Abstract
Introduction
Although dietary habits can affect colorectal cancer (CRC) survivors’ health, it is unclear how familiar survivors are with dietary guidelines, what they believe about healthy eating and alcohol consumption, and what hinders healthy dietary habits after cancer. This study assessed CRC survivors’ familiarity with dietary guidelines, their eating and drinking habits, and perceived facilitators and barriers to healthy eating after cancer, including social support and self-efficacy for maintaining a healthy diet and limiting alcohol.
Methods
A total of 593 individuals (50% female; mean age, 74 years) diagnosed with CRC approximately 6 years prior to study entry in early 2010 were identified through California Cancer Registry records and participated in a cross-sectional mailed survey assessing health behavior after cancer (46% adjusted response rate). Analyses were conducted in 2014–2015.
Results
Survivors were most familiar with—and most likely to follow—recommendations to choose low-fat foods; 15% had never heard of recommendations to limit alcohol. Survivors were more aware of recommendations involving messages to limit/avoid versus approach/choose certain foods. The most common barrier to a healthy diet involved the effort required (26%). Survivors received more family/friend support and provider recommendations for healthy eating than limiting alcohol.
Conclusions
Results provide an overview of awareness of and adherence to dietary recommendations among CRC survivors, highlighting the need for increasing awareness of recommendations that are especially relevant for survivors. Suggestions are made for modifying diet-related messages to facilitate comprehension and recall among CRC survivors, and increasing awareness among groups with the lowest awareness levels.
Introduction
Research has shown that diet, including alcohol, is associated with the development of colorectal cancer (CRC),1–3 and continues to affect health after diagnosis in the form of disease recurrence, physical functioning, and mortality.4–6 Among CRC survivors, high intake of red and processed meat have been correlated with poorer health outcomes4,7–9 as has the “Western dietary pattern,” defined by reliance upon red and processed meat, dairy, refined grains, and sweetened desserts.6,10 Diet is also indirectly implicated in the association between excess body weight and cancer risks.11,12
A growing body of research has incorporated diet into broader behavioral interventions with CRC survivors, showing benefits to health and well-being from encouraging healthier eating habits and regular exercise.13–15 Despite these successes, the reach of such efforts has been limited and there remains a need for widespread approaches to educating CRC survivors about the role diet and alcohol play in health. Currently, information is lacking on CRC survivors’ awareness and beliefs about dietary recommendations. Limited information from small studies outside the U.S. suggests that CRC survivors’ understanding of nutrition recommendations is poor16 and that many survivors do not consider diet to be an important factor in long-term health.17 More information is needed on what U.S. CRC survivors know about dietary recommendations, what they believe about the benefits of healthy eating and drinking habits, how much support they receive for these practices, and which barriers they encounter in understanding and following dietary recommendations. Collecting this information will better inform public health efforts to develop and disseminate information about diet to cancer survivors.
In the Prevention Among Colorectal Cancer Survivors study,18 the authors sought to assess the knowledge, attitudes, and practices of CRC survivors regarding preventive health behaviors after cancer. The present analysis examined survivors’ awareness and adherence to dietary guidelines. Specifically, the study aimed to identify which dietary recommendations are most and least well-known and practiced among CRC survivors, what the barriers are to healthy eating after cancer, and to identify characteristics of survivors with the lowest levels of awareness about recommendations.
Methods
Prevention Among Colorectal Cancer Survivors study methods have previously been described in detail.18 This was a cross-sectional survey study of CRC survivors 5–7 years post-diagnosis from the California Cancer Registry. Eligibility criteria included:
diagnosis of primary colon or rectal cancer, localized or regional stage, in 2003 or 2004 (approximately 5–7 years before study enrollment);
no history of cancer prior to the CRC diagnosis;
residing in California at diagnosis;
age ≥18 years at diagnosis;
no California Cancer Registry–related research participation within the previous 12 months (to reduce respondent burden);
no “do not contact” flag on record; and
ability to respond in English.
All recruitment and study methods were approved by IRBs of CDC, Public Health Institute, California Cancer Registry, and ICF International. Analyses for the present paper were conducted in 2014 and 2015.
