Abstract
Study Objectives:
The WatchPAT is a wrist-worn portable device that creates integration data regarding peripheral arterial tone (PAT), oxyhemoglobin saturation, heart rate, and actigraphy to diagnose or screen for obstructive sleep apnea (OSA). Previous studies have demonstrated the efficacy and validity of respiratory variables measured by the WatchPAT compared to those using polysomnography (PSG). However, the effects of arterial stiffness or atherosclerosis on WatchPAT parameters remain to be elucidated.
Methods:
Sixty-one consecutive patients with suspected OSA who underwent home-based testing with the WatchPAT 200, standard in-laboratory overnight polysomnography (PSG), and pulse wave velocity (PWV) as an index of arterial stiffness were studied. All PSG recordings were scored manually using the American Academy of Sleep Medicine criteria, whereas WatchPAT data were analyzed by an automatic algorithm. We evaluated how arterial stiffness affected respiratory event index data in WatchPAT (WP-AHI), because WP-AHI could be partly influenced by PAT, comparing WP-AHI and the apneahypopnea index measured by PSG (PSG-AHI) in consideration of PWV result.
Results:
Overall, WP-AHI was moderately correlated to PSG-AHI, but WP-AHI was significantly lower than PSG-AHI (28.4 ± 19.2 versus 53.6 ± 30.2 events/h, P < .0001). For the lower PWV group, there was a significant correlation and good agreement between the WP-AHI and PSG-AHI, but as the PWV increased, there was low correlation between the WP-AHI and PSG-AHI.
Conclusions:
Arterial stiffness may affect the respiratory variables measured by WatchPAT in patients with OSA.
Commentary:
A commentary on this article appears in this issue on page 301.
Citation:
Kinoshita T, Yahaba M, Terada J, Matsumura T, Sakurai Y, Nagashima K, Sakao S, Tatsumi K. Impact of arterial stiffness on WatchPAT variables in patients with obstructive sleep apnea. J Clin Sleep Med. 2018;14(3):319–325.
Keywords: apnea-hypopnea index, arterial stiffness, obstructive sleep apnea, pulse wave velocity, WatchPAT
BRIEF SUMMARY
Current Knowledge/Study Rationale: Although the current gold standard for diagnosing obstructive sleep apnea (OSA) is overnight polysomnography, the procedure is expensive and time consuming to administer. The WatchPAT is a portable device for which multiple studies have demonstrated good correlation with full polysomnography; however, the effects of arterial stiffness on WatchPAT variables still need to be elucidated.
Study Impact: This study demonstrates that arterial stiffness may affect the respiratory index measured by the WatchPAT in patients with OSA. Therefore, we believe that greater care should be taken when using the WatchPAT to diagnose OSA, especially in patients with severe arterial stiffness.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep-related breathing disorder characterized by repetitive complete or partial obstruction of the upper airway during sleep. The diagnosis and treatment of OSA are critical to avoid the potential risks of fatal cerebrovascular or cardiovascular events, and death from causes mainly related to the atherosclerotic process.1,2 However, at least 80% of moderate-to-severe OSA remains undiagnosed in middle-aged adults.3 The current gold standard for diagnosing OSA is in-laboratory overnight polysomnography (PSG),4 but despite providing the most accurate description of sleep disorders, it is limited by high costs, long waiting lists in some areas, and the requirement for overnight assessment. Therefore, alternative approaches are required.
The WatchPAT is a wrist-worn ambulatory device that is used to diagnose or screen for OSA, and is categorized as a type 3 monitoring device by the American Academy of Sleep Medicine (AASM).5 Multiple studies have demonstrated that the respiratory parameters calculated using the WatchPAT correlates well with that calculated by full PSG.6–8 In Japan, the WatchPAT has mainly been used to screen for OSA. The variables calculated by the WatchPAT algorithm are based on peripheral arterial tone (PAT), heart rate, oxyhemoglobin saturation, and actigraphy,9 allowing indirect detection of apnea and hypopnea events through PAT signal attenuation, heart rate increase, and desaturation.6,10,11 Given that the PAT measurement is based on a physiological signal reflecting changes in vascular volume in the digit using the finger-mounted plethysmograph,9 an impaired peripheral vascular response might affect the respiratory variables measured by the WatchPAT.
Indeed, patients using α-adrenergic receptor-blocking agents cannot be accurately evaluated by WatchPAT, because such agents are known to affect the PAT signal.12,13 The role of more common conditions, such as arterial stiffness or atherosclerosis, has not been adequately considered in the literature.
Brachial-ankle pulse wave velocity (baPWV) is a widely used modality for evaluating arterial stiffness.14 The baPWV can be noninvasively and indirectly assessed by calculating the time that it takes a pulse wave to travel along the vasculature between the brachium and ankle. Previous research has shown that baPWV is an independent predictor of the presence of coronary artery disease, myocardial injury, and future cardiovascular events.15,16 Therefore, we hypothesized that arterial stiffness (ie, a high pulse wave velocity [PWV]) would attenuate the accuracy of the respiratory variables measured by WatchPAT. In the current study, we investigated the effect of arterial stiffness, as measured by baPWV, on WatchPAT variables in patients with OSA.
