Abstract
Theories of grounded cognition propose that action verb knowledge relies in part on motor processing regions, including premotor cortex. Accordingly, impaired action verb knowledge in patients with amyotrophic lateral sclerosis (ALS) and Parkinson's Disease (PD) is thought to be due to motor system degeneration. Upper motor neuron disease in ALS degrades the motor cortex and related pyramidal motor system, while disease in PD is centered in the basal ganglia and can spread to frontostriatal areas that are important to language functioning. These anatomical distinctions in disease may yield subtle differences in the action verb impairment between patient groups. Here we compare verbs where the body is the agent of the action to verbs where the body is the theme. To examine the role of motor functioning in body verb production, we split patient groups into patients with high motor impairment (HMI) and those with low motor impairment (LMI), using disease-specific measures of motor impairment. Regression analyses assessed how verb production in ALS and PD was related to motor system atrophy. We find a dissociation between agent- and theme-body verbs in ALS: ALS HMI were impaired for agent body verbs but not theme verbs, compared to ALS LMI. This dissociation was not present in PD patients, who instead show depressed production for all body verbs. Although patients with cognitive impairment were excluded from this study, cognitive performance significantly correlated with the production of theme verbs in ALS and cognitive/stative verbs in PD. Finally, regression analyses related the agent-theme dissociation in ALS to grey matter atrophy of premotor cortex. These findings support the view that motor dysfunction and disease in premotor cortex contributes to the agent body verb deficit in ALS, and begin to identify some distinct characteristics of impairment for verbs in ALS and PD.
Keywords: Grounded cognition, Amyotrophic lateral sclerosis, Parkinson's Disease, Action semantics, Verb production
1. Introduction
Theories of grounded or embodied cognition propose that motor experience is integral to the representation of action semantics (Barsalou & Wiemer-Hastings, 2005; Binder & Desai, 2011; Pulvermüller, 2005). Although interpretations differ on the degree and manner whereby semantic representations depend upon sensorimotor experience, considerable evidence is consistent with the perspective that semantic knowledge is neither completely embodied nor entirely abstracted from perception or experience (Binder, Desai, Graves, & Conant, 2009; Kemmerer, 2015; Meteyard, Cuadrado, Bahrami, & Vigliocco, 2012). Instead, semantic representations may rely in part on both modal cortical regions, which process sensorimotor information, and hetero-modal convergence zones, which help to process high-level, abstracted knowledge. Thus, the motor system, including the primary motor and premotor cortex, is thought to partially support action verb comprehension and production (Pulvermüller, 2005). Functional imaging has shown that the primary motor and premotor cortex is activated when action verbs are presented as single words (Raposo, Moss, Stamatakis, & Tyler, 2009) and that generation of action words activates the premotor and supplementary motor area (Péran et al., 2010). Importantly, studies by Willems et al. (2009 Willems et al. (2010) distinguish between tasks that involve explicit mental imagery and automatic semantic processing. While the primary motor cortex is activated during mental imagery, they find that the premotor cortex is involved in the processing of action semantics, and they attribute this to the role of the premotor motor cortex in action planning (Hoshi & Tanji, 2000). This contribution of the motor system during action semantic processing is flexible, and depends on the cognitive demands of the semantic task (Kemmerer, 2015).
This link between action verb processing and cortical motor regions may help to explain specific deficits to action verb knowledge that have been observed in patients with motor system disorders, such as amyotrophic lateral sclerosis (ALS). ALS is characterized by progressive degeneration of upper and lower motor neurons, resulting in a loss of motor strength (Chiò et al., 2014; Strong et al., 2017). A hallmark feature of ALS is the presence of disease in primary motor cortex, which can extend to motor association regions (Bak & Chandran, 2012; Brettschneider et al., 2013; Chiò et al., 2014). Embodied cognition theories emphasize that motor cortex is functionally important to action semantic representations, and accordingly several studies have shown that ALS patients exhibit poor action verb knowledge, while object noun knowledge remains relatively preserved (Bak & Hodges, 2004; M. Grossman et al., 2008; York et al., 2014). However, the characterization of ALS as a pure motor disorder is no longer considered an accurate representation of this disease, and cognitive and executive impairment is now recognized as commonly co-occurring in ALS (Bak & Chandran, 2012; Phukan, Pender, & Hardiman, 2007; Strong et al., 2017). Thus, while evidence supports the view that disease to motor cortex impairs verb processing in ALS, cognitive dysfunction may also contribute to the selective deficit for action verbs compared to nouns (Abrahams et al., 2004).
Grounded cognition has also been put forward as a possible explanation for the action verb deficit reported in Parkinson's disease (PD). PD is a progressive movement disorder marked by akinesia and rigid movement caused by decreased dopaminergic signaling in the basal ganglia (Jankovic, 2008). Multiple studies have shown that PD patients also exhibit a selective action verb deficit, with intact object noun knowledge (Péran et al., 2003; Rodríguez-Ferreiro, Ménendez, Ribacoba, & Cuetos, 2009; Salmazo-Silva et al., 2017). However there is some debate about the cause of this deficit: while this action verb deficit is seen as evidence for the embodied representations of action verbs, motor slowing in PD is not associated disease in the pyramidal motor system. Anatomical studies of PD patients without cognitive impairment show only minimal cortical atrophy, with relatively preserved primary motor cortex and motor association cortex (González-Redondo et al., 2014; Mak et al., 2015). These studies also show that in PD patients with cognitive impairment, atrophy is widespread and also includes frontal, parietal and temporal cortical regions, causing decline in multiple cognitive domains that can affect language processing. Fronto-striatal pathophysiology in PD has been linked to reduced executive functioning and performance on action verb fluency (Obeso, Casabona, Bringas, Álvarez, & Jahanshahi, 2012; Piatt, Fields, Paolo, & Tröster, 1999; Salmazo-Silva et al., 2017). More so than motor cortex atrophy, PD is associated with disease and dysfunction of the basal ganglia, a structure which has been linked to the action verb deficits seen in PD (Cardona et al., 2013; Grossman, Stern, Gollomp, Vernon, & Hurtig, 1994; Silveri et al., 2012). While the traditional role of the basal ganglia has been seen as control of motor movement (Bohsali & Crosson, 2016), it has also been implicated in semantic selection and control processes that are important for verb processing (Crescentini, Lunardelli, Mussoni, Zadini, & Shallice, 2008; Crescentini, Shallice, & Macaluso, 2010). While disease in the basal ganglia and its projections may modulate functioning of the motor cortex during action semantic processing, its role in executive and language operations make the cause of action verb impairment in PD hard to disentangle. Thus, motor dysfunction may not be the sole or primary source of action verb deficits seen in PD, and it is possible that disease outside of the pyramidal motor system causes cognitive and linguistic impairments that can contribute to the verb deficit seen in PD.
A limitation of comparing nouns and verbs is that dissociations in impairment could be due to differences in grammatical and syntactic complexity, not in action content. Verbs are more syntactically complex and generally less concrete than nouns (Druks, 2002; Miller & Fellbaum, 1991) and are thus thought to rely more on executive functioning and semantic control processes (Mätzig, Druks, Masterson, & Vigliocco, 2009; Vigliocco, Vinson, Druks, Barber, & Cappa, 2011). Thus, the cognitive impairments experienced by both ALS and PD could contribute to their action verb deficit, especially for PD patients whose disease is typically not centered in the pyramidal motor system. Indeed, impaired verb generation has been related to cognitive functioning in non-demented PD patients, but not to their motor functioning (Crescentini, Mondolo, Biasutti, & Shallice, 2008; Péran et al., 2003).
