Abstract
Heterocyclic amines (HCAs) produced during high-temperature cooking have been studied extensively in terms of their genotoxic/genetic effects, but recent work has implicated epigenetic mechanisms involving non-coding RNAs. Colon tumors induced in the rat by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) have altered microRNA (miRNA) signatures linked to dysregulated pluripotency factors, such as c-Myc and Krüppel-like factor 4 (KLF4). We tested the hypothesis that dysregulated miRNAs from PhIP-induced colon tumors would provide a “PhIP signature” for use in other target organs obtained from a 1-year carcinogenicity bioassay in the rat. Downstream targets that were corroborated in the rat were then investigated in human cancer datasets. The results confirmed that multiple let-7 family members were downregulated in PhIP-induced skin, colon, lung, small intestine, and Zymbal’s gland tumors, and were associated with c-myc and Hmga2 upregulation. PhIP signature miRNAs with the profile mir-21high/mir-126low/mir-29clow/mir-215low/mir-145low were linked to reduced Klf4 levels in rat tumors, and in human pan-cancer and colorectal cancer. It remains to be determined whether this PhIP signature has predictive value, given that more than 20 different genotoxic HCAs are present in the human diet, plus other agents that likely induce or repress many of the same miRNAs. Future studies should define more precisely the miRNA signatures of other HCAs, and their possible value for human risk assessment.
Keywords: Colorectal cancer, MicroRNA, MYC, Pan-cancer, PhIP, TCGA
Introduction
Epidemiological evidence has linked the consumption of cooked and processed meat to increased risk for cardiovascular diseases (Micha et al. 2010), and for malignancies of the digestive system (Le et al. 2016), breast (Fu et al. 2011), and prostate (Cross et al. 2005). Heterocyclic amines (HCAs) found in high-temperature cooked meat can undergo metabolic activation to form bulky DNA adducts that are precursors to mutagenesis and carcinogenesis in various organs of the body (Alexander 1996; Adamson et al. 1996; Turesky et al. 1994). For this reason, HCAs have been investigated historically in terms of their genetic aspects linked to genotoxicity and tumor initiation in target tissues (Dashwood et al. 1998; David et al. 2016).
One of the most abundant HCAs found in cooked protein-rich foods is 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine(PhIP) (Nicken et al. 2013; Augustsson et al. 1997; Nagao et al. 1996). Metabolic intermediates of PhIP have been detected in various tissues, including serum, urine, and hair samples (Busquets et al. 2013; Le Marchand et al. 2016; Peng and Turesky 2011; Turteltaub et al. 1992), indicating wide systemic distribution in the human body. The US National Toxicology Program (NTP) classified PhIP as “reasonably anticipated to be a human carcinogen” (NTP 12th Report on Carcinogens), and the International Agency for Research on Cancer categorized PhIP as a Group 2B carcinogen (IARC 1997).
In preclinical models, PhIP serves as a multi-organ carcinogen (Ghoshal et al. 1994; Shirai et al. 1997; Ubagai et al. 2002), and can affect newborns through trans-placenta and neonatal exposure (Hasegawa et al. 1995). In PhIP-induced colon tumors, genetic changes in Ctnnb1 constitutively activate β-catenin and β-catenin/T-cell factor (Tcf) signaling, dysregulating the β-catenin/c-Myc/E2F1/Bcl-2 axis and downstream apoptosis pathways (Li et al. 2007; Wang et al. 2008a, b; Kwon et al. 2010). In rat colon and prostate carcinogenesis models, PhIP exposure has been linked to chronic inflammation via changes in molecular endpoints such as NADPH oxidase, nuclear factor kappa-B (NFκB) (Wang et al. 2011), cyclooxygenases (Wiese et al. 2001), and glutathione S-transferases (Nelson et al. 2001). PhIP-induced mammary carcinogenesis models identified changes in the expression of transcription factors, cyclins, annexins, matrix metalloproteinases, and extracellular signal- regulated (ERK) kinases (Choudhary et al. 2012).
In addition to the genetic aspect mentioned above, research with PhIP and other HCAs has moved into the epigenetic realm. Non-coding RNAs have gained increasing attention for their roles in epigenetic regulation, and as signatures of environmental exposure (Bolleyn et al. 2015; Wei et al. 2015; Yu and Cho 2015; Melis et al. 2014; Esteller 2011). For example, in human-derived prostaspheres, bisphenol A silenced small nucleolar RNAs with C/D motif (SNORDs) through histone modifications (Ho et al. 2015). Industrial exposure to metal-rich particulate matter altered microRNAs (miRNAs) linked to oxidative stress and inflammatory responses in blood (Bollati et al. 2010). It is well established that miRNAs are conserved, negative regulatory small RNAs comprising 21–23 nucleotides, which act via mRNA cleavage or translational inhibition to influence phenotypic outcomes such as cell proliferation, differentiation, autophagy, and apoptosis (Parasramka et al. 2012c; Vrijens et al. 2015).
Dysregulation of miRNAs has been linked to various pathologies, including cancer, neurodegeneration, obesity, and chronic inflammation (Dimmeler and Nicotera 2013). Consequently, altered miRNA signatures are regarded as possible biomarkers for disease diagnosis and prognosis, due to their relative stability and accessibility in various tissues and biofluids (Chen et al. 2008). For instance, mir-21 regulates phosphatase and tensin homolog (PTEN) and programmed cell death 4 (PDCD4) in tumors, leading to enhanced cell proliferation and stemness maintenance. In blood, mir-21 is viewed as a diagnostic and prognostic biomarker, whereas mir-141, a regulator of epithelial-to-mesenchymal transition, is a proposed circulating biomarker of late stage colorectal cancer (Yin et al. 2014). Circulating and tissue miRNAs also respond to environmental exposures, such as air pollution (Hou et al. 2016) and toxic chemicals (Zhang et al. 2010).
In cultured human breast cancer cells, PhIP treatment was reported to alter miRNAs regulating the response to xenoestrogen exposure (Papaioannou et al. 2014). In PhIP-induced rat colon tumors, dysregulated miRNAs were linked to the altered expression of c-Myc and other pluripotency factors, such as Sox2, Nanog, Oct-3/4, and Krüppel-like factor 4 (KLF4) (Parasramka et al. 2012b). We were intrigued by the possibility that miRNAs altered in PhIP-induced rat colon tumors might serve as biomarkers of PhIP exposure in other target organs for tumorigenesis, and by extension, might represent a ‘HCA miRNA signature’ in the general population. The present investigation reports, for the first time, on miRNAs and their validated downstream targets that are dysregulated in PhIP-induced tumors of the colon, small intestine, skin, lung, spleen, and Zymbal’s gland, and correlates with the corresponding human datasets.
