Abstract
Ischemia-reperfusion injury (IRI) during renal transplantation often initiates non-specific inflammatory responses that can result in the loss of kidney graft viability. However, the long-term consequence of IRI on renal grafts survival is uncertain. Here we review clinical evidence and laboratory studies, and elucidate the association between early IRI and later graft loss. Our critical analysis of previous publications indicates that early IRI does contribute to later graft loss through reduction of renal functional mass, graft vascular injury, and chronic hypoxia, as well as subsequent fibrosis. IRI is also known to induce kidney allograft dysfunction and acute rejection, reducing graft survival. Therefore, attempts have been made to substitute traditional preserving solutions with novel agents, yielding promising results.
Abbreviation: IRI, ischemia-reperfusion injury; MMP, matrix metalloproteinases; DAMP, Damage associated molecular pattern; MHC, major histocompatibility complex; IL-1, Interleukine-1
Keywords: Ischemia-reperfusion, Graft survival, Renal transplantation, Acute rejection, Th cells: T helper cells
Highlights
-
•
Ischaemia reperfusion injury (IRI) potentiates delayed renal graft function and causes reduction in renal graft survival
-
•
IRI causes innate immune system activation, hypoxic injury, inflammation and graft vascular disease
-
•
Reducing prolonged cold ischaemic time improves graft survival
-
•
Novel protective strategies include mesenchymal stem cells, machine perfusion, and ex vivo preservation solution saturated with gas.
-
•
Further studies are needed to investigate the long-term effects of novel ex vivo preservation agents
1. Introduction
Renal grafts inevitably experience ischemia from the moment they are separated from the donor blood supply. The insult begins following a period of transient surgical warm ischemia during donor organ extraction, followed by a lengthy cold ischemic period in hypothermal preserving solution before ending with warm ischemia during implantation in the recipient. After revascularization, blood flow in post-ischemic kidneys activates a sequence of events that aggravates renal injury. This pathological phenomenon is described as ischemia-reperfusion injury (IRI) and contributes to a high rate of morbidity. The severity of renal insult correlates strongly with early renal graft failure. However, most transplant patients completely recover from the initial IRI period. Clinical evidence demonstrates that transplanted kidneys with prolonged ischemic time are more susceptible to long-term deterioration. We review clinical studies, in order to elucidate the association between early IRI and later graft loss, and discuss important concepts relating to the pathology and prevention of graft dysfunction.
2. Survival of the Renal Grafts
With the introduction of potent immunosuppressive drugs such as calcineurin inhibitors, acute rejection rates have fallen dramatically, whilst allograft survival rates have risen steadily. Hariharan et al. (2000) analyzed graft survival for 93,934 renal transplantations performed in the USA between 1988 and 1996 and reported a marked improvement in the long-term survival of renal grafts from both living and cadaveric donors. Over this period, the half-life of living donor grafts increased from 12.7 to 21.6 years, and the half-life of cadaveric grafts increased 7.9 to 13.8 years. These significant improvements in graft survival have been attributed to refinement in kidney preservation and improvements in peri-operative care and immunosuppressive medication.
Despite the continuous progress in immunosuppressive and supportive therapy, improvement in graft survival has reached a plateau. The majority of grafts eventually develop chronic dysfunction which limits long-term graft survival (Chapman et al., 2005). Histologically, chronic failure is characterized by intimal thickening of arteries, glomerulosclerosis, tubular interstitial fibrosis, and tubular atrophy. Renal functional impairment is often found in combination with proteinuria and aggravation of de novo hypertension. These histological changes are thought to be the end result of cumulative damage to renal grafts associated with both immune and non-immune factors, however the precise etiological factors underlying these changes remain to be elucidated (Nankivell and Chapman, 2006). Renal graft IRI may be one of the critical factors contributing to deterioration in long-term graft survival.
3. Impact of IRI on Renal Graft Survival: Clinical Evidence
3.1. Ischemia Reperfusion Injury and Marginal Donor Organs
There are three main types of kidney donors: donation after brain death (DBD) donors, donation after cardiac death (DCD) donors and living donors. Another donor source is expanded criteria donation (ECD), which includes DCD donors and those with particular co-morbidites such as arterial hypertension or an age > 60 years (Iordanous et al., 2009).
DCD donors are commonly patients who have been unsuccessfully resuscitated or are awaiting cardiac death (Doyle et al., 2015). The commonest type of organ transplantation in the UK is donation after brain death (DBD), which is characterized by total and irreversible loss of brain function (Robey and Marcolini, 2013). DBD is generally preferred over DCD because the graft is perfused until the point of organ retrieval. Whilst organs from DCD donors are subject to prolonged warm ischaemic times, they may have a theoretic advantage. In DBD donors, also known as heart-beating brain dead donors, brain death results in a systemic inflammatory ‘cytokine storm’ (Vergoulas et al., 2009). The body responds by increasing the release of circulating catecholamines, resulting in a subsequent autonomic storm. This can precipitate pulmonary oedema, hypertension, severe myocardial damage and microvascular and parenchymal damage to the renal graft. Following this period of intense autonomic activity, there is a dramatic fall in circulating catecholamines, resulting in vasodilation, bradycardia and tissue hypoxia. Whilst DCD donors are also susceptible to this inflammatory response, due to the rapidity of neuronal damage this is often to a smaller extent than DBD donors (Mckeown et al., 2012). Due to a lack of donors and the prevalence of graft failure, there has been an increased use of marginal donors, such as DCD donors. Therefore, it is important to understand if there is a distinction between the vulnerability of DCD and DBD grafts to ischemia and reperfusion.
Summers et al. (2010) performed a retrospective analysis of the UK Transplant Registry in order to assess the factors that affect outcomes following kidney transplantation of 9134 deceased donor kidney transplants performed between January 1, 2000 and December 31, 2007 (Summers et al., 2010). 8289 (90.7%) were from DBD donors, and the remaining 845 transplants (9.3%) were extracted from donation after cardiac death (DCD) donors. The cohorts demonstrated no significant difference in 5-year kidney graft survival (76.4% DBD vs 76.2% DCD) or primary non-function rates (3% each). However, the incidence of delayed graft function (DGF: 24% DBD vs 49% DCD, P < 0.0001) was significantly higher in DCD grafts. DGF is a type of acute renal failure that causes post-transplantation oliguria, increased allograft immunogenicity and also increases the risk of acute rejection episodes (Gueler et al., 2015). Similarly, Gagandeep et al. also undertook an analysis of clinical outcomes but from US national data (Gagandeep et al., 2006). The authors reported that both allograft and recipient survival were similar between DCD and DBD cohorts. However, the risk of delayed graft function (DGF) was found to be 42 to 51% in DCD recipients in comparison to 24% in DBD grafts. Likewise, Doshi and Hunsicker also found no significant difference in 5-year patient survival (DCD vs. DBD 81.3 vs. 81.8%; P = 0.70) or allograft survival rates (DCD vs. DBD, 66.9 vs. 66.5%; P = 0.52). The risk of DGF, however, was higher in DCD grafts (DCD vs. DBD, 41 vs. 24%; P < 0.001). Therefore, studies have consistently demonstrated the increased prevalence of DGF in DCD kidney graft recipients. In the US, between 1985 and 1999, the rate of DGF was reported at 14.7% (Ojo et al., 1997). The incidence of DGF rose to 23% between 1998 and 2004 and was concurrent with the increased use of ECD (Tapiawala et al., 2010). DGF continues to be a major barrier for allograft survival and requires the compulsory return of the patient to dialysis.
Whilst both DCD and DBD grafts seem to possess similar rates of allograft survival and patient survival, higher rates of DGF in DCD organs may be due to these grafts being more susceptible to ischemia reperfusion injury (Gobe et al., 1999). Experimental studies have demonstrated that both ischemia and reperfusion in ischemically-damaged kidneys following prolonged periods of hypothermic preservation are involved in the development of delayed graft function (Koo et al., 1998). The generation of reactive oxygen species (ROS), release of inflammatory cytokines and adhesion and margination of leukocytes are all cellular events that result in renal injury and subsequent generation of DGF (Schroppel and Legendre, 2014).
3.2. Ischemia-Reperfusion Injury, Delayed Graft Function and Chronic Allograft Dysfunction
Delayed graft function (DGF) is defined as the failure of the transplanted kidney to function immediately, hence necessitating renal replacement therapy within the first week after surgery. Extended cold ischemia time is an independent risk factor for the development of delayed graft function (Quiroga et al., 2006). Prolonged cold ischemia promotes DGF and acute immune rejection which, in turn, can shorten long-term graft survival.
Studies investigating the effects of cold ischemia on delayed graft function (Table 1) have produced conflicting results when considering deceased, living and DCD donors. Earlier findings produced by Barba et al. (Barba et al., 2011) found that each hour of cold ischemia increased the risk of DGF in deceased donor grafts by 10%, however this relationship was only found beyond 18 h of cold ischemia. The authors found that, not only did cold ischemia under 18 h have little negative impact on DGF, but also had no negative affect on overall graft survival. These conclusions were evidenced by 5-year graft survival rates of 91% with cold ischemia <18 h and 84% with cold ischemia >18 h. Sert et al. (Sert et al., 2014) also demonstrated that cold ischemia time was an important modifiable risk factor in the development of DGF in deceased donor transplantations, with a higher incidence of DGF in grafts that had experienced longer cold ischemia time. Importantly, the authors found that these negative effects were particularly clearly observed in kidneys with a cold ischemia time between 20 and 30 h, and beyond 30 h. These findings support those produced by Barba et al. (Barba et al., 2011) in purporting that there is a specific threshold for cold ischemia to which the development of DGF is associated, with a subsequent increase in DGF for each additional hour of cold ischemia. When considering the effects of DGF on graft survival, it is interesting to note that there is evidence to suggest that DGF actually has no influence on patient or graft survival from deceased donors (Chaumont et al., 2015). In fact, graft survival was only significantly reduced when acute rejection occurred in combination with DGF. The authors also found that perioperative saline loading efficiently reduced the detrimental effects of cold ischemia, thus reducing the incidence of DGF.
Table 1.
