Significance
Copper is an important biological cofactor, but can also be toxic in excess. Members of the P1B-ATPase family of membrane transporters couple the energy of ATP hydrolysis to translocation of metal ions across membranes. P1B-ATPases have been classified into groups on the basis of sequence and metal ion specificity. Two subfamilies, the P1B-1-ATPases, which are linked to human diseases of copper metabolism, and the P1B-3-ATPases, found only in bacteria, have been assigned as Cu+ and Cu2+ transporters, respectively. Here we show that the P1B-3-ATPases are actually Cu+ transporters, necessitating revision of the classification scheme. These findings are consistent with the presence of only Cu+ in the cytoplasm, which eliminates the need for a Cu2+ efflux pump.
Keywords: P1B-ATPase, copper homeostasis, copper efflux, CopB, CopA
Abstract
The copper-transporting P1B-ATPases, which play a key role in cellular copper homeostasis, have been divided traditionally into two subfamilies, the P1B-1-ATPases or CopAs and the P1B-3-ATPases or CopBs. CopAs selectively export Cu+ whereas previous studies and bioinformatic analyses have suggested that CopBs are specific for Cu2+ export. Biochemical and spectroscopic characterization of Sphaerobacter thermophilus CopB (StCopB) show that, while it does bind Cu2+, the binding site is not the prototypical P1B-ATPase transmembrane site and does not involve sulfur coordination as proposed previously. Most important, StCopB exhibits metal-stimulated ATPase activity in response to Cu+, but not Cu2+, indicating that it is actually a Cu+ transporter. X-ray absorption spectroscopic studies indicate that Cu+ is coordinated by four sulfur ligands, likely derived from conserved cysteine and methionine residues. The histidine-rich N-terminal region of StCopB is required for maximal activity, but is inhibitory in the presence of divalent metal ions. Finally, reconsideration of the P1B-ATPase classification scheme suggests that the P1B-1- and P1B-3-ATPase subfamilies both comprise Cu+ transporters. These results are completely consistent with the known presence of only Cu+ within the reducing environment of the cytoplasm, which should eliminate the need for a Cu2+ P1B-ATPase.
Metal ions are required for critical cellular functions (1). In particular, copper is an essential cofactor in a multitude of proteins, but can be toxic, destroying iron–sulfur clusters and causing oxidative stress (2). Thus, regulating copper concentrations presents a major challenge to all organisms; 44% of the copper proteome has been assigned to copper homeostasis (3, 4). Proteins involved in copper homeostasis include metallosensors, metallochaperones, and membrane transporters (5). Among the transporters are the P1B-ATPases, a subset of the P-type ATPase family (6). P1B-ATPases are ubiquitous in nature and couple the energy of ATP hydrolysis to translocation of transition metal ions, including Cu+, Cu2+, Zn2+/Cd2+/Pb2+, and Co2+ (7–9). P1B-ATPases consist of six to eight transmembrane (TM) helices, an ATP-binding domain (ATPBD), an actuator domain, and in many cases, additional soluble metal-binding domains (MBDs), typically at the N terminus. Transport is accomplished via a classical Post-Albers cycle in which phosphorylation of a conserved aspartate residue in the ATPBD causes the enzyme to cycle between high (E1)- and low (E2)-affinity metal-binding states (10). In humans, defects in the Cu+-transporting P1B-ATPases ATP7A and ATP7B lead to Menkes syndrome and Wilson disease, respectively (11).
P1B-ATPases have been categorized into seven subtypes (P1B-1–P1B-7) based on conserved motifs in the TM domain, the presence of different types of MBDs, and biochemical and genetic data linking individual transporters to specific metal ions (7, 12, 13). One of the key TM motifs is a three-residue, cysteine-containing sequence in TM helix 4; other conserved residues in TM helix 6 have also been considered in developing the classification scheme. Of the subclasses, the P1B-2-ATPases transport Zn2+, Cd2+, and Pb2+ (CPC motif) (9, 14, 15), and the P1B-4-ATPases transport Co2+, Cd2+, Zn2+, and Fe2+ (SPC motif) (16–19). The metal specificities of the P1B-5 (PCP motif), P1B-6 (SCA motif), and P1B-7-ATPases (CSC motif) remain unclear, although some evidence links the P1B-5-ATPases to Ni2+ and Fe2+ (20, 21). The remaining two groups are the copper transporters. The P1B-1-ATPases, which include ATP7A and ATP7B, transport Cu+ (9, 22), whereas the P1B-3-ATPases are proposed to transport Cu2+ (23, 24). These two subfamilies differ from one another in several ways. First, the TM helix 4 motif is CPC in the P1B-1-ATPases and CPH in the P1B-3-ATPases. The presence of this histidine has been widely assumed to confer a preference for Cu2+ (12, 13, 23, 24). The P1B-3-ATPases, referred to as CopBs, are unique in that they contain a histidine-rich N-terminal extension that is proposed to be an MBD (23). This extension varies considerably in length, ranging from about 40–120 residues (SI Appendix, Fig. S1). By contrast, the P1B-1-ATPases, of which many are designated CopAs, usually have MBDs comprising one to six ferredoxin-like domains characterized by a conserved CXXC metal-binding motif that binds a single Cu+ ion (25).
The P1B-1-ATPases have been studied intensively due to the link to Wilson and Menkes diseases (11), with multiple solution structures of the MBDs (25), electron microscopy structures of Archaeoglobus fulgidus CopA (26) and ATP7B (27), and a crystal structure of CopA from Legionella pneumophila (LpCopA), albeit in the absence of bound copper or the MBD (28), available. In addition, the roles of the MBDs (29) and the nature of the TM Cu+-binding site (9, 29) have been probed by a range of biochemical, biophysical, and functional studies. By contrast, just a few studies of the P1B-3-ATPases, which are found only in bacteria, have been reported. While early work on Enterococcus hirae CopB (EhCopB) (30) suggested that it is a Cu+ transporter, subsequent studies of thermophilic CopBs from A. fulgidus (AfCopB) (23) and Thermus thermophilus (TtCopB) (31) indicated that CopBs are mainly Cu2+ transporters. Metal binding by the histidine-rich N-terminal region has not been investigated for any CopB. Thus, it remains unclear how the two classes of copper P1B-ATPases confer selectivity for Cu+ versus Cu2+.
