Abstract
Aims
To study the relation between body mass index (BMI) in young men and risk of early hospitalization with heart failure.
Methods and results
In a prospective cohort study, men from the Swedish Conscript Registry investigated 1968–2005 (n = 1 610 437; mean age, 18.6 years were followed 5–42 years (median, 23.0 years; interquartile range, 15.0–32.0), 5492 first hospitalizations for heart failure occurred (mean age at diagnosis, 46.6 (SD 8.0) years). Compared with men with a body mass index (BMI) of 18.5–20.0 kg/m2, men with a BMI 20.0–22.5 kg/m2 had an hazard ratio (HR) of 1.22 (95% CI, 1.10–1.35), after adjustment for age, year of conscription, comorbidities at baseline, parental education, blood pressure, IQ, muscle strength, and fitness. The risk rose incrementally with increasing BMI such that men with a BMI of 30–35 kg/m2 had an adjusted HR of 6.47 (95% CI, 5.39–7.77) and those with a BMI of ≥35 kg/m2 had an HR of 9.21 (95% CI, 6.57–12.92). The multiple-adjusted risk of heart failure per 1 unit increase in BMI ranged from 1.06 (95% CI, 1.02–1.11) in heart failure associated with valvular disease to 1.20 (95% CI, 1.18–1.22) for cases associated with coronary heart disease, diabetes, or hypertension.
Conclusion
We found a steeply rising risk of early heart failure detectable already at a normal body weight, increasing nearly 10-fold in the highest weight category. Given the current obesity epidemic, heart failure in the young may increase substantially in the future and physicians need to be aware of this.
Keywords: Heart failure, Adolescence, Hospital admission, Epidemiology
Heart failure, with a prognosis worse than that of many common forms of cancer,1 predominantly afflicts the elderly. However, we have recently documented an increasing incidence of heart failure in Sweden among persons younger than 45 years, while there was a fall among those aged 55 years and older.2
An obvious factor that coincides with the increase in heart failure among the young is a marked rise in body weight and obesity3 in Sweden and elsewhere. Obesity is a recognized risk factor for heart failure,4 starting at a high-normal or slightly elevated body mass index (BMI).5 In a study of Danish male conscripts,6 high BMI was associated with increased risk of heart failure but because there were only 106 cases the effect of body weight across a wide range could not be studied.
The Swedish registry of conscripts contains anthropometric data on >1.5 million young men. Through linkage to the Swedish national inpatient registry (IPR), we evaluated all cases of hospitalizations for heart failure in relation to their BMI over an extended period.
Methods and statistics
Participants
In a prospective cohort study, we used data from the Swedish Conscript registry.7–9 Until recently, Swedish law required that all 18-year-old Swedish men enlist. Exemptions were granted only for men serving time in prison, or with severe chronic medical or mental conditions (∼2–3% each year). From 1968 to 2005, all enlisted men (n = 1 810 348) underwent standardized physical and cognitive examinations at six conscription centres, where they were seen by a psychologist and a physician. After excluding men enlisting late (aged ≥25 years) (n = 49 208) and men with missing BMI (n = 150 703), 1 610 437 men were included. The Ethics Committee of the University of Gothenburg approved the study.
Swedish military service conscription register data and covariates from other sources
The 2-day examination involved measurement of weight, height, and blood pressure in the supine position after 5–10 min of rest. Height (m) was measured by use of wall-mounted stadiometers, and weight (kg) was measured by use of analogue or digital scales. Body mass index was calculated as weight/height2. Cardiovascular fitness was assessed using cycle ergometry.7 The cognitive performance tests and fitness tests8,9 are described in Supplementary material online.
The longitudinal integration database for health insurance and labour market studies (Swedish acronym LISA) held by Statistics Sweden provided information on parental education (80% coverage).
Follow-up procedures
Sweden has a universal healthcare system that provides low-cost hospital care to all citizens. Data from the Swedish Military Service Conscription Register was linked to the Swedish inpatient and death registries through the Swedish personal identification number.
Definition of heart failure and comorbidities
Because a large proportion of patients with heart failure had other primary discharge diagnoses (e.g. cardiomyopathy or congenital heart disease), a first-ever heart failure diagnosis code in any position was accepted. The International Classification of Diseases, Eighth Revision (ICD-8) was in use from 1968 to 1986, the ICD-9 from 1987 to 1996, and the ICD-10 since 1997. We defined heart failure by 427,00 and 427,10 for ICD-8, 428 for ICD-9, and I50 for ICD-10.
Diagnoses for concomitant or pre-existing comorbidity were included up to the point of the heart failure discharge diagnosis and are listed in the Supplement. We assigned mutually exclusive causes of heart failure in the following hierarchical order2: (i) congenital heart disease and valvulopathies, (ii) coronary heart disease (CHD) and/or diabetes and/or hypertension, (iii) cardiomyopathy, and (iv) other causes.
