Abstract
Objective
While medical professionals recommend lifestyle changes following a colorectal cancer (CRC) diagnosis to improve outcomes, such changes are not consistently implemented. This study examines whether higher distress is associated with lower likelihood of engaging in favorable behaviors after CRC diagnosis.
Methods
Women from the Nurses’ Health Study prospective cohort who completed anxiety (n=145) and depression (n=227) symptom scales within 4 years after receiving a CRC diagnosis were included. Measures of lifestyle (diet, physical activity, alcohol, smoking, BMI) were queried pre-diagnosis, when psychological symptoms were assessed (1988/1992), and then every four years thereafter until 2010. Women were categorized according to initial psychological symptoms levels and followed through 2010 or until last follow-up completed.
Results
Higher vs. lower anxiety symptoms were significantly related to unhealthier lifestyle scores throughout follow-up (β= −0.25, CI=−0.44, −0.05); however, the rate of change over time was similar across groups (pinteraction effect=0.41). Stratified analyses hinted that higher anxiety and depression symptoms were related to increased odds of reporting a future unhealthy lifestyle within 10-years post-diagnosis. Beyond 10 years, anxiety became statistically unrelated with future lifestyle, and higher depressive symptoms were associated with lower odds of subsequently having an unhealthy lifestyle, albeit non-statistically significant (OR=0.35, 95%CI=0.10, 1.24, p=0.10).
Conclusions
Among women with CRC, higher anxiety and depression symptoms were associated with subsequent unhealthier lifestyle in the 10 years following diagnosis. With replication, such findings may suggest that treating psychological symptoms early in the cancer trajectory may not solely reduce psychological distress but also promote healthier lifestyle.
Keywords: anxiety, depression, health behaviors, lifestyle, colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the most common cancers, with the majority of the cases occurring in individuals ages 50 and older (American Cancer Society, 2014; Ferlay et al., 2015). Despite an increase in the number of long-term survivors with CRC due to advances in early detection and treatment, 42% of patients are at high risk of mortality in the 10 years following diagnosis (American Cancer Society, 2014). Besides cancer stage and comorbid chronic conditions, tumor location also contributes to survival rates. Specifically, proximal tumors, more frequently found in women, are generally associated with poorer prognosis compared to distal tumors, which are more common in men (American Cancer Society, 2014; Hansen & Jess, 2012). Given the unalterable nature of such medical characteristics, there is a critical need to identify determinants of CRC survival that are modifiable, particularly for women over 50 years.
Unhealthy behaviors, such as smoking and physical inactivity, are related to risk of CRC incidence and poorer survival (Lee, Jeon, & Meyerhardt, 2015; Song & Giovannucci, 2016; Vijayvergia & Denlinger, 2015). Although individuals might reevaluate their health behaviors following a cancer diagnosis, any related changes are not always sustained (Newsom et al., 2012; Williams, Steptoe, & Wardle, 2013), with suboptimal levels of health behaviors noted in studies conducted between 2 and 7 years post-diagnosis (Bluethmann et al., 2015; Grimmett, Bridgewater, Steptoe, & Wardle, 2011; Schlesinger et al., 2014).
Anxiety and depression symptoms are among the most common forms of distress (Narrow, Rae, Robins, & Regier, 2002; Steel et al., 2014), and are often reported by patients with CRC (Custers, Gielissen, Janssen, de Wilt, & Prins, 2016; Dunn et al., 2013; Mosher, Winger, Given, Helft, & O’Neil, 2016). Further, they can co-occur among both general and oncological populations (Narrow et al., 2002; Steel et al., 2014; Trudel-Fitzgerald, Savard, & Ivers, 2014). While psychological distress levels vary across individuals within the first months after a cancer diagnosis (Trudel-Fitzgerald, Savard, & Ivers, 2013), approximately 50% of patients with CRC still reach the clinical level of psychological distress –a global score combining anxiety, depression and somatization symptoms– 5 years post-diagnosis (Dunn et al., 2013).
Biobehavioral theories have posited psychological symptoms as critical determinants of whether individuals adopt healthy behaviors (Ferrer, Green, & Barrett, 2015; Kubzansky, Winning, & Kawachi, 2014), suggesting that anxiety and depression symptoms might have an important impact on lifestyle habits, which are highly linked with CRC progression and survival. Most available research evaluating the role of psychological distress in health-related behavior change among individuals with cancer has been cross-sectional (Kanera et al., 2016; Park & Gaffey, 2007) or conducted within clinical trials (Hawkes, Patrao, Baade, Lynch, & Courneya, 2015; Schnoll et al., 2004; Vijayvergia & Denlinger, 2015), generally showing that higher symptom levels are related to poorer habits. Yet, whether distress affects the natural course of behaviors among patients with CRC is unclear. One prospective study conducted among 978 patients up to 3 years after cancer diagnosis found that each unit rise in anxiety levels was related to 11% higher likelihood of increased physical activity one year later; no association with depression was evident (Chambers, Lynch, Aitken, & Baade, 2009). However, in another study among 1,375 patients, higher anxiety and depression levels assessed approximately 5 years post-diagnosis were related to lower physical activity over the next 3 years (van Putten et al., 2016). These discrepant findings warrant further work to evaluate if anxiety and depression have different impacts on health behaviors, and if follow-up duration moderates these associations.
While most work considers behaviors singly, in fact, they tend to cluster. A recent review reported that low fruits and vegetables consumption frequently co-occurred with low physical activity (47–54% prevalence), as does alcohol misuse with smoking (9–14% prevalence) (Meader et al., 2016). Another systematic review showed that 50% of studies identified a similar cluster of risky health behaviors among their participants, and 81% of studies also noted a “healthy” cluster (favorable levels of multiple behaviors) (Noble, Paul, Turon, & Oldmeadow, 2015), supporting this idea that (un)healthy habits co-occur. Moreover, when health-related behaviors like smoking, physical inactivity, high alcohol consumption, poor diet and obesity are combined, they have a multiplicative impact on mortality compared to single habits. Accordingly, a meta-analysis showed a 66% reduced risk of death over an average of 13 years with the adoption of four out of these five lifestyle factors (Loef & Walach, 2012). Hence, it is critical to consider the contribution of psychological distress to shaping lifestyle trajectories using multiple time points among individuals with cancer (Park & Gaffey, 2007; Spring, King, Pagoto, Van Horn, & Fisher, 2015), particularly in patients with CRC who are at high risk of early mortality.
Thus, this study investigated whether anxiety and depression symptoms lead to worsening lifestyle (i.e., engaging in less healthy behaviors) over up to 20 years after CRC diagnosis or reduce the odds of having a healthy lifestyle at the end of follow-up among women with CRC. Associations of psychological symptoms with subsequent lifestyle were assessed over varying lengths of follow-up post-diagnosis and according to initial levels of lifestyle around the time of diagnosis. Based on prior work, it was hypothesized that both anxiety and depression symptoms would be related to unhealthier levels of lifestyle over time; it was further posited that associations would be stronger among women with initially unhealthier levels of habits and within the first years following diagnosis (for women with shorter follow-up periods).
