Abstract
Background
Clinical epidemiology data for young adults on renal replacement therapy (RRT) are lacking. While mostly transplanted, they have an increased risk of graft loss during young adulthood.
Methods
We combined the UK Renal Registry paediatric and adult databases to describe patient characteristics, transplantation and survival for young adults. We grouped patients 11–30 years of age starting RRT from 1999 to 2008 by age band and examined their course during 5 years of follow-up.
Results
The cohort (n = 3370) was 58% male, 79% white and 29% had glomerulonephritis. Half (52%) started RRT on haemodialysis (HD). Most (78%) were transplanted (18% pre-emptive, 61% as second modality); 11% were not listed for transplant. Transplant timing varied by age group. The deceased:living donor kidney transplant ratio was 2:1 for 11–<16 year olds and 1:1 otherwise. Median deceased donor transplant waiting times ranged from 6 months if <16 years of age to 17 months if ≥21 years. Overall 8% died, with being on dialysis and not transplant listed versus transplanted {hazard ratio [HR] 16.6 [95% confidence interval (CI) 10.8–25.4], P < 0.0001} and diabetes versus glomerulonephritis [HR 4.03 (95% CI 2.71–6.01), P < 0.0001] increasing mortality risk.
Conclusions
This study highlights the frequent use of HD and the importance of transplant listing and diabetes for young adults. More than half the young adults in our cohort started renal replacement therapy on HD. One in 10 young adults were not listed for transplant by 5 years and were ∼20 times more likely to die than those who were transplanted. Diabetes as a primary renal disease was common among young adults and associated with increased mortality. Overall, almost 1 in 10 young adults had died by 5 years from the start of RRT.
Keywords: clinical epidemiology, dialysis, kidney transplantation, registries, survival, young adult
INTRODUCTION
Comprehensive data focusing on young adults on renal replacement therapy (RRT) are lacking. The majority are managed with a kidney transplant; the latest UK data show 91.8% of prevalent 16–18 year olds in paediatric services and 72.9% of prevalent 18–24 year olds in adult services were transplant patients [1, 2]. Most children in the UK receiving RRT have a renal transplant [1], and those listed when <18 years of age receive greater priority on the national deceased donor transplant waiting list [3–5]. Although transplantation is seen as the treatment of choice, international data demonstrate a higher risk of graft loss during young adulthood [6], with those who are transplanted in childhood and transfer to adult services frequently studied [7, 8]. In a single-centre UK study, 8 of 20 kidney transplants in 18 year olds unexpectedly failed inside 3 years of transfer from paediatric to adult services [9]. Young adulthood is a time of increasing independence, experimentation and responsibility, when the brain and complex decision-making abilities are still maturing [10]. Clarity on management practices for young adults on RRT in the UK is necessary for improvements in the survival of these grafts, assuming that some of these losses are due to modifiable factors.
The UK Renal Registry (UKRR) reports on all established RRT patients. The first UKRR annual report was published in 1998 [11], with complete electronic coverage of adult units from 2008 [12]. Paediatric data were first reported in 1999 with complete coverage [13]. We aimed to describe the clinical epidemiology of young adults on RRT in the UK to determine changing characteristics between childhood and adulthood by combining paediatric and adult databases in order to capture all patients. We were specifically interested in transplantation practices for this group and wanted to identify high-risk groups with poor survival, providing potential targets for further service development.
MATERIALS AND METHODS
We created a dataset of young patients starting RRT over a 10-year period between 1999 and 2008 using UKRR data. This required extracting and merging data from separate adult and paediatric databases. We included all patients ≥11 years of age from the paediatric database and patients registered in adult renal centres reporting to the UKRR at the time of RRT start from the adult database (see Figure 1). We chose broad age cut-offs of 11 and 30 years because there is no clear consensus as to what age groups constitute young adulthood and we wanted overlap with paediatric and adult groups to see if any age-related trends were continuous across the age range. We divided the cohort into four bands according to age at RRT start; 11–<16, 16–<21, 21–<26 and 26–30 years. We examined 5 years of longitudinal data for each subject, so an individual starting RRT in 2008 had follow-up to the most recent registry data return on 31 December 2013, using 15 years of UKRR data (1999–2013).
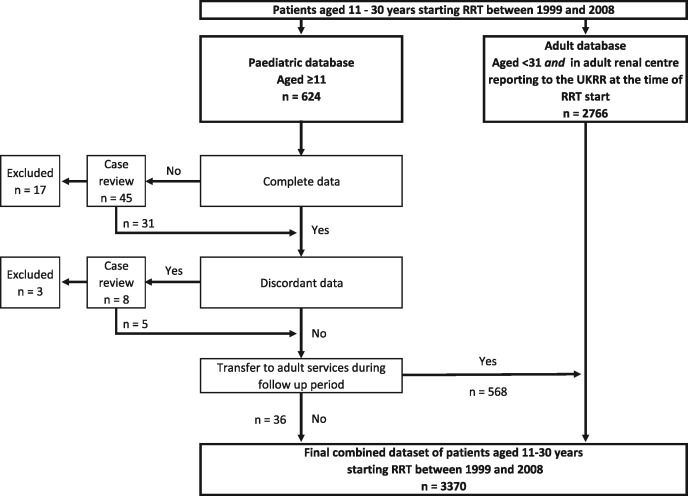
FIGURE 1.
