Abstract
Background
Singing is a complex physical activity dependent on the use of the lungs for air supply to regulate airflow and create large lung volumes. In singing, exhalation is active and requires active diaphragm contraction and good posture. Chronic obstructive pulmonary disease (COPD) is a progressive, chronic lung disease characterised by airflow obstruction. Singing is an activity with potential to improve health outcomes in people with COPD.
Objectives
To determine the effect of singing on health‐related quality of life and dyspnoea in people with COPD.
Search methods
We identified trials from the Cochrane Airways Specialised Register, ClinicalTrials.gov, the World Health Organization trials portal and PEDro, from their inception to August 2017. We also reviewed reference lists of all primary studies and review articles for additional references.
Selection criteria
We included randomised controlled trials in people with stable COPD, in which structured supervised singing training of at least four sessions over four weeks' total duration was performed. The singing could be performed individually or as part of a group (choir) facilitated by a singing leader. Studies were included if they compared: 1) singing versus no intervention (usual care) or another control intervention; or 2) singing plus pulmonary rehabilitation versus pulmonary rehabilitation alone.
Data collection and analysis
Two review authors independently screened and selected trials for inclusion, extracted outcome data and assessed risk of bias. We contacted authors of trials for missing data. We calculated mean differences (MDs) using a random‐effects model. We were only able to analyse data for the comparison of singing versus no intervention or a control group.
Main results
Three studies (a total of 112 participants) were included. All studies randomised participants to a singing group or a control group. The comparison groups included a film workshop, handcraft work, and no intervention. The frequency of the singing intervention in the studies ranged from 1 to 2 times a week over a 6 to 24 week period. The duration of each singing session was 60 minutes.
All studies included participants diagnosed with COPD with a mean age ranging from 67 to 72 years and a mean forced expiratory volume in one second (FEV1) ranging from 37% to 64% of predicted values. The sample size of included studies was small (33 to 43 participants) and overall study quality was low to very low. Blinding of personnel and participants was not possible due to the physical nature of the intervention, and selection and reporting bias was present in two studies.
For the primary outcome of health‐related quality of life, there was no statistically significant improvement in the St George's Respiratory Questionnaire total score (mean difference (MD) ‐0.82, 95% confidence interval (CI) ‐4.67 to 3.02, 2 studies, n = 58, low‐quality evidence). However, there was a statistically significant improvement in the SF‐36 Physical Component Summary (PCS) score favouring the singing group (MD 12.64, 95% CI 5.50 to 19.77, 2 studies, n = 52, low‐quality evidence). Only one study reported results for the other primary outcome of dyspnoea, in which the mean improvement in Baseline Dyspnoea Index (BDI) score favouring the singing group was not statistically significant (MD 0.40, 95% CI ‐0.65 to 1.45, 1 study, n = 30, very low‐quality evidence).
No studies examined any long‐term outcomes and no adverse events or side effects were reported.
Authors' conclusions
There is low to very low‐quality evidence that singing is safe for people with COPD and improves physical health (as measured by the SF‐36 physical component score), but not dyspnoea or respiratory‐specific quality of life. The evidence is limited due to the low number of studies and the small sample size of each study. No evidence exists examining the long‐term effect of singing for people with COPD. The absence of studies examining singing performed in conjunction with pulmonary rehabilitation precludes the formulation of conclusions about the effects of singing in this context. More randomised controlled trials with larger sample sizes and long‐term follow‐up, and trials examining the effect of singing in addition to pulmonary rehabilitation, are required to determine the effect of singing on health‐related quality of life and dyspnoea in people with COPD.
Plain language summary
Singing for COPD
Singing uses the lungs to provide airflow to produce musical words or sounds with the voice. Singing can require a lot of effort for muscle contraction and co‐ordination. This may benefit people with chronic obstructive pulmonary disease (COPD) in a manner similar to that of breathing exercises. Singing is said to be beneficial for health but we need evidence for this before it can be recommended specifically to address health conditions. We planned to examine whether singing had any effect on quality of life or breathlessness in people with COPD. We included three studies with a total of 112 participants. Participants were randomly assigned to singing training or to a non‐singing control group. The control groups were either a film workshop, handcraft work, or nothing at all. The singing was performed in groups, once to twice a week for one hour, for a minimum of six weeks. There was diversity in the results of the studies and we were unable to combine many results in 'meta‐analyses'. A meta‐analysis is a statistical analysis which combines the results of two or more separate studies to give a pooled result. Some studies showed improvements in some aspects of quality of life, while others showed no improvement. Breathlessness was only measured in one study and no improvement was found. The studies did not report whether any effects lasted for a long time after the singing training was completed. No studies reported any side effects from singing, so singing appears to be safe for people with COPD. The studies were of low quality due to the small number of participants and missing information about the methods and some of the outcomes. We were unable to find enough evidence to sufficiently determine the effect of singing in people with COPD. More studies are required and they should concentrate on enrolling larger numbers of people.
Summary of findings
for the main comparison.
| Singing compared with control for chronic obstructive pulmonary disease (COPD) | ||||||
|
Patient or population: people with stable COPD Settings: hospital and community Intervention: singing Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Singing | |||||
|
Health‐related quality of life (respiratory specific) St George's Respiratory Questionnaire (SGRQ; total score) Scale from 0‐100 Lower value post intervention is favourable, indicating improvement in health‐related quality of life Follow‐up: end of intervention (range 6 to 24 weeks) |
The mean change in SGRQ (total score) ranged across control groups from ‐5.0 to ‐0.4 | The mean change in SGRQ (total score) in the intervention groups was 0.8 units higher (3.0 units lower to 4.7 units higher) | 58 (2 studies) | ⊕⊕⊝⊝ low1,2 | The minimal important difference is 4 units lower | |
|
Health‐related quality of life (generic) SF‐36 (Physical Component Summary (PCS) score) Scale from 0‐100 Higher value post intervention is favourable, indicating improvement in health‐related quality of life Follow‐up: end of intervention (range 6 to 8 weeks) |
The mean change in SF‐36 (PCS score) ranged across control groups from ‐3.8 to ‐2.5 | The mean change in SF‐36 (PCS score) in the intervention groups was 12.6 units higher (5.5 units higher to 19.8 units higher) | 52 (2 studies) | ⊕⊕⊝⊝ low3,4 | The minimal important difference is 4 units higher | |
|
Health‐related quality of life (generic) SF‐36 (Mental Component Sumary (MCS) score) Scale from 0‐100 Higher value post intervention is favourable, indicating improvement in health‐related quality of life Follow‐up: end of intervention (range 6‐8 weeks) |
The mean change in SF‐36 (MCS score) ranged across control groups from ‐3.2 to 4.3 | The mean change in SF‐36 (MCS score) in the intervention groups was 5.4 units higher (3.9 units lower to 14.7 units higher) | 52 (2 studies) | ⊕⊕⊝⊝ low2,4 | The minimal important difference is 4 units higher | |
|
Dyspnoea Basal Dyspnea Index (BDI) (score) Scale from 0‐12 Higher value post intervention is favourable, indicating improvement in dyspnoea Follow‐up: end of intervention (24 weeks) |
The mean change in BDI (score) was 0.3 | The mean change in BDI (score) in the intervention groups was 0.4 units higher (0.7 units lower to 1.5 units higher) | 30 (1 study) | ⊕⊝⊝⊝ very low5,6 | The minimal important difference is 1 unit higher | |
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One study showed limitations in design: selection and detection bias unknown (risk of bias ‐1)
2 Meta‐anlaysis was limited to few studies with small sample sizes and wide confidence intervals (imprecision ‐1)
3 Meta‐analysis was limited to few studies with small sample sizes (imprecision ‐1)
4 One study showed reporting bias (risk of bias ‐1)
5 Study showed limitations in design: selection and detection bias unknown (risk of bias ‐1)
6 No meta‐analysis as only one study (imprecision ‐2)
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease characterised by airflow limitation that is not fully reversible and is associated with an abnormal inflammatory response of the lungs to noxious particles or gases (GOLD 2017). The prevalence of COPD has been reported as ranging from 0.2% to 37%, and varies widely across countries and populations (Rycroft 2012). Prevalence and incidence is greatest in men and those aged 75 years and over (Rycroft 2012). COPD is a major cause of morbidity and is the third most common cause of death globally (Lozano 2012). People with COPD have abnormal respiratory muscle function as a result of a mechanical disadvantage due to lung hyperinflation, diaphragmatic dysfunction and asynchronous breathing, and a reduction in respiratory muscle strength (Luce 1982). Symptoms of COPD include dyspnoea (breathlessness), cough and fatigue. Postures that enhance the function of the diaphragm may assist with dyspnoea relief (Luce 1982). Reduced functional capacity and physical inactivity are common features (Troosters 2010; Singer 2011), which significantly increase the risk for hospitalisation and mortality (Garcia‐Aymerich 2006). Pulmonary rehabilitation, encompassing exercise training, education and behaviour change (Spruit 2013), is an important component in the management of COPD and is beneficial in relieving dyspnoea and fatigue, and improving health‐related quality of life and exercise capacity (McCarthy 2015).