Measures
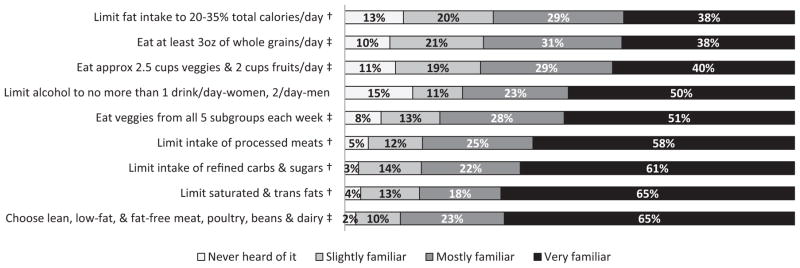
Familiarity with dietary and alcohol recommendations was measured using nine items developed to summarize recommendations current at the time of data collection (early 2010) issued by the U.S. DHHS and the U.S. Department of Agriculture as well as the World Cancer Research Fund and the American Institute for Cancer Research.1,19 Participants were asked to indicate how familiar they were with each dietary recommendation using a 4-point scale (Figure 1).
Figure 1.
Colorectal cancer survivors’ familiarity with dietary recommendations current at the time of data collection in January 2010.a
Note: Percentages do not include Don’t know or missing and are rounded to the closest integer.
aDietary recommendations were based on two publications current at the time of data collection [HHS/USDA, 2005; AIRC/WCRF, 2007].
†Items included in the “Avoid/Limit” factor.
‡Items included in the “Approach/Do eat” factor.
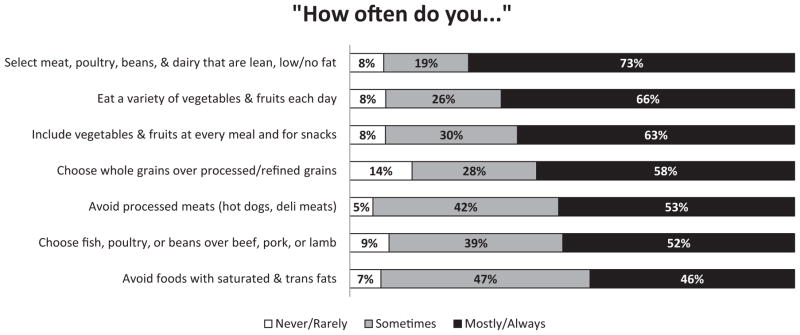
Current eating habits were measured using seven items summarizing concepts from the dietary recommendations. Respondents were asked how often they followed each of the recommendations using a 5-point scale (Figure 2). Two items assessed the frequency of consuming foods that recommendations specified limiting or avoiding (i.e., processed meats and saturated/trans fats). In analyses, the authors reverse-coded and interpreted these items as how often respondents avoided them.
Figure 2.
Colorectal cancer survivors’ dietary habits as measured against dietary recommendations current at the time of data collection.
Note: Percentages do not include Don’t know or missing responses and are rounded to the closest integer. Alcohol consumption is not included in this figure because it was measured using a different response format.
Alcohol consumption was assessed using a two-part question that has been used in the Behavioral Risk Factor Surveillance Survey.20 Respondents were also asked whether they currently drank alcohol less, the same amount, or more than before diagnosis, or whether they abstained pre- and post-diagnosis.
Based on theories of individual health behavior21 and previous research,22 the authors developed ten items (five barriers, five motivators) capturing beliefs that could impede or motivate adherence to a healthy diet. Responses used a 5-point scale from strongly disagree to strongly agree with a neutral midpoint.
Social support and self-efficacy for a healthy diet and limiting alcohol were measured using single items with 5-point response scales. Respondents were asked if, since finishing treatment for CRC, a doctor, nurse, or other healthcare provider had talked with them about healthy eating habits, and limiting or avoiding alcohol; responses included no, yes, or don’t know/can’t remember. They were also asked their agreement with the statement Close friends and family members think it’s important that I eat a healthy diet. Responses ranged from strongly disagree to strongly agree. Two statements asked whether survivors believed healthy eating habits and drinking alcohol were very harmful, somewhat harmful, neither, somewhat beneficial, or very beneficial for overall health and well-being.
Demographic characteristics were measured using standard questions on age, gender, race, ethnicity, marital status, and health insurance. One item recorded general health status using a 5-point scale ranging from poor to excellent. Height and weight were reported to calculate BMI.