In this study, we investigate the relationship between motor functioning and verb production in ALS and PD using the Cookie Theft Picture description task. By using the Cookie Theft picture, all subjects received a visual stimulus that contained the same action content (e.g. “stealing cookies”, “falling off the stool”, “washing dishes”, etc.) and helped guide the production of action/motor-related words across the participants. To minimize the potential confound that may be associated with non-language aspects of verb processing, we study patients who are not demented and do not have identifiable cognitive deficits. To account for grammatical complexity differences between stimuli, we adopt an alternative approach that compares motor to non-motor verbs (Fernandino et al., 2013; Kemmerer, Miller, Macpherson, Huber, & Tranel, 2013). We compare verbs that we hypothesize would be affected by degraded motor representations to those that would not. We expect that motor dysfunction will impair production of verbs that relate specifically to the body and muscle movements (e.g. runs in “the boy runs”), whereas verbs related to object motion (e.g. splashing in “the water is splashing”) and to cognition or states (e.g. knows in “she knows”) should be relatively preserved. Still, verbs within these categories are syntactically heterogeneous. We therefore perform a more fine-grained analysis that distinguishes between verbs where the body is the agent of the action (e.g. “the boy grabbed the cookie”) and verbs where the body is the theme/recipient of the action (e.g. “the boy is falling”). If action verb deficits are related to motor cortex disease, we expect to observe a dissociation between agent and theme verbs that are produced. Specifically, we reason that a motor system disorder should be more closely associated with the purposeful execution of an action, compared to the passive recipient of an action. While initiation and execution of a voluntary movement is difficult for patients with motor dysfunction, they may commonly experience involuntary or passive actions, such as falling, which may be less strongly dependent on representation in the motor system. Therefore, we expect that verbs where the body is the agent of the action will be impaired in individuals with a motor system disorder, whereas verbs where the body is the theme/recipient of the action will not. Moreover, to control for the effect that motor disease in ALS and PD can have on speech output, we calculate the proportion of verb types produced by each group, and we also examine whether speech rate affects any of our measures.
To directly test the relationship between motor functioning and body verb production, we divide patient groups into high motor impairment (HMI) and low motor impairment (LMI) groups. We divide groups based on assessments of motor functioning, using disease-specific assessments of motor functioning, including the revised Functional Rating Scale (ALS-FRS-R) in ALS and the Unified Parkinson's Disease Rating Scale (UPDRS III) in PD. While disease-specificity offers the advantage of sensitivity to the form of motor disorder that occurs in each disease, it should be acknowledged that clinical motor functioning scales are different in ALS and PD, and thus prevent a direct comparison between ALS and PD subgroups. However, by comparing HMI to LMI we can gain insight into how motor function affects the production of action-body verbs in each group.
Finally, theories of grounded cognition predict that primary motor and motor association cortices are important to the successful production of verbs. To test the functional role of motor regions on verb production, we examine how gray matter atrophy in bilateral primary motor cortex, premotor cortex, and supplementary motor area relate to agent body verb production in ALS and PD. Though not specified by theories of grounded cognition, we also include the basal ganglia in these analyses because of its central role in motor impairment in PD. If motor cortex regions are functionally important to production of verbs relating to motor actions of the body, we expect that disease to motor cortex regions in ALS patients would impair production of verbs where the body is the agent of the action. Unlike ALS, motor cortex atrophy in PD is secondary and is apparent only with widespread disease that also affects frontostriatal regions later in the disease process (González-Redondo et al., 2014; Mak et al., 2015). In combination with early disease to the basal ganglia, PD patients are vulnerable to executive and semantic control deficits (Bohsali & Crosson, 2016). Therefore, a verb production deficit in PD might not be specific to motor system dysfunction, and instead could relate to broader cognitive functioning. If so, we expect any deficit observed in PD to affect verbs more generally, and not be specific to verbs where the body is the agent of the action.
2. Methods
2.1. Subjects
Twenty-eight ALS patients, 21 PD patients, and 36 controls participated in the Cookie Theft picture description task (Table 1). All subjects were native speakers of English and participated in an informed consent procedure that was approved by the University of Pennsylvania and in accordance with the Declaration of Helsinki. ALS and PD diagnoses were based on published consensus criteria, and patients were without a diagnosed cognitive impairment (Brooks, Miller, Swash, & Munsat, 2000; Jankovic, 2008). Severity of motor impairment was measured in ALS using the Functional Rating Scale (FRS; Cedarbaum et al., 1999), and in PD using the Unified Parkinson's Disease Rating Scale motor subscale (UPDRS III; Goetz et al., 2008). Sixteen PD patients were treated with the dopaminergic precursor, levodopa, in combination with carbidopa, and 3 patients were on the dopaminergic agonist, rasagiline. In addition subjects underwent subsets of neuropsychological assessment that evaluated global cognitive functioning (Mini Mental State Exam, MMSE; Folstein, Folstein, & McHugh, 1975), semantic performance (Boston Naming Test and Pyramids and Palm Trees; Howard & Patterson, 1992; Kaplan, Goodglass, & Weintraub, 2001), executive performance (Visual Verbal and Reverse Digit Span Lezak, Howieson, & Loring, 1983; Wechsler & Stone, 1987), and fluency (category- and letter-guided fluency; Lezak et al., 1983). All patients had a MMSE score of 25 or higher, and there was no significant difference between patients and controls in MMSE. Composite semantic, executive, and fluency variables were created by standardizing test scores based on control performance, and averaging z-score values within each cognitive domain. There was no difference between ALS and PD in education, MMSE, semantic score, executive score, or fluency score.
Table 1. Demographic data for all participants.
| Control (n = 36) | ALS (n = 28) | PD (n = 21) | ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| M | SD | M | SD | M | SD | F | p | |
| Age (years) | 68.19 | 7.92 | 64.18 | 10.33 | 71.70 | 6.72 | 4.61* | .013 |
| Disease duration (months) | – | – | 44.14 | 25.38 | 90.26 | 57.86 | 13.95* | .001 |
| Education (years) | 15.89 | 2.52 | 15.96 | 3.02 | 16.52 | 2.62 | .40 | >.1 |
| MMSE (30) | 29.16 | 1.04 | 28.60 | 1.67 | 29.05 | 1.15 | 1.24 | >.1 |
| Composite semantic z-score | – | – | .18 | .76 | −.33 | 1.02 | 2.53 | >.1 |
| Composite executive z-score | – | – | −.01 | .94 | .00 | .84 | .004 | >.1 |
| Composite fluency z-score | .19 | .72 | − .25 | 1.10 | 2.15 | >.1 | ||
Mean (M) and standard deviations (SD) listed for healthy controls, ALS, and PD. Results of one-way ANOVA across all groups are listed: F-scores are listed in bold with asterisks for tests that reached significance (p < .05).
ALS is marked by a rapid disease progression and short survival span (Magnus et al., 2002). This means our ALS cohort was significantly younger and had a significantly shorter disease duration than our PD cohort, though age was not significantly associated with any of the behavioral measures discussed below. In addition, clinical assessment of ALS and PD uses different motor functioning scales. Because these scales cannot be compared, we do not make direct comparisons between ALS and PD motor dysfunction and body verb production. Instead, we divided both patient groups based on a median split into high motor impairment (HMI) and low motor impairment groups (LMI), and made statistical comparisons within patient groups. Median ALS-FRS-R score was 37 (on a scale from 0 to 48; 48 = normal motor function). ALS HMI patients had an ALS-FRS-R score of 37 or lower, and ALS LMI had an ALS-FRS-R score of higher than 37. Median UPDRS III score was 24 (on a scale from 0 to 56; 0 = normal function). PD HMI patients have a UPDRS III score of 24 or higher, and PD LMI have a score of lower than 24. Table 2 shows the demographic characteristics of HMI and LMI groups for ALS and PD patients. High and low motor groups for both ALS and PD patients are matched for age, education, MMSE, semantic score, executive score, and fluency score.
