Abstract
Despite accruing evidence showing that positive emotions facilitate stress recovery, the neural basis for this effect remains unclear. To identify the underlying mechanism, we compared stress recovery for people reflecting on a stressor while in a positive emotional context with that for people in a neutral context. While blood–oxygen-level dependent data were being collected, participants (N = 43) performed a stressful anagram task, which was followed by a recovery period during which they reflected on the stressor while watching a positive or neutral video. Participants also reported positive and negative emotions throughout the task as well as retrospective thoughts about the task. Although there was no effect of experimental context on emotional recovery, we found that ventromedial prefrontal cortex (vmPFC) activation during the stressor predicted more positive emotions during recovery, which in turn predicted less negative emotions during recovery. In addition, the relationship between vmPFC activation and positive emotions during recovery was mediated by decentering—the meta-cognitive detachment of oneself from one’s feelings. In sum, successful recovery from a stressor seems to be due to activation of positive emotion-related regions during the stressor itself as well as to their downstream effects on certain cognitive forms of emotion regulation.
Keywords: positive emotions, stress recovery, vmPFC, change-point analysis, emotion regulation
Introduction
In his elegant answer to why zebras do not get ulcers, Sapolsky (2007, p. 6) defined a stressor as ‘…anything in the outside world that knocks you out of homeostatic balance, and the stress response is what your body does to reestablish homeostasis’. Poorly managed responses to stressors add to cumulative biological burdens that make the organism more vulnerable to developing diseases or exacerbating existing disease symptoms (McEwen, 2000; Seeman et al., 2001). Negative emotions are essential components of stress responses (van Winkel et al., 2015; Abtahi and Kerns, 2017; Crosswell et al., 2017; Neubauer et al., 2017; Richardson, 2017). Via negative emotions, psychological stress is associated with cardiovascular diseases, acquired immune deficiency syndrome (AIDS), asthma and slowed wound healing (Cohen et al., 2007; Maple et al., 2015; Trueba et al., 2016). Stress also contributes to the development and exacerbation of mental illnesses imbued with negative emotions (Charles et al., 2013) including anxiety disorders, depression (Cohen et al., 2007; Miller et al., 2009), post-traumatic stress disorder, schizophrenia (Nugent et al., 2015) and substance abuse (Herman, 2012).
In addition to negative emotions, stress triggers dysregulation of positive emotions (Gilbert, 2012). Whereas low levels of positive emotions are related to depression, anxiety, eating disorders and borderline personality disorder (Gilbert, 2012; Carl et al., 2013; Jacob et al., 2013), high levels of positive emotions are found to be important in resilience and adaptive emotion regulation (Fredrickson et al., 2003; Zautra et al., 2005; Waugh et al., 2011; Trompetter et al., 2016). In a large Nova Scotian sample, researchers found increased positive emotions protective against 10 year incident coronary heart disease (Davidson et al., 2010). For AIDS patients, higher positive emotions were significantly associated with lower mortality with other risk factors controlled (Moskowitz, 2003).
One powerful avenue through which positive emotions influence stress responding is by facilitating stress recovery (Tugade et al., 2004; Waugh, 2014). Positive emotions have been found to ‘undo’ the physiological (Fredrickson and Levenson, 1998; Fredrickson et al., 2000) and cognitive (Hughes and Kendall, 2008; Falkenstern et al., 2009) residue from stressors, both of which are integral to mitigating the effects of stress on illness (Brosschot et al., 2006).
Positive emotions enhance cognitive flexibility and openness
One potential mechanism through which positive emotions facilitate stress recovery is by influencing information-processing style. Negative emotional states generally shift a person’s default tendency (such as holistic processing) to a detail-oriented and analytic information-processing style, thus narrowing attention and focus (Oatley et al., 2006; Clore and Palmer, 2009; Huntsinger et al., 2014). This narrowed attention can perpetuate or exacerbate negative states if unresolved, as is the case with rumination—an ineffective problem-solving strategy composed of inflexible and repetitive negative thoughts (Larsen and Prizmic, 2004).
In contrast, based on the broaden-and-build theory (Fredrickson, 2001), positive emotions have an immediate and short-term enhancing effect on the flexibility and openness of cognitive processes (Fredrickson and Cohn, 2008). With a flexible deployment of attention, positive emotions (especially low arousal ones) facilitate broadened cognitive style and better processing of salient information (Isen, 2008) as is evident in attention (Fredrickson and Branigan, 2005; Gable and Harmon-Jones, 2010), cognitive control (Xue et al., 2013), decision-making (Estrada et al., 1997), social perception (Waugh and Fredrickson, 2006), memory (Talarico et al., 2009) and visual perception (Johnson et al., 2010; Vanlessen et al., 2016).
This cognitive facilitation effect from positive emotions may contribute to faster stress recovery, specifically via enhancing the use of cognitively based emotion regulation strategies. Higher cognitive capacity (such as working memory) is associated with improved emotion regulation ability in both experimental and naturalistic settings (Schmeichel and Demaree, 2010). Higher cognitive capacity during stress recovery may augment the use of cognitively based emotion regulation strategies such as reappraisal, which refers to reframing the meaning of a situation to change one’s emotional response to it (Larsen and Prizmic, 2004; Gross, 2008), and/or decentering, which refers to the meta-cognitive strategy of viewing one’s experiences more objectively (Bernstein et al., 2015; Wolkin, 2015).
We further hypothesize that this cognitive facilitation mechanism for positive emotions most likely resides in the neural model of voluntary emotion regulation in which prefrontal regions regulate subcortical affective regions (Ochsner et al., 2012). The dorsal executive system (DES) plays an important role in the top–down control in cognitive reappraisal. Cognitive reappraisal involves the prefrontal cortex (PFC) [including dorsal lateral PFC (dlPFC) and ventral lateral PFC (vlPFC) and medial PFC (mPFC))] and cingulate system [including dorsal anterior cingulate cortex (dACC)], which together exert control and modulate activity in posterior and subcortical systems that generate emotional responses (Miller and Cohen, 2001; Ochsner et al., 2012; Morris et al., 2014). Within the DES, the dlPFC is important in cognitive control and maintenance of goal-relevant information (Iordan and Dolcos, 2017). It provides rich input to the dACC, which is related to the regulation of adaptive behavioral and autonomic responses to the environment (Pruessner et al., 2008). The dACC provides critical output to the amygdala (Ray and Zald, 2012), which is important in initiating fear learning (Schiller et al., 2008), processing emotionally salient signals from the environment and interacting with autonomic motor systems to express emotions (Ghashghaei and Barbas, 2002).
The anatomical connection from dACC to dlPFC and amygdala compensates for the sparse existence of direct neural fiber connections between the dlPFC and the amygdala (Ray and Zald, 2012). In this dlPFC-amygdala pathway, the ventromedial PFC (vmPFC) may serve as another mediator (Ochsner et al., 2012) through its connections to bilateral PFC regions and to limbic areas including the amygdala (Ghashghaei et al., 2007). Prefrontal activation during emotion regulation has been found to be positively correlated with vmPFC activation and negatively correlated with amygdala responses (Ochsner et al., 2012). Importantly, the vmPFC also provides positive emotion signals (Harris et al., 2007; Winecoff et al., 2013). Thus, the vmPFC may be a hub through which positive emotions may influence the dlPFC-dACC-amygdala cognitive regulation pathway.
Positive emotions bias and provide positive information
In addition to the cognitive facilitation route, positive emotions may improve stress recovery by influencing information selection. Based on the affect infusion model and emotion congruence theory, emotion influences attention, memory and judgment (Oatley et al., 2006; Clore and Palmer, 2009). People have a perceptual bias for emotion-congruent stimuli, which perpetuates the same emotional state cycle (Bartlett, 1932). People also tend to remember things that are more congruent with their present emotions and reconstruct their memory with emotionally congruent schemas and attitudes toward the event (Bartlett, 1932). In a stressor that induces negative emotions, this congruency tends to produce a negativity bias, in which negative emotional states enable additional processing that perpetuates the negative emotional state (Turowski et al., 2014).
During stress recovery, the introduction of positive emotions may make positive emotion-congruent memories more accessible and create incongruence in the negative emotional state, thus interrupting the self-perpetuating negative emotion cycle. This process resembles a psychological break from stress (Tugade et al., 2014) that can potentially short-circuit rumination (Folkman and Moskowitz, 2000). Indeed, simply having a break that gives people more time to think about alternatives can tilt a person’s negative bias toward ambivalent objects to be more positive (Neta and Tong, 2016).
Emotions not only bias information selection but also provide evaluative information about the value and importance of an object or situation (Clore and Palmer, 2009). In general, negative emotions signal negative attributes and events, ‘something is wrong’, whereas positive emotions signal positive attributes and events (Clore and Palmer, 2009; Turowski et al., 2014), such as ‘everything is safe’ (Gervais and Wilson, 2005). We therefore hypothesize that positive emotions may improve stress recovery by providing an informative signal that the environment is positive and/or safe.
