Abstract
Traumatic brain injury (TBI) and Alzheimer's disease (AD) are devastating neurological disorders, whose complex relationship is not completely understood. Cerebrovascular pathology, a key element in both conditions, could represent a mechanistic link between Aβ/tau deposition after TBI and the development of post concussive syndrome, dementia and chronic traumatic encephalopathy (CTE). In addition to debilitating acute effects, TBI-induced neurovascular injuries accelerate amyloid β (Aβ) production and perivascular accumulation, arterial stiffness, tau hyperphosphorylation and tau/Aβ-induced blood brain barrier damage, giving rise to a deleterious feed-forward loop. We postulate that TBI can initiate cerebrovascular pathology, which is causally involved in the development of multiple forms of neurodegeneration including AD-like dementias. In this review, we will explore how novel biomarkers, animal and human studies with a focus on cerebrovascular dysfunction are contributing to the understanding of the consequences of TBI on the development of AD-like pathology.
Keywords: Traumatic brain injury, Alzheimer's disease, Cerebrovascular pathology, Biomarkers, Aβ, Tau
Highlights
-
•
Cerebrovascular dysfunction (CVD) is emerging as a key element in the development of neurodegeneration after TBI.
-
•
We propose that TBI initiates CVD, accelerating Aβ/tau deposition and leading to neurodegeneration and dementias.
-
•
Clarifying this connection will support the development of novel biomarkers and therapeutic approaches for both TBI and AD.
1. Introduction
Traumatic brain injury (TBI) is a significant public health problem associated with both acute and long-term disabilities, which are mediated by multiple, not entirely understood, molecular cascades. In addition to debilitating acute effects, severe TBI, and especially repeated mild TBI (Blennow et al., 2016) can initiate long-term neurodegeneration processes leading to pathological features that have similarities with Alzheimer's disease (AD)(Washington et al., 2016; Blennow et al., 2016; Mendez, 2017).
The term ‘Punch drunk’ was introduced by Dr. Martland in 1928, when describing the variety of syndromes present in contact-sport players after repeated loss of consciousness, years after retiring from boxing. Symptoms reported were “facial characteristics of the parkinsonian syndrome” and “marked mental deterioration” (Martland, 1928). Martland described the presence of hemorrhages near the “corpora striata” and “corona radiata”, that were later replaced by gliosis and degenerative progressive lesions. Although he described his theory as “insusceptible of proof”, he already showed a relationship between vascular injury and the presence of lesions later in life in nearby brain locations. Since then, a large body of epidemiological studies has shown that having a history of previous TBIs is associated with the development of numerous types of dementia later in life (Mendez, 2017; Gardner et al., 2014). Other recent studies have suggested that TBI is not linked to AD but to other types of neurodegeneration such as Lewy body accumulation and Parkinsonism (Crane et al., 2016) (Weiner et al., 2017), highlighting the need to better understand the pathological mechanisms activated after TBI and their relationship with neurodegeneration.
Evidence that will be discussed throughout this review shows that cerebrovascular dysfunction (CVD) is a key element for the development of dementia after TBI. Exploring biomarkers of CVD will help understand the contribution of TBI-induced vascular damage to AD-like pathology and improve diagnostic and therapeutic approaches for both disorders. We propose a shift in focus from a “neuronal” to a “neurovascular” point of view, pointing to CVD after TBI as a possible causal contributor to Aβ/tau deposition, neurodegeneration, and early initiation of AD-like pathology.
2. Pathological Relationship Between TBI and Dementia
Multiple pathological processes link TBI with neurodegeneration and dementia. The term chronic traumatic encephalopathy (CTE) describes neuropathological changes that occur later in life in patients subjected to repeated concussive head injuries, and can present with symptoms and pathology mimicking neurological diseases including AD, Parkinson's disease (PD), frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). Some pathological features typically observed in AD have been found in postmortem brains of TBI and CTE (McKee et al., 2013), such as increases in hyperphosphorylated tau (P-Tau) and in some cases amyloid beta (Aβ) and TDP-43 deposits (Washington et al., 2014; Abisambra and Scheff, 2014). Indeed, recent consensus criteria consider CTE as a tauopathy (McKee et al., 2016).
2.1. Aβ Pathology in TBI and its Relationship With Vascular Dysfunction
Autopsies of relatively young TBI patients who died during the acute phase after injury show diffuse Aβ plaques similar to those found in AD patients located in the areas surrounding the lesion sites in both gray and white matter regions (reviewed in (Perry et al., 2016, Johnson et al., 2010)).
It is still unclear which mechanisms lead to Aβ accumulation in TBI. TBI, through vascular shear stress, can induce acute blood brain barrier (BBB) disruption, which is known to contribute to both ischemic damage and Aβ accumulation (Iadecola, 2013; Pluta et al., 2013). Hypoperfusion, vascular dysfunction and ischemia after TBI may all contribute to Aβ deposition (Iadecola, 2013; Pluta et al., 2013; De Silva and Faraci, 2016; Wolters et al., 2017). Previous studies showed a relationship between brain ischemic stress and Aβ-deposition (Wisniewski and Maslinska, 1996) and we previously reported that plasma Aβ42 levels are increased after transient hypoxia during static apnea in healthy subjects (Gren et al., 2016), indicating that general hypoxia may cause mild neuronal dysfunction or damage and stimulate Aβ production. Indeed, under blood flow reduction (hypoperfusion), β and γ-secretases are activated, leading to increased Aβ production (Gupta and Iadecola, 2015; Pluta et al., 2013). Additionally, trauma-induced brain heat/cooling alterations can modulate brain metabolism (Mrozek et al., 2012). Metabolic acidosis after TBI could also potentially contribute to Aβ accumulation, as it is known that Aβ, like other proteins, is prone to aggregation in a pH-dependent manner (Acharya et al., 2016). The formation Aβ aggregates in the perivascular spaces induced by these acute events after TBI may play a role in secondary injury cascades, including cerebrovascular damage, oxidative stress, mitochondrial damage, and endothelial cell dysfunction/death. Accordingly, our group has demonstrated the role of oligomeric species of Aβ in the activation of mitochondria- and death receptor-mediated pathways of endothelial cell stress and death (Fossati et al., 2010, Fossati et al., 2012a, Fossati et al., 2012b, Ghiso et al., 2014, Fossati et al., 2013). This cascade of neurovascular stress events induced by Aβ could be particularly exacerbated after repeated TBIs, and contribute to the development of AD-like pathology and dementia later in life.
2.2. Tau Pathology in TBI and its Relationship With Vascular Dysfunction
Tau is well known for its role in neurofibrillary tangle formation in AD. Neuropathological data indicates that CTE is a tauopathy intimately linked to CVD and characterized by the deposition of hyperphosphorylated tau protein as NFTs and pre-tangles in clusters, particularly around small blood vessels of the cortex, and typically in the depths of the sulci (Omalu et al., 2005; Goldstein et al., 2012; McKee et al., 2013; Smith et al., 2013; DeKosky et al., 2013; Blennow et al., 2012). NFTs in CTE typically follow the penetrating small cortical vessels as linear accumulations extending from the surface of the brain to the lowest layers of the cortical gray matter or, when observed in cross-section, as clusters, pre-tangles and dot-shaped and thread-like neuropils in a penumbra around small arterioles (McKee et al., 2015). Multiple forms of CVD are found in nearly all tauopathies (Michalicova et al., 2017). Interestingly, vessel wall remodeling, an early-onset process that precedes cerebral amyloid angiopathy (CAA), which may contribute to downstream microvascular pathology in AD, is also tau-associated (Merlini et al., 2016).
Studies in animal models show that acceleration/deceleration injury causes tau to become phosphorylated, misfolded, aggregated, and cleaved, generating neurotoxic tau peptide fragments (Huber et al., 2013; McKee et al., 2015). Recent data suggests that tau accumulation alone induces chronic dysfunction of the cerebral vasculature (Merlini et al., 2016; Blair et al., 2015), contributing to neurodegeneration.
