Abstract
Purpose
To assess the impact of hypoxia exposure on cellular radiation sensitivity and survival of tumor cells with diverse intrinsic radiation sensitivity under normoxic conditions.
Materials and methods
Three squamous cell carcinoma (SCC) cell lines, with pronounced differences in radiation sensitivity, were exposed to hypoxia prior, during or post irradiation. Cells were seeded in parallel for colony formation assay (CFA) and stained for γH2AX foci or processed for western blot analysis.
Results
Hypoxia during irradiation led to increased cellular survival and reduced amount of residual γH2AX foci in all the cell lines with similar oxygen enhancement ratios (OER SKX: 2.31, FaDu: 2.44, UT-SCC5: 2.32), while post-irradiation hypoxia did not alter CFA nor residual γH2AX foci. Interestingly, prolonged exposure to hypoxia prior to irradiation resulted in differential outcome, assessed as Hypoxia modifying factor (HMF) namely radiosensitization (SKX HMF: 0.76), radioresistance (FaDu HMF: 1.54) and no effect (UT SCC-5 HMF: 1.1). Notably, radiosensitization was observed in the ATM-deficient SKX cell line while UT SCC-5 and to a lesser extent also FaDu cells showed radiation- and hypoxia-induced upregulation of ATM phosphorylation. Across all the cell lines Rad51 was downregulated whereas phosphor-DNA-PKcs was enhanced under hypoxia for FaDu and UTSCC-5 and was delayed in the SKX cell line.
Conclusion
We herein report a key role of ATM in the cellular fitness of cells exposed to prolonged moderate hypoxia prior to irradiation. While DNA damage response post-irradiation seem to be mainly driven by non-homologous end joining repair pathway in these conditions, our data suggest an important role for ATM kinase in hypoxia-driven modification of radiation response.
Keywords: Cellular survival, Intrinsic radiation sensitivity, Hypoxia, γH2AX foci
Introduction
Tumor hypoxia is a well-established determinant of poor outcome especially after radiation therapy [1], [2] and has been intensively studied for example in head and neck squamous cell carcinoma (HNSCC) [2], [3], [4], [5]. It has been demonstrated that hypoxia induces resistance to radiation therapy through different mechanisms. Under hypoxic conditions less DNA damage including the most critical form, i.e. DNA double strand breaks (DSB), is induced [6]. Unrepaired DSBs severely compromise survival [7]. Key feature of the cellular response to radiation-induced damage, the so-called DNA Damage Response (DDR), is the activation of ATM, DNA-PKs and ATR kinases which mediate the interaction of DNA repair and checkpoint partners of DDR [8], [9], [10]. Crucial for the localization and amplification of the damage signal on the chromatin level is the phosphorylation of the histone H2AX which is potentiated by all the main three kinases of the DDR and can be targeted with phosphor-specific antibodies giving rise to nuclear substructures termed γH2AX foci [11], [12], [13], [14]. Due to the one – to – one correlation with the induced DSBs [12], [15] and the fact that once the break is repaired they are not detectable due to their de-phosphorylation [16], [17], [18], their utilization as markers of radiation-induced DSBs in translational and basic research has been rapidly increased [19], [20], [21], [22].
Apart from DNA damage induction other mechanisms of hypoxia-related treatment resistance have been implicated. For example, hypoxia promotes genetic instability and the acquisition of a more resistant phenotype through downregulation of homologous recombination (HR) DNA repair pathway [23], [24], [25]. Furthermore, several key regulators of DDR are subject to oxygen regulation including chromatin remodeling complexes [26], [27] and main kinases [28], [29]. Additional evidence comes from the fact that hypoxia has been found to be a strong inducer of epigenetic changes in the tumor cells [30], [31], [32], [33].
Previously, in two HNSCC tumor models grown as xenografts, we observed that the slope of the residual γH2AX foci dose response 24 h post-irradiation was two times higher in the radiosensitive model when the foci were counted in the well-oxygenated tumor cells. However, no difference could be observed between the two models when hypoxic tumor cells were considered [34]. The latter suggests that inter-tumoral heterogeneity in cellular radiosensitivity might be less pronounced under hypoxia than under normoxic conditions. However, an important caveat of this in vivo study represents the methodological limitation to control the oxygenation status in terms of extent, time and duration of hypoxia. Although the fact that the cells were hypoxic at the time of irradiation was assessed the impact of prolonged hypoxia exposure prior to irradiation was not feasible to be addressed. Therefore, it is difficult to conclude if the observed differences between oxic and hypoxic conditions arise from the lower induction of damage solely in each tumor model. Following these observations, we hypothesized that the duration and timing of hypoxia exposure has an impact on radiation response in a cell-line specific manner. To address this hypothesis, we have selected three different HNSCC cell lines, including the two of the former study, with pronounced differences in intrinsic radiosensitivity and applied hypoxia in different time-frames prior, post and/or during irradiation.