Statistical analysis
Incidence rates and corresponding 95% confidence intervals were calculated using Poisson regression. We used Cox proportional hazards models to assess the influence of BMI at the time of conscription and potential confounders on a first hospitalization for heart failure. Subjects were followed from the date of conscription until the time of (i) first heart failure hospitalization, (ii) death, (iii) emigration, or (iv) the end of follow-up (31 December 2010) (minimum, 5 years; maximum, 42 years at the time of the last follow-up). To avoid underestimating the true risk associated with high adolescent BMI, we refrained from adjusting for follow-up comorbidities such as CHD, diabetes, or hypertension, conditions which in themselves are strongly associated with an elevated BMI and constitute steps in the pathway toward heart failure. The proportional hazard assumptions were investigated using tests and plots based on weighted residuals.10 Diabetes and cardiovascular fitness showed signs of non-proportional hazards; hence, we stratified the models for these variables where applicable. Diastolic blood pressure also showed signs of non-proportional hazards, but further investigation by stratifying on quintiles or modelling its interaction with time showed a negligible effect on the estimates of BMI. Therefore, diastolic blood pressure was used in the models without any remedial measures. The year of conscription was modelled as a cubic restricted spline with four knots placed at the 5th, 35th, 65th, and 95th percentiles.11 We used the same approach in the analysis of BMI, where BMI was constrained to be linear in log hazard at a BMI of <18 and >27.5 kg/m2. Age at conscription was modelled as a categorical variable. To examine how strength, fitness, and IQ at the age of 18 years would impact the association between BMI and risk of heart failure in adulthood, we included these variables as covariates. Muscular strength, IQ, fitness stanines, and parental education were trichotomized as low (1–3), medium (4–6), and high (7–9). Due to the large number of observations, the P-values were very small (in all analyses when the 95% confidence interval was separated from 1, the P-values were <0.0001) and are therefore not reported.
Population-attributable risk, or the association of a specific risk factor with a specific disease as a proportion of all risk factors for that disease, was calculated using the method described by Natarajan.12 using the hazard ratios from the Cox proportional hazard regression models.
Statistical calculations were performed with SAS version 9.4 (SAS Institute, Cary, NC), with the exception of model checking, which was performed with R version 3.2.2 (http://www.R-project.org).
Results
Study population
Of the 1 610 437 men in the study (mean BMI, 21.9 kg/m2; standard deviation [SD], 3.1), 79.6% were of normal weight (BMI of 18.5–25.0 kg/m2), 10.0% were overweight (BMI of 25–30 kg/m2), and only 2.3% were obese (n = 36 608, of which 7723 had a BMI of ≥35.0 kg/m2) (Table 1). Baseline data for fitness, muscular strength, IQ, parental education, diagnoses at baseline and during follow-up are shown in Supplementary material online, Table S1.
Table 1.
Baseline characteristics, age at first heart failure discharge, incidence of heart failure and of heart failure from mutually exclusive associated conditions by body mass index category by body mass index category in 1 610 437 male conscripts
| All | BMI < 18.50 | BMI 18.50–19.99 | BMI 20.00–22.49 | BMI 22.50–24.99 | BMI 25.00–27.49 | BMI 27.50–29.99 | BMI 30.00–34.99 | BMI ≥ 35.00 | |
|---|---|---|---|---|---|---|---|---|---|
