Abstract
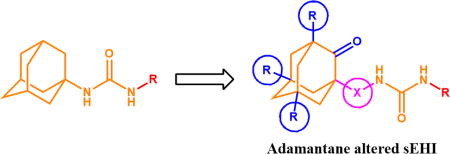
Adamantyl groups are widely used in medicinal chemistry. However, metabolism limits their usage. Herein, we report the first systematic study of adamantyl ureas and diureas bearing substituents in bridgehead positions of adamantane and/or spacers between urea groups and adamantane group, and tested their effects on soluble epoxide hydrolase inhibitor potency and metabolic stability. Interestingly, the effect on activity against human and murine sEH varied in opposite ways with each new methyl group introduced into the molecule. Compounds with three methyl substituents in adamantane were very poor inhibitors of murine sEH while still very potent against human sEH. In addition, diureas with terminal groups bigger than sEH catalytic tunnel diameter were still good inhibitors suggesting that the active site of sEH opens to capture the substrate or inhibitor molecule. The introduction of one methyl group leads to 4-fold increase in potency without noticeable loss of metabolic stability compared to the unsubstituted adamantane. However, introduction of two or three methyl groups leads to 8-fold and 98-fold decrease in stability in human liver microsomes for the corresponding compounds.
Keywords: soluble epoxide hydrolase, inhibitor, adamantane, isocyanate, urea
Graphical abstract
1. Introduction
The mammalian soluble epoxide hydrolase (sEH, E.C. 3.3.2.10) is involved in the metabolism of epoxy-fatty acids to vicinal diols through a catalytic addition of a water molecule.1, 2 Endogenous substrates for the sEH include epoxides of arachidonic acid (epoxyeicosatrienoic acids, or EETs), docosahexaenoic acid (EpDPEs), and other epoxides of fatty acids (EpFAs).3, 4 EETs and other EpFAs are important lipid mediators that have key roles in blood pressure regulation by exercising vasodilatory effects through the activation of the Ca2+-activated K+ channels in endothelial cells, which are beneficial in many renal and cardiovascular diseases.5, 6 Furthermore, the EETs also have anti-inflammatory and analgesic properties.7 Recently, EETs but not EpDPEs have been reported to promote angiogenesis in humans possibly due to their cyclooxygenase metabolites.8, 9 Conversion of EET to DHET by sEH produces a molecule that is more polar, readily conjugated and removed from the site of action. The inhibition of sEH in vivo by highly selective inhibitors results in an increase in the concentration of EETs, EpDPEs and other EpFAs and is accompanied by a reduction in angiotensin driven blood pressure, but also reduction of inflammation and pain, thereby suggesting that sEH is a promising target for the treatment of hypertension, inflammatory diseases and pain.10–12
Early on, small N,N’-disubstituted symmetric ureas, such as 1,3-dicyclohexyl urea (DCU) or 1,3-diadamantyl urea (DAU), were found to be very potent inhibitors of sEH.13–17 However, such compounds have poor solubility in many solvents. To improve solubility, asymmetric ureas with a flexible side chain, such as AUDA (12-(3-adamantylureido)-dodecanoic acid), were developed. While this class of sEH inhibitor shows biological effects when tested in vivo, they are rapidly metabolized, limiting their utility.18, 19 Therefore, to improve the metabolic stability, a third class of conformationally restricted inhibitors, such as TPPU 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea31 or t-AUCB (trans-4-((4-(3-adamantylureido)-cyclohexyl)oxy)-benzoic acid)19, were designed. This latest series includes very potent and more metabolically stable sEH inhibitors that permit in vivo studies. However, many of these compounds have in general poor water solubility and have a high melting point, making formulation difficult. Finally, a promising candidate produced by GlaxoSmithKlein, GSK2256294, has gone through phase I trials and phase II vascular trial and trial for the treatment of chronic obstructive pulmonary disorder (COPD) have been announced.20
Interestingly, existing adamantyl sEH inhibitors contain an unsubstituted adamantane in their structure. Compounds containing substituents in the adamantane part have never been systematically tested as sEH inhibitors. Also compounds having spacers between adamantane part and urea group were tested sporadically. Herein, we are testing the hypothesis that alterations in the adamantane part could regulate metabolic stability and water solubility of such inhibitors while maintaining good potency toward the target enzyme.
2. Results and Discussion
2.1 Synthesis of isocyanates
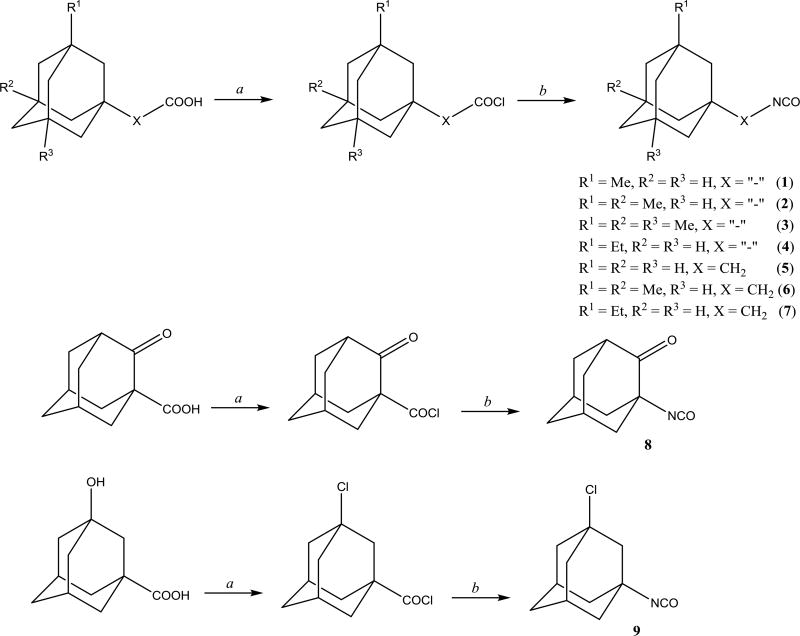
A convenient and efficient way used to synthesize unsymmetric ureas is the reaction of an isocyanate with an amine. Therefore, using synthetic scheme 1, we prepared a series of nine adamantyl containing isocyanates 1–9. The well-known Curtius rearrangement reaction was improved and applied to the corresponding acid chlorides.21 An improvement in the reaction led to the corresponding isocyanates through the domino reaction directly from their acid chlorides without isolation of the azide intermediates, which are potentially explosive.22 Adamantyl carboxylic acids with one to three methyl, ethyl or chlorine substituents in bridgehead positions as well as adamantyl acetic acids were chosen as starting materials for preparation of substituted adamantyl-isocyanates.
Scheme 1.
Synthesis of isocyanates 1–9. a. SOCl2, reflux, 1h; b. NaN3, toluene, acetonitrile, reflux, 2h.
2.2 Synthesis and evaluation of symmetrical diadamantyl ureas
The main route of in vivo metabolism of adamantyl containing sEH inhibitors is hydroxylation of the bridge and bridgehead positions of adamantane shell.23 Thus we hypothesized that introduction of substituents into the bridgehead positions of adamantane will help to directly protect the bridgehead position and increased polarity and steric hindrance will make it more difficult for P450’s to oxidize the bridge positions as well. Another benefit obtained from the substituents is increased solubility for oxygenated compounds and lower melting points. Adamantane melting point is 269.6–270.8 °C,24 1-methyladamantane – 100–101 °C,25 1,3-dimethyladamantane and 1,3,5-trimethyladamantane are liquid compounds with boiling points of 203.5 and 210.3 °C respectively.26 The same effect should be applied to the adamantane derivatives. Introduction of spacers between adamantane and functional groups also decreases melting points. For example, 1-isocyanatoadamantane melting point is 143–144 °C27 while 1-isocyanatomethyladamantane is a liquid compound. As predicted, compounds 1–7 appear as liquids and compound 9 has very low melting point of 29–30 °C.
It should be noted that introduction of HBDs or HBAs into adamantyl part of sEHI could lead to the significant loss of inhibitory activity. It was reported that bridgehead- and bridge-hydroxy analogs of 1-adamantan-1-yl-3-(5-(2-(2-ethoxyethoxy)ethoxy)pentyl)urea possess 62- and 34-fold lower activity.36 To improve water solubility of corresponding ureas compound 9, which contains a chlorine atom on a bridgehead position, was synthesized. Compound 9 has a lower clogP (1.53) compared 1-isocyanatoadamantane (2.99) and as it will be shown below, allows us to develop significantly more water soluble sEH inhibitors. With the new adamantyl containing isocyanates, we developed several series of disubstituted ureas, and diureas.
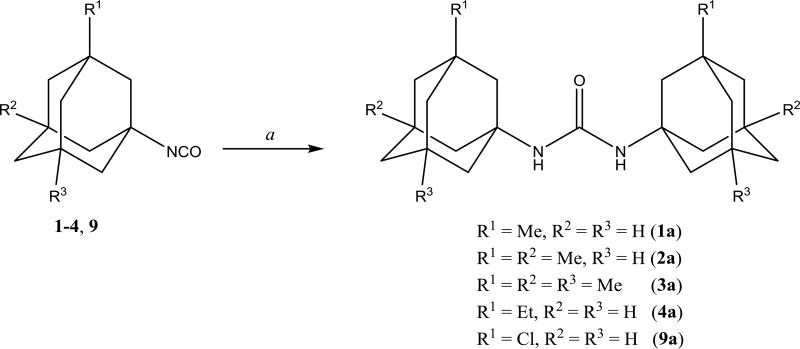
Symmetric ureas 1–4a, 9a were prepared (Scheme 2) as basic reference compounds. Symmetric ureas show high inhibitory activity and low water solubility (Table 1), and thus, such an impurity can noticeably affect properties of corresponding compounds.