The process leading to tau accumulation is unclear, however it has been proposed that mechanical stress beyond certain thresholds after TBI can disrupt microtubule networks within axons leading to diffuse axonal injury (DAI), tau release, hyper-phosphorylation and extracellular accumulation (Johnson et al., 2013; Kawata et al., 2016). While this option is plausible, other options should be considered, such as oxidative stress, which is known to modulate tau phosphorylation patterns in vitro (Katai et al., 2016). Studies in animal models of cerebral ischemia show altered tau gene expression in the infarct core after stroke (Ramos-Cejudo et al., 2012) or hyper-phosphorylated tau deposits in the hippocampus after middle cerebral artery occlusion (Xu et al., 2015). Accordingly, CSF tau levels are transiently increased in stroke patients (Hesse et al., 2001) as well as in TBI (Ost et al., 2006; Shahim et al., 2014) (Olivera et al., 2015). While increased brain, CSF and plasma tau levels after TBI could be merely the result of axonal injury, we propose that trauma-induced CVD also contributes to tau release, hyperphosphorylation and early accumulation after TBI. Indeed, recent literature directly implicates the endothelium and vascular factors in tau pathology (Iadecola, 2016), providing evidence that the endothelial isoform of nitric oxide (NO) synthase (eNOS) protects neurons from tau phosphorylation (Austin and Katusic, 2016).
Although the molecular events responsible for the development of cognitive impairment after TBI are not clear, combining recent findings for tau and Aβ pathology is possible to hypothesize that acute/transitory blood flow impairment and vascular damage after TBI may initiate a cascade of chronic capillary hypoperfusion, Aβ/tau accumulation, impairment of brain clearance, neuronal dysfunction and self-propagation of neurodegeneration. Nevertheless, further studies are needed to clarify how acute axonal injury, BBB opening, neuroinflammation and abnormally truncated and aggregated p-tau and Aβ develop into the progressive vascular processes observed in CTE, AD and other proteinopathies.
3. Cerebrovascular Damage in TBI: An Early Trigger of AD Pathology?
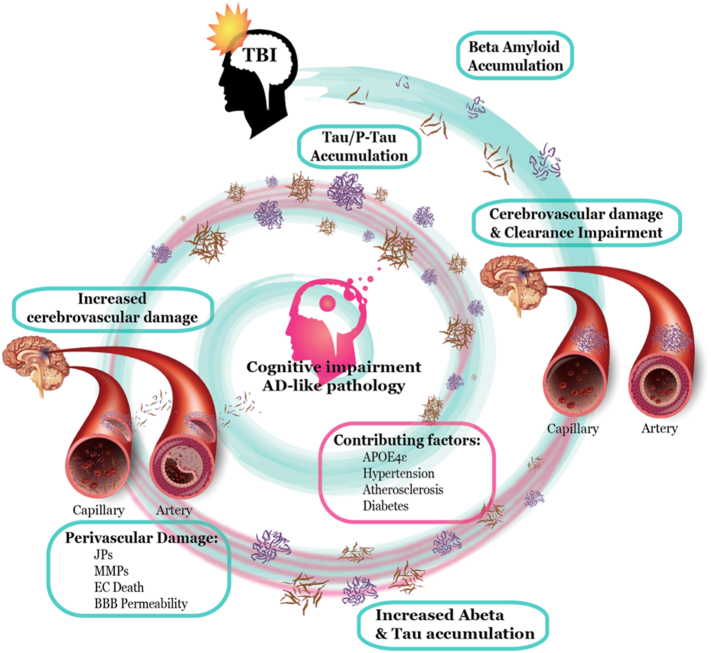
Cerebrovascular consequences of TBI include hemorrhages, edema, alterations in cerebral blood flow (CBF), vasospasms, BBB disruption, coagulopathy and chronic inflammation (recently reviewed in (Salehi et al., 2017)). TBI can be considered as a trigger, as well as a useful model for the study of certain pathological features of AD, such as Aβ and tau accumulation. Although TBI and AD have different etiologies, in both cases CVD is associated with Aβ and tau pathology. While Aβ and tau have been shown to induce CVD (Fossati et al., 2010, Fossati et al., 2012a, Fossati et al., 2012b, Ghiso et al., 2014, Fossati et al., 2013, Merlini et al., 2016, Blair et al., 2015), simultaneously, CVD appears to be responsible for Aβ and tau production/aggregation, abnormal inflammatory response, and a reduction of brain clearance (Iadecola, 2013; Pluta et al., 2013; De Silva and Faraci, 2016; Wolters et al., 2017; Tarasoff-Conway et al., 2015), establishing a feed-forward loop that may eventually lead to the development of dementia (Fig. 1).
Fig. 1.
TBI and AD are connected in a complex interplay.
Experimental data shows that Aβ and tau release leads to cerebrovascular injury and that their deposition around cerebral microvessels has a deleterious chronic effect. Secondarily, cerebrovascular injury is known to induce Aβ and tau deposition in a feedback loop that ultimately may lead to cognitive impairment and the development of AD-like pathology. Together with Aβ and tau accumulation, TBI induces endothelial cell (EC) damage, a modulation on junction proteins (JPs) and matrix metalloproteinase (MMPs) expression and ultimately an impairment of blood brain barrier (BBB) permeability. Because TBI is a relatively homogeneous disease compared to AD, analyzing biomarkers of TBI and their relationship with post-concussive symptoms and dementia offers a promising framework to better understand the relationship between cerebrovascular dysfunction (CVD) and the development of dementia.
Cerebrovascular events are a primary cause of several neurological disorders (Xing et al., 2012). Injury to endothelial cells alters CBF, reduces vascular reserves, BBB integrity and ultimately promotes global brain dysfunction and degeneration of the entire neurovascular unit (Iadecola, 2017). Additionally, comorbidities such as hypertension, atherosclerosis and diabetes contribute to damaging the cerebral microvasculature and increase the risk of neurodegenerative disorders like AD (Gupta and Iadecola, 2015).
Importantly, although most studies focused on the acute phase after the TBI event, CVD includes primary or secondary injury processes. Primary injury comprises mechanical deformation of the BBB endothelium by shear stress forces. Secondary injury processes include altered metabolism, fluctuations in CBF and clearance, and immune cell activation (Kawata et al., 2016). Results suggest that TBI-induced microvascular damage is associated with DAI, one of the major chronic pathological complications of TBI, and both are known to have an effect on clinical outcome. Together with the initial neuronal damage, CVD may play a causal role in the chronic post-concussive symptoms and neurodegeneration observed remotely after TBI (Kenney et al., 2016; Glushakova et al., 2014). This in line with the “hemorrhages later replaced by gliosis and degenerative progressive lesions” described by Martland in his studies in sport-contact players, mentioned in the introduction (Martland, 1928).
4. Endothelial Cell and BBB Damage in TBI and AD
Alterations in endothelial cell survival and BBB permeability seem to be early events after TBI and relevant contributors to pathology. The ability to maintain BBB integrity depends on adequate structural support of endothelial cells (EC) by tight junctions (TJ)-associated proteins, or junction proteins (JP), which include claudin 5, occludin and ZO-1. Previous studies showed a reduction in TJ with increased BBB permeability following TBI (Wen et al., 2014), Although the immediate effects are likely to be due to acute shear stress forces induced by TBI, it is possible to consider TBI as a vascular disorder with long-term consequences on cognitive function. Recent studies analyzing both acute and chronic time points after TBI in rats showed microvascular damage associated with inflammation, BBB disruption and progressive white matter lesions (Glushakova et al., 2014). TBI caused focal microbleeds that gradually increased over 3 months. Delayed focal BBB opening and early signs of localized inflammation preceded onset of further microbleeds. Electron microscopy performed at 7–30 days after TBI showed fibrous changes in the vascular wall, corrugated internal elastic lamina, apoptotic endothelial cells and degradation of the extracellular matrix (Danaila et al., 2013). Moreover, a study analyzing juvenile TBI (jTBI) in rats showed that Aβ is present and increased around cerebral microvessels after jTBI, and the diameter of those vessels is decreased by 25% and 34% at 2 and 6 months respectively (Jullienne et al., 2014).
Multiple studies link the vascular deposition of amyloid and the effects oligomeric species of Aβ to EC dysfunction and death. Work from our group indicates that direct exposure to oligomeric Aβ in vitro induces oxidative stress and is responsible for the specific and direct activation of apoptotic pathways in cerebral microvascular ECs. This pathways involve mitochondrial dysfunction and caspase activation, mediated by the engagement of the TRAIL (TNF-related apoptosis induced ligand) death receptors (DR4 and DR5), which trigger the extrinsic apoptotic pathway (Ghiso et al., 2014, Fossati et al., 2010, Fossati et al., 2012a, Fossati et al., 2012b, Fossati et al., 2016). Moreover, human brain microvascular ECs challenged with Aβ mutants associated in vivo with hemorrhagic manifestations, showed enhanced activation of matrix metalloproteinases (MMPs) like MMP-2 (Hernandez-Guillamon et al., 2010). Indeed, TBI has been reported to enhance production of reactive oxygen species (ROS), which activate MMPs. Enhanced MMP activity degrades extracellular matrix proteins and cerebral JPs, exacerbating BBB breakdown (Abdul-Muneer et al., 2013).
