Abstract
Myopia is a highly prevalent eye disease. There is limited information suggesting a relationship between myopia and inflammation. We found children with allergic conjunctivitis (AC) had the highest adjusted odds ratio (1.75, 95% confidence interval [CI], 1.72–1.77) for myopia among the four allergic diseases. A cohort study was conducted and confirmed that children with AC had a higher incidence and subsequent risk of myopia (hazard ratio 2.35, 95%CI 2.29–2.40) compared to those without AC. Lower refractive error and longer axial length were observed in an AC animal model. Myopia progression was enhanced by tumor necrosis factor (TNF)-α or interleukin (IL)-6 administration, two cytokines secreted by mast cell degranulation. The TNF-α or IL-6 weakened the tight junction formed by corneal epithelial (CEP) cells and inflammatory cytokines across the layer of CEP cells, which increased the levels of TNF-α, IL-6, and IL-8 secreted by retinal pigment epithelial cells. The expression levels of TNF-α, IL-6, IL-8, monocyte chemoattractant protein-1, and nuclear factor kappa B were up-regulated in eyes with AC, whereas IL-10 and the inhibitor of kappa B were down-regulated. In conclusion, the experimental findings in mice corroborate the epidemiological data showing that allergic inflammation influences the development of myopia.
Keywords: Myopia, Inflammation, Asthma, Atopic dermatitis, Allergic rhinitis, Allergic conjunctivitis, Population-based study
Highlights
-
•
Asthma, allergic rhinitis, atopic dermatitis, and allergic conjunctivitis are risk factors for myopia.
-
•
Children with allergic conjunctivitis had the highest adjusted odds ratio for myopia.
-
•
Up-regulation of allergic inflammation promotes the progression of myopia in the animal model.
Myopia is a major cause of visual impairment and has a huge impact on public healthcare systems and economies worldwide. The relationship between myopia and inflammation has seldom been discussed. We demonstrated that allergic inflammation promotes myopia progression.
1. Introduction
Myopia is a major cause of visual loss and has a tremendous impact on the public health systems and economies. It is estimated that 2.5 billion people will be affected by myopia in the next decade (Wojciechowski, 2011). In Taiwan, the prevalence of myopia is 9.4% in 6-year-old children and > 75% in 15-year-old adolescents. By the age of 18, the prevalence increases to 80–90%, and approximately 10–20% of these children are highly myopic (Lin et al., 2001). High myopia increases the risk of pathologic ocular changes such as premature cataract, glaucoma, retinal detachment, and myopic macular degeneration, all of which can cause irreversible visual loss (Saw et al., 2005, Leo and Young, 2011, Morgan et al., 2012).
Although the exact causes of myopia are unclear, multiple lines of evidence support that interactions between hereditary factors and environmental exposures play crucial roles in ocular growth and refractive development (Wojciechowski, 2011, Leo and Young, 2011, Morgan et al., 2012). Myopia primarily results from abnormal elongation of the vitreous chamber of the eye (Rada et al., 2006, Curtin, 1985). Results from clinical and experimental studies have clearly demonstrated that ocular elongation is associated with altered extracellular matrix (ECM) and remodeling of the connective tissue of the scleral shell (Rada et al., 2006, Harper and Summers, 2015). ECM composition and scleral remodeling are regulated by genes as well as environmental influences and individual behavioral factors (Rada et al., 2006, Harper and Summers, 2015, Hornbeak and Young, 2009). To date, the precise biological mechanisms by which environmental factors influence ocular refraction in humans are still unclear. Several reports have proposed a role for inflammation in myopia susceptibility (Kung et al., 2017, Herbort et al., 2011). Our recent population-based cohort study showed that patients with autoimmune diseases, such as type 1 diabetes mellitus; systemic lupus erytheomatosus, and uveitis, have higher risks of myopia compared to those without autoimmune diseases (Lin et al., 2016). Moreover, in an animal model of myopia, we found that inflammatory markers, such as c-Fos, nuclear factor kappa B (NFκB), interleukin (IL)-6, and tumor necrosis factor (TNF)-α were upregulated in myopic eyes and downregulated upon treatment with atropine and the immunosuppressive agent, cyclosporine A (CSA) (Lin et al., 2016). Therefore, both clinical and experimental results support that inflammation may contribute to myopia progression (Lin et al., 2016).
Allergic conjunctivitis (AC) is a group of inflammatory diseases affecting the ocular surface, caused by abnormal immune-hypersensitivity response to environmental allergens (Cordova et al., 2014). The immune mechanism of AC is characterized by immunoglobulin (Ig)-E-mediated mast cell degranulation and/or T-lymphocyte-mediated immune response. AC is characterized histologically by infiltration of the conjunctiva with inflammatory cells, including neutrophils, eosinophils, lymphocytes, and macrophages. Chronic AC may result in remodeling of the ocular surface tissues (Cordova et al., 2014). However, it is unknown whether the inflammatory process of AC may cause myopia progression. We hypothesized that allergic inflammation of the eye would mediate the development of myopia. Therefore, we first conducted a case-control study to investigate the prevalence of allergic diseases, including asthma, atopic dermatitis, allergic rhinitis, and AC, in children with and without myopia. Secondly, we conducted a cohort study to investigate the incidence and risk of myopia in children with AC compared to those without AC. Finally, we established an AC animal model to demonstrate the possible mechanisms underlying allergic inflammation as a risk factor of myopia.
2. Materials and Methods
2.1. Epidemiological Study Design
2.1.1. Study Population
The National Health Insurance (NHI) program was implemented in 1995 and has information about up to 99% of the 23.74 million people living in Taiwan. We compiled data files for children (aged < 18 years) from the NHI program, which were established and maintained by the National Health Research Institutes (NHRI). In this study, we used the registry for half of all insured children (age < 18 years) in Taiwan from 1996 to 2012. The disease criteria were defined and classified according to the diagnostic codes of the International Classifications of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The refraction power was measured after cycloplegia to avoid accommodative spasm. All patient identifications had been scrambled to protect privacy in the data linkage. This study was approved by the China Medical University ethical review committee (CRREC-103-048(CR-2)).
2.1.2. Case Control Study
We first conducted a case-control study to compare the prevalence of allergic diseases is compared between children with and without myopia (Table 1). We identified a total of 197,237 patients aged 1–18 years with newly diagnosed myopia (ICD-9 code 367.1x) during 2008–2012 as the myopia group, and the diagnosed date of myopia was considered as the index date. For each patient with myopia, one insured children without history of myopia were matched by sex, age (within 1-year intervals), parents' occupational status, urbanization of residential area, and year of index date, as the non-myopia group (Table 1). We identified subjects who were diagnosed with asthma (ICD-9-CM 493.xx), atopic dermatitis (ICD-9-CM 691.8x), allergic rhinitis (ICD-9-CM 477.xx), and AC (ICD-9-CM 372.05, 372.10, and 372.14). We used Pearson's chi-square test and Student's t-test to compare demographic data and allergic diseases between the myopia and non-myopia groups. Multiple logistic regression models to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for the association between allergic diseases and myopia after adjusting for sex, age, urbanization of residential area, parents' occupational status and allergic disease. A p value lower than 0.05 was deemed statistically significant. The data were analyzed using the SAS statistical package (version 9.3).
Table 1.
Demographic characteristics in children with or without myopia and odds ratios for myopia by the presence of specific allergic disease in multiple logistic regression models.
| Variables | Non-myopia group N = 197,237 |
Myopia group N = 197,237 |
Adjusted ORa (95% CI) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | p-Value | ||
| Sex | > 0.99 | |||||
| Girls | 98,517 | 49.9 | 98,517 | 49.9 | ||
| Boys | 98,720 | 50.1 | 98,720 | 50.1 | ||
| Age, years (SD)b, a | 8.71 | (2.54) | 8.73 | (2.51) | 0.07 | |
| Asthma | < 0.001 | |||||
| No | 154,251 | 78.2 | 148,073 | 75.1 | Ref | |
| Yes | 42,986 | 21.8 | 49,164 | 24.9 | 0.99 (0.98–1.01) | |
| Atopic dermatitis | < 0.001 | |||||
| No | 182,730 | 92.6 | 179,709 | 91.1 | Ref | |
| Yes | 14,507 | 7.36 | 17,528 | 8.89 | 1.06 (1.04–1.09)⁎⁎⁎ | |
| Allergic rhinitis | < 0.001 | |||||
| No | 127,333 | 64.6 | 110,529 | 56.0 | Ref | |
| Yes | 69,904 | 35.4 | 86,708 | 44.0 | 1.32 (1.30–1.34)⁎⁎⁎ | |
| Allergic conjunctivitis | < 0.001 | |||||
| No | 154,777 | 78.5 | 131,551 | 66.7 | Ref | |
| Yes | 42,460 | 21.5 | 65,686 | 33.3 | 1.75 (1.72–1.77)⁎⁎⁎ | |
Abbreviation: OR, odds ratio; CI, confidence interval; SD, standard deviation.
Model adjusted for sex, age (continuous), urbanization, parents' occupational status and allergic disease.
Student's t-test.
p < 0.001.
2.1.3. Cohort Study
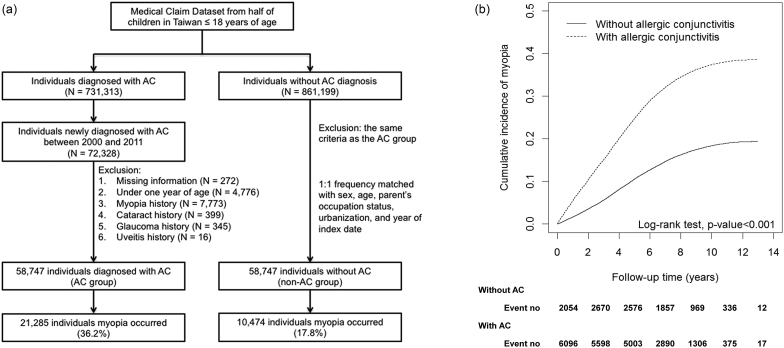
Secondly, we conducted a cohort study to examine the incidence rate and relative risk of myopia in the AC cohort compared to the non-AC cohort using a Cox proportional hazard regression. We identified patients aged 1–18 years with newly diagnosed allergic conjunctivitis (AC) in the year of 2000 as the AC group, and the diagnosed date of AC was considered as the index date. Then we randomly matched one child without AC to every patient with AC using frequency matching by sex, age (in 1-year intervals), parents' occupational status, urbanization of residential area, and year of index date, as the non-AC group. Subjects with a history of myopia before the index date, those lacking information on age, sex, parents' occupational status, or urbanization of residence area, those with history of uveitis (ICD-9-CM 360.11, 360.12, 362.18, 363.00, 363.01, 363.03, 363.05-363.08, 363.1x, 363.20, 363.21, 363.4x, 364.00-364.02, 364.04, 364.1x–364.3x), cataract (ICD-9-CM 366.xx and 743.3x) and glaucoma (ICD-9-CM 365.xx) before the index date were excluded. All subjects were followed up until myopia diagnosis, loss to follow-up, withdrawal from the insurance system, or the end of 2012 (Fig. 1a). The incidence rate (per 1000 person-years) of myopia was calculated by dividing the number of myopia incident by person-time at risk. We performed Kaplan–Meier analysis for the visual inspection of the cumulative incidence of myopia in the two cohorts. Multivariate Cox proportional hazards regression was used to examine the effect of AC on the risk of myopia, which was determined by the adjusted hazard ratio (HR) with a 95% confidence interval (CI). A P-value < 0.05 was deemed statistically significant. The data were analyzed using the SAS statistical package (version 9.3). Kaplan–Meier survival curves were plotted using the R software (version 2.14.1; R Development Core Team, Vienna, Austria).