Statistical Analysis
Descriptive statistics were used to examine all variables. All analyses were exploratory. BMI scores were dichotomized and recoded as not obese (BMI <30) or obese (BMI ≥30), as insufficient numbers in the underweight category prevented categorical analysis of all data. Cronbach’s alpha23 assessed whether all items on familiarity measured a single concept of awareness. To identify patterns of awareness about dietary recommendations, factor analysis was conducted, initially with the principal components method, then with Varimax rotation.24 Two factors were retained, which described two groups/clusters of awareness about recommendations: “approach/do eat foods” and “avoid/limit foods” (Figure 1). For ease of interpretation in analyzing awareness by survivor characteristics, the responses for each item were dichotomized into mostly familiar/very familiar (value=1) versus slightly familiar/never heard of it (value=0), and summed for awareness about “approach” and “avoid” guidelines. The distributions ranged from 0 to 4 (based on four items in each factor). Dichotomous scores were created for each factor representing being generally “aware” (summed scores ≥3) versus “not aware” (summed scores ≤2). Awareness of guidelines regarding alcohol was dichotomized by combining those who were mostly/ very familiar with guidelines versus slightly familiar/never heard of it. Dichotomous awareness scores for the “approach” and “avoid” recommendations were used as outcomes in logistic regressions. Results were presented as predicted margins, which can be interpreted as adjusted percentages.25 Differences were assessed with predicted marginal contrasts. Analyses were conducted using SAS Survey, version 9.3, and SUDAAN, version 10.1, to account for the complex sampling design and non-response. Weights were applied to generalize the results to the study population and account for non-response. Values of p<0.05 were considered statistically significant.
Results
A complete explanation of Prevention Among Colorectal Cancer Survivors study recruitment has been reported elsewhere.18 Briefly, survey packets were mailed to 1,414 survivors, yielding 593 completed surveys, and resulting in an adjusted response rate (which estimates ineligibility in non-response cases) of 46.3%. The cooperation rate, or participation rates among those with confirmed contact, was 64.0%. A separate analysis indicated that being divorced, widowed, or separated; non-Hispanic black, Hispanic, or Asian; and uninsured or having public insurance correlated with non-response (data not shown; JLR, unpublished observations, 2014). Conversely, no differences were found by age, gender, urban/rural residence, stage at diagnosis, or treatment received. Average time since primary diagnosis with CRC was 6.2 years (range, 5.2–7.2 years). As seen in Table 1, there were nearly equivalent male and female respondents, the majority of whom were non-Hispanic white, but sizable percentages of other race/ethnicities were represented. Because only nine participants described themselves as being “other” race, they were combined with the next smallest group, which was “Asian,” and this combined group became the “other” group for analytic purposes. Average age was 73.8 years.
Table 1.
Colorectal Cancer Survivors’ Awareness of “Approach” and “Avoid” Dietary Recommendations by Demographic and Health Status Characteristicsa
| Characteristics | Totalb | Approach/do eat | Avoid/limit | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| n (%)c | nb | Unadjusted, % (95% CI)c,d | Adjusted, % (95% CI)c,e | nb | Unadjusted, % (95% CI)c,d | Adjusted, % (95% CI)c,e | |
| Gender | p<0.05 | p<0.001 | p<0.001 | p<0.001 | |||
|
| |||||||
| Male | 295 (50.4) | 277 | 59.0 (52.2, 65.6) | 54.3 (46.8, 61.7) | 281 | 74.4 (67.9, 79.9) | 69.4 (61.6, 76.2) |
|
| |||||||
| Female | 296 (49.6) | 274 | 73.2 (66.8, 78.7) | 74.2 (67.5, 79.9) | 278 | 91.9 (87.9, 94.6) | 93.1 (89.4, 95.6) |
|
| |||||||
| Race/ethnicity | ns | p<0.05 | ns | ns | |||
|
| |||||||