Independently of the mechanism by which tau is accumulated in the brain after TBI, tau overexpression can also initiate BBB breakdown in vivo, as shown by recent data in transgenic mice expressing tau in a tetracycline-regulated system. Importantly, these features seem to be reversed when tau hyperproduction is interrupted by doxycycline (Blair et al., 2015). Taking into account that these results need to be replicated, they suggest that tau may play a direct and independent role in BBB dysfunction.
In addition, while pericyte dysfunction has been widely described in experimental models of AD(Sagare et al., 2013), acute pericyte loss and reactive perycitosis have been more recently described in TBI models (Zehendner et al., 2015).
Based on this evidence, in addition to the acute TBI-induced microvascular endothelial damage, the contribution of perivascular deposition of Aβ and tau to long-term BBB damage after TBI warrants further investigation.
5. Mitochondrial Dysfunction in the Vasculature in TBI and AD
Mitochondrial dysfunction has been suggested to be an early event in AD models and patients (Fossati et al., 2016; Swerdlow, 2017). Mitochondrial damage and failure of the cell energy-production systems are essential factors precipitating aging and inducing ROS overproduction, release of apoptotic factors, and other toxic mechanisms contributing to AD pathology and dementia (Silva et al., 2013). Previous work recognized mitochondrial dysfunction as a determining factor for microvascular dysfunction in AD (Fossati et al., 2010; Ghiso et al., 2014; Fossati et al., 2013). While TBI has been linked with mitochondrial dysfunction, mitochondrial effects of TBI at the vascular level have only recently started to be explored (Szarka et al., 2018), and their relationship with tau and Aβ deposits that are typically found near degenerating vessels needs to be further investigated.
6. Cerebrovascular Inflammation in TBI and AD
In animal models of TBI, delayed microvascular damage and focal microbleeds are temporally and regionally connected with BBB breakdown and progressive inflammatory responses (Glushakova et al., 2014). Microbleeds have been detected in both the acute and chronic stages following TBI (Kinnunen et al., 2011), and their prevalence has been associated with injury severity. In other forms of brain injury, microbleeds are known to be toxic to astrocytes, neurons, and endothelial cells (Lok et al., 2011) and are frequently observed surrounded by macrophages, which can initiate inflammatory and neurodegenerative cascades. Recent results show that perivascular macrophages (PVM) expressing CD36 and Nox2 are a major source of reactive oxygen species in mice overexpressing the Swedish mutation of the APP (Tg2576) and have been proposed as a therapeutic target in AD models (Park et al., 2017; Faraci, 2017). Persistent inflammation triggered by microbleeds and platelets accumulation after TBI might be responsible for the secondary activation of microglia, stimulation of gliosis, late complement activation and apoptosis, all processes closely associated with AD and dementia (Danaila et al., 2013; Kniewallner et al., 2016). Results from experimental animals models suggest that subclinical pathophysiological changes to the CNS caused by TBI persist after the TBI event, and dysregulation of glial activation as well as priming of the innate immune system might be additional contributing factors (Andreasson et al., 2016). The temporal pattern of the inflammatory response after TBI shows that cytokine/chemokine levels begin to rise within the first minutes to hours after the event and recruitment of peripheral immune cells to the brain occurs in a narrow window between 1 and 7 days after the injury (Andreasson et al., 2016). However, studies in patients using PET ligands for activated microglia found abnormal chronic inflammatory response up to 17 years after the TBI event (Ramlackhansingh et al., 2011), pointing to neuroinflammation as a chronic process which may affect neurovascular function for years, especially in the case of repeated TBIs.
7. Impairment of Clearance Systems in TBI and AD
The removal of waste produced by cell metabolism in the brain occurs through a variety of overlapping clearance systems that can be classified according to the compartment from which the waste is cleared and the compartment into which waste is cleared. Extracellular Aβ deposits can be removed from the brain by various clearance systems, including transport across the blood-brain barrier, astroglial-mediated interstitial fluid (ISF) bulk flow, known as the glymphatic system, and the meningeal lymphatic vessels. Because these clearance systems act together to drive waste removal from the brain, any alteration to their function could contribute to AD and to the development of dementia after TBI (as we reviewed extensively in (Tarasoff-Conway et al., 2015)). Clearance impairment may additionally contribute to Aβ accumulation, endothelial dysfunction, oxidative stress and inflammation (Gupta and Iadecola, 2015). An impairment of clearance systems occurring after TBI is responsible of Aβ and tau accumulation in rodents (reviewed in (Tarasoff-Conway et al., 2015)). Aquaporin-4 (AQP4) seems to play a fundamental role in maintaining paravascular solute clearance, as KO animals showed exacerbated pathology and neurodegeneration after TBI. Importantly, while it has been shown that S100B, NSE or GFAP serum levels are augmented after TBI, no increases are found in experimental models of TBI when the glymphatic system is interrupted by sleep deprivation, acetazolamide treatment, cisterna magna cisternotomy or in Aqp4−/− mice. We recently developed a novel method for measuring human CSF clearance through the nasal turbinate system in patients using dynamic PET (de Leon et al., 2017). We believe this method will be helpful to evaluate brain clearance alterations in AD as well as in TBI, and to better understand their contribution to the development of dementia after brain trauma. As paravascular clearance systems are known to be predominantly active during sleep (Lundgaard et al., 2017; Plog et al., 2015) and sleep management after TBI is challenging (Vermaelen et al., 2015), future longitudinal studies will also need to consider sleep alterations at different time points after TBI and their relative contribution to clearance and biomarkers levels.
8. Therapeutic Approaches for Modulating Cerebrovascular Function in TBI
The impact of TBI-induced CVD and its contribution to dementia can be inferred from pharmacological studies modulating cerebrovascular function. As an example, Lithium was proposed as a therapeutic approach for TBI due to its role in diminishing tau aggregation in transgenic mice (Perez et al., 2003), and recent data shows that lithium plays a role in stabilizing BBB structural integrity in TBI (Leeds et al., 2014; Bosche et al., 2016). Additionally, CBF restoring agents like brain natriuretic peptide or endothelin receptor antagonists have been proposed for TBI (James et al., 2010; Graves and Kreipke, 2015). Microvascular function can be also modulated by non-pharmacological approaches, including aerobic exercise. While exercise rehabilitation in TBI is controversial during the acute phase, studies inducing sustained aerobic exercise in animal models of Aβ and tau pathology show improved memory in functional tests, together with decreased BBB permeability, reduction of interstitial soluble brain levels of Aβ, limited oxidative stress and inflammation (Zhao et al., 2015; Herring et al., 2016) and CSF clearance (von Holstein-Rathlou et al., 2018). The effects of exercise programs in TBI patients have been explored, with positive results (Archer et al., 2012; Devine et al., 2016), however the explanation for their protective role, in addition to cerebrovascular modulation, may be linked to increased cognitive reserve or improved mood and stress (Weinstein et al., 2017).
9. Biomarker Studies in TBI and AD: Understanding and Diagnosing the Cerebrovascular Link
Biomarker studies are now integrating a wide range of molecular and neuroimaging techniques that offer a promising framework to better understand the relationship between CVD, Aβ/tau pathology, and the development of dementia after TBI (Fig. 2). Combining the information obtained from neuroimaging and molecular biomarkers in biofluids (CSF, serum and plasma) at different time points after TBI (acute phase, post-concussive syndrome, and chronic phase) is fundamental to understand the contribution of CVD to AD-like pathology presenting after TBI. The increase of CSF/Blood biomarkers after TBI may result from neuropathological processes other than CVD or may be simply the consequence of DAI and BBB damage. In order to obtain valuable information on the contribution of CVD to AD-like pathology through biomarker analysis, future research will need to pay a particular attention to study designs. First, longitudinal studies integrating neuroimaging and molecular biomarkers will be needed to clarify how variations in CSF/blood molecules reflect cerebrovascular pathology at different times after TBI. Additionally, combining experimental animal models with clinical research will be fundamental to elucidate the relationship between CVD and Aβ/tau deposition at the molecular level.
Fig. 2.
Biomarker studies to understand the cerebrovascular link between TBI and AD.
