Abstract
Human regulatory dendritic cells (DCreg) were generated from CD14 immunobead-purified or elutriated monocytes in the presence of vitamin D3 and IL-10. They exhibited similar, low levels of costimulatory CD80 and CD86, but comparatively high levels of co-inhibitory programed death ligand-1 (PD-L1) and IL-10 production compared to control immature DC (iDC). Following Toll-like receptor 4 ligation, unlike control iDC, DCreg resisted phenotypic and functional maturation and further upregulated PD-L1:CD86 expression. Whereas LPS-stimulated control iDC (mature DC; matDC) secreted pro-inflammatory tumor necrosis factor α, but no IL-10, the converse was observed for LPS-stimulated DCreg. DCreg weakly stimulated naïve and memory allogeneic CD4+ and CD8+ T cell proliferation and IFNγ, IL-17A and perforin/granzyme B production in MLR. Their stimulatory function was enhanced however, by blocking PD-1 ligation. High-throughput T cell receptor (TCR) sequencing revealed that, among circulating T cell subsets, memory CD8+ T cells contained the most alloreactive TCR clonotypes and that, while matDC expanded these alloreactive memory CD8 TCR clonotypes, DCreg induced more attenuated responses. These findings demonstrate the feasibility of generating highly-purified GMP-grade DCreg for systemic infusion, their influence on the alloreactive T cell response, and a key mechanistic role of the PD1 pathway.
Keywords: human, dendritic cells, immune regulation, cell therapy
1. Introduction
Currently, there is major interest in the potential of adoptive cell therapy with innate or adaptive immune cells for the control of allograft rejection [1, 2] and reduced patient dependence on pharmacologic immunosuppression. Based on extensive preclinical studies, both regulatory myeloid cells and regulatory T cells (Treg) offer considerable promise. Thus, dendritic cells (DC) with regulatory function (DCreg) maintain self-tolerance in the normal steady-state [3], terminate memory T cell responses [4] and regulate auto- and alloimmunity [5]. Ex vivo generation of DC in the presence of specific pharmacologic agents or anti-inflammatory cytokines inhibits their maturation, confers resistance to maturation and promotes their tolerogenicity [6]. These DCreg subvert naïve and memory T cell responses by various mechanisms [7]. Moreover, their administration as cell-based vectors inhibits autoimmune disorders [8], graft-versus-host disease [9] and organ transplant rejection in experimental animals [7, 10].
When infused into rodent allograft recipients before transplantation, with or without immunosuppressive agents, DCreg of donor origin promote long-term graft survival or tolerance [7]. Similar results have been obtained in mice or rats by infusing autologous DC [10, 11]. More recently, using a clinically-relevant, nonhuman primate (NHP) renal allograft model, we have shown [12] that pre-transplant infusion of DCreg of donor origin, together with a short course of minimal immunosuppression, prolongs graft survival, without evidence of host sensitization. Collectively, these findings raise expectations of the therapeutic potential of DCreg for promotion or restoration of tolerance in the clinic [13, 14].
Recently, phase 1 safety trials of autologous monocyte-derived DCreg, including those pulsed with autoAg and administered locally to patients with certain autoimmune diseases have been conducted [15–18]. The results indicate that DCreg are well-tolerated, and a phase 1/2 trial of autologous DCreg in live donor renal transplantation (NCT02252055) has begun as part of The ONE Study (www.onestudy.org) [19].
In preparation for first-in-human testing of donor-derived (allogeneic) DCreg in organ transplantation, we have characterized DCreg generated in vitamin (Vit)D3 and IL-10 from circulating monocytes isolated by either immunobead selection or elutriation. We show that adequate numbers of highly-purified DCreg that meet release criteria can be generated readily from elutriated monocytes under good manufacturing practice (GMP) conditions to meet envisioned target doses for systemic infusion. These DCreg resist maturation,- an important pre-requisite for their adoptive transfer into prospective graft recipients. They also express high levels of co-inhibitory programed death ligand-1 (PD-L1) relative to co-stimulatory CD86. Furthermore, PD1 pathway ligation plays a key role in regulating their allogeneic T cell stimulatory function.
2. Materials and Methods
2.1. DC generation and stimulation
Monocytes were isolated from either normal human buffy coat PBMC (LeukoPaks; Central Blood Bank of Pittsburgh) using CD14-specific immunobeads (Miltenyi, San Diego, CA), or obtained as the elutriated monocyte fraction of normal human volunteer leukapheresis products (Institute for Transfusion Medicine, Pittsburgh, PA) under an IRB-approved protocol. Immature DC (imDC) were generated as described [20], except that those generated from elutriated monocytes were cultured in DC serum-free media (Corning) without antibiotics using GMP grade reagents. DCreg were generated by addition of VitD3 (20mM; Sigma, St. Louis, MO) on days 0 and 4 and rhIL-10 (60ng/ml; Peprotech, Rocky Hill, NJ) on day 4 of culture. Lipopolysaccharide (LPS; Salmonella minnesota R595; 0.5 µg/ml, Enzo Life Sciences, Farmingdale, NY) was added to DC cultures on day 6 to evaluate their response to a potent maturation-inducing agent. Responses to soluble CD40 ligand (sCD40L; MegaCD40L, 100ng/ml, Enzo Life Sciences) or a pro-inflammatory cytokine cocktail (PCC) consisting of IL-6, TNFα, IL-1β (all 10ng/ml; Peprotech) and prostaglandin (PG)E2 (1µg/ml; Sigma) were also evaluated. Upon harvest, DC were washed thoroughly (1× PBS) before phenotypic and functional analyses.
2.2. Flow cytometric analysis
Cell surface marker and intracellular cytokine staining was performed as described [20]. Details of the panel of monoclonal Abs used are shown in Supplementary Table 1. Flow cytometry was performed on a BD Fortessa cytometer and data analyzed using FlowJo software (FlowJo, LLC Ashland, OR).
2.3. T cell proliferation assays
Allogeneic pan T cells were isolated (Miltenyi), labeled with carboxyfluorescein succinimidyl ester (CFSE; 2µM; Invitrogen, Waltham, MA) and co-cultured for 5 days at 37°C in 5% CO2 in air with DC (1DC:10T cell ratio). T cell proliferation was estimated by CFSE dilution. For some MLR, anti-PD-L1 (10µg/ml, Biolegend, San Diego, CA) and anti-PD-1 blocking Abs (10µg/ml; Affymetrix/eBioscience), anti-IL-6R blocking Ab (400µg/ml; Genentech, San Francisco, CA), anti-IL-10R blocking Ab (10µg/ml; Biolegend) or IgG1 isotype control (10µg/ml; Affymetrix/eBioscience) was added at the start of culture.