Scheme 2.
Preparation of symmetric disubstituted ureas 1–4a, 9a. a. THF, DBU, 3h
Table 1.
IC50 values for adamantyl urea based sEH inhibitors 1–4a, 9a, 1–5b, 8b, 9b, 2c, 4c, 5c, 7b
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| # | R1 | R2 | R3 | Human sEH IC50a (nM) |
Solubilityb (µM) |
Mp (°C) |
| 1a | Me | H | H | 0.5 ±0.1 | 20 ±5 | 290–292 |
| 2a | Me | Me | H | 1.1 ±0.2 | 25 ±5 | 277–278 |
| 3a | Me | Me | Me | 1.6 ±0.2 | 20 ±5 | 305–306 |
| 4a | Et | H | H | 4.0 ±0.2 | 15 ±5 | 248–251 |
| 9a | Cl | H | H | 0.6 ±0.1 | 140 ±10 | 133–135 |
| DAUc | H | H | H | 1.128 | 7 ±328 | 31230 |
| |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| # | R1 | R2 | R3 | X | Human sEH IC50 a (nM) |
Solubilityb (µM) |
Mp (°C) |
| 1b | Me | H | H | - | 0.5 ±0.1 | 200 ±10 | 238–239 |
| 2b | Me | Me | H | - | 0.8 ±0.1 | 180 ±10 | 231–232 |
| 3b | Me | Me | Me | - | 1.7 ±0.3 | 160 ±10 | 260–262 |
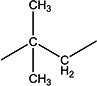
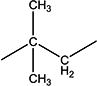
| 4b | Et | H | H | - | 1.8 ±0.1 | 50 ±10 | 218–219 |
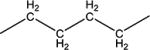
| 5b | H | H | H | -CH2- | 1.6 ±0.3 | 100 ±10 | 182–183 |
| 9b | Cl | H | H | - | 1.4 ±0.2 | 500 ±25 | 241–243 |
| 1471d | H | H | H | - | 2.019 | 160 ±20 | 250–255 |
|
| |||||||
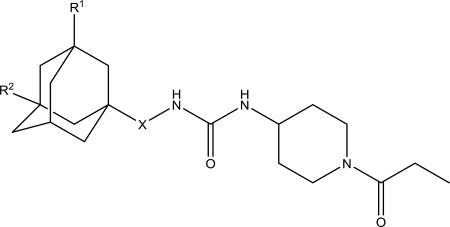
| 8b |
|
4.5 ±0.5 | 1000 ±50 | 252–254 | |||
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| # | R1 | R2 | X | Human she IC50a (nM) |
Solubilityb (µM) |
Mp (°C) |
| 2c | Me | Me | - | 3.3 ±0.3 | 210 ±10 | 181–182 |
| 4c | Et | H | - | 3.8 ±0.5 | 100 ±20 | 187–188 |
| 5c | H | H | -CH2- | 0.8 ±0.1 | 120 ±10 | 173–174 |
| 7b | Et | H | -CH2- | 1.8 ±0.2 | 150 ±20 | 169–170 |
| 1163e | H | H | - | 3.231 | 180 ±10 | 217–22131 |
|
| ||||||
| 1770f |
|
3.731 | 167 | 198–20131 | ||
As determined via a kinetic fluorescent assay. Results are means of three separate experiments.29
Solubilities were measured in sodium phosphate buffer (pH 7.4, 0.1 M) containing 1% of DMSO.
DAU – diadamantyl urea properties given for comparison.
1471– 4-((4-(3-(adamantan-1-yl)ureido)trans-cyclohexyl)oxy)benzoic acid properties given for comparison.
1163 – 1-(adamantan-1-yl)-3-(1-propionylpiperidin-4-yl)urea properties given for comparison.
1770 – 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea properties given for comparison.
Introduction of alkyl groups into bridgehead positions of adamantane surprisingly led to the 2–3 fold increase in water solubility of ureas 1–4a (Table 1). This could be explained by the decrease of intermolecular interaction between urea groups of such molecules due to the more bulky nature and less homogenous surface of substituted adamantane radicals. The difference in melting points corresponds to the difference between adamantane and its homologues. 1-Methyl substituent slightly lowers the melting point, a second methyl lowers it more and the melting point of an adamantane with three methyl substituents tends to rise. We found that inhibitory activity had no obvious correspondence to the physical properties, and for compound 1a the enzyme inhibition is 2-fold higher than basic diadamantyl urea (DAU, Table 1). Compound 2a and 3a have the same IC50 and 4a has a 4-fold lower activity. Introduction of ethyl group (4a, Table 1) if compared to adamantane with two methyl groups gives lower water solubility (which is still 2-fold better than DAU) and melting point along with 4-fold decrease in inhibitory activity on the recombinant human sEH.
Interestingly, introduction of chlorine into bridgehead position of adamantane compound 9a increased the activity (2-fold) but more importantly led to the more than 2-fold decrease in melting point and around 20-fold increase in water solubility. This makes isocyanate 9 a very promising reactant for the synthesis of urea-type sEH inhibitors.
2.3 Inhibitory activity of 1471 and 1163 homologues
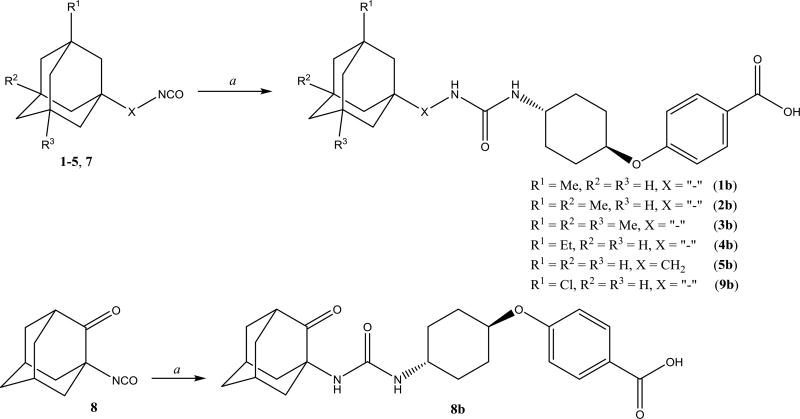
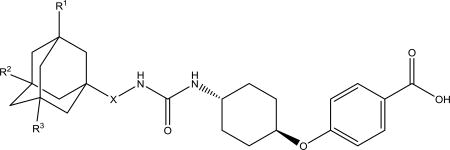
The next step was to investigate how adamantyl substituents affect the properties of known adamantyl containing sEH inhibitors. Compounds 1471 and 1163 were used for reference as two of the most promising among inhibitors bearing adamantane fragment and compound 1770 as their non-adamantane rference. trans-4-((4-aminocyclohexyl)oxy)benzoic acid was used as a starting amine to synthesize compounds 1–5b, 8b and 9b.
While, compared to the unsubstituted adamantane, the introduction of one and two methyl groups led to the slightly increased water solubility and decreased melting points, the presence of a third methyl substitution on the adamantane 3b yields physical properties similar to the ones of the unsubstituted compound (Table 1). Introduction of a methylene spacer between the adamantane and the urea group in the compound 5b gave the most significant decrease (70 °C) in melting point, probably due to the increased flexibility, accompanied with a 2-fold decrease in water solubility. Interestingly, compound 8b showed more than a 6-fold increase in water solubility while maintaining the same melting point. In terms of inhibitory potency, the same pattern is observed for the two series: 1471, 1–3b and DAU 1–3a studied (Table 1). Compounds with one methyl group yield the highest inhibitory potency (lowest IC50); the presence of a second methyl gives a 2-fold decrease in human sEH inhibitory activity yielding IC50s similar to the unsubstituted compounds, and the potency is decreased even more by the addition of a third methyl. Interestingly, the presence of the chloride atom yields a significantly higher water solubility (5–10-folds). Surprisingly, the analogs of compound 1163 (Table 1) did not follow the pattern described above (less difference in potency and water solubility). Compounds 2c and 4c possess human sEH inhibitory activity and water solubility similar to the 1163 but have moderately lower melting points. But compounds 5c and 7b differing only by the methylene spacer show 4-fold and 2-fold higher activity respectively. However, while existence of such spacer leads to certain melting point decrease it also decreases the water solubility up to 1.5-fold. Thus, introduction of methylene spacer between urea group and adamantane (or other bulky group) part could be used to increase sEH inhibitory activity for compounds which already possess moderate water solubility.