METHODS
Study Design
We enrolled adult patients with suspected OSA in whom the diagnosis was made at Chiba University Hospital between April 2015 and March 2016. Registrations were consecutively conducted at each patients' first visit at the Sleep Disordered Breathing Clinics in our hospital (if consent to participating in our study were obtained). Patients with suspected other sleep conditions (ie, nongeneral OSA such as heart failure with central sleep apnea, narcolepsy, or obvious neuromuscular disease) were not registered. Exclusion criteria for the study were history of permanent pacemaker, nonsinus cardiac arrhythmias, and use of α-adrenergic receptor-blocking agents or short-acting nitrates (24-hour washout period required). Before the study, patients completed a questionnaire concerning daytime sleepiness (Epworth Sleepiness Scale), medical history, and physical data (eg, weight and height). Patients first underwent home-based portable monitoring using a WatchPAT 200 (Itamar Medical Ltd., Caesarea, Israel) before visiting the hospital for standard in-laboratory full overnight PSG (mean duration between WatchPAT and PSG was 39.1 ± 26.5 days). On the same day as the patient underwent PSG, the baPWV was measured. Informed consent was obtained from all participants after the protocol was approved by the Institutional Review Board of Chiba University Hospital.
Home-Based Testing by WatchPAT
For home study, patients received instructions on how to use the WatchPAT device. These instructions addressed proper application of the device and were accompanied by a demonstration of correct use, and lasted 5 to 10 minutes. The WatchPAT is a four-channel, unattended, ambulatory monitoring device that records PAT signals, heart rate, oxygen saturation, and actigraphy. The WatchPAT calculates clinical parameters such as respiratory event index and 4% oxygen desaturation index (ODI) by its automated and proprietary algorithm (analyzed data reports are automatically made in the default setting). This device is worn around the wrist and has one finger probe that contains a PAT sensor for measuring PAT changes in peripheral arterial beds, a heart rate sensor, and an oximetry sensor for measuring arterial oxygen saturation. The wrist unit contains the actigraphy sensor for measuring wrist movements through a three-dimensional accelerometer. Using the Watch-PAT software (ZZZ PAT version 4.4.64.p, Itamar Medical Ltd., Caesarea, Israel), the resulting data were automatically analyzed to identify respiratory events and sleep states. The automated analysis used actigraphy to identify wake-sleep states. Respiratory events were detected as follows: (1) termination of respiratory disturbances led to a surge of sympathetic activity that influenced peripheral arterial vasoconstriction; (2) vasoconstriction of the peripheral arterial bed by alpha receptors resulted in an attenuation of PAT signal; and (3) PAT signal attenuation, increased heart rate, and oxygen desaturation were analyzed by the automatic computerized algorithm of the WatchPAT system.5 Manual editing of automated scoring is possible but was avoided in the current study to allow the assessment of only algorithm performance rather than that of the algorithm plus human operator. In addition, a previous study demonstrated the validation of automated scoring.5,7
In-Laboratory Testing by Polysomnography
All patients underwent standard, in-laboratory, full overnight PSG, using an E Series monitor (Compumedics, Victoria, Australia). The following were recorded: electroencephalography (EEG; C4-M1, O2-M1, C3-M2, O1-M2), electrooculography, submental and bilateral anterior tibial electromyography, electrocardiography, respiratory effort by thoracoabdominal piezoelectric belts, nasal airflow by nasal pressure cannula, nasal and oral flow by a thermistor, finger pulse oximetry, snoring recording by a neck microphone, and assessment of body posture by a thoracic belt sensor. The 2007 American Academy of Sleep Medicine alternative criteria was used to score the PSG.8 Apnea was defined as a reduction in nasal airflow to < 10% of the baseline for ≥ 10 seconds. Hypopnea was defined as a reduction in nasal airflow signal amplitude of ≥ 50% for ≥ 10 seconds in association with either an oxygen desaturation of ≥ 3% or arousal on EEG. OSA was defined as an apnea-hypopnea index (AHI) of ≥ 5 events/h, combined with predominantly obstructive respiratory events. OSA severity was classified according to the AHI, as follows: mild, 5–15; moderate, 15–30; and severe, ≥ 30 events/h. All PSG tests were scored by a single technician.
Brachial–Ankle Pulse Wave Velocity
The baPWV was measured in the supine position, using an automated device (Colin form; OMRON COLIN Co., Ltd. Tokyo, Japan) that measures arterial pulse waves in both the brachial and posterior tibial arteries by the oscillometric method.14 The pressure waveforms of the brachial and tibial arteries were obtained from occlusion and monitoring cuffs around the upper arm and the ankle. The transmission distance between the brachium and ankle was calculated automatically based on the height of the patient.17 The average of the left and right values was used for analysis. Several studies have reported the cutoff value of baPWV for cardiovascular disease. Maeda et al. compared 3,628 outpatients with diabetes and showed baPWV of 1400 cm/s as statistically adequate cutoffpoints for cardiovascular events and mortality.18 Kim et al. reported baPWV > 1700 cm/s was strongly associated with the presence and severity of cardiovascular disease.15 Based on these studies, we decided upon a baPWV threshold from 1300 cm/s to 1800 cm/s.
