Abstract
Colorectal cancer (CRC) is one of the most common malignant cancers and the leading cause of cancer-related deaths in worldwide. Although the monoclonal antibody therapy is prescribed for CRC, the metastasis resistant to therapy is the major cause of death of patients with CRC, which indicating the urgent demands for new therapeutic targets discovery. Aquaporin 8 (AQP8) has been identified alter expressed in several cancers including breast cancer, lung cancer and prostatic carcinoma. Our study demonstrated the functional significance of AQP8 in CRC cells growth and metastasis. Over-expression of AQP8 remarkably decreased growth, aggressiveness and colony formation in the CRC SW480 and HT-29 cells. Mechanistically, AQP8 over-expression inhibited tumorigenic phenotype by inactivating PI3K/AKT signaling and inhibiting PCDH7 expression. Furthermore, in vivo studies using nude mice xenograft and metastasis model identified the pivotal role of AQP8 in CRC cells growth and metastasis. Taken together, the present study verifies the vital role of the endogenous AQP8 in colorectal cancer progression.
Keywords: AQP8, colorectal cancer, metastasis, PCDH7
Introduction
Colorectal cancer is the most frequently diagnosed malignant cancer and is the third leading cause of cancer deaths globally [1]. The distant metastasis of colorectal cancer cells is a multistage process, in which cells escape from primary cancer sites and establish metastasis foci at distant tissues [2]. The metastasis of colorectal cancer cells is closely associated with aberrant activation of diversified signaling pathways which promote mobility, invasion and survival of tumor cells [3]. Currently, chemotherapeutics and many targeted agents including antibodies against epidermal growth factor receptor (EGFR) are utilized in the therapy of metastatic CRC [4]. Unfortunately, less than 20 percent of patients possess wild type KRAS benefit from monoclonal antibodies. Meanwhile, targeting several down-stream kinases of EGFR, including B-RAF and MEK, exhibits inconspicuous efficacy in CRC treatment [5]. Thus, it is important to reveal new mechanism which drives the progression of CRC.
Cancer cells growth and metastasis depend on nutrient-supply and metabolism [6]. Aquaporins (AQPs) compose of a family of membrane transport proteins, which are expressed in a variety of epithelial tissues [7]. 13 AQPs members have been identified and subdivided into two groups: AQP1, 2, 4, 5 and 8 functions as water-selective transporters, while AQP3, 7, 9 and 10 which transport water as well as other small solutes. Recent evidences suggest that the over-expression of several AQPs is implicated into tumorigenesis, including tumor cells migration, angiogenesis and tumor growth [8]. For example, AQP5 is over-expressed in cervical cancer, breast cancer and ovarian cancer [9]. In hepatocellular carcinoma, the reduced expression of AQP8 is associated with increased resistance to apoptosis [10]. Furthermore, the expression of AQP6 in uterine serous carcinoma was obviously decreased as compared with normal tissues [11]. Previous study has identified that AQP8 is expressed in all normal colon samples [12] but not, or to a less extent, in the colorectal tumors. However, the molecular functions of AQP8 in CRC growth and metastasis remain unexplored.
In the present study, we reanalyzed the GEO data set GSE32323 [13] and compared the expression pattern of differential genes between CRC tissues and corresponding normal tissues. The results revealed that AQP8 was significant decreased in CRC tissues which consistent with the previous study. Although, AQP8 is identified down-regulated in CRC, its role in CRC cells growth and metastasis remains unknown. Herein, we reported that AQP8 was a potential independent prognostic factor for patients with CRC. Over-expression of AQP8 inhibited CRC cells growth, mobility and aggressiveness in vitro. Mechanistically, we demonstrated down-regulation of PI3K and AKT expression in AQP8 over-expression CRC cells. The anti-cancer function of AQP8 was partly dependent on inhibiting PI3K/AKT signaling axis. Co-expression analysis identified the gene PCDH7 significantly co-expressed with AQP8 in CRC. Subsequently, we demonstrated over-expression of AQP8 promoted CRC migration and invasion dependent on PCDH7. Furthermore, up-regulation of AQP8 in CRC cells confers sensitivity for chemotherapeutic agents. Finally, in vivo xenograft models, we demonstrated that over-expression of AQP8 affected CRC cells growth and metastasis. Altogether, our results illustrated the functional of AQP8 in CRC progression and its effectiveness as a potential therapeutic target in CRC patients.
Materials and methods
Cell lines and tissue samples
CRC cell lines (SW480, HT-29, Colo-205, LOVO and HCT116), normal colorectal epithelial cell line HCoEpiC and murine colon epithelial cells, YAMC and MSIE were purchased from the Guang Zhou Jennio Biotech Co., Ltd (GuangZhou, China) and were cultured in 1640 or DMEM supplemented with 10% FBS. 47 paired of CRC and adjacent normal tissues were obtained from CRC patients who underwent surgery at the Guangdong General Hospital. None of CRC patients had been treated with chemotherapy before surgery. All tissues were snap-frozen in liquid nitrogen and stored at -196°C until protein and RNA extraction. Our study was approved by the Research Ethics Committee of the Guangdong General Hospital.
Transient or transfection of CRC cells
To over-express AQP8, PI3K or AKT, we transfected SW480 and HT-29 cells with pcDNA4-myc/his-AQP8, pcDNA4-myc/his-PI3K, pcDNA4-myc/his-AKT or control vectors. Stably transfected cells were selected using G418 (500 µg/ml; Life Technologies, Carlsbad, CA, USA). To down-expression PCDH7, SW480 and HT-29 cells were transfected with PCDH7 shRNA plasmid (CCGGGCTGGCATTATGACGGTGATTCTCGAGAATCACCGTCATAATGCCAGCTTTTTG) or with control shRNA.