We further hypothesize that this positive information route resides in the shared neural underpinnings between positive emotions and fear extinction. In rodent and human fear conditioning studies, fear extinction depends on the vmPFC (Ochsner et al., 2012). During reversal learning, the vmPFC provides a safety signal that facilitates fear inhibition by preventing fear response generalization and perseverance (Roy et al., 2012). The vmPFC may also facilitate fear inhibition by providing a reward signal due to negative reinforcement (Schiller et al., 2008). In addition to the vmPFC, the nucleus accumbens (NAcc) may also play a parallel role. Reward sensitivity, for example, is produced by the integration of information from the amygdala and NAcc in the vmPFC (Turowski et al., 2014). Thus, NAcc activation during positive emotions may provide input into the vmPFC to provide positive evaluations of events.
This study
Although the evidence shows that positive emotions facilitate stress recovery, the underlying neural mechanisms are not adequately understood. In this study, we conducted behavioral and functional magnetic resonance imaging (fMRI) experiments to test the routes through which positive emotions facilitate stress recovery. The participants performed a stressful anagram task in which their performance was negatively compared with the performance of others and then reflected on that stressor while watching either a positive-emotion-inducing or a neutral video. Positive and negative emotion ratings were collected throughout the stressor and recovery. After the recovery period, participants provided retrospective thoughts about the tasks including positive and negative thoughts about the stressor and about the video. They also completed a decentering and rumination measure (Fresco et al., 2007).
We hypothesized that reflecting on the stressor during the positive video would result in better positive and negative emotional recovery than would reflecting on the stressor during the neutral video. Also, positive emotions during recovery was hypothesized to be negatively correlated with negative emotions during recovery. As for neural activation, we hypothesized that the vmPFC would be the central hub for positive emotional recovery as it is expected to be linked with positive emotions and is likely involved in both the cognitive facilitation and information routes described earlier. For the cognitive facilitation route, we hypothesized that activation in the DES (dlPFC, dACC) would be related to better positive and/or negative emotional recovery and that activation in these regions as well as in the vmPFC would be related to increased decentering, and more positive (fewer negative) thoughts about the stressor, which may be due to reappraisal. For the positive information bias route, we hypothesized that activation in the NAcc and/or decreased activation in the amygdala would be related to better positive and/or negative emotional recovery and that activation in these regions as well as in the vmPFC would be related to decreased rumination, and more positive (and/or fewer negative) thoughts about the video, which may reflect positively biased information processing for emotional recovery.
Method
Participants
We recruited participants through the Be Involved clinical research service at Wake Forest Baptist Medical Center and flyers posted around Wake Forest University’s campus. Initially, 366 participants responded to the advertisement, of which 79 were eligible and interested (the most limiting criteria being dental braces and crowns). To be eligible, participants had to be between the ages of 18 and 64, native English speakers, right handed, have no history of psychiatric or neurological disorders, have no severe learning disabilities or head trauma or have any other physical limitations that prevented them from entering the MRI scanner. Eventually, 50 participants (18–57 years of age, M = 28.40 years, s.d. = 11.780, nFemale = 25, nWhite–non-hispanic = 27, nWhite-hispanic = 2, nAsian = 9, nAfrican–Amerian = 9, nMixed = 2, nUnknown = 1) completed the study. Informed consent was obtained from all participants, and all procedures were conducted in accordance with the Wake Forest Institutional Review Board ethical standards. Participants were paid $25 for their participation.
After the task but before debriefing, we used a structured interview to assess suspicion in the participants, because the stressor involved some deception to enhance the frustration. We asked the participants how they felt about the stressor and how they felt when learning about other participants’ performance. We excluded people who both expressed suspicion (e.g. ‘anagrams seemed unsolvable’ or ‘the statistics from other participants appeared fake’) and did not exhibit stress responses (increased negative emotions or decreased positive emotions). Based on these criteria, six participants’ data were excluded from data analyses (one from the positive video condition, five from the neutral condition). Another participant discontinued the study due to physical discomfort, so we analyzed the data for 43 participants (18–57 years of age, M = 28.19 years, s.d. = 11.843, nFemale = 23, nWhite–non-hispanic = 22, nWhite-hispanic = 1, nAsian = 9, nAfrican–Amerian = 8, nMixed = 2, nUnknown = 1).
Procedure
Pre-scanner preparation
Upon arrival, the participants were screened again for MRI eligibility. In the waiting room, the participants were trained to use the emotion rating scales and practiced an easy version of the anagram task programmed with E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA).
fMRI data acquisition
fMRI data were acquired using a 3T Siemens Skyra MRI scanner with a high-resolution, 32-channel head coil (Siemens Healthineers, Erlangen, Germany). To minimize head movement, padding was applied around the participant’s head and the participants were instructed to keep still. High-resolution T1-weighted anatomic images were obtained using a 3D volumetric MP-RAGE sequence with isotropic voxel-resolution of 1 mm (TR: 2300 ms, TE: 3 ms, TI: 900 ms, scan time: 5 min 30 s). Blood–oxygen-level dependent (BOLD) data were acquired with a single shot EPI from 36 slices (TR: 2 s, TE: 25 ms, 3.5 mm isotropic spatial resolution, FOV 22.4 × 22.4 cm2, 64 × 64 matrix size).
Scanner tasks
In the scanner, participants underwent a series of scans including the BOLD scan of interest (10 min 02 s, 299 volumes with 2 volumes discarded from scanner ramp-up), which included a baseline period, the anagram task and a recovery period. The other scans (Supplementary Figure S1) will not be addressed in this article.
Stressor task
During the baseline period, participants were instructed to relax for 3 min with their eyes open. Then, participants performed an adapted anagram task that was designed to cause frustration. For this task, we created two equally difficult lists of 15 anagrams, each of five unique letters. Each list consisted of 5 easy, 5 difficult and 5 unsolvable anagrams. The anagrams were selected (Bradshaw, 1984; Proctor and Vu, 1999), edited and tested for their difficulty in pilot studies. Participants were randomly assigned to either of the anagram lists.
In the stressor, participants first had 4 s to view an anagram, then 5 s to solve the anagram by typing on the keypad to reorder the scrambled letters on the screen (Figure 1). When time elapsed, they received immediate feedback on their accuracy with an artificially inflated statistic of other participants’ performance. We generated pseudorandom numbers so that the participants were guaranteed to receive a feedback percentage between 70 and 80 for the easy anagrams, between 60 and 70 for the hard anagrams and between 55 and 65 for the unsolvable anagrams. At the end of the 3 min stressor, the participants viewed their percentage correct along with a comment that 73% of the participants did better than them. Based on pilot data (Supplementary Information), this stressor has been shown to reliably induce negative emotions. In this sample, the average anagram accuracy was 3.26 out of 15 with a s.d. of 2.64.
Fig. 1.
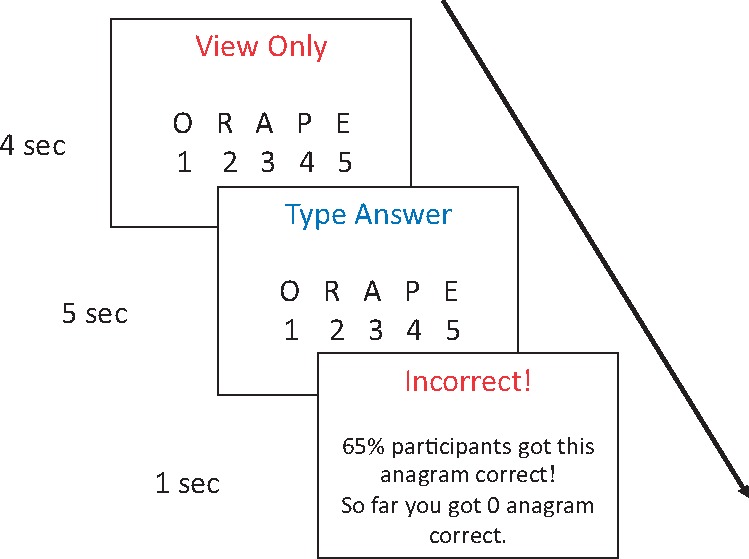
An example of a hard anagram from the first word list. The correct answer is 14 523 ‘opera’.
Post-stressor emotion induction
After the stressor, the participants rated their emotion and began a 3 min post-stressor recovery period. They were instructed to ‘think back to the anagram task’ and to ‘engage in any thoughts and feelings that come naturally’ while viewing a video. Participants in the positive emotion condition viewed a ‘Waves’ video with no sound. This video showed ocean waves, which was designed to induce contentment, a low arousal positive emotion that broadens cognition (Fredrickson and Branigan, 2005). Participants in the control condition viewed a neutral ‘Sticks’ video with no sound. This video showed brightly colored moving sticks, akin to an old computer screensaver. Both videos were selected from previous studies to induce expected emotions and a similar level of interest (Fredrickson and Levenson, 1998). Pilot data showed that participants who viewed the ‘Waves’ video reported higher positive emotions (but not lower negative emotions) during recovery than those who viewed the ‘Sticks’ video (Supplementary Figure S2).
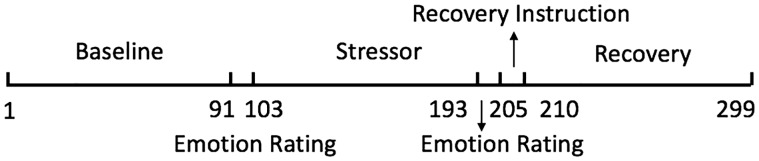
The timeline of the stress and recovery periods (Figure 2) is consistent with other stressors (Waugh et al., 2012) designed to investigate the neural responses associated with short-term emotional (Wager et al., 2009b) and physiological (Wager et al., 2009a) responses to and recovery from stress.