| NH white | 291 (64.7) | 277 | 68.3 (62.0, 74.1) | 65.9 (59.3, 71.8) | 286 | 86.3 (81.2, 90.2) | 84.1 (78.5, 88.4) |
|
| |||||||
| NH black | 101 (5.5) | 91 | 68.9 (58.2, 77.9) | 70.7 (59.2, 80.1) | 91 | 83.6 (73.8, 90.2) | 85.8 (75.7, 92.1) |
|
| |||||||
| NH otherf | 93 (13.9) | 85 | 56.3 (44.9, 67.1) | 47.9 (35.9, 60.1) | 88 | 78.3 (67.5, 86.2) | 69.9 (56.2, 80.8) |
|
| |||||||
| Hispanic | 107 (15.8) | 99 | 64.2 (53.8, 73.4) | 69.4 (58.1, 78.8) | 95 | 72.7 (62.3, 81.1) | 83.9 (75.3, 89.9) |
|
| |||||||
| Age group (years) | ns | p<0.05 | ns | ns | |||
|
| |||||||
| ≤69 | 277 (28.5) | 269 | 61.5 (55.4, 67.3) | 56.8 (49.1, 64.2) | 264 | 84.4 (79.4, 88.4) | 79.3 (72.6, 84.6) |
|
| |||||||
| ≥70 | 315 (71.5) | 283 | 68.1 (61.8, 73.7) | 67.5 (60.9, 73.5) | 296 | 82.5 (77.3, 86.7) | 83.6 (78.4, 87.6) |
|
| |||||||
| Marital status | ns | p<0.01 | ns | ns | |||
|
| |||||||
| Married/living together | 362 (63.0) | 350 | 66.4 (60.6, 71.8) | 69.4 (63.4, 74.8) | 353 | 82.5 (77.4, 86.6) | 84.2 (79.6, 88.0) |
|
| |||||||
| Divorced/widowed/separated/never married | 207 (37.0) | 188 | 63.6 (55.3, 71.3) | 54.2 (44.8, 63.2) | 191 | 84.9 (78.4, 89.8) | 78.0 (69.4, 84.8) |
|
| |||||||
| Education level | ns | ns | p<0.001 | p<0.001 | |||
|
| |||||||
| ≤High school/GED | 196 (35.0) | 174 | 59.7 (51.2, 67.7) | 60.4 (51.4, 68.7) | 177 | 72.8 (65.0, 79.5) | 71.3 (62.7, 78.5) |
|
| |||||||
| Some college | 191 (32.3) | 186 | 64.8 (56.4, 72.4) | 61.1 (52.1, 69.4) | 189 | 83.0 (75.4, 88.6) | 80.0 (71.9, 86.3) |
|
| |||||||
| College graduate | 180 (32.7) | 173 | 71.0 (62.6, 78.1) | 71.7 (62.8, 79.1) | 175 | 94.1 (88.7, 97.0) | 95.2 (90.6, 97.6) |
|
| |||||||
| Insurance status | ns | ns | p<0.05 | p<0.05 | |||
|
| |||||||
| Medicare/Medicaid/public assistance/none | 313 (63.1) | 289 | 66.0 (59.6, 71.9) | 61.9 (55.1, 68.3) | 298 | 79.7 (74.1, 84.3) | 79.6 (73.9, 84.3) |
|
| |||||||
| Private/military | 260 (36.9) | 249 | 66.6 (59.6, 73.0) | 68.0 (59.9, 75.1) | 247 | 88.6 (83.3, 92.4) | 87.4 (81.6, 91.6) |
|
| |||||||
| Health status | p<0.05 | p<0.01 | ns | ns | |||
|
| |||||||
| Excellent/very good/good | 447 (77.8) | 427 | 68.4 (63.1, 73.2) | 67.6 (62.0, 72.7) | 428 | 84.4 (80.0, 88.0) | 83.9 (79.3, 87.7) |
|
| |||||||
| Fair/poor | 124 (22.2) | 108 | 55.3 (44.3, 65.7) | 50.9 (39.4, 62.2) | 115 | 76.5 (66.9, 83.9) | 77.2 (67.2, 84.8) |
|
| |||||||
| BMI | ns | ns | ns | ns | |||
|
| |||||||
| Obese (BMI≥30) | 154 (26.4) | 146 | 60.2 (50.6, 59.2) | 58.2 (48.2, 67.6) | 145 | 79.4 (70.2, 86.3) | 80.4 (71.6, 87.0) |
|
| |||||||
| Not obese (<30) | 400 (73.6) | 378 | 67.6 (62.0, 72.8) | 66.7 (60.8, 72.0) | 383 | 84.0 (79.5, 87.7) | 83.3 (78.5, 87.3) |
Note: Boldface indicates statistical significance.
Dietary recommendations were based on factor analysis resulting in the following two factors: approach/do eat and avoid/do not eat (two outcome variables).
Total=number of responders in the survey sample; n=sample size associated with the respective model . Numbers do not add to the same total because of refuse or missing information.
Percentages are weighted to the study population.
p-values are based on a χ2 test.
p-values are based on a Satterthwaite adjusted F.
84 participants in the “other” category were NH Asian.
GED, General Educational Development test; NH, non-Hispanic; ns, nonsignificant.
As Figure 1 shows, familiarity with dietary guidelines varied, with 65% being very familiar with some recommendations to under 40% being very familiar with others. The guideline with the highest proportion having never heard of it involved limiting alcohol.
Reliability analysis demonstrated that all eight items assessing familiarity with guidelines fit well together as a measure of awareness (α=0.89). Individual item correlations with the total ranged from 0.56 to 0.72 (data not shown). Findings from the factor analysis showed that loadings for the “approach” factor ranged from 0.62 to 0.84 and loadings for the “avoid” factor ranged from 0.80 to 0.87. The variance explained by each factor was 2.57 and 3.09, respectively (data not shown).