Methods
Animal treatment
PhIP originally was defined as a prostate and colon carcinogen in male rats, and a mammary carcinogen in female rats, after continuous exposure in the diet for a year (Ito et al. 1997; Shirai et al. 1997). Subsequently, a modified protocol was devised that reduced the overall carcinogen exposure markedly, starting with short-term administration of PhIP followed by a high-fat diet (Ubagai et al. 2002; Wang et al. 2008a, b). For the current investigation, PhIP was given by daily oral gavage (40 mg/kg body weight) for 2 weeks, alternating with a high-fat diet for 4 weeks, and generated tumor incidence outcomes as follows: Zymbal’s gland 2%, liver 8%, spleen 8%, lung 10%, small intestine 27%, skin 38%, and colon 58% (Parasramka et al. 2012a). The 1-year carcinogenicity bioassay involved male F344 rats, purchased at 3–4 weeks of age from the National Cancer Institute. At termination, each rat (n = 40) was euthanized by CO2 inhalation, following a protocol that was approved by the Institutional Animal Care and Use Committee. After a thorough necropsy examination, tumor samples and other tissues were collected and flash-frozen before storage at −80 °C. Controls (n = 12 rat) that received vehicle by oral gavage for 2 weeks, alternating with a high-fat diet for 4 weeks, had no detectable tumors in any of the organs examined at the end of the study (Parasramka et al. 2012a).
RNA extraction and profiling
The key methodologies were described in previous studies that defined the major miRNAs altered in PhIP-induced rat colon tumors (Parasramka et al. 2012a). In brief, vehicle controls, PhIP-induced tumors, and tumor-matched normal tissues from colon, small intestine, skin, Zymbal’s gland, spleen, and lung were homogenized on ice. Total RNA was extracted in Trizol reagent (Life Technologies), whereas, miRNA was extracted using the miRNeasy kit (Qiagen, Valencia, CA, USA). RNA quantity and purity were verified by Nanodrop ND-1000, from the absorbance at 260 and 280 nm (260/280 ratio >1.9), as reported (Simonich et al. 2007; Jubert et al. 2009; Ertem et al. 2017). RNA (1 µg) from each rat tissue was reverse transcribed using the Superscript III RT kit (Life Technologies) in 10-µl reaction buffer, and diluted 10-fold before adding to a 10-µl reaction containing SYBR Green I Master mix (Roche Applied Science) and gene-specific primers. For miRNA analyses, 1-µg RNA from each rat tissue was reverse transcribed using the miScript II RT Kit (Qiagen) and diluted fivefold before adding to a 20-µl miScript Primer assay (Qiagen) containing primers specific for selected mature rat miRNAs. Real-time qPCR data were acquired on a Light-Cycler 480 II (Roche Applied Science). Relative expression levels of miRNAs and mRNAs were calculated using U6B and Gapdh as internal references for normalization, respectively.
Principle component analysis (PCA)
PCA used the online tool MetaboAnalyst 3.0 (Xia et al. 2015), and clustering was performed using Pearson correlation and average linkage.
Biological network analyses
MicroRNAs and their putative target mRNAs were examined via MetaCore pathway analysis (GeneGo Inc., St Joseph, MI, USA). For pathway enrichment analysis, p values were calculated using the formula for hypergeometric distribution, reflecting the probability for a pathway to arise by chance. Pathway maps were prioritized based on statistical significance, for tumors from animals given PhIP versus normal-looking tissue for each target organ. Interaction networks of miRNAs of interest were generated using Metacore (Thomson Reuters), as reported (Parasramka et al. 2012a). Functional annotation and subsequent analyses were conducted using DAVID (Huang et al. 2009) and TargetScan (Agarwal 2015).
Surveying the cancer genome atlas (TCGA)
Pan-cancer (PANCAN) miRNA (n = 11,010) and gene-specific RNA-seq data (n = 9755) were downloaded on 8 April 2016 from the UCSC Cancer Genomics Browser (Cline et al. 2013). To be consistent with the rat preclinical model, only primary tumor and solid normal tissue data were analyzed, whereas metastatic, advanced metastatic, and recurrent cancers were not included. The miRNA database contained 364 primary colon adenocarcinomas and 8 solid normal colon tissues, plus 8402 primary tumors and 635 normal tissues from other origins. The gene expression database for colon and rectal cancers contained 380 primary tumors and 50 solid normal tissues, plus 8079 primary tumors and 626 solid normal tissues from other origins. A survival plot was generated by Graphpad6.07 (GraphPad Software Inc.) according to the relative expression level of miRNAs in PANCAN datasets for solid primary tumors. Proportion views of miRNA expression were captured from the Cancer Browser, and statistical analyses were performed using Student’s t test with Benjamin–Hochberg correction, designating significantly upregulated (red) or downregulated (green) targets.
Statistical analyses
Unless indicated otherwise, results were presented as mean ± SEM with n = 3 per group. A paired two-tail Student’s t test was performed for tumor and tumor-matched normal tissue. Unpaired two-tail Student’s t test was performed for TCGA data, comparing primary tumor and normal tissue. An asterisk in the figure designates significance at *p < 0.05, **p < 0.01, ***p < 0.001, except when the exact p value is shown.
Results
Defining “PhIP signature” miRNAs in multiple target organs
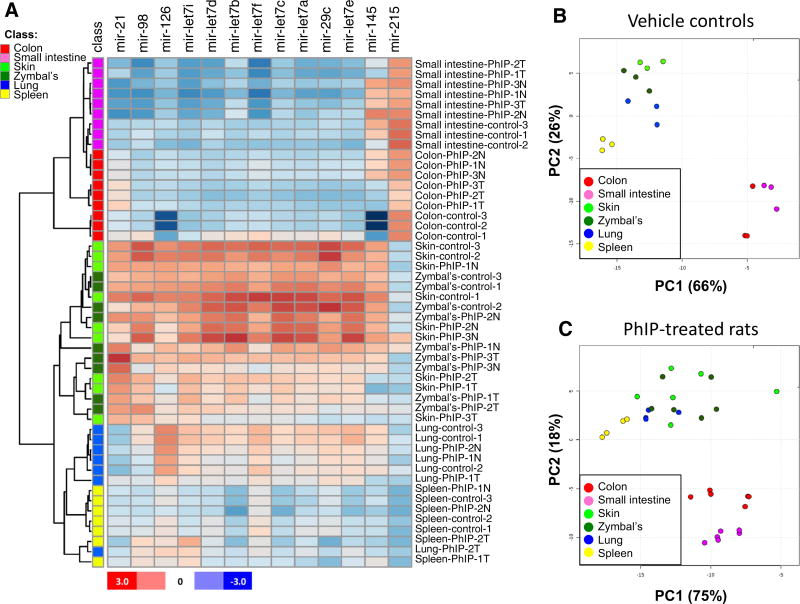
The miRNAs most highly dysregulated in PhIP-induced colon tumors (Parasramka et al. 2012a) were examined in other target tissues of the rat, namely the small intestine, skin, spleen, lung, and Zymbal’s gland. Hierarchical clustering of miRNAs grouped the colon and small intestine as separate from the other tissues (Fig. 1a, upper blue panels). The miRNA pattern was similar for skin and Zymbal’s gland (Fig. 1a, bold red panels at center), and clustered spleen and lung separately, except for replicate Lung-PhIP-2 T (Fig. 1a, lower panels).
Fig. 1.
