Abstract
Background
Many interventions delivered to improve health may benefit not only direct recipients but also people in close physical or social proximity. Our objective was to review all published literature about the spillover effects of interventions on health outcomes in low-middle income countries and to identify methods used in estimating these effects.
Methods
We searched 19 electronic databases for articles published before 2014 and hand-searched titles from 2010 to 2013 in five relevant journals. We adapted the Cochrane Collaboration’s quality grading tool for spillover estimation and rated the quality of evidence.
Results
A total of 54 studies met inclusion criteria. We found a wide range of terminology used to describe spillovers, a lack of standardization among spillover methods and poor reporting of spillovers in many studies. We identified three primary mechanisms of spillovers: reduced disease transmission, social proximity and substitution of resources within households. We found the strongest evidence for spillovers through reduced disease transmission, particularly vaccines and mass drug administration. In general, the proportion of a population receiving an intervention was associated with improved health. Most studies were of moderate or low quality. We found evidence of publication bias for certain spillover estimates but not for total or direct effects. To facilitate improved reporting and standardization in future studies, we developed a reporting checklist adapted from the CONSORT framework specific to reporting spillover effects.
Conclusions
We found the strongest evidence for spillovers from vaccines and mass drug administration to control infectious disease. There was little high quality evidence of spillovers for other interventions.
Keywords: Spillover effects; indirect effects; herd effects; herd immunity; diffusion; externalities; interference
Key Messages
Spillovers are the effects of an intervention on individuals who did not receive the intervention but who are connected to recipients through physical or social proximity.
Our systematic review found a wide range of terminology used to describe spillover effects, a lack of standardization among spillover measurement methods and poor reporting of spillover effects in many studies.
The strongest evidence for spillover effects exists for studies of vaccines and mass drug administration to control infectious disease. The evidence of spillover effects for other interventions is of limited or poor quality.
To facilitate improved reporting and standardization in future spillover studies, we developed a reporting checklist adapted from the CONSORT framework, specific to reporting of spillover effects.
Introduction
Interventions delivered to improve health are frequently targeted to specific populations. Such interventions may benefit not only direct recipients but also those who did not receive the intervention but are connected to recipients through physical or social proximity. Such effects, which we refer to as ‘spillovers’, are a component of the population-level impact of interventions. A wide range of terms has been used to describe spillovers in disciplines including economics, public health and political science: externalities,1,2 interference,3–7 contamination,8 herd immunity,9–11 stable unit treatment value assignment (SUTVA) violations,12 stability violations13 and indirect effects.14,15
A ‘positive’ spillover is an effect in the same direction as the treatment effect (on intervention recipients); conversely, a ‘negative’ spillover is an effect in the opposite direction of the treatment effect. If positive spillovers are present, studies that only estimate treatment effects without measuring spillover effects will underestimate the effectiveness of the intervention. In addition, cost-effectiveness calculations that exclude such spillovers may underestimate intervention benefits. Conversely, if negative spillovers are present, evaluations that do not measure spillover effects may overestimate health impacts and cost-effectiveness. Furthermore, negative spillovers could attenuate the effects of an otherwise beneficial intervention. For these reasons, when an intervention is capable of diffusing through a population, information about spillover effects is an important complement to estimates of treatment effects—when spillovers are found to be large and positive, such evidence may, for example, justify national scale-up of an intervention or a public subsidy.16 The well-documented evidence of spillovers (i.e. ‘herd effects’) of many vaccines justifies the cost-effective scale-up of immunization efforts to a global level via programmes such as the Global Alliance for Vaccines and Immunization.17,18
In the epidemiological literature focusing on trials of interventions other than vaccines, spillovers have motivated randomizing clusters rather than individuals in order to minimize the chance of spillovers into control units (i.e. ‘contamination’).8,19,20 Outside vaccine studies, epidemiologists consider spillovers in designing studies (e.g. the expectation of spillovers may motivate cluster-randomization instead of individual-randomization8,19,20), but they typically do not estimate spillovers explicitly alongside direct effects. Recently, spillovers have increasingly been framed as a quantity of interest themselves, particularly in economics where a growing literature describes spillovers of interventions including school-based deworming2 and insecticide-treated bed nets.21 On the whole, methods for estimating spillovers have developed independently within disciplines.
We conducted this systematic review in order to summarize the literature about spillover effects on health in low- and middle-income countries. We restrict our review to such countries because this review was supported by the International Initiative for Impact Evaluation (3ie), which focuses on low- and middle-income countries.22 Our objective is to provide a broad summary of the types of spillover effects that have been measured to date.
Methods
Protocol and registration
We attempted to register our protocol with the Campbell Coordination International Development Coordinating Group (IDCG). However, because our protocol included a synthesis of methods in addition to a systematic review, the IDCG did not accept our protocol. Instead, the International Initiative for Impact Evaluation (3ie), which funded this endeavour, supported the development of the protocol and provided both internal and external review.
Eligibility criteria
A complete description of eligibility criteria is available in Supplement 1, as Supplementary data at IJE online. Briefly, we included studies that: (i) were conducted in low- or middle-income countries as defined by the World Bank23 (as required by our funder); (ii) were quantitative studies evaluating an intervention; (iii) measured health outcomes; and (iv) included a comparison group with sufficient detail about the design and comparison group to determine whether there were serious threats to internal or external validity.
Information sources
We searched 19 electronic databases that contained articles on health, economics, social science, and other disciplines for articles published before 2014 (Supplement 2, available as Supplementary data at IJE online). In addition, we hand-searched all titles from 2010 through 2013 in the following journals, which we considered most likely to include relevant articles: Health Economics, The Journal of Development Effectiveness, The Lancet, PLoS Medicine and the World Bank Policy Research Working Papers.
Search
A detailed description of our search strategy is listed in Supplement 3, available as Supplementary data at IJE online. We searched reference lists of texts classified as eligible in the original search. We also identified records that cited included texts from the original search using Google Scholar. Following the search process, all records were merged, duplicates were removed and a unique ID was assigned to each record.
Study selection
At least one team member reviewed each record for relevance. Titles that were clearly not eligible for the review received no further review. We reviewed each available abstract that passed the title review for relevance. If an abstract was not available but a full text was, we reviewed the full text instead. Of the abstracts deemed relevant, we reviewed each full text for relevance. For records that were deemed not to be relevant, team members recorded the first reason for exclusion identified. If multiple versions of a paper were available, we included the most recent version of the paper.
Data collection process
We extracted data from included texts, and then a second team member independently checked all extracted data. In one case, spillover results were mentioned and disaggregated results were not listed in the publication, but the authors mentioned that results were available upon request.24 We contacted the authors to request these results but did not receive a reply.
Data items
We extracted information about: interventions; outcomes measured; study site; primary study design; study design used to estimate spillovers; purported spillover mechanism; scale of spillover (e.g. household versus village); average cluster-level treatment coverage; whether or not spillover measurement was pre-specified; and direct effect, total effect, overall effect and spillover effects reported numerically in tables or text. If multiple effects or model specifications were used to estimate the direct, total or overall effects, we chose the estimate that appeared to be the primary finding reported by the author and that allowed the greatest comparability of the effect with the spillover estimates. We considered spillovers to be pre-specified if spillover estimation methods were included in the original study protocol.
Risk of bias in individual studies
We classified specific criteria related to risk of bias for each study using criteria compiled from relevant fields.25–29 Duplicate assessment of risk of bias was performed for a 20% subsample. Classification was not blinded. Co-authors of this systematic review who had authored included studies did not participate in the classification of risk of bias criteria for any studies. For studies that performed secondary analyses, we attempted to obtain the original publication and incorporated information from the original publication(s) into our risk of bias assessment. We only assessed the risk of bias for the elements of the study that estimated effects on health outcomes. We also created an overall classification of risk of bias for individual studies by adapting the Cochrane GRADE approach30,31 to spillover estimation (Supplement 4, available as Supplementary data at IJE online). We developed these criteria through an iterative process in which we revised our classification system after the initial risk of bias assessment for each study and discussion with multiple reviewers. We then classified each study’s overall quality of evidence as ‘very low’, ‘low’, ‘medium’ or ‘high’.
Summary measures
Due to the wide range of interventions and outcomes evaluated in included studies, we did not consider it reasonable to assume that the studies included were independent and that a common treatment effect existed across all included studies.32 Thus, we did not calculate summary measures.
Synthesis of results
For spillover types for which a sufficient number of studies reported estimates, we standardized results for binary outcomes on the relative scale. We present results as the percentage reduction in outcomes attributable to intervention {[1- relative risk (RR)] x 100%}. For results reported only as risk differences, we calculated the percentage reduction by dividing the risk difference by the probability of the outcome in the control group. We chose the relative scale instead of the additive scale because the interpretation of risk differences depends upon the risk of the outcome among the untreated; given the wide range of interventions and outcomes included in this study, we consider the relative scale to be more appropriate because it facilitates direct comparison using a single measure. We did not synthesize results for continuous outcomes because very few studies measured the same continuous outcome. To generate forest plots, we converted estimates on the additive scale to the relative scale by dividing by the mean of the outcome among individuals not receiving treatment. In plots comparing estimates across studies, we presented 95% confidence intervals for the studies for which standard errors were reported or could be estimated on the relative scale. When possible, we used adjusted effect measures in these plots because many of the included studies used observational designs or used randomized designs that conditioned on a non-randomized variable (e.g. eligibility status) to measure spillovers. Thus, we consider adjusted estimates more appropriate because they are less likely to be biased than crude estimates. We excluded studies of low or very low quality from plots comparing results across studies.
