Abstract
Aim
The aim is to assess the effects of CDCs on heart structure, function, gene expression, and systemic parameters in aged rats. Diastolic dysfunction is characteristic of aged hearts. Cardiosphere-derived cell (CDC) therapy has exhibited several favourable effects on heart structure and function in humans and in preclinical models; however, the effects of CDCs on aging have not been evaluated.
Methods and Results
We compared intra-cardiac injections of neonatal rat CDCs to vehicle (phosphate-buffered saline, PBS) in 21.8 ± 1.6 month-old rats (mean ± standard deviation; n = 23 total). Ten rats 4.1 ± 1.5 months of age comprised a young reference group. Blood, echocardiographic, haemodynamic and treadmill stress tests were performed at baseline in all animals, and 1 month after treatment in old animals. Histology and the transcriptome were assessed after terminal phenotyping. For in vitro studies, human heart progenitors from older donors, or cardiomyocytes from aged rats were exposed to human CDCs or exosomes secreted by CDCs (CDC-XO) from paediatric donors. Transcriptomic analysis revealed that CDCs, but not PBS, recapitulated a youthful pattern of gene expression in the hearts of old animals (85.5% of genes differentially expressed, P < 0.05). Telomeres in heart cells were longer in CDC-transplanted animals (P < 0.0001 vs. PBS). Cardiosphere-derived cells attenuated hypertrophy by echo (P < 0.01); histology confirmed decreases in cardiomyocyte area (P < 0.0001) and myocardial fibrosis (P < 0.05) vs. PBS. Cardiosphere-derived cell injection improved diastolic dysfunction [lower E/A (P < 0.01), E/E’ (P = 0.05), end-diastolic pressure-volume relationship (P < 0.05) compared with baseline), and lowered serum brain natriuretic peptide (both P < 0.05 vs. PBS). In CDC-transplanted old rats, exercise capacity increased ∼20% (P < 0.05 vs. baseline), body weight decreased ∼30% less (P = 0.05 vs. PBS) and hair regrowth after shaving was more robust (P < 0.05 vs. PBS). Serum biomarkers of inflammation (IL-10, IL-1b, and IL-6) improved in the CDC group (P < 0.05 for each, all vs. PBS). Young CDCs secrete exosomes which increase telomerase activity, elongate telomere length, and reduce the number of senescent human heart cells in culture.
Conclusion
Young CDCs rejuvenate old animals as gauged by cardiac gene expression, heart function, exercise capacity, and systemic biomarkers.
Keywords: Aging, Diastolic dysfunction, Rejuvenation, Cell therapy, Cardiosphere-derived cells
Translational perspective
In senescent rats, we tested the idea that treatment with cardiosphere-derived cells (CDCs) from young donors might antagonize diastolic dysfunction and other consequences of aging. Allogeneic CDCs improved diastolic function and induced systemic functional improvements (notably, increased exercise tolerance) in old animals. Rejuvenating effects were likewise evident in human heart cells from older donors, co-cultured with young CDCs. Given that allogeneic CDCs are already in advanced clinical testing and have proven safe to date, progress to clinical testing is highly feasible.
Introduction
Cardiovascular disease increases markedly in prevalence with aging, creating a huge economic burden.1 Cell senescence underlies the aging process,2 and is characterized by progressive shortening of telomeres.3 Critical shortening of these protective ‘caps’ on the ends of linear chromosomes is associated with heart dysfunction and hypertrophy,4 impaired cardiomyocyte proliferation, and reduced regenerative capacity.5 The aged heart exhibits abnormal relaxation and/or increased stiffness,6,7 along with interstitial fibrosis and cardiomyocyte hypertrophy, increasing the risk of heart failure with preserved ejection fraction (HFpEF).8 Among rejuvenating strategies tested to date, parabiosis9 and cellular reprogramming10 seem promising, but none has addressed age-related heart dysfunction.
Cardiosphere-derived cells (CDCs) are cardiac progenitor cells which can differentiate into the three major cell types present in the heart, that is, cardiomyocytes, endothelial cells, and smooth muscle cells.11 Despite their progenitor status, CDCs work primarily indirectly12 (see Supplementary material online, Reference 1). They have been demonstrated to normalize left ventricular relaxation and diastolic pressure while improving survival in young Dahl salt-sensitive rats with hypertensive HFpEF.13 In this and other preclinical studies, CDCs mitigated inflammation, fibrosis and oxidative stress in diseased heart tissue.14,15 CDCs are also being tested clinically; data available to date indicate that they are safe, and may lead to improvements in post-ischaemic cardiac structure and function indicative of therapeutic regeneration.16 Based on this, we hypothesized that aged hearts characterized by fibrosis, myocardial hypertrophy, diastolic dysfunction, and chronic inflammation would benefit from therapy with CDCs. To test our hypothesis, we evaluated the effects of CDCs on age-related heart dysfunction and examined the broader rejuvenating potential of CDCs in a well-established rat model of senescence.
Methods
Animals and in vivo study protocol
Old Fisher 344 rats (21.8 ± 1.6 month old) were obtained from the National Institute of Aging. Younger Fishe 344 rats (4.1 ± 1.5 month old) were purchased from Envigo, Indianapolis, IN, USA. All F344 lines available in the USA are from the same origin (Columbia University), so share the same genetic background. All animals were studied in accordance with the local guidelines of the Animal Care and Use Committee as published by the National Institute of Health (NIH Publication No. 86-23, revised 1996).
Younger animals were used for the characterization of structural and functional changes related to aging. After initial phenotyping with echocardiography and exercise testing, old animals were divided into two groups that were matched prospectively for comparable baseline properties: Twelve rats were treated with CDCs, and 11 rats received phosphate-buffered saline (PBS, i.e. vehicle control). Prior to administration of CDCs or PBS, rats underwent invasive haemodynamic evaluation of left ventricular (LV) pressure and volume and aortic pressure. Old animals were evaluated at baseline and 1 month later by echocardiography, invasive haemodynamics, and exercise treadmill testing for structural and functional changes. Blood samples were collected at the same time points. After 1 month, animals were euthanized and hearts were harvested for further testing.