Target tissue miRNA profiling in the rat. a Heatmap showing the relative expression level of miRNAs, with Pearson average clustering of “PhIP signature miRNAs” for the different sub-groups indicated in the figure. For each tissue, n = 3 individual replicates, except for spleen and lung tumors, where n = 2. T tumor; N normal-looking tissue adjacent to tumor. b Principle component analysis (PCA) of miRNAs in normal tissues from control rats given vehicle but no PhIP. c The corresponding PCA data for tumor and adjacent normal-looking tissues from PhIP-treated rats
Principle component analysis (PCA) was conducted on triplicate samples of each normal tissue, obtained from control rats that had received vehicle but no PhIP (Fig. 1b). Colon and small intestine were clustered in one area, separate from spleen, and from skin, Zymbal’s gland and lung samples that were grouped together. When PCA was repeated for PhIP-treated animals (Fig. 1c), analyzing both tumor and adjacent normal-looking tissues, colon and small intestine continued to segregate from the other tissues examined. The results indicated that a panel comprising of mir-21, mir-126, mir-29c, mir-135, mir-215, plus eight members of the let-7 family could distinguish among the different target organs of PhIP-induced tumorigenesis in the rat.
The let-7 family is dysregulated in multiple target organs of PhIP tumorigenesis
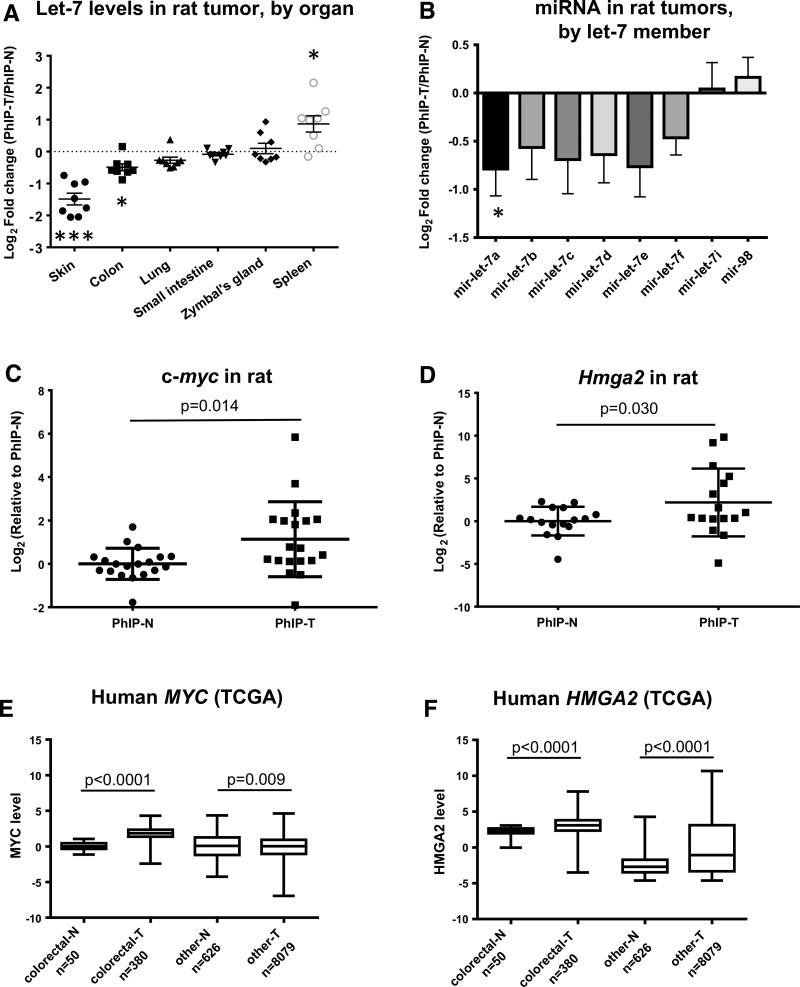
The let-7 family is highly conserved across species and function (Barh et al. 2010), and loss of let-7 expression during cancer development has been linked to changes in differentiation (Boyerinas et al. 2010) and oncogenic transformation (Iliopoulos et al. 2009). Using quantitative real-time RT-PCR, PhIP signature miRNAs that were examined in multiple target organs of the rat including let-7 family members let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7i, and mir-98. These miRNAs varied in expression among the various target tissues (Fig. 2a), with five members generally downregulated and two others marginally upregulated in PhIP-induced tumors (Fig. 2b).
Fig. 2.
Let-7 family is altered in multiple target organs of PhIP-induced tumorigenesis. a Expression of let-7 members according to tissue of origin. Each point represents an individual let-7 member in PhIP-induced tumor (T), normalized to the corresponding expression in adjacent normal-looking tissue (N). b Let-7 family members were downregulated in PhIP-induced tumors (all sites combined), with the exception of low-abundance members let-7i and mir-98. c, d qRT-PCR data for let-7 targets c-myc and Hmga2, normalized to Gapdh, in PhIP-induced tumors and adjacent normal tissue in the rat. e, f The corresponding human MYC and HMGA2 expression data from The Cancer Genome Atlas (TCGA), for Pan-Cancer primary tumors and normal tissue samples
Targets of let-7 were validated in tissue samples from PhIP-treated animals. When all six target organs were assessed, collectively, both c-myc and Hmga2 mRNA levels, normalized to Gapdh, were increased significantly in PhIP-induced tumors compared with matched normal tissue (Fig. 2c, d, respectively). Notably, when peripheral blood mononuclear cells (PBMCs) were collected as negative controls from the same study, representing a site that did not develop tumors, no marked differences were detected between PhIP- and vehicle-treated rats with respect to the relative expression levels of c-myc, Hmga2, and the PhIP signature miRNAs (data not shown).
In human colorectal cancers, and in “all other” cancers from the TCGA pan-cancer database, MYC and HMGA2 levels also were increased significantly compared with tissue-matched normal controls (Fig. 2e, f). The results are consistent with the tumor suppressor functions of let-7 family members, linked to upregulation of oncogenic targets in both rat and human primary tumors.
PhIP signature miRNAs predict worse prognosis
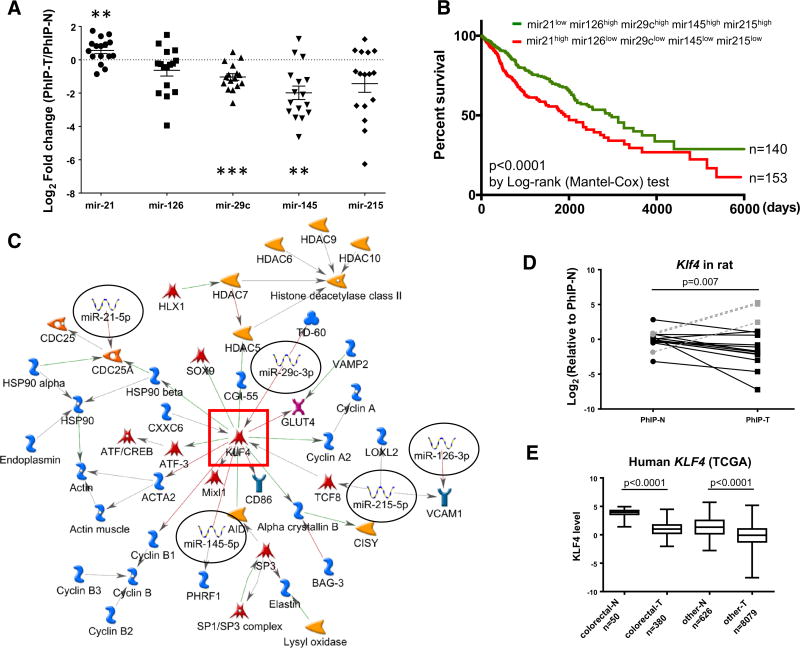
A second group of highly dysregulated PhIP signature miRNAs included mir-21, a well-known oncomiR (Buscaglia and Li 2011), and reported tumor suppressors mir-145, mir-126, mir-29c, and mir-215 (Sachdeva and Mo 2010; Parasramka et al. 2012a). When miRNAs were compared in PhIP-induced tumors (n = 16) versus adjacent normal-looking tissue (n = 16), there was a trend towards upregulation of mir-21 and downregulation of mir-126, mir-29c, mir-145, and mir-215 (Fig. 3a). Statistically significant differences were noted for mir-21 (p < 0.01), mir-29c (p < 0.001), and mir-145 (p < 0.01).