Data and Statistical Analysis
The following data were derived from in-laboratory PSG testing: total sleep time, rapid eye movement (REM) sleep, deep non-REM (NREM) sleep, 4% ODI, and AHI (PSG-AHI). The following data were derived from the home WatchPAT testing: total sleep time, REM sleep, deep NREM sleep, 4% ODI, and respiratory event index (WP-AHI). Demographic variables are presented as mean ± standard deviation, unless otherwise stated. Differences between the PSG and WatchPAT measurements were compared by paired t tests. Bland-Altman plots were used to determine the agreement between PSG and WatchPAT measurements in the study groups. Receiver operator characteristic (ROC) curves were constructed to illustrate the diagnostic performance of the WP-AHI, which was compared to the reference standard (ie, PSG-AHI ≥ 30 events/h for a positive result, and PSG-AHI < 30 events/h for a negative result). The area under the ROC curves, and the sensitivity and specificity for WP-AHI cutoff values were calculated. Pearson correlation coefficients between the PSG-AHI and WP-AHI were calculated; subgroup analyses according to different thresholds of the PWV values (ie, 1300, 1350, …, 1750, and 1800 cm/s) were also performed to explore the effect of arterial stiffness. Values of P < .05 by two-tailed tests were considered to denote statistical significance. All statistical analyses were done using JMP Pro Version 12.0 (SAS Institute, Cary, North Carolina, United States).
RESULTS
Participant Characteristics
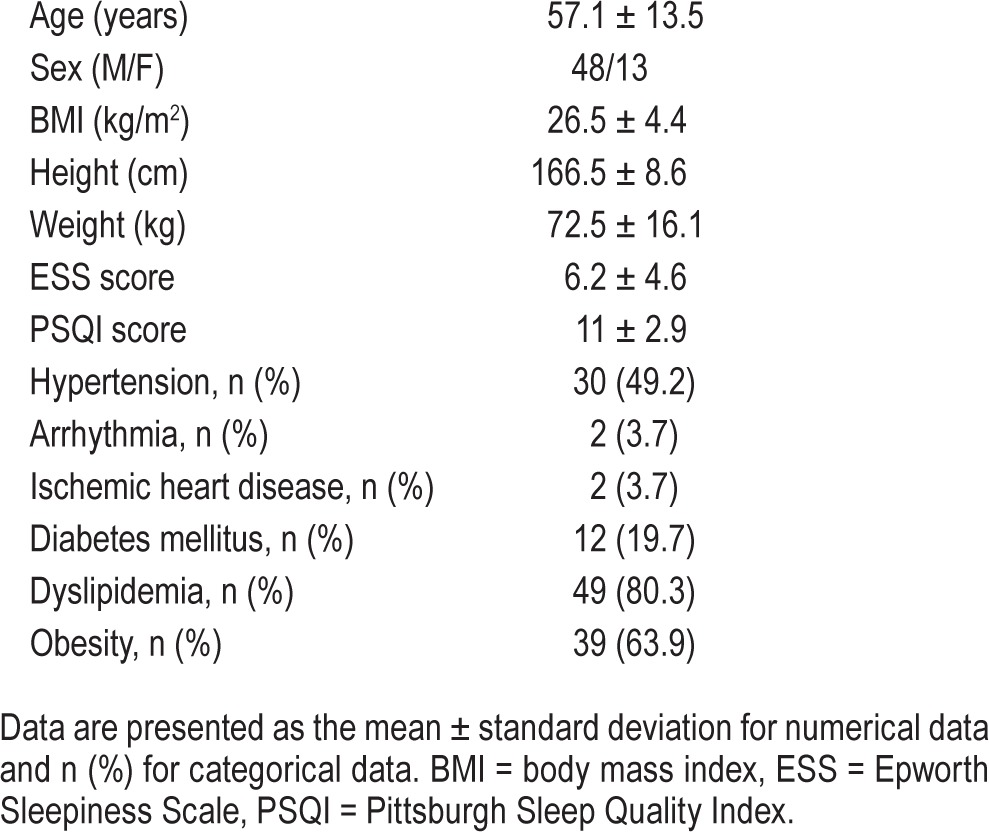
Characteristics of the study population are shown in Table 1. A total of 61 patients (48 males, 13 females) were studied, for whom the average age was 57.1 ± 13.5 years and the body mass index was 26.5 ± 4.4 kg/m2. Fifty-four patients (88.5%) had at least one comorbidity other than obesity, with the greatest percentages having hypertension (49.2%), diabetes mellitus (24.6%), or dyslipidemia (19.7%). All the patients recruited had OSA, with mild OSA in 6.6%, moderate OSA in 16.2%, and severe OSA in 77.2%.
Table 1.
Characteristics of study population (n = 61).