Risk of bias across studies
To assess publication bias, we produced funnel plots. We produced separate plots for studies estimating risk ratios (or 1-RR) and risk differences because insufficient information was reported to standardize measures on a single scale. Funnel plots only included studies that estimated effects for binary outcomes. We did not produce funnel plots for estimates using continuous outcomes because the number of different outcomes measured would not have allowed for comparison across a useful number of studies.
Additional analyses
We searched each included text for terms commonly used to describe spillovers and noted whether the terms appeared in each text.
Results
Study selection
We retrieved 49 749 records through our search process (Supplement 7 Figure 1, available as Supplementary data at IJE online). Following removal of duplicate records and records from non-bibliographical sources, we reviewed 31 622 titles for relevance. We reviewed relevant abstracts and full texts and classified 28 studies from the original search as eligible. We performed title, abstract and full-text review on the reference lists of the 28 eligible texts (n = 798 records) and identified one additional eligible text. We also reviewed records that cited the 28 original included texts (n = 1622 records) and identified an additional 25 eligible texts. A total of 54 records were included in this systematic review. Reasons for exclusion of full texts are listed in Supplement 5, available as Supplementary data at IJE online. We extracted data from 51 studies. We could not extract data for two studies that only reported spillover effects graphically33,34 or for one study that did not provide numerical results for spillover estimates.24
Study characteristics
Studies were conducted in 17 low- or middle-income countries. The most common study design was cluster-randomized trials (n = 13 studies; 24%) followed by re-analyses of cluster-randomized trials (n = 9; 17%) and re-analyses of individually randomized trials (n = 7; 13%). The most common interventions were vaccines (n = 22; 41%), mass drug administration for infectious disease control (n = 7; 13%) and health education (n = 5; 9%). Several programmes were commonly evaluated for spillovers: the maternal and child health programme in Matlab, Bangladesh;35,36 the PROGRESA programme, which offered conditional cash transfers in Mexico;37,38 cholera vaccines provided in Matlab Bangladesh;39–44 and the Primary School Deworming Program in Busia, Kenya.2,45–47 Studies estimated a variety of different statistical parameters to quantify spillovers; we define these parameters in Supplement 8, available as Supplementary data at IJE online.
Risk of bias within studies
Six studies (11%) had high quality evidence, 30 (56%) had moderate quality, 12 (22%) had low quality and six (11%) had very low quality evidence (Supplement 6, available as Supplementary data at IJE online). Of studies with high quality evidence, five used cluster-randomized43,48–51 designs and one used a household secondary attack rate study design.52 The proportion of studies with low or very low quality evidence was similar in studies that incorporated spillover measurement into the original design (35%) compared with those which did not pre-specify spillover estimation (36%). All high quality studies were peer- reviewed.
Spillover mechanisms
We identified three primary types of spillover mechanisms in included studies.
Reduced disease transmission (n = 28 studies): interventions may decrease the infectiousness of an intervention recipient, and in turn, the risk that non-recipients become ill may decrease.
Social proximity (n = 20): interventions may create spillovers when individuals change their behaviour as a result of intervention and in turn influence the behaviour of non-recipients with whom they are in social proximity. Family members, neighbours, classmates or even residents of the same village or city could be considered socially proximate with varying degrees of closeness.
Substitution (n = 3): when one household member receives additional resources as a result of intervention, spillovers may occur to other household members because additional resources are available to the household. For example, if one child receives free meals at school, more food may be available for siblings to eat at home.
Results by spillover mechanism
In this section, we summarize studies by spillover mechanisms because they influence spillover magnitude, scale of spillovers, and appropriate study designs for detecting spillovers. Within each mechanism we summarize studies by intervention type. Within each of these categories, we describe results by intervention types. We excluded very low quality studies (n = 6) from this summary.
Spillovers through reduced disease transmission
Studies of spillovers through reduced disease transmission included studies of vaccines (n = 21), mass drug administration to control infectious disease (n = 6), improved water and sanitation (n = 3) and insecticide treated nets (n = 1) (Tables 1–2; Supplement 7 Table 1, available as Supplementary data at IJE online). These studies evaluated spillovers through two approaches: analyses with group-level data (i.e. ecological analyses) and with individual-level data.
Table 2.
Spillover estimates from studies that estimated spillovers through reduced transmission using individual-level data and that measured spillovers as a function of distance to treated individuals
| Reference / country / quality of evidencea | Parameter type (in bold) and parameter description | Intervention | Distance / area over which spillovers were measured | Outcome / subgroup / time point | Estimate (95% CI) |
|---|---|---|---|---|---|
|
|
Cholera vaccine | Neighbourhood of 64 individuals | Cholera risk per 1000 persons |
|
|
|
Insecticide-treated nets | 0–900 m | Clinical malaria |
|
| High-density parasitaemia |
|
||||
| Moderate anaemia |
|
||||
| Haemogloblin level |
|
||||
| Child mortality |
|
||||
|
|
Improved water supply | 3 km | Diarrhoea |
|
|
|
School-based deworming | 0–3 km |
|
|
| 3–6 km |
|
|
|||
|
|
School-based deworming | 6 km | Self-reported health is ‘very good’ | Probability difference: 0.128 (−0.097, 0.353) |
| Height |
|
||||
| Body mass index |
|
||||
| Number of pregnancies |
|
||||
| Any miscarriages | Probability difference: −0.078 (−0.151, −0.005) | ||||
|
|
Mass azithromycin distribution | Not applicable | Trachoma | No quantitative estimates were reported, but they concluded that spillovers were present |
|
|
Polio vaccine | Not applicable | Poliovirus | No quantitative estimates were reported, but they found that the rate of excretion among household contacts increased following vaccination |
aThe quality of evidence reported here applies to each study as a whole even if multiple types of spillovers were estimated.
bWe estimated approximate cluster-treatment coverage using available information in each paper.
cConfidence intervals present a best-case scenario as they are not necessarily adjusted for clustering.
dWe report findings from a replication study of the original study, which revised estimates after correcting for coding errors in the original study. Aiken AM, Davey C, Hargreaves JR, Hayes RJ. Re-analysis of health and educational impacts of a school-based deworming programme in western Kenya: a pure replication. Int J Epidemiol 2015;44:1572–80.
eThis parameter was not explicitly estimated, but it could have been using the data collected in the study.
Table 1.
Spillover estimates from studies that estimated spillovers through reduced transmission using individual-level data measured within clusters
| Reference / country / quality of evidencea | Parameter type (in bold) and parameter description | Intervention | Cluster size and treatment coverageb | Outcome / subgroup / time point | Estimate (95% CI) |
|---|---|---|---|---|---|
|
|
Cholera vaccine |
|
Cholera | (1-RR) x 100%: 0% (−59%, 37%) |
|
|
Pertussis vaccine |
|
Pertussis |
|
|
Pertussis |
|
|||
|
|
Pertussis vaccine |
|
Pertussis |
|
|
|
School-based deworming |
|
Raven’s matrices score (measure of intelligence) | Mean difference: 0.220 (0.067, 0.373) |
| Height | Mean difference: 0.204 (−0.378, 0.786) | ||||
| Height-for-age | Mean difference: 0.029 (−0.057, 0.115) | ||||
| Stunting (height-for-age z-score < -2) | Risk difference: 0.007 (−0.024, 0.038) | ||||
|
|
Pneumococcal conjugate vaccine |
|
Pneumococcal nasopharyngeal carriage among children 2 to <5 years | Odds ratio: 0.28 (0.11, 0.70) |
| Pneumococcal nasopharyngeal carriage among children 5 to < 15 years | Odds ratio: 0.25 (0.14, 0.46) | ||||
| Pneumococcal nasopharyngeal carriage among children 15 years or older | Odds ratio: 0.43 (0.17, 1.10) | ||||
|
|
Pneumococcal conjugate vaccine |
|
Pneumococcal nasopharyngeal carriage among children 2.5 to < 5 years | Odds ratio: 0.15 (0.07, 0.33) |
| Pneumococcal nasopharyngeal carriage among children 5 to < 15 years | Odds ratio: 0.21 (0.10, 0.42) | ||||
| Pneumococcal nasopharyngeal carriage among children 15 years or older | Odds ratio: 0.02 (0.003, 0.18) | ||||
|
|
Typhoid vaccine |
|
Typhoid incidence |
|
|
|
Typhoid vaccine |
|
Typhoid incidence |
|
|
|
School-based deworming |
|
Moderate-heavy helminth infection | Risk difference: −0.18 (−0.32,−0.04) |
|
|
Pneumococcal conjugate vaccine |
|
Pneumococcal nasopharyngeal carriage among children ≥ 5 years | Prevalence ratio: 0.34 (0.18, 0.62) |
|
|
Mass azithromycin distribution |
|
Trachoma | (1-RR) x 100%: 35% (8%, 55%)e |
|
|
Mass azithromycin distribution |
|
Trachoma |
|
|
|
Pneumococcal conjugate vaccine |
|
Pneumococcal carriage | Hazard ratio: 0.39 (0.26, 0.58). |
aThe quality of evidence reported here applies to each study as a whole even if multiple types of spillovers were estimated.
bWe estimated approximate cluster-treatment coverage using available information in each paper.
cThe manuscript labels this parameter vaccine efficacy against transmission; however, we refer to it as vaccine efficacy for infectiousness based on the definition in Halloran E, Longini IM Jr, Struchiner CJ. Design and Analysis of Vaccine Studies. New York, NY: Springer, 2010.
dWe used estimates from the replication study published in Aiken AM, Davey C, Hargreaves JR, Hayes RJ. Re-analysis of health and educational impacts of a school-based deworming programme in western Kenya: a pure replication. Int J Epidemiol 2015;44:1572–80.
eConfidence intervals present a best-case scenario as they are not necessarily adjusted for clustering.