Cell proliferation assay
1 × 105 cells were cultured for 24 h, 48 h, 72 h and 96 h, respectively. At each time point, supernatant was removed and MTT (100 μl) was added into wells for additional 4 h incubation. The absorbance was determined at 490 nm.
Cell wound closure assay
1 × 106 CRC cells were seeded into six well plates until formed cell monolayer. A wound was generated using a 100 µl tip. After 24 h, the wounds were photographed, and percentage of wound closure was calculated [14].
Boyden invasion assay
1 × 104 cells were plated into the Boyden chamber inserts (Corning) which coated with basement membrane Matrix (BD, San Jose, CA, USA). Cells invasion analysis was conducted as described previously [15].
Colony formation analysis
CRC cells were cultured in 6 well plates (1000 cells per well). After CRC cells were cultured for two weeks, the cells colonies were stained with 0.1% crystal violet (Sigma-Aldrich, Dorset, USA) and counted.
Immunofluorescence assay
Cells were seeded in a chamber slide and staining was conducted with anti-AQP8, anti-PCDH7, anti-E-cadherin or anti-N-cadherin antibodies (Cell Signaling, Beverly, MA, USA) and secondary antibody (anti-human Alexa 488 or Alexa 647; Cell Signaling, Beverly, MA, USA). Cell nucleus was stained with DAPI (Beyotime Biotechnology, Nanjing, China).
Western blotting assay
Cell lysate was prepared using RIPA lysis buffer supplemented with proteinase inhibitors (Calbiochem, Darmstadt, Germany). Immunoblotting assay was conducted using anti-PI3K, anti-AKT or anti-GAPDH antibodies (Cell Signaling Technology, CA, USA). The signals were visualized using chemiluminescence system according to the instruction from manufacturer (GE Healthcare, Little Chalfont, USA).
Quantitative PCR
Total RNA was isolated from clinical samples or cultured cells with Trizol reagent (Invitrogen). The cDNA was synthesized from 1 ug total RNA using PrimeScript RT-polymerase (Thermo). qPCR reactions of target genes were conducted using SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA). GAPDH was used as an internal control. The comparative cycle threshold (Ct) method was applied to calculate the expression levels through calculating the 2(-ΔΔCt) method. Primer sequences were listed in the Supplementary Table 1.
Mouse xenograft experiment
BALB/c-nu mice (18-20 g) were purchased from SLAKE experimental animal limited company (Shanghai, China). 100 µl parental cells or AQP8 over-expressing CRC cells (5 × 106) were subcutaneously inoculated into the nude mice (n=6). Tumor growth was recorded every three days using digital calipers and tumor volumes were estimated using the formula: 0.5 × (L × W2), (L=length; W=width). In experimental metastasis model, (5 × 105) indicated SW480 cells were injected into nude mice via the lateral tail vein. Mice were sacrificed four weeks after inoculation and lung metastatic nodules were determined under a dissecting microscope [16]. All procedures were approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) of Guangdong General Hospital.
Statistical analysis
All values were presented as Mean ± SD. The differences were analyzed using Student’s t-test. P-value < 0.05 was considered significant.
Results
AQP8 is down-expressed in colon adenocarcinoma
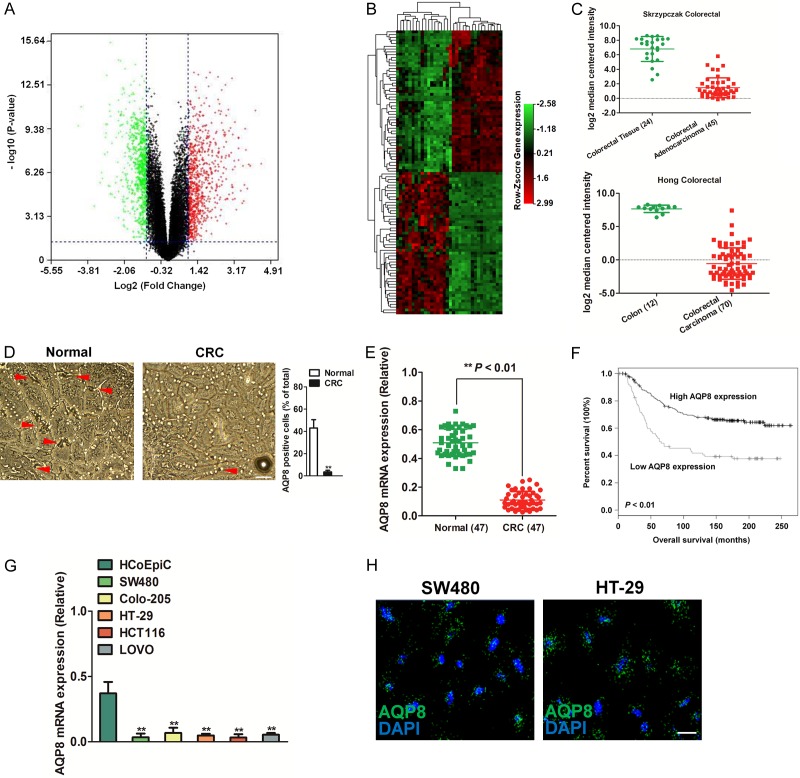
To identify the potential genes that were aberrantly expressed in human CRC, we analyzed the expression of diverse genes in cancerous and normal adjacent tissues using GEO data set GSE32323. A volcano plot showed up- or down-regulated genes from CRC samples compared with normal tissues (Figure 1A). The heat map generated using differential genes showed that AQP8 was remarkably down-regulated in CRC tissues (Figure 1B). Then, we queried the Oncomine database (http://oncomine.com) and found the over-expression of AQP8 in three CRC data-sets, namely, Skrzypczak et al [17] and Hong et al [18]. In both datasets, we observed significant AQP8 down-regulation in colon adenocarcinoma (Figure 1C). Analysis of other data-sets using TCGA and Kaiser et al [19] studies further supported the down-expression of AQP8 in CRC compared to normal tissues (Supplementary Figure 1A). In addition, we determined the level of AQP8 in 47 paired CRC tissues and corresponding normal tissues using immunohistochemistry (IHC) and qPCR analysis. The level of AQP8 was remarkably lower in CRC tissues than in adjacent normal tissues (Figure 1D and 1E). Kaplan-Meier survival analysis showed that patients with lower AQP8 expression had shorter overall survival (Figure 1F). Next, we assessed the expression of AQP8 in various colon adenocarcinoma cell lines. This identified SW480 cell line had the lowest AQP8 expression followed by HCT116, HT-29, LOVO and Colo-205 (Figure 1G). The expression of AQP8 protein in SW480 and HT-29 cells was confirmed by immunofluorescence (Figure 1H). All these results demonstrated that AQP8 was down-expressed in CRC.
