Abstract
The central amygdala (CeA) GABAergic system is hypothesized to drive the development of alcohol dependence, due to its pivotal roles in the reinforcing actions of alcohol and the expression of negative emotion, anxiety and stress. Recent work has also identified an important role for the CeA corticotropin-releasing factor (CRF) system in the interaction between anxiety/stress and alcohol dependence. We have previously shown that acute alcohol and CRF each increase action potential-independent GABA release in the CeA via their actions at presynaptic CRF type 1 receptors (CRF1s); however, the shared mechanism employed by these two compounds requires further investigation. Here we report that acute alcohol interacts with the CRF/CRF1 system, such that CRF and alcohol act via presynaptic CRF1s and P/Q-type voltage-gated calcium channels to promote vesicular GABA release and that both compounds occlude the effects of each other at these synapses. Chronic alcohol exposure does not alter P/Q-type voltage-gated calcium channel membrane abundance or this CRF1/P/Q-type voltage-gated calcium channel mechanism of acute alcohol-induced GABA release, indicating that alcohol engages this molecular mechanism at CeA GABAergic synapses throughout the transition to dependence. Thus, P/Q-type voltage-gated calcium channels, like CRF1s, are key regulators of the effects of alcohol on GABAergic signaling in the CeA.
Keywords: Alcohol/ethanol, Central amygdala, Corticotropin-releasing factor (CRF), Corticotropin-releasing factor type 1 receptor (CRF1), GABA, P/Q-type voltage-gated calcium channel, Alcohol dependence
1. Introduction
The central amygdala (CeA) is hypothesized to drive the development of alcohol dependence, due to its pivotal roles in the reinforcing actions of alcohol and the expression of negative emotion, anxiety and stress (Gilpin et al., 2015; Koob and Volkow, 2010). Alcoholics often cite anxiety and stress as strong motivators for drinking (Litman et al., 1977, 1983; Ludwig and Wikler, 1974; Sinha, 2009), and both cue-elicited craving and intoxication increase the amygdalar activity of alcohol-dependent patients (Koob and Volkow, 2010). The CeA comprises an interconnected network of γ-aminobutyric acid (GABA) interneurons and GABA projection neurons (Haubensak et al., 2010; Lopez de Armentia and Sah, 2004; Marek et al., 2013), and this inhibitory drive regulates the escalated alcohol intake and anxiety-like behavior of alcohol-dependent rats (Gilpin et al., 2015; Koob and Volkow, 2010; Rassnick et al., 1993).
Recent work has also identified an important role for the CeA corticotropin-releasing factor (CRF) system in the interaction between anxiety/stress and alcohol dependence (Gilpin et al., 2015; Rassnick et al., 1993; Roberto et al., 2010b). Notably, CRF is co-released with GABA in the CeA (Partridge et al., 2016), typically in response to neuronal burst firing (Rainnie et al., 1992; Yu and Shinnick-Gallagher, 1998), and the expression levels of CRF and its type 1 receptor (CRF1), as well as the basal concentration of GABA, are increased in the CeA of alcohol-dependent rats (Roberto et al., 2010b). Moreover, CeA-specific CRF1 antagonism reduced the alcohol intake (Funk et al., 2006; Varodayan et al., 2017b) and anxiety-like behavior (Rassnick et al., 1993) of alcohol-dependent rats. Critically, we have previously shown that acute alcohol and CRF each increase action potential-independent GABA release in the CeA via their actions at presynaptic CRF1s (Roberto et al., 2010b); however, the shared mechanism employed by these two compounds to activate CeA GABAergic synapses is not fully understood.
GABA release is strictly controlled by calcium influx through voltage-gated calcium channels, with the different channel subtypes displaying distinct distribution patterns based on their physiological roles (Catterall and Few, 2008). P/Q- and N-type voltage-gated calcium channels couple to presynaptic vesicles to promote neurotransmitter release, while L-type voltage-gated calcium channels are primarily somatodendritic (Hell et al., 1993; Sinnegger-Brauns et al., 2009). Previous studies have implicated voltage-gated calcium channels in several alcohol-related behaviors, including alcohol consumption and withdrawal syndrome (Newton et al., 2004; Varodayan et al., 2017b; Walter and Messing, 1999; Watson and Little, 2002). Multiple groups have also reported that alcohol both inhibits (Belia et al., 1995; Maldve et al., 2004; Mullikin-Kilpatrick and Treistman, 1993; Pietrzykowski et al., 2013; Xiao et al., 2005; Zucca and Valenzuela, 2010) and enhances (Belia et al., 1995; Pietrzykowski et al., 2013; Simasko et al., 1999) voltage-gated calcium channel activity, and alcohol’s actions on voltage-gated calcium channels regulate GABA release in several brain regions (Hirono et al., 2009; Varodayan et al., 2017b; Zucca and Valenzuela, 2010). Similarly, the CRF system can inhibit (Tao et al., 2008, 2009, 2006) or enhance (Yu and Shinnick-Gallagher, 1998) voltage-gated calcium channel activity to modulate CeA synaptic transmission (Krishnan et al., 2010; Pollandt et al., 2006).
Given the critical role of the CeA in the reinforcing actions of alcohol and the transition to dependence, a clearer understanding of the shared neurobiological mechanisms driving its activation by acute alcohol and the CRF/CRF1 system may provide insight into the development of this disease and promote therapeutic strategies for alcohol use disorders. Here we investigated the hypothesis that voltage-gated calcium channels may represent novel mechanisms by which acute alcohol and CRF co-stimulate CeA GABAergic synapses, and explored their potential neuroadaptation in the transition to alcohol dependence.
2. Material and methods
All the procedures in this study were approved by The Scripps Research Institute (TSRI) Institutional Animal Care and Use Committee and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.1. Chronic intermittent ethanol exposure
Male Sprague Dawley rats (n = 63; 329.8 ± 9.3 g) were purchased from Charles River Laboratories (Raleigh, NC). Chronic intermittent ethanol (CIE) rats (n = 17) were exposed daily to ethanol vapor (14 h vapor/10 h air) for 5–7 weeks. We, and others, have previously shown that rats that experience CIE become physically alcohol-dependent, with increased alcohol-drinking behavior, anxiety-like behavior, and reward deficits (Gilpin et al., 2008; O’Dell et al., 2004; Roberto et al., 2010b). Blood alcohol levels (BALs) were measured twice weekly by tail-bleeding and immediately prior to sacrifice. The mean BAL for all animals across the study was 206 ± 8 mg/dL. The naive rats (n = 46) were treated similarly, but received continuous air. CIE rats were taken directly from the ethanol-filled vapor chambers for sacrifice. However, electrophysiology slice preparation occurred in ethanol-free solutions, so all recordings were performed during an acute in vitro withdrawal period (1–8 h).
2.2. Electrophysiology
Rats were anesthetized (3–5% isoflurane) and decapitated, and the brains placed in oxygenated (95% O2/5% CO2), cold high-sucrose solution (pH 7.3–7.4; in mM): 206.0 sucrose; 2.5 KCl; 0.5 CaCl2; 7.0 MgCl2; 1.2 NaH2PO4; 26.0 NaHCO3; 5.0 glucose; 5.0 HEPES, as previously described (Herman et al., 2013; Herman and Roberto, 2016; Roberto et al., 2010b; Varodayan et al., 2016). The brains were coronally sliced (300 μm) and the tissue incubated (30 min at 37 °C, then 30 min at room temperature) in oxygenated artificial cerebrospinal fluid (aCSF; in mM): 130.0 NaCl; 3.5 KCl; 2 CaCl2; 1.25 NaH2PO4; 1.5 MgSO4; 24 NaHCO3; 10 glucose.