Flowchart showing how the cohort was constructed from the UK RR databases. At RRT start, patients were present in one database. During follow-up, data for paediatric patients moved to the adult database if they transferred to adult services. There were no data queries from the adult database. Further details around missing and discordant data are available in Supplementary Table S1. UKRR, UK Renal Registry; RRT, renal replacement therapy.
Where data were discordant between the databases, we selected the paediatric data, which tended to offer more detail. NHS Blood and Transplant provided missing ethnicity and transplant listing data. Queries were resolved with individual case review; Supplementary Table S1 details these.
Beginning with clinical characteristics, we examined sex, age, ethnicity and primary renal diagnosis (PRD), grouped using the 2012 ERA-EDTA coding [14]. We added subgroups in order to identify conditions of particular interest. We did not analyse demographics by time period due to incomplete UKRR coverage during the study period. We calculated a 2008 RRT incidence using Office for National Statistics (ONS) UK census data to obtain age-specific denominators [15].
For management, we investigated RRT start modality and pre-emptive transplantation, modality changes, transplant listing, first transplant type and time to transplant from listing for deceased donor grafts. In keeping with UKRR reports, we assumed that patients with a live transplant who either did not have a transplant listing date or had a date within 6 months of their transplant were listed for transplantation 6 months before their live transplant date, and modality changes between haemodialysis (HD) and peritoneal dialysis (PD) were defined as those lasting >90 days. We analysed graft loss by including all first and second transplants with 1 year of follow-up data and defining loss as return to dialysis, elective retransplantation or death. We assumed those lost to of follow-up had a functioning graft at 1 year. We also recorded starting unit type (paediatric or adult) and details of transfer to adult services where applicable.
Statistical analysis compared characteristics between patients in different age groups using tests appropriate to their data and distribution. We compared continuous data using the Kruskal–Wallis test for medians and the chi-square test for categorical variables.
For survival, we calculated crude and relative mortality rates per 1000 patient-years, comparing these with the 2001–14 (equivalent time period) average age-matched general population mortality data from the ONS [15]. We determined 95% confidence intervals (CIs) using Poisson regression. We looked at survival by age group using Kaplan–Meier plots and with a relative survival analysis [16], also using ONS data. A relative survival analysis adjusts the observed survival in the specific cohort for the background mortality risk seen in the general population so that the initial cumulative observed survival is converted to a cumulative relative survival. Hence, if worse survival observed in older compared with younger renal patients was a function of higher risk of dying in the general population, the relative survival would be more similar between the two groups than the observed survival. By using a multivariable Cox regression model, we explored the influence of age group adjusted by possible confounder variables in addition to age group on the risk of death after RRT start [hazard ratio (HR) (95% CI), P-value], having tested for the assumption of proportionality using visual plots, Schoenfeld residuals and testing for a log–time interaction. All subjects who remained alive after 5 years were censored at that time point. Censoring was also applied at recovery of renal function or if lost to follow-up. We analysed modality (transplant versus ‘dialysis, transplant listed’ and ‘dialysis, not transplant listed’) as a time-dependent variable, as this can change over the follow-up period. Each patient contributed to the survivor function of the treatment groups for the relevant time depending on the patient treatment timeline, and we attributed deaths to the current modality, with no crossover for the transplant to dialysis switch. As this analysis could not be adjusted for referral time due to missing data, a sensitivity analysis of survival after 90 days from RRT start was also performed, to remove any confounding effects of starting dialysis within 90 days of the first nephrology review. As some indication of non-proportionality was observed for diabetics versus non-diabetic patients, we also performed piecewise Cox regression stratifying by diabetic status.
RESULTS
The patient frequency, ethnic group and PRD group proportions by age group are displayed in Figures 2–4, respectively, with data including gender and PRD subgroups available in Supplementary Table S2. Overall data completeness was high. As shown in Supplementary Table S2, there was 1.0% missing ethnic data and only 0.7% of ethnic codes were discordant in patients registered in both paediatric and adult databases. There were similar missing data for PRD, with 1.9% missing and 1.3% discordant. We were able to link all but 15 patients (2.6%) who transferred from paediatric to adult services between the paediatric and adult databases, showing the ability to track patients longitudinally.
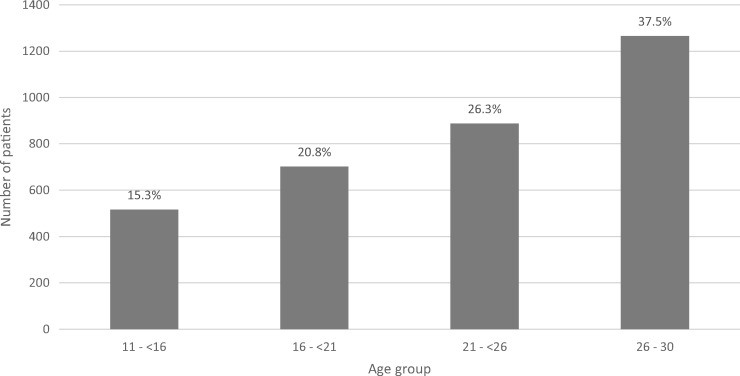
FIGURE 2.