Description of the intervention
Singing is the production of musical words or sounds with the voice (Oxford 2016). Singing can be performed individually or in a group (choir), and can be arranged or improvised. Singing is a much more complex physical activity than speaking due to the greater length of phrases and greater range of pitch required (Irons 2010). Singing is dependent on the use of the lungs for air supply. During normal tidal breathing, the diaphragm contracts for inhalation, while exhalation occurs passively. During singing, air flow must be regulated and larger lung volumes are required, thus exhalation is active and aided by the abdominal, internal intercostal and pelvic muscles. Singing requires a high degree of muscle co‐ordination by highly developed muscle reflexes. There are four stages of breathing with singing: inhalation; suspension; controlled exhalation (when phonation occurs); and recovery. A singer controls these stages consciously until they become conditioned reflexes (Mathis 2009).
Diaphragmatic breathing requires an increase in abdominal wall motion with a reduction in upper rib cage motion (Gosselink 2004), and is the method of breathing employed by singers, as the diaphragm can generate the greatest inspiratory muscle force to increase lung volumes and change subglottal pressures necessary for singing (Sundberg 1993). The subglottal air pressure requirements are much greater for singing tasks than for speaking tasks (Leanderson 1987; Leanderson 1988), as higher subglottal pressures are required for loudness and higher pitch (Sundberg 1993). Audible speech can be produced with subglottal pressures as low as 2 cmH2O (centimetre of water pressure), with ordinary speech ranging from 7 cmH2O to 10 cmH2O; however, singing can vary from 5 cmH2O to 40 cmH2O for soft to loud tones (Proctor 1980). An increase in subglottal pressure is achieved by decreasing the volume of the rib cage using muscular forces, elasticity forces and gravity (Sundberg 1993).
Posture can greatly affect the quantity of air, the capacity of the lungs and the ability to move air in and out when singing. Good posture facilitates an efficient breathing pattern and can influence the voice (Bunch 1995; Staes 2011). Trained singers have greater breathing efficiency and greater use of their lung capacity than non‐trained singers (Gould 1973; Salomoni 2016).
Mastery of diaphragmatic breathing is vital for singing. Data from Engen 2005 suggests a minimum of four half‐hour group singing sessions could be sufficient for people with emphysema to learn the diaphragmatic breathing technique correctly. Thus, singing needs to be performed for a sufficient duration, and most likely at a sufficient intensity in order to ensure an effective stimulus for learning this technique and for potentially having an effect on important health outcomes. The precise 'dosage' will likely vary for each person and may depend on their age, disease severity, and previous experience with singing (Irons 2010).
How the intervention might work
Singing is an activity that has the potential to improve health outcomes, such as relieving dyspnoea and enhancing quality of life, in people with COPD due to employment of diaphragmatic breathing, altered posture, and improved breathing co‐ordination. Qualitative studies of singing and health report that singing can enhance mood, provide social support and friendship, help develop self esteem and self confidence, relieve stress, promote good posture and distract attention from personal worries (MacDonald 2012). Singing in people with COPD has the potential to demonstrate similar effects due to the enjoyable and low‐risk nature of the activity (Engen 2005), and may have a positive impact on the distressing effects of COPD such as breathlessness, reduced quality of life and fatigue. The perceptions of people with COPD following a group singing programme support this (Morrison 2013; Skingley 2014).
Therapies that incorporate breathing manoeuvres, such as controlled breathing techniques including diaphragmatic breathing and active expiration (as performed during singing), have been shown to improve lung function (Esteve 1996), alleviate dyspnoea and improve quality of life (Gosselink 2003; Gosselink 2004), and improve functional exercise capacity (Holland 2012) in people with COPD. Singing requires great control to ensure a smooth and sustained exhalation. This exhalation is similar to that of pursed lip breathing and controlled breathing, which have been shown to reduce breathlessness in people with COPD (Gosselink 2003; Bianchi 2004).
Education on breathing and air support is fundamental in the process of learning to sing, and knowledge of the physical processes that make up the act of singing, and how those processes function (Mathis 2009), may improve breathing awareness and efficiency in people with COPD.
Poor posture (hyperkyphosis), which is common in people with COPD (Gaude 2014), can restrict the expansion of the rib cage and movement of the diaphragm. Singing requires the development of skills in controlling posture that may be transferable to activities in daily life for people with COPD (Lord 2010).
Why it is important to do this review
Singing may have the potential to improve health outcomes in people with COPD. Systematic reviews of research literature have been completed for singing in other chronic respiratory diseases such as bronchiectasis (Irons 2010), and cystic fibrosis (Irons 2016), and found an absence of randomised controlled trials (RCTs) to support or refute the benefits of singing. However, the authors of these reviews found studies that reported an improvement in quality of life in people with COPD, and a systematic review of singing for COPD has not yet been carried out. Furthermore, whilst pulmonary rehabilitation improves physical and psychosocial health outcomes in people with COPD (McCarthy 2015), the potential additional benefits of adding singing to pulmonary rehabilitation has not been examined.
Objectives
To determine the effect of singing on health‐related quality of life and dyspnoea in people with COPD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) reported as full‐text, published as abstract only, and unpublished data. We used data from studies published as abstract only where authors provided study data. Where the data were not available, we recorded the studies as awaiting classification.
Types of participants
We included studies that involved adults with COPD, diagnosed according to the investigators' definition, of any age or disease severity. The COPD was required to be stable (i.e. optimal and stable respiratory medications with no exacerbation or hospitalisation within the previous month). We included participants with COPD who used supplemental oxygen. Participants with and without a history of singing training could be included, and we recorded the singing training history wherever possible.
Types of interventions
We included studies examining structured, supervised singing training of at least four weeks' duration with a minimum of four sessions. Studies were included that compared:
singing versus no intervention (usual care) or another control intervention;
singing plus pulmonary rehabilitation versus pulmonary rehabilitation alone.
The singing could be performed individually or as part of a group (choir) facilitated by a singing leader, and inpatient and outpatient programmes were included. In the case of interventions combining one or more components of music therapy, for example instrumental and singing training, the singing needed to form the majority of the intervention. We recorded the precise nature of the singing facilitators' professional backgrounds, singing training and any pulmonary rehabilitation programme (frequency, duration, type, intensity), wherever possible.
Types of outcome measures
Primary outcomes
Health‐related quality of life, measured using total scores from either generic or respiratory‐specific quality‐of‐life questionnaires.
Dyspnoea, measured using a dyspnoea scale (e.g. Medical Research Council (MRC) dyspnoea scale (Bestall 1999)) or dyspnoea scores from a respiratory‐specific quality‐of‐life questionnaire (e.g. dyspnoea domain of the Chronic Respiratory Disease Questionnaire (Guyatt 1987)), or both.
Secondary outcomes
Respiratory muscle strength measured from a pressure gauge (e.g. maximal inspiratory and expiratory mouth pressures or maximal sniff nasal inspiratory pressure).
Pulmonary function measured by spirometry or plethysmography (e.g. forced expiratory volume in one second (FEV1) measured in litres or as per cent of predicted, forced vital capacity (FVC), FEV1/FVC ratio, total lung capacity (TLC), residual capacity (RC), functional residual capacity (FRC)).
Psychological status measured from generic psychological questionnaires or scales (e.g. Hospital Anxiety and Depression Scale (Zigmond 1983)).
Functional exercise capacity measured from a functional exercise test.
Peak exercise capacity measured from a peak exercise test.
Endurance exercise capacity measured from an endurance exercise test.
Healthcare utilisation recorded as hospitalisation or length of hospital stay, or both.
Physical activity level from objective measurement tools (e.g. pedometers, accelerometers, multi‐sensor devices).
Adverse events/side effects.