We recorded from neurons located in the medial subdivision of the central amygdala (CeA) using infrared differential interference contrast (IR-DIC) optics, a w60 water immersion objective (Olympus BX51WI, Tokyo, Japan) and a CCD camera (EXi Aqua, QImaging, Surrey, BC, Canada). Whole-cell voltage-clamp recordings were performed in gap-free acquisition mode with a sampling rate per signal of 10 kHz and low-pass filtered at 10 k Hz, using a Multiclamp 700B amplifier, Digidata 1440A and pClamp 10 software (Molecular Devices, Sunnyvale, CA). Recording pipettes (3–7 MΏ; King Precision, Claremont, CA) were filled with potassium-chloride internal solution (in mM): 145.0 KCl; 5.0 EGTA; 5.0 MgCl2; 10.0 HEPES; 2.0 Na+-ATP; 0.2 Na+-GTP. Miniature spontaneous GABAA–mediated inhibitory postsynaptic currents (mIPSCs) were isolated with 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 μM), DL-2-amino-5-phosphonovalerate (AP-5, 30 μM), CGP 55845A (1 μM) and tetrodotoxin (TTX, 0.5 μM). TTX is a voltage-gated sodium channel blocker and so prevents action potential generation/propagation, allowing for the mechanistic study of synaptic transmission at isolated synapses and the identification of pre vs. postsynaptic drug effects. The neurons were clamped at −60 mV and experiments with a series resistance >15 MΏ or a >20% change in series resistance, as monitored with a 10 mV pulse, were excluded. For all experiments involving altered aCSF calcium concentration, the aCSF magnesium levels were adjusted to compensate.
Recording frequencies, amplitudes and kinetics were analyzed over a 3 min interval using Mini Analysis (Synaptosoft Inc., Fort Lee, NJ) and visually confirmed, with mIPSC events <5 pA excluded and cells with less than 60 events/3 min interval excluded. To control for cell-to-cell variation in baseline electrophysiology properties, drug effects were normalized to their own neuron’s baseline prior to group analyses. Final values were analyzed (Prism 5.02; Graph-Pad, San Diego, CA) for independent significance using one-sample t-tests and compared using two-tailed t-tests. Data are presented as mean ± standard error of the mean (SEM), with the number of cells and animals used for each experiment reported in the figure legend.
2.3. Western blotting
Rats were anesthetized with isoflurane and decapitated. Naïve and CIE rat brains (n = 6 per group) were coronally sliced (2 mm) with a wire matrix and the CeA punched on a chilled stage with an 18-gage blunt needle. The 2 CeA from each animal were combined and the tissue stored at −80 °C.
The samples were homogenized with a rotor-stator homogenizer (Tissue Tearor, Cole-Parmer Instrument Co., Vernon Hills, IL) in 250 μL buffer A (in mM): 4.0 HEPES pH 7; 320.0 sucrose; 5.0 EDTA pH 8; phosphatase inhibitor cocktail (PhosSTOP; Roche Life Science, Basel, Switzerland); protease inhibitor cocktail (cOmplete, EDTA-free; Roche), and then enriched for membrane proteins ((Goebel-Goody et al., 2009), with slight modifications (Varodayan et al., 2017b)). Specifically, the homogenate was centrifuged twice (1000g, 10 min, 4 °C) and supernatant collected and combined. This total supernatant was then re-centrifuged (100,000g, 1 h, 4 °C) and the pellet re-suspended in 50 μL buffer A. 25 μg samples were loaded onto a 7.5% SDS polyacrylamide gel (Mini-PROTEAN TGX, Bio-rad Laboratories; Hercules, CA), electrophoresed (100 V, 2 h), and transferred to a PVDF membrane (100 mA, 22 h, 4 °C; Immobilon-P, EMD Millipore, Billerica, MA). The membranes were washed in Tris-buffered saline with 0.1% Tween-20 (TBST; Sigma-Aldrich, Inc., St. Louis, MO), blocked in 5% milk/TBST (2 h, room temperature), incubated in primary antibody (overnight, 4 °C; Cav2.1, 190 kDa, 1:500, Alomone Labs #ACC-001; Jerusalem, Israel) and incubated in HRP-conjugated secondary antibody (1 h, room temperature; donkey anti-rabbit, 1:5000, EMD Millipore AP182PMI). The protein was visualized using enhanced chemiluminescence (SuperSignal West Pico, Thermo Scientific Pierce, Pittsburgh, PA) and exposed to Hy-Blot CL film (Denville Scientific, South Plainfield, NJ). To obtain a loading control, the membranes were incubated for 15 min in 0.4% Coomassie stain (in 50% methanol, 10% acetic acid, 40% ddH2O; Coomassie Brilliant Blue R-250, Bio-Rad), de-stained (50% ddH2O, 43% methanol, 7% acetic acid) and dried (Lee et al., 2015). Digital images were acquired using light transmission (film) or reflective (membrane) scanning on a Scanjet G4050 (Hewlett-Packard Company, Palo Alto, CA).
Protein band optical densities (OD) and Coomassie staining were measured using Image Studio Lite (Li-Cor Biosciences, Lincoln, NE). To control for protein loading variation, each protein OD was normalized to its own lane’s Coomassie staining (50–150 kDa) (Welinder and Ekblad, 2011). To allow for sample comparison across blots, Coomassie-normalized protein values were expressed relative to the mean value for naïve rats on the same membrane. The final values were compared using two-tailed t-tests in Prism 5.02. Data are presented as mean ± SEM.
2.4. Drugs and chemicals
We purchased ω-Agatoxin TK, AP-5, CGP 55845A and DNQX from Tocris (Bristol, UK); 1,2-Bis(2-Aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), Nifedipine and TTX from Sigma (St. Louis, MO); ω-Conotoxin GVIA from AnaSpec (Fremont, CA); and ethanol from Remet (La Mirada, CA). CRF was synthesized by Dr. Jean Rivier at the Salk Institute for Biological Studies, and R121919 was synthesized by Dr. Kenner Rice at the Drug Design and Synthesis Section of the National Institute on Drug Abuse. Drugs were dissolved in aCSF and applied locally by Y-tubing (Murase et al., 1989) or bath perfusion.