Materials and methods
Cell lines and cell culture conditions
All three HNSCC cell lines have been previously described namely SKX, FaDu and UT SCC-5, both under in vitro and under in vivo conditions [34], [35], [36], [37], [38]. SKX exhibits high radiosensitivity due to functional inactivation of ATM [38], [39], FaDu shows moderate radiosensitivity [37] and UT SCC-5 cell line is characterized by high radioresistance [37]. During cultivation cells were kept in standard Dulbecco’s Minimum Essential Medium (DMEM) supplemented with 10% Fetal Calf Serum (FCS), 1% antibiotics (Penicillin/Streptomycin), 2% Hepes Buffer, 1% Sodium Pyruvate and 1% Non-Essential Amino Acids (all Biochrom GmbH, Germany) in 37 °C under humidified atmosphere. All cells were routinely checked for contamination with Mycoplasma. For the cause of the experiments, exponentially growing cells were seeded (5 × 105 cells; 20,000 cells/cm2) either in 25 cm2 cell culture flasks (Colony Formation Assay (CFA) and γH2AX assay) or in cell culture dishes (diameter: 6 cm, western blot analysis) until reaching 95% confluency (Table 1). At least 3 independent experiments were performed for CFA and at least 2 independent experiments were performed for γH2AX assay and Western Blot analysis.
Table 1.
Table showing the experimental settings and oxygen concentrations used for every condition tested.
| Oxygen concentration |
Experimental condition abbreviation | ||
|---|---|---|---|
| Growth period (4–5 days, Confluence level: 100000 cells/cm2) | Irradiation | Post irradiation (24 h) | |
| 21% | 21% | 21% | O–O–O |
| 21% | 0,1% | 21% | O–H–O |
| 21% | 21% | 1% | O–O–H |
| 21% | 0,1% | 1% | O–H–H |
| 1% | 21% | 21% | H–O–O |
| 1% | 21% | 1% | H–O–H |
| 1% | 0,1% | 21% | H–H–O |
| 1% | 0,1% | 1% | H–H–H |
Hypoxia and irradiation experiments
Cells were exposed to normoxia or moderate hypoxia within three distinct time frames according to the experimental setting applied as shown in Table 1. During growth phase, in the pre-irradiation period or post-irradiation treatment, cells were kept either under 21% or 1% oxygen (hypoxia incubator, Binder GmbH, Germany) for 4–5 days according to cell line until reaching confluency or for 24 h post-irradiation accordingly. During irradiation under hypoxic conditions, cells were transported and kept under severe hypoxia 0.1% O2 atmosphere, with the use of Gas Pouch system (BD GasPakTM-EZ-, Becton, Dickinson and Company, USA. Irradiation was performed as single doses (0, 1, 2, 4, 6, 8 Gy) (200 kV, 15 mA X-Rays; dose-rate: 0,91 Gy/min; RS 225 research system). For seeding in parallel of CFA and γH2AX assays, single cell suspension was prepared from the same flask and subsequently splitted for either seeding CFAs or used for cytospin (Shandon CytoSpin III Zytozentrifuge, Thermo Fisher Scientific Inc.) Preparation and immunofluorescent staining for γH2AX was performed as described previously [36]. For evaluating the magnitude of the hypoxic effect the OER based on the surviving fraction (SF) equal to 0.1 were calculated. In the case that irradiation was not performed during hypoxia treatment, the Hypoxia modification factor (HMF) was similarly calculated for the level of SF = 0.1.
Immunofluorescence and Image analysis
For the γH2AX assay, cells were centrifuged on glass slides using cytospin procedure (200 rpm, 2 min) and fixed in 4% formaldehyde (15 min). Subsequently, after cell membrane permeabilization step (Triton X100, 0.01% in PBS) and blocking with bovine serum albumin (BSA) (1% in PBS, 30 min, RT), application of the primary antibody for 1 h in 37 °C took place (1:1000 in BSA, 1 h) (anti-Histone γH2AX (phospho Ser139), clone JBW301, Merck Millipore, Upstate, Darmstadt, Germany). Alexa 488 fluorescent probe (1:400 in BSA, 45 min, RT) (Alexa flour 488 tyramid, Life technologies (Invitrogen)) was used as secondary antibody. Diamidino-2-phenylindole dihydrochloride (DAPI) (1:1000 in PBS, 10 min, RT) (Sigma Aldrich Co., USA) was used as a nucleus counterstaining and slides were mounted with fluorescent mounting medium (Dako Deutschland GmbH, Germany) to be ready for microscope observation.
For evaluation of γH2AX foci, fluorescent images were acquired under 400 magnification using an Axio Imager Z1 Apotome fluorescence microscope (Carl Zeiss Microscopy GmbH, Germany; monochrome digital camera (AxioCamMRm), Carl Zeiss Microscopy GmbH, Germany; motorized scanning stage, Märzhäuser, Wetzlar, Germany, EC Plan Neofluar). Images were taken in 0.5 µm planes along Z-axis and foci analysis was performed in maximal intensity projection Z-stacks. Evaluation of foci was performed manually, blinded using AxioVision software (Axio Imager Z1 Apotome fluorescence microscope) (LE 64, SP1, version: 4.9.1.0. for Windows) and was co-checked by two observers (FH, AM). Based on DAPI staining only cells with intact nuclei were evaluated and at least 150 cells per dose-group were randomly selected and number of foci were recorded.
