 11.0 years). Randomization resulted in 1081 infants in the casein hydrolysate group and 1078 in the control group. There were no differences in the demographics or the distribution of HLA genotypes between the 2 groups (Table 1).
Table 1. Demographic Characteristics, Dietary Exposure, and Autoantibody Status of the Trial Participants.
| Characteristic | Casein Hydrolysate (n = 1081a) |
Control Formula (n = 1078a) |
|---|---|---|
| Baseline Characteristics | ||
| HLA risk category, No. (%) | ||
| HLA-DQB1*0302/DQB1*02 [high risk] | 260 (24.1) | 256 (23.7) |
| HLA-DQB1*0302/x (x not DQB1*02, DQB1*0301, or DQB1*0602) [moderate risk] | 478 (44.2) | 475 (44.1) |
| HLA-DQA1*05-DQB1*02/y (y not DQA1*0201-DQB1*02, DQB1*0301, DQB1*0302, DQB1*0602, or DQB1*0603) [mild risk] | 332 (30.7) | 336 (31.2) |
| HLA-DQA1*03-DQB1*02/y (y not DQA1*0201-DQB1*02, DQB1*0301, DQB1*0302, DQB1*0602, or DQB1*0603) [rare mild risk] | 11 (1.0) | 11 (1.0) |
| Region, No. (%) | ||
| Finland | 212 (19.6) | 212 (19.7) |
| Canada | 265 (24.5) | 263 (24.4) |
| United States | 199 (18.4) | 196 (18.2) |
| Other | 405 (37.5) | 407 (37.8) |
| Maternal age, mean (SD), y | 30.7 (5.1) | 30.9 (4.9) |
| Female infants, No. (%) | 505 (46.7) | 516 (47.9) |
| Characteristics Obtained After Randomization | ||
| Relative with type 1 diabetes, No. (%) | ||
| Mother only | 530 (49.0) | 522 (48.4) |
| Father only | 355 (32.8) | 367 (34.0) |
| 1 Sibling only | 151 (14.0) | 157 (14.6) |
| >1 Family member | 45 (4.2) | 32 (3.0) |
| Breastfeeding duration, median (Q1-Q3), mo | 7.8 (2.1-9.0) | 7.1 (2.1-9.0) |
| No. of infants | 1071 | 1066 |
| Exclusive breastfeeding duration, median (Q1-Q3), wk | 0.29 (0.14-10.0) | 0.29 (0.14-7.0) |
| No. of infants | 1071 | 1065 |
| Age at first study formula intake, mean (SD), mo | 2.0 (2.3) | 1.8 (2.2) |
| No. of infants | 865 | 872 |
| Study formula duration, median (Q1-Q3), wk | 9.0 (0.4-18) | 10.0 (1.0-22) |
| No. of infants | 1071 | 1065 |
| Islet autoantibodies, No. (%)b | ||
| ICA+ | 394 (36.5) | 373 (34.8) |
| IAA+ | 183 (17.0) | 162 (15.1) |
| GADA+ | 207 (19.2) | 186 (17.3) |
| IA-2A+ | 115 (10.7) | 102 (9.5) |
| Duration of follow-up, mean (SD), y | 10.9 (2.8) | 11.0 (2.7) |
| Duration of follow-up, median (Q1-Q3), y | 11.5 (10.1-12.8) | 11.4 (10.2-12.8) |
Abbreviations: GADA, glutamic acid decarboxylase autoantibody; HLA, human leukocyte antigen; IAA, insulin autoantibody; IA-2A: tyrosine phosphatase–related insulinoma-associated 2 molecule autoantibody; ICA, islet cell antibody; Q, quartile.
Sample sizes are reported when they differ from the overall sample sizes.
Participants were considered positive for a specific autoantibody if they had 1 or more measurements greater than the specified threshold during follow-up (ICA >2.5 JDF units, IAA >2.80 RU, GADA >5.36 RU, and IA-2A >0.77 RU). Islet autoantibodies were measured at birth, 3 months, 6 months, 9 months, 12 months, and then annually to age 14 years.
