Abstract
Background
Fibrosis is a key pathological process in many chronic inflammatory disease states.
Aims
We hypothesized that tissue inhibitor metalloproteinase-1 and matrix metalloproteinase-9 (TIMP-1 and MMP-9), biomarkers of fibrosis, would predict all-cause mortality and we assessed the incremental value of these biomarkers when adjusting for clinical and other biomarkers.
Methods
The cohort included 5511 community-dwelling participants in the AGES-Reykjavik Study. The baseline Cox proportional hazards regression model was based on the Framingham Risk Score variables; we added TIMP-1, MMP-9, serum high-sensitivity C-reactive protein (hsCRP), and estimated glomerular filtration rate (eGFR). The primary outcome was all-cause 10-year mortality. Cause of death was categorized as cardiovascular death (CVD), cancer death, and other causes.
Results
Participants averaged 76 years and 43% were male. Ten-year mortality was 41% (2263 deaths). Of these, 915 (16.6%) died of cardiovascular disease (CVD), 543 (9.9%) with cancer, and 805 (14.6%) from other causes. For 10-year mortality, age was the strongest predictor (log likelihood χ2 = 798.7, P < 0.0001), followed by TIMP-1 (χ2 = 125.2, P < 0.0001), female gender, current smoker, diabetes mellitus, total cholesterol, eGFR (χ2 16.7, P < 0.0001), body mass index, and hsCRP (χ2 11.3, P = 0.0008) in that order. TIMP-1 and hsCRP had the highest continuous net reclassification improvement over the baseline model for 5-year survival [net reclassification index (NRI) 0.28 and 0.19, respectively, both P < 0.0001] and for 10-year survival (NRI 0.19 and 0.11, respectively, both statistically significant).
Conclusion
TIMP-1 is the strongest predictor of all-cause mortality after age. The metabolic pathways regulating extracellular matrix homeostasis and fibrogenic processes appear pathologically relevant and are prognostically important.
Introduction
Fibrosis is a key pathological outcome in many chronic inflammatory disease states.1 The extracellular matrix (ECM) is a highly dynamic structure, constantly undergoing remodelling. Abnormal ECM dynamics play a role in deregulated cell proliferation and in excessive tissue fibrosis.2 Ultimately, fibrosis can contribute to permanent scarring, organ malfunction, heart failure, and death.
Under normal conditions, tissue inhibitor metalloproteinases (TIMPs) and matrix metalloproteinases (MMPs) are actively involved in regulation and remodelling of the ECM.3 Imbalances or dysregulation of TIMPs and MMPs activate fibrotic pathways. Since TIMPs and MMPs are measurable in the serum, they may be able to detect subclinical disease and may aid in risk stratification.3,4
The current study is part of the Age, Gene/Environment Susceptibility-Reykjavik (AGES-Reykjavik) Study which examined 5764 survivors of the original Reykjavik Study cohort from 2002–2006.5,6 As a study focused on older, community-dwelling subjects, AGES-Reykjavik is an interesting population for addressing questions related to fibrosis and how they relate to mortality.
The specific aim of this study is to understand the prognostic significance of fibrotic processes in the AGES-Reykjavik cohort of older community-dwelling subjects. We hypothesize that the novel biomarkers TIMP-1 and MMP-9 provide prognostic information over risk factors and established biomarkers [high sensitivity C-reactive protein (hsCRP)7–12 and estimated glomerular filtration rate (eGFR)].13 The second aim is to assess the incremental value of the biomarkers for predicting mortality.
Methods
This study was approved by the National Bioethics Committee of Iceland, the institutional review board of the Intramural Research Program of the National Institute on Aging, and the Data Protection Authority in Iceland.
All subjects enrolled in the AGES-Reykjavik Study (N = 5764) were eligible for this study. We excluded subjects missing measurements of the biomarkers TIMP-1, MMP-9, hsCRP, and eGFR. We also excluded subjects missing any variables of the baseline model that define the variable within the Framingham Risk Score which included: gender, age, type 2 diabetes, smoking status (current and previous smoker), treated and untreated systolic blood pressure, total cholesterol, HDL cholesterol as well as statin use and body mass index (BMI). The final study size was 5511 participants (analysis cohort).