Fig. 1.
(a) Inclusion and exclusion criteria for the cohort study on allergic conjunctivitis. (b) The association between allergic conjunctivitis (AC) and myopia incidence. The cumulative incidence of myopia is shown for individuals with and without allergic conjunctivitis.
2.2. Experimental Study Design
2.2.1. AC Animal Model
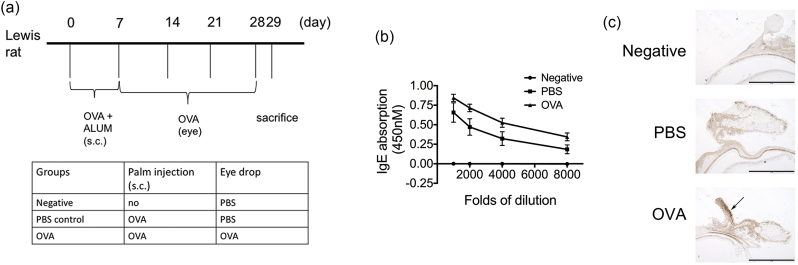
Three-week-old male Lewis rats (n = 10 animals each) were purchased from the National Laboratory Animal Center (Taipei, Taiwan) and maintained in a specific pathogen-free animal facility at China Medical University (Taichung, Taiwan). All animal care and experimental procedures conformed to institutional guidelines. Rats were injected with 100 μg of chicken ovalbumin (OVA; Sigma-Aldrich, St. Louis, MO) and with 200 μL of aluminum hydroxide (Sigma-Aldrich) (200 mg/mL) in their left hind footpad on days 0 and 7 as immunizations, and then challenged once in the conjunctival sac with 250 μg of OVA diluted in phosphate–buffered saline (PBS) on days 14, 21, and 28. There were two control groups. The rats in the “negative group” were given PBS in the immunization stages and PBS in challenge stages. On the other hand, the rats in the “PBS control group” were given OVA in the immunization stages and PBS in the challenge stages (Fig. 2a). Twenty-four hours after the final challenge with OVA, the refractive errors and axial lengths of the rats were measured and they were then given an overdose of Zoletil (Virbac, Taipei, Taiwan). After the animals were sacrificed, the eyelids and conjunctivae were collected. Blood was extracted from the ventricles and centrifuged for sera collection.
Fig. 2.
Ovalbumin (OVA) administration induced the development of allergic conjunctivitis. (a) The experimental protocol and grouping conditions. (b) Immunoglobulin (Ig)-E antibody secretion in the sera of OVA-sensitized mice. (c) Infiltration of eosinophils (arrow) into the conjunctiva induced by OVA.
2.2.2. Enzyme-linked Immunosorbent Assay (ELISA) for OVA-specific Immunoglobulin E (IgE) Antibodies and Cytokines
The 96-well immunoplates were coated with OVA (1 mg/mL) overnight at 4 °C. After blocking with 1% bovine serum albumin (BSA; Sigma-Aldrich) in PBS for 1 h at room temperature, serial dilutions of serum from the rats were added and incubated for 4 h at room temperature. The plates were then washed with PBS plus 0.05% Tween 20 (PBST) and incubated for 2 h at room temperature with horseradish peroxidase (HRP)-conjugated mouse anti-rat IgE antibody (Thermo Fisher Scientific Cat# MA5–16813 RRID:AB_2538296). After washing with PBST, the plates were developed using 3,3′,5,5′-tetramethylbenzidine (TMS; Sigma-Aldrich) and stopped with 0.1 N HCl, and then, the optical density (OD) of each well was read at 610 nm.
Culture supernatants were harvested and assayed for cytokine content using the commercially available enzyme-linked immunosorbent assay reagents for TNF-α, IL-6, and IL-8 and were measured according to the manufacturer's instructions (Duoset, R&D Systems, Minneapolis, MN).
2.2.3. Cell Culture
The primary corneal epithelium cells (HCEpiC; passages 3–5) and retinal pigment epithelial cells (HREpiC; passages 3–4) were cultured according to the instruction provided by ScienCell Research Laboratories (San Diego, CA). Cells were maintained at 37 °C and 5% CO2, with medium replacement every 2–3 days. HCEpiC cells were seeded in 6-well plates (1 × 105 cells/well) for 7 days to allow the formation of tight junctions and treated with TNF-α (10 ng/mL), IL-6 (10 ng/mL), TNF-α (10 ng/mL) + IL-6 (10 ng/mL), or PBS for 24 h. Cell lysates were collected for western blot analysis to determine the levels of tight junction proteins. HREpiC cells were seeded in 6-well plates (1 × 105 cells/well) and treated with basolateral media from HCEpiC treated with TNF-α (10 ng/mL), IL-6 (10 ng/mL), TNF-α (10 ng/mL) + IL-6 (10 ng/mL), or PBS for 24 h. Cell lysates were collected for quantitative (q)PCR to determine gene expression levels.
2.2.4. Ocular Biometry Assessment
Each eye was refracted to measure the refractive error using a hand-held streak retinoscope. The pupil of each eye was dilated with 1% tropicamide. Animals were anesthetized with 10% ether in O2. Ocular refraction was evaluated at the start and end of the experiment. At the end of the study, the animals were sacrificed by CO2 asphyxiation according to the guidelines of the Public Health Service, Office of Laboratory Animal Welfare, National Institutes of Health, and American Association of Veterinary Medicine. Eyes were enucleated using a razor blade on an ice plate under a surgical microscope (Topcon, Tokyo, Japan) by making a cut perpendicular to the anterior-posterior axis approximately 1-mm posterior to the ora serrata. The iris and ciliary body of the anterior segment of the eye were separated. The posterior sclera was excised using a 7-mm diameter trephine. The axial lengths were determined using A-scan ultrasonography (PacScan 300 Plus, New Hyde Park, NY). The average of 10 different measurements was used. The axial dimensions of the eyes were then measured by performing ultrasonography with a 10-MHz transducer (A-scan). The axial length of the eye was defined as the distance from the front of the cornea to the back of the sclera.
2.2.5. Immunofluorescence Staining
HCEpiC cells were seeded in chamber slides (2 × 104 cells/well) for 7 days to allow the formation of tight junctions and treated with TNF-α (10 ng/mL), IL-6 (10 ng/mL), TNF-α (10 ng/mL) + IL-6 (10 ng/mL), or PBS for 24 h. Cells were washed with Tris-buffered saline (TBS) and fixed with 4% paraformaldehyde and blocked with 1% bovine serum albumin, 0.1% Triton X-100 for 1 h. Cells were incubated with anti-ZO1 antibodies (Cell Signaling Technology, Danvers, MA) for 1 h. Cells were washed with TBS and incubated with goat anti-rabbit secondary antibody labeled with Alexa Fluor™ 594 (Invitrogen, Carlsbad, CA) and DAPI (4′,6-diamidino-2-phenylindole) DNA stain. After three washes with TBS, cells were imaged using confocal fluorescence microscopy. All experiments were performed at least in triplicate.
2.2.6. Transepithelial Electrical Resistance (TEER) Measurement
To evaluate the effect of inflammatory cytokines on the tight junction sealing of corneal epithelium, 2 × 104 HCEpiC cells (in 200 μL media) were plated into Millicell® 24-well cell culture inserts (1 μm) (Merck Millipore, Darmstadt, Germany). The 1300 μL media were put in the basolateral site. The media was changed every 3 days for a total of 14 days. Cells were treated with TNF-α (10 ng/mL), IL-6 (10 ng/mL), TNF-α (10 ng/mL) + IL-6 (10 ng/mL), or PBS for 24 h. Transepithelial electrical resistance were measured using an Millicell ERS-2 Voltohmmeter (Merck) according to the manufacturer's instructions. The transepithelial electrical resistance was calculated according to the formula: TEER (Ω cm2) = (resistance (Ω) − background resistance (Ω)) × membrane area (0.33 cm2). Changes in TEER for each treatment were calculated: change in TEER (%) = TEER (Ω cm2)/initial TEER (Ω cm2) − 100 (%). Apical and basolateral media were collected to measure the levels of TNF-α, IL-6, and IL-8. One milliliter of basolateral media was used to treat HREpiC seeded in 6-well plates.
2.2.7. Immunohistochemistry (IHC)
Eyelids and conjunctivae were fixed overnight in 4% paraformaldehyde in phosphate buffer and embedded in paraffin. Tissue blocks were sectioned at 8-μm thicknesses and collected on clean glass slides. Sections were deparaffinized, immersed in hydrogen peroxide for 30 min, and blocked for 1 h at room temperature in PBS containing 5% normal goat serum, and then incubated overnight at 4 °C with the specific primary antibody against eosinophil major basic protein (MBP; Bio-Rad / AbD Serotec Cat# MCA5751 RRID:AB_10671914), collagen type I (GeneTex Cat# GTX20292 RRID:AB_384293), transforming growth factor β (TGF-β) (Abcam Cat# ab66043 RRID:AB_1143428), matrix metalloproteinase-2 (MMP2) (Abcam Cat# ab37150 RRID:AB_881512), IL-6 (Abcam Cat# ab6672 RRID:AB_2127460), IL-8 (MyBioSource Cat# MBS551025), IL-10 (GeneTex Cat# GTX37657 RRID:AB_11172270), monocyte chemoattractant protein-1 (MCP-1) (Abcam Cat# ab9669 RRID:AB_2071551), TNF-α (Bioworld Cat# BS1857), nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) (Abcam Cat# ab16502), and inhibitor of kappa B (IκB) (GeneTex Cat# GTX50468 RRID:AB_11167876). This was followed by incubation with a secondary antibody conjugated to biotin for 1 h at room temperature. The slides were developed utilizing streptavidin-conjugated HRP with diaminobenzidine (DAB; Sigma-Aldrich) as the substrate and counterstained with hematoxylin.
3. Results
3.1. Children With AC Had the Highest Risk for Myopia Among Children With Allergic Diseases
For the case-control study, a total of 197,237 patients with myopia and 197,237 sex- and age- matched controls were identified (Table 1). In both groups, there were 50.1% boys and 49.9% girls and the mean (standard deviation) age at myopia diagnosis was 8.73 (2.51) years. The adjusted ORs of the following atopic diseases were significantly higher in the myopia group than in the non-myopia group: atopic dermatitis 1.06 (95% CI 1.04–1.09), allergic rhinitis 1.32 (95% CI 1.30–1.34), and AC 1.75 (95% CI 1.72–1.77) (Table 1). The OR of myopia was highest in children with AC than other allergic diseases. For the cohort study, 58,747 subjects in the AC group and 58,747 individuals in the non-AC group were enrolled (Fig. 1a). The results of the log-rank test and the cumulative incidence curve of myopia, as shown in Fig. 1b, indicated that patients with AC had a significantly higher incidence rate of myopia than those without AC (log-rank test, P < 0.001).