2.4. Cytokine cytometric bead array (CBA) assay
Cytokines in culture supernatants were quantified using BD Bioscience (San Jose, CA) Human Cytometric Bead Array (CBA) Flex kits, according to the manufacturer’s instructions.
2.5. Clonotypic analysis of alloreactive T cells
Naïve (CD45RA+) or memory (CD45RO+) CD4+ and CD8+ T cells were isolated from healthy controls by FACS. Sorted subsets were CFSE-labeled and incubated with allogeneic DCreg or matDC (1DC:10T cell ratio) in 5-day CFSE-MLR. Controls consisted of unstimulated T cell subsets or DC alone. Genomic DNA was extracted using the Qiagen DNeasy Blood & Tissue Kit (Cat No./ID: 69506; Qiagen, Gaithersburg, MD), quantified and shipped to Adaptive Biotechnologies (Seattle, WA) for T cell receptor (TCR)β complementarity determining region (CDR3) amplification, sequencing and data analysis. Briefly, a multiplexed two-step polymerase chain reaction (PCR) method was employed using a mixture of forward primers specific to TCR Vβ gene segments and reverse primers specific to TCR Jβ gene segments. V, D and J gene definitions were based on annotation in accordance with the IMGT database. TCR libraries were sequenced using an Illumina instrument according to the manufacturer’s instructions. The set of observed biological TCR β CDR3 sequences were normalized to correct for residual multiplex PCR amplification bias and quantified against a set of synthetic TCR β CDR3 sequence analogues with proprietary software (Adaptive Biotechnologies).
2.6. Statistical analyses
Results are expressed as means ± SEM. Differences between means were determined using a paired Student ‘t’-test, Wilcoxon signed-rank test or Mann-Whitney U test, as appropriate. ‘P’ values ≤ 0.05 were considered significant. For sequencing analysis, summary statistics were obtained from the immunoSEQ Analyzer toolset. Repertoire diversity was measured as one minus normalized Shannon’s entropy, commonly referred to as “clonality”. Clonal expansion was measured as a statistically significant change in clonal abundance using Fisher’s exact test. The Benjamini-Hochberg Procedure was used to control the false discovery rate with a ‘P’ value <0.01 for the two-tailed test considered significant.
3. Results
3.1. DCreg generation, purity and yield
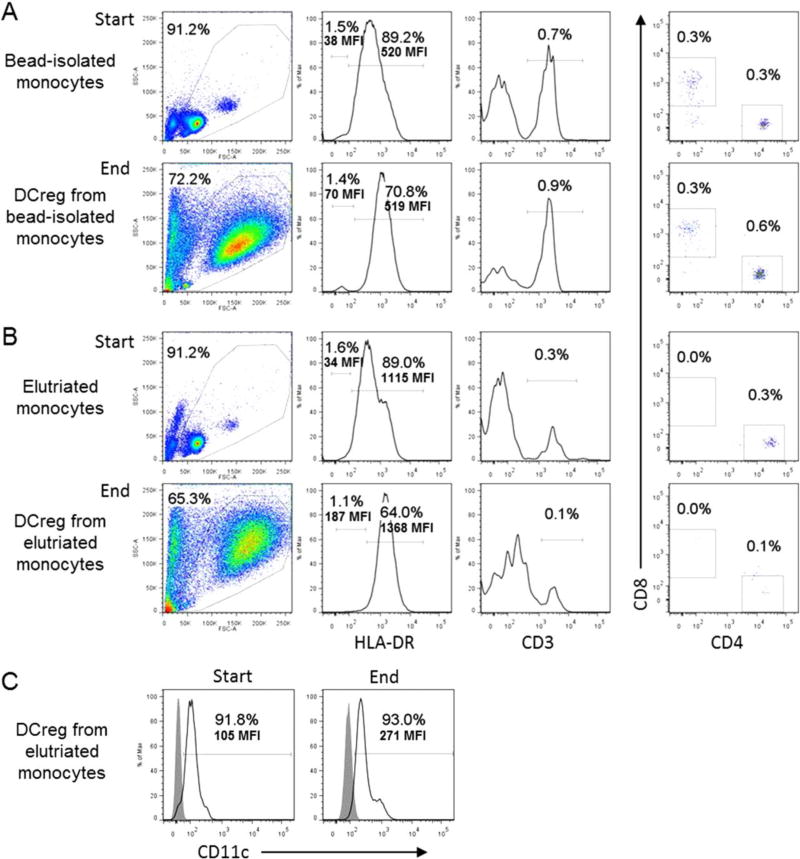
Initially, we generated DCreg in small-scale cultures from CD14 immunobead-purified monocytes isolated from 10 separate healthy individual buffy coats. We compared the results with those obtained subsequently following large scale-up production of DCreg from monocytes elutriated from 4 individual healthy donor leukapheresis products under GMP conditions. Table 1 shows the much greater number of DCreg generated under GMP scale-up conditions. The mean theoretical yield of DCreg from a single whole leukapheresis product was 593 [range 290–820] ± 266 × 106. This number of DCreg from a single donor leukapheresis product would be sufficient to infuse from 4.1 to 11.7 (mean 8.5) ×106/kg into a 70 kg recipient, a dose range equivalent to/in excess of that which we have shown [12] can prolong graft survival in NHP (3.5–10.106/kg). DCreg recovered from both sources of monocytes readily met release criteria: >80% viability, >85% purity (lin− HLA-DR+ CD11c+) and <0.3% contaminating T or B cells (release criteria <1% of each) (Fig. 1).
Table 1.
Yield of DCreg from bead-isolated and elutriated monocytes
| A | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| LeukoPak ID |
PBMC used for monocyte bead isolation (×106) |
Bead-isolated monocytes (×106) |
Cultured DCregBead recovered (×106) |
Total LeukoPak PBMC (×106) |
Theoretical # of cultured DCregBead (×106) |
|
| 3747 | 200 | 50 | 9 | 546 | 23 | |
| 3751 | 150 | 44 | 2 | 442 | 5 | |
| 3747 | 190 | 72 | 4 | 546 | 10 | |
| 6003 | 300 | 89 | 21 | 616 | 44 | |
| 5058 | 170 | 43 | 13 | 976 | 73 | |
| 774 | 160 | 34 | 5 | 564 | 17 | |
| 772 | 300 | 130 | 34 | 333 | 37 | |
| 1455 | 160 | 47 | 8 | 588 | 32 | |
| 1928 | 170 | 69 | 13 | 453 | 33 | |
| 1021 | 90 | 28 | 6 | 642 | 41 | |
|
| ||||||
| Mean(10) | 170 | 49 | 8 | 555 | 33 | |
| SEM | 21 | 10 | 3 | 54 | 6 | |
| B | |||||
|---|---|---|---|---|---|
|
| |||||
| Leukopheresis ID |
Elutriated monocytes (×106) |
Cultured DCregElut recovered (×106) |
Total elutriated monocytes (×106) |
Theoretical # of cultured DCregElut (×106) |
|
| CPL15–34 | 640 | 178 | 2900 | 812 | |
| CPL 15–39 | 620 | 255 | 2000 | 820 | |
| CPL16-1 | 640 | 93 | 2000 | 290 | |
| CPL16-15 | 960 | 350 | 2000 | 450 | |
|
| |||||
| Mean(4) | 715 | 219 | 2225 | 593 | |
| SEM | 82 | 55 | 225 | 133 | |
Fig. 1.