2.4 Synthesis and inhibitory activity of non-symmetric diadamantyl ureas
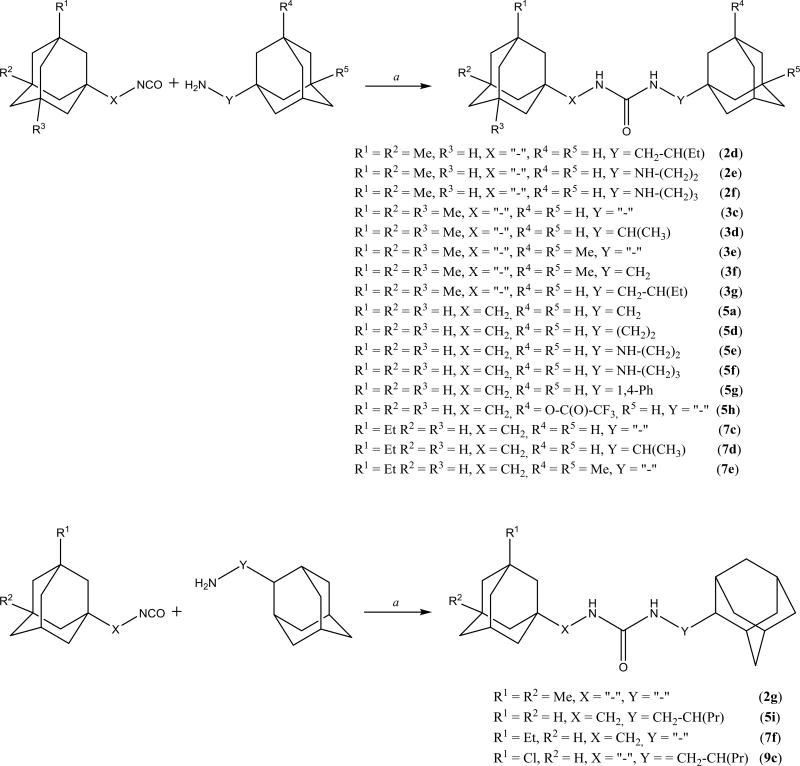
Next, to enlarge our dataset on the effects of substitution on the adamantane, a variety of non-symmetric diadamantyl ureas were synthesized (Scheme 5). Isocyanates 2–5, 7–9 were introduced into the reaction with adamantyl amine in order to synthesize this diverse series of ureas. In this series a 1,4-phenylene spacer between adamantane and urea group was used as well as spacers containing a secondary amino group (2e, 2f, 5e, 5f).
Scheme 5.
Preparation of non-symmetric diadamantyl disubstituted ureas. a. DMF, Et3N, 12h, double excess of Et3N used if initial amine used in form of hydrochloride.
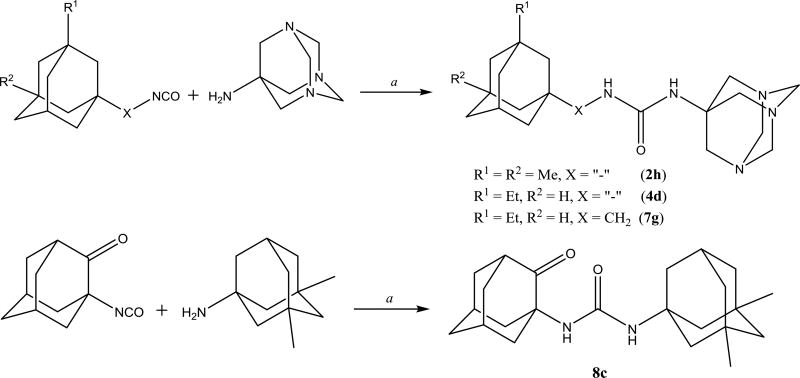
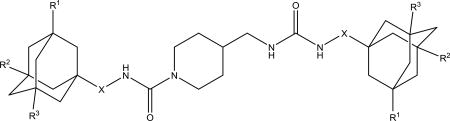
Compared to 2a or 5a, the introduction of spacers containing secondary amino groups (NH-(CH2)2 and NH-(CH2)3) in 2e, 2f, 5e and 5f leads to a marked decrease in activity accompanied by a significant increase in water solubility (Table 2). In the 5a series, replacement of one methylene spacer by 1,4-phenylene (5g) gives a roughly 2-fold lower inhibitory potency while maintaining melting point and water solubility. Further elongation of spacer from one to two methylene groups (5d) leads to further gain in potency and small decrease of melting point without a noticeable change in water solubility. Separately, introduction of larger branched spacers (2d, 3d, 3g, and 7d) usually led to a severe decrease in inhibitory activity and water solubility and increase in melting point when compared to the corresponding compounds with one methylene spacer. Attaching the urea on position 2 of the adamantane yielded compounds with higher melting point than the corresponding ureas at the 1-position. However, water solubility of both types of compounds is similar. As expected, changing one adamantane by 1,3,5-triazaadamantan-7-amine (2h, 4d and 7g) increased significantly the water solubility; however, it was accompanied by over 25-fold decrease in inhibitory activity rending such alteration impractical. Finally, compound 8c shows that adamantanone could be very promising alternative to adamantane since 8c possess high activity of 0.4 nM while having approximately 10-fold higher water solubility than most of diadamantyl ureas.
Table 2.
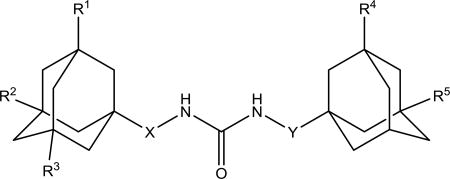
IC50 values for non-symmetric diadamantyl disubstituted ureas 2d-h, 3c-g, 4c, 5d-i, 7c-g, 8c, 9c
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| # | R1 | R2 | R3 | X | Y | R4 | R5 | Human sEH IC50a (nM) |
Solubilityb (µM) |
Mp (°C) |
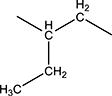
| 2d | Me | Me | H | - |
|
H | H | 13.8 | 40 ± 5 | 240–243 |
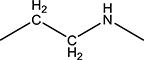
| 2e | Me | Me | H | - |
|
H | H | 119.9 | 210 ± 15 | 180–181 |
| 2f | Me | Me | H | - |
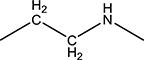
|
H | H | 79.6 | 1550 ± 50 | 174–175 |
| 3c | Me | Me | Me | -- | - | H | H | 6.1 | 35 ± 5 | 320–323 |
| 3d | Me | Me | Me | - |
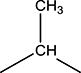
|
H | H | 25.4 | 12 ± 2 | 229–231 |
| 3e | Me | Me | Me | -- | - | Me | Me | 160.0 | 25 ± 5 | 304–305 |
| 3f | Me | Me | Me | - | -CH2- | Me | Me | 2.8 | 25 ± 5 | 287–288 |
| 3g | Me | Me | Me | - |
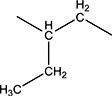
|
H | H | 22.9 | 20 ± 5 | 290–293 |
| 5a | H | H | H | -CH2- | -CH2- | H | H | 5.4 | 50 ± 5 | 137–139 |
| 5d | H | H | H | -CH2- |
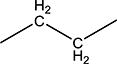
|
H | H | 4.4 | 45 ± 5 | 121–123 |
| 5e | H | H | H | -CH2- |
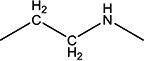
|
H | H | 28.5 | 375 ± 25 | 194–196 |
| 5f | H | H | H | -CH2- |
|
H | H | 17.2 | 2050 ± 50 | 184–187 |
| 5g | H | H | H | -CH2- |
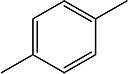
|
H | H | 12.2 | 50 ± 10 | 128–130 |
| 5h | H | H | H | -CH2- | - |
|
H | 0.4 | 50 ± 10 | 168–170 |
| 7c | Et | H | H | -CH2- | - | H | H | 2.4 | 12 ± 3 | 160–162 |
| 7d | Et | H | H | -CH2- |
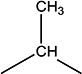
|
H | H | 12.8 | 17 ± 3 | 151–154 |
| 7e | Et | H | H | -CH2- | - | Me | Me | 1.5 | 8 ± 2 | 164–166 |
| |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| # | R1 | R2 | X | Y | Human sEH IC50a (nM) |
Solubilityb (µM) |
Mp (°C) |
| 2g | Me | Me | - | - | 9.6 | 20 ± 5 | 266–268 |
| 5i | H | H | -CH2- |
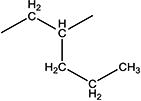
|
1992 | 60 ± 10 | 196–199 |
| 7f | Et | H | -CH2- | - | 2.7 | 16 ± 2 | 150–153 |
| 9c | Cl | H | - |
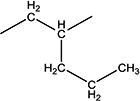
|
3.1 | 45 ± 5 | 163–164 |
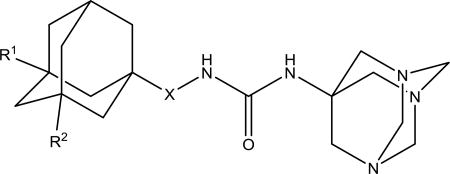
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| # | R1 | R2 | X | Human sEH IC50a (nM) |
Solubilityb (µM) |
Mp (°C) |
| 2h | Me | Me | - | 2894 | 110 ± 15 | 162–164 |
| 4d | Et | H | - | 268.6 | 190 ± 15 | 172–175 |
| 7g | Et | H | -CH2- | 60.6 | 110 ± 15 | 131–132 |
|
| ||||||
| 8c |
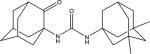
|
0.4 | 235 ± 15 | 202–204 | ||
As determined via a kinetic fluorescent assay. Results are means of three separate experiments. 29
Solubilities were measured in sodium phosphate buffer (pH 7.4, 0.1 M) containing 1% of DMSO.