Table 2. Demographic data for high and low motor impairment patients.
| ALS HMI (n = 17) | ALS LMI (n = 11) | t-test | PD HM I (n = 12) | PD LMI (n = 9) | t-test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| M | SD | M | SD | t | p | M | SD | M | SD | t | p | |
| Age | 64.00 | 11.40 | 64.45 | 8.96 | .11 | >.1 | 70.25 | 5.51 | 73.88 | 8.11 | 1.20 | >.1 |
| Disease duration | 53.12 | 28.44 | 30.27 | 9.82 | 3.04* | .01 | 97.18 | 65.50 | 80.75 | 47.97 | .60 | >.1 |
| Education | 15.57 | 3.08 | 16.45 | 3.01 | .72 | >.1 | 17.00 | 2.22 | 15.89 | 3.10 | .96 | >.1 |
| ALS-FRS-R (48) | 31.00 | 6.30 | 41.36 | 2.16 | 6.24* | .00 | – | – | – | – | – | |
| UPDRS III | – | – | – | – | – | 28.25 | 4.35 | 12.89 | 4.81 | 7.66* | .00 | |
| MMSE (30) | 27.90 | 2.02 | 29.30 | .82 | 2.03 | .07 | 28.92 | .90 | 29.25 | 1.49 | .63 | >.1 |
| Semantic z-score | .27 | .62 | .09 | .95 | .45 | >.1 | .05 | .54 | −.92 | 1.34 | 1.83 | >.1 |
| Executive z-score | .16 | .98 | −.28 | .85 | 1.24 | >.1 | .31 | .59 | −.38 | .98 | 1.86 | .08 |
| Fluency z-score | .17 | .85 | .21 | .62 | .10 | >.1 | −.19 | .98 | −.33 | 1.31 | .26 | >.1 |
Mean (M) and standard deviations (SD) listed for ALS and PD patients with high and low motor impairment (HMI/LMI). Results of independent t-tests between HMI and LMI patients are listed, and t-scores are listed in bold with asterisks for tests that reached significance (p < .05).
2.2. Behavioral task: Cookie Theft
Subjects were asked to verbally describe the Cookie Theft picture (Goodglass, Kaplan, & Barressi, 1983) and descriptions were digitally recorded, transcribed and coded. Verbs were identified based on dominant part of speech (Brysbaert, New, & Keuleers, 2012) and using sentential context. We calculated the proportion of verbs and content verbs (see below) produced for each subject by tallying each word type and dividing by the total number of words produced. Speech rate was calculated for each subject as the number of words produced per 60 sec of speech. Repetitions and dysfluencies were excluded from the counts.
2.2.1. Content verbs
Content verbs were identified as verbs that expressed the main action within the sentence, such as fall in “He is starting to fall”. This definition excludes copula and semi-copula verbs, such as “She is pretty”, or “She has shoes on”, as well as aspectual markers that denote how the main action is performed, such as finished in “She has finished washing the dishes”. Content verbs were identified as one of three types: body verbs (e.g. “He grabbed the cookie”), object motion verbs (e.g. “The water is spilling on the floor”), and cognitive or sta-tive verbs (e.g. “She is daydreaming”). We then calculated the proportion of body, motion, and stative verbs produced by tallying the type of verb and dividing by the total number of content verbs produced. In addition, concreteness ratings for each content verb produced were determined using published norms (Brysbaert, Warriner, & Kuperman, 2013), and the average concreteness of all content verbs was calculated for each subject. To compare patient groups, all content verb calculations and ratings (including body verb calculations; see below) were z-score converted based on control performance. To ensure this did not affect our results, statistical calculations were performed on both untransformed- and z-scores. All statistical results were consistent and did not change based on transformation.
2.2.2. Body verbs
By using naturalistic speech samples elicited by a known target (description of the Cookie Theft picture), we were able to use contextual information to determine the thematic roles associated with each verb produced. If impaired verb production is due to disease in motor association cortex, we expect verbs relating to the body to be most affected. We therefore focused analyses on the type of body verb produced. Body verbs tended to involve the hands (e.g. grabbing), the body (e.g. standing), the mouth (e.g. eating), and the eyes (e.g. watching). Given the motor deficits in PD and ALS, we hypothesize that patients would have difficulty with verbs relating to the hands, body, and mouth, but not the eyes. We also hypothesize that impairment would be related to verbs where the body is the agent of the action (“the boy is stealing cookies”), but not the theme (“the boy is falling”). We therefore identified the proportion of agent body verbs – where the body, hand or mouth were the agents of the action – and theme body verbs – where they were the recipients of the action. This was calculated by tallying the number of agent or theme body verbs and dividing by the total number of body verbs produced. Calculations were standardized based on control performance. Again, statistical tests were conducted on both untransformed- and z-scores, and there was no difference in significance between the two measures. As a control, we also analyzed the proportion of agent- and theme-eye verbs produced, since there was no expected effect of motor dysfunction on verbs relating to the eyes.
2.3. Imaging
Subsets of ALS (n = 11; HMI/LMI = 6/5) and PD (n = 13; HMI/ LMI = 8/5) patients participated in the imaging portion of this study and underwent magnetic resonance imaging (MRI). All subjects were right-handed, except one left-handed PD patient. Performance on Cookie Theft and neuropsychological evaluations were not significantly different from the full patient (all p-values > .05). T1-weighted images were collected on a Siemens 3T scanner, and acquisition used an 8-channel head coil with repetition time = 1620 msec, echo time = 3 msec, flip angle = 15°, matrix = 192 × 256, slice thickness = 1 mm, and in-plane resolution = .9 × .9 mm. Images were acquired within an average of 143 days (SD = 99.4) of the task for ALS and 148 days (SD = 88.5) for PD. Images were preprocessed and normalized to a standard space and segmented using the PipeDream interface (http://sourceforge.net/projects/neuropipedream/) and the Advanced Normalization Tools kit (http://www.picsl.upenn.edu/ANTS/). Gray matter volume was calculated, and images were transformed into Montreal Neurological Institute (MNI) space, down-sampled to 2 mm3 resolution, and smoothed using a 2-mm full-width half-maximum (FWHM) Gaussian kernel. Preprocessed images were compared in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). Regions of interest (ROIs) were selected based on a priori hypotheses proposed by theories of grounded cognition: primary motor cortex, premotor cortex and supplementary motor area. Because of its central role in movement dysfunction in PD, we also include the basal ganglia in these analyses. These regions were defined using the Mindboggle-101 labels (Klein & Tourville, 2012). To examine how atrophy in motor regions affected production of agent and theme body verbs, regression analyses related behavioral performance in ALS and PD to gray matter volume within selected ROIs. Regression analyses were accepted as significant using a height threshold of p < .05 (uncorrected) with a minimum cluster size of 10 voxels and a peak voxel threshold of p < .001. Cluster height thresholds were specified based on previous published work in ALS and PD (York et al., 2014).
3. Results
3.1. Behavioral results
Table 3 summarizes the behavioral data for controls and ALS groups and Table 4 summarizes the behavioral data for controls and PD groups. Independent sample t-tests show that there was no significant difference in the proportions of content verbs or all verbs produced among controls, ALS patients and PD patients (p > .1). However, controls had significantly faster speech rates (M = 143.17, SD = 39.63) than ALS patients (M = 120.54, SD = 29.36; t(62) = 2.53, p = .014), but did not differ from PD patients (M = 131.90, SD = 30.83; p > .1). Level of motor impairment also affected speech rate for ALS patients, which was slower for ALS HMI than for ALS LMI (Table 3; t(26) = 2.67, p = .013). Speech rate was slower for PD HMI than for PD LMI, but this did not reach significance (Table 4; p > .05). Despite a slower speech rate in ALS, speech rate is not significantly associated with any of the scores reported below for either ALS or PD patients (all p-values > .1).
Table 3. Cookie Theft production data for ALS, probability (P) of verb type.
| Control | ALS HMI | ALS LMI | t-test (HMI vs LMI) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| M | SD | M | SD | M | SD | t | p | |
| Speech Rate | ||||||||
| Average | 143.2 | 39.6 | 109.8 | 30.5 | 137.2 | 18.4 | 2.67* | .013 |
| P (Verb) | ||||||||
| Average | .24 | .04 | .24 | .05 | .25 | .06 | .64 | >.1 |
| P (Content Verb) | ||||||||
| Average | .11 | .02 | .11 | .04 | .11 | .03 | .11 | >.1 |
| P (Body) | ||||||||
| Average | .53 | .15 | .58 | .17 | .51 | .06 | ||
| z-score | – | – | .39 | 1.15 | .01 | .42 | 1.07 | >.1 |
| P (Object Motion) | ||||||||
| Average | .16 | .08 | .14 | .07 | .16 | .09 | ||
| z-score | – | – | −.16 | .94 | −.26 | 1.19 | .16 | >.1 |
| P (Cognitive/Stative) | ||||||||
| Average | .31 | .15 | .24 | .18 | .29 | .14 | ||
| z-score | – | – | −.58 | 1.22 | .13 | .97 | 1.60 | >.1 |
| P (Body as Agent) | ||||||||
| Average | .69 | .17 | .60 | .21 | .75 | .11 | ||
| z-score | – | – | −.49 | 1.23 | .35 | .68 | 2.08* | .047 |
| P (Body as Theme) | ||||||||
| Average | .19 | .12 | .28 | .19 | .16 | .11 | ||
| z-score | – | – | .74 | 1.51 | −.24 | .88 | 2.18* | .039 |
| P (Agent)–P (Theme) | ||||||||
| Average | .50 | .43 | .33 | .38 | .59 | .21 | 2.13* | .043 |
Mean (M) and standard deviations (SD) listed for healthy controls, and ALS patients with high and low motor impairment (HMI/LMI). Results of independent t-tests between HMI and LMI ALS patients are listed, and t-scores are in bold with asterisks for tests that reached significance (p < .05).