Isolation, expansion, and injection of cardiosphere-derived cells
Hearts were excised from 31 Sprague-Dawley neonatal rats to produce CDCs, as described.17 Rats in the CDC group received 1 × 106 passage 2 CDCs resuspended in 100 µL of PBS into the LV cavity with simultaneous aortic clamping (5 rats) or intramyocardially, divided among four injection sites (anterior, lateral, posterior walls and apex (7 rats). The same delivery strategies were used in the control group: intracavitary (LV, 5 rats) and intramyocardial (6 rats). No major differences were observed in the end points, and CDCs’ cardiac engraftment is similar (see Supplementary material online, Reference 2) with the two delivery strategies, so pooled results are presented.
Functional, histological, and molecular studies
Detailed descriptions of all the studies are available in the Supplementary Material online.
In vitro studies
When minced human heart tissue is grown in primary culture, it spontaneously gives rise to monolayers of cardiac stromal/progenitor cells (CSPCs).17 Cardiosphere-derived cells from human donors >55 years of age (old CSPCs) were used to assess human cardiac senescence in vitro. Cardiomyocytes were isolated from old rats (24 months) by enzymatic dissociation of the ventricles as described (see Supplementary material online, Reference 3). Human and rat cells were (separately) targeted with CDCs and exosomes secreted by CDCs or (CDC-XO) obtained from paediatric (<2 years of age) human donors (young CDCs or CDC-XO). For more detailed explanation, see Supplementary Material online.
Statistical analysis
All results are presented as mean ± standard deviation (± SEM in figures) or percentages, for continuous and categorical variables, respectively. Significance of differences was assessed by Student t-test or with one-way Analysis Of Variance (ANOVA) in case of multiple groups if the distribution of the variable was normal; otherwise, the Mann–Whitney or Kruskal–Wallis tests were used. Paired t-test was used to determine significance between baseline and end point in the same group of animals. Age-related changes in three age groups were estimated by linear regression analysis. Based on our previous results (∼10 ± 5% of differences in the functional tests between experimental groups), a sample size of 10 animals per group (assuming a 5% significance level and an 80% power level) was estimated, using a statistical software program (GB-Stat Version 10.0, Dynamic Microsystems Inc). Gene expression was analysed online, using QIAGEN data analysis centre [http://www.qiagen.com/us/shop/genes-and-pathways/data-analysis-center-overview-page/rt2-profiler-pcr-arrays-data-analysis-center (August 2017)]. All probability values reported are two-sided, with P < 0.05 considered significant. IBM SPSS Statistics 20 was used for all analyses. For in vitro studies, the lowest number of replicates per experiment was 3.
Results
We first characterized the animal model in terms of age-related structural and functional changes (see Supplementary material online, Figure S1). Aging was associated with progressive diastolic dysfunction (see Supplementary material online, Figure S1a–e and n) with preserved systolic function (see Supplementary material online, Figure S1h, i, and n), and steady increases of LV mass and LV diameter (see Supplementary material online, Figure S1f, g, and n). Blood pressure did not differ significantly among age groups (see Supplementary material online, Figure S1j and n), but exercise capacity decreased with aging (see Supplementary material online, Figure S1m and n).
Functional improvement of the heart: cardiosphere-derived cells decrease stiffness and improve relaxation of the left ventricle
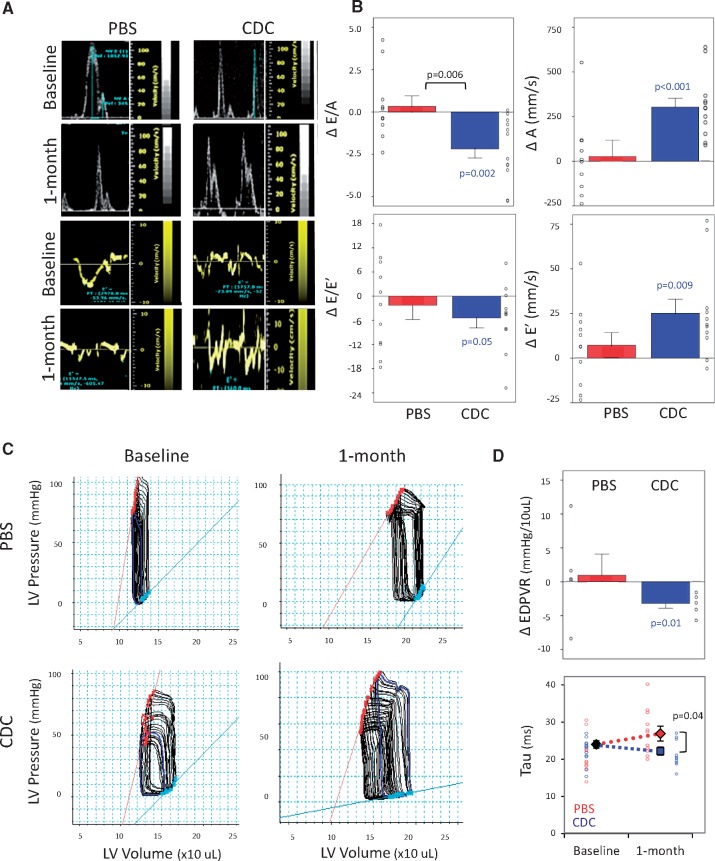
Figure 1A shows representative images of transmitral blood flow and tissue Doppler in PBS- and CDC-injected rats. After 1 month, old rats that had been transplanted with CDCs (but not those that had received only PBS) showed a decrease of E/A (from 4.2 ± 1.8 at baseline to 2.1 ± 0.4, P < 0.01) and E/E’ ratios (from 22.6 ± 5.2 at baseline to 16.3 ± 4.3, P = 0.05), back towards values seen in young rats (Figure 1B).
Figure 1.
Echocardiographic and haemodynamic changes in diastolic function. (A) Representative images of echo-Doppler transmitral flow and of tissue Doppler in a rat from the phosphate-buffered saline (PBS-control) and in a rat from the cardiosphere-derived cell (CDC) transplanted groups. (B) E/A and E/E’ ratios are decreased in CDC-treated rats after 1 month. (C) Representative images of left ventricular (LV) pressure-volume loops (PVL) in a rat from PBS-control and CDC-treated groups. (D) LV end-diastolic pressure-volume relationship (EDPVR) slopes are decreased in old CDC-treated group after 1 month and the time constant of relaxation, Tau is significantly lower in this group vs. control PBS. Number of animals: CDC-treated (n = 11) or PBS-injected (n = 11). P-values: all significant values are shown. Blue values (CDC group) represent the significance of the difference between baseline and end point within the group. Black values represent the significance between the groups.