Methods
Participants
The Nurses’ Health Study is an ongoing prospective cohort comprised of 121,700 U.S. female married nurses, ages 30–55 years at study inception in 1976 (Willett et al., 1987). They have completed biennial questionnaires on lifestyle, medical history and newly diagnosed medical conditions, with a high response rate of ≥ 86% since 1976 (Bao et al., 2016). The present study includes women diagnosed with CRC, with no prior cancer except non-melanoma skin cancer, who completed one assessment of anxiety (1988 or 2004) or depression (1992, 1996 or 2000) symptoms within four years following their CRC diagnosis, to capture distress once the treatments are mostly completed and psychological symptoms are likely more stable, in the context of longer term survival. Differences between eligible and ineligible women are presented in Appendix Table 1. Women who reported a CRC diagnosis during the study period were asked permission to obtain their medical records and pathology reports that a study physician, blinded to the study hypotheses, reviewed to confirm CRC diagnosis and to extract information on anatomic locations and tumor stage. Tumors were categorized as being either “proximal colon” or “distal colon and rectal” cancers.
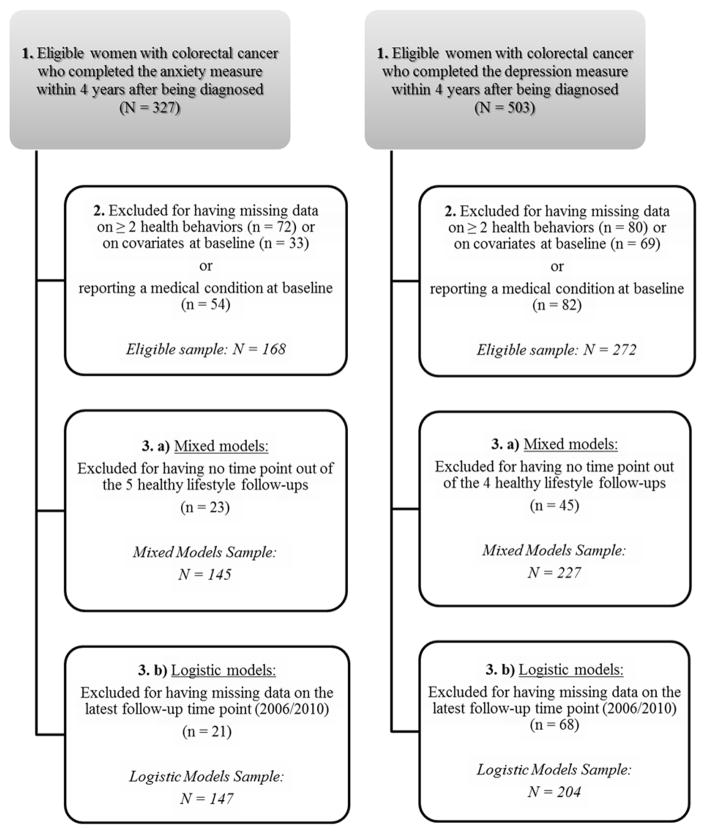
Figure 1 describes the samples used for the analyses with anxiety and depression symptoms, respectively. Eligible participants had to be free of diabetes, myocardial infarction or angina at the time the psychological measure was completed (to capture the specific impact of cancer diagnosis on lifestyle and because these chronic conditions may affect subsequent health behaviors) (Newsom et al., 2012), and had no missing data on health behaviors and measures of potential covariates when psychological symptom measures were completed. This resulted in 166 women with CRC for analyses with anxiety and 272 women for analyses with depression symptoms. In models considering changes in lifestyle score over time, women without data for lifestyle at follow-up time points after diagnosis were excluded (analytic sample sizes: nanxiety=145; ndepression=227). Thus, women with at least one follow-up assessment of lifestyle were included and most of them completed all available assessments after being diagnosed with CRC (range of completed lifestyle assessments: anxiety analyses = 2 to 6; depression analyses = 2 to 5). In logistic regression models testing the odds of reporting an unhealthy lifestyle at the study termination, women with missing data on lifestyle at the study termination were excluded. To make best use of the available sample, end of follow-up was defined as the lifestyle status in 2010, or in 2006 for participants who had missing data on the 2010 lifestyle score (analytic sample sizes: nanxiety=147; ndepression=204). Differences between excluded and included women on baseline characteristics for each analysis are detailed in Appendix Table 1. Appendix Figure 1 summarizes the different time assessments of anxiety, depression and lifestyle of the current longitudinal research. The study protocol was approved by the institutional review boards of Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health.
Figure 1.
Flowchart of the different analytic samples.
Measures
Anxiety and depression symptoms
Detailed information about the psychological measures is provided in Appendix Text 1. In brief, anxiety symptoms were assessed in 1988 and 2004 using the validated self-report Crown-Crisp Index (CCI) (Crown & Crisp, 1966). The 8 items are scored as 0 “never”, 1 “sometimes”, or 2 “always”, with a derived sum ranging from 0 (no anxiety) to 16 (high anxiety). Internal consistency reliability in the Nurses’ Health Study cohort is modest (α1988=0.57; α2004=0.57). Anxiety symptoms were dichotomized into high (≥4) vs. lower (<4) symptom levels (Albert, Chae, Rexrode, Manson, & Kawachi, 2005).
Depression symptoms were measured in 1992, 1996 and 2000 using the five-item Mental Health Index (MHI-5) from the Medical Outcomes Study Short-Form 36 Health Status Survey (Ware & Sherbourne, 1992). Items answered with a score ranging from 1 “all the time” to 6 “none of the time” were summed and the total score was scaled from 0 (high symptoms) to 100 (low symptoms), where a value ≤60 indicates higher vs. >60 lower depression symptoms (Rumpf, Meyer, Hapke, & John, 2001). In this cohort, internal consistency reliability for the depression scale is high (α1992=0.82; α1996=0.81; α2000=0.80). Because anxiety and depression measures were not queried at the same time assessment in the Nurses’ Health Study (minimum of 4 years apart) and because they are modestly correlated in this cohort (e.g., using the closest available measure of anxiety and depression, r=0.30), they were investigated separately.
Lifestyle score
Consistent with a lifestyle composite index used previously (Chiuve et al., 2008; Grimmett et al., 2011; Loef & Walach, 2012; Schlesinger et al., 2014) and with available prevention and survivorship guidelines for chronic disease (El-Shami et al., 2015; World Health Organization, 2014), the lifestyle score included five behavior-related factors: physical activity, diet, BMI, alcohol and tobacco consumption. Single behaviors, obtained via self-report at pre-diagnosis, in 1988 (anxiety sample) or 1992 (depression sample), and then every four years (last follow-up assessment available: 2010), were first dichotomized according to whether individuals were compliant with recommended guidelines or not (1/0). Component scores were then summed to create a lifestyle score, ranging from 0 “less healthy” to 5 “most healthy”. Lifestyle was also dichotomized at endorsement of 4 or 5 healthy behaviors, as this score is related to ~50% lower risk of chronic disease in this cohort (Chiuve et al., 2008).