Western blot
Total protein lysate was isolated as described before [40]. Following protein quantification using the Bio-RAD DC protein assay, samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE), and assessment of specific proteins was performed by Western blot analysis using specific antibodies. The following primary antibodies were used for analysis: anti-ATM (phospho Ser 1981), clone D6H9, anti-ATM, clone D2E2, (both cell signaling Technology, Inc., Danvers, MA, USA, dilution: 1: 1000, BSA); anti-actin, clone 103M4826V, Sigma Aldrich Co., St. Luis, USA (1: 2000, BSA); anti-DNA PK (phospho S2056), anti-DNA PK, clone 18–2, anti-Rad51, all abcam, Cambridge, UK (dilutions: 1: 1000, BSA, 1:500, milk, 1: 500, BSA respectively). Secondary antibodies used for analysis: Amersham ECL rabbit IgG, HRP linked whole Ab, clone NA934V; secondary AB anti-mouse, clone NAP31V, both GE Healthcare Life Science, USA dilutions: 1: 2000, BSA. Chemiluminescence was detected using Odyssey Fc imaging system (Li-cor Bioscience, USA) and digital images were evaluated with Image Studio Software (version: 4.0.21, Li-COR Bioscience GmbH, Lincoln, Nebrascka, USA).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 software (version: 5.03, GraphPad Software Inc., California, USA). Surviving fraction was estimated according to linear-quadratic model equation. Based on the equations:
| (1) |
and
| (2) |
The potential correlation between number of residual foci (γH2AX assay) and irradiation dose and the correlation between −lnSF(CFA) and mean number of residual foci (γH2AX assay) have been examined by fitting to linear regression model. For multiple comparisons of data obtained by CFA and by γH2AX assay one way analysis of variance (ANOVA) with Bonferroni correction was used and p-values below 0.05 were considered statistically significant.
We have estimated the mean number of lethal lesions expected by Poisson model per dose. From the linear regression analysis of −lnSF(CFA) with the mean number of residual foci the SF(γH2AX) per dose was recalculated for every condition based on the following equation:
| (3) |
where a is the intercept and b the slope of the linear regression, Mnfoci is the mean number of residual foci per dose level [36].
Results
Correlation between CFA and residual γH2AX foci data
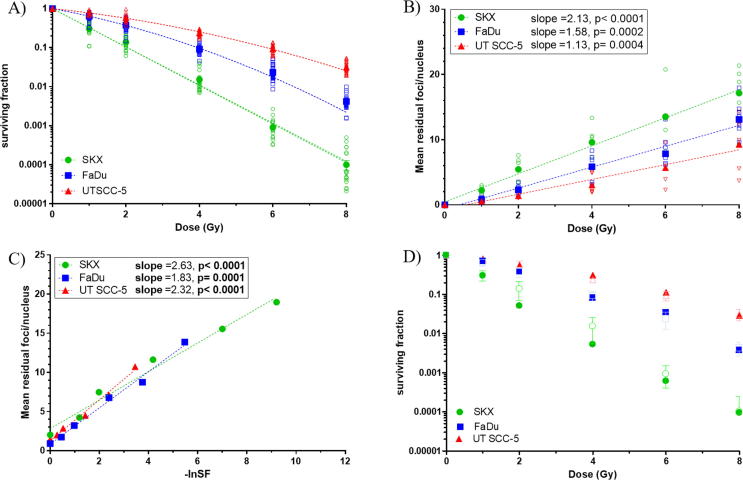
Under normoxic conditions (experimental group O–O–O) SKX, FaDu and UT SCC-5 showed clear-cut differences in clonogenic survival as well as in the corresponding slopes of residual γH2AX foci (Fig. 1, Supp. Table 1). The mean values of residual γH2AX foci plotted against the corresponding values of –ln SF obtained by CFA led to significant linear correlations for all cell lines. The latter, was observed over all conditions and cell lines tested, suggesting a strong correlation between clonogenic survival and number of residual foci not only under normoxic, as formally described, but also under hypoxic conditions (Fig. 1, Supp. Table 1). Cell survival curves derived from the γH2AX data were well in line with the ones from obtained from the CFA data for all cell lines and conditions tested (Fig.1D; Fig. 2; Suppl. Fig. 1).
Fig. 1.
Experimental group O–O–O (normoxia) A) Clonogenic cell survival curves obtained by CFA, B) Residual γH2AX foci dose response, C) mean number of residual γH2AX foci and –lnSF, D) Recalculation of the survival curves based on γH2AX data. Open symbols represent individual experimental values (A-C) or observed CFA points (D). Closed symbols indicate the mean of at least 8 independent experiments for CFA and at least 5 for γH2AX foci assay (A-C) and the calculated surviving fractions (D). Error bars depict the standard error of the mean (A-C) or standard deviation (D).
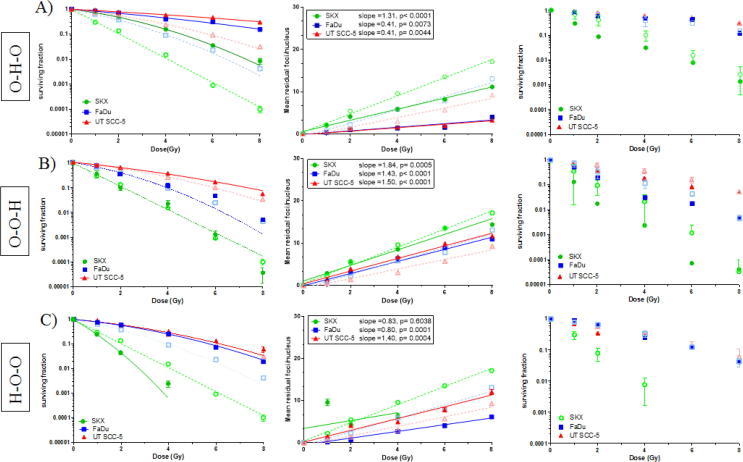
Fig. 2.