Eleven of the 13 studies that evaluated spillovers using group-level data found that the risk of illness declined as treatment coverage increased, suggesting that spillovers were present. Six studies evaluated spillovers of the cholera vaccine and found that cholera risk decreased among unvaccinated individuals as vaccine coverage increased (Supplement 7 Table 1 and Figure 2 Panel A).39–42,44,53–60 No such pattern was evident among vaccinated individuals, suggesting that spillover effects did not yield additional protection beyond that conferred by the vaccine itself (Supplement 7 Figure 2 Panel B).39,40,53,54 Five of these studies re-analysed data from the same trial, so their findings cannot be considered independent.39–43 There are two significant limitations to this type of analysis in assessing spillovers. First, in observational studies or randomized trials without perfect compliance, this type of ecological comparison is likely to be confounded by factors associated with both treatment compliance and the outcome. For example, vaccination coverage may have been higher in high-income areas with better access to care, which may have partially explained lower illness levels in these areas. Second, spillover findings are likely to be highly sensitive to the definition of the area in which treatment coverage and outcomes were measured; groups of different sizes or composition may have produced different results.61 Thus, overall, we consider these findings to be of lower quality than findings from studies analysing individual-level data.
Seventeen studies evaluated spillovers through reduced disease transmission using individual-level data (Tables 1–2). We separated these studies into two categories: those that measured spillovers within clusters (e.g. households, villages), and those that measured spillovers as a function of distance from treated individuals. Among the studies measuring spillovers in clusters, we expected that spillovers would be larger in smaller-sized clusters (e.g. households) because reductions in disease transmission are most likely to impact on individuals in close proximity. In general, we did not find this to be the case. Two out of four studies of spillovers in households found relatively large spillover effects;52,62 both studies estimated the reduction in risk associated with living in households with individuals diagnosed with pertussis who were vaccinated versus unvaccinated for pertussis (the vaccine efficacy for infectiousness); the study in Senegal estimated an 85% [95% confidence interval (CI) 46%, 95%] risk reduction,52 and the study in Brazil estimated a 61.6% (95% CI 12.8%, 83.1%) risk reduction.62 Three of the eight studies measuring spillovers in larger clusters (e.g. schools, villages) found evidence of large spillovers.51,63,64 We expected that spillovers would be larger at higher levels of treatment coverage, and we found this to be true in the relevant studies: of the four studies with cluster-level treatment coverage under 50%, two found no spillover effects.53,65 Of those with treatment coverage over 50%, all found evidence of spillovers, and in four studies, spillover effects were relatively large.51,52,63,64 Among studies that measured spillovers as a function of distance from treated individuals, the magnitude of spillovers was smaller than in studies evaluating spillovers within clusters. However, this finding may be explained by intervention type—none of these studies evaluated vaccines. Spillovers decayed with distance from treated individuals in two studies.2,48
Spillovers through social proximity
Seventeen studies evaluated spillovers through social proximity (Table 3). One found evidence of negative spillovers,46 eight found no evidence of spillovers66–73 and eight found evidence of spillovers for some but not all outcomes or conditions reported.21,37,38,50,74–77 These studies measured spillovers through four mechanisms among untreated individuals who were: (i) in areas where cash transfers were offered; (ii) in or near areas where subsidies or microloans were offered to promote certain health products or behaviours (e.g. subsidies for vaccines); (iii) socially connected to treated individuals; or (iv) in the same schools or areas as treated individuals, regardless of social links.
Table 3.
Spillover estimates from studies that estimated spillovers through social proximity
| Reference / country / quality of evidencea/ scale at which spillovers were measured | Parameter type (in bold) and parameter description | Intervention | Outcome / subgroup / time point | Estimate (95% CI) |
|---|---|---|---|---|
|
Spillovers of cash transfer interventions | ||||
|
|
Conditional cash transfers | Cervical cancer screening | Mean difference: 0.061 (0.022, 0.100) |
| Blood sugar screening | Mean difference: 0.010 (−0.025, 0.045) | |||
| Blood pressure screening | Mean difference: 0.025 (−0.010, 0.060) | |||
|
|
Conditional cash transfers | Child nutrition surveillance 6 months after programme initiation | Mean difference: 2.307 (0.817, 3.797) |
| Child nutrition surveillance 12 months after programme initiation | Mean difference: 6.846 (2.632, 11.060) | |||
|
|
Conditional cash transfers | Child growth monitoring visits |
|
|
|
Conditional and unconditional cash transfers | Psychological distress among all untreated girls during the intervention | Mean difference: 0.064 (0.007, 0.121) |
| Psychological distress among all untreated girls after the intervention | Mean difference: 0.007 (−0.056, 0.070) | |||
| Psychological distress among untreated girls in households without treated girls during the intervention | Mean difference: 0.099 (0.038, 0.160) | |||
| Psychological distress among untreated girls in households without treated girls after the intervention | Mean difference: 0.001 (−0.058, 0.060) | |||
| Psychological distress among untreated girls in households with treated girls during the intervention |
|
|||
| Psychological distress among untreated girls in households with treated girls after the intervention |
|
|||
|
|
Conditional cash transfers | Self-reported to be ill at 1 year | Mean difference: −0.030 (−0.060, 0.000) |
| Self-reported to be ill at 4 years |
|
|||
| In bed as a result of illness at 1 year | Mean difference: 0.017 (−0.046, 0.080) | |||
| In bed as a result of illness at 4 years | Mean difference: 0.042 (−0.028, 0.112) | |||
| Hospitalized in the previous year at 1 year |
|
|||
| Hospitalized in the previous year at 4 years |
|
|||
|
Spillovers of interventions with subsidies or microloans | ||||
|
|
Subsidized insecticide-treated nets | Probability of ITN use when < 50% eligible for the subsidy |
|
| Probability of ITN use when ≥ 50% eligible for the subsidy |
|
|||
|
|
Immunization campaign without incentives | Number of immunizations |
|
| Immunization campaign with incentives | Number of immunizations |
|
||
| Immunization campaign without incentives | Child received ≥ 1 immunization |
|
||
| Immunization campaign with incentives | Child received ≥ 1 immunization |
|
||
| Immunization campaign without incentives | Child has BCG scar |
|
||
| Immunization campaign with incentives | Child has BCG scar |
|
||
| Immunization campaign without incentives | Child was completely immunized |
|
||
| Immunization campaign with incentives | Child was completely immunized |
|
||
|
|
Free insecticide-treated nets | Recently acquired at least one ITN |
|
| Fraction of household members slept under ITN last night |
|
|||
| Recently acquired at least one bed net | Mean difference: 0.183 (−0.091, 0.457) | |||
| Fraction of household members slept under bed net last night | Mean difference: 0.157 (−0.014, 0.328) | |||
|
Average per capita bed nets owned by peers | Recently acquired at least one ITN |
|
|
| Fraction of household members slept under ITN last night | Mean difference: 0.020 (−0.145, 0.185) | |||
| Recently acquired at least one bed net | Mean difference: 0.042 (−0.350, 0.434) | |||
| Fraction of household members slept under bed net last night | Mean difference: 0.056 (−0.216, 0.328) | |||
| Average ITN usage the previous night among peers | Recently acquired at least one ITN |
|
||
| Fraction of household members slept under ITN last night | Mean difference: 0.007 (−0.115, 0.129) | |||
| Recently acquired at least one bed net |
|
|||
| Fraction of household members slept under bed net last night |
|
|||
|
|
Incentives for voluntary counselling and testing for HIV | Probability of learning HIV test results |
|
| Investigators assessed whether a 1% increase in the proportion of neighbours within 0–0.5 km who received incentives for learning their HIV test results was associated with choosing to learn one’s HIV test results. The authors stratified results by gender, the proportion of neighbours over increasing distances, distance to HIV testing centres and other variables. See the paper for the full set of results. | Probability of learning HIV test results |
|
||
|
Spillovers through social connections to treated individuals | ||||
|
|
Online sexual health education | Knowledge index at 1 week | Mean difference: 0.015 (−0.073, 0.103) |
| Knowledge index at 6 months | Mean difference: 0.013 (−0.148, 0.174) | |||
| Attitude index at 1 week | Mean difference: 0.026 (−0.066, 0.118) | |||
| Attitude index at 6 months | Mean difference: 0.023 (−0.077, 0.123) | |||
| Condom voucher redemption at 6 months | Mean difference: 0.040 (−0.031, 0.111) | |||
|
Knowledge index at 6 months |
|
||
| Attitude index at 6 months |
|
|||
| Condom voucher redemption at 6 months |
|
|||
|
|
Peer support intervention | Depression score of peers of intervention recipients |
|
| Depression score of peers of controls recipients |
|
|||
|
|
School-based deworming | Deworming consumption |
|
|
Spillovers through residence in the same area as treated individuals, regardless of social links | ||||
|
|
Women’s empowerment programme | Tuberculosis vaccine | Mean difference: 0.149 (0.053, 0.245) |
| DTP vaccine | Mean difference: 0.122 (0.024, 0.220) | |||
| Measles vaccine | Mean difference: 0.268 (0.170, 0.366) | |||
|
Tuberculosis vaccine | Mean difference: 0.108 (0.059, 0.157) | ||
| DTP vaccine | Mean difference: 0.090 (0.045, 0.135) | |||
| Measles vaccine | Mean difference: 0.089 (0.042, 0.136) | |||
|
|
Proportion of girls who received information about HIV transmission | Probability of using a condom during sex for girls | Mean difference: 0.476 (0.111, 0.841) |
| Probability of using a condom during sex for boys | Mean difference: 0.109 (−0.254, 0.472) | |||
| Proportion of boys who received information about HIV transmission | Probability of using a condom during sex for girls |
|
||
| Probability of using a condom during sex for boys | Mean difference: 0.042 (−0.387, 0.471) | |||
|
|
Women’s groups and health service strengthening | Neonatal mortality among women exposed to women’s groups who did not participate in them (cluster-level spillover effect conditional on exposure to treatment) |
|
| Neonatal mortality among women in the same villages where the groups took place, who had not heard of them (cluster-level spillover effect) |
|
|||
|
|
Nutrition education | Weight-for-age z-score |
|
|
|
Community monitoring and provision of health services | Rate of outpatient visits |
|
| Mean deliveries per facility per month |
|
|||
aThe quality of evidence reported here applies to each study as a whole even if multiple types of spillovers were estimated.