3.2. The AC Cohort Had a Higher Subsequent Risk of Myopia Compared to the Non-AC Cohort
The incidence of myopia was 2.35-fold higher in the AC cohort than in the non-AC cohort (45.3 vs. 18.9 per 1000 person-years; Table 2). A sex-specific analysis showed that the incidence rate of myopia was slightly higher in girls in both groups. By contrast, the adjusted HR (2.49; 95% CI: 2.41–2.57) for myopia was higher for boys in the AC group than for boys in the non-AC group. An age-specific analysis showed that the incidence of myopia significantly decreased with age in both groups. The adjusted HR for myopia increased from 2.30 (95% confidence interval [CI]: 2.24–2.36) for subjects aged 1–6 years to 2.56 (95% CI: 2.43–2.70) for those aged 7–12 years and to 6.08 (95% CI: 3.79–9.76) for those aged 13–18 years in the AC group compared to the non-AC group (p for interaction < 0.001). Urbanization level-specific analyses showed that the adjusted HR of myopia increased in the AC group, but not in the non-AC group, regardless of living in areas with the least urbanization.
Table 2.
Incidence rates and hazard ratios for myopia in children with allergic conjunctivitis (AC) compared with children without AC stratified by demographic factors in Cox proportional hazard regression.
| Variables | Non-AC group |
AC group |
aHRa (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Event no. | Person-years | IR | N | Event no. | Person-years | IR | ||
| All | 58,747 | 10,474 | 554,903 | 18.9 | 58,747 | 21,285 | 469,537 | 45.3 | 2.35 (2.29–2.40)⁎⁎⁎ |
| Sex | |||||||||
| Girl | 26,133 | 5105 | 238,398 | 21.4 | 26,133 | 9694 | 201,612 | 48.1 | 2.20 (2.13–2.28)⁎⁎⁎ |
| Boy | 32,614 | 5369 | 316,505 | 17.0 | 32,614 | 11,591 | 267,925 | 43.3 | 2.49 (2.41–2.57)⁎⁎⁎ |
| Age, years | |||||||||
| 1–6 | 37,148 | 8420 | 390,335 | 21.6 | 37,148 | 16,401 | 324,226 | 50.6 | 2.30 (2.24–2.36)⁎⁎⁎ |
| 7–12 | 19,641 | 2034 | 157,730 | 12.9 | 19,641 | 4766 | 138,759 | 34.4 | 2.56 (2.43–2.70)⁎⁎⁎ |
| 13–18 | 1958 | 20 | 6838 | 2.92 | 1958 | 118 | 6552 | 18.0 | 6.08 (3.79–9.76)⁎⁎⁎ |
| Urbanization | |||||||||
| Level 1 (highest) | 21,464 | 3690 | 203,245 | 18.2 | 21,464 | 7820 | 170,849 | 45.8 | 2.46 (2.37–2.56)⁎⁎⁎ |
| Level 2 | 18,754 | 3411 | 177,187 | 19.3 | 18,754 | 6788 | 150,290 | 45.2 | 2.30 (2.21–2.40)⁎⁎⁎ |
| Level 3 | 10,935 | 2023 | 102,113 | 19.8 | 10,935 | 3879 | 87,517 | 44.3 | 2.19 (2.07–2.31)⁎⁎⁎ |
| Level 4 (lowest) | 7594 | 1350 | 72,358 | 18.7 | 7594 | 2798 | 60,882 | 46.0 | 2.42 (2.27–2.58)⁎⁎⁎ |
Abbreviation: AC, allergic conjunctivitis; IR, incidence rates, per 1000 person-years; aHR, adjusted hazard ratio; CI, confidence interval.
Models mutually adjusted for sex, age (continuous), parents' occupational status, and urbanization.
p < 0.001.
Table 3 shows the association between the frequency of medical visits per year for AC and the risk of myopia. Compared to the non-AC group, the risk of myopia in the AC group increased from 1.46 (95% CI: 1.42–1.50) for those with < 1 visit/ per year to 8.90 (95% CI: 8.62–9.19) for those with > 2 visits/ per year (trend test, P < 0.001). Table 4 describes the risk of myopia stratified according to median follow-up duration. The risk of myopia was highest since the 1–2 year of AC diagnosis (HR = 3.43; 95% CI: 3.20–3.69) and the risk gradually decreased with time.
Table 3.
Incidence rates and hazard ratios of myopia in children with allergic conjunctivitis (AC) compared with children without AC stratified by frequency of medical visits for AC in Cox proportional hazard regression.
| Average frequency for medical visits, per year | N | Event no. | Person-years | IR | aHRa (95% CI) |
|---|---|---|---|---|---|
| Non-AC group | 58,747 | 10,474 | 554,903 | 18.9 | 1.00 |
| AC group | |||||
| < 1 | 39,282 | 10,030 | 358,670 | 28.0 | 1.46 (1.42–1.50)⁎⁎⁎ |
| 1–2 | 10,988 | 5247 | 76,255 | 68.8 | 3.52 (3.40–3.64)⁎⁎⁎ |
| > 2 | 8477 | 6008 | 34,612 | 174 | 8.90 (8.62–9.19)⁎⁎⁎ |
| p for trend | < 0.001 |
Abbreviation: AC, allergic conjunctivitis; IR, incidence rates, per 1000 person-years; aHR, adjusted hazard ratio; CI, confidence interval.
Model adjusted for sex, age (continuous), parents' occupational status, and urbanization.
p < 0.001.
Table 4.
Incidence rates and hazard ratios for myopia in children with allergic conjunctivitis (AC) compared with children without AC stratified by follow-up years in Cox proportional hazard regression.
| Follow-up years | Non-AC group |
AC group |
aHRa (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Event no. | Person-years | IR | Event no. | Person-years | IR | ||
| 1–2 | 957 | 114,988 | 8.32 | 3224 | 110,770 | 29.1 | 3.43 (3.20–3.69)⁎⁎⁎ |
| 2–3 | 2670 | 109,112 | 24.5 | 5586 | 98,345 | 56.8 | 2.32 (2.22–2.43)⁎⁎⁎ |
| 4–5 | 2576 | 100,540 | 25.6 | 5015 | 84,662 | 59.2 | 2.33 (2.22–2.44)⁎⁎⁎ |
| ≥ 6 | 3174 | 230,262 | 13.8 | 4588 | 175,761 | 26.1 | 1.92 (1.83–2.01)⁎⁎⁎ |
Abbreviation: AC, allergic conjunctivitis; IR, incidence rates, per 1000 person-years; aHR, adjusted hazard ratio; CI, confidence interval.
Models adjusted for sex, age (continuous), parents' occupational status, and urbanization.
p < 0.001.
3.2.1. Myopic Shift Occurred in Eyes With AC
To confirm the relationship between AC and myopia, an animal model of AC was established. Lewis rats were immunized subcutaneously with OVA twice and then challenged via the conjunctival sac with an OVA eye drop once a week for 3 weeks (Fig. 2a). To determine the effect of OVA immunization in rats, OVA-specific IgE antibodies in the sera were measured. OVA immunization led to a profound increase in OVA-specific IgE antibodies compared to the negative group (Fig. 2b). We also found a higher IgE level in rats treated with OVA in the conjunctiva. Eosinophil infiltration in the conjunctiva was determined by IHC using an eosinophil marker MBP. The conjunctivae of OVA-treated rats demonstrated greater eosinophil infiltration (Fig. 2c). Taken together, we successfully established an animal model of AC, which would be used to examine several ocular parameters.
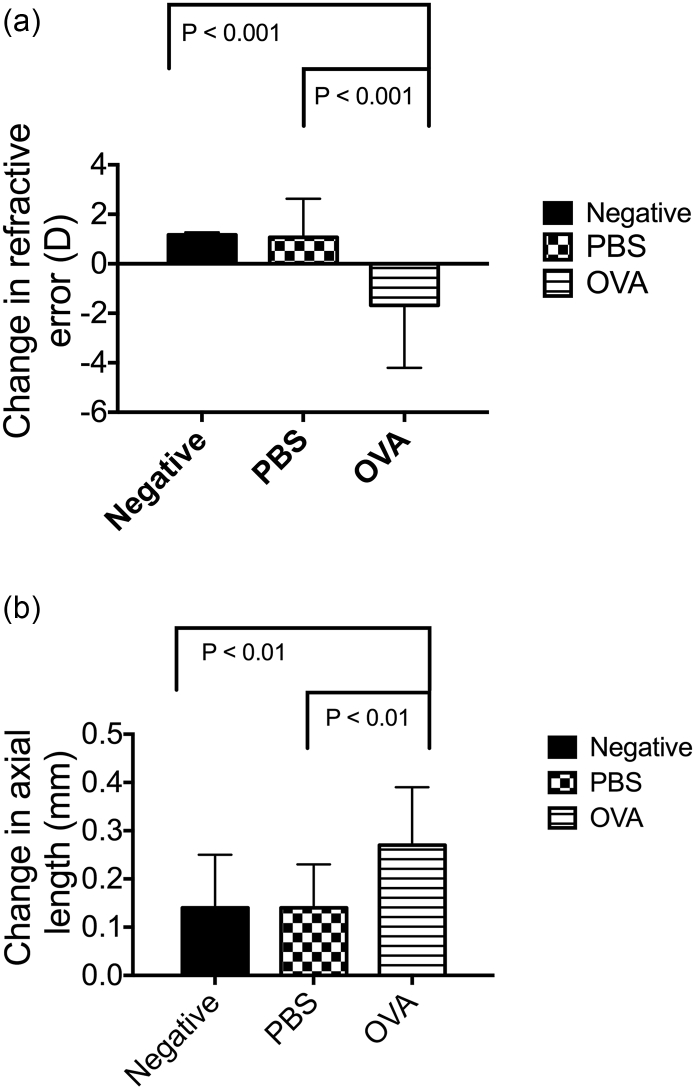
The refractive state and axial length of the eyes of the OVA-treated group, PBS control group, and negative group were measured on day 29, which was 24 h after the final OVA treatment (Fig. 2a). Following OVA treatment, the rats with AC had also developed myopia (change in refractive error (RE) = − 1.68 ± 2.52 D), whereas the rats in the negative group and PBS control group did not (change in RE = 1.17 ± 0.10 and 1.07 ± 1.56 D, respectively) (Fig. 3a). In addition, the axial lengths of AC eyes were significantly longer than those of the control eyes (change in axial length = 0.27 ± 0.12 vs. 0.14 ± 0.11 and 0.14 ± 0.09 mm, OVA, Negative, PBS, respectively) (Fig. 3b). Consequently, we confirmed that the relationship between allergic diseases and myopia observed in the case-control and cohort studies was also observed in the animal model.
Fig. 3.
Allergic conjunctivitis promotes myopia progression. (a) The RE was determined as the difference in diopter measurements taken before and after AC. An ANOVA was used to determine significant differences (P = 0.0011), and Dunnett's multiple comparisons tests were used for paired comparisons between negative, PBS-treated, and OVA-treated eyes. P < 0.05 was considered statistically significant. (b) The axial length was determined as the difference in axial length measurements taken before and after AC. ANOVA was used to determine significant differences (P = 0.0155) and Dunnett's multiple comparisons tests were used for paired comparisons between negative, PBS-treated, and OVA-treated eyes. P < 0.05 was considered statistically significant.