Fig. 2.
Timeline of experiment. x-axis is in TRs.
Materials
Emotion ratings
The participants self-reported their emotions throughout the study. In each instance of emotion ratings, they first saw a 1 s emotion rating reminder slide. They then reported their positive emotions (‘how pleasant do you feel right now’) and negative emotions (‘how unpleasant do you feel right now’) on separate rating scales. The positive emotion scale always preceded the negative emotion scale. The scale was from 1 (‘not at all’) to 5 (‘extremely’) with 0.5 increments. Participants were instructed to press the same number twice for a whole digit emotion rating (i.e. 11 equaled 1) and press one number and the adjacent larger number to reflect a 0.5 increment (i.e. 12 equaled 1.5). Participants were given 6.5 s to respond to each emotion rating scale and a 1 s fixation slide separated the two scales. For the emotion ratings right before and after the stressor during the continuous BOLD scan, the duration of the scales was increased to 11 s each to ensure that participants had enough time to respond. The experimenters also monitored the emotion rating process and collected the participants’ emotion ratings verbally if the participants failed to respond in time. Participants rated their emotions a total of three times: before the anagram task (baseline), after the anagram task (post-stressor) and after the emotion induction (recovery).
Post-task thought content questionnaires
At the end of the experiment, and after participants had exited the scanner (∼15 min elapsed after the stressor task had ended), participants filled out thought content and decentering questionnaires (and other personality scales that will not be analyzed here). They then received a post-study structured interview, debriefing and compensation.
Modified decentering questionnaire
The decentering questionnaire was based on the experiences questionnaire (Fresco et al., 2007) and consisted of two factors: rumination and decentering. We modified the items to measure in-the-moment stressor experience (state rumination and state decentering). Specifically, the participants were instructed to think about how much they endorsed those thoughts while watching the video, such as ‘I had the sense that I was fully aware of what was going on around me and inside me’. The 17 items were rated on a scale from 1 (‘not at all’) to 5 (‘very much’). In this sample, both subscales had acceptable reliability (state decentering: 11 items; α = 0.704; state rumination: 6 items; α = 0.662).
Thought content questionnaire
The thought content items were developed based on thought data collected from previous studies in the lab. The 14 items were grouped into 3 (positive, negative or irrelevant thoughts and feelings) × 2 (about the stressor and about the video) categories, such as ‘I thought that the video was calming’ (positive and about the video). The items were rated on a scale from 1 (‘not at all’) to 5 (‘very much’). In our sample, all subscales demonstrated acceptable internal consistency: positive thoughts about the stressor (7 items; α = 0.738), about the video (3 items; α = 0.530) and negative thoughts about the stressor (8 items; α = 885) and about the video (3 items; α = 0.744). Given the short length of the positive video thoughts scale, we also provided the mean of interitem correlations (Briggs and Cheek, 1986), which was an acceptable 0.499 (averaged from 0.505, 0.394 and 0.598).
Data analyses
fMRI data preprocessing
We applied a processing pipeline to preprocess the BOLD data in SPM12 (Wellcome Trust Centre for Neuroimaging). It is based upon the Wake Forest University data analysis pipeline (Maldjian et al., 2009), updated and briefly described, as follows. This automatic imaging preprocessing included data cleaning, realignment, coregistration, normalization to MNI space and spatially smoothing the data with an 8 × 8 × 10 mm full width at half maximum Gaussian smoothing kernel. We also regressed out six motion parameters and linearly detrended the data.
fMRI data analyses
Emotion mask
To investigate emotion-related brain regions, we used an emotion mask that was created from an automated meta-analysis of 790 studies with the keyword ‘emotion’ (Neurosynth.org, forward inference, 30 March 2017; Yarkoni, 2011; Yarkoni et al., 2011) consistent with previous fMRI studies on emotions (Waugh et al., 2017).
Change-point analysis
We conducted change-point analyses to identify brain regions responsive to the stressor and recovery tasks because this analytic technique provides a model-free method of detecting when and for how long regions are active during the session (Lindquist et al., 2007). This technique is particularly suitable for studying emotional states as they tend to have ‘uncertain onset times, temporal intensity profiles and durations’ (Lindquist et al., 2007, p. 1125). Researchers have recently highlighted this innovative technique as useful in analyzing the neural temporal dynamics of state anxiety (Robinson et al., 2010), acupuncture (Bai et al., 2010), stress responses within comorbid major depressive disorder and social anxiety disorders samples (Waugh et al., 2012) and resting state (Aston and Kirch, 2012). Unlike general linear modeling, change-point analyses can identify multiple peaks of activation within one epoch, which allows us to detect when during the stressor or recovery period the hypothesized region’s activation is associated with behavioral outcomes (Waugh et al., 2012). We first subtracted activation during the baseline period from activation during the stressor and recovery periods. Then, we calculated an exponentially weighted moving-average (EWMA) z for every time point after baseline in each voxel’s time-series (Lindquist et al., 2007).
For the group-level analyses, we first created regressors that represented the covariates of interest. For the emotion induction regressor, we used dummy variables corresponding to either the positive emotion or neutral induction. To quantify self-reported positive and negative emotional recovery, respectively, we regressed baseline and post-stressor levels of that emotion on its recovery level to create emotional recovery regressors. So more positive emotions during recovery indicates successful positive emotional recovery, whereas less negative emotions during recovery indicates successful negative emotional recovery. We also regressed baseline level of an emotion on the post-stressor level of that emotion to create its stress reactivity regressor. So less positive emotions post-stressor indicates stronger stress responses in positive emotions, whereas more negative emotions post-stressor indicates stronger stress responses in negative emotions. This analysis technique allowed us to identify brain regions in which activation corresponded specifically to emotional reactivity or recovery. These regressors were then dummy-coded as 1 (low) and 2 (high) according to a median split (because the custom scripts we used required binary predictors) and then mean-centered. These regressors were then separately entered into hierarchical EWMA (HEWMA) models to produce betas and t values that reflect each time points’ activation predicted by the regressor (Waugh et al., 2012). Then, we applied family-wise error (FWE) correction to obtain corrected t values across each voxel’s timeseries. We defined a change-point as the onset of three consecutive time points with above threshold t values. We then used 2D (spatial and temporal) k-mean clustering to derive clusters of voxels (k ≥ 10; P < 0.05 FWE corrected over time), and to correct over space, we retained only those clusters with at least one false discovery rate (FDR, q < 0.05) corrected peak.
Because the voxels within a cluster did not perfectly overlap in their individual time-series of activation, we devised a new way to represent the most consistent activation pattern of a cluster. Specifically, for a given cluster, we calculated, at each time point, the ratio of the voxels that had above threshold t values. To categorize the cluster activation results, we initially set three ratio criteria: 0.05 (meaning more than 5% of the voxels within the cluster had significant activation at that time point), 0.25 and 0.50. The results from the three ratios were highly similar, so we decided to only report the 0.05 ratio results because that ratio included the most voxels from the cluster and time points across the cluster. This approach provides a more conservative estimate of the cluster’s activation at each time-point than using the other ratios and reduces the likelihood of capitalizing on non-independence error.1 For reporting the results, we categorized the change-point onsets as occurring during the stressor (103–147 time points as the first half, 148–192 time points as the second half) and recovery (205–254 time points as the first half, 255–299 time points as the second half; Figure 2).
To examine the relationships between the activation in significant clusters and behavioral variables, we used weighted regression. For a particular cluster and for each participant, we first extracted the HEWMA z values of the time points when the cluster showed consistent (>0.05 ratio) above threshold activation. We then averaged HEWMA z values across those time points to produce a cluster HEWMA z value for each participant. We also averaged the voxel regression weights (assigned during the previous HEWMA analyses) across all the voxels to create a cluster regression weight for each participant. We then conducted weighted regression between this neural activation, the primary behavioral variables of interest and other continuous behavioral variables of interest (not median split as before).
Results
Behavioral data
Stress manipulation check
We conducted paired t-tests to check the effect of the stressor on positive emotions and negative emotions. Positive emotions significantly decreased from baseline (M = 2.791, s.d. = 1.264) to post-stressor (M = 2.244, s.d. = 1.093), t(42) = 3.588, P = 0.001, d = 0.547 and negative emotions significantly increased from baseline (M = 1.721, s.d. = 0.734) to post-stressor (M = 2.686, s.d. = 1.160), t(42) = −5.677, P < 0.001, d = 0.866, which suggest that we successfully manipulated stress.
Positive emotion induction check
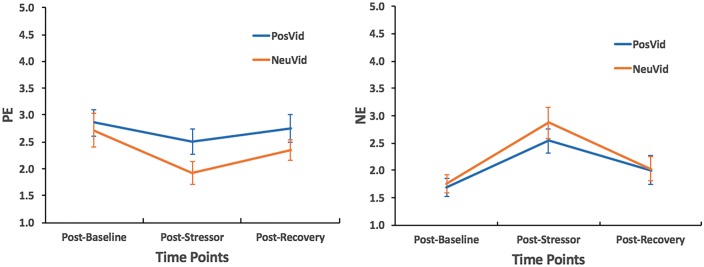
We next conducted an analysis of covariance (ANCOVA) with video condition (between-subject: positive emotion video and neutral video) as the independent variable and recovery levels of positive emotions and negative emotions as the dependent variables and with baseline and post-stressor levels of positive emotions and negative emotions as covariates.