Serial invasive haemodynamic measurements confirmed the echocardiographic findings. Figure 1C shows examples of pressure–volume loops before and 1 month after injection of PBS or CDCs in old animals. Such data were used to derive the slope of the LV end-diastolic pressure–volume relationship (EDPVR). Cardiosphere-derived cell treatment (but not PBS) decreased EDPVR after 1 month (Figure 1D, upper panel), indicating improved stiffness. The time constant of relaxation Tau (Figure 1D, lower panel) was abbreviated in CDC-transplanted rats 1 month post-injection (22.2 ± 3.5 ms vs. 26.9 ± 6.0 ms in PBS, P < 0.05, respectively). Minimum dP/dT values showed a similar trend as Tau, but did not reach statistical significance (see Supplementary material online, Figure S2).
No significant differences or evolving changes were observed in LV systolic function, which was normal in both groups at baseline (fractional shortening of ∼38% and ejection fraction of ∼66%, with no differences in the slope of the end-systolic pressure-volume relationship between groups; see Supplementary material online, Figure S2).
Structural changes of the heart: cardiosphere-derived cells regress left ventricular hypertrophy and are associated with less fibrosis
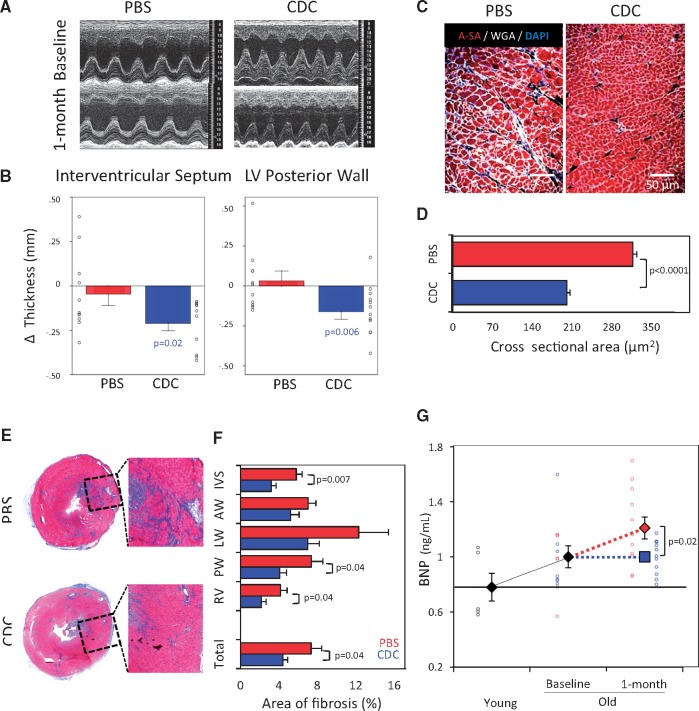
Figure 2A shows typical LV M-Mode images before and 1 month after injection of PBS or CDCs. After intervention in old rats, wall thickness decreased in the CDC group (P < 0.01 for interventricular septum and LV posterior wall) with borderline reduction of LV mass (Figure 2B, see Supplementary material online, Figure S2), but PBS injection had no evident effect. The echocardiographic changes were supported by the histological finding of smaller cross-sectional cardiomyocyte area after CDC vs. PBS injection (P < 0.0001, Figure 2C and D). Similar reductions in cardiomyocyte hypertrophy with CDCs have been observed in post-ischaemic heart failure.18
Figure 2.
Structural changes of the left ventricle and circulating levels of BNP. (A) Representative M-mode echocardiographic images from a rat from the phosphate-buffered saline group (PBS-control), and from a rat in the cardiosphere-derived cell (CDC) transplanted group. (B) CDC-injected rats (n = 11) had decreased echo-measured thickness of the interventricular septum and LV posterior wall. The control PBS rats (n = 11) showed an opposite trend. (C) Histological sections of myocardium from a rat in each group. (D) Pooled data for cardiomyocyte cross sectional area in CDC-injected (n = 6) vs. PBS-injected (n = 5) rats. (E) Representative heart sections stained with Masson’s trichrome. (F) CDC-group (n = 6) exhibited a decrease of fibrosis vs. control PBS (n = 5). (G) Serum levels of BNP in young (n = 5), old animals at baseline (n = 14) and after 1-month of treatment with CDC (n = 11) or PBS (n = 11). IVS: interventricular septum; LV-AW: left ventricular anterior wall; LV-LW: left ventricular lateral wall; LV-PW: left ventricular posterior wall; RV: right ventricular free wall. P-values: all significant values are represented. Blue values (CDC group) represent the significance of the difference between baseline and end point within the group. Black values represent the significance between the groups.
Additionally, cardiac fibrosis, assayed by Masson’s trichrome staining (Figure 2E and F), was reduced in rats transplanted with CDCs compared with control. Figure 2F shows that overall fractional fibrotic area dropped from 7.3% vs. 4.4% (P < 0.05), an effect which reflected reductions of scar throughout the heart. Finally, serum levels of brain natriuretic peptide (BNP), which is known to rise in human aging, increased with age in our study, but further increases post-injection were blunted by treatment with CDCs (Figure 2G).
