Abstract
Background
Autosomal dominant polycystic kidney disease (ADPKD) is a leading cause of end-stage renal disease, but estimates of its prevalence vary by >10-fold. The objective of this study was to examine the public health impact of ADPKD in the European Union (EU) by estimating minimum prevalence (point prevalence of known cases) and screening prevalence (minimum prevalence plus cases expected after population-based screening).
Methods
A review of the epidemiology literature from January 1980 to February 2015 identified population-based studies that met criteria for methodological quality. These examined large German and British populations, providing direct estimates of minimum prevalence and screening prevalence. In a second approach, patients from the 2012 European Renal Association‒European Dialysis and Transplant Association (ERA–EDTA) Registry and literature-based inflation factors that adjust for disease severity and screening yield were used to estimate prevalence across 19 EU countries (N = 407 million).
Results
Population-based studies yielded minimum prevalences of 2.41 and 3.89/10 000, respectively, and corresponding estimates of screening prevalences of 3.3 and 4.6/10 000. A close correspondence existed between estimates in countries where both direct and registry-derived methods were compared, which supports the validity of the registry-based approach. Using the registry-derived method, the minimum prevalence was 3.29/10 000 (95% confidence interval 3.27–3.30), and if ADPKD screening was implemented in all countries, the expected prevalence was 3.96/10 000 (3.94–3.98).
Conclusions
ERA–EDTA-based prevalence estimates and application of a uniform definition of prevalence to population-based studies consistently indicate that the ADPKD point prevalence is <5/10 000, the threshold for rare disease in the EU.
Keywords: autosomal dominant polycystic kidney disease, epidemiology, European Union, orphan disease, rare disease
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is a hereditary kidney disorder associated with ∼5% of the total end-stage renal disease (ESRD) [1, 2]. It is caused by mutations in either of two genes: PKD1 on chromosome 16 (∼85% of cases) or PKD2 on chromosome 4 (∼15% of cases) [3, 4]. The proteins encoded by PKD1 and PKD2—polycystin-1 and polycystin-2, respectively—associate with one another to form a complex that is localized to membranes in multiple cell types, including renal epithelial cells. Gene defects of ADPKD disrupt the normal differentiated phenotype of renal tubular epithelium. The mutations lead to increases in intracellular cyclic adenine monophosphate, resulting in a loss of mitotic polarity, increased cellular proliferation and apoptosis, and fluid secretion into cysts [5–8]. Cyst growth displaces and destroys normal kidney tissue, culminating in fibrosis, renal architectural derangement and ultimately kidney failure [5, 6].
ADPKD places substantial economic burden on the healthcare system, driven largely by ESRD expenditures [9]. The prevalence of ADPKD patients on renal replacement therapy (RRT) in the European Union (EU) has been estimated at 91.1/million population, with an associated cost (2010) of ∼651 million euro per year [10].
Estimating the prevalence of ADPKD is challenging for two reasons: first, at any given time, a proportion of cases are asymptomatic and thus undiagnosed. This feature reflects, in part, the progression of ADPKD over decades, with clinically detectable reductions in renal function typically appearing during the third and fourth decades of life [11]. It also reflects the complex genetics of ADPKD [3]. To date, >1270 and approximately 200 likely pathogenic mutations have been reported for PKD1 and PKD2, respectively [12]. There is a significant difference in rates of disease progression, with subjects with PKD1 mutations reaching ESRD at a younger age than those with PKD2 mutations [13, 14]. Even among subjects with a pathogenic PKD1 mutation, those with a truncating mutation have a more severe phenotype than those with a nontruncating mutation [13]. Second, general population screening for ADPKD is not feasible due to the high cost of genetic testing and diagnostic imaging, and expected low yields. Thus, population-based data are more limited for ADPKD than for more common conditions.
Point prevalence of ADPKD between 1/400 and 1/1000 is often linked to the seminal work of Dalgaard [15], who did not, in fact, ascertain the point prevalence of ADPKD. Dalgaard estimated the genetic prevalence of ADPKD at birth using Nyholm and Helweg-Larsen's [16] method for calculating morbid risk. He reported the figure of 0.8/1000 as an approximation of the theoretical risk of being ill from ADPKD during a lifetime of 80 years duration [15]. Despite Dalgaard's clear description, the figure of 1/1000 is often cited as the prevalence of ADPKD, rather than the theoretical lifetime risk. Point prevalence, lifetime prevalence and lifetime morbid risk (LMR) provide different information about disease occurrence. Point prevalence is the proportion of the population with disease at a specific time point, whereas lifetime prevalence measures the proportion of individuals who have had the disorder at some time in their life. LMR provides information on the proportion who have experienced the disorder plus the additional proportion who are expected to develop the disorder during a lifetime.
