Tourette syndrome is considered a basal ganglia disorder, but this model does not explain the natural history of tic expression, the sex disparity, and the character of tics. Albin proposes that Tourette syndrome reflects dysfunction of additional circuits interdigitating with the basal ganglia to comprise the Social Decision-Making Network.
Keywords: basal ganglia, tic disorder, habit-based behaviour, brain development, neuroendocrine system
Abstract
Tourette syndrome is a common neurodevelopmental disorder defined by characteristic involuntary movements, tics, with both motor and phonic components. Tourette syndrome is usually conceptualized as a basal ganglia disorder, with an emphasis on striatal dysfunction. While considerable evidence is consistent with these concepts, imaging data suggest diffuse functional and structural abnormalities in Tourette syndrome brain. Tourette syndrome exhibits features that are difficult to explain solely based on basal ganglia circuit dysfunctions. These features include the natural history of tic expression, with typical onset of tics around ages 5 to 7 years and exacerbation during the peri-pubertal years, marked sex disparity with higher male prevalence, and the characteristic distribution of tics. The latter are usually repetitive, somewhat stereotyped involuntary eye, facial and head movements, and phonations. A major functional role of eye, face, and head movements is social signalling. Prior work in social neuroscience identified a phylogenetically conserved network of sexually dimorphic subcortical nuclei, the Social Behaviour Network, mediating many social behaviours. Social behaviour network function is modulated developmentally by gonadal steroids and social behaviour network outputs are stereotyped sex and species specific behaviours. In 2011 O’Connell and Hofmann proposed that the social behaviour network interdigitates with the basal ganglia to form a greater network, the social decision-making network. The social decision-making network may have two functionally complementary limbs: the basal ganglia component responsible for evaluation of socially relevant stimuli and actions with the social behaviour network component responsible for the performance of social acts. Social decision-making network dysfunction can explain major features of the neurobiology of Tourette syndrome. Tourette syndrome may be a disorder of social communication resulting from developmental abnormalities at several levels of the social decision-making network. The social decision-making network dysfunction hypothesis suggests new avenues for research in Tourette syndrome and new potential therapeutic targets.
Introduction
Tourette syndrome is a common neurodevelopmental disorder defined by characteristic involuntary movements and vocalizations, tics, accompanied frequently by specific comorbid behavioural syndromes (Robertson, 2000, 2012, 2014; Jankovic, 2001a; Ganos, 2016; Robertson et al., 2017). The emergence of involuntary movements, vocalizations and subsequent natural history of Tourette syndrome follows a predictable pattern in the first two to three decades of life, suggesting that Tourette syndrome results from altered brain development trajectories. Discussions of brain system abnormalities underlying Tourette syndrome are dominated by speculations that basal ganglia abnormalities underlie the pathophysiology of Tourette syndrome (Albin, 2006; Albin and Mink, 2006; Felling and Singer, 2011; Ganos et al., 2013; Tremblay et al., 2015; Worbe et al., 2015b; Yael et al., 2015; Ganos, 2016). This concept is based on the response of tics to treatment with dopamine antagonists, the occurrence of tics in diseases with unequivocal striatal pathology, evidence from structural and functional imaging studies implicating basal ganglia associated circuits (see below), and a persuasive conceptualization of tics as a form of habits gone awry. Habit formation and maintenance are strongly implicated as primary functions of basal ganglia circuits (Yin and Knowlton, 2006; Smith and Graybiel, 2016). Basal ganglia circuits may be particularly important in assembling individual stimulus-response associations into more complex behavioural sequences that are executed as units. Dysregulation of this ‘chunking’ function is a plausible substrate for complex tics and obsessive compulsive behaviours (Smith and Graybiel, 2016).
Recent application of imaging methods in Tourette syndrome research disclosed evidence of widespread morphologic and functional changes in the CNS of Tourette syndrome subjects (see below), suggesting alternate hypotheses about the systems involved in Tourette syndrome. Abnormalities of neocortical development and/or function are suggested by some of these studies, a plausible hypothesis underpinned by the concept that an important function of some neocortical regions is to provide inhibitory control (Ganos et al., 2013; Worbe et al., 2015b; Yael et al., 2015).
These attractive and well supported ideas about the neurobiology of Tourette syndrome do not, however, account for some of the most conspicuous features of Tourette syndrome, specifically the marked sex disparity in Tourette syndrome prevalence, the natural history of the emergence and variation in tic severity, and the characteristic phenomenology of most tics. Recent developments in apparently unrelated areas of neuroscience related to sociality and emotion suggest additional brain systems that may be developmentally altered in Tourette syndrome. Here I explore the possibility that Tourette syndrome is characterized by abnormalities in a recently proposed brain system implicated in crucial aspects of social behaviour, the social decision-making network (SDM).
Tourette syndrome
Motor and phonic tics are repetitive, relatively stereotyped involuntary movements, usually brief in duration. Tics may be simple or complex. Simple tics are brief, meaningless movements, often involving a single muscle group. Complex tics are slower, and may involve apparently coordinated movements or vocalizations. Some simple tics (‘dystonic tics’) are more sustained. The relatively stereotyped character of tics differentiates tics from the random movements of the chorea-athetosis-ballism spectrum and most forms of myoclonus. Tics are generally not as rapid as typical myoclonus. Motor tics have a characteristic anatomic distribution. While truncal and limb tics are seen in clinical practice, there is a rostrocaudal gradient of tic expression with the majority of motor tics involving eye, facial, neck, and shoulder movements. Eye blinking, eye rolling, grimacing, nares flaring, and other facial movements are common motor tics, as are shoulder shrugging and stereotyped head-neck movements. Typical phonic tics include repetitive snorting, sniffing, throat-clearing, and coughing. Complex tics resemble organized movements and vocalizations, of which the most famous, albeit rare, example is coprolalia. Many tics are accompanied by characteristic subjective experiences with Tourette syndrome patients reporting a sense of discomfort, often in the affected region, relieved by tic performance. Some Tourette syndrome patients report an element of compulsion with their tics. Tics can typically be suppressed temporarily with conscious effort. Tic expression is modulated commonly by attentional loading; Tourette syndrome patients concentrating intently on a task often experience a decline in tics during task performance.
Tics are a common phenomenon, generally with onset in childhood, and often transient. Adult-onset tics are rare. Some individuals may have just a single persistent tic and some individuals exhibit only motor tics. When both motor and phonic tics are present, onset is below the age of 18 years, and tics are sustained for a period of longer than 1 year, Tourette syndrome is the diagnosis. Recent estimates highlight the relatively high prevalence of Tourette syndrome in children with epidemiology studies converging on estimates of 0.5% to 1.0% of children exhibiting Tourette syndrome. Estimated prevalence of Tourette syndrome among adults is much lower, by approximately a factor of 10, reflecting the distinctive natural history of Tourette syndrome (see below). Tourette syndrome exhibits a marked sex bias; the ratio of male to female Tourette syndrome patients is ∼3–4:1 (Knight et al., 2012; Scharf et al., 2015; Yang et al., 2016).
Tourette syndrome is accompanied frequently by comorbid psychiatric disorders, which may lead to more distress and disability than tics. Attention deficit hyperactivity disorder is common in Tourette syndrome, as are anxiety disorders. Many Tourette syndrome patients exhibit obsessive compulsive behaviours, often arising to the level of obsessive compulsive disorder (Hirschtritt et al., 2015). It is plausible to view simple tics and complex obsessions as opposite poles of a spectrum with compulsive behaviours occupying the middle of the spectrum. Differentiating complex tics from compulsions can be difficult (Worbe et al., 2010a).
Tics usually first manifest around the ages of 5 to 7 years. Tics generally fluctuate significantly in severity, distribution, and character. A common experience is for a child to exhibit one or more types of tics for weeks to months, with the specific tic type subsiding and replaced by another type of tic. Tic severity tends to peak between the ages of 10–14. Many Tourette syndrome patients subsequently experience declines in tic severity with some individuals experiencing tic remission as they enter adulthood. This relatively benign natural history accounts for lower prevalence of Tourette syndrome adults.
Tourette syndrome has a strongly heritable component. A high fraction of Tourette syndrome patients have a first degree relative with Tourette syndrome or tics. Efforts to define specific gene mutations associated with Tourette syndrome have been frustrating. Several large-scale association studies did not identify loci with strong connections to Tourette syndrome, indicating complex polygenic inheritance (Davis et al., 2013; Scharf et al., 2013; McGrath et al., 2014; Paschou et al., 2014; Yu et al., 2015; Bertelsen et al., 2016). Willsey et al. (2017) used whole exome sequencing and the strategy of studying ‘trios’ comprising an affected Tourette syndrome subject and unaffected parents to identify possible de novo coding variants associated with Tourette syndrome. Willsey et al. (2017) describe an excess of likely gene disrupting mutations associated with Tourette syndrome and identified four candidate genes. Rare pedigrees with apparent monogenic inheritance of Tourette syndrome were identified, most recently a family with a mutation in the gene encoding histidine decarboxylase (HDC) (Verkerk et al., 2003; Ercan-Sencicek et al., 2010; Lei et al., 2012; Karagiannidis et al., 2013; Dong et al., 2016). Further study of these genes may yield clues about the pathophysiology of more common forms of Tourette syndrome.
Treatment options for Tourette syndrome are limited. Dopamine antagonist agents will reduce tics, though usually not completely at clinically tolerated doses. Use of dopamine antagonists carries the risk of significant side effects including sedation, impaired cognition, cardiac arrhythmias, weight gain, and tardive movement disorders (Pringsheim et al., 2012). The monoamine depleting agent tetrabenazine, thought to have no risk of inducing tardive movement disorders, can be used. Side effects of reducing monoaminergic neurotransmission can limit clinical utility of this agent (Chen et al., 2012). α2-Adrenergic agonists such as clonidine and guanfacine are used in clinical practice and exhibit modest efficacy but generally good tolerability (Pringsheim et al., 2012). Behavioural interventions may play a larger role in treatment of Tourette syndrome. Solid trial data indicate that habit reversal therapy is useful for particularly bothersome tics (Piacentini et al., 2010). An extension of habit reversal therapy, the Comprehensive Behavioural Intervention for Tics, incorporating additional behavioural interventions, is effective in Tourette syndrome (Whittington et al., 2016). Appropriate behavioural interventions and psychiatric care, which may involve other classes of medications, are important for treating significant behavioural comorbidities. Some patients exhibit disabling tics for which conventional medical and behavioural therapies are inadequate. These unfortunate individuals often exhibit disabling behavioural co-morbidities (Cheung et al., 2007). For this population, deep brain stimulation (DBS) surgery may be a useful option (Schrock et al., 2015). Many children with Tourette syndrome exhibit mild-to-moderate tics and manageable behavioural co-morbidities. Education and reassurance of family and school personnel is often sufficient. A supportive family and social environment is often the best treatment for Tourette syndrome, particularly in view of the often relatively benign natural history.
