Abstract
The most common presentation of nephrolithiasis is idiopathic calcium stones in patients without systemic disease. Most stones are primarily composed of calcium oxalate and form on a base of interstitial apatite deposits, known as Randall’s plaque. By contrast some stones are composed largely of calcium phosphate, as either hydroxyapatite or brushite (calcium monohydrogen phosphate), and are usually accompanied by deposits of calcium phosphate in the Bellini ducts. These deposits result in local tissue damage and might serve as a site of mineral overgrowth. Stone formation is driven by supersaturation of urine with calcium oxalate and brushite. The level of supersaturation is related to fluid intake as well as to the levels of urinary citrate and calcium. Risk of stone formation is increased when urine citrate excretion is <400 mg per day, and treatment with potassium citrate has been used to prevent stones. Urine calcium levels >200 mg per day also increase stone risk and often result in negative calcium balance. Reduced renal calcium reabsorption has a role in idiopathic hypercalciuria. Low sodium diets and thiazide-type diuretics lower urine calcium levels and potentially reduce the risk of stone recurrence and bone diseas
Introduction
Kidney stones have increased in prevalence over the past 50 years in most countries, and as of 2010 the prevalence in the United States was 8.8%, compared with 5.5% in 19941. The prevalence is higher in men (10.6% among men compared with 7.1% among women in the recent United States data) and in Causasians compared with Africans1, and at least 35% of first time stone formers will have one or more recurrences. As stone disease often affects adults during their working years, the costs of stone disease include both medical intervention as well as time lost from work, school, or family care, and exceeds $10 billion dollars yearly in the United States2. The majority of stones (85%) contain primarily calcium oxalate (CaOx) admixed with some calcium phosphate (CaP) in the form of apatite or brushite, or occasionally uric acid; less commonly they may be composed primarily of CaP3. Non-calcium stones may be made of uric acid or cystine. Although many systemic diseases, such as primary hyperparathyroidism, bowel disease and renal tubular acidosis, can result in calcium stone formation, the majority of calcium stones are found in people with no systemic illness. Many idiopathic patients who form calcium stones have metabolic abnormalities that can be detected by 24 hour urine analysis but are not considered to be systemic disease. Among the most common of these abnormalities is idiopathic hypercalciuria4. This disorder has been shown to be heritable, but is also influenced by environmental factors such as diet.
Since most kidney stones seen in clinical practice will be idiopathic calcium stones, and the commonest predisposing factor in these patients is hypercalciuria, the remainder of this review will concentrate on these patients, and discuss the mechanisms of idiopathic calcium stone formation as well as potential therapeutic strategies to correct hypercalciuria and reduce the risk of stone formation.
Patient phenotyping
A traditional method of phenotyping patients with calcium stones includes analysis of stone crystal composition as well as consideration of the presence or absence of established diseases that could cause stones. Among idiopathic patients, those whose stones are predominantly composed of CaOx (>50%) are considered to be a separate group from those whose stones are predominantly composed of calcium phosphate (CaP) crystals such as apatite or brushite5.
Modern endoscopy techniques enable direct renal papillary visualization during stone surgery and this has added papillary morphology to our phenotyping. Direct biopsy of the papillum enables additional refinement of the stone phenotype but is unlikely to be used for patient care as it is a research tool at this time.
Formation of idiopathic calcium stones
A key question regarding calcium stone formation is whether these stones form in contact with renal tissue or in the bulk urine without a direct tissue interaction. The available evidence demonstrates that calcium stones do form in contact with tissue, but proof that formation in urine can also occur is lacking. If the predominant way in which stones form is on anchored sites in the renal papillae, then the nature of those sites, and the way in which they occur, is an important part of the story of stone pathogenesis.
Growth on interstitial apatite deposits
Clinically important CaOx stones can form as overgrowths on interstitial apatite deposits known as Randall’s plaque. During percutaneous or ureteroscopic stone removal surgery, CaOx stones — including stones of clinically significant size — can be seen growing on human renal papillae (Figure 1). These stones can be detached only with some effort, whereupon an attachment site can be seen on the stone. In some cases, the corresponding regions of attachment on the renal papillae are also visible to the surgeon.
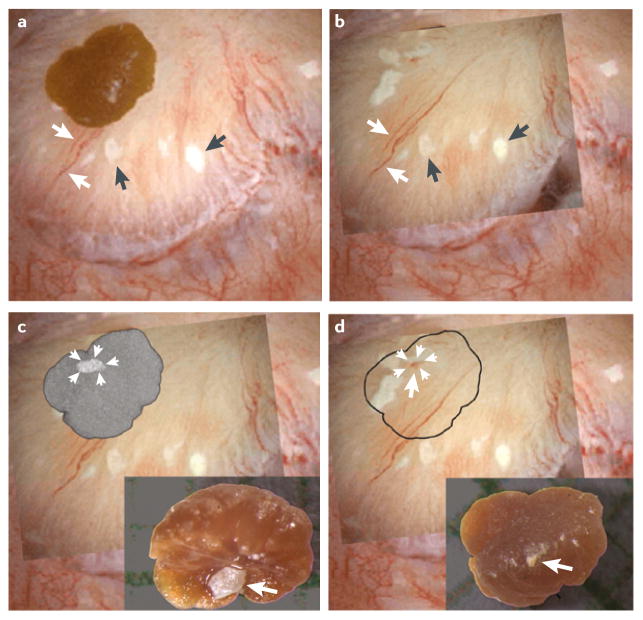
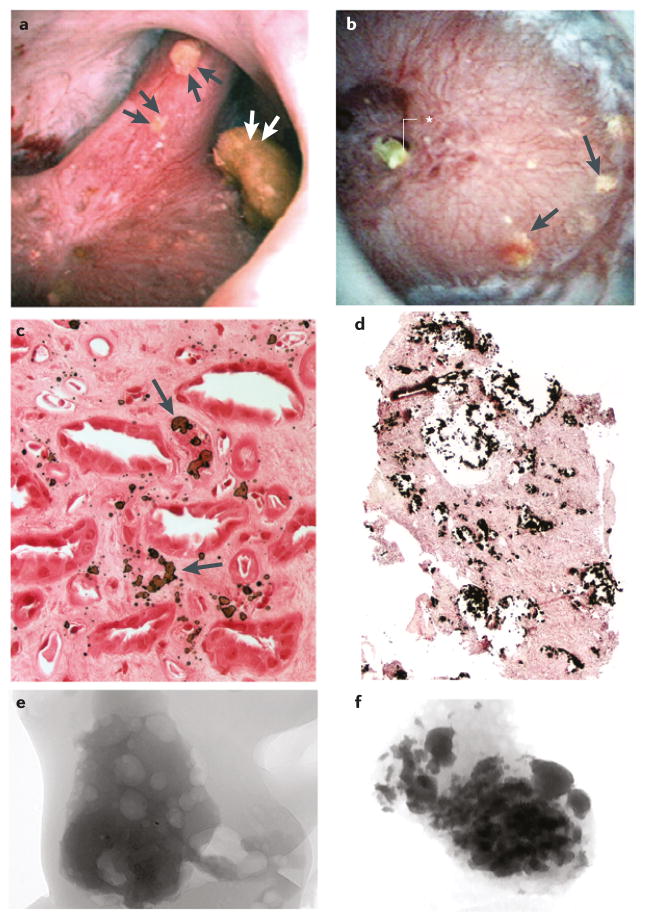
Figure 1. Interstitial plaque and corresponding attachment site on a CaOx stone.
a | Digital endoscopic image of a 3 mm CaOx stone attached to a papillum before stone removal by percutaneous nephrolithotomy. Several sites of interstitial plaque (arrowheads) are visible as well as blood vessels (arrows) that were used for image orientation. b | Following stone removal the papillum was reimaged — an overlay showing the sites of interstitial plaque and blood vessels after stone removal has been placed over the original endoscopic imagec | “Ghosted” CT image of the detached stone showing a site of calcium phosphate (arrowheads) on the papillary surface of the stone. The insert shows a light microscopic image of the papillary surface of the detached stone with the site of calcium phosphate (arrow). d | The site of calcium phosphate on the papillary surface of the stone (arrows) aligns with a region of interstitial plaque with a central blood spot (arrow) on the papillium, which is presumably the site of stone attachment. The insert shows the urinary surface of the detached stone.
In a study that included nine patients with idiopathic CaOx stones (those with systemic disease were excluded) we found that 78% of 115 stones visualized at surgery were attached to papillae, and of these 90% were attached over plaque, which itself occupied ≤5% of the papillary surface area.6 In a separate study of unattached stones in the same patients, evidence of an attachment site — an apatite deposit — was present on all of these stones, suggesting an origin on plaque.7
The percentage of patients without hypercalciuria who form CaOx stones on plaque is unknown. In an unselected population of 78 patients who underwent percutaneous nephrolithotomy (PNL), Linnes et al. identified 37 patients (of whom 26 were female) with idiopathic CaOx stones.8 These patients were not remarkably hypercalciuric (mean 24 h urinary calcium level of 210 mg). Although the researchers reported a mean plaque surface area coverage of 3.6%, they did not indicate whether or not the CaOx stones were found on plaque. In a subsequent study that included 42 idiopathic patients with CaOx stones who underwent PNL, abundant plaque (>5% surface area coverage) was found in 10 patients (of whom six were male).9 These patients had higher urinary calcium excretion (291 mg per day) than those with lower plaque abundance (of whom eight were male; 187 mg per day). In a third study, these researchers found no evidence of increased plaque or urine calcium in 95 patients who formed idiopathic CaOx stones (of whom 42 were male) compared with 19 healthy individuals or 23 patients who formed uric acid stones.10
Although these studies from a single group of investigators do not report the location in which the stones were formed, analysis of stones by micro-CT, showed that those from patients with >5% plaque surface area coverage were more likely to show evidence of growth on plaque than those from patients with <5% plaque surface area coverage. Future surgical series will clarify the frequency with which stone growth on plaque can be documented using endoscopy.
Formation of stones on plaque can also be investigated via careful analysis of the stones themselves. In a large series of stones analyzed in a French laboratory using infrared spectroscopy and morphoconstitutional analysis, 34% of 30,149 intact stones collected between 1989 and 2013 that were at least 85% CaOx showed evidence of having formed over Randall’s plaque11. Moreover, a marked increase in the frequency of nucleation of stones over plaque occurred between 1990 and 2010 in men and in women, particularly in young adults. Analysis of CaOx stones passed between 2009 and 2011 showed that among patients aged 20–29 years, approximately 60% of those passed by women and 50% of those passed by men had nucleated over plaque, compared with 20% of those passed by women and 25% of those passed by men aged ≥70 years. It is not possible to determine how many of these patients were idiopathic stone formers, but it is probable that the majority did not have systemic disease.
The phenomenon of CaOx stones growing on interstitial plaque has also been observed in patients with CaOx stones owing to primary hyperparathyroidism12, ileostomy13 and small bowel resection14 as well as in idiopathic patients whose stones are composed mainly of hydroxyapatite15 or brushite16. Patients who form idiopathic hypercalciuric CaOx stones are unique, however, because growth on plaque seems to be the only mechanism of stone formation and plaque is often the only renal tissue mineralization. In patients with systemic diseases and in patients who form hydroxyapatite or brushite stones, growth on plaque is found in combination with a different kind of tissue mineralization: plugging of inner medullary and papillary collecting ducts with crystals of hydroxyapatite, brushite or CaOx, depending on the clinical condition17 (discussed below).
