Abstract
Background
In spite of its invasive nature and risks, kidney biopsy is currently required for precise diagnosis of many chronic kidney diseases (CKDs). Here, we explored the hypothesis that analysis of the urinary proteome can discriminate different types of CKD irrespective of the underlying mechanism of disease.
Methods
We used data from the proteome analyses of 1180 urine samples from patients with different types of CKD, generated by capillary electrophoresis coupled to mass spectrometry. A set of 706 samples served as the discovery cohort, and 474 samples were used for independent validation. For each CKD type, peptide biomarkers were defined using statistical analysis adjusted for multiple testing. Potential biomarkers of statistical significance were combined in support vector machine (SVM)-based classifiers.
Results
For seven different types of CKD, several potential urinary biomarker peptides (ranging from 116 to 619 peptides) were defined and combined into SVM-based classifiers specific for each CKD. These classifiers were validated in an independent cohort and showed good to excellent accuracy for discrimination of one CKD type from the others (area under the receiver operating characteristic curve ranged from 0.77 to 0.95). Sequence analysis of the biomarkers provided further information that may clarify the underlying pathophysiology.
Conclusions
Our data indicate that urinary proteome analysis has the potential to identify various types of CKD defined by pathological assessment of renal biopsies and current clinical practice in general. Moreover, these approaches may provide information to model molecular changes per CKD.
Keywords: biomarkers, chronic kidney disease, peptides, proteome analysis, urine
INTRODUCTION
The prevalence of chronic kidney disease (CKD), defined as structural kidney damage or significant loss of glomerular filtration rate (GFR) (<60 mL/min/1.73 m2 for at least 3 months) [1], is estimated to be 8–16% worldwide, with an increasing trend [2, 3]. Therefore, CKD is now recognized as a global public health problem. The frequencies of the various types of CKD vary between countries, likely due to differences in genetically determined mechanisms of disease, environmental influences and criteria for performance of a kidney biopsy. A correct assessment of a CKD patient requires a precise diagnosis to guide the most appropriate treatment.
The diagnostic workup comprises assessment of clinical features (e.g. nephritic syndrome, isolated hematuria, rapidly progressive glomerulonephritis), histological findings [e.g. IgA nephropathy (IgAN)], biological mechanisms (e.g. hemolytic uremic syndrome) and possibly genetic factors (e.g. mutation in Col4a5). However, kidney biopsy is usually not applied to diagnose CKD in patients with diabetes and isolated hypertension as it is an invasive procedure with inherent risk and likely to provide no additional information for the clinical management [4]. As a consequence, misdiagnosis may occur. As an example, the existence of hypertensive nephropathy (nephrosclerosis) has been called into question [5]. With the exception of diabetes-associated CKD, the determination of the cause of renal disease is necessary and becomes more challenging. A variety of diagnostic tests may be pursued to refine the clinical diagnosis, with biopsy remaining the gold standard to assess diagnostic and prognostic histological features. However, kidney biopsy is an invasive procedure, and its diagnostic accuracy is sometimes limited [6]. Moreover, characterization of the urinary proteome may provide useful information about response to treatment. Recommendations for development of biomarkers using proteomics applicable for clinical care have recently been published [7, 8].
We have demonstrated before that analysis of the urinary proteome using capillary electrophoresis coupled to mass spectrometry (CE-MS) enables discrimination between patients with and without CKD [9], as well as prediction of progression of CKD, irrespective of the underlying disease mechanism [10]. The CE-MS technology allows the analysis of naturally occurring peptides (without tryptic digestion). This approach is often also called peptidomics, which is a subfield of proteomics. Using CE-MS, specific biomarkers for different types of CKD, such as ANCA-associated vasculitis, IgAN and diabetic nephropathy (DN), were defined [11–13]. In the present study, we assessed the value of the urinary proteome, as defined by CE-MS analysis, for the noninvasive discrimination of various types of CKD. Our results support the presence of urinary peptides with discriminatory power for different types of CKD. If the findings are validated in additional studies, such an approach to define CKD based on urinary profiles may be especially helpful at early stages of clinical disease, when a biopsy is not feasible due to small kidneys or comorbidities, or if a patient declines biopsy.
MATERIALS AND METHODS
Patient cohort
Urinary proteome datasets were extracted from the Human Urinary Proteome database [14–16], which currently includes data from analysis of more than 35 000 urine samples. All datasets of patients with CKD were selected irrespective of the diagnosis. However, if the specific CKD was represented by <30 datasets, patients with this type of CKD and the corresponding datasets were excluded. Following this selection procedure, 1180 datasets of patients representing eight major causes of CKD were extracted.
The study adhered to the regulations on the protection of individuals participating in medical research and was performed in accordance with the principles of the Declaration of Helsinki. All datasets had been anonymized. The study was approved by the local ethics committee in Hanover (No. 3096-2016).
The diagnoses were biopsy-proven except for DN and hypertensive nephrosclerosis (N). The patients from the cohort had been diagnosed at 32 different centers; the type of CKD had been established according to local clinical care and the best adherence to international guidelines. The patient cohorts have been described in greater detail in prior studies [9–12, 17–25] and included patients with primary focal segmental glomerulosclerosis (FSGS, n = 110), IgAN (n = 179), minimal-change disease (MCD, n = 35), membranous nephropathy (MN, n = 77), DN (n = 422) and N (n = 154), lupus nephritis (LN, n = 92, WHO Classes II, III and IV) and vasculitis-induced kidney disease (vasculitis, n = 111). A subset of the total dataset of 706 samples was used for discovery, and a separate group of 474 samples was used for validation.
Biomarker selection and classifier generation
For the definition of specific biomarkers for different types of CKD, peptides detected with a frequency of >30% in at least one of the groups were included. For statistical analysis, the non-parametric Wilcoxon test (R-based statistic software, version 2.15.3) was used. The calculated P-values were corrected using the false-discovery rate procedure introduced by Benjamini and Hochberg [26]. An adjusted P-value of 0.05 was set as the significance level. Potential biomarkers of statistical significance were combined in support vector machine (SVM)-based classifiers [27].
The sensitivity, specificity and area under the receiver operating characteristic (ROC) curve (AUC) of the generated classifiers were calculated using MedCalc version 12.7.5.0 (MedCalc Software bvba, Ostend, Belgium).