Basal ganglia changes and function in Tourette syndrome
Post-mortem, genetic and animal model studies
Efforts to understand the pathophysiology of Tourette syndrome are handicapped by the absence of gross pathologic changes in either the small number of autopsied brains or in routine imaging studies. The observation that dopamine antagonists ameliorate tics focused speculation about Tourette syndrome pathophysiology on the basal ganglia and specifically on the striatum. Additional lines of evidence support the concept that abnormalities of basal ganglia circuits are involved in Tourette syndrome pathophysiology. Tics are common in Huntington’s disease and other disorders with unequivocal striatal pathology (Jankovic, 2001b). In careful analyses of a small number of post-mortem Tourette syndrome brain specimens, Vaccarino’s group described changes of both pallidal and striatal neurons (Kalanithi et al., 2005; Kataoka et al., 2010; Lennington et al., 2016). Tourette syndrome brains exhibited reduced numbers of neurons in the external segment of the globus pallidus with a corresponding increase in the number of internal segment pallidal neurons (Kalanithi et al., 2005). In the same analysis, the number of striatal parvalbumin-immunoreactive interneurons was found to be reduced. In a subsequent analysis, both parvalbumin-immunoreactive and cholinergic striatal interneurons were reported as reduced in Tourette syndrome (Kataoka et al., 2010). Vaccarino and colleagues made the interesting suggestion that these changes were due to a neuronal migration defect during brain development. Because of, presumably, a paucity of suitable post-mortem material, these observations are not yet replicated.
Recently developed PET methods allow in vivo quantification of cholinergic terminal density by measuring regional vesicular acetylcholine transporter expression with 18F-fluoroethoxybenzovesamicol (18F-FEOBV; Petrou et al., 2014). Striatal 18F-FEOBV binding is not different between Tourette syndrome and control subjects, strongly suggesting normal striatal cholinergic neuron density in Tourette syndrome (Albin et al., 2017). The prior post-mortem report of diminished striatal cholinergic interneurons may reflect post-mortem changes, agonal effects, and/or medication effects (Albin et al., 2017).
The paucity of specific genes associated with Tourette syndrome inhibited efforts to develop animal models that might provide insight into the pathophysiology of Tourette syndrome. Information from a small number of models is consistent with basal ganglia dysfunction in Tourette syndrome. Verkerk et al. (2003) described a pedigree with Tourette syndrome and complex chromosomal rearrangements leading to loss of contactin-associated protein 2 (CNTNAP2), a member of the neurexin superfamily of transmembrane proteins. CNTNAP2 knockout mice manifest neuronal migration abnormalities, including reduced striatal parvalbumin containing interneurons (Peñagarikano et al., 2011). This line exhibits increased repetitive behaviours, including increasing grooming, reduced by dopamine antagonist treatment. Ercan-Sencicek et al. (2010) reported a pedigree in which tic occurrence is associated with HDC mutations. Affected individuals exhibit other abnormal features, including abnormal oropharyngeal anatomy and some cardiovascular abnormalities. HDC is responsible for the synthesis of histamine, the neurotransmitter of widely ramifying tuberomamillary neurons. These projections innervate the striatum and modulate dopaminergic neurotransmission. HDC knockout mice exhibit exacerbation of stereotyped behaviours and increased striatal dopamine release after stimulant treatments (Castellan Baldan et al., 2014). A potential problem with the paradigm of amphetamine induced stereotypies is that it may not mimic natural behavioural sequences such as grooming, weakening their relevance to Tourette syndrome (Berridge and Aldridge, 2000). Subsequent work with HDC knockout mice showed that this line exhibits excessive grooming under some stressful conditions, consistent with Tourette syndrome-related phenomena (Xu et al., 2015a).
There were also efforts to model Tourette syndrome in mice by duplicating the changes in striatal interneuron populations described by Kataoka et al. (2010), including selective depletion of striatal cholinergic interneurons (Xu et al., 2015b; Martos et al., 2017), parvalbumin-immunoreactive neurons (Xu et al., 2016), and combined depletion of striatal cholinergic and parvalbumin-immunoreactive neurons (Rapanelli et al., 2017). The in vivo imaging evidence for preserved striatal cholinergic interneurons (Albin et al., 2017) in Tourette syndrome subjects mentioned above undermines the relevance of models based on depletion of striatal cholinergic interneurons. These modelling experiments have implications for efforts to model Tourette syndrome in mice. These models have behavioural phenotypes and as they apparently result from striatal changes not found in Tourette syndrome, these behavioural assays may not be appropriate tools for evaluating putative Tourette syndrome models. In these experiments, striatal cholinergic interneuron depletion was performed in adult mice. Selective depletion of dorsal striatal cholinergic interneurons during brain development produces a markedly different phenotype (Pappas et al., 2015). Lesioning adult brain may not be appropriate for modelling a neurodevelopmental disorder such as Tourette syndrome.
Several non-human primate experiments support a key role for basal ganglia dysfunction in tic pathophysiology (François et al., 2004; Grabli et al., 2004; Worbe et al., 2009, 2011, 2013; Bronfeld et al., 2011, 2013a, b; McCairn et al., 2009, 2013, 2016; Godar et al., 2014; Israelashvili and Bar-Gad, 2015). Focal inhibition of striatal and external pallidal neurons by localized injections of the GABA-A receptor antagonist bicuculline produce involuntary movements and behavioural abnormalities consistent with tics and other phenomena characteristic of Tourette syndrome. Depending on the location of injection, these involuntary movements range from simple movements to complex behavioural sequences including stereotyped behaviours mimicking normal grooming behaviours. Involuntary movements following focal inhibition of neurons in the sensorimotor territories of the putamen are described as particularly faithful tic mimics. Vocal tics are reported to follow focal inhibition of nucleus accumbens neurons. The distinct involuntary movements and vocalizations following focal inhibition of different subregions of the striatum and pallidum reflect the anatomic and functional segregation of parallel specialized circuits that course through the basal ganglia (Alexander et al., 1986), a function of the topographic organization of corticostriate projections (Kemp and Powell, 1970). One study using similar approaches in rats reported similar phenomena with intrastriatal bicuculline injections (Bronfeld et al., 2013a, b).
Another potentially relevant rodent behavioural model implicating striatal dysfunction is grooming chains (Kalueff et al., 2016). Rodents groom in multi-component, relatively stereotyped behavioural sequences. Individual components of grooming chains appear to be ‘hardwired’ into lower motor centres in brainstem and spinal cord. The dorsal striatum is crucial for appropriate sequencing and completion of grooming chains (Cromwell and Berridge, 1996). The subpopulation of striatal projection (direct pathway) neurons expressing dopamine D1 receptors appears to be particularly important for grooming chain performance with administration of D1 agonists inducing excessive, highly repetitive grooming chains termed super-stereotypy (Berridge and Aldridge, 2000; Taylor et al., 2010). D1 receptor agonist induced super-stereotypy is reduced by concurrent administration of the D2 antagonist haloperidol (Taylor et al., 2010). These findings suggest that dopamine D1 receptors would be logical targets for treatment of tics (Albin and Mink, 2006). Consistent with this prediction, preliminary trial data suggest that tics are reduced by D1 antagonists and functional imaging data suggest increased activity of D1 expressing (direct pathway) striatal projection neurons in Tourette syndrome (Baym et al., 2008; Gilbert et al., 2014).
Clinical experience with DBS for treatment of severe Tourette syndrome is consistent also with basal ganglia circuit dysfunction. Some series report improvement with DBS. The most frequent DBS targets in Tourette syndrome are midline thalamic nuclei strongly interconnected with the basal ganglia, and the internal segment of the globus pallidus.
Imaging results
More direct demonstration of basal ganglia circuit abnormalities comes from application of modern imaging methods, particularly MRI methods. An important point about imaging studies in Tourette syndrome is that this is a confusing literature with papers reporting conflicting findings. Discrepant findings likely occur because of the many difficulties inherent in studying Tourette syndrome subjects. Important confounds include the difficulties of comparing subjects at different phases of brain development, the possibility that detected changes represent plastic adaptations to primary pathologies in Tourette syndrome, and potential effects of medications. Tourette syndrome study populations are heterogeneous, with varying mixtures of potentially relevant comorbidities and differing medication regimens. Recent useful reviews of imaging studies in Tourette syndrome highlight these issues but also provide some clarity about what appear to be abnormalities in Tourette syndrome with a consensus favouring abnormalities of basal ganglia structure and connectivity (Plessen et al., 2009; Felling and Singer, 2011; Neuner et al., 2013; Church and Schlagger, 2014). One apparently robust result, described best in a relatively large prospective study by Peterson and colleagues (Peterson et al., 2003; Bloch et al., 2005), is decreased caudate nucleus volume. Recent, relatively large morphometry studies in Tourette syndrome and control children, however, did not identify changes in caudate or putamen volumes in Tourette syndrome subjects (Greene et al., 2017; Forde et al., 2017). Greene et al. (2017) identified only volume changes in the thalamus, hypothalamus, and midbrain.
Recent structural and functional imaging studies identify changes in basal ganglia structure, connectivity, and function in Tourette syndrome. Worbe and colleagues (2012, 2015a) used MRI tractography and functional connectivity methods to identify abnormalities in connections within the basal ganglia and associated cortices. This group suggests that these abnormalities represent immature and atypical development of these circuits. In a particularly interesting study, this group demonstrated an increased propensity for subjects with Tourette syndrome to develop habits and correlated this tendency with abnormal connectivity of the putamen and supplementary motor cortex (Delorme et al., 2016). Other work supports abnormalities of basal ganglia circuit connections in Tourette syndrome and a recent study of brain control networks in Tourette syndrome also suggests immature development of these circuits (Church et al., 2009; Makki et al., 2009). A number of functional imaging studies of tic generation and suppression are consistent with basal ganglia circuit abnormalities in Tourette syndrome (Peterson et al., 1998; Wang et al., 2011; Neuner et al., 2014).
Studies in both Tourette syndrome children and adults describe thinner cortices in several regions, though some studies report increased regional cortical thickness, with suggestions that these are compensatory changes (Sowell et al., 2008; Draganski et al., 2010; Fahim et al., 2010; Muellner et al., 2015). Worbe et al. (2010b) suggest that differing patterns of cortical thinning correlate with Tourette syndrome subtypes. These structural studies are complemented by data suggesting abnormalities of cortical function in Tourette syndrome (Bohlhalter et al., 2006; Tinaz et al., 2014; Martín-Rodríguez et al., 2015).