bSubstitution is another possible mechanism of spillover in this paper.
c95% Confidence intervals could not be calculated due to insufficient information in the paper.
We hypothesized that spillovers would be stronger for interventions involving incentives or cash transfers than for those that did not because intervention uptake might be higher and the intervention might receive more attention from untreated individuals than interventions with no transfers or incentives. We also hypothesized that studies considering spillovers through social proximity might be more likely to detect spillovers if they considered social connections between treated and untreated individuals. However, we found neither of these to be true among the studies in this review. Three of the studies measuring spillovers of cash transfers found no evidence of spillovers,66–68 and two found evidence for some but not all outcomes measured.37,38 Even among the outcomes for which there was evidence of spillovers, the effect sizes were small. Two of four studies evaluating spillovers of subsidies or microloans for health products found evidence of spillovers.21,50,69,77 For example, in a study of incentives for immunization, Banerjee et al. estimated both total and spillover effects. The relative risk for the total effect on complete child immunization was 6.66 (95% CI 4.53, 8.80); for the spillover effect the relative risk was 3.47 (95% CI 2.18, 4.77).50 Two of the three studies that measured spillovers through social links to treatment recipients found no evidence of spillovers,70,71 and one found evidence of negative spillovers.46 Of the five studies measuring spillovers among untreated individuals in the same schools, villages or areas as treated individuals, one found evidence of spillovers,76 two found evidence for some but not all outcomes74,75 and two found no evidence of spillovers.72,73 Because the number of studies measuring spillovers through social proximity is relatively small and the types of interventions and outcomes measured varied widely, it is likely that the patterns we observed in this review do not necessarily generalize to the same interventions implemented in other contexts.
As for studies of spillovers through disease transmission, we also assessed whether spillover presence and effect sizes were associated with the size of the area in which spillovers were measured (e.g. household versus city). We did not find evidence of any patterns associated with area size.
Spillovers through substitution
Four studies measured spillovers through substitution (Table 4).24,78–80 Three studies measured whether siblings of children participating in school nutrition programmes or whose mothers participated in nutrition education programmes experienced improved growth as a result. None of these studies found evidence of spillovers.
Table 4.
Spillover estimates from studies that estimated spillovers through substitution
| Reference / country / quality of evidencea | Parameter type (in bold) and parameter description | Intervention | Outcome | Mean difference (95% CI) |
|---|---|---|---|---|
|
|
Information on infant nutrition and health | Height-for-age | −2.66 (−0.540, 0.008) |
| Weight-for-age | −0.142 (−0.456, 0.172) | |||
| Weight-for-height | −0.038 (−0.332, 0.256) | |||
| Diarrhoea | 0.004 (−0.055, 0.063) | |||
| Vomiting | −0.042 (−0.134, 0.050) | |||
| Fast breathing | −0.008 (−0.110, 0.094) | |||
| Fever | −0.018 (−0.130, 0.094) | |||
| Chills | −0.033 (−0.170, 0.104) | |||
|
|
School feeding programme | Weight-for-age | 0.031 (−0.230, 0.292) |
| Height-for-age | 0.094 (−0.218, 0.406) | |||
| Take-home rations | Weight-for-age | 0.445 (0.159, 0.731) | ||
| Height-for-age | 0.079 (−0.262, 0.420) | |||
|
|
HIV/AIDS treatment | Weight-for-height | 0.374 (−1.163, 1.911) |
|
|
School feeding and take home rations programme | Child growth and anaemia | The authors report that they found evidence of spillovers, but they did not present disaggregated spillover results. |
aThe quality of evidence reported here applies to each study as a whole even if multiple types of spillovers were estimated.
Results pooled across intervention
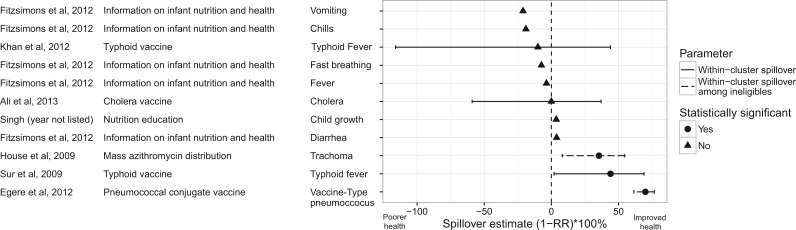
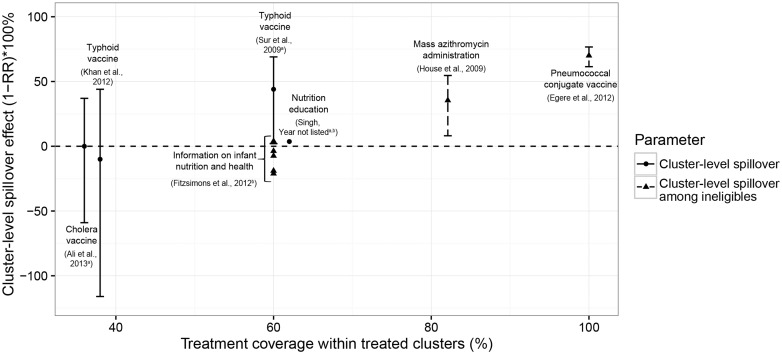
Because studies estimated a wide variety of types of spillovers, results from many studies were not directly comparable. However, a sufficient number of studies estimated within-cluster spillovers to allow for comparison of results across interventions. This type of spillover compares outcomes among untreated individuals in clusters with different proportions of treatment; most commonly, the effect compares untreated individuals in clusters in which some proportion of the cluster receives treatment with those in which no one receives treatment. The three interventions for which investigators reported positive spillover estimates were mass administration of azithromycin to control trachoma (35% decrease in trachoma),49 the typhoid vaccine in India (44% decrease in typhoid)81 and the pneumococcal conjugate vaccine (70% decrease in vaccine-type pneumococcus) (Figure 1).51 These spillovers were measured in studies with moderate and high quality. The remaining studies could not distinguish spillover estimates from the null. Within-cluster spillovers were stronger in studies in which the proportion of individuals treated within clusters was higher (Figure 2). The largest spillovers were present for a study evaluating spillovers of the pneumococcal vaccine by comparing outcomes in villages where 100% of individuals were vaccinated, with those in which only infants were vaccinated.51 Both studies with average cluster-level treatment coverage below 40% did not find evidence of spillovers.53,65
Figure 1.
Cluster-level spillover effects. On the x-axis, the cluster-level spillover effect is shown as the % change in outcome among the untreated in the treated cluster from the mean in the control group [i.e., (1-RR) x 100%, where RR is the relative risk]. Outcomes were recoded so that a greater value of the spillover effect indicates an improvement in health (e.g., higher vaccination coverage, lower mortality) and a smaller value indicates poorer health (e.g., lower vaccination coverage, higher mortality). This figure excludes studies of low or very low quality and studies that did not report information that allowed for standardization. Statistical significance was determined based on the measures presented in the paper for the parameter on its original scale. (a) Information required to convert standard errors for risk differences to standard errors for (1-RR) x 100% was not reported, thus 95% confidence intervals are not presented. (b) These studies were conducted in the same country (India) and are subject to dependence.
Figure 2.