Survivors were more aware of guidelines reflecting “avoid” dietary recommendations (83% aware, 95% CI=79.2%, 86.3%) than “approach” recommendations (66.1% aware, 95% CI=61.4%, 70.5%). Regarding alcohol guidelines, 73.5%, (95% CI=69.1%, 77.4%) were aware. Adjusted and unadjusted associations between demographic characteristics and awareness of recommendations are presented in Table 1. After adjusting for all other variables, greater awareness of “approach” guidelines was significantly associated with being female (74.2% vs 54.3%, p<0.001); age ≥70 years (67.5% vs 56.8%, p<0.05); part of a couple versus not coupled (69.4% vs 54.2%, p<0.01); and in good to excellent health (67.6% vs 50.9%, p<0.01). Additionally, the non-Hispanic other group versus all other race/ethnicity groups was significantly less familiar with “approach” guidelines (p=0.002).
Adjusted demographic characteristics significantly associated with greater awareness of “avoid” recommendations included being female (93.1% vs 69.4%, p<0.001); more educated (with increasing levels: 71.3%, 80.0%, 95.2%; p<0.001); and having private or military insurance (87.4%) versus public insurance (79.6%, p<0.05). Additionally, the non-Hispanic other group versus all other race/ethnicity groups was significantly less familiar with “avoid” guidelines (p=0.027).
The adjusted associations between demographic characteristics and awareness of alcohol recommendations revealed significantly greater awareness with increasing education (65% among less than high school/General Educational Development [GED] test, 75% among some college, 85% among college graduates; p<0.01) (data not shown). No other differences were statistically significant.
As Figure 2 shows, the dietary habit practiced always or most of the time by the largest percentage (72.7%) was choosing meat, poultry, beans, and dairy that are lean, low fat, or fat free. The habit with the highest percentage having never or rarely practiced it (13.9%) involved choosing whole grains instead of processed/refined grains.
More than half (53.5%) did not drink any alcohol in the past 30 days (data not shown). Of those who drank, 83.6% did so within recommended limits whereas 16.4% drank more than recommended. Compared with drinking habits at diagnosis, 23.2% reported currently drinking less, 31.6% the same amount, 0.9% more, and 44.2% abstained at both times.
Most survivors agreed with the statement Eating a healthy diet is important for my health (90.6%), and just more than half (56.5%) agreed that Eating a healthy diet makes me look good (Appendix Figure 1, available online). With regard to barriers, 26% agreed that Eating a healthy diet takes too much effort and only 8% agreed that Eating a healthy diet may cause injury or harm to my body.
Support, perceptions, and beliefs about healthy eating habits and limiting alcohol can be seen in Table 2. The majority (77%) agreed that friends and family supported healthy eating, and 70% said a provider had discussed diet with them. By contrast, 55% agreed that friends and family supported limiting or avoiding alcohol and 48% indicated that a provider had discussed alcohol consumption with them. The majority felt totally or mostly confident in their abilities to eat a healthy diet and avoid or limit alcohol. Most (93%) believed healthy eating habits were beneficial for overall health and well-being. However, 12% believed alcohol was beneficial for health, 58% believed it was harmful, and 30% believed it was neither beneficial nor harmful.
Table 2.
Support, Perceptions, and Beliefs Regarding Eating a Healthy Diet and Limiting Alcohol Consumption
| Healthy diet, % | Limiting alcohol, % | |
|---|---|---|
| Friends and family support this behavior | ||
| Strongly agree | 51 | 42 |
| Somewhat agree | 26 | 13 |
| Neither | 19 | 37 |
| Somewhat disagree | 2 | 2 |
| Strongly disagree | 2 | 6 |
| Provider discussed behavior with me | ||
| Yes | 70 | 48 |
| No | 22 | 40 |
| Can’t remember | 8 | 12 |
| Confidence in my ability to practice this behavior | ||
| Totally | 25 | 76 |
| Mostly | 40 | 14 |
| Moderately | 23 | 4 |
| Slightly | 9 | 3 |
| Not at all | 4 | 3 |
| Perceived harm or benefit of behavior | ||
| Very beneficial | 72 | 2a |
| Somewhat beneficial | 21 | 10a |
| Neither | 5 | 30a |
| Somewhat harmful | 1 | 25a |
| Very harmful | 0 | 33a |
Note: Missing/refused responses are not included. Percentages are rounded to the closest integer.
Refers to respondent beliefs about consuming alcohol rather than limiting it.