Human regulatory dendritic cells (DCreg) generated from bead-isolated or elutriated monocytes display highly purity, with very low levels of T cell contamination. Flow cytometric analysis of HLA-DR, CD3 and CD4+ versus CD8+ cells is shown before and after DCreg generation from (A) CD14+ bead-isolated monocytes (upper panels) or (B) elutriated monocytes (lower panels). (C) shows CD11c expression at the start and end of cultures (from elutriated monocytes). Data are representative of 2 individual normal donors.
3.2. DCreg are phenotypically immature and resist maturation following exposure to pro-inflammatory stimuli
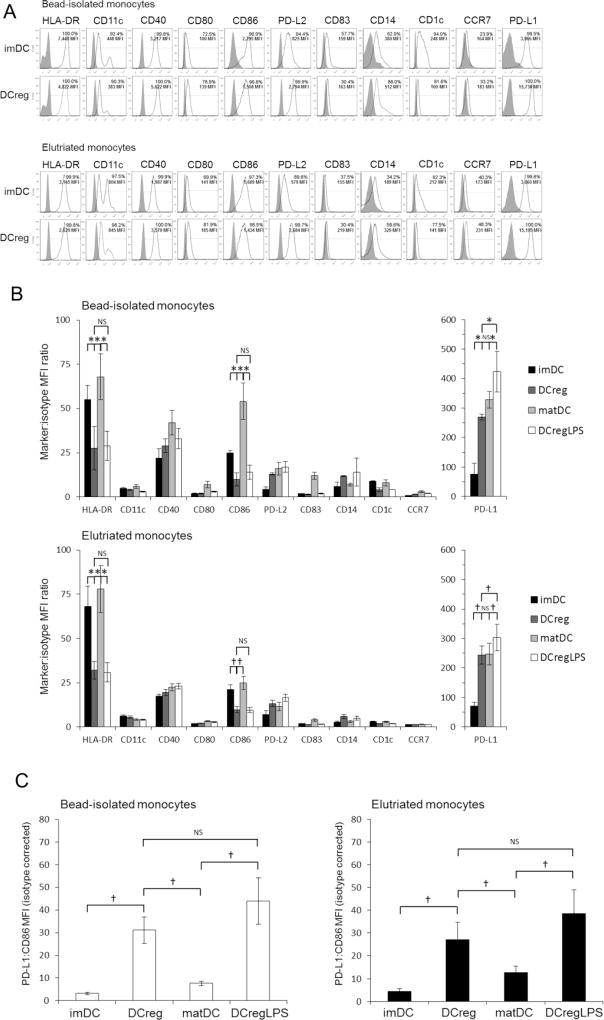
We compared the cell surface phenotype of DC populations generated from bead-isolated or elutriated monocytes. HLA-DR, CD11c, CD40, CD86, and PD-L1/2 were expressed on >90% of imDC and DCreg (Fig. 2A). However, DCreg expressed comparatively low levels (MFI) of HLA-DR and CD86, but much higher levels of PD-L1 compared with imDC (Fig. 2B). An important prerequisite of DCreg generated for therapeutic use, is the ability to resist maturation under pro-inflammatory conditions. Expression of HLA-DR, CD40, CD86, CD83 and CD14 on unstimulated DCreg were very similar to those on LPS-stimulated DCreg (Fig. 2B), indicating they resisted maturation induced by a potent Toll-like receptor agonist. The DCreg also resisted phenotypic maturation when cultured with a PCC or sCD40L (Supplementary Fig. 1). Interestingly, LPS-stimulated DCreg displayed similar, high levels of PD-L1 to unstimulated DCreg (Fig. 2B). Furthermore, although PD-L2 was expressed at lower levels than PD-L1, it followed the same pattern of expression as PD-L1 (LPS-stimulated DCreg compared with DCreg; Fig. 2B).
Fig. 2.
Cell surface phenotype of DCreg. Percent positive cells and mean fluorescence intensity (MFI) were determined for MHC class II (HLA-DR), the DC markers CD11c, CD1c and CD209 (= DC-SIGN; data not shown), T cell costimulatory (CD40, CD80 and CD86) and co-inhibitory (PD-L1 [CD274] and PD-L2 [CD273]) molecules, DC maturation (CD83) and myeloid cell (CD14) marker, and secondary lymphoid tissue homing receptor (CCR7; CD197). (A) Representative examples of cell surface marker expression by immature (im)DC and regulatory DC (DCreg) generated from CD14+ bead-isolated monocytes (upper panels) or elutriated monocytes (lower panels). (B) Cell surface marker expression (MFI) by DC populations generated from bead-isolated monocytes (n=9 individuals) or elutriated monocytes (n=7 individuals). Significances of differences were determined using Wilcoxon t-test. *p<0.01; †p<0.05’ NS = not significant. (C) PDL-1:CD86 MFI ratio of DC cultured from bead-isolated monocytes (n=8; left panel) or elutriated monocytes (n=8, right panel). Significances of differences were determined using Wilcoxon t-test. †p<0.05; NS = not significant. Data are means ±1SEM. matDC, LPS-stimulated imDC; DCreg+LPS, DCreg stimulated with LPS.
3.3. DCreg express a high PD-L1:CD86 ratio
The PD-L1:CD86 MFI ratio expressed by DCreg was significantly higher than that expressed by imDC (Fig. 2C). Importantly, this elevated PD-L1:CD86 ratio was maintained on DCreg generated from bead-isolated or elutriated monocytes following exposure to LPS (Fig. 2C), the PCC or sCD40L (Supplementary Fig. 1) and remained significantly higher than that observed for matDC. Similar observations were made for PD-L2 (Supplementary Fig. 2).