Multimodal biomarker studies can be used to better understand the complex interplay between TBI and the development of AD-like pathology. TBI induces early and subacute cerebrovascular function impairment that can be monitored by neuroimaging techniques. This includes blood flow impairment, hypoperfusion and ischemia, changes in brain metabolism and also an impairment of brain clearance systems. If these phenomena are not isolated but sustained because of repeated TBI events or severe TBI, secondary cerebrovascular damage can occur, including vascular damage, BBB abnormal permeability and microbleeds that can be also detected by neuroimaging techniques. Cerebrovascular function impairment and damage induce perivascular and parenchymal accumulation of tau and Aβ in the brain. All these processes act in a feed-forward loop, as an increase in perivascular accumulation of tau and Aβ induce vascular damage, limits vascular function and therefore impairment in blood flow and brain perfusion. These events induce changes in CSF, peripheral blood and biofluids molecules like tau, P-Tau, Aβ monomers/oligomers, metalloproteases and miRNAs, among others.
9.1. Neuroimaging Tools
Neuroimaging measures of CVD include CBF monitoring by MRI or Doppler as CBF alterations are known to compromise heat exchanges and metabolism, which can be temporarily interrupted after concussion (Mrozek et al., 2012). Another variable is vascular responsiveness to CO2 (VR-CO2), which is an indirect measurement of the degree of impairment in the ability of the cerebrovasculature to buffer against changes in arterial gases (vasoreactivity). A recent report showed that higher vasoreactivity was strongly associated with more severe headaches and worse cognitive symptoms after concussion ((Albalawi et al., 2017)). Our studies and others showed that VR-CO2 is impaired in neurodegenerative diseases like AD (Glodzik et al., 2014). Previous research in animal models found that hemodynamic depressions after TBI are related to an impairment of vasoconstriction and that Ca(2+) channels play a causative role in the loss of vasoreactivity (Maeda et al., 2005). In addition, other cerebrovascular hemodynamics measurements like pulsatility index (PI) and cerebral autoregulation (dCA) have been linked with white matter injury and will need to be considered for future TBI studies (Purkayastha et al., 2014).
Diffusion tensor imaging (DTI) data allow the tridimensional reconstruction of white matter fiber tracts and have been widely used after TBI (Herweh et al., 2016; Astafiev et al., 2015), leading to the observation that DAI co-localizes with microvascular injury and focal microbleeds. White matter hyperintensities are strongly correlated with cognitive dysfunction and are typically due to small vessel disease (Clark et al., 2016).
How changes in CBF, vascular reactivity and white matter abnormalities at different stages after TBI are linked with the overproduction and accumulation of biomarkers like Aβ, tau or neurofilament light chain (NFL) in brain and blood will need to be explored in the future. Studies integrating DTI with tau/Aβ determination may be key to understand the relationship between white matter disease and tau/Aβ accumulation after TBI. Another important resource for future biomarker studies will be the possibility to visualize Aβ/Tau deposits in vivo by PET together with microbleeds (imaged by T2*-MRI), clearance measures obtained using our novel dynamic PET methodology (de Leon et al., 2017), and blood biomarkers (by ultrasensitive assays like the Single Molecule Array (Simoa) technology) (Fossati et al., 2017), to clarify the relationship between CVD, clearance, brain Aβ/Tau pathology and peripheral biomarkers.
9.2. Molecular Biofluid Biomarkers
Nearly all processes taking place in the neurovascular unit after TBI can be analyzed directly or indirectly through molecular biomarkers in the CSF and blood (plasma and serum). Exploring biofluid biomarkers may help to understand the contribution of different molecular mechanisms of CVD to AD-like pathology after TBI (Fig. 3). Recent technologies such as Simoa allow the identification of biomarkers at very low concentrations in blood with promising results in TBI and AD for tau, Aβ, and NFL, among other biomarkers (Zetterberg and Blennow, 2016). Our group and others have previously analyzed biomarkers of vascular, amyloid and tau pathology in AD, MCI and CAA patients (Spiegel et al., 2016; Blennow et al., 2015; Hernandez-Guillamon et al., 2012), showing that Aβ40/42, tau and P-Tau, as well as MMPs and other cerebrovascular proteins, are modified in AD and CAA patients. The same biomarkers, which correlate with neuropathology and may predict future dementia, are modulated in biofluids after TBI (Zetterberg and Blennow, 2016).
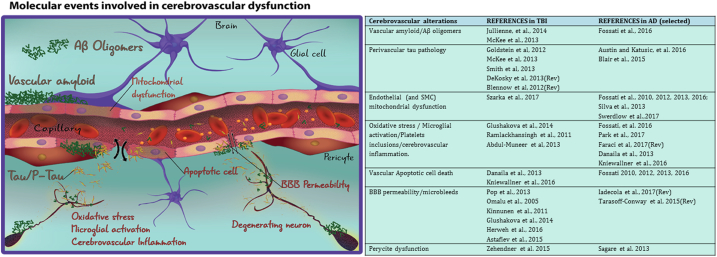
Fig. 3.
Molecular events involved in cerebrovascular dysfunction.
TBI and AD share certain pathological features where cerebrovascular dysfunction (CVD) plays a fundamental role. (Left) Cerebrovascular alterations taking place in the context of the neurovascular unit (NVU) in TBI and AD. (Right) Recent works in TBI and AD showing cerebrovascular alterations. Rev: Reviewed in.
Elevation in total plasma tau is found in concussed boxers, and in hockey players, as well as in military personnel deployed within the previous 18 months, particularly in the case of multiple TBIs (Olivera et al., 2015, Zetterberg and Blennow, 2016, Fossati et al., 2017). Tau may have an important value both as a biomarker and as a therapeutic target with anti-tau antibodies (Spiegel et al., 2016; Wisniewski and Goni, 2015). Our recent work in veterans with history of TBI with loss of consciousness (LOC), tested 1–10 years after the event, found elevated tau in subjects with 2 or more chronic TBI events when compared to individuals with 1 TBI (Fossati et al., 2017). Overall, tau and P-tau isoforms may increase with the number and severity of TBIs and can be potentially used as biomarkers of future risk of neurological decline. As an example, a recent report showed that elevated plasma tau levels within 6 h following sport-related concussions was related to premature return to play, indicating that plasma tau levels may be considered as a biomarker for readiness to return to play and CTE prevention (Gill et al., 2017). The presence of hyper-phosphorylated tau in plasma or serum of TBI patients still needs to be validated, and the optimization of novel assays allowing detection of P-tau isoforms in blood, currently undergoing in our lab and others (Rubenstein et al., 2017), will be an important next step. One particularly promising marker to detect ongoing neurodegeneration following multiple TBIs is NFL; increased NFL concentrations have been reported in both CSF and plasma in individuals with post-concussion syndrome (Shahim et al., 2016; Shahim et al., 2017).
To clarify the relationship between elevated blood/CSF biomarkers and cerebrovascular pathology, future biomarkers panels will require a combination of tau, Aβ, and NFL, with biomarkers specific for CVD like MMPs, endothelial microparticles (EMPs) or EC-derived exosomes, as well as tight junction proteins. Plasma concentrations of MMP-9 and fibronectin are modulated after severe TBI, predicting death and length of hospital stay (Copin et al., 2012). Experimental data shows that MMP-9 concentration in the CSF of TBI patients also correlates with neurological outcome, suggesting it may have prognostic value (Liu et al., 2014). It is hypothesized that the brain endothelium responds to mechanical injury by producing EMPs that contain brain endothelial proteins, including TJ proteins, and recent data demonstrated that injury of the cerebral endothelium induces EMPs production (Andrews et al., 2016; Curtis et al., 2013) (Nekludov et al., 2014). Mice subjected to TBI develop a hypercoagulable state within 3 h of the injury, induced by brain-derived microparticles transmigrating through the disrupted endothelial barrier in a platelet-dependent manner (Tian et al., 2015). EMPs are also found in the peripheral circulation in AD patients (Tenreiro et al., 2016).
We propose that combining panels of neuronal and vascular fluid biomarkers at multiple time-points after TBI, together with imaging biomarkers, will help to clarify the contribution and confirm the causal role of CVD in the development of AD-like pathology after TBI.
10. Conclusions
The link between TBI and AD-like dementia represents a fascinating field of research for scientists working on both diseases. Recent data suggests that CVD is a key element to understand the long-lasting effects of TBI and their association with dementia. In this review we described how CVD can be considered as a major contributor to AD-like pathology after TBI. Biofluid biomarker studies, coupled with neuroimaging tools in longitudinal designs after TBI, will offer a promising framework to elucidate this contribution. Future studies will need to dissect the relative contribution of CVD, DAI, inflammation and other genetic and environmental factors on AD-like pathology after TBI. While isolating each factor is challenging, the performance of TBI models on experimental animals with different backgrounds (i.e. cerebral amyloidosis, tauopathy, cerebrovascular pathology or neuroinflammation) will facilitate this goal. For human studies, in order to be able to evaluate the specific causal effects of TBI and CVD, we will need to account for all confounders. Therefore, considering both genetic (e.g. ApoE genotype) and metabolic/environmental factors such as age, BMI, cardiovascular risk, sleep, and diet will be important to provide valuable and solid results.