To investigate the basis for discrimination between Cu+ and Cu2+ by P1B-ATPases, we biochemically and spectroscopically characterized the CopB from Sphaerobacter thermophilus (StCopB), both with and without its 120-residue N-terminal histidine-rich domain. Contrary to prior reports and accepted dogma, our results indicate that StCopB is a Cu+ transporter. Binding of Cu2+ is observed, but mutagenesis and electron paramagnetic resonance (EPR) spectroscopic data indicate that it is not located in the proposed TM binding site. Instead, X-ray absorption spectroscopic (XAS) studies on the Cu+-bound protein define a sulfur-based coordination environment in the TM region. A reexamination of the bioinformatics analysis suggests that the P1B-1- and P1B-3-ATPases are subsets of the same class and provides a revised framework for overall P1B-ATPase classification. Finally, insights into the possible role of the N-terminal histidine-rich region are presented.
Results and Discussion
StCopB Does Not Bind Cu2+ in the Archetypal P1B-type ATPase TM Site.
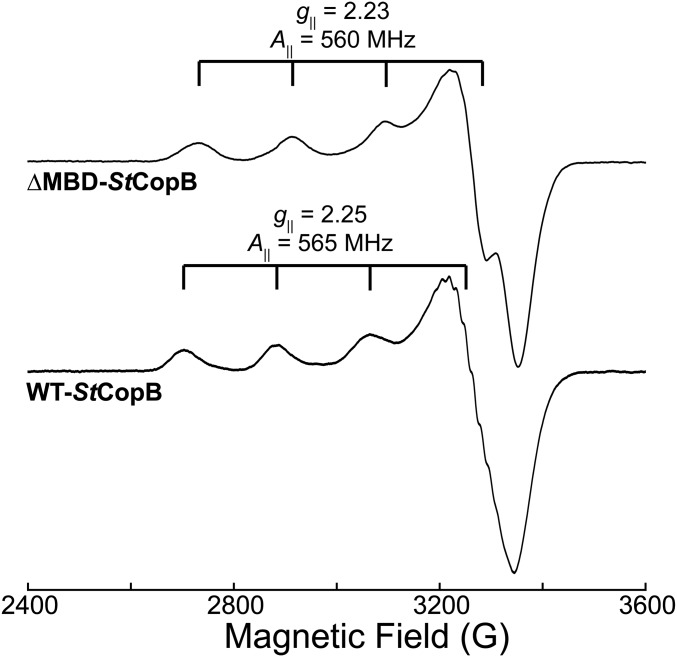
We initially assumed that StCopB is a Cu2+ transporter, as has been reported for AfCopB (23) and TtCopB (31). To probe Cu2+ binding by the CopB TM metal-binding site and to investigate whether the N-terminal histidine-rich regions bind metal ions, we expressed and purified both full-length StCopB (WT-StCopB, residues 1–785) and StCopB lacking the N-terminal region (ΔMBD-StCopB, residues 114–785) (SI Appendix, Fig. S2). After overnight incubation with varying amounts of Cu2+, followed by removal of excess copper, quantitation indicated that ΔMBD-StCopB binds a single Cu2+ ion even when incubated with a 10-fold excess (SI Appendix, Table S1). We first believed the Cu2+ to be bound at the TM-binding site reported in a recent study of AfCopB (24). Indeed, the EPR spectrum of ΔMBD-StCopB exhibited the type-2 Cu2+ signature (Fig. 1) reported for AfCopB (g|| = 2.23, g⊥ = 2.05, A|| = 560 MHz for ΔMBD-StCopB; g|| = 2.23, g⊥ = 2.06, A|| = 565 MHz for AfCopB) and attributed to nitrogen/oxygen (N/O) equatorial ligands on the basis of the Peisach–Blumberg correlation diagram (32). Consistent with this environment, electron-nuclear double resonance spectra collected on ΔMBD-StCopB identified at least one directly coordinated equatorial 14N ligand and suggested the presence of a coordinated HxO (SI Appendix, Fig. S3A).
Fig. 1.
EPR spectroscopic characterization of StCopB. Continuous wave (CW) X-band EPR spectra for (Top) ∆MBD-StCopB and (Bottom) 0.75 equiv Cu2+-loaded WT-StCopB. Spectra were scaled to unity. Conditions: 9.36–9.37 GHz microwave frequency, 320 ms time constant, 16 G modulation amplitude, 80 s scan time, and 20 K temperature.
Potential ligands to this presumed TM Cu2+-binding site were identified by aligning a homology model of WT-StCopB (33) with the crystal structure of LpCopA (28). In this model, the CPH motif of StCopB is in the same position as the CPC motif of LpCopA (SI Appendix, Fig. S4). To assess whether the Cu2+-binding site involves the CPH motif, as suggested for AfCopB (24), several variants (C404A, H406A, and C404A, H406A) of ΔMBD-StCopB were generated. Surprisingly, the EPR spectra of all three variants exhibited the same signal as ΔMBD-StCopB (SI Appendix, Fig. S3B), showing that the CPH motif is not the site of Cu2+ binding in StCopB.
We then investigated the Cu2+-binding properties of full-length WT-StCopB. Reconstitution of WT-StCopB with increasing amounts of Cu2+ [0–15 equivalent (equiv)] indicated that it can bind up to approximately eight Cu2+ ions (SI Appendix, Fig. S5). Since ΔMBD-StCopB can bind approximately one Cu2+ ion, no fewer than seven Cu2+ ions must bind to the MBD. In fact, none of the spectra at loading concentrations between 0.75 and 10 Cu2+ equiv show the spectrum of ΔMBD-StCopB (SI Appendix, Fig. S3C). Instead, WT-StCopB loaded with 0.75 equiv Cu2+ exhibits a type-2 Cu2+ EPR signal (g|| = 2.25, g⊥ = 2.05, A|| = 565 MHz) similar to, but distinct from, that of ΔMBD-StCopB, in particular with resolved 14N hyperfine along g⊥ (Fig. 1). The WT-StCopB spectrum shows a strong resemblance to that of Cu2+-(imidazole)4 (34). This similarity, combined with the abundance of histidine residues in the MBD, as well as the fact that this spectrum is distinct from that of ΔMBD-StCopB, suggests that histidine side chains in the MBD HXXH motifs (SI Appendix, Fig. S1) are the probable WT-StCopB Cu2+ ligands. Increasing the amount of Cu2+ increases the intensity of this signal without changing the Cu2+ g|| and A|| values, while the 14N hyperfine splitting along g⊥ is lost upon addition of 5 equiv Cu2+. This observation indicates that the multiple Cu2+ sites in the MBD have very similar coordination spheres with similar sets of N/O ligands, but with slight variations in their imposed coordination geometries.