Study Intervention
Eighty percent of infants in the casein hydrolysate group and 80.9% in the control group were exposed to the study formula during the intervention period. The mean (SD) ages of the infants at the time of study formula introduction were 2.0 (2.3) months in the hydrolysate group and 1.8 (2.2) months in the control group (difference, 0.2 months [95% CI, 0-0.42]). The mean (SD) duration of study-formula feeding was 10.2 (9.3) weeks in the casein hydrolysate group and 11.7 (9.7) weeks in the control group (difference, 1.5 weeks [95% CI, 0.7-2.3]; P < .001). As previously reported, the analysis of cow’s milk antibodies confirmed that the families adhered well to the dietary intervention, resulting in conspicuous differences in the antibody levels between the treatment groups.
Progression to Diabetes
The median age at initial seroconversion was 1.6 years (Q1-Q3, 1.0-3.0 years) in the casein hydrolysate group among those who progressed to clinical diabetes, whereas it was 1.5 years (Q1-Q3, 1.0-3.0 years; P = .38) among the progressors in the control group. The mean duration from seroconversion to clinical diabetes was 4.1 years (median, 3.5 years [Q1-Q3, 1.4-6.6]) in the casein hydrolysate group and 3.9 years (median, 3.1 years [Q1-Q3, 1.1-6.2]) in the control group (difference, 0.2 years [95% CI, −0.8 to 1.1]; P = .76). The number of participants who were positive for each specific autoantibody during the preclinical period is shown in Table 1. Five children (5.5%) in the casein hydrolysate group and 6 (7.3%) in the control group had no detectable autoantibodies before the diagnosis of diabetes (P = .62). At diagnosis, the number of autoantibody-negative participants had dropped to 4 (4.4%) and 5 (6.1%), respectively (difference, 1.7% [95% CI, −6.4% to 10.4%]).
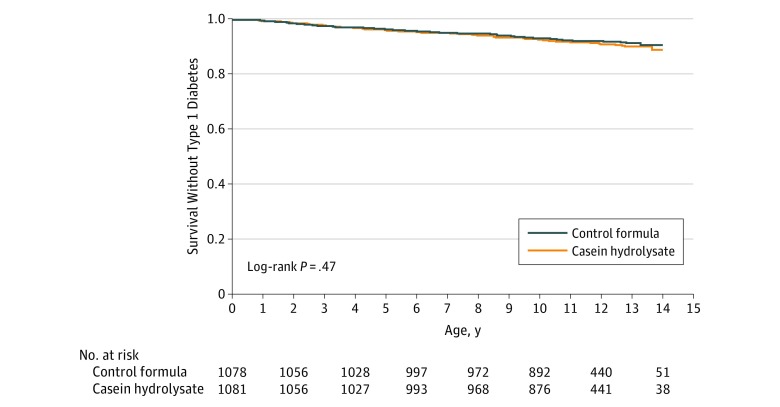
Diabetes
During follow-up, diabetes developed in 91 children in the casein hydrolysate group (8.4%) and in 82 in the control group (7.6%) (difference, 0.8% [95% CI, −1.6% to 3.2%]; P = .47; Figure 2). The hazard ratio for type 1 diabetes adjusted for HLA risk group, duration of breastfeeding, duration of study formula consumption, sex, and region, while treating study center as a random effect, was 1.1 (95% CI, 0.8-1.5; P = .46). There was no significant difference in the median age at diagnosis between the 2 groups (6.0 years [Q1-Q3, 3.1-8.9] vs 5.8 years [Q1-Q3, 2.6-9.1]; P = .75; difference, 0.2 years [95% CI, −0.9 to 1.2]). About one-fourth of the cases in each group were diagnosed without clinical symptoms (Table 2). Five children (5.5%) in the casein hydrolysate group and 3 (3.7%) in the control group presented with diabetic ketoacidosis (difference, 1.8% [95% CI, −6.3% to 9.8%]; P = .57). Comparisons between the treatment groups within HLA risk groups, according to the relationship to the affected family member (father, mother, or sibling with diabetes), geographic region associated with the clinical site of enrollment, or sex were not statistically significant (Table 3).
Figure 2. Cumulative Survival Without Type 1 Diabetes.