Clinical study of the impact of IRI on renal graft survival.
| Author (year) | Study design | Area of investigation | Conclusions |
|---|---|---|---|
| Barba et al. (2011) | Prospective analysis of 378 adult renal transplantations | Delayed graft function (DGF) and acute rejection | CI <18 h does not negatively impact graft survival. 91% graft survival with CI <18 h and 84% survival with CI >18 h. After 18 h, each hour of CI results in a 10% increase in DGF. |
| Sert et al. (2014) | Retrospective analysis of 111 deceased donor adult renal transplantations (1994–2009) | Delayed graft function and acute rejection | DGF prevalence was 54% and AR prevalence in the first year post-transplantation was 9.9%. Patients with DGF had higher serum creatinine levels at the first, third and fifth years. CI time is an important modifiable risk factor in the development of DGF. There was no correlation between CI time and AR. |
| Krishnan et al. (2016) | Retrospective analysis of 3717 living donor renal transplantations (1997–2012) in patients who had participated in the Australian Paired Kidney Exchange Program. | Delayed graft function and chronic allograft dysfunction | Donor age is an effect modifier between CI time and graft outcomes. For grafts obtained from donors >50 years, each additional hour of CI time was associated with adjusted odds of 1.28 for delayed graft function. |
| Kayler et al. (2017) | Retrospective cohort study of 6276 adult first-time kidney-only recipients of paired kidneys (derived from the same donor transplanted into different recipients) from donation after circulatory death (DCD) donors (1998–2013). | Delayed graft function | Prolonged CI time has limited bearing on long-term graft outcomes, in the setting of donation after circulatory death. Death censored graft survival is comparable between recipients of kidneys with higher CI time and lower CI time in DCD donor recipients. |
| Chaumont et al. (2015) | Retrospective analysis of 1784 deceased donor renal transplantations (1983–2014) | Delayed graft function and acute rejection | Absence of perioperative saline loading increases acute rejection incidence (OR = 1.9 [1.2–2.9]). Patient's residual diuresis ≤500 mL/d (OR = 2.3 [1.6–3.5]) and absence of perioperative saline loading (OR = 3.3 [2.0–5.4]) are risk factors for DGF. DGF has no influence on patient and graft outcome, unless occurring in combination with acute rejection, in which case there is a significant reduction in graft survival. |
| Kayler et al. (2011) | Retrospective analysis of 17,514 paired expanded criteria donor (ECD) renal transplantations (1995–2009) | Delayed graft function | Prolonged CI time is a risk factor for DGF among ECD renal transplants, however DGF has no significant effect on ECD graft survival. Prolonged CI time is associated with a significantly increased incidence of DGF in ECD kidneys (35% vs. 31%, p < 0.001) including substantially higher rates for CI time differences ≥ 15 h (42%). No significant difference in graft loss between groups with higher and lower CI (p = 0.47). |
| Hellegering et al. (2013) | Retrospective analysis of 472 adult living donor renal transplantations (1996–2010) | Early graft function (EGF) and chronic allograft dysfunction | Poor EGF had an incidence of 13.7% in living donor kidney allograft recipients. Patients with poor EGF experienced significantly lower rejection-free and long-term graft survival, compared to those with immediate graft function. |
| Perez Valdivia et al. (2011) | Retrospective analysis of 2525 non-combined cadaveric renal transplantations (2000–2008) | Early graft function and chronic allograft dysfunction | Duration of CI time was significantly associated with older donor and recipient age. Longer CI time was associated with poorer early graft function, independent of donor and recipient age. Prolonged CI time produces significant worse survival rates; CI time produce significantly worse survival rates for recipients (RR: 1.03, 1.005–1.05, P = 0.02) and grafts (RR: 1.03, 1.01–1.04, P = 0.002). |
Despite the apparent evidence to support the ‘threshold’ hypothesis of cold ischemia, there is also data to indicate that cold ischemia time below a defined threshold is also associated with DGF and chronic allograft dysfunction when considering living donors.
Krishnan et al. (Krishnan et al., 2016) found that, for recipients who had received kidneys from living older donors aged >50 years, every hour of cold ischemia time was associated with adjusted odds of 1.28 for DGF. Furthermore, cold ischemia time of >4–8 h was associated with adjusted hazards of 1.93 for overall graft loss and 1.91 for death-censored graft loss, compared to cold ischemia time of 1–2 h. These findings indicate that cold ischemia time significantly below the suggested threshold of approximately 20 h, as previously demonstrated by Barba and Sert, may also be associated with an increase in both DGF and chronic allograft dysfunction in living donor recipients. In addition, donor age also seems to be an effect modifier between cold ischemia time and graft outcomes, suggesting that efforts to reduce cold ischemia time, particularly involving older living donor kidneys, are necessary to improve graft survival.
To match the rising demand for organs within a limited donor pool, there has been an increasing use of suboptimal donors, including DCD donors and expanded criteria donor (ECD) grafts. With regards to the effects of cold ischemia time on DGF and chronic allograft dysfunction in DCD donor grafts, the findings have not correlated with those produced when investigating living and deceased donors. Kayler et al. (2017) found that prolonged cold ischemia time, up to 30 h, did not have any significant negative impact on long-term graft survival outcomes of kidneys from DCD donors. It is important to note that the authors did identify evidence for an association between prolonged cold ischemia time and primary non-function, however these findings were limited by event rates and differences between groups, thus warranting further investigation. Interestingly, these findings were similar when considering ECD grafts. ECD grafts are classically considered to be suboptimal, whilst the precise definition of an ECD remains elusive. An ECD is often thought to be any donor over the age of 60, or a donor over the age of 50 with two of the following co-morbidities: creatinine >1.5 mg/dl, hypertension or death following a stroke. Kayler et al. (2011) found that, whilst prolonged CI time was a risk factor for the development of DGF amongst ECD grafts, DGF alone had no significant effect on graft survival. These findings suggest that, as DGF seems to have no significant effect on graft survival within the ECD pool, ECD grafts that have traditionally been considered inadequate may, in fact, be viable. Both these studies are limited by the fact that rates of early rejection were not simultaneously investigated, as rejection has been shown to be intrinsically related to graft survival when considered in combination with DGF.
3.3. Ischemia-Reperfusion Injury and Acute Rejection
A wealth of clinical evidence has demonstrated that the severity of ischemia-reperfusion injury is positively associated with the frequency of acute rejection episodes. Barba et al. (2011) reported that >18 h of cold ischemia increased the incidence of acute rejection episodes as well as early onset of the first rejection episode.
The relationship between DGF and acute rejection remains controversial. Recently, Sert et al. (2014) found that there was no association between cold ischemia time and the incidence of acute rejection. A retrospective cohort study by Perez Valdivia et al. (2011) produced confounding results that indicated that longer cold ischemia time was associated with poorer early graft function, independent of donor and recipient age. Furthermore, prolonged cold ischemia time was found to reduce both patient and graft survival rates; longer cold ischemia time produces significantly worse survival rates for recipients (RR: 1.03, 1.005–1.05, P = 0.02) and grafts (RR: 1.03, 1.01–1.04, P = 0.002).
It is important to consider the relationship between acute rejection, DGF and long-term graft survival. Sert et al. (2014) demonstrated that, although the prevalence of acute rejection was higher in grafts with DGF, this association was not statistically significant, whilst, in fact, acute rejection demonstrated no negative effects on overall graft survival. Overall, the current literature is not conclusive regarding the effects of acute rejection and poor early graft function on long-term graft function. Most recently Hellegering et al. (2013), conducted a retrospective cohort study and found that patients with poor early graft function experienced significantly lower rejection-free and long-term graft survivals, compared to those with immediate graft function, as supported by two previous retrospective cohort studies (Brennan et al., 2004; Lee et al., 2010). However, two other retrospective cohort studies found no significant correlation between poor early graft function and graft survival (Nogueira et al., 2009; Tyson et al., 2010). As previously discussed, it is interesting to note that these confounding results may, at least in part, be explained by recent findings that indicate that acute rejection alone may not detrimentally effect graft survival unless occurring in combination with DGF (Chaumont et al., 2015).
Taking this evidence into account, it is possible that suppressing ischemia-reperfusion injury may decrease acute immune rejection episodes, whilst prevention of acute rejection within the first year may postpone later allograft failure. However, due to the confounding results within the literature, further investigation of the association between cold ischemia time, acute rejection and long-term graft survival is certainly warranted.
4. Impact of IRI on Renal Graft Survival: Hypothesis and Mechanisms
Whilst transplanted kidneys with prolonged ischemia time are more susceptible to long-term deterioration, the exact molecular mechanisms underlying this association remain to be characterized. We will review several hypotheses that seek to explain the relationship between early IRI and later graft loss.
4.1. Ischemia-Reperfusion Injury and Immunogenicity of the Renal Graft
The injury theory proposes that ischemia-reperfusion injury augments graft immunogenicity. This is based on the observed correlation between the degree of IRI insult and the subsequently increased disturbance of adaptive immunity and the greater possibility of immunologic recognition and graft rejection (Perico et al., 2004).
4.1.1. IRI and the Recognition of Allograft
Rejection of the renal allograft is initiated by immune recognition of foreign antigens. The immune recognition of allografts is mediated by T-cell receptors and alloantibodies (van der Merwe and Dushek, 2011). There are three distinct pathways of T-cell allorecognition. The first is the direct pathway which occurs when T-cells recognize endogenous peptides displayed in the context of donor MHC molecules on the surface of donor antigen-presenting cells (APCs). The second pathway is the indirect pathway, in which recipient T-cells recognize donor peptides presented on MHC molecules on the surface of recipient DCs (Benichou and Thomson, 2009). In this case, recipient DCs have taken up and processed antigens that have been released or shed by donor cells. The third pathway is the semi-direct pathway, which has been more recently identified as a mechanism by which recipient-derived APCs present allopeptides in the context of self-MHC (indirect pathway), in addition to acquired allo-MHC-peptide complexes (direct pathway).
The direct immune response against the graft is initiated by an inflammatory response that occurs when damaged or dying cells release endogenous adjuvants, referred to as damage-associated molecular patterns (DAMPs), and by the secretion of pro-inflammatory cytokines from distressed cells (Bianchi, 2007). Inflammatory DAMPs, including Heat-Shock proteins (HSP), HMGB-1 and DNA fragments, are recognized by specific receptors such as TLR-2 and TLR-4 which, in turn, trigger inflammatory and cytotoxic responses (Park et al., 2004). This frequently occurs in solid organ grafts after IRI because, as apoptotic cells are not rapidly cleared following the initial injury, secondary necrosis occurs due to loss of membrane integrity, resulting in the release of DAMPs (Dong et al., 2007). The massive release of pro-inflammatory cytokines, such as IL-1α and tumour necrosis factor (TNF), from damaged cells during ischemia-reperfusion injury causes donor DCs to become activated and migrate from the donor graft to the recipient lymph nodes and spleen, thus sensitizing recipient T-cells and stimulating T-cells via the direct pathway (Li and Okusa, 2010; Dong et al., 2007). A study by Dong et al. (2007) provides evidence that resident dendritic cells constitute the predominant first responders in the secretion of TNF during early renal ischemia-reperfusion injury.
The indirect pathway is initiated when recipient DCs take up material from dead and dying cells. This material is subsequently processed and presented to indirect-specific T-cells, which migrate into the graft and cause cellular damage, either by direct cytotoxicity (CD8), or by facilitating cytokine release from activated CD4+ cells. CD4+ T-helper cells can be subdivided into Th1 and Th2, which produce Th1-type cytokines and Th2-type cytokines respectively. Th1-type cytokines, such as interferon-α, induce pro-inflammatory responses, whilst Th2-type cytokines, such as IL-4, 5 and 13, are associated with fibrotic tissue deposition and interleukin-10, which is anti-inflammatory.