3. Results
3.1. Acute ethanol increased GABA release via P/Q-type voltage-gated calcium channels
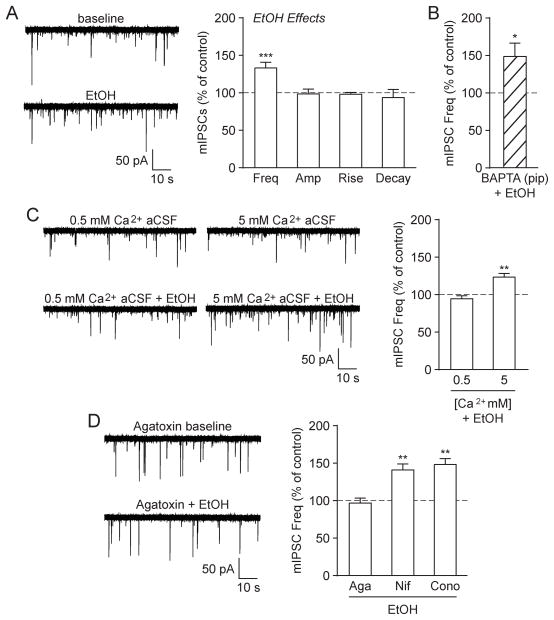
We first assessed the effects of acute alcohol on GABA transmission in the CeA of naïve rats by recording action potential-independent mIPSCs in the presence of the voltage-gated sodium channel blocker TTX (across the entire study the mean baseline mIPSC frequency = 0.34 ± 0.02 Hz, amplitude = 48.4 ± 1.7 pA, rise time = 2.40 ± 0.06 ms and decay time = 5.67 ± 0.26 ms). As previously reported (Roberto et al., 2004; Varodayan et al., 2016), here we found that application of a maximal effective concentration of ethanol (44 mM EtOH; see (Nie et al., 2009; Roberto et al., 2003, 2004) for concentration-effect curves on GABA transmission in the CeA) significantly increased the mIPSC frequency to 133.3 ± 7.5% of baseline ([t(13) = 4.46, p < 0.001 by one-sample t-test]; Fig. 1A). There were no significant changes in the mean mIPSC amplitude or kinetics, but ethanol increased mIPSC amplitudes in 3/14 neurons (to 130.7 ± 5.9% of baseline; [t(2) = 5.20, p < 0.05 by one-sample t-test]), with no effect in the remaining 11/14 neurons (similar to (Herman et al., 2013; Roberto et al., 2004; Varodayan et al., 2016)). As mIPSCs are action potential-independent, increased frequencies indicate higher GABA release probabilities and altered amplitudes/kinetics reflect changed GABAA receptor sensitivity (De Koninck and Mody, 1994; Otis et al., 1994). Therefore, acute ethanol increased GABA release at CeA synapses, without significantly affecting the region’s GABAA receptor composition or expression.
Fig. 1.
Acute alcohol increased GABA release in the naïve rat CeA via P/Q-type voltage-gated calcium channel activity. A: (Left) Representative mIPSC traces from a naïve rat CeA neuron in baseline conditions and during acute alcohol (44 mM EtOH) superfusion. (Right) EtOH significantly increased the mIPSC frequency, but had no effect on the mIPSC amplitude or kinetics (14 cells from 10 rats). B: EtOH significantly increased the mIPSC frequency in CeA neurons that were pre-loaded with 10 mM BAPTA (9 cells from 3 rats). C: (Left) Representative mIPSCs in low (0.5 mM) and high (5 mM) Ca2+ aCSF and during subsequent EtOH superfusion. (Right) EtOH significantly increased the mIPSC frequency in CeA neurons exposed to high Ca2+ aCSF, but not low Ca2+ aCSF (normalized to pre-alcohol baseline). For these extracellular calcium experiments, 6–7 cells from a minimum of 5 rats were used for each experimental group. D: (Left) Representative mIPSCs in the P/Q-type voltage-gated calcium channel blocker ω-Agatoxin TK (500 nM Aga) and during subsequent EtOH superfusion. (Right) EtOH’s enhancement of the mIPSC frequency was blocked in the presence of Aga (10 cells from 4 rats), but was unchanged by the L-type calcium channel blocker Nifedipine (10 μM Nif; 6 cells from 4 rats) or the N-type calcium channel blocker ω-Conotoxin GVIA (1 μM Cono; 6 cells from 5 rats).
Since calcium promotes synaptic vesicle fusion and neurotransmitter release (Catterall and Few, 2008), we suspected that ethanol’s actions on GABA release may involve changes in local calcium signaling. To investigate whether calcium in the postsynaptic recording cell mediates ethanol’s effects, we added the calcium chelator BAPTA (10 mM) to the recording pipette. Ethanol continued to increase the mIPSC frequency in these BAPTA-loaded neurons (147.2 ± 17.6%; [t(8) = 2.68, p < 0.05 by one-sample t-test]), indicating that its actions on GABA release do not require calcium signaling in the postsynaptic cell (Fig. 1B). We next assessed if ethanol-induced GABA release requires presynaptic neuronal calcium influx, by altering the extracellular calcium concentration (from the normal artificial cerebrospinal fluid (aCSF) calcium concentration of 2 mM to low (0.5 mM) or high (5 mM) calcium). Ethanol increased the mIPSC frequency in high Ca2+ aCSF (122.2 ± 4.9%; [t(5) = 4.57, p < 0.01 by one-sample t-test]), but had no effect in low Ca2+ aCSF (93.7 ± 3.9%; [t(6) = 1.61, p = n.s. by one-sample t-test]), revealing a role for extracellular calcium in ethanol-induced GABA release (Fig. 1C). There were no other ethanol- or calcium concentration-induced effects on mIPSC characteristics in these experiments. Thus, we find that ethanol requires presynaptic neuronal calcium influx in order to enhance CeA GABA release.
As calcium influx through voltage-gated calcium channels can directly stimulate neurotransmitter release (Catterall and Few, 2008), we investigated the possible role that voltage-gated calcium channels may play in ethanol’s enhancement of CeA GABA release. P/Q-type voltage-gated calcium channel blockade with ω-Agatoxin TK (500 nM) prevented ethanol’s effects on the mIPSC frequency (96.0 ± 6.5; [t(9) = 0.63, p = n.s. by one-sample t-test]), while L- and N-type voltage-gated calcium channel blockers had no effect (10 μM Nifedipine; [t(5) = 4.96, p < 0.01 by one-sample t-test] and 1 μM ω-Conotoxin GVIA; [t(5) = 6.13, p < 0.01 by one-sample t-test], respectively; Fig. 1D). ω-Agatoxin TK also had a per se effect on the mIPSC frequency (across the study it increased the mIPSC frequency to 134.4 ± 10.6% in nine cells; [t(8) = 3.25, p < 0.05 by one-sample t-test]), which was surprising given the role of these channels in regulating baseline GABA release (Catterall and Few, 2008; Hell et al., 1993; Sinnegger-Brauns et al., 2009). Of note, in three of these nine cells, ω-Agatoxin TK had no per se effect on the mIPSC frequency (105.0 ± 4.1%), but still prevented the ethanol/CRF facilitation (100.7 ± 1.4%; see section 3.2 for CRF facilitation), suggesting a lack of ceiling effect. Additionally, all three voltage-gated calcium channel blockers had no effects on mIPSC amplitudes or kinetics throughout the experiment. Collectively, these data reveal that acute ethanol acts via P/Q-type voltage-gated calcium channels to stimulate action potential-independent GABA release at naïve rat CeA synapses.