Patient frequency by age group in incident UK young adult RRT patients between 1999 and 2008. Percentages are shown for each group.
Clinical epidemiology
Figure 2 demonstrates increasing absolute patient numbers in the cohort with older age group. The UK 2008 incidence of RRT by age group at RRT start increased with age; the rate was 16.3 per million age-related population for the 11–<16 group, 20.0 for the 16–<21 group, 27.7 for the 21–<26 group and 46.1 for the 26–30 group. Young adults starting RRT in the UK were predominantly male (57.9%), with no difference in gender by age group.
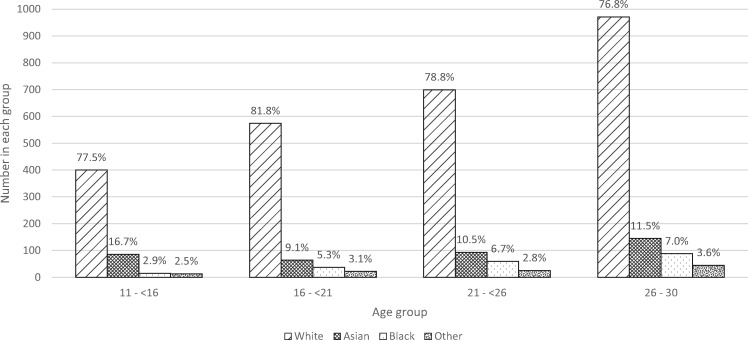
Figure 3 shows that the majority were of White ethnicity (78.5% overall), with variation in ethnic group proportions by age group (P < 0.05).
FIGURE 3.
Ethnic group by age group in incident UK young adult RRT patients between 1999 and 2008. Percentages are shown for each group. P < 0.05, chi-square test.
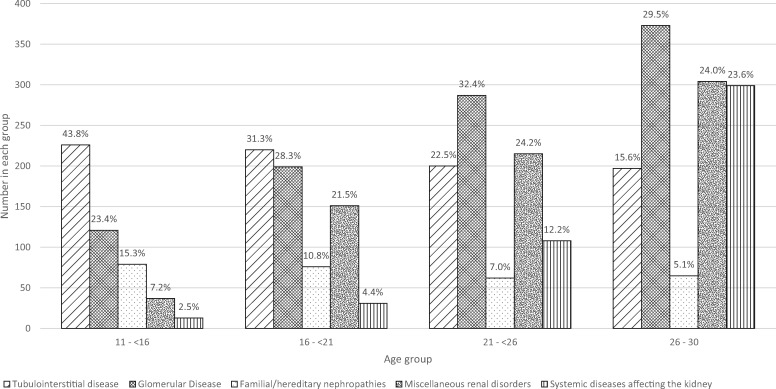
Figure 4 shows a decrease in tubulointerstitial and inherited PRDs and an increase in systemic diseases and miscellaneous conditions with increasing age group (P < 0.0001). As shown in Supplementary Table S2, there was a striking increase by age in diabetes causing end-stage renal disease (ESRD), with 17.4% of PRDs in the 26–30-year age group accounted for by diabetes.
FIGURE 4.
PRD groups by age group in incident young adult RRT patients between 1999 and 2008. Percentages are shown for each group. P < 0.0001, chi-square test. Percentages are shown for each group. PRD is grouped using the 2012 ERA-EDTA coding [12]; ‘Tubulointerstitial disease’ includes structural renal disorders. Subgroup data are available in Supplementary Table S2.
Management
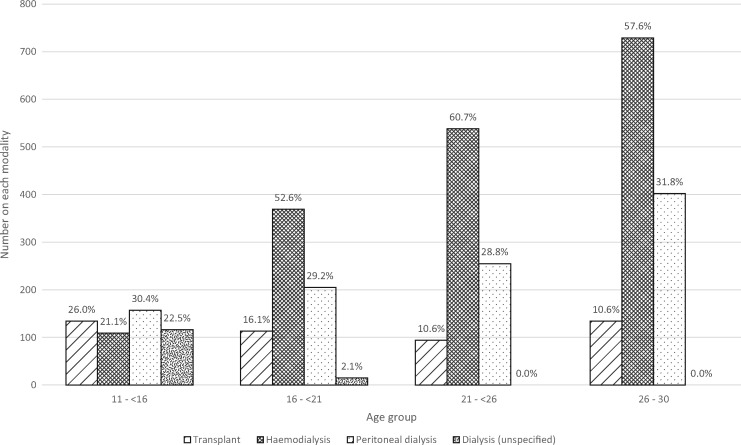
Start modality. Figures 5 and 6 show that half of young adults started RRT on HD, with more pre-emptive transplantation in the youngest age group. Overall, 51.8% received HD, 30.2% PD and 14.1% started RRT with a transplant, with strong statistical evidence of a difference in the RRT start modality by age group (P < 0.0001). Data regarding late referral were available for half the cohort and showed 29% had started dialysis within 90 days of the first nephrology review.