Reporting one or more of the outcomes listed here in the study was not an inclusion criterion for the review. We reviewed primary and secondary outcomes at baseline and immediately following the intervention period. If outcomes were also measured in the long term (e.g. six or 12 months after completion of intervention), we reviewed each of these time points in addition to immediately following the intervention period. The selected primary outcome measures are important to patients and clinicians, and all outcome measures were clinically relevant and could potentially be altered by a singing intervention.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the group. The Register contains studies identified from several sources:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org);
weekly searches of MEDLINE Ovid SP 1946 to date;
weekly searches of Embase Ovid SP 1974 to date;
monthly searches of PsycINFO Ovid SP 1967 to date;
monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) 1937 to date;
monthly searches of AMED EBSCO (Allied and Complementary Medicine);
handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings are in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/) and PEDro (www.pedro.org.au/). We searched all databases from their inception to 1 August 2017, and we imposed no restriction on language of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We searched for errata or retractions from included studies published in full‐text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (RJM, CE) independently screened titles and abstracts of all the potential studies we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports or publications and two review authors (RJM, CE) independently screened the full text and identified studies for inclusion. They also identified and recorded reasons for excluding ineligible studies. We resolved any disagreement through discussion or we consulted a third review author (ZJM). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and 'Characteristics of excluded studies' table (Moher 2009).
1.
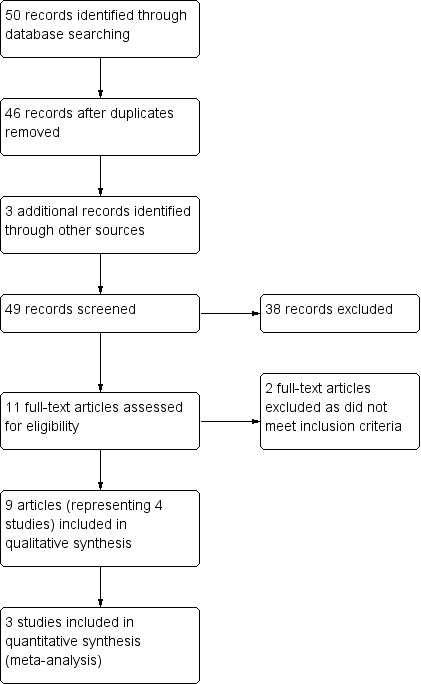
Study flow diagram.
Data extraction and management
We used a data collection form for study characteristics and outcome data. One review author (RJM) extracted study characteristics from included studies. A second review author (CE) spot‐checked study characteristics for accuracy against the trial report. We extracted the following study characteristics:
methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals and date of study;
participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria;
interventions: intervention, comparison, concomitant medications and excluded medications;
outcomes: primary and secondary outcomes specified and collected, and time points reported;
notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (RJM, CE) independently extracted outcome data from included studies. One review author (RJM) transferred data into Review Manager 5 (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports.
Assessment of risk of bias in included studies
Two review authors (RJM, CE) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted this review according to the published protocol (Differences between protocol and review).
Measures of treatment effect
We analysed continuous data as mean differences (MDs). We entered data presented as a scale with a consistent direction of effect.
We undertook meta‐analyses only where this was meaningful, that is, if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
We narratively described skewed data reported as medians and interquartile ranges.
Where multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons were combined in the same meta‐analysis, we halved the control group to avoid double counting.
Unit of analysis issues
We did not include cross‐over trials. If the search identified cluster‐randomised trials, the intention was to consult the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), however no cluster‐randomised trials were identified.
Dealing with missing data
We contacted investigators in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only).
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the studies in each analysis. Heterogeneity was considered significant if the P value was less than 0.10 (Higgins 2011).
Assessment of reporting biases
We were unable to pool more than 10 studies, however if more studies are included in future review updates we will create and examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We used a random‐effects model using Review Manager 5 (RevMan 2014) and used change from baseline results to final scores.
Where the outcomes were reported using adjusted analyses (such as ANOVA or ANCOVA), we used the generic inverse variance method to combine the results with other studies; where adjusted analyses were not available, we preferred change from baseline results to final scores.
'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: health‐related quality of life and dyspnoea. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade or upgrade the quality of studies using footnotes, and we have made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses if sufficient studies were retrieved:
severity of lung disease — severe (FEV1 % predicted less than 40%) versus not severe (FEV1 % predicted 40% predicted or greater);
mode of singing intervention — individual versus group (choir);
participant's experience with singing training — no previous history with singing training versus prior history of singing training;
singing facilitator's professional background — formally trained music or singing professional versus health or lay professional.
We planned to use the following outcomes in subgroup analyses:
health‐related quality of life;
dyspnoea.
We were unable to perform subgroup analyses due to the small number of studies. If more studies are included in future updates of this review, we will perform subgroup analyses using the formal test for subgroup interactions in Review Manager 5 (RevMan 2014).
Sensitivity analysis
We planned to carry out the following sensitivity analysis:
studies with a low risk of bias (to examine the effects of removing studies with a high risk of bias).
We were unable to conduct this sensitivity analysis because of the small number of studies. If more studies are included in future review updates, a sensitivity analysis will be performed to analyse the effects of studies with a low risk of bias for at least three of the following domains: random sequence generation, allocation concealment, blinding of outcome assessment, and incomplete outcome data.
Results
Description of studies
Refer to the Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification for the details of the studies included, excluded and awaiting classification.
Results of the search
Our search of the databases identified 50 citations. We identified three additional citations through handsearching. After removing duplicates, we reviewed 49 citation titles and abstracts, of which we excluded 38. We screened the full‐text versions of eleven citations for eligibility, and excluded two because they did not meet the review inclusion criteria. Nine citations were appropriate for inclusion in the review, however one citation was published in abstract form only and our attempts to contact the authors were unsuccessful. This study remains as a study awaiting classification. The remaining eight citations represented three studies. We created a PRISMA flow diagram to depict the search results (Figure 1). The review authors agreed on the inclusion of all citations, with a Cohen's kappa measurement of 1, indicating excellent agreement.
Included studies
We identified three studies (a total of 112 participants) which met the inclusion criteria for this review. They were represented by eight citations which were reviewed in full‐text. The full details of these studies can be found in the Characteristics of included studies table.
The sample size of studies ranged from 33 to 43 participants. All studies included participants diagnosed with chronic obstructive pulmonary disease (COPD), with a mean age ranging from 67 to 72 years and a mean forced expiratory volume in one second (FEV1) ranging from 37% to 64% of predicted values.
All studies randomised participants to a singing group or another control intervention. The frequency of the singing intervention in the studies ranged from 1 to 2 times a week over a 6 to 24 week period. The duration of the singing sessions was 60 minutes and they were conducted in groups led by a singing teacher. The singing sessions were structured in nature and included relaxation exercises, breathing exercises, vocalisation exercises, and singing. All studies began with relaxation exercises of the neck and upper limb muscles, or postural work and physical stretches. One study had participants perform singing‐related breathing exercises consisting of fast, deep inspirations, followed by slow, full or interrupted expirations; performing fast and deep respiratory incursions, paying attention to the upper abdominal movements; and generating breathing movements against, or with the help of, pressures generated by a hand placed on the upper abdominal region (Bonilha 2009). All studies performed vocal exercises, for example, pronouncing vowels such as "le", "la", "mi", "mu", and singing the melody of a familiar song using such vowels instead of actually singing the lyrics (Bonilha 2009). In all the studies, participants sang songs for 20 to 30 minutes. The comparison groups included a film workshop (Lord 2012), handcraft work (Bonilha 2009), and no intervention (Lord 2010).
The primary outcome of health‐related quality of life was measured in all three studies, using the St George's Respiratory Questionnaire total score (Bonilha 2009; Lord 2010), the SF‐36 Physical Component Summary (PCS) and Mental Component Summary (MCS) scores (Lord 2010; Lord 2012), and the COPD Assessment Test (CAT) total score (Lord 2012). The primary outcome of dyspnoea was assessed in one study using the Basal Dyspnea Index (BDI) (Bonilha 2009).
The following secondary outcomes were measured by the included studies: respiratory muscle strength (Bonilha 2009); pulmonary function (Bonilha 2009); psychological status (Lord 2010; Lord 2012); peak exercise capacity (Lord 2010; Lord 2012); and physical activity level (Lord 2012).
The following secondary outcomes were not measured by the included studies: functional exercise capacity; endurance exercise capacity; and healthcare utilisation.
Excluded studies
We excluded two citations (representing one study) from this review due to the intervention not meeting the inclusion criteria.
Risk of bias in included studies
Our assessment of the risk of bias in the included studies is presented in Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
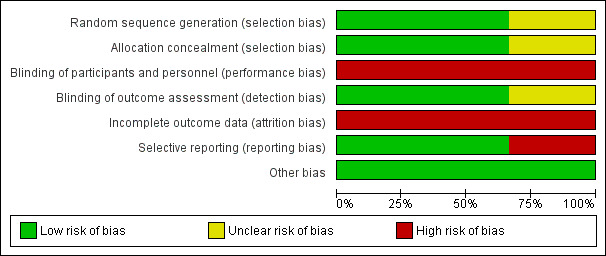
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All three studies reported participant randomisation, however one study did not provide sufficient information to determine the sequence generation or allocation concealment (Bonilha 2009).