Blood was drawn at the initial AGES-Reykjavik characterization and citrate plasma samples were frozen and stored at −80°C. MMP-9 and TIMP-1 were measured using ELISA assay kits. C-reactive protein concentration was measured in serum with a high-sensitivity assay (Roche Diagnostics) as an established biomarker. Serum creatinine was measured and estimated eGFR was calculated.13
The primary study outcome was all-cause mortality at 10 years based on the Icelandic National Roster. Complete mortality follow-up for this analysis went through to December 2006. Fact and cause of death were obtained from Statistics Iceland, which classified cause of death based on a nosologist review of medical and death records. An additional endpoint in this study was 5-year all-cause mortality. We also studied cardiovascular death (CVD), cancer-related deaths and ‘other’ causes of death. CV-related death included International Classification of Disease-10th revision codes I10-I25, I42-I52, I61, I63-I74 (hypertensive disease, myocardial infarction, coronary artery disease, ischaemic and haemorrhagic stroke, and aneurysms), Non-CV deaths included International Classification of Disease-10th revised codes for all neoplasms and ‘other’ encompassed airway disease, diseases of the nervous system other than cerebrovascular diseases, disease of the digestive system, disease of the genitourinary system, mental and behavioural disorders, and other causes.14
Statistics
Statistical analysis was performed using R within R-Studio and IBM SPSS Statistics® statistical software. The follow-up was the time from entering the study until death or 1 January 2016, whichever came first. The Cox proportional hazards regression model was used in survival analysis to estimate the association between all the predictors in the baseline model (Framingham Risk Score variables, BMI, and statin use) and the four biomarkers with mortality, and with CVD and cancer as causes of death. Time since entering the study was used as the time scale after also considering age as a time scale and age-group stratification in the survival models. The biomarkers TIMP-1 and MMP-9 were used on the original scale and hsCRP11 and eGFR (mL/min/1.73 m2) were used on the log-scale. Time dependency of the biomarker estimates was inspected using Schoenfeld residual plots and testing for interaction with time. The hazard ratios (HRs) associated with each biomarker were calculated for percentile ranges and for 1 standard deviation increments in each biomarker assuming log-linear relationship with the hazard for each end point. The log-linear relationships were inspected using cubic spline functions. All cause and cause specific HRs were estimated. The likelihood ratio statistic, as a χ2 value, from a test of significance of each biomarker in a multiple variable model, containing all the biomarkers, was used to compare the strength of the biomarkers as predictor variables. Continuous net reclassification index (NRI) and the integrated discrimination improvement (IDI) was calculated using the method of Pencina.15 Receiver operator characteristic (ROC) analysis and comparison of the area under ROC curves was performed using the method of Blanche for time dependent area under the curve (AUC).16 Internal validation of the AUC estimates was made using bootstrap methods.
Results
The baseline characteristics of our analysis cohort (N = 5511) can be seen in Table 1. The mean age was 76.8 years (range 68–98 years) and approximately 57% were female. Type 2 diabetes mellitus was present in 13% of the population. The mean systolic blood pressure in treated patients was 144 mmHg and 12% were current smokers. The mean body mass index was 27 kg/m2. The all-cause mortality was 16% (886 deaths) at 5-year follow-up and mortality was 41% (2263 deaths) at 10-year follow-up.
Table 1.
Demographics of the analysis cohort
| Demographics | Analysis cohort (N = 5511) number (proportion %) or mean (SD) |
|---|---|
| Age (years) | 76.81 (5.75) |
| Gender (females) | 3166 (57.4%) |
| Type 2 diabetes mellitus | 694 (12.6%) |
| Current smoker | 675 (12.2%) |
| Previous smoker | 2403 (43.6%) |
| Treated systolic blood pressure | 143.89 [21.15] |
| Untreated systolic blood pressure | 142.38 [20.54] |
| Total cholesterol | 217.75 [44.87] |
| HDL cholesterol | 61.31 [17.32] |
| Statin use | 1221 (22.2%) |
| Body mass index (kg/m2) | 27.03 [4.43] |
| Biomarkers | Median [interquartile] |
| MMP-9 (ng/mL) | 18.66 [15.05, 23.84] |
| TIMP-1 (ng/mL) | 224.6 [195.14, 261.90] |
| hsCRP (mg/L) | 1.9 [1.00, 3.90] |
| eGFR (mL/min/1.73 m2) | 63.92 [53.6, 74.96] |
Q1, quartile 1; Q3, quartile 3; SD, standard deviation; TIMP-1, tissue inhibitor metalloproteinase-1; matrix metalloproteinase-9, MMP-9; hsCRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate.
The median and interquartile ranges for biomarkers levels are summarized in Table 1. Of note, the median hsCRP is close to a level used to distinguish lower risk from higher risk. Similarly, the median eGFR is close to the level used to define normal renal clearance.