Discussion
Five to seven years after diagnosis, generally high levels of awareness about dietary guidelines were found among CRC survivors. The recommendation to choose lean, low-fat, and fat-free meat, poultry, beans, and dairy was the most familiar and most practiced by survivors. However, despite having particular relevance for CRC, recommendations regarding limiting processed meats and alcohol consumption were less well known. Survivors received less social support for limiting alcohol than for healthy eating and less than half of survivors recalled medical providers discussing alcohol consumption with them, a finding consistent with the modest estimates found in the general population.26 In addition, this study found interesting patterns of awareness. Recommendations involving specific quantities or proportions, such as limiting fat to “20%–35% of total calories” and eating “3 ounces” of whole grains each day tended to be less well known than were similar recommendations without specific quantities. Survivors were also more aware of recommendations that encouraged the avoidance of certain foods than those that encouraged certain foods. Regarding which survivors were in greatest need of dietary information, those who were male, single, aged ≤69 years, had less education, had public insurance, in worse health, and identified themselves as Asian or “other” race had the lowest awareness about dietary guidelines.
In a time when the long-term health and well-being of cancer survivors has been deemed a public health issue,27 and stakeholders are being called on to intervene using approaches with broader reach,28 this study offers valuable insights into further increasing awareness of dietary guidelines among CRC survivors. Perhaps the guideline in greatest need of increased awareness is that on limiting or avoiding alcohol; a sizable minority had never heard of this recommendation and many were unaware of alcohol’s potential harms to health. Given that alcohol has been deemed a “Group 1,” highest risk carcinogen for more than 25 years29 and is known to contribute specifically to CRC,30 greater awareness of this recommendation is needed among survivors, and also caregivers and medical care providers. Although it is encouraging that very few survivors reported drinking more than recommended, all survivors should be informed of the links between cancer and alcohol. By increasing awareness among several levels of social influence and by reducing confusion about alcohol’s potential health benefits, cancer survivors could become better informed and receive better support, when necessary.
The overarching patterns of awareness observed in this study align with research in health education and cognitive science. Barriers to comprehension and recall from detailed numerical information are inherent to the concepts of health literacy, numeracy, and cognition. The fuzzy trace theory,31 for instance, holds that people encode and recall information on gist traces, or the bottom-line meaning of messages, more than verbatim traces, or the exact details/numbers in messages. Likewise, finding that “avoid” recommendations were more familiar than “approach” recommendations is consistent with the negativity bias,32 or people’s tendency to attend to and remember negative over positive information. These findings highlight the importance of cross-disciplinary efforts and can help inform not only which messages need more attention but also how recommendations can be conveyed most effectively. For instance, in developing messages about healthy eating after cancer, high levels of health literacy and numeracy should be avoided33 in favor of clear, bottom-line messages that will be better understood and recalled.34 Future work could also explore methods for enhancing awareness about “approach” recommendations, perhaps by linking them with more attention-grabbing “avoid” messages or employing other strategies to bolster comprehension and recall.
This study found that awareness differed by survivor characteristics. Efforts to increase awareness among survivor subgroups known to be particularly unaware will need to consider the unique barriers (e.g., social support, gender roles, cost, access, culture, language) certain groups might face in acquiring information. To reach those in particular need, materials could be developed specifically to address known barriers, such as translating materials into other languages, using culturally appropriate dietary references, and tailoring materials for caregivers responsible for purchasing and preparing food. It would also be useful to refer survivors to free or low-cost diet-related resources available online or in the community, such as those available through non-profit organizations or self-management education programs.
Limitations
By stepping back to examine the areas in which cancer survivors lack information and support for healthy eating and which survivors are most in need of information, this study offers unique strengths to help inform new and promising approaches for public health intervention. However, it has some weaknesses. First, the study design was cross-sectional and is unable to describe how awareness and habits may change over time. Second, data were collected via self-report and responses on actual dietary habits were not validated. Third, although recruiting a diverse, older sample of CRC survivors from a state-based cancer registry was a primary strength of the study design, the findings might not generalize to the entire U.S. Additionally, analyses of non-response revealed systematic differences between responders and non-responders (i.e., marital status, race/ethnicity, insurance status) that could have affected the findings. However, notably, there were no differences in response status by other important characteristics such as age, gender, geographic region, stage at diagnosis, and treatments received.
Conclusions
The large and growing population of cancer survivors in the U.S. combined with the evidence linking diet and health outcomes after cancer has generated a need for wide-reaching approaches to informing cancer survivors about dietary recommendations. Though further work will need to identify effective strategies for widely disseminating up-to-date dietary recommendations—for instance, through registry correspondence, survivor-ship care plans, or self-management education programs—the findings provide a starting point in identifying which types of information and support survivors currently need. As a first step toward improving dietary habits, survivors could be better informed of dietary recommendations relevant to their particular cancer diagnosis. Priority groups for dietary education include men, Asians and other minority groups, singles, and those with less education and poor health. Finally, by using what we know from health education and other communication sciences about facilitating comprehension and recall of information, we may be in a better position to develop more effective messages about diet for cancer survivors.