Other work describes structural and functional changes in yet more regions, including the thalamus, amygdala, midbrain, and cerebellum (Garraux et al., 2006; Peterson et al., 2007; Ludolph et al., 2008; Makki et al., 2008; Miller et al., 2010; Neuner et al., 2010a; Tobe et al., 2010; Werner et al., 2011). A recent molecular imaging study of GABA-A receptor binding sites suggests widespread changes in GABA-A receptor expression in Tourette syndrome, including the ventral striatum, globus pallidus, thalamus, amygdala, cerebellum, some cortices, substantia nigra, and periaqueductal grey (PAG) (Lerner et al., 2012). The cumulative results of imaging studies suggest widespread developmental abnormalities in Tourette syndrome. This inference suggests that alterations in previously unexamined regions may be important in Tourette syndrome pathophysiology.
What basal ganglia dysfunction does not explain
There is convergent evidence for basal ganglia circuit abnormalities in Tourette syndrome, but there is no obvious way to connect basal ganglia circuit abnormalities with several salient features of Tourette syndrome. What accounts for the natural history of Tourette syndrome with the emergence of tics around ages 5 to 7 years and the subsequent peri-pubertal exacerbation? Why the marked sex disparity in prevalence? What accounts for the specific character of tics—specifically the expression of tics as eye movements, facial movements, head and neck movements, and involuntary phonations? A satisfactory account of the neurobiology of Tourette syndrome should account for the following cardinal features: (i) the characteristic distribution of tics with preferential expression of involuntary eye, facial, head, and shoulder movements; (ii) the presence and character of involuntary phonations; (iii) the stereotyped character of tics; (iv) the natural history of tic expression with tic emergence around ages 5 to 7 years and peri-pubertal exacerbation; (v) the sex disparity with male predominance of tic expression; and (vi) an important role for basal ganglia circuitry.
A successful account would also explain some other features of Tourette syndrome including: (i) the moderation of tic expression by task performance; and (ii) the high frequency of anxiety disorders in Tourette syndrome.
Unexplained cardinal features as clues to neurobiology of Tourette syndrome
While explaining these features of Tourette syndrome is challenging, they also provide clues suggesting an expanded view of Tourette syndrome neurobiology. A useful starting point is to consider one of the primary functions of eye, facial, and head movements—conspecific social signalling. A major, perhaps the major, function of these movements is non-verbal communication between conspecifics, particularly of emotional states. As demonstrated by psychologist Paul Ekman (2015), this aspect of non-verbal communication is both important and universal among humans. The use and interpretation of facial expressions, including eye position, is remarkably uniform across all cultures. This is likely also true of movements involving head position and shoulder movements, which contribute to aspects of emotional body language (de Gelder, 2015). Darwin (2009) famously argued that many of these aspects of non-verbal communication are not only universal among humans, but also phylogenetically conserved across a broad range of species. The concept that tics are distorted social signals explains why Tourette syndrome and related tic syndromes are perceived as disorders (Davis et al., 2004). Motor tics introduce confusion into an important and largely subliminal channel for social communication. The same is obviously true for involuntary phonations. A corollary of the concept that Tourette syndrome involves dysfunctional inter-conspecific social communication is that Tourette syndrome subjects should manifest abnormal social perceptions and socially inappropriate behaviours in addition to the disordered social signalling of tics. Emerging evidence indicates that this is the case, with research documenting altered social cognition, social disinhibition, and a phenomenon called non-obscene socially inappropriate symptoms (NOSIS) in subjects with Tourette syndrome (Kurlan et al., 1996; Channon et al., 2003, 2004, 2012; Eddy and Cavanna, 2013a, b; Hirschtritt et al., 2016). In their useful review of altered social cognition in Tourette syndrome, Eddy and Cavanna (2013b) offer the interesting interpretation that differences in performance on social cognitive tasks between Tourette syndrome and controls reflect inappropriate responses to social information.
Another clue follows from the period of tic onset and the peri-pubertal exacerbation of tics. These features suggest involvement of systems influenced by gonadal steroids, particularly androgens, as suggested some years ago by Cohen and colleagues (Peterson et al., 1992, 1994; Alexander and Peterson, 2004; Bortolato et al., 2013; Martino et al., 2013). An important role of androgens in the expression of tics is consistent with average tic onset around the ages of 5–7. This is approximately the period of the onset of adrenarche, when the development of the adrenal zona reticularis results in increasing production of the adrenal androgens dehydroepiandrosterone (DHEA) and DHEA sulphate (Auchus, 2011; Conley et al., 2012). Peri-pubertal exacerbation of tics has obvious correlations with rising gonadal steroid levels, including androgens. Dynamic changes in brain systems regulating social behaviours during sexual maturation is hardly surprising. Adolescence is a period of social experimentation necessary for the establishment of social independence, a necessary prelude to mating (Crone and Dahl, 2012; Sisk, 2016).
A final clue is the male predominance observed in Tourette syndrome. The marked difference in tic prevalence between sexes suggests developmental abnormalities of sexually dimorphic brain systems.
Aggregation of these clues suggests that we should be looking for a phylogenetically conserved brain system critical to social behaviour, influenced strongly by gonadal steroids, and exhibiting structural and/or functional sex dimorphism.
The social behaviour and the social decision-making networks
Newman (1999) synthesized a large array of behavioural, neurochemical, and anatomic data to propose the existence of a pan-mammalian social behaviour network (SBN; Fig. 1). The SBN consists of a densely interconnected group of subcortical nuclei regulating male and female mating behaviours, aggression, and parental behaviours. A defining feature of all SBN nuclei is expression of high levels of gonadal steroid receptors. SBN components include the medial amygdala-bed nucleus of the stria terminalis (MA-BNST; functionally conjoint structures part of the extended amygdala), the hypothalamic medial preoptic area (MPOA), the anterior hypothalamus, the ventromedial hypothalamus (VMH), the lateral septum, and the midbrain periaqueductal grey-central grey (PAG). Recent comparative studies describe this system as conserved across all vertebrate phyla (Goodson, 2005; O’Connell and Hofmann, 2011, 2012; Goodson and Kingsbury, 2013). SBN nuclei express relatively high levels of receptors for the nonapeptides oxytocin and arginine vasopressin, recognized as key modulators of social behaviour and social cognition (Donaldson and Young, 2008; Soares et al., 2010; Gabor et al., 2012). The paraventricular hypothalamus (PVH) is a major source of these nonapeptides and densely interconnected with the SBN nuclei identified by Newman (1999). Goodson and Kingsbury (2013) recently suggested classifying the PVH as one of the core nuclei of the SBN. Some of these nuclei also exhibit structural sex dimorphism (for reviews see Simerly, 2002; Dulac and Kimchi, 2007). A key point is that the SBN does not mediate exactly the same behaviours across all species and phyla. Rather, it is a crucial system for the expression and modulation of sex-specific and species-specific social behaviours. The SBN, for example, is critical to the expression of the wide variety of male and female parental behaviours seen in diverse vertebrate groups (Dulac et al., 2014).
Figure 1.
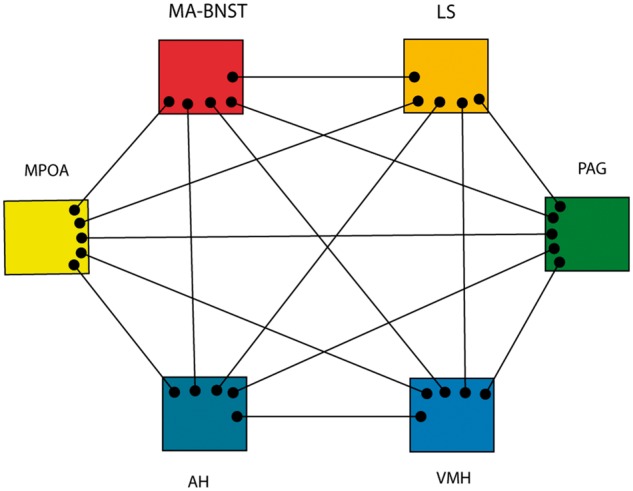
The Social Behaviour Network (SBN). Adapted from Newman (1999). This network mediates a large number of relatively stereotyped social behaviours, is modulated by gonadal steroids, and has sex dimorphic components. The PAG is the main efferent node, driving lower circuits responsible for specific behaviours. AH = anterior hypothalamus; MA = medial amgydala; LS = lateral septum.
Studies of a crucial female rodent sexual behaviour, lordosis, provides a nice example of SBN functions. Lordosis, a relatively complex but stereotyped motor behaviour, is the receptive posture adopted by female rats to facilitate successful sexual intercourse. Appropriate lordosis depends on gonadal steroid effects within the brain with the VMH as the crucial node for oestrogen effects (Harlan et al., 1984). The expression of lordosis depends on projections from the VMH to the PAG with the PAG controlling lower brainstem nuclei responsible for this stereotyped motor behaviour. Stolzenberg and Numan (2011) suggested an analogous pathway for maternal, female sexual, and male sexual behaviours with the MPOA and VMH as key SBN nodes for gonadal steroid action. MPOA/VMH projections to the PAG drive activation of lower circuits that generate these stereotyped behaviours. In a good example of functional sex dimorphism, the same circuit supports markedly different behaviours in male and female rats. In Newman’s model (1999), different patterns of activity in the interconnected SBN nuclei generate differing outputs to the PAG with PAG neurons ultimately driving different downstream effectors. In Tourette syndrome, aberrant PAG activity would result in generation of inappropriate activation of brainstem circuits needed for social signalling via stereotyped eye, face, head movements, and vocalizations (see below).
Recent studies using modern neurobiologic tools such as genetic manipulation and optogenetic stimulation of specific neuron populations within these nuclei confirm that SBN nuclei control a wide array of complex social and relatively stereotyped behaviours involving grooming, aggression, defensive behaviours, social submission, mating behaviours, feeding behaviours, predation behaviours, vocalizations, and parental care behaviours (Sternson, 2013; Lee et al., 2014; Dietrich et al., 2015; Wang et al., 2015; Anderson, 2016; Sakurai et al., 2016; Han et al., 2017). Control of these kinds of social behaviours is complex and depends partly on interactions of different neuronal populations within individual SBN nuclei. Hong et al. (2014), for example, describe optogenetic stimulation of a subpopulation of medial amygdala GABAergic neurons as eliciting aggressive behaviour, mating behaviours, and social grooming. Stimulation of closely adjacent medial amygdala glutamatergic neurons, in contrast, produced self-grooming in a non-social context. The functional behaviour of SBN nuclei is strongly influenced by gonadal steroids. Xu et al. (2012) demonstrated marked sexual dimorphisms in gonadal steroid regulation of gene expression within mouse SBN nuclei. Genetic manipulation of these gonadal steroid hormone regulated genes markedly altered sex specific social behaviours. Similarly, androgens have sex-specific effects on social behaviours via modulation of different populations of medial amygdala neurons (Unger et al., 2015).