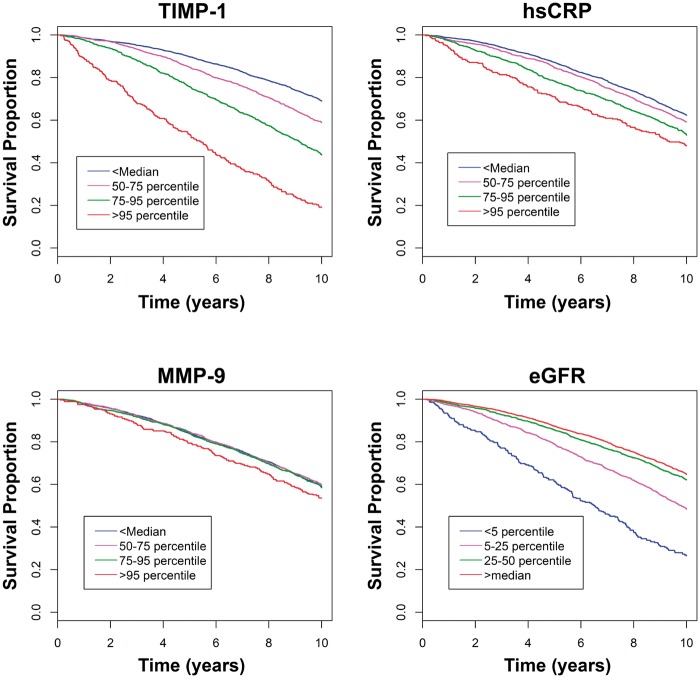
Kaplan–Meier analysis of the biomarkers when stratified by percentiles showed that the highest TIMP-1 percentile category (>95th percentile) had the worst survival for all biomarkers at both 5 and 10 years (Figure 1) with a HR 2.32 at 10 years (Table 2). The percent survival for subjects with TIMP-1 in the highest percentile category was 54% at 5 years and 19% at 10 years. For hsCRP, the percent survival was 72% and 47% at 5 and 10 years for the highest percentile category and the HR was 1.29 (Table 2). For eGFR, there was little separation in survival for the percentiles categories corresponding to most normal renal clearance (>median, 5–25%, 25–50%); only subjects with the renal function in the worst category had significantly increased HR. For MMP-9, no percentiles showed significant differences in survival.
Figure 1.
Kaplan–Meier curves for all-cause mortality related to biomarkers and percentiles.
Table 2.
Hazard ratios from Kaplan–Meier survival analysis when categorized by percentiles
| Biomarker | 50–75th percentile HR (95% CI) | 75–95th percentile HR (95% CI) | >95th percentile HR (95% CI) | Reference |
|---|---|---|---|---|
| TIMP-1 | 1.17 (1.05–1.30) | 1.34 (1.20–1.50) | 2.32 (1.97–2.74) | <median |
| hsCRP | 1.06 (0.96–1.18) | 1.27 (1.14–1.42) | 1.29 (1.08–1.55) | <median |
| MMP-9 | 0.93 (0.84–1.03) | 1.03 (0.92–1.15) | 1.02 (0.85–1.23) | <median |
| Biomarker | <5 percentile HR (95% CI) | 5–25th percentile HR (95% CI) | 25–50th percentile HR (95% CI) | Reference |
| eGFR | 1.42 (1.20–1.68) | 1.08 (0.96–1.20) | 0.92 (0.83–1.03) | >median |
TIMP-1, tissue inhibitor metalloproteinase-1; matrix metalloproteinase-9, MMP-9; hsCRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; HR, hazard ratio; CI, confidence interval.
The multivariable regression analysis (Table 3) was composed of the Framingham Risk Score variables, BMI and statin use, plus the four biomarkers for predicting 5- and 10-year follow-up. The HRs for 1 standard deviation difference in the biomarkers were based on assuming log-linear relationship with the hazard of death. This assumption held well for TIMP-1 and MMP-9 but there was a hint of a J-shape both for CRP and eGFR at extreme values upper values. However, over most of the range the relationship was log-linear. The χ2 values in Table 3 are likelihood ratio tests and are differences in likelihood ratio statistics between a model with and without the corresponding variable. For 10-year mortality, age was the strongest multivariable predictor (log likelihood χ2 = 798.7, P < 0.0001), followed by TIMP-1 (χ2 = 125.2, P < 0.0001), current smoker, female gender, diabetes mellitus, BMI, eGFR (χ2 = 16.7, P < 0.0001), total cholesterol and hsCRP (χ2 = 11.3, P = 0.0008) in that order. However, for 5-year mortality, the top three predictors were age, TIMP-1 (χ2 = 83.14, P < 0.0001) and current smoker (Table 3).
Table 3.