3.2.2. AC Altered the Expression of Myopia-related Makers
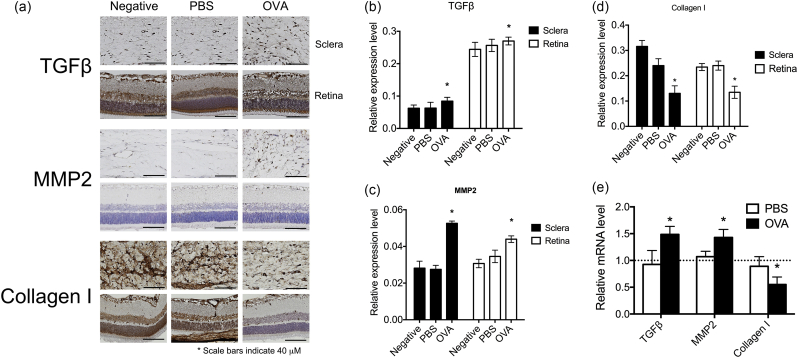
It has been known that the TGF-β expression level is elevated in the myopic eye, and continues to activate MMP2 expression. MMP2 is an enzyme that cleaves COL-I, the main extracellular matrix (ECM) in the sclera. Therefore, the expression level of COL-I would decrease in the myopic eye (Inamori et al., 2007, Guggenheim and McBrien, 1996, Hall et al., 2009, Norton and Rada, 1995, Lin et al., 2009, Lin et al., 2006). In order to confirm whether the animal model of AC expressed similar protein profiles as the myopic eye, IHC studies were performed. The IHC results from the retina and sclera indicated that the expression of TGF-β (Fig. 4a, b, and Supplementary Fig. 1) and MMP2 (Fig. 4a, c, and Supplementary Fig. 1) protein expressions were increased and COL-I (Fig. 4a, d and Supplementary Fig. 1) was decreased in OVA-treated sclerae and retinas as compared to the control groups. The mRNA expression levels of TGF-β and MMP2 in retinas were higher whereas COL-I was lower in OVA-treated eyes compared to the negative groups (Fig. 4e).
Fig. 4.
Allergic conjunctivitis increased the expression of myopia-related tissue-remodeling proteins. (a) Immunohistochemical analyses of TGF-β, MMP2, and COL-I in negative, PBS-treated, and OVA-treated eyes are shown. (b–d) The relative expression levels of TGF-β (b), MMP2 (c), and COL-I (d) were determined with the Image J software (Schindelin et al., 2012). (e) mRNA expression levels of TGF-β, MMP2, and COL-I in the retinas of PBS and OVA groups were compared to those in the negative group. ANOVA was used to determine significant differences (p < 0.0001) and Sidak's multiple comparisons tests were used for paired comparisons between PBS- and OVA-treated eyes. P < 0.05 was considered statistically significant. *: indicates a significant difference.
3.2.3. Up-regulation of Inflammation Promotes the Progression of Myopia
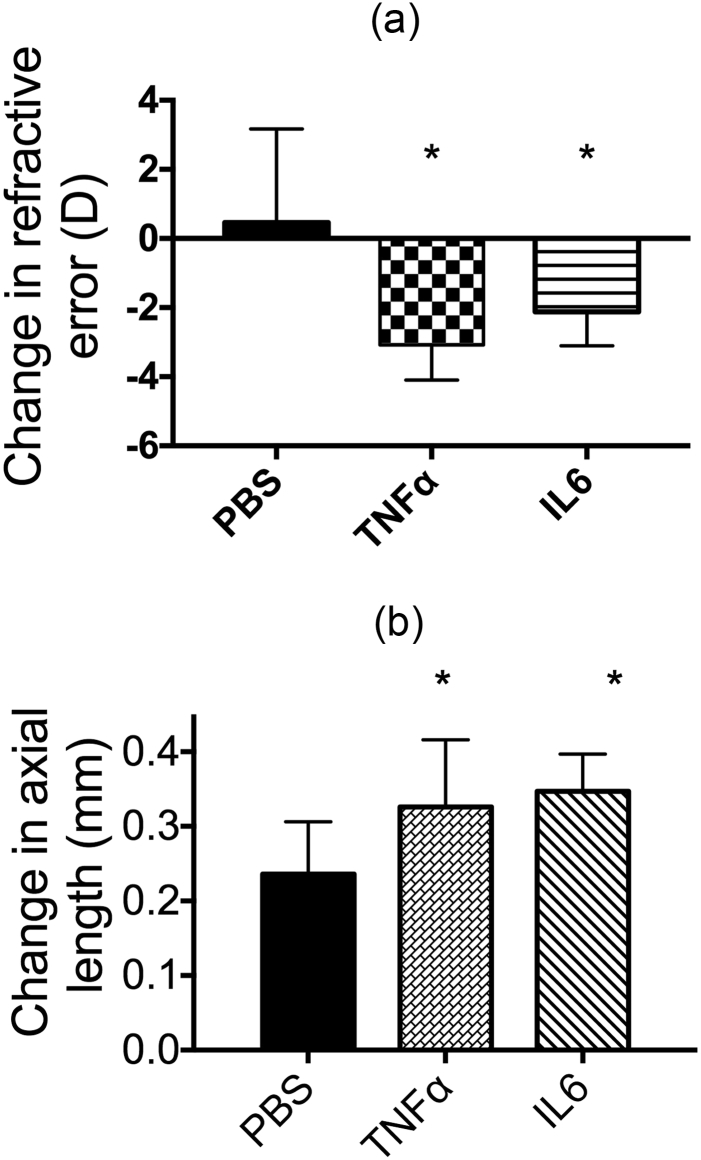
Mast cells play a key role in inducing AC signs and symptoms. Mast cells express FcεRI, a high-affinity IgE receptor. The binding of IgE with specific allergens (pollens, animal dander, or dust mite allergens) activates mast cells and induces degranulation. This causes the release of inflammatory mediators, IL-6, and TNF-α, inflammatory cytokines to initiate ocular inflammation. To determine whether increased inflammation in the ocular surface promotes myopia progression, TNF-α and IL-6 were applied to the eyes of hamsters every other day, and the RE and axial length were measured on day 21. After 21 days, the changes in RE and axial length in the PBS-treated group were 0.463 ± 2.71 D and 0.236 ± 0.07 mm, respectively. These values were reduced to − 3.083 ± 1.01 D and 0.326 ± 0.09 mm, respectively, after TNF-α treatment, and − 2.125 ± 0.98 D and 0.347 ± 0.05 mm, respectively, in IL-6-treated eyes; these differences were statistically significant with respect to the PBS-treated group (P < 0.05; Fig. 5a and b).
Fig. 5.
Effect of increasing inflammation in the eye on myopia progression. (a) The RE was determined as the difference in diopter measurements taken before and after treatment with 10 ng/mL TNF-α or IL-6 for 21 days. ANOVA was used to determine significant differences (p < 0.001), and Tukey's multiple comparisons tests were used for paired comparisons between PBS and TNF-α or IL-6-treated eyes. P < 0.05 was considered statistically significant. (b) The axial length was determined as the difference in axial length measurements taken before and after treatment with 10 ng/mL TNF-α or IL-6 for 21 days. An ANOVA was used to determine significant differences (p = 0.0043), and Tukey's multiple comparisons tests were used for paired comparisons between PBS and TNF-α or IL-6-treated eyes. P < 0.05 was considered statistically significant.
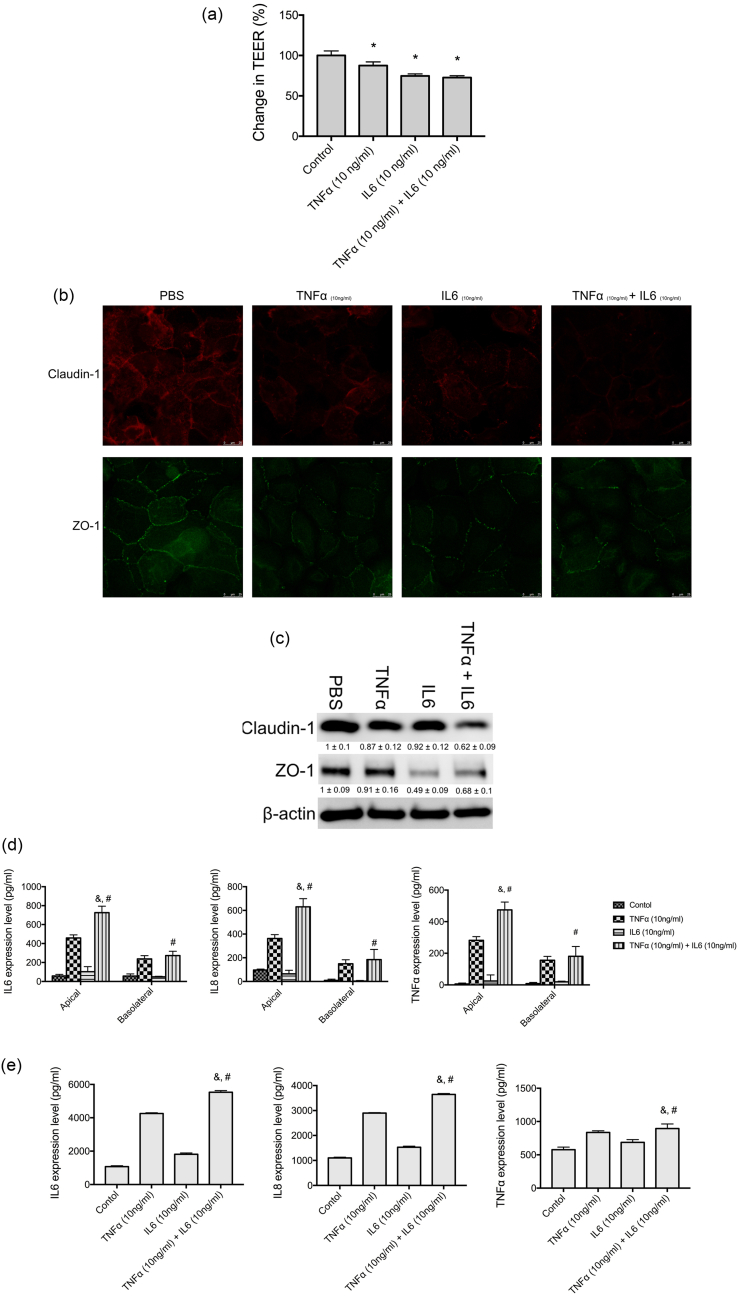
We found that treating corneal epithelial cells (CEP) with TNF-α or IL-6 lowered the TEER, and the TEER was further lowered with TNF-α and IL-6 co-treatment (Fig. 6a). TNF-α and IL-6 treatments reduced the levels of claudin-1 and ZO-1 tight junction proteins (Fig. 6b and c). In CEP, TNF-α treatment induced the expression of TNF-α, IL-6, and IL-8. IL-6 slightly increased the expression of TNF-α, IL-6, and IL-8. However, TNF-α and IL-6 co-treatment significantly promoted the expression of TNF-α, IL-6, and IL-8 in the apical site (Fig. 6d). We also found a significant increase in the expression of TNF-α, IL-6, and IL-8 in basolateral media (Fig. 6d). Human primary retinal pigment epithelial cells treated with basolateral media from TNF-α and IL-6-treated CEP exhibited even higher expression levels of TNF-α, IL-6, and IL-8 (Fig. 6e).