Participants in the positive emotion video condition (M = 2.750, s.d. = 1.285) did not report significantly higher positive emotions during recovery than those in the neutral video condition (M = 2.342, s.d. = 0.851), F(1, 39) = 0.007, P = 0.933 (Figure 3). Neither did they (M = 2.000, s.d. = 1.294) report significantly less negative emotions during recovery than those in the neutral video condition (M = 2.026, s.d. = 0.935), F(1, 39) = 0.148, P = 0.702 (Figure 3).
Fig. 3.
The effect of the stressor on positive emotion (PE) and negative emotion (NE) ratings. PosVid and NeuVid refer to positive and neutral video conditions, respectively.
Although behavioral data from a pilot study was promising (Supplemental Information), these results suggest that our experimental manipulations did not induce reliable changes in self-reported emotions during this fMRI study. Therefore, we were unable to test our hypotheses about the influence of emotional context on vmPFC activation and positive emotional recovery. Instead, for the remainder of the Results section, we only tested our hypotheses about the relationship between vmPFC activation and individual differences in positive and negative emotional recovery.
Correlations among positive emotional recovery, negative emotional recovery and thoughts about the stressor
As expected, positive emotional recovery was negatively correlated with negative emotional recovery, r = −0.651, P < 0.001. In addition, positive emotional recovery was significantly positively correlated with decentering and positive thoughts about the video, and marginally positively correlated with positive thoughts about the stressor (Table 1). Negative emotional recovery was negatively correlated with decentering, positive thoughts about the video and positive thoughts about the stressor (marginally). It was also positively correlated with negative thoughts about the stressor.
Table 1.
Correlations among positive/negative emotional recovery and modified experience and thought content questionnaire subscales
| Variables | Positive emotional recovery | Negative emotional recovery |
|---|---|---|
| Decentering | 0.524*** | −0.440** |
| Rumination | −0.131 | 0.323* |
| PosT(Stressor) | 0.258† | −0.293† |
| NegT(Stressor) | −0.160 | 0.331* |
| PosT(Video) | 0.318* | −0.441** |
| NegT(Video) | −0.228 | 0.103 |
Notes. Positive emotional recovery is the residual of post-recovery positive emotion (PE) controlled for baseline and post-stressor PE, so more PEs post-recovery indicates successful positive emotional recovery; Negative emotional recovery is the residual of post-recovery negative emotion (NE) controlled for baseline and post-stressor NE, so less NEs post-recovery indicates successful negative emotional recovery; PosT is positive thoughts; NegT is negative thoughts.
P < 0.10, *P < 0.05, **P < 0.01, ***P < 0.001.
fMRI data—change-point analyses
Overall intercept
Several brain regions exhibited significant activation throughout the stressor and recovery periods compared with the baseline period (Table 2). The middle temporal gyrus, which is associated with reading comprehension (St George et al., 1999), exhibited a time course corresponding to the onsets of reading instructions (stronger activation during the presentation of task instructions and anagram performance summary). The thalamus, which is associated with vigilance and attention as well as with effort in working memory (Engström et al., 2014), exhibited activation during the first half of the stressor. The right precentral gyrus, right parietal cortex or angular gyrus and supplementary motor area (SMA) clusters exhibited extended activation throughout the stressor. Right precentral gyrus is associated with hand motor activity and tactile sensations (Pishnamazi et al., 2016) and SMA is associated with volitional movement (Cunnington et al., 2003; Nguyen et al., 2014). In addition, right parietal cortex or angular gyrus is associated with numerical processing (Kadosh et al., 2012). The activation in this collection of regions may have been due to participants typing their emotion ratings and their responses to the anagrams.
Table 2.
Change-point analysis of the BOLD responses throughout the stressor—average (intercept) across all participants
| Region | L/R | x | y | z | voxels | vol | FDR peaks | Period |
|---|---|---|---|---|---|---|---|---|
| Activation ≥ baseline | ||||||||
| BA39; Temporal Mid | R | −52 | −56 | 16 | 50 | 3200 | 12 | S1; R1 |
| Thalamus; Thalamus | 0 | −12 | 4 | 27 | 1728 | 2 | S1 | |
| BA6; Precentral | R | −44 | 4 | 32 | 51 | 3264 | 17 | S |
| BA7; Angular | R | −28 | −60 | 48 | 16 | 1024 | 1 | S |
| BA6; Supp Motor | 0 | 12 | 52 | 21 | 1344 | 6 | S | |
Notes. Regions: based on the center voxel MNI coordinates referenced from MNI <-> Talairach with Brodmann Areas (1.09), Mricron under aal.nii.gz template; L/R: converted to L (left hemisphere) and R (right hemisphere) from the radiologist-view MNI coordinates; vol: volume (mm3); MaxZ: the largest corrected z value of all the voxels in a cluster; FDR peaks: number of peaks within the cluster that reached FDR correction; S: stressor; R: recovery; superscript 1: first half; superscript 2: second half.
Positive emotional recovery
Compared with participants who reported low positive emotions during recovery, those who reported high positive emotions exhibited stronger activation in a large medial frontal cortex cluster including the vmPFC, dorsal mPFC and ACC as well as in the thalamus and posterior cingulate (precuneus; Table 3). Notably, the activation in the medial frontal cortex cluster occurred during the stressor.
Table 3.
Change-point analysis of the BOLD responses—regression based on positive emotional recovery
| Region | L/R | x | y | z | voxels | vol | FDR peaks | Period |
|---|---|---|---|---|---|---|---|---|
| High PEs during recovery ≥ low PEs during recovery | ||||||||
| BA32; Cingulum Ant | R | −4 | 36 | 16 | 168 | 10 752 | 3 | S |
| Thalamus; Thalamus | 0 | −16 | 4 | 45 | 2880 | 13 | S; R1 | |
| BA31; Precuneus | R | −4 | −56 | 28 | 38 | 2432 | 8 | S; R |
Notes. Positive emotional recovery is the residual of post-recovery positive emotion (PE) controlled for baseline and post-stressor PE, so more PEs post-recovery indicates successful positive emotional recovery; Regions: based on the center voxel MNI coordinates referenced from MNI <-> Talairach with Brodmann Areas (1.09), Mricron under aal.nii.gz template; L/R: converted to L (left hemisphere) and R (right hemisphere) from the radiologist-view MNI coordinates; vol: volume (mm3); MaxZ: the largest corrected z value of all the voxels in a cluster; FDR peaks: number of peaks within the cluster that reached FDR correction; S: stressor; R: recovery; superscript 1: first half; superscript 2: second half.
vmPFC’s role in positive and negative emotional recovery
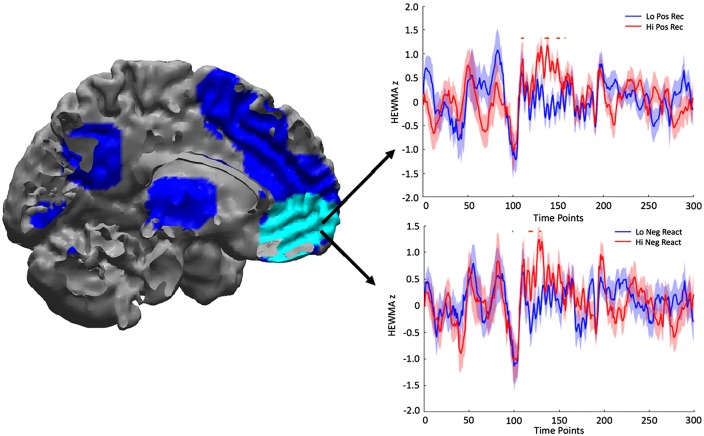
The medial frontal cortex cluster spanned several subregions including the vmPFC, a targeted region of analysis based on our hypotheses. Therefore, to conduct secondary vmPFC analyses, we isolated the significant voxels within the vmPFC by applying a vmPFC mask2 (Lancaster et al., 1997, 2000; Maldjian et al., 2003). The isolated vmPFC cluster had 42 voxels (MNI coordinates x = −4, y = 44, z = −8; Figure 4) including two FDR-corrected peaks.
Fig. 4.
Left: neural correlates of positive emotional recovery (Pos Rec) (all positive associations). The vmPFC ROI is in light blue. Right top: vmPFC activation time course based on Pos Rec groupings with significant differences between the two groups indicated by horizontal bars above the line plot. Right bottom: vmPFC activation time course based on Neg React (negative emotional stress reactivity) with significant differences between the two groups indicated by horizontal bars above the line plot. Red bar means high group had more vmPFC activation than low group and blue bar means low group had more vmPFC activation than high group.