2.5 Inhibitory activity of diadamantyl diureas
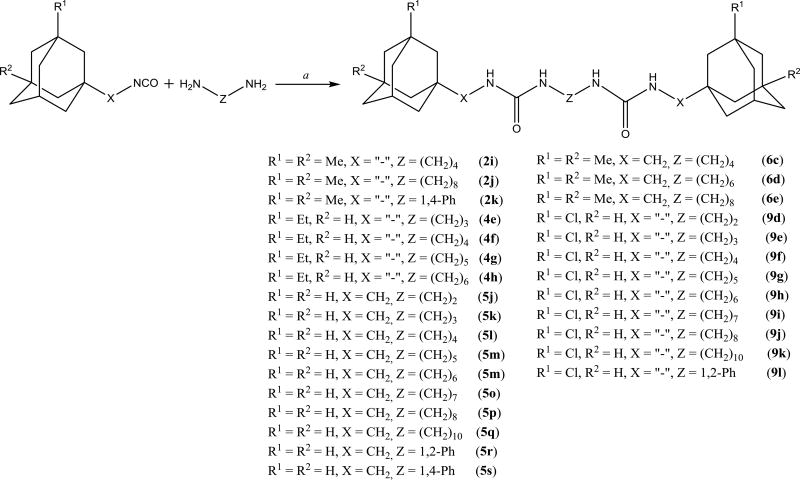
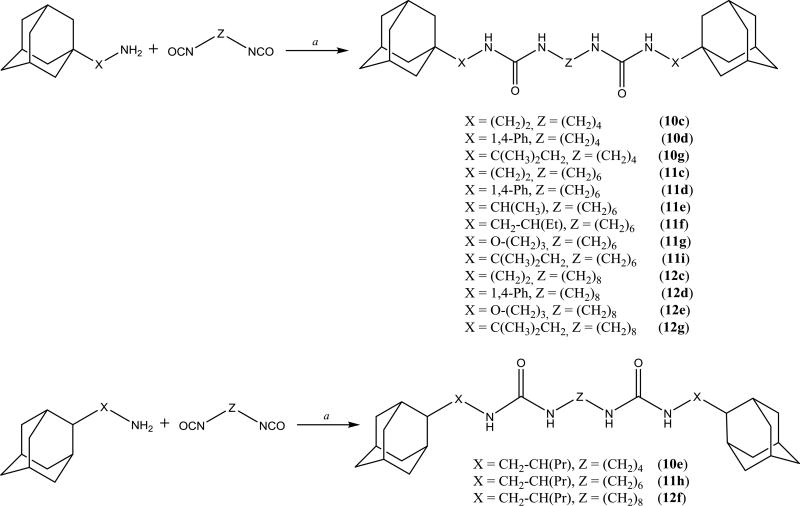
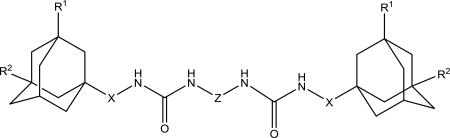
It was previously shown32 that symmetric diadamantyl diureas are potent sEH inhibitors with low metabolic stability and higher water solubility than corresponding symmetric diadamantyl ureas. High inhibitory activity of diureas was thought to result from additional hydrogen bond formation between oxygen atom of the second urea group and Ser 374 in sEH active site pocket.32 This assumption was later investigated by the thorough docking of various diureas.33 Another possibility is the presence of two reaction centers in one symmetric molecule which removes the need of proper orientation prior to the entering of sEH domain. In this case it is interesting to test the effect of substituents in adamantane, new spacers between adamantane and urea group and new linkers between urea groups on the properties of corresponding diureas. Two approaches were used to synthesize diadamantyl diureas. First approach (Scheme 6) is based on the reaction of adamantyl isocyanates 2, 4–6, 9 with diamines while the second (Scheme 7) is the opposite with adamantyl amines reacting with diisocyanates.
Scheme 6.
Preparation of symmetric diadamantyl disubstituted diureas. a. DMF, Et3N, 12h.
Scheme 7.
Preparation of symmetric diadamantyl disubstituted diureas. a. DMF, Et3N, 12h.
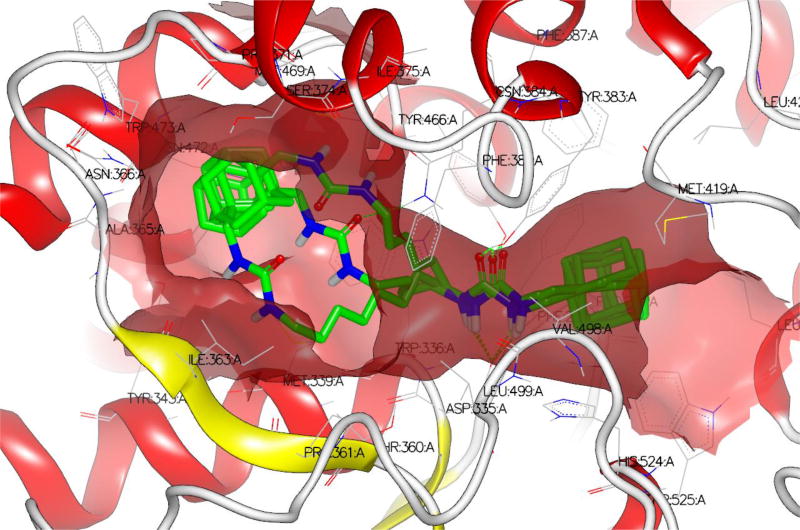
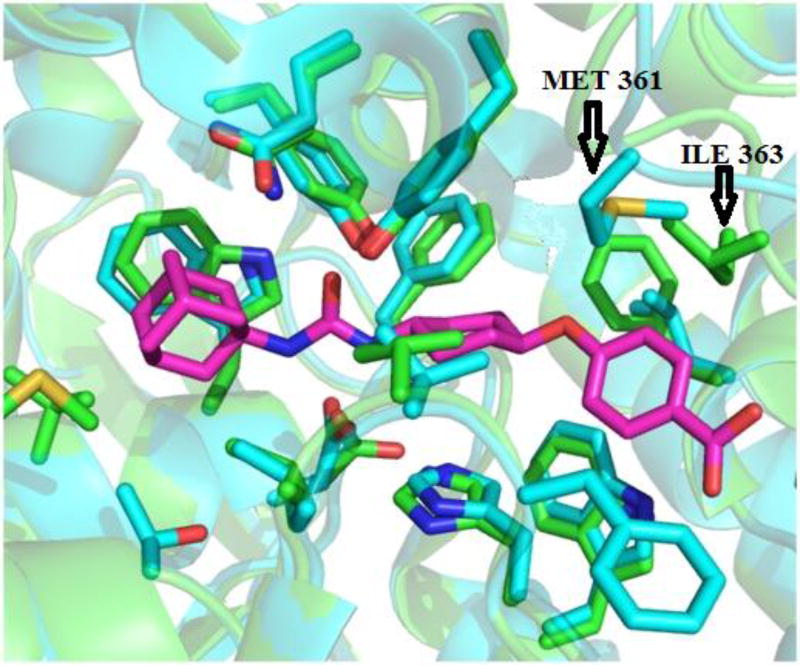
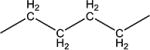
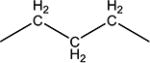
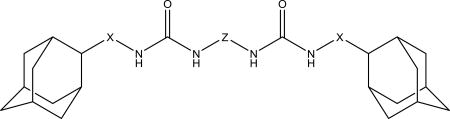
We decided to find if there is a minimal length of the linker between two urea groups which allows them to reach Ser 374 or CSN 384 and thus increase the activity. To optimize the length of the linker, compounds with 2 to 10 methylene groups without branching 5j–q and 9d–k were prepared. All of these compounds possess approximately the same IC50 except of compound 5j and 9d, which are much less active, suggesting that three methylene groups are the minimum for the best interaction with sEH domain. Docking of compounds 5l, 5n and 5p shows that there is enough space at the sEH domain even for C8 aliphatic chain between two urea groups (Fig. 1).
Figure 1.
Docking of compounds 5l, 5n and 5p shows the space capacity of sEH domain.