Row A) showing experimental group O–H–O (short-term hypoxia during irradiation), Row B) showing experimental group O–O–H (hypoxia after irradiation) and Row C) showing experimental group H–O–O (hypoxia prior to irradiation). For comparison data obtained under normoxic conditions (Fig. 1, O–O–O) are shown as dashed lines and open symbols. First column showing clonogenic cell survival curves obtained by CFA (solid lines and closed symbols), second column exhibits residual γH2AX foci dose response (solid line, closed symbols), and third column showing recalculation of the survival curves based on γH2AX data. The mean of at least 3 independent experiments for CFA and γH2AX foci assay are shown. Error bars depict the standard error of the mean (A-C) or standard deviation (D).
Hypoxia exposure during and after irradiation
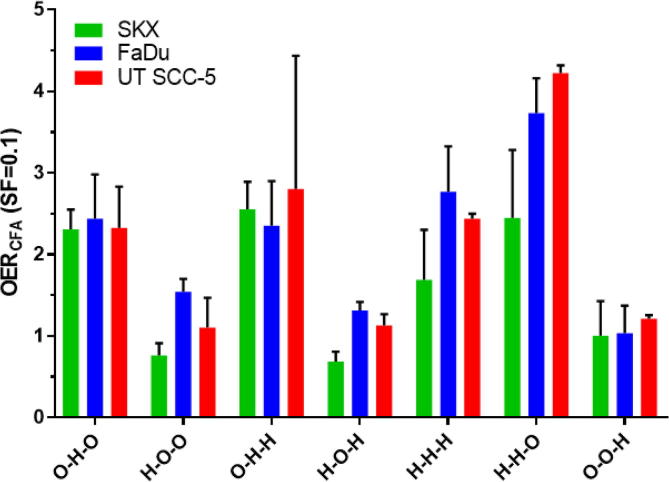
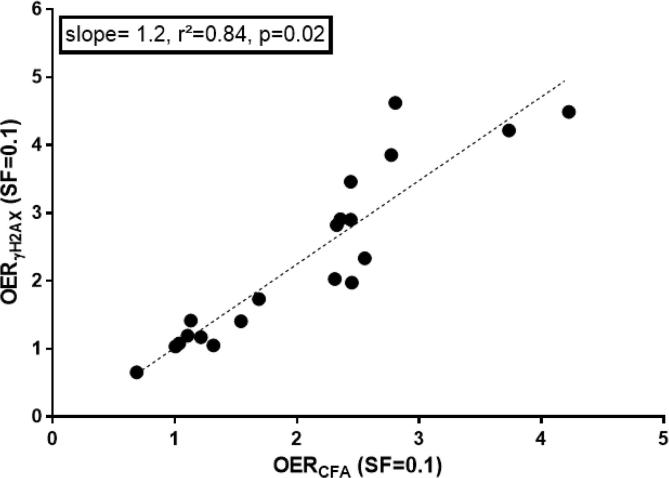
Exposure to hypoxia during irradiation (0.1%, experimental group O–H–O) resulted in higher cell survival and reduced γH2AX slopes (Fig.2A) with similar OER values (mean OER(CFA): 2.36, SD: 0.07) (Fig. 4; Supp. Table 1). As in all experimental groups, OER values calculated from cell survival and γH2AX data were highly concordant (r2 = 0.84, p = 0.02; Fig. 3). In all three cell lines exposure to hypoxia after irradiation (experimental group O–O–H) did change neither cell survival nor the slope of residual γH2AX foci (Fig.2B). This is further supported by corresponding HMF values between 1.0 and 1.2 under this condition (Fig. 4). Furthermore, combination of exposure to hypoxia both at the time and after irradiation (O–H–H) did also not significantly alter cellular survival compared to irradiation under hypoxia alone (Fig. 4; Suppl. Fig. 1B).
Fig. 4.
Bar graph of OER/HMF values calculated for 10% survival based on CFA data. Error bars indicating standard deviation of 3 individual experiments. Dashed line indicating an OER of 1.0, e.g. no disparity between normoxic control and condition tested.
Fig. 3.
Linear regression between OER/HMFs estimated from cell survival curves and γH2AX data at 10% survival.
Hypoxia exposure prior to irradiation
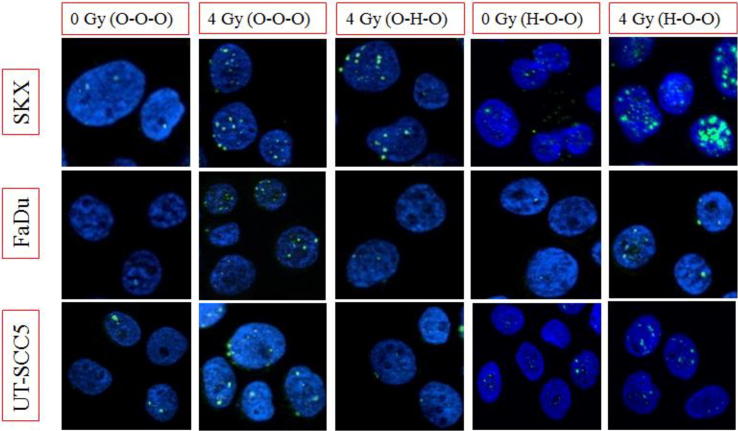
Long-term exposure to moderate hypoxia (1%) for four to five days prior to irradiation (Table 1, H–O–O) led to cell-line specific changes in radiosensitivity (Fig.2C). In SKX a remarkable radiosensitization was observed (HMF = 0,76) whereas FaDu showed radioresistance (HMF = 1,54) and no changes were detected in UT SCC-5 (HMF = 1,10) (Fig. 4, Supp. Table 1). Due to the pronounced increase of background γH2AX foci observed in SKX (Fig. 5) no data could be obtained under these conditions for irradiation doses higher than 4 Gy. Furthermore, it was not possible to perform the recalculation of the survival curve under this condition for SKX cell line, presumably due to lack of sufficient data to estimate the correlation between mean number of residual γH2AX foci and –lnSF. As in the experimental group H–O–O, similar observations were made in the other experimental groups when the cells were exposed to hypoxia prior to irradiation (experimental groups H–O–H, H–H–O, H–H–H; Fig. 4; Suppl. Fig. 1A, C, D; Supp. Table 1). The differential effect of pre-irradiation exposure to hypoxia on the three cell lines is depicted by OER/HMF values in Fig. 4. Notably, we consistently observed the lowest HMF in SKX and the highest in FaDu in the conditions were pre-irradiation hypoxia was included in the experimental plan. The only exception being the pronounced effect on the cellular survival of UTSCC-5 observed when hypoxia was present before and during irradiation with OER value of 4.2 (Fig.4; Suppl. Fig. 1C; Suppl. Table 1). In this condition, the β component of the linear quadratic model was substantially low, leading to the estimation of a hypothetical value for the SF(0.1) based on the curve fitting due to lack of an actual experimental point.
