This population register–based study assesses the association of genetics and rearing with parent-offspring resemblance for major depression in a national Swedish registry study.
Key Points
Question
How do effects of genetics and rearing each contribute to the transmission of risk for major depression from parents to children?
Findings
In this population register–based study of 2 269 552 offspring of intact, adoptive, not-lived-with father, stepfather, and triparental families from the general Swedish population, the effects of genes and rearing were approximately equal for parent-offspring resemblance for major depression. Genetic and rearing effects acted additively on offspring risk for major depression.
Meaning
Genetic and rearing effects are important in the cross-generational transmission of major depression.
Abstract
Importance
Twin studies have assessed sibling resemblance for major depression (MD) but cannot address sources of resemblance across generations.
Objective
To clarify the relative importance of genetic and rearing effects on the parent-offspring resemblance for MD.
Design
This Swedish population register–based study examined parents and children from the following 5 family types: intact (2 041 816 offspring), adoptive (14 104 offspring), not-lived-with (NLW) father (116 601 offspring), stepfather (67 826 offspring), and triparental (29 205 offspring). The 5 family types permitted quantification of parent-offspring resemblance for genes plus rearing, genes-only, and rearing-only associations. Treated MD was assessed from national primary care, specialist care, and inpatient registries. Data were collected from January 1, 1960, through December 31, 2016.
Exposure
Diagnosis of MD vs no diagnosis in parents.
Main Outcomes and Measures
Registration for MD.
Results
The study population included 2 269 552 offspring (51.5% male and 48.5% female; median age, 42; range, 26-56 years). The weighted tetrachoric correlations for MD across family types and across mothers and fathers were r = 0.17 (95% CI, 0.16-0.17) for genes plus rearing, r = 0.08 (95% CI, 0.06-0.09) for genes-only, and r = 0.08 (95% CI, 0.07-0.09) for rearing-only parent-child associations. Only the genes plus rearing association differed significantly between mothers (weighted tetrachoric correlation, r = 0.18; 95% CI, 0.18-0.18) and fathers (weighted tetrachoric correlation, r = 0.15; 95% CI, 0.15-0.16). In triparental families, the parent-offspring correlations for MD were estimated at r = 0.19 (95% CI, 0.17-0.22) for mothers in the genes plus rearing association, r = 0.10 (95% CI, 0.07-0.13) for NLW fathers in the genes-only association, and r = 0.08 (95% CI, 0.05-0.11) for stepfathers in the rearing-only association. In adoptive families, the effect of affected biological and affected adoptive parents on adoptee risk for MD was additive. In intact families, parental MD diagnosed by specialists in hospital or outpatient settings and primary care physicians affected equally the risk for MD in offspring.
Conclusions and Relevance
The parent-offspring resemblance for treated MD arises from genetic factors and rearing experiences to an approximately equal extent. Both forms of cross-generational transmission act additively on the risk for MD in the offspring.
Introduction
Twin studies have found that sibling resemblance for major depression (MD) is substantially influenced by genetic factors with little to no contribution from the shared family environment. However, twin studies examine members of the same generation and cannot address sources of resemblance across generations. The primary method for this assessment has been adoption studies. Although the twin literature on MD has increased in recent decades, 4 classic adoption studies of MD, all published from 1978 to 1986, had small sample sizes and substantial methodologic limitations and produced conflicting results. Two of these studies found significant transmission for MD from biological parents to adopted-away offspring but together identified only 15 cases of adoptee MD. The other 2 larger studies found no evidence of genetic transmission of MD across generations. One of these studies examined the association between MD in adoptive parents and their adoptive offspring and found no resemblance. More recently, a partial adoption study (lacking biological parents) found that MD in adoptive parents was significantly associated with MD in their adoptive adolescent offspring.
Renewed interest in adoption studies of psychiatric disorders has emerged largely owing to the availability of national record linkages in Scandinavia. However, to our knowledge, such studies have used psychiatric diagnoses from hospital admissions and sometimes specialist outpatient care. For MD, such samples are unlikely to be representative because most treated cases of MD are seen solely in outpatient primary care settings.
We herein report an expanded national Swedish adoption study of MD using a newly available primary care registry (PCR) that, when combined with hospital and psychiatric outpatient data, provides a representative sample of treated MD. We examine intact nuclear families, families with biological and adoptive parents from an adoption sample, families with not-lived-with (NLW) parents, families with stepparents, and triparental families to examine the following questions: (1) to what degree is the transmission of MD from parents to offspring the result of genetic vs rearing effects, and (2) how do genetic and rearing effects jointly contribute to the resemblance for MD between parents and their children? In particular, do they act additively or do they interact?