Cluster-level spillover effects by treatment coverage level. This figure plots cluster-level spillover estimates by the level of treatment coverage within treated clusters. We estimated treatment coverage using information available in each paper. On the y-axis, the cluster-level spillover effect is shown as the % change in outcome among the untreated in the treated cluster from the mean in the control group [i.e., (1-RR) x 100%, where RR is the relative risk]. Outcomes were recoded so that a greater value of the spillover effect indicates an improvement in health (e.g., higher vaccination coverage, lower mortality) and a smaller value indicates worse health (e.g., lower vaccination coverage, higher mortality). This figure excludes studies of low or very low quality and studies that did not report information that allowed for standardization. (a) These studies were conducted in the same country (India) and are subject to dependence. (b) Information required to convert standard errors for risk differences to standard errors for (1-RR) x 100% was not reported, thus 95% confidence intervals are not presented.
Risk of bias across studies
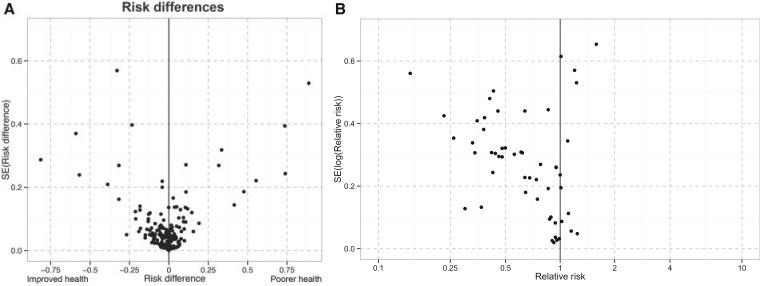
The funnel plots for total and direct effects suggest that publication bias was not present (Supplement 7 Figure 3). The funnel plot for spillover effects estimated with risk differences was balanced around 0, indicating minimal publication bias (Figure 3). For spillovers estimated with risk ratios, the plot was asymmetrical, with few studies producing estimates of negative spillover effects at any level of precision, indicating strong publication bias. Risk ratios were more common in public health studies of interventions that were unlikely to result in negative spillovers (e.g. vaccines). Conversely, risk differences were more common in economics studies of interventions for which the expected direction of spillover effects is less clear.
Figure 3.
Funnel plots for spillover effects. Panel A: This plot includes spillover estimates from 19 studies that reported risk differences for binary outcomes, of which all but one were from studies in the economics literature. These studies evaluated a wide range of interventions including women’s empowerment programs, mass drug administration for infectious disease control, peer group interventions, and nutrition programs. Panel B: This plot includes spillover estimates from 14 studies that reported risk ratios or protective efficacy ((1-RR) x 100%) for binary outcomes, all of which were from studies in the public health literature. These studies evaluated vaccines and mass drug administration for infectious disease control.
Additional analysis
We identified 15 terms commonly used to describe the concept of spillovers (Table 5). The most common terms were ‘indirect effect’ and ‘spillover’, followed by ‘externality/externalities’. ‘Indirect protection’ and ‘herd protection’ were other common terms.
Table 5.
Search terms related to spillover effects in included texts by academic fieldb
| Economics | Geography | Public health | Total | |
|---|---|---|---|---|
| Indirect effect*a | 12 | 2 | 13 | 27 |
| Spillover*a | 23 | 0 | 1 | 24 |
| Externalit*a | 19 | 0 | 0 | 19 |
| Seconda*a | 3 | 3 | 10 | 16 |
| Indirect protection | 0 | 4 | 11 | 15 |
| Herd protect*a | 0 | 2 | 12 | 14 |
| Diffusion | 7 | 1 | 3 | 11 |
| Herd immunity | 1 | 4 | 5 | 10 |
| Herd effect*a | 0 | 0 | 10 | 10 |
| Peer effect*a | 9 | 0 | 0 | 9 |
| Unexpected | 2 | 0 | 3 | 5 |
| Interference | 2 | 0 | 2 | 4 |
| Indirect protective | 0 | 0 | 4 | 4 |
| Contagion | 3 | 0 | 0 | 3 |
| Unexpected benefit*a | 0 | 0 | 1 | 1 |
aAsterisks at the end of search terms indicate wild-card characters allowed at the end of the search term. For example, ‘externalit*’ would retrieve search results for ‘externality’ and ‘externalities’.
bCounts allow for multiple terms per included text.
Discussion
Summary of evidence
To our knowledge, this is the first systematic review of health-related spillovers of interventions in low- and middle-income countries. Evidence of spillovers was strongest for spillovers through reduced disease transmission, and in particular for vaccines and mass drug administration for infectious disease control. There was also strong evidence of spillovers for insecticide-treated net use on health outcomes, but only one study evaluated this association. In studies of spillovers through social proximity, there was weak evidence of spillovers in most studies with a few exceptions: for example, there was evidence that an immunization campaign with incentives increased immunization coverage among non-participants in nearby villages. There was no evidence of spillovers through substitution effects in the three relevant studies.
There are several reasons why we believe we found the strongest evidence for spillovers through reduced disease transmission. First, spillovers through reduced disease transmission are mostly a function of physical proximity. Infectious disease theory suggests that spillovers occur through reduced disease transmission when susceptible and infected individuals come into contact;82 such contact can modify disease transmission across different populations and pathogens. Indeed, we found evidence of spillovers through reduced disease transmission across interventions, outcomes and populations. On the other hand, spillovers through social proximity may be a function of physical proximity as well as social dynamics that are highly dependent on culture and context, and these factors may vary by population, intervention and health outcome. The relative complexity of spillovers through social proximity may make spillovers less likely to occur through this mechanism than through reduced disease transmission. Similarly, for spillovers through substitution, although an intervention may free up a fixed amount of resources in a household, whether those resources support the health of non-intervention recipients may depend on complex factors, such as education level and culture, which vary across populations.
Second, study designs may have been more appropriate for detecting spillovers through reduced disease transmission than through social proximity or substitution. A rich literature has refined study designs to estimate spillovers of vaccines;4,5,7,13,15,82–86 these methods can easily be extended to studies of other interventions that produce spillovers through reduced disease transmission. For spillovers through social proximity or substitution, there is no equivalent methodological literature focused on empirical measurement. As a result, in this review, study designs for detecting spillovers through these mechanisms may have been suboptimal or biased. Indeed, the proportion of studies that we classified as moderate, low, or very low quality was greater among studies measuring spillovers through social proximity or substitution than among studies of spillovers through reduced disease transmission.
Finally, it is also possible that rigorous studies to measure spillovers through social proximity or substitution simply have not been conducted yet or were missed in our search. There were five high quality studies of spillovers through reduced disease transmission compared with only one for spillovers through social proximity and none for substitution. Thus, our findings do not necessarily reflect a lack of spillovers of any particular intervention. Rather, with the exception of vaccines and mass drug administration to control infectious disease, our findings show little evidence for health-related spillovers from currently published intervention studies conducted in low- and middle-income countries.
Quality of evidence
Most studies reported moderate or low quality evidence of spillovers, and there were two overarching sources of bias. First, many study designs did not adequately minimize unmeasured confounding of spillover estimates. Only two out of the 23 studies estimating within-cluster spillover effects used double-randomized designs67,70 which allow for the strongest inference for this type of spillover by minimizing selection bias and unmeasured confounding. In the 21 other such studies, untreated individuals in treated clusters may have been systematically different from individuals in control clusters, possibly because they were not eligible to receive the intervention or chose not to receive the intervention. Such systematic differences between the populations used to measure total effects versus spillover effects could result in biased spillover estimates relative to the estimates that would be obtained in a double-randomized design, in which measured and unmeasured confounders are balanced across both populations.
Second, in 33 out of 54 studies, spillover measurement was not pre-specified, which may have increased the chance that a study’s results were biased. We found evidence of publication bias for spillover estimates reported as risk ratios but not for total or direct effects. Pre-specification helps prevent publication bias. Without pre-specification, spillover parameters may be defined in a way that increases the chance of detecting positive spillovers, whether intentionally or not. For example, studies estimating spillovers conditional on treatment density within fixed areas may define areas in a way that increases the magnitude of spillover effects. In addition, when spillover measurement is not pre-specified, investigators may fail to measure spillovers altogether or they may be less likely to report null spillover findings.
Finally, because spillover effects are likely to be smaller than treatment effects in most cases, studies that do not pre-specify spillover measurement and incorporate them into sample size calculations may be underpowered to detect spillovers. Because spillovers tend to have smaller effect sizes relative to total or overall effects, typically larger sample sizes are required to detect them. As a result, they are more subject to selective reporting than direct effects.
The overall quality of evidence was lower for economics studies than public health studies. Most public health studies evaluated spillovers of vaccines, whereas many economics studies measured spillovers of complex interventions such as conditional cash transfer programmes. Our finding that studies of more complex interventions typically had lower quality ratings is consistent with other studies.87,88 There are several reasons for this pattern. First, complex, realistic interventions often cannot feasibly or ethically be randomized. As a result, many observational studies, some which employed innovative methods for measuring spillover effects in realistic settings, received lower quality ratings in our adapted GRADE framework.89 Second, evaluations of complex interventions often cannot blind participants and/or investigators, resulting in lower quality ratings. Third, many public health studies measured spillovers of outcomes directly targeted by an intervention (e.g. the impact of the cholera vaccine on cholera risk). Studies that measured outcomes indirectly affected by the intervention (e.g. the impact of childhood deworming on later miscarriage47) were more common in economics and received a lower quality rating due to concerns about indirectness of evidence.90 Finally, economics and other social science studies have different reporting norms compared with public health studies and do not always report information required to receive a high quality rating, such as whether randomized treatment allocation was concealed.