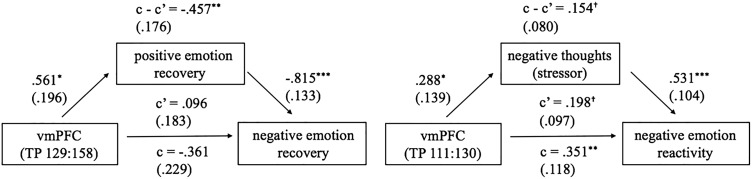
We next supported our data extraction technique by confirming that more vmPFC activation during the stressor significantly predicted higher positive emotions during recovery (Figure 5, left). Then, we tested our hypothesis about the neural model that the vmPFC is involved in positive emotional recovery, which in turn facilitates negative emotional recovery. In a mediation model, we examined the total path (bivariate associations) between vmPFC activation and negative emotional recovery as well as the indirect path in which the vmPFC may be associated with negative emotional recovery via positive emotional recovery. Despite a non-significant total path between vmPFC activation and negative emotional recovery, the indirect path between them was significant, t = −2.595, P = 0.009, indicating that more vmPFC activation during the stressor was associated with enhanced positive emotional recovery, and this association in turn improved negative emotional recovery (Figure 5, left).
Fig. 5.
Mediation between vmPFC activation and target variables. Left: positive emotional recovery mediates relationship between vmPFC activation during the middle part of the stressor and negative emotional recovery. Right: negative thoughts about the stressor mediates the relationship between vmPFC activation during the early part of the stressor and negative emotional stress reactivity. †P < 0.10, *P < 0.05, **P < 0.01, ***P < 0.001.
To explore the potential factors explaining individual differences in vmPFC activation, we examined the relationships between vmPFC activation and those behavioral variables already found to be associated with positive emotional recovery (decentering, positive thoughts about the stressor and positive thoughts about the video; Table 1). vmPFC activation was positively associated with decentering, positive thoughts about the stressor and positive thoughts about the video (Table 4, path a). We further tested if the behavioral variables mediated the positive association between vmPFC activation and positive emotional recovery. Among the behavioral variables, decentering significantly mediated the association between vmPFC activation and positive emotional recovery (Table 4, path c—c’).
Table 4.
Testing potential thought/cognition mediators between vmPFC activation and positive emotional recovery
| Path name | Association | Mediator: decentering | Mediator: PosT (stressor) | Mediator: PosT (video) |
|---|---|---|---|---|
| Path a | vmPFC -> mediator | 0.367** (0.141) | 0.297† (0.165) | 0.397† (0.201) |
| Path b | mediator -> Pos Rec (controlling for vmPFC) | 0.693*** (0.191) | 0.341† (0.180) | 0.398** (0.141) |
| Path c’ | vmPFC -> Pos Rec (without mediation) | 0.307 (0.186) | 0.460* (0.197) | 0.403* (0.190) |
| Path c—c’ | mediated path between vmPFC & Pos Rec | 0.254* (0.120) | 0.102 (0.078) | 0.157 (0.097) |
Notes. vmPFC: ventromedial prefrontal cortex, its activation was extracted from the vmPFC region of interest during the time points (129:158) that were positively associated with positive emotional recovery (Pos Rec). PosT: positive thoughts; Numbers in the parentheses: s.d.
P < 0.10; *P <0.05; **P < 0.01; ***P < 0.001.
Negative emotional recovery
Participants who reported lower negative emotions during recovery did not exhibit significantly different brain activation from those who reported higher negative emotions during recovery.
Stress reactivity—exploratory analyses of the vmPFC
Because we found that more vmPFC activation during the stressor was related to higher positive emotions during the recovery, we conducted exploratory follow-up analyses in which we examined whether the vmPFC activation during the stressor was also associated with stress responses in positive/negative emotions. In each case, we reconducted the HEWMA regression but only in this vmPFC cluster and with positive and negative emotional stress reactivity (post-stressor positive and negative emotional changes) as the regressors.
vmPFC’s role in positive emotional stress reactivity
vmPFC activation was not associated with more positive emotional stress reactivity (Table 5).
Table 5.
Change-point analysis of the BOLD responses—regression based on positive and negative emotional stress reactivity
| Region | L/R | x | y | z | voxels | vol | FDR peaks | Period |
|---|---|---|---|---|---|---|---|---|
| BA6; Precentral | Low positive emotional stress reactivity ≥ high positive emotional stress reactivity | |||||||
| R | −44 | 4 | 32 | 28 | 1792 | 1 | S1; R1 | |
| BA45; Frontal Inf Tri | High negative emotional stress reactivity ≥ low negative emotional stress reactivity | |||||||
| R | −48 | 24 | 8 | 73 | 4672 | 2 | S1 | |
Notes. Positive emotion reactivity is the residual of post-stressor positive emotion (PE) controlled for baseline PE, so less PEs post-stressor indicates stronger stress responses in PEs; Negative emotion reactivity is the residual of post-stressor negative emotion (NE) controlled for baseline NE, so less NEs post-recovery indicates successful negative emotional recovery; Regions: based on the center voxel MNI coordinates referenced from MNI <-> Talairach with Brodmann Areas (1.09), Mricron under aal.nii.gz template; L/R: converted to L (left hemisphere) and R (right hemisphere) from the radiologist-view MNI coordinates; vol: volume (mm3); MaxZ: the largest corrected z value of all the voxels in a cluster; FDR peaks: number of peaks within the cluster that reached FDR correction; S: stressor; R: recovery; superscript 1: first half; superscript 2: second half.
vmPFC role in negative emotional stress reactivity
We found that greater vmPFC activation during the first half of the stressor was associated with more negative emotional stress reactivity (this association was partially mediated through negative thoughts about the stressor; Figure 4, right bottom; Figure 5, right; Table 5).
The activation in the vmPFC that was associated with increased negative emotional stress reactivity mainly occurred during the beginning of the stressor (during the first five anagrams), whereas the vmPFC activation that was associated with increased positive emotional recovery mainly occurred during the middle part of the stressor (between the 6th and 10th anagrams). To clarify the vmPFC activation related to negative emotional stress reactivity and to positive emotional recovery, we categorized the participants into four groups via median split (high or low on negative emotional stress reactivity and on positive emotional recovery). Interesting profiles emerged (Figure 6). An ‘affectively flexible’ profile (high negative emotional stress reactivity, high positive emotional recovery) was characterized by extended duration of vmPFC activation throughout the stressor, whereas a ‘disengaged’ profile (low negative emotional stress reactivity, low positive emotional recovery) was characterized by low vmPFC activation throughout the stressor. In addition, ‘negative’ (high negative emotional stress reactivity, low positive emotional recovery) and ‘positive’ (low negative emotional stress reactivity, high positive emotional recovery) profiles were characterized by early and late vmPFC activation during the stressor, respectively.
Fig. 6.
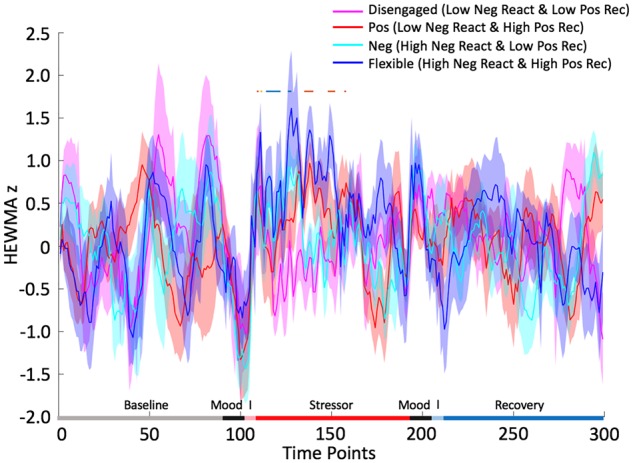
Negative emotional stress reactivity (Neg React) and positive emotional recovery (Pos Rec) in the vmPFC ROI. Horizontal bars above the line plot highlight the time points that there were significant between group differences in cluster activation. ‘I’ refers to the instructions from the following tasks. Blue bar suggests high Neg React group had greater activation than low Neg React group, orange suggests high Pos Rec group had greater activation than low Pos Rec group and yellow suggests the combination of blue and orange bars.
Stress-reactivity—other regions
For thoroughness, we examined whether negative and/or positive emotional stress reactivity predicted activations in other regions besides the vmPFC.
Positive emotional stress reactivity—other regions
Higher activation in right precentral gyrus during the first halves of the stressor period was related to lower positive emotional stress reactivity (Table 5).
Negative emotional stress reactivity—other regions
Higher activation in right vlPFC (frontal inferior triangularis) during the first half of the stressor was related to stronger negative emotional stress reactivity (Table 5). Right vlPFC activation during cognitive tasks has been positively correlated with negative emotions such as anxiety (Koric et al., 2012).
Discussion
This study contributes important findings to the recent literature examining the neural correlates of the effects of positive emotions on stress recovery (Speer and Delgado, 2017). Despite no evidence of a significant experimental effect on stress recovery, we demonstrated that positive emotions during recovery predicted decreased negative emotions. The change point analyses with the fMRI data also revealed the important roles of vmPFC activation during the stressor in impacting negative emotional recovery through positive emotional recovery.