Table 4. Cookie Theft production data for PD, probability (P) of verb type.
| Control | PDHMI | PD LMI | t-test (HMI vs LMI) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| M | SD | M | SD | M | SD | t | p | |
| Speech Rate | ||||||||
| Average | 143.2 | 39.6 | 114.2 | 136.4 | 10.3 | .30 | >.1 | |
| P (Verb) | ||||||||
| Average | .24 | .04 | .23 | .03 | .24 | .04 | 1.30 | >.1 |
| P (Content Verb) | ||||||||
| Average | .11 | .02 | .09 | .03 | .11 | .03 | 1.71 | >.1 |
| P (Body) | ||||||||
| Average | .53 | .15 | .50 | .14 | .39 | .15 | ||
| z-score | – | – | -.15 | .90 | −.93 | 1.00 | 1.75 | .097 |
| P (Object Motion) | ||||||||
| Average | .16 | .08 | .20 | .08 | .14 | .10 | ||
| z-score | – | – | .40 | 1.05 | −.16 | 1.33 | 1.27 | >.1 |
| P (Cognitive/Stative) | ||||||||
| Average | .31 | .15 | .30 | .14 | .46 | .11 | ||
| z-score | – | – | −.06 | .96 | 1.03 | .71 | 2.77* | .012 |
| P (Body as Agent) | ||||||||
| Average | .69 | .17 | .65 | .17 | .70 | .20 | ||
| z-score | – | – | −.23 | 1.01 | .09 | 1.21 | .57 | >.1 |
| P (Body as Theme) | ||||||||
| Average | .19 | .12 | .19 | .16 | .15 | .15 | ||
| z-score | – | – | .02 | 1.25 | −.27 | 1.18 | .47 | >.1 |
| P (Agent)–P (Theme) | ||||||||
| Average | .50 | .43 | .46 | .29 | .55 | .32 | .60 | >.1 |
Mean (M) and standard deviations (SD) listed for healthy controls, and PD patients with high and low motor impairment (HMI/LMI). Results of independent t-tests between HMI and LMI PD patients are listed, and t-scores in bold with asterisks for tests that reached significance (p < .05).
For content verbs, PD patients produced significantly fewer body verbs than ALS patients (t(47) = 2.73, p = .009), and significantly more cognitive verbs (t(47) = 2.23, p = .031). There was no difference in the proportion of motion verbs produced (p > .1). We next conducted exploratory analyses to determine if cognitive performance predicted body or cognitive verb production in PD or ALS. Examination of the cognitive variables (MMSE, semantic, executive, and fluency scores) revealed that MMSE score in PD was positively correlated with cognitive verb production (rho(20) = .443, p = .050), but not body verb production (p > .1). However, this moderate association between MMSE and cognitive verb production does not survive Bonferroni's correction for multiple comparisons (α = .0125). These relationships were not present in ALS (all p > .1).
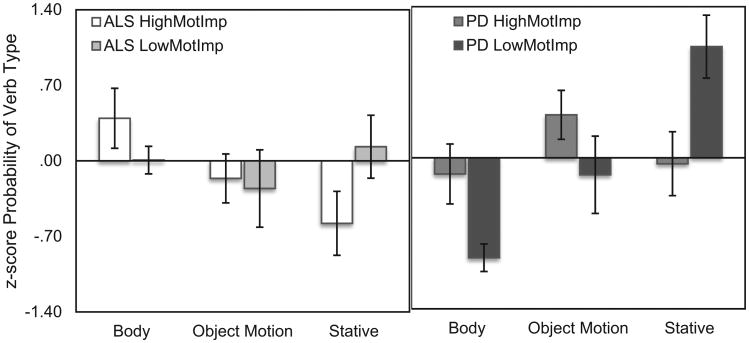
To examine the role of motor functioning in body verb production, we compared high and low motor impairment groups (Fig. 1). A repeated measures ANOVA reveals no interaction between verb-type (Body, Motion, Cognitive) and group (HMI, LMI) for ALS patients (p > .1). There was a main effect of verb-type (F(26) = 26.48, p < .001), but not of group (p > .1). T-tests confirm that there were no significant differences between HMI and LMI for proportion of body, object motion, or cognitive verbs produced by ALS patients (all p > .1). For PD patients, a repeated measures ANOVA reveals a significant interaction between verb-type and group (F(19) = 5.60, p = .029). There was no main effect of verb-type or group (p-values > .1). T-tests reveal that there were no significant differences in the proportion of body or object motion verbs produced by PD HMI and PD LMI (p > .1). However, PD HMI produced significantly fewer cognitive verbs than PD LMI patients (t(19) = 2.77, p = .012).
Fig. 1.
Proportion of Content Verbs Produced (z-score). The left panel shows the proportion of body, object motion and stative verbs produced by ALS HMI (white) and ALS LMI patients (light gray). The right panel shows the proportion of body, object motion and stative verbs produced by PD HMI (dark gray) and PD LMI patients (black).
These results do not indicate that decreased body verb production is related to impaired motor function in either ALS or PD. However, there was a positive association between MMSE and cognitive/stative verb production in PD (see above). In a post hoc analysis we further explored whether body verb production in patient groups could be modulated by concreteness, as verbs relating to the body tend to be the more concrete than those relating to a state or cognitive action. Indeed, for all subjects, increased concreteness of verb production was associated with increased body verb production (rho(84) = .63, p < .001), and ALS patients produced content verbs that were significantly more concrete than PD patients (t(47) = 2.19, p = .034).
3.1.1. Agent versus theme body verbs
We next conducted an analysis of thematic roles for body verbs produced by ALS and PD patients. For each body verb, we determined if the body was the agent of the action (e.g. “He grabbed it”) or the theme (e.g. “He is falling”). There was no significant difference between ALS and PD patients in proportion of agent-theme verbs produced (p > .1). A correlation between disease duration in ALS and production of agent-theme verbs revealed that a longer disease duration was associated with fewer agent verbs (rho(27) = − .460, p = .016) and increased theme body verbs (rho(27) = .453, p = .018). Of the cognitive variables (MMSE, semantic, executive and fluency scores), the proportion of theme verbs produced was significantly and positively correlated with semantic score (rho(15) = .671, p = .006) and with executive score (rho(28) = .507, p = .006) in ALS. No cognitive variables were correlated with the proportion of agent body verbs in ALS (all p > .1). No relationships between cognitive variables and agent or theme verb production were present for PD patients (all p > .1).