Methods
We collected information on individuals from Swedish population-based registers with national coverage linked using each person’s unique personal identification number, which was replaced with a serial number by Statistics Sweden to preserve confidentiality. We used the Multi-Generation Register, the Population and Housing censuses, the Swedish Hospital Discharge Register (national coverage, 1987-2012; partial coverage, 1969-1986), and the Outpatient Care Register (national coverage, 2001-2012). Furthermore, we used information from the new PCR, a research data set including individual-level information, such as diagnoses based on visits to primary health care centers, from the following Swedish counties: Blekinge (2009-2016), Värmland (2005-2015), Kalmar (2007-2016), Södermanland (1997-2017), Uppsala (2005-2015), Västernorrland (2008-2015), Norrbotten (2009-2016), Gävleborg (2010-2016), Halland (2007-2014), Jönköping (2008-2014), Kronoberg (2006-2016), Skåne (1998-2013), Östergötland (1997-2014), Stockholm (2003-2016), and Västergötland (2000-2013). The periods differ because of the timing of digitalizing of patient records. In 2016, these 15 counties (of 21) contained 87% of the Swedish population. We secured ethical approval for this study from the regional ethical review board of Lund University, Sweden, which waived the need for consent for this study of deidentified data.
We identified MD in the hospital discharge and outpatient (specialist) care registries and the PCR by the following codes: 296.2, 298.0, and 300.4 from the International Classification of Diseases, Eighth Revision (ICD-8); 296.2, 296.4, 298.0, and 300.4 from the International Classification of Diseases, Ninth Revision (ICD-9); and F32 and F33 from International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10). The MD diagnosis could be registered at any time. Individuals registered with schizophrenia (ICD-8 and ICD-9: 295; ICD-10: F20) and/or bipolar disorder (ICD-8: 296.1 and 296.3; ICD-9: 296.0, 296.1, and 296.4-8; ICD-10: F30 and F31) were censored from the sample.
The database was created by entering all individuals in the Swedish population born in Sweden from January 1, 1960, through December 31, 1990, and included the number of years from ages 0 to 15 years that individuals resided in the same household and geographical area as their biological mother, biological father, and possible stepfather. These individuals, the offspring in our study, were followed up through December 31, 2016, and were then aged 26 to 56 years. Geographical area was defined by small areas for market statistics (SAMS). Sweden has approximately 9200 SAMS, each with an average population of 1000 persons. From 1960 to 1985 (every fifth year), we used household identification and geographical status from the Population and Housing Census to define family types. The household identification includes all individuals living in the same dwelling. From 1986 onward (every year), we defined family type using the family identification and geographical status from the Total Population Register. The family identification is defined by related or married individuals registered at the same property. Furthermore, adults registered at the same property who have common children but are not married are registered in the same family. We created family types by investigating with whom the offspring shared the same household and family identification when they were aged 0 to 15 years. In the detection of stepparents during 1986 and onward, for an offspring living with his or her mother, we only captured the stepfather if he was married to the mother of the offspring and/or had a common child with the mother. For the years without this information, we approximated the household and geographical status with the information from the closest year.
We thereby defined 5 types of families. First, intact families that included offspring residing from ages 0 to 15 years in the same household with the biological mother and father. Second, families with NLW fathers included offspring who never resided in the same household or SAMS as the biological father. Third, families with stepfathers included offspring who did not reside from ages 0 to 15 years with the biological father and resided at least 10 years of this period with a nonbiologically related man 18 to 50 years older. Fourth, adoptive families included offspring adopted at younger than 5 years, with information available on both adoptive parents and at least 1 biological parent. Individuals adopted by biological relatives or an adoptive parent living with a biological parent were excluded from this sample, and to maximize our number of adoptees, we included offspring born from 1955 through 1990. The NLW fathers and stepfathers were defined so that their relationships with their offspring resembled those seen between an adoptee and his or her biological and adoptive parents, respectively. Fifth, triparental families included in a single family all 3 key parent-offspring relationships; offspring resided from ages 0 to 15 years in the same household with their biological mother, never resided with their biological father, and resided with a stepfather for at least 10 years. Triparental families are therefore not unique and represented an intersection of the families with NLW fathers and stepfamilies. To avoid redundancy, triparental families were not included in joint analyses of family types but were analyzed separately because of their particular informativeness.