| Number of men (% of total) | n = 1 610 437 (100%) | n = 130 423 (8.1%) | n = 289 857 (18.0%) | n = 648 830 (40.3%) | n = 342 960 (21.3%) | n = 119 469 (7.4%) | n = 42 290 (2.6%) | n = 29 385 (1.8%) | n = 7223 (0.4%) |
| Age (years) | 18.3 (0.7) | 18.3 (0.6) | 18.3 (0.6) | 18.3 (0.6) | 18.4 (0.8) | 18.4 (0.8) | 18.3 (0.8) | 18.3 (0.8) | 18.3 (0.7) |
| Height, mean (SD) | 1.79 (0.07) | 1.80 (0.07) | 1.79 (0.07) | 1.79 (0.07) | 1.79 (0.07) | 1.79 (0.07) | 1.79 (0.07) | 1.79 (0.07) | 1.79 (0.07) |
| Weight, mean (SD) | 70.5 (11.0) | 57.1 (4.9) | 62.3 (4.8) | 68.3 (5.4) | 75.6 (6.0) | 83.6 (6.6) | 91.9 (7.4) | 102.6 (9.1) | 121.2 (11.4) |
| BMI, mean (SD) | 21.9 (3.1) | 17.7 (0.7) | 19.3 (0.4) | 21.2 (0.7) | 23.5 (0.7) | 26.1 (0.7) | 28.6 (0.7) | 31.9 (1.4) | 37.8 (2.7) |
| Systolic BP, mean (SD) | 128.5 (10.9) | 125.6 (10.7) | 126.7 (10.7) | 128.2 (10.7) | 129.9 (10.7) | 131.3 (10.7) | 132.5 (11.1) | 133.9 (11.0) | 136.3 (11.4) |
| Diastolic BP, mean (SD) | 67.7 (9.7) | 67.5 (9.5) | 67.4 (9.6) | 67.4 (9.7) | 67.8 (9.8) | 68.5 (10.0) | 69.3 (10.3) | 70.8 (10.6) | 72.6 (10.9) |
| Age at heart failure diagnosis | 46.6 (8.0) | 47.4 (7.7) | 47.2 (7.8) | 46.9 (8.1) | 46.3 (8.1) | 46.4 (7.3) | 46.4 | 44.5 (8.6) | 42.8 (8.1) |
| Heart failure as a main diagnosis, n | 2576 | 209 | 392 | 819 | 537 | 298 | 132 | 143 | 46 |
| Cases per 100 000 observation years (95% CI) | 6.94 (6.68–7.22) | 6.31 (5.51–7.23) | 5.47 (4.96–6.04) | 5.39 (5.04–5.78) | 7.17 (6.59–7.80) | 12.09 (10.79–13.55) | 15.81 (13.33–18.75) | 26.40 (22.41–31.10) | 39.85 (29.85–53.20) |
| Heart failure in any diagnostic position, n | 5493 | 468 | 861 | 1781 | 1160 | 601 | 282 | 266 | 74 |
| Cases per 100 000 observation years (95% CI) | 14.81 (14.42–15.20) | 14.14 (12.92–15.48) | 12.03 (11.25–12.86) | 11.72 (11.19–12.28) | 15.50 (14.63–16.42) | 24.40 (22.53–26.43) | 33.81 (30.08–37.99) | 49.15 (43.59–55.43) | 64.18 (51.10–80.60) |
| Heart failure with congenital or acquired valvular disease, n | 704 | 77 | 132 | 257 | 146 | 48 | 25 | 16 | 3 |
| Cases per 100 000 observation years (95% CI) | 1.90 (1.76–2.04) | 2.33 (1.86–2.91) | 1.84 (1.55–2.19) | 1.69 (1.50–1.91) | 1.95 (1.66–2.29) | 1.95 (1.47–2.59) | 3.00 (2.03–4.44) | 2.96 (1.81–4.83) | 2.60 (0.84–8.07) |
| Heart failure with CHD, diabetes, or hypertension, n | 2731 | 201 | 384 | 791 | 596 | 357 | 177 | 179 | 46 |
| Cases per 100 000 observation years (95% CI) | 7.36 (7.09–7.64) | 6.07 (5.29–6.97) | 5.36 (4.85–5.93) 5.21 | 5.21 (4.86–5.58) | 7.96 (7.35–8.63) | 14.49 (13.07–16.08) | 21.22 (18.31–24.59) | 33.08 (28.57–38.30) | 39.89 (29.88–53.26) |
| Heart failure with cardiomyopathy, n | 803 | 81 | 142 | 285 | 153 | 73 | 33 | 25 | 11 |
| Cases per 100 000 observation years (95% CI) | 2.16 (2.02–2.32) | 2.45 (1.97–3.04) | 1.98 (1.68–2.34) | 1.88 (1.67–2.11) | 2.04 (1.74–2.40) | 2.96 (2.36–3.73) | 3.96 (2.81–5.56) | 4.62 (3.12–6.84) | 9.54 (5.28–17.23) |
| Heart failure, any other cause, n | 1255 | 109 | 203 | 448 | 265 | 123 | 47 | 46 | 14 |
| Cases per 100 000 observation years (95% CI) | 3.38 (3.20–3.57) | 3.29 (2.73–3.97) | 2.84 (2.47–3.25) | 2.95 (2.69–3.23) | 3.54 (3.14–3.99) | 4.99 (4.18–5.96) | 5.63 (4.23–7.50) | 8.50 (6.37–11.35) | 12.14 (7.19–20.50) |
Hospitalizations for heart failure
During follow-up lasting 5–42 years (median, 23.0 years; interquartile range, 15.0–32.0), we identified 5492 incident cases of heart failure (defined as a principal or secondary discharge diagnosis of heart failure) at a mean age of 46.6 years (SD, 8.0). Figure 1 shows a slightly J-shaped association between BMI and heart failure. The lowest risk was present at a BMI of ∼20 kg/m2, and a steep linear increase occurred with increasing body weight.
Figure 1.