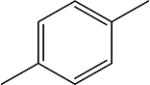
Spacers between adamantane and the urea group in diureas usually follow the same pattern as observed with simple ureas (DAU and 5a, 3e and 3f), a methylene spacer (2i 5l, 5n, 5p, 6c) leads to an increase in inhibitory activity (Table 3) accompanied by the better water solubility and lower (around 20–30°C) melting points which probably resulted from increased flexibility around the adamantane. Further increase of spacer to 1,2-ethylenes in compounds 10–12c results in a 2-fold decrease of activity while maintaining water solubility and melting points on the same level. Separately, a phenylene group introduced either between adamantane and urea group or between two urea groups (Table 3) leads to the significant decrease in human sEH inhibitory activity. In case of compounds 2k, 5r, 5s, 9l low activity could be the result of rotation restrictions. As for compounds 10–12d it could be attributed by the lack of space in the hydrophobic pocket of sEH and 6-fold increase of activity along with the extension of linker from four methylene groups (10d) to eight (12d) is a result of better ability of this diurea to bind to the Ser 374. Diureas 10e, 11h and 12f represent the biggest branched aliphatic spacer between adamantane and urea group. This 1,2-pentylene spacer apparently is too big or poorly orientated to fit into the sEH pocket. Compound 11f with a 1,2-butylene spacer has good inhibitory activity suggesting that it is the biggest branched spacer which allows to maintain a high level inhibitory activity. Finally, introduction of oxygen containing spacer –O–CH2–CH2–CH2– in compounds 11g and 12e although it leads to IC50 < 10 nM it have no positive impact on water solubility.
Table 3.
IC50 values for symmetric diadamantyl disubstituted diureas
| |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| # | R1 | R2 | X | Z | Human sEH IC50a (nM) |
Solubilityb (µM) |
Mp (°C) |
| 2i | Me | Me | - |
|
23.4 | n/a | 251–252 |
| 2j | Me | Me | - |
|
3.7 | n/a | 180–181 |
| 2k | Me | Me | - |
|
2475 | n/a | 327–329 |
| 4e | Et | H | - |
|
1.7 | 10 ± 2 | 243–244 |
| 4f | Et | H | - |
|
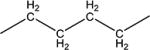
1.1 | 10 ± 2 | 260–262 |
| 4g | Et | H | - |
|
1.2 | 6 ± 1 | 258–259 |
| 4h | Et | H | - |
|
8.5 | 6 ± 1 | 247–249 |
| 5j | H | H | -CH2- |
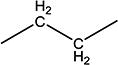
|
179.2 | 90 ± 10 | 211–212 |
| 5k | H | H | -CH2- |
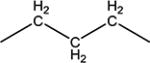
|
0.9 | 90 ± 10 | 225–226 |
| 5l | H | H | -CH2- |
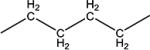
|
0.9 | 80 ± 10 | 232–234 |
| 5m | H | H | -CH2- |
|
0.9 | 75 ± 5 | 243–245 |
| 5n | H | H | -CH2- |
|
0.4 | 65 ± 5 | 218–219 |
| 5o | H | H | -CH2- |
|
0.6 | 70 ± 10 | 165–166 |
| 5p | H | H | -CH2- |
|
0.7 | 55 ± 10 | 163–164 |
| 5q | H | H | -CH2- |
|
1.2 | 45 ± 5 | 154–155 |
| 5r | H | H | -CH2- |
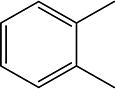
|
1364 | 45 ± 5 | 249–250 |
| 5s | H | H | -CH2- |
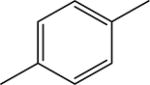
|
779 | 50 ± 5 | 239–240 |
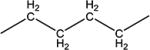
| 6c | Me | Me | -CH2- |
|
0.7 | 90 ± 5 | 244–246 |
| 6d | Me | Me | -CH2- |
|
1.0 | 75 ± 5 | 225–227 |
| 6e | Me | Me | -CH2- |
|
2.5 | 55 ± 5 | 188–190 |
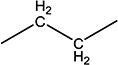
| 9d | Cl | H | - |
|
144.5 | 55 ± 5 | 204–205 |
| 9e | Cl | H | - |

|
1.7 | 75 ± 5 | 184–186 |
| 9f | Cl | H | - |
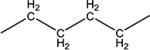
|
1.3 | 55 ± 5 | 192–193 |
| 9g | Cl | H | - |
|
1.8 | 60 ± 5 | 116–117 |
| 9h | Cl | H | - |
|
1.6 | 45 ± 5 | 113–115 |
| 9i | Cl | H | - |
|
1.4 | 75 ± 5 | 114–116 |
| 9j | Cl | H | - |
|
1.2 | 75 ± 5 | 110–112 |
| 9k | Cl | H | - |
|
0.9 | 75 ± 5 | 108–109 |
| 9l | Cl | H | - |
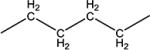
|
408.6 | 65 ± 5 | 186–188 |
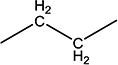
| 10c | H | H |
|
|
3.4 | 100 ± 10 | 229–230 |
| 11c | H | H |
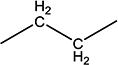
|
|
0.8 | 80 ± 10 | 193–195 |
| 12c | H | H |
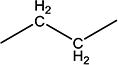
|
|
1.5 | 75 ± 5 | 167–168 |
| 10d | H | H |
|
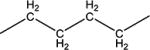
|
1792 | 75 ± 5 | 158–160 |
| 11d | H | H |

|
|
529.3 | 80 ± 5 | 183–185 |
| 12d | H | H |
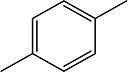
|
|
259.1 | 75 ± 5 | 167–168 |
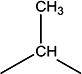
| 11e | H | H |
|
|
0.5 | 75 ± 5 | 164–165 |
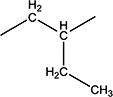
| 11f | H | H |
|
|
2.8 | 70 ± 5 | 175–176 |
| 11g | H | H |
|
|
9.4 | 45 ± 5 | 139–140 |
| 12e | H | H |
|
|
2.7 | 35 ± 5 | 224–225 |
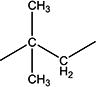
| 10g | H | H |
|
|
1.9 | n/a | 145–147 |
| 11i | H | H |
|
|
4.3 | n/a | 115–117 |
| 12g | H | H |
|
|
6.7 | n/a | 124–125 |
| |||||
|---|---|---|---|---|---|
|
| |||||
| # | X | Z | Human she IC50a (nM) |
Solubilityb (µM) |
Mp (°C) |
| 10e |
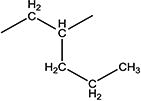
|
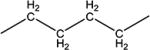
|
3299 | 55 ± 5 | 134–136 |
| 11h |
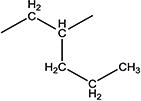
|
|
35000 | 75 ± 5 | 65–67 |
| 12f |
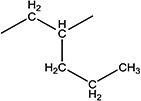
|
|
>100000 | 45 ± 5 | 74–75 |
As determined via a kinetic fluorescent assay. Results are means of three separate experiments.29
Solubilities were measured in sodium phosphate buffer (pH 7.4, 0.1 M) containing 1% of DMSO.
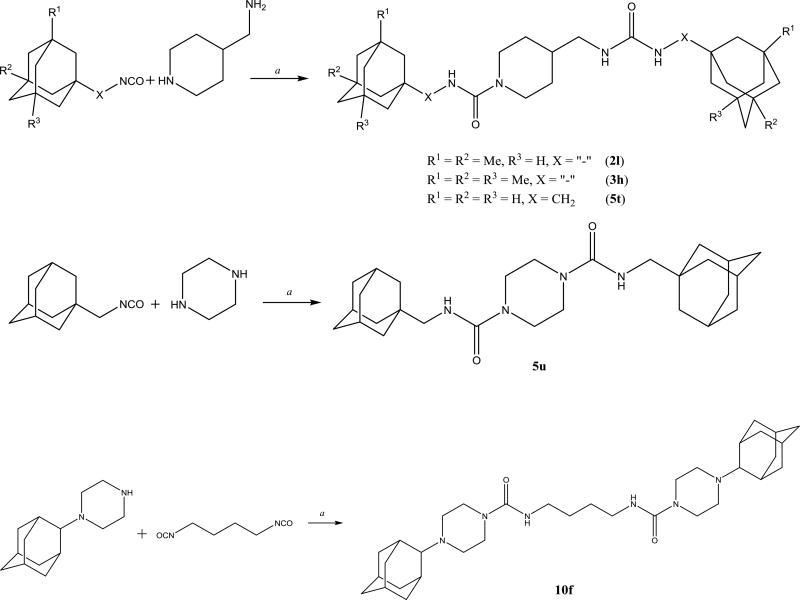
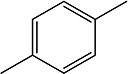
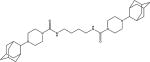
To obtain additional data on the benefit of substitution on the adamantane but away from the urea center, a series of compound containing 1,3,3-trisubstituted urea groups were developed (Scheme 8). Compounds 2l, 3h and 5t contain one disubstituted and one trisubstituted urea groups while compounds 5u and 10f have two trisubstituted urea groups.
Scheme 8.
Preparation of compounds bearing trisubstituted urea group. a. DMF, Et3N, 12h.
Compounds 2l, 3h and 5t have good inhibition potency due to the presence of one disubstituted urea group in its structure however their melting points are very high. The lower activity of 5u and 10f probably resulted from a less than optimal orientation in the active pocket.
2.6 Comparison of activity against human, murine and rat sEH
While human health application is the ultimate objective of this work, research work is conducted first in rodent animal models. Thus, to test if our observations above with the human sEH could extend to sEH enzymes from other species, a series of 18 compounds which could visualize the impact of methyl substituents in adamantane were selected and tested against the human, rat and mouse sEHs (Table 5). Compounds 10–12c were also tested as mouse sEH inhibitors to test the effect of additional methylene groups in linkers
Table 5.