RESULTS
The demographic patient data for the discovery cohort are given in Table 1. For biomarker definition in the discovery cohort, data for each CKD type were compared with that for all other CKDs in the cohort. Only peptides that remained significant after correction for multiple testing were retained. Using this approach, 287 disease-specific biomarkers were defined for FSGS, 291 for MCD, 311 for MN, 172 for LN, 509 for renal vasculitis and 116 for IgAN. Data for DN and N (DN&N) were pooled as these diseases were not typically diagnosed by biopsy; the patients do not receive cytotoxic or immunological agents but are treated to maintain blood pressure and glycemic control. In addition, the initial proteomics experiments for urine samples from patients with DN&N showed very similar results. Comparison of DN&N with the other CKDs resulted in the definition of a total of 619 DN&N-specific biomarkers. The biomarker candidates for all of the CKDs are listed in Supplementary data, Table S1.
Table 1.
Distribution of samples between the discovery and validation sets
| Disease | Discovery set (n = 706) |
Validation set (n = 474) |
||||||
|---|---|---|---|---|---|---|---|---|
| Sample number | Gender (% male) | Age (years) | eGFR (mL/min/1.73 m2) | Sample number | Gender (% male) | Age (years) | eGFR (mL/min/1.73 m2) | |
| FSGS | 79 | 62 | 41.3 ± 21.8 | 45.1 ± 26.7 | 31 | 55 | 29.1 ± 23.2 | 46.9 ± 32.7 |
| DN&N | 288 | 66 | 65.4 ± 13.8 | 40.0 ± 22.9 | 288 | 57 | 64.7 ± 10.7 | 55.6 ± 22.8 |
| IgAN | 122 | 65 | 42.6 ± 16.0 | 50.8 ± 29.8 | 57 | 63 | 37.0 ± 14.2 | 94.7 ± 30.0 |
| MCD | 25 | 72 | 35.1 ± 15.2 | 85.8 ± 35.9 | 10 | 40 | 45.7 ± 23.2 | 103.4 ± 53.9 |
| MN | 55 | 74 | 52.0 ± 15.2 | 68.5 ± 32.4 | 22 | 67 | 50.9 ± 16.4 | 89.6 ± 22.3 |
| LN | 63 | 17 | 39.8 ± 12.6 | 57.1 ± 23.5 | 29 | 13 | 35.6 ± 13.4 | 99.3 ± 17.6 |
| Vasculitis-induced kidney disease | 74 | 58 | 64.5 ± 10.3 | 41.3 ± 22.4 | 37 | 44 | 58.8 ± 14.6 | 70.2 ± 13.7 |
The ‘±’ values indicate standard deviation.
These discriminatory peptides for each type of CKD were compared with diagnostic peptide biomarkers for these diseases reported in earlier studies: specifically, a comparison was made to the previously identified CKD273 classifier that identifies CKD patients irrespective of diagnosis [9]. As shown (Supplementary data, Figure S1), 30% of the peptides overlapped between the CKD273 classifier and the DN&N-specific classifier. The CKD273 classifier includes peptides that overlap with peptide biomarkers of all seven types of CKD (Supplementary data, Figure S1), as expected for a classifier that identifies any type of CKD. A comparison of the peptides specific for the discrimination of vasculitis with those of a previous study [11] resulted in an overlap of 60%. Of the peptides specific for IgAN, 12% were also reported in a previous study by Julian et al. [13], and of the earlier reported DN biomarkers [12], 22% were identical to the DN&N-specific peptides in the present study.
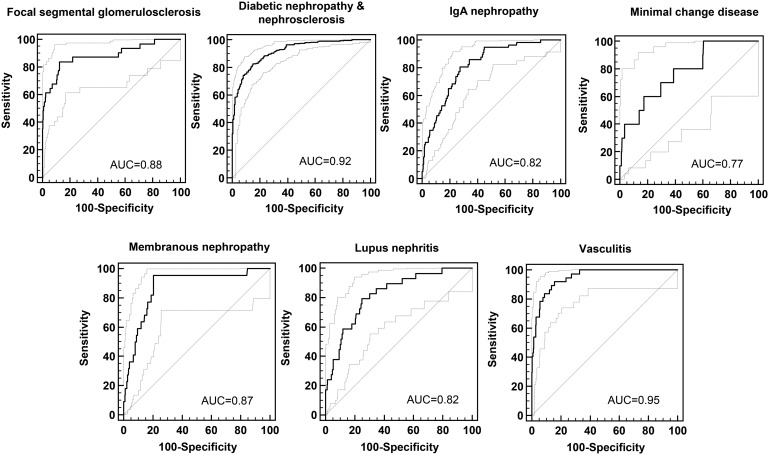
Next, we combined the defined biomarkers for each CKD into a disease-specific classifier, using an SVM algorithm. For the generation of the classifiers, only datasets of the discovery cohort were used. These seven different classifiers were applied to the datasets of the independent validation cohort (n = 474, Table 1), in each case comparing a single CKD with all other types of CKD. The AUCs of the ROC analyses of the different classifiers ranged from 0.77 to 0.95 (Table 2 and Figure 1). The highest AUCs were achieved in the diagnosis of DN&N and vasculitis (above 0.90), whereas the classifier for MCD had the lowest AUC (0.77).
Table 2.
Number of defined specific biomarkers and AUC in the validation set for each type of CKD
| Disease | Number of biomarkers (with sequencing information) | AUC (95% CI) |
|---|---|---|
| FSGS | 287 (107) | 0.88 (0.80–0.96) |
| DN&N | 619 (248) | 0.92 (0.89–0.94) |
| IgAN | 116 (71) | 0.82 (0.76–0.87) |
| MCD | 291 (121) | 0.77 (0.63–0.92) |
| MN | 311 (107) | 0.87 (0.80–0.95) |
| LN | 172 (70) | 0.82 (0.75–0.90) |
| Vasculitis-induced kidney disease | 509 (203) | 0.95 (0.92–0.98) |
FIGURE 1.
ROC analysis of the classification results for each developed classifier applied to independent samples from the validation set, for each type of CKD.
Amino acid sequences were obtained for 38% of the defined biomarkers (from 34.4% of the MN-specific biomarkers to 61.2% of the IgAN-specific biomarkers) (Supplementary data, Table S1). In total, 487 of the defined biomarkers were sequenced, 327 (67%) of which were significantly increased or decreased for a single CKD. Because of the low number (17%) of male patients in the LN group compared with the other groups, the 70 sequenced biomarkers were compared with previously defined gender-specific peptides [28] to rule out the possibility that the identified biomarkers for LN were female gender specific. An overlap was observed for only three collagen fragments, which therefore were excluded from further analysis.