Physical activity was assessed with a validated questionnaire (Chasan-Taber et al., 1996). A score of 1 was assigned when women reported ≥150 minutes per week of moderate-to-vigorous activity (e.g., brisk walking, running, bicycling). BMI was derived using women’s self-reported initial height and updated weight at each follow-up assessment. Previous work with the cohort has shown self-reported weight is highly correlated with weight measured by study staff (r = 0.96) (Rimm et al., 1990). Optimal weight (score of 1) was defined as BMI≤25 kg/m2. Dietary information was obtained from the 131 item Food Frequency Questionnaire, which has high reproducibility and validity when compared with 1-week diet records over a one-year period (Yuan et al., 2017). The summary score used here encompasses the following components of the Alternative Healthy Eating Index (McCullough et al., 2002): higher intakes of vegetables, fruit, nuts, soy, and cereal fiber; high ratio of chicken plus fish to red meat and polyunsaturated to saturated fat; low intake of trans fat; and multivitamin use. Each component score ranged from 0 to 10 (optimal dietary behavior). As done previously (Chiuve et al., 2008; Stampfer, Hu, Manson, Rimm, & Willett, 2000), a healthy diet (score of 1) was defined as a score in the top 40% of the current cohort distribution (updated at each follow-up assessment), which is related to a lower risk of several diseases, including stroke, diabetes and cancer (Chiuve et al., 2012). Healthy alcohol consumption (score of 1) was defined as drinking ≤1 drink/day (Kushi et al., 2012; Mosca et al., 2011). Lastly, women received a score of 1 if they reported currently being a non-smoker. Component and overall lifestyle scores were updated at each 4-year assessment.
Covariates
Selected potential covariates included age at CRC diagnosis, year of diagnosis, time between cancer diagnosis and completion of the anxiety or depression questionnaire, cancer stage, tumor location, marital status, education level, and pre-diagnosis lifestyle (within 4 years before diagnosis). Marital status was self-reported at the same time anxiety or depression symptoms were assessed (anxiety analyses=1988 or 2004; depression analyses=1992, 1996 or 2000), while education was queried only once in 1992.
Statistical analysis
Linear mixed models for repeated measures evaluated changes over time in lifestyle (outcome) based on the initial level of anxiety symptoms (exposure; all models detailed herein were also conducted separately with depression). Because preliminary analyses revealed a non-linear association of time with lifestyle, a categorical time variable (by follow-up assessments of lifestyle) was used. Anxiety symptoms were modeled either as a dichotomous (score of <4 vs. ≥4 categorized as higher vs. lower symptoms) or as a continuous (standardized) measure. The model with a dichotomous exposure estimated change in lifestyle score linked to higher vs. lower levels of anxiety symptoms; the model with a continuous exposure estimated if there was a monotonic effect of each one standard deviation (SD) change in anxiety symptoms on future lifestyle score. In both models, the time effect tested if the lifestyle score changed across follow-up assessments. A second set of models further included an interaction term to test, for instance, if lifestyle score changed (e.g., decreased) more rapidly among women with higher vs. lower anxiety level. A priori pairwise comparisons evaluated for which follow-up assessments discrepancies in lifestyle levels between women with higher vs. lower symptoms were evident over the study duration. Distinct sensitivity analyses 1) excluded the last completed lifestyle assessment of women who died two years after, as habits may change closer to death; 2) excluded women who completed the psychological measures > 2 years after being diagnosed with CRC, to capture anxiety and depression symptoms experienced closer to diagnosis; 3) included time-updated information on incident diabetes, myocardial infarction or angina across follow-up; and 4) excluded women with BMI values < 18.5 kg/m2 as it may reflect a prodromal disease or medical conditions, and may lead to deleterious outcomes among individuals with CRC (Kroenke et al., 2016; Song & Giovannucci, 2016). Of note, less than 4% and 8% of the anxiety and depression analytic samples, respectively, had a BMI value below 18.5 kg/m2 at any given follow-up assessment.
The association of baseline levels of psychological symptoms (exposure; dichotomous [higher vs. lower anxiety/depression symptoms] and continuous [standardized]) with the odds of reporting an unhealthy lifestyle at the end of follow-up (outcome; 2006 or 2010) was estimated with logistic regression models. An unhealthy lifestyle was defined as reporting ≤3 healthy behaviors at the end of follow-up. Similarly, as for the linear mixed models, distinct sensitivity analyses excluded women who 1) completed the psychological measures more than 2 years after being diagnosed with CRC, and 2) had lower BMI values (<18.5 kg/m2) as described above (the two other sensitivity analyses detailed above were not conducted here because they cannot be computed with only the two time points used in these logistic regression models). Finally, for the primary analyses, models stratified by either baseline lifestyle score (healthy/unhealthy) or by follow-up length (≤10 years/>10 years) were also evaluated.
As unhealthy women (e.g., with incident chronic condition or CRC recurrence) might be more likely to drop out of the study or die throughout follow-up, person- and time-specific inverse probability weights were created and included in the models (Hernan, Hernandez-Diaz, & Robins, 2004). Specifically, the probability of participating at each assessment was modeled based on the exposure and covariates of interest among women who were included, and then a weight that corresponded to the inverse probability of participating was created. As results from age-adjusted models were similar to those from fully-adjusted models, only the latter are shown. Analyses were conducted using SAS software version 9.3 with a 5% level of significance.
Results
Baseline Characteristics
At study baseline, women in the anxiety sample (n=145) were 66.4 years old on average (SD=8.6; range 42–83 years), most were registered nurses (67.6%) and in a relationship/married (75.2%). The mean time between CRC diagnosis and anxiety measure completion was 2.0 years (SD=1.2; range 0.08–3.92), with 74.5% of the women diagnosed with a non-advanced CRC (stages 0–II) and 49.7% with a proximal colon tumor. Most women reported low alcohol consumption (87.6%), were non-smokers (93.8%) and nearly half had a healthy BMI (i.e., ≤25 kg/m2; 47.8%). More than one out of four women engaged in at least 150 minutes of moderate-to-vigorous physical activity (27.5%). The derived lifestyle score at baseline was comparable to the pre-diagnosis score (meanbaseline=3.0, SD=1.0 vs. meanpre-diagnosis=2.9, SD=1.2). A quarter of the women reported high anxiety levels (26.2%) and 18 women died (12.4%) over the course of follow-up. Characteristics were similar in the depression sample (Appendix Text 2), although high depression levels were evident in 11.5% of women only. The distribution of covariates did not differ across levels of psychological symptoms (e.g., higher vs. lower anxiety symptoms).