Cardiosphere-derived cells induce biological rejuvenation of the heart
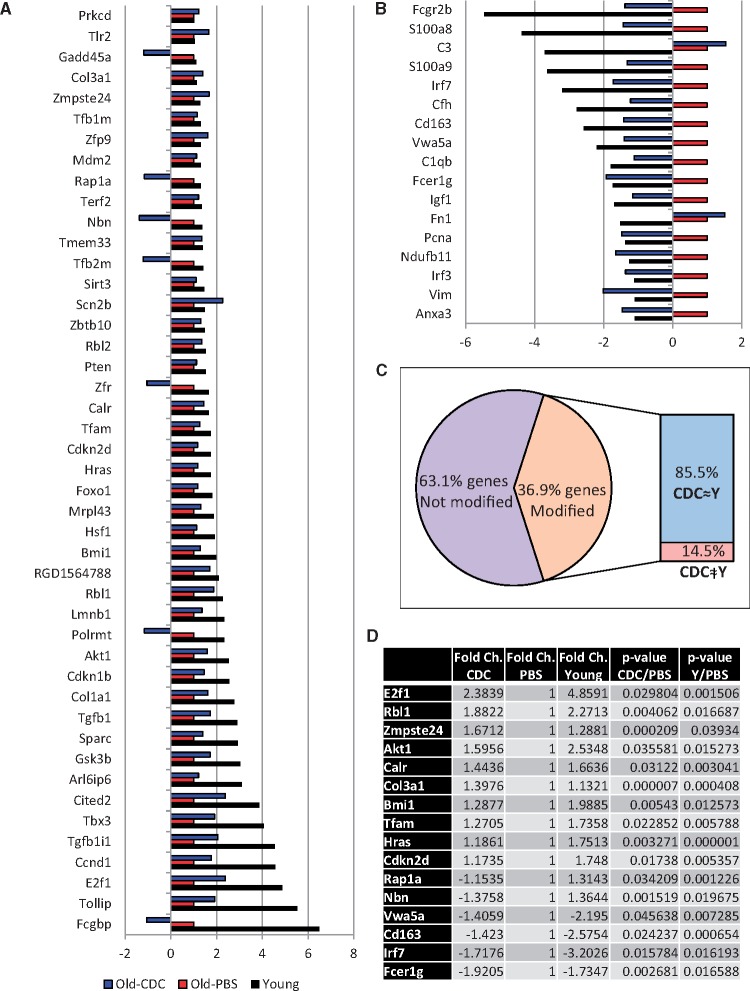
We analysed the expression of 168 genes implicated in tissue aging and cellular senescence pathways in whole-heart extracts from young rats, and from old rats treated with CDCs or PBS (Figure 3). Comparing the transcriptomes of CDC- and PBS-injected rats, significant differences were detected in 37% of the genes (Figure 3A and B). Most of the CDC-related changes (85.5%) directionally recapitulated the gene expression patterns of young animals (Figure 3C). Among the genes affected, those implicated in cell cycle control (e.g., E2f1 and Rbl1) and immune response (e.g., Fcer1g and Lrf7) figured most prominently (Figure 3D).
Figure 3.
Expression of aging and cellular senescence-related genes. Results are expressed as fold regulation vs. old rats injected with phosphate-buffered saline (old-PBS). (A) Significantly up-regulated genes in hearts of young and/or old-CDC animals compared with old-PBS group. (B) Significantly down-regulated genes in hearts of young and/or old-CDC animals compared with old-PBS group. (C) Diagram showing the proportion of differently-regulated genes (only those with significant differences) in young and/or old-CDC treated rats vs. old-PBS animals (62 genes of a total of 168 analysed). Of those 62 genes, 85.5% of the CDC-related changes recapitulated the gene expression pattern observed in young rats. (D) Genes with significant differences in both young and old-CDC rats vs. old-PBS animals. Ns: young rats (n = 4), old-PBS (n = 7), old-CDC (n = 8).
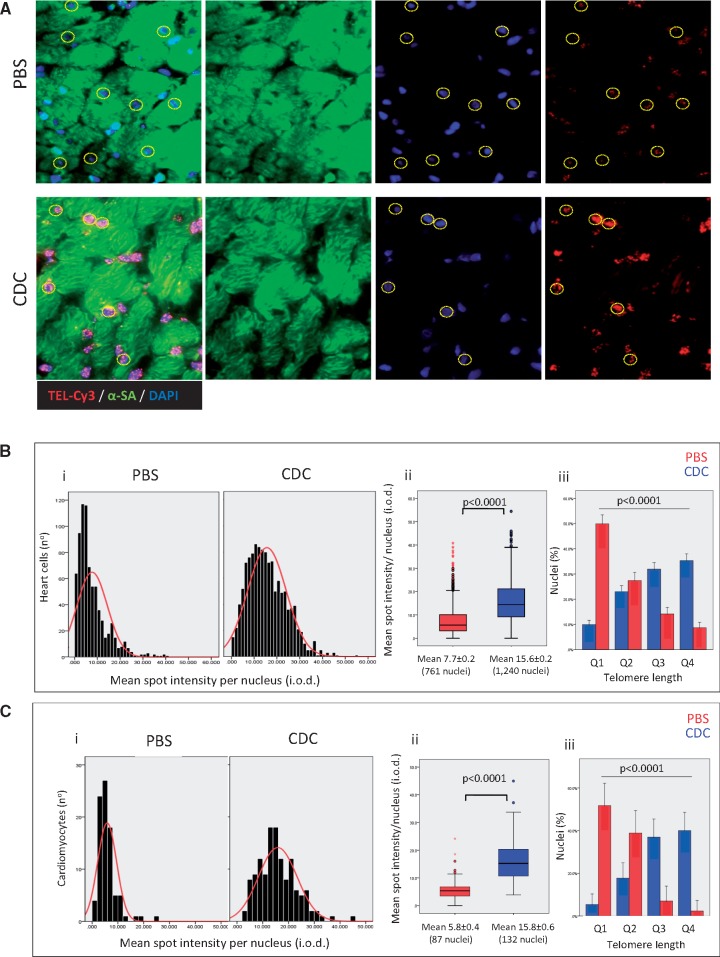
We found that cardiac telomeres were longer at study end-point in CDC-injected animals (P < 0.0001 vs. PBS controls; Figure 4A and B). About half of cells in the PBS group had extremely short telomeres (within the lowest quartile of length), compared with only 10% in CDC-injected rats (Figure 4Biii). In contrast, ∼40% of heart cells in the CDC group were within the highest quartile of telomere length (compared with <10% in PBS-injected animals). Findings were similar when telomere length was evaluated specifically in cardiomyocytes (Figure 4C), with longer mean length in CDC-injected rats (P < 0.0001 vs. PBS, Figure 4Cii) and an inverse telomere length distribution between the two groups (P < 0.0001, Figure 4Ciii).
Figure 4.