The Cox proportional hazards regression model was based on the Framingham Risk Score variables, BMI, statin use, plus the biomarkers TIMP-1, MMP-9, hsCRP, and eGFR
| Five-year multivariable analysis |
Ten-year multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| Wald χ2 | Hazard ratio (95% CI) | P-value | Wald χ2 | Hazard ratio (95% CI) | P-value | |
| Baseline model (Framingham variables + BMI and statin use) | ||||||
| Age (5 years) | 247.1 | 1.66 (1.56–1.76) | <0.0001 | 798.7 | 1.79 (1.73–1.87) | <0.0001 |
| Gender | 15.0 | 0.73 (0.63–0.85) | <0.0001 | 41.4 | 0.72 (0.65–0.80) | <0.0001 |
| Type 2 diabetes mellitus | 12.2 | 1.40 (1.17–1.67) | <0.0001 | 29.2 | 1.39 (1.24–1.56) | <0.0001 |
| Current smoker | 18.2 | 1.58 (1.28–1.94) | <0.0001 | 58.1 | 1.67 (1.46–1.90) | <0.0001 |
| Previous smoker | 1.2 | 1.10 (0.94–1.27) | 0.5 | 4.0 | 1.11 (1.01–1.21) | 0.42 |
| Treated systolic blood pressure (20 mmHg) | 5.0 | 0.94 (0.88–1.00) | 0.05 | 1.8 | 0.98 (0.94–1.02) | <0.05 |
| Untreated systolic blood pressure (20 mmHg) | 5.4 | 0.92 (0.86–0.99) | <0.001 | 1.9 | 0.97 (0.93–1.02) | 0.05 |
| Total cholesterol (50 mg/dL) | 4.1 | 0.93 (0.85–1.02) | <0.001 | 11.6 | 0.90 (0.85–0.96) | <0.0001 |
| HDL cholesterol (20 mg/dL) | 2.1 | 0.94 (0.86–1.03) | <0.001 | 0.5 | 0.99 (0.94–1.05) | 0.05 |
| Statin use | 4.9 | 0.82 (0.68–1.01) | <0.01 | 5.2 | 0.88 (0.78–0.99) | <0.05 |
| Body mass index (kg/m2) | 18.6 | 0.96 (0.95–0.98) | <0.0001 | 25.6 | 0.97 (0.96–0.98) | <0.0001 |
| Biomarkersa | ||||||
| MMP-9 (1 SD) | 0.1 | 1.01 (0.95–1.07) | 0.80 | 1.1 | 1.02 (0.98–1.06) | 0.31 |
| TIMP-1 (1 SD) | 83.1 | 1.32 (1.24–1.40) | <0.0001 | 125.2 | 1.28 (1.22–1.33) | <0.0001 |
| Log-hsCRP (1 SD) | 22.1 | 1.17 (1.10–1.26) | <0.0001 | 11.3 | 1.08 (1.03–1.12) | 0.0008 |
| Log-eGFR (1 SD) | 9.0 | 0.91 (0.85–0.97) | 0.003 | 16.7 | 0.91 (0.87–0.95) | <0.0001 |
Age and TIMP-1 were the two strongest multivariable predictors of all-cause mortality when adjusting for baseline model and other biomarkers at both 5 and 10 years of follow-up.
TIMP-1, tissue inhibitor metalloproteinase-1; matrix metalloproteinase-9, MMP-9; hsCRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; CI, confidence interval.
aBoth AGE and TIMP-1 are statistically significant (P < 0.0001) at 5 and 10 year mortality.
Cause of death analysis
For the overall cohort of 5511 individuals, 3248 (58.9%) were alive at 10 years or latest follow-up while 915 (16.6%) had died of cardiovascular disease (CVD), 543 (9.9%) had died from cancer, and 805 (14.6%) had died from other causes. Thus, 2263 (41.1%) had died by the 10-year follow-up.
The relative strength of the Framingham variables and the biomarkers was examined in a multivariable Cox regression model of cause-specific mortality (Table 4). After age, TIMP-1 was the second strongest predictor of CVD and the third strongest predictor of cancer death and other causes of death. Current smoking was the second strongest predictor of cancer death. Diabetes was the second strongest predictor of non-cardiovascular and non-cancer related deaths.
Table 4.
Relative strength of Framingham variables and the biomarkers for cause-specific death as assessed in multivariable Cox regression models
| All cause death |
Cardiovascular death |
Cancer death |
Other death |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | χ2 | P-value | Variable | χ2 | P-value | Variable | χ2 | P-value | Variable | χ2 | P-value |
| Age | 798.7 | <0.0001 | Age | 513.7 | <0.0001 | Age | 49.0 | <0.0001 | Age | 310.4 | <0.0001 |
| TIMP-1 | 125.2 | <0.0001 | TIMP-1 | 67.9 | <0.0001 | Current Smoker | 39.3 | <0.0001 | Diabetes | 29.0 | <0.0001 |
| Current Smoker | 58.1 | <0.0001 | Gender | 38.9 | <0.0001 | TIMP-1 | 37.7 | <0.0001 | TIMP-1 | 22.7 | <0.0001 |
| Gender | 41.4 | <0.0001 | eGFR | 25.2 | <0.0001 | hsCRP | 11.7 | 0.0006 | BMI | 18.1 | <0.0001 |
| Diabetes | 29.2 | <0.0001 | Current Smoker | 21.2 | <0.0001 | Gender | 10.2 | 0.0014 | Total Cholesterol | 15.0 | 0.0001 |
| BMI | 25.6 | <0.0001 | BMI | 7.7 | 0.0054 | Prior Smoker | 8.1 | 0.0044 | Statins | 10.4 | 0.0012 |
| eGFR | 16.7 | <0.0001 | hsCRP | 6.8 | 0.0092 | BMI | 2.2 | 0.14 | eGFR | 6.6 | 0.01 |
| Total cholesterol | 11.6 | 0.0007 | Diabetes | 5.6 | 0.018 | eGFR | 2.2 | 0.14 | Current Smoker | 6.3 | 0.01 |
| hsCRP | 11.3 | 0.0008 | Hypertension | 2.1 | 0.15 | Diabetes | 2.2 | 0.14 | MMP-9 | 3.1 | 0.08 |
| Statin use | 5.2 | 0.0222 | HDL | 1.1 | 0.30 | Statins | 1.7 | 0.19 | Gender | 2.8 | 0.10 |
| Prior smoker | 4.0 | 0.045 | Total Cholesterol | 0.7 | 0.40 | Hypertension | 1.4 | 0.24 | Prior Smoker | 2.7 | 0.10 |
| Hypertension | 1.9 | 0.16 | Statin Use | 0.4 | 0.51 | Total Cholesterol | 1.3 | 0.25 | HDL | 0.2 | 0.63 |
| MMP-9 | 1.1 | 0.31 | Prior Smoker | 0.2 | 0.68 | HDL | 0.4 | 0.51 | Hypertension | 0.0 | 0.95 |
| HDL | 0.5 | 0.47 | MMP-9 | 0.1 | 0.77 | MMP-9 | 0.1 | 0.77 | hsCRP | 0.0 | 0.99 |
‘Bold print’ demonstrates how the biomarkers ranked for cause specific mortality when compared to the baseline model.