Changes in Lifestyle Over Time
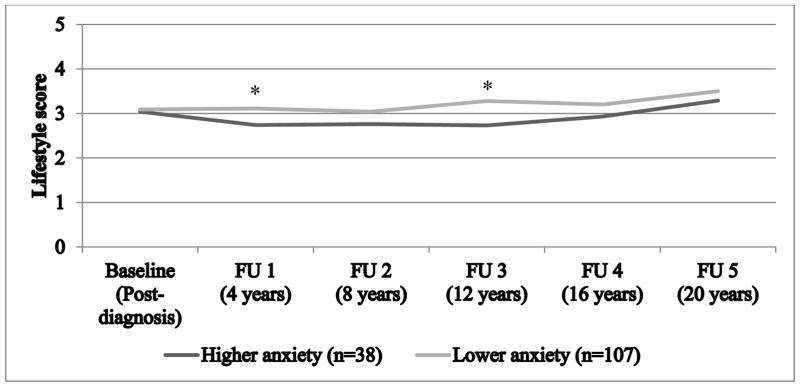
In the first model, there was a significant main effect of dichotomous anxiety only (βhigher vs. lower symptoms= −0.25, CI= −0.44, −0.05, panxiety group effect=0.01 [Figure 2]; ptime effect=0.09 [decomposition of time effect per time assessment in Appendix Table 2]), suggesting higher vs. lower anxiety symptoms were related to lower lifestyle scores throughout follow-up. This trend was also evident when considering single habits, whereby women with higher vs. lower anxiety levels tended to report poorer health-related behaviors, particularly diet and BMI (Appendix Table 4). Similarly, each 1-SD increase in anxiety levels was related to a significant decrease in lifestyle score across all time points (β= −0.18, CI= −0.27, −0.10, p1-SD increase<0.0001); no secular changes in lifestyle score were evident over the follow-up period (ptime effect=0.09). Further, the interaction term of anxiety symptoms with time assessments was not significant in either models (dichotomous symptoms: p=0.41; continuous symptoms: p=0.75), hinting that rate of change in lifestyle score over time did not depend on initial anxiety levels. As depicted in Figure 2, women reporting higher vs. lower anxiety symptoms exhibited a significantly lower (unhealthier) adjusted mean lifestyle score at 4 and 12 years (p=0.01 and p=0.02, respectively) after the anxiety assessment.
Figure 2. Adjusted means of lifestyle score throughout follow-up according to post-diagnosis anxiety symptoms.
Notes. The lifestyle score ranges from 0 (reaching the recommended guidelines for none of the 5 health behaviors) to 5 (reaching the recommended guidelines for all of the 5 health behaviors). Means adjusted for age at diagnosis (continuous), year of diagnosis (continuous), time between cancer diagnosis and completion of the anxiety questionnaire (continuous), cancer stage (advanced [stages III–IV] vs. non-advanced [stages 0–II]), tumor location (proximal colon vs. distal colon and rectal tumors), marital status (in a relationship vs. not), education level (registered nurses vs. university degree), and pre-diagnosis lifestyle score (ranges from 0 to 5). Post-diagnosis represents the time point when the anxiety measure was assessed. Asterisk (*) represents a significant difference between the lifestyle mean score of the two groups at a specific follow-up assessment (p≤0.05), while a superscript (a) represents a marginal difference (p≤0.10). FU = follow-up assessment.
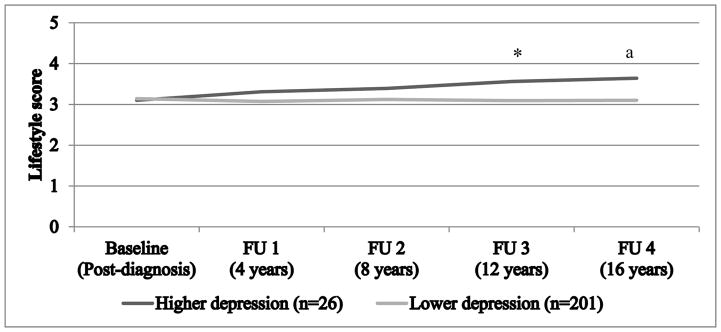
Conversely, results from models with dichotomized and continuous depression measures (Appendix Table 3, Models 1 and 2) showed that neither baseline depression symptoms nor time were significantly related to lifestyle mean score. Few differences were observed when single health behaviors were compared across depression levels, besides lower alcohol consumption among more depressed women (Appendix 4). In addition, no clear interaction effect emerged (dichotomous depression symptoms: p=0.29; continuous depression symptoms: p=0.31). Nonetheless, Figure 3 hints at a different rate of change in the adjusted mean lifestyle score throughout follow-up assessments depending on initial level of depression symptoms: women with higher symptoms reported a slight improvement in their lifestyle over time. Accordingly, pairwise comparisons revealed that having higher vs. lower depression symptoms at baseline was associated with healthier lifestyle score 12 and 16 years later (p=0.05 and p=0.10, respectively).
Figure 3. Adjusted means of lifestyle score throughout follow-up according to post-diagnosis depression symptoms.
Notes. The lifestyle score ranges from 0 (reaching the recommended guidelines for none of the 5 health behaviors) to 5 (reaching the recommended guidelines for all of the 5 health behaviors). Means adjusted for age at diagnosis (continuous), year of diagnosis (continuous), time between cancer diagnosis and completion of the depression questionnaire (continuous), cancer stage (advanced [stages III–IV] vs. non-advanced [stages 0–II]), tumor location (proximal colon vs. distal colon and rectal tumors), marital status (in a relationship vs. not), education level (registered nurses vs. university degree), and pre-diagnosis lifestyle score (ranges from 0 to 5). Post-diagnosis represents the time point when the depression measure was assessed. Asterisk (*) represents a significant difference between the lifestyle mean score of the two groups at a specific follow-up assessment (p≤0.05), while a superscript (a) represents a marginal difference (p≤0.10). FU = follow-up assessment.
Anxiety and depression findings were unchanged after either excluding the last lifestyle follow-up assessment among women who died within 2 years of that assessment, excluding women who completed the psychological measures within 2 years of CRC diagnosis, or including incident chronic conditions over follow-up (results not shown). Excluding women with lower BMI values (<18.5 kg/m2) yielded highly similar results (results not shown).
Odds of Having an Unhealthy Lifestyle at the End of Follow-Up
Every 1-SD increase in anxiety symptoms was related to 63% greater odds of reporting an unhealthy lifestyle at the study termination (odds ratios: OR=1.63, CI=1.06–2.52, p=0.03). A similar pattern, albeit not statistically significant, was noted with dichotomous anxiety (ORhigher vs. lower symptoms=2.03, CI=0.84–4.90, p=0.12). In analyses considering effect modification by initial lifestyle score or follow-up length, only the interaction term of dichotomized anxiety levels by follow-up length was significant (Table 1); higher vs. lower anxiety symptoms had a stronger impact on later unhealthy lifestyle within a shorter vs. longer follow-up period (≤10 years: OR=6.03, CI=1.39–26.17, p=0.02; >10 years: OR=0.80, CI=0.22–2.98, p=0.74; pinteraction=0.05).
Table 1.