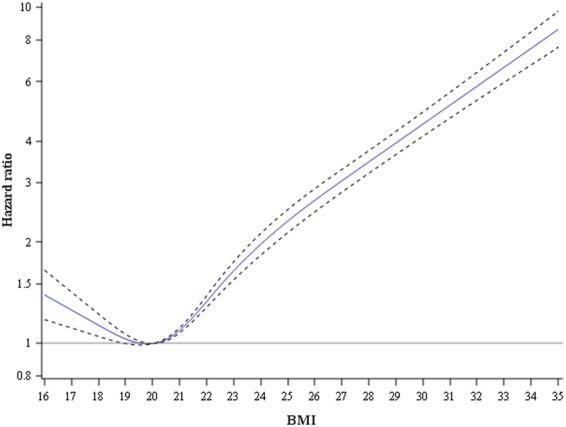
Association between body mass index at conscription and risk of hospitalization with a principal or secondary discharge diagnosis of heart failure. Adjusted for age at conscription, period, baseline comorbidities, and parental education. The number of events and men in different BMI categories are shown in Table 1.
Of the 5492 cases, 704 (12.8%) were associated with congenital or acquired valvular disease; 2731 (49.7%) with CHD, diabetes, or hypertension; and 803 (14.6%) with cardiomyopathy. One fourth of the cases (1255, 22.9%) did not have a registered associated diagnosis prior to, or concomitant with, the diagnosis of heart failure. For all categories, including cases with a principal diagnosis of heart failure (n = 2576) the two lowest normal weight categories had the lowest incidence (Table 1). For heart failure overall, these two categories had a risk of 12.03 and 11.72 per 100 000 person-years, respectively, increasing to 64.18 among men with baseline BMI of ≥35 kg/m2. Supplementary material online, Figures S1A–E, shows associations between BMI at the time of conscription and heart failure as a principal diagnosis, or associated with specific causes.
Using low-normal weight (BMI of 18.5–20.0 kg/m2) as the reference category, a moderate increase in risk was discernible at a slightly elevated normal weight (Table 2). Compared with men with low-normal weight men with a BMI 20.0–22.5 kg/m2 had an hazard ratio (HR) of 1.22 (95% CI, 1.10–1.35), after adjustment for age and year of conscription, comorbidities at baseline, parental education, systolic and diastolic blood pressure, IQ, muscle strength, and fitness. Men with a BMI of 22.5–25.0 kg/m2 had a corresponding adjusted HR of 1.90 (1.70–2.13). Hazard ratio for successively increasing BMI rose incrementally such that the HR for a BMI of 30–35 kg/m2 was 6.47 (5.39–7.77), while that for a BMI of ≥35 kg/m2 was 9.21 (6.57–12.92). The population-attributable risk for a BMI of ≥22.5 vs. <22.5 kg/m2 was 24.0% (95% CI, 21.4%–26.5%) in the fully adjusted model.
Table 2.
Hazard ratios for heart failure and mutually exclusive associated conditions of heart failurea by body mass index categoryb, and population-attributable risks for a body mass index of ≥22.5 vs. <22.5 kg/m2
| BMI category | Hazard ratio (95% CI) P-value |
||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Heart failure hospitalization (events/population) | 5492/1 610 352 | 4794/1 454 228 | 3791/1 116 880 |
| <18.5 | 1.08 (0.96–1.21) | 1.09 (0.96–1.23) | 0.96 (0.84–1.11) |
| 18.5 to <20.0 (reference) | 1.00 | 1.00 | 1.00 |
| 20.0 to <22.5 | 1.08 (1.00–1.17) | 1.07 (0.98–1.17) | 1.22 (1.10–1.35) |
| 22.5 to <25.0 | 1.59 (1.46–1.74) | 1.58 (1.43–1.73) | 1.90 (1.70–2.13) |
| 25.0 to <27.5 | 2.70 (2.43–3.00) | 2.58 (2.31–2.89) | 3.28 (2.88–3.74) |
| 27.5 to <30.0 | 4.00 (3.50–4.58) | 3.74 (3.23–4.32) | 4.27 (3.60–5.07) |
| 30.0 to <35.0 | 6.48 (5.64–7.45) | 5.59 (4.80–6.52) | 6.47 (5.39–7.77) |
| ≥35.0 | 10.38 (8.16–13.20) | 7.62 (5.78–10.06) | 9.21 (6.57–12.92) |
| Population-attributable risk, % (95% confidence interval)a | 22.6 (20.6–24.7) | 21.9 (19.7–24.2) | 24.0 (21.4–26.5) |