Figure 1.
The expression of AQP8 in CRC. A. Volcano Plot. Genes with fold change ≥ 2 and statistical significance were marked with green or red dots. B. The differentially expressed genes between CRC tissues and normal tissues were analyzed using GEO data-set GSE32323. C. AQP8 expression in CRC of two independent cohorts from Oncomine database. D. Immunohistochemical staining of AQP8 in CRC tissues and corresponding normal tissues. Scale bar: 200 μm. E. The mRNA level of AQP8 was determined by qPCR in 47 paired CRC tissues and normal tissues. F. Kaplan-Meier plots shown overall survival in CRC patients with different level of AQP8. G. Relative expression of AQP8 in a panel of colorectal cancer cell lines. **P < 0.01 compared to cells transfected with HCoEpic cells. H. Expression of AQP8 in SW480 cells and HT-29 cells was measured by immunofluorescence. Scale bar: 50 μm.
The role of AQP8 in CRC cells growth and invasion
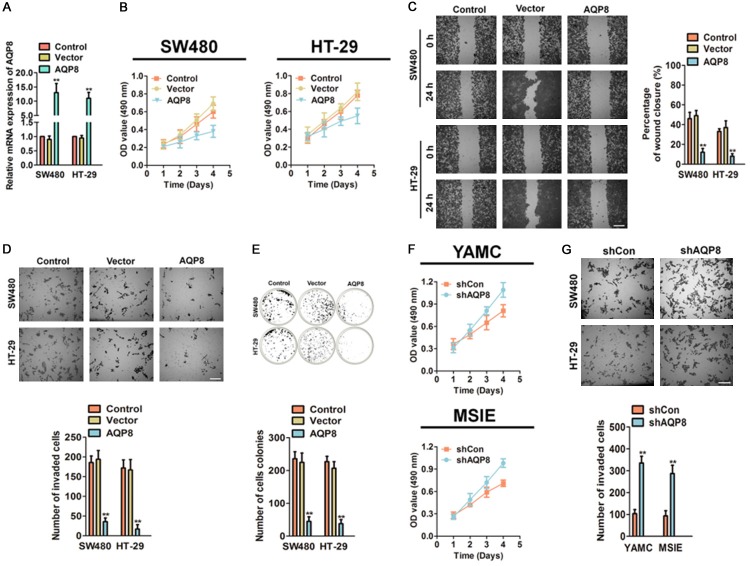
To identify the function role of AQP8 in CRC progression, AQP8 over-expressing SW480 and HT-29 cells were generated using pcDNA4-myc/his-AQP8. The transfection efficiency of AQP8 in both CRC cells was measured by qPCR (Figure 2A) compared with cells transfected with vector. To investigate the role of AQP8 in cell growth, we conducted MTT analysis with pooled AQP8 over-expressing SW480 and HT-29 as well as control cells. As shown in Figure 2B, AQP8 over-expression CRC cells exhibited significantly decreased proliferation as compared to control cells. To confirm the effect of AQP8 in cells migration and invasion, wound healing and Boyden chamber invasion analysis were conducted. Ectopic expression of AQP8 decreased both CRC cell lines migration (Figure 2C) and invasion (Figure 2D) as compared with control cells. Furthermore, the colonies formed by AQP8 over-expressing CRC cells were significantly decreased in soft agar assay as compared with the control cells (Figure 2E). To elaborate the effect of AQP8 in normal colon cells, immortalized colon epithelial cells were transfected with shRNA targeting AQP8 (shAQP8) and subjected to MTT and invasion analysis. AQP8 down-expression in YAMC and MSIE cells resulted in remarkably increase in growth (Figure 2F) and aggressive (Figure 2G). Altogether, these results demonstrated that AQP8 had an important role in CRC cell proliferation, mobility and invasion in vitro.
Figure 2.
Over-expression of AQP8 inhibits cells growth, invasion and colony formation. A. SW480 and HT-29 cells were transfected with pcDNA4-myc/his-AQP8 or pcDNA4-myc/his vector and the mRNA of AQP8 were analyzed by qPCR. B. Cell proliferation of control CRC cells and AQP8 over-expressing cells was determined by MTT analysis. C. The mobility of AQP8 over-expressing cells was assessed by wound healing analysis. Scale bar: 200 μm. D. Boyden invasion assay was conducted using control cells and CRC cells transfected with AQP8. Scale bar: 200 μm. E. Colony formation assay was conducted to evaluate the anchorage-independent growth of indicated cells. **P < 0.01 compared to control cells. F. AQP8 knocked-down accelerated cell proliferation in YAMC and MSIE cells as shown in MTT analysis. G. Cell invasion was determined by Boyden invasion assay using AQP8 knocked-down YAMC and MSIE cells. Scale bar: 200 μm. **P < 0.01 compared to cells transfected with shCon.