Fig. 5.
Representative images of γH2AX foci staining under different experimental conditions. For every cell line (row) and for different experimental conditions (columns) the nuclei are stained with dapi (blue) and the sites of phosphorylated histone H2AX is depicted as green spots. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Western blot analysis of DNA repair enzymes
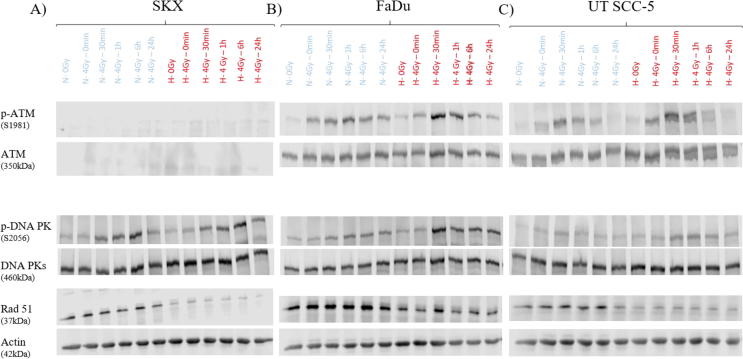
To elucidate the differential outcome of the three cell lines when prolonged hypoxia precedes irradiation we studied the kinetics of main DNA repair enzymes. Western blot analysis indicated that in all cell lines the homologous recombination pathways was downregulated as depicted by the levels of RAD51 protein (Fig. 6, Suppl. Fig. 2). For UT SCC-5 cell line and to lesser extent also for FaDu cells we observed a hypoxia-mediated, radiation-induced upregulation of p-ATM (S1981). DNA-PKcs phosphorylation at S2056, a mark of active non-homologous end joining pathway, was upregulated in response to irradiation and even more pronounced after exposure to hypoxia in both cell lines (H–O–O) (Fig. 6, Suppl. Fig. 2). Importantly, in the ATM-deficient SKX, ATM protein was not detected and a delayed phosphorylation of DNA-PKs was observed (Fig. 6, Suppl. Fig. 2).
Fig. 6.
Western blot analysis of DDR proteins under normoxia (N) or pre-irradiation long term hypoxia (H). Lysates were extracted at the different time points after irradiation. β-Actin and the un-phosphorylated form of the respective proteins served as loading controls.
Discussion
Based on previous data [34] the present in vitro study was conducted to address the hypothesis that functional differences in radiation sensitivity are less pronounced under hypoxic conditions and are depending on the duration that malignant cells are exposed to hypoxia prior to irradiation. For this, three HNSCC cell lines with known differences in intrinsic cellular radiosensitivity were selected (including the two used in the previous study), namely SKX (sensitive), FaDu (moderate), UT SCC-5 (radioresistant). We have applied hypoxia in three different time frames to unravel the potential impact of time-dependent relevance of hypoxia exposure to radiation sensitivity. We selected a moderate level of hypoxia (1%), previously shown to evoke accumulation of exogenous hypoxia marker (pimonidazole) without compromising cellular integrity [34]. Our endpoint was cellular survival and the correlation with residual γH2AX foci as marker of intrinsic radiation sensitivity. In parallel, we investigated the expression profile of DNA damage response enzymes. To avoid the previously reported cell-cycle dependent and damage-independent expression of γH2AX we used confluent cultures [41]. Based on the Poisson statistics implying that cellular survival post-irradiation is the probability of a cell to have zero lethal lesions, we observed strong linear correlation of residual γH2AX foci with –lnSFCFA for all cell lines and conditions tested. We were able to reproduce the cell survival curves as previously reported [36] and estimated OERs based on the γH2AX data, reported, to our knowledge, here for the first time (Fig 1,2,4; Suppl. Fig. 1; Suppl. Table 1). These strongly suggest the notion that residual γH2AX foci represent good indication of lethal radiation-induced DNA lesions and that they can be used as potential markers of intrinsic radiation sensitivity [20], [21], [22].