Diversity Among CopB MBDs and Characterization of the StCopB MBD.
Sequence alignments show that CopB-MBD sequences are diverse in length and sequence (SI Appendix, Fig. S1). A close examination of these sequences reveals a strong resemblance to hydrophilins, proteins characterized by high glycine content (>6%) and a high hydrophilicity index (>1.0) (35). The StCopB-MBD sequence, which is composed of ∼27% histidine residues located in repeated HXXH and HXH motifs, has >13% glycine residues and is highly hydrophilic with a grand average of hydropathicity (36) score of −1.5. Polar ϕ-segments are present throughout the StCopB MBD, although the canonical hydrophilin (Y-, S-, and K-) segments (37) are largely absent, similar to the histidine-rich plant dehydrins, which are involved in protecting cells from a variety of stress conditions, particularly osmotic and toxic metal stress (38).
To probe metal binding by the isolated StCopB MBD, recombinantly expressed StCopB MBD (residues 1–120, without added histidine tag) was purified using a Ni-NTA column (SI Appendix, Fig. S2). Consistent with secondary and tertiary structure predictions (SI Appendix, Fig. S6), the circular dichroism (CD) spectrum of StCopB MBD exhibits a large negative signal at 198 nm, indicative of random coil secondary structure (SI Appendix, Fig. S7). Addition of one equivalent of metal (Ni2+, Cu2+, Zn2+, or Ag+) did not produce any spectral changes. Further metal addition led to protein precipitation, reminiscent of the metal-induced aggregation reported for histidine-rich plant dehydrins (39). The CD spectrum collected in the presence of 33% 2,2,2-trifluoroethanol, known to stabilize secondary structure in proteins (40, 41), showed high helical content (208 and 222 nm), suggesting that StCopB MBD can form an ordered secondary structure (SI Appendix, Fig. S7). In addition, homology modeling of WT-StCopB predicts multiple different folds for the MBD within WT-StCopB, including a small ferredoxin-like fold or several helices (SI Appendix, Fig. S6). Thus, it might adopt a folded structure in the context of intact StCopB.
StCopB Is a Cu+-Specific P1B-ATPase.
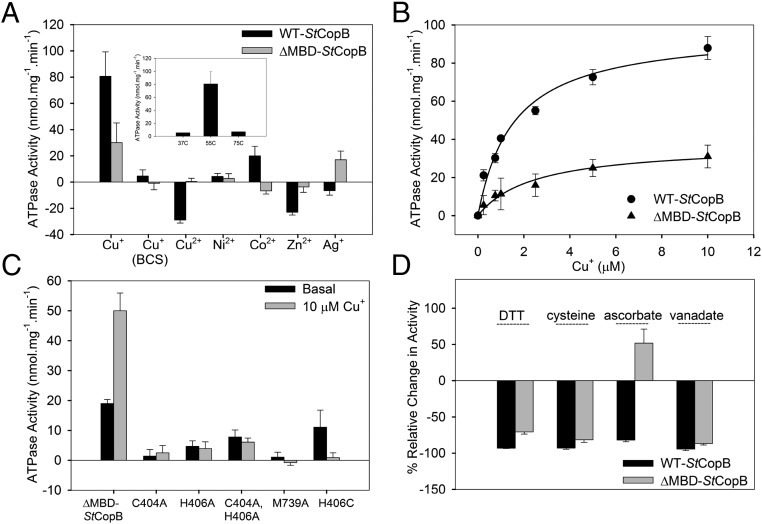
We next measured the ATP hydrolysis activity of WT-StCopB and ΔMBD-StCopB in the presence of various divalent metal ions (Cu2+, Ni2+, Co2+, or Zn2+). The majority of previously characterized P1B-3-ATPases, including AfCopB, TtCopB, and a CopB from Aquifex aeolicus (AaCtrA3), have been implicated in Cu2+ transport (23, 31, 42). Surprisingly, for both WT-StCopB and ΔMBD-StCopB, Cu2+-dependent stimulation of ATPase activity was not observed (Fig. 2A). Moreover, the basal ATPase activity for WT-StCopB was inhibited at ≥10 µM Cu2+, whereas Cu2+ addition did not affect the ΔMBD-StCopB basal activity. A similar effect was observed upon addition of Zn2+ to WT-StCopB and ΔMBD-StCopB (Fig. 2A). The observed inhibition could be due to binding of excess Cu2+ and Zn2+ by WT-StCopB (SI Appendix, Fig. S5). Conformational changes in the MBD might then hinder ATP hydrolysis by the ATPBD. WT-StCopB also exhibited some activity in the presence of Co2+, but not in the presence of Ag+, whereas ΔMBD-StCopB displayed the opposite trend with no activity stimulation by Co2+ and some activity upon Ag+ addition. Stimulation of ATPase activity and transport by Ag+ has been reported for AfCopB (23) and EhCopB (30).
Fig. 2.
Functional characterization of StCopB. (A) Metal-stimulated ATPase activity (nmol Pi mg−1⋅min−1) of WT-StCopB and ∆MBD-StCopB in the presence of 10 µM metal ions. Activity levels were corrected against basal activity in the absence of metal ions (WT-StCopB basal activity: 42.5 ± 7.2 nmol Pi mg−1⋅min−1 and ∆MBD-StCopB basal activity: 18.8 ± 2.0 nmol Pi mg−1⋅min−1). The maximal ATPase activity was observed in the presence of 10 µM Cu+ (WT-StCopB: 123 ± 17 nmol Pi mg−1⋅min−1; ∆MBD-StCopB: 50 ± 6 nmol Pi mg−1⋅min−1). (Inset) Maximal Cu+-stimulated WT-StCopB ATPase activity (basal corrected) at 37 °C, 55 °C, and 75 °C (representative values shown for 37 °C and 75 °C). (B) Specific ATPase activity (basal-corrected) of WT-StCopB (circles) and ∆MBD-StCopB (triangles) as a function of Cu+ concentration (µM) fitted to the equation y = (Vmax × x)/(Km + x). (C) Basal and Cu+-stimulated ATPase activity levels of ∆MBD-StCopB and its variants. (D) Relative effects of various reducing agents (DTT, cysteine, ascorbate) on maximal Cu+-stimulated ATP hydrolysis activity of WT-StCopB and ∆MBD-StCopB. Additionally, almost complete inhibition of ATPase activity was observed in the presence of 1 mM sodium orthovanadate, a phosphate analog and P-type ATPase competitive inhibitor. In all cases, error bars represent the SD of the average of at least three independent experiments.