3.2. CRF increased GABA release via P/Q-type voltage-gated calcium channels
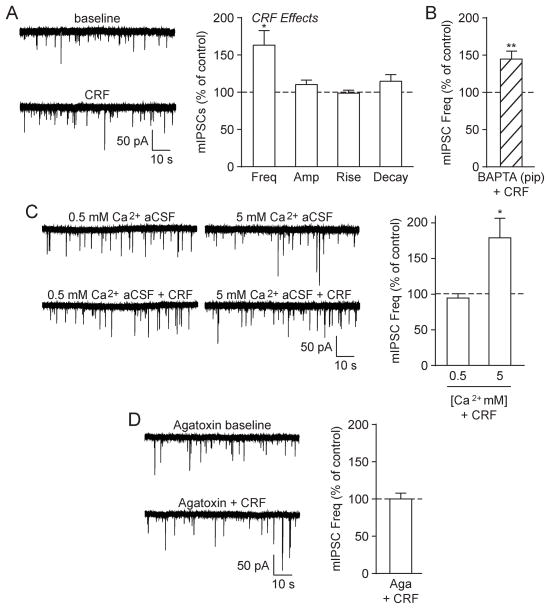
Ethanol’s actions in the CeA have previously been linked to the CRF1 (Roberto et al., 2010b), and so we next investigated the influence of CRF at CeA GABAergic synapses. Similar to acute ethanol, CRF (200 nM; see (Nie et al., 2009; Roberto et al., 2010b) for concentration-effect curves on GABA transmission in the CeA) significantly increased the mIPSC frequency to 163.2 ± 19.5% of baseline in CeA neurons from naïve rats ([t(7) = 3.24, p < 0.05 by one-sample t-test]; Fig. 2A). As before, there were no significant changes in the mean mIPSC amplitude or kinetics, although CRF did increase mIPSC amplitudes in 3/8 cells (to 126.7 ± 5.7% of baseline; [t(2) = 4.67, p < 0.05 by one-sample t-test]; similar to (Herman et al., 2013)). CRF continued to enhance the mIPSC frequency in BAPTA-loaded neurons (145.7 ± 10.7%; [t(5) = 4.27, p < 0.01 by one-sample t-test]; Fig. 2B) and in the presence of high Ca2+ aCSF (180.0 ± 27.4%; [t(5) = 2.92, p < 0.05 by one-sample t-test]), but not in low Ca2+ aCSF (95.0 ± 6.1%; [t(6) = 0.81, p = n.s. by one-sample t-test]; Fig. 2C). Moreover, ω-Agatoxin TK prevented CRF’s enhancement of the mIPSC frequency (100.2 ± 7.5%; [t(4) = 0.02, p = n.s. by one-sample t-test]; Fig. 2D). There were no additional effects on mIPSC characteristics in these experiments. Thus, CRF acts similarly to acute ethanol by employing P/Q-type voltage-gated calcium channels to induce CeA GABA release in naïve rats.
Fig. 2.
CRF increased GABA release in the naïve rat CeA via P/Q-type voltage-gated calcium channel activity. A: (Left) Representative mIPSCs from a naïve rat CeA neuron in baseline conditions and during CRF (200 nM) superfusion. (Right) CRF significantly increased the mIPSC frequency, but had no effect on the mIPSC amplitude or kinetics (8 cells from 5 rats). B: CRF significantly increased the mIPSC frequency in CeA neurons that were pre-loaded with 10 mM BAPTA (6 cells from 3 rats). C: (Left) Representative mIPSCs in low (0.5 mM) and high (5 mM) Ca2+ aCSF and during subsequent CRF superfusion. (Right) CRF significantly increased the mIPSC frequency in CeA neurons exposed to high Ca2+ aCSF, but not low Ca2+ aCSF (normalized to pre-CRF baseline). For these extracellular calcium experiments, 6–7 cells from a minimum of 4 rats were used for each experimental group. D: (Left) Representative mIPSCs in the P/Q-type voltage-gated calcium channel blocker ω-Agatoxin TK (500 nM Aga) and during subsequent CRF superfusion. (Right) CRF’s enhancement of the mIPSC frequency was blocked in the presence of Aga (5 cells from 3 rats).
3.3. Acute ethanol interacts with the CRF/CRF1 system to enhance GABA release
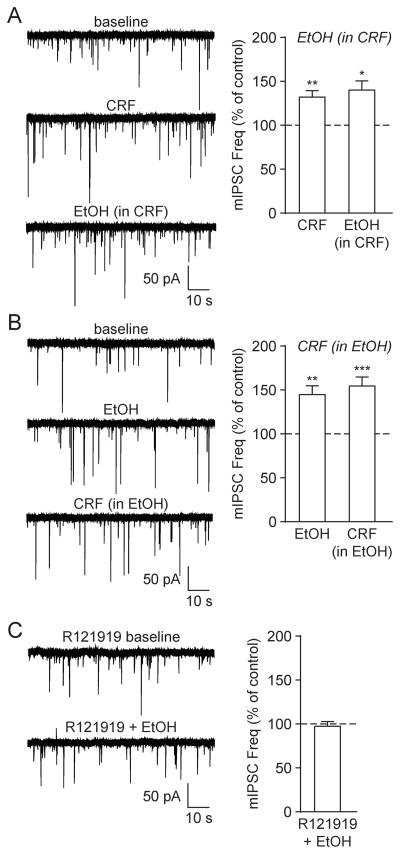
In order to clarify the relationship between ethanol and the CRF/CRF1 system in terms of their respective effects on GABA release in naïve rats, we next performed a series of interaction studies. We found that CRF prevented ethanol-induced GABA release ([t(5) = 1.94, p = n.s. by two-tailed paired t-test]; Fig. 3A). Specifically, the mIPSC frequency was potentiated by CRF to 132.1 ± 7.2% of baseline ([t(5) = 4.46, p < 0.01 by one-sample t-test]), and after the subsequent addition of ethanol (in the presence of CRF) it remained at 140.2 ± 10.2% of baseline ([t(5) = 3.92, p < 0.05 by one-sample t-test]). Similar to this interaction, ethanol occluded the effects of CRF on GABA release ([t(8) = 0.72, p = n.s. by two-tailed paired t-test]; Fig. 3B); ethanol increased the mIPSC frequency to 144.7 ± 10.1% of baseline ([t(8) = 4.43, p < 0.01 by one-sample t-test]), and CRF (in the presence of ethanol) maintained it at 154.6 ± 10.2% of baseline ([t(8) = 5.36, p < 0.001 by one-sample t-test]). Finally, the CRF1-specific antagonist R121919 (1 μM) prevented ethanol’s enhancement of the mIPSC frequency (96.4 ± 5.5%; [t(6) = 0.66, p = n.s. by one-sample t-test]; Fig. 3C), with no additional effects on mIPSC characteristics. Thus, alcohol interacts with the CRF/CRF1 system to enhance CeA GABA release in naïve rats.
Fig. 3.
Acute alcohol interacts with the CRF/CRF1 system to enhance GABA release. A: (Left) Representative mIPSCs from a naïve rat CeA neuron in baseline conditions, during CRF (200 nM) superfusion and following acute alcohol (44 mM EtOH) co-application in the continued presence of CRF. (Right) CRF significantly increased the mIPSC frequency, and EtOH (in CRF) had no further effect (6 cells from 4 rats). B: (Left) Representative mIPSCs in baseline conditions, during EtOH superfusion and following CRF + EtOH co-application. (Right) EtOH significantly increased the mIPSC frequency, and CRF (in EtOH) had no further effect (9 cells from 4 rats). C: (Left) Representative mIPSCs in the CRF1 antagonist R121919 (1 μM) and during subsequent EtOH superfusion. (Right) EtOH’s enhancement of the mIPSC frequency was blocked in the presence R121919 (7 cells from 3 rats).