FIGURE 5.
Start modality by age group in incident young adult RRT patients between 1999 and 2008. Percentages are shown for each group. Some paediatric patients were known to have received dialysis at RRT start but the type was unknown.
FIGURE 6.
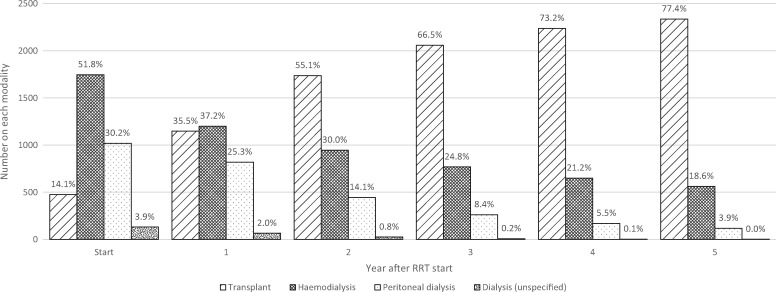
Overall modality proportions in the first 5 years of RRT in surviving UK incident young adults. Percentages are shown for each group. Some paediatric patients were known to have received dialysis at RRT start but the type was unknown.
Modality changes over the first 5 years of RRT. Figure 6 demonstrates transplantation was the major treatment for young adults, increasing rapidly in the first 3 years. HD was the next most frequently used modality, with PD least used.
Table 1 shows more detail about the use of transplantation in the first 5 years of RRT. Overall, 78.0% received a transplant within the first 5 years of starting RRT, with a higher proportion (93.0%) of the youngest patients receiving a transplant compared with the oldest (72.2%) (P < 0.0001). Of those transplanted, 18.1% were pre-emptive and 62.5% received a transplant after one dialysis modality. In terms of first transplant type, the proportion of living donor transplants increased with age (P < 0.0001), with a decreasing use of deceased donor transplants with increasing age, though this was non-linear, so the biggest change was seen between the 11–<16 and 16–<21 age groups. The overall median time to receiving a deceased donor transplant from listing was 13 months, and increased with age group (P < 0.0001). The difference in waiting time between the youngest and oldest groups was 1.67 times longer, or 11 months, reflecting preferential deceased donor allocation to younger patients. During the study period, 2522 of the 2696 (93.5%) grafts remained functioning at 1 year, with no difference by age group. These results demonstrate that in young adult patients starting RRT, transplantation occurs early and, in the majority, using a roughly equal proportion of living and deceased donors.
Table 1.
Transplantation in the first 5 years of RRT in incident UK young adults
| 11–< 16 years | 16–< 21 years | 21–< 26 years | 26–30 years | Total | |
|---|---|---|---|---|---|
| Transplant status, n (%) | |||||
| Transplanted in first 5 years of RRT | 480 (93.0) | 568 (80.9) | 669 (75.4) | 913 (72.2) | 2630 (78.0) |
| Listed and awaiting transplant | 20 (3.9) | 72 (10.3) | 107 (12.1) | 169 (13.4) | 368 (10.9) |
| Not listed for transplant | 16 (3.1) | 62 (8.8) | 111 (12.5) | 183 (14.5) | 372 (11.0) |
| Transplant timing*, n (%) | |||||
| Pre-emptive | 135 (28.1) | 113 (19.9) | 94 (14.1) | 134 (14.7) | 476 (18.1) |
| As second modality | 277 (57.7) | 342 (60.2) | 435 (65.0) | 591 (64.7) | 1645 (62.5) |
| As third modality | 52 (10.8) | 91 (16.0) | 106 (15.8) | 149 (16.3) | 398 (15.1) |
| ≥ fourth modality | 16 (3.3) | 22 (3.9) | 34 (5.1) | 39 (4.3) | 111 (4.2) |
| Transplant type, n (%) | |||||
| DBD | 317 (66.0) | 257 (45.2) | 286 (42.8) | 428 (46.9) | 1288 (49.0) |
| DCD | 4 (0.8) | 34 (6.0) | 63 (9.4) | 78 (8.5) | 179 (6.8) |
| Live | 159 (33.1) | 276 (48.6) | 315 (47.1) | 398 (43.6) | 1148 (43.7) |
| Missing | 0 (0.0) | 1 (0.2) | 5 (0.8) | 9 (1.0) | 15 (0.6) |
| Time to cadaveric transplant from listing (median years)* | 0.5 | 0.8 | 1.5 | 1.4 | |
| Transplant loss | |||||
| 1 year transplant failure, n (%) | 38 (7.5) | 40 (6.9) | 46 (6.7) | 50 (5.4) | 174 (6.5) |
| Total number with 1-year follow-up | 504 | 582 | 682 | 928 | 2696 |
DBD, donation after brain death; DCD, donation after circulatory death.
*P < 0.0001, chi-square test.