We examined the tetrachoric correlation for MD in our parent-offspring pairs because this measure of association is easy to interpret in a genetic-epidemiologic context and is insensitive to changes in base rates. To combine results from the different samples, we used the Olkin-Pratt meta-analytic approach. We calculated the combined correlations and the P values for the heterogeneity tests that evaluate the null hypothesis that effects are similar across samples.
Last, we investigated the interaction of genes and rearing. This analysis was performed in the adoptive sample in which we combined the effects of biological and adoptive parents to maximize power. To assess the interaction, we used a regression model, with MD in offspring as the dependent variable and MD in biological parents, MD in adoptive parents, and their interaction as independent variables using the identity link. The interaction term was then measured on the additive scale. Power was limited to detect subtle interactions. Two prior analyses of Swedish adoptive data attempted similar analyses, and one detected a significant interaction.
The statistical analyses were performed using SAS, version 9.4 (SAS Institute). We used Bonferroni corrections for comparisons of differences of correlations across the types of parent-offspring pairs.
Results
Sample
The study population included 2 269 552 offspring (51.5% male and 48.5% female; median age, 42; range, 25-56 years). Table 1 provides the sample size of offspring in our 5 informative family types and the prevalence of MD in offspring and parents. Prevalence rates of MD in offspring and biological parents were about 50% lower in the intact families than in the NLW father, stepfather, adoptive, and triparental families. However, prevalence rates for MD in stepparents and adoptive parents were similar to rates in intact families. The female to male prevalence ratio was similar in the offspring (mean, 1.84; SEM, 0.03) and parental generations (mean, 1.75; SEM, 0.03). Table 2 provides the sources of ascertainment of our cases of MD in the offspring and parents in our entire study population. Of note, 64.0% of affected offspring, 63.5% of mothers, and 54.5% of fathers were ascertained solely through the PCR.
Table 1. Prevalence of MD in Parents and Offspring From 5 Family Types.
| Family Member | Family Type, Prevalence of MD, % | ||||
|---|---|---|---|---|---|
| Intact (n = 2 041 816) |
NLW Father (n = 116 601) |
Stepfather (n = 67 826) |
Adoptive (n = 14 104) |
Triparental Father (n = 29 205) |
|
| All offspring | 10.2 | 17.0 | 15.4 | 15.6 | 16.0 |
| All female offspring | 13.7 | 21.8 | 19.9 | 20.4 | 21.0 |
| All male offspring | 7.1 | 12.4 | 11.0 | 11.3 | 11.1 |
| Biological mother | 11.7 | 21.2 | 17.3 | 16.1 | 18.1 |
| Biological father | 6.6 | 11.4 | 10.7 | 9.2 | 11.1 |
| Stepmother or adoptive mother | NA | NA | NA | 13.4 | NA |
| Stepfather or adoptive father | NA | NA | 8.1 | 6.6 | 8.1 |
Abbreviations: MD, major depression; NA, not applicable; NLW, not-lived-with.
Table 2. Sources of Ascertainment of Cases of MD in the Entire Population Sample.
| Registry | Percentage of Sample | ||
|---|---|---|---|
| Offspring | Mothers | Fathers | |
| Onlya | |||
| PCR + inpatient + specialist care | 3.6 | 3.5 | 4.0 |
| PCR + inpatient | 1.8 | 4.4 | 4.0 |
| PCR + specialist care | 13.1 | 7.7 | 7.3 |
| Inpatient + specialist care | 2.8 | 2.7 | 3.6 |
| PCR only | 64.0 | 63.5 | 54.5 |
| Inpatient only | 3.3 | 11.1 | 18.5 |
| Specialist only | 11.5 | 6.9 | 8.3 |
| Everb | |||
| PCR | 82.5 | 79.1 | 69.8 |
| Inpatient | 11.5 | 21.7 | 30.1 |
| Specialist care | 31.0 | 20.8 | 23.2 |
Abbreviations: MD, major depression; PCR, primary care register.
Cases were found only in the specific registries or combinations of registries; thus, total sums to near 100% (percentages have been rounded).
Cases were ever found by this register; thus, total sums to greater than 100%, given that many cases were detected by more than 1 register.