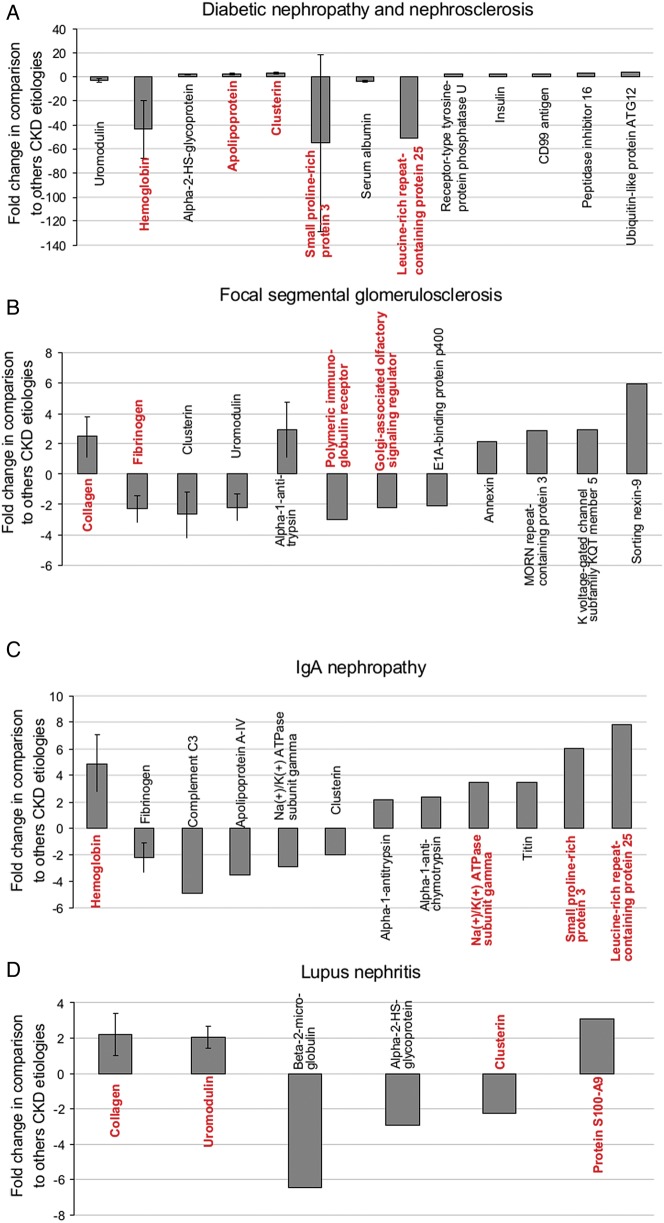
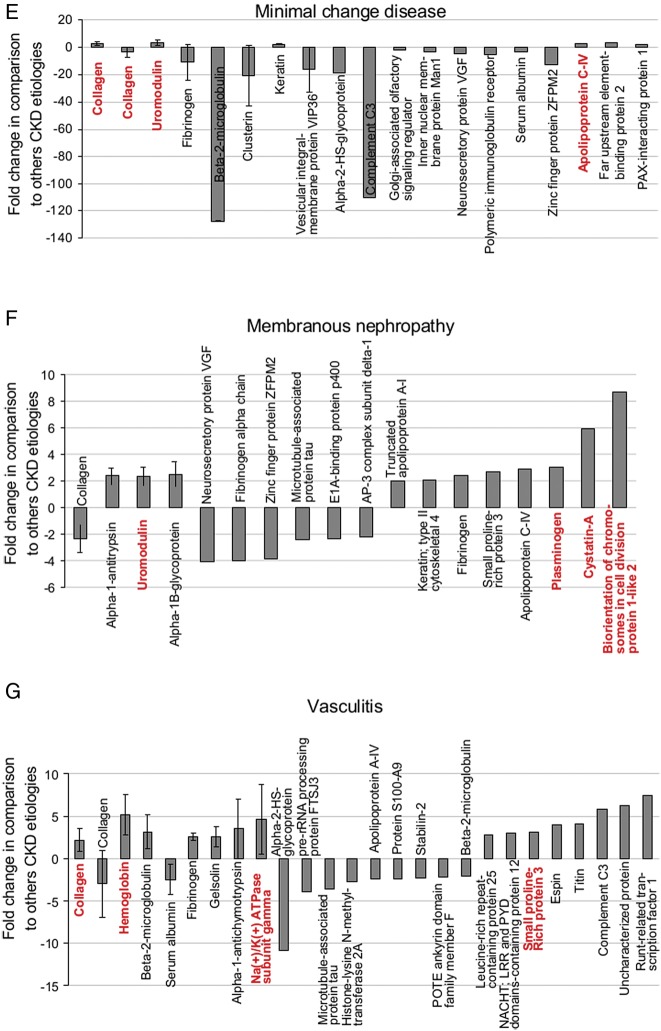
Next, we assessed the abundance of the sequenced markers for each CKD in more detail, using data from the discovery cohort. First, we investigated the abundance of individual peptides across all causes of CKD. To ease comparison, all fragments of the same protein with the same regulation direction (either increased or decreased abundance in one CKD versus the others) were combined. Using the signal intensity (amplitudes) of the individual biomarker peptides, a mean fold change for the respective protein and disease was calculated. Only proteins with a fold change of at least 2 were considered. The proteins for each CKD type that met these criteria are listed in Table 3, and the data are depicted in Figure 2 (the four lowest adjusted P-values are in red).
Table 3.