Since early studies of EhCopB indicated that it is a Cu+ transporter (30), and since three other CopBs [AfCopB (23), TtCopB (31), and AaCtrA3 (42)] were in fact significantly active in the presence of Cu+ as well as Cu2+ (25, 50, and 50% of Cu2+-stimulated activity, respectively), we considered the possibility that StCopB may be specific for Cu+ rather than Cu2+ and performed the ATPase activity assay in the presence of Cu+ generated by reduction of Cu2+ with 2-mercaptoethanol (2-ME). Unexpectedly, both WT-StCopB and ΔMBD-StCopB showed significant Cu+-stimulated ATPase activity with maximal values of 123 ± 17 nmol Pi mg−1⋅min−1 (basal activity: 42.5 ± 7.2 nmol Pi mg−1⋅min−1) and 50 ± 6 nmol Pi mg−1⋅min−1 (basal activity: 18.8 ± 2.0 nmol Pi mg−1⋅min−1), respectively, at 55 °C, which is the optimal growth temperature for the bacteria (43) (Fig. 2A, Inset). Lipids (0.01% asolectin) were required to observe this metal-stimulated ATPase activity. The measured Km values of 1.6 ± 0.3 µM and 2.5 ± 0.6 µM for Cu+ for WT-StCopB and ΔMBD-StCopB (Fig. 2B), respectively, are similar to previously reported Km values for CopB and CopA (31, 44, 45). Furthermore, Cu+-stimulated activity was eradicated by 1 mM bathocuproinedisulfonic acid (BCS) (Fig. 2A), a high-affinity Cu+-specific chelator. The pronounced activity enhancement in the presence of Cu+ compared with all other metal ions tested indicates that StCopB is a Cu+-specific transporter.
Having demonstrated that Cu2+ does not bind in the CPH TM site, we reasoned that this site might instead bind Cu+. Consistent with this hypothesis, the three CPH motif variants (C404A, H406A, and C404A,H406A) did not exhibit Cu+-stimulated ATPase activity (Fig. 2C). These results are at least in part due to disrupted binding of Cu+. Whereas WT-StCopB and ΔMBD-StCopB bind ∼1 molar equiv of Cu+, consistent with a single high-affinity Cu+-binding site and no Cu+ binding by the MBD (SI Appendix, Table S2), Cu+ binding by ΔMBD-StCopB variants C404A, H406A, and C404A,H406A is diminished by ∼20–50%. Mutation of the conserved methionine in the TM helix 6 MSXST (12, 13) motif (M739A) also abolished activity, similar to what was observed for the LpCopA M717V variant (46), although the M739A variant still bound close to 1 equiv of Cu+. The lack of ATPase activity likely results from disruption of the ion release pathway as proposed for LpCopA (46). Surprisingly, replacement of the conserved histidine residue in the CPH motif, H406, with cysteine to mimic the CPC motif in the Cu+-transporting P1B-1-ATPases resulted in similar basal ATPase activity to ΔMBD-StCopB, but absolutely no activity in the presence of Cu+. However, this H406C variant can be reconstituted with ∼2.1 molar equiv of Cu+ (SI Appendix, Table S2), suggesting that conversion of the CPH motif to a CPC motif introduces a second Cu+-binding site.
To probe the apparent difference in metal selectivity between AfCopB and StCopB, we purified full-length AfCopB (residues 1–690) (SI Appendix, Fig. S2) and tested it for Cu+-stimulated activity. Similar to what was reported by Meloni et al. (24), we observed no Cu2+- or Cu+-stimulated activity for purified AfCopB even in the presence of the lipids (0.01% asolectin). A Vmax value of 1.2 μmol Pi mg−1⋅h−1 (20 nmol Pi mg−1⋅min−1) was reported for AfCopB in the presence of 1 μM CuCl2, but may represent basal activity (24). We also tested AfCopB in Escherichia coli membranes prepared without any purification and detergent solubilization [similar to the procedure reported by Mana-Capelli et al. (23)] and observed very high basal activity at 75 °C with no detectable metal stimulation.
To further compare our results to previous work, we investigated the use of reductants other than 2-ME to generate Cu+, specifically DTT and cysteine, which were used in other studies of CopAs (28, 44) and CopBs (24). In contrast to 2-ME, no Cu+-stimulated ATPase activity for WT-StCopB or ΔMBD-StCopB was observed in the presence of DTT or cysteine (Fig. 2D). Similar levels of Cu+-stimulated ATPase activity were obtained in the presence of ascorbate for ΔMBD-StCopB, but no stimulation was observed for WT-StCopB, which could be due to Cu2+/ascorbate-mediated oxidation of the histidine residues in the MBD (47). It is important to note that DTT and cysteine can coordinate Cu+, DTT with a KD ≈ 10−15 M (48) and cysteine with a KD ≈ 10−10 M (49). Formation of these high-affinity complexes limits the availability of Cu+ and almost certainly accounts for the lack of Cu+-stimulated ATPase activity using these reductants. Consistent with this notion, DTT and cysteine also inhibited the metal-stimulated activity of the P1B-4-ATPAse CzcP (18). We confirmed the complexation of Cu+ by DTT and cysteine by monitoring Cu+ chelation with BCS at ∼485 nm (SI Appendix, Fig. S8). Immediate Cu+–BCS complex formation was observed upon addition of 2-ME or ascorbate, whereas reaction with DTT, cysteine, and tris(2-carboxyethyl)phosphine (TCEP) for 15 min failed to produce any significant Cu+–BCS complex, indicating that Cu+ is either sequestered by these reductants (DTT, cysteine) or not reduced (TCEP) and thus would not be available to stimulate ATPase activity. These findings explain the relative lack of Cu+-stimulated activity observed for AfCopB (23) and TtCopB (31), which were assayed in the presence of DTT. TtCopB was in fact suggested to use Cu+ in vivo (31).