TIMP-1, tissue inhibitor metalloproteinase-1; MMP-9, matrix metalloproteinase-9; hsCRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate.
Hazard ratios for the biomarkers for 10-year end point showed some variation depending on cause of death (Table 5). Based on HRs, TIMP-1 had the same strength of association with CVD death and cancer death at 10 years. hsCRP was slightly more strongly associated with cancer deaths than CVD but both were significant. Renal function, as measured by eGFR, was a moderately strong predictor of CVD and a weak but significant predictor of non-cancer/non-CVDs. MMP-9 was not significantly associated with any of the specific causes of death or all-cause mortality.
Table 5.
Hazard ratios for all-cause and cause-specific death with 95% confidence interval and Wald χ2 values for 10-year follow-up
| Ten-year multivariable analysis of all cause and cause specific mortality (the model includes biomarkers and Framingham variables, | |||||
|---|---|---|---|---|---|
| BMI, and statin use) | |||||
| Cause of death | TIMP-1 HR (95% CI) | Log-hsCRP HR (95% CI) | Log-eGFR HR (95% CI) | MMP-9 HR (95% CI) | |
| All cause | 1.28 (1.22–1.33) | 1.07 (1.03–1.12) | 0.91 (0.87–0.95) | 1.02 (0.98–1.06) | |
| CVD | 1.31 (1.23–1.39) | 1.10 (1.02–1.17) | 0.85 (0.79–0.90) | 0.99 (0.93–1.06) | |
| Cancer | 1.31 (1.20–1.43) | 1.16 (1.07–1.27) | 1.08 (0.98–1.19) | 1.01 (0.93–1.10) | |
| Other | 1.21 (1.12–1.30) | 1.00 (0.93–1.08) | 0.90 (0.84–0.98) | 1.06 (0.99–1.13) | |
| Cause of death | TIMP-1 Wald χ2 (P-value) | Log-hsCRP Wald χ2 | Log-eGFR Wald χ2 | MMP-9 Wald χ2 | |
| All cause | 125.2 (P<0.0001) | 11.3 (P = 0.0008) | 16.7 (P<0.0001) | 1.1 (P = 0.31) | |
| CVD | 67.9 (P<0.0001) | 6.8 (P<0.0092) | 25.2 (P<0.0001) | 0.1 (P = 0.77) | |
| Cancer | 37.7 (P<0.0001) | 11.7 (P = 0.0006) | 2.2 (P = 0.14) | 0.1 (P = 0.76) | |
| Other | 22.7 (P<0.0001) | 0.0 (P = 0.996) | 6.6 (P = 0.01) | 3.1 (P = 0.07) | |
Hazard ratios per 1 SD difference in each variable as assessed by multivariable Cox regression models.
BMI, body mass index; SD, standard deviation; HR, hazard ratio; TIMP-1, tissue inhibitor metalloproteinase-1; MMP-9, matrix metalloproteinase-9; hsCRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; CVD, cardiovascular death.
Net reclassification index and receiver operator characteristic analysis
TIMP-1 and hsCRP had the highest continuous net reclassification improvement indices when added to the baseline model for 5-year survival (NRI 0.28 and 0.19, respectively, both P < 0.0001, Table 6) and for 10-year survival (NRI 0.19 and 0.11, respectively, TIMP-1 P < 0.0001 and hsCRP P = 0.013, Table 6).
Table 6.