The objectives of the present study were to estimate two measures of point prevalence: (i) ‘minimum’ or point prevalence of diagnosed cases and (ii) point prevalence after screening (equal to minimum prevalence plus cases expected after population-based screening). These measures were then compared with the lifetime risk measures that are often cited in the literature.
MATERIALS AND METHODS
Minimum prevalence was defined as the point prevalence of diagnosed ADPKD cases divided by the population at a single point in time. This measure of disease burden has been applied in epidemiologic studies of Huntington's disease, another autosomal dominant genetic disorder [17, 18]. We also estimated ‘screening prevalence’ (equal to minimum prevalence plus those newly diagnosed cases detected after efforts to increase physician awareness and screen at risk patients). This yields the expected number of ADPKD patients in the EU who would be diagnosed if intensified screening were available in all countries. It provides an estimate of the burden of disease in the population, assuming intensified efforts to identify ADPKD patients.
Two approaches were used to estimate prevalence: first, a broad-based literature search was performed to identify high-quality population-based epidemiologic studies utilizing modern diagnostic methods to screen for ADPKD [19]. Second, the most recently available data (2012) from the European Renal Association–European Dialysis and Transplant Association (ERA–EDTA) Registry were used to estimate total ADPKD cases [20]. The total cases were then divided by the population covered by the Registry on 31 December 2012 to calculate minimum prevalence. Screening prevalence was estimated by inflating minimum prevalence by the screening yield obtained from large population-based studies.
Literature search strategy
Searches of the National Library of Medicine PubMed database were performed to identify high-quality epidemiologic studies published on ADPKD in EU countries. The search used the Medical Subject Heading terms (‘polycystic kidney’ or ‘autosomal dominant polycystic kidney disease’ or ‘polycystic kidney disease’) and (‘incidence study’ or ‘prevalence study’ or ‘epidemiologic study’) and encompassed all studies published between January 1980 and February 2015, as ultrasound and other modern diagnostic methods for ADPKD detection were available during this timeframe. Criteria used to select relevant studies included population-based studies and registry data, epidemiologic reviews and validity studies, and studies of clinical characteristics in large patient samples. In addition, an adequate sampling and power analysis, an appropriate denominator for prevalence estimates and a contemporary ADPKD definition were required [21]. In all, 2753 citations were identified, of which 2742 were excluded from further analysis because they were clinical studies based on small samples or methodologically flawed. Of the 11 population-based studies identified in the PubMed search, 9 were considered to have methodological flaws (Supplementary data, Table S1) [10, 22–31]. For example, the Heidland et al. study [24] was excluded due to low response rates and the use of proteinuria screening rather than more sensitive diagnostic procedures, such as ultrasound.
At the end of this selection process, two high-quality population-based studies on the ADPKD prevalence in EU populations remained:
Neumann et al. [27] presented results from the Else-Kroener-Fresenius-ADPKD-Study (EKFS–ADPKD), a population-based registry established in 2004 at the University Medical Center of Freiburg, Germany, that covered a population of 2 727 351. A prospective registration procedure to document patients with ADPKD began in 2009 and involved the cooperation of all nephrology centers, general practitioners, internists, urologists, human geneticists and neurosurgery centers in the region. Diagnosis of ADPKD followed internationally approved standard criteria and included clinical findings and family history. To optimize registration, each registrant was asked to inform affected relatives, and 6000 re-inquiries were sent to obtain the full data set of registrants. All registrants were offered genetic screening for germ-line mutations of PKD1 and PKD2 genes.
Patch et al. [28] performed a retrospective cohort study using data from the UK General Practice Research Database (GPRD), which included electronic medical records of >400 family practices and a registered population of 1.3–3.2 million. The GPRD was searched for all recorded medical codes for adult PKD from 1 January 1991 to 15 October 2008. The primary advantage of this design was that it was representative of the UK population as 98% of UK residents are registered with a family practice.
Estimating ADPKD prevalence in the EU via the ERA–EDTA Registry
Progressive impairment of renal function characterizes ADPKD [4], and most patients with the more common PKD1 mutation require RRT by age of 55–66 years [13]. Since renal failure is a frequent consequence of ADPKD, data from renal care registries were used to study geographic variation in the occurrence of ADPKD. The ERA–EDTA Registry is a well-respected source of epidemiologic data, and ∼200 peer-reviewed epidemiologic studies have been published using these data.
The 2012 ERA–EDTA annual report included data on the number of dialysis or transplant patients diagnosed with PKD from the following 18 EU/European Economic Area (EEA) countries: Austria, Belgium, Croatia, Denmark, Estonia, Finland, France, Greece, Latvia, the Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and the UK [20].