AQP8 inhibits PI3K/AKT signaling and EMT in CRC cells
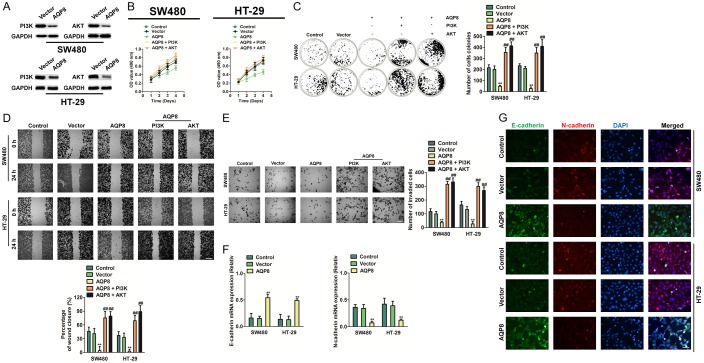
Phosphatidylinositol 3-kinase (PI3K/AKT) signaling pathway is known to drive cancer cells growth and survival [20]. Hence, to elucidate the function of AQP8 in CRC cell growth, we conducted western blotting for PI3K and AKT in stable AQP8 over-expression SW480 and HT-29 cells. We found significant down-expression of PI3K and AKT in AQP8 over-expression as compared with to control cells (Figure 3A). Furthermore, we sought to investigate whether recued PI3K/AKT in AQP8 over-expressing CRC cells could hamper AQP8-mediated effects. We transfected with both CRC cells with pcDNA4-myc/his-PI3K or pcDNA4-myc/his-AKT, respectively and found PI3K/AKT significantly rescued CRC cells proliferation and colony formation, when compared with SW480 transfected with AQP8 alone (Figure 3B, 3C). Consistently, significant induction in CRC cells mobility and invasion was observed on PI3K or AKT over-expression treatment (Figure 3D, 3E). Then, we carried out qPCR and cell immunofluorescence analyses with two cell lines. We demonstrated that the expression of AQP8 correlated with the levels of EMT markers: the level of the epithelial marker (E-cadherin) was higher in the AQP8 over-expression CRC cells than in control cells whereas the expression of the mesenchymal marker (N-cadherin) was lower in the AQP8 over-expression CRC cells than in control cells (Figure 3F, 3G).
Figure 3.
Over-expression of AQP8 inhibits PI3K/AKT signaling and alters the expression of E-cadherin/N-cadherin. A. SW480 and HT-29 cells were transfected with pcDNA4-myc/his-AQP8 or pcDNA4-myc/his vector and the expression of PI3K and AKT were determined by western blotting. B. Cell proliferation assay using cells co-transfected with AQP8 and PI3K or AKT. C. SW480 and HT-29 cells were co-transfected with pcDNA4-myc/his-AQP8 or and pcDNA4-myc/his-PI3K or pcDNA4-myc/his-AKT. Colony formation assays were performed to determine the effect of PI3K/AKT over-expression on CRC cells growth in vitro. D. pcDNA4-myc/his-PI3K or pcDNA4-myc/his-AKT was transfected into AQP8 over-expressing CRC cells and wound closure analysis was conducted. Scale bar: 200 μm. E. Boyden chamber Matrigel invasion assay using indicated cells. Scale bar: 200 μm. **P < 0.01 compared to control cells. ##P < 0.01 compared to AQP8 over-expressing cells. F. qPCR analysis of E-cadherin and N-cadherin protein expression in indicated cell lines. qPCR results were standardized in relation to GAPDH. G. Cell immunofluorescence staining for E-cadherin (green), N-cadherin (red) and nuclei (DAPI; blue). Scale bar: 50 μm. **P < 0.01 compared to control cells.
The co-expression of PCDH7 with AQP8 in CRC
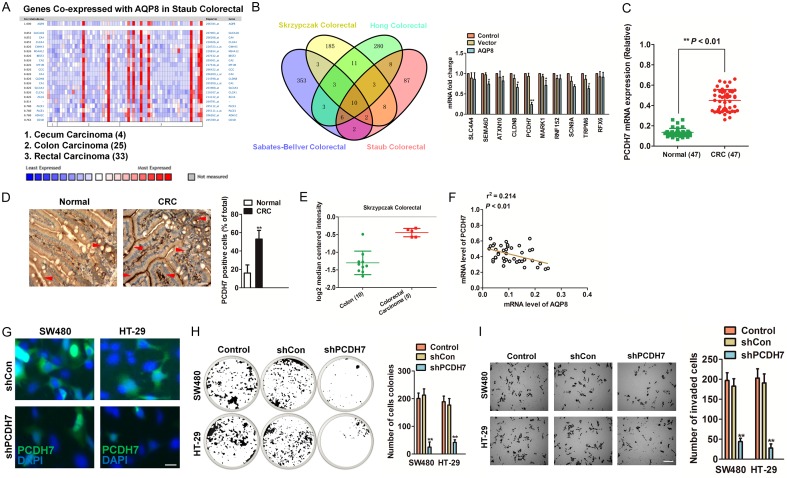
Co-expression analysis using four colorectal cancer datasets [17-19,21] from Oncomine was performed to identify a set of genes with co-expressed with AQP8 in CRC (Figure 4A and Supplementary Figure 2). qPCR assay was conducted to verify the common ten co-expressed genes that presents in the four co-expression datasets, and we found that PCDH7 was significantly decreased in AQP8 over-expressing SW480 cells as compared to the parental cells (Figure 4B). We future determined the PCDH7 level in the 47 cases of CRC samples and discovered that PCDH7 was higher in CRC than in normal tissues (Figure 4C, 4D). In Skrzypczak Colorectal [17] from Oncomine database (http://oncomine.com), the level of PDH7 was higher in CRC compared to normal (Figure 4E). To ascertain the co-expression of PCDH7 and AQP8 in CRC, we performed qPCR using CRC tissues. As expected, we find PCDH7 was negatively correlated with AQP8 in clinical samples (Figure 4F). To investigate the function of PCDH7 in CRC, we knocked-down PCDH7 by transfection of specific shRNA in both SW480 and HT-29 cells (Figure 4G). Colony formation and Transwell assay were performed to examine growth and invasion of CRC cells. In colonies formation assays, knocked-down of PCDH7 significantly reduced the number of colonies compared with the control (Figure 4H). Likewise, the number of invaded cells was markedly reduced in PCDH7-shRNA transfected SW480 and HT-29 cells (Figure 4I). All these data suggested that PCDH7 co-expression with AQP8 in CRC and PCDH7 knocked-down efficiently inhibit cells growth and aggressiveness.