Comparison of potency against Human, Rat and Mouse sEH.
| # | Structure | sEH IC50 (nM)a
|
||
|---|---|---|---|---|
| Human | Rat | Mouse | ||
| 10c |
|
3.4 | 6.0 | 21.4 |
| 11c |
|
0.8 | 2.1 | 5.8 |
| 5m |
|
0.9 | 2.8 | 5.0 |
| 6c |
|
0.7 | 2.8 | 11.0 |
| 9f |
|
1.3 | 3.5 | 5.8 |
| 1b |
|
0.5 | 1.6 | 2.0 |
| 2b |
|
0.8 | 2.7 | 3.8 |
| 3b |
|
1.7 | 11.0 | 34.5 |
| 1a |
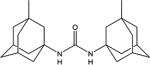
|
0.5 | 3.1 | 5.5 |
| 2c |
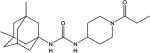
|
3.3 | 1.8 | 5.2 |
| 3a |
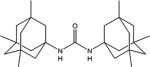
|
1.6 | 25.3 | 66.2 |
| 9a |
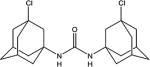
|
0.6 | 1.9 | 2.1 |
| 3c |
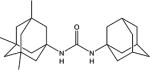
|
6.1 | 5.1 | 106.9 |
| 3e |
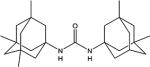
|
160.0 | 97.1 | 11376 |
| 3h |
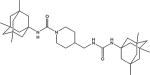
|
6.5 | 24.9 | 436 |
As determined via a kinetic fluorescent assay. Results are means of three separate experiments. 29
Using the Spearman’s ranking coefficient calculation, we observed a reasonable similarity in the pattern of inhibition between the human and the rat sEH (rho = 0.69) as well as with the mouse sEH (rho = 0.81). It has been reported that steric parameters have stronger effects on the potency of inhibitors against murine sEH rather than the human sEH.34 Beside comparable ranking, a similar steric effect was observed here, as the number of substitutions increased the effect on decreasing the inhibitory potency against the murine sEH was much stronger than against the human and rat sEH.
The significant difference in potency between the human and mouse sEH for compounds with trimethyladamantane moiety revealed by the fluorescent assay was confirmed by radioactive assay with [3H]trans-diphenyl-propene oxide (t-DPPO) as a substrate.35 This completely different assay shows the same differences in potency against human, mouse and rat enzymes. This gives us reason to believe that all other IC50 acquired by fluorescent method are reliable.
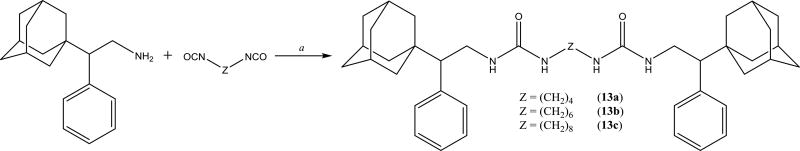
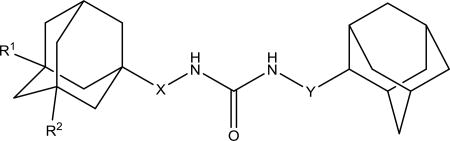
To understand the difference of selectivity between the human and murine enzyme, we compared the protein structures (Figure 2). When overlying the C-terminal active site of both enzymes, the only significant difference is that ILE 363 in the human sEH is substituted by MET 361 in the mouse sEH, which perturbs inside the catalytic tunnel and limits the size of the ligand that can fit into the murine enzyme.33 In addition, if the catalytic site of the sEH is a true tunnel, this residue can stop a ligand too big to reach catalytic residues. To test this later hypothesis, we used the available crystal structures to measure the diameter of the catalytic tunnel. Then, compounds 13a-c bearing terminal substituents bigger than sEH tunnel size evaluated by the X-Ray crystallography were synthesized (Scheme 9).
Figure 2.
Compound 1471 (red) docked into human (cyan) and mouse (green) sEH.
Scheme 9.
a. DMF, Et3N, 12h
If the sEH binding site is a stable tunnel, such compounds would be unable to get into it and its IC50 should be extremely high. However, IC50s for compounds 13a-c are below 1 μM for human and rat and below 4 μM for mouse sEH (Table 7). The fact that compounds with such large terminal groups possess such good inhibition potency, for example 13b has an IC50 < 100 nM human sEH, supports the ability of sEH to open in active site to capture the molecule of substrate or inhibitor.
Table 7.
Human, rat and mouse sEH IC50 for compounds 13a–c.
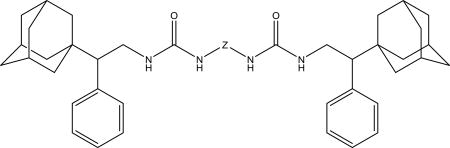
| ||||
|---|---|---|---|---|
|
| ||||
| # | Z | sEH IC50 (nM)a
|
||
| Human | Rat | Mouse | ||
| 13a |
|
605.0 | 951.3 | 3359.8 |
| 13b |
|
91.5 | 1014.0 | 1785.0 |
| 13c |
|
205.0 | 755.4 | 1866.1 |
As determined via a kinetic fluorescent assay. Results are means of three separate experiments. 29
2.7 Metabolic stability study
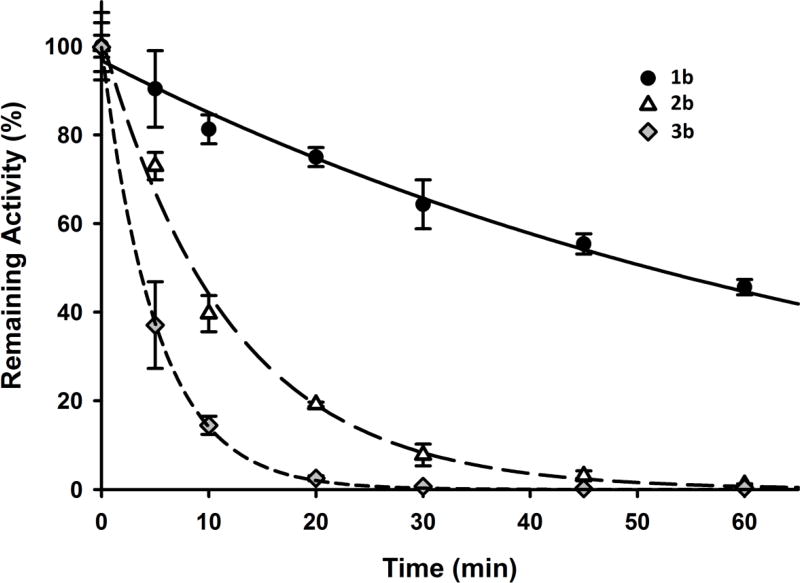
Finally, we tested the effect of methyl substituents in bridgehead positions of adamantane (1b, 2b and 3b) on metabolic stability (Figure 3). The intrinsic clearance was then calculated and compared to the unsubstituted compound 1471 (Table 8).
Figure 3.
Two and three methyl substituted 2b and 3b almost fully cleared after 60 min.
Table 8.
Metabolic stability of sEH inhibitors with substituents in bridgehead positions of adamantane against human microsomes (HLM).
| # | Amount remaining after 30 minutes in contact with HLM (%) |
T½ (min) | Clint* (µL.min−1.mg−1) |
|
|---|---|---|---|---|
| − NADPH | + NADPH | |||
| 147136 | 97 ± 8 | 69 ± 2 | 56 | 12 |
| 1b | 95 ± 3 | 64 ± 6 | 54 ± 3 | 13 ± 1 |
| 2b | 100 ± 3 | 8 ± 2 | 8 ± 1 | 84 ± 6 |
| 3b | 95 ± 4 | 0.7 ± 0.2 | 3.5 ± 0.3 | 196 ± 2 |
Intrinsic clearance
Because hydroxylation of bridge and bridgehead positions in adamantane is the main P450 dependent metabolism,36 and because bridgehead positions are also the most sensitive for the chemical reactivity, one would expect that the introduction of methyl groups into bridgehead position should protect the inhibitor from oxidation and reactivity. Surprisingly, the results obtained (Table 8) were the inverse of our initial assumption. There is almost no difference between 1471 and compound 1b bearing one methyl group. Introduction of a second methyl groups leads to 8-fold decrease of stability for compound 2b. Finally compound 3b in which all of the bridgehead carbons are tertiary and linked with methyl substituents is 98-fold less stable than parental 1471 (Figure 3). These results suggest a particular sensibility of the bridgehead methyl to enzymatic oxidation. This agrees with the metabolism of memantine (1-amino-3,5-dimethyladamantane),38 for which the main metabolic pathway is the hydroxylation of adamantane cage and methyl substituents while the amino group remains intact. Not only are the methyl groups likely to be good sites for hydroxylation, but in general as a compound overall becomes more hydrophobic its sensitivity to oxidation by cytochrome P450 enzyme increases.
3. Conclusions
In conclusion, substituted adamantyl ureas were synthesized via new isocyanates. Using sEH as a model system, we observed that the substitutions influence greatly the potency of the resulting ureas and diureas, as well as physical properties and metabolic stability. Interestingly, the best compromise is the presence of only one substitution, with either a methyl or chloride, on the bridgehead of the adamantane. In addition, the presence of a methylene between the adamantane and the urea function has positive effect on potency and physical properties. These results have implication for the design of further sEH inhibitors but also toward other targets because of the ubiquitous use of adamantane in drug design.