Fig. 6.
TNF-α and IL-6 altered the barrier function of corneal epithelial cells. (a) Relative transepithelial electrical resistance (TEER) of human primary corneal epithelial cells (CEP) incubated with PBS (control), TNF-α (10 ng/mL), IL-6 (10 ng/mL), or TNF-α (10 ng/mL) + IL-6 (10 ng/mL) for 24 h. ANOVA was used to determine significant differences (p < 0.0001), and Dunnett's multiple comparisons tests were used for paired comparisons between PBS and TNF-α or IL-6-treated groups. P < 0.05 was considered statistically significant. (b) CEP cells were cultured in chamber slides at 5 × 104 cells per well and incubated with PBS (control), TNF-α (10 ng/mL), IL-6 (10 ng/mL), or TNF-α (10 ng/mL) + IL-6 (10 ng/mL) for 24 h. Claudin-1 and ZO-1 were detected by immunofluorescence staining. (c) CEP cells were cultured in 6-well plates at 1 × 105 cells per well and incubated with PBS (control), TNF-α (10 ng/mL), IL-6 (10 ng/mL) or TNF-α (10 ng/mL) + IL-6 (10 ng/mL) for 24 h. Claudin-1 and ZO-1 were detected by western blot analysis. The relative expression levels were determined by densitometry analysis. Depicted western blots are one representative figure of three independent experiments. Results are the mean ± S.D. of three independent experiments. (d) CEP cells were seeded on transwell inserts with 0.4-μm pore size incubated with PBS (control), TNF-α (10 ng/mL), IL-6 (10 ng/mL) or TNF-α (10 ng/mL) + IL-6 (10 ng/mL) for 16 h and the levels of TNF-α, IL-6, and IL-8 in apical and basolateral compartments were determined with enzyme-linked immunosorbent assay (ELISA). ANOVA was used to determine significant differences (p < 0.0001), and Tukey's multiple comparisons tests were used for paired comparisons between TNF-α (&) or IL-6 (#) with TNF-α + IL-6-treated CEP cells. P < 0.05 was considered statistically significant. (e) The basolateral compartment media of CEP cells treated with PBS (control), TNF-α (10 ng/mL), IL-6 (10 ng/mL), or TNF-α (10 ng/mL) + IL-6 (10 ng/mL) for 16 h were collected. The basolateral compartment media were used to treat human primary retinal pigment epithelial cells for 16 h, and the levels of TNF-α, IL-6, and IL-8 were determined with ELISA. ANOVA was used to determine significant differences (p < 0.0001), and Tukey's multiple comparisons tests were used for paired comparisons between TNF-α (&) or IL-6 (#) with TNF-α + IL-6-treated retinal pigment epithelial cells. P < 0.05 was considered statistically significant.
3.2.4. AC Eyes Exhibited Higher Level of Inflammation
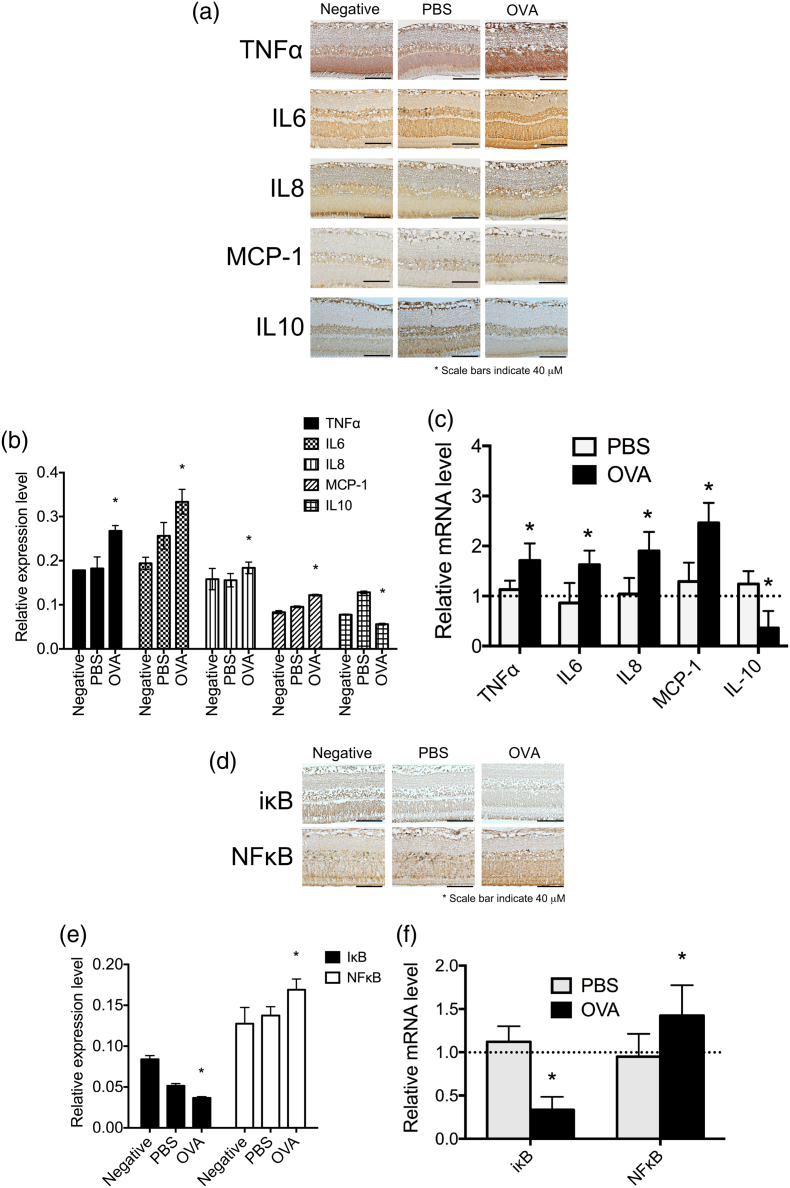
To validate that the OVA-treated eye was in a state of inflammation, several inflammatory-related cytokines were measured. The expression levels of pro-inflammatory cytokines TNF-α, IL-6, IL-8, and MCP-1 were up-regulated in AC eyes, whereas the expression levels of anti-inflammatory cytokines and IL-10 were down-regulated (Fig. 7a, b, and Supplementary Fig. 2). The mRNA expression levels of TNF-α, IL-6, IL-8, and MCP-1 in retinas were higher whereas IL-10 was lower in OVA-treated eyes compared to the negative groups (Fig. 7c). To further confirm the signaling pathway of allergic inflammation in myopia progression, the levels of NFκB and IκB were determined by IHC. The expression level of NFκB was more up-regulated in AC eyes than in control eyes, whereas the expression level of IκB was down-regulated (Fig. 7d, e, and Supplementary Fig. 3). The mRNA expression levels of NFκB in retinas were higher whereas IκB was lower in OVA-treated eyes compared to the negative groups (Fig. 7f). Taken together, these results indicate that allergic inflammation causes accelerated myopia (See Fig. 8).
Fig. 7.
Expression levels of inflammation-related transcription factors and cytokines in allergic conjunctivitis eyes. (a) Immunohistochemical analyses of TNF-α, IL-6, IL-8, MCP-1, and IL-10 expressions in negative, PBS-treated, and OVA-treated eyes. (b) The relative expression levels of TNF-α, IL-6, IL-8, MCP-1, and IL-10 were determined with the Image J software. (c) mRNA expression levels of TNF-α, IL-6, IL-8, MCP-1, and IL-10 in the retinas of the PBS and OVA groups were compared to those in the negative group. ANOVA was used to determine significant differences (p < 0.0001) and Sidak's multiple comparisons test was used for paired comparisons between PBS- and OVA-treated eyes. P < 0.05 was considered statistically significant. *: indicates a significant difference. (d) Immunohistochemical analyses of NFκB and IκB expressions in negative, PBS-treated, and OVA-treated eyes. (e) The relative expression levels of NFκB and IκB were determined with the Image J software. (f) mRNA expression levels of NFκB and IκB in the retinas of the PBS and OVA groups were compared to those in the negative group. ANOVA was used to determine significant differences (p < 0.0001) and Sidak's multiple comparisons test was used for paired comparisons between PBS- and OVA-treated eyes. P < 0.05 was considered statistically significant. *: indicates a significant difference.
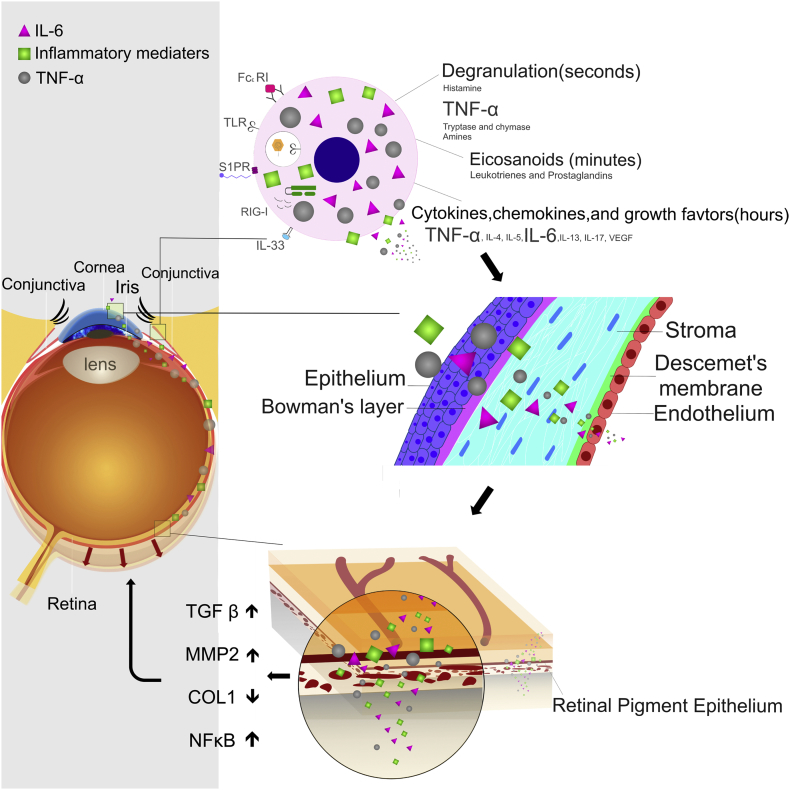
Fig. 8.
Schematic presentation of how allergic conjunctivitis inflammation modulates the signaling pathway to promote myopia.
4. Discussion
This is a population-based study describing the relationship between allergic diseases and myopia. In the case-control study, there was an increasing trend of an association for atopic dermatitis, allergic rhinitis, and AC, with adjusted ORs (95% CI) of 1.06 (1.04–1.09), 1.32 (1.30–1.34), and 1.75 (1.72–1.77), respectively. AC and allergic rhinitis had higher ORs than other allergic diseases, but AC had the highest association with myopia among all the allergic diseases investigated. Furthermore, patients with more than one kind of allergic disease were more prone to myopia (Supplementary Table 1). From our clinical experience, the association of allergic nasal and ocular symptoms (rhinoconjunctivitis) is common. Most children with AC have allergic rhinitis. Conjunctivitis symptoms are at least as severe as rhinitis symptoms in patients with hay fever and some have even generated the term of conjunctivorhinitis stressing the ocular symptoms.