Our findings indicate that post-irradiation hypoxia does not affect cellular outcome. The latter is consistent with previous observations where the kinetics of DNA repair have been evaluated in parallel in normoxic and hypoxic cells and despite the initial difference in the induced-damage, no difference in the kinetics of γH2AX foci disappearance was observed neither in vitro [42] nor in vivo [34]. Short-term hypoxia during the time of irradiation increased cellular survival and led to reduced amount of residual γH2AX foci in all cell lines. This observation corresponds well with the concept of reduced induction of DNA damage in the absence of oxygen at the time of irradiation [24], [43]. However, we observed a rather homogeneous increase in cellular survival as depicted by the OER values (Fig. 4) implying that the magnitude of the oxygen effect is independent from the intrinsic radiation sensitivity, thus contradicting our initial hypothesis that the magnitude of hypoxia effect during irradiation is variable among different cells lines. The apparent discrepancy between the in vitro and in vivo findings may be explained by the methodological limitations under in vivo situations with temporal and spatial heterogeneity in oxygenation [34], [44] further supporting the notion that the duration of hypoxia prior to irradiation is responsible for the observed differences.
Interestingly, long term exposure of cells to moderate hypoxia prior to irradiation yielded pronounced differences in their response to irradiation, implying a cell line-specific hypoxia adaptation effect. This cell-line specific heterogeneity is consistent with previous reports by others [45], [46], [47]. To elucidate potential mechanisms important proteins involved in DDR were investigated. In all cell lines, we observed a strong downregulation of Rad51, i.e. a key protein for the homologous-recombination (HR) repair pathway [9], [23], [24]. Downregulation, or reduced synthesis of HR proteins, including RAD51 protein in response to hypoxia exposure has been previously reported to be independent of cell cycle phases, p53 status and subsequent reoxygenation. Importantly, in these experiments the hypoxia-induced downregulation of HR proteins led to higher radiation sensitivity of tumor cells [25], [48], [49] and also lower OER values when cells carrying mutations in HR-proteins were irradiated under severe hypoxia [50].
Surprisingly, in our experiments we only observed increased radiation sensitivity in the ATM-deficient SKX cell line. One may speculate that in FaDu and UT SCC-5 hypoxia resulted in delayed but increased DNA-PKcs and ATM phosphorylation levels (Suppl. Fig. 2) which may at least in part compensate for the hypoxia-induced downregulation of Rad51. Upregulation of DNA-PKcs has been previously reported to be induced in response to hypoxia in a HIF 1α-dependent way and confer chemoresistance to hypoxic tumor cells [51]. On the other hand, ATM seems to play a very important role in mediating tumor cell response to prolonged hypoxia exposure. ATM has been shown to be activated in response to hypoxia and reoxygenation in tumor cell lines in a HIF 1α -independent way in lymphoblastoid cell lines even in the absence of induced DNA damage [28]. Intact ATM is required for the inhibition of mTORC1 by hypoxia, a process that promotes cellular survival under hypoxia for mouse embryonic fibroblasts [52], [53]. Furthermore, ATM has been shown to induce a CHK2 phosphorylation in response to hypoxia and ATM-knock out cells exhibit a reduced cellular survival in response to hypoxia due to suppression G2-checkpoint arrest [46], [47]. SKX cell line has a functional inactivation of ATM driven by a post-transcription regulation through overexpression of a micro-RNA leading to a profound effect in cellular radiation sensitivity [38], [39]. It comes as no surprise that ATM protein was not detectable in this cell line (Fig. 6). Additionally, this cell line exhibits high numbers of γH2AX foci in unirradiated controls and a delayed DNA-PKcs activation (Fig. 5, Fig. 6; Suppl. Fig. 2). The reasons for this are not fully understood and beyond the scope of the current study. In light of the described role of ATM in mediating radioresistance upon prolonged hypoxia exposure, it is intriguing to propose that ATM depletion could be the potential explanation for the induced radiosensitivity of SKX. Our observations are consistent with previously reported accumulation of γH2AX foci in cells exposed to prolonged severe hypoxia [49] and increased radiosensitivity of ATM-depleted cells upon exposure to prolonged hypoxia [46].
In summary, our study demonstrates in HNSCC a cell-line specific impact on radiation sensitivity of cells exposed to prolonged hypoxia prior to irradiation which may contribute to biological heterogeneity in tumor radiation response. Furthermore, our data suggest an important role for ATM in hypoxia-related modification of radiation response.
Financial support
This project was financially supported by IZKF Promotionskolleg, Medical Faculty, University Tuebingen (Project number E.05.00062).
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ctro.2017.06.001.