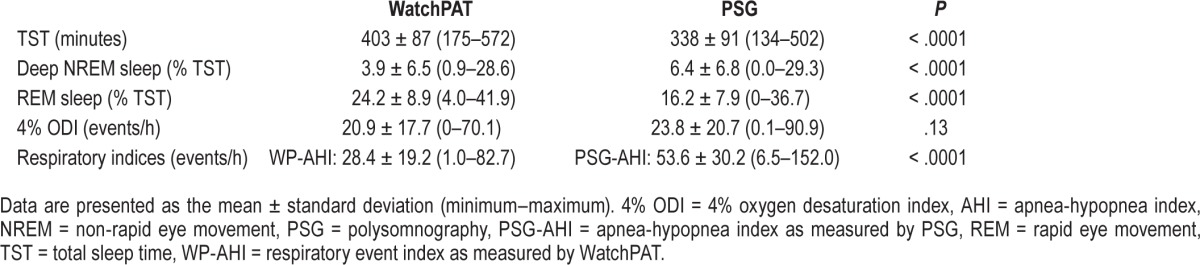
Comparison of Sleep and Breathing Variables Between PSG and WatchPAT
The comparison of sleep and respiratory variables between PSG and WatchPAT are shown in Table 2. The WP-AHI was significantly lower than the PSG-AHI. Total sleep time, deep NREM sleep, and REM sleep were significantly higher in WatchPAT than in PSG. However, there was no significant difference in the 4% ODI between the WatchPAT and PSG.
Table 2.
Sleep and breathing characteristics comparing polysomnography and WatchPAT (n = 61).
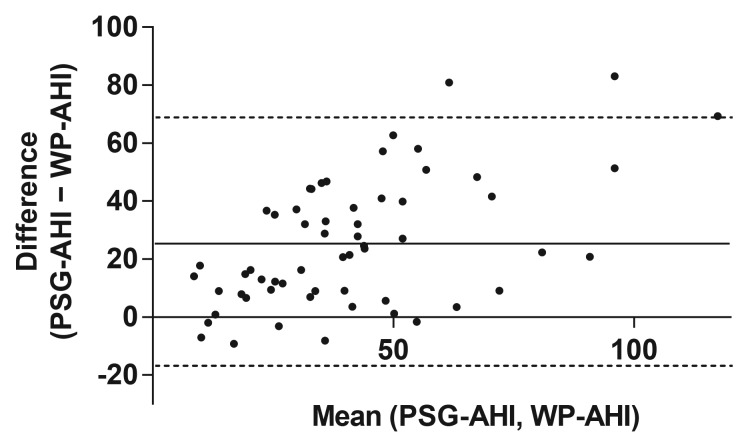
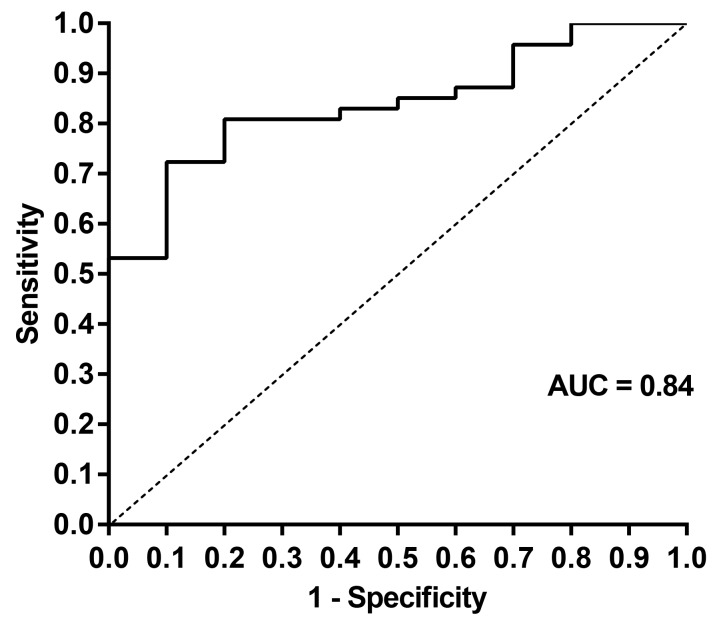
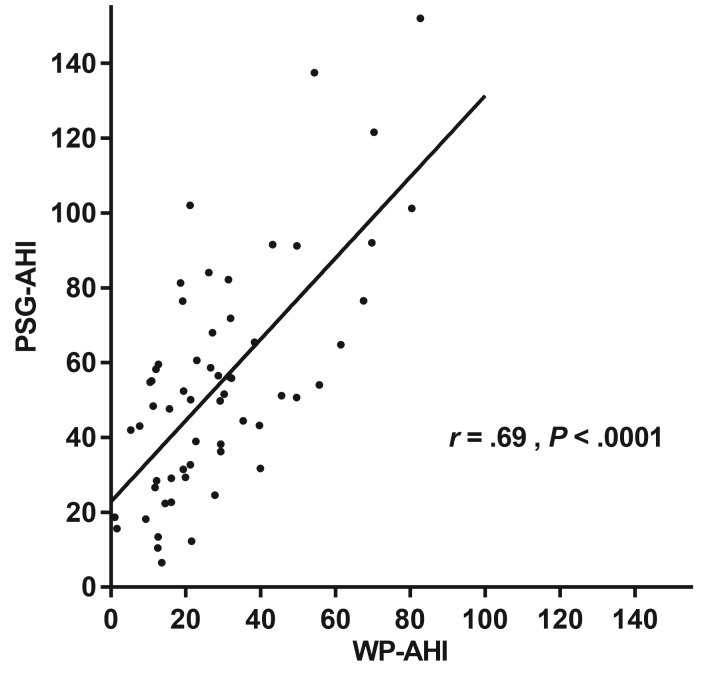
The Bland-Altman plot illustrated that as PSG-AHI increased, the WatchPAT tended to underestimate the respiratory event index, indicating that proportional bias existed in respiratory indices between WatchPAT and PSG, as shown in Figure 1. ROC curves for respiratory event indices are shown in Figure 2. The area under the curve for PSG-AHI ≥ 30 events/h was 0.84 (95% confidence interval [CI]: 0.73–0.95, P = .0008). When diagnosis of OSA was defined to WP-AHI cutoff values of 18.6 events/h, the sensitivity and specificity for the WatchPAT to identify PSGAHI ≥ 30 events/h were 0.79 (95% CI: 0.64–0.89) and 0.80 (95% CI: 0.44–0.97), respectively. As shown in Figure 3, there was a moderate correlation for the respiratory event indices between WatchPAT and PSG (r = .69, P < .0001).
