Abstract
Ulcerative colitis (UC) is a chronic and nonspecific intestinal inflammatory disease, which may increase the risk of colon cancer. Andrographolide is a main active component of Andrographis paniculata. The anti-inflammatory ability of andrographolide suggested its potential therapeutic effect against UC. In the present study, elevated serum concentrations of proinflammatory factors, including (TNF)-α, interleukin (IL)-1β, IL-6 and IL-23, as well as increased percentages of Th17 cells (IL-17+CD4+ cells) in CD4+ cells were detected in UC patients compared to that in healthy donors. These data suggested that Th17 immune responses may involve in the pathogenesis of UC. Experimental colitis mouse model was then established. The results of hematoxylin and eosin staining demonstrated the therapeutic effect of andrographolide on colitis. Enzyme-linked immunosorbent assay (ELISA), flow cytometry and western blotting analyses showed that andrographolide could decreased the levels of proinflammatory factors TNF-α, IL-1β, IL-6 and IL-17A in the serum and in the colon tissues, reduced the percentages of Th17 cells in CD4+ cells, and suppressed the levels of IL-23, IL-17A, ROR-γt (key transcription factor of Th17 cells) and p-STAT3 in the colon tissues. Further, peripheral blood mononuclear cells (PBMCs) were isolated from UC patients and treated with various concentrations of andrographolide (0, 10, 20 and 30 μg/ml). Andrographolide also showed inhibitory effects on the levels of proinflammatory factors, the percentages of Th17 cells and the expression of relative proteins. Similar results were obtained in lipopolysaccharide-treated normal PBMCs. These data suggested that andrographolide may inhibit Th17 immune response via STAT3 signaling. In conclusion, we demonstrated that andrographolide inhibited the activity of IL-23/IL-17 axis and down-stream pro-inflammatory factors so as to suppress inflammation response, resulting in the relieving of UC.
Keywords: Ulcerative colitis, andrographolide, inflammatory response, IL-23/IL-17 axis
Introduction
Ulcerative colitis (UC), a chronic, nonspecific intestinal inflammatory disease, usually occurs in young adults with high recurrence rate. The incidence rate of UC has been increasing in recent years [1]. Although the pathogenesis of UC is not completely elucidated, it is generally accepted that UC is caused by the disordered immune response of genetic susceptible host to intestinal flora. It has been demonstrated that proinflammatory factors including tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 are closely positive correlated with UC pathogenesis [2]. IL-23/IL-17 axis has been reported to regulate the proinflammatory factors TNF-α, IL-1β and IL-6, thus may play a key role in the pathogenesis of UC [3]. Studies have demonstrated that the mRNA levels of IL-23R and IL-17 are increased in intestinal mucosa of UC patients, and that their mRNA levels were correlated with the disease activity score in UC patients [4,5]. Zhang et al. found that mice with IL-17R knockout or with IL-17R IgG treatment were more resistant to 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis as compared to wild type mice [6]. Elson et al. indicated that the antibody against IL-23 subunit p19 could decrease the expression of proinflammatory factors in intestinal tract, resulting in the apoptosis of Th17 cells and the inhibition of inflammatory in intestinal tract [7]. These literatures indicate the important role of IL-23/IL-17 axis in the process of UC.
Current treatments against UC including mesalazine, hormone and immunosuppressive preparation are accompanied with such problems as complications, inefficacy for some patients and high recurrence rate [5,8]. Although biological agents present excellent curative effect, serious complications, such as tuberculosis or lymphoma, as well as the high medical costs seriously limit its applied range [9]. Therefore, it is of significant importance to explore a novel, safe, efficient and economic method for the treatment of UC.
Andrographis paniculata (AP) is a traditional Chinese medicine with the effect of heatclearing, detoxifying and anti-inflammatory. It is often used to treat gastrointestinal and respiratory infectious diseases clinically [10]. Andrographolide as the main effective constituent extracted from AP belongs to diterpene lactones. Andrographolide possess curative effect on acute lung injury and bronchial asthma by inhibiting inflammatory response [11]. Studies have demonstrated that andrographolide could down-regulated the levels of TNF-α, IL-1β and IL-6 [12]. AP extract (HMPL-004) [13,14] and andrographolide sulfonate [15], a derivative of andrographolide could be used to treat UC and experimental colitis, respectively. However, the effects of andrographolide on IL-23/IL-17 axis in UC patients were not completely elucidated.