Appendix A. Supplementary data
References
- 1.Colliez F., Gallez B., Jordan B.F. Assessing tumor oxygenation for predicting outcome in radiation oncology: a review of studies correlating tumor hypoxic status and outcome in the preclinical and clinical settings. Front Oncol. 2017;7:10. doi: 10.3389/fonc.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horsman M.R., Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J Radiat Res. 2016;57(Suppl. 1) doi: 10.1093/jrr/rrw007. i90-i8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordsmark M., Bentzen S.M., Rudat V., Brizel D., Lartigau E., Stadler P. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 4.Begg A.C. Predicting recurrence after radiotherapy in head and neck cancer. Semin Radiat Oncol. 2012;22:108–118. doi: 10.1016/j.semradonc.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Hill R.P., Bristow R.G., Fyles A., Koritzinsky M., Milosevic M., Wouters B.G. Hypoxia and predicting radiation response. Semin Radiat Oncol. 2015;25:260–272. doi: 10.1016/j.semradonc.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Gray L.H., Conger A.D., Ebert M., Hornsey S., Scott O.C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 7.Dikomey E., Brammer I. Relationship between cellular radiosensitivity and non-repaired double-strand breaks studied for different growth states, dose rates and plating conditions in a normal human fibroblast line. Int J Radiat Biol. 2000;76:773–781. doi: 10.1080/09553000050028922. [DOI] [PubMed] [Google Scholar]
- 8.Harper J.W., Elledge S.J. The DNA damage response: ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Hoeijmakers J.H. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 10.Goodarzi A.A., Noon A.T., Deckbar D., Ziv Y., Shiloh Y., Lobrich M. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell. 2008;31:167–177. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Hunt C.R., Ramnarain D., Horikoshi N., Iyengar P., Pandita R.K., Shay J.W. Histone modifications and DNA double-strand break repair after exposure to ionizing radiations. Radiat Res. 2013;179:383–392. doi: 10.1667/RR3308.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinner A., Wu W., Staudt C., Iliakis G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–5694. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogakou E.P., Pilch D.R., Orr A.H., Ivanova V.S., Bonner W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 14.van Attikum H., Gasser S.M. Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol. 2009;19:207–217. doi: 10.1016/j.tcb.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Sedelnikova O.A., Rogakou E.P., Panyutin I.G., Bonner W.M. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res. 2002;158:486–492. doi: 10.1667/0033-7587(2002)158[0486:qdoiid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Chowdhury D., Keogh M.C., Ishii H., Peterson C.L., Buratowski S., Lieberman J. Gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell. 2005;20:801–809. doi: 10.1016/j.molcel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury D., Xu X., Zhong X., Ahmed F., Zhong J., Liao J. A PP4-phosphatase complex dephosphorylates gamma-H2AX generated during DNA replication. Mol Cell. 2008;31:33–46. doi: 10.1016/j.molcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keogh M.C., Kim J.A., Downey M., Fillingham J., Chowdhury D., Harrison J.C. A phosphatase complex that dephosphorylates gammaH2AX regulates DNA damage checkpoint recovery. Nature. 2006;439:497–501. doi: 10.1038/nature04384. [DOI] [PubMed] [Google Scholar]
- 19.Bonner W.M., Redon C.E., Dickey J.S., Nakamura A.J., Sedelnikova O.A., Solier S. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivashkevich A., Redon C.E., Nakamura A.J., Martin R.F., Martin O.A. Use of the gamma-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 2012;327:123–133. doi: 10.1016/j.canlet.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olive P.L. Retention of gammaH2AX foci as an indication of lethal DNA damage. Radiother Oncol. 2011;101:18–23. doi: 10.1016/j.radonc.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 22.Banath J.P., Klokov D., MacPhail S.H., Banuelos C.A., Olive P.L. Residual gammaH2AX foci as an indication of lethal DNA lesions. BMC Cancer. 2010;10:4. doi: 10.1186/1471-2407-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luoto K.R., Kumareswaran R., Bristow R.G. Tumor hypoxia as a driving force in genetic instability. Genome Integrity. 2013;4:5. doi: 10.1186/2041-9414-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bristow R.G., Hill R.P. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 25.Chan N., Koritzinsky M., Zhao H., Bindra R., Glazer P.M., Powell S. Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res. 2008;68:605–614. doi: 10.1158/0008-5472.CAN-07-5472. [DOI] [PubMed] [Google Scholar]
- 26.Dastidar R.G., Hooda J., Shah A., Cao T.M., Henke R.M., Zhang L. The nuclear localization of SWI/SNF proteins is subjected to oxygen regulation. Cell Biosci. 2012;2:30. doi: 10.1186/2045-3701-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenneth N.S., Mudie S., van Uden P., Rocha S. SWI/SNF regulates the cellular response to hypoxia. J Biol Chem. 2009;284:4123–4131. doi: 10.1074/jbc.M808491200. [DOI] [PubMed] [Google Scholar]
- 28.Bencokova Z., Kaufmann M.R., Pires I.M., Lecane P.S., Giaccia A.J., Hammond E.M. ATM activation and signaling under hypoxic conditions. Mol Cell Biol. 2009;29:526–537. doi: 10.1128/MCB.01301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammond E.M., Dorie M.J., Giaccia A.J. Inhibition of ATR leads to increased sensitivity to hypoxia/reoxygenation. Cancer Res. 2004;64:6556–6562. doi: 10.1158/0008-5472.CAN-04-1520. [DOI] [PubMed] [Google Scholar]