A Sulfur-Containing Cu+ Coordination Environment.
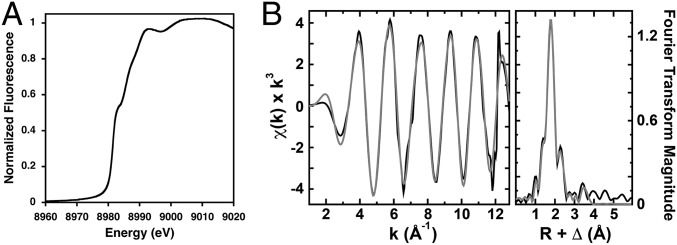
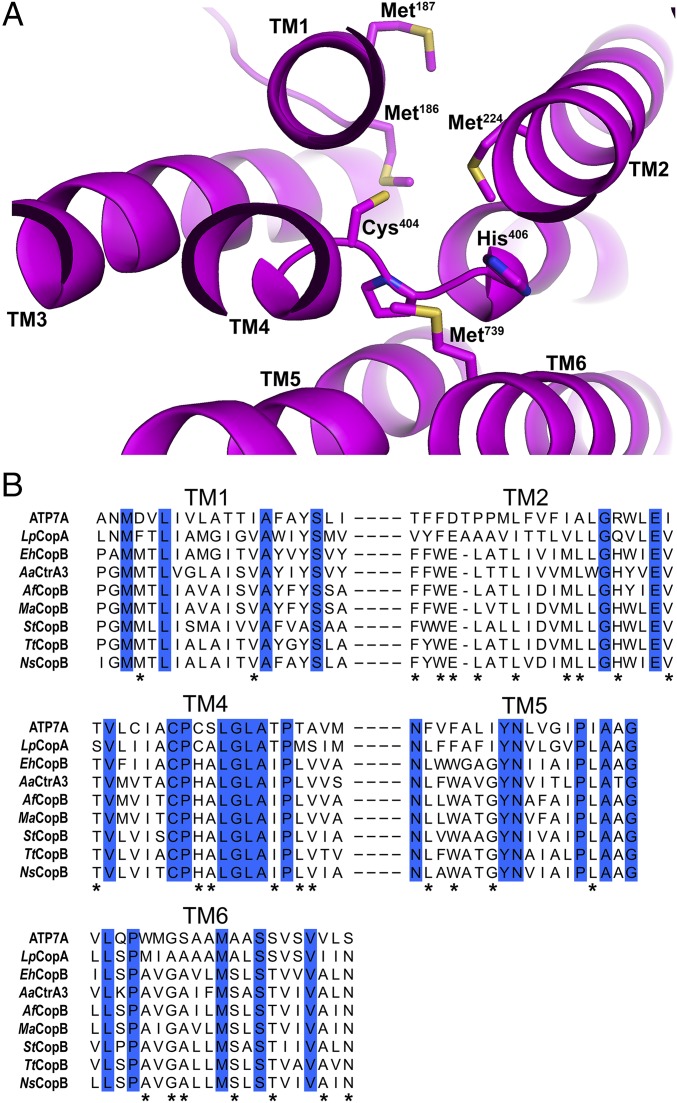
To elucidate the atomic details of StCopB Cu+ coordination within the TM region, we performed XAS analysis of Cu+-loaded WT-StCopB. The WT-StCopB XANES spectrum exhibits a 1s→4p transition at 8,983 eV (Fig. 3A), consistent with the presence of Cu+. Cu K-edge extended X-ray absorption fine structure (EXAFS) data of the Cu+-loaded WT-StCopB (Fig. 3B) were best fit with a nearest-neighbor environment composed of three S ligands at an average bond length of 2.27 Å and one S ligand at 2.48 Å, with no long-range scattering observed (SI Appendix, Table S3). There is no evidence for histidine coordination. Close inspection of the homology model and sequence alignment indicates the presence of a sulfur-lined inner channel that could be involved in Cu+ transport (Fig. 4A). The EXAFS data suggest a TM Cu+-binding site coordinated by Cys404 and potentially three methionine residues (Met186, Met187, Met224) in a tetrahedral fashion, although the presence of a chloride ligand cannot be ruled out. Similar sites involving Cu+ coordinated by Cys and Met residues in a trigonal planar geometry have been proposed for LpCopA (50) and human CTR1 (51). Importantly, all four proposed ligands are absolutely conserved among CopBs, with the methionine residues deriving from a PGMM motif in TM helix 1 and a MLLG motif in TM helix 2 (Fig. 4). These motifs, rather than the CopB CPH motif, likely confer metal specificity for Cu+.
Fig. 3.
X-ray absorption spectroscopic analysis of Cu+-loaded WT-StCopB. (A) Normalized K-edge Cu XANES spectra of WT-StCopB. The peak at 8,984 eV corresponds to a Cu+ 1s→4p transition. (B) Raw, unfiltered EXAFS data (black) and simulations (gray) (Left) and Fourier transforms of the raw EXAFS (black) and best-fit simulations (gray) (Right).
Fig. 4.
Proposed StCopB Cu+-binding site. (A) Close-up view of potential Cu+-binding site in the StCopB homology model. Residues that may be important in Cu+ binding and are absolutely conserved among CopBs include Met186 (TM1), Met187 (TM1), Met224 (TM2), and Cys404 (TM4). (B) Sequence alignment of the TM domains of human ATP7A, L. pneumophila CopA (LpCopA), E. hirae CopB (EhCopB), A. aeolicus copper transporter (AaCtrA3), A. fulgidus CopB (AfCopB), Methanosarcina acetivorans CopB (MaCopB), S. thermophilus CopB (StCopB), T. thermophilus CopB (TtCopB), and Nostoc sp. CopB (NsCopB) showing absolutely conserved residues (blue) among CopAs and CopBs. The residues absolutely conserved among CopBs are also highlighted (*).