Blinding
Blinding of personnel and participants was not possible due to the physical nature of the intervention. Two studies reported blinding of the outcome assessor (Lord 2010; Lord 2012).
Incomplete outcome data
All three studies reported dropouts and loss to follow‐up ranging from 22% to 30% (Bonilha 2009; Lord 2010; Lord 2012).
Selective reporting
Two studies reported all outcome measures as prespecified in the methods (Bonilha 2009; Lord 2010). One study did not report all outcomes at the post intervention time point as prespecified in the methods (Lord 2012).
Other potential sources of bias
All three studies appeared to be free of other sources of bias.
Effects of interventions
See: Table 1
See Table 1.
The Data and analyses table summarises the results of the meta‐analyses for the comparison of singing to a control group. All three studies reported the results as change from baseline measures.
Primary outcomes
Health‐related quality of life
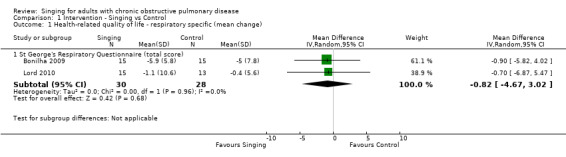
Two studies reported results which could be pooled for meta‐analysis for the St George's Respiratory Questionnaire (Bonilha 2009; Lord 2010). Results of the meta‐analysis are shown in Figure 4. There was no statistically significant improvement in the St George's Respiratory Questionnaire total score (mean difference (MD) ‐0.82, 95% confidence interval (CI) ‐4.67 to 3.02, n = 58). We assessed the quality of the evidence as low according to GRADE criteria (Table 1).
4.
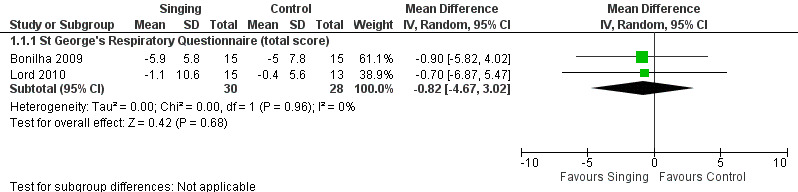
Forest plot of comparison: 1 Intervention — Singing vs Control, outcome: 1.1 Health‐related quality of life — respiratory specific (mean change).
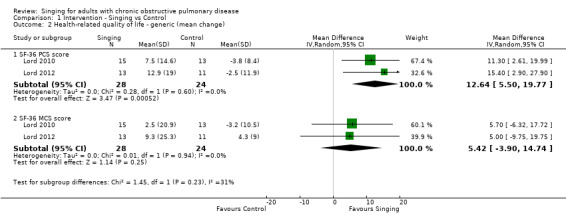
Two studies reported results which could be pooled for meta‐analysis for the SF‐36 (Lord 2010; Lord 2012). Results of the meta‐analysis are shown in Figure 5. There was a statistically significant improvement in the SF‐36 Physical Component Summary (PCS) score favouring the singing group (MD 12.64, 95% CI 5.50 to 19.77, n = 52). There was no statistically significant improvement in the SF‐36 Mental Component Summary (MCS) score, but the confidence interval is wide (MD 5.42, 95% CI ‐3.90 to 14.74, n = 52). We assessed the quality of the evidence as low according to GRADE criteria (Table 1).
5.
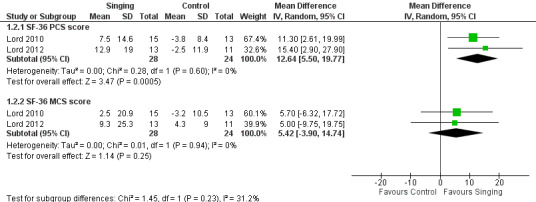
Forest plot of comparison: 1 Intervention — Singing vs Control, outcome: 1.2 Health‐related quality of life — generic (mean change).
Dyspnoea
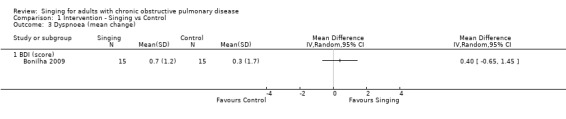
Only one study (Bonilha 2009) reported results for dyspnoea (Figure 6). The mean improvement in the Basal Dyspnoea Index (BDI) score favouring the singing group was not statistically significant (MD 0.40, 95% CI ‐0.65 to 1.45, n = 30). We assessed the quality of the evidence as very low according to GRADE criteria (Table 1).
6.

Forest plot of comparison: 1 Intervention — Singing vs Control, outcome: 1.3 Dyspnoea (mean change).
Secondary outcomes
Respiratory muscle strength
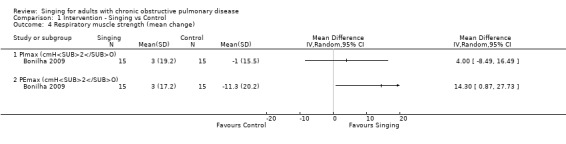
One study reported measures of respiratory muscle strength (Bonilha 2009). There was an improvement in maximal inspiratory pressure (PImax, cmH2O) favouring singing, but the confidence interval is too wide to exclude a possible reduction in inspiratory muscle pressure with the intervention (MD 4.00, 95% CI ‐8.49 to 16.49, n = 30).
There was a statistically significant improvement in maximal expiratory pressure (PEmax, cmH2O) favouring the singing group (MD 14.30, 95% CI 0.87 to 27.73, n = 30).
Pulmonary function
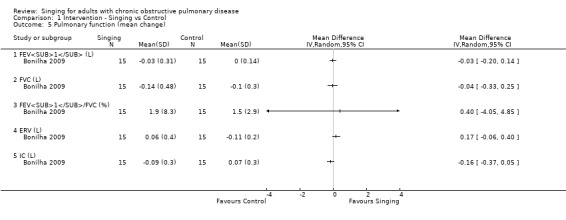
One study (Bonilha 2009) reported measures of pulmonary function in 30 participants, including forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, end respiratory volume (ERV), and inspiratory capacity (IC). There were no statistically significant differences between the singing group and control group for any of these measures (FEV1 litres (L) MD ‐0.03, 95% CI ‐0.20 to 0.14; FVC (L) MD ‐0.04, 95% CI ‐0.33 to 0.25; FEV1/FVC (%) MD 0.40, 95% CI ‐4.05 to 4.85; ERV (L) MD 0.17, 95% CI ‐0.06 to 0.40; IC (L) MD ‐0.16, 95% CI ‐0.37 to 0.05).
Psychological status
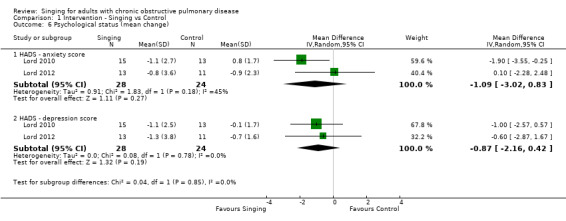
Two studies reported results which could be pooled for meta‐analysis for the Hospital Anxiety and Depression Scale (HADS) (Lord 2010; Lord 2012). There was no statistically significant improvement in the HADS anxiety score (MD ‐1.09, 95% CI ‐3.02 to 0.83, n = 52) or HADS depression score (MD ‐0.87, 95% CI ‐2.16 to 0.42, n = 52).
Functional exercise capacity
No studies measured functional exercise capacity.
Peak exercise capacity
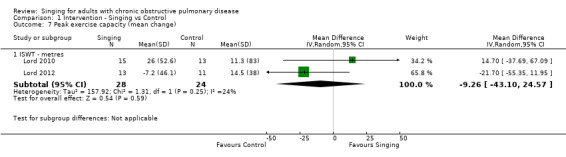
Two studies reported results which could be pooled for meta‐analysis using the Incremental Shuttle Walk Test (ISWT) (Lord 2010; Lord 2012). There is uncertainty due to imprecision about whether singing has an impact on ISWT distance (metres) compared to control (MD ‐9.26, 95% CI ‐43.10 to 24.57, n = 52).
Endurance exercise capacity
No studies measured endurance exercise capacity.
Healthcare utilisation
No studies measured healthcare utilisation or hospitalisation.