Telomere length of heart cells. (A) Representative detail of a confocal maximum projection images of telomere Q-FISH (TEL-Cy3) and alpha-sarcomeric actinin (α-SA) immunofluorescence in old animals treated with phosphate-buffered saline (PBS, n = 5) and old rats transplanted with cardiosphere-derived cells (CDC, n = 6). Cardiomyocyte nuclei were manually selected using the α-SA immunofluorescence image. Only unambiguously identified cardiomyocytes were considered for analysis. Dashed lines indicate cardiomyocytes with the telomere signal. (B) Telomere length distribution (i), mean telomere length (ii), and cell distribution according to quartiles (Q1—the shortest and Q4—the longest) of telomere length (iii) in entire population of heart cells. (C) Telomere length distribution (i), mean telomere length (ii), and cell distribution according to quartiles (Q1—the shortest and Q4—the longest) of telomere length (iii) in cardiomyocytes.
Cardiosphere-derived cells cause favourable systemic effects
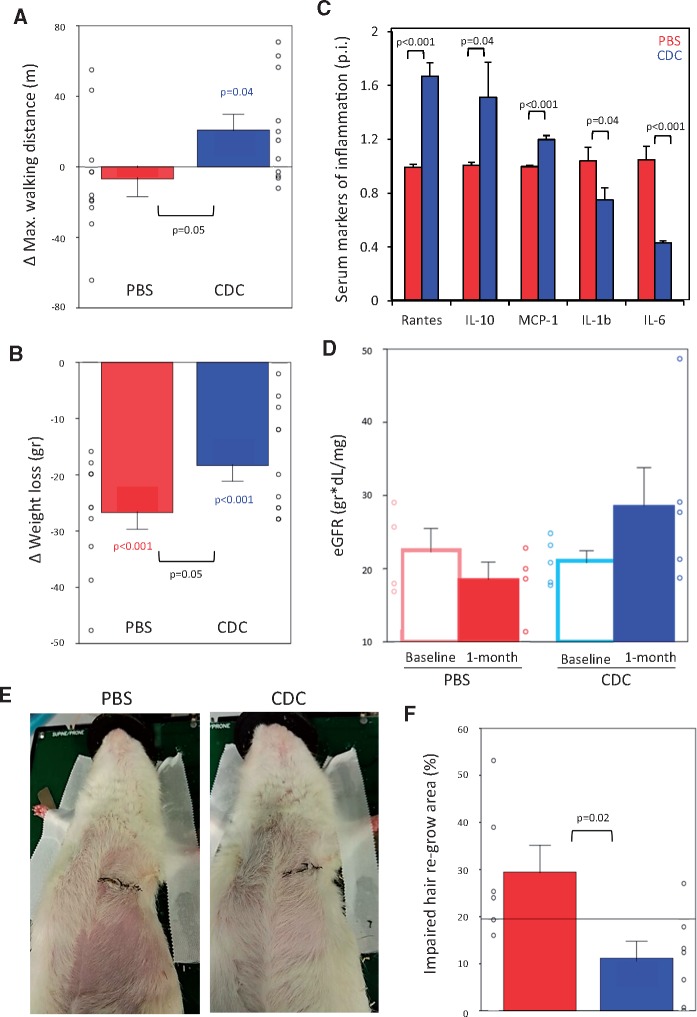
As described (see Supplementary material online, Reference 4), aging was associated with a marked decrease of exercise capacity (P < 0.001, see Supplementary material online, Figure S1m). In the aged rats, treadmill exercise capacity increased by ∼25% in the month after CDC injection (107.8 ± 25.5 m to 128.6 ± 12.4 m; P < 0.05), but changed little in PBS-injected old animals (Figure 5A). Weight loss secondary to sarcopenia or cachexia, compounded by surgery, also appeared to be responsive to CDCs: PBS-injected rats lost ∼30% more body weight in the post-operative month compared with CDC-transplanted animals (P = 0.05, Figure 5B). As systemic biomarkers of inflammation, interleukins (IL) 6, and 1β are known components of the senescence-associated secretory phenotype (SASP) which is implicated in propagation of the aging process.19 Serum levels of these ILs and other inflammatory cytokines were higher in old rats than in young animals (see Supplementary material online, Figure S4A), but CDC therapy lowered IL-1β and IL-6 levels (by 25% and 60%, respectively) compared with control rats at the study end point (Figure 5C). In eight animals, serum samples were available both at baseline and study end point, showing a five-fold decrease of IL-6 levels in CDC-treated rats. Heart and renal functions are closely correlated in the clinical entity of cardio-renal syndrome,20 which is prominent in elderly patients. Both serum creatinine (sCr) and blood urea nitrogen (BUN) levels were higher in old than in young rats (see Supplementary material online, Figure S4C and D). Levels of sCr decreased in CDC-injected old rats (P < 0.05) over the month post-intervention, despite less body weight loss than in PBS-injected animals. Taken together with the minimal decrease of BUN levels in the CDC group versus an increase in the control rats (see Supplementary material online, Figure S4B), estimated glomerular filtration rate increased in the CDC-transplanted animals (Figure 5D). Also, we observed an unexpected increase in the rate of hair regrowth after shaving in animals that had received CDCs relative to those injected with PBS (Figure 5E and F; see Supplementary material online, Figure S5). The improvements in exercise capacity, body mass, inflammatory cytokines, renal function and hair growth reveal that the rejuvenating benefits of CDCs are not limited to, or specific for, the cardiovascular system. Finally, serum levels of GDF 11, proposed as a systemic rejuvenating factor,9 were comparable in our experimental groups (see Supplementary material online, Figure S6), making it unlikely that this molecule plays an important role in CDC-associated rejuvenation.
Figure 5.
Systemic anti-aging effects. (A) Changes in maximal exercise capacity after 1 month of treatment show an increase in the cardiosphere-derived cell (CDC, n = 11) transplanted animals vs. a decrease in the phosphate-buffered saline (PBS, n = 11) group. CDC-related improvements amount to ∼20% of baseline functional capacity. (B) Body weight loss after 1 month of treatment. Weight decreased in both groups, but was less severe in CDC (n = 11) vs. control (n = 11) rats. (C) Serum markers of inflammation after 1 month of treatment. Fold changes in the CDC group vs. control rats; only cytokines with significant differences between the groups (n = 7 in each group) are presented. (D) Estimated glomerular filtration rate (eGFR) based on serum levels of creatinine (sCr), blood urea nitrogen (BUN) and weight. Although animals in the PBS group lost more weight than rats injected with CDCs, the latter experienced a greater decrease of sCr levels (P = 0.04) and BUN (BUN levels increased in the control rats). These changes translate into a 25% increase of eGFR in CDC-treated animals and an 11% decrease in PBS-injected animals. (E) Representative images of hair regrowth 3 weeks after shaving in old rats injected with CDC or PBS, showing more pronounced regrowth among CDC-treated animals. (F) Area of impaired hair regrowth in both groups (PBS, n = 6; CDC, n = 7). For A, B, and F: all significant P-values are shown. Coloured values (blue for the CDC group and red for the PBS group) are related with the changes in a parameter between baseline and end point within the group. Black values are related with the differences between the groups at the same time point.