Comparison of CopB and CopA sequences indicates that many key residues proposed for Cu+ transport by LpCopA (50, 52) are highly conserved among CopBs as well (Fig. 4B). In particular, a channel lined with residues Met148, Met717, Glu189, Glu205, and Asp337 in LpCopA corresponds to a proposed channel lined with Met186, Met739, Glu216, Glu231, and Asp362 in StCopB and is absolutely conserved among CopB and CopA sequences. In addition, StCopB Met224 is within the putative metal transport path and is strictly conserved among CopBs, but not in CopAs. This residue could potentially serve as a ligand in the absence of the second cysteine present in the CopA CPC motif, but not in the CopB CPH motif. The inhibitory effect of Cu+ on the CPC StCopB mutant might therefore derive from Cu+ binding in an incorrect conformation involving the second cysteine. These comparisons suggest that CopA and CopB utilize similar, but not identical, sets of residues to bind and export Cu+.
Finally, we probed the copper coordination environment of Cu2+-loaded ΔMBD-StCopB and the TM helix 4 CPH motif variants by XAS (SI Appendix, Fig. S9 and Table S4). Both the C404A and H406A variants displayed coordination environments similar to ΔMBD-StCopB, involving only N/O ligands and no S ligands. EXAFS fits with 3N/O and 1S ligands led to high Debye–Waller factors, indicating that an S ligand is not involved in Cu2+ binding. This result is in contrast to what was observed for AfCopB (24), but is consistent with the EPR analysis of these proteins (Fig. 1) as well as that of AfCopB (24). Additionally, the signature camelback feature observed in the Cu2+-loaded ΔMBD-StCopB EXAFS indicates histidine ligation.
Revising the P1B-ATPase Classification Scheme.
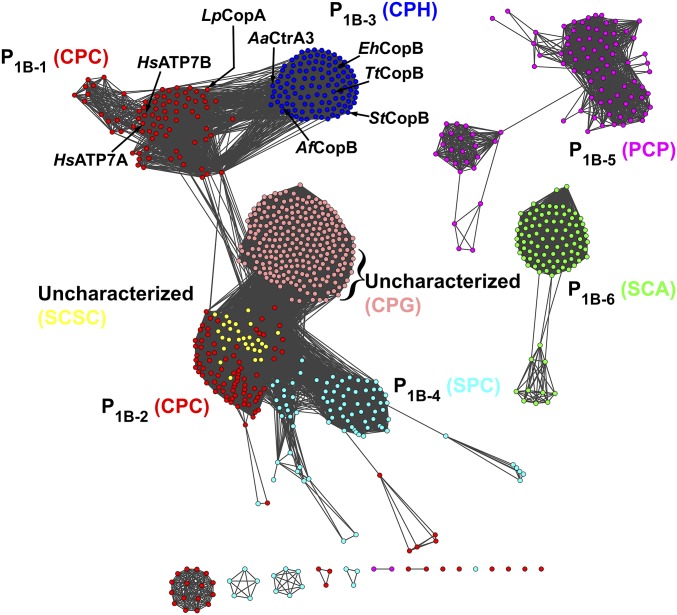
Given the strong evidence that StCopB and likely the other characterized CopBs are actually Cu+ transporters, we reexamined the classification of P1B-ATPases into the P1B-1–P1B-7 subfamilies (7, 12, 13). Notably, we identified several hundred P1B-ATPase sequences that belong to a subgroup characterized by a conserved CPG motif in TM helix 4. No member of this P1B-ATPase subfamily has been characterized. We then reinvestigated our previously generated P1B-ATPase sequence similarity network (13), incorporating 250 of these CPG-containing sequences and 22 sequences containing the SCSC TM helix 4 motif (previously classified as P1B-7-ATPases) into the clustering analysis (Fig. 5). Similar to the previous analysis, the sequences containing different TM helix 4 motifs clustered into distinct groups. The P1B-5 (PCP) and P1B-6 (SCA) subfamilies clustered separately, as previously observed. The CPG motif-containing sequences clustered strongly together, with some connections to the P1B-2 sequences containing the CPC motif. Importantly, the P1B-1 (CPC) and P1B-3 (CPH) subfamilies clustered separately as shown previously (13), but they are strongly connected when the network is visualized at 35% sequence identity, indicating that the two subfamilies are closely related. Moreover, they are more closely related to each other than both P1B-1 and P1B-2 sequences that contain the CPC motif. Finally, the SCSC motif-containing sequences clustered strongly within the P1B-2 subfamily, suggesting that these sequences are likely part of that subfamily rather than a separate subfamily.
Fig. 5.
The extended P1B-ATPase similarity network. Sequences are represented as nodes (colored circles), and the strength of their similarity is indicated by edges (lines connecting colored circles). Sequences are color coded and labeled by their signature TM helix 4 motifs. The pink cluster represents newly identified sequences containing a conserved CPG motif. Representative CopAs and CopBs discussed in the text are labeled.
Conclusions.
The combined results indicate that StCopB is a Cu+ transporter that binds a single Cu+ ion in the TM region using four sulfur ligands. It does bind Cu2+, but the mutagenesis data show that the TM Cu2+-binding site does not involve the TM helix 4 CPH motif as hypothesized previously. Instead, EPR and EXAFS data indicate that Cu2+ is coordinated by N/O ligands, including histidine. The location of the Cu2+-binding site remains unclear. The activity data clearly show that Cu+, but not Cu2+, stimulated ATP hydrolysis by StCopB, and previous reports of no Cu+-stimulated activity can be attributed to the use of Cu+-complexing agents as reductants. While the hydrophilin-like StCopB MBD is necessary for maximal activity, it surprisingly does not bind Cu+. Instead, binding of approximately eight Cu2+ or Zn2+ ions inhibits basal ATPase activity, perhaps by interfering with the ATPBD. Thus, the MBD may have multiple functions, stabilizing the ATPBD during Cu+ efflux and sequestering metal ions other than Cu+ under conditions of stress, as observed in the similar plant dehydrins (38). The overall conservation of residues proposed to be important for copper transport by CopAs and CopBs is striking, and, taken together with the revised sequence similarity network, suggests that these two P1B-ATPase subfamilies represent related solutions for Cu+ transport rather than the existence of specific Cu+ and Cu2+ transporters. This conclusion resolves a long-standing conundrum in the field: copper within the reducing environment of the cytoplasm should be Cu+ (4, 31, 53, 54), obviating the need for a Cu2+ P1B-ATPase.