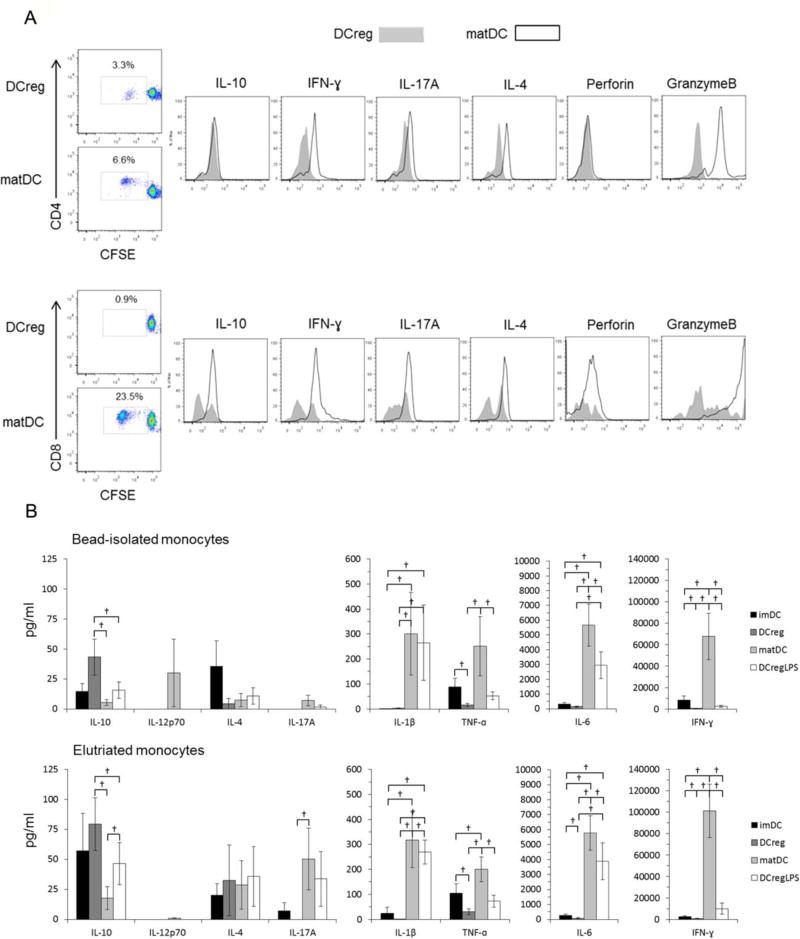
3.4. DCreg secrete high levels of IL-10 but not TNFα
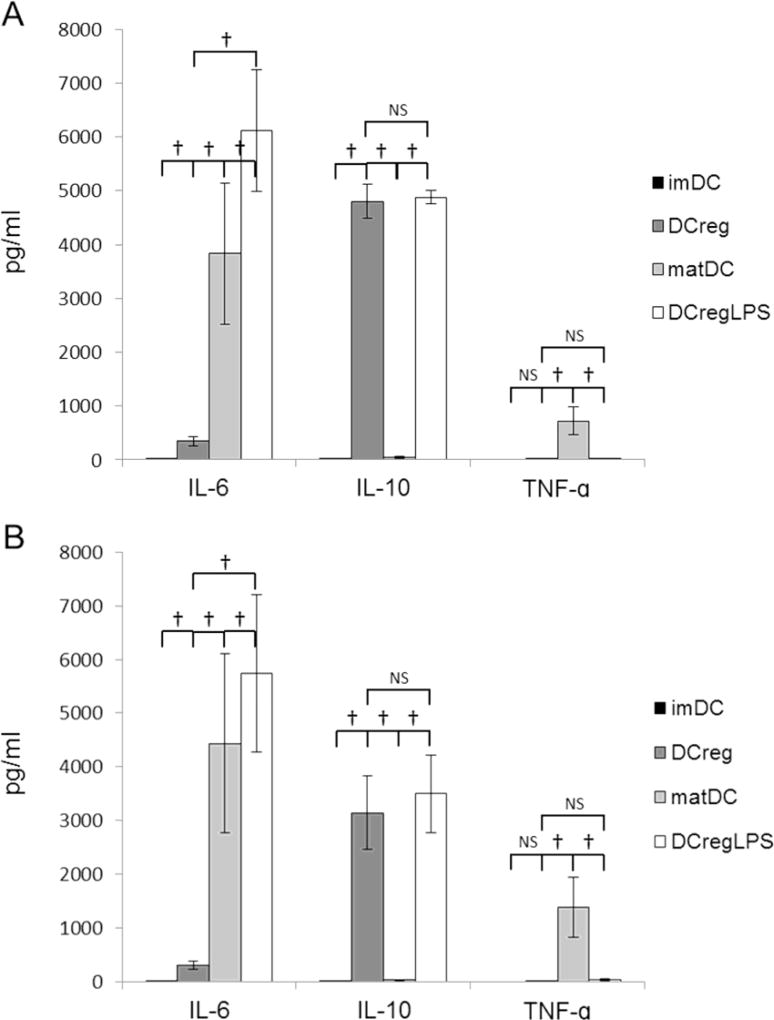
We quantified levels of multiple cytokines in 7-day DC cultures and in 5-day allogeneic MLR supernatants. IL-6, IL-10 and TNFα production profiles of DC generated from beaded or elutriated monocytes was very similar, with no significant differences in cytokine concentrations between these populations (Fig. 3). Notably, IL-10 was produced by DCreg, but not by control imDC. IL-1β, IL-4 and IL-12p70 were not detected under any culture conditions. Cytokine levels in DCreg culture supernatants were also compared before and after LPS stimulation. Highly variable concentrations of IL-6, increased levels of TNFα and profoundly decreased concentrations of IL-10 (compared with DCreg) were observed in matDC cultures, whereas LPS-stimulated DCreg supernatants exhibited similar high levels of IL-10 and minimal TNFα (high IL-10:TNFα ratios) to those of control, unstimulated DCreg. LPS-stimulated DCreg exhibited similar, variable levels of IL-6 production to matDC (Fig. 3). Linear regression analysis (Excel) was performed to determine whether IL-6 production by bead-derived or elutriated monocyte-derived DCreg during co-culture with allogeneic T cells correlated with T cell proliferation or DCreg IL-10 production levels. No correlation was observed between IL-6 production levels and T cell proliferation or DCreg IL-10 production.
Fig. 3.
Cytokine secretion by DCreg. DC populations were generated from (A) CD14+ bead-isolated (n=8 individuals) or (B) elutriated monocytes (n=7 individuals) then cultured overnight in the absence or presence of LPS. Cytokine levels in supernatants were determined by cytometric bead array assay. Data are means ±1SEM. imDC, immature DC; DCreg, regulatory DC; matDC, imDC stimulated with LPS; DCreg + LPS, DCreg stimulated with LPS. †, p<0.05; NS = not significant.