Cross-Generational Transmission of Risk to MD
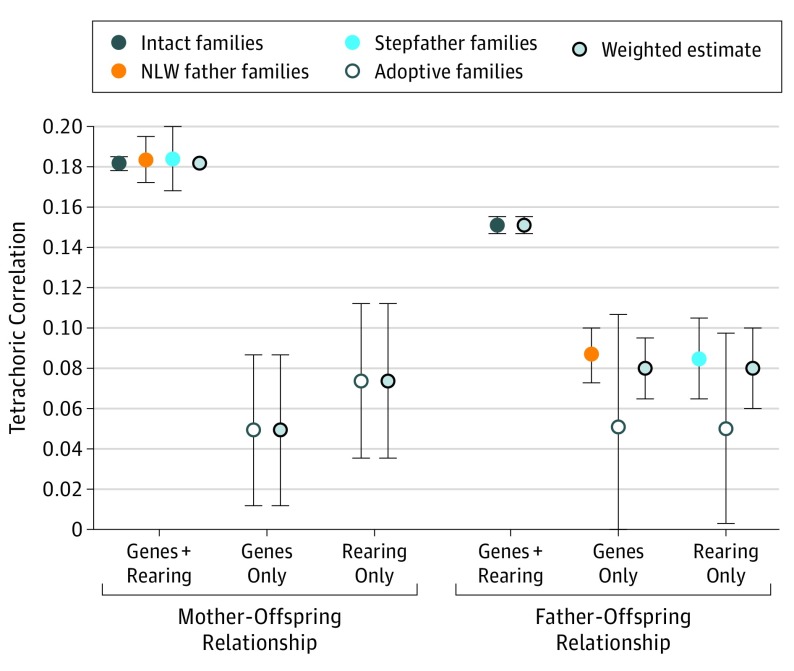
Parent-offspring tetrachoric correlations for MD across our 4 independent family types (excluding triparental families) are seen in Table 3 and the Figure. We had 3 informative samples for the genes plus rearing mother-offspring association, all with correlations r = 0.18 (95% CIs for intact, NLW father, and stepfather: 0.18-0.19, 0.17-0.19, and 0.17-0.20, respectively). However, only 1 estimate was available for fathers (from intact families), r = 0.15 (95% CI, 0.15-0.16). For genes-only and rearing-only associations with mothers, we had 1 informative sample each from the adoptive families estimated at r = 0.05 (95% CI, 0.01-0.09) and r = 0.08 (95% CI, 0.04-0.11). For fathers, 2 samples each were informative for the genes-only and rearing-only associations, with correlations of r = 0.05 (95% CI, −0.01 to 0.11 and 0.00 to 0.10) for adoptive families, r = 0.09 (95% CI, 0.07-0.11; genes-only association) for NLW father families, and r = 0.09 (95% CI, 0.07-0.11; rearing-only association) for stepfamilies.
Table 3. Parent-Offspring Tetrachoric Correlations for MD Across the Major Family Types.
| Relationship by Source of Resemblance | Family Type, Tetrachoric Correlation (95% CI) | Weighted Estimate Across Families, Tetrachoric Correlation (95% CI) | P Value for Test of Heterogeneity Across Families | |||
|---|---|---|---|---|---|---|
| Intact | NLW Father | Stepfather | Adoptive | |||
| Mother-offspring | ||||||
| Genes + rearing | 0.18 (0.18 to 0.19) | 0.18 (0.17 to 0.19) | 0.18 (0.17 to 0.20) | NA | 0.18 (0.18 to 0.18) | .91 |
| Genes only | NA | NA | NA | 0.05 (0.01 to 0.09) | 0.05 (0.01 to 0.09) | NA |
| Rearing only | NA | NA | NA | 0.08 (0.04 to 0.11) | 0.08 (0.04 to 0.11) | NA |
| Father-offspring | ||||||
| Genes + rearing | 0.15 (0.15 to 0.16) | NA | NA | NA | 0.15 (0.15 to 0.16) | NA |
| Genes only | NA | 0.09 (0.07 to 0.10) | NA | 0.05 (−0.01 to 0.11) | 0.08 (0.07 to 0.10) | .18 |
| Rearing only | NA | NA | 0.09 (0.07 to 0.11) | 0.05 (0.00 to 0.10) | 0.08 (0.06 to 0.10) | .18 |
Abbreviations: MD, major depression; NA, not applicable; NLW, not-lived-with.
Figure. Parent-Offspring Tetrachoric Correlations for Major Depression.
Correlations are shown across the major family types and include weighted estimates. NLW indicates not-lived-with.
Table 3 and the Figure also present weighted correlation estimates for associations for which we had more than 1 sample. None were statistically heterogeneous. For mothers, the genes plus rearing association was estimated at r = 0.18 (95% CI, 0.18-0.18). The father-offspring genes-only association was estimated at r = 0.08 (95% CI, 0.07-0.10); the rearing-only association, r = 0.08 (95% CI, 0.06-0.10).