In addition to the direct and indirect pathways, it is also important to consider the role of the semi-direct pathway of T-cell allorecognition. Following engraftment, recipient-derived APCs infiltrate the allograft and, as well as presenting allopeptides indirectly, also present allogeneic MHC-peptide complexes directly by acquiring donor complexes from donor-derived cells or tissues (Smyth et al., 2008). This process is known as ‘cross-dressing’ and is thought to encourage a continued direct alloimmune response for a prolonged period following death of donor-derived APCs. It has recently been demonstrated that acquired allo-MHC-peptide complexes on recipient dendritic cells are able to promote allograft rejection indefinitely, even following the termination of the direct pathway and in the absence of cross-presentation (Smyth et al., 2017). Nonetheless, the precise role of the semi-direct pathway in the development of allograft rejection remains to be elucidated.
Laboratory studies have shown that renal IRI results in the long-term infiltration of activated and effector-memory T-lymphocytes, whilst the expression of the effector-memory phenotype by infiltrated lymphocytes indicates that these lymphocytes are responding to an injury-associated antigen (Ascon et al., 2009). The presence of infiltrating T cells could be due to the direct, indirect or semi-direct pathway of allorecognition. The direct pathway predominates in early acute allograft rejection, as there are a large number of donor DCs expressing a high level of MHC molecules and direct allospecific T-cells present in the graft. Later, as the number of donor DCs is gradually depleted, the T-cell mediated indirect pathway begins to dominate and is considered the main pathway in chronic rejection (Benichou et al., 2011).
IRI can influence these processes in various ways. Firstly, graft ischemia-reperfusion injury can promote indirect allo-recognition by providing antigens that may be readily taken up and processed in the indirect pathway.
Secondly, DC migration is increased during ischemia-reperfusion injury, which promotes the migration of T-cells (Jurewicz et al., 2010). T-cells migrate into the renal transplant graft and differentiate into effector T-cells, which include CD8+ cytotoxic T-cells and CD4+ T helper cells.
Thirdly, IRI may promote a shift in cytokine profiles at the moment of engraftment, which could critically affect renal allograft rejection and tolerance (Strom, 2004) through activating different T-cell populations. During IRI, activated macrophages infiltrating the renal graft are capable of producing IL-1, IL-6, IL-12 and IL-23 (Li and Okusa, 2006), so it is naturally postulated that the pro-rejection response is shifted, which augments graft damage. It is clear that ischemia-reperfusion manifests a particular type of immune response, which shifts the balance towards a pro-inflammatory Th1 and Th17 response, rather than an anti-inflammatory regulatory T-cell (Treg) response. Th17 cells are capable of producing pro-inflammatory cytokines, such as IL-17A, IL-17F and IL-21, and chemokines, such as CXCL1, CXCL2, CCL2 (Tesmer et al., 2008), whilst Tregs are immunosuppressive lymphocytes and their actions are mediated by the production of anti-inflammatory cytokines, such as IL-10 or TGF-β (Kinsey et al., 2009). Activation and predomination of Th1 and Th17 responses over the T-reg response results in the recruitment of other inflammatory cells, including neutrophils and monocytes, at the site of inflammation, therefore contributing to allograft rejection (Chadha et al., 2011).
Finally, it has also recently been demonstrated that renal IRI may promote the humoral immune response by amplifying antibody mediated rejection (Fuquay et al., 2013). Therefore, following IRI, an antigen-specific amplified IgG response may be generated in the presence of an alternative functional complement pathway.
Taken together, the initial vigorous cell death initiated by IRI causes the massive release of DAMPs, and induces a heavy influx of inflammatory cells, such as neutrophils and macrophages. This in turn results in tubular cell injury and produces further cellular components that are readily presented by MHC molecules on dendritic cells and macrophages, accelerating antigen processing and presentation, and facilitating T-cell recognition. The inflammatory milieu established during reperfusion injury attracts more T-cells to the ‘danger’ zone and therefore promotes their activation, differentiation and cytotoxic actions (Fig. 1).
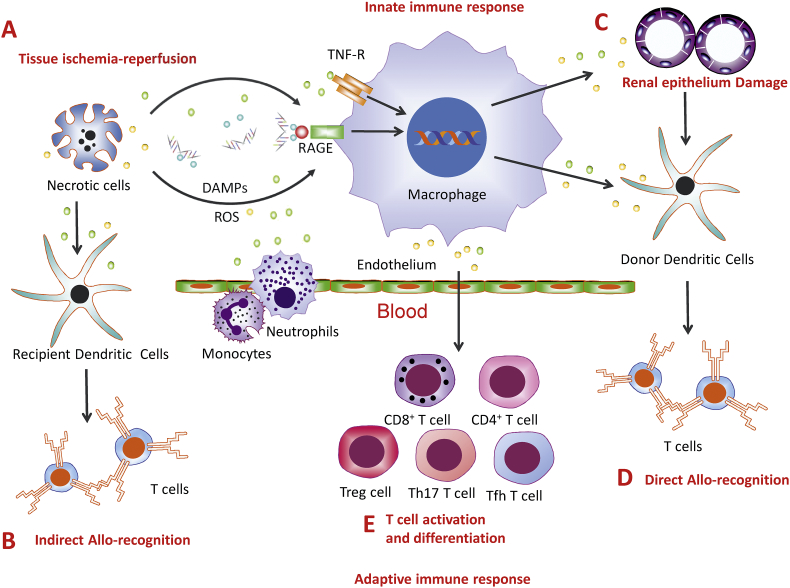
Fig. 1.
Ischemia-reperfusion injury and allo-immune response. During renal graft ischemia-reperfusion injury (IRI), (A) renal cell necrosis releases DAMP molecules such as High-mobility group box 1 protein (HMGB1), which are recognised by receptors, such as receptor for advanced glycation end products (RAGE) and Toll-like receptors-2, 4, and 9 (TLR-2, 4 and 9), reactive oxygen species (ROS) and inflammatory cytokines, such as TNF, leading to immune cell activation. (B) Inflammatory cells produce and release pro-inflammatory chemokines (e.g. CXCL1, CXCL2) and cytokines (e.g. IL-1 and 6) and promote tissue inflammation, infiltration of monocyte and neutrophils. (C) Infiltrating monocytes produce ROS and inflammatory cytokines such as TNF, enhance the necroptosis in the epithelial cells (D) Renal cells undergoing necrosis could lead to the release of donor antigens. They are presented by donor antigen presenting cells (APC) and stimulated T-cells. This is direct allo-recognition. (E) As the number of donor antigen presenting cells gradually decreases, the recipient APCs process and present the antigens to T-cells, leading to indirect allo-recognition. (F) The inflammatory milieu formed during IRI influences the activation and differentiation of T-cells, such as CD8+ T-cells, CD4+ Th1 and Th2 cells, Treg cells, Tfh cells and Th17 cells, which contribute to either rejection or tolerance of the renal grafts.
4.2. Ischemia-Reperfusion Injury and Loss of Renal Mass
The hyperfiltration theory by Brenner et al. (1996) highlighted the role of chronic hemodynamic changes in renal failure. Glomerular hyperfiltration has been defined as GFR of more than two standard deviations above the mean GFR of healthy individuals (Sasson and Cherney, 2012). The threshold for glomerular hyperfiltration ranges from 125 ml/min/1.73 m2 to 175 ml/min/1.73 m2 (Dahlquist et al., 2001; Amin et al., 2005). Increased filtration per nephron is an adaptive response of the remaining nephrons to meet the excess load. Studies have demonstrated that functional absence of one kidney results in an adaptive increase in contralateral renal blood flow and a decrease in renal vascular resistance; these hemodynamic changes might be mediated by the NO system (Sigmon et al., 2004). As such, a 30–70% increase in glomerular filtration rate above control values is usually observed in the remaining kidney (Shohat et al., 1991). After loss of renal mass through injury, the remaining renal tissue compensates with hyperplasia (increase in cell number) and hypertrophy (increase in cell volume). It has been demonstrated that the compensatory growth response is initiated immediately after unilateral nephrectomy (Helal et al., 2012). Studies in rodents have demonstrated that the extent of compensatory growth correlates closely with the amount of renal tissue that is surgically removed (Santos et al., 2006), which has important implications in the case of IRI with severe reduction in the size of the nephron population. An increase in glomerular filtration rate at the level of single nephrons has been demonstrated following gradual reduction of renal mass (Metcalfe, 2007). Accumulating clinical evidence demonstrates that glomerular hyperfiltration occurs in the majority of grafts (Seun Kim et al., 2002) in later years. Giral et al. (2005) investigated the effects of graft mass on the long-term outcome of cadaveric kidney grafts of 1142 recipients. Patients with moderate renal mass reduction demonstrated renal hyperfiltration, whilst the kidney grafts with optimal renal mass improved their filtration rate continuously for the entire 4-year period.
Reduction of the functional nephron population by ischemia-reperfusion injury results in an elevated workload for the remaining renal mass, and compensatory hyperplasia and hypertrophy in the renal graft (Fiorina et al., 2005). These compensatory morphological and functional changes are temporarily adequate to maintain electrolyte and water balance and prevent the development of renal functional insufficiency (Devarajan, 2006). However, functional overloading could easily exhaust the remaining nephrons and result in sub-optimal efficiency of blood filtration. Gradual accumulation of toxic metabolites would elevate the oxidative stress and inflammatory reactions within the grafts, in turn resulting in further reduction of functional renal mass and further functional overloading. Chronic renal functional impairment then develops until end-stage graft failure occurs (Fig. 2).
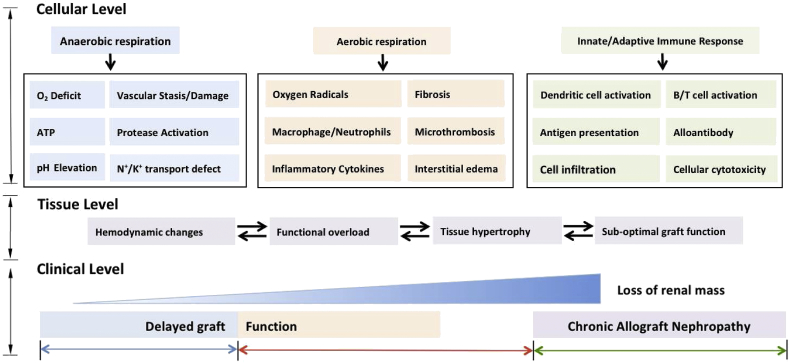
Fig. 2.