Proteins with a minimal fold change of 2 for each type of CKD in comparison with other types of CKD
| Protein name | Number of protein fragments | P-value (adjusted) | Mean fold change |
|---|---|---|---|
| DN&N | |||
| Uromodulin | 11 | 2.33E-08 | −2.71 (±1.40) |
| Hemoglobins | 5 | <0.1E-13 | −43.53 (±23.86) |
| Alpha-2-HS-glycoprotein | 4 | 1.33E-06 | 2.16 (±0.49) |
| Apolipoprotein | 3 | 8.57E-12 | 2.67 (±0.88) |
| Clusterin | 3 | 8.59E-12 | 3.32 (±1.06) |
| Small proline-rich protein 3 | 2 | <0.1E-13 | −54.96 (±73.57) |
| Serum albumin | 2 | 6.19E-03 | −3.68 (±0.51) |
| Leucine-rich repeat-containing protein 25 | 1 | <0.1E-13 | −50.80 |
| Receptor-type tyrosine-protein phosphatase U | 1 | 8.68E-03 | 2.24 |
| Insulin | 1 | 3.96E-03 | 2.54 |
| CD99 antigen | 1 | 4.00E-09 | 2.69 |
| Peptidase inhibitor 16 | 1 | 4.06E-09 | 3.06 |
| Ubiquitin-like protein ATG12 | 1 | 1.65E-10 | 4.13 |
| Focal segmental glomerulosclerosis | |||
| Collagens | 41 | 3.91E-05 | 2.47 (±1.33) |
| Fibrinogens | 5 | 2.09E-04 | −2.31 (±0.88) |
| Clusterin | 2 | 2.85E-02 | −2.67 (±1.52) |
| Uromodulin | 2 | 1.25E-02 | −2.19 (±0.88) |
| Alpha-1-antitrypsin | 2 | 4.91E-03 | 2.92 (±1.81) |
| Polymeric immunoglobulin receptor | 1 | 1.14E-03 | −2.97 |
| Golgi-associated olfactory signaling regulator | 1 | 1.49E-04 | −2.23 |
| E1A-binding protein p400 | 1 | 2.76E-03 | −2.09 |
| Annexin | 1 | 1.78E-02 | 2.11 |
| MORN repeat-containing protein 3 | 1 | 2.16E-03 | 2.90 |
| Potassium voltage-gated channel subfamily KQT member 5 | 1 | 2.37E-02 | 2.96 |
| Sorting nexin-9 | 1 | 3.64E-02 | 5.96 |
| IgAN | |||
| Hemoglobins | 20 | 2.72E-12 | 4.91 (±2.15) |
| Fibrinogens | 2 | 2.78E-02 | −2.19 (±2.10) |
| Complement C3 | 1 | 7.43E-03 | −4.87 |
| Apolipoprotein A-IV | 1 | 3.37E-02 | −3.53 |
| Sodium/potassium-transporting ATPase subunit gamma | 1 | 3.50E-02 | −2.86 |
| Clusterin | 1 | 2.94E-02 | −2.00 |
| Alpha-1-antitrypsin | 1 | 1.96E-02 | 2.20 |
| Alpha-1-antichymotrypsin | 1 | 3.99E-02 | 2.40 |
| Sodium/potassium-transporting ATPase subunit gamma | 1 | 1.82E-06 | 3.46 |
| Titin | 1 | 8.44E-06 | 3.51 |
| Small proline-rich protein 3 | 1 | 6.50E-12 | 6.04 |
| Leucine-rich repeat-containing protein 25 | 1 | 4.06E-09 | 7.80 |
| LN | |||
| Collagens | 55 | 2.14E-11 | 2.21 (±1.22) |
| Uromodulin | 4 | 7.44E-04 | 2.08 (±0.63) |
| Protein S100-A9 | 1 | 8.65E-04 | 3.07 |
| Clusterin | 1 | 3.13E-02 | −2.25 |
| Beta-2-microglobulin | 1 | 3.26E-02 | −6.45 |
| Alpha-2-HS-glycoprotein | 1 | 3.42E-02 | −2.93 |
| MCD | |||
| Collagens | 73 | 1.11E-05 | 2.53 (±1.15) |
| Collagens | 15 | 2.59E-04 | −3.76 (±3.56) |
| Uromodulin | 10 | 2.17E-05 | 3.46 (±2.01) |
| Fibrinogen alpha chain | 4 | 7.74E-04 | −10.94 (±13.24) |
| Beta-2-microglobulin | 2 | 6.63E-03 | −127.65 (±0.53) |
| Clusterin | 2 | 6.91E-03 | −20.83 (±22.14) |
| Keratin | 2 | 6.63E-03 | 2.43 (±0.28) |
| Vesicular integral-membrane protein VIP36 | 2 | 1.02E-02 | −16.39 (±16.92) |
| Alpha-2-HS-glycoprotein | 1 | 4.35E-02 | −18.17 |
| Complement C3 | 1 | 4.04E-02 | −110.40 |
| Golgi-associated olfactory signaling regulator | 1 | 4.85E-02 | −2.10 |
| Inner nuclear membrane protein Man1 | 1 | 3.21E-02 | −3.67 |
| Neurosecretory protein VGF | 1 | 1.55E-02 | −4.65 |
| Polymeric immunoglobulin receptor | 1 | 3.75E-03 | −4.99 |
| Serum albumin | 1 | 7.95E-03 | −3.21 |
| Zinc finger protein ZFPM2 | 1 | 1.36E-03 | −12.93 |
| Apolipoprotein C-IV | 1 | 4.45E-04 | 2.82 |
| Far upstream element-binding protein 2 | 1 | 9.95E-03 | 3.22 |
| PAX-interacting protein 1 | 1 | 1.73E-02 | 2.08 |
| MN | |||
| Collagens | 28 | 1.07E-04 | −2.31 (±1.01) |
| Uromodulin | 8 | 1.40E-05 | 2.35 (±0.70) |
| Alpha-1-antitrypsin | 8 | 1.94E-04 | 2.37 (±0.60) |
| Alpha-1B-glycoprotein | 4 | 1.73E-04 | 2.48 (±0.87) |
| Cystatin-A | 1 | 1.77E-11 | 5.91 |
| Biorientation of chromosomes in cell division protein 1 | 1 | 3.80E-09 | 8.72 |
| Plasminogen | 1 | 3.58E-05 | 3.03 |
| Neurosecretory protein VGF | 1 | 4.45E-05 | −4.11 |
| Small proline-rich protein 3 | 1 | 9.01E-05 | 2.69 |
| Fibrinogen beta chain | 1 | 7.43E-04 | 2.45 |
| E1A-binding protein p400 | 1 | 8.46E-04 | −2.30 |
| Microtubule-associated protein tau | 1 | 1.39E-03 | −2.41 |
| Apolipoprotein C-IV | 1 | 2.81E-03 | 2.90 |
| Fibrinogen alpha chain | 1 | 1.43E-02 | −4.06 |
| Zinc finger protein ZFPM2 | 1 | 1.43E-02 | −3.84 |
| Truncated apolipoprotein A-I | 1 | 1.43E-02 | 2.01 |
| Keratin; type II cytoskeletal 4 | 1 | 2.61E-02 | 2.10 |
| AP-3 complex subunit delta-1 | 1 | 2.79E-02 | −2.22 |
| Vasculitis-induced kidney disease | |||
| Collagens | 77 | 4.25E-11 | 2.16 (±1.35) |
| Collagens | 49 | 3.16E-09 | −2.96 (±3.95) |
| Hemoglobins | 22 | 1.99E-14 | 5.15 (±2.44) |
| Beta-2-microglobulin | 7 | 1.51E-07 | 3.17 (±1.96) |
| Fibrinogens | 3 | 1.78E-04 | 2.55 (±0.42) |
| Serum albumin | 3 | 1.85E-03 | −2.51 (±1.79) |
| Sodium/potassium-transporting ATPase subunit gamma | 2 | 2.83E-13 | 4.62 (±4.10) |
| Gelsolin | 2 | 1.60E-06 | 2.57 (±1.19) |
| Alpha-1-antichymotrypsin | 2 | 1.50E-04 | 3.56 (±3.51) |
| Small proline-rich protein 3 | 1 | 2.71E-10 | 3.12 |
| Runt-related transcription factor 1 | 1 | 1.41E-09 | 7.45 |
| Espin | 1 | 1.28E-08 | 4.03 |
| NACHT; LRR and PYD domains-containing protein 12 | 1 | 4.11E-08 | 2.97 |
| Titin | 1 | 1.15E-07 | 4.06 |
| Beta-2-microglobulin | 1 | 1.51E-07 | −2.12 |
| Stabilin-2 | 1 | 8.62E-05 | −2.29 |
| Microtubule-associated protein tau | 1 | 1.40E-04 | −3.64 |
| Alpha-2-HS-glycoprotein | 1 | 1.61E-04 | −10.84 |
| Apolipoprotein A-IV | 1 | 2.33E-04 | −2.43 |