Logistic regression models evaluating the association of post-diagnosis anxiety and depression symptoms with the odds of having an unhealthy lifestyle at the end of follow-up (either 2006 or 2010)
| Stratified Analyses | ||||
|---|---|---|---|---|
| Anxiety | Depression | |||
| Healthy | Unhealthy | Healthy | Unhealthy | |
| (Higher vs. lower symptoms) | (Higher vs. lower symptoms) | (Higher vs. lower symptoms) | (Higher vs. lower symptoms) | |
|
|
||||
| Sample size | 53 | 94 | 76 | 128 |
| (number of women with unhealthy lifestyle at follow-up) | (21) | (81) | (26) | (109) |
| Odds Ratio | 2.42 | 5.30a | 0.36 | 0.32a |
| (95% CI) | (0.69, 8.50) | (0.81, 34.82) | (0.05, 2.55) | (0.10, 1.04) |
|
| ||||
| Interaction: p=0.73 | Interaction: p=0.81 | |||
| Anxiety | Depression | |||
| ≤10 years of FU | > 10 years of FU | ≤10 years of FU | > 10 years of FU | |
| (Higher vs. lower symptoms) | (Higher vs. lower symptoms) | (Higher vs. lower symptoms) | (Higher vs. lower symptoms) | |
|
|
||||
| Sample size | 102 | 45 | 92 | 112 |
| (number of women with unhealthy lifestyle at follow-up) | (71) | (31) | (61) | (74) |
| Odds Ratio | 6.03* | 0.80 | 1.44 | 0.35a |
| (95% CI) | (1.39, 26.17) | (0.22, 2.98) | (0.28, 7.37) | (0.10, 1.24) |
|
| ||||
| Interaction: p=0.05 | Interaction: p=0.08 | |||
Notes. Sensitivity analyses using only the 2010 lifestyle score as the outcome led to highly similar estimates but with larger confidence intervals compared to results obtained with the 2006–2010 assessments combined. To ensure more stable estimates and make best use of the available sample, only the latter results are presented. Models adjusted for age at diagnosis (continuous), year of diagnosis (continuous), time between cancer diagnosis and completion of the anxiety or depression questionnaire (continuous), cancer stage (advanced [stages III–IV] vs. non-advanced [stages 0–II]), tumor location (proximal colon vs. distal colon and rectal tumors), marital status (in a relationship vs. not), education level (registered nurses vs. university degree), and pre-diagnosis lifestyle score (ranges from 0 to 5). CI=confidence intervals, FU=follow-up;
p≤0.10,
p≤0.05,
p≤0.01,
p≤0.001.
Although the association did not reach statistical significance, women with higher vs. lower depression symptoms had 51% reduced odds (OR=0.49, CI=0.20–1.23, p=0.13) of having an unhealthy lifestyle at the end of follow-up. Every SD increase in depression symptoms was also related to a non-significant 19% reduced odds (OR=0.81, CI=0.61–1.07, p=0.14) of reporting an unhealthy lifestyle later on. While the interaction term testing if baseline levels of lifestyle altered effects of depression symptoms on future lifestyle score was not significant (Table 1; p=0.81), follow-up length may moderate effects of depression symptoms on future lifestyle. More specifically, while women with higher vs. lower symptoms had non-significantly increased odds of reporting a less healthy lifestyle within 10 years of follow-up (OR=1.44, CI=0.28–7.37, p=0.66), higher depression symptoms were related to marginally decreased odds after 10 years of follow-up (OR=0.35, CI=0.10–1.24, p=0.10; pinteraction=0.08). For both anxiety and depressive symptoms, estimates derived from analyses conducted only among women who completed the psychological measures within 2 years of CRC diagnosis or only after excluding those with lower BMI values provided estimates that were nearly identical to those derived with the more inclusive sample (results not shown).
Discussion
To our knowledge, this study is the first to examine whether anxiety and depression symptoms affect lifestyle after a CRC diagnosis among midlife women over up to 20 years of follow-up. As hypothesized, more anxious women showed an overall unhealthier lifestyle throughout follow-up compared to their less anxious counterparts, even after adjusting for relevant covariates including pre-diagnosis levels of habits. Moreover, every SD increase in anxiety symptoms was significantly related to decreasing lifestyle score (unhealthier) over two decades and to 63% increased odds of having an unhealthy lifestyle at the study termination. These results suggest a dose-response relationship, whereby even less severe anxiety symptoms may lead to alterations in health-related behaviors. Conversely, results from the main analyses revealed no clear evidence that initial levels of depression symptoms impact lifestyle over time.
However, findings from the stratified analyses suggested that follow-up length may be an important moderator of the relationship between psychological distress and future lifestyle. In fact, by leveraging the unique follow-up covering two decades, results showed that anxiety and to a lesser extent depression symptoms were related to increased odds of having an unhealthy lifestyle when measured within 10 years after diagnosis (e.g., a six-fold increase in women with higher anxiety symptoms). This result is consistent with our initial hypothesis positing a deleterious impact of psychological symptoms on the subsequent endorsement of a healthy lifestyle. It is also consistent with prior work assessing psychological symptoms within the first years following diagnosis among individuals with cancer (Park & Gaffey, 2007; van Putten et al., 2016; Vijayvergia & Denlinger, 2015) and with results obtained from cancer-free adults (McKenzie & Harris, 2013; Trudel-Fitzgerald, Tworoger, Poole, Williams, & Kubzansky, 2016).
Interestingly, when lifestyle was assessed more than 10 years post-diagnosis, effects of psychological symptoms were largely attenuated or in the case of depression, associated with reduced odds of having a future unhealthy lifestyle. Until the current study, limited longitudinal research has characterized health behaviors over such a long period of time, and it was unclear whether psychological symptoms might adversely impact lifestyle habits even a decade after a CRC diagnosis. In this sample, an apparent protective effect of baseline depression symptoms on subsequent likelihood of adopting an unhealthy lifestyle was found. This could be explained by mechanisms relevant primarily among longer-term survivors. For instance, patients who are in remission 10 years after being diagnosed with CRC may regain a sense of control over their health or enhance their sense of self-efficacy by engaging in healthy behaviors over time (Kanera et al., 2016; Park & Gaffey, 2007; Schnoll et al., 2004). It is also possible that survivors replace maladaptive strategies elicited by the cancer diagnosis (e.g., avoidance, rumination, disengagement) with more adaptive strategies over time, particularly after reaching a remission stage (e.g., problem-solving, reappraisal, use of social support). In fact, while maladaptive strategies are associated with higher levels of psychological symptoms among individuals with cancer, adaptive strategies are related to healthier behaviors in this population (Dempster, Howell, & McCorry, 2015; Park & Gaffey, 2007; Trudel-Fitzgerald et al., 2017). Alternatively, other authors have noted that higher baseline depression scores were related to greater exercise levels among women with breast cancer over a 12-month follow-up period; they explained this unexpected finding by speculating that women with a cancer diagnosis may develop an increased awareness of the beneficial impact of exercise on mood (Pinto, Trunzo, Reiss, & Shiu, 2002). A similar mechanism may be at play here, whereby women with higher initial depression symptoms may engage in healthier behaviors over time in an attempt to improve their mood.