To estimate minimum point prevalence rates that could be generalizable across the EU, two calculations were applied to the ADPKD patient subset of the ERA−EDTA Registry: first, the total number of diagnosed patients was estimated from the population of PKD patients receiving RRT [22]. Second, EU screening prevalence was calculated by determining how many additional previously undiagnosed patients could be identified through intensive family- and population-based screening using pre- and post-data from contemporary screening studies [22, 24].
Renal replacement therapy rates in the literature
Prevalence of RRT in patients diagnosed with ADPKD has been reported in the literature across various time periods. Neumann et al. [27] calculated an RRT prevalence of 32% in a population of 2.7 million for the period of 2004‒09, while Davies et al. [22] calculated a prevalence of 23% (70/303) in a population of 2.1 million for 1970–90; that is, 209 families with 303 members alive and 70 members on RRT. This establishes a range of estimates for RRT prevalence in the EU/EEA over a period of four decades. The higher prevalence reported by Neumann et al. [27] for 2010 is consistent with the findings of Spithoven et al. [10], who compared intervals of ERA–EDTA RRT data between 1991 and 2010 and concluded that the RRT prevalence had increased during this period due, in part, to reduced cardiovascular mortality rates.
Screening yield rates in the literature
The difference between the numbers of patients diagnosed with ADPKD before and after intensified screening represents the additional percentage of ADPKD patients who could be diagnosed with real-world screening in an average EU/EEA country. Real-world screening for ADPKD was performed in an area in Germany with a population of ∼2.7 million and in an area in Wales with a population of 2.1 million [22, 27]. In Germany, registration of known ADPKD patients was expanded by contacting all registered internists, general practitioners, urologists and medical geneticists in this geographic area, and affected relatives of known cases were sought by sending 6000 re-inquiries. In addition, physician awareness of ADPKD was increased through survey questions regarding diagnostic criteria for ADPKD. Using this approach, an additional 8% of cases were identified in southwest Germany [27]. Similarly, Davies et al. [22] contacted all physicians and nephrologists in south and mid-Wales, obtained detailed family histories, examined autopsy reports, made home visits to family members of known cases and recommended screening to all adults at risk. After screening was offered, these efforts yielded an additional 20%. (For full details of the screening methods utilized by Neumann et al. [27] and Davies et al. [22], please refer to pages 2, 4 and 5 and pages 478 and 479, respectively).
The percent age of ADPKD patients who were diagnosed before screening is likely to be higher in Germany than in other European populations because working-age adults are screened for hypertension, a key clinical feature of ADPKD [26, 28, 32–35]. In contrast, the percent age of cases known before screening from the Davies study [22] is probably too low due to improvements in diagnostic technology since the early 1980s when the study was conducted. A midrange of these percentages (85%) was, therefore, applied to estimate that an additional 15% could be identified after population-based screening. This percent age does not represent all undiagnosed cases in the population as it is accepted that some patients with family history of ADPKD delay testing for fear of societal or economic prejudices. The estimate reflects the practical limitations of screening programs but is based on the assumption that real-world ADPKD screening is extended throughout the EU.
Sensitivity analyses for renal registry estimates
To examine the sensitivity of the renal registry estimates, the effect of varying the upper and lower bounds of the percents of patients receiving RRT (23 and 32%, respectively) was explored. Similarly, the effect of varying the screening yield rates was also explored by testing the upper and lower bounds of the published rates (80 and 90%, respectively).
RESULTS
Point prevalence of ADPKD from population studies
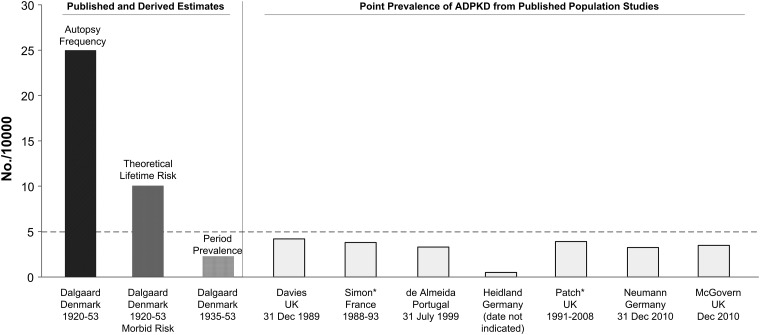
Eleven population-based studies were identified, of which seven provided estimates of ADPKD prevalence (Figure 1). Two of the 11 European population-based studies met the prespecified criteria for acceptable high-quality epidemiologic data. The first study, based on data from the EKFS–ADPKD registry in southwestern Germany, identified 891 ADPKD cases (658 index cases plus 233 relatives) in a population of 2 727 351. The minimum prevalence of ADPKD in this population was 2.41/10 000 and the point prevalence after screening was 3.3/10 000 on 31 December 2010 [27].