11. Outstanding Questions
This review highlights the relevance of CVD as a key contributor to AD-like pathology after TBI. Future studies are required to explore the effects of acute or chronic CBF alterations and perivascular deposition of Aβ and tau, alone and in combination, on endothelial mitochondrial dysfunction and BBB damage after TBI and their relationship with neurovascular dysfunction and cognitive impairment. Also, longitudinal study designs in patients integrating neuroimaging data (PET, MRI, CBF, VR-CO2) with biofluid biomarkers (including Aβ, tau, NFL and CVD-associated molecules), and accounting for multiple confounders, will help us to understand the contribution of TBI-induced impairment of neurovascular function to AD-like pathology.
12. Search Strategy and Selection Criteria
Research included in this review was collected through PubMed and recent academic conferences using the search terms “tau”, “amyloid-beta”, “cerebrovascular function”, “TBI”, “AD”, “ischemia”, “csf”, “blood”, “plasma”, “serum”, “cerebral blood flow”, “pathology”. Information was included when related directly to the relationship between TBI and AD and cerebrovascular dysfunction. Mostly articles published in English between 2012 and 2017 were included. An exception was made for Martland's paper “PUNCH DRUNK” (Martland, 1928) for its historical relevance considering the scope of the review.
Author's contributions
SF conceived the review out of recent unpublished work. JRC and SF designed the review outline, wrote and reviewed the review, did the literature search, data extraction and interpretation. TW, CM, HZ, KB and MJDL provided critical reviews, revised the manuscript and provided relevant insights and edits. All authors read and approved the final version of the manuscript.
Disclosures
MDL has several imaging and CSF based patents that are managed by New York University. KB has served as a consultant or at advisory boards for Alzheon, BioArctic, Biogen, Eli Lilly, Fujirebio Europe, IBL International, Merck, Pfizer, and Roche Diagnostics, HZ and KB are co-founders of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. The other authors declare no conflict of interest.
Acknowledgements
This work was supported by the American Heart Association Grant 13SDG16860017, the Blas Frangione Foundation New Investigator Grant (2014) and the Leon Levy Fellowship in Neuroscience (2016) awarded to SF; MDL's grants NIH AG022374, AG013616, AG012101, AG008051, RF1057570; grants awarded to CM and MDL by Cohen Veterans Bioscience; and NIH grants AG008051 and NS073502 awarded to TW. We thank Ludovic Debure for figures design and editing.
References
- Abdul-Muneer P.M., Schuetz H., Wang F., Skotak M., Jones J., Gorantla S., Zimmerman M.C., Chandra N., Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic. Biol. Med. 2013;60:282–291. doi: 10.1016/j.freeradbiomed.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abisambra J.F., Scheff S. Brain injury in the context of tauopathies. J. Alzheimers Dis. 2014;40:495–518. doi: 10.3233/JAD-131019. [DOI] [PubMed] [Google Scholar]
- Acharya S., Srivastava K.R., Nagarajan S., Lapidus L.J. Monomer dynamics of Alzheimer peptides and kinetic control of early aggregation in Alzheimer's disease. ChemPhysChem. 2016;17:3470–3479. doi: 10.1002/cphc.201600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albalawi T., Hamner J.W., Lapointe M., Meehan W.P.R., Tan C.O. The relationship between cerebral vasoreactivity and post-concussive symptom severity. J. Neurotrauma. 2017;34:2700–2705. doi: 10.1089/neu.2017.5060. [DOI] [PubMed] [Google Scholar]
- Andreasson K.I., Bachstetter A.D., Colonna M., Ginhoux F., Holmes C., Lamb B., Landreth G., Lee D.C., Low D., Lynch M.A., Monsonego A., O'banion M.K., Pekny M., Puschmann T., Russek-Blum N., Sandusky L.A., Selenica M.L., Takata K., Teeling J., Town T., VAN Eldik L.J. Targeting innate immunity for neurodegenerative disorders of the central nervous system. J. Neurochem. 2016;138:653–693. doi: 10.1111/jnc.13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews A.M., Lutton E.M., Merkel S.F., Razmpour R., Ramirez S.H. Mechanical injury induces brain endothelial-derived microvesicle release: implications for cerebral vascular injury during traumatic brain injury. Front. Cell. Neurosci. 2016;10:43. doi: 10.3389/fncel.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer T., Svensson K., Alricsson M. Physical exercise ameliorates deficits induced by traumatic brain injury. Acta Neurol. Scand. 2012;125:293–302. doi: 10.1111/j.1600-0404.2011.01638.x. [DOI] [PubMed] [Google Scholar]
- Astafiev S.V., Shulman G.L., Metcalf N.V., Rengachary J., Macdonald C.L., Harrington D.L., Maruta J., Shimony J.S., Ghajar J., Diwakar M., Huang M.X., Lee R.R., Corbetta M. Abnormal white matter blood-oxygen-level-dependent signals in chronic mild traumatic brain injury. J. Neurotrauma. 2015;32:1254–1271. doi: 10.1089/neu.2014.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S.A., Katusic Z.S. Loss of endothelial nitric oxide synthase promotes p25 generation and tau phosphorylation in a murine model of Alzheimer's disease. Circ. Res. 2016;119:1128–1134. doi: 10.1161/CIRCRESAHA.116.309686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair L.J., Frauen H.D., Zhang B., Nordhues B.A., Bijan S., Lin Y.C., Zamudio F., Hernandez L.D., Sabbagh J.J., Selenica M.L., Dickey C.A. Tau depletion prevents progressive blood-brain barrier damage in a mouse model of tauopathy. Acta Neuropathol. Commun. 2015;3:8. doi: 10.1186/s40478-015-0186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K., Hardy J., Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76:886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Blennow K., Dubois B., Fagan A.M., Lewczuk P., DE Leon M.J., Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer's disease. Alzheimers Dement. 2015;11:58–69. doi: 10.1016/j.jalz.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K., Brody D.L., Kochanek P.M., Levin H., Mckee A., Ribbers G.M., Yaffe K., Zetterberg H. Traumatic brain injuries. Nat. Rev. Dis. Primers. 2016;2:16084. doi: 10.1038/nrdp.2016.84. [DOI] [PubMed] [Google Scholar]
- Bosche B., Molcanyi M., Rej S., Doeppner T.R., Obermann M., Muller D.J., Das A., Hescheler J., Macdonald R.L., Noll T., Hartel F.V. Low-dose lithium stabilizes human endothelial barrier by decreasing MLC phosphorylation and universally augments cholinergic vasorelaxation capacity in a direct manner. Front. Physiol. 2016;7:593. doi: 10.3389/fphys.2016.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A.L., Sorg S.F., Schiehser D.M., Luc N., Bondi M.W., Sanderson M., Werhane M.L., Delano-Wood L. Deep white matter hyperintensities affect verbal memory independent of PTSD symptoms in veterans with mild traumatic brain injury. Brain Inj. 2016;30:864–871. doi: 10.3109/02699052.2016.1144894. [DOI] [PubMed] [Google Scholar]
- Copin J.C., Rebetez M.M., Turck N., Robin X., Sanchez J.C., Schaller K., Gasche Y., Walder B. Matrix metalloproteinase 9 and cellular fibronectin plasma concentrations are predictors of the composite endpoint of length of stay and death in the intensive care unit after severe traumatic brain injury. Scand. J. Trauma Resusc. Emerg. Med. 2012;20:83. doi: 10.1186/1757-7241-20-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane P.K., Gibbons L.E., Dams-O'connor K., Trittschuh E., Leverenz J.B., Keene C.D., Sonnen J., Montine T.J., Bennett D.A., Leurgans S., Schneider J.A., Larson E.B. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 2016;73:1062–1069. doi: 10.1001/jamaneurol.2016.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis A.M., Edelberg J., Jonas R., Rogers W.T., Moore J.S., Syed W., Mohler E.R., 3RD Endothelial microparticles: sophisticated vesicles modulating vascular function. Vasc. Med. 2013;18:204–214. doi: 10.1177/1358863X13499773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaila L., Popescu I., Pais V., Riga D., Riga S., Pais E. Apoptosis, paraptosis, necrosis, and cell regeneration in posttraumatic cerebral arteries. Chirurgia (Bucur) 2013;108:319–324. [PubMed] [Google Scholar]