Net reclassification index and integrated discrimination improvement statistics for individual biomarkers over Framingham risk variables, body mass index, and statin use
| Five-year mortality |
Ten-year mortality |
|||||
|---|---|---|---|---|---|---|
| Index | 95% CI | P-value | Index | 95% CI | P-value | |
| TIMP-1 | ||||||
| NRI | 0.28 | 0.21–0.36 | <0.0000 | 0.19 | 0.14–0.26 | <0.0000 |
| NRI for events | 0.02 | −0.04 to 0.08 | 0.01 | −0.03 to 0.04 | ||
| NRI for non-events | 0.27 | 0.22–0.30 | 0.19 | 0.15–0.23 | ||
| IDI | 0.027 | 0.019–0.036 | <0.0000 | 0.02 | 0.01–0.02 | <0.0000 |
| hsCRP | ||||||
| NRI | 0.19 | 0.12–0.28 | <0.0000 | 0.11 | 0.05–0.17 | 0.013 |
| NRI for events | 0.07 | 0.02–0.15 | 0.01 | −0.02 to 0.04 | ||
| NRI for non-events | 0.12 | 0.08–0.16 | 0.10 | 0.06–0.14 | ||
| IDI | 0.006 | 0.002–0.01 | <0.0000 | 0.003 | 0.001–0.007 | 0.003 |
| eGFR | ||||||
| NRI | 0.12 | 0.04–0.20 | 0.0014 | 0.07 | 0.01–0.13 | 0.02 |
| NRI for events | −0.05 | −0.12 to 0.01 | −0.05 | −0.08 to − 0.02 | ||
| NRI for non-events | 0.17 | 0.13–0.22 | 0.12 | 0.08–0.16 | ||
| IDI | 0.12 | 0.007–0.02 | <0.0000 | 0.007 | 0.004–0.01 | <0.0000 |
| MMP-9 | ||||||
| NRI | 0.01 | −0.07 to 0.07 | 0.8590 | 0.01 | −0.06 to 0.06 | 0.87 |
| NRI for events | −0.27 | −0.33 to − 0.20 | −0.25 | −0.28 to − 0.20 | ||
| NRI for non-events | 0.27 | 0.23–0.31 | 0.25 | 0.19–0.28 | ||
| IDI | 0.0006 | −0.1 to 0.08 | 0.32 | 0.0004 | −0.0001 to 0.001 | 0.17 |
NDI, net reclassification index; IDI, integrated discrimination improvement.
For TIMP-1, the NRI was dominated by an improved classification of participants who did not die within 5 years, and had almost no change in classification of participants that died (Table 6). There was a similar pattern for the TIMP-1 NRI regarding 10-year mortality in subjects with and without events (Table 6). The NRI for hsCRP improved as a result of reclassification of both participants that died or did not die by 5-year follow-up. For 10-year follow-up, the hsCRP NRI was mostly limited to survivors that were reclassified towards lower risk. For eGFR, the NRI was negatively affected among subjects that died by 5-year follow-up and was weakly significant for 10-year mortality. The overall continuous NRI for MMP-9 was not statistically significant and neither improved nor impaired re-classification.
The IDI index was used as a tool to evaluate the relative strength of variables to predict an outcome of interest. TIMP-1 had the highest IDI indices when added to the baseline model for 5-year survival (IDI 0.027, P < 0.0001, Table 6) and for 10-year survival (IDI 0.017, P < 0.0001) but these were relatively weak changes. For hsCRP at 5-year and 10-year survival, the IDI was significant (P < 0.0001 and P = 0.0008, Table 3) but modest strength.
Starting with the area under the receiver operator characteristic (ROC) curve for the baseline model (0.7349 and 0.7694 for 5-year mortality and 10-year mortality), TIMP-1 increased the AUC for both 5-year and 10-year mortality prediction over the baseline model (P < 0.0001 for both end points, see Supplementary material online, Addendum TableS2) although the magnitude of change was small. MMP-9 did not significantly change the AUC relative to the baseline model. The change in AUC for the biomarker eGFR was borderline significant for 5-year mortality and not significant for 10-year mortality. Finally, hsCRP statistically increased the AUC for 5-year outcome but not 10-year mortality (see Supplementary material online, Addendum TableS2) but the magnitude of change was small. The bias in the AUC estimates was found to be less than 1%.
The calibration of the baseline model and other models were assessed (see Supplementary material online, Addendum FigureS1).
Addendum Figure demonstrates 2 Kaplan Meier Curves for all-cause mortality related to biomarkers and quartiles. Addendum Table 1 demonstrates the hazard ratios for the survival analysis based on quartiles.
Beyond pre-specified analyses, we added Troponin-T (TnT) and Troponin-I (TnI) to our multivariable model that included all risk factors and the other biomarkers (see Supplementary material online, Addendum TableS3). TIMP-1 remained a strong and significant predictor all-cause mortality and CV mortality at 10 years after adding TnT and TnI to the multivariable model. The effect size of TIMP-1 was attenuated by 20% for all-cause mortality and 23.9% for CVD mortality. The χ2 for TnT and TIMP-1 for 10-year all-cause mortality and for CVD mortality were all individually significant but comparisons between TnT and TIMP-1 were not statistically significant.
Discussion
In this study, TIMP-1 is the strongest predictor of all-cause mortality in the AGES-Reykjavik cohort after age when also considering the variables used to define the Framingham Risk score and statin use. The biomarker, hsCRP, was also a strong predictor of 5-year all-cause mortality but a weaker predictor of 10-year risk. The metabolic pathways regulating homeostasis in the ECM appear pathologically relevant and fibrogenic processes are prognostically important in this Icelandic population older than 66 years of age.