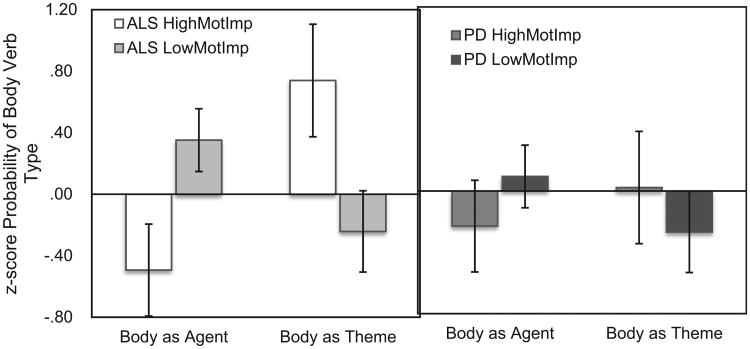
To examine the relationship between motor dysfunction and agent body verb production, we compared HMI and LMI groups in ALS and PD (Fig. 2). A repeated-measures ANOVA reveals a significant interaction between thematic role (Agent, Theme) and group (HMI, LMI), for ALS patients (F(26) = 4.49, p = .044). There was a main effect of thematic role (F(26) = 52.89, p < .001), but not of group (p < .1). T-tests reveal that ALS HMI produced significantly fewer agent-body verbs than ALS LMI (t(26) = 2.08, p = .047), and significantly more theme verbs (t(25.8) = 2.18, p = .039). Confirming the disassociation between agent and theme verbs produced, the difference score between Agent and Theme Verbs (Agent – Theme) was also significantly lower for ALS HMI than ALS LMI (t(26) = 2.13, p = .043). ALS HMI also had a lower Agent-Theme score than controls, though this did not reach significance (F(51) = 1.97, p = .054). For PD patients, there was no interaction between thematic role and group (p > .1). There was a main effect of thematic role (F(19) = 54.59, p < .001), but not of group (p < .1). T-tests show no differences between high and low motor impairment for PD patients (all p-values > .1). As a control, we examined agent and thematic role for verbs related to the eye, a body part not hypothesized to be affected by motor dysfunction in either ALS or PD. There was no significant difference (all p-values > .1) between the proportion of agent- or theme-eye verbs produced by ALS HMI (Agent Eye: M = .07, SD = .096; Theme Eye: .04, SD = .095) and ALS LMI (Agent Eye: M = .03, SD = .051; Theme Eye: .07, SD = .089), or between PD HMI (Agent Eye: M = .13, SD = .099; Theme Eye: .04, SD = .062) and PD LMI (Agent Eye: M = .07, SD = .110; Theme Eye: .07, SD = .127).
Fig. 2.
Proportion of Agent and Theme Verbs Produced (z-score). The left panel shows the proportion of body agent and body theme verbs produced by ALS HMI (white) and ALS LMI patients (light gray). The right panel shows the proportion of body agent and body theme verbs produced by PD HMI (dark gray) and PD LMI patients (black).
3.2. Imaging results
Behavioral results revealed that motor impairment modulates the proportion of Agent-Theme verbs produced, with ALS HMI patients producing significantly fewer agent body verbs and more theme body verbs than ALS LMI. Though not significant, PD HMI also had a lower Agent-Theme score than PD LMI. To examine how atrophy in motor regions affects this dissociation, we performed regression analyses which related gray matter volume to the Agent-Theme score in ALS and PD. Analyses were constrained to a priori ROIs: left and right primary motor cortex, premotor cortex, supplementary motor area, and basal ganglia. Results in Table 5 and Fig. 3 revealed a significant cluster in the left premotor cortex (BA 6) for ALS (z = 3.27, p < .001). The same analysis in PD revealed no significant clusters.
Table 5. Regression of agent-theme score with gray matter volume in ALS.
| BA | MNI Coordinates | z-score | p | Cluster size (voxels) | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| Left prefrontal cortex | 6 | −10 | 2 | 46 | 3.27 | <.001 | 12 |
Fig. 3.
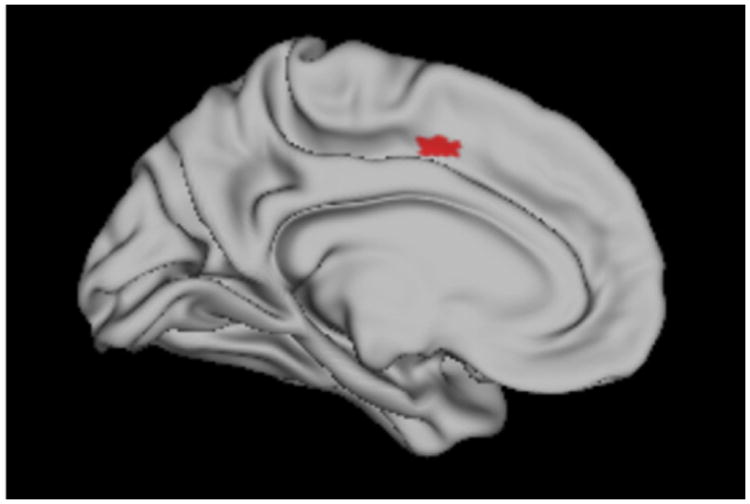
Regression of Agent-Theme score with Gray Matter Volume in ALS (red).
4. Discussion
Here we examined the relationship between action semantics and motor cortical structures by assessing body verb production in ALS and PD patients. While both patient groups have a motor disorder that has been previously shown to selectively impair action verb processing compared to object nouns, it is uncertain the extent to which these effects are driven by the major grammatical category differences between nouns and verbs. To avoid the confound of grammatical class, we focused our analyses on verb subtypes. By using semi-structured naturalistic speech samples, we were able to use sentential context to assess the thematic matrix associated with each body verb. We therefore compared production of verbs where the body was the agent of the action and verbs where the body was the recipient/ theme of the action. In addition, we compared production of verbs that are related to the body to production of verbs related to objects or cognitive states.
4.1. Agent versus theme body verbs
In a fine-grained analysis designed to help us understand the specific role of motor impairment in action verb processing, we examined the proportion of agent- and theme-body verbs produced by patients. We reasoned that disease in the motor system might degrade the representation of verbs implicated in the voluntary execution of an action, compared to verbs associated with the passive recipient of an action. In ALS patients, our results revealed a dissociation between agent and theme verbs, with ALS HMI producing significantly fewer agent and significantly more theme verbs than ALS LMI. Imaging results confirmed that this difference in agent and theme verb production in ALS was related to gray matter atrophy in premotor cortex (BA 6). This specific deficit for agent body verbs in ALS is in accordance with weak theories of grounded cognition, which propose that premotor cortex plays an important role in action verb processing and predicts a mild to moderate deficit in populations with motor cortex lesions or disease (Meteyard et al., 2012). An important point in the dissociation between agent and theme verbs is that it cannot be attributed to a pure output problem: this measure normalizes for quantity of speech by examining how often a verb is produced that involves a voluntary motor movement of the body. Moreover, this is not due to a grammatical impairment for agent verbs, as this dissociation between agent and theme verbs is not present for eye-related verbs, which are not expected to be affected by ALS. Thus, the finding that ALS patients with HMI are more likely to describe involuntary rather than voluntary bodily actions is a revealing aspect of their disease, highlighting the specificity of their impairment.
In addition to motor functioning, analyses also revealed that cognitive performance contributed to the magnitude of this effect, as better semantic and executive performance significantly correlated with increased theme body verb production in ALS. This may be due in part to the possibility that theme verbs are more complex than agent verbs because of the relatively opaque identification of the agent. Importantly, the direction of this relationship indicates that a cognitive or executive impairment leads to reduced theme verb production. This is the opposite pattern expected from a motor impairment and is the opposite pattern to the impairment we observe in ALS. These results thus suggest that the relative difficulty with the bodily action verbs involving an explicit agent component, compared to theme, is multifactorial in nature: disease in motor association regions in ALS can impair agent body verb production while mild cognitive limitations can impair theme body verb production. These results in ALS are in line with new diagnostic criteria for ALS-frontotemporal spectrum disorders (Strong et al., 2017), which recognize that verb deficits associated with atrophy to the prefrontal and motor cortex can be a marker of cognitive impairment in ALS.