Based on the high association between AC and myopia in the case-control study, we subsequently conducted a cohort study to examine the incidence rates and relative risk of myopia in the AC cohort compared to the non-AC cohort. Overall, the incidence of myopia was 2.35-fold greater in the AC cohort than in the non-AC cohort at the end of the follow-up period. The increased AC to non-AC HRs of myopia was consistent, regardless of age, sex, and urbanization. Our data showed that the trends of ORs and relative risks were consistent. The risk of myopia was greatest 1–2 years after the diagnosis of AC. In addition, children with AC who had frequent AC-related medical visits had relatively higher risks of myopia development. Thus, physicians need to be vigilant for myopia when children are diagnosed with AC. Most cohort studies previously published have not investigated candidate risk factors and have struggled to explain the mechanisms involved. To ensure the strength of association between AC and the incidence of myopia, an AC animal model was established. This study shows that rats with AC were simultaneously observed to have lower refractive errors and longer axial lengths. The experimental findings in mice corroborate the epidemiological data that AC is a potential risk factor of myopia.
Acute inflammation of the sclera and choroid would lead to myopization. Long-term corticosteroid therapy, including sulfonamides and acetazolamide, may also cause myopia. On the contrary, myopia itself predisposed subjects to ocular inflammatory conditions, for example, multifocal choroiditis. All the cases mentioned above provide various examples that inflammation may be a cause or consequence of myopia. In our previous studies, we observed a higher incidence of myopia in patients with inflammatory diseases such as systemic lupus erythematosus (3.5%), uveitis (5.2%), or type 1 diabetes mellitus (7.9%) compared to patients without inflammatory diseases (P < 0.001). These findings provide experimental and clinical evidence that inflammation may potentially influence the development of myopia (Lin et al., 2016).
Allergic diseases are another promising route to investigate the relationship between inflammation and myopia. This is because both allergy and myopia are chronic diseases, have long-term progression, and there is a high prevalence of both diseases in Asia. Clinically, ocular allergy is one of the clinical symptoms experienced. Being constantly exposed to the environment renders the eye a common site of allergic inflammation, which usually occurs in the conjunctiva. AC is an IgE hypersensitivity reaction that is triggered when the ocular surface encounters environmental antigens such as pollen, animal dander, or other airborne antigens. Because it is often comorbid with rhinitis and asthma, AC is generally underdiagnosed. The prevalence of AC has been estimated to range from 15% to 40% of the population. Mast cells play a key role in inducing AC signs and symptoms. The binding of IgE with specific allergens (pollens, animal dander, or dust mite allergens) activates mast cells and induces degranulation. This causes the release of inflammatory mediators, histamines, proteases, prostaglandin D2, and leukotriene C4, which cause inflammation. Activated mast cells also express IL-6 and TNF-α inflammatory cytokines to initiate ocular inflammation.
Previous studies have shown that the levels of inflammatory cytokines, such as TNF-α, were higher in patients with AC in both the sera and tears (Oray and Toker, 2013, Leonardi et al., 2003, Pelikan, 2014). Moreover, in a rabbit AC animal model, the permeability of the blood-conjunctival and blood-aqueous barriers was increased (Lapalus et al., 1986). Accordingly, AC would also cause an increase in the expression levels of inflammatory cytokines intraocularly. In our study, we found that MCP-1, IL-6, IL-8, and TNF-α were higher in allergic eyes, whereas IL-10 was lower. Compared to the high myopic cataract (HMC) study, the expression of MCP-1 in the aqueous humor of patients with HMC was increased (Zhu et al., 2016). The cytokine expression profiles were similar to those in the current study. MCP-1 expression has been detected in vitreous samples and is thought to be related to the development of diabetic retinopathy (Matsumoto et al., 2002). TNF-α and IL-8 are also associated with inflammatory disorders in the eyes, including endotoxin-induced uveitis (de Vos et al., 1994, Verma et al., 1999). TNF-α increases the expression level of IL-6 (Kurokouchi et al., 1998). The expression of IL-8 can be up-regulated by TNF-α and IL-6, while IL-10 inhibits its expression (DeForge et al., 1993, Xie, 2001). The level of IL-8 is further increased in CPE cells treated with combined TNF-α and IL-6. We also found that CPE cells treated with IL-6 had increased expression of TNF-α. In a transwell study, we also found an increase in the levels of TNF-α and IL-6 in the basolateral site, which may have resulted from disruption of the tight junction or have been secreted into the basolateral site by CPE cells. Thus, a vicious cycle develops between continuously induced TNF-α and IL-6 inflammatory responses in the cornea that would affect the levels of TNF-α, IL-6, and IL-8 intraocularly, which would induce retinal inflammation and promote myopia progression. TNF-α activating NFκB would also increase the expression of MMP2, an important molecule in promoting tissue remodeling in the eye (Fig. 8).
The sclera is a dynamic tissue in the development of an organism. Ocular size and refraction were regulated by ECM composition and its biomechanical properties (Rada et al., 2006). In a genetic linkage study, genome-wide association study, and two-dimensional gel electrophoresis, many genes correlated with myopia were discovered with known or unknown functions. Among these candidate genes, those coding for a variety of ECM growth and remodeling pathways have repeatedly been mentioned. High myopia was characterized by scleral thinning and resulted from a reduction in COL-I, the major structural protein in the sclera (Inamori et al., 2007, Norton and Rada, 1995). The degradation of COL-I in the myopic eye was due to increased synthesis of matrix metalloproteinases (MMPs), such as the active form of MMP2 (Guggenheim and McBrien, 1996, Hall et al., 2009). Furthermore, MMP2 was induced by TGF-β through NFκB (Wang et al., 2011) and demonstrated higher levels of expression in high myopia (Lin et al., 2009, Lin et al., 2006). In this study, the allergic rats expressed higher MMP2 and TGF-β and lower COL-I. This was the same as the expression pattern in the myopic eye (Fig. 4). Together with the refractive error and axial length data, we have demonstrated that AC is a possible cause of the induction of myopia in an animal model. To sum up, the up-regulation of pro-inflammatory cytokines and down-regulation of anti-inflammatory cytokines indicates a pro-inflammatory state in the allergic eye, which could then influence the progression of myopia.
It has been shown that AC patients with a specific IgE to indoor antigens exhibit higher myopia than individuals without allergies (Mimura et al., 2009a). Myopia usually begins at an age between 6 and 12 years and may develop/worsen quickly during the teenage years. Some teenagers have to change glasses within only a few months. The progression of myopia generally stops by age 20. Mimura et al. found that patients with SAC were more myopic than control individuals. The average age was 47.5 ± 20.2 years for SAC patients and 51.4 ± 22.4 years for control individuals (Mimura et al., 2009b). The study included subjects over 20 years of age, which indicates that they were in the stable stage of myopia development. Therefore, this study can only conclude that myopia may increase the risk of AC, but not that AC is a risk factor for myopia. Since we have no information on the age at which the patients began to demonstrate AC, it is difficult to determine the most important risk factor. Thus, while these studies revealed a correlation between AC and myopia, they did not clearly demonstrate a cause-effect relationship. In this study, we used an animal model of AC to support the viewpoint that patients with AC were prone to myopia, but not the contrary.
Genetic epidemiology data have revealed that myopia is associated with both environmental and genetic factors. Genome-wide association studies (GWAS) have identified single nucleotide polymorphisms associated with myopia (Solouki et al., 2010, Nakanishi et al., 2009, Shi et al., 2011, Kiefer et al., 2013). We found several genes linked with inflammation as well as immune-privileged pathways: e.g., the 70-kD membrane protein CD55 inhibits the C3 convertase step to inhibit complement activation, thereby protecting against complement mediated tissue damage. CD55 is important in modulating autoimmune diseases and inflammation. In immune-privileged tissues, such as the placenta, cornea, and testis, CD55 is overexpressed and lowers inflammatory responses in these tissues (Streilein, 2003, Sohn et al., 2007).
Activation of the complement system is strongly regulated in the human body to avoid overstimulation and damage resulting from inflammation. The complement system is dysregulated in several eye diseases, including glaucoma, autoimmune uveitis, diabetic retinopathy, and age-related macular degeneration (Clark and Bishop, 2017). The complement system is also involved in the pathogenesis of myopia. Expression levels of C3 (P = 0.004) and CH50 (P < 0.001) are significantly increased in patients with pathologic myopia (− 8 D to − 25 D) (Long et al., 2013). The levels of C1q, C3, and C5b-9 are significantly upregulated in the sclera of guinea pigs with negative lens-defocused myopia (Gao et al., 2015). Activation of the complement system may promote remodeling of the extracellular matrix and development of myopia (Gao et al., 2015). In a meta-analysis of eight transcriptome databases for lens-induced or form-deprivation myopia, the authors found significant activation of the complement system in chick models of myopia (Riddell and Crewther, 2017). It has also been noted that the eyes are a hotspot for complement dysregulation (Riddell and Crewther, 2017, Skeie and Mahajan, 2014). Accordingly, complement-activation induced inflammation may play a part in the pathogenesis of myopia.
In addition to genetic factors, environmental risk factors are known to play significant roles in the development of myopia. Education or near work activities has been considered a risk factor for which the total amount of time of reading and the duration of continuous reading have been shown to correlate to myopia (Morgan et al., 2018, Li et al., 2015, Goss, 2000). Asthenopia induced by extended use of eye may cause symptoms such as photophobia, burning sensation, ophthalmalgia, strain, hyperemia, dryness, headache, and blurred vision (Sheedy et al., 2003, Zhao et al., 2017). These symptoms might be associated with mild inflammation in the eye. There is still lack of evidence that asthenopia-induced/associated inflammation is associated with myopia progression. The recent gene/environment interaction has provided a potential link to inflammation. In a meta-analysis on the single nucleotide polymorphism v.s. education interaction effects on refractive error among 40,036 adults from European ancestry and 10,315 individuals of Asian ancestry, three different genetic loci, namely amphiregulin (AREG), gamma-aminobutyric acid (GABA) C receptor p1 (GABRR1) and phosphodiesterase 10A (PDE10A) were found to have strong interactions with education in Asian populations but not in European populations. The results indicate that the complex interactions between environment and gene lead to the diversity of myopia incidence (Fan et al., 2016). AREG is a low-affinity ligand of the epidermal growth factor receptor and is known to regulate inflammation through the interaction with regulatory T cells (Zaiss et al., 2015, Lu et al., 2006, Zaiss et al., 2013). Moreover, neutralizing antibody against AREG inhibits myopia in guinea pigs (Jiang et al., 2017). The GABAergic system inhibits the T cell-mediated immunity through modulating the expression of proinflammatory cytokines as well as the activation of NF-κB pathway (Wu et al., 2017). Inhibiting GABAc receptor reduces the IL-1β induced biphasic effect on rat major pelvic ganglia neurons (Akasu and Tsurusaki, 1999). Treating R264.7 macrophages with PDE10A inhibitors decreases the production of nitrite activated by lipopolysaccharide administration (Garcia et al., 2017). The environment stress initiated by education may eventually alter these gene expression to promote the development of myopia in Asian populations.