Physical activity level
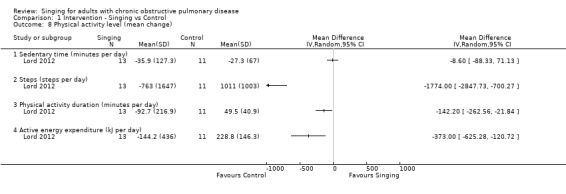
One study (Lord 2012) reported measures of physical activity for 24 participants, including steps (steps per day), sedentary time (minutes per day), physical activity duration (minutes per day), and active energy expenditure (kJ per day). There were no statistically significant differences between the singing group and control group for sedentary time (minutes per day), but the confidence interval is wide (MD ‐8.60, 95% CI ‐88.33 to 71.13). There were statistically significant differences in the remaining measures of physical activity favouring the control group (steps (steps per day) MD ‐1774.00, 95% CI ‐2847.73 to ‐700.27; physical activity duration (minutes per day) MD ‐142.20, 95% CI ‐262.56 to ‐21.84; active energy expenditure (kJ per day) MD ‐373.00, 95% CI ‐625.28 to ‐120.72).
Adverse events and side effects
No adverse events or side effects were reported by any of the included studies, and participant withdrawal reasons (where provided) were unrelated to the singing intervention. The study by Bonilha 2009 reported that the vocal exercises and singing was well tolerated by the participants, with no complaints of severe dyspnoea, chest pain, regurgitation or dizziness (Bonilha 2009). In the study by Lord 2010, no participants reported any negative effects from the singing (Lord 2010). Lord 2012 did not report on this outcome (Lord 2012).
Discussion
Summary of main results
We included three studies with a total of 112 participants in this review. The sample size of studies ranged from 33 to 43 participants. All studies included participants diagnosed with chronic obstructive pulmonary disease (COPD) with a mean age ranging from 67 to 72 years and mean forced expiratory volume in one second (FEV1) ranging from 37% to 64% of predicted values. All studies randomised participants to a singing or a control group. The comparison groups included a film workshop (Lord 2012), handcraft work (Bonilha 2009), and no intervention (Lord 2010). The frequency of the singing intervention in the studies ranged from 1 to 2 times a week over a 6 to 24 week period. The duration of the singing sessions was 60 minutes.
Results for health‐related quality of life were diverse. There was no significant change in the St George's Respiratory Questionnaire total score between groups, however a statistically significant improvement in the SF‐36 Physical Component Summary (PCS) score favouring the singing group was found (MD 12.64, 95% CI 5.50 to 19.77, 2 studies, n = 52) which surpassed the minimal important difference of 4 units (Hays 2001). No change in dyspnoea was demonstrated.
Measures of pulmonary function and inspiratory muscle strength were only measured in one study and showed no significant differences between the singing group and control group. There was a statistically significant improvement in expiratory muscle pressure favouring the singing group, although this improvement was not clinically significant. No improvement in anxiety, depression, exercise capacity or physical activity level following singing were found. Healthcare utilisation was not measured by any studies and no adverse effects from singing were reported. There are no data to draw conclusions about the long‐term effects of singing in people with COPD.
The main results show few statistically or clinically significant health outcomes. There were baseline imbalances between the studies, especially in lung function. This, along with the small number of participants, may have affected the precision around the mean differences. There is a clear need for larger trials with longer duration of follow‐up to gain a better understanding of the effects of singing in people with COPD.
Overall completeness and applicability of evidence
Applicability of evidence includes consideration of whether people with COPD would be willing or motivated to participate in singing. Participants in the included studies were recruited from hospital respiratory clinics and no information was provided on previous experience of COPD management, such as pulmonary rehabilitation, or indeed of singing. A recent qualitative study found that people with COPD perceive that pulmonary rehabilitation does not fit their perception of health and that participation may be time‐consuming and conflict with daily activities (Mathar 2017). It is not known whether singing might be considered similarly and what the impact of this might be on recruitment to a randomised controlled trial. Nonetheless, qualitative research studies have reported high satisfaction with singing by people with COPD, including self‐reported improvements in both breathing and psychological outcomes (Goodridge 2013; Pacheco 2014; McNaughton 2016).
This review included people with stable COPD of moderate to severe disease severity, therefore the results cannot be extrapolated to people with unstable disease such as during or following an exacerbation, or to people with milder COPD. The studies also only reported the short‐term effects of singing. Without long‐term studies, the effect of singing over a longer period of time cannot be determined. Furthermore, no healthcare utilisation data was reported in any of the selected studies which is particularly important in relation to commissioning and potential for incorporation of findings into healthcare guidelines in the future.
Two studies randomised participants to an active comparison group which matched the time and attention of the singing group. However, the comparison group of one study provided no intervention. With an insufficient number of trials randomising participants to active and non‐active comparison groups, we cannot determine whether any improvements in health outcomes were simply a result of participation in a group with support from a leader and fellow participants.
From the data in this review, we cannot determine the optimal delivery mode or dosage of singing required to achieve positive health outcomes in people with COPD. The singing in each study was delivered by a singing teacher, however all studies delivered the singing in groups, so the effect of individual singing lessons in people with COPD cannot be ascertained. The frequency and duration of the singing programs ranged from once to twice a week, and from 6 to 24 weeks in length. It is unclear what frequency and duration is sufficient to provide an effective stimulus for learning the technique of singing and to have an effect on our health outcomes of interest. In all studies participants were instructed to practice their singing at home, however compliance was not measured, therefore it is unknown whether more frequent singing than the supervised group sessions may have contributed to some of the outcomes.
There was general consistency in direction of effects in most outcomes. However, the outcome of anxiety showed an opposite effect. The results from Lord 2010 favoured singing, whilst Lord 2012 results favoured the control group. This inconsistency may be explained by the clinical heterogeneity of the two trials with baseline differences in lung function, anxiety, quality of life and exercise capacity, or it may simply be explained by the impact of chance on such small numbers of participants in each group.
Whilst there was one clinically significant result for singing demonstrating an improvement in the SF‐36 PCS score, the diversity in results for health‐related quality of life may be explained by the methods employed by the studies. A primary outcome and sample size calculation was only reported by one of the included studies (Lord 2012). Therefore, we cannot determine whether two of the three included studies had an adequate sample size calculation or were adequately powered to determine a significant change for the outcome of health‐related quality of life. There was a clinically significant change in physical activity favouring the control group in one study (Lord 2012) which is difficult to explain. No other health outcome was clinically significant, although the improvement in anxiety following singing showed a trend towards the minimal important difference (Puhan 2008). The small number of studies and small sample sizes are most likely the major reasons why the changes in outcomes in this review were so variable.
Quality of the evidence
The overall quality of the evidence for the studies included in this review was very low to low. The major methodological shortcoming was the small sample size of the studies. The quality of the evidence was also impacted by the inability to blind the population and personnel due to the physical nature of the intervention. An unknown randomisation process and lack of blinding of the outcome assessor compromised the quality of one study (Bonilha 2009), whilst a reporting bias was present in another study (Lord 2012).
Potential biases in the review process
We adhered to the standard Cochrane methodological procedures to minimise bias, including having two authors independently screen trials, extract trial data and perform the 'Risk of bias' assessment. Attempts were made to contact trial authors where missing information was identified and for a study published in abstract form only, however data were not provided.
Agreements and disagreements with other studies or reviews
This findings of this Cochrane review are consistent with the overall findings of a recent review of singing for lung health in people with COPD (Lewis 2016). Cohort and qualitative data were also examined as part of the narrative literature review which found that participants generally reported positive impacts of singing on their activities of daily living such as housework, their ability to manage their breathlessness, and improved well‐being (Lewis 2016). These findings are in agreement with the positive effects shown in this review for one aspect of health‐related quality of life, even though the results of the meta‐analyses with this small population did not demonstrate clinical significance for all health‐related quality of life measures.
Authors' conclusions
Implications for practice.
There is limited low‐quality evidence that singing is safe for people with moderate to severe COPD and improves physical health (as measured by the SF‐36 physical component score). Whilst singing may be appealing and subjectively beneficial to some people with COPD, there is currently insufficient evidence to advocate singing as an effective intervention to achieve clinically significant health outcomes above and beyond no intervention or group‐based recreational activities.
Implications for research.
More randomised controlled trials are required to determine the effect of singing on health‐related quality of life and dyspnoea in people with COPD; trials examining the effect of singing in addition to pulmonary rehabilitation are also needed. In particular, large studies with long‐term follow‐up are necessary. Future studies need to incorporate important methodological features such as adequate sample sizes, randomisation, allocation concealment and blinding of outcome assessors, as well as longer follow‐up, to ensure high‐quality evidence is available on the effectiveness of singing in people with COPD.