| Heart failure hospitalization with congenital heart disease/valvulopathy (events/population) | 704/1 610 352 | 625/1 454 228 | 462/1 116 ,880 |
| <18.5 | 1.15 (0.87–1.52) | 1.30 (0.96–1.75) | 1.06 (0.74–1.52) |
| 18.5 to <20.0 (reference) | 1.00 | 1.00 | 1.00 |
| 20.0 to <22.5 | 1.03 (0.83–1.27) | 1.11 (0.88–1.39) | 1.11 (0.85–1.44) |
| 22.5 to <25.0 | 1.30 (1.03–1.65) | 1.43 (1.11–1.84) | 1.38 (1.01–1.88) |
| 25.0 to <27.5 | 1.43 (1.02–1.99) | 1.55 (1.09–2.20) | 1.68 (1.12–2.54) |
| 27.5 to <30.0 | 2.33 (1.52–3.58) | 2.28 (1.44–3.63) | 2.30 (1.33–3.98) |
| 30.0 to <35.0 | 2.63 (1.56–4.43) | 2.66 (1.54–4.60) | 1.78 (0.81–3.91) |
| ≥35.0 | 3.00 (0.95–9.44) | 1.04 (0.14–7.50) | 1.54 (0.21–11.26) |
| Population-attributable risk, % (95% CI)a | 9.6 (4.3–15.3) | 10.1 (4.3–16.3) | 9.7 (2.8–17.0) |
| Heart failure hospitalization with CHD, diabetes, or hypertension (events/population) | 2731/1 610 352 | 2357/1 454 228 | 1900/1 116 880 |
| <18.5 | 1.02 (0.86–1.21) | 1.00 (0.83–1.21) | 0.93 (0.75–1.15) |
| 18.5 to <20.0 (reference) | 1.00 | 1.00 | 1.00 |
| 20.0 to <22.5 | 1.09 (0.97–1.24) | 1.07 (0.94–1.22) | 1.32 (1.14–1.54) |
| 22.5 to <25.0 | 1.90 (1.67–2.16) | 1.83 (1.60–2.11) | 2.43 (2.07–2.86) |
| 25.0 to <27.5 | 3.74 (3.24–4.33) | 3.50 (2.99–4.10) | 4.95 (4.13–5.92) |
| 27.5 to <30.0 | 5.93 (4.96–7.09) | 5.35 (4.40–6.49) | 6.81 (5.43–8.54) |
| 30.0 to <35.0 | 10.37 (8.67–12.40) | 8.68 (7.12–10.59) | 11.38 (9.01–14.38) |
| ≥35.0 | 15.55 (11.39–21.23) | 10.44 (7.25–15.03) | 14.75 (9.64–22.59) |
| Population-attributable risk, % (95% CI)a | 31.7 (28.7–34.6) | 30.6 (27.4–33.9) | 33.4 (29.7–37.0) |
| Heart failure hospitalization with cardiomyopathy (events/population) | 803/1 610 352 | 726/1 454 228 | 569/1 116 880 |
| <18.5 | 1.18 (0.90–1.55) | 1.26 (0.95–1.67) | 1.07 (0.76–1.49) |
| 18.5 to <20.0 (reference) | 1.00 | 1.00 | 1.00 |
| 20.0 to <22.5 | 1.01 (0.83–1.24) | 0.97 (0.79–1.20) | 1.11 (0.87–1.41) |
| 22.5 to <25.0 | 1.18 (0.94–1.49) | 1.12 (0.88–1.43) | 1.32 (0.99–1.74) |
| 25.0 to <27.5 | 1.82 (1.37–2.41) | 1.64 (1.21–2.22) | 1.76 (1.23–2.52) |
| 27.5 to <30.0 | 2.56 (1.75–3.74) | 2.39 (1.61–3.56) | 2.25 (1.37–3.68) |
| 30.0 to <35.0 | 3.24 (2.11–4.97) | 2.53 (1.57–4.07) | 2.26 (1.22–4.17) |
| ≥35.0 | 7.88 (4.25–14.60) | 6.96 (3.63–13.36) | 6.86 (2.93–16.02) |
| Population-attributable risk, % (95% CI)a | 12.1 (6.8–17.5) | 10.1 (4.5–16.0) | 10.4 (4.0–17.2) |
| Heart failure hospitalization, all other (events/population) | 1255/1 610 352 | 1086/1 454 228 | 860/1 116 880 |
| <18.5 | 1.08 (0.86–1.37) | 1.00 (0.77–1.29) | 0.92 (0.68–1.25) |
| 18.5 to <20.0 (reference) | 1.00 | 1.00 | 1.00 |
| 20.0 to <22.5 | 1.14 (0.96–1.34) | 1.11 (0.93–1.33) | 1.18 (0.96–1.44) |
| 22.5 to <25.0 | 1.50 (1.25–1.80) | 1.53 (1.26–1.87) | 1.74 (1.38–2.18) |
| 25.0 to <27.5 | 2.26 (1.80–2.83) | 2.26 (1.77–2.88) | 2.65 (2.01–3.49) |
| 27.5 to <30.0 | 2.70 (1.97–3.72) | 2.73 (1.94–3.82) | 2.79 (1.86–4.19) |
| 30.0 to <35.0 | 4.49 (3.26–6.19) | 4.17 (2.93–5.95) | 4.63 (3.04–7.07) |
| ≥35.0 | 7.91 (4.59–13.62) | 6.85 (3.70–12.70) | 6.68 (2.90–15.39) |
| Population-attributable risk, % (95% CIl)a | 16.7 (12.5–21.1) | 17.7 (13.1–22.5) | 19.3 (13.9–24.8) |
Model 1: Adjusted for age at conscription, conscription year, test center, and comorbidities at baseline.