In the present study, we determined the curative effect of andrographolide on TNBS-induced colitis in mice from the histopathological level, and also investigated the possible mechanisms involved in the therapy effects of andrographolide. Our data may provide experimental and theoretical foundation to the exploration of novel therapy method against UC.
Materials and methods
Clinic samples and isolation of Peripheral blood mononuclear cells (PBMCs)
The study protocol was approved by the independent Ethics Committee of Zhejiang Hospital. Informed and written consent was obtained from all patients or their advisers according to the ethics committee guidelines. A total of 90 patients with UC admitted to Department of gastroenterology, Zhejiang Hospital and 90 age matched healthy donors were enrolled in this study. Serum samples were obtained from all the participants to measure the concentrations of TNF-α, IL-1β, IL-6 and IL-23. PBMCs were isolated from whole blood samples of 30 UC patients and 30 healthy donors by gradient centrifugation with Lymphocyte cell separation media (Cedarlane, Hornby, Ontario, Canada). The percentages of CD4+IL-17+ cells were detected in isolated PBMCs by flow cytometry analysis.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of TNF-α, IL-1β, IL-6 and IL-23 were determined by using ELISA Kits (Bio-swap, Shanghai, China) following the manufacturer’s procedures. Absorbance value (OD value) was measured at 450 nm and the concentrations of cytokines were calculated by using standard curve.
Cytokine staining and flow cytometry analysis
PBMCs were resuspended in RPMI-1640 (Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA), 100 ng/ml PMA (Sigma, St. Louis, MO, USA), 1 μg/ml ionomycin (Sigma) and 1 μg/ml monesine (Aladdin, Shanghai, China), and cultured on 24-well plates (0.5 × 105 cells/well) in a 37°C incubator with 5% CO2 for 4 h. Then, the PBMCs were labelled with anti-CD4-FITC (Biolegend, San Diego, CA, USA) for 1 h at 4°C. After fixing with 2% formaldehyde and permeabilizing with 0.1% Triton X-100 in PBS, the PBMCs were stained with anti-IL-17-PE (Biolegend) for 1 h and analyzed on a flow cytometry (BD Bioscience, Franklin Lakes, NJ, USA). Cells presented CD4+ and IL-17+ were determined as Th17 cells.
TNBS-induced colitis in mice
All animal experiments were approved by the Institutional Animal Care and Use Committee of Zhejiang Hospital. Specific pathogen-free (SPF) 5- to 6-week-old C57BL/6 mice purchased from the Shanghai Experimental Animal Center (Shanghai, China) were allocated into 4 groups (n=6/group) including Control group, UC group, experiment group (UC+AG) and positive control group (UC+ Mesalazine). Colitis was induced in UC group, experiment group and positive control group as previously described [16]. The mice were fasted overnight and anesthetized by intraperitoneal injection of sodium pentobarbital. 2.5% (w/v) TNBS solution in 50% ethanol was injected using a catheter through anus (100 μl/mice). The Control group was injected with 100 μl of 50% ethanol through the same technique. Two days after TNBS or ethanol injection, the Control and UC groups were treated with gastric perfusion of 0.4 mL/d distilled water. The experiment and positive control groups were given 0.1 g/kg/d of andrographolide (AG, Sigma) and 0.5 g/kg/d of mesalazine, respectively. After a continuous delivery for 7 d, mice were sacrificed at 24 h after the last delivery. Blood samples were collected for ELISA and flow cytometry analyses. Colon tissues were collected for histological, ELISA and western blot analyses.
Histological analysis
Colon tissues were fixed in 10% formalin for 48 h. Tissues were dehydrated by ethyl alcohol and transparency by xylene, then embedded in paraffin and cut into 5 μm-thick sections. The sections were deparaffinized, dehydrated, and stained with hematoxylin and eosin (HE).