| Leucine-rich repeat-containing protein 25 | 1 | 1.21E-03 | 2.74 |
| POTE ankyrin domain family member F | 1 | 1.63E-03 | −2.26 |
| pre-rRNA processing protein FTSJ3 | 1 | 3.17E-03 | −3.96 |
| Uncharacterized protein | 1 | 4.91E-03 | 6.27 |
| Complement C3 | 1 | 7.12E-03 | 5.78 |
| Protein S100-A9 | 1 | 1.64E-02 | −2.43 |
| Histone-lysine N-methyltransferase 2A | 1 | 2.42E-02 | −2.69 |
Data listed are the number of significant protein fragments for each protein, the lowest observed P-value and the estimated mean fold change (± standard deviation).
FIGURE 2.
Regulation of the specific proteins in individual types of CKD. The mean fold change and standard deviation (when more than one fragment was observed) of proteins with a mean fold change of >2 based on discovery data for the DN&N group (A), FSGS (B), IgAN (C), LN (D), MCD (E), MN (F) and vasculitis-induced kidney disease (G) are shown. The four proteins (except panel A where five proteins are marked because two proteins have equal P-values) for which the fragments had the lowest adjusted P-values are marked in red.
As shown for DN&N, a decrease of hemoglobin was observed in comparison to the findings for the other types of CKD. Five different hemoglobin peptide fragments were significantly decreased in the DN&N group displaying low adjusted P-values (<1.0 × 10−14, Figure 2A). In addition, a decrease of small proline-rich protein 3 and leucine-rich repeat-containing protein 25 and an increase of clusterin and apolipoprotein fragments (with equal P-values) were observed in DN&N (Figure 2A). For FSGS, increase of collagen fragments and decrease of fibrinogen, polymeric immunoglobulin receptor and Golgi-associated olfactory signaling regulator were observed (Figure 2B). In contrast to the DN&N group, for IgAN, the most characteristic feature was the increase of hemoglobin, leucine-rich repeat-containing protein 25 and small proline-rich protein fragments, while sodium/potassium-ATPase was also increased (Figure 2C). The lowest P-values for the LN cohort were observed for increased collagen, uromodulin and protein S100-A9 fragments, and for decrease of clusterin (Figure 2D). In the MCD group, the lowest P-values were observed for multiple increased (n = 73) and to a lesser extent decreased (n = 15) fragments of collagen, but also increased fragments of uromodulin, and apolipoprotein C-IV (Figure 2E and Table 3). Also, a strong decrease of two fragments of beta-2-microglobulin (mean fold change of −128.0) was observed. In the MN group (Figure 2F), the lowest P-values were calculated for increased fragments of cystatin-A, biorientation of chromosomes in cell division protein 1, uromodulin and plasminogen. In the vasculitis group, the lowest P-values were observed for the increased hemoglobin, sodium/potassium-ATPase, collagen and small proline-rich protein 3 fragments (Figure 2G).
When analyzing the data in such a way, multiple differences and similarities between the different types of CKD became apparent (Supplementary data, Table S1). For example, fragments of hemoglobin, small proline-rich protein 3 and leucine-rich repeat-containing protein 25 were strongly decreased (fold changes >40) with the lowest P-values in the DN&N group (P < 1 × 10−14), whereas the abundance of fragments of the same proteins was increased in the IgAN and vasculitis groups. Further similarities between CKD patients with vasculitis or IgAN were observed, including increases in abundance of 16 hemoglobin fragments with markedly low P-values (P = 2 × 10−14 and 2.7 × 10−12) and of peptides derived from titin, sodium/potassium-ATPase subunit gamma and alpha-1-antichymotrypsin. In contrast, the abundance of fibrinogen and complement C3 fragments showed different trends in IgAN and vasculitis samples. The amounts of fibrinogen and complement C3 fragments were decreased in the IgAN group and increased in the vasculitis group. Decreased abundance of fragments of fibrinogen and Golgi-associated olfactory signaling regulator was observed for MCD and FSGS. Increase in abundance of clusterin fragments was observed in the MCD, MN and LN groups.
DISCUSSION
CE-MS analysis has previously led to the identification of a multipeptide urinary classifier (CKD273) for the diagnosis of CKD irrespective of the underlying mechanism of disease [9, 10, 23, 29]. Additional studies have shown that CKD273 is superior to albuminuria in early prediction of CKD progression [10, 18, 22, 25]. Further studies using CE-MS urinary proteome analysis have also shown that biomarkers can display a response to treatment: in DN a response to angiotensin receptor blockers [30, 31] or in ANCA-associated vasculitis a response to immunosuppression [11]. In the study presented here, we have tested the hypothesis that urinary peptides hold information on disease etiology and can be employed to differentiate between distinct types of CKD. This hypothesis also dictated the study design: focusing on comparing one group with all other CKD types, without involving healthy controls. This also explains to some extent the varying degree of overlap (ranging from 12 to 60%) of the presented discriminatory peptides with previously reported biomarker findings for these diseases (11, 50–52; discussed also below). Interestingly, a substantial overlap between the peptides identified here and the CKD273 [32] classifier could be detected for all types of disease (Supplementary data, Figure S1), further supporting that the CKD273 classifier is a general classifier for the diagnosis of all CKD types.