Recent evidence suggests that time spent outdoors is an important protective factor of myopia (Rose et al., 2016, Wu et al., 2016). The protective effect may be due to high light intensity outdoors, the chromaticity of daylight or increased vitamin D levels. Based on the findings from animal models and epidemiological studies, it is hypothesized that high light levels outdoors or rapid luminance changes trigger the release of dopamine. It has been shown that retinal dopamine is an important regulator of normal eye growth (mostly as a stop signal) and functions in the development of myopia (Bergen et al., 2016, Stone et al., 1989). Dopamine is also important for modulating the immune responses and lowering inflammation that connect the nervous and immune systems (Basu and Dasgupta, 2000, Beck et al., 2004, Sarkar et al., 2010). Dopamine modulates immune response by binding to the dopaminergic D1 (D1/D5) and D2 (D2/D3/D4) receptors. By binding to D1 receptors, dopamine activates adenylate cyclase to increase the levels of cAMP, which then activates protein kinase A (PKA) and cAMP-responsive element binding protein (CREB). Activated PKA and CREB inhibit the activation of NFκB, which reduce the levels of IL-6, IL-8, MCP-1, and TNF-α (Beck et al., 2004, Platzer et al., 2000, Bacic et al., 1991, Neumann et al., 1995, Abraham et al., 2001). Activation of D2 receptors can also promote cAMP production to inhibit NFκB activation (Yang et al., 2003). Therefore, longer outdoor activities might increase the level of dopamine to lower inflammation in the eye and inhibit myopia progression. Moreover, increased blood vitamin D levels secondary to daylight exposure are inversely related to myopia (Mutti and Marks, 2011, Yazar et al., 2014). Many studies reported that insufficient vitamin D intake is association with childhood atopy and food allergies (Bozzetto et al., 2012). Therefore, the underlying myopia protective effect of time spent outdoors may be partly due to reducing eye inflammation and allergic symptoms from increasing dopamine and vitamin D level.
Several limitations should be noted and the results should be interpreted with caution. First, the NHIRD does not provide detailed information on patients such as time spent outdoors, time spent near work, parental smoking, birth season and post-natal light levels, birth order, and maternal age, all of which have been associated with myopia. Second, the clinical details and laboratory data are not available in the NHIRD, which means that IgE data were not available for our study. To ensure the validity of disease diagnosis, we used the previously reported ICD-9-CM codes to identify children with allergic disease to compare to other surveys based on the questionnaire designed by the International Study of Asthma and Allergies in Childhood (ISAAC), which show similar prevalence rates of allergic disorders for children in Taiwan (Zaiss et al., 2015). Third, the prevalence of AC may also be underestimated, particularly in children with mild symptoms of AC, and those with AC treated with over-the-counter medications or eye drops. However, both myopia and non-myopia cohorts have underestimated AC, and this random error can be overcome and minimized by a large sample size. Fourth, the current study was conducted in a specific population, and the correlation between AC and myopia in other countries requires further investigation to determine if the incidence rate could be referenced in other populations. Fifth, there are no data on diopter and axial length in the NHRID. Therefore, we were unable to evaluate the development of refractive error over time. A clinical trial to track the effect of AC on the progression of refractive error requires further investigation. Sixth, in Taiwan, most children checked their refractive error at the ophthalmologic department after cycloplegia. Therefore, most of the transient index myopia could be prevented. However, we could not exclude the condition completely. Readers should take this issue into consideration when they interpret the results.
In conclusion, AC-induced retinal inflammation may promote myopia progression. From epidemiological observations, children with AC had a higher risk for myopia. In an animal model, ocular surface inflammation resulting from mast cell degranulation, altered the tight junction of the cornea, initiated the secretion of inflammatory cytokines in the cornea, and subsequently caused retinal inflammation, which in turn promoted myopia progression (Fig. 8). Inflammation in the etiopathogenesis of myopia remains under-estimated. In this study, through a population-based study and an animal model, we demonstrated a different mechanism of the development of myopia from previous research. Future epidemiologic studies in different geographic regions and experimental studies will be needed to validate the findings of this study and to provide a more comprehensive understanding of the underlying mechanisms of myopia.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Author Contribution
Conceived and designed the experiments: LW and HJL. NHRID data retrieval and analysis: HYC and HJC. Performed the experiments: CCW, YJK, CSC, CYC, CJL and PTT. Analyzed the data: YSH, CCW, LW, and HJL. Interpreted the data: CCW, LW and HJL. Wrote the paper: HJL, CCW, and LW.
Acknowledgements
This study was supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW106-TDU-B-212-113004), China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM10601010036), NRPB Stroke Clinical Trial Consortium (MOST 106-2321-B-039-005), Ministry of Science and Technology, Taiwan, R.O.C. (MOST103-2314-B-039-035-MY3 and MOST105-2628-B-039-008-MY3), China Medical University Hospital, Taichung, Taiwan (DMR-106-149), and China Medical University, Taichung, Taiwan (CMU106-ASIA-24), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, Katsuzo and Kiyo Aoshima Memorial Funds, Japan, and the Bureau of Health Promotion, Department of Health, R.O.C. (Taiwan) (DOH99-HP-1205). This work was also supported by CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.01.024.
Contributor Information
Hui-Ju Lin, Email: d2396@mail.cmuh.org.tw.
Lei Wan, Email: leiwan@mail.cmu.edu.tw.
Appendix A. Supplementary data
Supplementary material
References
- Abraham E., Arcaroli J., Shenkar R. Activation of extracellular signal-regulated kinases, NF-kappa B, and cyclic adenosine 5′-monophosphate response element-binding protein in lung neutrophils occurs by differing mechanisms after hemorrhage or endotoxemia. J. Immunol. 2001;166(1):522–530. doi: 10.4049/jimmunol.166.1.522. [DOI] [PubMed] [Google Scholar]
- Akasu T., Tsurusaki M. Interleukin-1beta causes a biphasic response in neurons of rat major pelvic ganglia. Neurosci. Lett. 1999;272(2):119–122. doi: 10.1016/s0304-3940(99)00583-2. [DOI] [PubMed] [Google Scholar]
- Bacic F., Uematsu S., McCarron R.M., Spatz M. Dopaminergic receptors linked to adenylate cyclase in human cerebromicrovascular endothelium. J. Neurochem. 1991;57(5):1774–1780. doi: 10.1111/j.1471-4159.1991.tb06380.x. [DOI] [PubMed] [Google Scholar]
- Basu S., Dasgupta P.S. Dopamine, a neurotransmitter, influences the immune system. J. Neuroimmunol. 2000;102(2):113–124. doi: 10.1016/s0165-5728(99)00176-9. [DOI] [PubMed] [Google Scholar]
- Beck G., Brinkkoetter P., Hanusch C. Clinical review: immunomodulatory effects of dopamine in general inflammation. Crit. Care. 2004;8(6):485–491. doi: 10.1186/cc2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen M.A., Park H.N., Chakraborty R. Altered refractive development in mice with reduced levels of retinal dopamine. Invest. Ophthalmol. Vis. Sci. 2016;57(10):4412–4419. doi: 10.1167/iovs.15-17784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzetto S., Carraro S., Giordano G., Boner A., Baraldi E. Asthma, allergy and respiratory infections: the vitamin D hypothesis. Allergy. 2012;67(1):10–17. doi: 10.1111/j.1398-9995.2011.02711.x. [DOI] [PubMed] [Google Scholar]
- Clark S.J., Bishop P.N. The eye as a complement dysregulation hotspot. Semin. Immunopathol. 2017 doi: 10.1007/s00281-017-0649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova C., Gutierrez B., Martinez-Garcia C. Oleanolic acid controls allergic and inflammatory responses in experimental allergic conjunctivitis. PLoS One. 2014;9(4):e91282. doi: 10.1371/journal.pone.0091282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin Brian J. vol. xv. Harper & Row; Philadelphia: 1985. The Myopias: Basic Science and Clinical Management; p. 495. [Google Scholar]
- de Vos A.F., Klaren V.N., Kijlstra A. Expression of multiple cytokines and IL-1RA in the uvea and retina during endotoxin-induced uveitis in the rat. Invest. Ophthalmol. Vis. Sci. 1994;35(11):3873–3883. [PubMed] [Google Scholar]
- DeForge L.E., Preston A.M., Takeuchi E., Kenney J., Boxer L.A., Remick D.G. Regulation of interleukin 8 gene expression by oxidant stress. J. Biol. Chem. 1993;268(34):25568–25576. [PubMed] [Google Scholar]
- Fan Q., Verhoeven V.J., Wojciechowski R. Meta-analysis of gene-environment-wide association scans accounting for education level identifies additional loci for refractive error. Nat. Commun. 2016;7 doi: 10.1038/ncomms11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T.T., Long Q., Yang X. Complement factors C1q, C3 and C5b-9 in the posterior sclera of guinea pigs with negative lens-defocused myopia. Int. J. Ophthalmol. 2015;8(4):675–680. doi: 10.3980/j.issn.2222-3959.2015.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A.M., Brea J., Gonzalez-Garcia A. Targeting PDE10A GAF domain with small molecules: a way for allosteric modulation with anti-inflammatory effects. Molecules. 2017;22(9) doi: 10.3390/molecules22091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss D.A. Nearwork and myopia. Lancet. 2000;356(9240):1456–1457. doi: 10.1016/S0140-6736(00)02864-6. [DOI] [PubMed] [Google Scholar]
- Guggenheim J.A., McBrien N.A. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest. Ophthalmol. Vis. Sci. 1996;37(7):1380–1395. [PubMed] [Google Scholar]
- Hall N.F., Gale C.R., Ye S., Martyn C.N. Myopia and polymorphisms in genes for matrix metalloproteinases. Invest. Ophthalmol. Vis. Sci. 2009;50(6):2632–2636. doi: 10.1167/iovs.08-2427. [DOI] [PubMed] [Google Scholar]
- Harper A.R., Summers J.A. The dynamic sclera: extracellular matrix remodeling in normal ocular growth and myopia development. Exp. Eye Res. 2015;133:100–111. doi: 10.1016/j.exer.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbort C.P., Papadia M., Neri P. Myopia and inflammation. J. Ophthal. Vision Res. 2011;6(4):270–283. [PMC free article] [PubMed] [Google Scholar]