Loss of functional mass during renal graft ischemia-reperfusion injury. Renal graft ischemia-reperfusion injury promotes abnormal cellular changes that result in the gradual loss of functional kidney mass. The pathophysiology of ischemia-reperfusion injury is complex and can potentiate graft damage both acutely and chronically due to a variety of cellular and tissue changes that can manifest clinically. On a cellular level, ischemia results in the predominance of anaerobic processes and subsequent cellular damage via processes such as protease activation and N+/K+ transport defects. The associated reperfusion injury occurs due to the restoration of oxygen supply to the graft, resulting in a potpourri of cellular changes including the generation of reactive oxygen species and the upregulation of inflammatory cytokine pathways. The cellular responses during ischemia and reperfusion injury result in abnormal changes within renal tissue, with many of these processes acting synergistically to cause the gradual loss of functional renal mass. The cellular and tissue responses result in graft damage that is exhibited clinically in the form of acute renal necrosis, vasculitis and failure. A less familiar clinical feature of ischemia-reperfusion injury is chronic allograft dysfunction, which predominantly occurs due to abnormal immune activation and the subsequent deterioration in functional graft mass.
4.3. Ischemia-Reperfusion Injury and Chronic Renal Graft Hypoxia
Studies in renal grafts have shown that tubulointerstitial damage, rather than glomerular change, determines graft functional decline. Nangaku (2006) proposed chronic hypoxia in the tubulointerstitium as a common pathway for renal failure. This chronic hypoxic theory states that chronic oxygen deprivation to the tubulointerstitial compartment, caused by compromise of the postglomerular capillary circulation, is responsible for the scarring process. In the case of kidney transplantation, microvasculature damage and dysfunction, initiated during ischemia-reperfusion injury, creates a hypoxic environment that triggers a fibrotic response in tubulointerstitial cells which ultimately results in graft failure.
4.3.1. Ischemia-Reperfusion Injury and Graft Vascular Damage
The renal cortex has an average pO2 of approximately 30 mmHg, whilst oxygen tension in the renal medulla does not rise above 10 mmHg (Chen et al., 2009). The effects of limited O2 supply are aggravated by the high O2 demand associated with the high tubular O2 consumption necessary for solute exchange and the high rate of aerobic glycolysis. Under pathological conditions, the delicate balance of oxygen supply compared to demand is disturbed. As a result, the kidney is highly susceptible to hypoxic injury.
Adequate reperfusion in the graft is vital for renal functional recovery by reducing hypoxic tissue injury. Upon reperfusion, it has been demonstrated that the renal blood flow is only partially restored, with a consistent reduction in total flow of up to 50% after reperfusion (Sutton et al., 2002; Molitoris and Sutton, 2004). The failure of blood to reperfuse a temporarily ischemic area after the physical obstruction has been removed or bypassed was described as the “no-reflow” phenomenon (Rezkalla and Kloner, 2002). It was suggested that an uncontrolled and inappropriate release of various inflammatory mediators, leading to direct cytotoxic effects on endothelial cells, impairs microvascular autoregulation of blood flow. In addition, it was reported that in the post-ischemic kidney, increased oxidative stress leads to an imbalance of vasodilatory and vasoconstrictive mediators in the endothelium and epithelium of the kidney (Legrand et al., 2008). Renal arterioles exhibit impaired vasorelaxation in response to vasodilators, such as acetylcholine (ACh), and increased responsiveness to vasoconstrictive agents including endothelin, angiotensin II, thromboxane A2, leukotriene C4 and endothelium-derived prostaglandin H2. The tonic modulation of renal flow is also disturbed through excessive vascular constriction (Legrand et al., 2008). Damage to both endothelial and smooth muscle cells occurs rapidly after ischemic insult. Upon reperfusion, blood flow can be impaired in peritubular capillaries due to endothelial cell rupture or congestion. The interstitial oedema compresses capillaries, narrows the vascular lumen and increases blood flow resistance (Basile et al., 2001). Leukocyte activation and accumulation augment endothelial damage by sustaining the inflammatory and cytotoxic response. Pro-inflammatory cytokines released during IRI activate the coagulation pathways, which contributes to microcirculatory dysfunction by capillary congestion, resulting in severe compromise of local and global renal perfusion (Hoffmann et al., 2005; Sharfuddin et al., 2009).
In addition to hypoperfusion, hypovascularization is another consequence of ischemia-reperfusion-associated vascular damage. It was demonstrated that the number of peritubular capillaries in human renal biopsies declines with progressive loss of renal function (Steegh et al., 2011). Capillary density declines rapidly within days of the initial insult and persists for weeks, in parallel with an increase in peritubular matrix deposition (Steegh et al., 2011). The inflammatory response and oxidative stress-induced endothelial cell death are responsible for vascular regression and increased deposition of fibrotic tissues due to hypoperfusion. This could also compress and damage the microvasculature.
In the long-term survival of grafts, pathological examination of graft vasculature has revealed a broad spectrum of abnormalities, ranging from fibrous intimal thickening to complicated atherosclerotic plaques; this is termed graft vascular disease (Mitchell, 2009). Shortly after transplantation, focal fibrous intimal thickening and vasculitis predominates. This develops into chronic allograft vasculopathy, manifested as focal atherosclerotic plaques or diffuse intimal thickening. The cellular components of intimal proliferative lesions consist of endothelial cells, smooth muscle cells, macrophages and T-lymphocytes (Skaro et al., 2005; Kitchens et al., 2007). Graft vascular disease usually involves the entire length of the transplanted arterial vasculature, including penetrating intraparenchymal arterioles (Mitchell, 2009). Blood flow resistance increases 16-fold, with a 50% reduction in the luminal radius (Mitchell and Libby, 2007). Therefore, arterial remodelling could severely compromise graft perfusion, whilst occlusion of arterioles could result in stellate infarcts, which may develop into fibrotic lesions (Huibers et al., 2011). Studies have shown that ischemia-reperfusion injury accelerates the development of graft vascular disease and the early ischemia-associated recruitment of neutrophils. In addition, these studies demonstrate that macrophages can provide a pro-inflammatory environment that supports the allograft-specific immune response against the vasculature. Macrophages and T-cells in the alloresponse stage produce additional cytokine mediators and, in combination with antibody-mediated responses, induce widespread endothelial and medial smooth muscle injury (Wehner et al., 2007). Interferon-α, produced by infiltrating macropahges, exerts potent effects on inflammatory and smooth muscle cell activation and recruitment.
4.3.2. Ischemia-Reperfusion Injury and Hypoxia-Fibrosis Response
The decreased vascular density and increased vascular dysfunction associated with ischemia-reperfusion injury promotes chronic renal graft hypoperfusion, which causes persistent regional hypoxia. Hypoxia is a potent profibrogenic stimulus for tubular epithelial cells, interstitial fibroblasts and renal microvascular endothelial cells (Norman et al., 2000). In response to a low pO2, there is an accumulation of extracellular matrix (ECM) proteins as a result of both increased matrix synthesis and decreased matrix degradation, mediated by a decreased expression of matrix metalloproteinases (MMPs) (Fine et al., 2000). Hypoxia induces complex changes in the expression of a large number of genes involved in cell survival and adaptation. In tubular epithelial cells, hypoxia also induces the expression of fibrogenic factors, including Transforming Growth Factors and Endothelin-1 (Yamashita et al., 2001). Hypoxia promotes a fibrogenic phenotype in fibroblasts, which is characterized by increased proliferation, enhanced myofibroblast differentiation and contraction and altered ECM metabolism (Norman et al., 2000). In addition to increased ECM production, hypoxia suppresses matrix degradation by decreasing the activity of matrix metalloproteinases, in particular interstitial collagenase matrix metalloproteinase-1 (Fine and Norman, 2008).
Chronic hypoxia may potentiate the inflammatory response by providing the chemotactic stimulus that drives immune cell recruitment and migration at sites of injury (Nizet and Johnson, 2009). In addition, it has been suggested that inflammatory cells, such as macrophages, contribute to pathological extracellular matrix accumulation (Ko et al., 2008). Although the mechanisms of cell recruitment and retention within the kidney have not been clearly defined, a role for hypoxia seems plausible as inflammatory cells preferentially migrate to hypoxic sites (Li et al., 2010).
Histologic analysis of chronically scarred kidneys reveals the loss of microvasculature in the fibrotic bands of collagen and ECM (Norman, 2006). The hypovascularised and hypoperfused regions are formed after the initial ischemia-reperfusion injury. The cells around the edges of such regions are subjected to prolonged hypoxia and develop mitochondrial functional impairment. This results in persistent energy deficits and subsequently causes cells to be replaced by connective tissue after undergoing apoptosis. The vicious cycle of fibrogenic response is established, leading to the obliteration of more capillaries and more fibroid lesions (Fig. 3).
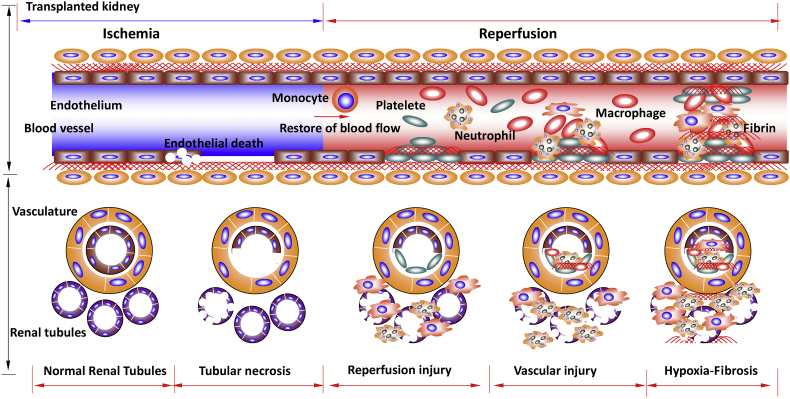
Fig. 3.
Graft vascular injury during renal graft ischemia-reperfusion injury. Renal graft vascular injury is a common occurrence following renal transplantation due to ischemia-reperfusion injury. Endothelial vasoconstriction and necrosis occurs during periods of prolonged ischemia. Vasoactive hormones may be responsible for the haemodynamic abnormalities during ischemia, due to an increase in the intrarenal activity of vasoconstrictors and a deficiency of vasodilators. This ultimately results in a reduction in renal blood flow and persistent intrarenal hypoxia, thus exacerbating tubular injury and potentiating acute tubular necrosis within the transplanted kidney. During reperfusion, pro-coagulant pathways predominate, resulting in fibrin deposition and platelet aggregation in peritiubular and glomerular capillaries. These processes can cause microthromboses, leading to impaired renal perfusion and decreased glomerular filtration rate. Red blood cells and leukocytes adhere to the vascular endothelium, further contributing to the blockage of renal blood vessels. The formation of platelet plugs containing fibrin can cause tubular obstruction and increased tubular pressure, it is thought that the exposure of tissue factor to the subendothelium following microvasculature damage promotes the coagulation cascade during reperfusion. Thromboses within the renal vasculature of the transplanted graft promote tubular necrosis and graft failure.