PD patients showed no significant difference in agent or theme verb production between HMI and LMI. While not significant, the effect of motor impairment in PD was in the same direction as in ALS: PD HMI produced slightly fewer agent body verbs and slightly more theme body verbs than PD LMI. We acknowledge three important caveats when interpreting this negative finding in PD. First and most importantly, the motor ratings for patient groups were not the same. Therefore, while we found that ALS HMI produced significantly fewer agent-body verbs, we do not interpret a lack of an effect of motor impairment on body verb production in PD as evidence against any relationship in PD. Second, most of our PD patients were on antiparkinsonian medication at the time of testing, and this may have affected the magnitude of the verb deficit observed. However, despite patients being on treatment, our population demonstrated variance in motor ability; 12 of our PD patients had moderate to severe motor dysfunction and were included in the PD HMI group. A third caveat is that the Cookie Theft picture has a limited amount of action material. Use of the Cookie Theft description task somewhat controlled the action/motor content across subjects' speech samples, allowing us to more easily compare verb production across individuals. However, this restriction may have also reduced our ability to detect a body or agent verb impairment in patients. Finally, the PD patients in this study had a significantly longer disease duration than the ALS patients. Disease duration is truncated in ALS due to its relatively short survival period (Magnus et al., 2002). It could be that the longer disease duration in PD results in more global deficits that can affect both agent-and theme-body verbs, and thus obfuscates any specific relationship to motor dysfunction. However, we observed no significant association between disease duration and body verb production in PD, though disease duration is positively correlated with the agent-theme dissociation in ALS. With these caveats in mind, we found no relationship between impaired agent or body verb production and motor dysfunction in PD. This absence may indicate that motor dysfunction may not be the sole or primary cause of impaired body verb production in PD (discussed below). Indeed, despite a consistent finding of action verb impairment in PD, multiple studies (including this one) fail to find a direct link between motor impairment (as measured by UPDRS III) and action verb impairment (Bocanegra et al., 2017; Péran et al., 2009, 2013). If however motor dysfunction is a contributing factor to the action verb deficit in PD, it may be that the UPDRS III is not be sensitive to an impairment of motor representations.
Because our patients were without overt cognitive impairment, we did not expect to find, nor did we find a significant relationship between verb production and cognitive factors in PD. However, previous work has shown that even a subthreshold cognitive impairment may affect verb production; in a task requiring non-demented PD patients to generate verbs from noun or verb cues, impaired verb generation was related to cognitive functioning, not motor functioning (Péran et al., 2003; see also; Koerts, Leenders, & Brouwer, 2009). Other studies have also shown that executive function and control processes in PD contribute significantly to their verb processing deficit (Colman et al., 2009; Crescentini, Mondolo, et al., 2008; Crescentini et al., 2010; Silveri et al., 2012). On the other hand, others have found no relationship between executive functioning and the action verb impairment in PD (Bocanegra et al., 2015). Together with the results of our study, these mixed findings highlight the heterogeneity of executive and language impairments in PD (Biundo, Weis, & Antonini, 2016) and indicate that one system may not be solely responsible for the action verb deficit in PD. Indeed, the basal ganglia are highly connected with multiple cortical regions and play an important supervisory role to many cognitive and motor functions (Bohsali & Crosson, 2016; Rowe & Rittman, 2016). Thus, progressive neurodegeneration in non-demented PD patients may affect multiple neuronal systems – including motor, frontal and parietal – that contribute to a deficit for body verb production.
4.2. Body versus stative verbs
In agreement with previous studies showing impaired action verb knowledge in PD, we found that PD patients produced significantly fewer body verbs and more cognitive verbs, compared to ALS patients. However contrary to our predictions, our results did not reveal a straightforward deficit for body verbs in patients with ALS and PD who have a motor impairment; when examining how body verb production related to motor impairment, both ALS and PD patients with HMI produced a higher proportion of body verbs than patient groups with LMI. This effect of increased body verb/decreased stative verb production was significant for PD HMI patients, compared to PD LMI. Thus, unlike the agent and theme dissociation, this trend is in the opposite direction of that expected by grounded cognition.
While our results do not evince an effect of motor impairment on body verb production, the increased production of body verbs and decreased stative verbs for HMI groups could be due in part to a concreteness effect. Body verbs, like kick and grab tend to be more concrete than cognitive or stative verbs like think and know, and in this study increased concreteness was positively correlated with increased body verb production for all subjects. It is therefore possible that the trend for increased body verb and decreased stative verb production in HMI, compared to LMI groups, may be related to a “concreteness effect” (Holcomb, Kounios, Anderson, & West, 1999; Jessen et al., 2000) – the result of impaired processing for abstract words. Cognitive/stative verbs that tend to be abstract may require more cognitive resources to support their processing, compared to the relatively concrete body verbs. Indeed, increased concreteness of speech has been previously linked to atrophy of the frontal regions and the caudate (Cousins, Ash, Irwin, & Grossman, 2017). In addition, we found that increased production of cognitive/stative verbs, which are typically more abstract, was related to higher MMSE scores in PD, although this did not survive correction for multiple comparisons. These results suggest that cognitive functioning may play an important role in the dissociation we see for body and stative verb production between ALS and PD. While our participants were selected to be free of dementia, many ALS patients may have subtle deficits in executive and semantic performance (Abrahams et al., 2004; Strong et al., 2017), and we therefore cannot rule out that some ALS patients may have subtle cognitive deficits that could play a role in difficulty with abstract, stative verbs.
Finally, there are limitations of the current study which should be explored in future investigations. First, Cookie Theft descriptions by both patients and controls included metaphorical uses of verbs that might also rely on motor experience (Jamrozik, Mcquire, Cardillo, & Chatterjee, 2015). For example, participants describe cups that “sit” on counters and paths that “run” to the garage. Thus our delineations between content verbs that involve the body versus an object versus a state may obscure the role of motor system in processing metaphorical actions. Second, patients who participated in this study were in relatively mild stages of disease. This was a necessary condition, so they could undergo MRI, which required patients to lie still and supine for an extended period of time. Yet, it may be that action verb impairments related to motor disease are even more apparent with increased dysfunction. Longitudinal assessments are needed to see how declining motor function and increasing disease in motor cortical regions affect body verb production in ALS and PD. Another limitation of our study is that we could not directly compare motor impairment effects between patients with ALS and PD because of the different nature of these conditions and the disease-specific metrics used, although we were able to perform within-group comparisons based on the relative amount of motor disability. ALS patients were also significantly younger than PD patients, though age did not significantly predict any of the measures of behavioral performance. While the different profiles of verb impairment we observe between ALS and PD are in line with hypotheses based on anatomical pathology, future work that controls for demographic differences is needed to confirm these distinctions. Finally, the goal of this study was specifically to investigate the effect of motor impairment on body verb production, and we found evidence that atrophy in premotor cortex in ALS is related to impaired production of verbs where the body is the agent of the action. However, our results indicate that in addition to motor functioning, cognitive functioning also affected verb production. Moreover, this effect was revealed even though the patients in this study were without apparent dementia. Previous studies in PD have linked action verb impairment to disease in frontal cortical and subcortical structures, which causes declines in executive functioning and selection mechanisms, and our results here indicate that executive and semantic processing in ALS also affect the production of theme body verbs. Rather than solely due to motor dysfunction, our results suggest that disease in ALS and PD affects multiple systems, including motor and cognitive, and that future work is needed to examine how these processes interact to impair processing specific aspects of action verbs.
5. Conclusion
Grounded cognition theories propose a critical link between motor experience and action semantics. To test the functional role of motor cortical regions, we examined the production of body verbs in two disorders of the motor system, ALS and PD. We divided each patient group into subgroups with high and low motor impairment to investigate the relationship between verb production and motor functioning. In ALS HMI compared to ALS LMI, we found a selective deficit for verbs where the body was the agent of the action. Regression analyses revealed that relatively decreased production of agent verbs to theme verbs in ALS was related to decreased gray matter volume in premotor cortex. Although both patient groups were non-demented, cognitive functioning also contributed to the effects we find: better cognitive functioning was moderately associated with increased production of theme verbs in ALS and increased cognitive verb production in PD. These results indicate a complex, multifactorial relationship between production of specific verb classes and motor and cognitive functioning.
Footnotes
This work was supported in part by NIH (NS053488 and AG017586) and the ALS Foundation. The authors have no conflicts of interest Q1 to report.