The wider clinical use of urinary proteomics and the increasing body of knowledge showing that it is fulfilling its expectations as an informative tool in establishing diagnosis and prognosis, and also guiding the approach to treatment in patients with renal diseases, have brought some investigators to opposite opinions regarding the value of urinary proteomics versus kidney biopsy in clinical practice [33, 34]. Despite contrasting viewpoints expressed in these papers, Glassock [33] and Mischak [34] agreed that biomarker-based analyses should focus on the correct diagnosis of a specific kidney disease among patients with similar manifestations rather than the discrimination between CKD patients and healthy individuals [33]. The value of CE-MS-based proteomics to differentiate types of CKD etiologies has been shown, but mostly in small patient populations. Julian et al. demonstrated signatures specific to IgAN (n = 10 patients) compared with other renal diseases [13], Haubitz et al. were able to differentiate IgAN (n = 45 patients) from MN (n = 13 patients) and healthy controls [35], and Rossing et al. described specific urinary peptides enabling discrimination of DN (n = 44 patients) from other types of CKD [12]. Also, biomarkers specific for ANCA-associated vasculitis (n = 10 patients) have been identified [11]. Of note, many of the biomarker peptides identified in the present study for the diagnosis of specific types of CKD were also identified in these previous studies (between 12 and 60%), further validating the value of some of these urinary peptides as specific biomarkers. The present study was designed with the aim to identify specific urinary peptide markers for the main types of CKD, using datasets from a large cohort of 1180 individuals with CKD. These urinary biomarkers for each CKD type were combined into diagnostic classifiers that allowed separation of one CKD type from the others. The DN&N group was distinguished from all other causes of CKD with an AUC of 0.92. DN&N are typically diagnosed without biopsy in clinical practice, and the therapeutic approach is based on nonspecific approaches for nephroprotection [4]. The diagnosis, which is based on albuminuria and other microvascular complications, may not be correct [36]. Therefore, it is important to differentiate this diagnosis from other types of renal disease that currently are defined by biopsy and require more disease-specific therapeutic interventions. In cases for which the benefit of a biopsy may not outweigh the risk, the approach developed in this study could change the diagnostic strategy, by introducing a urinary proteome-based test before deciding whether to perform a biopsy. Our findings support performance of a prospective study of urine-based proteomics to improve the diagnostic strategy in kidney diseases.
Although the classifiers defined in this study are composites of multiple peptides specific for a single CKD type, similarities in the individual biomarkers between some CKDs were observed. Such information may be helpful to describe disease progression and development of kidney damage in each specific CKD type, on a molecular level, and, especially if complemented by supporting tissue data, also for the development of new therapeutic approaches potentially targeting several types of kidney disease.
Different clusterin fragments seem to play an important role in the discrimination between DN&N and other types of CKD. Three fragments of clusterin are increased in the DN&N group and appear to be specific. Chu et al. have shown that urinary levels of clusterin discriminated patients with DN from healthy controls [37]. In the FSGS, IgAN, LN and MCD groups, all clusterin fragments were decreased, with a mean fold change of >2. Schanstra et al. observed a negative correlation between the urinary level of clusterin fragments and estimated glomerular filtration rate (eGFR) [10]. This finding may reflect that more than 50% of the patients in that study had DN. Ghiggeri et al. analyzed levels of clusterin in serum and urine; in both specimen types, clusterin levels were reduced in active MN and FSGS and in children with nephrotic syndrome [38]. Also, a study of patients with systemic lupus erythematosus (SLE) showed low serum levels of clusterin, especially in those with proteinuria [39].
Also characteristic for the DN&N group is the lower levels of hemoglobin fragments compared with those in patients with other types of CKD. On the other hand, higher urinary levels of hemoglobin fragments were observed in the IgAN and vasculitis groups, as expected in patients with hematuria. Characteristically, higher urinary levels of hemoglobin fragments in vasculitis patients have been previously described by Haubitz et al. [11]. We observed similar patterns for hemoglobin fragments in patients with IgAN. More similarities in the profiles of IgAN and ANCA-associated groups in comparison with other types of CKD were observed. These findings may reflect that patients with severe IgAN frequently exhibit histological features similar to those of patients with ANCA-associated vasculitis, such as prominent glomerular inflammation that may include crescents [11, 40].
MCD and FSGS also showed similarities in their proteomic signatures. A decreased amount of fibrinogen appears to be common to both diseases. MCD and FSGS share some ultrastructural features, such as foot process effacement, yet their immunofluorescence features and pathophysiologies likely have important differences. MCD typically has no glomerular scarring or immunofluorescence staining, whereas FSGS has segmental glomerulosclerosis and positive IgM and C3 immunofluorescence [41]. MCD and FSGS are primary podocytopathies and thus may belong to the same clinical spectrum in which a more severe injury leads to loss of podocytes in FSGS. However, a renal biopsy early in the clinical course of patients with FSGS may not demonstrate the glomerular scarring typical of FSGS, leading to an incorrect diagnosis of MCD. Glucocorticoid therapy is much more effective for patients with MCD than for patients with FSGS [42]. Therefore, novel therapeutic approaches for patients presenting with nephrotic syndrome typical of MCD or FSGS would be welcome [43] as a reliable diagnostic tool. Our data confirmed some similarities between the urinary proteomic patterns for MCD and FSGS; nonetheless, we confirmed molecular differences (e.g. the strong down-regulation of beta-2-microglobulin in MCD or up-regulation of alpha-1-antitrypsin in FSGS [32, 44], Table 3). Future studies should explore whether the urinary proteome differentiates patients with glucocorticoid-responsive disease from those who are glucocorticoid resistant.
One of the most specific peptides that distinguished LN from other types of CKD was the S100-A9 fragment. This calcium- and zinc-binding protein plays a prominent role in the regulation of inflammatory processes and the immune response. Significantly higher levels of this protein as well as of S100-A8 in serum samples of SLE patients compared with healthy controls have been reported by Soyfoo et al. [45].
Collagen fragments have been reported as major constituents of diagnostic classifiers for a majority of CKD types (11, 44, 50–52, 66). Even though significant changes in abundance of many collagen fragments were observed, the main CKD type-specific associated fragments were not part of the collagen family (Table 3). This may be explained by the differences in the design of the present study (focusing on differences between patients with various types of CKD) versus the previous ones (focusing on differences between patients with CKD and healthy controls). Furthermore, the present study focused mostly on analysis of samples from patients at advanced stages of CKD (where biopsy data are typically available). In this regard, recent studies support that urinary collagen fragments are early biomarkers for CKD (in patients with GFR >60 mL/min/1.73 m2) and are not among the most prominent biomarkers in more advanced stages [46]. Collectively, these observations explain the modest biomarker value of collagen peptides in the present context of discriminating different CKD types.