5. Summary of the Hypotheses
The renal graft needs to carry the workload of two previously intact kidneys. However, this workload must be fulfilled despite a rapidly diminishing pool of functioning nephrons due to ischemia-reperfusion injury, as well as episodes of acute rejection and hypoperfusion-induced hypoxic injuries, in addition to inflammation and graft vascular disease. Various pathological processes act collectively to contribute to the development of graft failure. Activation of the immune response results in the loss of renal function, which culminates in significant haemodynamic stress. This, in turn, causes additional cellular injury and inflammation, acting in a vicious cycle to further promote immune activation.
Extensive ablation of renal mass initiates a rapidly progressive course of hemodynamically-mediated glomerular injury and renal functional impairment. Thus, we propose that the critical effect of ischemia-reperfusion injury on graft survival is its initiation of renal mass ablation by increasing renal parenchymal destruction, enhancing graft immunogenicity, increasing vascular damage and expanding fibrotic lesions. Therefore, protecting the renal graft against ischemia-reperfusion injury would slow the tempo of chronic renal graft failure by preserving the nephron supply, decreasing graft immunogenicity and protecting the intra-organ microvasculature.
6. Impact of IRI on Renal Graft Survival: Novel Protective Strategies
Recently, new strategies targeting renal graft IRI have shown promising results in extending the survival of renal grafts after transplantation.
6.1. Machine Perfusion
In order to ensure the best possible function of the organ following transplantation, two methods of organ preservation are currently used in clinical practice: static cold storage and machine perfusion. Recent studies investigating machine perfusion have demonstrated its beneficial effects in suppressing renal graft IRI.
6.1.1. Hypothermic Machine Perfusion
An alternative to ex vivo hypothermic ischemic preservation is the use of hypothermic machine perfusion (HMP). This process involves the introduction of a cooled perfusion solution into an enclosed system through the organ's vascular bed under a defined pressure, therefore partially emulating natural perfusion. Many studies have been performed to assess the renoprotective efficacy of cold storage vs. HMP. Moers et al. conducted a multi-centre, international randomized study in a group of 336 kidney pairs, one of which was preserved using cold storage and the other with HMP (Moers et al., 2009). The study showed a significant reduction in DGF risk from 26.5% to 20.8% with HMP, as well as an increase in one-year graft survival from 90% to 94% compared to cold storage. In patients who received a kidney following cold storage, DGF significantly reduced three-year transplant survival compared to kidneys stored with HMP, whilst a DGF episode had a much smaller effect on overall transplant survival. Wszola et al. conducted a prospective study whereby 69 kidney biopsy specimens were collected with a 5-year patient follow-up (Wszola et al., 2014). The study demonstrated that machine perfusion has the ability to mediate hypoxia-related gene expression, thus potentially improving long-term graft survival. As discussed in a recent review by de Deken et al. (2016), there is still considerable debate regarding the optimum settings for hypothermic machine perfusion. Despite this, there is significant evidence to support its use in clinical practice, particularly in relation to expanded criteria donors (ECDs) (Moers et al., 2009; Treckmann et al., 2011).
6.1.2. Normothermic Machine Perfusion
A novel method of organ preservation that has recently been studied is normothermic machine perfusion (NMP). Theoretically, NMP has the benefit of reducing hypothermia-related graft damage, as well as enabling good assessment of ischemic organ damage. Whilst progress has been made in NMP, mainly in experimental settings, there are limited clinical studies investigating whether these findings can be translated in human organs. Hosgood and Nicholson (2011) published the first report on NMP of a human kidney graft and, in their subsequent paper of 18 kidneys from ECDs (Nicholson and Hosgood, 2013), demonstrated that NMP-stored grafts possessed a significantly lower rate of DGF (5.6%) compared to the cold storage control group (36.2%). Since then, Hosgood and Nicholson subsequently transplanted a pair of DCD kidneys previously rejected for transplantation by all UK transplant centres due to poor perfusion (Hosgood et al., 2016). Patchy areas were cleared in both kidneys during 60 min of NMP and the grafts were eventually transplanted, resulting in immediate graft function. Following these successful studies, Hosgood has begun conducting a UK-based phase II multicentre trial recruiting 400 patients in order to compare pre-transplant NMP and conventional cold storage, with results expected by 2020 (Hosgood et al., 2017).
6.2. Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are self-renewing, multipotent cells, with numerous studies indicating that exogenous administration of MSCs has the ability to attenuate renal ischemia reperfusion injury and promote renal repair (Peired et al., 2016). MSCs are thought to facilitate various cellular processes by mediating the release of soluble factors (Galderisi and Giordano, 2014). These processes include immunomodulation via IL-6, IL-8, and MCP-1 release (Casiraghi et al., 2014; Squillaro et al., 2016; Liechty et al., 2000), extracellular matrix remodelling via the modulation of fibronectin, collagen and metalloproteinase-mediated processes (Ries et al., 2007; Togel et al., 2005b; Goransson et al., 2004; Liu et al., 2013), and alterations in growth factors and regulators, including vascular endothelial growth factor (VEGF) (Togel et al., 2009; Schrijvers et al., 2004), insulin-like growth factor 1 (IGF-1) (Imberti et al., 2007; Zhang et al., 2004), and hepatocyte growth factor (HGF) (Chen et al., 2011). MSC-mediated microvesicle release has also been shown to enhance cell survival and attenuate programmed cell death processes (Bruno et al., 2012, 2009). Overall, numerous studies have demonstrated that exogenous administration of MSCs attenuates renal ischemia-reperfusion injury (Du et al., 2013; Bruno et al., 2009; Togel et al., 2005a; Jiang et al., 2015; Duffield and Bonventre, 2005; Monteiro Carvalho Mori Da Cunha et al., 2015; Duffield et al., 2005) and, therefore, may be a potential future renoprotective strategy.
6.3. Gas as Ex Vivo Preservation Supplement
Ex vivo preservation is commonly performed in hypothermic conditions in the presence of preserving solutions, such as University of Wisconsin (UW) solution saturated with nitrogen. However, in recent years, efforts have been made to limit ischemia-reperfusion injury in renal grafts during ex vivo preservation by using preserving solutions saturated with alternative gases.
6.3.1. Argon
Argon is an inert noble gas that has demonstrated neuroprotective and, more recently, renoprotective properties (Loetscher et al., 2009). Irani et al. found that cold-storage with argon improved kidney recovery in comparison to mediums containing nitrogen or air (Irani et al., 2011). It has, therefore, been suggested that argon may provide an organoprotective environment for kidney transplantation by preserving organ quality and function.
6.3.2. Xenon
Ma et al. (2009) have demonstrated that xenon preconditioning has the potential to confer renoprotection against IRI. Xenon, which shares similar molecular and chemical characteristics with argon, demonstrated an increased efficiency of HIF-1α translation via modulation of the mammalian target of rapamycin pathway. Xenon provided morphologic and functional renoprotection in a model of renal ischemia-reperfusion injury, and the study's results suggested that xenon preconditioning acted as a natural inducer of HIF-1α. Therefore, the administration of xenon prior to renal ischemia could potentially prevent acute renal failure and be beneficial in the clinical setting during procedures that temporarily interrupt renal perfusion. In addition to preconditioning, it has also demonstrated the ability for xenon-saturated preserving solution to protect renal grafts during ex vivo preservation (Zhao et al., 2013). Grafts stored in xenon-saturated preservative solution possessed significantly less severe histopathologic changes and reduced B-cell lymphoma (Bcl-2) and heat shock protein expression. Following engraftment in Lewis rat recipients, there was a reduction in macrophage infiltration, fibrosis and a significant improvement in renal function in the xenon-treated grafts. Clinically, xenon has the potential to enhance the marginal donor pool of renal grafts by improving graft survival following ischemia during ex vivo preservation.
6.3.3. Carbon Monoxide
Nakao et al. (2008) demonstrated the ability of carbon monoxide (CO) in preserving solution to reduce donor-kidney injury after cold storage (Nakao et al., 2008). The findings suggest that CO prevents an increase in heme content within the kidney by inhibiting the degradation of cytochrome P450 heme proteins, thus eliminating the inflammatory effects of excessive heme within the graft. Long-term effects of CO exposure remain to be elucidated and further studies are necessary to evaluate long-term graft survival post-treatment.
6.3.4. Hydrogen Sulphide
Recent evidence suggests that hydrogen sulphide (H2S) has a protective role against renal ischemia-reperfusion injury. The premise behind the renoprotective effects of H2S are linked to its intrinsic role in renal physiology, such as mediating urinary sodium excretion. Numerous in vivo and ex vivo studies have proposed that H2S induces a variety antiapoptotic (Xu et al., 2009; Tripatara et al., 2008; Lobb et al., 2014), anti-inflammatory (Hunter et al., 2012; Tripatara et al., 2008), and antioxidant effects (Bos et al., 2013; Simon et al., 2011; Xu et al., 2009), thus attenuating renal ischemia reperfusion injury associated with pathophysiological processes such as acute kidney injury and following renal transplantation. Whilst there is significant evidence to indicate the renoprotective effects of H2S against renal ischemia-reperfusion injury, further studies are required to elicit whether H2S ex vivo preservation would be a viable option in clinical practice, with regards to both efficacy and safety.
7. Conclusion
Ischemia-reperfusion injury during renal transplantation continues to be a significant challenge in maintaining organ function and preserving graft viability. Despite improvements in immunosuppression, long-term graft survival rates remain far from ideal. This suggests that immune rejection is not the sole cause of chronic graft failure, but can also be precipitated by factors such as IRI. An intrinsic link between prolonged ischemic times and delayed graft failure has been established, as well as an association between the severity of IRI and the frequency of acute rejection episodes.
8. Outstanding Questions
Despite improvements in graft survival rates, overall long-term survival still remains modest, particularly when considering the increasing demand for renal allografts within an already depleted donor pool. Whilst the association between delayed graft failure and poor long-term graft survival is well-established, due to confounding results within the current literature, further investigation of the relationship between cold ischemia time, acute rejection and long-term graft survival is warranted. In addition, although investigation of alternative renoprotective agents such as xenon, argon, carbon monoxide and hydrogen sulphide has produced promising results, further pre-clinical studies are required in order to enhance our understanding of the long-term effects of these agents on both the transplanted organ and body's systemic response to them. Furthermore, promising novel therapies such as the exogenous administration of mesenchymal stem cells also require significant further investigation. Obtaining a better understanding of the efficacy and safety profile of these agents and therapies should facilitate the progression of pre-clinical to well-powered, high quality clinical studies.
9. Search Strategy and Selection Criteria
This is a narrative review exploring the long-term effects of ischemia reperfusion injury and prolonged cold ischemic times on renal graft survival. Pubmed was used as database for literature search and kidney graft ischemia-reperfusion injury were used as key words. Studies investigating the effects of cold ischemia on renal graft rejection, delayed graft failure and overall graft survival within the last six years were eligible for inclusion in the review of clinical data. Abstracts were not considered unless full study reports were available.