Prior studies have correlated markers of fibrosis with inflammation17 or cardiovascular risk factors.18 Hansson et al.19 demonstrated that higher levels of circulating MMP-9 or TIMP-1 were associated with higher risk of death and higher TIMP-1 levels were related to higher risk of stroke and cardiovascular mortality. TIMP-1 was a predictor for future CVD in patients with known CAD in two other studies.20,21
The four TIMPs are the natural endogenous inhibitors of the matrix metalloproteinases (MMPs), of which 22 MMPs are found in humans. Of the TIMPs, TIMP-1 is relatively unique as being present in the plasma and thus accessible as a biomarker. The MMPs regulate the turnover of ECM by regulation of bioactive molecules, chemokines, and growth factors. There are more than 20 zinc (II)-dependent metalloproteinases (MMPs). In our study, we focused on the gelatinase (MMP-9), which regulates pathological remodelling processes that involve inflammation and fibrosis, since TIMP-1 closely associates with MMP-9 and inhibits the protease. Since MMP-9 regulates fibrosis in the extracellular space, it is possible that the circulating levels of MMP-9 do not reflect the biological activity in this population as well as tissue levels or tissue activity.
The fibrotic process is regulated and may be reversible.22 Initiation of fibrogenesis involves injury and inflammatory activation of monocytes and chemokines that result in profibrotic macrophages. TGF-β plays a central role in activating and promoting proliferation of myofibroblasts. Two processes regulate progression towards healing of fibrosis. Monocyte-derived macrophages play important roles in the resolution of hepatic fibrosis.23 In addition, MMP-13, MMP-9, TRAIL (TNF-related apoptosis-inducing ligand), and low levels of TIMP-1 result in degradation of the ECM (i.e. scar resolution). Excess levels of TIMP-1inhibit MMPs and result in ECM deposition and fibrotic scar.
There are multiple potential targets for treatment of fibrosis.1,24 Twenty-five potential treatments to modulate fibrosis are already in clinical trials.1 Four of these medications are already approved for use in patients, albeit for other indications than modulating fibrosis. Our Kaplan–Meier survival analysis suggests there may be an opportunity to treat patients that is several years in duration. Even the most severe elevations in TIMP-1, it took many years to reach 50% mortality.
In the absence of a specific currently recognized disease, targeting inflammation might have significant effects on the fibrotic process. Acute and chronic inflammatory reactions play an important part in triggering fibrosis in many organ systems. Future clinical trials might take an approach comparable to the JUPITER trial.11 Statins, anti-inflammatory, or anti-fibrotic agents might be logical choices to reduce fibrosis.
Elevated TIMP-1 is not specific for CVD. TIMP-1 and fibrotic pathways appear important for cancer and potentially other diseases. TIMP-1 and inhibition of MMP-9 may influence anti-apoptotic activity in cancer, tumour growth, angiogenesis, and tissue invasion.25 TIMP-1 promotes cell proliferation and in many forms of diverse cancers. TIMP-1 is overexpressed in several types of human cancers.26 As a cytokine and key regulator of ECM degradation, TIMP-1 has multiple functions associated with the tumour micro-environment and cancer progression. Higher levels of TIMP-1 expression in patients with TNBC patients were associated with a poor prognosis.26 Inhibiting TIMP-1 prevented tumour growth in mice.
Per the European Society Cardiology Guidelines on CVD prevention,27 circulating and urinary biomarkers have no or only limited value when added to the SCORE system and state that hsCRP contributes little to CV risk assessment.27 The guidelines do not address TIMP-1 which is a stronger predictor of all-cause mortality and CVD death than hsCRP.
Limitations
Our cohort focused on an aging population. This Icelandic cohort is a Caucasian or European population and thus may not reflect other ethnicities.
Despite being the strongest predictor of mortality after age in this study, the NRI, IDI, and AUC statistics need to be interpreted cautiously. With a large sample size, small changes in re-classification become detectable with high-statistical confidence. Positive likelihood ratios assess the relative strength of elements within the multivariable models.
It is prudent to consider fibrotic pathways important from a pathophysiological perspective. Further work will be needed to understand the best way to use the biomarker or whether the biomarker may help select patients that might benefit from fibrosis modifying therapies.
The concept of treatment of fibrosis based on TIMP-1 or other similar biomarkers will need to be proven by clinical trials. Even with these cautions, it is still quite intriguing that TIMP-1 is as powerful or more powerful predictor of mortality than the other biomarkers and patient characteristics studied.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
National Heart, Lung, and Blood Institute Intramural Research Program (ZIA HL006136-06); the National Institute on Aging Intramural Research Program (N01-AG-12100); Hjartavernd (the Icelandic Heart Association); and the Althingi (the Icelandic Parliament). The study was approved by the Icelandic National Bioethics Committee (VSN: 00-063) and the Medstar Research Institute (project 2003-145).
Conflict of interest: none declared.