Nonetheless, caution is required when interpreting results from the current study. Although these findings might suggest somewhat different patterns of anxiety and depression symptoms on future lifestyle among patients with CRC, these differences may also be due to methodological artifacts. The smaller sample size and shorter length of follow-up for the anxiety vs. the depression sample might affect the associations with lifestyle and their directionality. The marginal significance of some results combined with the occasionally large confidence intervals may also reflect unstable estimates. Moreover, given the particularly low level of depression symptoms in study participants and the fairly small analytic sample sizes, replication of these results using larger samples is needed before drawing firm conclusions.
If replicated in future research, the changes in lifestyle score evident herein, both positive and negative, are non-negligible. Numerous studies have documented suboptimal levels of diet, physical activity, BMI, alcohol and tobacco consumption among individuals with cancer shortly after diagnosis (Bluethmann et al., 2015; Grimmett et al., 2011; Schlesinger et al., 2014). These habits are also well-documented risk factors for other illnesses, including cardiovascular disease (Chiuve et al., 2008; Newsom et al., 2012), whereas adopting at least 4 of those behaviors would strongly protect against early death (Loef & Walach, 2012). Given the cardiotoxicity of cancer treatments (Hong, Iimura, Sumida, & Eager, 2010), patients exhibiting unfavorable behaviors are at an even higher risk of fatal cardiovascular diseases. Thus, adopting a healthy lifestyle may have a considerable positive impact on various health outcomes throughout their survivorship.
Limited research has evaluated feasibility of lifestyle interventions targeting multiple behaviors in patients with CRC, although preliminary findings are suggestive. For example, a 12-week telephone-based lifestyle intervention conducted among 29 patients with CRC who had completed treatment within the prior 6 months showed significant improvements on objectively measured daily step counts (p = 0.001) and physical activity (p = 0.004), as well as on various self-reported components of diet (e.g., fruits/vegetables and meat intake; p< 0.05) (Grimmett, Simon, Lawson, & Wardle, 2015); similar findings were observed among another sample of 18 patients with CRC who were randomized to a 12-week in person intervention (Bourke et al., 2011). However, a feasibility study conducted among 18 patients with CRC revealed that improvements in diet, but not in physical activity, which occurred during the 3-month lifestyle intervention were maintained at the study termination one month later (Anderson, Caswell, Wells, Steele, & Macaskill, 2010). Given the short follow-up period considered in studies to date, the longer-term impact of modifying lifestyle on health among individuals with cancer is yet to be examined. Treating psychological symptoms shortly after diagnosis and documenting any subsequent changes on lifestyle might be another promising direction for future studies.
This study has some limitations. Given these observational data are obtained in a cohort of professional women, findings may not generalize to other populations. In the current study, data on anxiety and depression symptoms pre-diagnosis were too limited to be considered; however, accounting for these pre-diagnosis symptoms would likely lead to similar findings, given preliminary evidence suggesting that psychological states are largely stable over long periods of time, even when individuals are diagnosed with cancer in the intervening period (Jones et al., 2015; Schumacher et al., 2013). Other forms of distress that are common in oncology settings, like fear of recurrence (Custers et al., 2016), were not assessed in this cohort, whereas the anxiety measure used herein has some limitations including somewhat low internal consistency reliability and greater emphasis on potential anxiety-provoking situations rather than anxiety symptomatology per se. Further, distress and lifestyle measures queried closer in time to the cancer diagnosis may provide additional insight on psychological and behavioral changes that occur during that period (Bluethmann et al., 2015; Dennis, Waring, Payeur, Cosby, & Daudt, 2013) and guide earlier interventions. Moreover, while the current results were robust after adjusting for incident chronic conditions over the study period, residual confounding related to issues that are specific to CRC (e.g., recurrence, ostomy) may still exist, given the observational design; however, such information were not queried in this cohort. Lastly, self-reported habits may be vulnerable to social desirability, which can lead to biased estimation of health behaviors (Adams et al., 2005; Hebert et al., 2002). Yet, even if absolute levels are not reported completely accurately, behaviors would likely still be categorized appropriately as either healthy/unhealthy.
Strengths of this study are the use of a longitudinal study design with multiple lifestyle assessments that capture natural health behaviors changes over 20 years. By leveraging data from a well-characterized prospective cohort, this is the first research to describe associations of psychological symptoms with future lifestyle in women with CRC that are independent of pre-diagnosis levels of health behaviors. Using inverse probability weights reduced the likelihood of selection bias, while the composite lifestyle score facilitates a comprehensive perspective on how anxiety and depression symptoms impact multiple habits that jointly matter for health.
Overall, the current findings suggest that anxiety symptoms are significantly related to subsequent unhealthier lifestyle in midlife women diagnosed with CRC; effects of depression are less clear-cut. Results from more nuanced analyses hinted that anxiety and depression may be related to an unhealthier lifestyle in the first 10 years after CRC diagnosis while the detrimental effects of initially high levels of distress on habits seem to either fade or even be related to lower likelihood of having an unhealthy lifestyle in women who survive at least 10 years past diagnosis. Despite the use of a weighting strategy to account for dropouts across the follow-up period, a residual “healthy worker survivor effect” (Shah, 2009) may explain these longer term results, whereby only healthier women who are more likely to engage in favorable habits survived at least 10 years. If replicated, our findings suggest that a deeper understanding of the role of psychological factors on lifestyle may allow more targeted identification of survivors who would most benefit from making behavioral changes. Moreover, treating anxiety and depression symptoms early in the cancer trajectory may not solely reduce cancer-related psychological distress but also promote healthier lifestyle, hence improving overall quality of life and survival.
Supplementary Material
Acknowledgments
Acknowledgments & Financial Support
We would like to thank the participants and the staff of the Nurses’ Health Study for their valuable contributions as well as the following American state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. This work was supported the National Institute of Health (grant number R01 CA163451) and Department Of Defense (grant number W81XWH-13-1-0493). The Nurses’ Health Study is supported by the National Institute of Health (grants number UM1 CA186107 and P01 CA87969). CTF received postdoctoral fellowships from the Canadian Institutes of Health Research and from the Fonds de Recherche du Québec - Santé.