Transfer to adult services. As shown in Supplementary Table S3, during the study period 568 patients started RRT in paediatric centres and transferred to an adult centre. Overall 82.1% started RRT in an adult renal unit, but this partially reflects our choice of age groups to include in the analysis. Of those 16–18 years of age, 176 (63%) started in an adult unit and 103 (37%) started in a paediatric unit. The median age of transfer was 18 years.
Survival
Of the young adults who started RRT during the study period, 283 (8.3%) died within 5 years. The median time to death was 1.72 years (interquartile range 0.66–2.98). Supplementary Figure S1 shows the Kaplan–Meier survival plot by age group. Survival was worse with increasing age; the proportion of patients that died was 3.3, 6.6, 9.0 and 11.1%, respectively, for age groups 11–<16, 16–<21, 21–<26 and 26–30 years (P < 0.0001). Mortality and survival data shown in Table 2 demonstrate a higher crude mortality with older age group but no linear trend in standardized mortality ratios (SMR). The overall crude mortality was 18.0 (95% CI 16.0–20.2) and the SMR was 45.1. The cumulative observed survival decreased with older age group and was 0.91 overall. This was hardly changed in the cumulative relative survival (improved by 0.003, or 3%). Therefore, survival was worse for older age groups with or without adjustment for background general population mortality risk.
Table 2.
Mortality and survival by age group in incident young adult RRT patients compared with the general population
| Crude mortality rate/1000 patient-years |
Standardized mortality ratio | Cumulative survival |
|||
|---|---|---|---|---|---|
| Young adult RRT patients (95% CI) | Age-matched general populationa | Observed survival in young adult RRT patients | Relative survivalb (95% CI) | ||
| Age group (years) | |||||
| 11–< 16 | 6.77 (2.90–15.8) | 0.14 | 49.9 | 0.961 | 0.962 (0.938–0.978) |
| 16–< 21 | 13.8 (10.3–18.4) | 0.38 | 36.0 | 0.924 | 0.926 (0.901–0.946) |
| 21–< 26 | 19.4 (10.1–37.3) | 0.48 | 40.8 | 0.899 | 0.902 (0.877–0.921) |
| 26–30 | 24.2 (13.0–45.1) | 0.58 | 41.9 | 0.876 | 0.899 (0.857–0.898) |
| Overall | 18.0 (16.0–20.2) | 0.40 | 45.1 | 0.905 | 0.908 (0.896–0.918) |
Using 2001–14 mortality data from the ONS, an equivalent period to the study follow-up (2000–13).
Cumulative relative survival accounts for the background risk of dying in the age-matched general population [14].
Table 3 displays a multivariable Cox regression model analysing the influence of other variables in addition to age group on the risk of death in incident young adults on RRT. Univariate Cox regression (data shown in text only) showed a strong linear effect of age group at RRT start on risk of death, with an HR of 2.33 (95% CI 1.25–4.34), 3.34 (1.85–6.01) and 4.02 (2.27–7.11), respectively, for age groups 16–<21, 21–<26 and 26–30 compared with 11–<16 (P < 0.0001). Adjusting for confounders reduced the effect of age group. The strongest effect was seen with modality; being on dialysis and not listed for transplant drastically increased the risk of death compared with transplantation [HR 16.6 (95% CI 10.8–25.4) P < 0.0001]. PRD had a major effect on survival; compared with glomerulonephritis, diabetics were nearly five times more likely to die [HR 4.03 (95% CI 2.71–6.01), P < 0.0001]. Other tubulointerstitial disorders [HR 6.49 (95% CI 4.07–10.4), P < 0.0001], miscellaneous renal conditions [HR 1.88 (95% CI 1.28–2.76), P = 0.001] and other systemic diseases [HR 1.93 (95% CI 1.08–3.48), P = 0.03] also increased the risk of death. Late presentation of ESRD did not explain the findings, as excluding deaths in the first 90 days did not affect the HRs, and there were similar effects between HD and PD, with low power due to reduced sample size.
Table 3.
Cox regression model on the effect of age group at RRT start on mortality, adjusting age group by other variables
| Variable | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| Age group (years) | |||
| 11–<16 | 1.00 | ||
| 16–<21 | 1.87 | 0.95–3.67 | 0.07 |
| 21–<26 | 2.12 | 1.11–4.05 | 0.02 |
| 26–30 | 2.04 | 1.07–3.87 | 0.03 |
| Sex | |||
| Male | 1.00 | ||
| Female | 1.13 | 0.88–1.46 | 0.3 |
| Ethnicity | |||
| White | 1.00 | ||
| Asian | 0.59 | 0.36–0.98 | 0.04 |
| Black | 0.71 | 0.43–1.15 | 0.2 |
| Other | 0.64 | 0.30–1.38 | 0.3 |
| Year of start | |||
| 1999-2002 | 1.35 | 1.00–1.83 | 0.05 |
| 2003-2005 | 0.97 | 0.71–1.33 | 0.9 |
| 2006-2008 | 1.00 | ||
| Modality | |||
| Transplant | 1.00 | ||
| Dialysis, transplant listed | 4.20 | 2.61–6.75 | <0.0001 |
| Dialysis, not transplant listed | 16.6 | 10.8–25.4 | <0.0001 |
| Primary renal diagnosis | |||
| Glomerular Diseasea | 1.00 | ||
| Familial/hereditary nephropathies | |||
| Other familial/hereditary nephropathies | 0.59 | 0.21–1.66 | 0.3 |
| Polycystic kidney disease | 1.07 | 0.33–3.44 | 0.9 |
| Miscellaneous renal disorders | 1.88 | 1.28–2.76 | 0.001 |
| Systemic diseases affecting the kidney | |||
| Diabetesb | 4.03 | 2.71–6.01 | <0.0001 |
| Other systemic diseasesc | 1.93 | 1.08–3.48 | 0.03 |
| Tubulointerstitial disease | |||
| Obstructive | 1.37 | 0.78–2.40 | 0.3 |
| Renal dysplasia ± reflux | 0.43 | 0.20–0.97 | 0.04 |
| Other tubulointerstitial diseased | 6.49 | 4.07–10.4 | <0.0001 |
Data based on 3243 patients and 248 events and excludes those with a missing ethnicity or PRD.