Western blot assay
Total proteins were extracted from tissue samples or PBMCs in radioimmune precipitation buffer (RIPA; Byeotime, Shanghai, China) according to the manufacturer’s protocols. Protein concentrations were quantified by using bicinchoninic acid (BCA) method. Equal amounts of protein were loaded on 10% sodium dodecylsulphate (SDS)-polyacrylamide gels. After electrophoresis, proteins on the gel were transferred onto a nitrocellulose blotting membrane (Millipore, Bredford, MA, USA) and incubated with 5% skim milk for 30 min at room temperature. The membranes were incubated with STAT3 (Cell Signaling Technology, Danvers, MA, USA), p-STAT3 (Abcam, Cambridge, MA, USA), ROR-γt (Abcam), IL-17A (Abcam), IL-23 (Abcam) and GAPDH (Cell Signaling Technology) antibodies at 4°C overnight. Then the membranes were washed by Tris-buffered saline/Triton X-100 (TBST) and incubated with horseradish peroxidase conjugated secondary antibody for 1 h. The protein bands were detected with an Enhanced Chemiluminescence kit (BioRad, Richmond, CA, USA) and quantified by ImageJ software (http://rsb.info.nih.gov/ij/, Bethesda, MD, USA).
PBMCs treatment
PBMCs were isolated from healthy donors and UC patients, and plated onto 12-well plates (1 × 106 cells/well). PBMCs from UC patients were treated with 10 μg/mL AG490 (Selleck Chemicals, Houston, TX, USA), or 10, 20 and 30 μg/mL of andrographolide (AG). In addition, PBMCs isolated from healthy donors were pre-treated with three concentrations of andrographolide (10, 20 and 30 μg/mL) or 10 μg/mL AG490 for 2 h. Then cells except control group were treated with 1 μg/mL lipopolysaccharide (LPS, Sigma) to active IL-23/IL-17 pathway. After 48 h of culture, culture media were collected for ELISA analysis, and PBMCs were harvested for flow cytometry and western blot analyses.
Statistical analysis
Statistical analysis was performed with Graphpad Prism software (version 6.0, San Diego, CA, USA). Student’s t test and one-way analysis of variance with post-hoc test were used to evaluate differences between two groups and among more than two groups, respectively. A p value less than 0.05 was considered statistically significant.
Results
Higher concentrations of pro-inflammatory factors and higher percentage of Th17 cells in UC patients
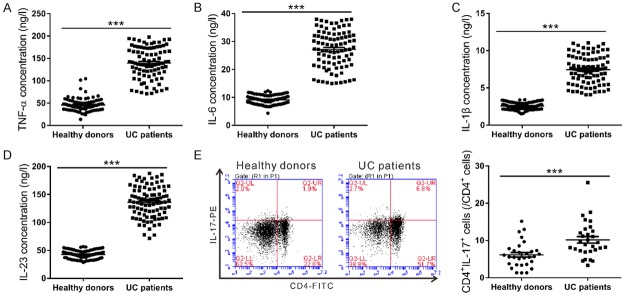
ELISA and flow cytometry were employed to measure the serum concentrations of proinflammatory factors (TNF-α, IL-1β, IL-6 and IL-23), and the percentage of Th17 cells in CD4+ T cells, respectively. As the result shown in Figure 1A-D, the concentrations of proinflammatory factors TNF-α, IL-1β, IL-6 and IL-23 significantly increased in UC patients compared with those in healthy donors (n=90, P<0.001). Compared to the healthy donors, UC patients showed a remarkable increased percentage of Th17 cells (IL-17+CD4+) in CD4+ T cells (Figure 1E, P<0.001). The elevated concentrations of those proinflammatory factors and increased percentage of Th17 cells indicated the increased inflammatory response and the activated IL-23/IL-17 axis in UC patients.
Figure 1.
The serum concentrations of proinflammatory factors and the percentages of Th17 cells in CD4+ cells from UC patients and healthy donors. (A-D) Proinflammatory factors TNF-α (A), IL-6 (B), IL-1β (C) and IL-23 (D) were detected up-regulated in UC patients compared with those in healthy donors as measured by ELISA (n=90, ***P<0.001). (E) PBMCs isolated from the whole blood samples of 30 UC patients and 30 healthy donors were labelled with anti-CD4-FITC and anti-IL-17-PE, and then analyzed on a flow cytometry. Higher percentage of CD4+IL-17+ cells in CD4+ cells was detected in UC patients as assessed by flow cyometry analysis (n=30, ***P<0.001).