The vmPFC was associated with increased positive emotions during recovery, which was in turn associated with decreased negative emotions during recovery. Notably, this vmPFC activation occurred during the stressor instead of during the recovery period and did not predict positive emotional reactivity to the stressor. We speculate that given vmPFC’s role in producing positive emotional experiences (Doré et al., 2017) and attenuating negative emotions (Seo et al., 2014), the vmPFC may provide ‘real-time’ positive appraisals of the stressor, as is supported by the finding that this activation was correlated with more positive thoughts about the stressor. In the midst of the stressor, these positive appraisals may not have as much influence as negative appraisals on participants’ gestalt emotional response to the stressor (Zautra et al., 2000; Baumeister et al., 2001). Given that stimuli high in arousal, negative valence and relevance garner more attention (Mazzietti and Koenig, 2014), negative appraisals, which are more congruent and relevant with the participants’ mood state of anxiety and frustration, may capture more attention than positive appraisals. Or negative appraisals may be differentially weighted in their impact on overall emotional responses. However, after the stressor, when the immediacy of the threat is over, these positive appraisals may become more powerful predictors of the participants’ emotional response (Waugh, 2014). Indeed, vmPFC activation during the stressor was related to increased positive emotional recovery (in response to the video). The effect of these positive appraisals on the overall emotional response may also be due in part to curtailing the no-longer relevant negative appraisals as is evidenced by the finding that the relationship between vmPFC and positive emotional recovery was mediated by decentering. Decentering reflects experiential acceptance and non-judgmental thinking about the stressor (Lo et al., 2014).
One caveat to this formulation is that this vmPFC activation occurred during the middle of the stressor and vmPFC activation during the early part of the stressor was associated with increased negative emotional stress reactivity. One possible explanation of this finding is that participants engaged the vmPFC early on because they were quick to evaluate the stressor experience as negative, which is supported by the result that the significant association between the vmPFC and negative emotion stress reactivity was partially mediated by negative thoughts about the stressor. Early vmPFC activation may serve as part of an attempt to regulate negative emotional responses to the stressor. Those participants who continued to recruit the vmPFC throughout the stressor were able to produce positive appraisals that would facilitate successful stress recovery. This is consistent with evidence emphasizing the role of the vmPFC in successful positive reappraisal (Doré et al., 2017). The engagement of the vmPFC and positive reappraisal during the stressor may thus impact memory encoding about the stressor. When participants later reflected on the stressful experience, negative schemata may be less accessible or activated (Raedt and Koster, 2010). This formulation is fairly speculative, however, and needs to be tested in future investigations.
In investigating the neural mechanisms through which positive emotions influence stress recovery, we found evidence for both the cognitive facilitation route and the positive information bias route. For the cognitive facilitation route, although we found no evidence that higher activation in DES regions during recovery were associated with successful stress recovery, we did find indirect support that positive emotions may have facilitated cognitively based emotion regulation strategies. Decentering, one such strategy, mediated the relationship between vmPFC activation and positive emotional recovery. Cognitive reappraisal (measured with positive thoughts about the stressor) is another such strategy and was associated (albeit weakly) with both vmPFC activation during the stressor and positive emotional recovery.
The evidence was weaker for the positive information bias route. We found neither amygdala nor NAcc activation associated with negative or positive emotional recovery. Additionally, the critical vmPFC activation occurred during the stressor period, suggesting that it was responding in real-time to the stressor and not necessarily providing positive emotional input during the recovery period. The only evidence for the information bias route was that positive thoughts about the video, which did presumably occur during the recovery period, were positively associated with both vmPFC activation and positive emotional recovery. Our explanation is that vmPFC activation during the stressor, although no longer active during the recovery period, did bias some systems to respond more positively to stimuli during the recovery period. For example, as part of a network associated with positive autobiographical memories (Speer and Delgado, 2017), the vmPFC could have biased participants’ encoding of thoughts that they had during the video, which then reflected a positive recall bias later. Notably, positive memories, via this neural network, have been shown to dampen cortisol and reduce negative emotions during stress recovery (Speer and Delgado, 2017).
We recognize several limitations to this study. The positive emotion induction was not as strong as we expected, which precluded our ability to draw causal inferences that positive emotional recovery caused more successful negative emotional recovery, or caused changes in participants’ thoughts. More powerful positive inductions in future experiments will strengthen our ability to make valid causal explanations. Another limitation was that the thought content questionnaires were retrospective and susceptible to participants’ later emotional state. Future investigations might attempt to assess in-the-moment thoughts as is done for mind-wandering studies (Christoff et al., 2009). Relatedly, in-the-moment assessments of emotional responses throughout the stressor and recovery periods would also offer more insight into whether positive emotions really do mediate/precede negative emotions during recovery and whether vmPFC is associated with that process. As for analyses, existing HEWMA scripts dichotomize the continuous regressors, which may have decreased the power to detect subtler individual differences. Another limitation is our measurement of stress responses. In this study, we focused on the emotional changes post-stressor with underlying assumptions that decreases in positive emotions and increases in negative emotions are essential components of stress responses. We also examined relatively short-term emotional recovery, however, other aspects of stress recovery [e.g. physiology (Janson and Rohleder, 2017) and stress-related thoughts (Gianferante et al., 2014)] may persist past what we measured. Indeed, some stress responses with longer timescales (such as cortisol) might later feedback and influence neural responding. Future investigations should complement these emotion ratings with more objective, physiological stress response measures and extend the measurement of the associated neural responses to more fully characterize the processes associated with stress recovery.
In conclusion, the findings advance our understanding of biobehavioral models of positive emotions and stress regulation by providing evidence that vmPFC activation during the stressor may impact positive emotions and confer benefits on stress regulation not seen until recovery. Both the cognitive facilitation route and information bias route may work in conjunction to incorporate positive emotions in stress recovery through positive reappraisal and decentering. Importantly, we were only able to demonstrate these effects because we used change-point analyses that allowed us to detect when during the task activation of these regions occurred. Results from change-point analyses revealed that vmPFC activation at different points during the stressor may have different functions or at least predict different outcomes (negative emotional stress reactivity vs positive emotional recovery). In this study, we examined overall levels of activation in the hypothesized brain regions. In future investigations, it will be necessary to investigate changes in functional connectivity among these regions throughout the stressor to more adequately develop neural models of the effect of positive emotions on stress regulation.
Funding
This study was supported by a grant from the National Institutes of Health (MH106928) to Christian Waugh and Kateri McRae.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Supplementary Material
Acknowledgements
The authors thank the AACT lab at the University of Denver and Tor Wager for their help in the early stages of study development. They thank Rae-ling Lee, Emolab at Wake Forest University and Debra Fuller for fMRI data collection. They also thank Ben Wagner and Joseph Maldjian for their help preprocessing the fMRI data and Michael Tobia for assisting with the fMRI data analyses. Katelyn Garcia is now at the NIH.
Footnotes
Computing the regressions between neural activation and the original behavioral covariate upon which that neural activation was based may capitalize on non-independence error. It is true that these analyses are not completely orthogonal, although they are not completely redundant as well because we are extracting data from all the voxels in a cluster, each of which exhibited significant correlations with the behavioral variable but at different time points. To address this, we only compute these betas to use in the path analyses, and do not interpret the P-values on their own.
The vmPFC mask was generated by combining Talairach Daemon (TD) Brodmann regions 10, 11, 25 and 32 with a box size of 20, 90 and 20 centering at 0, −20 and −20 (MNI coordinates).