Glomerular disease was chosen as a comparator, as it was the most frequent diagnosis.
There was a significant non-proportionality over time between those with and without diabetes, with the effect seen only after 230 days and no effect on other HRs when using a piecewise Cox regression analysis; this model presents the overall HR for the entire follow-up period.
PRD codes in the ‘other systemic diseases’ group include amyloid, haemolytic uraemic syndrome, renovascular diseases and hypertension. Of those with amyloid (n = 15), 33.3% died, while the proportion of death from other conditions was 6.6, 8.6 and 6.5%, respectively.
PRD codes in the ‘other tubulointerstitial disease’ group include drug-induced tubulopathies and interstitial nephritis; of those with drug-induced tubulopathies (n = 65), 32.3% died and of those with interstitial nephritis (n = 45), 15.6% died.
DISCUSSION
These new national UK results provide information on an understudied population and show how the clinical epidemiology, treatment and survival of young adults on RRT change with age. We have created a unique resource linking two separate datasets, bridging the gap between paediatric and adult registries. Applying this approach to other disease registers could yield similar comprehensive data for young adulthood. Linkage was successful, with almost all paediatric cases being traced into the adult database. Our long time period enabled us to collate a relatively large population sample and gave us power to test a variety of hypotheses.
We highlight several points: first, half the cohort started RRT on HD, with about a third having started dialysis within 90 days of the first nephrology review. Second, 1 in 10 young adults were not listed for transplant by 5 years and were ∼20 times more likely to die than those transplanted. Almost 1 in 10 young adults died by 5 years from RRT start, a rate similar to that of malignant melanoma [17]. We need to better understand the reasons for these observations. Third, a diabetic PRD confers additional risk with a 5-fold higher risk of death compared with glomerulonephritis. Since 17% of 26–30 year olds had ESRD due to diabetes, this is a key group for further research.
Our report would benefit from items we were unable to report, including comorbidity (low completeness), reasons patients were unfit for transplant and cause of death (missing in 46% of cases and coded as ‘Other’ in a further 18%). Future work includes linking to ONS data to evaluate cause of death and to report dialysis access and peritonitis rates, which may add important context to the treatment modality trends. Our dataset was not designed to evaluate longer-term graft loss, as the transplant follow-up time was unequal, but we were able to report the 1-year rate. Further, assessing the impact of social deprivation using postcode data collected by the UKRR would be valuable.
Our data have delineated how PRD distributions change with age in young adults. The decrease in tubulointerstitial diseases is likely due to the reduction in structural renal disorders with advancing age, and we clearly see the emergence of end-organ failure due to diabetes in the 26–30-year age group. The proportion of ESRD due to diabetes in this age group (17.4%) was very similar to that of UK adults in 2014 (16.1%) [2]. Miscellaneous renal disorders include unknown/uncertain aetiology diagnoses, and the increase in these may reflect a higher frequency of these with advancing age. Of this group, 56% were male, which may suggest the presence of undiagnosed structural problems. Due to different PRD groupings, direct comparison between UK paediatric and adult PRD data is restricted; however, our results show the changing pattern from predominant structural renal disease in childhood through to increasing diabetes, hypertension and renovascular diseases in late adulthood. The PRD data are consistent with previous evidence in prevalent UK young adults 0–39 years of age [18]. UK paediatric RRT data contribute 20% of European paediatric registry data, suggesting the same patterns are likely to be seen [19]. From an international perspective, the age, sex and PRD ratios are comparable with a global cohort of young adult HD patients 18–30 years of age [20].
Although pre-emptive transplant rates clearly decrease with increasing age, the high proportion of unspecified dialysis in the 11–<16-year age group clouds comparison of trends in the use of HD and PD at RRT start. Nevertheless, the use of PD at RRT start was stable at ∼30% across the older age groups. Compared with adults in the UK in 2014, where 8.2% started RRT with a transplant and 20.0% started on PD, pre-emptive transplantation and PD rates are higher in young adults [21], perhaps due to PD being seen as more compatible with a young person’s lifestyle. A higher proportion (29.3%) of cases was known to have started dialysis within 90 days of the first nephrology review, compared with 25.5% of children from 1996 to 2012 [22] and 18.0% of UK adults in 2014 [21]. However, this is based on limited ‘first seen date’ data (50% completeness). Assuming that an anticipated RRT start would preferentially involve a transplant or PD over HD would suggest high numbers of unexpected starts in our dataset, with more than half the cases starting RRT on HD. Our data in Figure 6 show PD is the least used modality overall, and it would be helpful to better understand this.