Exosomes as mediators of anti-senescent effects of cardiosphere-derived cells
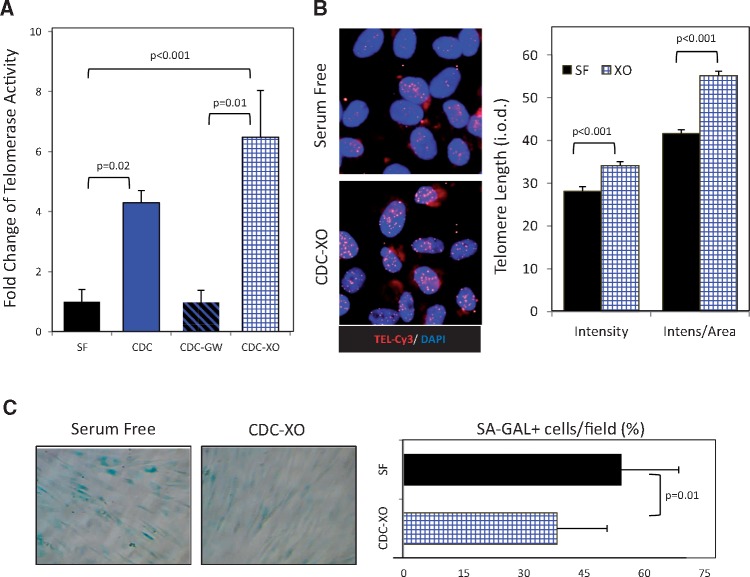
Our data are consistent with the hypothesis that telomerase activation and related telomere elongation underlie the anti-senescent effects observed here in old rats. We wanted to test this idea, and to do so in human cells in order to exclude rodent-specific effects. To assay the potential anti-aging effects of young CDCs on old human heart cells, we primed CSPCs obtained from >55-year-old donors with young (donors <2 years of age) CDCs, resuspended in serum-free conditioned media, using a transwell co-culture system. Telomerase was almost inactive in old CSPCs but its activity increased four-fold after 96 h of transwell culture with young CDCs, compared with control (Figure 6A). Many, if not most, effects of CDCs are mediated by secreted exosomes. A blocker of exosome release, GW4869 (20 uM), abrogated the telomerase-activating effect of CDCs (Figure 6A). Furthermore, old CSPCs primed with exosomes isolated from young CDCs (CDC-XO) exhibited a six-fold increase of telomerase activity (Figure 6A). Both lines of evidence support the notion that exosomes mediate young CDC-induced telomerase activation.
Figure 6.
Exosome-mediated activation of telomerase-telomere axis and decrease of cell senescence by young CDCs in old human cardiac stromal-progenitor cells (CSPCs). (A) Telomerase activity in extracts of CSPCs from old human donors after 96 h was determined following telomeric repeat amplification protocol (TRAP) in four groups: the control group incubated with serum-free media (SF); cells co-cultured with young donor CDCs alone or together with GW4869 inhibitor of exosome release (CDC and CDC-GW, respectively), using transwell membranes; cells co-cultured with young CDC exosomes (CDC-XO) resuspended in serum-free media. (B) Representative images of cells subjected to telomere Q-FISH analysis. Nuclei are stained with DAPI and telomeres with specific CY3-labeled probe (red). Telomere length was analysed by measuring the integrated optical density (i.o.d.) of the Cy3-channel within the nuclear borders after subtracting the background i.o.d. Results adjusted to the nuclear area are presented as well. (C) Histochemistry images for senescence-associated β-galactosidase (SA-GAL) (blue). Proportion of senescent, SA-GAL+ cells after 96 h co-incubation time period with young CDC-XO or SF.
One of the main functions of telomerase is telomere lengthening with associated cellular rejuvenation.21 We further tested both effects in old CSPCs. Telomeres were longer (Figure 6B) and the number of senescence-associated β-galactosidase positive (SA-GAL+) CSPCs were lower (Figure 6C) in young CDC-XO primed cells compared with control cells after 96 h.
To investigate whether these effects are seen in working myocardial cells, we tested the effects of young CDC-XO on ventricular cardiomyocytes isolated from old (24 month old) rats (see Supplementary material online, Figure S7). The proportion of senescent SA-GAL+ cells was lower in CDC-XO-primed cardiomyocytes than in control cells (P < 0.05; see Supplementary material online, Figure S7A), accompanied by a two-fold increase of borderline statistical significance in telomerase activity after 72 h (see Supplementary material online, Figure S7B). We analysed short- and long-term survival of α-sarcomeric actinin positive cardiomyocytes and observed a progressive decrease in the number of cells in the control group after 24 h, but preservation of the initial number of plated cardiomyocytes in the CDC-XO-primed group (see Supplementary material online, Figure S7C).
Discussion
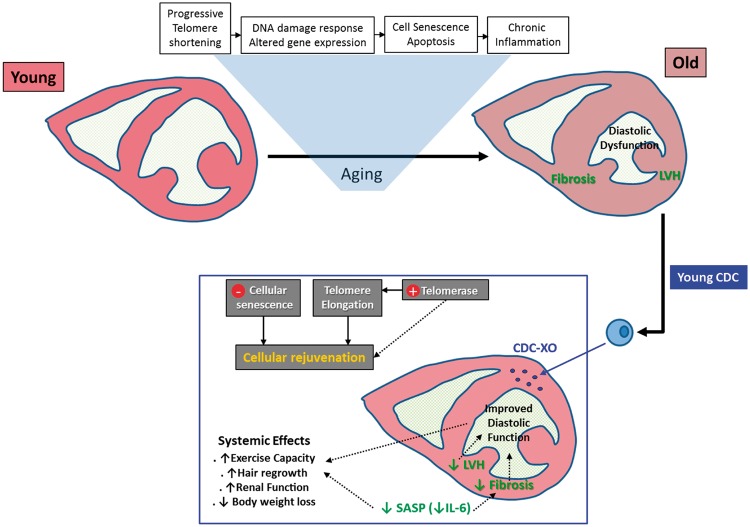
Here we documented the ability of cell therapy to attenuate age-related diastolic dysfunction and structural changes. Unexpectedly, we also found broadly favourable systemic effects of CDCs in old animals, despite the fact that the cells were delivered to the heart. The in vivo findings are supported by in vitro demonstration of novel exosome-mediated anti-senescent properties, yielding mechanistic insights into the anti-aging effects of young CDCs (Figure 7).