Given considerable empirical data indicating that basal ganglia circuit abnormalities are associated with Tourette syndrome, and a plausible theoretical rationale for suspecting involvement of the SBN in Tourette syndrome, a satisfactory conception of Tourette syndrome neurobiology requires incorporation of both systems into models of Tourette syndrome pathophysiology. In a detailed comparative analysis, O’Connell and Hofmann (2011) proposed the existence of a phylogenetically conserved larger network for adaptive social behaviours; the SDM (Fig. 2). As defined by O’Connell and Hofmann (2011), the SDM consists of much of the basal ganglia circuitry and their connections, with an emphasis on the mesolimbic component of the basal ganglia and including the basolateral amygdala and hippocampal formation, plus the nuclei of the SBN. Key nodes for interactions between the basal ganglia component of the SDM and the SBN component are the lateral septum and the MA-BNST (Fig. 2). In addition to these proposed interaction nodes, there are also significant direct projections from SBN nuclei to midbrain dopaminergic neurons, notably the ventral tegmental area (VTA; Watabe-Uchida et al., 2012; Beier et al., 2015). SBN interactions with the VTA and the ventral striatum (including nucleus accumbens) may be particularly important given the key role of the VTA and ventral striatum in the regulation of motivated behaviours, including identification of salient environmental stimuli and appropriate action selection (Robinson and Berridge, 1993; Salamone and Correa, 2012). Further research is required to determine if O’Connell and Hofmann’s ambitious concept is valid for all vertebrae taxa, but one recent re-evaluation of the SDM concept concluded that it is well supported in mammals (Goodson and Kingsbury, 2013). Results of recent functional studies of social behaviours in non-human primates and monogamous voles are consistent with the SDM concept (Amadei et al., 2017; Sliwa and Freiwald, 2017). There is also interesting convergence between the SDM concept and models put forward in recent discussions of the ‘emotional brain’ (LeDoux, 2012; Etkin et al., 2015). LeDoux (2012) reconceptualized emotion in terms of functions and circuits crucial to survival. This conceptualization of emotion certainly overlaps with the SDM concept and the implicated circuits overlap considerably with much of the circuitry proposed as parts of the SDM.
Figure 2.
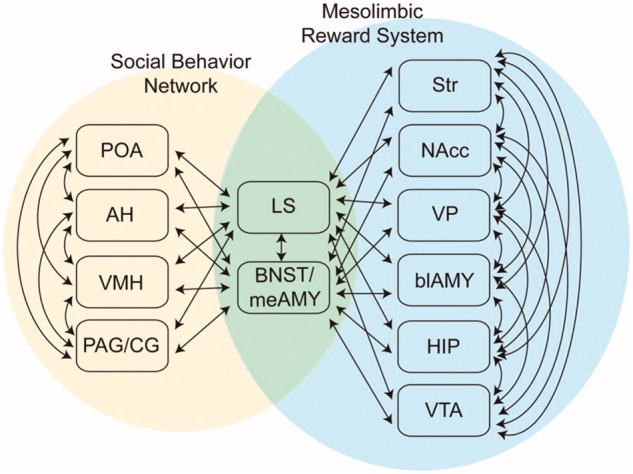
The Social Decision-making Network (SDM). Reprinted with permission from O’Connell and Hofmann (2011). This network combines crucial basal ganglia circuits with the SBN to form a greater network mediating evaluation of social signals, selection of appropriate social behaviours, and the initiation of relevant social behaviours. This cartoon omits direct projections from SBN hypothalamic nuclei to the VTA. AH = anterior hypothalamus; blAMY = basolateral amygdala; HIP = hippocampal formation; LS = lateral septum; meAMY = medial amygdala; NAcc = nucleus accumbens; PAG/CG = periaqueductal grey/central grey; POA = medial preoptic area; Str = striatum; VP = ventral pallidum.
Two limbs of the social decision-making network
In their discussion of MPOA/VMH control of maternal and male sexual behaviours, Stolzenberg and Numan (2011) proposed an interesting model suggesting that the basal ganglia circuit and SBN components of the SDM have complementary functions (Fig. 3). For many behaviours, psychologists distinguish an appetitive phase involving actions bringing animals into contact with a goal stimulus and a consummatory phase in which animals actually interact with the desired object. For some rat reproductive behaviours, Stolzenberg and Numan (2011) suggest that the consummatory phase is mediated by output from the MPOA/VMH to the PAG, with the latter controlling lower circuits producing the actual motor acts. The appetitive phase results from MPOA interactions with dopaminergic systems, particularly the VTA. In this model, MPOA outputs to the VTA modulate dopaminergic inputs from the VTA to the ventral striatum, a key basal ganglia circuit node regulating motivational states. McHenry et al. (2017) recently described a sophisticated partial validation of this model. MPOA neurons of female mice are critical for appropriate responses to male reproductive social cues such as male urine odours. This response is modulated powerfully by circulating oestradiol levels. McHenry et al. (2017) demonstrated that optogenetic stimulation of oestrogen receptor expressing MPOA neurons projecting to the VTA evoked ventral striatal dopamine release and enhanced female attraction to males in a gonadal steroid dependent manner. Generalizing the Stolzenberg and Numan (2011) model to all SDM functions, the basal ganglia component of the SDM may be responsible for the evaluation and valuation of relevant stimuli and actions with the SBN component responsible for driving social actions. Normal social function would require appropriate integration of the functions of both SDM limbs.
Figure 3.
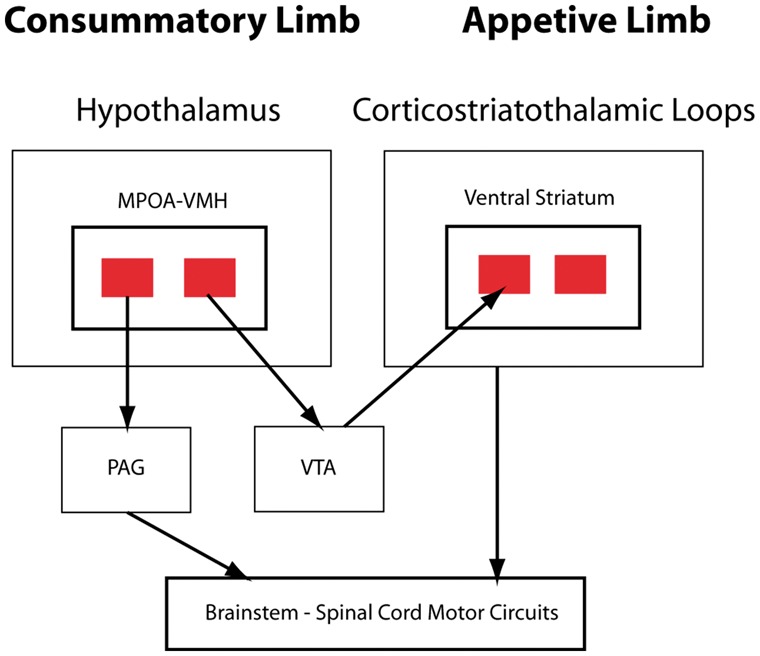
Two limb model of the SDM for maternal, and male and female sexual behaviours. Projections from VMH and MPOA to PAG are responsible for consummatory behaviours. projections from the MPOA to the VTA, with subsequent dopaminergic signalling in the ventral striatum, are responsible for appetitive behaviours, including evaluation of the motivational significance of stimuli. Adapted from Stolzenberg and Numan (2011).
The generalized Stolzenberg and Numan (2011) SDM model is sensible in view of the widely accepted role of midbrain dopaminergic neurons and the basal ganglia as critical components of brain reward and valuation systems. Baez-Mendoza and Schultz (2013, 2016) and Baez-Mendoza et al. (2016) emphasize the critical role of the striatal complex and associated circuits in a wide variety of social behaviours including evaluating social rewards, trust of other social agents, assessing inequities, evaluating actions of conspecifics, pair-bond formation, and maternal behaviours.
The existence of excitatory projections from SBN hypothalamic nuclei to midbrain dopaminergic neurons is the basis for an alternative model of SBN and basal ganglia integration. The Stolzenberg and Numan SDM model emphasizes functional division with ventral striatum involved in evaluation of and the SBN responsible for execution of social behaviours. In this model the PAG plays a particularly important role as the initiator of socially relevant, stereotyped motor acts via its action on relevant brainstem and spinal circuits, a plausible explanation for nature of and pattern of expression of tics. The primate basal ganglia microstimulation studies discussed above, however, suggest that tics can be initiated from focal basal ganglia disinhibition (François et al., 2004; Grabli et al., 2004; Worbe et al., 2009, 2011, 2013; Bronfeld et al., 2011, 2013a, b; McCairn et al., 2013, 2016; Godar et al., 2014; Israelashvili and Bar-Gad, 2015). Caligiore et al. (2017) developed a computational model of one of these tic models (McCairn et al., 2013). In this model, increasing dopaminergic input is a key feature of tic initiation. The primate nigrostriatal projection is somewhat topographically organized (Haber et al., 2000). SBN hypothalamic nuclei might project specifically to dopaminergic midbrain neurons innervating striatal subregions particularly important for control of eye, facial, vocal, and head-neck movements. SBN hypothalamic neuron activity might produce increased dopaminergic inputs to striatal subregions initiating tics. This model also provides an explanation for the character and pattern of tic expression. A hybrid model is also plausible. In normal social functioning, SBN hypothalamic nuclei might coordinate the activity of relevant striatal subregions and the PAG. In Tourette syndrome, this coordination might be disrupted.
A persuasive hypothesis suggests that evolution of unusually large brains among primates, particularly hominins, is driven by needs for complex computations intrinsic to elaborate social lives (Dunbar and Shultz, 2007). Comparative studies indicate that both the SBN and the basal ganglia are phylogenetically ancient features of the CNS. Their close functional integration is likely to be particularly important for highly social humans.
The role of the amygdala
If efficient functional integration of the basal ganglia and SBN components of the SDM is important in social behaviour, then interaction nodes should be associated with social functions. As described above, this appears to be the case for SBN to VTA connections. In the SDM model, the amygdala is a major interaction node with the MA-BNST as an interface between the basal ganglia and the SBN (Fig. 3). The amygdala is a complex structure consisting of several nuclei and receiving a wide variety of cortical and subcortical inputs (for concise overview see Benarroch, 2015). Amygdala nuclei are strongly interconnected, the MA-BNST, for example, receives input from the basolateral nucleus, and these interconnections represent important channels for basal ganglia–SBN interactions.