Plaque formation
The initial sites of plaque formation are the basement membranes of the thin limbs of the loops of Henle, in which plaque appears as microspherules of alternating organic and apatite crystal laminations (Figure 2)18. Subsequently, either by extension or additional formation, plaque appears in the papillary interstitium. Plaque that forms in this location is an amalgam of apatite crystals in an organic matrix that contains osteopontin19, inter-α-trypsin inhibitor heavy chain 320, and other molecules that have yet to be determined.
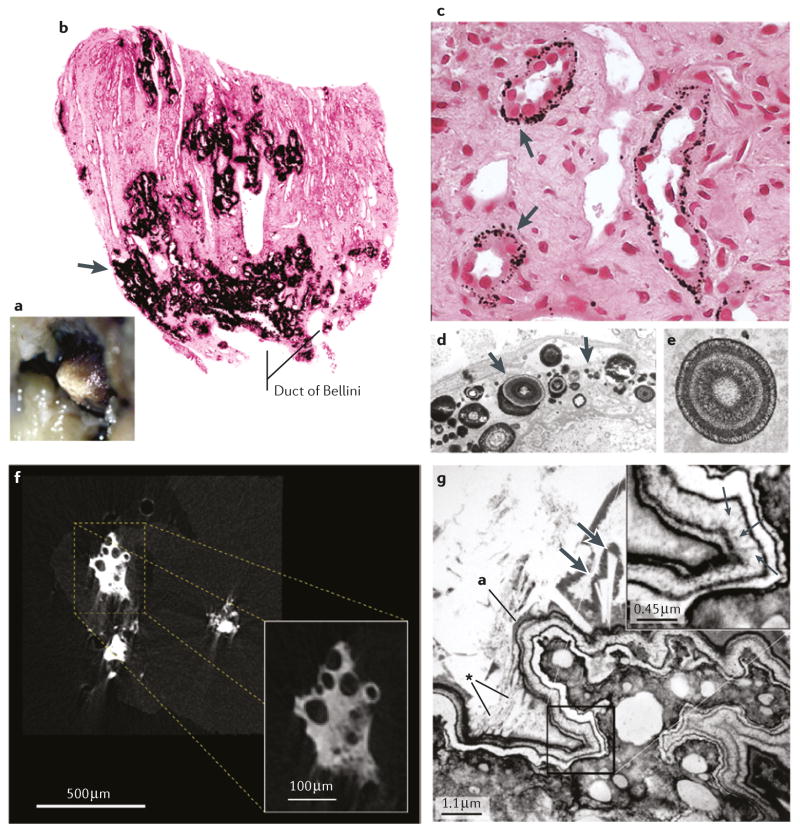
Figure 2. Sites of interstitial plaque in idiopathic CaOx stone formers.
a | Endoscopic image showing irregular whitish regions of interstitial plaque covering the papillary tip. b | A biopsy from this region showing black Yasue-stained material (arrow) within the interstitial space of the inner medulla. c | Light microscopy and d | transmission electron microscopy images showing regions of plaque in the basement membrane of the thin loops of Henle only, suggesting that plaque originates at this site. e | The plaque is composed of laminated spheres with up to nine alternating light (hydroxyapatite) and dark (matrix) layers. f | Micro CT image of plaque that has filled the interstitium and generated islands that encompass nearby tubules with no evidence of intraluminal deposits. g | Transmission electron microscopy imageshowing a multilayered ribbon-like structure separating a region of interstitial plaque (lower right) from an overgrowth of developing stone (upper left). In the thickest region of the white lamina of the ribbon (insert), tiny thin spicules run perpendicular to the surface adjacent to voids containing tightly packed crystals (small arrows). Numerous small crystals have grown into the outer border of the ribbon (asterisk) and merged with large crystals within the urinary space at the developing overgrowth. The double arrows indicate a large in-growing crystal.
High resolution CT imaging of plaque within intraoperative tissue biopsy samples from patients with idiopathic CaOx stones supports the idea of plaque extension from the thin limbs of the loops of Henle into the interstitium. In some images mineral can be seen forming along the long axis of a thin limb and merging smoothly with the plate-like interstitial plaque21. An analysis of papillary biopsy samples from 12 patients with idiopathic CaOx stones who underwent PNL identified plaque in all patients22. The plaque deposits were located in the interstitium and in the basement membrane of the tubular epithelium; the specific tubule segments involved were not reported. X-ray dispersive microanalysis showed that the deposits were composed of calcium and phosphate. In the interstitium, plaque deposits were mixed in with long thin fibers of collagen. The collagen fibers act as a scaffolding for the heterogeneous nucleation of CaP crystal as is seen in bone formation. These findings are confirmatory of our work.
The mechanisms that lead to the formation of interstitial plaque are unknown. A role of osteogenesis can be excluded because the critical bone genes RUNX2 and SP7 (also known as Osterix) were not expressed at sites of plaque in biopsy samples from nine patients with idiopathic CaOx stones23. By contrast, these genes were expressed in samples from 10 of 12 patients with medullary sponge kidney disease23. The rather primitive papillary interstitial cells of patients with medullary sponge kidney do express RUNX2 and SP7, but the sites of expression are never the sites of mineral deposition. In patients with medullary sponge kidney, tiny stones can be found within dilated cystic terminal collecting ducts, but bone genes are not expressed at these sites.
We have observed that plaque coverage is a direct function of urine calcium excretion and an inverse function of urine pH and urine volume24. The mechanism by which urine calcium influences plaque formation may be related to renal tubule calcium reabsorption, which is decreased in stone formers with hypercalciuria. In particular, less calcium is reabsorbed in the proximal tubules of hypercalciuric stone formers, and more is delivered into the distal nephron. In the outer renal medulla, the vascular bundles are surrounded by a ring of thick ascending limb (TAL), which reabsorb calcium independently of water25. Calcium reabsorption in the TAL occurs mainly via the paracellular pathway26 and is driven by the transepithelial voltage difference between tubule lumen and the blood. As delivery of calcium to the TAL increases, reabsorption of calcium will tend to increase at the same rate, and as water is not reabsorbed the concentration of calcium within the vascular bundles will increase. The descending vasa recta provide blood to the papillary capillary network that surrounds the thin limbs of the Loop of Henle in which plaque particles originate18. In principle increased proximal tubule delivery of calcium can, therefore, foster papillary plaque formation via a mechanism that we have called ‘vas washdown’27. This idea is compatible with what is known about plaque formation and renal physiology, but has yet to be proven experimentally. Supersaturations in the papillary interstitium that might affect plaque formation are largely independent of urine supersaturation, and these two types of supersaturation have different physiological causes.
Stoller and colleagues published arguments in favour of plaque genesis in the vasa recta, based on a study of cadaveric kidneys from 50 consecutive autopsies (including those of two individuals who were stone formers) in which papillary calcifications were radiologically detected in 57% of kidneys. Calcifications were found in the interstitium around tubules, and hypertension was the factor most strongly associated with such calcifications. Epidemiologic data linking stone formation to cardiovascular disease28 is cited as supporting the link to vascular disease, however histologic. evidence of a vascular site of origin is not evident in the majority of data on stone formation.
Two animal models of plaque formation have been produced: the NHERF-1 knockout mouse and a Tamm-Horsfall protein knockout mouse29. These models might prove to be a promising tool for further investigations into the pathogenesis of renal stones.
Stone formation over plaque
The process of CaOx stone formation over plaque cannot proceed unless the urothelium is compromised such that urine supersaturations can drive new crystal formation. The exposed surface of plaque is covered by a multilayered ribbon of alternating crystals and organic matrix, which faces the urinary space (Figure 2). Given that plaque is exposed to urine, the formation of an inner organic layer seems to be the first event. As this layer contains uromodulin (also known as Tamm-Horsfall protein) it must be of urine origin30.
Successive layers of hydroxyapatite nucleation and organic overlays create the multilaminar covering ribbon over the exposed plaque. The form of apatite in the ribbon is amorphous30;it does not have the exact structure of mature apatite and is rather in the form of tiny highly hydrated crystals. This form transitions to mature apatite at the urinary surface, which creates larger and more angular spiky crystals that build up the apatite–matrix base of the new stone. This base can be seen when stones are removed, or when unattached stones that have formed on plaque are carefully inspected31. The processes that convert the orderly laminations of the ribbon into the less regular and more bulky stone base, and convert apatite nucleation into CaOx nucleation to make up the bulk of the final stone, are unknown but presumably involve conversion of amorphous apatite into mature apatite.
Growth on tubule plugs
Plugging of Bellini ducts and inner medullary collecting ducts occurs in some patients with idiopathic CaOx stones8, 10, in virtually all patients with idiopathic brushite or hydroxyapatite stones15 and in all stone formers with systemic diseases such as primary hyperparathyroidism12, bowel diseases that include ileostomy13, small bowel resection14, obesity bypass procedures32, renal tubular acidosis33, cystinuria34, and primary hyperoxaluria35. The location of Bellini duct plugs means that they are exposed to the tubule fluid on their proximal surface and the urine on their distal surfaces,. They can grow upward into the nephron and outwards into the bulk urine (Figure 3).
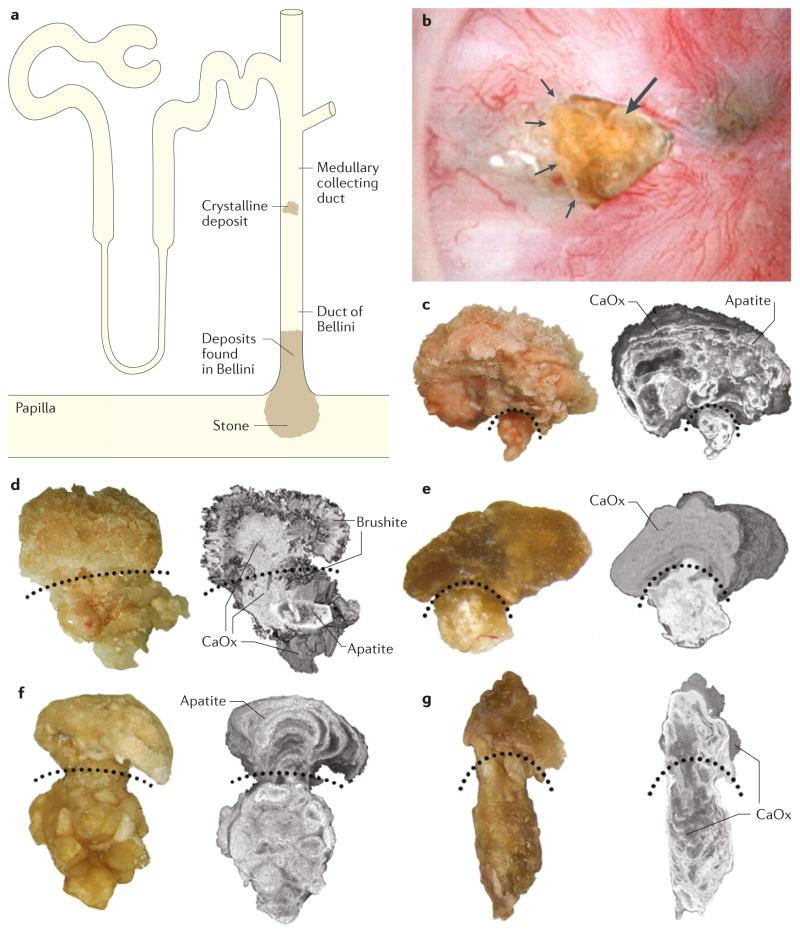
Figure 3. Bellini duct plugs with overgrowths in patients who formed calcium stones.
a | Sites of crystalline deposits that form plugged Bellini ducts (BD) with an overgrowth region protruding into the urinary space. b | Endoscopic image of a plug (arrow) protruding from a dilated BD (arrowheads). BD plugs with overgrowths visible by light microscopy and micro-CT from c | a patient with idiopathic hydroxyapatite stones, d | a patient with idiopathic brushite stones e | a patient with ileostomy, f | a patient with primary hyperparathyroidism and g | a medullary sponge kidney. The curved, dotted lines divide the BD plugs from the overgrowth regions.