Fig. 3.
PhIP signature miRNAs predict poor prognosis. a Expression profile of five major PhIP signature miRNAs in tumors compared with the corresponding adjacent normal tissue; n = 16, **p < 0.01, ***p < 0.001. b Survival curves of patients listed with primary tumors in the Pan-Cancer TCGA database, grouped according to the relative expression of PhIP signature miRNAs indicated in the figure. c Metacore pathway analysis of five PhIP signature miRNAs defined a central node involving KLF4 (red square). d In qRT-PCR analyses, Klf4 was downregulated in PhIP-induced tumors compared with adjacent normal tissue in the rat, except for skin tumor samples, where the reverse trend was observed (gray dotted lines). e TCGA data showing downregulation of KLF4 in human colorectal cancer and pan-cancer primary tumors (T), compared with normal tissues (N)
This miRNA signature was used to profile the human pan-cancer database in TCGA, and predicted a significantly worse overall survival in cancer patients (Fig. 3b, red line). Metacore analysis for the corresponding miRNAs revealed a network of interactions centered on KLF4 (Fig. 3c, red square). Subsequent investigation confirmed that the predicted target was significantly lower in the majority of PhIP-induced rat tumors (Fig. 3d), and in human primary colorectal and “other” cancers (Fig. 3e). Thus, tumors harboring a PhIP signature miRNA profile predicted worse overall prognosis in cancer patients.
Overlapping PhIP signature miRNAs in human primary tumors
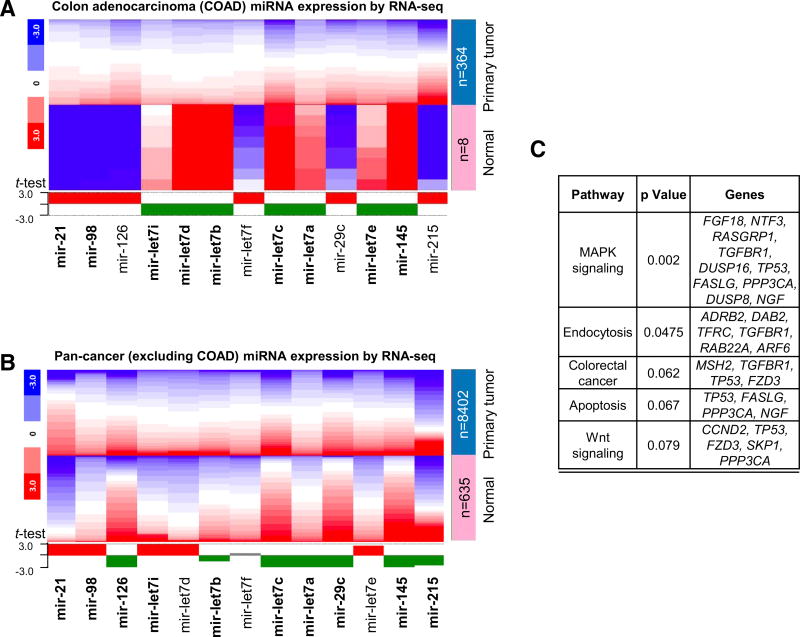
When TCGA data were examined in more detail, among the 13 most highly dysregulated miRNAs in PhIP-induced rat tumors, 9 were similarly changed in human colon adenocarcinomas (Fig. 4a), and 10 were correspondingly altered in human pan-cancer datasets (Fig. 4b). Functional annotation identified key pathways for these miRNAs, including MAPK signaling, endocytosis, apoptosis, Wnt signaling, and colorectal cancer (Fig. 4c).
Fig. 4.
PhIP miRNA signature from rats recapitulated in human primary tumors. a TCGA data were mined for PhIP signature miRNA levels in human colon primary adenocarcinomas (COAD) compared with normal tissue. The heatmap is color-coded to indicate the relative gene expression level within each dataset. The expression profile of each miRNA that recapitulated the corresponding outcome in rat is shown in bold. b Corresponding data for pan-cancer, excluding the colon adenocarcinoma (COAD) data already analyzed in a. c Functional annotation of gene targets of PhIP signature miRNAs. Mir-21, mir-145, and let-7 family members were entered into TargetScan (http://www.targetscan.org), which identified 180 predicted target genes. These predicted targets were uploaded into DAVID, and the functional annotation results for the top five KEGG pathway terms were listed, as shown
Discussion
Environmental factors can impact epigenetic mechanisms that regulate the crosstalk between DNA methylation, histone modifications, and non-coding RNAs (Rajendran et al. 2011a, b; Parasramka et al. 2012a; Hou et al. 2012; Tellez-Plaza et al. 2014; Johnson et al. 2016). Historically, HCAs have been investigated in terms of their genotoxic/genetic influences and various prevention strategies (Hayashi et al. 1985; Hernaez et al. 1998; Dashwood et al. 1998; Xu and Dashwood 1999; Kim et al. 2016), but few studies to date have explored the possible epigenetic aspects linked to human intake of PhIP and structurally related compounds in the diet.
Starting with the colon tumors, we performed an unbiased screen and validated the most highly dysregulated miRNAs and their downstream targets in the rat (Parasramka et al. 2012a). The current investigation builds upon those initial observations, testing the hypothesis that PhIP signature miRNAs in colon tumors might be applicable to other target organs of the rat. Indeed, a relatively small and focused 13-panel miRNA profile clustered colon and small intestine separately from skin, Zymbal’s gland, spleen, and lung. Similar groupings were reported following genome-wide miRNA profiling of 55 different organs and tissues in the rat (Minami et al. 2014).
Dysregulated miRNAs in tumors trigger upregulation or downregulation of mRNA targets, depending on the circumstances. Overexpression of mir-21, for example, causes loss of tumor suppressors PDCD4 and PTEN to promote cell survival (Li et al. 2014), epithelial-to-mesenchymal transition (Ferraro et al. 2014), and chemoresistance (Chao et al. 2013). In addition to targeting PDCD4, PTEN, and FASL to inhibit apoptosis (Buscaglia and Li 2011), mir-21 can coordinate with mir-145 in regulating colon cancer stemness and chemoresistance through CD44, SOX-2, and β-catenin (Yu et al. 2015). Interestingly, both mir-21 and mir-145 were among the PhIP signature miRNAs dysregulated in multiple target organs of the rat.