References
- Abrahams S, Goldstein LH, Simmons A, Brammer M, Williams SCR, Giampietro V, et al. Word retrieval in amyotrophic lateral sclerosis: A functional magnetic resonance imaging study. Brain: a Journal of Neurology. 2004;127(Pt 7):1507–1517. doi: 10.1093/brain/awh170. http://doi.org/10.1093/brain/awh170. [DOI] [PubMed] [Google Scholar]
- Bak TH, Chandran S. What wires together dies together: Verbs, actions and neurodegeneration in motor neuron disease. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2012;48(7):936–944. doi: 10.1016/j.cortex.2011.07.008. http://doi.org/10.1016/j.cortex.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Bak TH, Hodges JR. The effects of motor neurone disease on language: Further evidence. Brain and Language. 2004;89(2):354–361. doi: 10.1016/S0093-934X(03)00357-2. http://dx.doi.org/10.1016/S0093-934X(03)00357-2. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Wiemer-Hastings K. Grounding cognition: The role of perception and action in memory, language, and thinking (2005) 2005. Situating Abstract Concepts. [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. http://doi.org/10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and metaanalysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. http://doi.org/10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biundo R, Weis L, Antonini A. Cognitive decline in Parkinson's disease: The complex picture. Npj Parkinson's Disease. 2016;2(April):16018. doi: 10.1038/npjparkd.2016.18. http://doi.org/10.1038/npjparkd.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocanegra Y, García AM, Lopera F, Pineda D, Baena A, Ospina P, et al. Cuetos F. Unspeakable motion: Selective action-verb impairments in Parkinson's disease patients without mild cognitive impairment. Brain and Language. 2017;168:37–46. doi: 10.1016/j.bandl.2017.01.005. http://doi.org/10.1016/j.bandl.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Bocanegra Y, Garcia AM, Pineda D, Buritica O, Villegas A, Lopera F, et al. Ibanez A. Syntax, action verbs, action semantics, and object semantics in Parkinson's disease: Dissociability, progression, and executive influences. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2015;69:237–254. doi: 10.1016/j.cortex.2015.05.022. http://doi.org/10.1016/j.cortex.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Bohsali A, Crosson B. The basal ganglia: Novel perspectives on motor and cognitive functions. Switzerland: Springer International Publishing; 2016. The basal ganglia and language: A tale of two loops; pp. 217–242. http://doi.org/10.1007/978-3-319-42743-0_10. [Google Scholar]
- Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, et al. Trojanowski JQ. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Annals of Neurology. 2013;74(1):20–38. doi: 10.1002/ana.23937. http://doi.org/10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders: Official Publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases. 2000;1(5):293–299. doi: 10.1080/146608200300079536. http://doi.org/10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Brysbaert M, New B, Keuleers E. Adding part-of-speech information to the SUBTLEX-US word frequencies. Behavior Research Methods. 2012:991–997. doi: 10.3758/s13428-012-0190-4. http://doi.org/10.3758/s13428-012-0190-4. [DOI] [PubMed]
- Brysbaert M, Warriner AB, Kuperman V. Concreteness ratings for 40 thousand generally known English word lemmas. Gianico-Relyea & Altarriba; Paivio: 2013. [DOI] [PubMed] [Google Scholar]
- Cardona JF, Gershanik O, Gelormini-Lezama C, Houck AL, Cardona S, Kargieman L, et al. Ibanez A. Action-verb processing in Parkinson's disease: New pathways for motor-language coupling. Brain Structure & Function. 2013;218(6):1355–1373. doi: 10.1007/s00429-013-0510-1. http://doi.org/10.1007/s00429-013-0510-1. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. Journal of the Neurological Sciences. 1999;169(1–2):13–21. doi: 10.1016/s0022-510x(99)00210-5. http://dx.doi.org/10.1016/S0022-510X(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Chiò A, Pagani M, Agosta F, Calvo A, Cistaro A, Filippi M. Neuroimaging in amyotrophic lateral sclerosis: Insights into structural and functional changes. The Lancet Neurology. 2014;13(12):1228–1240. doi: 10.1016/S1474-4422(14)70167-X. http://dx.doi.org/10.1016/S1474-4422(14) [DOI] [PubMed] [Google Scholar]
- Colman KSF, Koerts J, van Beilen M, Leenders KL, Post WJ, Bastiaanse R. The impact of executive functions on verb production in patients with Parkinson's disease. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2009;45(8):930–942. doi: 10.1016/j.cortex.2008.12.010. http://doi.org/10.1016/j.cortex.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Cousins KAQ, Ash S, Irwin DJ, Grossman M. Dissociable substrates underlie the production of abstract and concrete nouns. Brain and Language. 2017;165:45–54. doi: 10.1016/j.bandl.2016.11.003. http://doi.org/10.1016/j.bandl.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescentini C, Lunardelli A, Mussoni A, Zadini A, Shallice T. A left basal ganglia case of dynamic aphasia or impairment of extra-language cognitive processes? Neurocase: Case Studies in Neuropsychology, Neuropsychiatry, and Behavioural Neurology. 2008;14(2):184–203. doi: 10.1080/13554790802108380. http://doi.org/10.1080/13554790802108380. [DOI] [PubMed] [Google Scholar]
- Crescentini C, Mondolo F, Biasutti E, Shallice T. Supervisory and routine processes in noun and verb generation in nondemented patients with Parkinson's disease. Neuropsychologia. 2008;46(2):434–447. doi: 10.1016/j.neuropsychologia.2007.08.021. http://doi.org/10.1016/j.neuropsychologia.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Crescentini C, Shallice T, Macaluso E. Item retrieval and competition in noun and verb generation: An FMRI study. Journal of Cognitive Neuroscience. 2010;22(6):1140–1157. doi: 10.1162/jocn.2009.21255. http://doi.org/10.1162/jocn.2009.21255. [DOI] [PubMed] [Google Scholar]
- Druks J. Verbs and nouns - a review of the literature. Journal of Neurolinguistics. 2002;15(3–5):289–315. http://dx.doi.org/10.1016/S0911-6044(01)00029-X. [Google Scholar]
- Fernandino L, Conant LL, Binder JR, Blindauer K, Hiner B, Spangler K, et al. Parkinson's disease disrupts both automatic and controlled processing of action verbs. Brain and Language. 2013;127(1):65–74. doi: 10.1016/j.bandl.2012.07.008. http://doi.org/10.1016/j.bandl.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. http://dx.doi.org/10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Zweig RM. Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Movement Disorders. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. http://doi.org/10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- González-Redondo R, García-García D, Clavero P, Gasca-Salas C, García-Eulate R, Zubieta JL, et al. Rodríguez-Oroz MC. Grey matter hypometabolism and atrophy in Parkinson's disease with cognitive impairment: A two-step process. Brain: a Journal of Neurology. 2014;137(8):2356–2367. doi: 10.1093/brain/awu159. http://doi.org/10.1093/brain/awu159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barressi B. Cookie Theft picture. Boston diagnostic aphasia examination. Philadelphia: Lea Febiger. In: Levinson D, Darrow C, Klein E, Levinson M, McKee B, editors. The seasons of a man's life. NY: Knopf; 1983. [Google Scholar]
- Grossman M, Anderson C, Khan A, Avants B, Elman L, McCluskey L. Impaired action knowledge in amyotrophic lateral sclerosis. Neurology. 2008;71(18):1396–1401. doi: 10.1212/01.wnl.0000319701.50168.8c. http://doi.org/10.1212/01.wnl.0000319701.50168.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Stern MB, Gollomp S, Vernon G, Hurtig HI. Verb learning in Parkinson's disease. Neuropsychology. 1994;8(3):413–423. [Google Scholar]
- Holcomb PJ, Kounios J, Anderson JE, West WC. Dual-coding, context-availability, and concreteness effects in sentence comprehension: An electrophysiological investigation. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25(3):721–742. doi: 10.1037//0278-7393.25.3.721. http://doi.org/10.1037/0278-7393.25.3.721. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Integration of target and body-part information in the premotor cortex when planning action. Nature. 2000;408(6811):466–470. doi: 10.1038/35044075. http://doi.org/10.1038/35044075. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson KE. The pyramids and palm trees test: A test of semantic access from words and pictures. Thames Valley Test Company; 1992. [Google Scholar]