Figure 7.
Schematic depiction of heart aging and proposed mechanisms whereby young CDCs exert anti-senescent effects. The process of aging is depicted in the upper row. Transplanted CDCs secrete exosomes (CDC-XO) which lead to cellular rejuvenation. In the heart, left ventricular hypertrophy (LVH) is attenuated and fibrosis is decreased, leading to improved diastolic function. Systemically, a reduction of the senescence-associated secretory phenotype (SASP) contributes to systemic benefits. Although not shown here, we cannot rule out remote effects of CDCs or CDC-secreted exosomes on target tissues, independent of SASP.
Cardiac and vascular aging prominently affect diastolic function.22 Left ventricular diastolic stiffness increases with normal aging in humans, even in the absence of hypertension,23 a phenomenon also observed here and previously in aging rats. Longitudinal echocardiographic assessments reveal prominent age-related myocardial hypertrophy.24 Aging hearts exhibit myocyte hypertrophy, increased myocyte apoptosis, interstitial and subendocardial fibrosis, and amyloid deposition as consequences of multiple senescence-related pathways.25 Cardiosphere-derived cells increased telomere length, recapitulated a young gene expression pattern, decreased interstitial fibrosis and attenuated hypertrophy. Meanwhile, diastolic function improved, in association with lower levels of circulating BNP in CDC-injected rats.
Telomere shortening is a biomarker of lifetime stress, and telomere attrition is responsible for accelerated aging.3,21 Moreover, telomere dysfunction is a popular target for interventions to augment the regenerative capacity of mammalian hearts, given that telomeres figure prominently in cardiomyocyte cell-cycle arrest after birth.5 Here, telomere length of heart cells globally, and of cardiomyocytes specifically, was greater in old animals transplanted with CDCs obtained from very young rats compared with placebo (PBS). Telomerase is activated in the neonatal mammalian heart26 and is required for heart regeneration in zebrafish.27 We found that telomerase activity was increased in old human heart progenitor cells and in senescent rat cardiomyocytes primed with young CDCs. These effects were mediated in a paracrine manner by CDC-secreted exosomes. Telomerase activation was associated with telomere elongation, decreased cell senescence and cardioprotection in culture. Our results indicate that telomere elongation with CDCs is not exclusive to cardiomyocytes. Although non-cardiomyocyte heart cell populations were not specifically characterized in this study, rejuvenation of any type of cell, defined by the presence of longer telomeres, will likely be beneficial from a functional point of view. Specifically, senescence of fibroblasts may contribute to diastolic dysfunction by favouring their transformation into proinflammatory myofibroblasts, with consequent adverse extracellular matrix remodelling and secretion of inflammatory cytokines.28
Circulating levels of inflammatory cytokines IL-1β and IL-6 were decreased, accompanied by an increase of anti-inflammatory IL-10 levels in old CDC-treated animals. Thus, CDCs antagonize the SASP, which contributes to a spiral of increasing inflammation, dysfunction, and age-related diseases.29 Attenuation of SASP may underlie the observed improvements in exercise capacity, sarcopenia, hair regrowth and renal function. The systemic benefits of CDC therapy discovered here, notably the sizable increase of functional capacity, are remarkable in that they transcend mere localized correction of single-organ dysfunction. The marked systemic benefits on hair regrowth, renal function and exercise capacity appear disproportionate to the improvements in cardiac performance. The systemic improvements are all the more striking for having occurred remote from the focused site of delivery of the CDCs into the heart. While we have not explored the mechanism of the remote effects, it is not unreasonable to hypothesize contributions from CDCs that may have leaked into the systemic circulation, and/or secretion of exosomes from embedded CDCs. Systemic spread of exosomes has been implicated in various long-range signalling processes, including the skeletal myopathy that accompanies heart failure.30 In our scenario, the proposed long-range signalling differs in that it would be salutary rather than detrimental.
Study limitations
Maximum life span in this rat strain is ∼2.5 years, and we studied ∼22-month-old animals. Thus, our 1-month follow-up does not suffice to determine if the observed changes are maintained over time, or whether longevity is affected. In addition, extrapolation to humans should be made only cautiously. Although we selected a naturally-aged animal model containing many similarities to normal human aging,8,9 we did not consider frequent comorbidities such as hypertension, and associated treatments, which may influence the response to cell therapy. We did, however, confirm that CDCs and their exosomes antagonize human heart cell aging in vitro. Additionally, although all of the functional studies were performed sequentially, allowing us to evaluate CDC-induced changes in the same animal, histopathological evaluation was done only at study end-point, so only differences between groups at that specific time point could be assessed. The detailed specific antifibrotic and antihypertrophic mechanisms were not evaluated, being beyond the scope of this article, but the implication of exosomes does tie in mechanistically to previous work showing exosome-mediated reductions in cardiac fibrosis and hypertrophy.12,31 Finally, the design of this study does not establish if the rejuvenating properties shown here are seen only with young CDCs, so the efficacy of adult cells, demonstrated in other studies (see Supplementary material online, References 5 and 6) should be further tested.
Conclusions
The present findings motivate further translational studies of rejuvenation by CDC therapy. If such follow-up studies are promising, progress to clinical testing may be highly feasible, given that allogeneic CDCs are already in advanced clinical testing and have proven safe to date.11
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
L.G-S. conceived and designed the research, performed statistical analysis, drafted the manuscript; E.M. conceived and designed the research, handled funding and supervision, and made critical revisions of the manuscript for key intellectual content; W.L., S.F., R.M., J.V., J.H.C., and L.G. helped in acquiring the data.