Table 4 presents weighted estimates of our results across mothers and fathers, with a heterogeneity test. One significant difference was found. The genes plus rearing association was significantly stronger for mother-offspring than for father-offspring relationships. The aggregate-weighted estimates for the correlation of MD between parents and offspring were r = 0.17 (95% CI, 0.16-0.17) for genes plus rearing, r = 0.08 (95% CI, 0.06-0.09) for genes-only, and r = 0.08 (95% CI, 0.07-0.09) for rearing-only associations. Of interest, we found suggestive evidence that genetic associations were stronger in father-offspring than mother-offspring relationships.
Table 4. Comparison of Mother-Offspring and Father-Offspring Tetrachoric Correlations for MD Across Major Family Types.
| Family Type by Source of Resemblance | Tetrachoric Correlation (95% CI) | Uncorrected P Value for Test of Heterogeneity | ||
|---|---|---|---|---|
| Mothers | Fathers | Weighted Estimate | ||
| Intact | ||||
| Gene + rearing | 0.18 (0.18-0.19) | 0.15 (0.15-0.16) | 0.17 (0.16-0.17) | <.001a |
| NLW father | ||||
| Gene + rearing | 0.18 (0.17-0.19) | NA | 0.18 (0.17-0.19) | NA |
| Genes only | 0.09 (0.07-0.10) | 0.09 (0.07-0.10) | NA | |
| Stepfamilies | ||||
| Gene + rearing | 0.18 (0.17-0.20) | NA | 0.18 (0.17-0.20) | NA |
| Rearing only | NA | 0.09 (0.07-0.11) | 0.09 (0.07-0.11) | NA |
| Adoptive | ||||
| Genes only | 0.05 (0.01-0.09) | 0.05 (-0.01-0.11) | 0.05 (0.02-0.08) | .96 |
| Rearing only | 0.08 (0.04-0.11) | 0.05 (0.00-0.10) | 0.07 (0.04-0.10) | .40 |
| Weighted estimate across all family types | ||||
| Gene + rearing | 0.18 (0.18-0.18) | 0.15 (0.15-0.16) | 0.17 (0.16-0.17) | <.001 |
| Genes only | 0.05 (0.01-0.09) | 0.08 (0.07-0.10) | 0.08 (0.06-0.09) | .02 |
| Rearing only | 0.08 (0.04-0.11) | 0.08 (0.06-0.10) | 0.08 (0.07-0.09) | .99 |
Abbreviations: MD, major depression; NA, not applicable; NLW, not-lived-with.
Significance threshold after Bonferroni correction for 5 tests was P < .01.
Nature of the Relationship Between Genetic and Rearing Effects
Our best sample for assessing the additivity of genetic and rearing effects was triparental families that contained, in a single family unit, the 3 key parent-offspring relationships. In these families, parent-offspring correlations for MD were r = 0.19 (95% CI, 0.17-0.22) for mother-offspring relationships, which included genes plus rearing; r = 0.10 (95% CI, 0.07-0.13) for NLW father-offspring relationships, which included genes only; and r = 0.08 (95% CI, 0.05-0.11) for stepfather-offspring relationships, which reflected a rearing-only effects.
Our best sample for assessing gene and rearing interactions was adoptive families. Using an additive scale and combining the effects of biological and adoptive parents to maximize power, we found significant genetic (β = 0.021; 95% CI, 0.005-0.038) and rearing (β = 0.036; 95% CI, 0.019-0.054) associations but no significant interaction (β = −0.002; 95% CI, −0.040 to 0.036).
Discussion
Our goal was to address in, to our knowledge, the first large-scale adoption study of MD, 2 key questions about the magnitude and nature of the cross-generational transmission of this disorder.
Genes and Environment in Parent-Offspring Transmission
In accordance with numerous prior studies, we found substantial resemblance for MD in parents and children from intact families. Consistent with 2 of the 4 prior MD adoption studies, we also found evidence of genetic transmission of MD risk across generations. However, contrary to the single prior classic adoption study that examined this question but consistent with the findings in adolescents by Tully et al, we found significant resemblance for MD between adoptive parents (and stepparents) and the children who they reared.
Our evidence for environmental transmission of risk for clinically diagnosed MD is also supported indirectly by a number of prior studies. The literature has documented the adverse psychiatric effects of being reared by parents with depression. More critically, 2 different genetic-epidemiologic designs have provided evidence of environmental contributions to the cross-generational transmission of depression. Three studies using a children-of-twins design (methodologic details are found in McAdams et al) found evidence of parent-offspring environmental transmission of depression: 1 for DSM-III-R major depression and 2 for depressive symptoms. Using a novel assisted-conception design, Lewis et al and Harold et al found substantial correlations for depressive symptoms among genetically unrelated mother-child dyads. Finally, an adoption study found significant correlations between the levels of neuroticism in adoptive mothers and depressive symptoms in their offspring during middle childhood.