FIGURE 1.
Point prevalence of ADPKD from published population studies [22–24, 26–28, 30] compared with estimates derived by autopsy frequency and theoretical lifetime risk [17]. *Period prevalence estimates provided by Patch et al. [28] and Simon et al. [30] were recalculated to estimate total point prevalence. Hatched line at 5/10 000 indicates criterion used for orphan designation in EU. Estimates based on Dalgaard's [15] autopsy data represent frequency of polycystic kidneys at autopsy (black bar). Genetic frequency from hospital records was derived by Nyholm and Helweg-Larsen's [16] method for calculating morbid risk (dark gray bar). Period prevalence was calculated using autopsy and hospital records (hatched gray bar). Point prevalence values from published population studies are plotted to right (light gray bars).
The second study, based on GPRD data, identified 1238 diagnosed ADPKD cases in a population of 3.2 million from 1991 to 2008, yielding a minimum prevalence of 3.89/10 000 [28]. Assuming that an additional 15% could be identified after screening [22, 27], the total number of ADPKD patients in this British population was estimated at 1456, resulting in a screening prevalence of 4.6/10 000. Thus, both minimum and screening prevalence estimates from a population of 5 927 351 demonstrated a prevalence of <5/10 000.
Prevalence calculation based on renal registry data
The estimate for EU/EEA minimum prevalence of ADPKD was 3.29/10 000 (95% confidence interval 3.27–3.30). This estimate was derived by combining RRT registry data from 18 countries with Neumann et al.'s [27] published prevalence for Germany. The estimate for EU/EEA point prevalence after screening was 3.96/10 000 (95% confidence interval 3.94–3.98). This rate yields the expected number of ADPKD patients in the EU who would be diagnosed if intensive screening was available in all countries, based on renal registry data from a total population of 407 428 518 (81% of EU population; Table 1).
Table 1.
Minimum prevalence of ADPKD in 19 EU countries estimated using renal registry data and published population-based data
| EU country | Population covered by ERA–EDTA Registry: 1 January 2013 (n) | Point prevalent cases from EU renal registries: 31 December 2012 (n) | Estimated diagnosed cases (n)a |
|---|---|---|---|
| Austria | 8 451 860 | 561 | 2439 |
| Belgium | 11 161 642 | 1198 | 5209 |
| Croatia | 4 262 140 | 260 | 1130 |
| Denmark | 5 602 628 | 434 | 1887 |
| Estonia | 1 320 174 | 66 | 287 |
| Finland | 5 426 674 | 551 | 2396 |
| France | 65 578 819 | 6932 | 30 139 |
| Germany | 80 523 746 | 19 406b | |
| Greece | 11 062 508 | 983 | 4274 |
| Latvia | 2 023 825 | 105 | 457 |
| The Netherlands | 16 779 575 | 1269 | 5517 |
| Poland | 38 533 299 | 1468 | 6383 |
| Portugal | 10 487 289 | 706 | 3070 |
| Romania | 20 020 074 | 885 | 3848 |
| Slovakia | 5 410 836 | 171 | 743 |
| Slovenia | 2 058 821 | 188 | 817 |
| Spain | 45 263 418 | 4530 | 19 696 |
| Sweden | 9 555 893 | 888 | 3861 |
| UK | 63 905 297 | 5137 | 22 335 |
| Total | 407 428 518 | 133 893 | |
| Total point prevalence | 3.29/10 000 (95% CI 3.27–3.30) |
aCalculated using RRT estimates from the published literature of 23% (Davies et al. [22]).
bDoes not include registry estimates from Germany.
CI, confidence interval.
Validation of the renal registry method for prevalence estimates
Registry-derived estimates for individual EU countries were compared with published prevalence rates from McGovern et al. [26], Neumann et al. [27] and Patch et al. [28]. Results showed a close correspondence between the two methods, supporting the validity of the renal registry approach for estimating ADPKD prevalence in countries without published data (Table 2).
Table 2.