As plugs open onto the urinary space their distal ends are exposed to the supersaturations present in urine. These open ends often have overgrowths31, which are sometimes described as ‘microliths’. Although the hypothesis is attractive, no proof yet exists that plug overgrowths can increase in size to form clinically relevant stones. Plugging occurs within the renal epithelial compartments, causes obvious local injury, and is an integral part of the complex mineralization of the kidneys of patients who form stones.
Brushite stones
When brushite is present in stones it is usually the predominant crystal component, although stones may also contain some CaOx or apatite. Patients who form brushite stones show a much wider range of plugging severity that those who form other types of stones. Some papillae can be pristine apart from scattered interstitial apatite plaque (Figure 4), whereas others show tubule plugging with crystals, dilated Bellini duct openings, pitting and retraction16. Histological changes in plugged tubules include massive dilation, loss of epithelium and peritubular fibrosis. Plugging can be visualized during surgery; dilation of the Bellini duct is can be clearly seen, whereas plugged inner medullary collecting ducts are visible through the urothelium as elongate yellow cylinders that reflect crystal casts.
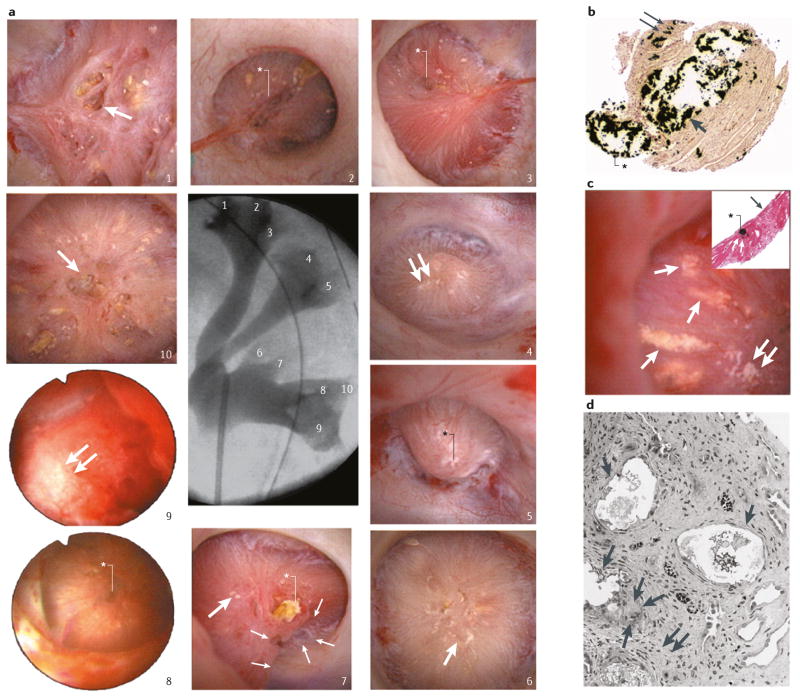
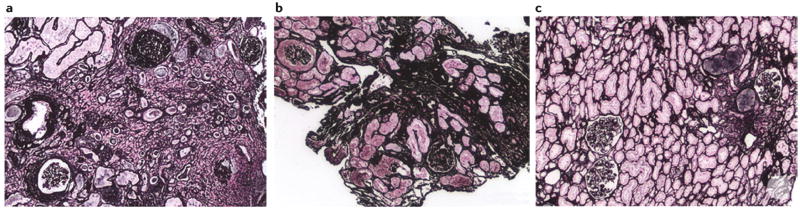
Figure 4. Renal papilla of a patient who formed brushite stones.
a | Endoscopic images of all ten papilla. The numbering corresponds to the x-ray image of the collection system. Papilla 1, 6, 7 and 10 show severe morphological changes including pitting (large arrows), flattening, plugs (black asterisk, papilla 7) and yellow plaque. Papilla 2, 3, 5 and 8 show small pits (white asterisks) whereas papilla 4, 7 and 9 show small sites of interstitial plaque (double arrows). b | Scattered inner medullary collecting ducts (IMCDs) and Bellini duct (BD) filled with yellow crystalline deposits that have dilated the tubular lumens (arrow) and protrude from the opening of the BD (asterisk). Small sites of interstitial plaque are also visible (double arrows). c | Sites of yellow plaque (arrows) in the lumens of IMCD (asterisk in insert) just beneath the urothelium (arrow in insert) and near sites of interstitial plaque (double arrows). d | showing extensive injury in the lining cells of IMCDs clogged with mineral deposits (arrows). Regions of extensive interstitial fibrosis surround the plugged IMCDs (double arrows) and entrapped and injured adjacent thin loops of Henle (asterisk). Giant cells (arrowheads) were occasionally found near damaged IMCDs.
Apatite stones
These stones, which are particularly common among women, are composed predominantly (> 50%) of apatite; the remainder is CaOx. Much like patients who form brushite stones, patients who form apatite stones also form apatite plugs in their Bellini ducts and inner medullary collecting ducts (Figure 5). These plugs are generally smaller and far more numerous than those of patients who form brushite stones. In addition, interstitial calcifications of a kind not seen in other stone formers are also present15, which are irregular randomly distributed
Figure 5. Renal papilla of patients who formed idiopathic apatite stones.
Patients who form apatite stones can be categorized into two groups on the basis of endoscopy images: those with a | normal appearing papillae and those with b | severe changes that resemble those of patients who form distal renal acidosis stones. Panel a | shows a papilla with three separate attached stones (double arrowheads) and no yellow plaque or Bellini duct plugs, whereas panel b | shows a papilla with numerous regions of yellow plaque (arrows) and a large BD plug (asterisk). Light microscopy images showing c | novel interstitial plaque structures (arrow) — a form of plaque that is unique to patients who form apatite stones — characterized by irregular, large and randomly distributed laminar structures of hydroxyapatite crystal and matrix, and d | numerous plugged inner medullary collecting ducts surrounding an area of extensive interstitial fibrosis in a papilla with severe changes. Micro-CT images of tubular deposits in biopsy samples from e | a normal appearing papilla with no deposits and f | a severely damaged papilla with numerous small deposits.
laminar deposits of interstitial HA and matrix, without microspherules, and with a very high crystal-to-matrix ratio (Figure 5). The significance of these calcifications, which we have termed ‘novel interstitial plaque structures’, is not yet known.
As intratubular apatite deposits are so numerous, the tissue of some papillae can be almost completely replaced by crystal plugs, and nephrocalcinosis may be apparent on radiographs. In this respect the papillae are similar in appearance to those found in patients with distal renal tubular acidosis, and careful interpretation of blood and urine testing is necessary to avoid misdiagnosis.
Plug composition
Although few plugs have been harvested for study, their diversity is remarkable (Figure 3). A plug isolated from a patient with idiopathic hydroxyapatite stones had a tubule segment composed of hydroxyapatite and an overgrowth of hydroxyapatite and CaOx whereas a patient with idiopathic brushite stones produced a plug with a tubule segment that contained brushite, hydroxyapatite and CaOx and an overgrowth with a center of CaOx and an outer region of brushite15. A plug from a patient with stones resulting from ileostomy had an hydroxyapatite tubule segment with a pure CaOx overgrowth31, and a plug from a patient with primary hyperparathyroidism had an intratubular portion of hydroxyapatite with an overgrowth of hydroxyapatite 31. In our analysis of 12 patients with medullary sponge kidney disease we found a single plug, which was composed of a mixture of hydroxyapatite and CaOX in the tubule and overgrowth regions23. We also found multiple examples of tiny (500 μm diameter) round microliths made of lamina of hydroxyapatite and CaOx in the dilated and pathological inner medullary collecting ducts of these patients. How these microliths form is unknown.
Cortical changes
As only ~350 Bellini ducts drain the nephrons of a single kidney25, one might expect to find changes in the cortical tissue of patients with substantial plugging in comparison to that of patients in whom plugging is not found. Among patients with idiopathic CaOx stones who did not have plugs, cortex (Figure 6) looked similar to that of people without kidney disease15. Cortex from patients who formed brushite or hydroxyapatite stones showed scattered regions of scarring and nephron loss15. Given this scattered distribution it is unsurprising that such scarring rarely results in a reduction in renal function15, 16, 36.
Figure 6. Renal cortical changes in patients who formed calcium stones.
Histopathology images showing a | moderate changes in the cortex of a patient who formed brushite stones, b | patchy change in the cortex of a patient who formed apatite stones, and c | minimal interstitial fibrosis and glomerulosclerosis in a patient who formed idiopathic CaOx stones.
Remaining questions
We know from observation of human kidneys that growth on plaque occurs and is widespread among patients who form calcium stones, whether idiopathic or secondary to systemic disease. Tubule plugs give rise to overgrowths that resemble kidney stones in their composition, but overgrowths observed so far are much too small to produce clinical disease. The natural assumption is that these overgrowths increase in size and become clinically relevant stones, but this mechanism remains to be proven. We have not yet found stones of clinically important size attached to plugs, suggesting that overgrowths do not increase in size to form stones in this location. Such growth might occur in the crevasses of the calyces, but no method yet exists to test this hypothesis.
The frequencies with which plaque and plugging occur will gradually be defined as larger populations are studied in the future. It is important that such studies clearly document the locations in which stones are forming, so that mechanisms of stone formation can be clarified.
Formation in free solution is commonly suggested as a mechanism of kidney stone formation37, but proof that this mechanism occurs is lacking. Stones that grow from a plug overgrowth or form in the bulk urine might look the same. The organic stones, cystine and uric acid, might theoretically form in free solution, but no experimental test currently exists to prove this hypothesis in humans. The microliths of patients with medullary sponge kidneys are perhaps the most obvious candidates for formation in free solution23. These round, unattached, whorls of hydroxyapatite and CaOx form in the lumens of dilated inner medullary collecting ducts but no proof exists that they grow into clinically significant stones.
Finally, the relationship between cortical changes in patients who form brushite or hydroxyapatite stones and clinically important renal disease is unknown. Some evidence supports an increased risk of chronic kidney disease among patients who form stones38, which perhaps reflects our findings of cortical scarring in patients with inner medullary duct plugs, but further research is needed.