Mir-21 is one of a cadre of miRNAs altered by environmental exposures such as cigarette smoking, air pollution, diesel exhaust, nanoparticles, particulate matter, toxic metals, and diverse chemicals (Vrijens et al. 2015). Environmental pollutants such as bisphenol A, polychlorinated biphenyls, arsenic, mercury, lead, and cadmium also downregulate members of the let-7 family of suppressor miRNAs (Li et al. 2015), which can impact IL6-STAT3 signaling and epigenetic transformation (Iliopoulos et al. 2009).
Multiple let-7 family members were among the PhIP signature miRNAs, with let-7a, let-7b, let-7c, let-7d, led-7e, and let-7f being downregulated in the different target organs of PhIP tumorigenesis. In accordance with the tumor suppressor functions ascribed to let-7 members (Wang et al. 2012), we confirmed upregulation of their oncogenic targets, c-myc and Hmga2, in the corresponding rat tumors. Interestingly, human breast cancer cells treated with bisphenol A or dichlorodiphenyltrichloroethane (DDT) also had attenuated let-7 levels (Tilghman et al. 2012), highlighting a potential broader impact of environmental influences on these suppressor miRNAs.
Four additional suppressor miRNAs were downregulated at multiple tumor sites in PhIP-treated rats, namely mir-126, mir-29c, mir-145, and mir-215. In human stage II and III colon cancers, mir-215 is decreased and is associated with poor prognosis (Karaayvaz et al. 2011), but mir-215 also plays a role in chemoresistance (Song et al. 2010). As discussed above, mir-215 can coordinate with mir-21 to regulate CD44, SOX2, and β-catenin in colon cancer cells (Yu et al. 2015). Mir-126 inhibits pancreatic (Hamada et al. 2012) and breast cancer progression and metastasis (Zhu et al. 2011) by targeting ADAM9 and PI3K, whereas downregulation of mir-126 is associated with poor prognosis in non-small cell lung cancer, due to dysregulated EGFL7 (Liu et al. 2009). The mir-29 family regulates DNA methylation and metastasis in lung cancer and nasopharyngeal carcinoma by targeting DNA methyltransferases (Fabbri et al. 2007; Sengupta et al. 2008).
We postulated that the miRNA profile prioritized in PhIP-induced rat tumors might serve as a “fingerprint” of environmental HCA exposure in the human population. Insufficient miRNA data were available in TCGA to interrogate the entire panel of 13 PhIP signatures miRNAs, collectively. However, a focused panel comprising of mir-21high/ mir-126low/mir-29clow/mir-215low/mir-145low predicted significantly worse overall patient survival (Fig. 3b). Interestingly, this subgroup consisted of 153/9723 available cases, giving an estimated frequency of 1.57% of pancancers in the human population. However, we are cautious not to over-interpret these findings as being directly associated with PhIP exposure. There are more than 20 different genotoxic HCAs present in the diet (Adamson et al. 1996; Nagao et al. 1996). Also, polycyclic aromatic hydrocarbons, N-nitroso compounds, lipid peroxides, and reactive oxygen species are thought to contribute to meat-associated DNA damage and cancer etiology (Knize et al. 1999; Hamidi et al. 2016), and might induce or repress the same miRNAs as PhIP.
Nonetheless, we were interested in the fact that PhIP signature miRNAs defined KLF4 as a central player (Fig. 3c), a transcription factor with known links to cell proliferation, autophagy, transformation, metastasis, and pluripotency (Farrugia et al. 2016). Expression of KLF4 was reduced significantly in human primary colorectal and human pan-cancer, and Klf4 also was decreased in the majority of PhIP-induced rat tumors. In PhIP-induced skin tumors, however, Klf4 was overexpressed rather than downregulated. It is known that KLF4 can serve as a bivalent transcription factor, with activating or repressive functions on gene expression according to the circumstances (Rowland et al. 2005). In the skin, nuclear KLF4 was linked to squamous epithelial dysplasia (Foster et al. 2005), but KLF4 deficiency also correlated with increased cell proliferation and enhanced tumor outcomes in a classical mouse skin carcinogenesis model (Li et al. 2012). Cross-talk with other transcription factors, such as the glucocorticoid receptor (Sevilla et al. 2015), likely contributes to the divergent actions of KLF4 in the skin.
Finally, when PhIP signature miRNAs were compared, as a group, with TCGA data for colon adenocarcinoma and pan-cancer, most of the miRNAs in human subjects were upregulated or downregulated in the same direction as in the rat tumors. Bioinformatics analyses implicated gene ontology pathways linked to endocytosis, apoptosis, MAPK signaling, Wnt signaling, and colorectal cancer. We did observe a relatively modest change in c-myc and Hmga2 expression in PhIP-induced tumors, compared with adjacent normal tissue, whereas the expression range of these proteins was larger in human tumors from the same organs. The wide range of expression in humans probably reflects different types of environmental exposure. Thus, it is difficult to make firm conclusions about the relative abundance of these proteins and attribute them specifically to PhIP, or other HCAs, in humans. Nonetheless, the current investigation has laid the groundwork for follow-up studies on other environmental HCAs, their miRNA signatures, and the downstream targets implicated in cancer development.
Acknowledgments
This research was supported in part by NIH Grants CA090890, CA122959, ES00210, and ES023512, the John S. Dunn Foundation, and a Chancellor’s Research Initiative.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no actual or potential competing financial interests.