4. Experimental
4.1 Chemistry
4.1.1 General experimental details
All reagents and solvent were purchased from commercial suppliers and were used directly without further purifications unless otherwise specified. All syntheses were carried out in a dry nitrogen atmosphere unless otherwise specified.
1H NMR spectra were recorded on a Bruker DRX-500 spectrometer with deuterated chloroform (CDCl3; δ = 7.24 ppm) or deuterated dimethyl sulfoxide (DMSO-d6) containing TMS an internal standard. 13C NMR spectra were recorded on a Bruker DRX-500 spectrometer at 125 MHz. Mass spectra were registered on Agilent GC 5975/MSD 7820 or Finnigan MAT INCOS 50. The purity of the inhibitors reported in this manuscript was determined by elemental analysis using Perkin-Elmer Series II 2400.
4.1.2 General Procedure for the synthesis of isocyanates 1–9
First step. To 1 equiv. of corresponding carboxylic acid was added 1.2 equiv. of thionyl chloride at room temperature. The reaction mass was refluxed for 1.5 h. After cooling, thionyl chloride was removed in vacuo. The residue was weighted and dissolved in anhydrous toluene and was used in the next step without further purification. Second step. Solution of acid chloride from first step was added dropwise for 1 h to the suspension of 1.1 equiv. of sodium azide in 20 equiv. of anhydrous toluene. The reaction mass was refluxed for 1 h. After cooling, reaction mass was filtered through the sodium chloride and toluene was removed in vacuo. The residue was purified by distillation in vacuo or crystallization from ethanol.
4.1.3 General Procedure for the synthesis of symmetric ureas 1–4a and 9a
To 1 equiv. of corresponding isocyanate in 40 equiv. of THF was added 0.05 equiv. of 1,8-Diazabicyclo[5.4.0]undec-7-ene at room temperature. Reaction mass was stirred at room temperature overnight. After adding 1N HCl and water, the resulting white precipitates were collected by suction filtration.
4.1.4 General Procedure for the synthesis of ureas 1–5b, 8b and 9b
To 1 equiv. of corresponding isocyanate in 40 equiv. of DMF was added 1.5 equiv. of trans-4-((4-aminocyclohexyl)oxy)benzoic acid and 1.5 equiv. of Et3N at 0 °C. Reaction mass was stirred at room temperature overnight. After adding 1N HCl and water, the resulting white precipitates were collected by suction filtration. Other ureas obtained by the methods similar to the above described methods and described in detail in supplementary materials.
4.2 Determination of inhibitory potency (IC50)
The IC50 values reported herein were determined using either a fluorescent based assay28 or a radioactive based assay.34
Fluorescent assay
Enzymes (~2nM mouse sEH, ~1nM rat sEH, or ~1 nM human sEH) were incubated at 30 °C with inhibitors ([I]final = 0.4 – 100,000 nM) for 5 min in 100 mM sodium phosphate buffer (200 µL, pH 7.4) containing 0.1 mg/mL of BSA and 1% of DMSO. The substrate (cyano(2-methoxynaphthalen-6-yl)methyl trans-(3-phenyloxyran-2-yl)methylcarbonate, CMNPC) was then added ([S]final = 5 µM). Activity was assessed by measuring the appearance of the fluorescent 6-methoxynaphthaldehyde product (λem = 330 nm, λex = 465 nm) at 30 °C during a 10 min incubation (Spectramax M2; Molecular Device, Inc., Sunnyvale, CA). The IC50 values that are the concentrations of inhibitors that reduce activity by 50% were calculated from at least five different concentrations, each in triplicate, with at least 2 on either side of 50% activity mark.
Radioactive assay
Enzymes (2 nM mouse sEH or 3 nM human sEH 5nM rat sEH) were incubated with inhibitors ([I]final = 1 – 100,000 nM) at 30° C for 5 min in sodium phosphate buffer (pH 7.4) containing 0.1 mg/mL of BSA and 1% of DMSO, prior to substrate introduction ([S]final: 50 µM; ~12,000 dpm/assay). The enzymes were incubated at 30°C for 10 min, and the reaction was quenched by the addition of 60 µL methanol and 200 µL isooctane, which extracts the remaining epoxide from the aqueous phase. The activity was followed by measuring the quantity of radioactive diol formed in the aqueous phase using a liquid scintillation counter (Tri-Carb 2810TR, Perkin Elmer, Waltham, MA). Under the conditions used, rates were linear with both time and enzyme concentration and resulted in at least 5% but not more than 30% hydrolysis of the substrate. Assays were performed in triplicate. The IC50 was determined by regression of at least five data points with a minimum of two points in the linear region of the curve on either side of the IC50.
4.3 Microsomal stability
The stability of the sEH inhibitors was determined using human liver microsomes as described.9 The inhibitors ([I]final = 1 uM) to a suspension of human liver microsomes ([E]final= 1 mg/mL) in potassium phosphate buffer (0.1M pH7.4) containing 3mM MgCl2 and 1 mM EDTA. After 5 minutes incubation at 37 °C, the reaction was started by the addition of NADPH generating system (buffer was added in the control tubes). After 30 minutes at 37 °C, the reaction was stopped by the addition of one volume of methanol containing CUDA (200 nM) as surrogate. The amount of remaining inhibitors were determine by LC/MS/MS. The stability is reported as a percentage of compound remaining after 30 minutes in these conditions. Results are triplicate average.
Mass spectrometry analysis were performed using a Waters Quattro Premier triple quadrupole tandem mass spectrometer (Micromass, Manchester, UK) interfaced to an electrospray ionization (ESI) source. The MS was coupled with a Waters Acquity UPLC (Waters, Milford, MA, USA). A Varian Pursuit5 C18 RP HPLC column (150 mm × 2.1 mm, particle size 5 µm) was used to separate the analytes. The ESI was performed following HPLC in the positive mode at 2.51 kV capillary voltage. The source and the desolvation temperatures were set at 120 and 300°C, respectively. Cone gas (N2) and desolvation gas (N2) were maintained at flow rates of 10 and 700 L/h, respectively. Dwell time was set to 0.1 s. A regression curve for each compound was obtained from at least six different concentrations of standard stock solutions (R2 > 0.99).
Supplementary Material
Scheme 3.
Preparation of disubstituted ureas 1–5b, 8b, 9b. a. 4-((4-aminocyclohexyl)oxy) benzoic acid, DMF, Et3N, 12h.
Scheme 4.
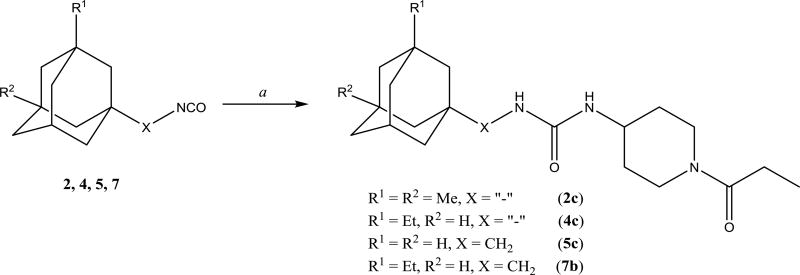
Preparation of disubstituted ureas 2c, 4c, 5c, 7b. a. 1-(4-aminopiperidin-1-yl)propan-1-one, DMF, Et3N, 12h.
Table 4.
IC50 values for compounds 2l, 3h, 5t, 5u, 10f bearing trisubstituted urea groups
| |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| # | R1 | R2 | R3 | X | Human sEH IC50a (nM) |
Solubilityb (µM) |
Mp (°C) |
| 2l | Me | Me | H | - | 1.9 | 30 ± 5 | 232–234 |
| 3h | Me | Me | Me | - | 6.5 | 20 ± 5 | 265–268 |
| 5t | H | H | H | -CH2- | 39.7 | 15 ± 5 | 127–128 |
|
| |||||||
| 5u |
|
2006 | 90 ± 10 | 275–276 | |||
|
| |||||||
| 10f |
|
16800 | 25 ± 5 | 195–197 | |||
As determined via a kinetic fluorescent assay. Results are means of three separate experiments. 29
Solubilities were measured in sodium phosphate buffer (pH 7.4, 0.1 M) containing 1% of DMSO.
Table 6.
Difference between human and mouse sEH potency for compounds 3b, 3c, 3e determined by fluorescent and radioactive assays.
| # | Human sEH IC50 | Rat sEH IC50 | Mouse sEH IC50 | |||
|---|---|---|---|---|---|---|
| Fluorescent | Radioactive | Fluorescent | Radioactive | Fluorescent | Radioactive | |
| 3b | 1.7 | 8.8 | 11.0 | 28.1 | 34.5 | 363.0 |
| 3c | 6.1 | 122.0 | 5.1 | 16.2 | 106.9 | 597.7 |
| 3e | 160.0 | 5930 | 97.1 | 180.5 | 11379 | >10000 |
IC50 values are dependent of the assay conditions. Because the substrate concentration in the radioactive assay ([S] = 50 µM) is 10-fold higher than in the fluorescent assay ([S] = 5 µM), one could expect that the inhibition potency will be larger with the radioactive assay than the fluorescent one.