- Jamrozik A, Mcquire M, Cardillo ER, Chatterjee A. Metaphor: Bridging embodiment to abstraction. Psychonomic Bulletin & Review. 2015;23(4):1080–1089. doi: 10.3758/s13423-015-0861-0. http://doi.org/10.3758/s13423-015-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J. Parkinson's disease: Clinical features and diagnosis. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79(1957):368–376. doi: 10.1136/jnnp.2007.131045. http://doi.org/10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jessen F, Heun R, Erb M, Granath DO, Klose U, Papassotiropoulos A, et al. The concreteness effect: Evidence for dual coding and context availability. Brain and Language. 2000;74(1):103–112. doi: 10.1006/brln.2000.2340. http://doi.org/10.1006/brln.2000.2340. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston naming test. Pro-ed; 2001. [Google Scholar]
- Kemmerer D. Are the motor features of verb meanings represented in the precentral motor cortices? Yes, but within the context of a flexible, multilevel architecture for conceptual knowledge. Psychonomic Bulletin & Review. 2015;22(4):1068–1075. doi: 10.3758/s13423-014-0784-1. http://doi.org/10.3758/s13423-014-0784-1. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Miller L, Macpherson MK, Huber J, Tranel D. An investigation of semantic similarity judgments about action and non-action verbs in Parkinson's disease: Implications for the embodied cognition framework. Frontiers in Human Neuroscience. 2013;7(April):146. doi: 10.3389/fnhum.2013.00146. http://doi.org/10.3389/fnhum.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Tourville J. 101 labeled brain images and a consistent human cortical labeling protocol. Frontiers in Neuroscience. 2012;6(Dec):1–12. doi: 10.3389/fnins.2012.00171. http://doi.org/10.3389/fnins.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerts J, Leenders KL, Brouwer WH. Cognitive dysfunction in non-demented Parkinson's disease patients: Controlled and automatic behavior. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2009;45(8):922–929. doi: 10.1016/j.cortex.2009.02.014. http://doi.org/10.1016/j.cortex.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Lezak M, Howieson DB, Loring DW. Neuropsychological assessment. New York: Oxford University Press; 1983. [Google Scholar]
- Magnus T, Beck M, Giess R, Puls I, Naumann M, Toyka KV. Disease progression in amyotrophic lateral sclerosis: Predictors of survival. Muscle & Nerve. 2002;25(5):709–714. doi: 10.1002/mus.10090. http://doi.org/10.1002/mus.10090. [DOI] [PubMed] [Google Scholar]
- Mak E, Su L, Williams GB, Firbank MJ, Lawson RA, Yarnall AJ, et al. O'Brien JT. Baseline and longitudinal grey matter changes in newly diagnosed Parkinson's disease: ICICLE-PD study. Brain: a Journal of Neurology. 2015;138(10):2974–2986. doi: 10.1093/brain/awv211. http://doi.org/10.1093/brain/awv211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mätzig S, Druks J, Masterson J, Vigliocco G. Noun and verb differences in picture naming: Past studies and new evidence. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2009;45(6):738–758. doi: 10.1016/j.cortex.2008.10.003. http://doi.org/10.1016/j.cortex.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Meteyard L, Cuadrado SR, Bahrami B, Vigliocco G. Coming of age: A review of embodiment and the neuroscience of semantics. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2012;48(7):788–804. doi: 10.1016/j.cortex.2010.11.002. http://doi.org/10.1016/j.cortex.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Miller GA, Fellbaum C. Semantic networks of English. Cognition. 1991;41:197–229. doi: 10.1016/0010-0277(91)90036-4. [DOI] [PubMed] [Google Scholar]
- Obeso I, Casabona E, Bringas ML, Álvarez L, Jahanshahi M. Semantic and phonemic verbal fluency in Parkinson's disease: Influence of clinical and demographic variables. Behavioural Neurology. 2012;25(2):111–118. doi: 10.3233/BEN-2011-0354. http://doi.org/10.3233/BEN-2011-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péran P, Cardebat D, Cherubini A, Piras F, Luccichenti G, Peppe A, et al. Sabatini U. Object naming and action-verb generation in Parkinson's disease: A fMRI study. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2009;45(8):960–971. doi: 10.1016/j.cortex.2009.02.019. http://doi.org/10.1016/j.cortex.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Péran P, Démonet JF, Cherubini A, Carbebat D, Caltagirone C, Sabatini U. Mental representations of action: The neural correlates of the verbal and motor components. Brain Research. 2010;1328:89–103. doi: 10.1016/j.brainres.2010.02.082. http://doi.org/10.1016/j.brainres.2010.02.082. [DOI] [PubMed] [Google Scholar]
- Péran P, Nemmi F, Méligne D, Cardebat D, Peppe A, Rascol O, et al. Sabatini U. Effect of levodopa on both verbal and motor representations of action in Parkinson's disease: A fMRI study. Brain and Language. 2013;125(3):324–329. doi: 10.1016/j.bandl.2012.06.001. http://doi.org/10.1016/j.bandl.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Péran P, Rascol O, Demonet JF, Celsis P, Nespoulous JL, Dubois B, et al. Deficit of verb generation in nondemented patients with Parkinson's disease. Movement Disorders. 2003;18(2):150–156. doi: 10.1002/mds.10306. http://doi.org/10.1002/mds.10306. [DOI] [PubMed] [Google Scholar]
- Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. The Lancet Neurology. 2007;6(11):994–1003. doi: 10.1016/S1474-4422(07)70265-X. http://dx.doi.org/10.1016/S1474-4422(07)70265-X. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Tröster AI. Action (verb naming) fluency as an executive function measure: Convergent and divergent evidence of validity. Neuropsychologia. 1999;37(13):1499–1503. doi: 10.1016/s0028-3932(99)00066-4. http://dx.doi.org/10.1016/S0028-3932(99)00066-4. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nature Reviews Neuroscience. 2005;6(July):576–582. doi: 10.1038/nrn1706. http://doi.org/10.1038/2201358a0. [DOI] [PubMed] [Google Scholar]
- Raposo A, Moss HE, Stamatakis EA, Tyler LK. Modulation of motor and premotor cortices by actions, action words and action sentences. Neuropsychologia. 2009;47(2):388–396. doi: 10.1016/j.neuropsychologia.2008.09.017. http://doi.org/10.1016/j.neuropsychologia.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ferreiro J, Menéndez M, Ribacoba R, Cuetos F. Action naming is impaired in Parkinson disease patients. Neuropsychologia. 2009;47(14):3271–3274. doi: 10.1016/j.neuropsychologia.2009.07.007. http://doi.org/10.1016/j.neuropsychologia.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Rowe J, Rittman T. Oxford textbook of cognitive neurology and dementia. UK: Oxford University Press Oxford; 2016. The basal ganglia in cognitive disorders; pp. 69–80. [Google Scholar]
- Salmazo-Silva H, Parente MAdeMP, Rocha MS, Baradel RR, Cravo AM, Sato JR, et al. Carthery-Goulart MT. Lexical-retrieval and semantic memory in Parkinson's disease: The question of noun and verb dissociation. Brain and Language. 2017;165:10–20. doi: 10.1016/j.bandl.2016.10.006. http://doi.org/10.1016/j.bandl.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Ciccarelli N, Baldonero E, Piano C, Zinno M, Soleti F, et al. Daniele A. Effects of stimulation of the subthalamic nucleus on naming and reading nouns and verbs in Parkinson's disease. Neuropsychologia. 2012;50(8):1980–1989. doi: 10.1016/j.neuropsychologia.2012.04.023. http://doi.org/10.1016/j.neuropsychologia.2012.04.023. [DOI] [PubMed] [Google Scholar]
- Strong MJ, Abrahams S, Goldstein LH, Woolley S, Mclaughlin P, Snowden J, et al. Turner MR. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotrophic Lateral Sclerosis & Frontotemporal Degeneration. 2017:1–22. doi: 10.1080/21678421.2016.1267768. http://doi.org/10.1080/21678421.2016.1267768. [DOI] [PMC free article] [PubMed]
- Vigliocco G, Vinson DP, Druks J, Barber H, Cappa SF. Nouns and verbs in the brain: A review of behavioural, electrophysiological, neuropsychological and imaging studies. Neuroscience and Biobehavioral Reviews. 2011;35(3):407–426. doi: 10.1016/j.neubiorev.2010.04.007. http://doi.org/10.1016/j.neubiorev.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Stone CP. Wechsler memory scale-revised. Psychological Corporation; 1987. [Google Scholar]
- York C, Olm C, Boller A, McCluskey L, Elman L, Haley J, et al. Grossman M. Action verb comprehension in amyotrophic lateral sclerosis and Parkinson's disease. Journal of Neurology. 2014;261(6):1073–1079. doi: 10.1007/s00415-014-7314-y. http://doi.org/10.1007/s00415-014-7314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]