The study presented here has certain shortcomings: first, the urine data/samples were selected retrospectively from previous studies. Second, the diagnosis of DN&N relied on clinical criteria rather than on a kidney biopsy, and the criteria differed somewhat between the clinical centers. However, this approach corresponds to the standard clinical practice, and it is fair to assume that only a minority of samples may be misclassified. It appears interesting to investigate if the use of the classifier provides information on whether the particular patient fits into the ‘DN&N’ standard or whether a renal biopsy should be recommended in search of other causes of CKD. However, this must be assessed in a separate study. In addition, data on treatment at the time of sampling as well as information on the disease class/subtype within each group of CKD patients (e.g. in some LN, the WHO class was not specified) were not always available. However, the large number of subjects in the study ensures that missing data in some patients should not have a significant impact on the interpretation of the results. There was a significant difference in the renal function between the patients in the discovery and the validation sets, with patients in the former having more advanced CKD. This design, however, ensures that the disease in the discovery phase would have sufficient impact on the selection of biomarkers, which may not be the case at an early stage of disease. The fact that the classifiers also performed well in patients with earlier stages of disease (as evident from the validation set) may be seen as a further strength of the approach.
The study also has several important strengths. Most importantly, the large number of subjects (1180) in the study allows for a comprehensive analysis of urine samples from patients with a wide range of manifestations of various types of CKD. The results were verified in an independent validation set, further supporting the weight of the findings. In addition, several of the identified biomarkers are associated with apparent mechanisms of disease.
In conclusion, urinary proteome analysis clearly differentiated various types of CKD. These findings indicate the value of these urine peptide-based signature panels in the differential diagnosis of certain types of CKD. They may also serve as an excellent basis for bioinformatic assessment of the different types of CKD, to understand molecular pathophysiology and identify the best-suited therapeutic targets [47, 48]. In contrast to kidney biopsy, urinary proteome analysis offers the possibility of being applied early in the course of the disease when the benefit of intervention is optimal and of being repeatable without any risk for the patient and, thus, can be used to monitor progression of disease and/or treatment response. A proteomic analysis of urine may be of immediate use to guide treatment of specific patients, especially at an early point of disease initiation and progression. A potential next step to be taken may be the assessment of the exact benefit of this approach in a multicenter randomized controlled trial.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org
Supplementary Material
ACKNOWLEDGEMENTS
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 279277 (PRIORITY) and grant agreement no. 608332 (iMODE-CKD).
A.O. was supported by Spanish Government ISCIII initiatives FEDER funds PI13/00047, REDinREN/RD012/0021, Programa Intensificación Actividad Investigadora. B.A.J. and J.N. have been supported in part by a grant from the National Institutes of Health DK078244 and by a gift from the IGA Nephropathy Foundation of America.
CONFLICT OF INTEREST
H.M. is cofounder and a shareholder of Mosaiques Diagnostics GmbH. J.S., C.P. and P.Z. are employees of Mosaiques Diagnostics GmbH.
REFERENCES
- 1. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–266 [PubMed] [Google Scholar]
- 2. Jha V, Garcia-Garcia G, Iseki K. et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382: 260–272 [DOI] [PubMed] [Google Scholar]
- 3. Eckardt KU, Coresh J, Devuyst O. et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 2013 [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez Suarez ML, Thomas DB, Barisoni L. et al. Diabetic nephropathy: is it time yet for routine kidney biopsy? World J Diabetes 2013; 4: 245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freedman BI, Sedor JR. Hypertension-associated kidney disease: perhaps no more. J Am Soc Nephrol 2008; 19: 2047–2051 [DOI] [PubMed] [Google Scholar]
- 6. Beck L, Bomback AS, Choi MJ. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis 2013; 62: 403–441 [DOI] [PubMed] [Google Scholar]
- 7. Mischak H, Apweiler R, Banks RE. et al. Clinical proteomics: a need to define the field and to begin to set adequate standards. Proteomics Clin Appl 2007; 1: 148–156 [DOI] [PubMed] [Google Scholar]
- 8. Mischak H, Allmaier G, Apweiler R. et al. Recommendations for biomarker identification and qualification in clinical proteomics. Sci Transl Med 2010; 2: 46ps42. [DOI] [PubMed] [Google Scholar]
- 9. Good DM, Zürbig P, Argiles A. et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics 2010; 9: 2424–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schanstra JP, Zurbig P, Alkhalaf A. et al. Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol 2015; 26: 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haubitz M, Good DM, Woywodt A. et al. Identification and validation of urinary biomarkers for differential diagnosis and evaluation of therapeutic intervention in anti-neutrophil cytoplasmic antibody-associated vasculitis. Mol Cell Proteomics 2009; 8: 2296–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rossing K, Mischak H, Dakna M. et al. Urinary proteomics in diabetes and CKD. J Am Soc Nephrol 2008; 19: 1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Julian BA, Wittke S, Novak J. et al. Electrophoretic methods for analysis of urinary polypeptides in IgA-associated renal diseases. Electrophoresis 2007; 28: 4469–4483 [DOI] [PubMed] [Google Scholar]
- 14. Coon JJ, Zürbig P, Dakna M. et al. CE-MS analysis of the human urinary proteome for biomarker discovery and disease diagnostics. Proteomics Clin Appl 2008; 2: 964–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siwy J, Mullen W, Golovko I. et al. Human urinary peptide database for multiple disease biomarker discovery. Proteomics Clin Appl 2011; 5: 367–374 [DOI] [PubMed] [Google Scholar]