Amygdala function is strongly implicated in different aspects of social behaviours. Starting with the pioneering lesion work of Kluver and Bucy in the 1930s, a large body of data indicates a significant role for amygdala function in social behaviours (Fusar-Poli et al., 2009; Adolphs, 2010; Bickart et al., 2012, 2014; Chang et al., 2015; Rutishauser et al., 2015). Probably the best known aspect of amygdala function in social behaviour is the role of the amygdala in perception of the significance of facial expressions, but components of the amygdala likely play a role in social actions. Based in part on functional connectivity MRI studies and review of a wide range of other work, Bickart et al. (2012, 2014) proposed that the amygdala is a hub for a variety of related social functions. Bickart et al. (2012, 2014) propose that different amygdala nuclei are central nodes in at least three major social function networks; one for social perception (evaluating social signals), one for social affiliative behaviours (prosocial acts), and one for social aversion behaviours (social avoidance). The affiliative network is centred on the medial amygdala. The dense interconnections of amygdala nuclei allow these networks to interact.
There are considerable data that amygdala function and structure changes during puberty, very likely under the influence of gonadal steroids (Scherf et al., 2013; Herting et al., 2014; Mills et al., 2014; Herting and Sowell, 2017). Some functional and structural changes may evolve over relatively prolonged intervals. Chung et al. (2002) suggested that sexual differentiation of the human BNST extends into adulthood. As mentioned above, morphometric imaging work suggests abnormal volume of the amygdala in Tourette syndrome and Lerner et al. (2012) documented abnormal amygdala GABA-A receptor expression in Tourette syndrome. These results are consistent with abnormal amygdala function in Tourette syndrome. In the context of social perception, Neuner et al. (2010b) described abnormal amygdala activity in subjects with Tourette syndrome viewing emotional facial expressions.
Amygdala abnormalities might contribute to other features of Tourette syndrome. Emerging data suggest that amygdala abnormalities, particularly within the central nucleus of the amygdala and the BNST, are important substrates of anxiety (Kim et al., 2013; Tovote et al., 2015; Marcinkiewcz et al., 2016; Shackman and Fox, 2016). The suggestion that SDM dysfunction, specifically dysfunction of functional social circuits centred on the amygdala, has another potential correlate in Tourette syndrome. Bickart et al. (2014) point out that their social affiliative network, centred on the medial amygdala, overlaps significantly with the brain default mode network, and that their social aversion network, centred on the dorsal amygdala, overlaps with the brain salience network. The participation of different brain regions in the default mode and salience networks, and their functions, are altered by attention and task engagement. It is plausible, then, that attentional loading and task engagement could alter the function of amygdala nuclei that participate in the social function networks centred on the amygdala. This might explain the modulation of tic expression by task engagement.
The periaqueductal grey, vocalizations, and facial expressions
A basic feature of the SDM concept is that the PAG is the key node for the activation of lower circuits that result in the expression of relatively stereotyped social behaviours. This aspect of the SDM concept is consistent with data indicating that the PAG controls or influences a variety of motor behaviours, including many crucial for survival (Zhang et al., 1994; Koutsikou et al., 2015; Deng et al., 2016; Tovote et al., 2016). These behaviours can be relatively complex, involving freezing, flight, running, and predation. Older literature on the PAG also indicates that it controls a variety of socially significant motor behaviours relevant to tic characteristics. Stimulation of the PAG in decerebrate cats, for example, produces natural sounding vocalizations (Zhang et al., 1994). The PAG controls a wide variety of lower circuits that regulate speech, coughing, sneezing, and respiratory movements, as well as facial movements (Holstege, 2002, 2014; Holstege and Subramanian, 2016). The PAG is a key node in Holstege’s emotional motor system, a set of pathways that coordinate motor acts and regulation of autonomic functions in the expression of emotional behaviours. Holstege’s work particularly emphasizes the role of the emotional motor system and PAG in speech production, which involves control and coordination of brainstem nuclei regulating facial movements, tongue movements, pharyngeal function, laryngeal function, and diaphragm movements. SDM dysfunction leading to abnormal or uncoordinated PAG regulation of these downstream components of Holstege’s emotional motor system is a plausible explanation for the presence and character of vocal tics.
There is less detailed literature on PAG function and control of facial movements; however, Holstege emphasizes the importance of PAG inputs to premotor brainstem nuclei that innervate the facial nucleus. In addition, the primate facial nucleus itself receives substantial direct projections from the BNST and other amygdala subregions (Müri, 2016). The implication of these studies is that the PAG is an efferent node of SDM for control of facial expression. This inference is consistent with a suggestion made by Devinsky (1983) that the PAG and associated midbrain tegmentum are crucial for tic expression. Devinsky (1983) pointed to tic-like phenomena associated with encephalitis lethargica, a disorder characterized by PAG pathology. As mentioned above, some prior MRI morphometry data describe midbrain abnormalities in Tourette syndrome (Garraux et al., 2006) and Lerner et al. (2012) identified the PAG as an area of aberrant GABA-A receptor expression in Tourette syndrome.
Conclusions and potential implications
Conceiving of Tourette syndrome as a disorder of SDM function resulting in abnormal social communication appears to explain several previously cryptic cardinal features of Tourette syndrome. The SDM dysfunction hypothesis accounts for the characteristic distribution of tics, the presence and character of vocal tics, their stereotyped quality, the natural history of tic expression, the male sex predominance, and the role of the basal ganglia. This hypothesis may also explain other features of Tourette syndrome such as the modulation of tic expression by task engagement and the high frequency of anxiety disorders in Tourette syndrome. Recent imaging data (Lerner et al., 2012; Greene et al., 2017) are consistent with the SDM hypothesis.
The SDM dysfunction hypothesis suggests new directions for Tourette syndrome research. In general, exploring all components of the SDM in Tourette syndrome subjects may prove rewarding. Altered developmental trajectories of SDM components is a plausible general explanation for Tourette syndrome. There could, for example, be discordant development of the two limbs (basal ganglia and SBN) of the SDM. Systematic, prospective, longitudinal neuroimaging and neuroendocrinologic studies of Tourette syndrome and control children would be a logical approach to evaluating this hypothesis. While many of the SBN nuclei hypothesized to be involved may be too small for conventional morphometric analysis, Greene et al. (2017) identified increased volume of the hypothalamus as one of the few abnormalities in their morphometric study of Tourette syndrome children. More recently developed functional connectivity and network methods might be a way of addressing this hypothesis. For example, is there altered connectivity between the PAG and other SDM structures in Tourette syndrome? Do Tourette syndrome subjects exhibit differences from control subjects in the amygdala centred social networks described by Bickart et al. (2014)? Do these networks develop normally in Tourette syndrome subjects? As described above, social neurobiology is a fertile research field where the combination of modern tools and well established behavioural paradigms is allowing precise dissection of SDM pathways involving a variety of stereotyped social behaviours. As more detailed understanding of SDM function emerges, insights from this work might be extended to human experiments. More detailed knowledge of SDM circuitry might allow application of molecular imaging methods to identify potentially abnormal pathways in the same way that Lerner et al. (2012) used GABA-A receptor ligand molecular imaging to identify abnormalities in the PAG and other structures. At a minimum, this knowledge might be applied to putative animal models of Tourette syndrome as a ways of improving model validation and outcome measures for preclinical intervention studies.
The SDM dysfunction hypothesis may also have implications for other aspects of Tourette syndrome research. Tourette syndrome genetics research has been frustrating. Despite an apparent high heritability, association studies are largely barren. The SDM is a rather large network, encompassing many nuclei and connected to many other regions, such as cortical regions found in morphometric studies to be abnormal in Tourette syndrome. This raises the possibility that abnormal development of many, many structures could result in a Tourette syndrome phenotype. Tourette syndrome would be less a syndrome than a meta-syndrome. As a large number of genes affect the developmental trajectories of the many structures composing the SDM, it is plausible that small effects involving many genes would be responsible for Tourette syndrome. It would also be plausible that there would considerable heterogeneity of such genes among Tourette syndrome subjects, though many would be predicted to be involved in brain development. These inferences from the SDM model are consistent with the recent work of Willsey et al. (2017). With statistical modelling, this group estimated that >400 genes are involved in Tourette syndrome. The available information about all four candidate genes identified in their study suggest involvement in brain development (Willsey et al., 2017). Candidate genes discovered in these kinds of association studies might be further evaluated in rodents to determine if they influence the development of SDM nuclei and circuits. Those that do could be prioritized for further investigation.
The SDM hypothesis might also be useful in guiding the search for Tourette syndrome subtypes. Hirschtritt et al. (2016) suggest the existence of a subgroup characterized by social disinhibition. Different subgroups of Tourette syndrome might be characterized by abnormalities in different limbs of the SDM. Correlation of social behaviour measures with structural, connectional, and functional MRI measures of SDM components might assist the identification of biologically valid Tourette syndrome subgroups.
Dopaminergic signalling is a significant focus of Tourette syndrome research. There is little evidence of abnormalities of striatal dopaminergic innervation in Tourette syndrome (Albin et al., 2009). Functional studies of striatal dopamine release in Tourette syndrome subjects yielded conflicting results though one study suggested increased amphetamine-evoked dopamine release in the ventral striatum, possibly consistent with increased ventral striatal dopaminergic signalling driven by SBN activation of VTA neurons (Wong et al., 2008; Denys et al., 2013). Definition of the precise projection targets of SBN neurons projecting to the midbrain dopaminergic neuron complex would be interesting. Do they project primarily to the VTA, as suggested in the two limb SDM model discussed above, or do they project also to substantia nigra dopaminergic neurons that preferentially innervate striatal subregions involved in head, face, and eye movements, and vocalizations? Studies in non-human primates addressing this question might cast light on the specific mechanisms generating tics.
The SDM dysfunction hypothesis, however, suggests alternative important sites of dopaminergic signalling in Tourette syndrome. Dopaminergic signalling within the MPOA, which receives dopaminergic innervation from the incerto-hypothalamic projection, regulates maternal behaviour in rats (Numan and Stolzenberg, 2009). The amygdala itself receives a significant dopaminergic innervation from midbrain dopaminergic neurons. Recent functional imaging studies suggest that dopaminergic signalling within the amygdala modulates the affiliative social network centred on the medial amygdala (Atzil et al., 2017). Recent data also indicate the existence of a significant projection from the VTA to the lateral septum, another interaction node between the basal ganglia and SBN components of the SBN (Khan et al., 2017).
In terms of potential therapies, it is plausible that the burgeoning and increasingly detailed understanding of the circuitry underlying social behaviours will suggest new pharmacologic approaches to Tourette syndrome. In the short term, the SDM hypothesis suggests new targets for DBS in Tourette syndrome. The amygdala, as a key interaction node for the two major components of the SDM and as a major actor in social behaviours, is a plausible target for DBS in Tourette syndrome. The PAG, as the main output structure of the SDM and key regulator of social motor behaviours, may be a good target for treatment of particularly troublesome tics. Both the amygdala and PAG (Satpute et al., 2013) have complex architecture and it is plausible that improving DBS technologies, or more speculatively, clinical use of optogenetic methods, could target amygdala and PAG subdivisions to produce useful clinical effects.