Our findings appear to be inconsistent with twin studies that have not detected a role for the shared environment in MD. However, shared environment from twin studies only assesses parental behaviors that are consistent across children (key parenting behaviors are only moderately correlated within twin pairs) and includes any other environmental factors (eg, peer, sibling, and community) that affect twin resemblance. Adoption studies provide a more direct assessment of the effect of parental behaviors on offspring. Furthermore, because of the substantial genetic effects, twin studies are not well powered to detect moderate shared environmental effects.
Joint Impact of Genes and Environment
We took 2 approaches to understanding how the genetic and rearing influences from parents acted together in affecting risk for MD in their children. First, we examined a natural experiment, triparental families, which contained all 3 kinds of parent-child relationship reflecting genes plus rearing (mother), genes-only (NLW father), and rearing-only (stepfather). Expressed as tetrachoric correlations, the effects of genes and rearing environment were additive; the sum of the NLW fathers (r = 0.10) and stepfathers (r = 0.08) nearly equaled that of the rearing biological mother (r = 0.19). These results were similar to those found in our global analysis of our 4 family types, in which the summary correlations for parent-offspring relationships reflecting genes only (r = 0.08) and rearing only (r = 0.08) were nearly identical to that obtained for genes plus rearing (r = 0.17). These analyses suggest that the parental genetic and environmental contributions to risk for MD in their offspring act largely additively.
Second, the best natural experiment for statistically evaluating interactions of genetics by rearing environment was the adoptive family. In these analyses, conducted on an additive scale, we found main effects for genes and main effects for parental rearing effects, but no evidence of interactions between them. Both our lines of evidence suggest that the effect impact of parental genes and rearing on risk for MD in their offspring is an additive one.
Other Concerns
Most cases of MD in our study in parents and offspring were ascertained through the PCR. We previously examined the validity of these diagnoses and found them to be supported by (1) expected patterns of comorbidity (eg, high co-occurrence with anxiety disorders); (2) a high proportion of cases treated with antidepressants (79.4%); (3) a tetrachoric correlation in full siblings (r = 0.15) similar to that found for lifetime MD assessed by structured psychiatric interview in female-female, male-male, and male-female Swedish dizygotic twins (r = 0.16, r = 0.11, and r = 0.11, respectively); and (4) expected epidemiologic associations with the female sex, lower parental educational attainment, reduced educational attainment and rates of marriage, and increased risk for divorce. To evaluate whether MD diagnosed in the PCR was less familial than MD diagnosed in specialist or inpatient care, we examined in intact families whether the risk for MD in the offspring was increased if the parent had an MD diagnosis recorded in the specialist or inpatient registries vs in the PCR. No such pattern was seen in mothers or fathers (odds ratios, 1.00 [95% CI, 0.98-1.02] and 0.96 [95% CI, 0.93-0.99], respectively).
Because narrow sense heritability can be estimated by doubling the genes-only parent-offspring correlation, our study estimated this form of heritability of MD to be only approximately 16%, lower than that calculated from twin studies. Other studies showed substantially lower heritability estimates from adoption compared with twin studies for alcohol use disorder, externalizing antisocial behaviors, and personality. Many possible causes exist for this difference, one of which is a gene and age interaction or a gene and cohort interaction, because twins are the same age and parents and offspring will always differ by several decades in age and are often exposed to different historical conditions.
Limitations
These results should be interpreted in the context of 5 potentially important methodologic limitations. First, this study is restricted to persons with treated depression in Sweden and may not extrapolate to other samples. Second, we obtained our participants from hospital, specialist, and primary care registries. Registry data require neither participant cooperation nor accurate recall. However, these data can produce false-negative and false-positive diagnoses, the nature of which is difficult to estimate. Sweden has no large-scale, interview-based psychiatric epidemiologic study with which to compare our treated prevalence rates. However, an epidemiologic survey in Norway provided a lifetime estimate of MD of 17.8%, and 42 161 interviewed twins from the birth certificate–based Swedish twin registry produced an estimated lifetime prevalence of MD of 19.5%. Determining whether such incomplete ascertainment is more likely to attenuate or upwardly bias estimates of parent-child resemblance is difficult.