What's new
| Date | Event | Description |
|---|---|---|
| 6 February 2019 | Amended | Three potentially eligible studies added to 'studies awaiting classification'. These studies have not been fully incorporated into the review. |
History
Protocol first published: Issue 7, 2016 Review first published: Issue 12, 2017
| Date | Event | Description |
|---|---|---|
| 19 February 2018 | Amended | Added acknowledgement to grant from the National Center for Complementary and Integrative Health (NCCIH). |
Acknowledgements
The review authors are grateful for the support of the Cochrane Airways group.
Christian Osadnik was the editor for this review and commented critically on the review.
The Background and Methods sections of this protocol are based on a standard template used by Cochrane Airways.
This work was partially funded by Grant Number R24 AT001293 from the National Center for Complementary and Integrative Health (NCCIH). The contents of this systematic review are solely the responsibility of the authors and do not necessarily represent the official views of the NCCIH or the National Institutes of Health.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane infrastructure funding to Cochrane Airways. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
We will adapt the MEDLINE strategy and RCT filter to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the CAGR
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD OR AECOPD):TI,AB,KW
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 (sing or singing or singer* or song*):ti,ab,kw
#8 (voice* or vocal*) NEAR (exercis* or train*)
#9 diaphragm* NEAR2 breath*
#10 MeSH DESCRIPTOR Music Therapy
#11 choir*:ti,ab,kw
#12 #7 or #8 or #9 or #10 or #11
#13 #6 AND #12
[in search line #4, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, COPD]
Data and analyses
Comparison 1. Intervention ‐ Singing vs Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Health‐related quality of life ‐ respiratory specific (mean change) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 St George's Respiratory Questionnaire (total score) | 2 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐4.67, 3.02] |
| 2 Health‐related quality of life ‐ generic (mean change) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 SF‐36 PCS score | 2 | 52 | Mean Difference (IV, Random, 95% CI) | 12.64 [5.50, 19.77] |
| 2.2 SF‐36 MCS score | 2 | 52 | Mean Difference (IV, Random, 95% CI) | 5.42 [‐3.90, 14.74] |
| 3 Dyspnoea (mean change) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 BDI (score) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Respiratory muscle strength (mean change) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 PImax (cmH2O) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 PEmax (cmH2O) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pulmonary function (mean change) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 FEV1 (L) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 FVC (L) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 FEV1/FVC (%) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 ERV (L) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 IC (L) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Psychological status (mean change) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 HADS ‐ anxiety score | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐3.02, 0.83] |
| 6.2 HADS ‐ depression score | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐2.16, 0.42] |
| 7 Peak exercise capacity (mean change) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 ISWT ‐ metres | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐9.26 [‐43.10, 24.57] |
| 8 Physical activity level (mean change) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Sedentary time (minutes per day) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Steps (steps per day) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Physical activity duration (minutes per day) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Active energy expenditure (kJ per day) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Intervention ‐ Singing vs Control, Outcome 1 Health‐related quality of life ‐ respiratory specific (mean change).
1.2. Analysis.
Comparison 1 Intervention ‐ Singing vs Control, Outcome 2 Health‐related quality of life ‐ generic (mean change).
1.3. Analysis.
Comparison 1 Intervention ‐ Singing vs Control, Outcome 3 Dyspnoea (mean change).
1.4. Analysis.
Comparison 1 Intervention ‐ Singing vs Control, Outcome 4 Respiratory muscle strength (mean change).
1.5. Analysis.
Comparison 1 Intervention ‐ Singing vs Control, Outcome 5 Pulmonary function (mean change).
1.6. Analysis.
Comparison 1 Intervention ‐ Singing vs Control, Outcome 6 Psychological status (mean change).
1.7. Analysis.
Comparison 1 Intervention ‐ Singing vs Control, Outcome 7 Peak exercise capacity (mean change).
1.8. Analysis.
Comparison 1 Intervention ‐ Singing vs Control, Outcome 8 Physical activity level (mean change).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bonilha 2009.
| Methods | Study design: randomised controlled trial | |
| Participants |
Participants: n = 43 Included: COPD (according to GOLD criteria); stable for 2 months; ex‐smoker Excluded: severe comorbidities; oxygen therapy; smoker Baseline characteristics: Intervention group — singing (n = 15)
Control group — handcraft (n = 15)
|
|
| Interventions |
Intervention characteristics: Intervention group — singing
Control group — handcraft
|
|
| Outcomes |
Health‐related quality of life — St George's Respiratory Questionnaire, total score
Dyspnoea — Basal Dyspnoea Index (BDI), score
Respiratory muscle strength — PImax, cmH2O
Respiratory muscle strength — PEmax, cmH2O
Lung function — FVC, L
Lung function — FEV1, L
Lung function — FEV1/FVC, %
Lung function — ERV, L
Lung function — IC, L
|
|
| Identification |
Country: Brazil Setting: hospital Authors name: Amanda Gimenes Bonilha Institution: University of Sao Paulo Email: jabmarti@fmrp.usp.br Address: Internal Medicine Department, Avenida Bandeirantes 3900, CEP: 14048‐800, Ribeirao Preto, Sao Paulo, Brazil |
|
| Notes | Authors were contacted for further information, with no response. Sponsorship source: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not specified, but due to the physical nature of the intervention it is unlikely the participants were able to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes measured reported for all participants completing post intervention assessment High dropout rate from singing group (35%) |
| Selective reporting (reporting bias) | Low risk | All outcome measures listed in methods were reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Lord 2010.
| Methods | Study design: randomised controlled trial | |
| Participants |
Participants: n = 36 Included: COPD (diagnosed according to the GOLD guidelines and attending respiratory clinics) Baseline characteristics Intervention group — singing (n = 15)
Control group — no intervention (n = 13)
|
|
| Interventions |
Intervention characteristics: Intervention group — singing
Control group — no intervention |
|
| Outcomes |
Health‐related quality of life — SF‐36 Physical Component Summary (PCS), score
Quality of life — SF‐36 Mental Component Summary (MCS), score
Psychological status — Hospital Anxiety and Depression Scale (HADS) ‐ anxiety subscale, score
Psychological status — Hospital Anxiety and Depression Scale (HADS) ‐ depression subscale, score
Peak exercise capacity — Incremental Shuttle Walk Test (ISWT), m
|
|
| Identification |
Country: United Kingdom Setting: hospital Authors name: Victoria M Lord Institution: Royal Brompton & Harefield NHS Foundation Trust Email: n.hopkinson@imperial.ac.uk Address: Royal Brompton Hospital, Sydney Street, London SW3 6NP, UK |
|
| Notes | Prior to randomisation, all study participants received a 30‐minute standard session on breathing control and techniques to manage breathlessness, delivered by a respiratory physiotherapist. Pursed lip breathing and nose breathing were also discussed in relation to managing episodes of shortness of breath. Each participant received a standard Royal Brompton Hospital “Help Yourself ‐ physiotherapy for people with respiratory symptoms” and was advised to practice the techniques at home. Sponsorship source: Royal Brompton and Harefeld Arts (rb&hArts) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Consecutive sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not specified, but due to the physical nature of the intervention it is unlikely the participants were able to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes measured reported for all participants completing post intervention assessment High dropout rate from singing group (25%) |
| Selective reporting (reporting bias) | Low risk | All outcome measures listed in methods were reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Lord 2012.