In conclusion, the SDM dysfunction hypothesis provides a cogent and potentially fruitful framework for future Tourette syndrome research.
Acknowledgements
I thank Leon Dure, Kent Berridge, Kirk Frey, and Bill Dauer for critical readings of earlier versions of this essay.
Funding
Supported by National Institutes of Health – National Institute of Neurological Disorders and Stroke grants R21NS088302 and P50NS091856.
Glossary
Abbreviations
- BNST
bed nucleus of the stria terminalis
- MPOA
medial preoptic area
- PAG
periaqueductal grey
- SBN
social behaviour network
- SDM
social decision-making network
- VMH
ventromedial hypothalamus
- VTA
ventral tegmental area
References
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci 2010; 1191: 42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL. Neurobiology of basal ganglia and Tourette syndrome: striatal and dopamine function. Adv Neurol 2006; 99: 99–106. [PubMed] [Google Scholar]
- Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends Neurosci 2006; 29: 175–82. [DOI] [PubMed] [Google Scholar]
- Albin RL, Koeppe RA, Wernette K, Zhuang W, Nichols T, Kilbourn MR, et al. Striatal [11C]dihydrotetrabenazine and [11C]methylphenidate binding in Tourette syndrome. Neurology 2009; 72: 1390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Minderovic C, Koeppe RA. Normal vesicular acetylcholine transporter expression in Tourette syndrome. eNeuro 2017, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MD, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9: 357–81. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Peterson BS. Testing the prenatal hormone hypothesis of tic-related disorders: gender identity and gender role behavior. Dev Psychopathol 2004; 16: 407–20. [DOI] [PubMed] [Google Scholar]
- Amadei EA, Johnson ZV, Kwon YJ, Shpiner AC, Saravanan V, et al. , Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature 2017; 546: 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ. Circuit modules linking internal states and social behaviour in flies and mice. Nat Rev Neurosci 2016; 17: 692–704. [DOI] [PubMed] [Google Scholar]
- Atzil S, Touroutoglou A, Rudy T, Salcedo S, Feldman R, Hooker JM, et al. Dopamine in the medial amygdala network mediates human bonding. Proc Natl Acad Sci USA 2017; 114: 2361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchus RJ. The physiology and biochemistry of adrenarche. Endocr Dev 2011; 20: 20–7. [DOI] [PubMed] [Google Scholar]
- Baez-Mendoza R, Schultz W. The role of the striatum in social behavior. Front Neurosci 2013; 7: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez-Mendoza R, Schultz W. Performance error-related activity in monkey striatum during social interactions. Sci Rep 2016; 6: 37199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez-Mendoza R, van Coeverden JR, Schultz W. A neuronal reward inequity signal in primate striatum. J Neurophysiol 2016; 115: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baym CL, Corbett BA, Wright SB, Burge SA. Neural correlates of tic severity and cognitive control in children with Tourette syndrome. Brain 2008; 131: 165–79. [DOI] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 2015; 162: 622–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. The amygdala: functional organization and involvement in neurologic disorders. Neurology 2015; 84: 313–24. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW. Super-stereotypy I: enhancement of a complex movement sequence by systemic dopamine D1 agonists. Synapse 2000; 37: 194–204. [DOI] [PubMed] [Google Scholar]
- Bertelsen B, Stefánsson H, Riff Jensen L, Melchior L, Mol Debes N, Groth C, et al. Association of AADAC deletion and Gilles de la Tourette syndrome in a large European Cohort. Biol Psychiatry 2016; 79: 383–91. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J Neurosci 2012; 32: 14729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Dickerson BC, Barrett LF. The amygdala as a hub in brain networks that support social life. Neuropsychologia 2014; 63: 235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology 2005; 65: 1253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain 2006; 129: 2029–37. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Godar SC, Mosher LJ, Paba S, Marrosu F, et al. The implication of neuroactive steroids in Tourette's syndrome pathogenesis: a role for 5α-reductase? J Neuroendocrinol 2013; 25: 1196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfeld M, Belelovsky K, Bar-Gad I. Spatial and temporal properties of tic-related neuronal activity in the cortico-basal ganglia loop. J Neurosci 2011; 31: 8713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfeld M, Yael D, Belelovsky K, Bar-Gad I. Motor tics evoked by striatal disinhibition in the rat. Front Syst Neurosci 2013a; 7: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfeld M, Israelashvili M, Bar-Gad I. Pharmacological animal models of Tourette syndrome. Neurosci Biobehav Rev 2013b; 37: 1101–19. [DOI] [PubMed] [Google Scholar]
- Caligiore D, Mannella F, Arbib MA, Baldassare G. Dysfunctions of the basal ganglia-cerebellar-thalamo-cortical system produce motor tics in Tourette syndrome. PLoS Comput Biol 2017; 13: e1005395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellan Baldan L, Williams KA, Gallezot JD, Pogorelov V, Rapanelli M, Crowley M, et al. Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice. Neuron 2014; 81: 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, Fagan NA, Toda K, Utevsky AV, Pearson JM, Platt ML. Neural mechanisms of social decision-making in the primate amygdala. Proc Natl Acad Sci USA 2015; 112: 16012–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon S, Crawford S, Vakili K, Robertson MM. Real-life-type problem solving in Tourette syndrome. Cogn Behav Neurol 2003; 16: 3–15. [PubMed] [Google Scholar]
- Channon S, Sinclair E, Waller D, Healey L, Robertson MM. Social Cognition in Tourette syndrome: intact theory of mind and impaired inhibitory functioning. J Autism Dev Dis 2004; 34: 669–77. [DOI] [PubMed] [Google Scholar]
- Channon S, Drury H, Gafson L, Stern J, Robertson MM. Judgements of social appropriateness in adults with Tourette’s syndrome. Cogn Neuropsychiatry 2012; 17: 246–51. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Ondo WG, Dashtipour K, Swope DM. Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. Clin Ther 2012; 34: 1487–504. [DOI] [PubMed] [Google Scholar]
- Cheung MY, Shahed J, Jankovic J. Malignant Tourette syndrome. Mov Disord 2007; 22: 1743–50. [DOI] [PubMed] [Google Scholar]
- Chung WC, De Vries GJ, Swaab DF. Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. J Neurosci 2002; 22: 10227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Fair DA, Dosenbach NU, Cohen AL, Miezin FM, Petersen SE, et al. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain 2009; 132: 225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Schlaggar BL. Pediatric Tourette syndrome: insights from recent neuroimaging studies. J Obsessive Compuls Relat Disord 2014; 3: 386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley AJ, Bernstein RM, Nguyen AD. Adrenarche in nonhuman primates: the evidence for it and the need to redefine it. J Endocrinol 2012; 214: 121–31. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax. J Neurosci 1996; 16: 3444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci 2012; 13: 636–50. [DOI] [PubMed] [Google Scholar]
- Darwin C. The expression of emotions in man and animals. New York; Oxford University Press; 2009. [Google Scholar]
- Davis KD, Davis JS, Dowler L. In motion, out of place: the public spaces of Tourette syndrome. Soc Sci Med 2004; 59: 103–12. [DOI] [PubMed] [Google Scholar]
- Davis LK, Yu D, Keenan CL, Gamazon ER, Konkashbaev AI, Derks EM, et al. Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS Genet 2013; 9: e1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gelder B, de Borst AW, Watson R. The perception of emotion in body expressions. Wiley Interdiscip Rev Cogn Sci 2015; 6: 149–58. [DOI] [PubMed] [Google Scholar]
- Delorme C, Salvador A, Valabrègue R, Roze E, Palminteri S, Vidailhet M, et al. Enhanced habit formation in Gilles de la Tourette syndrome. Brain 2016; 139: 605–15. [DOI] [PubMed] [Google Scholar]
- Deng H, Xiao X, Wang Z. Periaqueductal gray neuronal activities underlie different aspects of defensive behaviors. J Neurosci 2016; 36: 7580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys D, de Vries F, Cath D, Figee M, Vulink N, Veltman DJ, et al. Dopaminergic activity in Tourette syndrome and obsessive-compulsive disorder. Eur Neuropsychopharmacol 2013; 23: 1423–31. [DOI] [PubMed] [Google Scholar]
- Devinsky O. Neuroanatomy of Gilles de la Tourette syndrome: possible midbrain involvement. Arch Neurol 1983; 40: 508–14. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Zimmer MR, Bober J, Horvath TL. Hypothalamic AGRP neurons drive stereotypic behaviors beyond feeding. Cell 2015; 160: 1222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 2008; 322: 900–4. [DOI] [PubMed] [Google Scholar]
- Dong H, Liu W, Liu M, Xu L, Li Q, Zhang R, et al. Investigation of a possible role for the histidine decarboxylase gene in tourette syndrome in the chinese han population: a family-based study. PLoS One 2016; 11: e0160265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Martino D, Cavanna AE, Hutton C, Orth M, Robertson MM, et al. Multispectral brain morphometry in Tourette syndrome persisting into adulthood. Brain 2010; 133: 3661–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, Kimchi T. Neural mechanisms underlying sex-specific behaviors in vertebrates. Curr Opin Neurobiol 2007; 17: 675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, O'Connell LA, Wu Z. Neural control of maternal and paternal behaviors. Science 2014; 345: 765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RI, Shultz S. Evolution in the social brain. Science 2007; 317: 1344–7. [DOI] [PubMed] [Google Scholar]
- Eddy CM, Cavanna AE. On being your own worst enemy: an investigation of socially inappropriate symptoms in Tourette syndrome. J Psychiatr Res 2013a; 47: 1259–63. [DOI] [PubMed] [Google Scholar]
- Eddy CM, Cavanna AE. Altered social cognition in Tourette syndrome: nature and implications. Behav Neurol 2013b; 27: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P. Emotion in the human face. Los Altos, CA: Malor; 2015. [Google Scholar]
- Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O'Roak BJ, Mason CE, et al. L-histidine decarboxylase and Tourette's syndrome. N Engl J Med 2010; 362: 1901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci 2015; 16: 693–700. [DOI] [PubMed] [Google Scholar]
- Fahim C, Yoon U, Das S, Lyttelton O, Chen J, Arnaoutelis R, et al. Somatosensory-motor bodily representation cortical thinning in Tourette: effects of tic severity, age and gender. Cortex 2010; 46: 750–60. [DOI] [PubMed] [Google Scholar]
- Felling RJ, Singer HS. Neurobiology of Tourette syndrome: current status and need for further investigation. J Neurosci 2011; 31: 12387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde NJ, Zwiers MP, Naaijen J, Akkermans SEA, Openneer TJC, et al. Basal ganglia structure in Tourette’s disorder and/or attention-deficit/hyperactivity disorder. Mov Disord 2017; 32: 601–4. [DOI] [PubMed] [Google Scholar]
- François C, Grabli D, McCairn K, Jan C, Karachi C, Hirsch EC, et al. Behavioural disorders induced by external globus pallidus dysfunction in primates II. Anatomical study. Brain 2004; 127: 2055–70. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 2009; 34: 418–32. [PMC free article] [PubMed] [Google Scholar]
- Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav Neurosci 2012; 126: 97–109. [DOI] [PubMed] [Google Scholar]
- Ganos C, Roessner V, Münchau A. The functional anatomy of Gilles de la Tourette syndrome. Neurosci Biobehav Rev 2013; 37: 1050–62. [DOI] [PubMed] [Google Scholar]
- Ganos C. Tics and tourette's: update on pathophysiology and tic control. Curr Opin Neurol 2016; 29: 513–18. [DOI] [PubMed] [Google Scholar]
- Garraux G, Goldfine A, Bohlhalter S, Lerner A, Hanakawa T, Hallett M. Increased midbrain gray matter in Tourette's syndrome. Ann Neurol 2006; 59: 381–5. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Budman CL, Singer HS, Kurlan R, Chipkin RE. A D1 receptor antagonist, ecopipam, for treatment of tics in Tourette syndrome. Clin Neuropharmacol 2014; 37: 26–30. [DOI] [PubMed] [Google Scholar]
- Godar SC, Mosher LJ, Di Giovanni G, Bortolato M. Animal models of tic disorders: a translational perspective. J Neurosci Methods 2014; 238: 54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav 2005; 48: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kingsbury MA. What's in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm Behav 2013; 64: 103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabli D, McCairn K, Hirsch EC, Agid Y, Féger J, François C, et al. Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain 2004; 127: 2039–54. [DOI] [PubMed] [Google Scholar]
- Greene DJ, Williams AC, Koller JM, Schlaggar BL, Black KJ, et al. Brain structure in pediatric Tourette syndrome. Mol Psychiatry 2017; 22: 972–80. doi: 10.1038/mp.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, MacFarland NR. Nigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 2000; 20: 2369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Rangel MJ Jr, Motta SC, Zhang X, Perez IO, et al. Integrated control of predatory hunting by the central nucleus of the amygdala. Cell 2017; 168: 311–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan RE, Shivers BD, Pfaff DW. Lordosis as a sexually dimorphic sexual function. Prog Brain Res 1984; 61: 239–55. [DOI] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, Sowell ER. The role of testosterone and estradiol in brain volume changes across adolescence: a longitudinal structural MRI study. Hum Brain Mapp 2014; 35: 5633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Sowell ER. Puberty and structural brain development in humans. Front Neuroendocrinol 2017; 44: 122–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry 2015; 72: 325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschtritt ME, Darrow SM, Illmann C, Osiecki L, Grados M, Sandor P, et al. Social disinhibition is a heritable subphenotype of tics in Tourette syndrome. Neurology 2016; 87: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G. Emotional innervation of facial musculature. Mov Disord 2002; 17: S12–6. [DOI] [PubMed] [Google Scholar]
- Holstege G. The periaqueductal gray controls brainstem emotional motor systems including respiration. Prog Brain Res 2014; 209: 379–405. [DOI] [PubMed] [Google Scholar]
- Holstege G, Subramanian HH. Two different motor systems are needed to generate human speech. J Comp Neurol 2016; 524: 1558–77. [DOI] [PubMed] [Google Scholar]
- Hong W, Kim DW, Anderson DJ. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 2014; 158: 1348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelashvili M, Bar-Gad I. Corticostriatal divergent function in determining the temporal and spatial properties of motor tics. J Neurosci 2015; 35: 16340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J. Tourette's syndrome. Tics and Tourette's: update on pathophysiology and tic control. N Engl J Med 2001a; 345: 1184–92. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Differential diagnosis and etiology of tics. Adv Neurol 2001b; 85: 15–29. [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci USA 2005; 102: 13307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci 2016; 17: 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol 2010; 518: 277–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannidis I, Dehning S, Sandor P, Tarnok Z, Rizzo R, Wolanczyk T, et al. Support of the histaminergic hypothesis in Tourette syndrome: association of the histamine decarboxylase gene in a large sample of families. J Med Genet 2013; 50: 760–4. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The cortico-striate projection in the monkey. Brain 1970; 93: 525–46. [DOI] [PubMed] [Google Scholar]
- Khan S, Stott SRW, Chabrat A, Truckenbrodt AM, Spencer-Dene B, Nave K-A, et al. Survival of a novel subset of midbrain dopaminergic neurons projecting to the lateral septum is dependent on NeuroD proteins. J Neurosci 2017; 37: 2305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 2013; 496: 219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol 2012; 47: 77–90. [DOI] [PubMed] [Google Scholar]
- Koutsikou S, Watson TC, Crook JJ, Leith JL, Lawrenson CL, Apps R, et al. The periaqueductal gray orchestrates sensory and motor circuits at multiple levels of the neuraxis. J Neurosci 2015; 35: 14132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlan R, Daragjati C, Como PG, McDermott MP, Trinidad KS, et al. Non-obscene complex socially inappropriate behavior in Tourette syndrome. J Neuropsychiatry Clin Neurosci 1996; 8: 311–17. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Rethinking the emotional brain. Neuron 2012; 73: 653–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim DW, Remedios R, Anthony TE, Chang A, Madisen L, et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 2014; 509: 627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Deng X, Zhang J, Su L, Xu H, Liang H, et al. Mutation screening of the HDC gene in Chinese Han patients with Tourette syndrome. Am J Med Genet B Neuropsychiatr Genet 2012; 159B: 72–6. [DOI] [PubMed] [Google Scholar]
- Lennington JB, Coppola G, Kataoka-Sasaki Y, Fernandez TV, Palejev D, Li Y, et al. Transcriptome analysis of the human striatum in Tourette syndrome. Biol Psychiatry 2016; 79: 372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A, Bagic A, Simmons JM, Mari Z, Bonne O, Xu B, et al. Widespread abnormality of the γ-aminobutyric acid-ergic system in Tourette syndrome. Brain 2012; 135: 1926–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludolph AG, Pinkhardt EH, Van Elst TL, Libal G, Ludolph AD, Fegert JM, et al. Are amygdala volume alterations in children with Tourette syndrome due to ADHD comorbidity. Dev Med Child Neurol 2008; 50: 524–9. [DOI] [PubMed] [Google Scholar]
- Makki MI, Behen M, Bhatt A, Wilson B, Chugani HT. Microstructural abnormalities of striatum and thalamus in children with Tourette syndrome. Mov Disord 2008; 23: 2349–56. [DOI] [PubMed] [Google Scholar]
- Makki MI, Govindan RM, Wilson BJ, Behen ME, Chugani HT. Altered fronto-striato-thalamic connectivity in children with Tourette syndrome assessed with diffusion tensor MRI and probabilistic fiber tracking. J Child Neurol 2009; 24: 669–78. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Mazzone CM, D'Agostino G, Halladay LR, Hardaway JA, DiBerto JF, et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 2016; 537: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Rodríguez JF, Ruiz-Rodríguez MA, Palomar FJ, Cáceres-Redondo MT, Vargas L, Porcacchia P, et al. Aberrant cortical associative plasticity associated with severe adult Tourette syndrome. Mov Disord 2015; 30: 431–5. [DOI] [PubMed] [Google Scholar]
- Martino D, Macerollo A, Leckman JF. Neuroendocrine aspects of Tourette syndrome. Int Rev Neurobiol 2013; 112: 239–79. [DOI] [PubMed] [Google Scholar]
- Martos YV, Braz BP, Beccaria JP, Murer MG, Belforte JE. Compulsive social behavior emerges after selective ablation of striatal cholinergic interneurons. J Neurosci 2017; 37: 2849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn KW, Bronfeld M, Belelovsky K, Bar-Gad I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain 2009; 132: 2125–38. [DOI] [PubMed] [Google Scholar]
- McCairn KW, Iriki A, Isoda M. Global dysrhythmia of cerebro-basal ganglia-cerebellar networks underlies motor tics following striatal disinhibition. J Neurosci 2013; 33: 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn KW, Nagai Y, Hori Y, Ninomiya T, Kikuchi E, Lee JY, et al. A primary role for nucleus accumbens and related limbic network in vocal tics. Neuron 2016; 89: 300–7. [DOI] [PubMed] [Google Scholar]
- McGrath LM, Yu D, Marshall C, Davis LK, Thiruvahindrapuram B, Li B, et al. Copy number variation in obsessive-compulsive disorder and tourette syndrome: a cross-disorder study. J Am Acad Child Adolesc Psychiatry 2014; 53: 910–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, et al. Hormonal gain control of a medial preoptic area social reward circuit. Nat Neurosci 2017; 20: 449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Bansal R, Hao X, Sanchez-Pena JP, Sobel LJ, Liu J, et al. Enlargement of thalamic nuclei in Tourette syndrome. Arch Gen Psychiatry 2010; 67: 955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ. The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci 2014; 36: 147–60. [DOI] [PubMed] [Google Scholar]
- Muellner J, Delmaire C, Valabrégue R, Schüpbach M, Mangin JF, Vidailhet M, et al. Altered structure of cortical sulci in Gilles de la Tourette syndrome: further support for abnormal brain development. Mov Disord 2015; 30: 655–61. [DOI] [PubMed] [Google Scholar]
- Müri RM. Cortical control of facial expression. J Comp Neurol 2016; 524: 1578–85. [DOI] [PubMed] [Google Scholar]
- Neuner I, Kupriyanova Y, Stoecker T, Huang R, Posnansky O, Schneider F, et al. White-matter abnormalities in Tourette syndrome extend beyond motor pathways. Neuroimage 2010a; 51: 1184–93. [DOI] [PubMed] [Google Scholar]
- Neuner I, Kellermann T, Stocker T, Kircher T, Habel J, Shah JN, et al. Amygdala hypersensitivity in response to emotional faces in Tourette’s patients. World J Biol Psychiatry 2010b; 11: 858–72. [DOI] [PubMed] [Google Scholar]
- Neuner I, Schneider F, Shah NJ. Functional neuroanatomy of tics. Int Rev Neurobiol 2013; 112: 35–71. [DOI] [PubMed] [Google Scholar]
- Neuner I, Werner CJ, Arrubla J, Stöcker T, Ehlen C, Wegener HP, et al. Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Front Hum Neurosci 2014; 8: 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci 1999; 877: 242–57. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Fron Neuroendocrinol 2009; 30: 46–64. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol 2011; 519: 3599–639. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science 2012; 336: 1154–7. [DOI] [PubMed] [Google Scholar]