Andrographolide exhibited therapy effect against experimental colitis
In order to evaluate the therapeutic effect of andrographolide on UC, a mouse model of TNBS-induced colitis was established. The mice were allocated into 4 groups (n=6/group) including Control group, UC group, experiment group (UC+AG) and positive control group (UC+ Mesalazine). HE staining was performed to evaluate the damage in colon (Figure 2). UC group presented cell swelling, necrosis and degeneration with massive neutrophil infiltration, while less severe cell necrosis and normal architectural were observed after treatment with andrographolide or Mesalazine, indicating protective effect of andrographolide on experimental colitis.
Figure 2.
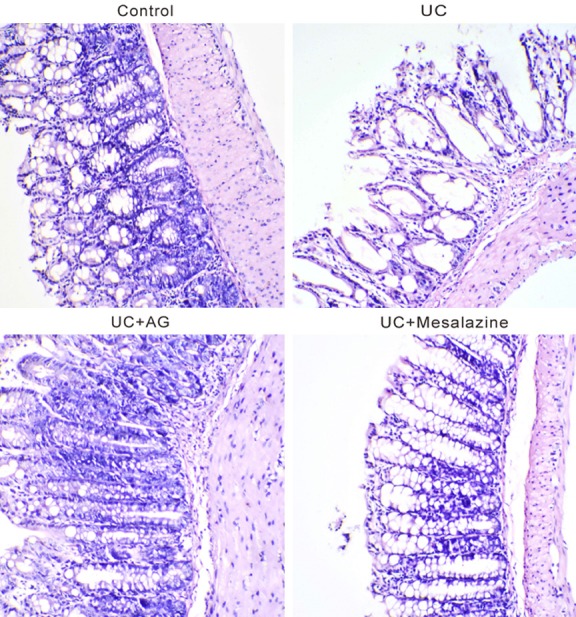
Histologic analysis of colon tissues. TNBS-induced colitis mouse model was established and then treated with 0.1 g/kg/d of andrographolide (AG) or 0.5 g/kg/d of Mesalazine for 7 d. At 24 h after the last delivery, the mice were killed and colon tissues were subjected to hematoxylin and eosin staining. Magnification: × 100.
Effects of andrographolide on inflammatory response and activation of IL-23/IL-17 in experimental colitis
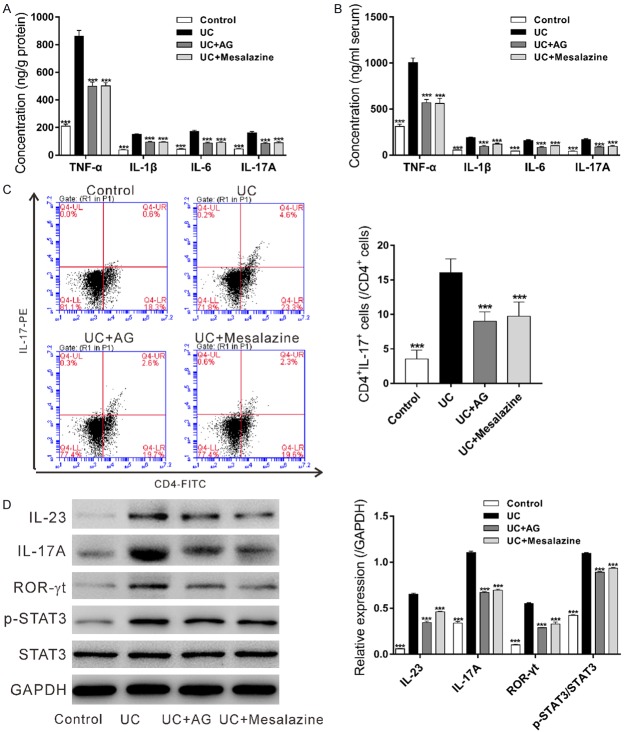
The concentrations of TNF-α, IL-1β, IL-6 and IL-23 in the colon tissues (Figure 3A) and the serum (Figure 3B) were also measured by ELISA. All the detected proinflammatory factors were significantly increased in UC group as compared to the Control group, and notably decreased by andrographolide or Mesalazine treatment. Andrographolide had a comparable effect as mesalazine. These data indicated that andrographolide could suppress inflammatory response in TNBS-induced colitis.