References
- Abtahi M.M., Kerns K.A. (2017). Attachment and emotion regulation in middle childhood: changes in affect and vagal tone during a social stress task. Attachment & Human Development, 19(3), 221–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston J.A.D., Kirch C. (2012). Evaluating stationarity via change-point alternatives with applications to fMRI data. The Annals of Applied Statistics, 6(4), 1906–48. [Google Scholar]
- Bai L., Tian J., Zhong C., et al. (2010). Acupuncture modulates temporal neural responses in wide brain networks: evidence from fMRI study. Molecular Pain, 6, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett F.C. (1932). Remembering: A Study in Experimental and Social Psychology. New York, NY: Cambridge University Press. [Google Scholar]
- Baumeister R.F., Bratslavsky E., Finkenauer C., Vohs K.D. (2001). Bad is stronger than good. Review of General Psychology, 5(4), 323–70. [Google Scholar]
- Bernstein A., Hadash Y., Lichtash Y., Tanay G., Shepherd K., Fresco D. M. (2015). Decentering and Related Constructs: A Critical Review and Meta-Cognitive Processes Model. Perspectives on Psychological Science, 10(5), 599–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw J.L. (1984). A guide to norms, ratings, and lists. Memory & Cognition, 12(2), 202–6. [DOI] [PubMed] [Google Scholar]
- Briggs S.R., Cheek J.M. (1986). The role of factor analysis in the development and evaluation of personality scales. Journal of Personality, 54(1), 106–48. [Google Scholar]
- Brosschot J.F., Gerin W., Thayer J.F. (2006). The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research, 60(2), 113–24. [DOI] [PubMed] [Google Scholar]
- Carl J.R., Soskin D.P., Kerns C., Barlow D.H. (2013). Positive emotion regulation in emotional disorders: a theoretical review. Clinical Psychology Review, 33(3), 343–60. [DOI] [PubMed] [Google Scholar]
- Charles S.T., Piazza J.R., Mogle J., Sliwinski M.J., Almeida D.M. (2013). The wear and tear of daily stressors on mental health. Psychological Science 24(5), 733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K., Gordon A.M., Smallwood J., Smith R., Schooler J.W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences, 106(21), 8719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G.L., Palmer J. (2009). Affective guidance of intelligent agents: how emotion controls cognition. Cognitive Systems Research, 10(1), 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Janicki-Deverts D., Miller G.E. (2007). Psychological stress and disease. JAMA, 298(14), 1685–7. [DOI] [PubMed] [Google Scholar]
- Crosswell A.D., Moreno P.I., Raposa E.B., et al. (2017). Effects of mindfulness training on emotional and physiologic recovery from induced negative affect. Psychoneuroendocrinology, 86(Suppl. C), 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R., Windischberger C., Deecke L., Moser E. (2003). The preparation and readiness for voluntary movement: a high-field event-related fMRI study of the Bereitschafts-BOLD response. NeuroImage, 20(1), 404–12. [DOI] [PubMed] [Google Scholar]
- Davidson K.W., Mostofsky E., Whang W. (2010). Don’t worry, be happy: positive affect and reduced 10-year incident coronary heart disease: the Canadian Nova Scotia Health Survey. European Heart Journal, 31(9), 1065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré B.P., Boccagno C., Burr D., et al. (2017). Finding positive meaning in negative experiences engages ventral striatal and ventromedial prefrontal regions associated with reward valuation. Journal of Cognitive Neuroscience, 29(2), 235–44. [DOI] [PubMed] [Google Scholar]
- Engström M., Karlsson T., Landtblom A.-M. (2014). Thalamic activation in the Kleine-Levin syndrome. Sleep: Journal of Sleep and Sleep Disorders Research, 37(2), 379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada C.A., Isen A.M., Young M.J. (1997). Positive affect facilitates integration of information and decreases anchoring in reasoning among physicians. Organizational Behavior and Human Decision Processes, 72(1), 117–35. [Google Scholar]
- Falkenstern M., Schiffrin H.H., Nelson S.K., Ford L., Keyser C. (2009). Mood over matter: can happiness be your undoing? The Journal of Positive Psychology, 4(5), 365–71. [Google Scholar]
- Folkman S., Moskowitz J.T. (2000). Positive affect and the other side of coping. American Psychologist, 55(6), 647–54. [DOI] [PubMed] [Google Scholar]
- Fredrickson B.L. (2001). The role of positive emotions in positive psychology: the broaden-and-build theory of positive emotions. American Psychologist, 56(3), 218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson B.L., Branigan C. (2005). Positive emotions broaden the scope of attention and thought-action repertoires. Cognition and Emotion, 19(3), 313–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson B.L., Cohn M.A. (2008). Positive emotions In Lewis M., Haviland-Jones J.M., Barrett L.F., editors. Handbook of Emotions, 3rd edn, pp. 777–96. New York, NY: Guilford Press. [Google Scholar]
- Fredrickson B.L., Levenson R.W. (1998). Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cognition and Emotion, 12(2), 191–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson B.L., Mancuso R.A., Branigan C., Tugade M.M. (2000). The undoing effect of positive emotions. Motivation and Emotion, 24(4), 237–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson B.L., Tugade M.M., Waugh C.E., Larkin G.R. (2003). What good are positive emotions in crisis? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. Journal of Personality and Social Psychology, 84(2), 365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco D.M., Moore M.T., van Dulmen M.H.M., et al. (2007). Initial psychometric properties of the experiences questionnaire: validation of a self-report measure of decentering. Behavior Therapy, 38(3), 234–46. [DOI] [PubMed] [Google Scholar]
- Gable P., Harmon-Jones E. (2010). The blues broaden, but the nasty narrows: attentional consequences of negative affects low and high in motivational intensity. Psychological Science, 21(2), 211–5. [DOI] [PubMed] [Google Scholar]
- Gervais M., Wilson D.S. (2005). The evolution and functions of laughter and humor: a synthetic approach. The Quarterly Review of Biology, 80(4), 395–430. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H.T., Barbas H. (2002). Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience, 115(4), 1261–79. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H.T., Hilgetag C.C., Barbas H. (2007). Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage, 34(3), 905–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianferante D., Thoma M.V., Hanlin L., et al. (2014). Post-stress rumination predicts HPA axis responses to repeated acute stress. Psychoneuroendocrinology, 49, 244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert K.E. (2012). The neglected role of positive emotion in adolescent psychopathology. Clinical Psychology Review, 32(6),467–81. [DOI] [PubMed] [Google Scholar]
- Gross J.J. (2008). Emotion regulation In Lewis M., Haviland-Jones J.M., Barrett L.F., editors. Handbook of Emotions, 3rd edn, pp. 497–512. New York, NY: Guilford Press. [Google Scholar]
- Harris L.T., McClure S.M., van den Bos W., Cohen J.D., Fiske S.T. (2007). Regions of the MPFC differentially tuned to social and nonsocial affective evaluation. Cognitive, Affective & Behavioral Neuroscience, 7(4), 309–16. [DOI] [PubMed] [Google Scholar]
- Herman J.P. (2012). Neural pathways of stress integration. Alcohol Research : Current Reviews, 34(4), 441–7. [PMC free article] [PubMed] [Google Scholar]
- Hughes A.A., Kendall P.C. (2008). Effect of a positive emotional state on interpretation bias for threat in children with anxiety disorders. Emotion, 8(3), 414–8. [DOI] [PubMed] [Google Scholar]
- Huntsinger J.R., Isbell L.M., Clore G.L. (2014). The affective control of thought: malleable, not fixed. Psychological Review, 121(4), 600–18. [DOI] [PubMed] [Google Scholar]
- Iordan A.D., Dolcos F. (2017). Brain activity and network interactions linked to valence-related differences in the impact of emotional distraction. Cerebral Cortex, 27(1), 731–49. [DOI] [PubMed] [Google Scholar]
- Isen A.M. (2008). Some ways in which positive affect influences decision making and problem solving In Lewis M., Haviland-Jones J.M., Barrett L.F., editors. Handbook of Emotions, 3rd edn, pp. 548–73. New York, NY: Guilford Press. [Google Scholar]
- Jacob G.A., Ower N., Buchholz A. (2013). The role of experiential avoidance, psychopathology, and borderline personality features in experiencing positive emotions: a path analysis. Journal of Behavior Therapy and Experimental Psychiatry, 44(1), 61–8. [DOI] [PubMed] [Google Scholar]
- Janson J., Rohleder N. (2017). Distraction coping predicts better cortisol recovery after acute psychosocial stress. Biological Psychology, 128, 117–24. [DOI] [PubMed] [Google Scholar]
- Johnson K.J., Waugh C.E., Fredrickson B.L. (2010). Smile to see the forest: facially expressed positive emotions broaden cognition. Cognition and Emotion, 24(2), 299–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosh R.C., Bien N., Sack A. (2012). Automatic and intentional number processing both rely on intact right parietal cortex: a combined fMRI and neuronavigated TMS study. Frontiers in Human Neuroscience, 6(2),1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koric L., Volle E., Seassau M.. et al. (2012). How cognitive performance-induced stress can influence right VLPFC activation: an fMRI study in healthy subjects and in patients with social phobia. Human Brain Mapping, 33(8), 1973–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Summerln J.L., Rainey L.. et al. (1997). The Talairach Daemon, a database server for Talairach Atlas Labels. NeuroImage, 5, S633. [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M.. et al. (2000). Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping, 10(3), 120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R.J., Prizmic Z. (2004). Affect regulation In Baumeister R.F., Vohs K.D., Baumeister R.F., Vohs K.D., editors. Handbook of Self-Regulation: Research, Theory, and Applications, pp. 40–61. New York, NY: Guilford Press. [Google Scholar]
- Lindquist M.A., Waugh C., Wager T.D. (2007). Modeling state-related fMRI activity using change-point theory. NeuroImage, 35(3), 1125–41. [DOI] [PubMed] [Google Scholar]
- Lo C.S.L., Ho S.M.Y., Yu N.K.K., Siu B.P.Y. (2014). Decentering mediates the effect of ruminative and experiential self-focus on negative thinking in depression. Cognitive Therapy and Research, 38(4), 389–96. [Google Scholar]
- Maldjian J.A., Baer A.H., Kraft R.A., Laurienti P.J., Burdette J.H. (2009). Fully automated processing of fMRI data in SPM: from MRI scanner to PACS. Neuroinformatics, 7(1), 57–72. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti, P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. Neuroimage, 19(3), 1233–9. [DOI] [PubMed] [Google Scholar]