The median follow-up time was 11.5 years (quartile [Q] 1-Q3, 10.1-12.8 years) in the casein hydrolysate group and 11.4 years (Q1-Q3, 10.2-12.8 years) in the control group.
Table 2. Characteristics of the Participants Who Progressed to Type 1 Diabetes.
| Characteristic | Casein Hydrolysate (n = 91a) |
Control Formula (n = 82a) |
Between-Group Difference (95% CI) | P Value |
|---|---|---|---|---|
| Male, No. (%) | 49 (53.8) | 33 (40.2) | 13.6 (−2.1 to 28.4) | .07 |
| Age at diagnosis, median (Q1-Q3), y | 6.0 (3.1-8.9) | 5.8 (2.6-9.1) | 0.2 (−0.9 to 1.2) | .75 |
| Maximum No. of autoantibodies before diagnosis, median (Q1-Q3) | 4 (3-4) | 4 (3-4) | 0 (−0.3 to 0.3) | .55 |
| Participants with detectable autoantibodies before diagnosis, No. (%) | ||||
| 1 Autoantibody | 1 (1.1) | 2 (2.4) | 1.3 (−4.7 to 8.3) | .56 |
| 2 Autoantibodies | 7 (7.7) | 4 (4.9) | 2.8 (−6.1 to 11.5) | |
| 3 Autoantibodies | 29 (31.9) | 20 (24.4) | 7.5 (−6.8 to 21.2) | |
| 4 Autoantibodies | 52 (57.1) | 52 (63.4) | 6.3 (−9.0 to 21.1) | |
| Clinical symptoms at diagnosis, No. (%) | 70 (76.9) | 61 (74.4) | 2.5 (−10.9 to 16.1) | |
| Diabetic ketoacidosis at diagnosis, No. (%) | 5 (5.5) | 3 (3.7) | 1.8 (−6.3 to 9.8) | .57 |
| Hemoglobin A1c at diagnosis, median (Q1-Q3) | ||||
| % (DCCT unit) | 7.9 (6.5-9.4) | 8.1 (7.0-9.3) | 0.2 (−0.5 to 0.7) | .77 |
| No. of infants | 80 | 72 | ||
| mmol/mol | 62.4 (47.8-79.1) | 65.0 (53.0-78.1) | 2.6 (−5.3 to 7.7) | .80 |
| No. of infants | 80 | 72 | ||
| Family history of type 1 diabetes, No. (%) | ||||
| Mother | 30 (33.0) | 29 (35.4) | 2.4 (−12.3 to 17.1) | .54 |
| Father | 32 (35.2) | 28 (34.2) | 1.0 (−13.8 to 15.6) | |
| Sibling | 18 (19.8) | 20 (24.4) | 4.6 (−8.5 to 17.8) | |
| >1 Sibling | 11 (12.1) | 5 (6.1) | 6.0 (−3.9 to 15.7) | |
| Breastfeeding >6 mo, No. (%) | 60 (65.9) | 48 (58.5) | 7.4 (−7.7 to 22.2) | .32 |
| No breastfeeding, No. (%) | 4 (4.4) | 4 (4.9) | 0.5 (−7.4 to 8.9) | .88 |
Abbreviation: Q, quartile.
Sample sizes are reported when they differ from the overall sample sizes.
Table 3. Development of Type 1 Diabetes: Unadjusted Treatment Effect in Subgroups.