Figure 3.
Effects of andrographolide on inflammatory response and activation of IL-23/IL-17 in experimental colitis. TNBS-induced colitis mouse model was established and then treated with 0.1 g/kg/d of andrographolide (AG) or 0.5 g/kg/d of Mesalazine for 7 d. At 24 h after the last delivery, proinflammatory factors TNF-α, IL-1β, IL-6 and IL-23 in colonic tissue (A) and serum (B) were measured by ELISA assay. PMBCs were isolated from the whole blood samples of mice and the percentages of CD4+IL-17+ cells in CD4+ cells (C) were assessed by antibody labeling and flow cytometry analysis. The protein levels of IL-23, IL-17A, ROR-γt, p-STAT3 and STAT3 in colon tissues (D) were detected by western blot. ***P<0.001, compared with the UC group.
As the result shown in Figure 3C, UC group had a higher percentage of Th17 cells in CD4+ cells than the Control group. Andrographolide-treated group or Mesalazine-treated group presented significant lower percentage of Th17 cells than UC group. These data suggested that andrographolide could inhibited the activation of IL-23/IL-17 axis in PBMCs from the mouse model of colitis.
In addition, western blot analyses were used to measure the expression of IL-23, IL-17A, ROR-γt (Th17 lineage-specific transcription factor [17]), p-STAT3 and STAT3 in colon. The levels of IL-23, IL-17A, ROR-γt and p-STAT3 were increased in UC group, and decreased by andrographolide or Mesalazine treatment (Figures 3D and S1). These data suggested the inhibitory effects of andrographolide on the activation of IL-23/IL-17 axis and STAT3 signaling in the colon tissues of the mouse model of colitis.
Effects of andrographolide in UC PBMCs
To further examine the effect of andrographolide on UC, isolated UC PBMCs were treated with various concentrations of andrographolide and AG490, a STAT3 inhibitor. As the results shown in Figure 4, UC PBMCs presented up-regulated expression of proinflammatory factors, high percentage of Th17 cells, and increased expression of IL-23, IL-17A, ROR-γt and p-STAT3 (Figures 4C and S2) as compared with normal Control PBMCs. Further, andrographolide treatment significantly reduced the concentrations of cytokines and the activated IL-23/IL-17 axis in a dose-dependent manner. AG490 showed similar effects as andrographolide.
Figure 4.
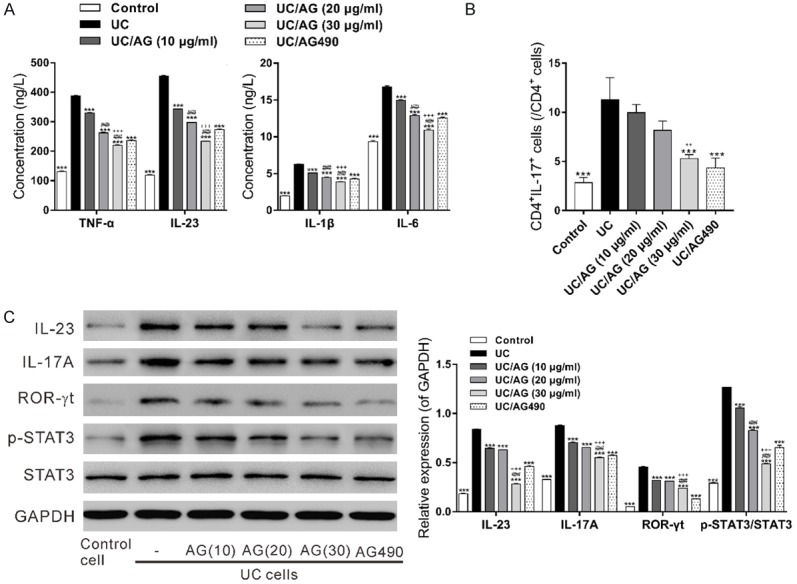
Effects of andrographolide in UC PBMCs. PBMCs from UC patients were treated with 10 μg/mL AG490, or 10, 20 and 30 μg/mL of andrographolide (AG) for 48 h. Control cells were isolated from healthy donors and did not receive any treatment. A: Proinflammatory factors TNF-α, IL-1β, IL-6 and IL-23 in the culture medium were measured by ELISA assay. B: The percentages of CD4+IL-17+ cells in CD4+ cells were assessed by antibody labeling and flow cytometry analysis. C: The protein levels of IL-23, IL-17A, ROR-γt, p-STAT3 and STAT3 in the PBMCs were detected by western blot. ***P<0.001, compared with the UC group; ###P<0.001 compared with the UC/AG (10 μg/ml) group; ++ P<0.01, +++ P<0.001 compared with the UC/AG (20 μg/ml) group.