Acknowledgments
Acknowledgments
This work was supported by the British Medical Research Council (MRC), The Developmental Pathway Funding Scheme (DPFS) program (project grant G802392) and BJA/RCoA Research Fellowship grant.
Author Contributions
HZ and DM designed the study, HZ produced the figures, and all authors contribute to the preparation of the manuscript.
We thank Dr. Qian Chen and Dr. Rele Ologunde for their critical comments on the manuscript.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures were created originally for this article by Dr. Hailin Zhao.
References
- Amin R., Turner C., van Aken S., Bahu T.K., Watts A., Lindsell D.R., Dalton R.N., Dunger D.B. The relationship between microalbuminuria and glomerular filtration rate in young type 1 diabetic subjects: the Oxford Regional Prospective Study. Kidney Int. 2005;68:1740–1749. doi: 10.1111/j.1523-1755.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- Ascon M., Ascon D.B., Liu M., Cheadle C., Sarkar C., Racusen L., Hassoun H.T., Rabb H. Renal ischemia-reperfusion leads to long term infiltration of activated and effector-memory T lymphocytes. Kidney Int. 2009;75:526–535. doi: 10.1038/ki.2008.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba J., Zudaire J.J., Robles J.E., Tienza A., Rosell D., Berian J.M., Pascual I. Is there a safe cold ischemia time interval for the renal graft? Actas Urol. Esp. 2011;35:475–480. doi: 10.1016/j.acuro.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Basile D.P., Donohoe D., Roethe K., Osborn J.L. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Renal Physiol. 2001;281:F887–99. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- Benichou G., Thomson A.W. Direct versus indirect allorecognition pathways: on the right track. Am. J. Transplant. 2009;9:655–656. doi: 10.1111/j.1600-6143.2009.02572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benichou G., Yamada Y., Yun S.H., Lin C., Fray M., Tocco G. Immune recognition and rejection of allogeneic skin grafts. Immunotherapy. 2011;3:757–770. doi: 10.2217/imt.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M.E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Bos E.M., Wang R., Snijder P.M., Boersema M., Damman J., Fu M., Moser J., Hillebrands J.L., Ploeg R.J., Yang G., Leuvenink H.G., Van Goor H. Cystathionine gamma-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J. Am. Soc. Nephrol. 2013;24:759–770. doi: 10.1681/ASN.2012030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan T.V., Freise C.E., Fuller T.F., Bostrom A., Tomlanovich S.J., Feng S. Early graft function after living donor kidney transplantation predicts rejection but not outcomes. Am. J. Transplant. 2004;4:971–979. doi: 10.1111/j.1600-6143.2004.00441.x. [DOI] [PubMed] [Google Scholar]
- Brenner B.M., Lawler E.V., Mackenzie H.S. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774–1777. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- Bruno S., Grange C., Deregibus M.C., Calogero R.A., Saviozzi S., Collino F., Morando L., Busca A., Falda M., Bussolati B., Tetta C., Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno S., Grange C., Collino F., Deregibus M.C., Cantaluppi V., Biancone L., Tetta C., Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casiraghi F., Remuzzi G., Perico N. Mesenchymal stromal cells to promote kidney transplantation tolerance. Curr. Opin. Organ Transplant. 2014;19:47–53. doi: 10.1097/MOT.0000000000000035. [DOI] [PubMed] [Google Scholar]
- Chadha R., Heidt S., Jones N.D., Wood K.J. Th17: contributors to allograft rejection and a barrier to the induction of transplantation tolerance? Transplantation. 2011;91:939–945. doi: 10.1097/TP.0b013e3182126eeb. [DOI] [PubMed] [Google Scholar]
- Chapman J.R., O'connell P.J., Nankivell B.J. Chronic renal allograft dysfunction. J. Am. Soc. Nephrol. 2005;16:3015–3026. doi: 10.1681/ASN.2005050463. [DOI] [PubMed] [Google Scholar]
- Chaumont M., Racape J., Broeders N., EL Mountahi F., Massart A., Baudoux T., Hougardy J.M., Mikhalsky D., Hamade A., LE Moine A., Abramowicz D., Vereerstraeten P. Delayed graft function in kidney transplants: time evolution, role of acute rejection, risk factors, and impact on patient and graft outcome. J. Transp. Secur. 2015;2015:163757. doi: 10.1155/2015/163757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Layton A.T., Edwards A. A mathematical model of O2 transport in the rat outer medulla. I. Model formulation and baseline results. Am. J. Physiol. Renal Physiol. 2009;297:F517–36. doi: 10.1152/ajprenal.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Qian H., Zhu W., Zhang X., Yan Y., Ye S., Peng X., Li W., Xu W. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev. 2011;20:103–113. doi: 10.1089/scd.2009.0495. [DOI] [PubMed] [Google Scholar]
- Dahlquist G., Stattin E.L., Rudberg S. Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy; a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrol. Dial. Transplant. 2001;16:1382–1386. doi: 10.1093/ndt/16.7.1382. [DOI] [PubMed] [Google Scholar]
- de Deken J., Kocabayoglu P., Moers C. Hypothermic machine perfusion in kidney transplantation. Curr. Opin. Organ Transplant. 2016;21:294–300. doi: 10.1097/MOT.0000000000000306. [DOI] [PubMed] [Google Scholar]
- Devarajan P. Update on mechanisms of ischemic acute kidney injury. J. Am. Soc. Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- Dong X., Swaminathan S., Bachman L.A., Croatt A.J., Nath K.A., Griffin M.D. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 2007;71:619–628. doi: 10.1038/sj.ki.5002132. [DOI] [PubMed] [Google Scholar]
- Doyle M.B., Collins K., Vachharajani N., Lowell J.A., Shenoy S., Nalbantoglu I., Byrnes K., Garonzik-Wang J., Wellen J., Lin Y., Chapman W.C. Outcomes using grafts from donors after cardiac death. J. Am. Coll. Surg. 2015;221:142–152. doi: 10.1016/j.jamcollsurg.2015.03.053. [DOI] [PubMed] [Google Scholar]
- Du T., Zou X., Cheng J., Wu S., Zhong L., Ju G., Zhu J., Liu G., Zhu Y., Xia S. Human Wharton's jelly-derived mesenchymal stromal cells reduce renal fibrosis through induction of native and foreign hepatocyte growth factor synthesis in injured tubular epithelial cells. Stem Cell Res Ther. 2013;4:59. doi: 10.1186/scrt215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield J.S., Bonventre J.V. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int. 2005;68:1956–1961. doi: 10.1111/j.1523-1755.2005.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield J.S., Park K.M., Hsiao L.L., Kelley V.R., Scadden D.T., Ichimura T., Bonventre J.V. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J. Clin. Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine L.G., Norman J.T. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- Fine L.G., Bandyopadhay D., Norman J.T. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int. Suppl. 2000;75:S22–6. [PubMed] [Google Scholar]
- Fiorina P., Venturini M., Folli F., Losio C., Maffi P., Placidi C., LA Rosa S., Orsenigo E., Socci C., Capella C., DEL Maschio A., Secchi A. Natural history of kidney graft survival, hypertrophy, and vascular function in end-stage renal disease type 1 diabetic kidney-transplanted patients: beneficial impact of pancreas and successful islet cotransplantation. Diabetes Care. 2005;28:1303–1310. doi: 10.2337/diacare.28.6.1303. [DOI] [PubMed] [Google Scholar]
- Fuquay R., Renner B., Kulik L., Mccullough J.W., Amura C., Strassheim D., Pelanda R., Torres R., Thurman J.M. Renal ischemia-reperfusion injury amplifies the humoral immune response. J. Am. Soc. Nephrol. 2013;24:1063–1072. doi: 10.1681/ASN.2012060560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagandeep S., Matsuoka L., Mateo R., Cho Y.W., Genyk Y., Sher L., Cicciarelli J., Aswad S., Jabbour N., Selby R. Expanding the donor kidney pool: utility of renal allografts procured in a setting of uncontrolled cardiac death. Am. J. Transplant. 2006;6:1682–1688. doi: 10.1111/j.1600-6143.2006.01386.x. [DOI] [PubMed] [Google Scholar]
- Galderisi U., Giordano A. The gap between the physiological and therapeutic roles of mesenchymal stem cells. Med. Res. Rev. 2014;34:1100–1126. doi: 10.1002/med.21322. [DOI] [PubMed] [Google Scholar]
- Giral M., Nguyen J.M., Karam G., Kessler M., Hurault De Ligny B., Buchler M., Bayle F., Meyer C., Foucher Y., Martin M.L., Daguin P., Soulillou J.P. Impact of graft mass on the clinical outcome of kidney transplants. J. Am. Soc. Nephrol. 2005;16:261–268. doi: 10.1681/ASN.2004030209. [DOI] [PubMed] [Google Scholar]
- Gobe G., Willgoss D., Hogg N., Schoch E., Endre Z. Cell survival or death in renal tubular epithelium after ischemia-reperfusion injury. Kidney Int. 1999;56:1299–1304. doi: 10.1046/j.1523-1755.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- Goransson V., Johnsson C., Jacobson A., Heldin P., Hallgren R., Hansell P. Renal hyaluronan accumulation and hyaluronan synthase expression after ischaemia-reperfusion injury in the rat. Nephrol. Dial. Transplant. 2004;19:823–830. doi: 10.1093/ndt/gfh003. [DOI] [PubMed] [Google Scholar]
- Gueler F., Shushakova N., Mengel M., Hueper K., Chen R., Liu X., Park J.K., Haller H., Wensvoort G., Rong S. A novel therapy to attenuate acute kidney injury and ischemic allograft damage after allogenic kidney transplantation in mice. PLoS One. 2015;10:e0115709. doi: 10.1371/journal.pone.0115709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan S., Johnson C.P., Bresnahan B.A., Taranto S.E., Mcintosh M.J., Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N. Engl. J. Med. 2000;342:605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- Helal I., Fick-Brosnahan G.M., Reed-Gitomer B., Schrier R.W. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 2012;21:293–300. doi: 10.1038/nrneph.2012.19. [DOI] [PubMed] [Google Scholar]