Comparison of minimum prevalence estimates from ERA–EDTA Registry data versus population-based studies
| Country | Registry year | Diagnosed patients, n |
|
|---|---|---|---|
| Registry method | Population-based study | ||
| Germany | 2010 | 23 054 | 23 912a |
| UK | 2010 2012 |
20 522 22 335 |
23 668b 24 923c |
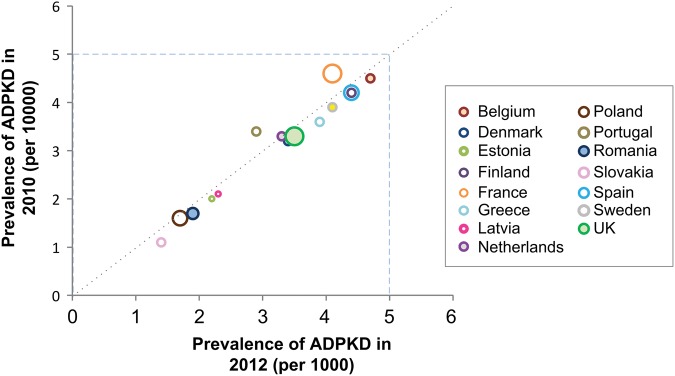
To assess reliability over time, the correlation between country-specific, registry-based estimates was evaluated in 2010 and 2012 (Figure 2) [20, 36]. Point prevalence estimates were highly correlated and appeared to be stable over time.
FIGURE 2.
Correlation between country-specific minimum prevalence estimates in 2010 and 2012. Estimates from ERA–EDTA Registry in 2010 and 2012 were calculated assuming a 23% RRT rate [20, 36]. Estimates were not adjusted for between-country differences in population age structure. Prevalence data for Austria (3.39), Croatia (3.12) and Slovenia (4.67) were available only for 2012; data for Germany (3.32) and Italy (2.80) were available only for 2010.
Sensitivity analysis for renal registry-based estimates
The two assumptions used to estimate point prevalence were subjected to sensitivity analyses. Each assumption was tested separately by fixing one assumption and varying the other across a range based on available evidence. Thus, the assumption for the proportion of ADPKD patients on RRT varied between the published ranges of 23 and 32%, and the screening yield varied between the published ranges of 80 and 90%. This sensitivity analysis resulted in a screening prevalence ranging between 2.90 and 4.16/10 000 at the two most extreme assumptions (Table 3).
Table 3.
Sensitivity analyses: estimates of ADPKD screening prevalence by variation in key assumptions
| Proportion on RRT | Prevalence by % of cases diagnosed prescreening |
||
|---|---|---|---|
| 80% | 85% | 90% | |
| 23%a | 4.16 | 3.96 | 3.77 |
| 32%b | 3.18 | 3.03 | 2.90 |
DISCUSSION
In this study, measures of ADPKD point prevalence estimated with two different methods showed consistent results. First, high-quality, population-based studies of large German and British populations provided estimates of ADPKD minimum prevalence of 2.4 and 3.9/10 000, respectively. Estimates of screening prevalence were 3.3 and 4.6/10 000, respectively. Although population-based studies are considered the gold standard for estimating disease prevalence, the total combined population from these two studies represented only a limited proportion of the total EU population. In the second approach, RRT patient counts from national and regional registries and literature-based assumptions about disease severity and screening rates were used to determine a more generalizable prevalence estimate (covering 19 EU countries and 81% of the EU population). This approach yielded a minimum ADPKD point prevalence on 31 December 2012 of 2.41/10 000 and an estimated screening prevalence of 3.96/10 000. Both results support the classification of medicines to treat ADPKD as orphan medicinal products in the EU [37].
The renal registry approach had several potential limitations. First, the ADPKD prevalence estimate reflects the quality of the underlying database. Hommel et al. [38] crosschecked 3020 incident RRT patients in the Danish National Registry on Regular Dialysis and Transplantation (NRDT) with the same patients in the Danish National Patient Registry, which contains information on hospital admissions and treatments. Results indicated 97% completeness in the NRDT and high validity of the start date of RRT. The validity of common renal diagnoses was also high, e.g. diagnosis of adult PKD as measured by inter-rater agreement was excellent (Κ = 0.95). Thus, completeness of ADPKD patient registration in NRDT was highly acceptable. Although not all renal registries publish validity studies, the available information supports a high degree of confidence in these data [39, 40]. Second, the registry-based estimate of ADPKD prevalence was dependent on the validity of the inflation factors used in the calculations. The validity of these values was directly tested by comparing predicted numbers of ADPKD cases from the registry model with independent population-based estimates. A close correspondence existed between the numbers of diagnosed cases estimated with each method, validating the renal registry approach. Finally, the registries did not typically differentiate between RRT patients with ADPKD and autosomal recessive polycystic kidney disease (ARPKD). The degree of overestimation is likely to be very small, however, because few ARPKD patients survive to adulthood [41–44]. In the ERA–EDTA 2012 data, diagnosis information was included for 133 893 PKD cases and 99% were attributed to ADPKD.