References
- Adamson RH, Thorgiersson UP, Sugimura T. Extrapolation of heterocyclic amine carcinogenesis data from rodents and nonhuman primates to humans. Arch Toxicol Suppl. 1996;18:303–318. doi: 10.1007/978-3-642-61105-6_29. [DOI] [PubMed] [Google Scholar]
- Agarwal V, Bell GW, Nam J-W, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife Aug. 2015;12:4. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. Formation and processing of reactive metabolites of the heterocyclic amines. Arch Toxicol Suppl. 1996;18:275–285. doi: 10.1007/978-3-642-61105-6_27. [DOI] [PubMed] [Google Scholar]
- Augustsson K, Skog K, Jagerstad M, Steineck G. Assessment of the human exposure to heterocyclic amines. Carcinogenesis. 1997;18:1931–1935. doi: 10.1093/carcin/18.10.1931. [DOI] [PubMed] [Google Scholar]
- Barh D, Malhotra R, Ravi B, Sindhurani P. MicroRNA let-7: An emerging next-generation cancer therapeutic. Curr Oncol. 2010;17:70–80. doi: 10.3747/co.v17i1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollati V, Marinelli B, Apostoli P, Bonzini M, Nordio F, Hoxha M, et al. Exposure to metal-rich particulate matter modifies the expression of candidate microRNAs in peripheral blood leukocytes. Environ Health Perspect. 2010;118:763–768. doi: 10.1289/ehp.0901300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolleyn J, De Kock J, Rodrigues RM, Vinken M, Rogiers V, Vanhaecke T. MicroRNAs as key regulators of xenobiotic biotransforamtion and drug response. Arch Toxicol. 2015;89:1523–1541. doi: 10.1007/s00204-014-1314-7. [DOI] [PubMed] [Google Scholar]
- Boyerinas B, Park SM, Hau A, Murmann AE, Peter ME. The role of let-7 in cell differentiation and cancer. Endocr Relat Cancer. 2010;17:F19–36. doi: 10.1677/ERC-09-0184. [DOI] [PubMed] [Google Scholar]
- Buscaglia LE, Li Y. Apoptosis and the target genes of micro-RNA-21. Chin J Cancer. 2011;30:371–380. doi: 10.5732/cjc.011.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets R, Frandsen H, Jonsson JA, Puignou L, Galceran MT, Skog K. Biomonitoring of dietary heterocyclic amines and metabolites in urine by liquid phase microextraction:2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), a possible biomarker of exposure to dietary phip. Chem Res Toxicol. 2013;26:233–240. doi: 10.1021/tx3003966. [DOI] [PubMed] [Google Scholar]
- Chao TF, Xiong HH, Liu W, Chen Y, Zhang JX. mir-21 mediates the radiation resistance of glioblastoma cells by regulating PDCD4 and HMSH2. J Huazhong Univ Sci Technolog Med Sci. 2013;33:525–529. doi: 10.1007/s11596-013-1153-4. [DOI] [PubMed] [Google Scholar]
- Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- Choudhary S, Sood S, Donnell RL, Wang HC. Intervention of human breast cell carcinogenesis chronically induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Carcinogenesis. 2012;33:876–885. doi: 10.1093/carcin/bgs097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MS, Craft B, Swatloski T, Goldman M, Ma S, Haussler D, et al. Exploring TCGA pan-cancer data at the UCSC cancer genomics browser. Sci Rep. 2013;3:2652. doi: 10.1038/srep02652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AJ, Peters U, Kirsh VA, Andriole GL, Reding D, Hayes RB, et al. A prospective study of meat and meat mutagens and prostate cancer risk. Cancer Res. 2005;65:11779–11784. doi: 10.1158/0008-5472.CAN-05-2191. [DOI] [PubMed] [Google Scholar]
- Dashwood RH, Suzui M, Nakagama H, Sugimura T, Nagao M. High frequency of beta-catenin (Ctnnb1) mutations in the colon tumors induced by two heterocyclic amines in the F344 rat. Cancer Res. 1998;58:1127–1129. [PubMed] [Google Scholar]
- David R, Ebbels T, Gooderham N. Synergistic and antagonistic mutation responses of human MCL-5 cells to mixtures of benzo[a]pyrene and 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine: Dose-related variation in the joint effects of common dietary carcinogens. Environ Health Perspect. 2016;124:88–96. doi: 10.1289/ehp.1409557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Nicotera P. MicroRNAs in age-related diseases. EMBO Mol Med. 2013;5:180–190. doi: 10.1002/emmm.201201986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertem FU, Zhang W, Chang K, Dashwood W, Rajendran P, Sun D, Abudayyeh A, Vilar E, Abdelrahim M, Dashwood RH. Oncogenic targets Mmp7, S100a9, Nppband Aldh1a3 from transcriptome profiling of FAP and Pirc adenomas are downregulated in response to tumor suppression by Clotam. Int J Cancer. 2017;140:460–468. doi: 10.1002/ijc.30458. [DOI] [PubMed] [Google Scholar]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3a and 3b. Proc Natl Acad Sci USA. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia MK, Vanderbilt DB, Salkeni MA, Ruppert JM. Kruppel-like pluripotency factors as modulators of cancer cell therapeutic responses. Cancer Res. 2016;76:1677–1682. doi: 10.1158/0008-5472.CAN-15-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro A, Kontos CK, Boni T, Bantounas I, Siakouli D, Kosmidou V, et al. Epigenetic regulation of mir-21 in colorectal cancer: ITGB4 as a novel mir-21 target and a three-gene network (mir-21-ITGBETA4-PDCD4) as predictor of metastatic tumor potential. Epigenetics. 2014;9:129–141. doi: 10.4161/epi.26842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KW, Liu Z, Nail CD, Li X, Fitzgerald TJ, Bailey SK, et al. Induction of KLF4 in basal keratinocytes blocks the proliferation- differentiation switch and initiates squamous epithelial dysplasia. Oncogene. 2005;24:1491–1500. doi: 10.1038/sj.onc.1208307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Deming SL, Fair AM, Shrubsole MJ, Wujcik DM, Shu XO, et al. Well-done meat intake and meat-derived mutagen exposures in relation to breast cancer risk: the nashville breast health study. Breast Cancer Res Treat. 2011;129:919–928. doi: 10.1007/s10549-011-1538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal A, Preisegger KH, Takayama S, Thorgeirsson SS, Snyderwine EG. Induction of mammary tumors in female sprague-dawley rats by the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and effect of dietary fat. Carcinogenesis. 1994;15:2429–2433. doi: 10.1093/carcin/15.11.2429. [DOI] [PubMed] [Google Scholar]
- Hamada S, Satoh K, Fujibuchi W, Hirota M, Kanno A, Unno J, et al. mir-126 acts as a tumor suppressor in pancreatic cancer cells via the regulation of ADAM9. Mol Cancer Res. 2012;10:3–10. doi: 10.1158/1541-7786.MCR-11-0272. [DOI] [PubMed] [Google Scholar]
- Hamidi EN, Hajeb P, Selamat J, Abdull Razis AF. Polycyclic aromatic hydrocarbons (PAHs) and the bioaccessibility in meat: a tool for assessing human cancer risk. Asian Pac J Cancer Prev. 2016;17:15–23. doi: 10.7314/apjcp.2016.17.1.15. [DOI] [PubMed] [Google Scholar]
- Hasegawa R, Kimura J, Yaono M, Takahashi S, Kato T, Futakuchi M, et al. Increased risk of mammary carcinoma development following transplacental and trans-breast milk exposure to a food-derived carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), in sprague-dawley rats. Cancer Res. 1995;55:4333–4338. [PubMed] [Google Scholar]
- Hayashi S, Moller ME, Thorgeirsson SS. Genotoxicity of heterocyclic amines in the Salmonella/hepatocyte system. Jpn J Cancer Res. 1985;76:835–845. [PubMed] [Google Scholar]
- Hernaez JF, Xu M, Dashwood RH. Antimutagenic activity of tea towards 2-hydroxyamino-3-methylimidazo[4,5-f]quinoline: effect of tea concentration and brew-time on electrophile scavening. Mutat Res. 1998;402:299–306. doi: 10.1016/s0027-5107(97)00309-6. [DOI] [PubMed] [Google Scholar]
- Ho SM, Cheong A, Lam HM, Hu WY, Shi GB, Zhu X, et al. Exposure of human prostaspheres to bisphenol a epigenetically regulates snord family noncoding RNAs via histone modification. Endocrinology. 2015;156:3984–3995. doi: 10.1210/en.2015-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Zhang X, Wang D, Baccarelli A. Environmental chemical exposures and human epigenetics. Int J Epidemiol. 2012;41:79–105. doi: 10.1093/ije/dyr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Barupal J, Zhang W, Zheng Y, Liu L, Zhang X, et al. Particulate air pollution exposure and expression of viral and human microRNAs in blood: the Beijing truck driver air pollution study. Environ Health Perspect. 2016;124:344–350. doi: 10.1289/ehp.1408519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-κB, Lin28, let-7 microRNA, and IL-6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine)(Group 2B) 1997 http://www.inchem.org/documents/iarc/vol56/08-phip.html.