3.4. Alcohol dependence does not alter the effects of acute ethanol at CeA GABAergic synapses
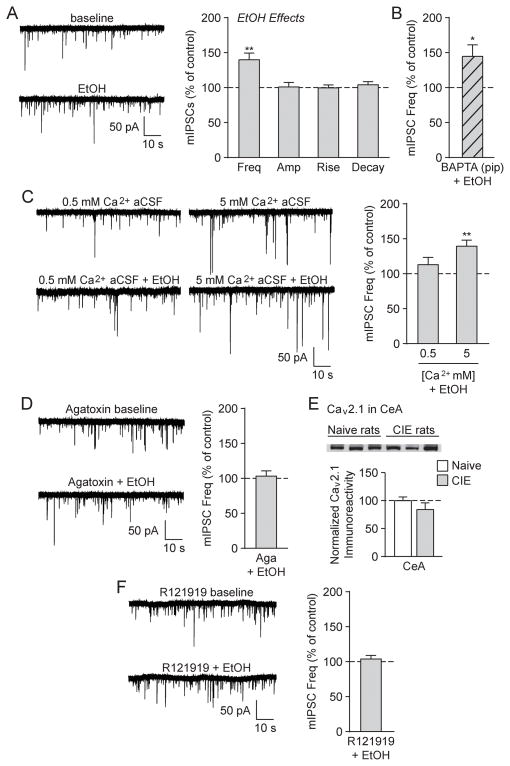
To investigate whether chronic alcohol exposure alters the response of CeA GABAergic synapses to acute ethanol, we next exposed rats to chronic intermittent alcohol exposure (CIE) to induce physical dependence (O’Dell et al., 2004; Roberto et al., 2010b). We found that CeA neurons from CIE rats had a significantly higher baseline mIPSC frequency vs. naïve rats ([t(103) = 3.05, p < 0.01 by unpaired two-tailed t-test] (Roberto et al., 2004)), with a mean CIE baseline mIPSC frequency = 0.46 ± 0.03 Hz, amplitude = 49.7 ± 1.7 pA, rise time = 2.35 ± 0.06 ms and decay time = 5.64 ± 0.21 ms. CIE rats also displayed a similar increase in the mean mIPSC frequency (139.8 ± 9.7%; [t(5) = 4.12, p < 0.01 by one-sample t-test]; Fig. 4A) after acute ethanol application as observed in naïve rats, with no significant changes in the mean mIPSC amplitude or kinetics (though acute ethanol increased mIPSC amplitudes in 2/6 cells to 117.7 ± 4.2% of baseline; [p=n.s. by one-sample t-test]; similar to (Roberto et al., 2004)). Therefore, chronic alcohol exposure does not produce tolerance to ethanol’s acute actions at CeA GABAergic synapses.
Fig. 4.
Alcohol dependence does not alter the effects of acute alcohol at CeA GABAergic synapses. A: (Left) Representative mIPSCs from a CIE rat CeA neuron in baseline conditions and during acute alcohol (44 mM EtOH) superfusion. (Right) EtOH significantly increased the mIPSC frequency, but had no effect on the mIPSC amplitude or kinetics (6 cells from 5 rats). B: EtOH significantly increased the mIPSC frequency in CeA neurons that were pre-loaded with 10 mM BAPTA (6 cells from 3 rats). C: (Left) Representative mIPSCs in low (0.5 mM) and high (5 mM) Ca2+ aCSF and during subsequent EtOH superfusion. (Right) EtOH significantly increased the mIPSC frequency in CIE CeA neurons exposed to high Ca2+ aCSF, but not low Ca2+ aCSF (normalized to pre-alcohol baseline). For these extracellular calcium experiments, 6 cells from a minimum of 4 rats were used for each experimental group. D: (Left) Representative mIPSCs in the P/Q-type voltage-gated calcium channel blocker ω-Agatoxin TK (500 nM Aga) and during subsequent EtOH superfusion. (Right) EtOH’s enhancement of the mIPSC frequency was blocked in the presence of Aga (5 cells from 3 rats). E: (Top) Representative western blot image of P/Q-type voltage-gated calcium channel (Cav2.1) membrane abundance from the CeA of naïve and CIE rats. (Bottom) Quantification revealed no difference in CeA Cav1.2 membrane expression in CIE vs. naïve rats (6 rats were used for each experimental group). F: (Left) Representative mIPSCs in the CRF1 antagonist R121919 (1 μM) and during subsequent EtOH superfusion. (Right) EtOH’s enhancement of the mIPSC frequency was blocked in the presence of R121919 (6 cells from 3 rats).
Similar to its effects in the naïve rats, acute ethanol enhanced the mIPSC frequency in CIE rat CeA neurons that were loaded with BAPTA (143.2 ± 16.5; [t(5) = 2.61, p < 0.05 by one-sample t-test]; Fig. 4B) or exposed to high Ca2+ aCSF (139.6 ± 8.7%; [t(5) = 4.58, p < 0.01 by one-sample t-test]), but not low Ca2+ aCSF (113.2 ± 10.3%; [t(5) = 1.28, p = n.s. by one-sample t-test]; Fig. 4C). Moreover, as in the naïve rats, ω-Agatoxin TK had a per se effect on the mIPSC frequency (138.4 ± 10.3% in 5 cells from 3 CIE rats; [t(4) = 3.75, p < 0.05 by one-sample t-test]), and prevented ethanol’s enhancement of the mIPSC frequency in CIE rats (102.4 ± 7.3; [t(4) = 0.33, p = n.s. by one-sample t-test]; Fig. 4D). In accordance with these findings, western blotting studies revealed that chronic alcohol exposure did not change the CeA membrane abundance of P/Q-type voltage calcium channels (Cav2.1; [t(10) = 1.18, p = n.s. by unpaired two-tailed t-test]; Fig. 4E). Finally, CRF1 antagonism by R121919 blocked ethanol’s actions on the mIPSC frequency (102.9 ± 5.0%; [t(5) = 0.58, p = n.s. by one-sample t-test]; Fig. 4F). There were no additional effects on mIPSC characteristics in these experiments. Thus, acute ethanol acts similarly in the CeA of both naïve and alcohol-dependent rats by employing CRF1s and P/Q-type voltage-gated calcium channels to induce GABA release.
4. Discussion
Collectively, these data indicate that acute alcohol interacts with the CRF/CRF1 system to increase CeA GABA release, via P/Q-type voltage-gated calcium channel activity. As the CeA is primarily GABAergic, alcohol- and CRF-induced inhibition of local interneurons can lead to the disinhibition of CeA inhibitory projection neurons and thus, the inhibition of downstream brain regions (e.g. the bed nucleus of the stria terminalis (BNST), hypothalamus, midbrain and brainstem (Alheid, 2003; Herman and Roberto, 2016)). Chronic alcohol exposure does not alter P/Q-type voltage-gated calcium channel membrane abundance or this CRF1/P/Q-type voltage-gated calcium channel mechanism of acute alcohol-induced GABA release, so alcohol’s stimulation of CeA synaptic function with each and every alcohol exposure may contribute to the region’s over-activation during dependence (Gilpin et al., 2015; Koob and Volkow, 2010).
Notably, basal GABA release was enhanced in the CeA of CIE rats compared to naïve rats, suggesting greater local inhibition of CeA neurons in alcohol-dependent rats. CRF1 and P/Q-type voltage-gated calcium channel activity did not mediate this change, as there were no differences in the per se effects of their respective antagonists on CeA GABA transmission in naïve vs. alcohol-dependent rats. Additionally, both alcohol and CRF increased CeA GABA release, and in a subset of CeA neurons, enhanced GABAA receptor function. While we identified a shared presynaptic mechanism in this study, future work is needed to elucidate whether alcohol and CRF’s postsynaptic actions occur within the same neuronal populations and share common mechanisms of action. Finally, acute alcohol increased CeA GABA release in alcohol-dependent rats to a similar magnitude as in naïve rats, indicating a lack of functional tolerance to acute alcohol’s actions at CeA GABAergic synapses (Roberto et al., 2004, 2010b). Others have reported alcohol-induced GABA release in the basolateral amygdala (BLA), brainstem, cerebellum, hippocampus, substantia nigra and ventral tegmental area (VTA) of naïve rodents (Hirono et al., 2009; Kelm et al., 2011; Qi et al., 2010; Theile et al., 2009; Weiner and Valenzuela, 2006). Multiple mechanisms likely govern these effects, but only a few intracellular pathways have been identified. Specifically, alcohol increased GABA release in cerebellar interneurons through protein kinase A (PKA), protein kinase C (PKC) and intracellular calcium pathways (Hirono et al., 2009; Kelm et al., 2011) and in VTA neurons via 5-hydroxytryptomine-2C (5HT-2C) receptors and intracellular calcium stores (Theile et al., 2009). Therefore, alcohol has widespread effects on GABA transmission throughout many brain regions, but only a few intracellular pathways have been identified and these mechanisms appear to be region-specific.