Of note is the variability in prevalence between countries (Figure 2). Some variation may be related to geographic clustering of families in which ADPKD is present. For example, higher prevalence rates are evident in Belgium and France, which is consistent with an early study by Simon et al. [30] that reported higher prevalence in France than in other European countries. Differences in access to healthcare resources may also be a factor as recent evidence indicates that early treatment of hypertension may delay the progression of ADPKD [24, 45]. It is also important to note that variability in estimates for some countries may be related to smaller population size as random error is more likely to affect these estimates.
Much of the nearly 10-fold variation in the reported prevalence of ADPKD is due to differences in prevalence definitions or the misinterpretation of autopsy data [10, 15, 22, 3, 25–31]. ADPKD prevalence is often cited as 1 in 773, but this is an inaccurate citation as Dalgaard [15] clearly reports that this figure is the percent of cases found at autopsy. In the early 20th century, ADPKD patients were more likely to have died from unexplained causes as ultrasound technology was not available for detection and treatment options were limited and less effective. Thus, ADPKD patients would be more likely to die at an early age, be autopsied and be over-represented in these data.
Dalgaard's [15] estimate of the LMR of ADPKD (0.8/1000) is also cited as the prevalence of ADPKD, although it is more accurately a theoretical estimate of lifetime risk. The LMR method used by Dalgaard assumed complete survival of the population to the age of 80 years and is known to produce an overestimate of actual risk when the competing risk of mortality is not taken into account [46]. Alternative methods adjust the incidence rate by the all-cause mortality rate in the population and provide the actual risk of developing the disease before dying of another cause [47]. Future research using contemporary cohorts and alternative methods to estimate LMR for ADPKD is needed. Contemporary data may yield different results as the accuracy of LMR calculations depends on the stability of age-of-onset distributions over generations and this is likely to have changed since these data were collected in 1920–53. In addition, the accuracy of diagnosis and age-of-onset information is likely to be improved.
The large difference between often-cited and contemporary prevalence rates can be further clarified by calculating period prevalence from the Dalgaard study [15] using both autopsied and hospitalized cases. Dalgaard reported population-based data on the number of cases detected in clinical settings over an 18-year period. When these patients are added to autopsied cases and divided by the population, the result is a period prevalence of 3.26/10 000, which is nearly identical to the point prevalence rate of 3.25/10 000 recently reported in Europe [27]. Thus, results of this early study are not inconsistent with recent data after similar prevalence definitions are applied.
From a public health perspective, we emphasize the utility of our prevalence estimate of 3.96/10 000 as it measures the point prevalence of ADPKD in the general population, inclusive of both known cases and those expected to be diagnosed after population-based screening. Although it is not inclusive of all patients who inherited ADPKD-related mutations, it measures burden of disease and treatment need, assuming increased awareness and surveillance of ADPKD in the EU.
In conclusion, indirect prevalence estimates, such as these, provide much needed epidemiologic data for national policies on rare disease. The validity of indirect prevalence estimates is, however, dependent on careful selection of valid and generalizable studies for key assumptions, validation strategies to assess assumptions and realistic sensitivity analyses. In this case, ERA–EDTA-based prevalence estimates, alternative assumption analyses and application of uniform prevalence definitions to population-based ADPKD studies over six decades consistently indicated that ADPKD point prevalence was <5/10 000, which is the threshold for a rare disease in the EU. This finding has important policy implications for governmental and research agencies, professional organizations, academia, clinical research programs, industry and rare-disease patient groups.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
C.J.W. and A.K.H. are consultants to Otsuka. J.D.B., H.B.K., A.J.M. and F.S.C. are employees of Otsuka. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank David Norris, PhD, Ecosse Medical Communications, LLC, Falmouth, MA, USA, for editorial assistance provided during the preparation of this review funded by Otsuka Pharmaceutical Development and Commercialization, Inc. The authors also thank Anneke Kramer, PhD, of the ERA–EDTA Registry for assistance with questions related to ERA–EDTA Registry data. Funding for statistical analyses of epidemiologic data and study oversight was provided by Otsuka Pharmaceutical Development & Commercialization, Inc.