- Ito N, Hasegawa R, Imaida K, Tamano S, Hagiwara A, Hirose M, Shirai T. Carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the rat. Mutat Res. 1997;376:107–114. doi: 10.1016/s0027-5107(97)00032-8. [DOI] [PubMed] [Google Scholar]
- Johnson GS, Li J, Beaver LM, Dashwood WM, Sun D, Rajendran P, Williams DE, Ho E, Dashwood RH. A functional pseudogene, NMRAL2P, is regulated by Nrf2 and serves as a coactivator of NQO1 in sulforaphane-treated colon cancer cells. Mol Nutr Food Res. 2016 doi: 10.1002/mnfr.201600769. doi: 10.1002/mnfr.201600769 (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- Jubert C, Mata J, Bench G, Dashwood R, Pereira C, Tracewell W, Turteltaub K, Williams D, Bailey G. Effects of chlorophyll and chlorophyllin on low-dose aflatoxin B(1) pharmacokinetics in human volunteers. Cancer Prev Res (Phila.) 2009;2:1015–1022. doi: 10.1158/1940-6207.CAPR-09-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaayvaz M, Pal T, Song B, Zhang C, Georgakopoulos P, Mehmood S, et al. Prognostic significance of mir-215 in colon cancer. Clin Colorectal Cancer. 2011;10:340–347. doi: 10.1016/j.clcc.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Guo J, O’Sullivan MG, Gallaher DD, Turesky RJ. Comparative DNA adduct formation and induction of colonic aberrant crypt foci in mice exposed to 2-amino-9h-pyrido[2,3-b] indole, 2-amino-3,4-dimethylimidazo[4,5-f]quinoline, and azoxymethane. Environ Mol Mutagen. 2016;57:125–136. doi: 10.1002/em.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knize MG, Salmon CP, Pais P, Felton JS. Food heating and the formation of heterocyclic amine and polycyclic aromatic hydrocarbon mutagens/carcinogens. Adv Exp Med Biol. 1999;459:179–193. doi: 10.1007/978-1-4615-4853-9_12. [DOI] [PubMed] [Google Scholar]
- Kwon IK, Wang R, Thangaraju M, Shuang H, Liu K, Dashwood R, Dulin N, Ganapathy V, Browning DD. PKG inhibits TCF signaling in colon cancer cells by blocking β-catenin expression and activating FOXO4. Oncogene. 2010;29:3423–3434. doi: 10.1038/onc.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le NT, Michels FA, Song M, Zhang X, Bernstein AM, Giovannucci EL, et al. A prospective analysis of meat mutagens and colorectal cancer in the nurses’ health study and health professional follow-up study. Environ Health Perspect. 2016;124:1529–1536. doi: 10.1289/EHP238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand L, Yonemori K, White KK, Franke AA, Wilkens LR, Turesky RJ. Dose validation of PhIP hair level as a biomarker of heterocyclic aromatic amines exposure: a feeding study. Carcinogenesis. 2016;37:685–691. doi: 10.1093/carcin/bgw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Dashwood WM, Zhong X, Nakagama H, Dashwood RH. Bcl-2 overexpression in PhIP-induced colon tumors: cloning of the rat Bcl-2 promoter and characterization of a pathway involving beta-catenin, c-myc and E2f1. Oncogene. 2007;26:6194–6202. doi: 10.1038/sj.onc.1210438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zheng H, Yu F, Yu T, Liu C, Huang S, et al. Deficiency of the Kruppel-like factor KLF4 correlates with increased cell proliferation and enhanced skin tumorigenesis. Carcinogenesis. 2012;33:1239–1246. doi: 10.1093/carcin/bgs143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xin S, He Z, Che X, Wang J, Xiao X, et al. MicroRNA-21 (mir-21) post-transcriptionally downregulates tumor suppressor PDCD4 and promotes cell transformation, proliferation, and metastasis in renal cell carcinoma. Cell Physiol Biochem. 2014;33:1631–1642. doi: 10.1159/000362946. [DOI] [PubMed] [Google Scholar]
- Li Q, Kappil MA, Li A, Dassanayake PS, Darrah TH, Friedman AE, et al. Exploring the associations between microRNA expression profiles and environmental pollutants in human placenta from the national children’s study. Epigenetics. 2015;10:793–802. doi: 10.1080/15592294.2015.1066960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Peng XC, Zheng XL, Wang J, Qin YW. Mir-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer. 2009;66:169–175. doi: 10.1016/j.lungcan.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Melis JP, Derks KW, Pronk TE, Wackers P, Schaap MM, Zwart E, van Ijcken WF, Jonker MJ, Breit TM, Pothof J, van Steeg H, Luijten M. In vivo murine hepatic microRNA and mRNA expression signatures predicting the (non-)genotoxic carcinogenic potential of chemicals. Arch Toxicol. 2014;88:1023–1034. doi: 10.1007/s00204-013-1189-z. [DOI] [PubMed] [Google Scholar]
- Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121:2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami K, Uehara T, Morikawa Y, Omura K, Kanki M, Horinouchi A, et al. miRNA expression atlas in male rat. Sci Data. 2014;1:140005. doi: 10.1038/sdata.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M, Wakabayashi K, Ushijima T, Toyota M, Totsuka Y, Sugimura T. Human exposure to carcinogenic heterocyclic amines and their mutational fingerprints in experimental animals. Environ Health Perspect. 1996;104(Suppl 3):497–501. doi: 10.1289/ehp.96104s3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CP, Kidd LC, Sauvageot J, Isaacs WB, De Marzo AM, Groopman JD, et al. Protection against 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine cytotoxicity and DNA adduct formation in human prostate by glutathione S-transferase P1. Cancer Res. 2001;61:103–109. [PubMed] [Google Scholar]
- Nicken P, Schröder B, von Keutz A, Breves G, Steinberg P. The colon carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) is actively secreted in the distal colon of the rat: an integrated view on the role of PhIP transport and metabolism in PhIP-induced colon carcinogenesis. Arch Toxicol. 2013;87:895–904. doi: 10.1007/s00204-012-1006-0. [DOI] [PubMed] [Google Scholar]
- NTP 12th Report on Carcinogens. Report on Carcinogens. 12 2011. U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program. [Google Scholar]
- Papaioannou MD, Koufaris C, Gooderham NJ. The cooked meat-derived mammary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) elicits estrogenic-like microRNA responses in breast cancer cells. Toxicol Lett. 2014;229:9–16. doi: 10.1016/j.toxlet.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Parasramka MA, Dashwood WM, Wang R, Abdelli A, Bailey GS, Williams DE, et al. MicroRNA profiling of carcinogen-induced rat colon tumors and the influence of dietary spinach. Mol Nutr Food Res. 2012a;56:1259–1269. doi: 10.1002/mnfr.201200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasramka MA, Dashwood WM, Wang R, Saeed HH, Williams DE, Ho E, et al. A role for low-abundance miRNAs in colon cancer: the mir-206/Krüppel-like factor 4 (Klf4) axis. Clin Epigenetics. 2012b;4:16. doi: 10.1186/1868-7083-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasramka MA, Ho E, Williams DE, Dashwood RH. Micro-RNAs, diet, and cancer: new mechanistic insights on the epigenetic actions of phytochemicals. Mol Carcinog. 2012c;51:213–230. doi: 10.1002/mc.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Turesky RJ. Mass spectrometric characterization of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine N-oxidized metabolites bound at Cys34 of human serum albumin. Chem Res Toxicol. 2011;24:2004–2017. doi: 10.1021/tx2003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran P, Wiliams DE, Ho E, Dashwood RH. Metabolism as a key to histone deacetylase inhibition. Crit Rev Biochem Mol Biol. 2011a;46:181–199. doi: 10.3109/10409238.2011.557713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran P, Ho E, Williams DE, Dashwood RH. Dietary phytochemicals, HDAC inhibition, and DNA damage/repair defects in cancer cells. Clin Epigen. 2011b;3:4. doi: 10.1186/1868-7083-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland BD, Bernards R, Peeper DS. The Klf4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol. 2005;7:1074–1082. doi: 10.1038/ncb1314. [DOI] [PubMed] [Google Scholar]