Here we report that CRF1 antagonism prevented alcohol-induced GABA release in the CeA of naïve rats (as previously described in (Herman et al., 2013)) and alcohol-dependent rats. Moreover, pretreatment with either acute alcohol or CRF occluded the effect of the other compound in naïve rats, indicating a clear interaction between alcohol and the CRF/CRF1 system. While the direct site of alcohol’s actions remains unknown, the most parsimonious explanation is that alcohol activates CRF1s to induce CeA GABA release. Alternatively, alcohol may activate intracellular pathways (see below), or may induce CRF release from the lateral subdivision of the CeA (Veening et al., 1984) (though the lateral CeA CRF input to the medial CeA seems to be minor in rats (Pomrenze et al., 2015)) or from distal inputs from other known CRF-expressing regions, such as the BNST or paraventricular hypothalamic nucleus (Gafford et al., 2012; Merchenthaler, 1984; Wang et al., 2011). Other G protein-coupled receptors (GPCRs) have also been implicated in alcohol-induced CeA GABA release, including the type 1 cannabinoid receptor (CB1) (Roberto et al., 2010a; Varodayan et al., 2016), δ-opioid receptor (Kang-Park et al., 2009) and neuropeptide Y receptor (Gilpin et al., 2011). Additionally, CB1 and GABAB receptors mediate alcohol’s potentiation of GABA release in the BLA and cerebellum (Kelm et al., 2011; Talani and Lovinger, 2015; Varodayan et al., 2017a), while in the VTA, μ-opioid and 5HT-2C receptors are involved (Theile et al., 2009). Therefore, alcohol’s actions on CeA GABA release via CRF1 do not occur in isolation, and future studies must identify which alcohol-induced GPCR pathways interact and predominate, and whether they are dysregulated after chronic ethanol exposure.
CRF1s also regulate P/Q-type voltage-gated calcium channel activity (Kuryshev et al., 1996; Ritchie et al., 1996), and here we demonstrated that both alcohol- and CRF-induced GABA release were mediated by P/Q-type voltage-gated calcium channels. P/Q-type voltage-gated calcium channels closely interact with synaptic vesicle fusion machinery proteins so that their activation produces a calcium influx that can directly trigger GABA release (Catterall and Few, 2008). CRF1 activity can also induce the adenylyl cyclase (AC)/PKA and phospholipase C/PKC pathways (Blank et al., 2003; Gutknecht et al., 2009; Riegel and Williams, 2008), and our laboratory has previously reported a role for AC7, PKA and PKCε in alcohol- and CRF-induced GABA release in the rodent CeA (Bajo et al., 2008; Cruz et al., 2011, 2012). These 2nd messenger systems have significant crosstalk, and both can interact with voltage-gated calcium channels (Catterall, 2000; Catterall and Few, 2008; Cens et al., 2006; Dai et al., 2009). Therefore, alcohol’s site of action is difficult to determine in the present study, as it may directly act upon any combination of these signaling molecules. Nonetheless, the most likely scenario from the present work is that acute alcohol acts on CRF1s to produce downstream changes in PKA/PKC signaling that modulate P/Q-type voltage-gated calcium channel activity leading to action potential-independent GABA release.
Interestingly, we recently reported that acute alcohol also enhances action potential-dependent GABA release in the CeA of naïve rats, but this effect occurs via an L-type voltage-gated calcium channel mechanism (Varodayan et al., 2017b). It is important to note that action potential-dependent neurotransmitter release results from neuronal activity across the entire synaptic network to produce classical neural communication, whereas the addition of the voltage-gated sodium channel blocker TTX (as in the current study) blocks action potential generation/propagation to reveal action potential-independent neurotransmitter release that can maintain homeostasis and mediate plasticity at mature synapses (Kavalali, 2015). Critically, P/Q-type voltage-gated calcium channel blockade did not alter alcohol-induced action potential-dependent GABA release in the naïve rat CeA (Varodayan et al., 2017b), while in the present study L-type voltage-gated calcium channel blockade did not alter alcohol-induced action potential-independent GABA release. Therefore, these two types of GABA release represent different forms of CeA neurotransmission that are regulated by alcohol via distinct voltage-gated calcium channel mechanisms. Action potential-dependent and -independent neurotransmission are also differently regulated by several other molecules/compounds (e.g. presynaptic metabotropic glutamate receptors (Glitsch, 2006), nitric oxide species (Pan et al., 1996), cholesterol (Wasser et al., 2007), antimalarial drugs (McArdle et al., 2006), γ-secretase (Pratt et al., 2011) and methyl CpG binding protein 2 (MeCP2) (Nelson et al., 2006, 2008)), leading to the growing consensus that different vesicle populations govern action potential-dependent and -independent neurotransmitter release, possibly via distinct vesicle fusion machinery, spatial segregation of the vesicle and/or retrograde signaling (Kavalali, 2015).
The CeA integrates emotionally salient sensory information about fearful and anxiety-inducing stimuli to produce the appropriate behavioral and physiological responses (Gilpin et al., 2015). Its activity promotes alcohol drinking and anxiety-like behaviors, and its over-activation is considered a hallmark of the transition to alcohol dependence (Gilpin et al., 2015; Koob and Volkow, 2010). Collectively, our work highlights the diversity of acute alcohol’s actions on CeA voltage-gated calcium channels (P/Q-vs. L-type voltage-gated calcium channels), revealing its intricate control over different types of GABA release (action potential-independent vs. -dependent release). Moreover, acute alcohol interacts with the CRF/CRF1 system to produce action potential-independent CeA GABA release. This CRF1/P/Q-type voltage-gated calcium channel mechanism can still be engaged after chronic alcohol exposure, indicating that alcohol uses this molecular mechanism at CeA GABAergic synapses throughout the transition to dependence. Therefore, our data identify P/Q-type voltage-gated calcium channels, like CRF1s, as critical regulators of acute alcohol’s actions on CeA synaptic transmission, presenting a novel locus for therapeutic development to ameliorate alcohol use disorders.
Acknowledgments
This is manuscript number 29493 from The Scripps Research Institute. We thank Dr. Matthew Buczynski, Maury Cole and Dr. Miranda Staples for their technical support and Dr. Dean Kirson for valuable comments on the manuscript. CRF was synthesized by Dr. Jean Rivier at the Salk Institute for Biological Studies, and R121919 was synthesized by Dr. Kenner Rice at the Drug Design and Synthesis Section of the National Institute on Drug Abuse. This work was supported by the National Institutes of Health [AA015566, AA021491, AA017447, AA006420, AA013498, AA021802]. The authors declare no competing financial interests.