3.5. DCreg are weak stimulators of allogeneic T cell proliferation
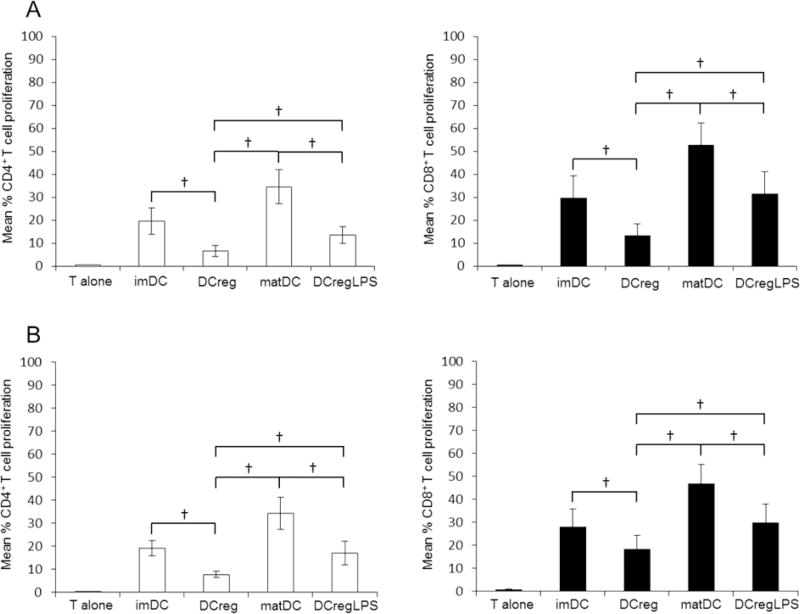
DCreg induced significantly lower levels of CD4+ and CD8+ T cell proliferation than imDC and matDC (Fig. 4). Further, following LPS stimulation, DCreg induced significantly lower levels of CD4+ and CD8+ T cell proliferation than control LPS-stimulated matDC. These results characterize DCreg as weak allostimulatory antigen-presenting cells and further demonstrate their ability to resist functional maturation. The poor T cell stimulatory ability of DCreg compared to imDC and matDC was almost identical, further showing that DCreg with similar function can be generated from CD14+ or elutriated monocytes (Fig. 4).
Fig. 4.
Proliferation of allogeneic T cells stimulated with DCreg. DC were generated from (A) bead-isolated monocytes (n=7 individuals) or (B) elutriated monocytes (n=7 individuals). CFSE-labeled allogeneic T cells were co-cultured for 5 days with various populations of DC (1DC:10T cells). imDC or DCreg were stimulated for 18 to 20 hr with LPS (0.2µg/ml) to generate matDC and DCregLPS, respectively. T cell proliferation was measured by CFSE dilution. Data are means ±1SEM. Significances of differences were determined using Wilcoxon t-test. †, p<0.05; NS = not significant.
3.6. DCreg induce minimal levels of proinflammatory cytokines and cytotoxic effector molecule production, but enhanced IL-10 production in MLR
As shown in Fig. 5A, IFNγ, IL-17A, IL-4 and granzyme B expression by alloactivated CD4+ and CD8+ T cells stimulated by DCreg was markedly attenuated compared with T cells stimulated with control matDC. Levels of secreted IL-12p70, IL-1β, IL-6, TNFα and IFNγ in DCreg-MLR culture supernatants were much lower than those in matDC-MLR supernatants. IL-10 was the only cytokine present at increased levels in DCreg-MLR compared to matDC-MLR supernatants (Fig. 5B). LPS-stimulated DCreg were associated with lower levels of IL-12p70, IL-6, TNFα, IL-17A and IFNγ, and an increased concentration of secreted IL-10 compared to matDC.
Fig. 5.
Cytokine expression by allogeneic T cells co-cultured with DCreg. (A) Intracellular cytokine expression by allogeneic CD4+ (upper panels) and CD8+ T cells (lower panels) stimulated with DCreg or matDC generated from bead-isolated monocytes. CFSE-labeled allogeneic pan T cells were co-cultured for 5 days with DC (1DC:10T cells). The DC-stimulated T cells were harvested, then stimulated for 5 h with αCD2,CD3,CD28 microbeads in the presence of Golgi-plug. (B) Cytokine production in MLR cultures of allogeneic T cells stimulated with DC populations generated from bead-isolated monocytes (n=5 individuals; upper panels) or elutriated monocytes (n=7 individuals; lower panels). Data are means ±1SEM. †, p<0.05.
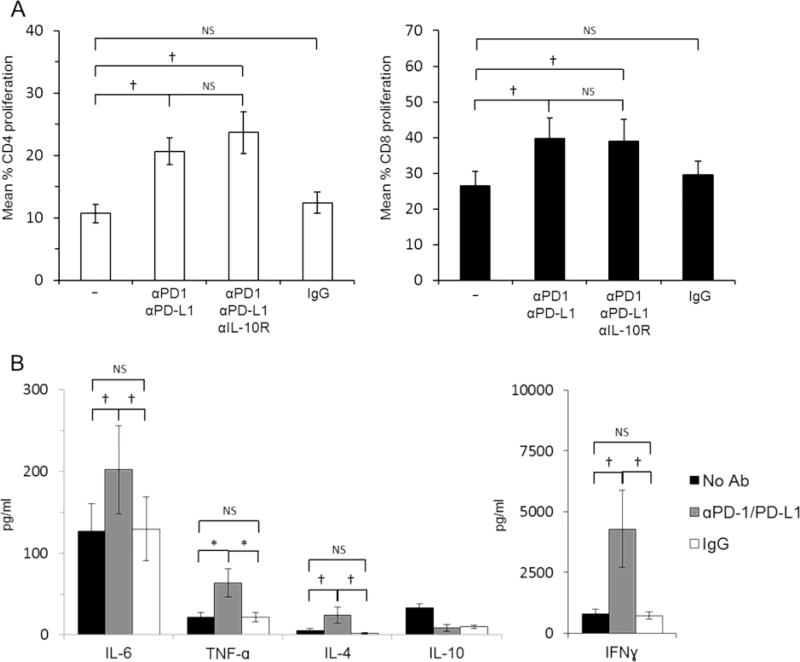
3.7 High PD-L1 expression by DCreg attenuates allogeneic T cell proliferation and IFNγ production
The PD-L1/PD-L2-PD1 pathway plays an important role in maintaining T cell homeostasis and in induction/maintenance of peripheral tolerance [21, 22]. To ascertain whether PD-L1 expression at markedly elevated levels by DCreg was responsible for their poor T cell stimulatory function, we added PD-L1 and PD-1 blocking Abs at the start of DCreg-allogeneic T cell co-cultures. This significantly increased CD4+ and CD8+ T cell proliferation (Fig. 6A), markedly enhanced IFNγ secretion, and increased IL-6 and TNFα levels when compared to co-cultures to which no blocking Abs were added (Fig. 6B). To test the role of IL-10, that was secreted by unstimulated and LPS-stimulated DCreg, but not by imDC or matDC, anti-IL-10R Ab was added along with PD-L1 and PD-1 blocking Ab, but its addition did not further affect T cell proliferation. Thus, PD-L1 expression by DCreg plays a key role in restraining allogeneic T cell proliferation and IFNγ production.