Model 2: Additionally adjusted for parental education, systolic and diastolic blood pressure.
Model 3: Additionally adjusted for IQ, muscle strength, and fitness.
a Mutually exclusive causes of heart failure were assigned in the following hierarchical order: (i) congenital heart disease and valvulopathies, (ii) coronary heart disease (CHD) and/or diabetes and/or hypertension, (iii) cardiomyopathy, and (iv) other causes (Supplementary material online, Appendix p. 5).
b Incidence rates are given in Table 1.
Cases associated with CHD, diabetes, or hypertension demonstrated very high multiple-adjusted HRs of 11.38 (9.01–14.38) for a BMI of 30 to <35 kg/m2 and 14.75 (9.64–22.59) for a BMI of ≥35 kg/m2. For heart failure associated with cardiomyopathy, risk increased to a multiple-adjusted HR of 6.86 (2.93–16.02) for a BMI of ≥35 kg/m2. The fully adjusted population-attributable risk for a BMI of ≥22.5 vs. <22.5 kg/m2 ranged from 9.7 (2.8–17.0), for heart failure associated with valvular disease, to 33.4 (29.7–37.0) for heart failure associated with CHD, diabetes, or hypertension. Associations were similar for heart failure as a main diagnosis (Supplementary material online, Table S2), but less strong for acute myocardial infarction, stroke, cardiovascular death, and all-cause mortality. Table 3 shows the HRs associated with heart failure per 1-unit increase in BMI in men with BMI of ≥20 kg/m2, with multiple-adjusted estimates ranging from 1.06 (1.02–1.11) (valvular disease) to 1.20 (1.18–1.22) (CHD, diabetes, or hypertension).
Table 3.
Adjusted hazard ratios associated with heart failure and mutually exclusive associated conditions of heart failurea per 1-unit increase in BMI in men with a BMI of ≥20 kg/m2b
| HR per 1-kg/m2 increase (95% CI) |
|||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Heart failure in any diagnostic position (events/population) | 4163/1 190 092 | 3650/1 076 443 | 2910/825 376 |
| 1.17 (1.16–1.18) | 1.15 (1.14–1.16) | 1.16 (1.15–1.17) | |
| Heart failure as a main diagnosis (events/population) | 1975/1 190 092 | 1730/1 076 443 | 1378/825 376 |
| 1.18 (1.17–1.19) | 1.17 (1.15–1.18) | 1.18 (1.16–1.20) | |
| Heart failure with congenital or acquired valvular disease (events/population) | 495/1 190 092 | 448/1 076 443 | 327/825 376 |
| 1.09 (1.06–1.12) | 1.08 (1.04–1.11) | 1.06 (1.02–1.11) | |
| Heart failure with CHD, diabetes, or hypertension (events/population) | 2145/1 190 092 | 1860/1 076 443 | 1515/825 376 |
| 1.20 (1.19–1.22) | 1.19 (1.17–1.20) | 1.20 (1.18–1.22) | |
| Heart failure with cardiomyopathy (events/population) | 580/1 190 092 | 517/1 076 443 | 412/825 376 |
| 1.13 (1.10–1.15) | 1.11 (1.09–1.14) | 1.09 (1.06–1.13) | |
| Heart failure, any other cause (events/population) | 943/1 190 092 | 825/1 076 443 | 656/825 376 |
| 1.13 (1.11–1.15) | 1.13 (1.10–1.15) | 1.13 (1.10–1.16) | |
Discussion
The present data set enabled us to investigate the association between body weight across a wide range and early heart failure. We documented an increase in risk that was almost 10-fold among the very obese, and detectable already at body weight levels that are considered to be normal. The effect was broadly similar across our predefined categories of heart failure, and for heart failure as a main diagnosis.