Figure 4.
CST4 decreases PCDH7 expression in CRC. A. Co-expression data from Oncomine (www.oncomine.org). We used the following filters: gene ‘AQP8’ Analysis Type: ‘Co-expression analysis’ Cancer Type: ‘CRC’. The color changed according to a weaker (blue) or higher (red) expression. B. Venn graph represented the number of candidate co-expression genes determined by four datasets (left panel). qPCR analysis of genes in parental SW480 cells and the AQP8 over-expression SW480 cells (right panel). C. The relative mRNA expression of PCDH7 was evaluated by qPCR in 47 paired human normal colorectal tissues and CRC tissues. D. The level of PCDH7 was evaluated by IHC in normal colorectal tissues and CRC tissues. Scale bar: 200 μm. **P < 0.01 compared to normal. E. Box plots derived from gene expression data in Oncomine comparing expression of PCDH7 in normal and CRC tissue. F. The negative correlation analysis of PCDH7 and AQP8 in colorectal cancer tissues. G. SW480 and HT-29 cells were transfected with shRNA targeting PCDH7 (shPCDH7) and the expression of PCDH7 were analyzed by immunofluorescence. Scale bar: 50 μm. H. Colony formation assay was conducted to evaluate the anchorage-independent growth of indicated cells. I. Boyden chamber Matrigel invasion assay using control cells and CRC cells transfected with shPCDH7. Scale bar: 200 μm. *P < 0.05 compared to control cells, **P < 0.01 compared to control cells.
The function of AQP8 is dependent on PCDH7
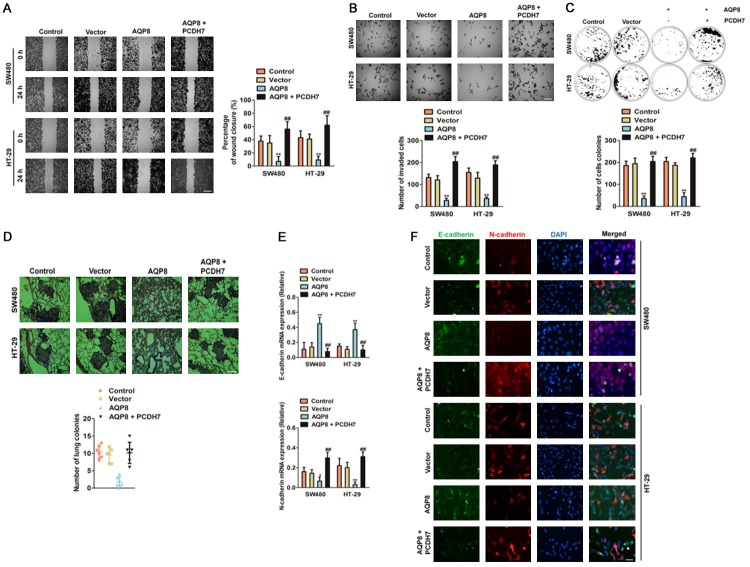
Encouraged by the above findings, we decided to investigate whether targeting PCDH7 abrogated the function of AQP8. We treated SW480 and HT-29 cells with PCDH7, and found that over-expression of PCDH7 rescued the cells migration (Figure 5A) and invasion (Figure 5B) that inhibited by AQP8. As expected, cells co-transfected with PCDH7 and AQP8 exhibited more number of cells colonies compared with cells transfected AQP8 (Figure 5C). To reveal the role of AQP8/PXDH7 in the CRC cells metastasis in vivo, the experimental metastasis analysis was conducted. Parental cells, AQP8 over-expressing SW480 cells, PCDH7 over-expressing SW480 cells and cells co-transfected with AQP8/PCDH7 were injected into nude mice via the lateral tail vein, respectively. We found that over-expression of PCDH7 significantly rescued the pulmonary metastasis of CRC cells, which briefly transfected with AQP8 (Figure 5D). Finally, we performed qPCR and cell immunofluorescence analyses to whether ectopic expression of PCDH7 rescued the expression of EMT associated markers. As expected, PCDH7 over-expression decreased the epithelial marker (E-cadherin) and increased the mesenchymal marker (N-cadherin) in the CRC cells transfected with AQP8 (Figure 5E, 5F). These results implied that AQP8/PXDH7 axis is associated the metastasis of CRC cells and the anti-cancer function of AQP8 was dependent on PCDH7.
Figure 5.