The majority (81.9%) of young adults starting RRT experienced dialysis prior to transplantation, which highlights the need for appropriate dialysis preparation. The use of donation after circulatory death (DCD) kidney transplants emerges during young adulthood. The source of donor kidneys used in transplantation was roughly equal between donation after brainstem death (DBD) (49%) and living kidney transplants (44%), with the remainder being DCD (7%) for this group of young adult patients in the first 5 years of RRT. This ratio is similar to that seen with prevalent UK children in 2014 [1], but with increasing use of DCD kidneys in young adults. In adults in 2012, the ratio of patients receiving DBD (36%) and living kidney transplants (38%) was equal, with a much higher use of DCD kidneys (26%) [23]. Those in the two older age groups waited 11 months longer than those in the youngest group for a deceased donor transplant, reflecting UK allocation policy [3].
Almost 1 in 10 young adults had died by 5 years from RRT start, with an inverse survival relationship with age. This may be because the 26–30 year olds had the highest proportions of dialysis use, not being transplanted by 5 years and diabetes. The lower pre-emptive transplant rates and increased use of dialysis in the older age groups may suggest a suboptimal pre-ESRD period, and the cause of renal failure may explain barriers to transplantation. This evidence would benefit from further research, including psychosocial aspects. Other studies using US registry data have shown an SMR of ∼35 for 10–15 year olds and 15 for 16–21 year olds in the first year post-transplant [24]. Our data in the first 5 years for all RRT showed SMRs of ∼50 and 36 for comparable age groups. The HR we present for those on dialysis and transplant listed (4.2) is similar to that of previous studies in patients <21 years of age receiving a first transplant (4.85) [25]. Compared with our data, higher overall mortality of 38.6 per 1000 patient-years for those 5–< 21 years of age has been reported [26], although this study censored at transplantation and included younger children who have poorer survival. Our relative survival analysis showed that the cumulative observed survival and survival relative to the general population were broadly similar and indicate that any excess death is likely to be renal related, taking into account increasing mortality with age in the general population.
We found an increased risk of death for those with diabetes, other tubulointerstitial disease, other systemic diseases and miscellaneous renal disorders. The increased HR seen in the Cox regression model confirms other European data where patients with multisystem disorders and rare diseases had a significantly higher risk of death compared with other groups [27]. The other tubulointerstitial disease group HR result was influenced by deaths in those with drug-induced tubulopathies, where a third of cases died—possibly explained by malignancy and treatment with platinum-based chemotherapy. The other systemic diseases group result was similarly influenced by deaths in those with amyloid, where again a third of cases died—perhaps related to the disease itself or potentially toxic treatment regimens. Survival of incident young adults starting RRT appears worse than in children but better than in adults and older adults, showing poorer survival with advancing age. Of our cohort, 91.7% were alive at 5 years. Of children <16 years of age starting RRT in the UK (2000–13), 6% died over 3.5-years median follow-up [1]. In 2014, the unadjusted 5-year survival of UK incident adult patients 18–64 years of age was 71.1%, and 32.9% for those ≥65 years of age [28].
In summary, more than half the young adults in our cohort started RRT on HD. In a Cox regression model, 1 in 10 young adults were not listed for transplant by 5 years and were ∼20 times more likely to die than those who were transplanted. Diabetes as a primary renal disease was common among young adults and associated with increased mortality. Overall, almost 1 in 10 young adults had died by 5 years from the start of RRT.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank all the patients contributing data to the UKRR, the UKRR staff and all the participating centres. They gratefully acknowledge the help of NHS Blood and Transplant, Dr Sian Griffin, Sheila Tickle, Jodi Paget, Julie McEntee and Hayley Stark in providing missing data. They also thank the British Kidney Patient Association and Kidney Research UK, whose contribution through the Tony Wing award contributed to this work.
FUNDING STATEMENT
This study was supported by SP/TWA/2013 (A.H.) from the BKPA and Kidney Research UK.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose. We declare that the results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Hamilton AJ, Braddon F, Casula A. et al. UK Renal Registry 18th Annual Report: Chapter 4 demography of patients receiving renal replacement therapy in paediatric centres in the UK in 2014. Nephron 2016; 132(Suppl 1): 99–110 [DOI] [PubMed] [Google Scholar]
- 2. MacNeill SJ, Casula A, Shaw C. et al. UK Renal Registry 18th Annual Report: Chapter 2 UK renal replacement therapy prevalence in 2014: national and centre-specific analyses. Nephron 2016; 132(Suppl 1): 41–68 [DOI] [PubMed] [Google Scholar]
- 3. Zalewska K. Kidney Transplantation: Deceased Donor Organ Allocation http://www.odt.nhs.uk/pdf/kidney_allocation_policy.pdf (1 September 2016, date last accessed).