References
- Adams SA, Matthews CE, Ebbeling CB, Moore CG, Cunningham JE, Fulton J, Hebert JR. The effect of social desirability and social approval on self-reports of physical activity. Am J Epidemiol. 2005;161(4):389–398. doi: 10.1093/aje/kwi054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111(4):480–487. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Colorectal Cancer Facts & Figures 2014–2016. 2014. Retrieved from Atlanta. [Google Scholar]
- Anderson AS, Caswell S, Wells M, Steele RJ, Macaskill S. “It makes you feel so full of life” LiveWell, a feasibility study of a personalised lifestyle programme for colorectal cancer survivors. Support Care Cancer. 2010;18(4):409–415. doi: 10.1007/s00520-009-0677-4. [DOI] [PubMed] [Google Scholar]
- Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE, Chavarro JE. Origin, methods, and evolution of the three Nurses’ Health Studies. Am J Public Health. 2016;106(9):1573–1581. doi: 10.2105/AJPH.2016.303338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DM, Murphy JM, Goldman PA, Jr, WJE, Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Medical Care. 1991;29(2):169–176. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- Bluethmann SM, Basen-Engquist K, Vernon SW, Cox M, Gabriel KP, Stansberry SA, … Demark-Wahnefried W. Grasping the ‘teachable moment’: Time since diagnosis, symptom burden and health behaviors in breast, colorectal and prostate cancer survivors. Psychooncology. 2015 doi: 10.1002/pon.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke L, Thompson G, Gibson DJ, Daley A, Crank H, Adam I, … Saxton J. Pragmatic lifestyle intervention in patients recovering from colon cancer: A randomized controlled pilot study. Arch Phys Med Rehabil. 2011;92(5):749–755. doi: 10.1016/j.apmr.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Chambers SK, Lynch BM, Aitken J, Baade P. Relationship over time between psychological distress and physical activity in colorectal cancer survivors. J Clin Oncol. 2009;27(10):1600–1606. doi: 10.1200/JCO.2008.18.5157. [DOI] [PubMed] [Google Scholar]
- Chang SC, Wang W, Pan A, Jones RN, Kawachi I, Okereke OI. Racial variation in depression risk factors and symptom trajectories among older women. American Journal of Geriatric Psychiatry. 2016;24(11):1051–1062. doi: 10.1016/j.jagp.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, … Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, … Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB. Primary prevention of stroke by healthy lifestyle. Circulation. 2008;118(9):947–954. doi: 10.1161/CIRCULATIONAHA.108.781062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown S, Crisp AH. A short clinical diagnostic self-rating scale for psychoneurotic patients: The Middlesex Hospital Questionnaire (M.H.Q.) Br J Psychiatry. 1966;112(490):917–923. doi: 10.1192/bjp.112.490.917. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Smits N, Donker T, ten Have M, de Graaf R. Screening for mood and anxiety disorders with the five-item, the three-item, and the two-item Mental Health Inventory. Psychiatry Research. 2009;168(3):250–255. doi: 10.1016/j.psychres.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Custers JA, Gielissen MF, Janssen SH, de Wilt JH, Prins JB. Fear of cancer recurrence in colorectal cancer survivors. Support Care Cancer. 2016;24(2):555–562. doi: 10.1007/s00520-015-2808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster M, Howell D, McCorry NK. Illness perceptions and coping in physical health conditions: A meta-analysis. J Psychosom Res. 2015;79(6):506–513. doi: 10.1016/j.jpsychores.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Dennis DL, Waring JL, Payeur N, Cosby C, Daudt HM. Making lifestyle changes after colorectal cancer: Insights for program development. Curr Oncol. 2013;20(6):e493–511. doi: 10.3747/co.20.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J, Ng SK, Holland J, Aitken J, Youl P, Baade PD, Chambers SK. Trajectories of psychological distress after colorectal cancer. Psychooncology. 2013;22(8):1759–1765. doi: 10.1002/pon.3210. [DOI] [PubMed] [Google Scholar]
- El-Shami K, Oeffinger KC, Erb NL, Willis A, Bretsch JK, Pratt-Chapman ML, … Cowens-Alvarado RL. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J Clin. 2015;65(6):428–455. doi: 10.3322/caac.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, … Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Ferrer RA, Green PA, Barrett LF. Affective science perspectives on cancer control: Strategically crafting a mutually beneficial research agenda. Perspect Psychol Sci. 2015;10(3):328–345. doi: 10.1177/1745691615576755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmett C, Bridgewater J, Steptoe A, Wardle J. Lifestyle and quality of life in colorectal cancer survivors. Qual Life Res. 2011;20(8):1237–1245. doi: 10.1007/s11136-011-9855-1. [DOI] [PubMed] [Google Scholar]
- Grimmett C, Simon A, Lawson V, Wardle J. Diet and physical activity intervention in colorectal cancer survivors: A feasibility study. Eur J Oncol Nurs. 2015;19(1):1–6. doi: 10.1016/j.ejon.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen IO, Jess P. Possible better long-term survival in left versus right-sided colon cancer: A systematic review. Dan Med J. 2012;59(6):A4444. [PubMed] [Google Scholar]
- Hawkes AL, Patrao TA, Baade P, Lynch BM, Courneya KS. Predictors of physical activity in colorectal cancer survivors after participation in a telephone-delivered multiple health behavior change intervention. J Cancer Surviv. 2015;9(1):40–49. doi: 10.1007/s11764-014-0389-8. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Ebbeling CB, Matthews CE, Hurley TG, Ma Y, Druker S, Clemow L. Systematic errors in middle-aged women’s estimates of energy intake: comparing three self-report measures to total energy expenditure from doubly labeled water. Ann Epidemiol. 2002;12(8):577–586. doi: 10.1016/s1047-2797(01)00297-6. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- Hong RA, Iimura T, Sumida KN, Eager RM. Cardio-oncology/onco-cardiology. Clin Cardiol. 2010;33(12):733–737. doi: 10.1002/clc.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine J, Firestone J, Ong L, Cribbie R, Dorian P, Harris L, et al. A randomized controlled trial of cognitive behavior therapy tailored to psychological adaptation to an implantable cardioverter defibrillator. Psychosomatic Medicine. 2011;73(3):226–233. doi: 10.1097/PSY.0b013e31820afc63. [DOI] [PubMed] [Google Scholar]
- Jones SM, LaCroix AZ, Li W, Zaslavsky O, Wassertheil-Smoller S, Weitlauf J, … Danhauer SC. Depression and quality of life before and after breast cancer diagnosis in older women from the Women’s Health Initiative. J Cancer Surviv. 2015;9(4):620–629. doi: 10.1007/s11764-015-0438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanera IM, Bolman CA, Mesters I, Willems RA, Beaulen AA, Lechner L. Prevalence and correlates of healthy lifestyle behaviors among early cancer survivors. BMC Cancer. 2016;16:4. doi: 10.1186/s12885-015-2019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CH, Neugebauer R, Meyerhardt J, Prado CM, Weltzien E, Kwan ML, … Caan BJ. Analysis of body mass index and mortality in patients with colorectal cancer using causal diagrams. JAMA Oncol. 2016;2(9):1137–1145. doi: 10.1001/jamaoncol.2016.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Winning A, Kawachi I. Affective states and health. In: Berkman LF, Kawachi I, Glymour MM, editors. Social Epidemiology. 2. New York: Oxford University Press; 2014. pp. 320–364. [Google Scholar]
- Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, … Gansler T. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- Lee J, Jeon JY, Meyerhardt JA. Diet and lifestyle in survivors of colorectal cancer. Hematol Oncol Clin North Am. 2015;29(1):1–27. doi: 10.1016/j.hoc.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: A systematic review and meta-analysis. Prev Med. 2012;55(3):163–170. doi: 10.1016/j.ypmed.2012.06.017. [DOI] [PubMed] [Google Scholar]
- McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, … Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261–1271. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- McKenzie SH, Harris MF. Understanding the relationship between stress, distress and healthy lifestyle behaviour: A qualitative study of patients and general practitioners. BMC Fam Pract. 2013;14:166. doi: 10.1186/1471-2296-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meader N, King K, Moe-Byrne T, Wright K, Graham H, Petticrew M, … Sowden AJ. A systematic review on the clustering and co-occurrence of multiple risk behaviours. BMC Public Health. 2016;16:657. doi: 10.1186/s12889-016-3373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, … Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57(12):1404–1423. doi: 10.1016/j.jacc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher CE, Winger JG, Given BA, Helft PR, O’Neil BH. Mental health outcomes during colorectal cancer survivorship: A review of the literature. Psychooncology. 2016;25(11):1261–1270. doi: 10.1002/pon.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narrow WE, Rae DS, Robins LN, Regier DA. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry. 2002;59(2):115–123. doi: 10.1001/archpsyc.59.2.115. [DOI] [PubMed] [Google Scholar]
- Newsom JT, Huguet N, McCarthy MJ, Ramage-Morin P, Kaplan MS, Bernier J, … Oderkirk J. Health behavior change following chronic illness in middle and later life. J Gerontol B Psychol Sci Soc Sci. 2012;67(3):279–288. doi: 10.1093/geronb/gbr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble N, Paul C, Turon H, Oldmeadow C. Which modifiable health risk behaviours are related? A systematic review of the clustering of Smoking, Nutrition, Alcohol and Physical activity (‘SNAP’) health risk factors. Prev Med. 2015;81:16–41. doi: 10.1016/j.ypmed.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Oldroyd JC, Cyril S, Wijayatilaka BS, O’Neil A, McKenzie DP, Zavarsek S, et al. Evaluating the impact of depression, anxiety & autonomic function on health related quality of life, vocational functioning and health care utilisation in acute coronary syndrome patients: the ADVENT study protocol. BMC Cardiovasc Disord. 2013;13:103. doi: 10.1186/1471-2261-13-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CL, Gaffey AE. Relationships between psychosocial factors and health behavior change in cancer survivors: An integrative review. Ann Behav Med. 2007;34(2):115–134. doi: 10.1007/BF02872667. [DOI] [PubMed] [Google Scholar]
- Pinto BM, Trunzo JJ, Reiss P, Shiu SY. Exercise participation after diagnosis of breast cancer: Trends and effects on mood and quality of life. Psychooncology. 2002;11(5):389–400. doi: 10.1002/pon.594. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- Rumpf HJ, Meyer C, Hapke U, John U. Screening for mental health: validity of the MHI-5 using DSM-IV Axis I psychiatric disorders as gold standard. Psychiatry Res. 2001;105(3):243–253. doi: 10.1016/s0165-1781(01)00329-8. [DOI] [PubMed] [Google Scholar]
- Schlesinger S, Walter J, Hampe J, von Schonfels W, Hinz S, Kuchler T, … Nothlings U. Lifestyle factors and health-related quality of life in colorectal cancer survivors. Cancer Causes Control. 2014;25(1):99–110. doi: 10.1007/s10552-013-0313-y. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Rothman RL, Newman H, Lerman C, Miller SM, Movsas B, … Cheng J. Characteristics of cancer patients entering a smoking cessation program and correlates of quit motivation: Implications for the development of tobacco control programs for cancer patients. Psychooncology. 2004;13(5):346–358. doi: 10.1002/pon.756. [DOI] [PubMed] [Google Scholar]
- Schumacher JR, Palta M, Loconte NK, Trentham-Dietz A, Witt WP, Heidrich SM, Smith MA. Characterizing the psychological distress response before and after a cancer diagnosis. J Behav Med. 2013;36(6):591–600. doi: 10.1007/s10865-012-9453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. Healthy worker effect phenomenon. Indian J Occup Environ Med. 2009;13(2):77–79. doi: 10.4103/0019-5278.55123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Giovannucci E. Preventable incidence and mortality of carcinoma associated with lifestyle factors among White adults in the United States. JAMA Oncol. 2016;2(9):1154–1161. doi: 10.1001/jamaoncol.2016.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory: Bibliography. 2. Palo Alto, CA: Consulting Psychologists Press; 1989. [Google Scholar]
- Spring B, King AC, Pagoto SL, Van Horn L, Fisher JD. Fostering multiple healthy lifestyle behaviors for primary prevention of cancer. Am Psychol. 2015;70(2):75–90. doi: 10.1037/a0038806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343(1):16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- Steel Z, Marnane C, Iranpour C, Chey T, Jackson JW, Patel V, Silove D. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980–2013. Int J Epidemiol. 2014;43(2):476–493. doi: 10.1093/ije/dyu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel-Fitzgerald C, Chen Y, Singh A, Okereke OI, Kubzansky LD. Psychiatric, psychological, and social determinants of health in the Nurses’ Health Study cohorts. American Journal of Public Health. 2016;106(9):1644–1649. doi: 10.2105/AJPH.2016.303318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel-Fitzgerald C, Savard J, Ivers H. Evolution of cancer-related symptoms over an 18-month period. J Pain Symptom Manage. 2013;45(6):1007–1018. doi: 10.1016/j.jpainsymman.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Trudel-Fitzgerald C, Savard J, Ivers H. Longitudinal changes in clusters of cancer patients over an 18-month period. Health Psychol. 2014;33(9):1012–1022. doi: 10.1037/a0033497. [DOI] [PubMed] [Google Scholar]
- Trudel-Fitzgerald C, Savard J, Slim LM, Roy RC, Flett GL, Hewitt PL, Ivers H. The relationship of perfectionism with psychological symptoms in cancer patients and the contributing role of hyperarousability and coping. Psychol Health. 2017;32(4):381–401. doi: 10.1080/08870446.2016.1273354. [DOI] [PubMed] [Google Scholar]
- Trudel-Fitzgerald C, Tworoger SS, Poole EM, Williams DR, Kubzansky LD. Prospective changes in healthy lifestyle among midlife women: When psychological symptoms get in the way. Am J Prev Med. 2016;51(3):327–335. doi: 10.1016/j.amepre.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Putten M, Husson O, Mols F, Luyer MD, van de Poll-Franse LV, Ezendam NP. Correlates of physical activity among colorectal cancer survivors: Results from the longitudinal population-based profiles registry. Support Care Cancer. 2016;24(2):573–583. doi: 10.1007/s00520-015-2816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayvergia N, Denlinger CS. Lifestyle factors in cancer survivorship: Where we are and where we are headed. J Pers Med. 2015;5(3):243–263. doi: 10.3390/jpm5030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- Willett WC, Green A, Stampfer MJ, Speizer FE, Colditz GA, Rosner B, … Hennekens CH. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med. 1987;317(21):1303–1309. doi: 10.1056/NEJM198711193172102. [DOI] [PubMed] [Google Scholar]
- Williams K, Steptoe A, Wardle J. Is a cancer diagnosis a trigger for health behaviour change? Findings from a prospective, population-based study. Br J Cancer. 2013;108(11):2407–12. doi: 10.1038/bjc.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global status report on noncommunicable diseases. 2014. [Google Scholar]
- Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, … Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570–584. doi: 10.1093/aje/kww104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.