Ureas with alteration in adamantane fragment were systematically studied as sEH inhibitors.
94 adamantyl ureas with substituents in bridge and bridgehead positions were synthesized.
Difference of activity against human, rat and murine sEH was investigated.
Dependence of substituents number in adamantane on metabolic stability was studied.
Acknowledgments
This work was partially supported by Russian Foundation for Basic Research (project no. 16-43-340116 r_a) and by the Ministry of Education and Science of the Russian Federation (base part of state assignment for 2017–2019; project no. 4.7491.2017/BCh), National Institute of Environmental Health Sciences (NIEHS) grant R01 ES002710, and NIEHS Superfund Research Program grant P42 ES004699.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data containing detailed NMR spectra on synthesized compounds can be found in online version.
References
- 1.Arand M, Grant DF, Beetham JK, Friedberg T, Oesch F, Hammock BD. Sequence similarity of mammalian epoxide hydrolases to the bacterial haloalkane dehalogenase and other related proteins. Implication for the potential catalytic mechanism of enzymatic epoxide hydrolysis. FEBS Lett. 1994;338:251–256. doi: 10.1016/0014-5793(94)80278-5. [DOI] [PubMed] [Google Scholar]
- 2.Oesch F. Mammalian epoxide hydrases: inducible enzymes catalysing the inactivation of carcinogenic and cytotoxic metabolites derived from aromatic and olefinic compounds. Xenobiotica. 1973;3:305–340. doi: 10.3109/00498257309151525. [DOI] [PubMed] [Google Scholar]
- 3.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 5.Imig JD. Epoxyeicosatrienoic acids, 20-hydroxyeicosatetraenoic acid, and renal microvascular function. Prostaglandins Other Lipid Mediat. 2013;104–105:2–7. doi: 10.1016/j.prostaglandins.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imig JD. Eicosanoids and renal damage in cardiometabolic syndrome. Expert Opin Drug Metab Toxicol. 2008;4:165–174. doi: 10.1517/17425255.4.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 8.Panigrahy D, Greene ER, Pozzi A, Wang DW, Zeldin DC. EET signaling in cancer. Cancer Metastasis Rev. 2011;30:525–540. doi: 10.1007/s10555-011-9315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rand AA, Barnych B, Morisseau C, Cajka T, Lee KSS, Panigrahy D, Hammock BD. Cyclooxygenase-derived proangiogenic metabolites of epoxyeicosatrienoic acids. Proc Natl Acad Sci USA. 2017;114:4370–4375. doi: 10.1073/pnas.1616893114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiecker M, Liao JK. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch Biochem Biophys. 2005;433:413–420. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Imig JD, Zhao X, Capdevilla JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 12.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46:975–981. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, Sanborn JR, Hammock BD. Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci USA. 1999;96:8849–8854. doi: 10.1073/pnas.96.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McElroy NR, Jurs PC, Morisseau C, Hammock BD. QSAR and classification of murine and human soluble epoxide hydrolase inhibition by urea-like compounds. J Med Chem. 2003;46:1066–1080. doi: 10.1021/jm020269o. [DOI] [PubMed] [Google Scholar]
- 15.Kim IH, Morisseau C, Watanabe T, Hammock BD. Design, synthesis, and biological activity of 1,3-disubstituted ureas as potent inhibitors of the soluble epoxide hydrolase of increased water solubility. J Med Chem. 2004;47:2110–2122. doi: 10.1021/jm030514j. [DOI] [PubMed] [Google Scholar]
- 16.Kim IH, Heirtzler FR, Morisseau C, Nishi K, Tsai HJ, Hammock BD. Optimization of amide-based inhibitors of soluble epoxide hydrolase with improved water solubility. J Med Chem. 2005;48:3621–3629. doi: 10.1021/jm0500929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang SH, Wecksler AT, Zhang G, Morisseau C, Nguyen LV, Fu SH, Hammock BD. Synthesis and biological evaluation of sorafenib- and regorafenib-like sEH inhibitors. Bioorg Med Chem Lett. 2013;23:3732–3737. doi: 10.1016/j.bmcl.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GlaxoSmithKline. ClinialTrials.gov [Internet] Bethesda (MD): 2000–2015. A Study to Assess the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Single Doses of GSK2256294 in Healthy Volunteers, and Single and Repeat Doses of GSK2256294 in Adult Male Moderately Obese Smokers. Available from: http://clinicaltrials.gov/ct2/show/NCT01762774 NLM Identifier: NCT01762774. [Google Scholar]
- 21.Khardin AP, Gureev NG, Radchenko SS. Curtius rearrangement of adamantane derivatives. J Org Chem USSR (English Translation) 1980;16:57. [Google Scholar]
- 22.Butov GM, Burmistrov VV, Pitushkin DA. One-step preparation method for adamantyl-containing isocyanates, precursors of epoxide hydrolase inhibitors. Russ J Org Chem. 2017;53:673–678. [Google Scholar]
- 23.Ulu A, Davis BB, Tsai HJ, Kim IH, Morisseau C, Inceoglu B, Fiehn O, Hammock BD, Weiss RH. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in apolipoprotein e-knockout mouse model. J Cardiovasc Pharmacol. 2008;52(4):314–323. doi: 10.1097/FJC.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schleyer PR, Donaldson MM. The relative stability of bridged hydrocarbons. II endo- and exo-Trimethylenenorbornane. The formation of adamantane. J Am Chem Soc. 1960;82(17):4645–4651. [Google Scholar]
- 25.Applequist J, Rivers P, Applequist DE. Theoretical and experimental studies of optically active bridgehead-substituted adamantanes and related compounds. J Am Chem Soc. 1969;91(21):5705–5711. [Google Scholar]
- 26.Varushchenko RM, Pashchenko LL, Druzhinina AI, Abramenkov AV. Thermodynamics of vaporization of some alkyladamantanes. J Chem Thermodynamics. 2001;33:733–744. [Google Scholar]
- 27.Butov GM, Mokhov VM, Burmistrov VV, Saad KR, Pitushkin DA. Reactions of 1,3-dehydroadamantane with inorganic oxygen-free acids. Russ J Org Chem. 2014;50:1276–1278. [Google Scholar]
- 28.Morisseau C, Goodrow MH, Newman JW, Wheelock CE, Dowdy DL, Hammock BD. Structural refinement of inhibitors of urea-based soluble epoxide hydrolases. Biochem Pharm. 2002;63:1599–1608. doi: 10.1016/s0006-2952(02)00952-8. [DOI] [PubMed] [Google Scholar]
- 29.Jones PD, Wolf NM, Morisseau C, Whetstone P, Hock B, Hammock BD. Fluorescent substrates for soluble epoxide hydrolase and application to inhibition studies. Anal Biochem. 2005;343:66–75. doi: 10.1016/j.ab.2005.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stetter H, Wulff C. Über verbindungen mit urotropin-struktur, XXIV1) derivate des 1-amino-adamantans. Chem Ber. 1962;95:2302–2304. [Google Scholar]
- 31.Rose TE, Morisseau C, Liu JY, Inceoglu B, Jones PD, Sanborn JR, Hammock BD. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010;53:7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burmistrov V, Morisseau C, Lee KSS, Shihadih DS, Harris TR, Butov GM, Hammock BD. Symmetric adamantyl-diureas as soluble epoxide hydrolase inhibitors. Bioorg Med Chem Lett. 2014;24:2193–2197. doi: 10.1016/j.bmcl.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burmistrov VV, Butov GM, Karlov DS, Palyulin VA, Zefirov NS, Morisseau C, Hammock BD. Synthesis and properties of diadamantyl-containing symmetric diureas as target-oriented inhibitors of human soluble epoxide hydrolase. Russ J Bioorg Chem. 2016;42(4):404–414. [Google Scholar]
- 34.Gomez GA, Morisseau C, Hammock BD, Christianson DW. Structure of human epoxide hydrolase reveals mechanistic inferences on bifunctional catalysis in epoxide and phosphate ester hydrolysis. Biochemistry. 2004;43:4716–4723. doi: 10.1021/bi036189j. [DOI] [PubMed] [Google Scholar]
- 35.Morisseau C, Hammock BD. Current protocols in toxicology. Supplement 33. 2007;4:23. doi: 10.1002/0471140856.tx0423s33. [DOI] [PubMed] [Google Scholar]
- 36.Liu JY, Tsai HJ, Morisseau C, Lango J, Hwang SH, Watanabe T, Kim IH, Hammock BD. In vitro and in vivo metabolism of N-adamantyl substituted urea-based soluble epoxide hydrolase inhibitors. Biochem Pharmacol. 2015;98(4):718–731. doi: 10.1016/j.bcp.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shihadih DS, Harris TR, Yang J, Merzlikin O, Lee KS, Hammock BD, Morisseau C. Identification of potent inhibitors of the chicken soluble epoxide hydrolase. Bioorg Med Chem Lett. 2015;25:276–279. doi: 10.1016/j.bmcl.2014.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wanka L, Iqbal K, Schreiner PR. The lipophilic bullet hits the targets: medicinal chemistry of adamantane derivatives. Chem Rev. 2013;113:3516–3604. doi: 10.1021/cr100264t. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.