Reporting recommendations
We found a wide range of terminology used to describe spillovers, a lack of standardization among spillover methods, and poor reporting of spillovers in many studies. Very few studies clearly defined the specific spillover effect estimated, and in many studies insufficient information was available to compare spillover effects with direct effects or with spillover effects in other studies. More standardized, systematic reporting across disciplines, particularly in the social sciences, would increase comparability across studies and allow for more careful assessment of risk of bias in studies.91 To facilitate such standardization, we propose a checklist specific to reporting of spillover effects, adapted from the CONSORT and STROBE frameworks92,93 (Table 6). This checklist is focused only on reporting spillover effects and is meant to complement the CONSORT92 and STROBE94 checklists. We provide an explanation and examples for each item in the checklist in Supplement 9, available as Supplementary data at IJE online. By including items in the checklist that apply to both randomized and observational studies, our objective is to foster more consistent reporting of spillovers across academic disciplines in future studies.
Table 6.
Reporting checklist for studies estimating spillovers
| Section/topic | No. | Checklist item |
|---|---|---|
| Title and abstract | ||
| Title and abstract | 1 | If spillovers were measured as a primary outcome of a study, mention them in the title and/or abstract. Use the term ‘spillovers’ or ‘indirect effects’ to refer to spillovers |
| Introduction | ||
| Background | 2 | Use the term ‘spillovers’ or ‘indirect effects’ to refer to spillovers |
| Methods | ||
| Study design | 3 | Indicate whether spillover estimation was pre-specified |
| 4 | Describe whether buffers existed between treatment and control units, whether in physical or social distance | |
| 5 | If treatment or outcome density was measured within areas, describe the rationale for and method of defining these areas | |
| 6 | Describe the scale on which spillovers are expected (e.g. household, village etc.) | |
| 7 | For study designs used to estimate spillovers other than the double-randomized or the cluster-randomized design, provide a clear description of the assumptions required to estimate valid statistical parameters if SUTVA is violated | |
| Participants | 8 | Provide a clear description of treatment eligibility criteria |
| 9 | State whether individuals enrolled to measure spillovers were eligible for the treatment or not | |
| Interventions | 10 | Provide a clear description of how treatment was allocated to groups and individuals |
| 11 | Describe whether untreated individuals in treated areas were randomly assigned to not receive treatment, if they opted out of treatment, if they were ineligible for treatment or if there were other reasons they were not treated | |
| 12 | State whether the level of treatment allocation was chosen in order to measure spillovers | |
| 13 | Describe the mechanism of spillovers hypothesized and assessed for each treatment | |
| 14 | Describe whether a buffer zone was created between treatment and control units | |
| Outcomes | 15 | If outcomes measured to estimate direct, total or overall effects differed from outcomes measured to estimate spillover effects, provide a rationale for the difference |
| Study size | 16 | Describe any calculations conducted to determine the sample size needed to estimate spillover parameters. If none, state that none were conducted |
| Statistical methods | 17 | Define the specific spillover parameter(s) estimated for each intervention |
| 18 | Describe the statistical analysis methods used to estimate spillover effects | |
| 19 | Indicate whether spillovers were estimated among individuals allocated to not receive treatment vs those that chose not to take treatment (i.e. indicate whether the spillover analysis was intention-to-treat) | |
| Results | ||
| Participant flow | 20 | If using a clustered design to measure spillovers, provide the number of clusters allocated to treatment and control that were included in the assessment of spillovers |
| 21 | If using a clustered design to measure spillovers, provide the number of individuals that received and did not receive treatment within treatment and control clusters | |
| 22 | If using a clustered design to measure spillovers conditional on eligibility status, provide the number of individuals eligible to receive treatment in treated clusters and the total number of individuals in treated clusters | |
| 23 | If using a clustered design to measure spillovers, provide the number of individuals allocated to treatment within treatment clusters, allocated to not receive treatment within treated clusters, and allocated to control clusters | |
| 24 | If using a clustered design to measure spillovers, provide information about the proportion of individuals receiving treatment within each cluster | |
| 25 | If measurement occurred in buffer zones between treatment and control clusters, provide the number of individuals who did and did not receive treatment in buffer zones | |
| 26 | Describe whether loss to follow-up rates were similar among individuals measured for spillover vs direct/total/overall effects and whether the characteristics of those lost to follow-up for spillover measurement differed from those who were not lost to follow-up | |
| Recruitment | 27 | If dates of data collection for spillover measures differed from dates for direct, total or overall effect measures, explain the discrepancy |
| Main results | 28 | Clearly label which results estimate each spillover parameter |
| 29 | If multiple spillover mechanisms were hypothesized, label results according to the hypothesized spillover mechanism | |
| 30 | Present direct, total, overall and spillover effects in the same population subgroups to allow for assessment of the proportion of the total and overall effects attributable to spillovers | |
| 31 | Report whether there was any evidence that untreated individuals in the treatment or control group were exposed to treatment (e.g. if untreated individuals had heard of the intervention or knew individuals who received it) | |
| 32 | Describe any evidence of contamination of the control group | |
| Discussion | ||
| Summary of findings / key results | 33 | Present theory or evidence supporting the proposed mechanism of spillover. |
| Limitations | 34 | Discuss any potential biases that may be present for spillover parameters Discuss whether these biases may also be present for direct or total effect parameters. This includes contamination of the control group |
| 35 | Articulate whether any analyses conducted to estimate spillovers were not pre-specified | |
| Generalizability | 36 | Comment on external validity of findings and whether any methods used to estimate spillover effects may have compromised external validity (e.g. matching of untreated in the treatment group to untreated in the control group) |
SUTVA, stable unit treatment value assignment.
Limitations
Our search and review process was subject to several limitations. Although we made every effort to conduct a comprehensive search, since the concept of spillovers is poorly indexed, it is possible that we missed relevant articles. Greater consistency in the use of terms that describe spillovers would improve future efforts to identify relevant papers by searching electronic databases. We excluded studies from high-income countries from this review since our focus was on interventions relevant to populations in low- and middle-income countries. This focus was a requirement of our funder. However, there are relevant papers measuring health spillovers from high-income countries, many of which evaluate vaccines.95–97 Some relevant papers which may have been eligible came to our attention after we completed our search process, so we did not include them.98–101 In addition, some of the databases we searched (e.g. Google Scholar) do not allow for repeatable searches, so our complete search results cannot be fully replicated. During the review process, some titles and abstracts could only be reviewed by one team member, and duplicate risk of bias assessment was only possible in a subset of studies. It is possible that there was misclassification that would have been prevented by complete duplicate review.
Our synthesis of results was also subject to several limitations. The information needed to convert standard errors from the additive to the relative scale was not available in the included studies, so our comparison of estimates across studies did not take precision into account. Since there were very few studies measuring spillovers of the same intervention, our ability to summarize results by intervention type was limited. For papers on vaccines and mass drug administration for infectious disease control, results from studies included in the review may have been dependent because many studies re-analysed data from the same study populations or from the same country. Evidence of spillovers for these interventions in other populations would strengthen the generalizability of these findings.
Conclusions
This review of spillover effects on health outcomes in low- and middle-income countries found a wide range of terminology used to describe spillovers, a lack of standardization among spillover methods and poor reporting of spillovers in many studies. The strongest evidence for spillover effects was found in studies evaluating vaccines and mass drug administration to control infectious disease. There was little evidence available for other types of interventions, and the quality of evidence was moderate or poor in most studies. Future studies would benefit from incorporation of spillover measurement in the design phase and standardized reporting of spillover estimation methods and spillover findings.
Funding
Funding for this study was provided by the International Initiative for Impact Evaluation (3ie) (SR4-1084).
Supplementary Material
Acknowledgements
We are grateful to John Eyers at the International Initiative for Impact Evaluation (3ie) for his thoughtful input on our search strategy.
Conflict of interest: None declared.