Third, our 3 registries were active during different time frames, with the PCR covering the shortest period. Therefore, the parent and offspring samples in our study are not completely comparable because a higher proportion of parents were ascertained through inpatient care. Fourth, our adoptive sample was slightly older than our other samples. We examined whether restricting this sample to the years of birth of the other samples (1960-1990) systematically changed our results; it did not. Fifth, we cannot rule out that NLW fathers had contact with their offspring that might have increased their resemblance for MD.
Conclusions
Using an extended adoption design applied to the Swedish general population and nearly complete ascertainment of treated MD in parents and offspring, we found that parent-offspring transmission of risk for MD is the result of genetic factors and rearing experiences to an approximately equal degree. These 2 forms of cross-generational transmission appear to act additively on offspring MD risk. Although much of the genetic research on MD has recently focused on the role of molecular variants, our results suggest that environmental processes also affect appreciably the tendency for MD to aggregate within families. A complete understanding of the familial transmission of MD will require projects that assess carefully genetic and familial-environmental processes.
References
- 1.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552-1562. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163(1):109-114. [DOI] [PubMed] [Google Scholar]
- 3.Wender PH, Kety SS, Rosenthal D, Schulsinger F, Ortmann J, Lunde I. Psychiatric disorders in the biological and adoptive families of adopted individuals with affective disorders. Arch Gen Psychiatry. 1986;43(10):923-929. [DOI] [PubMed] [Google Scholar]
- 4.Cadoret RJ, O’Gorman TW, Heywood E, Troughton E. Genetic and environmental factors in major depression. J Affect Disord. 1985;9(2):155-164. [DOI] [PubMed] [Google Scholar]
- 5.von Knorring AL, Cloninger CR, Bohman M, Sigvardsson S. An adoption study of depressive disorders and substance abuse. Arch Gen Psychiatry. 1983;40(9):943-950. [DOI] [PubMed] [Google Scholar]
- 6.Cadoret RJ. Evidence for genetic inheritance of primary affective disorder in adoptees. Am J Psychiatry. 1978;135(4):463-466. [DOI] [PubMed] [Google Scholar]
- 7.Tully EC, Iacono WG, McGue M. An adoption study of parental depression as an environmental liability for adolescent depression and childhood disruptive disorders. Am J Psychiatry. 2008;165(9):1148-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kendler KS, Sundquist K, Ohlsson H, et al. Genetic and familial environmental influences on the risk for drug abuse: a national Swedish adoption study. Arch Gen Psychiatry. 2012;69(7):690-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendler KS, Ji J, Edwards AC, Ohlsson H, Sundquist J, Sundquist K. An extended Swedish national adoption study of alcohol use disorder. JAMA Psychiatry. 2015;72(3):211-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song J, Bergen SE, Kuja-Halkola R, Larsson H, Landén M, Lichtenstein P. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;17(2):184-193. [DOI] [PubMed] [Google Scholar]
- 11.Petersen L, Sørensen TI, Kragh Andersen P, Mortensen PB, Hawton K. Genetic and familial environmental effects on suicide attempts: a study of Danish adoptees and their biological and adoptive siblings. J Affect Disord. 2014;155:273-277. [DOI] [PubMed] [Google Scholar]
- 12.Hjalmarsson R, Lindquist MJ. The origins of intergenerational associations in crime: lessons from Swedish adoption data. Labour Econ. 2013;20:68-81. [Google Scholar]
- 13.Olfson M, Blanco C, Marcus SC. Treatment of adult depression in the United States. JAMA Intern Med. 2016;176(10):1482-1491. [DOI] [PubMed] [Google Scholar]
- 14.Sundquist J, Ohlsson H, Sundquist K, Kendler KS. Common adult psychiatric disorders in Swedish primary care where most mental health patients are treated. BMC Psychiatry. 2017;17(1):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendler KS, Ohlsson H, Sundquist J, Sundquist K. Triparental families: a new genetic-epidemiological design applied to drug abuse, alcohol use disorders, and criminal behavior in a Swedish national sample. Am J Psychiatry. 2015;172(6):553-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falconer DS. Introduction to Quantitative Genetics. 3rd ed New York, NY: Wiley; 1989. [Google Scholar]
- 17.Babchishin KM, Helmus LM. The influence of base rates on correlations: an evaluation of proposed alternative effect sizes with real-world data. Behav Res Methods. 2016;48(3):1021-1031. [DOI] [PubMed] [Google Scholar]