Fig. 6.
The PD1 pathway plays a key role in DCreg function. (A) CFSE-labelled allogeneic T cells were co-cultured with DCreg for 5 days (1DC:10T cells). T cell proliferation was determined by CFSE dilution. PD-L1 and PD-1 blocking antibodies (10µg/ml) ± anti-IL-10R antibody (10µg/ml) or IgG1 isotype control (10µg/ml) were added at the start of culture. (B) Cytokine levels in MLR cultures. Data are means ±1SEM. Significances of differences were determined by using Wilcoxon t-test. *, p<0.01; †, p<0.05. NS = not significant.
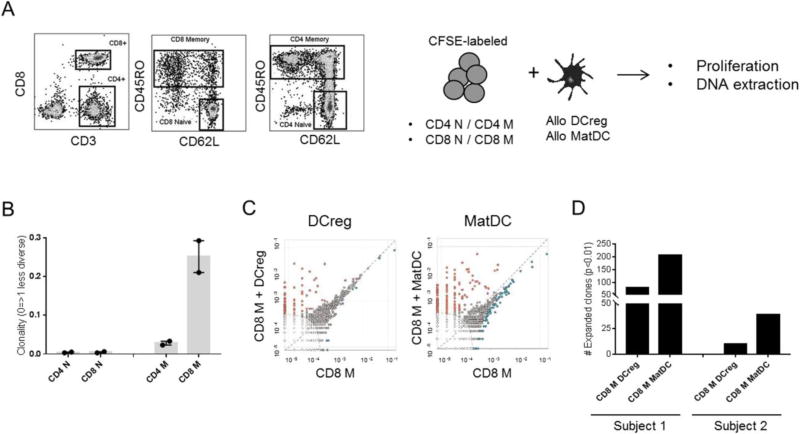
3.8. DCreg allostimulation induces more limited TCR clonotype expansion of memory CD8+ T cells than matDC
We used high throughput TCR immune-sequencing with DNA purified from flow-sorted naïve and memory CD4+ and CD8+ T cells of two healthy control subjects before and after allo-stimulation with either DCreg or matDC in CFSE-MLR (Fig. 7A). This allowed us assess the overall TCR clonal repertoire within resting T cells and the frequency of alloreactive TCR clonotypes before and after exposure to allogeneic DC. Circulating naïve CD4+ and CD8+ T cells displayed highly diverse (polyclonal) TCR repertoires, whereas memory T cells were less diverse, with CD8+ T cells being the most oligoclonal (Fig. 7B). Resting (unstimulated) memory CD8+ T cells (among all T cell subsets tested) contained most alloreactive TCR clonotypes that were shared and correlated positively with alloreactive TCR clonotypes after DCreg or matDC stimulation (Fig. 7C). For both subjects, allostimulation triggered clonal expansion, however DCreg induced more limited alloreactive memory CD8+ T cell clonal expansion. For both DC types, the existing repertoire of expanded clones was largely undisturbed, and there was no evidence of clones disappearing from the CD8+ memory repertoire upon stimulation.
Fig. 7.
Clonotypic analysis of alloreactive T cells stimulated with DCreg. (A) Gating strategy used to FACS sort naïve (N) and memory (M) CD4 and CD8 T cell subsets. Each sorted population was cultured with allogeneic DCreg or matDC in 5-day CFSE-MLR. DNA purified from each condition was amplified by PCR amplified and sequenced for the TCRVβ locus using the Adaptive Technologies platform. (B) TCR repertoire diversity within unstimulated T cell subsets (n=2 healthy individuals). (C) Comparison of shared alloreactive TCR clonotypes between unstimulated M CD8+ T cells (x-axis) and DC-stimulated M CD8+ T cells (y-axis). Dots on the axes represent the frequency of TCR clonotypes that were found in one population but not the other, while those close to the diagonal dotted line represent frequency equality. Significantly expanded clones are highlighted in orange. The elliptical dotted line signifies the threshold for statistical comparison. Representative data from Subject 1. (D) Numbers of alloreactive clones expanded after 5-day MLR stimulation with allogeneic DCreg or matDC. Data from both subjects are shown.
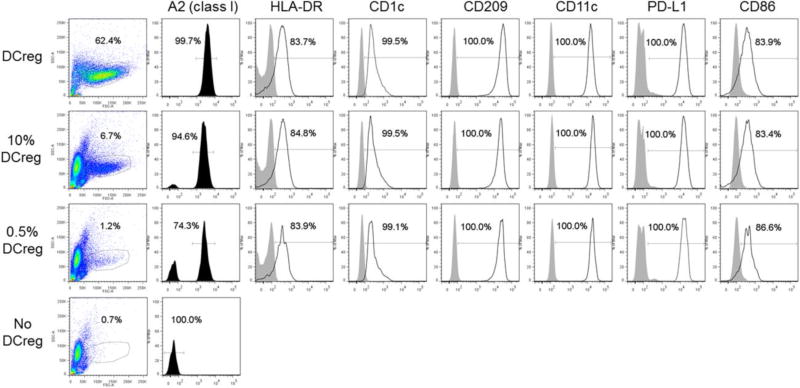
3.9. Mock infusion of DCreg and their detection in allogeneic whole blood
DCreg harvested from two GMP scale-up preparations were placed (350×106) in infusion medium in a transfer bag, held at 4°C for 4 hr, then mock infusion performed slowly over 1 hr at RT using an infusion set (190 cm line) incorporating a 180 µm filter (CareFusion, San Diego CA). In each case, ≥80% of the ‘infused’ cells passed through the entire infusion set, including the filter. Additionally, we examined the presence and phenotype of DCreg following their addition to fresh allogeneic whole blood. As shown in Fig. 8, the allogeneic DCreg were readily detected using mAb directed against MHC class I Ag not shared with the ‘recipient’.
Fig. 8.