Materials and Methods
Detailed procedures for preparation of StCopB proteins and variants, protein metal loading and quantitation, and ATPase activity assays are included in SI Appendix, SI Materials and Methods. Also described are computational methods for structure prediction, homology modeling, and sequence similarity network generation. Standard methods were used for collection of circular dichroism, EPR, and XAS spectroscopic data; instrument specifics are given in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants GM58518 (to A.C.R.), GM118035 (to A.C.R.), DK068139 (to T.L.S.), GM111097 (to B.M.H.), and 5T32GM008382 (to M.O.R.). Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource (SSRL), a directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the US Department of Energy Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the Department of Energy Office of Biological and Environmental Research and by NIH–National Institute of General Medical Sciences (including Grant P41GM103393).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721783115/-/DCSupplemental.
References
- 1.Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Metalloproteins and metal sensing. Nature. 2009;460:823–830. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- 2.Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci USA. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreini C, Bertini I, Rosato A. Metalloproteomes: A bioinformatic approach. Acc Chem Res. 2009;42:1471–1479. doi: 10.1021/ar900015x. [DOI] [PubMed] [Google Scholar]
- 4.Rensing C, McDevitt SF. 2013. The copper metallome in prokaryotic cells: Metallomics and the cell, Metal Ions in Life Sciences, ed Banci L (Springer, Dordrecht, The Netherlands), Vol 12, pp 417–450.
- 5.Festa RA, Thiele DJ. Copper: An essential metal in biology. Curr Biol. 2011;21:R877–R883. doi: 10.1016/j.cub.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmgren MG, Nissen P. P-type ATPases. Annu Rev Biophys. 2011;40:243–266. doi: 10.1146/annurev.biophys.093008.131331. [DOI] [PubMed] [Google Scholar]
- 7.Argüello JM, Eren E, González-Guerrero M. The structure and function of heavy metal transport P1B-ATPases. Biometals. 2007;20:233–248. doi: 10.1007/s10534-006-9055-6. [DOI] [PubMed] [Google Scholar]
- 8.Argüello JM, González-Guerrero M, Raimunda D. Bacterial transition metal P1B-ATPases: Transport mechanism and roles in virulence. Biochemistry. 2011;50:9940–9949. doi: 10.1021/bi201418k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sitsel O, et al. Structure and function of Cu(I)- and Zn(II)-ATPases. Biochemistry. 2015;54:5673–5683. doi: 10.1021/acs.biochem.5b00512. [DOI] [PubMed] [Google Scholar]
- 10.Albers RW. Biochemical aspects of active transport. Annu Rev Biochem. 1967;36:727–756. doi: 10.1146/annurev.bi.36.070167.003455. [DOI] [PubMed] [Google Scholar]
- 11.Bull PC, Cox DW. Wilson disease and Menkes disease: New handles on heavy-metal transport. Trends Genet. 1994;10:246–252. doi: 10.1016/0168-9525(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 12.Argüello JM. Identification of ion-selectivity determinants in heavy-metal transport P1B-type ATPases. J Membr Biol. 2003;195:93–108. doi: 10.1007/s00232-003-2048-2. [DOI] [PubMed] [Google Scholar]
- 13.Smith AT, Smith KP, Rosenzweig AC. Diversity of the metal-transporting P1B-type ATPases. J Biol Inorg Chem. 2014;19:947–960. doi: 10.1007/s00775-014-1129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma R, Rensing C, Rosen BP, Mitra B. The ATP hydrolytic activity of purified ZntA, a Pb(II)/Cd(II)/Zn(II)-translocating ATPase from Escherichia coli. J Biol Chem. 2000;275:3873–3878. doi: 10.1074/jbc.275.6.3873. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, et al. Structure and mechanism of Zn2+-transporting P-type ATPases. Nature. 2014;514:518–522. doi: 10.1038/nature13618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zielazinski EL, Cutsail GE, III, Hoffman BM, Stemmler TL, Rosenzweig AC. Characterization of a cobalt-specific P1B-ATPase. Biochemistry. 2012;51:7891–7900. doi: 10.1021/bi3006708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raimunda D, Long JE, Padilla-Benavides T, Sassetti CM, Argüello JM. Differential roles for the Co2+/Ni2+ transporting ATPases, CtpD and CtpJ, in Mycobacterium tuberculosis virulence. Mol Microbiol. 2014;91:185–197. doi: 10.1111/mmi.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith AT, Barupala D, Stemmler TL, Rosenzweig AC. A new metal binding domain involved in cadmium, cobalt and zinc transport. Nat Chem Biol. 2015;11:678–684. doi: 10.1038/nchembio.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel SJ, et al. Fine-tuning of substrate affinity leads to alternative roles of Mycobacterium tuberculosis Fe2+-ATPases. J Biol Chem. 2016;291:11529–11539. doi: 10.1074/jbc.M116.718239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Traverso ME, et al. Identification of a hemerythrin-like domain in a P1B-type transport ATPase. Biochemistry. 2010;49:7060–7068. doi: 10.1021/bi100866b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zielazinski EL, et al. Sinorhizobium meliloti Nia is a P(1B-5)-ATPase expressed in the nodule during plant symbiosis and is involved in Ni and Fe transport. Metallomics. 2013;5:1614–1623. doi: 10.1039/c3mt00195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenzweig AC, Arguello JM. 2012. Toward a molecular understanding of metal transport by P1B-type ATPases: Metal transporters, Current Topics in Membranes, eds Lutsenko S, Argüello JM (Elsevier Academic Press, San Diego), Vol 69, pp 113–136.