- 18.Schulze R. Meta-analysis: A Comparison of Approaches. Gottingen, Germany: Hogrefe & Huber Publishers; 2004. [Google Scholar]
- 19.SAS Institute, Inc SAS/STAT User’s Guide, Version 9.3. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 20.Abdi H. Bonferroni and Sidak corrections for multiple comparisons In: Salkind NJ, ed. Encyclopedia of Measurement and Statistics. Thousand Oaks, CA: Sage; 2007:103-107. [Google Scholar]
- 21.Weissman MM, Gammon GD, John K, et al. Children of depressed parents: increased psychopathology and early onset of major depression. Arch Gen Psychiatry. 1987;44(10):847-853. [DOI] [PubMed] [Google Scholar]
- 22.Weissman MM, Warner V, Wickramaratne P, Moreau D, Olfson M. Offspring of depressed parents: 10 years later. Arch Gen Psychiatry. 1997;54(10):932-940. [DOI] [PubMed] [Google Scholar]
- 23.Radke-Yarrow M, Martinez P, Mayfield A, Ronsaville D. Children of Depressed Mothers: From Early Childhood to Maturity. New York, NY: Cambridge University Press; 1998. [Google Scholar]
- 24.Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychol Rev. 1999;106(3):458-490. [DOI] [PubMed] [Google Scholar]
- 25.Natsuaki MN, Shaw DS, Neiderhiser JM, et al. Raised by depressed parents: is it an environmental risk? Clin Child Fam Psychol Rev. 2014;17(4):357-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAdams TA, Neiderhiser JM, Rijsdijk FV, Narusyte J, Lichtenstein P, Eley TC. Accounting for genetic and environmental confounds in associations between parent and child characteristics: a systematic review of children-of-twins studies. Psychol Bull. 2014;140(4):1138-1173. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. Revised Third Edition Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 28.Singh AL, D’Onofrio BM, Slutske WS, et al. Parental depression and offspring psychopathology: a Children of Twins study. Psychol Med. 2011;41(7):1385-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silberg JL, Maes H, Eaves LJ. Genetic and environmental influences on the transmission of parental depression to children’s depression and conduct disturbance: an extended Children of Twins study. J Child Psychol Psychiatry. 2010;51(6):734-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAdams TA, Rijsdijk FV, Neiderhiser JM, et al. The relationship between parental depressive symptoms and offspring psychopathology: evidence from a children-of-twins study and an adoption study. Psychol Med. 2015;45(12):2583-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis G, Rice F, Harold GT, Collishaw S, Thapar A. Investigating environmental links between parent depression and child depressive/anxiety symptoms using an assisted conception design. J Am Acad Child Adolesc Psychiatry. 2011;50(5):451-459.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harold GT, Rice F, Hay DF, Boivin J, van den Bree M, Thapar A. Familial transmission of depression and antisocial behavior symptoms: disentangling the contribution of inherited and environmental factors and testing the mediating role of parenting. Psychol Med. 2011;41(6):1175-1185. [DOI] [PubMed] [Google Scholar]
- 33.Eley TC, Deater-Deckard K, Fombonne E, Fulker DW, Plomin R. An adoption study of depressive symptoms in middle childhood. J Child Psychol Psychiatry. 1998;39(3):337-345. [PubMed] [Google Scholar]
- 34.Kendler KS. Parenting: a genetic-epidemiologic perspective. Am J Psychiatry. 1996;153(1):11-20. [DOI] [PubMed] [Google Scholar]
- 35.Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. Behav Genet. 1994;24(3):239-258. [DOI] [PubMed] [Google Scholar]
- 36.Kessler RC, Berglund P, Demler O, et al. ; National Comorbidity Survey Replication . The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095-3105. [DOI] [PubMed] [Google Scholar]
- 37.Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62(10):1097-1106. [DOI] [PubMed] [Google Scholar]
- 38.Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med. 2015;45(5):1061-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychol Bull. 2002;128(3):490-529. [PubMed] [Google Scholar]
- 40.Vukasović T, Bratko D. Heritability of personality: a meta-analysis of behavior genetic studies. Psychol Bull. 2015;141(4):769-785. [DOI] [PubMed] [Google Scholar]
- 41.Kringlen E, Torgersen S, Cramer V. A Norwegian psychiatric epidemiological study. Am J Psychiatry. 2001;158(7):1091-1098. [DOI] [PubMed] [Google Scholar]
- 42.Horn JM, Loehlin JC, Willerman L. Intellectual resemblance among adoptive adoptive and biological relatives: the Texas adoption project. Behav Genet. 1979;9(3):177-201. [DOI] [PubMed] [Google Scholar]
- 43.Hyde CL, Nagle MW, Tian C, et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet. 2016;48(9):1031-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]