REFERENCES
- 1. Levy M, Feingold J. Estimating prevalence in single-gene kidney diseases progressing to renal failure. Kidney Int 2000; 58: 925–943 [DOI] [PubMed] [Google Scholar]
- 2. US Renal Data System. 2011 USRDS Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; http://www.usrds.org/2011/pdf/v1_00_intro_11.pdf (15 March 2016, date last accessed) [Google Scholar]
- 3. Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 2008; 359: 1477–1485 [DOI] [PubMed] [Google Scholar]
- 4. Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 2007; 369: 1287–1301 [DOI] [PubMed] [Google Scholar]
- 5. Belibi FA, Reif G, Wallace DP. et al. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int 2004; 66: 964–973 [DOI] [PubMed] [Google Scholar]
- 6. Torres VE, Wang X, Qian Q. et al. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 2004; 10: 363–364 [DOI] [PubMed] [Google Scholar]
- 7. Wang X, Gattone V 2nd, Harris PC. et al. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 2005; 16: 846–851 [DOI] [PubMed] [Google Scholar]
- 8. Yamaguchi T, Pelling JC, Ramaswamy NT. et al. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int 2000; 57: 1460–1471 [DOI] [PubMed] [Google Scholar]
- 9. Lentine KL, Xiao H, Machnicki G. et al. Renal function and healthcare costs in patients with polycystic kidney disease. Clin J Am Soc Nephrol 2010; 5: 1471–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spithoven EM, Kramer A, Meijer E. et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival—an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant 2014; 29(Suppl 4): iv15–iv25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2011; 7: 556–566 [DOI] [PubMed] [Google Scholar]
- 12. PKD Foundation. Autosomal Dominant Polycystic Kidney Disease Mutation Database: PKDB. Available at: http://pkdb.mayo.edu (12 February 2016, date last accessed)
- 13. Cornec-Le Gall E, Audrézet MP, Chen JM. et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 2013; 24: 1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hateboer N, v Dijk MA, Bogdanova N. et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 1999; 353: 103–107 [DOI] [PubMed] [Google Scholar]
- 15. Dalgaard OZ. Bilateral polycystic disease of the kidneys; a follow-up of two hundred and eighty-four patients and their families. Acta Med Scand Suppl 1957; 328: 1–255 [PubMed] [Google Scholar]
- 16. Nyholm M, Helweg-Larsen HF. On the computation of morbid risk (disease expectancy). Acta Genet 1954; 5: 25–38 [DOI] [PubMed] [Google Scholar]
- 17. Morrison PJ, Harding-Lester S, Bradley A. Uptake of Huntington disease predictive testing in a complete population. Clin Genet 2011; 80: 281–286 [DOI] [PubMed] [Google Scholar]
- 18. Tassicker RJ, Teltscher B, Trembath MK. et al. Problems assessing uptake of Huntington disease predictive testing and a proposed solution. Eur J Hum Genet 2009; 17: 66–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pei Y, Obaji J, Dupuis A. et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 2009; 20: 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ERA-EDTA Registry: ERA-EDTA Registry Annual Report 2012. Amsterdam, The Netherlands: Academic Medical Center, Dept of Medical Informatics, 2014. http://www.era-edta-reg.org/files/annualreports/pdf/AnnRep2012.pdf (10 February 2016, date last accessed) [Google Scholar]
- 21. Barua M, Pei Y. Diagnosis of autosomal-dominant polycystic kidney disease: an integrated approach. Semin Nephrol 2010; 30: 356–365 [DOI] [PubMed] [Google Scholar]
- 22. Davies F, Coles GA, Harper PS. et al. Polycystic kidney disease re-evaluated: a population-based study. Q J Med 1991; 79: 477–485 [PubMed] [Google Scholar]
- 23. de Almeida E, Sousa A, Pires C. et al. Prevalence of autosomal-dominant polycystic kidney disease in Alentejo, Portugal. Kidney Int 2001; 59: 2374. [DOI] [PubMed] [Google Scholar]
- 24. Heidland A, Bahner U, Deetjen A. et al. Mass-screening for early detection of renal disease: benefits and limitations of self-testing for proteinuria. J Nephrol 2009; 22: 249–254 [PubMed] [Google Scholar]
- 25. Martinez V, Comas J, Arcos E. et al. Renal replacement therapy in ADPKD patients: a 25-year survey based on the Catalan registry. BMC Nephrol 2013; 14: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGovern AP, Jones S, van Vlymen J. et al. Identification of people with autosomal dominant polycystic kidney disease using routine data: a cross sectional study. BMC Nephrol 2014; 15: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neumann HP, Jilg C, Bacher J. et al. Epidemiology of autosomal-dominant polycystic kidney disease: an in-depth clinical study for south-western Germany. Nephrol Dial Transplant 2013; 28: 1472–1487 [DOI] [PubMed] [Google Scholar]