- 16. Stalmach A, Albalat A, Mullen W. et al. Recent advances in capillary electrophoresis coupled to mass spectrometry for clinical proteomic applications. Electrophoresis 2013; 34: 1452–1464 [DOI] [PubMed] [Google Scholar]
- 17. Alkhalaf A, Zürbig P, Bakker SJ. et al. Multicentric validation of proteomic biomarkers in urine specific for diabetic nephropathy. PLoS One 2010; 5: e13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Argiles A, Siwy J, Duranton F. et al. CKD273, a new proteomics classifier assessing CKD and its prognosis. PLoS One 2013; 8: e62837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glorieux G, Mullen W, Duranton F. et al. New insights in molecular mechanisms involved in chronic kidney disease using high-resolution plasma proteome analysis. Nephrol Dial Transplant 2015; 30: 1842–1852 [DOI] [PubMed] [Google Scholar]
- 20. Julian BA, Wittke S, Haubitz M. et al. Urinary biomarkers of IgA nephropathy and other IgA-associated renal diseases. World J Urol 2007; 25: 467–476 [DOI] [PubMed] [Google Scholar]
- 21. Ovrehus MA, Zurbig P, Vikse BE. et al. Urinary proteomics in chronic kidney disease: diagnosis and risk of progression beyond albuminuria. Clin Proteomics 2015; 12: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roscioni SS, de Zeeuw D, Hellemons ME. et al. A urinary peptide biomarker set predicts worsening of albuminuria in type 2 diabetes mellitus. Diabetologia 2012; 56: 259–267 [DOI] [PubMed] [Google Scholar]
- 23. Siwy J, Schanstra JP, Argiles A. et al. Multicentre prospective validation of a urinary peptidome-based classifier for the diagnosis of type 2 diabetic nephropathy. Nephrol Dial Transplant 2014; 29: 1563–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snell-Bergeon JK, Maahs DM, Ogden LG. et al. Evaluation of urinary biomarkers for coronary artery disease, diabetes, and diabetic kidney disease. Diabetes Technol Ther 2009; 11: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zürbig P, Jerums G, Hovind P. et al. Urinary proteomics for early diagnosis in diabetic nephropathy. Diabetes 2012; 61: 3304–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B (Methodological) 1995; 57: 125–133 [Google Scholar]
- 27. Weissinger EM, Wittke S, Kaiser T. et al. Proteomic patterns established with capillary electrophoresis and mass spectrometry for diagnostic purposes. Kidney Int 2004; 65: 2426–2434 [DOI] [PubMed] [Google Scholar]
- 28. Dakna M, Harris K, Kalousis A. et al. Addressing the challenge of defining valid proteomic biomarkers and classifiers. BMC Bioinformatics 2010; 11: 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Molin L, Seraglia R, Lapolla A. et al. A comparison between MALDI-MS and CE-MS data for biomarker assessment in chronic kidney diseases. J Proteomics 2012; 75: 5888–5897 [DOI] [PubMed] [Google Scholar]
- 30. Andersen S, Mischak H, Zürbig P. et al. Urinary proteome analysis enables assessment of renoprotective treatment in type 2 diabetic patients with microalbuminuria. BMC Nephrol 2010; 11: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rossing K, Mischak H, Parving HH. et al. Impact of diabetic nephropathy and angiotensin II receptor blockade on urinary polypeptide patterns. Kidney Int 2005; 68: 193–205 [DOI] [PubMed] [Google Scholar]
- 32. Argiles A, Mourad G, Mion C. et al. Two-dimensional gel electrophoresis of urinary proteins in kidney diseases. Contrib Nephrol 1990; 83: 1–8 [DOI] [PubMed] [Google Scholar]
- 33. Glassock RJ. Con: Kidney biopsy: an irreplaceable tool for patient management in nephrology. Nephrol Dial Transplant 2015; 30: 528–531 [DOI] [PubMed] [Google Scholar]
- 34. Mischak H. Pro: Urine proteomics as a liquid kidney biopsy: no more kidney punctures! Nephrol Dial Transplant 2015; 30: 532–537 [DOI] [PubMed] [Google Scholar]
- 35. Haubitz M, Wittke S, Weissinger EM. et al. Urine protein patterns can serve as diagnostic tools in patients with IgA nephropathy. Kidney Int 2005; 67: 2313–2320 [DOI] [PubMed] [Google Scholar]
- 36. Fiorentino M, Bolignano D, Tesar V. et al. Renal biopsy in 2015 – from epidemiology to evidence-based indications. Am J Nephrol 2016; 43: 1–19 [DOI] [PubMed] [Google Scholar]
- 37. Chu L, Fu G, Meng Q. et al. Identification of urinary biomarkers for type 2 diabetes using bead-based proteomic approach. Diabetes Res Clin Pract 2013; 101: 187–193 [DOI] [PubMed] [Google Scholar]
- 38. Ghiggeri GM, Bruschi M, Candiano G. et al. Depletion of clusterin in renal diseases causing nephrotic syndrome. Kidney Int 2002; 62: 2184–2194 [DOI] [PubMed] [Google Scholar]
- 39. Newkirk MM, Apostolakos P, Neville C. et al. Systemic lupus erythematosus, a disease associated with low levels of clusterin/apoJ, an antiinflammatory protein. J Rheumatol 1999; 26: 597–603 [PubMed] [Google Scholar]
- 40. Moreno JA, Martin-Cleary C, Gutierrez E. et al. AKI associated with macroscopic glomerular hematuria: clinical and pathophysiologic consequences. Clin J Am Soc Nephrol 2012; 7: 175–184 [DOI] [PubMed] [Google Scholar]
- 41. Fogo AB. Minimal change disease and focal segmental glomerulosclerosis. Nephrol Dial Transplant 2001; 16(Suppl 6): 74–76 [DOI] [PubMed] [Google Scholar]
- 42. Moura LR, Franco MF, Kirsztajn GM. Minimal change disease and focal segmental glomerulosclerosis in adults: response to steroids and risk of renal failure. J Bras Nefrol 2015; 37: 475–480 [DOI] [PubMed] [Google Scholar]
- 43. Malaga-Dieguez L, Bouhassira D, Gipson D. et al. Novel therapies for FSGS: preclinical and clinical studies. Adv Chronic Kidney Dis 2015; 22: e1–e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith A, L'Imperio V, De SG. et al. alpha-1-Antitrypsin detected by MALDI imaging in the study of glomerulonephritis: its relevance in chronic kidney disease progression. Proteomics 2016; 16: 1759–1766 [DOI] [PubMed] [Google Scholar]
- 45. Soyfoo MS, Roth J, Vogl T. et al. Phagocyte-specific S100A8/A9 protein levels during disease exacerbations and infections in systemic lupus erythematosus. J Rheumatol 2009; 36: 2190–2194 [DOI] [PubMed] [Google Scholar]
- 46. Pontillo C, Jacobs L, Staessen J. et al. A urinary proteome-based classifier for the early detection of decline in glomerular filtration. Nephrol Dial Transplant 2016; in press [DOI] [PubMed] [Google Scholar]
- 47. Molina F, Dehmer M, Perco P. et al. Systems biology: opening new avenues in clinical research. Nephrol Dial Transplant 2010; 25: 1015–1018 [DOI] [PubMed] [Google Scholar]
- 48. Cisek K, Krochmal M, Klein J. et al. The application of multi-omics and systems biology to identify therapeutic targets in chronic kidney disease. Nephrol Dial Transplant 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.