AQP8 inhibits the growth and metastasis of CRC cells dependent on PCDH7. A. HT-29 and SW480 cells were co-transfected with the AQP8 and the PCDH7 plasmid, the mobility of cells was determined by wound healing assays. Scale bar: 200 μm. B. HT-29 and SW480 cells were co-transfected with the AQP8 and the PCDH7 plasmid, the invasion of cells was determined by Transwell invasion assays. Scale bar: 200 μm. C. PCDH7 over-expression counteracted the inhibition effects of colony formation in AQP8 over-expression CRC cells. D. SW480 cells were co-transfected with lentiviral particles containing AQP8 and PCDH7. The metastasis of cells was analyzed in vivo metastasis xenograft experiment. E. Over-expression of PCDH7 reverse the effect of the AQP8 up-regulation on expression of EMT associated markers in CRC cells. **P < 0.01 compared to control cells, ##P < 0.01 compared to AQP8 over-expression cells. F. HT-29 and SW480 cells were co-transfected with the AQP8 and the PCDH7 plasmid, the expression of E-cadherin and N-cadherin were determined by immunofluorescence. Scale bar: 50 μm.
Up-regulation of AQP8 inhibits CRC growth in vivo and increases sensitivity to chemotherapeutic drugs
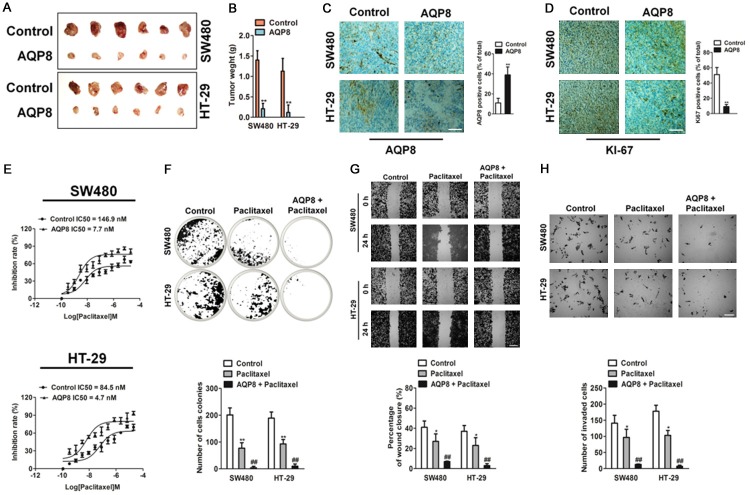
To investigate whether AQP8 affected the growth of CRC cells in vivo, parental and AQP8-overexpressing CRC cells were subcutaneously injected into nude mice. The results suggested that AQP8-overexpressing markedly suppressed tumor growth in vivo (Figure 6A). Tumors with AQP8 over-expression exhibited lower weight than control tumors (Figure 6B). The over-expression of AQP8 was future confirmed by immunohistochemical staining (Figure 6C), and tumors formed by AQP8 overexpression cells exhibited lower positive percentage of Ki67 than control tumors (Figure 6D). Then, we explored whether the alterations of AQP8 confer sensitivity toward chemotherapeutic agent, thus we conducted growth inhibition analysis with AQP8 over-expressing CRC cells under paclitaxel treatment. Interestingly, AQP8 over-expressing cells were found to be more sensitive to paclitaxel than control cells (Figure 6E). Next, we investigated the relative effect of paclitaxel on the colony formation, migration and invasion of AQP8 overexpression cells. Paclitaxel treatment resulted in more significantly inhibition in AQP8 over-expression cells colony formation as compared with the inhibition in control cells treated with paclitaxel (Figure 6F). Likewise, the percent inhibition of CRC cells migration and cells invasion with paclitaxel treatment was significantly higher in AQP8 over-expression cells that control cells treated with paclitaxel (Figure 6G, 6H). Collectively these results suggest that AQP8 over-expression inhibited CRC tumor growth in vivo and conferred sensitivity to paclitaxel in colon cancer cells.
Figure 6.
Over-expression of AQP8 inhibits colon cancer cells growth in vivo and confers sensitivity to paclitaxel. A. AQP8 overexpression inhibited tumor growth in vivo. Tumor pictures from mice inoculated with stable AQP8 overexpression CRC cell lines. Growth curve of tumor volume measured on indicated days. B. Tumor weight at the end of experiment. C. Photographs exhibited the IHC staining for AQP8 in tumors (left panel). Number of AQP8 positive cells in control and AQP8 stable expression tumors (right panel). Scale bar: 200 μm. D. Photographs exhibited the IHC staining for Ki-67 in tumors. Number of Ki-67 positive cells in control and AQP8 stable expression tumors (right panel). Scale bar: 200 μm. **P < 0.01 compared to control. E. In vitro paclitaxel-induced cytotoxicity assay using AQP8 over-expression colon cancer cells. Cells were cultured in the presence of paclitaxel for indicated time and followed by MTT assay. IC50 value was calculated. F. Cells were cultured in the presence of paclitaxel and colony formation was analysis. G. Wound healing assay was conducted using indicated cells treated with paclitaxel (50 nM). Scale bar: 200 μm. H. Boyden chamber Matrigel invasion assay using indicated cells treated with paclitaxel (50 nM). Scale bar: 200 μm. *P < 0.01 compared to control cells, **P < 0.01 compared to control cells, ##P < 0.01 compared to control cells treated with paclitaxel alone.
Discussion
Colorectal carcinoma (CRC) is the digestive malignant cancer with high risk of cancer-associated death for its highly metastatic potential [22]. Recently, both the incident and mortality rate of patient with CRC continue to climb. Nevertheless, there are no efficacious therapeutic strategies for metastatic CRC owing to the complicated mechanisms behind CRC metastasis [23]. Herein, we identified that aquaporin 8 (AQP8) was remarkably decreased in CRC tissues as compared with that in corresponding normal tissues. Additionally, we investigated the prognostic effect of AQP8 in the survival of CRC patients. The Kaplan-Meier analysis suggested that CRC patients with low expression levels possess poor survival. Altogether, our findings suggested that AQP8 was closely associated with CRC progression and might function as an prognostic factor for the survival of patients.