| Subgroup | Casein Hydrolysate | Control Formula | Hazard Ratio (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| Total No. | No. With Diabetes | No. Without Diabetes at End of Studya | Total No. | No. With Diabetes | No. Without Diabetes at End of Studya | ||
| Overall | 1081 | 91 | 960 | 1078 | 82 | 968 | 1.116 (0.828-1.504) |
| HLA risk group | |||||||
| 1 | 260 | 37 | 212 | 256 | 36 | 213 | 1.019 (0.644-1.612) |
| 2 | 478 | 39 | 433 | 475 | 25 | 438 | 1.549 (0.938-2.560) |
| 3 | 332 | 15 | 305 | 336 | 19 | 309 | 0.797 (0.405-1.570) |
| 4 | 11 | 0 | 10 | 11 | 2 | 8 | 0 |
| Proband | |||||||
| Sibling only | 151 | 18 | 130 | 157 | 20 | 135 | 0.934 (0.493-1.769) |
| Parent only | 901 | 66 | 808 | 902 | 59 | 817 | 1.134 (0.798-1.611) |
| Sibling and parent | 27 | 6 | 21 | 17 | 3 | 14 | 1.297 (0.324-5.187) |
| Other | 2 | 1 | 1 | 2 | 0 | 2 | |
| Region | |||||||
| Finland | 212 | 20 | 191 | 212 | 14 | 198 | 1.421 (0.718-2.814) |
| United States | 199 | 14 | 176 | 196 | 18 | 168 | 0.720 (0.358-1.449) |
| Canada | 265 | 20 | 233 | 263 | 26 | 229 | 0.763 (0.426-1.367) |
| Other | 405 | 37 | 360 | 407 | 24 | 373 | 1.610 (0.963-2.691) |
| Male | 576 | 42 | 520 | 562 | 49 | 502 | 0.820 (0.543-1.238) |
| Female | 505 | 49 | 440 | 516 | 33 | 466 | 1.575 (1.013-2.448) |
Abbreviation: HLA, human leukocyte antigen.
Does not include 58 participants (30 in the casein hydrolysate group and 28 in the control group) who died or became lost to follow-up prior to the end of the study.
The prespecified per-protocol analysis was defined to include those who were not exposed to any nonallowed foods containing cow’s milk and had exposure to study formula for at least 60 days. The hazard ratio for type 1 diabetes in this subpopulation (n = 1177), adjusted for HLA risk group, duration of breastfeeding, duration of study formula consumption, sex, and region, while treating study center as a random effect, was 1.1 (95% CI, 0.7-1.7; P = .63).
As noted previously, the 2 treatment groups did not differ according to the characteristics of participants who developed diabetes (Table 2) or when analyzed within prespecified subgroups (Table 3). As a post-hoc analysis, the hazard ratio of the treatment groups was estimated after adjusting for the age at which multiple autoantibodies appeared (median, 3.2 years [Q1-Q3, 1.6-6.3] in the casein hydrolysate group and 3.0 years [Q1-Q3, 1.5-6.1] in the control group; P = .42) with little effect on the overall results (hazard ratio, 0.95 [95% CI, 0.70-1.28]; P = .95).
Adverse Events
The frequency of any infection was 0.90 events/year in the hydrolysate group and 0.93 events/year in the control group. The corresponding frequencies of upper respiratory infections were 0.48 and 0.50, respectively. The rate of other adverse events was of the same magnitude in the 2 groups (eTable in Supplement 2). Similar linear growth and weight gain were observed in both groups.
Discussion
In this international randomized trial in children with an HLA genotype conferring increased risk for type 1 diabetes and an affected first-degree relative, weaning to a highly hydrolyzed formula during infancy did not reduce the incidence of type 1 diabetes compared with cow’s milk–based formula. This outcome is consistent with the report of this trial that showed no difference between the study groups in the appearance of islet autoantibodies, but is not consistent with data from the pilot study, which reported that weaning to an extensively hydrolyzed formula in infancy was associated with a decrease in the frequency of disease-associated autoantibodies by the age of 7.5 years. That study was conducted in 230 Finnish children, while the current trial included 2159 high-risk children from 15 different countries, most participants being from Canada, Finland, and the United States. The larger number of participants in this study provides substantially greater statistical power in a more heterogeneous study population compared with the pilot study and, therefore, provides a more definitive answer to whether weaning to an extensively hydrolyzed formula is protective of diabetes.
Overall, 173 participants (8.0%) progressed to type 1 diabetes during the follow-up for 11.5 years. This is close to an expected rate of 7.5% by the age of 10 years in the control group, on which the sample size estimate was based. For unknown reasons, the rate of diabetes was higher, although not significantly so, among females compared with males in the casein hydrolysate group. About 49% of the participants had a mother affected by type 1 diabetes, while only around 35% of those who presented with clinical disease had an affected mother. This reflects the well-known fact that offspring of mothers with type 1 diabetes have a reduced disease risk compared with offspring of affected fathers.