- Hellegering J., Visser J., Kloke H.J., D'ancona F.C., Hoitsma A.J., van der Vliet J.A., Warle M.C. Poor early graft function impairs long-term outcome in living donor kidney transplantation. World J. Urol. 2013;31:901–906. doi: 10.1007/s00345-012-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J.N., Vollmar B., Laschke M.W., Fertmann J.M., Jauch K.W., Menger M.D. Microcirculatory alterations in ischemia-reperfusion injury and sepsis: effects of activated protein C and thrombin inhibition. Crit. Care. 2005;9(Suppl. 4):S33–7. doi: 10.1186/cc3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosgood S.A., Nicholson M.L. First in man renal transplantation after ex vivo normothermic perfusion. Transplantation. 2011;92:735–738. doi: 10.1097/TP.0b013e31822d4e04. [DOI] [PubMed] [Google Scholar]
- Hosgood S.A., Saeb-Parsy K., Hamed M.O., Nicholson M.L. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am. J. Transplant. 2016;16:3282–3285. doi: 10.1111/ajt.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosgood S.A., Saeb-Parsy K., Wilson C., Callaghan C., Collett D., Nicholson M.L. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibers M., de Jonge N., van Kuik J., Koning E.S., van Wichen D., Dullens H., Schipper M., de Weger R. Intimal fibrosis in human cardiac allograft vasculopathy. Transpl. Immunol. 2011;25:124–132. doi: 10.1016/j.trim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Hunter J.P., Hosgood S.A., Patel M., Rose R., Read K., Nicholson M.L. Effects of hydrogen sulphide in an experimental model of renal ischaemia-reperfusion injury. Br. J. Surg. 2012;99:1665–1671. doi: 10.1002/bjs.8956. [DOI] [PubMed] [Google Scholar]
- Imberti B., Morigi M., Tomasoni S., Rota C., Corna D., Longaretti L., Rottoli D., Valsecchi F., Benigni A., Wang J., Abbate M., Zoja C., Remuzzi G. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J. Am. Soc. Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- Iordanous Y., Seymour N., Young A., Johnson J., Iansavichus A.V., Cuerden M.S., Gill J.S., Poggio E., Garg A.X. Recipient outcomes for expanded criteria living kidney donors: the disconnect between current evidence and practice. Am. J. Transplant. 2009;9:1558–1573. doi: 10.1111/j.1600-6143.2009.02671.x. [DOI] [PubMed] [Google Scholar]
- Irani Y., Pype J.L., Martin A.R., Chong C.F., Daniel L., Gaudart J., Ibrahim Z., Magalon G., Lemaire M., Hardwigsen J. Noble gas (argon and xenon)-saturated cold storage solutions reduce ischemia-reperfusion injury in a rat model of renal transplantation. Nephron Extra. 2011;1:272–282. doi: 10.1159/000335197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M.H., Li G., Liu J., Liu L., Wu B., Huang W., He W., Deng C., Wang D., Li C., Lahn B.T., Shi C., Xiang A.P. Nestin(+) kidney resident mesenchymal stem cells for the treatment of acute kidney ischemia injury. Biomaterials. 2015;50:56–66. doi: 10.1016/j.biomaterials.2015.01.029. [DOI] [PubMed] [Google Scholar]
- Jurewicz M., Takakura A., Augello A., Naini S.M., Ichimura T., Zandi-Nejad K., Abdi R. Ischemic injury enhances dendritic cell immunogenicity via TLR4 and NF-kappa B activation. J. Immunol. 2010;184:2939–2948. doi: 10.4049/jimmunol.0901889. [DOI] [PubMed] [Google Scholar]
- Kayler L.K., Magliocca J., Zendejas I., Srinivas T.R., Schold J.D. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis. Am. J. Transplant. 2011;11:2647–2656. doi: 10.1111/j.1600-6143.2011.03741.x. [DOI] [PubMed] [Google Scholar]
- Kayler L., Yu X., Cortes C., Lubetzky M., Friedmann P. Impact of cold ischemia time in kidney transplants from donation after circulatory death donors. Transplant. Direct. 2017;3:e177. doi: 10.1097/TXD.0000000000000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey G.R., Sharma R., Huang L., Li L., Vergis A.L., Ye H., Ju S.T., Okusa M.D. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2009;20:1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchens W.H., Chase C.M., Uehara S., Cornell L.D., Colvin R.B., Russell P.S., Madsen J.C. Macrophage depletion suppresses cardiac allograft vasculopathy in mice. Am. J. Transplant. 2007;7:2675–2682. doi: 10.1111/j.1600-6143.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- Ko G.J., Boo C.S., Jo S.K., Cho W.Y., Kim H.K. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol. Dial. Transplant. 2008;23:842–852. doi: 10.1093/ndt/gfm694. [DOI] [PubMed] [Google Scholar]
- Koo D.D., Welsh K.I., Roake J.A., Morris P.J., Fuggle S.V. Ischemia/reperfusion injury in human kidney transplantation: an immunohistochemical analysis of changes after reperfusion. Am. J. Pathol. 1998;153:557–566. doi: 10.1016/S0002-9440(10)65598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A.R., Wong G., Chapman J.R., Coates P.T., Russ G.R., Pleass H., Russell C., He B., Lim W.H. Prolonged ischemic time, delayed graft function, and graft and patient outcomes in live donor kidney transplant recipients. Am. J. Transplant. 2016;16:2714–2723. doi: 10.1111/ajt.13817. [DOI] [PubMed] [Google Scholar]
- Lee S.Y., Chung B.H., Piao S.G., Kang S.H., Hyoung B.J., Jeon Y.J., Hwang H.S., Yoon H.E., Choi B.S., Kim J.I., Moon I.S., Kim Y.S., Choi Y.J., Yang C.W. Clinical significance of slow recovery of graft function in living donor kidney transplantation. Transplantation. 2010;90:38–43. doi: 10.1097/TP.0b013e3181e065a2. [DOI] [PubMed] [Google Scholar]
- Legrand M., Mik E.G., Johannes T., Payen D., Ince C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol. Med. 2008;14:502–516. doi: 10.2119/2008-00006.Legrand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Okusa M.D. Blocking the immune response in ischemic acute kidney injury: the role of adenosine 2A agonists. Nat. Clin. Pract. Nephrol. 2006;2:432–444. doi: 10.1038/ncpneph0238. [DOI] [PubMed] [Google Scholar]
- Li L., Okusa M.D. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin. Nephrol. 2010;30:268–277. doi: 10.1016/j.semnephrol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Cohen A., Hudson T.E., Motlagh D., Amrani D.L., Duffield J.S. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation. 2010;121:2211–2220. doi: 10.1161/CIRCULATIONAHA.109.928796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechty K.W., Mackenzie T.C., Shaaban A.F., Radu A., Moseley A.M., Deans R., Marshak D.R., Flake A.W. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat. Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- Liu N., Tian J., Cheng J., Zhang J. Migration of CXCR4 gene-modified bone marrow-derived mesenchymal stem cells to the acute injured kidney. J. Cell. Biochem. 2013;114:2677–2689. doi: 10.1002/jcb.24615. [DOI] [PubMed] [Google Scholar]
- Lobb I., Zhu J., Liu W., Haig A., Lan Z., Sener A. Hydrogen sulfide treatment ameliorates long-term renal dysfunction resulting from prolonged warm renal ischemia-reperfusion injury. Can. Urol. Assoc. J. 2014;8:E413–8. doi: 10.5489/cuaj.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher P.D., Rossaint J., Rossaint R., Weis J., Fries M., Fahlenkamp A., Ryang Y.M., Grottke O., Coburn M. Argon: neuroprotection in in vitro models of cerebral ischemia and traumatic brain injury. Crit. Care. 2009;13:R206. doi: 10.1186/cc8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Lim T., Xu J., Tang H., Wan Y., Zhao H., Hossain M., Maxwell P.H., Maze M. Xenon preconditioning protects against renal ischemic-reperfusion injury via HIF-1alpha activation. J. Am. Soc. Nephrol. 2009;20:713–720. doi: 10.1681/ASN.2008070712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckeown D.W., Bonser R.S., Kellum J.A. Management of the heartbeating brain-dead organ donor. Br. J. Anaesth. 2012;108(Suppl. 1):i96–107. doi: 10.1093/bja/aer351. [DOI] [PubMed] [Google Scholar]
- Metcalfe W. How does early chronic kidney disease progress? A background paper prepared for the UK Consensus Conference on early chronic kidney disease. Nephrol. Dial. Transplant. 2007;22(Suppl. 9):ix26–30. doi: 10.1093/ndt/gfm446. [DOI] [PubMed] [Google Scholar]
- Mitchell R.N. Graft vascular disease: immune response meets the vessel wall. Annu. Rev. Pathol. 2009;4:19–47. doi: 10.1146/annurev.pathol.3.121806.151449. [DOI] [PubMed] [Google Scholar]
- Mitchell R.N., Libby P. Vascular remodeling in transplant vasculopathy. Circ. Res. 2007;100:967–978. doi: 10.1161/01.RES.0000261982.76892.09. [DOI] [PubMed] [Google Scholar]
- Moers C., Smits J.M., Maathuis M.H., Treckmann J., van Gelder F., Napieralski B.P., van Kasterop-Kutz M., van der Heide J.J., Squifflet J.P., van Heurn E., Kirste G.R., Rahmel A., Leuvenink H.G., Paul A., Pirenne J., Ploeg R.J. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2009;360:7–19. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
- Molitoris B.A., Sutton T.A. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 2004;66:496–499. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- Monteiro Carvalho Mori Da Cunha M.G., Zia S., Oliveira Arcolino F., Carlon M.S., Beckmann D.V., Pippi N.L., Luhers Graca D., Levtchenko E., Deprest J., Toelen J. Amniotic fluid derived stem cells with a renal progenitor phenotype inhibit interstitial fibrosis in renal ischemia and reperfusion injury in rats. PLoS One. 2015;10:e0136145. doi: 10.1371/journal.pone.0136145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A., Faleo G., Shimizu H., Nakahira K., Kohmoto J., Sugimoto R., Choi A.M., Mccurry K.R., Takahashi T., Murase N. Ex vivo carbon monoxide prevents cytochrome P450 degradation and ischemia/reperfusion injury of kidney grafts. Kidney Int. 2008;74:1009–1016. doi: 10.1038/ki.2008.342. [DOI] [PubMed] [Google Scholar]
- Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- Nankivell B.J., Chapman J.R. Chronic allograft nephropathy: current concepts and future directions. Transplantation. 2006;81:643–654. doi: 10.1097/01.tp.0000190423.82154.01. [DOI] [PubMed] [Google Scholar]
- Nicholson M.L., Hosgood S.A. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am. J. Transplant. 2013;13:1246–1252. doi: 10.1111/ajt.12179. [DOI] [PubMed] [Google Scholar]
- Nizet V., Johnson R.S. Interdependence of hypoxic and innate immune responses. Nat. Rev. Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira J.M., Haririan A., Jacobs S.C., Weir M.R., Hurley H.A., Al-Qudah H.S., Phelan M., Drachenberg C.B., Bartlett S.T., Cooper M. The detrimental effect of poor early graft function after laparoscopic live donor nephrectomy on graft outcomes. Am. J. Transplant. 2009;9:337–347. doi: 10.1111/j.1600-6143.2008.02477.x. [DOI] [PubMed] [Google Scholar]