- 4. Oniscu GC, Schalkwijk AA, Johnson RJ. et al. Equity of access to renal transplant waiting list and renal transplantation in Scotland: cohort study. BMJ 2003; 327: 1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dudley CR, Johnson RJ, Thomas HL. et al. Factors that influence access to the national renal transplant waiting list. Transplantation 2009; 88: 96–102 [DOI] [PubMed] [Google Scholar]
- 6. Foster BJ, Dahhou M, Zhang X. et al. Association between age and graft failure rates in young kidney transplant recipients. Transplantation 2011; 92: 1237–1243 [DOI] [PubMed] [Google Scholar]
- 7. Foster BJ. Heightened graft failure risk during emerging adulthood and transition to adult care. Pediatr Nephrol 2015; 30: 567–576 [DOI] [PubMed] [Google Scholar]
- 8. Van Arendonk KJ, James NT, Boyarsky BJ. et al. Age at graft loss after pediatric kidney transplantation: exploring the high-risk age window. Clin J Am Soc Nephrol 2013; 8: 1019–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watson AR. Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol 2000; 14: 469–472 [DOI] [PubMed] [Google Scholar]
- 10. Giedd JN. The teen brain: insights from neuroimaging. J Adolesc Health 2008; 42: 335–343 [DOI] [PubMed] [Google Scholar]
- 11. Ansell D, Feest T.. The First Annual Report—The UK Renal Registry—September 1998. Bristol: The Renal Association, 1998, 1–150 [Google Scholar]
- 12. Ansell D. UK. Renal Registry 11th Annual Report (December 2008): Chapter 1 Summary of findings in the 2008 UK Renal Registry Report. Nephron 2009; 111(Suppl 1): c1–2 [DOI] [PubMed] [Google Scholar]
- 13. Ansell D, Feest T, Will E.. The Second Annual Report of the UK Renal Registry. Bristol: The Renal Association, 1999, 1–123 [Google Scholar]
- 14. Venkat-Raman G, Tomson CR, Gao Y. et al. New primary renal diagnosis codes for the ERA-EDTA. Nephrol Dial Transplant 2012; 27: 4414–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Office for National Statistics. http://www.ons.gov.uk/. (7 January 2016, date last accessed).
- 16. Dickman PW, Coviello E.. Estimating and modeling relative survival. Stata J 2015; 15: 186–215 [Google Scholar]
- 17.Cancer Research UK. Cancer Survival for Common Cancers http://www.cancerresearchuk.org/health-professional/cancer-statistics/survival/common-cancers-compared#heading-Zero. (7 January 2016, date last accessed).
- 18. Neild GH. Primary renal disease in young adults with renal failure. Nephrol Dial Transplant 2010; 25: 1025–1032 [DOI] [PubMed] [Google Scholar]
- 19. Schaefer F, Groothoff J, Coppo R. et al. ESPN/ERA-EDTA Registry Paediatric Data 2012. Amsterdam: ESPN/ERA-EDTA Registry, 2014. [Google Scholar]
- 20. Ferris M, Gibson K, Plattner B. et al. Hemodialysis outcomes in a global sample of children and young adult hemodialysis patients: the PICCOLO MONDO cohort. Clin Kidney J 2016; 9: 295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gilg J, Caskey F, Fogarty D.. UK Renal Registry 18th Annual Report: Chapter 1 UK renal replacement therapy incidence in 2014: national and centre-specific analyses. Nephron 2016; 132(Suppl 1): 9–40 [DOI] [PubMed] [Google Scholar]
- 22. Pruthi R, Casula A, Inward C. et al. Early requirement for RRT in children at presentation in the United Kingdom: association with transplantation and survival. Clin J Am Soc Nephrol 2016; 11: 795–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson RJ, Bradbury LL, Martin K. et al. Organ donation and transplantation in the UK—the last decade: a report from the UK national transplant registry. Transplantation 2014; 97(Suppl 1): S1–S27 [DOI] [PubMed] [Google Scholar]
- 24. Foster BJ, Dahhou M, Zhang X. et al. Change in mortality risk over time in young kidney transplant recipients. Am J Transplant 2011; 11: 2432–2442 [DOI] [PubMed] [Google Scholar]
- 25. Laskin BL, Mitsnefes MM, Dahhou M. et al. The mortality risk with graft function has decreased among children receiving a first kidney transplant in the United States. Kidney Int 2015; 87: 575–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitsnefes MM, Laskin BL, Dahhou M. et al. Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990–2010. JAMA 2013; 309: 1921–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kramer A, Stel VS, Tizard J. et al. Characteristics and survival of young adults who started renal replacement therapy during childhood. Nephrol Dial Transplant 2009; 24: 926–933 [DOI] [PubMed] [Google Scholar]
- 28. Steenkamp R, Rao A, Fraser S.. UK Renal Registry 18th Annual Report (December 2015) Chapter 5: Survival and causes of death in UK adult patients on renal replacement therapy in 2014: National and centre-specific analyses. Nephron 2016; 132(Suppl 1): 111–144 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.