- De Leon M.J., Li Y., Okamura N., Tsui W.H., Saint-Louis L.A., Glodzik L., Osorio R.S., Fortea J., Butler T., Pirraglia E., Fossati S., Kim H.J., Carare R.O., Nedergaard M., Benveniste H., Rusinek H. Cerebrospinal fluid clearance in Alzheimer disease measured with dynamic PET. J. Nucl. Med. 2017;58:1471–1476. doi: 10.2967/jnumed.116.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva T.M., Faraci F.M. Microvascular dysfunction and cognitive impairment. Cell. Mol. Neurobiol. 2016;36:241–258. doi: 10.1007/s10571-015-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky S.T., Blennow K., Ikonomovic M.D., Gandy S. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat. Rev. Neurol. 2013;9:192–200. doi: 10.1038/nrneurol.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine J.M., Wong B., Gervino E., Pascual-Leone A., Alexander M.P. Independent, community-based aerobic exercise training for people with moderate-to-severe traumatic brain injury. Arch. Phys. Med. Rehabil. 2016;97:1392–1397. doi: 10.1016/j.apmr.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Faraci F.M. Disease highlights the cellular diversity of neurovascular units: sign in stranger. Circ. Res. 2017;121:203–205. doi: 10.1161/CIRCRESAHA.117.311386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati S., Cam J., Meyerson J., Mezhericher E., Romero I.A., Couraud P.O., Weksler B.B., Ghiso J., Rostagno A. Differential activation of mitochondrial apoptotic pathways by vasculotropic amyloid-beta variants in cells composing the cerebral vessel walls. FASEB J. 2010;24:229–241. doi: 10.1096/fj.09-139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati S., Ghiso J., Rostagno A. Insights into caspase-mediated apoptotic pathways induced by amyloid-beta in cerebral microvascular endothelial cells. Neurodegener. Dis. 2012;10:324–328. doi: 10.1159/000332821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati S., Ghiso J., Rostagno A. TRAIL death receptors DR4 and DR5 mediate cerebral microvascular endothelial cell apoptosis induced by oligomeric Alzheimer's Abeta. Cell Death Dis. 2012;3 doi: 10.1038/cddis.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati S., Todd K., Sotolongo K., Ghiso J., Rostagno A. Differential contribution of isoaspartate post-translational modifications to the fibrillization and toxic properties of amyloid-beta and the asparagine 23 Iowa mutation. Biochem. J. 2013;456:347–360. doi: 10.1042/BJ20130652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati S., Giannoni P., Solesio M.E., Cocklin S.L., Cabrera E., Ghiso J., Rostagno A. The carbonic anhydrase inhibitor methazolamide prevents amyloid beta-induced mitochondrial dysfunction and caspase activation protecting neuronal and glial cells in vitro and in the mouse brain. Neurobiol. Dis. 2016;86:29–40. doi: 10.1016/j.nbd.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati S., Ramos-Cejudo J., Debure L., Pirraglia E., Glodzik L., Osorio R.S., Chen J., Provost A., Jeromin A., Haas M., Marmar C., Deleon M. Differential value of plasma tau as a biomarker for Alzheimer's disease and chronic traumatic brain injury. Alzheimers Dement. 2017;13:1307. [Google Scholar]
- Gardner R.C., Burke J.F., Nettiksimmons J., Kaup A., Barnes D.E., Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71:1490–1497. doi: 10.1001/jamaneurol.2014.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiso J., Fossati S., Rostagno A. Amyloidosis associated with cerebral amyloid angiopathy: cell signaling pathways elicited in cerebral endothelial cells. J. Alzheimers Dis. 2014;42(Suppl. 3):S167–76. doi: 10.3233/JAD-140027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J., Merchant-Borna K., Jeromin A., Livingston W., Bazarian J. Acute plasma tau relates to prolonged return to play after concussion. Neurology. 2017;88:595–602. doi: 10.1212/WNL.0000000000003587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glodzik L., Rusinek H., Pirraglia E., Mchugh P., Tsui W., Williams S., Cummings M., Li Y., Rich K., Randall C., Mosconi L., Osorio R., Murray J., Zetterberg H., Blennow K., De Leon M. Blood pressure decrease correlates with tau pathology and memory decline in hypertensive elderly. Neurobiol. Aging. 2014;35:64–71. doi: 10.1016/j.neurobiolaging.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glushakova O.Y., Johnson D., Hayes R.L. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. J. Neurotrauma. 2014;31:1180–1193. doi: 10.1089/neu.2013.3080. [DOI] [PubMed] [Google Scholar]
- Goldstein L.E., Fisher A.M., Tagge C.A., Zhang X.L., Velisek L., Sullivan J.A., Upreti C., Kracht J.M., Ericsson M., Wojnarowicz M.W., Goletiani C.J., Maglakelidze G.M., Casey N., Moncaster J.A., Minaeva O., Moir R.D., Nowinski C.J., Stern R.A., Cantu R.C., Geiling J., Blusztajn J.K., Wolozin B.L., Ikezu T., Stein T.D., Budson A.E., Kowall N.W., Chargin D., Sharon A., Saman S., Hall G.F., Moss W.C., Cleveland R.O., Tanzi R.E., Stanton P.K., Mckee A.C. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012;4:134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J.C., Kreipke C.W. Endothelin, cerebral blood flow, and traumatic brain injury: implications for a future therapeutic target. In: Kobeissy F.H., editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. CRC Press/Taylor & Francis.; Boca Raton (FL): 2015. Chapter 37. Frontiers in Neuroengineering. [PubMed] [Google Scholar]
- Gren M., Shahim P., Lautner R., Wilson D.H., Andreasson U., Norgren N., Blennow K., Zetterberg H. Blood biomarkers indicate mild neuroaxonal injury and increased amyloid beta production after transient hypoxia during breath-hold diving. Brain Inj. 2016;30:1226–1230. doi: 10.1080/02699052.2016.1179792. [DOI] [PubMed] [Google Scholar]
- Gupta A., Iadecola C. Impaired Abeta clearance: a potential link between atherosclerosis and Alzheimer's disease. Front. Aging Neurosci. 2015;7:115. doi: 10.3389/fnagi.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Guillamon M., Mawhirt S., Fossati S., Blais S., Pares M., Penalba A., Boada M., Couraud P.O., Neubert T.A., Montaner J., Ghiso J., Rostagno A. Matrix metalloproteinase 2 (MMP-2) degrades soluble vasculotropic amyloid-beta E22Q and L34V mutants, delaying their toxicity for human brain microvascular endothelial cells. J. Biol. Chem. 2010;285:27144–27158. doi: 10.1074/jbc.M110.135228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Guillamon M., Martinez-Saez E., Delgado P., Domingues-Montanari S., Boada C., Penalba A., Boada M., Pagola J., Maisterra O., Rodriguez-Luna D., Molina C.A., Rovira A., Alvarez-Sabin J., Ortega-Aznar A., Montaner J. MMP-2/MMP-9 plasma level and brain expression in cerebral amyloid angiopathy-associated hemorrhagic stroke. Brain Pathol. 2012;22:133–141. doi: 10.1111/j.1750-3639.2011.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A., Munster Y., Metzdorf J., Bolczek B., Krussel S., Krieter D., Yavuz I., Karim F., Roggendorf C., Stang A., Wang Y., Hermann D.M., Teuber-Hanselmann S., Keyvani K. Late running is not too late against Alzheimer's pathology. Neurobiol. Dis. 2016;94:44–54. doi: 10.1016/j.nbd.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Herweh C., Hess K., Meyding-Lamade U., Bartsch A.J., Stippich C., Jost J., Friedmann-Bette B., Heiland S., Bendszus M., Hahnel S. Reduced white matter integrity in amateur boxers. Neuroradiology. 2016;58:911–920. doi: 10.1007/s00234-016-1705-y. [DOI] [PubMed] [Google Scholar]
- Hesse C., Rosengren L., Andreasen N., Davidsson P., Vanderstichele H., Vanmechelen E., Blennow K. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci. Lett. 2001;297:187–190. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- Huber B.R., Meabon J.S., Martin T.J., Mourad P.D., Bennett R., Kraemer B.C., Cernak I., Petrie E.C., Emery M.J., Swenson E.R., Mayer C., Mehic E., Peskind E.R., Cook D.G. Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. J. Alzheimers Dis. 2013;37:309–323. doi: 10.3233/JAD-130182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Untangling neurons with endothelial nitric oxide. Circ. Res. 2016;119:1052–1054. doi: 10.1161/CIRCRESAHA.116.309927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James M.L., Wang H., Venkatraman T., Song P., Lascola C.D., Laskowitz D.T. Brain natriuretic peptide improves long-term functional recovery after acute CNS injury in mice. J. Neurotrauma. 2010;27:217–228. doi: 10.1089/neu.2009.1022. [DOI] [PubMed] [Google Scholar]