- Pappas SS, Darr K, Holley SM, Cepeda C, Mabrouk OS, et al. Forebrain deletion of the dystonia protein torsinA causes dystonic-like movements and loss of striatal cholinergic neurons. Elife 2015; 4: e08352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschou P, Yu D, Gerber G, Evans P, Tsetsos F, Davis LK, et al. Genetic association signal near NTN4 in Tourette syndrome. Ann Neurol 2014; 76: 310–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 2011; 147: 235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Leckman JF, Scahill L, Naftolin F, Keefe D, Charest NJ, et al. Steroid hormones and CNS sexual dimorphisms modulate symptom expression in Tourette's syndrome. Psychoneuroendocrinolog 1992; 17: 553–63. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Leckman JF, Scahill L, Naftolin F, Keefe D, Charest NJ, et al. Steroid hormones and Tourette's syndrome: early experience with antiandrogen therapy. J Clin Psychopharmacol 1994; 14: 131–5. [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry 1998; 55: 326–33. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, et al. Basal Ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry 2003; 60: 415–24. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Choi HA, Hao X, Amat JA, Zhu H, Whiteman R, et al. Morphologic features of the amygdala and hippocampus in children and adults with Tourette syndrome. Arch Gen Psychiatry 2007; 64: 1281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou M, Frey KA, Kilbourn MA, Scott PJ, Raffel DM, et al. In vivo imaging of human cholinergic nerve terminals with (-)-5-(18)F-fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med 2014; 55: 396–404. [DOI] [PubMed] [Google Scholar]
- Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA 2010; 303: 1929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Peterson BS. Imaging evidence for anatomical disturbances and neuroplastic compensation in persons with Tourette syndrome. J Psychosom Res 2009; 67: 559–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim T, Doja A, Gorman D, McKinlay D, Day L, Billinghurst L, et al. Canadian guidelines for the evidence-based treatment of tic disorders: pharmacotherapy. Can J Psychiatry 2012; 57: 133–43. [DOI] [PubMed] [Google Scholar]
- Rapanelli M, Frick LR, Xu M, Groman SM, Jindachomthong K, et al. Targeted interneuron depletion in the dorsal striatum produces autism-like behavioral abnormalities in male but not female mice. Biol Psychiatry 2017; 82: 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain 2000; 123: 425–62. [DOI] [PubMed] [Google Scholar]
- Robertson MM. The Gilles de la Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed 2012; 97: 166–75. [DOI] [PubMed] [Google Scholar]
- Robertson MM. Movement disorders: Tourette syndrome–beyond swearing and sex? Nat Rev Neurol 2014; 10: 6–8. [DOI] [PubMed] [Google Scholar]
- Robertson MM, Eapen V, Singer HS, Martino D, Scharf JM, Paschou P, et al. Gilles de la Tourette syndrome. Nat Rev Dis Primers 2017; 3: 16097. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 1993; 18: 247–91. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Mamelak AN, Adolphs R. The primate amygdala in social perception—insights from electrophysiological recordings and stimulation. Trends Neurosci 2015; 38: 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, Zhao S, Takatoh J, Rodriguez E, Lu J, Leavitt AD, et al. Capturing and manipulating activated neuronal ensembles with CANE delineates a hypothalamic social-fear circuit. Neuron 2016; 92: 739–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron 2012; 76: 470–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute AB, Wager TD, Cohen-Adad J, Bianciardi M, Choi JK, Buhle JT, et al. Identification of discrete functional subregions of the human periaqueductal gray. Proc Natl Acad Sci USA 2013; 110: 17101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf JM, Yu D, Mathews CA, Neale BM, Stewart SE, Fagerness JA, et al. Genome-wide association study of Tourette's syndrome. Mol Psychiatry 2013; 18: 721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Ben-Shlomo Y. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord 2015; 30: 221–8. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Smyth JM, Delgado MR. The amygdala: an agent of change in adolescent neural networks. Horm Behav 2013; 64: 298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrock LE, Mink JW, Woods DW, Porta M, Servello D, Visser-Vandewalle V, et al. Tourette syndrome deep brain stimulation: a review and updated recommendations. Mov Disord 2015; 30: 448–71. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS. Contributions of the central extended amygdala to fear and anxiety. J Neurosci 2016; 36: 8050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL. Hormone-dependent adolescent organization of socio-sexual behaviors in mammals. Curr Opin Neurobiol 2016; 38: 63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 2002; 25: 507–36. [DOI] [PubMed] [Google Scholar]
- Sliwa J, Freiwald WA. A dedicated network for social interaction processing in the primate brain. Science 2017; 356: 745–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Graybiel AM. Habit formation. Dialogues Clin Neurosci 2016; 18: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MC, Bshary R, Fusani L, Goymann W, Hau M, Hirschenhauser K, et al. Hormonal mechanisms of cooperative behaviour. Philos Trans R Soc Lond B Biol Sci 2010; 365: 2737–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Kan E, Yoshii J, Thompson PM, Bansal R, Xu D, et al. Thinning of sensorimotor cortices in children with Tourette syndrome. Nat Neurosci 2008; 11: 637–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM. Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron 2013; 77: 810–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, Numan M. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci Biobehav Rev 2011; 35: 826–47. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Rajbhandari AK, Berridge KC, Aldridge JW. Dopamine receptor modulation of repetitive grooming actions in the rat: potential relevance for Tourette syndrome. Brain Res 2010; 1322: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinaz S, Belluscio BA, Malone P, van der Veen JW, Hallett M, et al. Role of the sensorimotor cortex in Tourette syndrome using multimodal imaging. Hum Brain Mapp 2014; 35: 5834–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe RH, Bansal R, Xu D, Hao X, Liu J, Sanchez J, et al. Cerebellar morphology in Tourette syndrome and obsessive-compulsive disorder. Ann Neurol 2010; 67: 479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci 2015; 16: 317–31. [DOI] [PubMed] [Google Scholar]
- Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, et al. Midbrain circuits for defensive behaviour. Nature 2016; 534: 206–12. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Worbe Y, Thobois S, Sgambato-Faure V, Féger J. Selective dysfunction of basal ganglia subterritories: from movement to behavioral disorders. Mov Disord 2015; 30: 1155–70. [DOI] [PubMed] [Google Scholar]
- Unger EK, Burke KJ Jr, Yang CF, Bender KJ, Fuller PM, Shah NM. Medial amygdala aromatase neurons regulate aggression in both sexes. Cell Rep 2015; 10: 453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, Mathews CA, Joosse M, Eussen BH, Heutink P, Oostra BA, et al. CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics 2003; 82: 1–9. [DOI] [PubMed] [Google Scholar]
- Wang Z, Maia TV, Marsh R, Colibazzi T, Gerber A, Peterson BS. The neural circuits that generate tics in Tourette's syndrome. Am J Psychiatry 2011; 168: 1326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen IZ, Lin D. Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron 2015; 85: 1344–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 2012; 74: 858–73. [DOI] [PubMed] [Google Scholar]
- Werner CJ, Stoecker T, Kellerman T, Wegener HP, Schneider F, Shah NJ, et al. Altered amygdala functional connecitivity in adult Tourette’s syndrome. Eur Arch Psychiatry Clin Neurosci 2011; 260: 95–9. [DOI] [PubMed] [Google Scholar]
- Whittington C, Pennant M, Kendall T, Glazebrook C, Trayner P, Groom M, et al. Practitioner Review: treatments for Tourette syndrome in children and young people—a systematic review. J Child Psychol Psychiatry 2016; 57: 988–1004. [DOI] [PubMed] [Google Scholar]
- Willsey AJ, Fernandez TV, Yu D, King RA, Dietrich A, et al. De novo coding variants are strongly associated with Tourette disorder. Neuron 2017; 94: 486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Brasić JR, Singer HS, Schretlen DJ, Kuwabara H, Zhou Y, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacol 2008; 33: 1239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y, Baup N, Grabli D, Chaigneau M, Mounayar S, McCairn K, et al. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb Cortex 2009; 19: 1844–56. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Mallet L, Golmard JL, Béhar C, Durif F, Jalenques I, et al. Repetitive behaviours in patients with Gilles de la Tourette syndrome: tics, compulsions, or both? PLoS One 2010a; 5: e12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y, Gerardin E, Hartmann A, Valabrégue R, Chupin M, Tremblay L, et al. Distinct structural changes underpin clinical phenotypes in patients with Gilles de la Tourette syndrome. Brain 2010b; 133: 3649–60. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Epinat J, Féger J, Tremblay L. Discontinuous long-train stimulation in the anterior striatum in monkeys induces abnormal behavioral states. Cereb Cortex 2011; 21: 2733–41. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Malherbe C, Hartmann A, Pélégrini-Issac M, Messé A, Vidailhet M, et al. Functional immaturity of cortico-basal ganglia networks in Gilles de la Tourette syndrome. Brain 2012; 135: 1937–46. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Sgambato-Faure V, Epinat J, Chaigneau M, Tandé D, François C, et al. Towards a primate model of Gilles de la Tourette syndrome: anatomo-behavioural correlation of disorders induced by striatal dysfunction. Cortex 2013; 49: 1126–40. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Marrakchi-Kacem L, Lecomte S, Valabregue R, Poupon F, Guevara P, et al. Altered structural connectivity of cortico-striato-pallido-thalamic networks in Gilles de la Tourette syndrome. Brain 2015a; 138: 472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y, Lehericy S, Hartmann A. Neuroimaging of tic genesis: present status and future perspectives. Mov Disord 2015b; 30: 1179–83. [DOI] [PubMed] [Google Scholar]
- Xu M, Li L, Ohtsu H, Pittenger C. Histidine decarboxylase knockout mice, a genetic model of Tourette syndrome, show repetitive grooming after induced fear. Neurosci Lett 2015a; 595: 50–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Kobets A, Du JC, Lennington J, Li L, Banasr M, et al. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc Natl Acad Sci USA 2015b; 112: 893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Li L, Pittenger C. Ablation of fast-spiking interneurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neurosci 2016; 324: 321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, et al. Modular genetic control of sexually dimorphic behaviors. Cell 2012; 148: 596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yael D, Vinner E, Bar-Gad I. Pathophysiology of tic disorders. Mov Disord 2015; 30: 1171–8. [DOI] [PubMed] [Google Scholar]
- Yang J, Hirsch L, Martino D, Jette N, Roberts J, Pringsheim T. The prevalence of diagnosed Tourette syndrome in Canada: a national population-based study. Mov Disord 2016; 31: 1658–63. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci 2006; 7: 464–76. [DOI] [PubMed] [Google Scholar]
- Yu D, Mathews CA, Scharf JM, Neale BM, Davis LK, Gamazon ER, et al. Cross-disorder genome-wide analyses suggest a complex genetic relationship between Tourette's syndrome and OCD. Am J Psychiatry 2015; 172: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SP, Davis PJ, Bandler R, Carrive P. Brain stem integration of vocalization: role of the midbrain periaqueductal gray. J Neurophysiol 1994; 72: 1337–56. [DOI] [PubMed] [Google Scholar]