Effects of andrographolide in LPS-treated PBMCs
In addition, to study the effects of andrographolide on PBMCs with active IL-23/IL-17 pathway, PBMCs isolated from healthy donors were treated with LPS to form inflammation model. As illustrated in Figure 5, LPS treatment significantly increased the concentrations of detected cytokines, the percentages of Th17 cells and the expression of relative proteins (Figures 5C and S3), which were significantly reduced by pretreatment with andrographolide or AG490.
Figure 5.
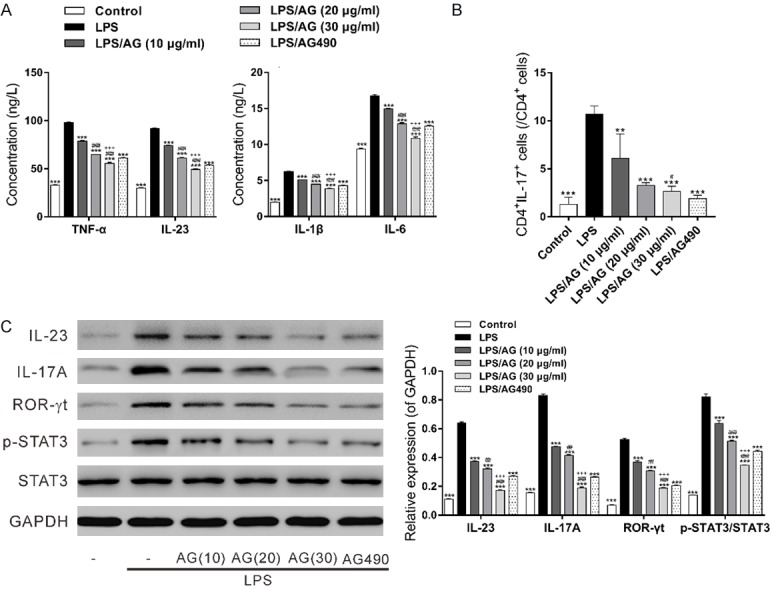
Effects of andrographolide in LPS-treated normal PBMCs. PBMCs isolated from healthy donors were pre-treated with 10, 20 and 30 μg/mL of andrographolide (AG) or 10 μg/mL AG490 for 2 h, and then treated with 1 μg/mL LPS for another 48 h. Control cells received no treatment. A: Proinflammatory factors TNF-α, IL-1β, IL-6 and IL-23 in the culture medium were measured by ELISA assay. B: The percentages of CD4+IL-17+ cells in CD4+ cells were assessed by antibody labeling and flow cytometry analysis. C: The protein levels of IL-23, IL-17A, ROR-γt, p-STAT3 and STAT3 in PMBCs were detected by western blot. **P<0.01, ***P<0.001, compared with the LPS group; #P<0.05, ##P<0.01, ###P<0.001 compared with the LPS/AG (10 μg/ml) group; +++ P<0.001 compared with the LPS/AG (20 μg/ml) group.
Discussion
The disordered immune response has been suggested as a main cause of UC [2]. Emerging evidences have supported the important role of IL-23/IL-17 axis in the process of UC [4-7]. In the present study, increased concentration of TNF-α, IL-1β, IL-6 and IL-23 were detected in the serum sample from UC patients compared with those from healthy donors, indicating the increased inflammatory response in UC patients. The percentages of Th17 (IL-17+CD4+) cells in CD4+ cells from the PBMCs of UC patients were higher than those from healthy donors, suggesting that Th17 immune responses may involve in the pathogenesis of UC (Figure 1).