Additional strengths of the current trial include a very high retention rate of participants and dietary adherence. The fact that the study was performed in 15 countries on 3 continents also supports the generalizability of the results. This study was planned to have 2 end points, namely (1) positivity for 2 autoantibodies by the age of 6 years and (2) clinical diabetes by the age of 10 years. While the previous report of this study showed no benefit in terms of a reduction in seroconversion to autoantibody positivity, the follow-up of the trial participants to 10 to 14 years of age enabled the study to evaluate the possible effect of the treatment on progression from autoimmunity to diabetes.
The study was not designed to test the effect of breastfeeding because random assignment of infants to breastfeeding or formula feeding was not considered ethical. However, no effect of exclusive breastfeeding was seen on progression to seroconversion or diabetes. Some prospective studies assessing the associations between infant feeding patterns and the development of β-cell autoimmunity in children who are at genetic risk for type 1 diabetes have not observed any associations between the duration of either exclusive or total breastfeeding and β-cell autoimmunity. However, a recently published study involving children from the general population showed that no breastfeeding was related to an increased risk of diabetes compared with infants with history of any breastfeeding.
The casein-based formula used as the intervention modality in this study was highly hydrolyzed and did not contain intact proteins. Less than 0.3% of the peptides had a molecular weight exceeding 2000 Da. Accordingly, the formula should be free of intact bovine insulin, which is present in cow’s milk. Vaarala et al showed that infants fed a conventional cow’s milk–based formula before the age of 3 months developed a strong immune response to bovine insulin, which differs from human insulin by 3 amino acids. Infants developing early signs of β-cell autoimmunity lacked the capacity to mount oral tolerance to bovine insulin. It has been speculated that sustained bovine insulin immunity might contribute to prediabetes progression, as weaning to an insulin-free formula reduced the cumulative incidence of autoantibodies by more than half in young children at genetic risk for type 1 diabetes. The current data do not, however, support the bovine insulin hypothesis.
To our knowledge, this is the first trial to test with adequate power whether eliminating exposure to foreign intact protein in the infant diet could prevent type 1 diabetes in a genetically high-risk population. This trial suggests that cow’s milk does not play a critical role in the development of type 1 diabetes.
Limitations
The results of this study are not directly generalizable to the background population because participants were selected based on a positive family history for type 1 diabetes and an HLA genotype conferring risk for type 1 diabetes. In addition, the outcome is not necessarily applicable to children with other HLA genotypes.
Conclusions
Among infants at risk for type 1 diabetes, weaning to a hydrolyzed formula compared with a conventional formula did not reduce the cumulative incidence of type 1 diabetes after a median follow-up for 11.5 years. These findings do not support a need to revise the current dietary recommendations for infants at increased risk for type 1 diabetes.
Trial Protocol
eTable. Reported Adverse Events in the Two Arms of the TRIGR Study.