References
- 1. Duflo E, Glennerster R, Kremer M. Using randomization in development economics research: A toolkit. Handb Dev Econ 2007;4:3895–62. [Google Scholar]
- 2. Miguel E, Kremer M. Worms: Identifying Impacts on Education and Health in the Presence of Treatment Externalities. Econometrica 2004;72:159–217. [Google Scholar]
- 3. Cox DR. Planning of Experiments. Oxford, UK: Wiley, 1958. [Google Scholar]
- 4. Hudgens MG, Halloran ME. Toward Causal Inference With Interference. J Am Stat Assoc 2008;103:832–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tchetgen EJT, VanderWeele TJ. On causal inference in the presence of interference. Stat Methods Med Res 2012;21:55–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenbaum PR. Interference Between Units in Randomized Experiments. J Am Stat Assoc 2007;102:191–200. [Google Scholar]
- 7. VanderWeele TJ, Tchetgen Tchetgen EJ. Effect partitioning under interference in two-stage randomized vaccine trials. Stat Probab Lett 2011;81:861–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayes R, Alexander ND, Bennett S, Cousens SN. Design and analysis issues in cluster-randomized trials of interventions against infectious diseases. Stat Methods Med Res 2000;9:95–116. [DOI] [PubMed] [Google Scholar]
- 9. John TJ, Samuel R. Herd immunity and herd effect: new insights and definitions. Eur J Epidemiol. 2000;16:601–06. [DOI] [PubMed] [Google Scholar]
- 10. Fine PE. Herd immunity: history, theory, practice. Epidemiol Rev 1993;15:265–302. [DOI] [PubMed] [Google Scholar]
- 11. Fox JP, Elveback L, Scott W, Gatewood L, Ackerman E. Herd immunity: basic concept and relevance to public health immunization practices; 1971. Am J Epidemiol 1995;141:187–97. [DOI] [PubMed] [Google Scholar]
- 12. Rubin DB. Formal mode of statistical inference for causal effects. J Stat Plan Inference 1990;25:279–92. [Google Scholar]
- 13. Halloran ME, Struchiner CJ. Causal Inference in Infectious Diseases. Epidemiology 1995;6:142–51. [DOI] [PubMed] [Google Scholar]
- 14. Halloran ME, Haber M, Longini IM Jr, Struchiner CJ. Direct and indirect effects in vaccine efficacy and effectiveness. Am J Epidemiol 1991;133:323–31. [DOI] [PubMed] [Google Scholar]
- 15. VanderWeele T, Tchetgen Tchetgen E, Halloran M. Components of the Indirect Effect in Vaccine Trials: Identification of Contagion and Infectiousness Effects. Epidemiology 2012;23:751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dybvig PH, Spatt CS. Adoption externalities as public goods. J Public Econ 1983;20:231–47. [Google Scholar]
- 17. Ozawa S, Mirelman A, Stack ML, Walker DG, Levine OS. Cost-effectiveness and economic benefits of vaccines in low- and middle-income countries: A systematic review. Vaccine 2012;31:96–108. [DOI] [PubMed] [Google Scholar]
- 18. Edejer TT-T, Aikins M, Black R, Wolfson L, Hutubessy R, Evans DB. Cost effectiveness analysis of strategies for child health in developing countries. BMJ 2005;331:1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell MJ, Donner A, Klar N. Developments in cluster randomized trials and Statistics in Medicine. Stat Med 2007;26:2–19. [DOI] [PubMed] [Google Scholar]
- 20. Donner A, Klar N. Design and Analysis of Cluster Randomization Trials in Health Research. Oxford, UK: Wiley, 2010. [Google Scholar]
- 21. Bhattacharya D, Dupas P, Kanaya S. Estimating the Impact of Means-tested Subsidies under Treatment Externalities with Application to Anti-Malarial Bednets. Cambridge, MA: National Bureau of Economic Research, 2013. [Google Scholar]
- 22. Benjamin-Chung J, Abedin J, Berger D. et al. The identification and measurement of health-related spillovers in impact evaluations: a systematic review.London: International Initiative for Impact Evaluation (3ie), 2015. [Google Scholar]
- 23. World Bank. How We Classify Countries. 2012. http://data.worldbank.org/about/country-classifications (1 March 2012, date last accessed).
- 24. Buttenheim A, Alderman H, Friedman J. Impact evaluation of school feeding programmes in Lao People’s Democratic Republic. J Dev Eff 2011;3:520–42. [Google Scholar]
- 25. Higgins J, Greene S. Cochrane Handbook for Systematic Reviews of Interventions. Lonon: Cochrane Collaboration, 2011. [Google Scholar]
- 26. Coalition for Evidence-Based Policy. Checklist For Reviewing a Randomized Controlled Trial of a Social Program or Project, To Assess Whether It Produced Valid Evidence.Washington, DC: Coalition for Evidence-Based Policy, 2010. [Google Scholar]
- 27. Effective Practice and Organisation of Care (EPOC) Group. Risk of bias for studies with a separate control group.London: Cochrane Review Group, 2009. [Google Scholar]
- 28. Gertler PJ. Impact Evaluation in Practice. New York, NY: World Bank Publications, 2011. [Google Scholar]
- 29. Lee DS, Lemieux T. Regression Discontinuity Designs in Economics. J Econ Lit 2010;48:281–355. [Google Scholar]
- 30. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guyatt GH, Oxman AD, Vist GE. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berk R, Freedman D. Statistical assumptions as empirical commitments. In: Collier D, Sekhon J, Stark P (eds). Statistical Models and Causal Inference. Cambridge, UK: Cambridge University Press, 2010. [Google Scholar]
- 33. Shekhawat N, Mkocha H, Munoz B. et al. Cohort and Age Effects of Mass Drug Administration on Prevalence of Trachoma: A Longitudinal Study in Rural Tanzania. Invest Ophthalmol Vis Sci 2014;55:2307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paul JR, Horstmann DM, Riordan JT. et al. An oral poliovirus vaccine trial in Costa Rica. Bull World Health Organ 1962;26:311–29. [PMC free article] [PubMed] [Google Scholar]
- 35. Chaudhuri A. Intra-household spillover effects of a maternal and child health program: evidence from rural Bangladesh. Dissertation. Department of Economics, San Francisco State University, 2005. [Google Scholar]
- 36. Joshi S, Schultz TP. Family Planning and Women’s and Children’s Health: Long-Term Consequences of an Outreach Program in Matlab, Bangladesh. Demography 2013;50:149–80. [DOI] [PubMed] [Google Scholar]
- 37. Avitabile C. Spillover Effects in Healthcare Programs: Evidence on Social Norms and Information Sharing. Washington, DC: Inter-American Development Bank, 2012. [Google Scholar]
- 38. Handa S, Huerta M-C, Perez R, Straffon B. Poverty, Inequality, and Spillover in Mexico’s Education, Health, and Nutrition Program. Washington, DC: International Food Policy Research Institute, 2001. [Google Scholar]
- 39. Ali M, Emch M, von Seidlein L. et al. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet 2005;366:44–49. [DOI] [PubMed] [Google Scholar]
- 40. Emch M, Ali M, Root ED, Yunus M. Spatial and environmental connectivity analysis in a cholera vaccine trial. Soc Sci Med 2009;68:631–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ali M, Emch M, Yunus M. et al. Vaccine Protection of Bangladeshi Infants and Young Children Against Cholera: Implications for Vaccine Deployment and Person-to-Person Transmission. Pediatr Infect Dis J 2008;27:33–37. [DOI] [PubMed] [Google Scholar]
- 42. Emch M, Ali M, Park J-K, Yunus M, Sack DA, Clemens JD. Relationship between neighbourhood-level killed oral cholera vaccine coverage and protective efficacy: evidence for herd immunity. Int J Epidemiol 2006;35:1044–50. [DOI] [PubMed] [Google Scholar]
- 43. Perez-Heydrich C, Hudgens MG, Halloran ME, Clemens JD, Ali M, Emch ME. Assessing effects of cholera vaccination in the presence of interference. Biometrics 2014;70:731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Root ED, Giebultowicz S, Ali M, Yunus M, Emch M. The Role of Vaccine Coverage within Social Networks in Cholera Vaccine Efficacy. PLoS One. 2011;6:e22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ozier O. Exploiting Externalities to Estimate the Long-term Benefits of Early Childhood Deworming. Working Paper Series. New York, NY: World Bank, 2014. [Google Scholar]
- 46. Kremer M, Miguel E. The illusion of sustainability. Q J Econ 2007;122:1007–65. [Google Scholar]
- 47. Baird S, Hicks JH, Kremer M, Miguel E. Worms at Work: Public Finance Implications of a Child Health Investment. Cambridge, MA: Poverty Action Lab, 2013. [Google Scholar]
- 48. Hawley WA, Phillips-Howard PA, Kuile FOT. et al. Community-Wide Effects of Permethrin-Treated Bed Nets on Child Mortality and Malaria Morbidity in Western Kenya. Am J Trop Med Hyg 2003;68(Suppl 4):121–27. [PubMed] [Google Scholar]
- 49. House JI, Ayele B, Porco TC. et al. Assessment of herd protection against trachoma due to repeated mass antibiotic distributions: a cluster-randomised trial. Lancet 2009;373:1111–18. [DOI] [PubMed] [Google Scholar]
- 50. Banerjee AV, Duflo E, Glennerster R, Kothari D. Improving immunisation coverage in rural India: clustered randomised controlled evaluation of immunisation campaigns with and without incentives. BMJ 2010;340:c2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Egere U, Townend J, Roca A. et al. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal carriage in newborns in rural Gambia: a randomised controlled trial. PloS One 2012;7:e49143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Préziosi M-P, Halloran ME. Effects of pertussis vaccination on transmission: vaccine efficacy for infectiousness. Vaccine 2003;21:1853–61. [DOI] [PubMed] [Google Scholar]
- 53. Ali M, Sur D, You YA. et al. Herd protection by a bivalent-killed-whole-cell oral cholera vaccine in the slums of Kolkata, India. Clin Infect Dis 2013;56:1123–31. [DOI] [PubMed] [Google Scholar]