| Methods | Study design: randomised controlled trial | |
| Participants |
Participants: n = 33 Included: COPD (diagnosed according to the GOLD guidelines and attending respiratory clinics) Baseline characteristics: Intervention group — singing (n = 18)
Control group — film workshop (n = 15)
|
|
| Interventions |
Intervention characteristics: Intervention group — singing
Control group — film workshop
|
|
| Outcomes |
Health‐related quality of life — SF‐36 Physical Component Summar (PCS), score
Health‐related quality of life — SF‐36 Mental Component Summary (MCS), score
Health‐related quality of life — COPD Assessment Test (CAT), score
Psychological status — Hospital Anxiety and Depression Scale (HADS) — anxiety subscale, score
Psychological status — Hospital Anxiety and Depression Scale (HADS) ‐ depression subscale, score
Peak exercise capacity — Incremental Shuttle Walk Test (ISWT), m
Physical activity — steps per day, n
Physical activity — sedentary time per day, min
Physical activity — physical activity duration per day, min
Physical activity — active energy expenditure per day, kJ
|
|
| Identification |
Country: United Kingdom Setting: hospital Authors name: Victoria M Lord Institution: Royal Brompton & Harefield NHS Foundation Trust Email: n.hopkinson@ic.ac.uk Address: Royal Brompton Hospital, Sydney Street, London SW3 6NP, UK |
|
| Notes | Prior to randomisation, all study participants received a 30‐minute standard session on breathing control and techniques to manage breathlessness, delivered by a respiratory physiotherapist. Pursed‐lip breathing and nose breathing were also discussed in relation to managing episodes of shortness of breath. Each participant received a standard Royal Brompton Hospital “Help Yourself ‐ physiotherapy for people with respiratory symptoms” and was advised to practice the techniques at home. Sponsorship source: Royal Brompton and Harefield Arts (rb&hArts) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence was developed by an author who was not involved with the day to day conduct of the trial |
| Allocation concealment (selection bias) | Low risk | Consecutive sequentially numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not specified, but due to the physical nature of the intervention it is unlikely the participants were able to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes measured reported for all participants completing post intervention assessment High dropout rate from singing group (28%) |
| Selective reporting (reporting bias) | High risk | Spirometry and exacerbation rate were not reported post intervention |
| Other bias | Low risk | Study appears to be free of other sources of bias |
cmH2O: centimetre of water
COPD: chronic obstructive pulmonary disease
ERV: end respiratory volume
FEV1: forced expiratory volume in one second
FVC: forced vital capacity
GOLD: Global Initiative for Chronic Obstructive Lung Disease
IC: inspiratory capacity
L: litre
min: minute
PImax: maximal inspiratory pressure
PEmax: maximal expiratory pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Canga 2015 | Intervention did not meet study inclusion criteria of singing forming the majority of the intervention |
Characteristics of studies awaiting assessment [ordered by study ID]
Kaasgaard 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Liu 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Miyahara 2001.
| Methods | Study design: randomised controlled trial |
| Participants |
Participants: n = 20 Included: COPD (moderate to severe) Baseline characteristics:
|
| Interventions |
Intervention characteristics: Intervention group — Japanese traditional "Shigin" singing programme
Control group — no training |
| Outcomes |
Pulmonary function Respiratory muscle strength Peak exercise capacity Health‐related quality of life — Chronic Respiratory Disease Questionnaire, score |
| Notes | Abstract only Authors were contacted for further information, with no response |
S 2017.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
COPD: chronic obstructive pulmonary disease
FEV1: forced expiratory volume in one second
Characteristics of ongoing studies [ordered by study ID]
Kaasgaard 2017.
| Trial name or title | The effects of singing training for patients with chronic obstructive pulmonary disease |
| Methods | Cluster‐randomised controlled trial |
| Participants | People with COPD |
| Interventions | Singing group versus pulmonary rehabilitation group; 90 minute sessions twice weekly for 10 weeks |
| Outcomes | Not known |
| Starting date | Not known |
| Contact information | Mette Kaasgaard (mk@clin.au.dk) |
| Notes | Researcher was contacted for further information, with no response |
COPD: chronic obstructive pulmonary disease
Differences between protocol and review
We added clarification regarding the types of interventions: the criterion 'in the case of interventions combining one or more components of music therapy, for example instrumental and singing training, the singing must form the majority of the intervention' was added.
Summary of findings table: only the primary outcomes of health‐related quality of life and dyspnoea were reported. Secondary outcomes of respiratory muscle strength and adverse events were not added due to only one study and no data, respectively, being available.
Subgroup and sensitivity analyses were not performed due to the small number of trials included.
Heterogeneity was considered significant if the P value was less than 0.10 (Higgins 2011).
Contributions of authors
RJM: protocol initiation, development and writing; additional literature search; retrieval of papers; study screening, quality appraisal and data extraction; author contact; data entry; data analysis; data interpretation; manuscript writing.
CE: protocol development and writing; additional literature search; retrieval of papers; study screening, quality appraisal and data extraction; data analysis; data interpretation; manuscript writing.
EC: protocol development and writing; data interpretation; manuscript writing; manuscript review.
ZJM: protocol development and writing; data interpretation; manuscript review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Cochrane Complementary Medicine Field bursary, USA.
Financial support received by RJM to facilitate completion of the review
Declarations of interest
RJM: none known.
CE: none known.
EC: none known.
ZJM: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Bonilha 2009 {published data only}
- Bonilha AG, Onofre F, Vieira ML, Almeida Prado MY, Martinez JA. Effects of singing classes on pulmonary function and quality of life of COPD patients. International Journal of Chronic Obstructive Pulmonary Disease 2009;4(1):1‐8. [PMC free article] [PubMed] [Google Scholar]
- Martinez JB, Bonilha AG, Onofofre F, Vieira ML, Almeida Prado MY. Effects of singing on pulmonary function and quality of life of COPD patients [abstract]. American Thoracic Society International Conference, 2008 May 16‐21, Toronto 2008, (Meeting Abstracts):A663[#F97]. [Google Scholar]
Lord 2010 {published data only}
- Lord VM, Cave P, Hume V, Flude L, Evans A, Kelly JL, et al. Singing for breathing effects of singing lessons in patients with COPD ‐ a randomised control trial [abstract]. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2012;24(Meeting Abstracts):308. [Google Scholar]
- Lord VM, Cave P, Hume VJ, Flude EJ, Evans A, Kelly JL, et al. Singing teaching as a therapy for chronic respiratory disease ‐ a randomised controlled trial and qualitative evaluation. BMC Pulmonary Medicine 2010;10:41. [DOI: 10.1186/1471-2466-10-41] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord VM, Hume V, Cave P, Flude LJ, Kelly JL, Hopkinson NS. Effect of singing lessons in patients with COPD a randomised controlled trial [abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A5134. [Google Scholar]
Lord 2012 {published data only}
- Lord VM, Hume VJ, Kelly JL, Cave P, Silver J, Waldman M. Effects of "Singing For Breathing" TM in patients with chronic obstructive pulmonary disease (COPD) ‐ a randomized control trial [abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A5788. [Google Scholar]
- Lord VM, Hume VJ, Kelly JL, Cave P, Silver J, Waldman M, et al. Erratum to: Singing classes for chronic obstructive pulmonary disease: a randomized controlled trial [BMC Pulm Med, 12(1), 2012, 69]. BMC Pulmonary Medicine 2014;14(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord VM, Hume VJ, Kelly JL, Cave P, Silver J, Waldman M, et al. Singing classes for chronic obstructive pulmonary disease: a randomized controlled trial. BMC Pulmonary Medicine 2012;12(69):69. [DOI: 10.1186/1471-2466-12-69] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Canga 2015 {published data only}
- Beth Israel Medical Center. Music therapy in the treatment of chronic obstructive pulmonary disease. ClinicalTrials.gov 2008.
- Canga B, Azoulay R, Raskin J, Loewy J. AIR: advances in respiration ‐ music therapy in the treatment of chronic pulmonary disease. Respiratory Medicine 2015;109:1532‐9. [DOI: 10.1016/j.rmed.2015.10.001] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kaasgaard 2018 {published data only}
- Kaasgaard M. Is singing efficient in COPD rehabilitation?. European Clinical Respiratory Journal, 7th Scandinavian COPD Research Symposium. Norway. 5 (Supplement 1). 2016.
Liu 2019 {published data only}
- Liu H, Song M, Zhai Z‐H, Shi R‐J, Zhou X‐L. Group singing improves depression and life quality in patients with stable COPD: a randomized community‐based trial in China. Quality of Life Research 2019. [DOI: 10.1007/s11136-018-2063-5] [DOI] [PMC free article] [PubMed]
Miyahara 2001 {published data only (unpublished sought but not used)}
- Miyahara N, Eda R, Kunichika N, Takeyama H. Benefits of singing training in chronic obstructive pulmonary disease patients. American Journal of Respiratory and Critical Care Medicine 2001;163:5 Suppl. [Google Scholar]
S 2017 {published data only}
- Jamaly S, Leidag M, Schneider HW, Domanksi U, Rasche K, Schroder M, et al. The effect of singing therapy compared to standard physiotherapeutic lung sport in COPD. Pneumologie. Kongress der Deutschen Gesellschaft fur Pneumologie und Beatmungsmedizin e.V.. Germany (Supplement 1). 2017; Vol. 71.
References to ongoing studies
Kaasgaard 2017 {unpublished data only}
- The effects of singing training for patients with chronic obstructive pulmonary disease. Ongoing study Not known.