Figure 1. Bland-Altman plot of respiratory event indices.
Differences were calculated as the PSG-AHI minus WP-AHI. Means for the PSG and WatchPAT findings are on the x-axis, and differences are on the y-axis. Solid lines on the y-axis are drawn from 0, mean difference. Dotted lines on the y-axis are drawn from mean ± 1.96 × standard deviation of the differences. PSG = polysomnography, PSGAHI = apnea-hypopnea index as measured by PSG, WP-AHI = respiratory event index as measured by WatchPAT.
Figure 2. ROC curve identifying pathologic apnea and hypopnea events from sleep for the WatchPAT and PSG.
The comparison threshold was set at PSG-AHI ≥ 30 events/h on PSG. PSG = polysomnography, PSG-AHI = apnea-hypopnea index as measured by PSG, ROC = receiver operating characteristic.
Figure 3. Scatterplot of PSG-AHI by WP-AHI.
A moderate correlation was found (r = .69, P < .0001, n = 61) between PSG-AHI and WP-AHI. PSG-AHI = apnea-hypopnea index as measured by polysomnography, WP-AHI = respiratory event index as measured by WatchPAT.
The Effect of Arterial Stiffness on the Relationship Between WatchPAT and PSG
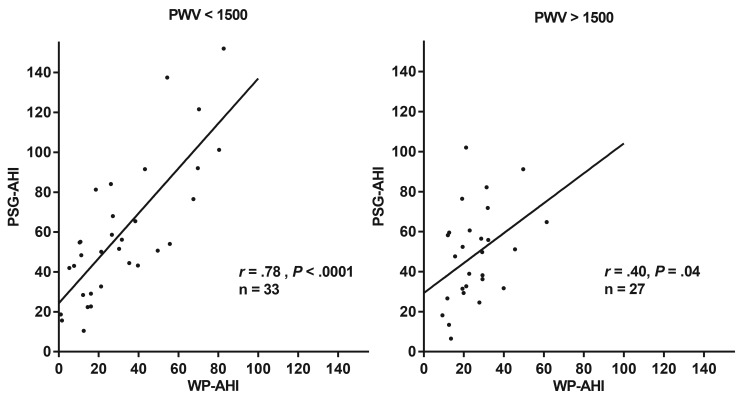
To evaluate the effect of arterial stiffness, we compared the correlations between WP-AHI and PSG-AHI groups with PWV results lower and higher than the selected PWV (range 1300–1800 cm/s), as shown in Table 3. In patients with lower PWV values than each cutoff value (from 1300 to 1800 cm/s), there were very high correlations for the respiratory event indices between the WatchPAT and the PSG groups. Among them, patients with a PWV under 1300 cm/s has the highest correlation between PSG-AHI and WP-AHI (r = .811, P = .0001). As the PWV cutoff value increased, the correlation became lower. Conversely, patients with a PWV over 1550 cm/s have no statistical correlation for the respiratory event indices between the WatchPAT and the PSG. Figure 4 shows the representative difference classified by a PWV of 1500 cm/s (lower; n = 33, r = .78, P < .0001 higher; n = 27, r = .40, P = .04).
Table 3.
Correlation coefficients between PSG-AHI and WP-AHI according to different thresholds of the PWV values.