- 54. Khatib AM, Ali M, von Seidlein L. et al. Effectiveness of an oral cholera vaccine in Zanzibar: findings from a mass vaccination campaign and observational cohort study. Lancet Infect Dis 2012;12:837–44. [DOI] [PubMed] [Google Scholar]
- 55. Huq A, Yunus M, Sohel SS. et al. Simple sari cloth filtration of water is sustainable and continues to protect villagers from cholera in Matlab, Bangladesh. mBio 2010;1:e00034–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haile M, Tadesse Z, Gebreselassie S. et al. The Association between Latrine Use and Trachoma: A Secondary Cohort Analysis from a Randomized Clinical Trial. Am J Trop Med Hyg 2013;89:717–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cooper E, Fitch L. Pertussis: Herd Immunity and Vaccination Coverage in St. Lucia. Lancet 1983;322:1129–32. [DOI] [PubMed] [Google Scholar]
- 58. Forleo-Neto E, de Oliveira CF, Maluf EMCP. et al. Decreased point prevalence of Haemophilus influenzae Type b (Hib) oropharyngeal colonization by mass immunization of Brazilian children less than 5 years old with Hib polyribosylribitol phosphate polysaccharide—tetanus toxoid conjugate vaccine in combination with diphtheria-tetanus toxoids—pertussis vaccine. J Infect Dis 1999;180:1153–58. [DOI] [PubMed] [Google Scholar]
- 59. Chen W-J, Moulton LH, Saha SK, Mahmud AA, Arifeen SE, Baqui AH. Estimation of the herd protection of Haemophilus influenzae type b conjugate vaccine against radiologically confirmed pneumonia in children under 2 years old in Dhaka, Bangladesh. Vaccine 2014;32:944–48. [DOI] [PubMed] [Google Scholar]
- 60. Root ED, Lucero M, Nohynek H. et al. Distance to health services affects local-level vaccine efficacy for pneumococcal conjugate vaccine (PCV) among rural Filipino children. Proc Natl Acad Sci U S A 2014;111:3520–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Openshaw S. Ecological fallacies and the analysis of areal census data. Environ Plan A 1984;16:17–31. [DOI] [PubMed] [Google Scholar]
- 62. Baptista PN, Magalhães V, Rodrigues LC, Rocha MW, Pimentel AM. Pertussis vaccine effectiveness in reducing clinical disease, transmissibility and proportion of cases with a positive culture after household exposure in Brazil. Pediatr Infect Dis J 2006;25:844–46. [DOI] [PubMed] [Google Scholar]
- 63. Chidambaram JD, Melese M, Alemayehu W. et al. Mass Antibiotic Treatment and Community Protection in Trachoma Control Programs. Clin Infect Dis 2004;39:e95–e97. [DOI] [PubMed] [Google Scholar]
- 64. Hammitt LL, Akech DO, Morpeth SC. et al. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health 2014;2:e397–e405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Khan MI, Soofi SB, Ochiai RL. et al. Effectiveness of Vi capsular polysaccharide typhoid vaccine among children: A cluster randomized trial in Karachi, Pakistan. Vaccine 2012;30:5389–95. [DOI] [PubMed] [Google Scholar]
- 66. Ribas RP, Soares FV, Teixeira CG, Silva E, Hirata GI. Externality and behavioural change effects of a non-randomised CCT programme: Heterogeneous impact on the demand for health and education. Working Paper No. 2011‐1. Brasilia: International Policy Centre for Inclusive Growth, 2011. [Google Scholar]
- 67. Baird S, De Hoop J, Özler B. Income shocks and adolescent mental health. J Hum Resour 2013;48:370–403. [Google Scholar]
- 68. Contreras D, Maitra P. Health spillover effects of a conditional cash transfer program [Internet]. Monash University Working Paper, 2012. [Google Scholar]
- 69. Tontarawongsa C, Mahajan A, Tarozzi A. (Limited) diffusion of health-protecting behaviors: evidence from non-beneficiaries of a public health program in Orissa (India). Department of Economics, Duke University, 2011. [Google Scholar]
- 70. Chong A, Gonzalez-Navarro M, Karlan D, Valdivia M. Effectiveness and Spillovers of Online Sex Education: Evidence from a Randomized Evaluation in Colombian Public Schools. Cambridge, MA: National Bureau of Economic Research, 2013. F [Google Scholar]
- 71. German D, Sutcliffe CG, Sirirojn B. et al. Unanticipated Effect of a Randomized Peer Network Intervention on Depressive Symptoms Among Young Methamphetamine Users in Thailand. J Community Psychol 2012;40:799–813. [Google Scholar]
- 72. Singh P. Spillovers in learning and behavior: Evidence from a nutritional information campaign in urban slums. Munich Personal RePEc Archive 33362, 2011. [Google Scholar]
- 73. Björkman M, Svensson J. Power to the people: evidence from a randomized field experiment on community-based monitoring in Uganda. Q J Econ 2009;124:735–69. [Google Scholar]
- 74. Dupas P. Relative risks and the market for sex: Teenagers, sugar daddies and HIV in Kenya. Munich Personal RePEc Archive 248, 2005 [Google Scholar]
- 75. Azad K, Barnett S, Banerjee B. et al. Effect of scaling up women’s groups on birth outcomes in three rural districts in Bangladesh: a cluster-randomised controlled trial. Lancet 2010;375:1193–202. [DOI] [PubMed] [Google Scholar]
- 76. Janssens W. Measuring externalities in program evaluation. Discussion paper. Tinbergen Institute, 2005. [Google Scholar]
- 77. Godlonton S, Thornton R. Peer effects in learning HIV results. J Dev Econ 2012;97:118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fitzsimons E, Malde B, Mesnard A, Vera-Hernández M. Household responses to information on child nutrition: experimental evidence from Malawi. Institute for Fiscal Studies, 2012. [Google Scholar]
- 79. Kazianga H, Levy D, Linden LL, Sloan M. The Effects of ‘Girl-Friendly’ Schools: Evidence From the BRIGHT School Construction Program in Burkina Faso. Cambridge, MA: National Bureau of Economic Research, 2012. [Google Scholar]
- 80. Zivin JG, Thirumurthy H, Goldstein M. AIDS treatment and intrahousehold resource allocation: Children’s nutrition and schooling in Kenya. J Public Econ 2009;93:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sur D, Ochiai RL, Bhattacharya SK. et al. A Cluster-Randomized Effectiveness Trial of Vi Typhoid Vaccine in India. N Engl J Med 2009;361:335–44. [DOI] [PubMed] [Google Scholar]
- 82. Halloran E, Longini IM Jr, Struchiner CJ. Design and Analysis of Vaccine Studies. New York, NY: Springer, 2010. [Google Scholar]
- 83. Halloran ME, Struchiner CJ. Study Designs for Dependent Happenings. Epidemiology 1991;2:331–38. [DOI] [PubMed] [Google Scholar]
- 84. Longini IM, Sagatelian K, Rida WN, Halloran ME. Optimal vaccine trial design when estimating vaccine efficacy for susceptibility and infectiousness from multiple populations. Stat Med 1998;17:1121–36. [DOI] [PubMed] [Google Scholar]
- 85. Clemens J, Shin S, Ali M. New approaches to the assessment of vaccine herd protection in clinical trials. Lancet Infect Dis 2011;11:482–87. [DOI] [PubMed] [Google Scholar]
- 86. Halloran ME. The Minicommunity Design to Assess Indirect Effects of Vaccination. Epidemiol Methods 2012;1:83–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Moversusisyan A, Melendez-Torres GJ, Montgomery P. Outcomes in systematic reviews of complex interventions never reached ‘high’ GRADE ratings when compared with those of simple interventions. J Clin Epidemiol 2016;78:22–33. [DOI] [PubMed] [Google Scholar]
- 88. Moversusisyan A, Melendez-Torres GJ, Montgomery P. Users identified challenges in applying GRADE to complex interventions and suggested an extension to GRADE. J Clin Epidemiol 2016;70:191–99. [DOI] [PubMed] [Google Scholar]
- 89. Durrheim DN, Reingold A. Modifying the GRADE framework could benefit public health. J Epidemiol Community Health 2010;64:387. [DOI] [PubMed] [Google Scholar]
- 90. Guyatt GH, Oxman AD, Kunz R. et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol 2011;64:1303. [DOI] [PubMed] [Google Scholar]
- 91. Miguel E, Camerer C, Casey K. et al. Promoting Transparency in Social Science Research. Science 2014;343:30–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schulz K, Altman D, Moher D; the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. von Elm E, Altman DG, Egger M. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vandenbroucke JP, von Elm E, Altman DG. et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Metlay JP, Fishman NO, Joffe M, Edelstein PH. Impact of pediatric vaccination with pneumococcal conjugate vaccine on the risk of bacteremic pneumococcal pneumonia in adults. Vaccine 2006;24:468–75. [DOI] [PubMed] [Google Scholar]
- 96. Piedra PA, Gaglani MJ, Kozinetz CA. et al. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine 2005;23:1540–48. [DOI] [PubMed] [Google Scholar]
- 97. de Heer HD, Koehly L, Pederson R, Morera O. Effectiveness and Spillover of an After-School Health Promotion Program for Hispanic Elementary School Children. Am J Public Health 2011;101:1907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Spears D. Essays in the Economics of Sanitation and Human Capital in Developing Countries PhD thesis. Department of Economics, Princeton University, 2013. [Google Scholar]
- 99. Baird S, Bohren A, McIntosh C, Ozler B. Designing experiments to measure spillover and threshold effects. PIER Working Paper No. 14–006. Department of Economics, University of Pennsylvania, 2014. [Google Scholar]
- 100. Barham T. Enhancing Cognitive Functioning: Medium-Term Effects of a Health and Family Planning Program in Matlab. Am Econ J Appl Econ 2012;4:245–73. [Google Scholar]
- 101. Duflo E. Child Health and Household Resources in South Africa: Evidence from the Old Age Pension Program. Am Econ Rev 2000;90:393–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.