Detection of allogeneic DCreg in whole blood. DCreg were generated from elutriated monocytes of an A2 (MHC class 1) positive individual, incubated in 5% goat serum for 15 min on ice, then added to 50µL fresh whole blood (~5.105 WBC) of an A2 negative individual, so that the final % of allogeneic DCreg was 10% or 5%. An Ab cocktail containing FITC anti-A2 Ab, together with Abs against DC and costimulatory (CD86) and coinhibitory (PD-L1) markers was then added for 20 min at 4°C in the dark. Two ml of 1×BD lysis/fixation buffer was added. After 10 min at 4°C in the dark, the cells were washed thoroughly before flow cytometric analysis.
4. Discussion
Crucial to the manufacture of human DCreg for infusion into organ allograft recipients,- whether from healthy donors or patients with end-stage organ failure (recipient-derived DCreg), is their generation in sufficient purity and quantity under GMP conditions to achieve safe and effective target cell doses. DCreg must exhibit functional stability and resistance to maturation in the face of inflammatory stimuli to minimize any potential risk of undesired immunostimulatory effects in vivo (host sensitization). While there is no current consensus regarding the optimal choice of agents for generation of clinical grade DCreg [15–18, 23], our previous studies in NHP, including those given renal transplants, provide a strong rationale for generation of DCreg using VitD3 and IL-10. Thus, these DCreg induce functional unresponsiveness in alloreactive T cells and inhibit allograft rejection [12, 24]. While each agent alone inhibits DC maturation and confers tolerogenic properties on DC [6], we found by comparing VitD3 and IL-10 with other agents, that their combination resulted in the optimal yield of maturation-resistant DCreg. The present report shows that this approach readily allows the generation of human DCreg from monocytes purified either from peripheral blood buffy coats using CD14 immunobeads, or from monocytes elutriated from non-mobilized leukapheresis products. The immunophenotype, high level of purity (<1% T or B cell contamination) and function of DCreg that we generated from both sources was very similar. Since elutriation is the less expensive procedure, it is both an economically (and logistically) preferable choice for human DCreg generation in sufficient quantities for their systemic administration.
In recent phase 1 studies of local adoptive transfer of DCreg into patients with autoimmune disease, doses in the range 1–10 × 106 have been administered either intradermally (type-1 diabetes), intradermally or arthroscopically (rheumatoid arthritis) or intraperitoneally (Crohn’s disease) [15–18], with no evidence of significant adverse effects. Such doses of DCreg given locally can silence effector T cells and induce Ag-specific Treg in human volunteers [25, 26] and immunoregulatory and anti-inflammatory effects in patients with autoimmune disease [17]. Systemic infusion of related, donor-derived (allogeneic) regulatory myeloid cells (regulatory macrophages; 7 or 8 × 106 cells/kg, via central line) in renal transplant patients is also well-tolerated [27], with patients maintained on low dose tacrolimus immunosuppression three years post-transplant. The present findings demonstrate our ability to generate adequate numbers of monocyte-derived DCreg from a single, non-mobilized donor leukapheresis product under GMP conditions to allow infusion of up to 5 × 106 DCreg /kg. This is within the therapeutic range that has proved efficacious in safely prolonging graft survival in minimally-immunosuppressed NHP transplant recipients [12].
The DCreg we generated expressed high levels of PD-L1, that played a key role in attenuating their T cell stimulatory ability. They also exhibited much higher PD-L1:CD86 ratios than control DC. This high ratio was preserved following DCreg stimulation with the potent myeloid DC activating agent LPS, a PCC or sCD40L. In addition, the DCreg secreted high levels of IL-10, but little or no IL-12. In addition to adequate cell number, viability, purity and sterility, each of which each constitute critical product release criteria, we propose that the PD-L1: CD86 ratio be considered as a potential additional release criterion, or provide the basis of a DCreg potency assay based on experience gained manufacturing these cells for use in patients.
Evidence from rodent and NHP studies suggests that, even though their survival may be limited in allogeneic hosts, donor-derived DCreg have the capacity to downregulate T cell responses via the direct, indirect and semi-direct pathways, leading to decreased alloAg-specific Tmem responses that may reflect clonal regulation/exhaustion [28, 29]. The human DCreg generated in the present study expressed high levels of PD-L1 that transmits an inhibitory signal via PD1 that, in turn, reduces proliferation of activated CD8+ T cells and promotes the development, maintenance and function of induced Treg [22]. Moreover, the DCreg also secreted anti-inflammatory IL-10, that potently suppresses DC, T cell and NK cell effector functions and promotes CD4+ type-1 Treg [30].
With respect to our TCR repertoire sequencing data, there is considerable heterogeneity within the human alloreactive memory CD8T cell pool that can be ascribed to genetic differences between individuals and epigenetic “instructions” gained by human T cells after exposure to viruses, bacteria, alloAg etc, throughout life. We also understand that these differences may contribute to heterogeneous modulation of alloreactive T cell clonotype responses by DCreg. Indeed, the results of our two TCR immunosequencing experiments suggest just this. Overall, they show that, compared with matDC, DCreg induce more limited alloreactive memory CD8+ T cell clonal expansion. Notably, donor-derived VitD3/IL-10 DCreg attenuate alloreactive CD8+ T memory cell responses in renal-allografted NHP [12, 29]. We plan to set up TCR immunosequencing each time we infuse donor-derived DCreg into a transplant patient, and thus obtain the personalized mechanism/signature of donor-specific T cell hyporesponsiveness that we postulate will be induced.
5. Conclusion
In conclusion, DCreg meeting criteria for systemic infusion can be generated under GMP conditions and display properties that may be conducive to therapeutic efficacy in organ transplantation.
Supplementary Material
Highlights.
GMP grade regulatory dendritic cells can be generated from human blood monocytes in adequate numbers for systemic infusion
DCreg exhibit high levels of PD-L1 expression and IL-10 production
DCreg resist phenotypic and functional maturation
Blocking PD-1 ligation upregulates their T cell stimulatory function
High throughput TCR sequencing shows DCreg induce attenuated memory T cell responses
Acknowledgments
The authors’ work was supported by National Institutes of Health (NIH) Clinical Trial Planning Grant R34 AI 123033 (AWT), CTSI grant NIH U01 TR000005 (SR) and by the Starzl Transplantation Institute Hammill Family and Robert and Courtney Bost Funds. We thank Veronica Sutherland and Misty DeRiggi for technical support in the DCreg scale-up studies and acknowledge the generous participation of the volunteers without whom the study could not have been undertaken. The study used the University of Pittsburgh Cancer Institute CCSG-supported Immunologic Monitoring and Cellular Products Laboratory core facility, which is supported by NIH grant P30 CA047904-24.