- 28. Patch C, Charlton J, Roderick PJ. et al. Use of antihypertensive medications and mortality of patients with autosomal dominant polycystic kidney disease: a population-based study. Am J Kidney Dis 2011; 57: 856–862 [DOI] [PubMed] [Google Scholar]
- 29. Shaw C, Simms RJ, Pitcher D. et al. Epidemiology of patients in England and Wales with autosomal dominant polycystic kidney disease and end-stage renal failure. Nephrol Dial Transplant 2014; 29: 1910–1918 [DOI] [PubMed] [Google Scholar]
- 30. Simon P, Le Goff JY, Ang KS. et al. Epidemiologic data, clinical and prognostic features of autosomal dominant polycystic kidney disease in a French region. Nephrologie 1996; 17: 123–130 [PubMed] [Google Scholar]
- 31. World MJ. Military nephrology: a review of cases 1985–2011. J R Army Med Corps 2012; 158: 300–304 [DOI] [PubMed] [Google Scholar]
- 32. Helal I, McFann K, Reed B. et al. Serum uric acid, kidney volume and progression in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant 2013; 28: 380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kelleher CL, McFann KK, Johnson AM. et al. Characteristics of hypertension in young adults with autosomal dominant polycystic kidney disease compared with the general U.S. population. Am J Hypertens 2004; 17: 1029–1034 [DOI] [PubMed] [Google Scholar]
- 34. Kocyigit I, Yilmaz MI, Unal A. et al. A link between the intrarenal renin angiotensin system and hypertension in autosomal dominant polycystic kidney disease. Am J Nephrol 2013; 38: 218–225 [DOI] [PubMed] [Google Scholar]
- 35. Perrone R, Miskulin DC. Making an earlier diagnosis of ADPKD: implications for the treatment of hypertension. Nephrol News Issues 2006; 20: 32, 35–36 [PubMed] [Google Scholar]
- 36. ERA-EDTA Registry: ERA-EDTA Registry Annual Report 2010. Amsterdam, The Netherlands: Academic Medical Center, Dept of Medical Informatics, 2012. http://www.era-edta-reg.org/files/annualreports/pdf/AnnRep2010.pdf (10 February 2016, date last accessed) [Google Scholar]
- 37. Regulation (EC) No. 141/2000 of the European Parliament and of the Council of 16 December 1999 on orphan medicinal products. http://ec.europa.eu/health/files/eudralex/vol-1/reg_2000_141_cons-2009-07/reg_2000_141_cons-2009-07_en.pdf. (15 December 2015, date last accessed)
- 38. Hommel K, Rasmussen S, Madsen M. et al. The Danish Registry on Regular Dialysis and Transplantation: completeness and validity of incident patient registration. Nephrol Dial Transplant 2010; 25: 947–951 [DOI] [PubMed] [Google Scholar]
- 39. Fotheringham J, Fogarty D, Jacques R. et al. Chapter 13 The linkage of incident renal replacement therapy patients in England (2002–2006) to hospital episodes and national mortality data: improved demography and hospitalisation data in patients undergoing renal replacement therapy. Nephron Clin Pract 2012; 120(Suppl 1): c247–c260 [DOI] [PubMed] [Google Scholar]
- 40. Nordio M, Antonucci F, Feriani M. et al. Reliability of administrative databases in epidemiological research: the example of end-stage renal disease requiring renal replacement therapy in patients with diabetes. G Ital Nefrol 2009; 26(Suppl 45): S7–S11 [PubMed] [Google Scholar]
- 41. Capisonda R, Phan V, Traubuci J. et al. Autosomal recessive polycystic kidney disease: outcomes from a single-center experience. Pediatr Nephrol 2003; 18: 119–126 [DOI] [PubMed] [Google Scholar]
- 42. Parfrey PS. Autosomal-recessive polycystic kidney disease. Kidney Int 2005; 67: 1638–1648 [DOI] [PubMed] [Google Scholar]
- 43. Roy S, Dillon MJ, Trompeter RS. et al. Autosomal recessive polycystic kidney disease: long-term outcome of neonatal survivors. Pediatr Nephrol 1997; 11: 302–306 [DOI] [PubMed] [Google Scholar]
- 44. Zerres K, Rudnik-Schoneborn S, Deget F. et al. Autosomal recessive polycystic kidney disease in 115 children: clinical presentation, course and influence of gender. Arbeitsgemeinschaft fur Padiatrische, Nephrologie. Acta Paediatr 1996; 85: 437–445 [DOI] [PubMed] [Google Scholar]
- 45. Schrier RW, McFann KK, Johnson AM. Epidemiological study of kidney survival in autosomal dominant polycystic kidney disease. Kidney Int 2003; 63: 678–685 [DOI] [PubMed] [Google Scholar]
- 46. Schouten LJ, Straatman H, Kiemeney LA. et al. Cancer incidence: life table risk versus cumulative risk. J Epidemiol Community Health 1994; 48: 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lim HJ, Zhang X, Dyck R. et al. Methods of competing risks analysis of end-stage renal disease and mortality among people with diabetes. BMC Med Res Methodol 2010; 10: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.