Supplementary Material
Acknowledgments
Publication of this article was supported by the Centers for Disease Control and Prevention, Division of Cancer Prevention and Control.
The Prevention Among Colorectal Cancer Survivors study was funded by CDC. We would like to thank Dr. Isabelle Romieu for her review of an early draft of the manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
Appendix. Supplementary data
Supplementary data associated with this article can be found at, http://dx.doi.org/10.1016/j.amepre.2015.08.012.
Footnotes
No financial disclosures were reported by the authors of this paper.
References
- 1.World Cancer Research Fund and American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 2.Baan R, Straif K, Grosse Y, et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8(4):292–293. doi: 10.1016/s1470-2045(07)70099-2. http://dx.doi.org/10.1016/S1470-2045(07)70099-2. [DOI] [PubMed] [Google Scholar]
- 3.Baena R, Salinas P. Diet and colorectal cancer. Maturitas. 2015;80(3):258–264. doi: 10.1016/j.maturitas.2014.12.017. http://dx.doi.org/10.1016/j.maturitas.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 4.McCullough ML, Gapstur SM, Shah R, Jacobs EJ, Campbell PT. Association between red and processed meat intake and mortality among colorectal cancer survivors. J Clin Oncol. 2013;31(22):2773–2782. doi: 10.1200/JCO.2013.49.1126. http://dx.doi.org/10.1200/JCO.2013.49.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J, Jeon JY, Meyerhardt JA. Diet and lifestyle in survivors of colorectal cancer. Hematol Oncol Clin North Am. 2015;29(1):1–27. doi: 10.1016/j.hoc.2014.09.005. http://dx.doi.org/10.1016/j.hoc.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298(7):754–764. doi: 10.1001/jama.298.7.754. http://dx.doi.org/10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 7.Zell JA, Ignatenko NA, Yerushalmi HF, et al. Risk and risk reduction involving arginine intake and meat consumption in colorectal tumorigenesis and survival. Int J Cancer. 2007;120(3):459–468. doi: 10.1002/ijc.22311. http://dx.doi.org/10.1002/ijc.22311. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, Wu H, Wang PP, et al. Dietary patterns and colorectal cancer recurrence and survival: a cohort study. BMJ Open. 2013;3(2) doi: 10.1136/bmjopen-2012-002270. http://dx.doi.org/10.1136/bmjopen-2012-002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan DS, Lau R, Aune D, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011;6(6):e20456. doi: 10.1371/journal.pone.0020456. http://dx.doi.org/10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makambi KH, Agurs-Collins T, Bright-Gbebry M, Rosenberg L, Palmer JR, Adams-Campbell LL. Dietary patterns and the risk of colorectal adenomas: the Black Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2011;20(5):818–825. doi: 10.1158/1055-9965.EPI-10-1213. http://dx.doi.org/10.1158/1055-9965.EPI-10-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson TM, Park Y, Robien K, et al. Body mass index and risk of second obesity-associated cancers after colorectal cancer: a pooled analysis of prospective cohort studies. J Clin Oncol. 2014;32(35):4004–4011. doi: 10.1200/JCO.2014.56.8444. http://dx.doi.org/10.1200/JCO.2014.56.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedirko V, Romieu I, Aleksandrova K, et al. Pre-diagnostic anthropometry and survival after colorectal cancer diagnosis in Western European populations. Int J Cancer. 2014;135(8):1949–1960. doi: 10.1002/ijc.28841. http://dx.doi.org/10.1002/ijc.28841. [DOI] [PubMed] [Google Scholar]
- 13.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301(18):1883–1891. doi: 10.1001/jama.2009.643. http://dx.doi.org/10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winger JG, Mosher CE, Rand KL, Morey MC, Snyder DC, Demark-Wahnefried W. Diet and exercise intervention adherence and health-related outcomes among older long-term breast, prostate, and colorectal cancer survivors. Ann Behav Med. 2014;48(2):235–245. doi: 10.1007/s12160-014-9598-7. http://dx.doi.org/10.1007/s12160-014-9598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawkes AL, Pakenham KI, Chambers SK, Patrao TA, Courneya KS. Effects of a multiple health behavior change intervention for colorectal cancer survivors on psychosocial outcomes and quality of life: a randomized controlled trial. Ann Behav Med. 2014;48(3):359–370. doi: 10.1007/s12160-014-9610-2. http://dx.doi.org/10.1007/s12160-014-9610-2. [DOI] [PubMed] [Google Scholar]