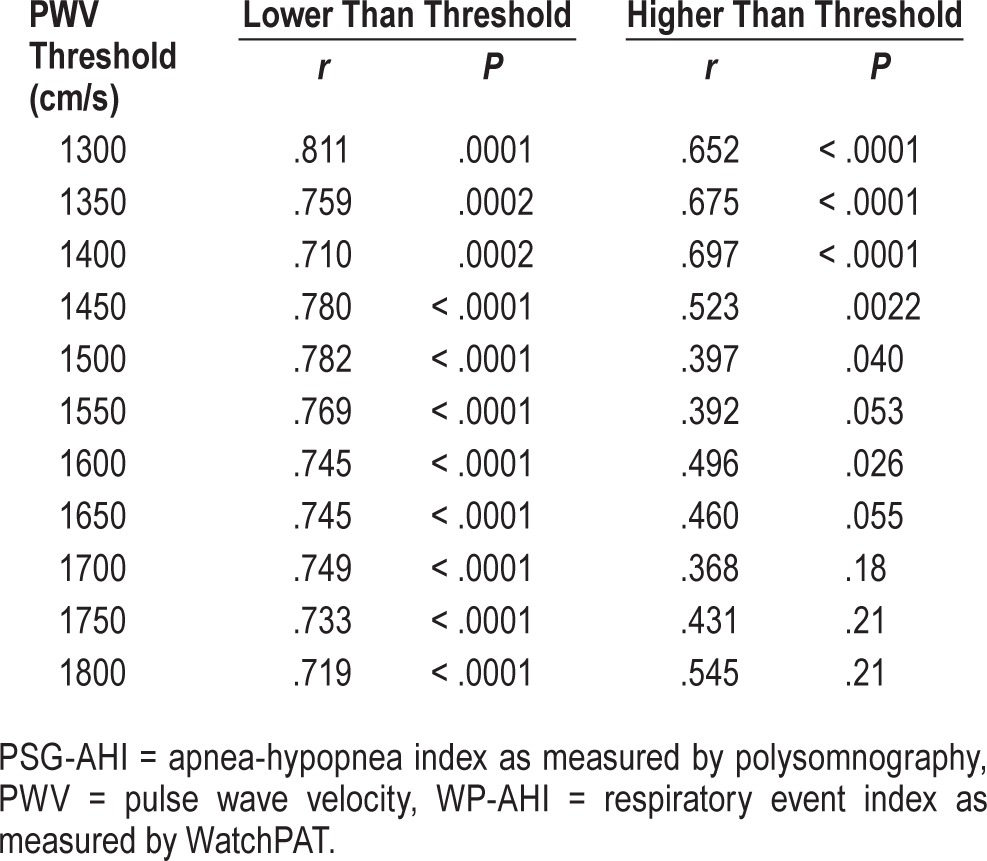
Figure 4. Scatterplots of PSG-AHI by WP-AHI based on the PWV threshold of 1500 cm/s.
Data are shown for patients with a PWV under 1500 cm/s (A) and a PWV over 1500cm/s (B). The Pearson correlation coefficients were .78 (P < .0001) and .40 (P < .04), respectively. PSG-AHI = apnea-hypopnea index as measured by polysomnography, PWV = pulse wave velocity, WP-AHI = respiratory event index as measured by WatchPAT.
DISCUSSION
This is the first study, to our knowledge, to have used baPWV to evaluate how arterial stiffness affects the respiratory variables measured by WatchPAT in patients with OSA. We showed that overall correlation and agreement were good between WPAHI and PSG-AHI, and that this correlation was strongest among patients with low arterial stiffness. By contrast, there was low correlation between WP-AHI and PSG-AHI among patients with high arterial stiffness. These results indicate that arterial stiffness should be considered, especially in patients suspected of arterial stiffness, when evaluating by WatchPAT. The mean WP-AHI was also lower than the mean PSG-AHI in this study, suggesting that the WatchPAT may underestimate the respiratory event index, partially because of the effect of arterial stiffness.
The Effect of Arterial Stiffness Measured by PWV on WatchPAT Variables
In the current study, the WP-AHI in the high PWV group had worse correlation with PSG-AHI than that in the lower group. This indicated that reduction in the extensibility of the peripheral arteries due to increased stiffness was related to the estimated number of apnea and hypopnea events analyzed by the WatchPAT. Arterial stiffness reflects physiological changes in the mechanical and structural properties of the vascular wall that lead to reduced arterial compliance. In turn, variation in arterial stiffness has been correlated with cardiovascular outcomes, and is an emerging prognostic factor with hypertension, diabetes, obesity, and dyslipidemia.19 Given that the PAT is a major component variable in assessments using the Watch-PAT, it was anticipated that arterial stiffness would affect the related respiratory index, especially for patients in clinical settings (eg, diversity of medications, complications, age, and OSA severity). Most previous studies of the WatchPAT have excluded patients with diabetes mellitus, peripheral neuropathy, vasculopathy, or cardiac disease because these diseases may theoretically affect peripheral blood flow.7 Few studies have focused on the effect of arterial impairment on results from the WatchPAT. Aging is also associated with impaired peripheral vascular tone and can affect the WatchPAT data, but Onder et al. demonstrated that aging did not negatively affect the PAT-recorded respiratory index.20 However, they did show that there was a significant difference between young and old groups in PAT- and PSG-recorded stage N3 sleep.20 This finding may be attributed to aging and impaired vascular tone.
WP-AHI Compared With PSG-AHI
We also showed that the mean WP-AHI was lower than the mean PSG-AHI, even though most studies that validated the WatchPAT demonstrated good agreement with PSG for the respiratory event index. This discordance can be explained by the differences in study populations. The Brand-Altman plot illustrated that as PSG-AHI increased, the WatchPAT tended to underestimate the respiratory event index. Thus, consistent with research by other authors,6,21 we observed proportional bias when using the WatchPAT in this study. This may be explained, in part, by the fact that the WatchPAT had more difficulty detecting individual events when multiple events occurred over a brief period.21 It was also notable that our research included patients with severe OSA (mean AHI 53.6 events/h, and severe OSA in 77%), whereas most previous studies have tended to include patients with mild-to-moderate OSA. This important difference in the study population may also have caused the results to be underestimated. Given that most symptomatic patients who consult their doctor have severe OSA (eg, daytime sleepiness or severe snoring), it is important to know that the WatchPAT might underestimate the respiratory variables in these patients.