Abbreviations
- 5HT-2C
5-hydroxytryptomine-2C
- aCSF
artificial cerebrospinal fluid
- AC
adenylyl cyclase
- AP-5
DL-2-amino-5-phosphonovalerate
- BLA
basolateral amygdala
- BAL
blood alcohol level
- BAPTA
1,2-Bis(2-Aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid
- Cav2.1
P/Q-type voltage-gated calcium channel
- CB1
type 1 cannabinoid receptor
- CeA
central amygdala
- CIE
chronic intermittent ethanol vapor exposed
- CRF
corticotropin-releasing factor
- CRF1
corticotropin-releasing factor type 1 receptor
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- EtOH
ethanol
- GABA
γ-aminobutyric acid
- GPCR
G-protein coupled receptor
- mIPSC
miniature GABAA–mediated inhibitory postsynaptic current
- OD
optical density
- PKA
protein kinase A
- PKC
protein kinase C
- SEM
standard error
- TTX
tetrodotoxin
- VTA
ventral tegmental area
References
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci U S A. 2008;105:8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belia S, Mannucci R, Lisciarelli M, Cacchio M, Fano G. Double effect of ethanol on intracellular Ca2+ levels in undifferentiated PC12 cells. Cell Signal. 1995;7:389–395. doi: 10.1016/0898-6568(94)00092-p. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23:700–707. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Cens T, Rousset M, Leyris JP, Fesquet P, Charnet P. Voltage- and calcium-dependent inactivation in high voltage-gated Ca(2+) channels. Prog Biophys Mol Biol. 2006;90:104–117. doi: 10.1016/j.pbiomolbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Cruz MT, Bajo M, Maragnoli ME, Tabakoff B, Siggins GR, Roberto M. Type 7 adenylyl cyclase is involved in the ethanol and CRF sensitivity of GABAergic synapses in mouse central amygdala. Front Neurosci. 2011;4:207. doi: 10.3389/fnins.2010.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, Roberto M. Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biol Psychiatry. 2012;71:666–676. doi: 10.1016/j.biopsych.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y, Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J Neurophysiol. 1994;71:1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford GM, Guo JD, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)alpha1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci U S A. 2012;109:16330–16335. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry. 2015;77:859–869. doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M. Neuropeptide Y opposes alcohol effects on gamma-aminobutyric acid release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry. 2011;69:1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 2008;Chapter 9(Unit 9.29) doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch M. Selective inhibition of spontaneous but not Ca2+-dependent release machinery by presynaptic group II mGluRs in rat cerebellar slices. J Neurophysiol. 2006;96:86–96. doi: 10.1152/jn.01282.2005. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Gutknecht E, Van der Linden I, Van Kolen K, Verhoeven KF, Vauquelin G, Dautzenberg FM. Molecular mechanisms of corticotropin-releasing factor receptor-induced calcium signaling. Mol Pharmacol. 2009;75:648–657. doi: 10.1124/mol.108.050427. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Kallupi M, Luu G, Oleata CS, Heilig M, Koob GF, Ciccocioppo R, Roberto M. Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: alcohol and CRF effects. Neuropharmacology. 2013;67:337–348. doi: 10.1016/j.neuropharm.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Roberto M. Cell-type-specific tonic GABA signaling in the rat central amygdala is selectively altered by acute and chronic ethanol. Addict Biol. 2016;21:72–86. doi: 10.1111/adb.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono M, Yamada M, Obata K. Ethanol enhances both action potential-dependent and action potential-independent GABAergic transmission onto cerebellar Purkinje cells. Neuropharmacology. 2009;57:109–120. doi: 10.1016/j.neuropharm.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Kang-Park MH, Kieffer BL, Roberts AJ, Roberto M, Madamba SG, Siggins GR, Moore SD. Mu-opioid receptors selectively regulate basal inhibitory transmission in the central amygdala: lack of ethanol interactions. J Pharmacol Exp Ther. 2009;328:284–293. doi: 10.1124/jpet.108.140749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci. 2015;16:5–16. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Ethanol-enhanced GABA release: a focus on G protein-coupled receptors. Brain Res Rev. 2011;65:113–123. doi: 10.1016/j.brainresrev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B, Centeno M, Pollandt S, Fu Y, Genzer K, Liu J, Gallagher JP, Shinnick-Gallagher P. Dopamine receptor mechanisms mediate corticotropin-releasing factor-induced long-term potentiation in the rat amygdala following cocaine withdrawal. Eur J Neurosci. 2010;31:1027–1042. doi: 10.1111/j.1460-9568.2010.07148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryshev YA, Childs GV, Ritchie AK. Corticotropin-releasing hormone stimulates Ca2+ entry through L- and P-type Ca2+ channels in rat corticotropes. Endocrinology. 1996;137:2269–2277. doi: 10.1210/endo.137.6.8641175. [DOI] [PubMed] [Google Scholar]
- Lee AM, Wu DF, Dadgar J, Wang D, McMahon T, Messing RO. PKCepsilon phosphorylates alpha4beta2 nicotinic ACh receptors and promotes recovery from desensitization. Br J Pharmacol. 2015;172:4430–4441. doi: 10.1111/bph.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman GK, Eiser JR, Rawson NS, Oppenheim AN. Towards a typology of relapse: a preliminary report. Drug Alcohol Depend. 1977;2:157–162. doi: 10.1016/0376-8716(77)90023-0. [DOI] [PubMed] [Google Scholar]
- Litman GK, Stapleton J, Oppenheim AN, Peleg M, Jackson P. Situations related to alcoholism relapse. Br J Addict. 1983;78:381–389. doi: 10.1111/j.1360-0443.1983.tb02526.x. [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P. Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. J Neurophysiol. 2004;92:1285–1294. doi: 10.1152/jn.00211.2004. [DOI] [PubMed] [Google Scholar]
- Ludwig AM, Wikler A. “Craving” and relapse to drink. Q J Stud Alcohol. 1974;35:108–130. [PubMed] [Google Scholar]
- Maldve RE, Chen X, Zhang TA, Morrisett RA. Ethanol selectively inhibits enhanced vesicular release at excitatory synapses: real-time visualization in intact hippocampal slices. Alcohol Clin Exp Res. 2004;28:143–152. doi: 10.1097/01.ALC.0000106304.39174.AD. [DOI] [PubMed] [Google Scholar]
- Marek R, Strobel C, Bredy TW, Sah P. The amygdala and medial prefrontal cortex: partners in the fear circuit. J Physiol. 2013;591:2381–2391. doi: 10.1113/jphysiol.2012.248575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ, Sellin LC, Coakley KM, Potian JG, Hognason K. Mefloquine selectively increases asynchronous acetylcholine release from motor nerve terminals. Neuropharmacology. 2006;50:345–353. doi: 10.1016/j.neuropharm.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I. Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution Peptides. 1984;5(Suppl 1):53–69. doi: 10.1016/0196-9781(84)90265-1. [DOI] [PubMed] [Google Scholar]
- Mullikin-Kilpatrick D, Treistman SN. Electrophysiological studies on calcium channels in naive and ethanol-treated PC12 cells. Alcohol Alcohol Suppl. 1993;2:385–389. [PubMed] [Google Scholar]
- Murase K, Ryu PD, Rancid M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989;103:56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- Nelson ED, Kavalali ET, Montague LM. MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr Biol. 2006;16:710–716. doi: 10.1016/j.cub.2006.02.062. [DOI] [PubMed] [Google Scholar]