Body weight is increasing in Sweden.13 Recent estimates of obesity, which are fairly typical for Europe, are ∼4% of those <20 years of age and just <20% of adults,14 with considerably higher estimates of obesity found in other Western countries, such as the USA. In a study of the present cohort of adolescent men, the prevalence of moderate obesity (BMI 30.0–34.9 kg/m2) almost quintupled from the early 1970s and over the next 35 years, while the prevalence of BMI ≥35 kg/m2 increased 10-fold.3
We found that risk of heart failure started to increase at body weights that are normal and considered desirable (BMI of 22.5–25.0 kg/m2). Most studies use a cut-off of 25 kg/m2, which will underestimate the risk of heart failure associated with elevated weight. Although high body weight has long been linked to cardiomegaly, cardiomyopathy, and sudden death,15 these conditions were thought only to be associated with severe obesity. However, Kenchaiah et al.16 demonstrated that the risk of heart failure began to increase at much lower BMI levels, with a 5% and 7% increase in the risk per 1-unit BMI increase men and women, respectively, who were middle-aged or older. Our estimate (16% per 1-unit BMI increase) is markedly higher.
The mechanisms linking obesity to cardiac structural and functional abnormalities are not well understood. A complex interplay exists between body weight and haemodynamic, neurohormonal, and metabolic factors as well as inflammation and oxidative stress.17–20 These factors contribute to the development of cardiac hypertrophy, interstitial fibrosis,17 and other forms of cardiac dysfunction.18–20 Bariatric surgery reportedly dramatically reverses the disturbances in left ventricular function.21 Importantly, obesity early in life is not only associated with cardiometabolic risk factors22 but also with impaired cardiovascular structure and function.23 Even so, the greatest population burden of heart failure among the young and middle-aged is driven by the high-normal weight or overweight categories because these categories are the most highly represented.
The association between body weight and heart failure was strongest in patients with conditions well known to be associated with heart failure (CHD, diabetes, or hypertension) and weakest, but still highly significant, in patients with valvular disease. There was also a strong association for heart failure attributed to cardiomyopathy, although this is a highly heterogeneous condition.24
With respect to limitations to our findings, diagnoses of heart failure, and other conditions were collected for administrative reasons, cases were not formally validated, and there was no data on cardiac function or other clinical characteristics. Nonetheless, a hospital diagnosis of heart failure in Sweden has been shown to have high validity, particularly as a main diagnosis,25 and so have other major cardiovascular diagnoses.26 In our study, risk estimates were similar regardless of whether cases were assigned a primary diagnosis or not. In a recent unpublished validation study that we performed on 964 hospital records with a discharge diagnosis of heart failure (mean age 78 years) the diagnosis was definite or probable in 94.4%, the most common reason for assigning a probable and not definite diagnosis being that an echocardiography had not been done. This would apply in very few cases below the age of 65, where a careful evaluation of any case of suspected heart failure would be mandatory. Accordingly, it is unlikely that the strong association that we found would be due to misclassification of heart failure.
A second limitation is the lack of information on subsequent weight development, and on other risk factors in adult life such as dyslipidaemia. A mildly elevated weight at the age of 18 years might be a marker of an increased risk of developing subsequent obesity.27 The extent to which weight gain in adulthood contributes to the development of early heart failure cannot be established from our data. A third limitation was that the findings of the present study are limited to Swedish men and may not be applicable to women or to other settings. The main strength of the present study is the uniquely large number of cases occurring at a young age.
In conclusion, we found a steep increase in the risk of early heart failure associated with increasing body weight starting at levels that are considered normal. Given the global trend in adolescent overweight and obesity, early heart failure might well become a major threat worldwide, an aspect that has not received much attention in current prevention guidelines.28 The strong association that we found between body weight and early heart failure serves to underline the urgent need for global action to curb the obesity epidemic.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
L. S. performed statistical analysis; A. R., M. Å., K. T. handled funding and supervision; M. Å. acquired the data; A. R., M. Å., K. T. conceived and designed the research; A. R., J. R., M. Å., K. T., L. S. drafted the manuscript; M. S., M. W., D. Å., G. K., L. S. made critical revision of the manuscript for key intellectual content.
Funding
This work was supported by grants from: the Swedish state under an agreement between the Swedish Government and the County Councils Concerning Economic Support of Research and Education of Doctors (ALFGBG-427301); the Swedish Society for Physicians, the Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, Sweden, the Swedish Heart and Lung Foundation (2015-0438); the Swedish Research Council (2013-5187, 2013-4236); and the Swedish Council for Health, Working Life and Welfare (FORTE) (2007-2280, 2013-0325).
Conflict of interest: none declared.