AP extract (HMPL-004) [13,14] and andrographolide sulfonate [15] were efficient for the treatment of UC and experimental colitis, respectively. In the present study, we investigated the therapeutic effect of andrographolide in TNBS-induced experimental colitis. Mesalazine, which is widely used for UC treatment [8], was served as positive control. The results of HE staining indicated the protective effect of andrographolide and Mesalazine in TNBS-induced colon damage (Figure 2). Subsequently, ELISA and flow cytometry analyses demonstrated the inhibitory effect of andrographolide and Mesalazine against inflammation response. It is worth noting that andrographolide has comparable effects as Mesalazine. Our study suggests that andrographolide may be a promising agent in the treatment of UC.
Moreover, we investigated the effects of andrographolide on immune response and IL-23/IL-17 axis. Andrographolide significantly decreased the concentration of TNF-α, IL-1β, IL-6 and IL-17A in colon (Figure 3A) and serum samples (Figure 3B), which was induced by TNBS. The percentages of Th17 (IL-17+CD4+) cells in CD4+ cells were also reduced by andrographolide treatment (Figure 3C). These data demonstrated that andrographolide inhibited Th17 immune response. ROR-γt is the key transcription factor in the differentiation of Th17 cells [17]. Bacterial antigens could active macrophages to secrete proinflammatory factor IL-23, which induces the activation of ROR-γt through STAT3 pathway in CD4+ T cells [18]. STAT3 signaling pathway, stimulated by cytokines, plays critical role in cell proliferation, differentiation, apoptosis, cell cycle and immunoregulation [19]. Here, the levels of IL-23, IL-17A, ROR-γt and p-STAT3 in the colon were increased in UC animal model as compared to Control. Andrographolide treatment significantly reduced the levels of above proteins (Figure 3D). These data suggested that andrographolide may inhibit Th17 immune response via STAT3 signaling.
To further explore the possible mechanism of andrographolide, we used PBMCs with various treatments to investigate the mechanisms involved in the inhibition effect of andrographolide against IL-23/IL-17 axis. Corresponding to our expectation, andrographolide treatment decreased the ratio of Th17 cells in CD4+ cells and the concentrations of down-stream cytokines in UC PBMCs in a dose-dependent manner (Figure 4A and 4B). In addition, IL-23/IL-17 axis related proteins in UC PBMCs were also dose-dependently down-regulated by andrographolide treatment (Figure 4C). These suggested the correspondence between the concentration of andrographolide and IL-23/IL-17 activity. Further, PBMCs from healthy donors were treated with LPS to form an inflammation model and similar results were obtained after the treatment of andrographolide (Figure 5). AG490, a JAK2 inhibitor, was used as positive control [20]. Higher concentration of andrographolide presented similar results compared to AG490 group. Therefore, andrographolide suppresses inflammation response through the inhibition of IL-23/IL-17 axis and the down-stream signaling pathway.
In conclusion, we demonstrated that andrographolide inhibits the activation of IL-23/IL-17 axis and down-stream pro-inflammatory factors so as to suppress inflammation response, resulting in the reliving of UC. We provide experimental and theoretical foundation to the investigation of andrographolide as a novel therapy method against UC.