- Nelson ED, Kavalali ET, Montague LM. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci. 2008;28:395–406. doi: 10.1523/JNEUROSCI.3796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PM, Orr CJ, Wallace MJ, Kim C, Shin HS, Messing RO. Deletion of N-type calcium channels alters ethanol reward and reduces ethanol consumption in mice. J Neurosci. 2004;24:9862–9869. doi: 10.1523/JNEUROSCI.3446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, Rice KC, Roberto M, Siggins GR. Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. Scientific World Journal. 2009;9:68–85. doi: 10.1100/tsw.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci U S A. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZH, Segal MM, Lipton SA. Nitric oxide-related species inhibit evoked neurotransmission but enhance spontaneous miniature synaptic currents in central neuronal cultures. Proc Natl Acad Sci U S A. 1996;93:15423–15428. doi: 10.1073/pnas.93.26.15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Forcelli PA, Luo R, Cashdan JM, Schulkin J, Valentino RJ, Vicini S. Stress increases GABAergic neurotransmission in CRF neurons of the central amygdala and bed nucleus stria terminalis. Neuropharmacology. 2016;107:239–250. doi: 10.1016/j.neuropharm.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Ortiz-Miranda S, Knott TK, Custer E, Puig S, Lemos JR, Treistman SN. Molecular tolerance of voltage-gated calcium channels is evident after short exposures to alcohol in vasopressin-releasing nerve terminals. Alcohol Clin Exp Res. 2013;37:933–940. doi: 10.1111/acer.12057. [DOI] [PubMed] [Google Scholar]
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006;24:1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, Dadgar J, Kharazia V, De Guglielmo G, Crawford E, Janak PH, George O, Rice KC, Messing RO. A transgenic rat for investigating the anatomy and function of corticotrophin releasing factor circuits. Front Neurosci. 2015;9:487. doi: 10.3389/fnins.2015.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Zhu P, Watari H, Cook DG, Sullivan JM. A novel role for {gamma}-secretase: selective regulation of spontaneous neurotransmitter release from hippocampal neurons. J Neurosci. 2011;31:899–906. doi: 10.1523/JNEUROSCI.4625-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi SH, Liu Y, Hao LY, Guan QH, Gu YH, Zhang J, Yan H, Wang M, Zhang GY. Neuroprotection of ethanol against ischemia/reperfusion-induced brain injury through decreasing c-Jun N-terminal kinase 3 (JNK3) activation by enhancing GABA release. Neuroscience. 2010;167:1125–1137. doi: 10.1016/j.neuroscience.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Fernhout BJ, Shinnick-Gallagher P. Differential actions of corticotropin releasing factor on basolateral and central amygdaloid neurones, in vitro. J Pharmacol Exp Ther. 1992;263:846–858. [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Williams JT. CRF facilitates calcium release from intracellular stores in midbrain dopamine neurons. Neuron. 2008;57:559–570. doi: 10.1016/j.neuron.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie AK, Kuryshev YA, Childs GV. Corticotropin-releasing hormone and calcium signaling in corticotropes. Trends Endocrinol Metab. 1996;7:365–369. doi: 10.1016/s1043-2760(96)00168-3. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology. 2010a;35:1962–1972. doi: 10.1038/npp.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010b:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simasko SM, Boyadjieva N, De A, Sarkar DK. Effect of ethanol on calcium regulation in rat fetal hypothalamic cells in culture. Brain Res. 1999;824:89–96. doi: 10.1016/s0006-8993(99)01188-9. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, Hoda JC, Sartori SB, Striessnig J. Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol. 2009;75:407–414. doi: 10.1124/mol.108.049981. [DOI] [PubMed] [Google Scholar]
- Talani G, Lovinger DM. Interactions between ethanol and the endocannabinoid system at GABAergic synapses on basolateral amygdala principal neurons. Alcohol. 2015;49:781–794. doi: 10.1016/j.alcohol.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Hildebrand ME, Liao P, Liang MC, Tan G, Li S, Snutch TP, Soong TW. Activation of corticotropin-releasing factor receptor 1 selectively inhibits CaV3.2 T-type calcium channels. Mol Pharmacol. 2008;73:1596–1609. doi: 10.1124/mol.107.043612. [DOI] [PubMed] [Google Scholar]
- Tao J, Zhang Y, Huang H, Jiang X. Activation of corticotropin-releasing factor 2 receptor inhibits Purkinje neuron P-type calcium currents via G(o) alpha-dependent PKC epsilon pathway. Cell Signal. 2009;21:1436–1443. doi: 10.1016/j.cellsig.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Tao J, Zhang Y, Soong TW, Li S. Expression of urocortin 2 and its inhibitory effects on intracellular ca2+ via L-type voltage-gated calcium channels in rat pheochromocytoma (PC12) cells. Neuropsychopharmacology. 2006;31:2600–2609. doi: 10.1038/sj.npp.1301123. [DOI] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Role of 5-hydroxytryptamine2C receptors in Ca2+-dependent ethanol potentiation of GABA release onto ventral tegmental area dopamine neurons. J Pharmacol Exp Ther. 2009;329:625–633. doi: 10.1124/jpet.108.147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Bajo M, Soni N, Luu G, Madamba SG, Schweitzer P, Roberto M. Chronic alcohol exposure disrupts CB1 regulation of GABAergic transmission in the rat basolateral amygdala. Addict Biol. 2017a;22:766–778. doi: 10.1111/adb.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, de Guglielmo G, Logrip ML, George O, Roberto M. Alcohol dependence disrupts amygdalar L-type voltage-gated calcium channel mechanisms. J Neurosci. 2017b;37:4593–4603. doi: 10.1523/JNEUROSCI.3721-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Soni N, Bajo M, Luu G, Madamba SG, Schweitzer P, Parsons LH, Roberto M. Chronic ethanol exposure decreases CB1 receptor function at GABAergic synapses in the rat central amygdala. Addict Biol. 2016;21:788–801. doi: 10.1111/adb.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Walter HJ, Messing RO. Regulation of neuronal voltage-gated calcium channels by ethanol. Neurochem Int. 1999;35:95–101. doi: 10.1016/s0197-0186(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Wang L, Goebel-Stengel M, Stengel A, Wu SV, Ohning G, Tache Y. Comparison of CRF-immunoreactive neurons distribution in mouse and rat brains and selective induction of Fos in rat hypothalamic CRF neurons by abdominal surgery. Brain Res. 2011;1415:34–46. doi: 10.1016/j.brainres.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser CR, Ertunc M, Liu X, Kavalali ET. Cholesterol-dependent balance between evoked and spontaneous synaptic vesicle recycling. J Physiol. 2007;579:413–429. doi: 10.1113/jphysiol.2006.123133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson WP, Little HJ. Selectivity of the protective effects of dihydropyridine calcium channel antagonists against the ethanol withdrawal syndrome. Brain Res. 2002;930:111–122. doi: 10.1016/s0006-8993(02)02236-9. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Welinder C, Ekblad L. Coomassie staining as loading control in Western blot analysis. J Proteome Res. 2011;10:1416–1419. doi: 10.1021/pr1011476. [DOI] [PubMed] [Google Scholar]
- Xiao ZM, Li LJ, Yu SZ, Lu ZN, Li CY, Zheng JQ. Effects of extracellular Ca(2+) influx and intracellular Ca(2+) release on ethanol-induced cytoplasmic Ca(2+) overload in cultured superior cervical ganglion neurons. Neurosci Lett. 2005;390:98–103. doi: 10.1016/j.neulet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Yu B, Shinnick-Gallagher P. Corticotropin-releasing factor increases dihydropyridine- and neurotoxin-resistant calcium currents in neurons of the central amygdala. J Pharmacol Exp Ther. 1998;284:170–179. [PubMed] [Google Scholar]
- Zucca S, Valenzuela CF. Low concentrations of alcohol inhibit BDNF-dependent GABAergic plasticity via L-type Ca2+ channel inhibition in developing CA3 hippocampal pyramidal neurons. J Neurosci. 2010;30:6776–6781. doi: 10.1523/JNEUROSCI.5405-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]