- Norman J.T. Protecting the microvasculature: a tie-ght connection to ameliorating chronic kidney disease? J. Am. Soc. Nephrol. 2006;17:2353–2355. doi: 10.1681/ASN.2006070741. [DOI] [PubMed] [Google Scholar]
- Norman J.T., Clark I.M., Garcia P.L. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- Ojo A.O., Wolfe R.A., Held P.J., Port F.K., Schmouder R.L. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968–974. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- Park J.S., Svetkauskaite D., He Q., Kim J.Y., Strassheim D., Ishizaka A., Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Peired A.J., Sisti A., Romagnani P. Mesenchymal stem cell-based therapy for kidney disease: a review of clinical evidence. Stem Cells Int. 2016;2016:4798639. doi: 10.1155/2016/4798639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Valdivia M.A., Gentil M.A., Toro M., Cabello M., Rodriguez-Benot A., Mazuecos A., Osuna A., Alonso M. Impact of cold ischemia time on initial graft function and survival rates in renal transplants from deceased donors performed in Andalusia. Transplant. Proc. 2011;43:2174–2176. doi: 10.1016/j.transproceed.2011.06.047. [DOI] [PubMed] [Google Scholar]
- Perico N., Cattaneo D., Sayegh M.H., Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004;364:1814–1827. doi: 10.1016/S0140-6736(04)17406-0. [DOI] [PubMed] [Google Scholar]
- Quiroga I., Mcshane P., Koo D.D., Gray D., Friend P.J., Fuggle S., Darby C. Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol. Dial. Transplant. 2006;21:1689–1696. doi: 10.1093/ndt/gfl042. [DOI] [PubMed] [Google Scholar]
- Rezkalla S.H., Kloner R.A. No-reflow phenomenon. Circulation. 2002;105:656–662. doi: 10.1161/hc0502.102867. [DOI] [PubMed] [Google Scholar]
- Ries C., Egea V., Karow M., Kolb H., Jochum M., Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- Robey T.E., Marcolini E.G. Organ donation after acute brain death: addressing limitations of time and resources in the emergency department. Yale J. Biol. Med. 2013;86:333–342. [PMC free article] [PubMed] [Google Scholar]
- Santos L.S., Chin E.W., Ioshii S.O., Tambara Filho R. Surgical reduction of the renal mass in rats: morphologic and functional analysis on the remnant kidney. Acta Cir. Bras. 2006;21:252–257. doi: 10.1590/s0102-86502006000400012. [DOI] [PubMed] [Google Scholar]
- Sasson A.N., Cherney D.Z. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J. Diabetes. 2012;3:1–6. doi: 10.4239/wjd.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers B.F., Flyvbjerg A., de Vriese A.S. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- Schroppel B., Legendre C. Delayed kidney graft function: from mechanism to translation. Kidney Int. 2014;86:251–258. doi: 10.1038/ki.2014.18. [DOI] [PubMed] [Google Scholar]
- Sert I., Colak H., Tugmen C., Dogan S.M., Karaca C. The effect of cold ischemia time on delayed graft function and acute rejection in kidney transplantation. Saudi J. Kidney Dis. Transpl. 2014;25:960–966. doi: 10.4103/1319-2442.139865. [DOI] [PubMed] [Google Scholar]
- Seun Kim Y., Soo Kim M., Suk Han D., Kee Kim D., Min Myoung S., Kim S., II, Park K. Evidence that the ratio of donor kidney weight to recipient body weight, donor age, and episodes of acute rejection correlate independently with live-donor graft function. Transplantation. 2002;74:280–283. doi: 10.1097/00007890-200207270-00021. [DOI] [PubMed] [Google Scholar]
- Sharfuddin A.A., Sandoval R.M., Berg D.T., Mcdougal G.E., Campos S.B., Phillips C.L., Jones B.E., Gupta A., Grinnell B.W., Molitoris B.A. Soluble thrombomodulin protects ischemic kidneys. J. Am. Soc. Nephrol. 2009;20:524–534. doi: 10.1681/ASN.2008060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohat J., Erman A., Boner G., Rosenfeld J. Mechanisms of the early and late response of the kidney to contralateral nephrectomy. Ren. Physiol. Biochem. 1991;14:103–111. doi: 10.1159/000173393. [DOI] [PubMed] [Google Scholar]
- Sigmon D.H., Gonzalez-Feldman E., Cavasin M.A., Potter D.L., Beierwaltes W.H. Role of nitric oxide in the renal hemodynamic response to unilateral nephrectomy. J. Am. Soc. Nephrol. 2004;15:1413–1420. doi: 10.1097/01.asn.0000130563.67384.81. [DOI] [PubMed] [Google Scholar]
- Simon F., Scheuerle A., Groger M., Stahl B., Wachter U., Vogt J., Speit G., Hauser B., Moller P., Calzia E., Szabo C., Schelzig H., Georgieff M., Radermacher P., Wagner F. Effects of intravenous sulfide during porcine aortic occlusion-induced kidney ischemia/reperfusion injury. Shock. 2011;35:156–163. doi: 10.1097/SHK.0b013e3181f0dc91. [DOI] [PubMed] [Google Scholar]
- Skaro A.I., Liwski R.S., O'neill J., Vessie E.L., Zhou J., Hirsch G.M., Lee T.D. Impairment of recipient cytolytic activity attenuates allograft vasculopathy. Transpl. Immunol. 2005;14:27–35. doi: 10.1016/j.trim.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Smyth L.A., Harker N., Turnbull W., El-Doueik H., Klavinskis L., Kioussis D., Lombardi G., Lechler R. The relative efficiency of acquisition of MHC:peptide complexes and cross-presentation depends on dendritic cell type. J. Immunol. 2008;181:3212–3220. doi: 10.4049/jimmunol.181.5.3212. [DOI] [PubMed] [Google Scholar]
- Smyth L.A., Lechler R.I., Lombardi G. Continuous acquisition of MHC:peptide complexes by recipient cells contributes to the generation of anti-graft CD8(+) T cell immunity. Am. J. Transplant. 2017;17:60–68. doi: 10.1111/ajt.13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squillaro T., Peluso G., Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- Steegh F.M., Gelens M.A., Nieman F.H., van Hooff J.P., Cleutjens J.P., van Suylen R.J., Daemen M.J., van Heurn E.L., Christiaans M.H., Peutz-Kootstra C.J. Early loss of peritubular capillaries after kidney transplantation. J. Am. Soc. Nephrol. 2011;22:1024–1029. doi: 10.1681/ASN.2010050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom T.B. Is transplantation tolerable? J. Clin. Invest. 2004;113:1681–1683. doi: 10.1172/JCI22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D.M., Johnson R.J., Allen J., Fuggle S.V., Collett D., Watson C.J., Bradley J.A. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet. 2010;376:1303–1311. doi: 10.1016/S0140-6736(10)60827-6. [DOI] [PubMed] [Google Scholar]
- Sutton T.A., Fisher C.J., Molitoris B.A. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- Tapiawala S.N., Tinckam K.J., Cardella C.J., Schiff J., Cattran D.C., Cole E.H., Kim S.J. Delayed graft function and the risk for death with a functioning graft. J. Am. Soc. Nephrol. 2010;21:153–161. doi: 10.1681/ASN.2009040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer L.A., Lundy S.K., Sarkar S., Fox D.A. Th17 cells in human disease. Immunol. Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togel F., Hu Z., Weiss K., Isaac J., Lange C., Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol. Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- Togel F., Isaac J., Hu Z., Weiss K., Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- Togel F., Zhang P., Hu Z., Westenfelder C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J. Cell. Mol. Med. 2009;13:2109–2114. doi: 10.1111/j.1582-4934.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treckmann J., Moers C., Smits J.M., Gallinat A., Maathuis M.H., van Kasterop-Kutz M., Jochmans I., Homan Van Der Heide J.J., Squifflet J.P., Van Heurn E., Kirste G.R., Rahmel A., Leuvenink H.G., Pirenne J., Ploeg R.J., Paul A. Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transpl. Int. 2011;24:548–554. doi: 10.1111/j.1432-2277.2011.01232.x. [DOI] [PubMed] [Google Scholar]
- Tripatara P., Patel N.S., Collino M., Gallicchio M., Kieswich J., Castiglia S., Benetti E., Stewart K.N., Brown P.A., Yaqoob M.M., Fantozzi R., Thiemermann C. Generation of endogenous hydrogen sulfide by cystathionine gamma-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab. Investig. 2008;88:1038–1048. doi: 10.1038/labinvest.2008.73. [DOI] [PubMed] [Google Scholar]
- Tyson M., Castle E., Andrews P., Heilman R., Mekeel K., Moss A., Mulligan D., Reddy K. Early graft function after laparoscopically procured living donor kidney transplantation. J. Urol. 2010;184:1434–1439. doi: 10.1016/j.juro.2010.06.013. [DOI] [PubMed] [Google Scholar]
- van der Merwe P.A., Dushek O. Mechanisms for T cell receptor triggering. Nat. Rev. Immunol. 2011;11:47–55. doi: 10.1038/nri2887. [DOI] [PubMed] [Google Scholar]
- Vergoulas G., Boura P., Efstathiadis G. Brain dead donor kidneys are immunologically active: is intervention justified? Hippokratia. 2009;13:205–210. [PMC free article] [PubMed] [Google Scholar]
- Wehner J., Morrell C.N., Reynolds T., Rodriguez E.R., Baldwin W.M., 3RD Antibody and complement in transplant vasculopathy. Circ. Res. 2007;100:191–203. doi: 10.1161/01.RES.0000255032.33661.88. [DOI] [PubMed] [Google Scholar]
- Wszola M., Kwiatkowski A., Domagala P., Wirkowska A., Bieniasz M., Diuwe P., Rafal K., Durlik M., Chmura A. Preservation of kidneys by machine perfusion influences gene expression and may limit ischemia/reperfusion injury. Prog. Transplant. 2014;24:19–26. doi: 10.7182/pit2014384. [DOI] [PubMed] [Google Scholar]
- Xu Z., Prathapasinghe G., Wu N., Hwang S.Y., Siow Y.L., O K. Ischemia-reperfusion reduces cystathionine-beta-synthase-mediated hydrogen sulfide generation in the kidney. Am. J. Physiol. Renal Physiol. 2009;297:F27–35. doi: 10.1152/ajprenal.00096.2009. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Discher D.J., Hu J., Bishopric N.H., Webster K.A. Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP. J. Biol. Chem. 2001;276:12645–12653. doi: 10.1074/jbc.M011344200. [DOI] [PubMed] [Google Scholar]
- Zhang J., Li Y., Chen J., Yang M., Katakowski M., Lu M., Chopp M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004;1030:19–27. doi: 10.1016/j.brainres.2004.09.061. [DOI] [PubMed] [Google Scholar]
- Zhao H., Ning J., Savage S., Kang H., Lu K., Zheng X., George A.J., Ma D. A novel strategy for preserving renal grafts in an ex vivo setting: potential for enhancing the marginal donor pool. FASEB J. 2013;27:4822–4833. doi: 10.1096/fj.13-236810. [DOI] [PubMed] [Google Scholar]