Formation of crystals
Role of supersaturation
Supersaturation is accepted on physical chemistry grounds as the prime source of free energy that drives the nucleation and growth of crystals39, 40. The supersaturation of urine with respect to the relevant crystal phases in stones can be estimated using a well-established set of measurements41, 42 and computer programs that calculate the simultaneous ion equilibria in solution of these stone salts, comparing them to the known thermodynamic solubility constants to derive estimates of supersaturation. [ref: Finlayson B. “Calcium Stones: Some Physical and Clinical Aspects”, in Calcium Metabolism in Renal Failure and Nephrolithiaisis, David DS, ed. John Wiley and Sons, New York 1977. pp 337–382]. At least two supersaturations are always of concern in patients who form calcium stones: CaOx and CaP. Even patients who form the purest idiopathic CaOx stones over plaque and have no tubule plugging require that hydroxyapatite form as the base of these stones30. In almost all patients with idiopathic calcium stones who also have tubule plugging, hydroxyapatite and brushite as well as CaOx can be found in plugs and stones14, 31. Whether or not specific attempts to regulate both CaP and CaOx supersaturation will lead to improved stone prevention could perhaps be evaluated in future trials. Given the current lack of trial evidence, and little obvious risk, monitoring and controlling both supersaturation values seems prudent as a clinical approach.
With perhaps the exception of minor oxalate secretion in some patients who form idiopathic calcium stones43, the main stone crystal components — calcium, phosphate and oxalate — are filtered and reabsorbed so supersaturation of urine cannot exceed that of blood except through water extraction along the nephron. Urine supersaturation is essentially the stored energy produced by water extraction, which can be dissipated by driving phase change40. This well established paradigm emphasizes the remarkable power of simple hydration; by reducing water reabsorption, fluids reverse the primary engine of crystallization.
Inhibition by urine proteins
Modern techniques have identified >1000 urine proteins44, 45, many of which have long been known for their abilities to kinetically retard calcium crystal nucleation, growth and aggregation46, 47. Stones contain many such proteins, presumably because their crystals have adsorbed them from urine as they formed48.
Urine proteins are the main reason why urine can remain stable for days despite supersaturation; simple salt solutions with a similar level of supersaturation are unstable40. Essentially, the proteome alters the link between supersaturation and crystallization. In simple solutions supersaturation and crystallization are tightly related, whereas in urine supersaturation is necessary to achieve crystal formation and prevent crystal dissolution, but a particular level of supersaturation cannot be predicted to result in new crystal formation due to the presence of an unknown number of inhibitors. The idea of comparing the urine supersaturation of healthy individuals and patients who form stones, and identifying a level of supersaturation that will reliably distinguish between the two groups is, therefore, flawed.
Crystal interactions
Brushite
In urine, when nucleation is induced in vitro by addition of calcium, the initial phase that forms is usually brushite49. Oxalate can bind to calcium atoms on the surface of brushite and begin to form CaOx crystals. As CaOx crystals are less soluble than brushite they are able to cannibalize the brushite provided the solution is supersaturated with respect to CaOx40, 50. In the presence of osteocalcin40, hydroxyapatite can begin to form on the surface of brushite with liberation of a proton as the monohydrogen phosphate of brushite is taken up into the hydroxyapatite lattice as trivalent negative phosphate ions.
As a result, brushite is usually a transient phase in urine, and one would not generally expect to find brushite in kidney stones. Indeed, large series of stones have reported that only approximately 1% contain brushite51, 52.. Mechanisms of brushite persistence might involve specific patterns of the urine proteome, but this hypothesis has not yet been investigated.
Citrate
Citrate ions bind calcium53 so reduce the amount of calcium that is available to bind with oxalate or phosphate and thereby lower supersaturation with respect to all of the calcium stone crystals54. Binding of citrate to brushite crystals widens their growth plates so slows their growth and inhibits the formation of new brushite as oxalate and apatite use it as a source of ions40. Citrate also binds to growth ridges on CaOx crystals and slows their growth40, 55.
It is important to note that brushite crystal cannibalization has been shown to occur in vitro rather than in whole urine. Likewise the effects of citrate on crystals have only been proven in simple solutions. All crystals follow the same laws of physics, but given the huge urine proteome with its crystal active molecules, the interactions of crystals in urine and with citrate might not be fully predicted by in vitro studies.
Idiopathic hypercalciuria
It is the molarity of calcium, oxalate, phosphate, and citrate, along with urine pH, that mainly determine SS for CaOx and brushite, and molarities reflect the relationship between solute excretion and urine flow. Hypercalciuria is associated with higher levels of supersaturation at any given urine flow when compared to normal calcium excretion. Idiopathic hypercalciuria is familial [ref], found in up to 50% of idiopathic calcium stone formers and is often of considerable magnitude, so it has long occupied the attention of clinicians and scientists56. Increases in oxalate excretion and reductions in citrate excretion might well be of equal importance to hypercalciuria in terms of stone formation but are not discussed in this Review.
Definition
Although hypercalciuria can result in renal stones and probably also bone mineral loss and fractures57, it is not a disease per se, but rather represents the upper tail of a continuous distribution, similar to height, weight or blood pressure.
As urine calcium excretion is a continuously distributed variable it is not ideal or even reasonable to choose a single cut-off point to define ‘normal’ levels. Rather, similar to blood pressure and body weight, urine calcium excretion should be considered a graded risk factor, and the calcium excretion rate of the individual patient should be considered along with other factors that affect urine supersaturation when making treatment decisions.
Data from epidemiological studies enable correlation of various levels of urine calcium excretion with the relative risk of stone formation58. For both sexes, the lower 95% confidence interval (CI) for a relative risk ratio for stone formation of >1 (that is, increased risk) is present at urine calcium levels of about 200 mg daily. This value could, therefore, be considered the lower limit of clinical hypercalciuria and the threshold for consideration of treatment measures aimed at lowering urine calcium excretion. Of course, being a graded risk factor, lowering of urine calcium levels to <200 mg might be appropriate in certain patients, such as those with persistent stone formation.
Mechanisms
Increased gut calcium absorption
In individuals with normal urinary calcium excretion, intestinal calcium absorption rises with dietary intake; on average around 25% of dietary calcium is absorbed in both men and women. By contrast, around 30% of dietary calcium is absorbed in patients with idiopathic hypercalciuria.59–70
Gastrointestinal calcium absorption is mediated by both epithelial transport and passive paracellular absorption71. The increased fraction of dietary calcium that is absorbed in patients with idiopathic hypercalciuria suggests that epithelial transport of calcium is increased in these patients. As the vitamin D hormone system increases epithelial calcium transport, increased calcium absorption in patients with idiopathic hypercalciuria could be the result of increased serum calcitriol levels, increased tissue vitamin D receptor expression or a combination of these factors. Indeed, the serum calcitriol levels of individuals with idiopathic hypercalciuria are generally higher than those of individuals with normal urinary calcium excretion72, suggesting that activation of the vitamin D hormone system might be an integral component of idiopathic hypercalciuria73. Inbred hypercalciuric rats have normal serum calcitriol levels, but high intestinal and renal vitamin D receptor expression and enhanced intestinal epithelial calcium absorption74.
Increased renal calcium loss
Two mechanisms exist by which renal calcium losses might be increased in patients with hypercalciuria: increased filtered load of calcium or reduced tubule calcium reabsorption. Under controlled dietary conditions, the serum concentrations of ultrafilterable calcium in patients with idiopathic hypercalciuria and individuals with normal urinary calcium levels overlap both fasting and fed 75. Filtered loads of calcium do not differ, and show no tendency to increase with meals in either group75. By contrast, the urinary calcium levels of patients with idiopathic hypercalciuria exceeds that of normal individuals during fasting, and increased greatly above normal levels in the fed state76. This increase occurs because of a marked fall in overall tubule calcium reabsorption75.
Although the levels of insulin and parathyroid hormone both change with meals and can differ between individuals with and without hypercalciuria, a decrease in calcium reabsorption in these groups occurs at overlapping levels of these hormones77. Likewise, the levels of fractional sodium reabsorption overlap in these the two groups75. Tubule calcium reabsorption in patients with idiopathic hypercalciuria and those with normal calcium excretion, therefore, differs independently of insulin levels, parathyroid hormone levels and sodium handling77, 78.
The delivery of calcium out of the proximal tubule into more distal nephron segments can be calculated using endogenous lithium clearance as a marker for proximal tubule reabsorption. Using this technique we found that distal calcium delivery was increased in patients with idiopathic hypercalciuria compared with individuals with normal calcium excretion79. This finding indicates that the proximal nephron segments contribute to the increase in urine calcium excretion in these patients.
Bone mineral balance
Most patients with idiopathic hypercalciuria seem to have increased intestinal calcium absorption coupled with decreased renal calcium reclamation. However, urinary calcium excretion often exceeds gut calcium absorption in balance studies, and in fact, when dietary calcium intake is very low, patients with idiopathic hypercalciuria can excrete more calcium than they ingest81.
These data suggest that idiopathic hypercalciuria will cause bone disease when patients are challenged with a low calcium diet for stone prevention81. Consistent with this hypothesis, many studies have shown reduced bone mineral density and an increased incidence of fractures among patients with idiopathic hypercalciuria57. This is one reason why low calcium diets are not advisable for treatment of stone formers with idiopathic hypercalciuria. As patients with idiopathic hypercalciuria seem to have a generalized alteration of both gut and renal calcium handling, and are at risk of bone loss, attempting to separate patients into those with primarily absorptive or renal hypercalciuria is not clinically useful.
Patient management
Management of supersaturation
The urine supersaturation of a patient who is actively forming new stones is clearly too high with respect to the crystals in the stones that are being formed. Our current stone treatments aim at decreasing urinary supersaturation with respect to the stone mineral by a significant margin, perhaps by half. Lowering urine supersaturation often requires multiple interventions, which may include increased fluid intake, dietary counseling, and medications. Interventions should be targeted at the factors which are most likely to be raising supersaturation in a given patient, uncovered by 24 hour urines done prior to treatment. Depending on stone (and supersaturation) type, these could include inadequate urine volume, elevated urine calcium, oxalate, or uric acid, or low urine citrate. A general approach to stone prevention is outlined in Box 1. Although no trials have specifically investigated whether management of supersaturation is more effective than routine drug therapy without such management82 for prevention of stone formation, we believe that management of supersaturation is justified and clinical trials of such an approach are not unreasonable. Patients should always be followed up with 24 hour urine measurements 4–6 weeks after treatment is initiated, to assess the success of treatment at lowering supersaturation. If supersaturation persists, treatment can be adjusted according to the persistent risk factors.
Box 1. Management of patients who form idiopathic calcium stones.
Daily fluid intake should be high (>3 l on average) to achieve at least 2 l of urine daily; fluid intakes ≥3.5 l daily to achieve urine volumes of ≥≥2.5 l daily are advisable with adjustments for higher fluid needs depending on work, climate, and lifestyle. Accurate measurement of urine volume during treatment is essential.
In patients who are hypercalciuric, dietary calcium levels should be at least 1,200 mg daily (calcium should be obtained preferably from food rather than supplements) and dietary sodium levels should be restricted to ≤ 1.5 g daily.
We suggest using thiazide diuretics when high urine volume and reduced sodium intake fail to reduce supersaturations to half of their original values, when this end point has been reached but new stones form, and in patients who are unable or unwilling to alter their fluid and sodium intake sufficiently to reduce stone risk.