AQP8 gene located on chromosome 16 p12, encodes a 261 amino acid protein, which participates into water metabolism. AQP8 is involved into the pathogenesis of inflammatory disease [9]. So far, the function of AQP8 in carcinogenesis is controversial. Previous studies show that AQP8 regulates esophageal cancer cells migration via the EGFR-ERK1/2 signaling pathway [24]. In addition, the increased AQP8 expression may play a role in transformation of cervical intraepithelial neoplasia (CIN) into cervical cancer, and in early invasion and lymphatic metastasis of cervical cancer [11]. By contrast, AQP8 is primarily expressed in paraneoplastic normal tissues and rarely existed in colorectal carcinoma [12]. Meanwhile, the molecular functions of AQP8 in CRC growth and metastasis remain unexplored.
Our research indicated that AQP8 had vital roles in CRC growth and metastasis. We demonstrated that AQP8 over-expression abrogated colorectal cancer cell line SW480 and HT-29 proliferation, aggressive and colony formation in vitro. Furthermore, colon epithelial cells exhibited significant increase in cell growth and invasion upon transfected with shAQP8. Our results also indicated that AQP8 over-expression in CRC cells remarkably suppressed the expression of PI3K and AKT. Finally, over-expression of the PI3K/AKT rescued CRC cell proliferation and invasion, which inhibited by AQP8 over-expression.
To precisely explore the mechanisms by which AQP8 contributed to CRC growth and invasion, we analyzed Oncomine databases and found the correlation coefficient for AQP8 and PCDH7 was high. PCDH7 is a member of protocadherins (PCDHs) family, belonging to cadherin superfamily [25]. The human PCDH7 gene is localized in chromosome 4p15, which encodes the membrane protein that is believed to function in cell-cell recognition, adhesion and signal transduction [26]. The protocadherin family can be classified into two groups: clustered PCDHs (PCDH α, β and γ family) and non-clustered PCDHs, based on their genomic structure [27]. PCDHs have been shown to be over-expressed in several malignancies and are correlated with cancer cells metastasis [28]. Here, we found that over-expression AQP8 inhibited CRC cell growth and metastasis through decreasing PCDH7 expression. Finally, we explored the possible function of AQP8 in CRC growth and metastasis in vivo. Consistent with the results in vitro, ectopic expression of AQP8 expression inhibited CRC tumor growth and lung metastasis in vivo. In conclusion, our study provided the molecular evidences, which support the vital role of a novel AQP8-PCDH7 signaling axis in growth and metastasis of colorectal carcinoma.
Acknowledgements
Clinical analysis of radiotherapy in the operation of local advanced colorectal cancer; The clinical application of 3D-laparoscopic gastro-cavity surgical technique (3D-ligs) in endogenous gastric mesenchymal tumors around cardia.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Tiwari R, Pandey SK, Goel S, Bhatia V, Shukla S, Jing X, Dhanasekaran SM, Ateeq B. SPINK1 promotes colorectal cancer progression by downregulating Metallothioneins expression. Oncogenesis. 2015;4:e162. doi: 10.1038/oncsis.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ro SH, Xue X, Ramakrishnan SK, Cho CS, Namkoong S, Jang I, Semple IA, Ho A, Park HW, Shah YM, Lee JH. Tumor suppressive role of sestrin2 during colitis and colon carcinogenesis. Elife. 2016;5:e12204. doi: 10.7554/eLife.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S, Han B, Zhang Q, Dou J, Wang F, Lin W, Sun Y, Peng G. Vasohibin-1 suppresses colon cancer. Oncotarget. 2015;6:7880–7898. doi: 10.18632/oncotarget.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D, Wei P, Peng Z, Huang C, Tang H, Jia Z, Cui J, Le X, Huang S, Xie K. The critical role of dysregulated FOXM1-PLAUR signaling in human colon cancer progression and metastasis. Clin Cancer Res. 2013;19:62–72. doi: 10.1158/1078-0432.CCR-12-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y, Mao F, Zuo X, Moussalli MJ, Elias E, Xu W, Shureiqi I. 15-LOX-1 suppression of hypoxia-induced metastatic phenotype and HIF-1alpha expression in human colon cancer cells. Cancer Med. 2014;3:472–484. doi: 10.1002/cam4.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu P, Zhao N, Sheng D, Hou J, Hao C, Yang X, Zhu B, Zhang S, Han Z, Wei L, Zhang L. Inhibition of growth and metastasis of colon cancer by delivering 5-fluorouracil-loaded Pluronic P85 copolymer micelles. Sci Rep. 2016;6:20896. doi: 10.1038/srep20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walz T, Fujiyoshi Y, Engel A. The AQP structure and functional implications. Handb Exp Pharmacol. 2009:31–56. doi: 10.1007/978-3-540-79885-9_2. [DOI] [PubMed] [Google Scholar]