- Hornbeak D.M., Young T.L. Myopia genetics: a review of current research and emerging trends. Curr. Opin. Ophthalmol. 2009;20(5):356–362. doi: 10.1097/ICU.0b013e32832f8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamori Y., Ota M., Inoko H. The COL1A1 gene and high myopia susceptibility in Japanese. Hum. Genet. 2007;122(2):151–157. doi: 10.1007/s00439-007-0388-1. [DOI] [PubMed] [Google Scholar]
- Jiang W.J., Song H.X., Li S.Y. Amphiregulin antibody and reduction of axial elongation in experimental myopia. EBioMedicine. 2017;17:134–144. doi: 10.1016/j.ebiom.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer A.K., Tung J.Y., Do C.B. Genome-wide analysis points to roles for extracellular matrix remodeling, the visual cycle, and neuronal development in myopia. PLoS Genet. 2013;9(2) doi: 10.1371/journal.pgen.1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung Y.J., Wei C.C., Chen L.A. Kawasaki disease increases the incidence of myopia. Biomed. Res. Int. 2017;2017:2657913. doi: 10.1155/2017/2657913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokouchi K., Kambe F., Yasukawa K. TNF-alpha increases expression of IL-6 and ICAM-1 genes through activation of NF-kappaB in osteoblast-like ROS17/2.8 cells. J. Bone Miner. Res. 1998;13(8):1290–1299. doi: 10.1359/jbmr.1998.13.8.1290. [DOI] [PubMed] [Google Scholar]
- Lapalus P., Moulin G., Bayer V., Fredj-Reygrobellet D., Elena P.P. Effects of a new anti-allergic agent: the magnesium salt of N-acetyl-aspartyl-glutamic acid on experimental allergic inflammation of the rabbit eye. Curr. Eye Res. 1986;5(7):517–522. doi: 10.3109/02713688608996374. [DOI] [PubMed] [Google Scholar]
- Leo S.W., Young T.L. An evidence-based update on myopia and interventions to retard its progression. Am. Assoc. Pediatric Ophthalmol. Strabismus. 2011;15(2):181–189. doi: 10.1016/j.jaapos.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi A., Brun P., Tavolato M., Plebani M., Abatangelo G., Secchi A.G. Tumor necrosis factor-alpha (TNF-alpha) in seasonal allergic conjunctivitis and vernal keratoconjunctivitis. Eur. J. Ophthalmol. 2003;13(7):606–610. doi: 10.1177/112067210301300702. [DOI] [PubMed] [Google Scholar]
- Li S.M., Li S.Y., Kang M.T. Near work related parameters and myopia in Chinese children: the Anyang Childhood Eye Study. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0134514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.L., Shih Y.F., Hsiao C.K., Chen C.J., Lee L.A., Hung P.T. Epidemiologic study of the prevalence and severity of myopia among schoolchildren in Taiwan in 2000. J. Formos. Med. Assoc. 2001;100(10):684–691. [PubMed] [Google Scholar]
- Lin H.J., Wan L., Tsai Y. The TGFbeta1 gene codon 10 polymorphism contributes to the genetic predisposition to high myopia. Mol. Vis. 2006;12:698–703. [PubMed] [Google Scholar]
- Lin H.J., Wan L., Tsai Y. Sclera-related gene polymorphisms in high myopia. Mol. Vis. 2009;15:1655–1663. [PMC free article] [PubMed] [Google Scholar]
- Lin H.J., Wei C.C., Chang C.Y. Role of chronic inflammation in myopia progression: clinical evidence and experimental validation. EBioMedicine. 2016;10:269–281. doi: 10.1016/j.ebiom.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q., Ye J., Li Y., Wang S., Jiang Y. C-reactive protein and complement components in patients with pathological myopia. Optom. Vis. Sci. 2013;90(5):501–506. doi: 10.1097/OPX.0b013e31828daa6e. [DOI] [PubMed] [Google Scholar]
- Lu L.F., Lind E.F., Gondek D.C. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442(7106):997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Takahashi M., Ogata M. Relationship between glycoxidation and cytokines in the vitreous of eyes with diabetic retinopathy. Jpn. J. Ophthalmol. 2002;46(4):406–412. doi: 10.1016/s0021-5155(02)00508-7. [DOI] [PubMed] [Google Scholar]
- Mimura T., Yamagami S., Usui T. Relationship between myopia and allergen-specific serum IgE levels in patients with allergic conjunctivitis. Clin. Exp. Ophthalmol. 2009;37(7):670–677. doi: 10.1111/j.1442-9071.2009.02130.x. [DOI] [PubMed] [Google Scholar]
- Mimura T., Mimura Y., Arimoto A. Relationship between refraction and allergic conjunctivitis. Eye (Lond) 2009;23(1):63–66. doi: 10.1038/sj.eye.6702999. [DOI] [PubMed] [Google Scholar]
- Morgan I.G., Ohno-Matsui K., Saw S.M. Myopia. Lancet. 2012;379(9827):1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- Morgan I.G., French A.N., Ashby R.S. The epidemics of myopia: aetiology and prevention. Prog. Retin. Eye Res. 2018;62:134–149. doi: 10.1016/j.preteyeres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Mutti D.O., Marks A.R. Blood levels of vitamin D in teens and young adults with myopia. Optom. Vis. Sci. 2011;88(3):377–382. doi: 10.1097/OPX.0b013e31820b0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H., Yamada R., Gotoh N. A genome-wide association analysis identified a novel susceptible locus for pathological myopia at 11q24.1. PLoS Genet. 2009;5(9) doi: 10.1371/journal.pgen.1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M., Grieshammer T., Chuvpilo S. RelA/p65 is a molecular target for the immunosuppressive action of protein kinase A. EMBO J. 1995;14(9):1991–2004. doi: 10.1002/j.1460-2075.1995.tb07191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton T.T., Rada J.A. Reduced extracellular matrix in mammalian sclera with induced myopia. Vis. Res. 1995;35(9):1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- Oray M., Toker E. Tear cytokine levels in vernal keratoconjunctivitis: the effect of topical 0.05% cyclosporine a therapy. Cornea. 2013;32(8):1149–1154. doi: 10.1097/ICO.0b013e31828ffdf8. [DOI] [PubMed] [Google Scholar]
- Pelikan Z. Cytokines in tears during the secondary keratoconjunctival responses induced by allergic reaction in the nasal mucosa. Ophthalmic Res. 2014;52(1):32–42. doi: 10.1159/000358200. [DOI] [PubMed] [Google Scholar]
- Platzer C., Docke W., Volk H., Prosch S. Catecholamines trigger IL-10 release in acute systemic stress reaction by direct stimulation of its promoter/enhancer activity in monocytic cells. J. Neuroimmunol. 2000;105(1):31–38. doi: 10.1016/s0165-5728(00)00205-8. [DOI] [PubMed] [Google Scholar]
- Rada J.A., Shelton S., Norton T.T. The sclera and myopia. Exp. Eye Res. 2006;82(2):185–200. doi: 10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Riddell N., Crewther S.G. Novel evidence for complement system activation in chick myopia and hyperopia models: a meta-analysis of transcriptome datasets. Sci. Rep. 2017;7(1):9719. doi: 10.1038/s41598-017-10277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K.A., French A.N., Morgan I.G. Environmental factors and myopia: paradoxes and prospects for prevention. Asia Pac. J. Ophthalmol. (Phila) 2016;5(6):403–410. doi: 10.1097/APO.0000000000000233. [DOI] [PubMed] [Google Scholar]
- Sarkar C., Basu B., Chakroborty D., Dasgupta P.S., Basu S. The immunoregulatory role of dopamine: an update. Brain Behav. Immun. 2010;24(4):525–528. doi: 10.1016/j.bbi.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw S.M., Gazzard G., Shih-Yen E.C., Chua W.H. Myopia and associated pathological complications. Ophthalmic Physiol. Opt. 2005;25(5):381–391. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy J.E., Hayes J.N., Engle J. Is all asthenopia the same? Optom. Vis. Sci. 2003;80(11):732–739. doi: 10.1097/00006324-200311000-00008. [DOI] [PubMed] [Google Scholar]
- Shi Y., Qu J., Zhang D. Genetic variants at 13q12.12 are associated with high myopia in the Han Chinese population. Am. J. Hum. Genet. 2011;88(6):805–813. doi: 10.1016/j.ajhg.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeie J.M., Mahajan V.B. Proteomic landscape of the human choroid-retinal pigment epithelial complex. JAMA Ophthalmol. 2014;132(11):1271–1281. doi: 10.1001/jamaophthalmol.2014.2065. [DOI] [PubMed] [Google Scholar]
- Sohn J.H., Bora P.S., Jha P., Tezel T.H., Kaplan H.J., Bora N.S. Complement, innate immunity and ocular disease. Chem. Immunol. Allergy. 2007;92:105–114. doi: 10.1159/000099261. [DOI] [PubMed] [Google Scholar]
- Solouki A.M., Verhoeven V.J., van Duijn C.M. A genome-wide association study identifies a susceptibility locus for refractive errors and myopia at 15q14. Nat. Genet. 2010;42(10):897–901. doi: 10.1038/ng.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone R.A., Lin T., Laties A.M., Iuvone P.M. Retinal dopamine and form-deprivation myopia. Proc. Natl. Acad. Sci. U. S. A. 1989;86(2):704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein J.W. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 2003;3(11):879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- Verma M.J., Mukaida N., Vollmer-Conna U., Matsushima K., Lloyd A., Wakefield D. Endotoxin-induced uveitis is partially inhibited by anti-IL-8 antibody treatment. Invest. Ophthalmol. Vis. Sci. 1999;40(11):2465–2470. [PubMed] [Google Scholar]
- Wang Y., Tang Z., Xue R. TGF-beta1 promoted MMP-2 mediated wound healing of anterior cruciate ligament fibroblasts through NF-kappaB. Connect. Tissue Res. 2011;52(3):218–225. doi: 10.3109/03008207.2010.516849. [DOI] [PubMed] [Google Scholar]
- Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clin. Genet. 2011;79(4):301–320. doi: 10.1111/j.1399-0004.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P.C., Huang H.M., Yu H.J., Fang P.C., Chen C.T. Epidemiology of myopia. Asia Pac. J. Ophthalmol. (Phila) 2016;5(6):386–393. doi: 10.1097/APO.0000000000000236. [DOI] [PubMed] [Google Scholar]
- Wu C., Qin X., Du H., Li N., Ren W., Peng Y. The immunological function of GABAergic system. Front. Biosci. (Landmark Ed) 2017;22:1162–1172. doi: 10.2741/4539. [DOI] [PubMed] [Google Scholar]
- Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12(4):375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- Yang M., Zhang H., Voyno-Yasenetskaya T., Ye R.D. Requirement of Gbetagamma and c-Src in D2 dopamine receptor-mediated nuclear factor-kappaB activation. Mol. Pharmacol. 2003;64(2):447–455. doi: 10.1124/mol.64.2.447. [DOI] [PubMed] [Google Scholar]
- Yazar S., Hewitt A.W., Black L.J. Myopia is associated with lower vitamin D status in young adults. Invest. Ophthalmol. Vis. Sci. 2014;55(7):4552–4559. doi: 10.1167/iovs.14-14589. [DOI] [PubMed] [Google Scholar]
- Zaiss D.M., van Loosdregt J., Gorlani A. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38(2):275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss D.M.W., Gause W.C., Osborne L.C., Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42(2):216–226. doi: 10.1016/j.immuni.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.L., Jiang J., Yu J., Xu H.M. Role of short-wavelength filtering lenses in delaying myopia progression and amelioration of asthenopia in juveniles. Int. J. Ophthalmol. 2017;10(8):1261–1267. doi: 10.18240/ijo.2017.08.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Zhang K., He W. Proinflammatory status in the aqueous humor of high myopic cataract eyes. Exp. Eye Res. 2016;142:13–18. doi: 10.1016/j.exer.2015.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material