Urine citrate levels should be increased to >400 mg daily using potassium citrate or other oral potassium-based alkali.
In patients with urinary citrate ≥400 mg daily or with calcium phosphate stones, the use of alkali supplements has not been specifically tested and theoretical considerations suggest a possible adverse effect in the latter instance.
Fluid intake
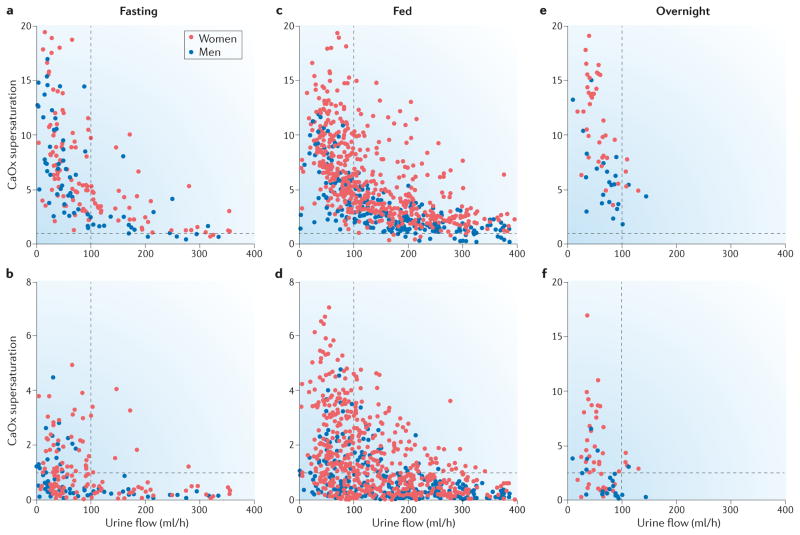
Levels of urine supersaturation vary with urine flow rate and food intake (figure 7), in both normal subjects and hypercalciuric calcium stone formers fed identical diets and studied over the course of a day. During fasting, supersaturations of CaOx and CaP increase steeply as urine flow rate falls below 100 ml/h (Figure 7)76. The relationship between supersaturation and urine flow is less regular for CaP than for CaOx because urine pH strongly influences CaP supersaturation52 but not CaOx supersaturation. However it is clear that fluid intake markedly decreases supersaturation for both stone salts. A urine flow of 100 ml/h during fasting and overnight, and 125 ml/h when fed would be predicted to minimize urine supersaturation. Given an estimate of 0.9 l per day (40 ml/h) insensible water losses83 among patients who are not exposed to an extreme of climate, fluid intakes of 140 ml/h during fasting and overnight and 165 ml/h when fed would be expected to achieve this urine flow. Lifestyle habits, climate, working conditions, and the use of therapies that lower urine calcium or oxalate excretion might alter these requirements. Our calculations suggest an ideal fluid intake of about 3.5 l per day to reduce the risk of stone formation; however, an intake of this volume might not be practical. As patients might not accurately know their urine volumes, measurement during treatment is essential.
Figure 7. Effect of urine flow rate on calcium oxalate and calcium phosphate supersaturations.
Urine supersaturations of calcium oxalate (CaOx) and calcium phosphate (CaP) varied inversely with urine flow rate in normal subjects (blue) and hypercalciuric idiopathic calcium stone formers (red) on the same diet. During fasting both a | CaOx and b |[ the figure is mislabeled, the y axis says CaOx, but should say CaP] CaP supersaturation values were generally high when urine flow was <100 ml/h. When urine flow was >100 ml/h many CaP supersaturation values were less than one, indicating that crystal formation will not occur. A much higher urine flow rate was required to reduce CaOx supersaturations to a similar level. When the participants were fed the distributions of c | CaOx and d | CaP supersaturations shifted to the right because of increased urinary excretion of calcium and oxalate. A urine flow rate of around 125 ml/h seems to be an appropriate clinical goal to prevent large increases in CaOx and CaP supersaturations. e and f | Although each participant provided only one urine sample overnight the general pattern of supersaturation and urine flow is similar to that seen during fasting. A goal of 100 ml/h urine flow overnight seems reasonable for stone prevention. Data from Bergsland et al. Am. J. Physiol Renal Physiol 297, F1017–F1023 (2009)76.
Validation of the importance of fluids is provided by a randomized trial of water intake done in 200 idiopathic calcium stone formers who had formed a single stone. After five years, 12% of the treated patients, who increased their urine volume from 1068 ml at baseline to 2061 ml during treatment, had a recurrent kidney stone, compared to 27% of the controls, whose baseline urine volume of 1008 ml did not change during the study (p=0.008). Supersaturations for both calcium oxalate and calcium phosphate decreased significantly in the treated arm.
Low sodium diet
Urine calcium excretion is linearly related to urine sodium excretion, in both normal subjects and stone formers, and lowering sodium intake can lower urine calcium. A number of controlled experiments have documented the effects of changes in dietary sodium intake on urinary calcium excretion in healthy individuals84–92 and in patients with idiopathic hypercalciuria87, 91–97. Moreover observational studies have shown changes in urine calcium levels in these groups when dietary sodium levels have changed spontaneously94, 98–100.
A study of men with idiopathic hypercalciuria and calcium stones compared the effects of a low calcium, low oxalate diet to those of a diet with 1,200 mg calcium, reduced sodium and oxalate protein101. In this study, 23 of 51 patients on the low calcium diet and 12 of 52 patients on the reduced sodium and protein diet formed a new stone over 5 years (P <0.01). The relative contributions of the lowered sodium and protein intakes on stone formation cannot be determined. However, given its remarkable ability to lower urine calcium, a trial of low sodium diet alone versus thiazide might be justified.
No data are available on the effect of sodium on bone balance in patients with idiopathic hypercalciuria, but the findings of a study of dietary sodium and calcium intakes in menopausal women suggest that a reduced sodium diet might benefit bone balance in the setting of idiopathic hypercalciuria88. As expected, calcium absorption was higher in women who received high calcium diets than in those on low calcium diets and calcium balance was negative in the low calcium diet groups. In the low sodium, high calcium diet phase, bone balance became positive because calcium absorption was the same as for high sodium, high calcium diet, but fecal and urine calcium losses were reduced with reduced dietary sodium.
In patients who are hypercalciuric, dietary calcium should be ample (≥1200 mg) and dietary sodium should ideally be restricted to < 1.5 g (65 mmol) per day as recommended for the US adult population by the Centers for Disease Control and Prevention102. This low sodium diet has the potential to reduce urine calcium levels and possibly maintain or even increase bone mineral stores. It is preferable to obtain calcium from foods rather than supplements, but if supplements are used they should be taken with meals.
Thiazide and thiazide-like diuretics
Thiazide diuretics lower urinary calcium excretion103 and have been used to prevent the formation of calcium stones104. The effectiveness of this therapy is presumed to arise from the hypocalciuric action of thiazide, although this hypothesis has not been rigorously tested.
Among a welter of shorter trials of thiazide diuretics for stone prevention, three stand out for being randomized controlled trials with a treatment period of 3 years. First, in 1984, Laerum et al. showed that treatment with hydrochlorothiazide (25 mg twice daily) reduced stone recurrence in patients with urolithiasis; 10 of 25 patients in the placebo group formed a new stone compared with only four of 23 patients in the hydroclorothiazide group (P <0.01)105. In 1988 Ettinger et al. reported that treatment with chlorthalidone (25 mg daily) reduced the recurrence of CaOx stones; 12 of 26 patients in the placebo group versus 4 of 28 patients in the treatment group formed a new stone (P <0.01)106. Finally in 1993, Borghi et al. showed that treatment with the nonthiazide diuretic indapamide (2 mg daily) reduced stone recurrence in patients with calcium nephrolithiasis and hypercalciuria; nine of 21 patients in the placebo group and only six of 43 patients in the treatment group formed a new stone during the treatment period (P <0.01)107. Although all three studies were limited by incomplete follow up of lost participants, and another randomized trial might be justified, these data suggest that thiazide is effective for stone prevention.
In four men, we performed a complete analysis of tubule reabsorption before and after 6 months of treatment with chlorthalidone 25 mg daily108. Thiazide reduced urine calcium excretion, fractional calcium excretion, and fractional lithium excretion, indicating that its effect involves reduced distal delivery of calcium out of the proximal tubule. These results suggest that thiazide might not only reduce stone formation, but also formation of interstitial plaque, but this hypothesis remains to be tested experimentally.
Data from balance experiments has demonstrated that thiazide therapy can improve bone mineral balance in idiopathic hypercalciuric stone formers. We found that chlorthalidone therapy (25 mg daily for 6 months) lowered net intestinal calcium absorption to a lesser extent than urine calcium excretion, so net bone balance increased109. Additional studies have shown increases in BMD110 and reduction of fracture rates111 with thiazide agents. We tend to treat patients with thiazide diuretics when high urine volume and reduced sodium intake have failed to bring urine supersaturations to half of their original values, when this end point has been reached but new stones occur and in patients who cannot or will not alter their fluid and sodium intake sufficiently to reduce stone risk.
Potassium citrate
Given its multiple roles in calcium binding and as an inhibitor of the formation of all three types of common stone crystal species, citrate is theoretically an important defense against stone formation. Consistent with this hypothesis, reduced levels of citrate correlated with new onset of stones in long-term observational studies of men and women58. For both sexes, the upper 95% confidence interval for the relative risk ratio of becoming a stone former is <1 when citrate excretion is >400 mg daily; lower levels of citrate excretion confer progressively increased risk of stone formation58. Treatment with potassium citrate or another oral potassium-based alkali should, therefore, be used to increase urine citrate levels to >400 mg daily and reduce the risk of stone formation in patients with idiopathic calcium stones.
Oral citrate intake increases urine citrate levels by imposing an alkali load as the citrate is metabolized as citric acid112, 113. Perhaps as a mechanism of removing alkali without raising urine pH the kidneys reduce renal citrate reabsorption via effects on the main citrate transporter in the proximal tubule114. Urine bicarbonate excretion often increases in response to oral citrate intake, resulting in increases in urine pH and CaP supersaturation112.
Potassium citrate — or potassium alkali of any kind — lowers urine calcium excretion in patients who form stones115 or in patients with bone diseases116. This effect probably occurs because the alkali neutralizes chronic dietary acid load116. As metabolic acidosis decreases proximal tubule sodium reabsorption117, 118 via a number of mechanisms119, 120, reversing the chronic acid load from diet will likely increase sodium reabsorption, which will help to conserve filtered calcium and decrease calcium excretion. Distal effects of diet acid load and its reversal have not yet been studied in humans or proven in experimental models.