- 8.Maugeri R, Schiera G, Di Liegro CM, Fricano A, Iacopino DG, Di Liegro I. Aquaporins and Brain Tumors. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17071029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verkman AS, Hara-Chikuma M, Papadopoulos MC. Aquaporins--new players in cancer biology. J Mol Med (Berl) 2008;86:523–529. doi: 10.1007/s00109-008-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo X, Sun T, Yang M, Li Z, Li Z, Gao Y. Prognostic value of combined aquaporin 3 and aquaporin 5 overexpression in hepatocellular carcinoma. Biomed Res Int. 2013;2013:206525. doi: 10.1155/2013/206525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang H, Shi Y, Tuokan T, Chen R, Wang X. Expression of aquaporin 8 and phosphorylation of Erk1/2 in cervical epithelial carcinogenesis: correlation with clinicopathological parameters. Int J Clin Exp Pathol. 2014;7:3928–3937. [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer H, Stenling R, Rubio C, Lindblom A. Differential expression of aquaporin 8 in human colonic epithelial cells and colorectal tumors. BMC Physiol. 2001;1:1. doi: 10.1186/1472-6793-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khamas A, Ishikawa T, Shimokawa K, Mogushi K, Iida S, Ishiguro M, Mizushima H, Tanaka H, Uetake H, Sugihara K. Screening for epigenetically masked genes in colorectal cancer Using 5-Aza-2’-deoxycytidine, microarray and gene expression profile. Cancer Genomics Proteomics. 2012;9:67–75. [PubMed] [Google Scholar]
- 14.Geng R, Tan X, Wu J, Pan Z, Yi M, Shi W, Liu R, Yao C, Wang G, Lin J, Qiu L, Huang W, Chen S. RNF183 promotes proliferation and metastasis of colorectal cancer cells via activation of NF-kappaB-IL-8 axis. Cell Death Dis. 2017;8:e2994. doi: 10.1038/cddis.2017.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G, Zhao R, Zhao X, Chen XI, Wang D, Jin Y, Liu XI, Zhao CI, Zhu Y, Ren C, Li M, Jin X, Zhang F, Zhong Z, Wang T, Li X. MicroRNA-181a enhances the chemotherapeutic sensitivity of chronic myeloid leukemia to imatinib. Oncol Lett. 2015;10:2835–2841. doi: 10.3892/ol.2015.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding C, Luo J, Li L, Li S, Yang L, Pan H, Liu Q, Qin H, Chen C, Feng J. Gab2 facilitates epithelial-to-mesenchymal transition via the MEK/ERK/MMP signaling in colorectal cancer. J Exp Clin Cancer Res. 2016;35:5. doi: 10.1186/s13046-015-0280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skrzypczak M, Goryca K, Rubel T, Paziewska A, Mikula M, Jarosz D, Pachlewski J, Oledzki J, Ostrowski J. Modeling oncogenic signaling in colon tumors by multidirectional analyses of microarray data directed for maximization of analytical reliability. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong Y, Downey T, Eu KW, Koh PK, Cheah PY. A ‘metastasis-prone’ signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clin Exp Metastasis. 2010;27:83–90. doi: 10.1007/s10585-010-9305-4. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser S, Park YK, Franklin JL, Halberg RB, Yu M, Jessen WJ, Freudenberg J, Chen X, Haigis K, Jegga AG, Kong S, Sakthivel B, Xu H, Reichling T, Azhar M, Boivin GP, Roberts RB, Bissahoyo AC, Gonzales F, Bloom GC, Eschrich S, Carter SL, Aronow JE, Kleimeyer J, Kleimeyer M, Ramaswamy V, Settle SH, Boone B, Levy S, Graff JM, Doetschman T, Groden J, Dove WF, Threadgill DW, Yeatman TJ, Coffey RJ Jr, Aronow BJ. Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biol. 2007;8:R131. doi: 10.1186/gb-2007-8-7-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen-Solal KA, Boregowda RK, Lasfar A. RUNX2 and the PI3K/AKT axis reciprocal activation as a driving force for tumor progression. Mol Cancer. 2015;14:137. doi: 10.1186/s12943-015-0404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staub E, Groene J, Heinze M, Mennerich D, Roepcke S, Klaman I, Hinzmann B, Castanos-Velez E, Pilarsky C, Mann B, Brummendorf T, Weber B, Buhr HJ, Rosenthal A. An expression module of WIPF1-coexpressed genes identifies patients with favorable prognosis in three tumor types. J Mol Med (Berl) 2009;87:633–644. doi: 10.1007/s00109-009-0467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binefa G, Rodriguez-Moranta F, Teule A, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol. 2014;20:6786–6808. doi: 10.3748/wjg.v20.i22.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson CM, Wei C, Ensor JE, Smolenski DJ, Amos CI, Levin B, Berry DA. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24:1207–1222. doi: 10.1007/s10552-013-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang H, Shi YH, Talaf TK, Lin C. Aquaporin-8 mediates human esophageal cancer Eca-109 cell migration via the EGFR-Erk1/2 pathway. Int J Clin Exp Pathol. 2014;7:7663–7671. [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SY, Yasuda S, Tanaka H, Yamagata K, Kim H. Non-clustered protocadherin. Cell Adh Migr. 2011;5:97–105. doi: 10.4161/cam.5.2.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Xiang H, Zhang Y, Wang J, Yu G. Loss of PCDH9 is associated with the differentiation of tumor cells and metastasis and predicts poor survival in gastric cancer. Clin Exp Metastasis. 2015;32:417–428. doi: 10.1007/s10585-015-9712-7. [DOI] [PubMed] [Google Scholar]
- 27.Lin YL, Wang YL, Fu XL, Li WP, Wang YH, Ma JG. Low expression of protocadherin7 (PCDH7) is a potential prognostic biomarker for primary non-muscle invasive bladder cancer. Oncotarget. 2016;7:28384–28392. doi: 10.18632/oncotarget.8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beukers W, Hercegovac A, Vermeij M, Kandimalla R, Blok AC, van der Aa MM, Zwarthoff EC, Zuiverloon TC. Hypermethylation of the polycomb group target gene PCDH7 in bladder tumors from patients of all ages. J Urol. 2013;190:311–316. doi: 10.1016/j.juro.2013.01.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.