- Maple H., Chilcot J., Lee V., Simmonds S., Weinman J., Mamode N. (2015). Stress predicts the trajectory of wound healing in living kidney donors as measured by high-resolution ultrasound. Brain, Behavior, and Immunity, 43, 19–26. [DOI] [PubMed] [Google Scholar]
- Mazzietti A., Koenig O. (2014). The relevance bias: valence-specific, relevance-modulated performance in a two-choice detection task. Cognition and Emotion, 28(1), 143–52. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. (2000). Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology, 22(2), 108–24. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202. [DOI] [PubMed] [Google Scholar]
- Miller G., Chen E., Cole S.W. (2009). Health psychology: developing biologically plausible models linking the social world and physical health. Annual Review of Psychology, 60(1), 501–24. [DOI] [PubMed] [Google Scholar]
- Morris J.A., Leclerc C.M., Kensinger E.A. (2014). Effects of valence and divided attention on cognitive reappraisal processes. Social Cognitive and Affective Neuroscience, 9(12), 1952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz J.T. (2003). Positive affect predicts lower risk of AIDS mortality. Psychosomatic Medicine, 65(4), 620–6. [DOI] [PubMed] [Google Scholar]
- Neta M., Tong T.T. (2016). Don’t like what you see? Give it time: longer reaction times associated with increased positive affect. Emotion, 16(5), 730–9. [DOI] [PubMed] [Google Scholar]
- Neubauer A.B., Smyth J.M., Sliwinski M.J. (2017). When you see it coming: stressor anticipation modulates stress effects on negative affect. Emotion. Doi: 10.1037/emo0000381. [DOI] [PubMed] [Google Scholar]
- Nguyen V.T., Breakspear M., Cunnington R. (2014). Reciprocal interactions of the SMA and cingulate cortex sustain premovement activity for voluntary actions. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(49), 16397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent K.L., Chiappelli J., Rowland L.M., Hong L.E. (2015). Cumulative stress pathophysiology in schizophrenia as indexed by allostatic load. Psychoneuroendocrinology, 60, 120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley K., Keltner D., Jenkins J.M. (2006). Understanding Emotions, 2nd edn.Malden: Blackwell Publishing. [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251(1), E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pishnamazi M., Nojaba Y., Ganjgahi H., Amousoltani A., Oghabian M.A. (2016). Neural correlates of audiotactile phonetic processing in early-blind readers: an fMRI study. Experimental Brain Research, 234(5), 1263–77. [DOI] [PubMed] [Google Scholar]
- Proctor R.W., Vu K.-P.L. (1999). Index of norms and ratings published in the Psychonomic Society journals. Behavior Research Methods, Instruments & Computers, 31(4), 659–67. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Dedovic K., Khalili-Mahani N., et al. (2008). Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry, 63(2), 234–40. [DOI] [PubMed] [Google Scholar]
- Raedt R.D., Koster E.H.W. (2010). Understanding vulnerability for depression from a cognitive neuroscience perspective: a reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective, & Behavioral Neuroscience, 10(1), 50–70. [DOI] [PubMed] [Google Scholar]
- Ray R.D., Zald D.H. (2012). Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience and Biobehavioral Reviews, 36(1), 479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C.M.E. (2017). Emotion regulation in the context of daily stress: impact on daily affect. Personality and Individual Differences, 112(Suppl. C), 150–6. [Google Scholar]
- Robinson L.F., Wager T.D., Lindquist M.A. (2010). Change point estimation in multi-subject fMRI studies. NeuroImage, 49(2), 1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M., Shohamy D., Wager T.D. (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M. (2007). Why zebras don’t get ulcers: stress, metabolism, and liquidating your assets In Monat A., Lazarus R.S., Reevy G., editors. The Praeger Handbook on Stress and Coping, Vol. 3, pp. 181–97. Westport, CT: Praeger Publishers/Greenwood Publishing Group. [Google Scholar]
- Schiller D., Levy I., Niv Y., LeDoux J.E., Phelps E.A. (2008). From fear to safety and back: reversal of fear in the human brain. The Journal of Neuroscience, 28(45), 11517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel B.J., Demaree H.A. (2010). Working memory capacity and spontaneous emotion regulation: high capacity predicts self-enhancement in response to negative feedback. Emotion, 10(5), 739–44. [DOI] [PubMed] [Google Scholar]
- Seeman T.E., McEwen B.S., Rowe J.W., Singer B.H. (2001). Allostatic load as a marker of cumulative biological risk: macArthur studies of successful aging. Proceedings of the National Academy of Sciences of the United States of America, 98(8), 4770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D., Olman C.A., Haut K.M., Sinha R., MacDonald A.W. III, Patrick C.J. (2014). Neural correlates of preparatory and regulatory control over positive and negative emotion. Social Cognitive and Affective Neuroscience, 9(4), 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer M.E., Delgado M.R. (2017). Reminiscing about positive memories buffers acute stress responses. Nature Human Behaviour, 1(5), 0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George M., Kutas M., Martinez A., Sereno I.M. (1999). Semantic integration in reading: engagement of the right hemisphere during discourse processing. Brain, 122(7), 1317–25. [DOI] [PubMed] [Google Scholar]
- Talarico J.M., Berntsen D., Rubin D.C. (2009). Positive emotions enhance recall of peripheral details. Cognition and Emotion, 23(2), 380–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompetter H.R., Kleine E., Bohlmeijer E.T. (2016). Why does positive mental health buffer against psychopathology? An exploratory study on self-compassion as a resilience mechanism and adaptive emotion regulation strategy. Cognitive Therapy and Research . Doi: 10.1007/s10608-016-9774-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueba A.F., Simon E., Auchus R.J., Ritz T. (2016). Cortisol response to acute stress in asthma: moderation by depressive mood. Physiology & Behavior, 159, 20–6. [DOI] [PubMed] [Google Scholar]
- Tugade M.M., Devlin H.C., Fredrickson B.L. (2014). Infusing positive emotions into life: the broaden-and-build theory and a dual-process model of resilience In Tugade M.M., Shiota M.N., Kirby L.D., editors. Handbook of Positive Emotions, pp. 28–43. New York, NY: Guilford Press. [Google Scholar]
- Tugade M.M., Fredrickson B.L., Barrett L.F. (2004). Psychological resilience and positive emotional granularity: examining the benefits of positive emotions on coping and health. Journal of Personality, 72(6), 1161–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turowski T.K., Man V.Y., Cunningham W.A. (2014). Positive emotion and the brain: the neuroscience of happiness In Gruber J., Moskowitz J.T., editors. Positive Emotion: Integrating the Light Sides and Dark Sides, pp. 95–115. New York, NY: Oxford University Press. [Google Scholar]
- van Winkel M., Nicolson N.A., Wichers M., Viechtbauer W., Myin-Germeys I., Peeters F. (2015). Daily life stress reactivity in remitted versus non-remitted depressed individuals. European Psychiatry, 30(4), 441–7. [DOI] [PubMed] [Google Scholar]
- Vanlessen N., De Raedt R., Koster E.H.W., Pourtois G. (2016). Happy heart, smiling eyes: a systematic review of positive mood effects on broadening of visuospatial attention. Neuroscience and Biobehavioral Reviews, 68, 816–37. [DOI] [PubMed] [Google Scholar]
- Wager T.D., van Ast V.A., Hughes B.L., Davidson M.L., Lindquist M.A., Ochsner K.N. (2009). Brain mediators of cardiovascular responses to social threat, Part II: prefrontal-subcortical pathways and relationship with anxiety. NeuroImage, 47(3), 836–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Waugh C.E., Lindquist M., Noll D.C., Fredrickson B.L., Taylor S.F. (2009). Brain mediators of cardiovascular responses to social threat: part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage, 47(3), 821–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh C.E. (2014). The regulatory power of positive emotions in stress: a temporal-functional approach In Kent M., Davis M.C., Reich J.W., editors. The Resilience Handbook: Approaches to Stress and Trauma, pp. 73–85. New York, NY: Routledge/Taylor & Francis Group. [Google Scholar]
- Waugh C.E., Fredrickson B.L. (2006). Nice to know you: positive emotions, self-other overlap, and complex understanding in the formation of a new relationship. The Journal of Positive Psychology, 1(2), 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh C.E., Hamilton J.P., Chen M.C., Joormann J., Gotlib I.H. (2012). Neural temporal dynamics of stress in comorbid major depressive disorder and social anxiety disorder. Biology of Mood & Anxiety Disorders, 2, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh C.E., Shing E.Z., Avery B.M., Jung Y., Whitlow C.T., Maldjian J.A. (2017). Neural predictors of emotional inertia in daily life. Social Cognitive and Affective Neuroscience, 12(9), 1448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh C.E., Thompson R.J., Gotlib I.H. (2011). Flexible emotional responsiveness in trait resilience. Emotion, 11(5), 1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winecoff A., Clithero J.A., Carter R.M., Bergman S.R., Wang L., Huettel S.A. (2013). Ventromedial prefrontal cortex encodes emotional value. The Journal of Neuroscience, 33(27), 11032–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkin J.R. (2015). Cultivating multiple aspects of attention through mindfulness meditation accounts for psychological well-being through decreased rumination. Psychology Research and Behavior Management, 8, 171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S., Cui J., Wang K., Zhang S., Qiu J., Luo Y. (2013). Positive emotion modulates cognitive control: an event‐related potentials study. Scandinavian Journal of Psychology, 54(2), 82–8. [DOI] [PubMed] [Google Scholar]
- Yarkoni T. (2011). Neurosynth.org. from neurosynth.org [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra A.J., Johnson L.M., Davis M.C. (2005). Positive affect as a source of resilience for women in chronic pain. Journal of Consulting and Clinical Psychology, 73(2), 212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra A.J., Reich J.W., Davis M.C., Potter P.T., Nicolson N.A. (2000). The role of stressful events in the relationship between positive and negative affects: evidence from field and experimental studies. Journal of Personality, 68(5), 927–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.