‘Calcium’ stones
Potassium citrate treatment has been shown to reduce the formation of new calcium stones, but no trial has specifically targeted patients who form CaP stones; the majority of participants in the published studies made CaOx stones. In the first of two double-blind randomized controlled trials, 16 of 20 patients in the placebo group who completed 3 years of follow-up formed a new calcium stone compared to five of 18 patients who received potassium citrate121. In the second trial122, 16 of 25 patients in the placebo group and two of 16 patients in the treatment group formed a new calcium stone after 3 years of follow up. In both of these trials the reduction in stone formation with therapy was statistically significant. A third randomized controlled trial that was not double blinded showed no significant difference in stone formation between the study groups; six of 22 patients in the placebo group and five of 16 patients in the treatment group produced new stones over 3 years.123 The difference from the other two trials is clearly the low rate of recurrence among the placebo patients.. In two randomized controlled trials that followed patients after shock wave lithotripsy, those treated with potassium citrate had lower rates of retained fragment growth and new stone formation than untreated controls after 12 months of follow up 124, 125
Brushite and apatite stones
The effect of potassium citrate on formation of brushite and apatite stones is unclear. On the one hand, inhibition of brushite and hydroxyapatite formation, reduction of urine calcium excretion, and reduction of free calcium ions in urine should reduce CaP supersaturation and formation of CaP stones. On the other hand, higher urine pH directly increases supersaturation with respect to brushite52, 115 and fosters hydroxyapatite formation by providing increased receptors to take up the protons that are liberated as brushite is converted to hydroxyapatite126. The effect of potassium citrate on the formation of brushite or hydroxyapatite stones has not yet been evaluated in a clinical trial. In the meantime, physicians who use citrate might mitigate the risk of stone formation by closely monitoring the increases in urine pH, CaP supersaturation and citrate excretion after starting treatment, and adjusting treatment accordingly. If stone recurrence persists, use of citrate should be re-evaluated.
Conclusions
Many patients who form idiopathic calcium stones are hypercalciuric. Supersaturations of CaP and CaOx in the urine and local to the papillary interstitium foster the formation of interstitial apatite plaque, overgrowth of amorphous apatite on exposed plaque, and the formation of the main CaOx body of attached stones. Citrate and some urine proteins kinetically slow the process of stone formation, but plaque overgrowth might eventually create stones despite these inhibitors. Supersaturations also create tubule plugs but exactly how this process occurs and whether the tiny overgrowths found on plugs become clinically significant stones is unknown. Idiopathic hypercalciuria is a renal disorder that results from reduced tubule calcium reabsorption. Further research is needed to clarify whether activation of the vitamin D hormone system with abnormally high intestinal calcium absorption occurs secondary to calcium losses in patients with hypercalciuria or is a primary component of the renal disorder. Importantly, low calcium diets are inappropriate and can cause harmful losses of bone mineral in these patients. Despite the remaining questions, the available data suggest that high fluid intake, low sodium diet and treatment with potassium citrate and thiazide diuretics are practical and effective approaches for stone prevention in idiopathic patients.
Key points.
Idiopathic calcium stones are always accompanied by mineral deposits: interstitial deposits of apatite in patients with calcium oxalate (CaOx) stones or calcium phosphate (CaP) plugs in those with CaP stones
Overgrowth of CaOx stones on plaque depends on the formation of an initial CaP phase; urine saturations of CaP and CaOx might, therefore, be equally important
Microliths form on the open ends of tubule plugs but proof that these can grow into clinically relevant stones is lacking
Patients with tubule plugs who form CaP stones show varying degrees of cortical fibrosis and nephron loss
Trial data support the use of high fluid intake, potassium citrate, thiazide diuretic agents and a reduced sodium diet for prevention of recurrent calcium renal stones. [
As idiopathic hypercalciuria arises from reduced renal tubule calcium reabsorption and is associated with negative calcium balance and bone disease, management with a low calcium diet is contraindicated
Acknowledgments
The authors’ work is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (PO1 DK56788).
Biographies
Fredric Coe MD is currently a Professor of Medicine in the Nephrology Section at the University of Chicago, where he founded the Kidney Stone Program and has led it for 40 years. He has authored over 350 books, chapters and peer-reviewed papers on the subjects of the pathogenesis and treatment of kidney stones and other aspects of mineral metabolism.
Elaine Worcester MD is a currently a Professor of Medicine in the Nephrology Section at the University of Chicago. Her research focuses on pathogenesis of hypercalciuria in patients with kidney stones and the interaction between altered renal tubular calcium handling and mineral deposits in the kidney tissue of stone formers.
Andrew Evan PhD is Chancellor’s Professor of Anatomy and Cell Biology at Indiana University School of Medicine in Indianapolis. His research has demonstrated the mechanisms by which many kidney stones form in human tissue, and the links between kidney stones and specific types of mineral deposits in the renal papillae of stone formers.
Footnotes
Competing interests statement
The authors declare no competing interests.
Reference List
- 1.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkali Z, Rasooly R, Star RA, Rodgers GP. Urinary Stone Disease: Progress, Status, and Needs. Urol. 2015;86:651–653. doi: 10.1016/j.urology.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worcester EM, Coe FL. Nephrolithiasis. Prim Care. 2008;35:369–91. vii. doi: 10.1016/j.pop.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worcester EM, Coe FL. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol. 2008;28:120–132. doi: 10.1016/j.semnephrol.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parks JH, Worcester EM, Coe FL, Evan AP, Lingeman JE. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int. 2004;66:777–785. doi: 10.1111/j.1523-1755.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller NL, et al. A formal test of the hypothesis that idiopathic calcium oxalate stones grow on Randall’s plaque. BJU Int. 2009;103:966–971. doi: 10.1111/j.1464-410X.2008.08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller NL, et al. In idiopathic calcium oxalate stone-formers, unattached stones show evidence of having originated as attached stones on Randall’s plaque. BJU Int. 2010;105:242–245. doi: 10.1111/j.1464-410X.2009.08637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linnes MP, et al. Phenotypic characterization of kidney stone formers by endoscopic and histological quantification of intrarenal calcification. Kidney Int. 2013;84:818–825. doi: 10.1038/ki.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, et al. Distinguishing characteristics of idiopathic calcium oxalate kidney stone formers with low amounts of Randall’s plaque. Clin J Am Soc Nephrol. 2014;9:1757–1763. doi: 10.2215/CJN.01490214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viers BR, et al. Endoscopic and Histologic Findings in a Cohort of Uric Acid and Calcium Oxalate Stone Formers. Urology. 2015 doi: 10.1016/j.urology.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letavernier E, et al. Demographics and characterization of 10,282 Randall plaque-related kidney stones: a new epidemic? Medicine (Baltimore) 2015;94:e566. doi: 10.1097/MD.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evan AE, et al. Histopathology and surgical anatomy of patients with primary hyperparathyroidism and calcium phosphate stones. Kidney Int. 2008;74:223–229. doi: 10.1038/ki.2008.161. [DOI] [PubMed] [Google Scholar]
- 13.Evan AP, et al. Intra-tubular deposits, urine and stone composition are divergent in patients with ileostomy. Kidney Int. 2009;76:1081–1088. doi: 10.1038/ki.2009.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evan AP, et al. Renal histopathology and crystal deposits in patients with small bowel resection and calcium oxalate stone disease. Kidney Int. 2010;78:310–317. doi: 10.1038/ki.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evan AP, et al. Contrasting histopathology and crystal deposits in kidneys of idiopathic stone formers who produce hydroxy apatite, brushite, or calcium oxalate stones. Anat Rec (Hoboken ) 2014;297:731–748. doi: 10.1002/ar.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evan AP, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005;67:576–591. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 17.Coe FL, Evan AP, Lingeman JE, Worcester EM. Plaque and deposits in nine human stone diseases. Urol Res. 2010;38:239–247. doi: 10.1007/s00240-010-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evan AP, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evan AP, et al. Apatite plaque particles in inner medulla of kidneys of calcium oxalate stone formers: osteopontin localization. Kidney Int. 2005;68:145–154. doi: 10.1111/j.1523-1755.2005.00388.x. [DOI] [PubMed] [Google Scholar]
- 20.Evan AP, et al. Renal inter-alpha-trypsin inhibitor heavy chain 3 increases in calcium oxalate stone-forming patients. Kidney Int. 2007;72:1503–1511. doi: 10.1038/sj.ki.5002569. [DOI] [PubMed] [Google Scholar]
- 21.Williams JC, Jr, Lingeman JE, Coe FL, Worcester EM, Evan AP. Micro-CT imaging of Randall’s plaques. Urolithiasis. 2015;43(Suppl 1):13–17. doi: 10.1007/s00240-014-0702-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan SR, Rodriguez DE, Gower LB, Monga M. Association of Randall plaque with collagen fibers and membrane vesicles. J Urol. 2012;187:1094–1100. doi: 10.1016/j.juro.2011.10.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evan AP, et al. Biopsy Proven Medullary Sponge Kidney: Clinical Findings, Histopathology, and Role of Osteogenesis in Stone and Plaque Formation. Anat Rec (Hoboken ) 2015 doi: 10.1002/ar.23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo RL, et al. Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int. 2003;64:2150–2154. doi: 10.1046/j.1523-1755.2003.00316.x. [DOI] [PubMed] [Google Scholar]
- 25.Kriz W, Kaissling B. Structural Organization of the Mammalian Kidney. In: Alpern RJ, Moe OW, Caplan M, editors. Seldin and Giebisch’s The Kidney: Physiology and Pathophysiology. Elsevier; Amsterdam: 2013. pp. 595–691. [Google Scholar]