Clinical Implications
The WatchPAT is a simple and patient-friendly portable device for assessing suspected OSA, providing suitably accurate assessment of respiratory disturbance during sleep with minimal technical effort and low cost. The device has been widely used in clinical settings and trials already (eg, screening in certain diseases and occupations, determination of treatment effect).10,22–24 However, our results demonstrated a note of caution that arterial stiffness may affect the respiratory index measured by the WatchPAT, and that it might be necessary to consider the grade of arterial stiffness when assessing test results using this device. This is especially important because, compared with earlier studies, our population was closer to that typically seen in clinical settings. Indeed, we included patients with more severe OSA and with several comorbidities, including hyper-tension, diabetes mellitus, and dyslipidemia, whereas previous studies of the WatchPAT have mainly included patients with mild-to-moderate OSA and no comorbidities.7 In real-word clinical situations, we therefore think that the WatchPAT might underestimate the respiratory event index when compared with PSG. Given this, clinicians might need to assess the merits of the WatchPAT device over full PSG before performing an assessment for patients with OSA using WatchPAT.
Limitations
There are several limitations to our study. First, this study included a small number of patients with OSA and it was conducted at a single hospital. Second, there is a possibility of night-to-night variation because the WatchPAT and PSG assessments were not performed on the same nights (the duration between WatchPAT and PSG was 39.1 ± 26.5 days). This may affect our results to some extent, whereas our study originally intended to evaluate the efficacy and accuracy of home-based WatchPAT in practical use in actual clinical situations (ie, a portable modality for use in screening or for the diagnosis of in-home situations) compared to that of in-laboratory PSG. However, other studies have demonstrated good correlation for respiratory disturbance between the WatchPAT and PSG when recorded on different nights, so we do not think that this will have had a major influence on the relationship between the WPAHI and PSG-AHI.5,25 Third, in our study, we found that total sleep time was significantly longer in WatchPAT than in PSG. Possible reasons include the following: (1) because WatchPAT does not monitor EEG, total sleep time may have been underestimated in our subjects, and (2) the first-night effect during in-hospital PSG testing may decrease the total sleep time (versus home testing). Additionally, regarding longer deep sleep time in WatchPAT than that in PSG, O'Brien et al. showed poor relationships in sleep stage characteristics between WatchPAT and PSG; in particular, agreement for deep sleep was not well achieved.22 In the current study, it remains uncertain whether WatchPAT underestimated deep sleep time. Fourth, it should be noted that the baPWV mainly represents large arteries, whereas WatchPAT represents peripheral arteries. Nevertheless, baPWV is known to be partially influenced by peripheral arteries.26 Activation of α-sympathetic nerves, which is essential for detecting the respiratory index when using the WatchPAT, has been demonstrated to reduce forearm vascular and fingertip vascular resistance when using α-adrenergic agonist.13 Therefore, we consider it reasonable to use baPWV to assess arterial stiffness as a factor that can change fingertip blood flow. Finally, regarding body mass index and Epworth Sleepiness Scale score, the averages of our study were quite low (26.5 ± 4.4 kg/m2 and 6.2 ± 4.6, respectively) compared to other general previous reports for OSA. We believe that this disagreement may be because of population difference (eg, East Asian versus Western countries) regardless of the presence of severe OSA. Therefore, some differences may exist between our study population and those of others.
CONCLUSIONS
In conclusion, we have shown that there was a strong correlation between WP-AHI and PSG-AHI as PWV values decreased, but that there was low or no correlation as PWV increased, indicating that arterial stiffness may affect the respiratory event index measured by the WatchPAT. The results of the current study suggest exercising caution when using the WatchPAT to diagnose OSA in patients with severe arterial stiffness.