- 16.Anderson AS, Caswell S, Wells M, Steele RJ, Macaskill S. “It makes you feel so full of life” LiveWell, a feasibility study of a personalised lifestyle programme for colorectal cancer survivors. Support Care Cancer. 2010;18(4):409–415. doi: 10.1007/s00520-009-0677-4. http://dx.doi.org/10.1007/s00520-009-0677-4. [DOI] [PubMed] [Google Scholar]
- 17.Pullar JM, Chisholm A, Jackson C. Dietary information for colorectal cancer survivors: an unmet need. N Z Med J. 2012;125(1356):27–37. [PubMed] [Google Scholar]
- 18.Hawkins NA, Berkowitz Z, Rodriguez JL, Miller JW, Sabatino SA, Pollack LA. Examining adherence with recommendations for follow-up in the Prevention among Colorectal Cancer Survivors study. Oncol Nurs Forum. 2015;42(3):233–240. doi: 10.1188/15.ONF.233-240. http://dx.doi.org/10.1188/15.ONF.233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. DHHS, U.S. Department of Agriculture. Dietary Guidelines for Americans. 2005. HHS Publication No. HHS-ODPHP-2005-01-DGA-A. [Google Scholar]
- 20.CDC. Behavioral Risk Factor Surveillance System website. www.cdc.gov/brfss/questionnaires.htm.
- 21.Glanz KR, Rimer BK, Viswanath K. Health Behavior and Health Education: Theory, Research, and Practice. 4. San Francisco, CA: Jossey-Bass; 2008. [Google Scholar]
- 22.Falk LW, Bisogni CA, Sobal J. Food choice processes of older adults: a qualitative investigation. J Nutr Educ. 1996;28(5):257–265. http://dx.doi.org/10.1016/S0022-3182(96)70098-5. [Google Scholar]
- 23.Cronbach L. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. http://dx.doi.org/10.1007/BF02310555. [Google Scholar]
- 24.Pedhazur EJ, Schmelkin LP. Measurement, Design, and Analysis: An Integrated Approach. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. [Google Scholar]
- 25.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55(2):652–659. doi: 10.1111/j.0006-341x.1999.00652.x. http://dx.doi.org/10.1111/j.0006-341X.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 26.D’Amico EJ, Paddock SM, Burnam A, Kung FY. Identification of and guidance for problem drinking by general medical providers: results from a national survey. Med Care. 2005;43(3):229–236. doi: 10.1097/00005650-200503000-00005. http://dx.doi.org/10.1097/00005650-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Smith JL, Pollack LA, Rodriguez JL, et al. Assessment of the status of a National Action Plan for Cancer Survivorship in the USA. J Cancer Surviv. 2013;7(3):425–438. doi: 10.1007/s11764-013-0276-8. http://dx.doi.org/10.1007/s11764-013-0276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frieden TR. A framework for public health action: the health impact pyramid. Am J Public Health. 2010;100(4):590–595. doi: 10.2105/AJPH.2009.185652. http://dx.doi.org/10.2105/AJPH.2009.185652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.IARC. Alcohol drinking. IARC Monogr Eval Carcinog Risks Hum. 1988;44:1–378. [PMC free article] [PubMed] [Google Scholar]
- 30.IARC. Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum. 2010;96:1–1440. [PMC free article] [PubMed] [Google Scholar]
- 31.Reyna VF, Brainerd CJ. Fuzzy-trace theory: an interim synthesis. Learn Indiv Diff. 1995;7:1–75. http://dx.doi.org/10.1016/1041-6080(95)90031-4. [Google Scholar]
- 32.Peeters GCJ. Positive-negative asymmetry in evaluations: the distinction between affective and informational negativity effects. In: Stroebe W, Hewstone M, editors. European Review of Social Psychology. Chichester, UK: Wiley; 1990. pp. 33–60. http://dx.doi.org/10.1080/14792779108401856. [Google Scholar]
- 33.Health.gov. America’s health literacy: why we need accessible health information. An issue brief from the U.S. Department of Health and Human Services. www.health.gov/communication/literacy/issuebrief. Published 2008.
- 34.Brust-Renck PG, Royer CE, Reyna VF. Communicating numerical risk: human factors that aid understanding in health care. Rev Hum Factors Ergon. 2013;8(1):235–276. doi: 10.1177/1557234X13492980. http://dx.doi.org/10.1177/1557234X13492980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.