- 26.Bernardo JF, Friedman PA. Renal calcium metabolism. In: Alpern RJ, Caplan MJ, Moe OW, editors. Seldin and Giebisch’s The Kidney:Physiology and Pathophysiology. Academic Press; Amsterdam: 2013. pp. 2225–2247. [Google Scholar]
- 27.Coe FL, Evan A, Worcester E. Pathophysiology-based treatment of idiopathic calcium kidney stones. Clin J Am Soc Nephrol. 2011;6:2083–2092. doi: 10.2215/CJN.11321210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoller ML, Shami GS, McCormick VD, Kerschmann RL. High resolution radiography of cadaveric kidneys: unraveling the mystery of Randall’s plaque formation. Journal of Urology. 1996;156:1263–1266. doi: 10.1016/s0022-5347(01)65565-4. [DOI] [PubMed] [Google Scholar]
- 29.Evan AP, et al. Comparison of the pathology of interstitial plaque in human ICSF stone patients to NHERF-1 and THP-null mice. Urol Res. 2010;38:439–452. doi: 10.1007/s00240-010-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evan AP, et al. Mechanism of formation of human calcium oxalate renal stones on Randall’s plaque. Anat Rec (Hoboken ) 2007;290:1315–1323. doi: 10.1002/ar.20580. [DOI] [PubMed] [Google Scholar]
- 31.Evan AP, Worcester EM, Coe FL, Williams J, Jr, Lingeman JE. Mechanisms of human kidney stone formation. Urolithiasis. 2015;43(Suppl 1):19–32. doi: 10.1007/s00240-014-0701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evan AP, et al. Renal intratubular crystals and hyaluronan staining occur in stone formers with bypass surgery but not with idiopathic calcium oxalate stones. Anat Rec (Hoboken ) 2008;291:325–334. doi: 10.1002/ar.20656. [DOI] [PubMed] [Google Scholar]
- 33.Evan AP, et al. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int. 2007;71:795–801. doi: 10.1038/sj.ki.5002113. [DOI] [PubMed] [Google Scholar]
- 34.Evan AP, et al. Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int. 2006;69:2227–2235. doi: 10.1038/sj.ki.5000268. [DOI] [PubMed] [Google Scholar]
- 35.Worcester EM, et al. A test of the hypothesis that oxalate secretion produces proximal tubule crystallization in primary hyperoxaluria type I. Am J Physiol Renal Physiol. 2013;305:F1574–F1584. doi: 10.1152/ajprenal.00382.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worcester EM, Parks JH, Evan AP, Coe FL. Renal function in patients with nephrolithiasis. J Urol. 2006;176:600–603. doi: 10.1016/j.juro.2006.03.095. [DOI] [PubMed] [Google Scholar]
- 37.Coe FL, Evan AP, Worcester EM, Lingeman JE. Three pathways for human kidney stone formation. Urol Res. 2010;38:147–160. doi: 10.1007/s00240-010-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rule AD, Krambeck AE, Lieske JC. Chronic kidney disease in kidney stone formers. Clin J Am Soc Nephrol. 2011;6:2069–2075. doi: 10.2215/CJN.10651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coe FL, Parks JH. Physical Chemistry of Calcium Stone Disease. In: Coe FL, Parks JH, editors. Nephrolithiasis: Pathogenesis and Treatment. Year Book Medical Publishers; Chicago: 1988. pp. 38–58. [Google Scholar]
- 40.Qiu SR, Orme CA. Dynamics of biomineral formation at the near-molecular level. Chem Rev. 2008;108:4784–4822. doi: 10.1021/cr800322u. [DOI] [PubMed] [Google Scholar]
- 41.Finlayson B. Calcium stones: some physical and clinical aspects. In: David DS, editor. Calcium metabolism in renal failure and nephrolithiasis. John Wiley & Sons; New York: 1977. pp. 337–382. [Google Scholar]
- 42.Parks JH, Coward WM, Coe FL. Correspondence between stone composition and urine supersaturation in nephrolithiasis. Kidney Int. 1997;51:894–900. doi: 10.1038/ki.1997.126. [DOI] [PubMed] [Google Scholar]
- 43.Bergsland KJ, Zisman AL, Asplin JR, Worcester EM, Coe FL. Evidence for net renal tubule oxalate secretion in patients with calcium kidney stones. Am J Physiol Renal Physiol. 2011;300:F311–F318. doi: 10.1152/ajprenal.00411.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santucci L, et al. Urinary proteome in a snapshot: normal urine and glomerulonephritis. J Nephrol. 2013;26:610–616. doi: 10.5301/jn.5000233. [DOI] [PubMed] [Google Scholar]
- 45.Wright CA, et al. Label-free quantitative proteomics reveals differentially regulated proteins influencing urolithiasis. Mol Cell Proteomics. 2011;10:M110. doi: 10.1074/mcp.M110.005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar V, Lieske JC. Protein regulation of intrarenal crystallization. Curr Opin Nephrol Hypertens. 2006;15:374–380. doi: 10.1097/01.mnh.0000232877.12599.f4. [DOI] [PubMed] [Google Scholar]
- 47.Aggarwal KP, Narula S, Kakkar M, Tandon C. Nephrolithiasis: molecular mechanism of renal stone formation and the critical role played by modulators. Biomed Res Int. 2013;2013:292953. doi: 10.1155/2013/292953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canales BK, et al. Proteome of human calcium kidney stones. Urol. 2010;76:1017–1020. doi: 10.1016/j.urology.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Pak CYC. Physicochemical Basis for Formation of Renal Stones of Calcium Phosphate Origin: Calculation of the Degree of Supersaturation of Urine with Respect to Brushite. J Clin Invest. 1969;48:1914–1922. doi: 10.1172/JCI106158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nancollas GH, Henneman ZJ. Calcium oxalate: calcium phosphate transformations. Urol Res. 2010;38:277–280. doi: 10.1007/s00240-010-0292-3. [DOI] [PubMed] [Google Scholar]
- 51.Costa-Bauza A, et al. Type of renal calculi: variation with age and sex. World J Urol. 2007;25:415–421. doi: 10.1007/s00345-007-0177-4. [DOI] [PubMed] [Google Scholar]
- 52.Parks JH, Worcester EM, Coe FL, Evan AP, Lingeman JE. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int. 2004;66:777–785. doi: 10.1111/j.1523-1755.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 53.Joseph NR. The dissociation constants of organic calcium complexes. J Biol Chem. 1946;164:529–541. [PubMed] [Google Scholar]
- 54.Rodgers AL, Allie-Hamdulay S, Jackson GE, Sutton RA. Enteric hyperoxaluria secondary to small bowel resection: use of computer simulation to characterize urinary risk factors for stone formation and assess potential treatment protocols. J Endourol. 2014;28:985–994. doi: 10.1089/end.2014.0077. [DOI] [PubMed] [Google Scholar]
- 55.De Yoreo JJ, Qiu SR, Hoyer JR. Molecular modulation of calcium oxalate crystallization. Am J Physiol Renal Physiol. 2006;291:F1123–F1131. doi: 10.1152/ajprenal.00136.2006. [DOI] [PubMed] [Google Scholar]
- 56.Albright F, HENNEMAN P, Benedict PH, Forbes AP. Idiopathic hypercalciuria: a preliminary report. Proc R Soc Med. 1953;46:1077–1081. [PMC free article] [PubMed] [Google Scholar]
- 57.Heilberg IP, Weisinger JR. Bone disease in idiopathic hypercalciuria. Curr Opin Nephrol Hypertens. 2006;15:394–402. doi: 10.1097/01.mnh.0000232880.58340.0c. [DOI] [PubMed] [Google Scholar]
- 58.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int. 2008;73:489–496. doi: 10.1038/sj.ki.5002708. [DOI] [PubMed] [Google Scholar]
- 59.Liberman UA, et al. Metabolic and calcium kinetic studies in idiopathic hypercalciuria. J Clin Invest. 1968;47:2580–2590. doi: 10.1172/JCI105940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heaney RP, Skillman TG. SECRETION AND EXCRETION OF CALCIUM BY THE HUMAN GASTROINTESTINAL TRACT. J Lab Clin Med. 1964;64:29–41. [PubMed] [Google Scholar]
- 61.Nassim JR, Higgins BA. Control of Idiopathic Hypercalciuria. Br Med J. 1965;1:675–681. doi: 10.1136/bmj.1.5436.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edwards NA, Hodgekinson A. Metabolic Studies in Patients with Idiopathic Hypercalciuria. Clin Sci. 1965;29:143–157. [PubMed] [Google Scholar]
- 63.Henneman PH, Benedict PH, Forbes AP, Dudley HR. Idiopathic Hypercalciuria. N Engl J Med. 1958;259:802–807. doi: 10.1056/NEJM195810232591702. [DOI] [PubMed] [Google Scholar]
- 64.Jackson WPU, Dancaster C. A consideration of the hypercalciuria in sarcoidosis, idiopathic hypercalciuria, and that produced by vitamin D. A new suggestion regarding calcium metabolism. J Clin Endocrinol Metab. 1959;19:658. doi: 10.1210/jcem-19-6-658. [DOI] [PubMed] [Google Scholar]
- 65.Harrison AR. Some results of metabolic investigations in cases of renal stone. Br J Urol. 1959;31:398. doi: 10.1111/j.1464-410x.1959.tb09438.x. [DOI] [PubMed] [Google Scholar]
- 66.Dent CE, Harper CM, Parfitt AM. The Effect of Cellulose Phosphate on Calcium Metabolism in Patients with Hypercalciuria. Clin Sci. 1964;27:417–425. [PubMed] [Google Scholar]
- 67.Parfitt AM, Higgins BA, Nassim JR, Collins JA, Hilb A. Metabolic Studies in Patients with Hypercalciuria. Clin Sci. 1964;27:463–482. [PubMed] [Google Scholar]
- 68.Yendt ER, Gagne RJA, Cohanim M. The effects of thiazides in idiopathic hypercalciuria. Transactions of the American Clinical and Climatological Assoc. 1966;77:96–110. [PMC free article] [PubMed] [Google Scholar]
- 69.Dent CE, Watson L. METABOLIC STUDIES IN A PATIENT WITH IDIOPATHIC HYPERCALCIURIA. Br Med J. 1965;2:449–452. doi: 10.1136/bmj.2.5459.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson J, Lee HA, Tomlinson RW. Some metabolic aspects of idiopathic hypercalciuria. Nephron. 1967;4:129–138. doi: 10.1159/000179579. [DOI] [PubMed] [Google Scholar]
- 71.Khanal RC, Nemere I. Regulation of intestinal calcium transport. Annu Rev Nutr. 2008;28:179–196. doi: 10.1146/annurev.nutr.010308.161202. [DOI] [PubMed] [Google Scholar]
- 72.Worcester EM, Coe FL. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol. 2008;28:120–132. doi: 10.1016/j.semnephrol.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Insogna KL, Broadus AE, Dreyer BE, Ellison AF, Gertner JM. Elevated production rate of 1,25-dihydroxyvitamin D in patients with absorptive hypercalciuria. J Clin Endocrinol Metab. 1985;61:490–495. doi: 10.1210/jcem-61-3-490. [DOI] [PubMed] [Google Scholar]
- 74.Bushinsky DA, Frick KK, Nehrke K. Genetic hypercalciuric stone-forming rats. Curr Opin Nephrol Hypertens. 2006;15:403–418. doi: 10.1097/01.mnh.0000232881.35469.a9. [DOI] [PubMed] [Google Scholar]
- 75.Worcester EM, et al. Evidence that postprandial reduction of renal calcium reabsorption mediates hypercalciuria of patients with calcium nephrolithiasis. Am J Physiol Renal Physiol. 2007;292:F66–F75. doi: 10.1152/ajprenal.00115.2006. [DOI] [PubMed] [Google Scholar]