- 30.Chen H.F., Wu K.J. Epigenetics, TET proteins, and hypoxia in epithelial-mesenchymal transition and tumorigenesis. Biomedicine (Taipei) 2016;6:1. doi: 10.7603/s40681-016-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melvin A., Rocha S. Chromatin as an oxygen sensor and active player in the hypoxia response. Cell Signal. 2012;24:35–43. doi: 10.1016/j.cellsig.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mimura I., Nangaku M., Kanki Y., Tsutsumi S., Inoue T., Kohro T. Dynamic change of chromatin conformation in response to hypoxia enhances the expression of GLUT3 (SLC2A3) by cooperative interaction of hypoxia-inducible factor 1 and KDM3A. Mol Cell Biol. 2012;32:3018–3032. doi: 10.1128/MCB.06643-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Beucken T., Koch E., Chu K., Rupaimoole R., Prickaerts P., Adriaens M. Hypoxia promotes stem cell phenotypes and poor prognosis through epigenetic regulation of DICER. Nat Commun. 2014;5:5203. doi: 10.1038/ncomms6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menegakis A., Eicheler W., Yaromina A., Thames H.D., Krause M., Baumann M. Residual DNA double strand breaks in perfused but not in unperfused areas determine different radiosensitivity of tumours. Radiother Oncol. 2011;100:137–144. doi: 10.1016/j.radonc.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Eicheler W., Zips D., Dorfler A., Grenman R., Baumann M. Splicing mutations in TP53 in human squamous cell carcinoma lines influence immunohistochemical detection. J Histochem Cytochem. 2002;50:197–204. doi: 10.1177/002215540205000207. [DOI] [PubMed] [Google Scholar]
- 36.Menegakis A., Yaromina A., Eicheler W., Dorfler A., Beuthien-Baumann B., Thames H.D. Prediction of clonogenic cell survival curves based on the number of residual DNA double strand breaks measured by gammaH2AX staining. Int J Radiat Biol. 2009;85:1032–1041. doi: 10.3109/09553000903242149. [DOI] [PubMed] [Google Scholar]
- 37.Yaromina A., Thames H., Zhou X., Hering S., Eicheler W., Dorfler A. Radiobiological hypoxia, histological parameters of tumour microenvironment and local tumour control after fractionated irradiation. Radiother Oncol. 2010;96:116–122. doi: 10.1016/j.radonc.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Kasten-Pisula U., Menegakis A., Brammer I., Borgmann K., Mansour W.Y., Degenhardt S. The extreme radiosensitivity of the squamous cell carcinoma SKX is due to a defect in double-strand break repair. Radiother Oncol. 2009;90:257–264. doi: 10.1016/j.radonc.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Mansour W.Y., Bogdanova N.V., Kasten-Pisula U., Rieckmann T., Kocher S., Borgmann K. Aberrant overexpression of miR-421 downregulates ATM and leads to a pronounced DSB repair defect and clinical hypersensitivity in SKX squamous cell carcinoma. Radiother Oncol. 2013;106:147–154. doi: 10.1016/j.radonc.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 40.Toulany M., Dittmann K., Baumann M., Rodemann H.P. Radiosensitization of Ras-mutated human tumor cells in vitro by the specific EGF receptor antagonist BIBX1382BS. Radiother Oncol. 2005;74:117–129. doi: 10.1016/j.radonc.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 41.McManus K.J., Hendzel M.J. ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol Biol Cell. 2005;16:5013–5025. doi: 10.1091/mbc.E05-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olive P.L., Banath J.P. Phosphorylation of histone H2AX as a measure of radiosensitivity. Int J Radiat Oncol Biol Phys. 2004;58:331–335. doi: 10.1016/j.ijrobp.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 43.Hall E.J., Giaccia A.J. 7th ed. Radiobiology for the Radiologist: Lippincott Williams & Wilkins; 2012. Oxygen effect and reoxygenation. [Google Scholar]
- 44.Rademakers S.E., Span P.N., Kaanders J.H., Sweep F.C., van der Kogel A.J., Bussink J. Molecular aspects of tumour hypoxia. Mol Oncol. 2008;2:41–53. doi: 10.1016/j.molonc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zolzer F., Streffer C. Increased radiosensitivity with chronic hypoxia in four human tumor cell lines. Int J Radiat Oncol Biol Phys. 2002;54:910–920. doi: 10.1016/s0360-3016(02)02963-2. [DOI] [PubMed] [Google Scholar]
- 46.Freiberg R.A., Krieg A.J., Giaccia A.J., Hammond E.M. Checking in on hypoxia/reoxygenation. Cell Cycle. 2006;5:1304–1307. doi: 10.4161/cc.5.12.2811. [DOI] [PubMed] [Google Scholar]
- 47.Gibson S.L., Bindra R.S., Glazer P.M. Hypoxia-induced phosphorylation of Chk2 in an ataxia telangiectasia mutated-dependent manner. Cancer Res. 2005;65:10734–10741. doi: 10.1158/0008-5472.CAN-05-1160. [DOI] [PubMed] [Google Scholar]
- 48.Bindra R.S., Gibson S.L., Meng A., Westermark U., Jasin M., Pierce A.J. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597–11604. doi: 10.1158/0008-5472.CAN-05-2119. [DOI] [PubMed] [Google Scholar]
- 49.Kumareswaran R., Ludkovski O., Meng A., Sykes J., Pintilie M., Bristow R.G. Chronic hypoxia compromises repair of DNA double-strand breaks to drive genetic instability. J. Cell Sci. 2012;125:189–199. doi: 10.1242/jcs.092262. [DOI] [PubMed] [Google Scholar]
- 50.Sprong D., Janssen H.L., Vens C., Begg A.C. Resistance of hypoxic cells to ionizing radiation is influenced by homologous recombination status. Int J Radiat Oncol Biol Phys. 2006;64:562–572. doi: 10.1016/j.ijrobp.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 51.Um J.H., Kang C.D., Bae J.H., Shin G.G., Kim D.W., Kim D.W. Association of DNA-dependent protein kinase with hypoxia inducible factor-1 and its implication in resistance to anticancer drugs in hypoxic tumor cells. Exp Mol Med. 2004;36:233–242. doi: 10.1038/emm.2004.32. [DOI] [PubMed] [Google Scholar]
- 52.Cam H., Easton J.B., High A., Houghton P.J. MTORC1 signaling under hypoxic conditions is controlled by ATM-dependent phosphorylation of HIF-1alpha. Mol Cell. 2010;40:509–520. doi: 10.1016/j.molcel.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olcina M.M., Grand R.J., Hammond E.M. ATM activation in hypoxia – causes and consequences. Mol Cell Oncol. 2014;1:e29903. doi: 10.4161/mco.29903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.