Supplementary Material
Acknowledgements
Thanks to Lisa Trahan for valuable editorial assistance and to Dr Roberta Gottlieb for the use of a fluorescent microscope.
Funding
NIH R01HL124074 to E.M.; and by the Cedars-Sinai Board of Governors Heart Stem Cell Center.
Conflict of interest: E.M. owns founder’s equity in, and serves as unpaid scientific advisor to, Capricor Inc. The other authors report no conflicts.
References
- 1. Terzic A, Waldman S.. Chronic diseases: the emerging pandemic. Clin Transl Sci 2011;4:225–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Erusalimsky JD, Kurz DJ.. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp Gerontol 2005;40:634–642. [DOI] [PubMed] [Google Scholar]
- 3. Lopez-Otin C, Blasco M, Partridge L, Serrano M, Kroemer G.. The hallmarks of aging. Cell 2013;153:1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F, Blasco M.. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J 2003;22:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aix E, Gutierrez O, Sanchez-Ferrer C, Aguado T, Flores I.. Postnatal telomere dysfunction induces cardiomyocyte cell-cycle arrest through p21 activation. J Cell Biol 2016;213:571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Senni M, Paulus WJ, Gavazzi A, Fraser AG, Díez J, Solomon SD, Smiseth OA, Guazzi M, Lam CS, Maggioni AP, Tschöpe C, Metra M, Hummel SL, Edelmann F, Ambrosio G, Stewart Coats AJ, Filippatos GS, Gheorghiade M, Anker SD, Levy D, Pfeffer MA, Stough WG, Pieske BM.. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J 2014;35:2797–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Camichi GG, Savarese G, Akhmedov A, Luscher TF.. Molecular mechanism of endothelial and vascular aging: implications for cardiovascular disease. Eur Heart J 2015;36:3392–3403. [DOI] [PubMed] [Google Scholar]
- 8. Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr, Jacobsen SJ, Rodeheffer RJ.. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011;306:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loffredo F, Steinhauser M, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT.. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 2013;153:828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E, Araoka T, Vazquez-Ferrer E, Donoso D, Roman JL, Xu J, Rodriguez Esteban C, Nuñez G, Nuñez Delicado E, Campistol JM, Guillen I, Guillen P, Izpisua Belmonte JC.. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 2016;167:1719–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marban E. Breakthroughs in cell therapy for heart disease: focus on cardiosphere-derived cells. Mayo Clin Proc 2014;89:850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ibrahim AG, Cheng K, Marban E.. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2014;8:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gallet R, de Couto G, Simsolo E, Valle J, Sun B, Liu W, Tseliou E, Zile MR, Marbán E.. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction in rats by decreasing fibrosis and inflammation. JACC Basic Transl Sci 2016;1:14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tseliou E, de Couto G, Terrovitis J, Sun B, Weixin L, Marban L, Marban E.. Angiogenesis, cardiomyocyte proliferation and anti-fibrotic effects underlie structural preservation postinfarction by intramyocardially-injected cardiospheres. PLoS One 2014;9:e88590.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aminzadeh MA, Tseliou E, Sun B, Cheng K, Malliaras K, Makkar RR, Marbán E.. Therapeutic efficacy of cardiosphere-derived cells in a transgenic mouse model of non-ischaemic dilated cardiomyopathy. Eur Heart J 2015;36:751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E.. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 2012;379:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith R, Barile L, Cheol Cho H, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E.. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007;115:896–908. [DOI] [PubMed] [Google Scholar]
- 18. de Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, Arditi M, Marbán E.. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest 2015;125:3147–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lasry A, Ben-Neriah Y.. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol 2015;36:217–228. [DOI] [PubMed] [Google Scholar]
- 20. Bock JS, Gottlieb SS.. Cardiorenal syndrome: new perspectives. Circulation 2010;121:2592–2600. [DOI] [PubMed] [Google Scholar]
- 21. Madonna R, De Caterina R, Willerson J, Geng Y.. Biological function and clinical potential of telomerase and associated proteins in cardiovascular tissue repair and regeneration. Eur Heart J 2011;32:1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borlaug B. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2014;11:507–515. [DOI] [PubMed] [Google Scholar]
- 23. Fujimoto N, Hastings L, Bhella S, Shibata S, Gandhi K, Carrick-Ranson G, Levine BD.. Effect of ageing on left ventricular compliance and distensibility in healthy sedentary humans. J Physiol 2012;590:1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hees PS, Fleg JL, Lakatta EG, Shapiro EP.. Left ventricular remodeling with age in normal men versus women: novel insights using three-dimensional magnetic resonance imaging. Am J Cardiol 2002;90:1231–1236. [DOI] [PubMed] [Google Scholar]
- 25. Waller BF. The old-age heart: normal aging changes which can produce or mimic cardiac disease. Clin Cardiol 1988;11:513–517. [DOI] [PubMed] [Google Scholar]
- 26. Bär C, Bernardes de Jesus B, Serrano R, Tejera A, Ayuso E, Jimenez V, Formentini I, Bobadilla M, Mizrahi J, de Martino A, Gomez G, Pisano D, Mulero F, Wollert KC, Bosch F, Blasco MA.. Telomerase expression confers cardioprotection in the adult mouse heart after acute myocardial infarction. Nat Commun 2014;5:5863.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bednarek D, González-Rosa JM, Guzmán-Martínez G, Gutiérrez-Gutiérrez Ó, Aguado T, Sánchez-Ferrer C, Marques IJ, Galardi-Castilla M, de Diego I, Gómez MJ, Cortés A, Zapata A, Jiménez-Borreguero LJ, Mercader N, Flores I.. Telomerase is essential for zebrafish heart regeneration. Cell Rep 2015;12:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coppé JP, Desprez P, Krtolica A, Campisi J.. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010;5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland J.. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013;123:966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murach KA, McCarthy JJ.. MicroRNAs, heart failure, and aging: potential interactions with skeletal muscle. Heart Fail Rev 2017; 22:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tseliou E, Fouad J, Reich H, Slipczuk L, de Couto G, Aminzadeh M, Middleton R, Valle J, Weixin L, Marbán E.. Fibroblasts rendered antifibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. J Am Coll Cardiol 2015;66:599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.