- 76.Bergsland KJ, Coe FL, Gillen DL, Worcester EM. A test of the hypothesis that the collecting duct calcium-sensing receptor limits rise of urine calcium molarity in hypercalciuric calcium kidney stone formers. Am J Physiol Renal Physiol. 2009;297:F1017–F1023. doi: 10.1152/ajprenal.00223.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Worcester EM, Bergsland KJ, Gillen DL, Coe FL. Evidence for increased renal tubule and parathyroid gland sensitivity to serum calcium in human idiopathic hypercalciuria. Am J Physiol Renal Physiol. 2013;305:F853–F860. doi: 10.1152/ajprenal.00124.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Worcester EM, et al. Evidence for increased postprandial distal nephron calcium delivery in hypercalciuric stone-forming patients. Am J Physiol Renal Physiol. 2008;295:F1286–F1294. doi: 10.1152/ajprenal.90404.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Worcester EM, et al. Evidence for increased postprandial distal nephron calcium delivery in hypercalciuric stone-forming patients. Am J Physiol Renal Physiol. 2008;295:F1286–F1294. doi: 10.1152/ajprenal.90404.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, F.a.N.B.I.o.M. Dietary Reference Intakes for Calcium and Vitamin D. National Academy Press; Washington, DC: 2010. [Google Scholar]
- 81.Coe FL, et al. Effects of low-calcium diet on urine calcium excretion, parathyroid function and serum 1,25(OH)2D3 levels in patients with idiopathic hypercalciuria and in normal subjects. Am J Med. 1982;72:25–32. doi: 10.1016/0002-9343(82)90567-8. [DOI] [PubMed] [Google Scholar]
- 82.Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD. Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:659–667. doi: 10.7326/M13-2908. [DOI] [PubMed] [Google Scholar]
- 83.Pak CYC, Sakhaee K, Crowther C, Brinkley L. Evidence Justifying a High Fluid Intake in Treatment of Nephrolithiaisis. Annals of Internal Medicine. 1980;93:36–39. doi: 10.7326/0003-4819-93-1-36. [DOI] [PubMed] [Google Scholar]
- 84.Breslau NA, Mcguire JL, Zerwekh JE, Pak CYC. The role of dietary sodium on renal excretion and intstinal absorption of calicum and on vitamin D metabolism. J Clin Endocrinol Metab. 1982;55:369–373. doi: 10.1210/jcem-55-2-369. [DOI] [PubMed] [Google Scholar]
- 85.Sakhaee K, Harvey JA, Padalino P, Whitson P, Pak CYC. The Potential Role of Salt Abuse on the Risk for Kidney Stone Formation. Journal of Urology. 1993;150:310–312. doi: 10.1016/s0022-5347(17)35468-x. [DOI] [PubMed] [Google Scholar]
- 86.McCarron DA, et al. Urinary Calcium Excretion at Extremes of Sodium Intake in Normal Man. Am J Nephrol. 1981;1:84–90. doi: 10.1159/000166496. [DOI] [PubMed] [Google Scholar]
- 87.Phillips MJ, Cooke JNC. Relation between urinary calcium and sodium in patients with idiopathic hypercalciuria. Lancet. 1967;1:1354–1357. doi: 10.1016/s0140-6736(67)91763-1. [DOI] [PubMed] [Google Scholar]
- 88.Teucher B, et al. Sodium and bone health: impact of moderately high and low salt intakes on calcium metabolism in postmenopausal women. J Bone Miner Res. 2008;23:1477–1485. doi: 10.1359/jbmr.080408. [DOI] [PubMed] [Google Scholar]
- 89.McParland BE, Goulding A, Campbell AJ. Dietary salt affects biochemical markers of resorption and formation of bone in elderly women. BMJ. 1989;299:834–835. doi: 10.1136/bmj.299.6703.834-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frings-Meuthen P, Baecker N, Heer M. Low-grade metabolic acidosis may be the cause of sodium chloride-induced exaggerated bone resorption. J Bone Miner Res. 2008;23:517–524. doi: 10.1359/jbmr.071118. [DOI] [PubMed] [Google Scholar]
- 91.Nascimento L, Oliveros FH, Cunningham E. Renal handling of sodium and calcium in hypercalciuria. Clin Pharmacol Ther. 1984;35:342–347. doi: 10.1038/clpt.1984.41. [DOI] [PubMed] [Google Scholar]
- 92.Pak CY, et al. Effect of dietary modification on urinary stone risk factors. Kidney Int. 2005;68:2264–2273. doi: 10.1111/j.1523-1755.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- 93.Muldowney FP, Freaney R, KMoloney MF. Importance of Dietary Sodium in the Hypercalciuria Syndrome. Kidney Int. 1982;22:292–296. doi: 10.1038/ki.1982.168. [DOI] [PubMed] [Google Scholar]
- 94.Silver J, Rubinger D, Friedlaender MM, Popovtzer MM. Sodium-dependent idiopathic hypercalciuria in renal-stone formers. Lancet. 1983;2:484–486. doi: 10.1016/s0140-6736(83)90513-5. [DOI] [PubMed] [Google Scholar]
- 95.Nouvenne A, et al. Diet to reduce mild hyperoxaluria in patients with idiopathic calcium oxalate stone formation: a pilot study. Urol. 2009;73:725–30. 730. doi: 10.1016/j.urology.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 96.Nouvenne A, et al. Effects of a low-salt diet on idiopathic hypercalciuria in calcium-oxalate stone formers: a 3-mo randomized controlled trial. Am J Clin Nutr. 2010;91:565–570. doi: 10.3945/ajcn.2009.28614. [DOI] [PubMed] [Google Scholar]
- 97.Burtis WJ, Gay L, Insogna KL, Ellison A, Broadus AE. Dietary hypercalciuria in patients with calcium oxalate kidney stones. Am J Clin Nutr. 1994;60:424–429. doi: 10.1093/ajcn/60.3.424. [DOI] [PubMed] [Google Scholar]
- 98.Blackwood AM, Sagnella GA, Cook DG, Cappuccio FP. Urinary calcium excretion, sodium intake and blood pressure in a multi-ethnic population: results of the Wandsworth Heart and Stroke Study. J Hum Hypertens. 2001;15:229–237. doi: 10.1038/sj.jhh.1001171. [DOI] [PubMed] [Google Scholar]
- 99.Taylor EN, Curhan GC. Demographic, dietary, and urinary factors and 24-h urinary calcium excretion. Clin J Am Soc Nephrol. 2009;4:1980–1987. doi: 10.2215/CJN.02620409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Damasio PC, et al. The role of salt abuse on risk for hypercalciuria. Nutr J. 2011;10:3. doi: 10.1186/1475-2891-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Borghi L, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 102.U.S. Department of Agriculture and U.S. Department of Health and HumanServices. Dietary Guidelines for Americans. U.S. Government Printing Office; Washington, DC: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yendt ER, Gagne RJA, Cohanim M. The Effects of Thiazides in Idiopathic Hypercalciuria. Am J Med Sci. 1966;261:449–460. doi: 10.1097/00000441-196604000-00009. [DOI] [PubMed] [Google Scholar]
- 104.Fink HA, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med. 2013;158:535–543. doi: 10.7326/0003-4819-158-7-201304020-00005. [DOI] [PubMed] [Google Scholar]
- 105.Laerum E, Larsen S. Thiazide Prophylaxis of Urolithiasis: A double-blind study in general practice. Acta Med Scand. 1984;215:383–389. [PubMed] [Google Scholar]
- 106.Ettinger B, Citron JT, Livermore B, Dolman LI. Chlorthalidone reduces calcium oxalate calculous recurrence but magnesium hydroxide does not. Journal of Urology. 1988;139:679–684. doi: 10.1016/s0022-5347(17)42599-7. [DOI] [PubMed] [Google Scholar]
- 107.Borghi L, Meschi T, Guerra A, Novarini A. Randomized prospective study of a nonthiazide diuretic, indapamide, in preventing calcium stone recurrences. J Cardiovascular Pharmacology. 1993;22(Suppl 6):S78–S86. [PubMed] [Google Scholar]
- 108.Bergsland KJ, Worcester EM, Coe FL. Role of proximal tubule in the hypocalciuric response to thiazide of patients with idiopathic hypercalciuria. Am J Physiol Renal Physiol. 2013;305:F592–F599. doi: 10.1152/ajprenal.00116.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coe FL, Parks JH, Bushinsky DA, Langman CB, Favus MJ. Chlorthalidone promotes mineral retention in patients with idiopathic hypercalciuria. Kidney Int. 1988;33:1140–1146. doi: 10.1038/ki.1988.122. [DOI] [PubMed] [Google Scholar]
- 110.Bolland MJ, et al. The effect of treatment with a thiazide diuretic for 4 years on bone density in normal postmenopausal women. Osteoporos Int. 2007;18:479–486. doi: 10.1007/s00198-006-0259-y. [DOI] [PubMed] [Google Scholar]
- 111.Aung K, Htay T. Thiazide diuretics and the risk of hip fracture. Cochrane Database Syst Rev. 2011:CD005185. doi: 10.1002/14651858.CD005185.pub2. [DOI] [PubMed] [Google Scholar]
- 112.Caudarella R, Vescini F, Buffa A, Stefoni S. Citrate and mineral metabolism: kidney stones and bone disease. Front Biosci. 2003;8:s1084–s1106. doi: 10.2741/1119. [DOI] [PubMed] [Google Scholar]
- 113.Curthoys NP, Moe OW. Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol. 2014;9:1627–1638. doi: 10.2215/CJN.10391012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Halperin ML, Cheema DS, Kamel KS. Physiology of acid-base balance: links with kidney stone prevention. Semin Nephrol. 2006;26:441–446. doi: 10.1016/j.semnephrol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 115.Pinheiro VB, Baxmann AC, Tiselius HG, Heilberg IP. The effect of sodium bicarbonate upon urinary citrate excretion in calcium stone formers. Urol. 2013;82:33–37. doi: 10.1016/j.urology.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 116.Moseley KF, Weaver CM, Appel L, Sebastian A, Sellmeyer DE. Potassium citrate supplementation results in sustained improvement in calcium balance in older men and women. J Bone Miner Res. 2013;28:497–504. doi: 10.1002/jbmr.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Menegon LF, Figueiredo JF, Gontijo JA. Effect of metabolic acidosis on renal tubular sodium handling in rats as determined by lithium clearance. Braz J Med Biol Res. 1998;31:1269–1273. doi: 10.1590/s0100-879x1998001000006. [DOI] [PubMed] [Google Scholar]
- 118.Safirstein R, Glassman VP, DiScala VA. Effects of an NH4Cl-induced metabolic acidosis on salt and water reabsorption in dog kidney. Am J Physiol. 1973;225:805–809. doi: 10.1152/ajplegacy.1973.225.4.805. [DOI] [PubMed] [Google Scholar]
- 119.Wang T, Egbert AL, Jr, Aronson PS, Giebisch G. Effect of metabolic acidosis on NaCl transport in the proximal tubule. Am J Physiol. 1998;274:F1015–F1019. doi: 10.1152/ajprenal.1998.274.6.F1015. [DOI] [PubMed] [Google Scholar]
- 120.Balkovetz DF, Chumley P, Amlal H. Downregulation of claudin-2 expression in renal epithelial cells by metabolic acidosis. Am J Physiol Renal Physiol. 2009;297:F604–F611. doi: 10.1152/ajprenal.00043.2009. [DOI] [PubMed] [Google Scholar]
- 121.Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak CYC. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. Journal of Urology. 1993;150:1761–1764. doi: 10.1016/s0022-5347(17)35888-3. [DOI] [PubMed] [Google Scholar]
- 122.Ettinger B, et al. Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. Journal of Urology. 1997;158(6):2069–2073. doi: 10.1016/s0022-5347(01)68155-2. [DOI] [PubMed] [Google Scholar]
- 123.Hofbauer J, Hobarth K, Szabo N, Marberger M. Alkali citrate prophylaxis in idiopathic recurrent calcium oxalate urolithiasis--a prospective randomized study. Br J Urol. 1994;73:362–365. doi: 10.1111/j.1464-410x.1994.tb07597.x. [DOI] [PubMed] [Google Scholar]
- 124.Soygur T, Akbay A, Kupeli S. Effect of potassium citrate therapy on stone recurrence and residual fragments after shockwave lithotripsy in lower caliceal calcium oxalate urolithiasis: a randomized controlled trial. J Endourol. 2002;16:149–152. doi: 10.1089/089277902753716098. [DOI] [PubMed] [Google Scholar]
- 125.Lojanapiwat B, et al. Alkaline citrate reduces stone recurrence and regrowth after shockwave lithotripsy and percutaneous nephrolithotomy. Int Braz J Urol. 2011;37:611–616. doi: 10.1590/s1677-55382011000500007. [DOI] [PubMed] [Google Scholar]
- 126.Johnsson MS, Nancollas GH. The role of brushite and octacalcium phosphate in apatite formation. Crit Rev Oral Biol Med. 1992;3:61–82. doi: 10.1177/10454411920030010601. [DOI] [PubMed] [Google Scholar]