- Sachdeva M, Mo YY. Mir-145-mediated suppression of cell growth, invasion and metastasis. Am J Transl Res. 2010;2:170–180. [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, den Boon JA, Chen IH, Newton MA, Stanhope SA, Cheng YJ, et al. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci USA. 2008;105:5874–5878. doi: 10.1073/pnas.0801130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla LM, Latorre V, Carceller E, Boix J, Vodak D, Mills IG, et al. Glucocorticoid receptor and Klf4 co-regulate anti-inflammatory genes in keratinocytes. Mol Cell Endocrinol. 2015;412:281–289. doi: 10.1016/j.mce.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Shirai T, Sano M, Tamano S, Takahashi S, Hirose M, Futakuchi M, et al. The prostate: a target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997;57:195–198. [PubMed] [Google Scholar]
- Simonich MT, Egner PA, Roebuck BD, Orner GA, Jubert C, Pereira C, Groopman JD, Kensler TW, Dashwood RH, Williams DE, Bailey GS. Natural chlorophyll inhibits aflatoxin B1-induced multi-organ carcinogenesis in the rat. Carcinogenesis. 2007;28:1294–1302. doi: 10.1093/carcin/bgm027. [DOI] [PubMed] [Google Scholar]
- Song B, Wang Y, Titmus MA, Botchkina G, Formentini A, Kornmann M, et al. Molecular mechanism of chemoresistance by mir-215 in osteosarcoma and colon cancer cells. Mol Cancer. 2010;9:96. doi: 10.1186/1476-4598-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Plaza M, Tang WY, Shang Y, Umans JG, Francesconi KA, Goessler W, et al. Association of global DNA methylation and global DNA hydroxymethylation with metals and other exposures in human blood DNA samples. Environ Health Perspect. 2014;122:946–954. doi: 10.1289/ehp.1306674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman SL, Bratton MR, Segar HC, Martin EC, Rhodes LV, Li M, et al. Endocrine disruptor regulation of microRNA expression in breast carcinoma cells. PLoS One. 2012;7:e32754. doi: 10.1371/journal.pone.0032754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesky RJ, Gross GA, Stillwell WG, Skipper PL, Tannenbaum SR. Species differences in metabolism of heterocyclic aromatic amines, human exposure, and biomonitoring. Environ Health Perspect. 1994;102(Suppl 6):47–51. doi: 10.1289/ehp.94102s647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turteltaub KW, Vogel JS, Frantz CE, Shen N. Fate and distribution of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in mice at a human dietary equivalent dose. Cancer Res. 1992;52:4682–4687. [PubMed] [Google Scholar]
- Ubagai T, Ochiai M, Kawamori T, Imai H, Sugimura T, Nagao M, et al. Efficient induction of rat large intestinal tumors with a new spectrum of mutations by intermittent administration of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in combination with a high fat diet. Carcinogenesis. 2002;23:197–200. doi: 10.1093/carcin/23.1.197. [DOI] [PubMed] [Google Scholar]
- Vrijens K, Bollati V, Nawrot TS. MicroRNAs as potential signatures of environmental exposure or effect: a systematic review. Environ Health Perspect. 2015;123:399–411. doi: 10.1289/ehp.1408459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Dashwood WM, Löhr CV, Fischer KA, Nakagama H, Williams DE, et al. Beta-catenin is strongly elevated in rat colonic epithelium following short-term intermittent treatment with 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and a high-fat diet. Cancer Sci. 2008a;99:1754–1759. doi: 10.1111/j.1349-7006.2008.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Dashwood WM, Löhr CV, Fischer KA, Pereira CB, Louderback M, Nakagama H, Bailey GS, Williams DE, Dashwood RH. Protective versus promotional effects of white tea and caffenine on PhIP-induced tumorigenesis and β-catenin expression in the rat. Carcinogenesis. 2008b;29:834–839. doi: 10.1093/carcin/bgn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yamamoto JF, Caberto C, Saltzman B, Decker R, Vogt TM, et al. Genetic variation in the bioactivation pathway for polycyclic hydrocarbons and heterocyclic amines in relation to risk of colorectal neoplasia. Carcinogenesis. 2011;32:203–209. doi: 10.1093/carcin/bgq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cao L, Wang Y, Wang X, Liu N, You Y. Regulation of let-7 and its target oncogenes (review) Oncol Lett. 2012;3:955–960. doi: 10.3892/ol.2012.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Li F, Yang J, Liu X, Cho WC. MicroRNAs as regulators of airborne pollution-induced lung inflammation and carcinogenesis. Arch Toxicol. 2015;89:677–685. doi: 10.1007/s00204-015-1462-4. [DOI] [PubMed] [Google Scholar]
- Wiese FW, Thompson PA, Kadlubar FF. Carcinogen substrate specificity of human COX-1 and COX-2. Carcinogenesis. 2001;22:5–10. doi: 10.1093/carcin/22.1.5. [DOI] [PubMed] [Google Scholar]
- Xia J, Sinelnikov IV, Han B, Wishart DS. Metaboanalyst 3.0— making metabolomics more meaningful. Nucleic Acids Res. 2015;43:W251–W257. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Dashwood RH. Chemoprevention studies of heterocyclic amine-induced colon carcinogenesis. Cancer Lett. 1999;143:179–183. doi: 10.1016/s0304-3835(99)00121-4. [DOI] [PubMed] [Google Scholar]
- Yin J, Bai Z, Song J, Yang Y, Wang J, Han W, et al. Differential expression of serum mir-126, mir-141 and mir-21 as novel biomarkers for early detection of liver metastasis in colorectal cancer. Chin J Cancer Res. 2014;26:95–103. doi: 10.3978/j.issn.1000-9604.2014.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HW, Cho WC. The role of microRNAs in toxicology. Arch Toxicol. 2015;89:319–325. doi: 10.1007/s00204-014-1440-2. [DOI] [PubMed] [Google Scholar]
- Yu Y, Nangia-Makker P, Farhana L, S GR, Levi E, Majumdar AP. mir-21 and mir-145 cooperation in regulation of colon cancer stem cells. Mol Cancer. 2015;14:98. doi: 10.1186/s12943-015-0372-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, et al. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem. 2010;56:1830–1838. doi: 10.1373/clinchem.2010.147850. [DOI] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Xie H, Zhou Z, Chen H, Hu T, et al. Endothelial- specific intron-derived mir-126 is down-regulated in human breast cancer and targets both VEGFA and PIK3R2. Mol Cell Biochem. 2011;351:157–164. doi: 10.1007/s11010-011-0723-7. [DOI] [PubMed] [Google Scholar]