Abbreviations
- DC(s)
dendritic cell(s)
- DCreg
regulatory DC
- GLP
good manufacturing practice
- imDC
immature DC
- matDC
mature DC
- PCC
pro-inflammatory cytokine cocktail
- PD-(L)1
programed death (ligand)1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12:417–430. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson JA, Geissler EK. Now or never? The case for cell-based immunosuppression in kidney transplantation. Kidney Int. 2015;87:1116–1124. doi: 10.1038/ki.2015.50. [DOI] [PubMed] [Google Scholar]
- 3.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J. Exp. Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenna TJ, Thomas R, Steptoe RJ. Steady-state dendritic cells expressing cognate antigen terminate memory CD8+ T-cell responses. Blood. 2008;111:2091–2100. doi: 10.1182/blood-2007-07-103200. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–35. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 7.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 8.Hilkens CM, Isaacs JD, Thomson AW. Development of dendritic cell-based immunotherapy for autoimmunity. Int. Rev. Immunol. 2010;29:156–183. doi: 10.3109/08830180903281193. [DOI] [PubMed] [Google Scholar]
- 9.Stenger EO, Turnquist HR, Mapara MY, Thomson AW. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood. 2012;119:5088–5103. doi: 10.1182/blood-2011-11-364091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beriou G, Peche H, Guillonneau C, Merieau E, Cuturi MC. Donor-specific allograft tolerance by administration of recipient-derived immature dendritic cells and suboptimal immunosuppression. Transplantation. 2005;79:969–972. doi: 10.1097/01.tp.0000158277.50073.35. [DOI] [PubMed] [Google Scholar]
- 11.Garrovillo M, Ali A, Depaz HA, Gopinathan R, Oluwole OO, Hardy MA, Oluwole SF. Induction of transplant tolerance with immunodominant allopeptide-pulsed host lymphoid and myeloid dendritic cells. Am J Transplant. 2001;1:129–137. [PubMed] [Google Scholar]
- 12.Ezzelarab MB, Zahorchak AF, Lu L, Morelli AE, Chalasani G, Demetris AJ, Lakkis FG, Wijkstrom M, Murase N, Humar A, Shapiro R, Cooper DK, Thomson AW. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am J Transplant. 2013;13:1989–2005. doi: 10.1111/ajt.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vassalli G. Dendritic cell-based approaches for therapeutic immune regulation in solid-organ transplantation. Journal of transplantation. 2013;2013:761429. doi: 10.1155/2013/761429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreau A, Alliot-Licht B, Cuturi MC, Blancho G. Tolerogenic dendritic cell therapy in organ transplantation. Transpl. Int. 2016 doi: 10.1111/tri.12889. [DOI] [PubMed] [Google Scholar]
- 15.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026–2032. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jauregui-Amezaga A, Cabezon R, Ramirez-Morros A, Espana C, Rimola J, Bru C, Pino-Donnay S, Gallego M, Masamunt MC, Ordas I, Lozano M, Cid J, Panes J, Benitez-Ribas D, Ricart E. Intraperitoneal administration of autologous tolerogenic dendritic cells for refractory Crohn's Disease: A Phase I study. Journal of Crohn's & colitis. 2015;9:1071–1078. doi: 10.1093/ecco-jcc/jjv144. [DOI] [PubMed] [Google Scholar]
- 17.Benham H, Nel HJ, Law SC, Mehdi AM, Street S, Ramnoruth N, Pahau H, Lee BT, Ng J, ME GB, Hyde C, Trouw LA, Dudek NL, Purcell AW, O'Sullivan BJ, Connolly JE, Paul SK, Le Cao KA, Thomas R. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci Transl Med. 2015;7:290ra287. doi: 10.1126/scitranslmed.aaa9301. [DOI] [PubMed] [Google Scholar]
- 18.Bell GM, Anderson AE, Diboll J, Reece R, Eltherington O, Harry RA, Fouweather T, MacDonald C, Chadwick T, McColl E, Dunn J, Dickinson AM, Hilkens CM, Isaacs JD. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann. Rheum. Dis. 2017;76:227–234. doi: 10.1136/annrheumdis-2015-208456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geissler EK. The ONE Study compares cell therapy products in organ transplantation: introduction to a review series on suppressive monocyte-derived cells. Transplantation research. 2012;1:11. doi: 10.1186/2047-1440-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macedo C, Turnquist HR, Castillo-Rama M, Zahorchak AF, Shapiro R, Thomson AW, Metes D. Rapamycin augments human DC IL-12p70 and IL-27 secretion to promote allogeneic Type 1 polarization modulated by NK Cells. Am J Transplant. 2013;13:2322–2333. doi: 10.1111/ajt.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naranjo-Gomez M, Raich-Regue D, Onate C, Grau-Lopez L, Ramo-Tello C, Pujol-Borrell R, Martinez-Caceres E, Borras FE. Comparative study of clinical grade human tolerogenic dendritic cells. J Transl Med. 2011;9:89. doi: 10.1186/1479-5876-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zahorchak AF, Kean LS, Tokita D, Turnquist HR, Abe M, Finke J, Hamby K, Rigby MR, Larsen CP, Thomson AW. Infusion of stably immature monocyte-derived dendritic cells plus CTLA4Ig modulates alloimmune reactivity in rhesus macaques. Transplantation. 2007;84:196–206. doi: 10.1097/01.tp.0000268582.21168.f6. [DOI] [PubMed] [Google Scholar]
- 25.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174–177. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, Oberg HH, Pascher A, Lutzen U, Janssen U, Broichhausen C, Renders L, Thaiss F, Scheuermann E, Henze E, Volk HD, Chatenoud L, Lechler RI, Wood KJ, Kabelitz D, Schlitt HJ, Geissler EK, Fandrich F. Cutting Edge: Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J. Immunol. 2011;187:2072–2078. doi: 10.4049/jimmunol.1100762. [DOI] [PubMed] [Google Scholar]
- 28.Divito SJ, Wang Z, Shufesky WJ, Liu Q, Tkacheva OA, Montecalvo A, Erdos G, Larregina AT, Morelli AE. Endogenous dendritic cells mediate the effects of intravenously injected therapeutic immunosuppressive dendritic cells in transplantation. Blood. 2010;116:2694–2705. doi: 10.1182/blood-2009-10-251058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezzelarab MB, Lu L, Guo H, Zahorchak AF, Shufesky WF, Cooper DK, Morelli AE, Thomson AW. Eomesoderminlo CTLA4hi alloreactive CD8+ memory T cells are associated with prolonged renal transplant survival induced by regulatory dendritic cell infusion in CTLA4 immunoglobulin-treated nonhuman primates. Transplantation. 2016;100:91–102. doi: 10.1097/tp.0000000000000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.