- Johnson V.E., Stewart W., Smith D.H. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer's disease? Nat. Rev. Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V.E., Stewart W., Smith D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013;246:35–43. doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullienne A., Roberts J.M., Pop V., Paul Murphy M., Head E., Bix G.J., Badaut J. Juvenile traumatic brain injury induces long-term perivascular matrix changes alongside amyloid-beta accumulation. J. Cereb. Blood Flow Metab. 2014;34:1637–1645. doi: 10.1038/jcbfm.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katai E., Pal J., Poor V.S., Purewal R., Miseta A., Nagy T. Oxidative stress induces transient O-GlcNAc elevation and tau dephosphorylation in SH-SY5Y cells. J. Cell. Mol. Med. 2016;20:2269–2277. doi: 10.1111/jcmm.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata K., Liu C.Y., Merkel S.F., Ramirez S.H., Tierney R.T., Langford D. Blood biomarkers for brain injury: what are we measuring? Neurosci. Biobehav. Rev. 2016;68:460–473. doi: 10.1016/j.neubiorev.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney K., Amyot F., Haber M., Pronger A., Bogoslovsky T., Moore C., Diaz-Arrastia R. Cerebral vascular injury in traumatic brain injury. Exp. Neurol. 2016;275(Pt 3):353–366. doi: 10.1016/j.expneurol.2015.05.019. [DOI] [PubMed] [Google Scholar]
- Kinnunen K.M., Greenwood R., Powell J.H., Leech R., Hawkins P.C., Bonnelle V., Patel M.C., Counsell S.J., Sharp D.J. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniewallner K.M., Wenzel D., Humpel C. Thiazine Red(+) platelet inclusions in Cerebral Blood Vessels are first signs in an Alzheimer's Disease mouse model. Sci. Rep. 2016;6:28447. doi: 10.1038/srep28447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds P.R., Yu F., Wang Z., Chiu C.T., Zhang Y., Leng Y., Linares G.R., Chuang D.M. A new avenue for lithium: intervention in traumatic brain injury. ACS Chem. Neurosci. 2014;5:422–433. doi: 10.1021/cn500040g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.L., Chen C.C., Lee H.C., Cho D.Y. Matrix metalloproteinase-9 in the ventricular cerebrospinal fluid correlated with the prognosis of traumatic brain injury. Turk. Neurosurg. 2014;24:363–368. doi: 10.5137/1019-5149.JTN.8551-13.0. [DOI] [PubMed] [Google Scholar]
- Lok J., Leung W., Murphy S., Butler W., Noviski N., Lo, E. H. Intracranial hemorrhage: mechanisms of secondary brain injury. Acta Neurochir. Suppl. 2011;111:63–69. doi: 10.1007/978-3-7091-0693-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I., Lu M.L., Yang E., Peng W., Mestre H., Hitomi E., Deane R., Nedergaard M. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J. Cereb. Blood Flow Metab. 2017;37:2112–2124. doi: 10.1177/0271678X16661202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Lee S.M., Hovda D.A. Restoration of cerebral vasoreactivity by an L-type calcium channel blocker following fluid percussion brain injury. J. Neurotrauma. 2005;22:763–771. doi: 10.1089/neu.2005.22.763. [DOI] [PubMed] [Google Scholar]
- Martland H.S. Punch drunk. JAMA. 1928;91:1103–1107. [Google Scholar]
- McKee A.C., Stern R.A., Nowinski C.J., Stein T.D., Alvarez V.E., Daneshvar D.H., Lee H.S., Wojtowicz S.M., Hall G., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W., Cantu R.C. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A.C., Stein T.D., Kiernan P.T., Alvarez V.E. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25:350–364. doi: 10.1111/bpa.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A.C., Cairns N.J., Dickson D.W., Folkerth R.D., Keene C.D., Litvan I., Perl D.P., Stein T.D., Vonsattel J.P., Stewart W., Tripodis Y., Crary J.F., Bieniek K.F., Dams-O'Connor K., Alvarez V.E., Gordon W.A., Group, TC The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75–86. doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M.F. What is the relationship of traumatic brain injury to dementia? J. Alzheimers Dis. 2017;57:667–681. doi: 10.3233/JAD-161002. [DOI] [PubMed] [Google Scholar]
- Merlini M., Wanner D., Nitsch R.M. Tau pathology-dependent remodelling of cerebral arteries precedes Alzheimer's disease-related microvascular cerebral amyloid angiopathy. Acta Neuropathol. 2016;131:737–752. doi: 10.1007/s00401-016-1560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalicova A., Banks W.A., Legath J., Kovac A. Tauopathies-focus on changes at the neurovascular unit. Curr. Alzheimer Res. 2017;14:790–801. doi: 10.2174/1567205014666170203143336. [DOI] [PubMed] [Google Scholar]
- Mrozek S., Vardon F., Geeraerts T. Brain temperature: physiology and pathophysiology after brain injury. Anesthesiol. Res. Pract. 2012;2012:989487. doi: 10.1155/2012/989487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekludov M., Mobarrez F., Gryth D., Bellander B.M., Wallen H. Formation of microparticles in the injured brain of patients with severe isolated traumatic brain injury. J. Neurotrauma. 2014;31:1927–1933. doi: 10.1089/neu.2013.3168. [DOI] [PubMed] [Google Scholar]
- Olivera A., Lejbman N., Jeromin A., French L.M., Kim H.S., Cashion A., Mysliwiec V., Diaz-Arrastia R., Gill J. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 2015;72:1109–1116. doi: 10.1001/jamaneurol.2015.1383. [DOI] [PubMed] [Google Scholar]
- Omalu B.I., Dekosky S.T., Minster R.L., Kamboh M.I., Hamilton R.L., Wecht C.H. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57:128–134. doi: 10.1227/01.neu.0000163407.92769.ed. (discussion 128-34) [DOI] [PubMed] [Google Scholar]
- Ost M., Nylen K., Csajbok L., Ohrfelt A.O., Tullberg M., Wikkelso C., Nellgard P., Rosengren L., Blennow K., Nellgard B. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology. 2006;67:1600–1604. doi: 10.1212/01.wnl.0000242732.06714.0f. [DOI] [PubMed] [Google Scholar]
- Park L., Uekawa K., Garcia-Bonilla L., Koizumi K., Murphy M., Pistik R., Younkin L., Younkin S., Zhou P., Carlson G., Anrather J., Iadecola C. Brain perivascular macrophages initiate the neurovascular dysfunction of Alzheimer Abeta peptides. Circ. Res. 2017;121:258–269. doi: 10.1161/CIRCRESAHA.117.311054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M., Hernandez F., Lim F., Diaz-Nido J., Avila J. Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. J. Alzheimers Dis. 2003;5:301–308. doi: 10.3233/jad-2003-5405. [DOI] [PubMed] [Google Scholar]
- Perry D.C., Sturm V.E., Peterson M.J., Pieper C.F., Bullock T., Boeve B.F., Miller B.L., Guskiewicz K.M., Berger M.S., Kramer J.H., Welsh-Bohmer K.A. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J. Neurosurg. 2016;124:511–526. doi: 10.3171/2015.2.JNS14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plog B.A., Dashnaw M.L., Hitomi E., Peng W., Liao Y., Lou N., Deane R., Nedergaard M. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J. Neurosci. 2015;35:518–526. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta R., Furmaga-Jablonska W., Maciejewski R., Ulamek-Koziol M., Jablonski M. Brain ischemia activates beta- and gamma-secretase cleavage of amyloid precursor protein: significance in sporadic Alzheimer's disease. Mol. Neurobiol. 2013;47:425–434. doi: 10.1007/s12035-012-8360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha S., Fadar O., Mehregan A., Salat D.H., Moscufo N., Meier D.S., Guttmann C.R., Fisher N.D., Lipsitz L.A., Sorond F.A. Impaired cerebrovascular hemodynamics are associated with cerebral white matter damage. J. Cereb. Blood Flow Metab. 2014;34:228–234. doi: 10.1038/jcbfm.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlackhansingh A.F., Brooks D.J., Greenwood R.J., Bose S.K., Turkheimer F.E., Kinnunen K.M., Gentleman S., Heckemann R.A., Gunanayagam K., Gelosa G., Sharp D.J. Inflammation after trauma: microglial activation and traumatic brain injury. Ann. Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Ramos-Cejudo J., Gutierrez-Fernandez M., Rodriguez-Frutos B., Exposito Alcaide M., Sanchez-Cabo F., Dopazo A., Diez-Tejedor E. Spatial and temporal gene expression differences in core and periinfarct areas in experimental stroke: a microarray analysis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein R., Chang B., Yue J.K., Chiu A., Winkler E.A., Puccio A.M., Diaz-Arrastia R., Yuh E.L., Mukherjee P., Valadka A.B., Gordon W.A., Okonkwo D.O., Davies P., Agarwal S., Lin F., Sarkis G., Yadikar H., Yang Z., Manley G.T., Wang K.K.W., The T.-T.B.I.I., Cooper S.R., Dams-O'connor K., Borrasso A.J., Inoue T., Maas A.I.R., Menon D.K., Schnyer D.M., Vassar M.J. Comparing plasma phospho tau, total tau, and phospho tau-total tau ratio as acute and chronic traumatic brain injury biomarkers. JAMA Neurol. 2017;74:1063–1072. doi: 10.1001/jamaneurol.2017.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare A.P., Bell R.D., Zhao Z., Ma Q., Winkler E.A., Ramanathan A., Zlokovic B.V. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat. Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Salehi A., Zhang J.H., Obenaus A. Response of the cerebral vasculature following traumatic brain injury. J. Cereb. Blood Flow Metab. 2017;37:2320–2339. doi: 10.1177/0271678X17701460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahim P., Tegner Y., Wilson D.H., Randall J., Skillback T., Pazooki D., Kallberg B., Blennow K., Zetterberg H. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 2014;71:684–692. doi: 10.1001/jamaneurol.2014.367. [DOI] [PubMed] [Google Scholar]
- Shahim P., Tegner Y., Gustafsson B., Gren M., Arlig J., Olsson M., Lehto N., Engstrom A., Hoglund K., Portelius E., Zetterberg H., Blennow K. Neurochemical aftermath of repetitive mild traumatic brain injury. JAMA Neurol. 2016;73:1308–1315. doi: 10.1001/jamaneurol.2016.2038. [DOI] [PubMed] [Google Scholar]
- Shahim P., Zetterberg H., Tegner Y., Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology. 2017;88:1788–1794. doi: 10.1212/WNL.0000000000003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva D.F., Selfridge J.E., Lu J., E. L., Roy N., Hutfles L., Burns J.M., Michaelis E.K., Yan S., Cardoso S.M., Swerdlow R.H. Bioenergetic flux, mitochondrial mass and mitochondrial morphology dynamics in AD and MCI cybrid cell lines. Hum. Mol. Genet. 2013;22:3931–3946. doi: 10.1093/hmg/ddt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.H., Johnson V.E., Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel J., Pirraglia E., Osorio R.S., Glodzik L., Li Y., Tsui W., Saint Louis L.A., Randall C., Butler T., Xu J., Zinkowski R.P., Zetterberg H., Fortea J., Fossati S., Wisniewski T., Davies P., Blennow K., De Leon M.J. Greater specificity for cerebrospinal fluid P-tau231 over P-tau181 in the differentiation of healthy controls from Alzheimer's disease. J. Alzheimers Dis. 2016;49:93–100. doi: 10.3233/JAD-150167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow R.H. Mitochondria and mitochondrial cascades in Alzheimer's disease. J. Alzheimers Dis. 2017 Oct 7 doi: 10.3233/JAD-170585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarka N., Pabbidi M.R., Amrein K., Czeiter E., Berta G., Pohoczky K., Helyes Z., Ungvari Z., Koller A., Buki A., Toth P. Traumatic brain injury impairs myogenic constriction of cerebral arteries: role of mitochondria-derived H2O2 and TRPV4-dependent activation of BKCa channels. J. Neurotrauma. 2018 Jan 12 doi: 10.1089/neu.2017.5056. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasoff-Conway J.M., Carare R.O., Osorio R.S., Glodzik L., Butler T., Fieremans E., Axel L., Rusinek H., Nicholson C., Zlokovic B.V., Frangione B., Blennow K., Menard J., Zetterberg H., Wisniewski T., DE Leon M.J. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 2015;11:457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenreiro M.M., Ferreira R., Bernardino L., Brito M.A. Cellular response of the blood-brain barrier to injury: potential biomarkers and therapeutic targets for brain regeneration. Neurobiol. Dis. 2016;91:262–273. doi: 10.1016/j.nbd.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Tian Y., Salsbery B., Wang M., Yuan H., Yang J., Zhao Z., Wu X., Zhang Y., Konkle B.A., Thiagarajan P., Li M., Zhang J., Dong J.F. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood. 2015;125:2151–2159. doi: 10.1182/blood-2014-09-598805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaelen J., Greiffenstein P., Deboisblanc B.P. Sleep in traumatic brain injury. Crit. Care Clin. 2015;31:551–561. doi: 10.1016/j.ccc.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Von Holstein-Rathlou S., Petersen N.C., Nedergaard M. Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci. Lett. 2018;662:253–258. doi: 10.1016/j.neulet.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington P.M., Morffy N., Parsadanian M., Zapple D.N., Burns M.P. Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer's disease mouse model. J. Neurotrauma. 2014;31:125–134. doi: 10.1089/neu.2013.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington P.M., Villapol S., Burns M.P. Polypathology and dementia after brain trauma: does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? Exp. Neurol. 2016;275(Pt 3):381–388. doi: 10.1016/j.expneurol.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M.W., Crane P.K., Montine T.J., Bennett D.A., Veitch D.P. Traumatic brain injury may not increase the risk of Alzheimer disease. Neurology. 2017;89:1923–1925. doi: 10.1212/WNL.0000000000004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein A.A., Chin L.M., Collins J., Goel D., Keyser R.E., Chan L. Effect of aerobic exercise training on mood in people with traumatic brain injury: a pilot study. J. Head Trauma Rehabil. 2017;32:E49–E56. doi: 10.1097/HTR.0000000000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J., Qian S., Yang Q., Deng L., Mo, Y. & Yu, Y. Overexpression of netrin-1 increases the expression of tight junction-associated proteins, claudin-5, occludin, and ZO-1, following traumatic brain injury in rats. Exp. Ther. Med. 2014;8:881–886. doi: 10.3892/etm.2014.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T., Goni F. Immunotherapeutic approaches for Alzheimer's disease. Neuron. 2015;85:1162–1176. doi: 10.1016/j.neuron.2014.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski H.M., Maslinska D. Beta-protein immunoreactivity in the human brain after cardiac arrest. Folia Neuropathol. 1996;34:65–71. [PubMed] [Google Scholar]
- Wolters F.J., Zonneveld H.I., Hofman A., VAN DER Lugt A., Koudstaal P.J., Vernooij M.W., Ikram M.A., Heart-Brain Connection Collaborative Research, G Cerebral perfusion and the risk of dementia: a population-based study. Circulation. 2017;136:719–728. doi: 10.1161/CIRCULATIONAHA.117.027448. [DOI] [PubMed] [Google Scholar]
- Xing C., Hayakawa K., Lok J., Arai K., Lo E.H. Injury and repair in the neurovascular unit. Neurol. Res. 2012;34:325–330. doi: 10.1179/1743132812Y.0000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C.S., Liu A.C., Chen J., Pan Z.Y., Wan Q., Li Z.Q., Wang Z.F. Overactivation of NR2B-containing NMDA receptors through entorhinal-hippocampal connection initiates accumulation of hyperphosphorylated tau in rat hippocampus after transient middle cerebral artery occlusion. J. Neurochem. 2015;134:566–577. doi: 10.1111/jnc.13134. [DOI] [PubMed] [Google Scholar]
- Zehendner C.M., Sebastiani A., Hugonnet A., Bischoff F., Luhmann H.J., Thal S.C. Traumatic brain injury results in rapid pericyte loss followed by reactive pericytosis in the cerebral cortex. Sci. Rep. 2015;5:13497. doi: 10.1038/srep13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H., Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat. Rev. Neurol. 2016;12:563–574. doi: 10.1038/nrneurol.2016.127. [DOI] [PubMed] [Google Scholar]
- Zhao G., Liu H.L., Zhang H., Tong X.J. Treadmill exercise enhances synaptic plasticity, but does not alter beta-amyloid deposition in hippocampi of aged APP/PS1 transgenic mice. Neuroscience. 2015;298:357–366. doi: 10.1016/j.neuroscience.2015.04.038. [DOI] [PubMed] [Google Scholar]