Additional references
Bestall 1999
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54(7):581‐6. [DOI: 10.1136/thx.54.7.581] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bianchi 2004
- Bianchi R, Gigliotti F, Romagnoli I, Lanini B, Castellani C, Grazzini M, et al. Chest wall kinematics and breathlessness during pursed‐lip breathing in patients with COPD. Chest 2004;125(2):459‐65. [DOI: 10.1378/chest.125.2.459] [DOI] [PubMed] [Google Scholar]
Bunch 1995
- Bunch MA. Dynamics of the Singing Voice. Vienna: Springer, 1995. [DOI: 10.1007/978-3-7091-2065-1] [DOI] [Google Scholar]
Engen 2005
- Engen RL. The singer's breath: implications for treatment of persons with emphysema. Journal of Music Therapy 2005;42(1):20‐48. [DOI: 10.1093/jmt/42.1.20] [DOI] [PubMed] [Google Scholar]
Esteve 1996
- Esteve F, Blanc‐Gras N, Gallego J, Benchetrit G. The effects of breathing pattern training on ventilatory function in patients with COPD. Biofeedback and Self‐regulation 1996;21(4):311‐21. [DOI: 10.1007/BF02214431] [DOI] [PubMed] [Google Scholar]
Garcia‐Aymerich 2006
- Garcia‐Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006;61:772‐8. [DOI: 10.1136/thx.2006.060145] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaude 2014
- Gaude GS, Savadatti R, Hattiholi J. Postural correction for kyphosis improves the dyspnea index and pulmonary functions in patients with chronic obstructive pulmonary disease: a randomized trial over 12 weeks. International Journal of Health & Allied Sciences 2014;3(1):44‐51. [DOI: 10.4103/2278-344X.130615] [DOI] [Google Scholar]
GOLD 2017
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Pocket guide to COPD diagnosis, management and prevention 2017. www.goldcopd.org (accessed 4 September 2017).
Goodridge 2013
- Goodridge D, Nicol JJ, Horvey KJ, Butcher S. Therapeutic singing as an adjunct for pulmonary rehabilitation participants with COPD: outcomes of a feasibility study. Music and Medicine 2013;5(3):169‐76. [DOI: 10.1177/1943862113493012] [DOI] [Google Scholar]
Gosselink 2003
- Gosselink R. Controlled breathing and dyspnea in patients with chronic obstructive pulmonary disease (COPD). Journal of Rehabilitation Research and Development 2003;40(5 Suppl 2):25‐34. [DOI] [PubMed] [Google Scholar]
Gosselink 2004
- Gosselink R. Breathing techniques in patients with chronic obstructive pulmonary disease (COPD). Chronic Respiratory Disease 2004;1(3):163‐72. [DOI: 10.1191/1479972304cd020rs] [DOI] [PubMed] [Google Scholar]
Gould 1973
- Gould WJ, Okamura H. Static lung volumes in singers. Annals of Otology, Rhinology and Laryngology 1973;82(1):89‐95. [DOI: 10.1177/000348947308200118] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 18 February 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guyatt 1987
- Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;42:773‐8. [DOI: 10.1136/thx.42.10.773] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hays 2001
- Hays RD, Morales LS. The RAND‐36 measure of health‐related quality of life. Annals of Medicine 2001;33(5):350‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holland 2012
- Holland AE, Hill CJ, Jones AY, McDonald CF. Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD008250.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Irons 2010
- Irons JY, Kenny DT, Chang AB. Singing for children and adults with bronchiectasis. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD007729.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Irons 2016
- Irons JY, Kenny DT, Chang AB. Singing as an adjunct therapy for children and adults with cystic fibrosis. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD008036.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leanderson 1987
- Leanderson R, Sundberg J, Euler C. Breathing muscle activity and subglottal pressure dynamics in singing and speech. Journal of Voice 1987;1(3):258‐61. [DOI: 10.1016/S0892-1997(87)80009-7] [DOI] [Google Scholar]
Leanderson 1988
- Leanderson R, Sundberg J. Breathing for singing. Journal of Voice 1988;2(1):2‐12. [DOI: 10.1016/S0892-1997(88)80051-1] [DOI] [Google Scholar]
Lewis 2016
- Lewis A, Cave P, Stern M, Welch L, Taylor K, Russell J, et al. Singing for lung health ‐ a systematic review of the literature and consensus statement. npj Primary Care Respiratory Medicine 2016;26:16080. [DOI: 10.1038/npjpcrm.2016.80] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lozano 2012
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380(9859):2095‐128. [DOI: 10.1016/S0140-6736(12)61728-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luce 1982
- Luce JM, Culver BH. Respiratory muscle function in health and disease. Chest 1982;81:82‐90. [DOI: 10.1378/chest.81.1.82] [DOI] [PubMed] [Google Scholar]
MacDonald 2012
- MacDonald R, Kreutz G, Mitchell L. Music, health, and wellbeing. Oxford: Oxford University Press, 2012. [Google Scholar]
Mathar 2017
- Mathar H, Fastholm P, Lange P, Larsen NS. Why do patients decline participation in offered pulmonary rehabilitation? A qualitative study. Clinical Rehabilitation 2017;31(12):1674‐83. [DOI: 10.1177/0269215517708821] [DOI] [PubMed] [Google Scholar]
Mathis 2009
- Mathis DR. Melodic sculpturing: the art and science of singing. Bloomington, IN: AuthorHouse, 2009. [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD003793.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McNaughton 2016
- McNaughton A, Aldington S, Williams G, Levack WM. Sing your lungs out: a qualitative study of a community singing group for people with chronic obstructive pulmonary disease (COPD). BMJ Open 2016;6:e012521. [DOI: 10.1136/bmjopen-2016-012521] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 2013
- Morrison I, Clift S, Page S, Salisbury I, Shipton M, Skingley A, et al. A UK feasibility study on the value of singing for people with chronic obstructive pulmonary disease (COPD). UNESCO Observatory Multi‐Disciplinary Journal in the Arts 2013;3(3):1‐19. [Google Scholar]
Oxford 2016
- Oxford University Press. Oxford dictionary 2016. www.oxforddictionaries.com/definition/english/sing (accessed 21 January 2016).
Pacheco 2014
- Pacheco CM, Costa A, Amado J, Almeida P. Singing in chronic obstructive pulmonary diease patients: a pilot study in Portugal. Revista portuguesa de pneumologia 2014;20:225‐8. [DOI: 10.1016/j.rppneu.2014.02.009] [DOI] [PubMed] [Google Scholar]
Proctor 1980
- Proctor D. Breathing speech and song. New York: Springer Verlag, 1980. [Google Scholar]
Puhan 2008
- Puhan MA, Frey M, Buchi S, Schunemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health and Quality of Life Outcomes 2008;6:46. [DOI: 10.1186/1477-7525-6-46] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rycroft 2012
- Rycroft CE, Heyes A, Lanza L, Becker K. Epidemiology of chronic obstructive pulmonary disease: a literature review. International Journal of COPD 2012;7:457‐94. [DOI: 10.2147/COPD.S32330] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salomoni 2016
- Salomoni S, Hoorn W, Hodges P. Breathing and singing: objective characterization of breathing patterns in classical singers. PLoS One 2016;11(5):e0155084. [DOI: 10.1371/journal.pone.0155084] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singer 2011
- Singer J, Yelin EH, Katz PP, Sanchez G, Iribarren C, Eisner MD, et al. Respiratory and skeletal muscle strength in COPD: impact on exercise capacity and lower extremity function. Journal of Cardiopulmonary Rehabilitation and Prevention 2011;31(2):111‐9. [DOI: 10.1097/HCR.0b013e3182033663] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skingley 2014
- Skingley A, Page S, Clift S, Morrison I, Coulton S, Treadwell P, et al. "Singing for breathing": participants' perceptions of a group singing programme for people with COPD. Arts & Health: an International Journal for Research, Policy and Practice 2014;6(1):59‐74. [DOI: 10.1080/17533015.2013.840853] [DOI] [Google Scholar]
Spruit 2013
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2013;188(8):e13‐e64. [DOI: 10.1164/rccm.201309-1634ST] [DOI] [PubMed] [Google Scholar]
Staes 2011
- Staes FF, Jansen L, Vilette A, Coveliers Y, Daniels K, Decoster W. Physical therapy as a means to optimize posture and voice parameters in student classical singers: a case report. Journal of Voice 2011;25(3):e91‐101. [DOI: 10.1016/j.jvoice.2009.10.012] [DOI] [PubMed] [Google Scholar]
Sundberg 1993
- Sundberg J. Breathing behavior during singing. National Journal 1993;Jan/Feb:4‐9, 49‐51. [Google Scholar]
Troosters 2010
- Troosters T, Sciurba F, Battaglia S, Langer D, Rao Valluri S, Martino L, et al. Physical inactivity in patients with COPD, a controlled multi‐centre pilot‐study. Respiratory Medicine 2010;104(7):1005‐11. [DOI: 10.1016/j.rmed.2010.01.012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI: 10.1111/j.1600-0447.1983.tb09716.x] [DOI] [PubMed] [Google Scholar]