- 23.Mana-Capelli S, Mandal AK, Argüello JM. Archaeoglobus fulgidus CopB is a thermophilic Cu2+-ATPase: Functional role of its histidine-rich-N-terminal metal binding domain. J Biol Chem. 2003;278:40534–40541. doi: 10.1074/jbc.M306907200. [DOI] [PubMed] [Google Scholar]
- 24.Meloni G, Zhang L, Rees DC. Transmembrane type-2-like Cu2+ site in the P1B-3-type ATPase CopB: Implications for metal selectivity. ACS Chem Biol. 2014;9:116–121. doi: 10.1021/cb400603t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boal AK, Rosenzweig AC. Structural biology of copper trafficking. Chem Rev. 2009;109:4760–4779. doi: 10.1021/cr900104z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen GS, Wu CC, Cardozo T, Stokes DL. The architecture of CopA from Archeaoglobus fulgidus studied by cryo-electron microscopy and computational docking. Structure. 2011;19:1219–1232. doi: 10.1016/j.str.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayakanthan S, Braiterman LT, Hasan NM, Unger VM, Lutsenko S. Human copper transporter ATP7B (Wilson disease protein) forms stable dimers in vitro and in cells. J Biol Chem. 2017;292:18760–18774. doi: 10.1074/jbc.M117.807263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gourdon P, et al. Crystal structure of a copper-transporting PIB-type ATPase. Nature. 2011;475:59–64. doi: 10.1038/nature10191. [DOI] [PubMed] [Google Scholar]
- 29.Lutsenko S, Gupta A, Burkhead JL, Zuzel V. Cellular multitasking: The dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance. Arch Biochem Biophys. 2008;476:22–32. doi: 10.1016/j.abb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solioz M, Odermatt A. Copper and silver transport by CopB-ATPase in membrane vesicles of Enterococcus hirae. J Biol Chem. 1995;270:9217–9221. doi: 10.1074/jbc.270.16.9217. [DOI] [PubMed] [Google Scholar]
- 31.Schurig-Briccio LA, Gennis RB. Characterization of the PIB-type ATPases present in Thermus thermophilus. J Bacteriol. 2012;194:4107–4113. doi: 10.1128/JB.00849-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peisach J, Blumberg WE. Structural implications derived from the analysis of electron paramagnetic resonance spectra of natural and artificial copper proteins. Arch Biochem Biophys. 1974;165:691–708. doi: 10.1016/0003-9861(74)90298-7. [DOI] [PubMed] [Google Scholar]
- 33.Wu CC, Rice WJ, Stokes DL. Structure of a copper pump suggests a regulatory role for its metal-binding domain. Structure. 2008;16:976–985. doi: 10.1016/j.str.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva KI, Michael BC, Geib SJ, Saxena S. ESEEM analysis of multi-histidine Cu(II)-coordination in model complexes, peptides, and amyloid-β. J Phys Chem B. 2014;118:8935–8944. doi: 10.1021/jp500767n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battaglia M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 2008;148:6–24. doi: 10.1104/pp.108.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 37.Rorat T. Plant dehydrins–Tissue location, structure and function. Cell Mol Biol Lett. 2006;11:536–556. doi: 10.2478/s11658-006-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garay-Arroyo A, Colmenero-Flores JM, Garciarrubio A, Covarrubias AA. Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J Biol Chem. 2000;275:5668–5674. doi: 10.1074/jbc.275.8.5668. [DOI] [PubMed] [Google Scholar]
- 39.Mu P, et al. Cu2+ triggers reversible aggregation of a disordered His-rich dehydrin MpDhn12 from Musa paradisiaca. J Biochem. 2011;150:491–499. doi: 10.1093/jb/mvr082. [DOI] [PubMed] [Google Scholar]
- 40.Roccatano D, Colombo G, Fioroni M, Mark AE. Mechanism by which 2,2,2-trifluoroethanol/water mixtures stabilize secondary-structure formation in peptides: A molecular dynamics study. Proc Natl Acad Sci USA. 2002;99:12179–12184. doi: 10.1073/pnas.182199699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sönnichsen FD, Van Eyk JE, Hodges RS, Sykes BD. Effect of trifluoroethanol on protein secondary structure: An NMR and CD study using a synthetic actin peptide. Biochemistry. 1992;31:8790–8798. doi: 10.1021/bi00152a015. [DOI] [PubMed] [Google Scholar]
- 42.Chintalapati S, Al Kurdi R, van Scheltinga ACT, Kühlbrandt W. Membrane structure of CtrA3, a copper-transporting P-type-ATPase from Aquifex aeolicus. J Mol Biol. 2008;378:581–595. doi: 10.1016/j.jmb.2008.01.094. [DOI] [PubMed] [Google Scholar]
- 43.Pati A, et al. Complete genome sequence of Sphaerobacter thermophilus type strain (S 6022) Stand Genomic Sci. 2010;2:49–56. doi: 10.4056/sigs.601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandal AK, Cheung WD, Argüello JM. Characterization of a thermophilic P-type Ag+/Cu+-ATPase from the extremophile Archaeoglobus fulgidus. J Biol Chem. 2002;277:7201–7208. doi: 10.1074/jbc.M109964200. [DOI] [PubMed] [Google Scholar]
- 45.Solioz M, Stoyanov JV. Copper homeostasis in Enterococcus hirae. FEMS Microbiol Rev. 2003;27:183–195. doi: 10.1016/S0168-6445(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 46.Andersson M, et al. Copper-transporting P-type ATPases use a unique ion-release pathway. Nat Struct Mol Biol. 2014;21:43–48. doi: 10.1038/nsmb.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Requena JR, et al. Copper-catalyzed oxidation of the recombinant SHa(29-231) prion protein. Proc Natl Acad Sci USA. 2001;98:7170–7175. doi: 10.1073/pnas.121190898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao Z, et al. Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins: Detection probes and affinity standards. J Biol Chem. 2011;286:11047–11055. doi: 10.1074/jbc.M110.213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rigo A, et al. Interaction of copper with cysteine: Stability of cuprous complexes and catalytic role of cupric ions in anaerobic thiol oxidation. J Inorg Biochem. 2004;98:1495–1501. doi: 10.1016/j.jinorgbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Mattle D, et al. A sulfur-based transport pathway in Cu+-ATPases. EMBO Rep. 2015;16:728–740. doi: 10.15252/embr.201439927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci USA. 2009;106:4237–4242. doi: 10.1073/pnas.0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grønberg C, Sitsel O, Lindahl E, Gourdon P, Andersson M. Membrane snchoring and ion-entry dynamics in P-type ATPase copper transport. Biophys J. 2016;111:2417–2429. doi: 10.1016/j.bpj.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foster AW, Osman D, Robinson NJ. Metal preferences and metallation. J Biol Chem. 2014;289:28095–28103. doi: 10.1074/jbc.R114.588145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cotruvo JA, Jr, Aron AT, Ramos-Torres KM, Chang CJ. Synthetic fluorescent probes for studying copper in biological systems. Chem Soc Rev. 2015;44:4400–4414. doi: 10.1039/c4cs00346b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.