Supplementary Material
References
- 1. Wynn TA, Ramalingam TR.. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012;18:1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu P, Takai K, Weaver VM, Werb Z.. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 2011;3:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Braunwald E. Biomarkers in heart failure. N Engl J Med 2008;358:2148–2159. [DOI] [PubMed] [Google Scholar]
- 4. Morrow DA, de Lemos JA.. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation 2007;115:949–952. [DOI] [PubMed] [Google Scholar]
- 5. Sigurdsson E, Thorgeirsson G, Sigvaldason H, Sigfusson N.. Prevalence of coronary heart disease in Icelandic men 1968-1986. The Reykjavik Study. Eur Heart J 1993;14:584–591. [DOI] [PubMed] [Google Scholar]
- 6. Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V.. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007;165:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ridker PM, Hennekens CH, Buring JE, Rifai N.. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–843. [DOI] [PubMed] [Google Scholar]
- 8. Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB.. Production of C-reactive protein and risk of coronary events in stable and unstable Angina. European Concerted Action on Thrombosis and, Disabilities Angina Pectoris Study Group. Lancet 1997;349:462–466. [DOI] [PubMed] [Google Scholar]
- 9. Ridker PM, Glynn RJ, Hennekens CH.. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation 1998;97:2007–2011. [DOI] [PubMed] [Google Scholar]
- 10. Ridker PM. The JUPITER trial: results, controversies, and implications for prevention. Circ Cardiovasc Qual Outcomes 2009;2:279–285. [DOI] [PubMed] [Google Scholar]
- 11. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 12. Sabatine MS, Morrow DA, de Lemos JA, Omland T, Sloan S, Jarolim P, Solomon SD, Pfeffer MA, Braunwald E.. Evaluation of multiple biomarkers of cardiovascular stress for risk prediction and guiding medical therapy in patients with stable coronary disease. Circulation 2012;125:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stevens LA, Coresh J, Greene T, Levey AS.. Assessing kidney function–measured and estimated glomerular filtration rate. N Engl J Med 2006;354:2473–2483. [DOI] [PubMed] [Google Scholar]
- 14. Van Elderen SS, Zhang Q, Sigurdsson S, Haight TJ, Lopez O, Eiriksdottir G, Jonsson P, de Jong L, Harris TB, Garcia M, Gudnason V, van Buchem MA, Launer LJ.. Brain volume as an integrated marker for the risk of death in a community-based sample: age gene/environment susceptibility–Reykjavik Study. J Gerontol A Biol Sci Med Sci 2016;71:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pencina MJ, D' Agostino RB, D' Agostino RB, Vasan RS.. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 16. Blanche P, Dartigues JF, Jacqmin-Gadda H.. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med 2013;32:5381–5397. [DOI] [PubMed] [Google Scholar]
- 17. Allal-Elasmi M, Zayani Y, Zidi W, Zaroui A, Feki M, Mourali S, Mechmeche R, Kaabachi N.. The measurement of circulating matrix metalloproteinase-8 and its tissue inhibitor and their association with inflammatory mediators in patients with acute coronary syndrome. Clin Lab 2014;60:951–956. [DOI] [PubMed] [Google Scholar]
- 18. Garvin P, Nilsson L, Carstensen J, Jonasson L, Kristenson M, Pockley G.. Circulating matrix metalloproteinase-9 is associated with cardiovascular risk factors in a middle-aged normal population. PLoS One 2008;3:e1774.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansson J, Vasan RS, Arnlov J, Ingelsson E, Lind L, Larsson A, Michaelsson K, Sundstrom J.. Biomarkers of extracellular matrix metabolism (MMP-9 and TIMP-1) and risk of stroke, myocardial infarction, and cause-specific mortality: Cohort Study. PLoS ONE 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lubos E, Schnabel R, Rupprecht HJ, Bickel C, Messow CM, Prigge S, Cambien F, Tiret L, Munzel T, Blankenberg S.. Prognostic value of tissue inhibitor of metalloproteinase-1 for cardiovascular death among patients with cardiovascular disease: results from the AtheroGene study. Eur Heart J 2006;27:150–156. [DOI] [PubMed] [Google Scholar]
- 21. Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L; AtheroGene Investigators. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 2003;107:1579–1585. [DOI] [PubMed] [Google Scholar]
- 22. Ramachandran P, Iredale JP.. Macrophages: central regulators of hepatic fibrogenesis and fibrosis resolution. J Hepatol 2012;56:1417–1419. [DOI] [PubMed] [Google Scholar]
- 23. Karlmark KR, Zimmermann HW, Roderburg C, Gassler N, Wasmuth HE, Luedde T, Trautwein C, Tacke F.. The fractalkine receptor CX(3)CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 2010;52:1769–1782. [DOI] [PubMed] [Google Scholar]
- 24. Rockey DC, Bell PD, Hill JA.. Fibrosis–A common pathway to organ injury and failure. N Engl J Med 2015;373(1):96. [DOI] [PubMed] [Google Scholar]
- 25. Muller V, Riethdorf S, Rack B, Janni W, Fasching PA, Solomayer E, Aktas B, Kasimir-Bauer S, Zeitz J, Pantel K, Fehm T, group Ds. Prospective evaluation of serum tissue inhibitor of metalloproteinase 1 and carbonic anhydrase IX in correlation to circulating tumor cells in patients with metastatic breast cancer. Breast Cancer Res 2011;13:R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng G, Fan X, Hao M, Wang J, Zhou X, Sun X.. Higher levels of TIMP-1 expression are associated with a poor prognosis in triple-negative breast cancer. Mol Cancer 2016;15:30.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM, Authors/Task Force Members. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steyerberg EW, Vergouwe Y.. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J 2014;35: 1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.