References
- 1.Knip M. Can we predict type 1 diabetes in the general population? Diabetes Care. 2002;25(3):623-625. [DOI] [PubMed] [Google Scholar]
- 2.Knip M, Siljander H, Ilonen J, Simell O, Veijola R. Role of humoral beta-cell autoimmunity in type 1 diabetes. Pediatr Diabetes. 2016;17(suppl 22):17-24. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler A-G, Rewers M, Simell O, et al. . Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libman IM, LaPorte RE. Changing trends in epidemiology of type 1 diabetes mellitus throughout the world: how far have we come and where do we go from here. Pediatr Diabetes. 2005;6(3):119-121. [DOI] [PubMed] [Google Scholar]
- 5.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G; EURODIAB Study Group . Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027-2033. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler A-G, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48(3):460-468. [DOI] [PubMed] [Google Scholar]
- 7.Kimpimäki T, Kupila A, Hämäläinen A-M, et al. . The first signs of β-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. J Clin Endocrinol Metab. 2001;86(10):4782-4788. [DOI] [PubMed] [Google Scholar]
- 8.Ilonen J, Kiviniemi M, Lempainen J, et al. ; Finnish Pediatric Diabetes Register . Genetic susceptibility to type 1 diabetes in childhood: estimation of HLA class II associated disease risk and class II effect in various phases of islet autoimmunity. Pediatr Diabetes. 2016;17(suppl 22):8-16. [DOI] [PubMed] [Google Scholar]
- 9.Kostic AD, Gevers D, Siljander H, et al. ; DIABIMMUNE Study Group . The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17(2):260-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virtanen SM, Räsänen L, Aro A, et al. ; Childhood Diabetes in Finland Study Group . Infant feeding in Finnish children less than 7 yr of age with newly diagnosed IDDM. Diabetes Care. 1991;14(5):415-417. [DOI] [PubMed] [Google Scholar]
- 11.Norris JM, Barriga K, Klingensmith G, et al. . Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290(13):1713-1720. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler A-G, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290(13):1721-1728. [DOI] [PubMed] [Google Scholar]
- 13.Virtanen SM, Kenward MG, Erkkola M, et al. . Age at introduction of new foods and advanced beta cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes. Diabetologia. 2006;49(7):1512-1521. [DOI] [PubMed] [Google Scholar]
- 14.Knip M, Virtanen SM, Åkerblom HK. Infant feeding and the risk of type 1 diabetes. Am J Clin Nutr. 2010;91(5)(suppl):1506S-1513S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knip M, Åkerblom HK, Becker D, et al. ; TRIGR Study Group . Hydrolyzed infant formula and early β-cell autoimmunity: a randomized clinical trial. JAMA. 2014;311(22):2279-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Åkerblom HK, Krischer J, Virtanen SM, et al. ; TRIGR Study Group . The Trial to Reduce IDDM in the Genetically at Risk (TRIGR) Study: recruitment, intervention and follow-up. Diabetologia. 2011;54(3):627-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorkio S, Cuthbertson D, Bärlund S, et al. ; TRIGR Study Group . Breastfeeding patterns of mothers with type 1 diabetes: results from an infant feeding trial. Diabetes Metab Res Rev. 2010;26(3):206-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parkkola A, Härkönen T, Ryhänen SJ, Ilonen J, Knip M; Finnish Pediatric Diabetes Register . Extended family history of type 1 diabetes and phenotype and genotype of newly diagnosed children. Diabetes Care. 2013;36(2):348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications, part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539-553. [DOI] [PubMed] [Google Scholar]
- 20.Goggins WB, Finkelstein DM. A proportional hazards model for multivariate interval-censored failure time data. Biometrics. 2000;56(3):940-943. [DOI] [PubMed] [Google Scholar]
- 21.Lan KK, DeMets DL. Changing frequency of interim analysis in sequential monitoring. Biometrics. 1989;45(3):1017-1020. [PubMed] [Google Scholar]
- 22.Knip M, Virtanen SM, Seppä K, et al. ; Finnish TRIGR Study Group . Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010;363(20):1900-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warram JH, Krolewski AS, Gottlieb MS, Kahn CR. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med. 1984;311(3):149-152. [DOI] [PubMed] [Google Scholar]
- 24.Familial risk of type I diabetes in European children: the Eurodiab Ace Study Group and the Eurodiab Ace Substudy 2 Study Group. Diabetologia. 1998;41(10):1151-1156. [DOI] [PubMed] [Google Scholar]
- 25.Lund-Blix NA, Dydensborg Sander S, Størdal K, et al. . Infant feeding and risk of type 1 diabetes in two large Scandinavian birth cohorts. Diabetes Care. 2017;40(7):920-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaarala O, Knip M, Paronen J, et al. . Cow’s milk formula feeding induces primary immunization to insulin in infants at genetic risk for type 1 diabetes. Diabetes. 1999;48(7):1389-1394. [DOI] [PubMed] [Google Scholar]
- 27.Vaarala O, Ilonen J, Ruohtula T, et al. . Removal of bovine insulin from cow’s milk formula and early initiation of beta-cell autoimmunity in the FINDIA pilot study. Arch Pediatr Adolesc Med. 2012;166(7):608-614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Reported Adverse Events in the Two Arms of the TRIGR Study.