Supplementary Material
References
- 1. Stewart S, Ekman I, Ekman T, Odén A, Rosengren A. Population impact of heart failure and the most common forms of cancer: a study of 1,162,309 hospital cases in Sweden (1988–2004). Circ Cardiovasc Qual Outcomes 2010;3:573–580. [DOI] [PubMed] [Google Scholar]
- 2. Barasa A, Schaufelberger M, Lappas G, Swedberg K, Dellborg M, Rosengren A. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur Heart J 2014;35:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neovius M, Teixeira-Pinto A, Rasmussen F. Shift in the composition of obesity in young adult men in Sweden over a third of a century. Int J Obes (Lond) 2008;32:832–836. [DOI] [PubMed] [Google Scholar]
- 4. Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, Vatten LJ. Body mass index, abdominal fatness and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation 2016;133:639–649. [DOI] [PubMed] [Google Scholar]
- 5. Björck L, Novak M, Schaufelberger M, Giang KW, Rosengren A. Body weight in midlife and long-term risk of developing heart failure-a 35-year follow-up of the primary prevention study in Gothenburg, Sweden. BMC Cardiovasc Disord 2015;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt M, Bøtker HE, Pedersen L, Sørensen HT. Young adulthood obesity and risk of acute coronary syndromes, stable angina pectoris, and congestive heart failure: a 36-year cohort study. Ann Epidemiol 2014;24:356–361. [DOI] [PubMed] [Google Scholar]
- 7. Nordesjö LO, Schéle R. Validity of an ergometer cycle test and measures of isometric muscle strength when prediction some aspects of military performance. Swedish Journal of Defence Medicine 1974;10:11–23. [Google Scholar]
- 8. Nyberg J, Aberg MA, Schioler L, Nilsson M, Wallin A, Torén K, Kuhn HG. Cardiovascular and cognitive fitness at age 18 and risk of early-onset dementia. Brain 2014;137:1514–1523. [DOI] [PubMed] [Google Scholar]
- 9. Aberg MA, Pedersen NL, Toren K, Svartengren M, Bäckstrand B, Johnsson T, Cooper-Kuhn CM, Aberg ND, Nilsson M, Kuhn HG. Cardiovascular fitness is associated with cognition in young adulthood. Proc Natl Acad Sci USA 2009;106:20906–20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–526. [Google Scholar]
- 11. Harrell FE., Jr Regression modeling strategies. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 12. Natarajan S, Lipsitz SR, Rimm E. A simple method of determining confidence intervals for population attributable risk from complex surveys. Stat Med 2007;26:3229–3239. [DOI] [PubMed] [Google Scholar]
- 13. Neovius K, Johansson K, Kark M, Tynelius P, Rasmussen F. Trends in self-reported BMI and prevalence of obesity 2002–10 in Stockholm County, Sweden. Eur J Public Health 2013;23:312–315. [DOI] [PubMed] [Google Scholar]
- 14. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bray GA. Pathophysiology of obesity. Am J Clin Nutr 1992;55(2 Suppl.):488S–494S. [DOI] [PubMed] [Google Scholar]
- 16. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 17. Mahajan R, Lau DH, Sanders P. Impact of obesity on cardiac metabolism, fibrosis, and function. Trends Cardiovasc Med 2015;25:119–126. [DOI] [PubMed] [Google Scholar]
- 18. Wong CY, O'Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 2004;110:3081–3087. [DOI] [PubMed] [Google Scholar]
- 19. Leichman JG, Aguilar D, King TM, Vlada A, Reyes M, Taegtmeyer H. Association of plasma free fatty acids and left ventricular diastolic function in patients with clinically severe obesity. Am J Clin Nutr 2006;84:336–341. [DOI] [PubMed] [Google Scholar]
- 20. Kishi S, Armstrong AC, Gidding SS, Colangelo LA, Venkatesh BA, Jacobs DR Jr, Carr JJ, Terry JG, Liu K, Goff DC Jr, Lima JA. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the CARDIA study (Coronary Artery Risk Development in Young Adults). JACC Heart Fail 2014;2:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leichman JG, Wilson EB, Scarborough T, Aguilar D, Miller CC III, Yu S, Algahim MF, Reyes M, Moody FG, Taegtmeyer H. Dramatic reversal of derangements in muscle metabolism and left ventricular function after bariatric surgery. Am J Med 2008;121:966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med 2015;373:1307–1317. [DOI] [PubMed] [Google Scholar]
- 23. Ayer J, Charakida M, Deanfield JE, Celermajer DS. Lifetime risk: childhood obesity and cardiovascular risk. Eur Heart J 2015;36:1371–1376. [DOI] [PubMed] [Google Scholar]
- 24. Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000;342:1077–1084. [DOI] [PubMed] [Google Scholar]
- 25. Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Failure. 2005;7:787–791. [DOI] [PubMed] [Google Scholar]
- 26. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Juhola J, Magnussen CG, Viikari JS, Kähönen M, Hutri-Kähönen N, Jula A, Lehtimäki T, Åkerblom HK, Pietikäinen M, Laitinen T, Jokinen E, Taittonen L, Raitakari OT, Juonala M. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr 2011;159:584–590. [DOI] [PubMed] [Google Scholar]
- 28. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F. European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