DISCLOSURE STATEMENT
Work for this study was performed at Chiba University Hospital. All the authors have read and approved this manuscript. This work was supported, in part, by a research grant to the Intractable Respiratory Diseases and Pulmonary Hypertension Research Group, the Ministry of Health, Labor and Welfare, the Japan Agency for the Medical Research and Development (AMED), JSPS KAKENHI (Grant number 16K09528). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Reiko Kunii (Department of Laboratory Medicine, Chiba
University) for the technical support for this research.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- baPWV
brachial-ankle pulse wave velocity
- EEG
electroencephalography
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PAT
peripheral arterial tone
- PSG
polysomnography
- PSG-AHI
apnea-hypopnea index as measured by polysomnography
- REM
rapid eye movement
- WP-AHI
respiratory event index as measured by WatchPAT
REFERENCES
- 1.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 3.Watson NF. Health care savings: the economic value of diagnostic and therapeutic care for obstructive sleep apnea. J Clin Sleep Med. 2016;12(8):1075–1077. doi: 10.5664/jcsm.6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an american academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JH, Kim EJ, Kim YS, et al. Validation study of portable device for the diagnosis of obstructive sleep apnea according to the new AASM scoring criteria: Watch-PAT 100. Acta Otolaryngol. 2010;130(7):838–843. doi: 10.3109/00016480903431139. [DOI] [PubMed] [Google Scholar]
- 6.Bar A, Pillar G, Dvir I, Sheffy J, Schnall RP, Lavie P. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest. 2003;123(3):695–703. doi: 10.1378/chest.123.3.695. [DOI] [PubMed] [Google Scholar]
- 7.Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343–1350. doi: 10.1001/jamaoto.2013.5338. [DOI] [PubMed] [Google Scholar]
- 8.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 9.Schnall RP, Shlitner A, Sheffy J, Kedar R, Lavie P. Periodic, profound peripheral vasoconstriction--a new marker of obstructive sleep apnea. Sleep. 1999;22(7):939–946. [PubMed] [Google Scholar]
- 10.Yuceege M, Firat H, Demir A, Ardic S. Reliability of the Watch-PAT 200 in detecting sleep apnea in highway bus drivers. J Clin Sleep Med. 2013;9(4):339–344. doi: 10.5664/jcsm.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pillar G, Bar A, Betito M, et al. An automatic ambulatory device for detection of AASM defined arousals from sleep: the WP100. Sleep Med. 2003;4(3):207–212. doi: 10.1016/s1389-9457(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 12.Zou D, Grote L, Eder DN, Peker Y, Hedner J. Obstructive apneic events induce alpha-receptor mediated digital vasoconstriction. Sleep. 2004;27(3):485–489. doi: 10.1093/sleep/27.3.485. [DOI] [PubMed] [Google Scholar]
- 13.Grote L, Zou D, Kraiczi H, Hedner J. Finger plethysmography--a method for monitoring finger blood flow during sleep disordered breathing. Respir Physiol Neurobiol. 2003;136(2-3):141–152. doi: 10.1016/s1569-9048(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 14.Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25(3):359–364. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Rhee MY, Kim YS, et al. Brachial-ankle pulse wave velocity for the prediction of the presence and severity of coronary artery disease. Clin Exp Hypertens. 2014;36(6):404–409. doi: 10.3109/10641963.2013.846354. [DOI] [PubMed] [Google Scholar]
- 16.Turin TC, Kita Y, Rumana N, et al. Brachial-ankle pulse wave velocity predicts all-cause mortality in the general population: findings from the Takashima study, Japan. Hypertens Res. 2010;33(9):922–925. doi: 10.1038/hr.2010.103. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka H, Munakata M, Kawano Y, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27(10):2022–2027. doi: 10.1097/HJH.0b013e32832e94e7. [DOI] [PubMed] [Google Scholar]
- 18.Maeda Y, Inoguchi T, Etoh E, et al. Brachial-ankle pulse wave velocity predicts all-cause mortality and cardiovascular events in patients with diabetes: the Kyushu Prevention Study of Atherosclerosis. Diabetes Care. 2014;37(8):2383–2390. doi: 10.2337/dc13-1886. [DOI] [PubMed] [Google Scholar]
- 19.Liao J, Farmer J. Arterial stiffness as a risk factor for coronary artery disease. Curr Atheroscler Rep. 2014;16(2):387. doi: 10.1007/s11883-013-0387-8. [DOI] [PubMed] [Google Scholar]
- 20.Onder NS, Akpinar ME, Yigit O, Gor AP. Watch peripheral arterial tonometry in the diagnosis of obstructive sleep apnea: influence of aging. Laryngoscope. 2012;122(6):1409–1414. doi: 10.1002/lary.23233. [DOI] [PubMed] [Google Scholar]
- 21.Ayas NT, Pittman S, MacDonald M, White DP. Assessment of a wrist-worn device in the detection of obstructive sleep apnea. Sleep Med. 2003;4(5):435–442. doi: 10.1016/s1389-9457(03)00111-4. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien LM, Bullough AS, Shelgikar AV, Chames MC, Armitage R, Chervin RD. Validation of Watch-PAT-200 against polysomnography during pregnancy. J Clin Sleep Med. 2012;8(3):287–294. doi: 10.5664/jcsm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pittman SD, Pillar G, Berry RB, Malhotra A, MacDonald MM, White DP. Follow-up assessment of CPAP efficacy in patients with obstructive sleep apnea using an ambulatory device based on peripheral arterial tonometry. Sleep Breath. 2006;10(3):123–131. doi: 10.1007/s11325-006-0058-x. [DOI] [PubMed] [Google Scholar]
- 24.Park CY, Hong JH, Lee JH, et al. Clinical usefulness of watch-PAT for assessing the surgical results of obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(1):43–47. doi: 10.5664/jcsm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittman SD, Ayas NT, MacDonald MM, Malhotra A, Fogel RB, White DP. Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation. Sleep. 2004;27(5):923–933. doi: 10.1093/sleep/27.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugawara J, Hayashi K, Yokoi T, et al. Brachial-ankle pulse wave velocity: an index of central arterial stiffness? J Hum Hypertens. 2005;19(5):401–406. doi: 10.1038/sj.jhh.1001838. [DOI] [PubMed] [Google Scholar]