Acknowledgements
This study was supported by Natural Science Foundation of Zhejiang Province (LQ15H030005), and Medical and health science and technology plan of Zhejiang Province (2015KYA011).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Hindryckx P, Baert F, Hart A, Magro F, Armuzzi A, Peyrin-Biroulet L. Clinical trials in ulcerative colitis: a historical perspective. J Crohns Colitis. 2015;9:580–588. doi: 10.1093/ecco-jcc/jjv074. [DOI] [PubMed] [Google Scholar]
- 2.Scarpa M, Castagliuolo I, Castoro C, Pozza A, Scarpa M, Kotsafti A, Angriman I. Inflammatory colonic carcinogenesis: a review on pathogenesis and immunosurveillance mechanisms in ulcerative colitis. World J Gastroenterol. 2014;20:6774–6785. doi: 10.3748/wjg.v20.i22.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fragoulis GE, Siebert S, McInnes IB. Therapeutic targeting of IL-17 and IL-23 cytokines in immune-mediated diseases. Annu Rev Med. 2016:337–353. doi: 10.1146/annurev-med-051914-021944. [DOI] [PubMed] [Google Scholar]
- 4.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi T, Fuse S, Sakamoto N, Mikami M, Ogawa S, Hamaoka K, Arakaki Y, Nakamura T, Nagasawa H, Kato T, Jibiki T, Iwashima S, Yamakawa M, Ohkubo T, Shimoyama S, Aso K, Sato S, Saji T. A new Z score curve of the coronary arterial internal diameter using the lambda-mu-sigma method in a pediatric population. J Am Soc Echocardiogr. 2016;29:794–801. doi: 10.1016/j.echo.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 7.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann F, Stein J. Clinical trial: controlled, open, randomized multicentre study comparing the effects of treatment on quality of life, safety and efficacy of budesonide or mesalazine enemas in active left-sided ulcerative colitis. Aliment Pharmacol Ther. 2010;32:368–376. doi: 10.1111/j.1365-2036.2010.04354.x. [DOI] [PubMed] [Google Scholar]
- 9.Iskandar HN, Dhere T, Farraye FA. Ulcerative colitis: update on medical management. Curr Gastroenterol Rep. 2015;17:44. doi: 10.1007/s11894-015-0466-9. [DOI] [PubMed] [Google Scholar]
- 10.Yu Z, Lu B, Sheng Y, Zhou L, Ji L, Wang Z. Andrographolide ameliorates diabetic retinopathy by inhibiting retinal angiogenesis and inflammation. Biochim Biophys Acta. 2015;1850:824–831. doi: 10.1016/j.bbagen.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Parichatikanond W, Suthisisang C, Dhepakson P, Herunsalee A. Study of anti-inflammatory activities of the pure compounds from an-drographis paniculata (burm. f.) nees and their effects on gene expression. Int Immunopharmacol. 2010;10:1361–1373. doi: 10.1016/j.intimp.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Mohammadi M, Hayatbakhsh MM, Zahedi MJ, Jalalpour MR, Pakgohar A. Serum interleukin-23 levels in patients with ulcerative colitis. Iran J Immunol. 2011;8:183–188. [PubMed] [Google Scholar]
- 13.Sandborn WJ, Targan SR, Byers VS, Rutty DA, Mu H, Zhang X, Tang T. Andrographis paniculata extract (HMPL-004) for active ulcerative colitis. Am J Gastroenterol. 2013;108:90–98. doi: 10.1038/ajg.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang T, Targan S, Li ZS, Xu C, Byers V, Sandborn W. Randomised clinical trial: herbal extract HMPL-004 in active ulcerative colitis-a double-blind comparison with sustained release mesalazine. Aliment Pharmacol Ther. 2011;33:194–202. doi: 10.1111/j.1365-2036.2010.04515.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Guo W, Guo L, Gu Y, Cai P, Xie N, Yang X, Shu Y, Wu X, Sun Y. Andrographolide sulfonate ameliorates experimental colitis in mice by inhibiting Th1/Th17 response. Int Immunopharmacol. 2014;20:337–345. doi: 10.1016/j.intimp.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Scheiffele F, Fuss IJ. Induction of TNBS colitis in mice. Curr Protoc Immunol. 2002 doi: 10.1002/0471142735.im1519s49. Chapter 15: Unit 15.19. [DOI] [PubMed] [Google Scholar]
- 17.Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, Valmori D. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the TH17 lineage-specific transcription factor RORγt. Proc Natl Acad Sci U S A. 2009;106:8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu FY, Huang SG, Zhang HY, Ye H, Chi HG, Zou Y, Lv RX, Zheng XB. Effects of baicalin in CD4+ CD29+ T cell subsets of ulcerative colitis patients. World J Gastroenterol. 2014;20:15299–15309. doi: 10.3748/wjg.v20.i41.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang QC, Zhang YJ, Lu R, Chen YX, Fang JY. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008;10:287–297. doi: 10.1593/neo.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JS, Lee J, Lim MA, Kim EK, Kim SM, Ryu JG, Lee JH, Kwok SK, Park KS, Kim HY, Park SH, Cho ML. JAK2-STAT3 blockade by AG490 suppresses autoimmune arthritis in mice via reciprocal regulation of regulatory T Cells and Th17 cells. J Immunol. 2014;192:4417–4424. doi: 10.4049/jimmunol.1300514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.